Abstract
Rationale: Uninvolved normal-appearing airway epithelium has been shown to exhibit specific mutations characteristic of nearby non–small cell lung cancers (NSCLCs). Yet, its somatic mutational landscape in patients with early-stage NSCLC is unknown.
Objectives: To comprehensively survey the somatic mutational architecture of the normal airway epithelium in patients with early-stage NSCLC.
Methods: Multiregion normal airways, comprising tumor-adjacent small airways, tumor-distant large airways, nasal epithelium and uninvolved normal lung (collectively airway field), matched NSCLCs, and blood cells (n = 498) from 48 patients were interrogated for somatic single-nucleotide variants by deep-targeted DNA sequencing and for chromosomal allelic imbalance events by genome-wide genotype array profiling. Spatiotemporal relationships between the airway field and NSCLCs were assessed by phylogenetic analysis.
Measurements and Main Results: Genomic airway field carcinogenesis was observed in 25 cases (52%). The airway field epithelium exhibited a total of 269 somatic mutations in most patients (n = 36) including key drivers that were shared with the NSCLCs. Allele frequencies of these acquired variants were overall higher in NSCLCs. Integrative analysis of single-nucleotide variants and allelic imbalance events revealed driver genes with shared “two-hit” alterations in the airway field (e.g., TP53, KRAS, KEAP1, STK11, and CDKN2A) and those with single hits progressing to two in the NSCLCs (e.g., PIK3CA and NOTCH1).
Conclusions: Tumor-adjacent and tumor-distant normal-appearing airway epithelia exhibit somatic driver alterations that undergo selection-driven clonal expansion in NSCLC. These events offer spatiotemporal insights into the development of NSCLC and, thus, potential targets for early treatment.
Keywords: normal airway, cancerization field, early-stage non–small cell lung cancer, deep targeted sequencing, allelic imbalance
At a Glance Commentary
Scientific Knowledge on the Subject
“Field cancerization” in non–small cell lung cancer (NSCLC) was originally coined based on observations of histologic abnormalities in tumor-adjacent normal tissues. Work thus far has identified gene expression modifications, chromosomal aberrations, and, to a limited extent, single-gene mutations in tumor-adjacent and distant airway epithelium. We sought to comprehensively study the somatic mutation landscape of normal-appearing airway epithelia in patients with early-stage NSCLC, which currently remains largely unexplored.
What This Study Adds to the Field
This work identifies somatic driver mutational processes in the adjacent and distant normal-appearing airway of patients with early-stage NSCLC. We have developed computational innovations to identify somatic DNA alterations in these normal tissues that exist at low mutant cell fractions. Our study of multiple tissues from individual patients points toward the future of genomic investigations in medicine, where evolutionary trajectories can be inferred from diverse spatial or temporal sampling. The molecular changes we detect in these normal tissues present as early alterations in the transition to the malignant phenotype of NSCLC and thus serve as potential targets for early detection and treatment.
A cancerization field was first proposed by Slaughter and colleagues (1) following the observation that histologic abnormalities were widespread with an epithelial field adjacent to and surrounding tumors. The cancerized field was shown to comprise normal-appearing cells exhibiting tumor-associated molecular alterations that are, thus, highly pertinent to tumor initiation or progression. Although this phenomenon was first described in oral cancer, accumulating evidence supports the presence of cancerized fields in other malignancies, such as of the stomach, skin, esophagus, liver, cervix, and lung (2–4).
Lung cancer, particularly non–small cell lung cancer (NSCLC), is the leading cause of cancer-related deaths worldwide (5). There are very few targeted strategies to treat NSCLC at its primitive stages largely because of poor understanding of early molecular aberrations in development of its two major subtypes, lung adenocarcinoma (LUAD) and squamous cell carcinoma (LUSC) (6). Previous studies described expression profiles in the cancerization field of normal-appearing airway including those shared with adjacent NSCLCs (6, 7). A study of histologically normal, premalignant and malignant epithelia of LUSCs identified multiple sequentially occurring chromosomal deletions, such as loss of heterozygosity (LOH) of 3p and 9q, in the multistage progression of LUSCs (6). Deletions at chromosome arms 2q and 12p were also identified in the bronchial epithelium of smokers (8). Mutations in known lung cancer drivers, such as EGFR and KRAS, were described in normal respiratory epithelium and nonmalignant lung tissues (9, 10). A recent multiregion study by our group provided evidence for chromosomal alterations, leading to allelic imbalance (AI), including frequent and recurrent events on chromosome 9 in the airway epithelium of patients with early-stage NSCLC (11).
Although these studies shed light on molecular alterations in histologically normal tissues, the landscape of somatic driver mutations in the normal-appearing airway in NSCLC still remains largely unknown. Here, we surveyed the somatic mutational landscape of spatially distributed multiregion samples comprising the normal-appearing airway cancerization field and paired NSCLCs. We observed genomic airway field carcinogenesis in about half of the patients studied. We also report somatic single-nucleotide variant (SNV) and AI profiles common to both normal airways and NSCLCs including driver alterations that likely denote the molecular evolution of NSCLC.
