Abstract
Rationale: Daily high-dose aspirin therapy benefits many patients with aspirin-exacerbated respiratory disease but provides no benefit for aspirin-tolerant patients with asthma. Type 2 inflammation characterizes aspirin-exacerbated respiratory disease.
Objectives: To determine whether high-dose aspirin therapy changes biomarkers of type 2 inflammation in aspirin-exacerbated respiratory disease.
Methods: Forty-two subjects with aspirin-exacerbated respiratory disease underwent an aspirin desensitization and were placed on high-dose aspirin (1,300 mg daily). Fifteen aspirin-tolerant subjects with asthma were also placed on high-dose aspirin. Biologic specimens and clinical parameters were collected at baseline and after 8 weeks on aspirin. Urinary eicosanoids, plasma tryptase and cytokine levels, platelet–leukocyte aggregates, and granulocyte transcripts were assessed.
Measurements and Main Results: Eight weeks of high-dose aspirin decreased nasal symptoms and urinary prostaglandin E metabolite (P < 0.05) and increased urinary leukotriene E4 (P < 0.01) levels in subjects with aspirin-exacerbated respiratory disease, but not in those with aspirin-tolerant asthma. Urinary prostaglandin D2 and thromboxane metabolites decreased in both groups. Only in subjects with aspirin-exacerbated respiratory disease, exhaled nitric oxide (P < 0.05), plasma tryptase (P < 0.01), and blood eosinophil (P < 0.01) and basophil (P < 0.01) counts increased and plasma tryptase correlated with eosinophil counts (Pearson r = 0.514; P < 0.01) on aspirin. After correction for eosinophil counts, aspirin-induced changes in blood granulocyte transcripts did not differ between groups. Aspirin had no effect on platelet–leukocyte aggregates, platelet activation markers, or plasma cytokines in either group.
Conclusions: High-dose aspirin therapy for 8 weeks paradoxically increases markers of type 2 inflammation in subjects with aspirin-exacerbated respiratory disease, despite reducing nasal symptoms. This effect of aspirin is unique to aspirin-exacerbated respiratory disease and not observed in subjects with aspirin-tolerant asthma.
Keywords: aspirin-exacerbated respiratory disease, type 2 inflammation, aspirin-tolerant asthma, mast cell, cysteinyl leukotrienes
At a Glance Commentary
Scientific Knowledge on the Subject
High-dose aspirin therapy is the only targeted therapy available for aspirin-exacerbated respiratory disease, a phenotype of asthma characterized by mast cell activation and cysteinyl leukotriene production, yet the mechanism of clinical benefit is poorly understood.
What This Study Adds to the Field
In 42 subjects with aspirin-exacerbated respiratory disease, 8 weeks of aspirin 1,300 mg daily paradoxically increased markers of type 2 inflammation, mast cell activation, cysteinyl leukotriene production, peripheral blood eosinophilia, and exhaled nitric oxide, findings not observed in 15 aspirin-tolerant subjects with asthma. These observations support that the exquisite dependence on prostaglandin E2 inhibition for mast cell activation and 5-lipooxygenase activity is a central defect in aspirin-exacerbated respiratory disease and reveal that the therapeutic benefit of high-dose aspirin is independent of a global change in the prostaglandin E2 pathway.
Aspirin-exacerbated respiratory disease (AERD) is characterized by late-onset asthma, chronic rhinosinusitis with nasal polyposis, tissue eosinophilia, and pathognomonic acute respiratory reactions upon exposure to all nonselective cyclooxygenase (COX) inhibitors. Aspirin desensitization followed by daily high-dose aspirin therapy slows nasal polyp regrowth and improves respiratory symptoms after 6–12 months and can be offered as a therapeutic option to most patients with AERD (1, 2). Aspirin therapy provides no benefit for patients with aspirin-tolerant asthma (ATA) (3). Despite a working understanding of the chronic baseline and acute COX-1 inihibitor–induced reactions in AERD, the mechanism by which daily aspirin provides therapeutic benefit in AERD and the early biomarkers of clinical response are poorly understood.
At baseline, AERD is characterized by dysregulated eicosanoid pathways leading to profound type 2 inflammation. Impairments in respiratory tract prostaglandin (PG) E2 production and/or signaling lead to mast cell activation and 5-lipoxygenase activation. Elevated baseline urinary cysteinyl leukotriene (cysLT) and PGD2 metabolite (PGD-M) levels may reflect markers of an impaired PGE2 pathway. Platelet–leukocyte aggregates, which are increased at baseline in subjects with AERD, serve as an additional source of cysLTs and may facilitate recruitment of granulocytes to the tissue (4, 5). On COX-1 inhibition, metabolites of the cysLT and PGD2 pathways, leukotriene E4 (LTE4) and PGD-M, respectively, surge above their elevated baselines and correspond with profound upper and lower respiratory symptoms. Reactions also involve mast cell activation (indicated by increases in nasal fluid and/or serum/plasma tryptase [6, 7]) and effector cell (eosinophil and innate lymphoid type 2 cell) recruitment from the peripheral blood into the respiratory tract (8, 9). The impact of high-dose aspirin therapy on these known biomarkers of AERD has not been systematically studied and compared with the response to high-dose aspirin in aspirin-tolerant control subjects.
