Abstract
Rationale: Current diagnostic criteria for bronchopulmonary dysplasia rely heavily on the level and duration of oxygen therapy, do not reflect contemporary neonatal care, and do not adequately predict childhood morbidity.
Objectives: To determine which of 18 prespecified, revised definitions of bronchopulmonary dysplasia that variably define disease severity according to the level of respiratory support and supplemental oxygen administered at 36 weeks’ postmenstrual age best predicts death or serious respiratory morbidity through 18–26 months’ corrected age.
Methods: We assessed infants born at less than 32 weeks of gestation between 2011 and 2015 at 18 centers of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network.
Measurements and Main Results: Of 2,677 infants, 683 (26%) died or developed serious respiratory morbidity. The diagnostic criteria that best predicted this outcome defined bronchopulmonary dysplasia according to treatment with the following support at 36 weeks’ postmenstrual age, regardless of prior or current oxygen therapy: no bronchopulmonary dysplasia, no support (n = 773); grade 1, nasal cannula ≤2 L/min (n = 1,038); grade 2, nasal cannula >2 L/min or noninvasive positive airway pressure (n = 617); and grade 3, invasive mechanical ventilation (n = 249). These criteria correctly predicted death or serious respiratory morbidity in 81% of study infants. Rates of this outcome increased stepwise from 10% among infants without bronchopulmonary dysplasia to 77% among those with grade 3 disease. A similar gradient (33–79%) was observed for death or neurodevelopmental impairment.
Conclusions: The definition of bronchopulmonary dysplasia that best predicted early childhood morbidity categorized disease severity according to the mode of respiratory support administered at 36 weeks’ postmenstrual age, regardless of supplemental oxygen use.
Keywords: infant chronic lung disease, supplemental oxygen, mechanical ventilation
At a Glance Commentary
Scientific Knowledge on the Subject
Bronchopulmonary dysplasia (BPD), a chronic neonatal lung disease, is among the most common and severe sequelae of preterm birth. However, the current diagnostic criteria for BPD, which rely heavily on the level and duration of oxygen therapy, do not reflect contemporary neonatal care or adequately predict childhood morbidity.
What This Study Adds to the Field
In this study of very preterm infants (N = 2,677), the diagnostic criteria that best predicted late death or serious respiratory morbidity through 18–26 months’ corrected age defined the presence and severity of BPD according to the mode of respiratory support administered at 36 weeks’ postmenstrual age, regardless of the prior duration or current level of oxygen therapy. The optimal diagnostic criteria were no BPD, no support; grade 1 BPD, nasal cannula ≤2 L/min; grade 2 BPD, nasal cannula >2 L/min or noninvasive positive airway pressure; and grade 3 BPD, invasive mechanical ventilation.
Bronchopulmonary dysplasia (BPD), a chronic neonatal lung disease, is among the most common and severe sequelae of preterm birth. On the basis of current criteria, BPD affects over half of extremely premature infants, predisposes survivors to adverse neurodevelopment and cardiorespiratory health, and is associated with substantial resource use and cost (1–4). Despite improvements in neonatal care over the past 30 years, BPD rates have not declined, and few evidence-based therapies to prevent and treat the disease exist (1, 5, 6). One barrier to reducing the burden of BPD is the inability of existing diagnostic criteria to reliably establish disease presence and severity (7–10).
The first published diagnostic criteria defined BPD, in part, as the continued use of supplemental oxygen at 28–30 days of age (11, 12). These initial criteria provided a much-needed diagnostic framework, but they were not designed to predict later outcomes (11, 12). Shennan and colleagues addressed this limitation in 1988, when they demonstrated that supplemental oxygen use at 36 weeks’ postmenstrual age (PMA) better predicted adverse pulmonary health during early childhood than use at 28 days (13). However, this dichotomous definition does not characterize the wide spectrum of BPD severity (10). To better stratify disease acuity, a consensus conference sponsored by the NIH proposed a categorical definition of BPD in 2001 (14). This definition classifies BPD as mild, moderate, or severe according to the amount of supplemental oxygen (<30% vs. ≥30%) and the mode of respiratory support administered at 36 weeks’ PMA to very preterm infants treated with supplemental oxygen for at least 28 days (14). Unfortunately, these widely used criteria are no longer valid (9). They do not reflect contemporary neonatal respiratory care, including the widespread use of high-flow nasal cannula, and may not adequately predict childhood outcomes in contemporary very preterm infants (9).
We therefore conducted the present study to develop a modern, evidence-based definition of BPD. Our aim was to determine which of several revised, severity-graded diagnostic criteria best predict clinically meaningful respiratory and neurodevelopmental outcomes measured at 18–26 months’ corrected age in a recent, multicenter cohort of very preterm infants. Some of the results of this study have been reported previously in the form of abstracts (15, 16).
Methods
Population and Data Source
Study data were obtained from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. The Neonatal Research Network uses predefined protocols to prospectively collect medical and developmental outcome data from birth through the first 2 years of age on all infants with gestational ages <27 weeks. The same follow-up data are collected on infants with higher gestational ages enrolled in Neonatal Research Network studies that measure neurodevelopmental outcomes. Before July 1, 2012, postdischarge outcome data were collected at 18–22 months’ corrected age. Thereafter, these data were collected at 22–26 months’ corrected age. The present study evaluated infants born at <32 weeks of gestation at 18 Neonatal Research Network centers between April 1, 2011, and April 1, 2015. Eligible infants survived to 36 weeks’ PMA (gestational age in wk + age after birth in wk) and either completed 18–26 months of follow-up or died before anticipated follow-up. Infants with severe congenital malformations or syndromes and those missing key study data were excluded. The institutional review board at each center approved collection of study data during the initial hospitalization and follow-up.