Some of the results of this study have been previously reported in the form of abstracts (12–14).
Methods
Normal Airway and NSCLC Samples and Cohort
Multiregion samples comprising tumor-adjacent small airways, tumor-distant ipsilateral large airways, nasal epithelium, normal lung tissue, peripheral blood cells, and NSCLC tumors were obtained from 48 early-stage patients (stages IA–IIIA; 37 LUADs and 11 LUSCs; 42 ever-smokers and 6 nonsmokers) who were evaluated at The University of Texas MD Anderson Cancer Center. All 48 patients did not receive neoadjuvant therapy or any therapy for at least a year before surgery. Demographic and clinicopathologic data for all cases are summarized in Table E1 in the online supplement. The study was approved by the institutional review board and all patients provided written informed consent. A breakdown of samples obtained from each patient is summarized in Table E2. Detailed procedures on sample collection and evaluation are provided in the online supplement.
Deep-targeted DNA Sequencing
DNA was extracted from fresh-frozen samples using QIAamp DNA kit from Qiagen according to the manufacturer’s instructions. Sequencing of 409 cancer-associated genes in the Ion AmpliSeq Comprehensive Cancer Panel was performed in the manner we previously reported using the Ion Torrent Proton platform (15). Sequencing data files have been deposited in the sequence read archive under Bioproject accession PRJNA453609.
Somatic Mutation Detection and Analysis
Somatic mutations were rigorously identified in the manner we recently reported (15, 16) based on four different callers. For a mutation to be admissible, it must have been identified by at least two different callers in at least one sample for a patient. SNVs in exonic, splicing, and untranslated regions within the cancer gene panel were assessed. SNVs were also studied for patterns in base substitutions and mutational signatures using the R package deconstructSigs. Nonsynonymous mutations were also studied a priori in bona fide NSCLC (17, 18) and cancer driver genes (19).
Allelic Imbalance Detection and Analysis
We reported AI profiles on 39 of the 48 early-stage NSCLC cases (11). AI profiles in the additional nine patients were analyzed using hapLOH and chromosomal aberrations were characterized in the manner we previously reported (11). SNVs from the sequencing data were then integrated with AI profiles.
Statistical and Phylogenetic Analysis of the Cancerization Field in the Normal-Appearing Airway
For each patient, similarity measures were computed based on shared SNVs and AI between NSCLCs and each matched airway field sample. Normal field samples were sorted based on this measure and assigned equidistant numeric values. A field cancerization area under the curve measure (FCAUC) was then computed for all cases using DescTools package. All patients with a nonzero FCAUC were identified as potential exhibitors of field effects. Of these, cases with a visually pronounced FCAUC were interrogated for specific patterns. The presence of a spatial gradient of variant allele frequencies (VAFs) in each patient was also tested. Samtools was used to obtain forced VAFs for field tissues from allelic depths at each mutated locus in the matched tumor. A “field effect” slope was calculated by regressing VAFs on arbitrarily assigned distances for the patient’s field samples (ordered by distance from tumor: S1–S5, L, N, and Na). The mean slope across all 48 patients was calculated. This mean was then tested for statistical significance using permutation analysis by altering the field tissue order in each iteration. Because of the lack of a natural null distribution for our test statistic, a conservative randomization approach robust to model misspecification was used. Phylogenetic trees were constructed from somatic SNVs and AIs. Distance matrices were generated using dist.gene from the ape R package after which unrooted neighbor joining trees were drawn. Trees for each patient were also constructed separately for SNVs and AI events.
Additional details are provided in the online supplement.
Results
Deep Sequencing and Genotype Profiling of Multiregion Normal-Appearing Airway Epithelia in Early-Stage NSCLC
We surveyed mutational field processes in multiregion airway samples from 48 patients with early-stage (stages IA–IIIA) NSCLC (37 LUADs and 11 LUSCs) (see Table E1) by deep targeted DNA sequencing of a panel (n = 409) of cancer-associated genes and by high-resolution SNP array profiling. This cohort was mainly comprised of smokers (42 ever-smokers and 6 nonsmokers) (see Table E1). We studied 498 multiregion samples (450 somatic, 48 germline/control subjects) (see Table E2). These spatially distributed samples comprised the following: primary NSCLCs (T) in all but one patient; three to eight multiregion NSCLC biopsies (core needle biopsies [CNBs]) in 28 patients; normal-appearing airways adjacent to the tumor for all 48 patients (S1–S5; S1, relatively closest to NSCLC and S5, relatively farthest), and brushings of centrally located large airways (L) and of nasal epithelia (Na) for a subset of patients (21 and 24, respectively). Targeted DNA sequencing resulted in an average depth of 1,278.4× across all samples. Sequencing quality metrics for all 498 samples are summarized in Table E3. To enhance our focus on normal-appearing samples, we pooled the T and CNB samples collectively as an NSCLC set and S, L, N, and Na (airway) samples were denoted as the cancerization field.