Previous studies of high-dose aspirin therapy in patients with AERD propose type 1 cysLT receptor downregulation (10), PGD2 inhibition (8), and IL-4/STAT-6 inhibition (11, 12) as potential mechanisms of the clinical benefit. Despite clear clinical benefits, small studies of subjects with AERD demonstrate that high-dose aspirin may paradoxically increase levels of serum tryptase and urinary LTE4 (8, 11). In a clinical trial of high-dose aspirin (650 mg twice daily) in subjects with ATA and AERD we tested the hypothesis that the clinical benefit of daily high-dose aspirin in AERD is independent of a reduction in mast cell activation and cysLT production. The results from this phase of the clinical trial have not been previously reported. The primary endpoints from the prasugrel crossover phase of the clinical trial (Figure 1A) (NCT01597375) were negative and were previously reported (13).
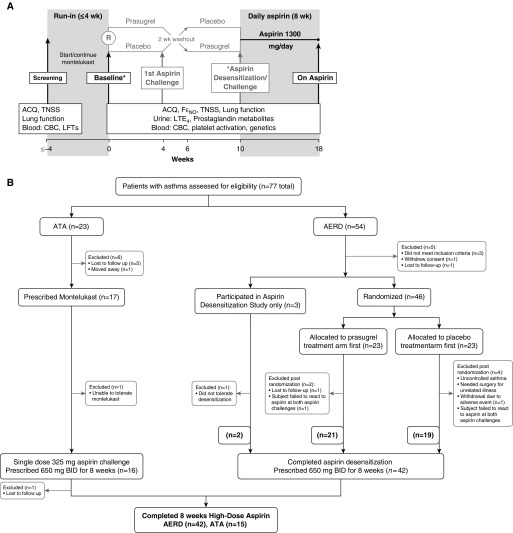
Figure 1.
Trial design. (A) Overview of the trial design and procedures. The period between screening and V1 was a minimum of 24 hours (for patients already taking montelukast) and a maximum of 4 weeks. At the baseline visit, baseline disease symptoms including asthma control questionnaire and total nasal symptom scores, lung function, and fractional exhaled nitric oxide were assessed, and blood and urine samples were collected. Most patients with aspirin-exacerbated respiratory disease (AERD) then underwent randomization to prasugrel or placebo and underwent two aspirin challenge/desensitization visits (gray font) and completed desensitization at Week 10. *The remainder completed aspirin challenge (all patients with aspirin-tolerant asthma [ATA]) and desensitization (remaining patients with AERD) at the baseline visit at Week 0. All subjects who successfully completed challenge and/or desensitization returned on high-dose aspirin 8 weeks later. (B) Summary of the numbers of patients involved in screening, randomization, and study completion for subjects with AERD and ATA. ACQ = asthma control questionnaire; BID = twice a day; CBC = complete blood count; FeNO = fractional exhaled nitric oxide; LFT = liver function test; LTE4 = leukotriene E4; TNSS = total nasal symptom scores.
Methods
After a 4-week run-in period on stable asthma treatment and montelukast (10 mg daily), subjects with ATA and AERD were placed on 650 mg of aspirin twice daily for 8 weeks. Subjects with AERD underwent a 1-day aspirin desensitization protocol as previously reported (13) and ATA subjects underwent a single-dose 325-mg aspirin challenge. Each subject’s baseline asthma treatment regimen including montelukast at stable dosages was continued throughout the duration of the study period. Before exposure to aspirin, baseline blood, urine, and lung function were collected in all subjects, and fractional exhaled nitric oxide (FeNO) was collected in subjects with AERD (Figure 1A). Symptomatic response to high-dose aspirin therapy was assessed by the seven-item asthma control questionnaire (ACQ-7) with lower values denoting better control and a minimally important difference of 0.5 (14, 15) and the total nasal sinus symptom (TNSS) score, a composite score of nasal congestion, runny nose, itchy nose, sneezing, itchy eyes, teary eyes, itchy ears or throat, or eye redness (0–5 for each question, maximum 40 with a lower value denoting less severe symptoms) (13).