Evaluation of Severity-graded Definitions of BPD
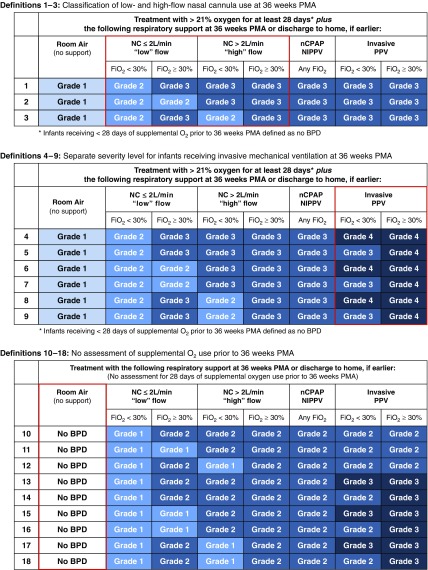
We evaluated 18 prespecified, severity-graded BPD definitions (Figure 1). Definition 1 was constructed to closely resemble the 2001 NIH consensus definition, with the modification that currently unclassifiable infants receiving high-flow (>2 L/min) nasal cannula at 36 weeks’ PMA were grouped with those treated with noninvasive positive airway pressure (14). The remaining 17 definitions modified these diagnostic criteria to address the following knowledge gaps: 1) how best to classify BPD severity among infants receiving low-flow (≤2 L/min) versus high-flow (>2 L/min) nasal cannula at 36 weeks’ PMA, 2) whether inclusion of a distinct severity level for infants receiving invasive mechanical ventilation at 36 weeks’ PMA improves prediction of adverse childhood outcomes, and 3) whether eliminating the current requirement that infants must receive at least 28 days of supplemental oxygen before 36 weeks’ PMA to establish a diagnosis of BPD affects prognostic accuracy. Of note, the oxygen reduction test was not used to determine oxygen dependency in this analysis, because only 57% (452 of 791) of eligible babies underwent testing (17).
Figure 1.
The 18 prespecified severity-graded definitions of bronchopulmonary dysplasia (BPD). Definitions 1–3 establish the initial framework. Key components of the definition evaluated in each subset are highlighted by the red boxes. All infants treated with head box oxygen are included with those receiving nasal cannula (NC) at ≤2 L/min flow. The mode of respiratory support and FiO2 were recorded as the highest level administered at 36 weeks’ postmenstrual age (PMA). Any temporary increases in FiO2 for desaturation events, apnea, bradycardia, or procedures were disregarded if the infant returned to the previous FiO2 within 2 hours. Supplemental oxygen administered only during feedings was not included for infants who did not receive oxygen therapy at other times during the day. nCPAP = nasal continuous positive airway pressure; NIPPV = nasal intermittent positive pressure ventilation; PPV = positive pressure ventilation.
Outcomes
We determined how accurately each of the 18 prespecified diagnostic criteria predicted the composite primary outcome of death between 36 weeks’ PMA and 18- to 26-month follow-up (late death) or serious respiratory morbidity, defined as the occurrence of at least one of the following: tracheostomy placed any time before follow-up; continued hospitalization for respiratory reasons at or beyond 50 weeks’ PMA; use of supplemental oxygen, respiratory support, or respiratory monitoring (e.g., pulse oximeter or apnea monitor) at follow-up; or two or more rehospitalizations for respiratory reasons before follow-up. Continued hospitalization at 50 weeks’ PMA is approximately 2 SD above the mean age at discharge for extremely preterm infants included in Neonatal Research Network studies. Two or more rehospitalizations represents the upper 75th percentile for rehospitalization number among Neonatal Research Network babies. Postdischarge respiratory outcome data were ascertained from parents during the follow-up visit. The evaluated respiratory endpoints are consistent with several prior studies exploring postdischarge respiratory morbidity in very preterm infants and represent established adverse outcomes that are meaningful to parents and healthcare providers (2, 18–22).
The secondary study outcome was the composite of late death or moderate to severe neurodevelopmental impairment at 18–26 months’ corrected age, assessed by neurologic examination, and defined as a Bayley Scales of Infant and Toddler Development, Third Edition, cognitive or motor composite score less than 85, a Gross Motor Function Classification System level greater than or equal to 2, bilateral blindness, and/or severe hearing impairment that cannot be corrected with amplification (23, 24). Growth restriction and measures of healthcare use at follow-up were assessed as additional secondary outcomes.
Statistical Analyses
The strength of the association between each BPD definition and the two composite study outcomes was assessed using multivariable logistic regression. The study outcome was included in each model as a dichotomous dependent variable, and the BPD definition was included as a categorical independent variable. All regression models were adjusted for the following prespecified perinatal characteristics known to be associated with the study outcomes: gestational age, birth weight, sex, small for gestational age (defined as a birth weight <10th percentile for gestational age and sex [25]), race/ethnicity, treatment with antenatal corticosteroids, treatment with antenatal magnesium, maternal education, insurance type, primary caregiver marital status, and study center. Further adjustment for follow-up performed at 18–22 versus 22–26 months’ corrected age did not significantly affect model performance and was not included in the final regression models. A concordance (c)-statistic (equivalent to the area under the receiver operating characteristic curve) was calculated for each regression model. This value quantifies the model predictive accuracy, specifically how well each definition discriminates between babies who did and did not develop the study outcome. The optimal diagnostic criteria were defined a priori as the BPD definition resulting in the highest c-statistic for the primary study outcome. The accuracy for predicting the secondary study outcome was intended to resolve any equivalent results for the primary outcome. c-Statistic values were compared using methods described by DeLong and colleagues (26).