Somatic Mutation Signatures in the Uninvolved Normal-Appearing Airway of Patients with Early-Stage NSCLC
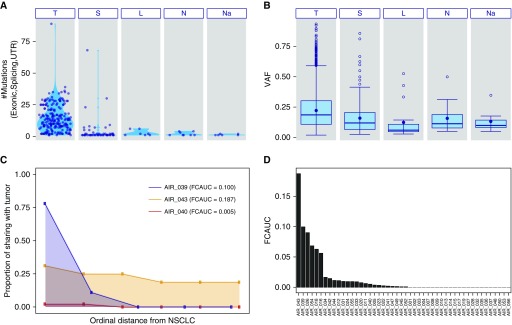
We identified 3,286 somatic mutations in 285 samples, mostly in NSCLCs (T or CNB; 3,017 mutations in 209 samples from 48 patients) (see Table E4). The mean somatic mutational burden was significantly higher in NSCLCs (13.9 mutations per sample) compared with the airway field (1.2 mutations per sample) (Figure 1A). We identified 269 somatic mutations in 76 airway field samples from 36 patients, most of which were observed in airways adjacent to the tumor (226 out of 269 field mutations) (see Table E4). The overall airway field mutation burden decreased as distance from tumors increased (S, 226; L, 19; N, 16; Na, 8 mutations), a classical “field” phenotype (Figure 1A). Further interrogation of 27 cases with both multiregion CNBs and tumor sections identified 266 mutations unique to CNBs that were missed in whole tumor sections (see Table E4). Of these, 70 mutations from five cases were shared with the airway field (see Table E4). Although mutation burdens in lifetime smoker NSCLCs were significantly higher than in nonsmokers (P < 10−15), we observed marginal evidence for this pattern in the airway field (see Figure E1). Smoker NSCLCs exhibited more tobacco-associated (signature 4 [20]) C > A base substitutions compared with nonsmoker tumors (see Figure E2). Enrichment of this signature was also observed in smoker airway field samples albeit to a lesser extent (see Figure E2). Overall, VAFs of mutations in airway samples decreased as distances from matched NSCLCs increased (Figure 1B; see Table E4). Statistical analysis (see Methods section) of the airway field VAFs showed that the net mean slope across all patients was not only negative (−0.003) but also statistically significant (P = 0.03) (see Figure E3) suggesting the presence of an overall spatial field effect, with clonal mosaicism enriched closer to NSCLC.
Figure 1.
Somatic mutation profiles in the genomic cancerization field of the normal airway in early-stage non–small cell lung cancer (NSCLC). Deep DNA sequencing and SNP array profiling analyses of 450 multiregion normal-appearing airway epithelia and NSCLCs, along with 48 germline samples, from 48 patients with early-stage NSCLC, were performed as described in the Methods and online supplement. (A) The total number of somatic single-nucleotide variants (SNVs) across the airway field comprising multiregion samples from tumor-adjacent small airways (S), distant large airways (L), nasal epithelium (Na), and uninvolved normal lung tissue (N) and their matched NSCLCs (T) are depicted. Each point represents a single sample and plots within each sample type show somatic SNV burden distributions. (B) Box plots demonstrate variant allele frequency distributions of the identified SNVs across the different types of samples. (C) Genomic airway field cancerization was quantified based on shared SNV and allelic imbalance profiles as described in the Methods and online supplement sections and summarized as field cancerization area under the curve (FCAUC: 0, lack of airway cancerization evidence and no sharing of alterations in the airway field with the tumor; 1, complete sharing of alterations between all airway field samples and matched NSCLCs). Shown here are three representative cases with relatively varied FCAUCs. The x-axis denotes an ordinal distance of airway field tissues from its matched NSCLC (0–1), and y-axis denotes the proportion of shared aberrations with the matched NSCLC (0 indicates no shared events, to 1 for complete sharing). The area under the curve (i.e., FCAUC) for each of the three cases are shaded. (D) A bar plot of FCAUCs for each patient profiled. UTR = untranslated region; VAF = variant allele frequency.
We next called chromosomal mutations (“events”) leading to AI in all cases using hapLOH (21), to infer subtle AI at a whole-genome scale (see Table E5), and integrated those with the identified somatic SNVs. NSCLCs exhibited a relatively high abundance of somatic SNVs and AI with an overall concordance in burden of these mutation types (ρ = 0.43; P < 10−10, Spearman rank test) (see Figure E4, right). Matched airway field samples also exhibited a positive relationship between AI and SNVs, albeit to a lesser extent (ρ = 0.22; P < 10−3) (see Figure E4, left). We then attempted to construct a genomic airway field phenotype (see Methods; Figure 1C). We identified 25 cases that exhibited a nonzero FCAUC, indicating evidence for shared somatic aberrations between normal-appearing airway samples and matched NSCLCs (Figure 1D).