Internally validated c-statistics were calculated to further assess the performance of each definition. These values were determined by rerunning the regression models on 100 bootstrap samples consisting of 2,677 infants randomly selected from among the original cohort with replacement. The difference between the original and bootstrap c-statistics is an estimate of the optimism of the model performance (27). The average of these values subtracted from the full cohort c-statistic defines the internally validated c-statistic (27). Sensitivity, specificity, likelihood ratios, and positive and negative predictive values were calculated using multivariable logistic regression and receiver operating characteristic curves in the full cohort.
We performed three post hoc analyses. The first compared the predictive accuracy of the optimal diagnostic criteria with one that graded BPD severity among infants receiving nasal continuous positive airway pressure or nasal intermittent positive pressure ventilation and ≥30% oxygen at 36 weeks’ PMA as equivalent to those receiving invasive mechanical ventilation. The second analysis rank ordered the predictive accuracy of the 18 prespecified definitions using c-statistics calculated from regression models adjusting for center alone. This characterized how well each definition predicted the study outcomes according to BPD severity averaged over all study centers without adjusting for the distribution of other important predictor variables. The third compared the predictive accuracy of the optimal diagnostic criteria with a definition that is similar to one proposed at a recent Eunice Kennedy Shriver National Institute of Child Health and Human Development BPD workshop (Table E1 in the online supplement) (28). All analyses were performed with SAS version 9.4 software (SAS Institute).
Results
Characteristics of the Study Population
A total of 2,677 infants were included in this analysis, after 742 who were lost to follow-up or missing study data were excluded (Figure E1). Relative to the included infants, those who were excluded demonstrated modest but statistically significant differences in birth weight, race and ethnicity, insurance type, and level of maternal education (Table E2). The characteristics of the included infants are summarized in Table 1. In total, 683 (26%) died between 36 weeks’ PMA and anticipated 18- to 26-month follow-up or developed serious respiratory morbidity. Late death or moderate to severe neurodevelopmental impairment occurred in 1,305 (49%) (25).
Table 1.
Characteristics and Outcomes of the Study Infants
| Characteristic | Full Study Cohort (N = 2,677) |
|---|---|
| Gestational age, wk, mean (SD) | 25.2 (1.3) |
| <27 wk, n (%) | 2,380 (89) |
| 27 wk to 31 wk and 6 d, n (%) | 297 (11) |
| Birth weight, g, mean (SD) | 765 (168) |
| Sex, male, n (%) | 1,356 (51) |
| Small for gestational age, n (%)* | 219 (8) |
| Race, n (%) | |
| Black | 1,225 (46) |
| White | 1,312 (49) |
| Other | 140 (5) |
| Ethnicity, n (%) | |
| Hispanic | 322 (12) |
| Non-Hispanic | 2,355 (88) |
| Corrected age at follow-up, mo, mean (SD) | 23.3 (3.0) |
| Follow-up at 18–22 mo corrected age, n (%)† | 719 (28) |
| Follow-up at 22–26 mo corrected age, n (%)† | 1,849 (72) |
| Antenatal corticosteroids, n (%) | 2,397 (90) |
| Antenatal magnesium, n (%) | 2,077 (78) |
| Maternal marital status, married, n (%) | 1,126 (42) |
| Insurance type, n (%) | |
| Medicaid | 1,528 (57) |
| Private | 1,012 (38) |
| Self-pay/uninsured | 101 (4) |
| Other | 36 (1) |
| Maternal level of education less than high school, n (%) | 499 (19) |
| Outcome | |
| Late death or serious respiratory morbidity, n (%) | 683 (26) |
| Late death or moderate to severe NDI, n (%) | 1,305 (49) |
Definition of abbreviation: NDI = neurodevelopmental impairment.
Defined as a birth weight below the 10th percentile for gestational age and sex (25).
Calculated for the 2,568 infants who survived to follow-up testing.
Identification and Performance of the Optimal BPD Definition
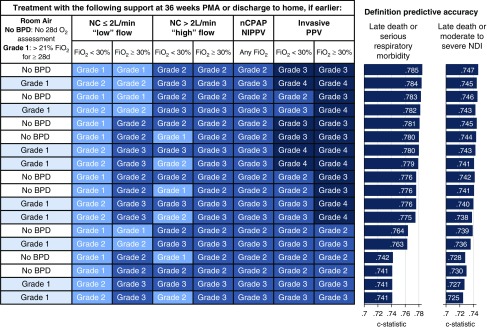
Figure 2 shows the 18 prespecified BPD definitions ordered from highest to lowest accuracy for predicting the primary study outcome. The optimal definition (number 15; Figure 1) categorized BPD severity according to the mode of respiratory support administered at 36 weeks’ PMA, regardless of the prior duration or current level of oxygen therapy. On the basis of these diagnostic criteria, infants breathing in room air at 36 weeks’ PMA did not have BPD. Disease severity among the remaining infants was classified according to treatment with the following support: grade 1, nasal cannula at flow rates ≤2 L/min; grade 2, nasal cannula at flow rates >2 L/min or noninvasive positive airway pressure; and grade 3, invasive mechanical ventilation. This definition produced the highest predictive accuracy using internally validated c-statistics (Table E3) and in a post hoc analysis adjusting for center alone. Consideration of supplemental oxygen use among infants receiving nasal continuous positive airway pressure or nasal intermittent positive pressure ventilation at 36 weeks’ PMA did not improve prediction of either study outcome. The definition most similar to the 2001 NIH consensus definition (number 1; Figure 1) was among the least accurate predictors of both study outcomes (Figure 2) and produced significantly lower c-statistic values than the optimal definition (P < 0.001 for both composite outcomes) (14). The optimal definition also outperformed a slightly modified version of the 2018 Eunice Kennedy Shriver National Institute of Child Health and Human Development workshop definition (P ≤ 0.002 for both composite outcomes) (Table E1) (28).