Driver Mutated Genes in the Cancerization Field of the Normal-Appearing Airway
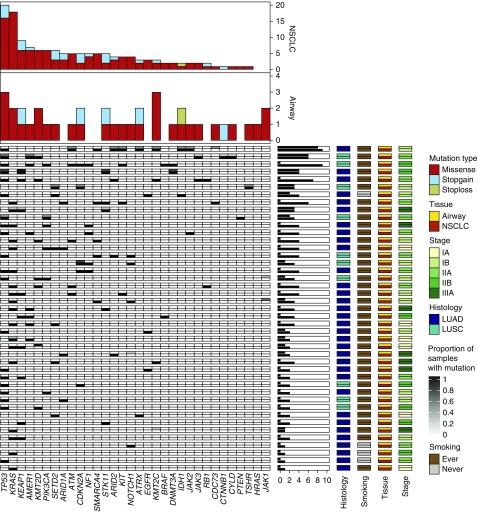
We found 28 mutated bona fide drivers (17–19), among other genes, displaying protein-damaging mutations in the airway field samples (see Table E6). TP53, KRAS, KEAP1, KMT2D, KMT2C, STK11, ATRX, IDH1, and JAK1 were mutated in normal-appearing airway samples from more than one case, with KMT2C and TP53 being most recurrently mutated in the airway field (n = 3 cases) (Figure 2; see Tables E4 and E6). Most (19 out of 28) of these genes exhibited the same mutation in matched NSCLCs, some of which were shared within multiple field samples for the patient (Figure 2; see Tables E4 and E6). NSCLC-adjacent small airways (21 samples from 13 cases) exhibited mutations in 22 driver genes. Most (17 out of 22) of these genes were also mutated in the matched NSCLCs (see Table E6). Relatively more distant large airways (n = 2) showed mutations in five genes (CDKN2A, PIK3CA, SETD2, TP53, and TSHR); all were also mutated in matched NSCLCs (see Table E6). Uninvolved normal lung tissue (n = 4) and nasal epithelium (n = 4) showed mutations in RB1, RET, TSHR, and ATK1, respectively, none of which were shared with matched NSCLCs (see Table E6). Airway field samples comprised mutations consistent with previously characterized variants specific to LUADs (KRAS, STK11, and KEAP1) (18) and LUSCs (CDKN2A, PIK3CA, and KMT2D) (17) or to both (TP53) (see Figure E5). For genes mutated in both NSCLCs and airway field samples, the observed VAFs in the tumors were higher than in field samples (see Figure E6), suggestive of selection-driven clonal expansion of the airway field.
Figure 2.
Landscape of somatic driver variants in the non–small cell lung cancer (NSCLC)-adjacent and NSCLC-distant normal airway epithelium. Deep DNA sequencing of a cancer gene panel (n = 409) and identification of somatic nonsynonymous (e.g., missense, nonsense, and stoploss) variants in all airway field and matched NSCLCs was performed as described in the Methods section. Mutated genes previously implicated as drivers in NSCLC or other malignancies (17–19) are shown for the airway field and tumor samples. Columns denote genes and rows represent individual patients. Each patient is denoted by a row with the airway field presented on the top half of the cell and the matched NSCLC in the bottom half. Mutated genes are color coded based on the proportion of airway samples carrying a variant within the gene (proportion range 0–1; white to black; right panel) and presence in the matched NSCLC (white, absent; black, present; right panel). The number of patients with the indicated driver mutated genes in the airway field and NSCLC are shown as bar plots (top panels). Annotations for stage, histology, smoking, and tissue type (airway and NSCLCs) for all patients are shown in the right panels. Patients were ordered, top to bottom, based on airway field and NSCLC somatic mutation burdens (middle horizontal barplots). LUAD = lung adenocarcinoma; LUSC = lung squamous cell carcinoma.
Early Two-Hit Oncophenotypic Alterations in the Airway Field and NSCLC
We then probed for somatic “two-hit” alterations (genes with both SNVs and within AI events). Airway field of four cases exhibited two hits in known NSCLC drivers, such as TP53/17p focal or whole-arm loss, KRAS/12p focal gain, KEAP1/19p arm loss, STK11/19p arm loss, CDKN2A/9p arm loss, and SETD2/3p arm loss (Figure 3) and that were also shared with matched NSCLCs (Figure 3). We expanded the analysis to include other known cancer-associated genes (see Figure E7) and identified additional two-hit genes in the airway field, such as CYLD/16q loss and DNMT3A/2p gain (see Table E7). We also noted 12 cases whose airway field samples exhibited single hits (AI or SNV) for driver genes, and where the matched NSCLCs exhibited two hits for the same genes resulting in “first shared hit/second tumor hit” pairs (see Table E8). These included CDKN2A/9p copy-neutral LOH (cn-LOH), PIK3CA/3q gain, TP53/17p cn-LOH, KRAS/12p focal gain, and IDH1/2q gain where the first (shared) hit is an SNV with a presumably subsequent AI event observed in matched NSCLCs; and pairs, such as 9q cn-LOH/NOTCH1, 9q cn-LOH/PTCH1, 9q cn-LOH/ABL1, 16q loss/CDH1, and 2p gain/MSH2, where the first (shared) hit is an AI event with a presumably subsequent SNV in matched NSCLCs (Figures 3; see Figure E7 and Table E8). We identified other examples exhibiting this pattern involving subtle (i.e., from a low fraction of cells exhibiting the alteration) AI events in the genome that span mutated genes, such as ARID2, ATM, CDKN2A, IDH1, KIT, KMT2D, KMT2C, JAK2, and NOTCH2 (see Table E8). These somatic AIs and SNVs can offer insights into NSCLC pathogenesis.