Figure 2.
Rank order of the 18 evaluated definitions of bronchopulmonary dysplasia (BPD). The definitions are ordered from highest (top) to lowest (bottom) accuracy for predicting death between 36 weeks’ postmenstrual age (PMA) and 18- to 26-month follow-up or serious respiratory morbidity. Concordance (c) statistic values were calculated using logistic regression, adjusting for gestational age, birth weight, sex, small for gestational age, race/ethnicity, treatment with antenatal corticosteroids, treatment with antenatal magnesium, maternal level of education, insurance type, primary caregiver marital status, and study center. NC = nasal cannula; nCPAP = nasal continuous positive airway pressure; NDI = neurodevelopmental impairment; NIPPV = nasal intermittent positive pressure ventilation; PPV = positive pressure ventilation.
The measures of diagnostic performance (e.g., sensitivity and specificity) for the optimal definition varied, depending on the adjusted outcome probability used to predict the presence or absence of the study outcomes. Setting this cut point at 51% for death or serious respiratory morbidity (i.e., assuming all infants predicted by the multivariable model to have a >51% chance of developing this adverse outcome will actually develop the outcome) and 47% for death or moderate to severe neurodevelopmental impairment correctly classified the true outcome status in the highest proportion of infants: 81% for late death or serious respiratory morbidity (sensitivity, 36%; specificity, 96%) and 69% for late death or poor neurodevelopment (sensitivity, 69%; specificity, 68%) (Figure E2).
Outcome Rates according to the Optimal BPD Definition
A total of 773 infants (29%) were breathing in room air at 36 weeks’ PMA and did not have BPD (Table 2). Grade 1 BPD was present in 1,038 infants (39%), grade 2 in 617 (23%), and grade 3 in 249 (9%). Unadjusted rates of the primary and secondary composite outcomes increased in a stepwise manner as BPD severity increased (Table 2). The frequency of late death or serious respiratory morbidity rose from 10% among infants without BPD to 77% among those with grade 3 BPD. Rates of late death or moderate to severe neurodevelopmental impairment increased from 33% among those without BPD to 79% among those with grade 3 disease. Growth restriction and postdischarge health care use were also more common among infants with greater BPD severity (Table 3).
Table 2.
Composite and Individual Outcome Rates according to the Optimal Bronchopulmonary Dysplasia Definition
| Outcome | No BPD (n = 773) | Grade 1 BPD (n = 1,038)* | Grade 2 BPD (n = 617) | Grade 3 BPD (n = 249) |
|---|---|---|---|---|
| Late death or serious respiratory morbidity† | 76 (10) | 199 (19) | 216 (35) | 192 (77) |
| Death after 36 wk PMA | 14 of 773 (2) | 25 of 1,038 (2) | 20 of 617 (3) | 50 of 249 (20) |
| Tracheostomy | 2 of 759 (0.3) | 7 of 1,013 (0.7) | 28 of 596 (5) | 62 of 199 (31) |
| NICU hospitalization beyond 50 wk PMA for respiratory reasons | 6 of 759 (0.8) | 25 of 1,013 (2) | 90 of 596 (15) | 101 of 199 (51) |
| Supplemental O2 use at follow-up | 6 of 759 (0.8) | 32 of 1,013 (3) | 52 of 596 (9) | 58 of 199 (29) |
| Ventilator or CPAP at follow-up | 4 of 759 (0.5) | 5 of 1,013 (0.5) | 18 of 596 (3) | 28 of 199 (14) |
| Respiratory monitor use at follow-up | 7 of 759 (0.9) | 41 of 1,013 (4) | 71 of 596 (12) | 81 of 199 (41) |
| ≥2 hospitalizations for respiratory reasons | 51 of 759 (7) | 132 of 1,013 (13) | 129 of 595 (22) | 57 of 199 (29) |
| Late death or moderate to severe NDI | 257 (33) | 480 (46) | 372 (60) | 196 (79) |
| Death after 36 wk PMA | 14 of 773 (2) | 25 of 1,038 (2) | 20 of 617 (3) | 50 of 249 (20) |
| Bayley-3 cognitive or motor composite score <85 | 173 of 757 (23) | 316 of 1,004 (31) | 260 of 585 (44) | 123 of 194 (63) |
| GMFCS level ≥2 | 36 of 759 (5) | 76 of 1,011 (8) | 93 of 597 (16) | 67 of 199 (34) |
| Blindness | 2 of 759 (0.3) | 12 of 1,012 (1) | 15 of 595 (3) | 12 of 198 (6) |
| Deafness | 14 of 759 (2) | 26 of 1,013 (3) | 17 of 595 (3) | 14 of 199 (7) |
Definition of abbreviations: Bayley-3 = Bayley Scales of Infant Development, Third Edition; BPD = bronchopulmonary dysplasia; CPAP = continuous positive airway pressure; GMFCS = Gross Motor Function Classification System; NDI = neurodevelopmental impairment; NICU = neonatal ICU; PMA = postmenstrual age.
Data are shown as n (%).
Includes 50 infants treated with supplemental oxygen administered by head box at 36 weeks’ PMA.
Data for nonmortality outcomes are shown for children who underwent follow-up at 18–26 months’ corrected age.
Table 3.