Figure 3.
Somatic “two-hit” aberrations in the adjacent and distant normal airway epithelium of patients with early-stage non–small cell lung cancer (NSCLC). Data from deep DNA sequencing and SNP array profiling were integrated to identify NSCLC-associated drivers that comprised either somatic single-nucleotide variants (SNVs) or allelic imbalance (AI) and genes with two-hit aberrations (both SNVs and AI) in the airway field and NSCLC samples. Columns and rows represent patients and NSCLC-associated driver genes, respectively. Each column denotes a patient with the left half of the cell corresponding to the airway field (gray) and right half (black) to its matched NSCLC. Genes with two-hit aberrations are depicted in red and genes comprising either single SNVs or AI events are depicted in orange and yellow, respectively. The detected AI events were annotated as gain (brown), loss (blue), copy-neutral loss-of-heterozygosity (cn-LOH, green), and undeterminable (gray) events. AI events exhibiting intratumoral heterogeneity within multiregion tumor samples (e.g., one biopsy with a cn-LOH and another biopsy within the same tumor showing a copy gain for the same chromosomal region: cn-LOH, gain) are annotated separately. Stacked bars showing the distribution of different types of AI events for each gene are shown in the right panels for both the airway fields and NSCLCs. NSCLC-associated driver genes are ordered top to bottom based on overall two-hit and single-hit patterns in the airway field and NSCLC; and the cases (columns) are ordered left to right based on overall burden of somatic hits across these genes. LUAD = lung adenocarcinoma; LUSC = lung squamous cell carcinoma.
Phylogenetic Evaluation of the Cancerization Field in the Uninvolved Normal-Airway Epithelium
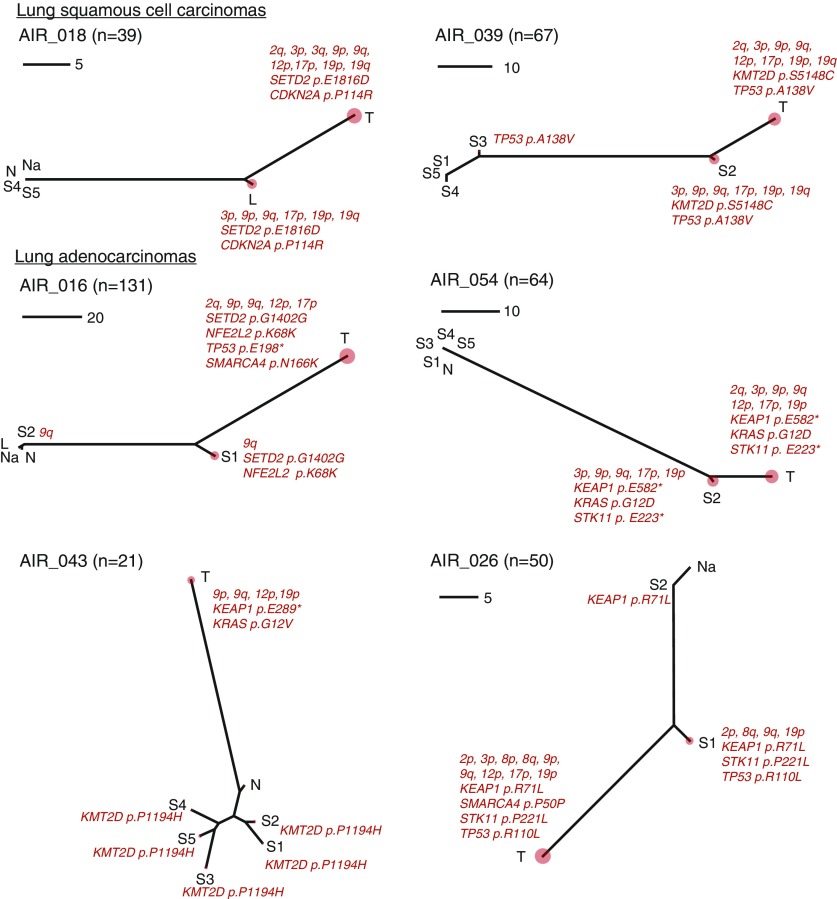
We performed phylogenetic analysis to further interrogate relationships between the field samples and matched NSCLCs. Phylogenetic trees for all cases are shown in the online supplement. For most patients (30/48), we observed a concordance of tree topologies between their separately constructed SNV and AI profiles (see online supplement). Six patients (four LUADs and two LUSCs) exhibited phylogenetic tree structures with relatively pronounced airway field carcinogenesis phenotypes (Figure 4); the same patients statistically determined previously (Figure 1D) to markedly display field effects. These phylogenetic trees represent a serial inclusion of mutations, especially within airway epithelia, that might imply an order of events in airway field cancerization and NSCLC pathogenesis. For example, cases AIR_039, AIR_016, and AIR_026 exhibited multiple airway specimens with differential mutation loads. Case AIR_039 likely presented an early TP53 mutation (S3) that was sequentially followed by additional hits comprising a KMT2D mutation, and chromosomal aberrations, such as 3p loss and 17p loss in the relatively closer airway epithelium (S2) that eventually acquired additional mutations to progress to NSCLCs (e.g., 12p gain). Similarly, AIR_026 exhibited an early mutation in KEAP1, with subsequent STK11 and TP53 point mutations and 9q loss; the matched NSCLC exhibited additional events including SMARCA4 mutation, 17p loss, and a subtle 12p AI event. These trees also encompass driver genes with two-hit alterations, such as a driver with a shared mutation in both the airway field and NSCLC (e.g., TP53 in AIR_026) but with an additional NSCLC-specific hit (e.g., 17p loss in AIR_026), therefore alluding to the two-hit model of progression of normal airway epithelia to NSCLC development described previously. Overall, this analysis highlighted potential sequential patterns of mutations and key drivers in progression of the field to NSCLC.