Rates of Additional Secondary Outcomes according to the Optimal Bronchopulmonary Dysplasia Definition
| Outcome | No BPD (n = 773) | Grade 1 BPD (n = 1,038) | Grade 2 BPD (n = 617) | Grade 3 BPD (n = 249) |
|---|---|---|---|---|
| PMA at NICU discharge, wk, mean (SD) | 38.5 (3.5) | 41.2 (5.8) | 45.3 (7.6) | 53.5 (11.2) |
| Growth at follow-up*† | ||||
| Weight <10% | 128 of 755 (17) | 180 of 1,009 (18) | 151 of 597 (25) | 56 of 199 (28) |
| Length <10% | 201 of 752 (27) | 296 of 1,006 (29) | 233 of 595 (39) | 97 of 199 (49) |
| Head circumference <10% | 120 of 750 (16) | 179 of 1,007 (18) | 153 of 588 (26) | 74 of 197 (38) |
| Healthcare use assessed at follow-up* | ||||
| Gastrostomy/feeding tube | 22 of 759 (3) | 70 of 1,013 (7) | 97 of 597 (16) | 94 of 199 (47) |
| Independently feeds self | 706 of 759 (93) | 860 of 1,013 (85) | 446 of 597 (75) | 95 of 199 (48) |
| Inhaled pulmonary medication use within 3 mo of follow-up | 198 of 759 (26) | 371 of 1,013 (37) | 275 of 596 (46) | 131 of 199 (66) |
| Anti–gastroesophageal reflux medication use within 3 mo of follow-up‡ | 54 of 759 (7) | 119 of 1,013 (12) | 121 of 596 (20) | 87 of 199 (44) |
| Pulmonary specialist | 65 of 759 (9) | 234 of 1,013 (23) | 213 of 597 (36) | 127 of 199 (64) |
| Home nurse | 2 of 759 (0.3) | 26 of 1,013 (3) | 41 of 597 (7) | 61 of 199 (31) |
| Rehospitalization (any cause)* | ||||
| Rehospitalization | 283 of 759 (37) | 506 of 1,013 (50) | 357 of 597 (60) | 141 of 199 (71) |
| No. of rehospitalizations per patient, mean (SD) | 0.64 (1.17) | 1.06 (1.61) | 1.51 (2.12) | 2.29 (2.67) |
| Rehospitalization in an ICU | 73 of 759 (10) | 153 of 1,013 (15) | 138 of 597 (23) | 65 of 199 (33) |
| No. of ICU admissions per patient, mean (SD) | 0.10 (0.33) | 0.22 (0.63) | 0.43 (1.04) | 0.62 (1.12) |
| Neurodevelopment* | ||||
| Bayley-3 cognitive or motor composite score <70 | 38 of 757 (5) | 95 of 1,004 (9) | 95 of 585 (16) | 77 of 194 (40) |
| Bayley-3 cognitive or motor composite score 70–84 | 135 of 757 (18) | 221 of 1,004 (22) | 165 of 585 (28) | 46 of 194 (24) |
| GMFCS level ≥4 | 9 of 759 (1) | 18 of 1,011 (2) | 25 of 597 (4) | 24 of 199 (12) |
| GMFCS level 2–3 | 27 of 759 (4) | 58 of 1,011 (6) | 68 of 597 (11) | 43 of 199 (22) |
Definition of abbreviations: BPD = bronchopulmonary dysplasia; Bayley-3 = Bayley Scales of Infant and Toddler Development, Third Edition; GMFCS = Gross Motor Function Classification System; NICU = neonatal ICU; PMA = postmenstrual age.
Data are shown as n (%) unless otherwise specified.
Data are shown for children who underwent follow-up at 18–26 months’ corrected age.
Growth percentiles were defined using the World Health Organization Child Growth Standards.
Defined as a proton pump inhibitor, histamine 2 receptor antagonist, or gastrointestinal prokinetic medication.
Discussion
Our objective was to develop modern diagnostic criteria for BPD that accommodate current respiratory care practices and accurately predict important childhood morbidity. The definition that best predicted death after 36 weeks’ PMA or adverse respiratory and neurodevelopmental outcomes at 18–26 months’ corrected age classified BPD severity according to the mode of respiratory support administered at 36 weeks’ PMA. Consideration of supplemental oxygen use before or at 36 weeks’ PMA did not improve the accuracy for predicting either study outcome.
These diagnostic criteria are appealing for several reasons. Most important, they were derived from a data-driven approach rather than expert consensus. Second, a definition that reliably predicts meaningful long-term outcomes is essential for the development and regulatory approval of new therapies intended for BPD prevention or treatment (10). Poor respiratory health in childhood, including the need for rehospitalization and home oxygen therapy, is a noted concern of parents and healthcare providers (18, 19, 29). The optimal definition demonstrated good discriminatory power and correctly predicted the presence or absence of late death or serious respiratory morbidity in over 80% of study infants (30). Finally, the definition is pragmatic. Its ease of application makes it suitable for bedside clinical care, research, and disease benchmarking in large, international registries. The definition removes the need to assess supplemental oxygen use before and at 36 weeks’ PMA (14). Accurate evaluation of daily oxygen therapy is time consuming and inconsistently performed (7, 9). Moreover, intercenter variability in oxygen administration can bias the classification of BPD presence and severity (31, 32). Although the oxygen reduction test was designed to help overcome this practice variability, the utility of the test, even in research settings, is limited by low testing rates among eligible babies (9, 17).
The 12 definitions with the highest accuracy for predicting late death or serious respiratory morbidity demonstrated only slight differences in discriminatory ability. However, each of these definitions used a higher BPD severity level for infants receiving invasive mechanical ventilation at 36 weeks’ PMA. This result, coupled with the twofold higher rates of late death, serious respiratory morbidity, and moderate to severe neurodevelopmental impairment among infants receiving invasive rather than noninvasive positive airway pressure at 36 weeks’ PMA, strongly supports the distinct classification of infants treated with invasive mechanical ventilation at 36 weeks’ PMA.
Although most adverse respiratory outcomes occurred rarely among infants breathing in room air at 36 weeks’ PMA, 7% of these children were rehospitalized for respiratory reasons two or more times during the first 2 years of life. Moreover, one-fourth received a respiratory medication within 3 months of follow-up. These findings agree with previous observations that some very preterm infants without BPD exhibit abnormalities in respiratory health during their first years of life (33). Subtle deficits in lung function combined with postdischarge exposure to viral infection, secondary smoke inhalation, and socioeconomic deprivation may contribute to the adverse respiratory outcomes observed in this subset of infants (34–38).
The optimal BPD definition demonstrated higher accuracy for predicting adverse respiratory outcomes than for predicting poor neurodevelopment. This finding is not surprising. Multiple nonrespiratory factors contribute to adverse development in very preterm infants (39, 40). Nonetheless, the observed stepwise increase in death or moderate to severe neurodevelopmental impairment from 33% among infants without BPD to 79% among those with grade 3 BPD further substantiates the important relationship between BPD severity and poor neurodevelopment (2, 41, 42).
We classified BPD severity according to the respiratory support administered at 36 weeks' PMA, similar to other commonly used definitions (13, 14). Several studies have investigated whether diagnosing BPD at a later time point improves prediction of childhood outcomes (13, 20, 21, 43). These produced conflicting results (13, 20, 21, 43). The Neonatal Research Network does not routinely collect detailed respiratory data at weekly intervals surrounding term equivalent gestation. As such, we are unable to determine whether the definition identified in our analysis would perform better at an alternative PMA. However, any potential benefit of diagnosing BPD at a later age must be balanced against the inability to accurately assess the respiratory support needs of infants who are transferred to other institutions before that time point (44).
Our study cohort consisted primarily of infants born at less than 27 weeks of gestation, those at the highest risk for BPD and childhood morbidity. It is uncertain whether the evaluated definitions would perform similarly in a cohort consisting predominately of more mature preterm babies. We also limited our analysis to infants who survived to 36 weeks’ PMA. As many as half of all deaths occurring before 36 weeks’ PMA in extremely preterm infants are pulmonary related (9, 45). A valid definition of early, evolving BPD may help inform the design of future studies aimed at reducing mortality and respiratory morbidity among these critically ill infants.
We did not evaluate the predictive accuracy of several possible permutations of respiratory support and supplemental oxygen level at 36 weeks’ PMA. We limited this study to 18 prespecified definitions to balance the need to address key questions regarding the diagnostic criteria for BPD with minimizing the possibility of spurious findings resulting from multiple comparisons. We acknowledge that the evaluated definitions do not distinguish the pathophysiologic reasons for each infant’s respiratory support needs. Further research is needed to characterize BPD phenotypes and validate the diagnostic tools used to provide this information. Finally, we recognize that respiratory support and monitoring device use may depend, in part, on the preferences of the family and prescribing care provider. There is also a possibility of bias in our study results owing to incomplete follow-up of some eligible infants.
In conclusion, existing BPD definitions do not adequately classify the presence and severity of BPD among all very preterm infants (9). In this study, we demonstrate the data-driven development of a pragmatic definition of BPD that accounts for contemporary respiratory care practices and has good discriminatory power for predicting early childhood morbidity. Although future studies should further assess the validity of these new diagnostic criteria, our results provide a strong rationale for using the definition described in this analysis as the modern, severity-based criteria for BPD.
Supplementary Material
Acknowledgments
Acknowledgment
The authors are indebted to their medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The authors dedicate this report to Barbara Schmidt, M.D., M.Sc., F.R.C.P.(C.), C.M., on the occasion of her retirement and in recognition of her contribution to neonatal medicine.
Footnotes
Supported by grants from the NIH and the Eunice Kennedy Shriver National Institute of Child Health and Human Development for the Neonatal Research Network, including for the follow-up study, and by NHLBI grant K23HL136843 (E.A.J.).
Data sharing: Data reported in this paper may be requested through a data use agreement. Further details are available at https://neonatal.rti.org/index.cfm?fuseaction=DataRequest.Home.