Figure 4.
Molecular spatial and temporal relationships between the normal airway cancerization field and early-stage non–small cell lung cancer (NSCLC). For every patient, the single-nucleotide variants and allelic imbalances detected (n) across airway field and NSCLC tissues were integrated to generate unrooted neighbor-joining phylogenetic trees to study intrapatient multiregion samples as described in the Methods section. Six cases (two LUSC and four LUAD) previously identified by an independent statistical analysis of field cancerization (Figure 1D) are shown. The phylogenetic trees were annotated with mutations in known cancer-associated genes and large chromosomal aberrations previously implicated in NSCLC pathogenesis. Each tree is accompanied by a scale to denote the number of mutations. The relative somatic burden for each tissue in a tree is denoted by a correspondingly sized red circle. The distances among the multiple points of a tree correspond to the extent of shared and disparate mutational events among samples of a patient. The tree topologies for cases with evidence for genomic field cancerization differ from a typically straight line that would be expected if only NSCLCs presented mutations (see online supplement). Phylogenetic trees for the remaining cases are provided in the online supplement. L = distant large airways; LUAD = lung adenocarcinoma; LUSC = lung squamous cell carcinoma; N = uninvolved normal lung tissue; Na = nasal epithelium; S = tumor-adjacent small airways; T = matched NSCLC.
Discussion
We present, to our knowledge, the most comprehensive analysis of genomic aberrations in normal-appearing airway epithelium from a rich cohort of 498 samples comprising multiregion and spatially distributed airway and NSCLC specimens, along with germline samples, from 48 patients to identify the landscape of somatic point mutations and AI in the normal-appearing airway. The adjacent and distant-to-tumor uninvolved normal-appearing airway field comprised somatic mutations in key drivers that were present at higher allele frequencies in the matched NSCLCs. We also identified key driver genes with shared two-hit alterations (both SNVs and AI) in the airway field and those with single hits in the field coupled with NSCLC-specific mutations. Findings from our study offer insights into a continuum of alterations with plausible spatiotemporal properties in the normal-appearing airway epithelium and nearby NSCLC.
Previously, somatic mutations in the EGFR oncogene have been identified in normal epithelium of EGFR-mutant LUADs (9) and KRAS variants have been detected in lung tissue adjacent to resected tumors (10). Yet, the spectrum of somatic driver mutations and genes in the normal-appearing airway cancerization field is not known. Here, we found that genomic airway field cancerization phenotypes were identified in more than 50% of the cases suggesting that airway field carcinogenesis is not uncommon in early-stage NSCLC. It is important to note that, given our focus on somatic genomic changes, we contrasted genomic profiles in airways and tumors to peripheral blood cells or distant normal lung parenchyma in each patient, thus enabling us to focus on likely pathogenic changes in the lungs of NSCLC patients.
This study design differs from previous reports (6) that focused on messenger/total RNA changes in the airway field cancerization in which expression profiles were compared and contrasted directly between airways of smokers with and without lung cancer. Also, most of the subjects in our study comprised multiple samples from both the cancerization field and the NSCLC itself, thus adding more confidence to the identified genomic airway cancerization events. Additionally, we not only pinpointed driver alterations (e.g., KRAS and PIK3CA mutations) in the normal airway epithelium (both adjacent and distant) but also demonstrated that these changes were shared with the NSCLC with many occurring sequentially, lending further confidence to the probable role of these genomic changes in pathogenesis of this malignancy from the epithelial cancerization field. These changes may hint at mechanisms that underlie tumor recurrence or development of second primary tumors in the remaining lung following surgical treatment with curative intent. Interestingly, recent studies suggested that driver mutations are likely to be found in preconditioned epithelial fields of phenotypically healthy individuals (22, 23). Future studies, perhaps integrating our efforts with current surveys of control subjects (e.g., airway brushings from lung cancer-free smokers) and/or incorporating other possible factors of field mutagenesis (e.g., alternative exposures), may improve the understanding of field cancerization changes involved in lung cancer pathogenesis.
In our study, we present complementary findings on genomic airway cancerization that allude to potential clonally selected changes in the transition of normal airway field to NSCLC: overall increased somatic VAFs in NSCLCs relative to the airway field, shared mutated driver genes between the airway field and NSCLCs, and acquisition of additional driver events in the NSCLCs themselves along with overall increased driver gene VAF in the tumors. We surmise that these genomic airway field cancerization changes provide insights into spatial and temporal development of NSCLCs. If so, further studies that include longitudinally profiled airway field samples from lung cancer patients and/or smokers who are free of lung cancer would bear this out. Mutations in key drivers that we find here to be shared between the airway field and NSCLC were previously described as truncal mutations in intratumoral heterogeneity studies of NSCLC (24, 25). It is noteworthy that our present analysis of multiregion NSCLC biopsies allowed us to capture more truncal tumoral mutations that are shared with the field. Future studies concurrently probing intratumoral heterogeneity and intrafield heterogeneity could create a genomic roadmap to NSCLC pathogenesis.
It is noteworthy that several driver genes we found to be mutated in the airway field have been previously implicated in lung preneoplasia. For instance, our previous study (15) and that of Izumchenko and colleagues (26) identified mutations in TP53 and KRAS in atypical adenomatous hyperplasia (AAH), the precursor lesion to LUAD. Specific to our recent genomic survey of AAH (15, 27), we found that KEAP1 and KMT2D were mutated in AAHs both of which we find here to be mutated in the airway field and matched NSCLCs, indicating that, under certain selective pressures, the cancerized field may evolve to preneoplastic and ultimately to malignant lesions (2, 28). It is, however, plausible that airway field mutations may not be clonally selected in progression to lung premalignant and malignant phenotypes. For instance, previous studies have described driver mutations (e.g., BRAF and KRAS) that are relatively more frequent in AAHs compared with LUADs (15, 27). Future studies and models that concurrently interrogate matched airway fields, AAHs, and adenocarcinomas may aid in identifying field changes that are clonally selected in progression to premalignant and malignant lung lesions.
In conclusion, our deep sequencing and profiling analysis of a rich set of spatially distributed multiregion normal airway epithelia and early-stage NSCLCs illuminated somatic variants of key driver mutated genes in the airway field that were mostly shared with matched NSCLCs. Overall, these somatic variants were positively selected in the tumors suggestive of clonal expansion in NSCLC. Our study not only points to early mutational processes that likely demarcate key events in the emergence of NSCLC from the normal-appearing cancerization field but can also pave the way for similar interrogations in other malignancies. These airway field changes may comprise potential targets for early treatment (e.g., adjuvant therapy to prevent tumor recurrence) of NSCLCs.
Supplementary Material
Footnotes
Supported in part by Cancer Prevention and Research Institute of Texas (grant RP150079, P.S. and H.K.); NIH (grant R01HG005859, P.S.); Department of Defense lung cancer program (grant W81XWH-10-1-1007, H.K., S.D., A.E.S., and I.I.W.); The University of Texas Lung Specialized Programs of Research Excellence (grant P50CA70907); MD Anderson’s Institutional Tissue Bank Award (P30CA016672); and The University of Texas MD Anderson Cancer Center Core Support Grant, Tissue Biorepository and Pathology Facility (P30CA16672).
Author Contributions: H.K. and P.S. conceived, designed, and supervised the study. H.K., W.L., T.M., Z.W., C.B., G.E.D., N.K., C.M., R.E.-Z., R.M., S.G.S., J. Fujimoto, E.A.E., and P.S. helped with data acquisition. H.K., S.S., Y.J., F.A.S.L., J.W., J. Fowler, and P.S. analyzed the data. H.K., S.S., Y.J., F.A.S.L., J.W., J.Z., J. Fowler, J.V.H., S.D., A.E.S., I.I.W., and P.S. interpreted the results. H.K., S.S., and P.S. wrote the manuscript. All authors approved and reviewed the submitted manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201806-1178OC on March 21, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 2.Curtius K, Wright NA, Graham TA. An evolutionary perspective on field cancerization. Nat Rev Cancer. 2018;18:19–32. doi: 10.1038/nrc.2017.102. [DOI] [PubMed] [Google Scholar]
- 3.Kadara H, Wistuba II. Field cancerization in non-small cell lung cancer: implications in disease pathogenesis. Proc Am Thorac Soc. 2012;9:38–42. doi: 10.1513/pats.201201-004MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braakhuis BJ, Tabor MP, Kummer JA, Leemans CR, Brakenhoff RH. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727–1730. [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 6.Kadara H, Scheet P, Wistuba II, Spira AE. Early events in the molecular pathogenesis of lung cancer. Cancer Prev Res (Phila) 2016;9:518–527. doi: 10.1158/1940-6207.CAPR-15-0400. [DOI] [PubMed] [Google Scholar]