Author Contributions: E.A.J. conceptualized and designed the study, drafted the initial and final manuscripts, and collaborated with M.G.G. and S.M. to perform and review the statistical analyses. K.D. and S.B.D. conceptualized and designed the study, provided interpretation of data, critically reviewed and revised the manuscript for important intellectual content, and approved the final manuscript as submitted. M.G.G. and S.M. participated in the design of the study, conducted all analyses and provided interpretation of data, and reviewed and approved the final manuscript as submitted. N.A.B., M.K., H.K., M.M.L., B.B.P., A.F.D., B.A.Y., and E.C.E. participated in the design of the study and the interpretation of the data, and they revised the article for important intellectual content and approved the final manuscript as submitted.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201812-2348OC on April 17, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network
References
- 1.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314:1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. National Institute of Child Health and Human Development Neonatal Research Network. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt B, Roberts RS, Davis PG, Doyle LW, Asztalos EV, Opie G, et al. Caffeine for Apnea of Prematurity (CAP) Trial Investigators; Caffeine for Apnea of Prematurity CAP Trial Investigators. Prediction of late death or disability at age 5 years using a count of 3 neonatal morbidities in very low birth weight infants. J Pediatr. 2015;167:982–986.e2. doi: 10.1016/j.jpeds.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 4.Álvarez-Fuente M, Arruza L, Muro M, Zozaya C, Avila A, López-Ortego P, et al. The economic impact of prematurity and bronchopulmonary dysplasia. Eur J Pediatr. 2017;176:1587–1593. doi: 10.1007/s00431-017-3009-6. [DOI] [PubMed] [Google Scholar]
- 5.Poets CF, Lorenz L. Prevention of bronchopulmonary dysplasia in extremely low gestational age neonates: current evidence. Arch Dis Child Fetal Neonatal Ed. 2018;103:F285–F291. doi: 10.1136/archdischild-2017-314264. [DOI] [PubMed] [Google Scholar]
- 6.Horbar JD, Edwards EM, Greenberg LT, Morrow KA, Soll RF, Buus-Frank ME, et al. Variation in performance of neonatal intensive care units in the United States. JAMA Pediatr. 2017;171:e164396. doi: 10.1001/jamapediatrics.2016.4396. [DOI] [PubMed] [Google Scholar]
- 7.Hines D, Modi N, Lee SK, Isayama T, Sjörs G, Gagliardi L, et al. International Network for Evaluating Outcomes (iNeo) of Neonates. Scoping review shows wide variation in the definitions of bronchopulmonary dysplasia in preterm infants and calls for a consensus. Acta Paediatr. 2017;106:366–374. doi: 10.1111/apa.13672. [DOI] [PubMed] [Google Scholar]
- 8.Jobe AH, Steinhorn R. Can we define bronchopulmonary dysplasia? J Pediatr. 2017;188:19–23. doi: 10.1016/j.jpeds.2017.06.064. [DOI] [PubMed] [Google Scholar]
- 9.Poindexter BB, Feng R, Schmidt B, Aschner JL, Ballard RA, Hamvas A, et al. Prematurity and Respiratory Outcomes Program. Comparisons and limitations of current definitions of bronchopulmonary dysplasia for the Prematurity and Respiratory Outcomes Program. Ann Am Thorac Soc. 2015;12:1822–1830. doi: 10.1513/AnnalsATS.201504-218OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinhorn R, Davis JM, Göpel W, Jobe A, Abman S, Laughon M, et al. International Neonatal Consortium. Chronic pulmonary insufficiency of prematurity: developing optimal endpoints for drug development. J Pediatr. 2017;191:15–21, e1. doi: 10.1016/j.jpeds.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Bancalari E, Abdenour GE, Feller R, Gannon J. Bronchopulmonary dysplasia: clinical presentation. J Pediatr. 1979;95:819–823. doi: 10.1016/s0022-3476(79)80442-4. [DOI] [PubMed] [Google Scholar]
- 12.Tooley WH. Epidemiology of bronchopulmonary dysplasia. J Pediatr. 1979;95:851–858. doi: 10.1016/s0022-3476(79)80451-5. [DOI] [PubMed] [Google Scholar]
- 13.Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. 1988;82:527–532. [PubMed] [Google Scholar]
- 14.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 15.Jensen EA, Dysart K, Gantz M, McDonald S, Bamat N, Keszler M, et al. Development of a revised severity based definition of bronchopulmonary dysplasia: accounting for modern respiratory care [abstract]. Presented at the 7th Congress of the European Academy of Paediatric Societies. October 30–November 3, 2018, Paris, France. Abstract EAPS8-0965. [Google Scholar]
- 16.Jensen EA, Dysart K, Gantz M, McDonald S, Bamat N, Keszler M, et al. Development of a revised severity based definition of bronchopulmonary dysplasia: accounting for modern respiratory care [abstract]. Presented at Pediatric Academic Societies Meeting; May 5–8 2018, Toronto, ON, Canada. Abstract 1417.1151. [Google Scholar]
- 17.Walsh MC, Wilson-Costello D, Zadell A, Newman N, Fanaroff A. Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. J Perinatol. 2003;23:451–456. doi: 10.1038/sj.jp.7210963. [DOI] [PubMed] [Google Scholar]
- 18.Janvier A, Farlow B, Baardsnes J, Pearce R, Barrington KJ. Measuring and communicating meaningful outcomes in neonatology: a family perspective. Semin Perinatol. 2016;40:571–577. doi: 10.1053/j.semperi.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Jaworski M, Janvier A, Lefebvre F, Luu TM. Parental perspectives regarding outcomes of very preterm infants: toward a balanced approach. J Pediatr. 2018;200:58–63, e1. doi: 10.1016/j.jpeds.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Isayama T, Lee SK, Yang J, Lee D, Daspal S, Dunn M, et al. Canadian Neonatal Network and Canadian Neonatal Follow-Up Network Investigators. Revisiting the definition of bronchopulmonary dysplasia: effect of changing panoply of respiratory support for preterm neonates. JAMA Pediatr. 2017;171:271–279. doi: 10.1001/jamapediatrics.2016.4141. [DOI] [PubMed] [Google Scholar]