- 7.Kadara H, Fujimoto J, Yoo S-Y, Maki Y, Gower AC, Kabbout M, et al. Transcriptomic architecture of the adjacent airway field cancerization in non-small cell lung cancer. J Natl Cancer Inst. 2014;106:dju004. doi: 10.1093/jnci/dju004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grepmeier U, Dietmaier W, Merk J, Wild PJ, Obermann EC, Pfeifer M, et al. Deletions at chromosome 2q and 12p are early and frequent molecular alterations in bronchial epithelium and NSCLC of long-term smokers. Int J Oncol. 2005;27:481–488. [PubMed] [Google Scholar]
- 9.Tang X, Shigematsu H, Bekele BN, Roth JA, Minna JD, Hong WK, et al. EGFR tyrosine kinase domain mutations are detected in histologically normal respiratory epithelium in lung cancer patients. Cancer Res. 2005;65:7568–7572. doi: 10.1158/0008-5472.CAN-05-1705. [DOI] [PubMed] [Google Scholar]
- 10.Nelson MA, Wymer J, Clements N., Jr Detection of K-ras gene mutations in non-neoplastic lung tissue and lung cancers. Cancer Lett. 1996;103:115–121. doi: 10.1016/0304-3835(96)04202-4. [DOI] [PubMed] [Google Scholar]
- 11.Jakubek Y, Lang W, Vattathil S, Garcia M, Xu L, Huang L, et al. Genomic landscape established by allelic imbalance in the cancerization field of a normal appearing airway. Cancer Res. 2016;76:3676–3683. doi: 10.1158/0008-5472.CAN-15-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sivakumar S, Jakubek Y, Anthony San Lucas F, Lang W, McDowell C, Weber Z, et al. Somatic mutational processes in the cancerization field of the normal-appearing airway reveal early drivers in the development of non-small cell lung cancer [abstract] Cancer Res. 2018;78:A3997. [Google Scholar]
- 13.Sivakumar S, Jakubek Y, Lang W, McDowell T, Garcia MM, Chow C-W, et al. Mutational landscape in the normal-appearing airway cancerization field of early-stage non-small cell lung cancer [abstract] Cancer Res. 2017;77:A1434. [Google Scholar]
- 14.Scheet P, Sivakumar S, McDowell CL, Jakubek Y, Lang W, Xu L, et al. Landscape of somatic mutations in the normal-appearing airway cancerization field of early-stage non-small cell lung cancer [abstract] Am J Hum Genet. 2016;A:2830W. [Google Scholar]
- 15.Sivakumar S, Lucas FAS, McDowell TL, Lang W, Xu L, Fujimoto J, et al. Genomic landscape of atypical adenomatous hyperplasia reveals divergent modes to lung adenocarcinoma. Cancer Res. 2017;77:6119–6130. doi: 10.1158/0008-5472.CAN-17-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deshpande A, Lang W, McDowell T, Sivakumar S, Zhang J, Wang J, et al. Strategies for identification of somatic variants using the Ion Torrent deep targeted sequencing platform. BMC Bioinformatics. 2018;19:5. doi: 10.1186/s12859-017-1991-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [Published erratum appears in Nature 491:288.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [Published erratum appears in Nature 559:E12.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexandrov LB, Jones PH, Wedge DC, Sale JE, Campbell PJ, Nik-Zainal S, et al. Clock-like mutational processes in human somatic cells. Nat Genet. 2015;47:1402–1407. doi: 10.1038/ng.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vattathil S, Scheet P. Haplotype-based profiling of subtle allelic imbalance with SNP arrays. Genome Res. 2013;23:152–158. doi: 10.1101/gr.141374.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martincorena I, Roshan A, Gerstung M, Ellis P, Van Loo P, McLaren S, et al. Tumor evolution: high burden and pervasive positive selection of somatic mutations in normal human skin. Science. 2015;348:880–886. doi: 10.1126/science.aaa6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martincorena I, Fowler JC, Wabik A, Lawson ARJ, Abascal F, Hall MWJ, et al. Somatic mutant clones colonize the human esophagus with age. Science. 2018;362:911–917. doi: 10.1126/science.aau3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Bruin EC, McGranahan N, Mitter R, Salm M, Wedge DC, Yates L, et al. Spatial and temporal diversity in genomic instability processes defines lung cancer evolution. Science. 2014;346:251–256. doi: 10.1126/science.1253462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Fujimoto J, Zhang J, Wedge DC, Song X, Zhang J, et al. Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science. 2014;346:256–259. doi: 10.1126/science.1256930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izumchenko E, Chang X, Brait M, Fertig E, Kagohara LT, Bedi A, et al. Targeted sequencing reveals clonal genetic changes in the progression of early lung neoplasms and paired circulating DNA. Nat Commun. 2015;6:8258. doi: 10.1038/ncomms9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yatabe Y, Borczuk AC, Powell CA. Do all lung adenocarcinomas follow a stepwise progression? Lung Cancer. 2011;74:7–11. doi: 10.1016/j.lungcan.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abbosh C, Venkatesan S, Janes SM, Fitzgerald RC, Swanton C. Evolutionary dynamics in pre-invasive neoplasia. Curr Opin Syst Biol. 2017;2:1–8. doi: 10.1016/j.coisb.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.