- 21.Davis PG, Thorpe K, Roberts R, Schmidt B, Doyle LW, Kirpalani H Trial Indomethacin Prophylaxis in Preterms Investigators. Evaluating “old” definitions for the “new” bronchopulmonary dysplasia. J Pediatr. 2002;140:555–560. doi: 10.1067/mpd.2002.123291. [DOI] [PubMed] [Google Scholar]
- 22.Keller RL, Feng R, DeMauro SB, Ferkol T, Hardie W, Rogers EE, et al. Prematurity and Respiratory Outcomes Program. Bronchopulmonary dysplasia and perinatal characteristics predict 1-year respiratory outcomes in newborns born at extremely low gestational age: a prospective cohort study. J Pediatr. 2017;187:89–97.e3. doi: 10.1016/j.jpeds.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayley N. Bayley Scales of Infant and Toddler Development. 3rd ed. San Antonio, TX: Harcourt Assessment, Inc.; 2006. [Google Scholar]
- 24.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 25.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 26.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 27.Steyerberg EW, Harrell FE, Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–781. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 28.Higgins RD, Jobe AH, Koso-Thomas M, Bancalari E, Viscardi RM, Hartert TV, et al. Bronchopulmonary dysplasia: executive summary of a workshop. J Pediatr. 2018;197:300–308. doi: 10.1016/j.jpeds.2018.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gage S, Kan P, Oehlert J, Gould JB, Stevenson DK, Shaw GM, et al. Determinants of chronic lung disease severity in the first year of life: a population based study. Pediatr Pulmonol. 2015;50:878–888. doi: 10.1002/ppul.23148. [DOI] [PubMed] [Google Scholar]
- 30.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed. New York: John Wiley and Sons; 2000. Assessing the fit of the model; pp. 143–202. [Google Scholar]
- 31.Ambalavanan N, Walsh M, Bobashev G, Das A, Levine B, Carlo WA, et al. NICHD Neonatal Research Network. Intercenter differences in bronchopulmonary dysplasia or death among very low birth weight infants. Pediatrics. 2011;127:e106–e116. doi: 10.1542/peds.2010-0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al. National Institute of Child Health and Human Development Neonatal Research Network. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114:1305–1311. doi: 10.1542/peds.2004-0204. [DOI] [PubMed] [Google Scholar]
- 33.Stevens TP, Finer NN, Carlo WA, Szilagyi PG, Phelps DL, Walsh MC, et al. SUPPORT Study Group of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Respiratory outcomes of the Surfactant Positive Pressure and Oximetry Randomized Trial (SUPPORT) J Pediatr. 2014;165:240–249.e4. doi: 10.1016/j.jpeds.2014.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gergen PJ, Fowler JA, Maurer KR, Davis WW, Overpeck MD. The burden of environmental tobacco smoke exposure on the respiratory health of children 2 months through 5 years of age in the United States: Third National Health and Nutrition Examination Survey, 1988 to 1994. Pediatrics. 1998;101:E8. doi: 10.1542/peds.101.2.e8. [DOI] [PubMed] [Google Scholar]
- 35.Friedrich L, Stein RT, Pitrez PM, Corso AL, Jones MH. Reduced lung function in healthy preterm infants in the first months of life. Am J Respir Crit Care Med. 2006;173:442–447. doi: 10.1164/rccm.200503-444OC. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez-Solis M, Garcia-Marcos L, Bosch-Gimenez V, Pérez-Fernandez V, Pastor-Vivero MD, Mondéjar-Lopez P. Lung function among infants born preterm, with or without bronchopulmonary dysplasia. Pediatr Pulmonol. 2012;47:674–681. doi: 10.1002/ppul.21609. [DOI] [PubMed] [Google Scholar]
- 37.Drysdale SB, Wilson T, Alcazar M, Broughton S, Zuckerman M, Smith M, et al. Lung function prior to viral lower respiratory tract infections in prematurely born infants. Thorax. 2011;66:468–473. doi: 10.1136/thx.2010.148023. [DOI] [PubMed] [Google Scholar]
- 38.Laugier O, Garcia P, Boucékine M, Daguzan A, Tardieu S, Sambuc R, et al. Influence of socioeconomic context on the rehospitalization rates of infants born preterm. J Pediatr. 2017;190:174–179.e1. doi: 10.1016/j.jpeds.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Linsell L, Malouf R, Morris J, Kurinczuk JJ, Marlow N. Prognostic factors for cerebral palsy and motor impairment in children born very preterm or very low birthweight: a systematic review. Dev Med Child Neurol. 2016;58:554–569. doi: 10.1111/dmcn.12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linsell L, Malouf R, Morris J, Kurinczuk JJ, Marlow N. Prognostic factors for poor cognitive development in children born very preterm or with very low birth weight: a systematic review. JAMA Pediatr. 2015;169:1162–1172. doi: 10.1001/jamapediatrics.2015.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singer L, Yamashita T, Lilien L, Collin M, Baley J. A longitudinal study of developmental outcome of infants with bronchopulmonary dysplasia and very low birth weight. Pediatrics. 1997;100:987–993. doi: 10.1542/peds.100.6.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang H, Dysart K, Kendrick DE, Li L, Das A, Hintz SR, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Prolonged respiratory support of any type impacts outcomes of extremely low birth weight infants. Pediatr Pulmonol. 2018;53:1447–1455. doi: 10.1002/ppul.24124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malavolti AM, Bassler D, Arlettaz-Mieth R, Faldella G, Latal B, Natalucci G. Bronchopulmonary dysplasia-impact of severity and timing of diagnosis on neurodevelopment of preterm infants: a retrospective cohort study. BMJ Paediatr Open. 2018;2:e000165. doi: 10.1136/bmjpo-2017-000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Rossem MC, van de Loo M, Laan BJ, de Sonnaville ES, Tamminga P, van Kaam AH, et al. Accuracy of the diagnosis of bronchopulmonary dysplasia in a referral-based health care system. J Pediatr. 2015;167:540–544.e1. doi: 10.1016/j.jpeds.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Patel RM, Kandefer S, Walsh MC, Bell EF, Carlo WA, Laptook AR, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015;372:331–340. doi: 10.1056/NEJMoa1403489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.