Participants with AERD had a history of physician-diagnosed asthma and nasal polyposis, and clinical reactions to oral aspirin with features of upper and/or lower airway involvement confirmed by formal aspirin challenge (13). They met clinical qualifications for high-dose aspirin therapy with respiratory symptoms that were not adequately responsive to other standard therapies, including inhaled and intranasal corticosteroids and leukotriene modifiers. Participants with ATA had physician-diagnosed asthma, no current nasal polyposis, and no history of adverse reaction to any nonselective COX inhibitor. Both groups had stable asthma, defined as a post-bronchodilator FEV1 of greater than or equal to 70% of predicted, no increase in baseline dose of oral glucocorticoids for at least 3 months, and no history of hospitalization or emergency room visits for asthma for at least the prior 6 months. Patients were excluded if they had current severe gastroesophageal reflux disease, a history of peptic ulcer disease, gastrointestinal bleed or bleeding diathesis, or current use of anticoagulant or any antiplatelet drugs. All patients were between the ages of 18 and 65 years old, were nonpregnant, non-breast-feeding, were not current smokers, and signed informed consent. This was a single-site study, conducted at the Asthma Research Center of the Brigham and Women’s Hospital and approved by the local institutional review board.
Urinary Measurements
All urine samples were stored at −80°C and were analyzed by gas chromatography–mass spectrometry at Vanderbilt University as previously described (4). Concentrations of LTE4, the major urinary thromboxane metabolite 11-dehydrothromboxane B2 (TXB-M) (16), the major PGD2 metabolite 9a,11b-dihydroxy-15-oxo-2,3,18,19-tetranorprost-5-ene-1,20-dioic acid (PGD-M) (17), and the PGE metabolite 9,15-dioxo-11a-hydroxy-13,14-dihydro-2,3,4,5-tetranor-prostan-1,20-dioic acid (PGE-M) were measured and reported as picomoles per milligram of creatinine.
Flow Cytometry
Peripheral blood was drawn into heparinized tubes, kept at room temperature, and processed or assayed within 1 hour of collection. Complete blood counts with differential were processed at a reference laboratory (LabCorp). Platelet-rich plasma was obtained from the top layer after a 20-minute centrifuge at 200 × g. Platelet-rich plasma was incubated with directly conjugated antibodies specific for CD61 and CD62P and whole blood was incubated with directly conjugated antibodies specific for CD61, CD45, CD14, and CCR3, or appropriate isotype controls (BD Biosciences). Cells were fixed in 1% paraformaldehyde and at least 20,000 CD45+ cells for whole blood analyses or 50,000 platelets for platelet-rich plasma analyses were recorded for each sample an a FACSAria flow cytometer (BD Biosciences) and analyzed using FlowJo Version 10 (TreeStar).
Plasma Protein Measurements
Plasma processed within 1 hour of collection was assayed for total and mature tryptase analysis performed at Virginia Commonwealth University by UniCAP (18, 19). Plasma samples were assayed for granulocyte-macrophage colony–stimulating factor, IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-9, IL-10, IL-12p70, IL-13, IL-17A, monocyte chemoattractant protein 1, macrophage inflammatory protein (MIP) 1α, MIP1β, and tumor necrosis factor-α using a multiplex immunoassay (Fireplex, Abcam) and total IgE by an ELISA (Invitrogen).
Peripheral Blood Granulocyte RNA Sequencing
The 75-bp paired-end reads RNA sequencing of patient-derived peripheral blood granulocytes was performed at the Partners Personalized Medicine Translational Genomics Core. Libraries were prepared using the Illumina TruSeq RNA library preparation kit (Illumina, Inc.) and run on the Illumina HiSeq system. Following adapter trimming with Skewer, reads were aligned to the GRCH38 human genome build with STAR. Quality control for the sequencing reads was done using FastQC. Samples were excluded if they had less than 10 million total reads or there was a discrepancy between XIST and Y chromosome expression and reported sex. Differential expression analysis of autosomal transcripts was performed using the “DESeq2” Bioconductor package. Statistical significance was declared at a Benjamini-Hochberg adjusted P value less than 0.05.
Outcome Measures
Primary outcomes included change in urinary LTE4, PGD-M, PGE-M, TXB2, FeNO, plasma tryptase, peripheral blood granulocyte counts, platelet–leukocyte aggregates, and COX-2 expression in peripheral blood granulocytes from baseline after 8 weeks of 1,300 mg of aspirin daily, compared between AERD and ATA groups. Clinical outcomes included change in FEV1% of predicted, TNSS, and ACQ-7 in both groups. Exploratory outcomes included association among urinary eicosanoid levels, mast cell activation as measured by plasma tryptase, and markers of eosinophilic inflammation including peripheral blood granulocyte counts and FeNO.
Statistical Analyses
Data are expressed as means ± SD unless otherwise noted. Two-sided paired Student’s t test assessed change on high-dose aspirin from baseline for both groups. A Student’s t test assessed for a difference in the change on aspirin between the AERD and ATA groups. Correlation between biomarkers on high-dose aspirin was assessed using Pearson correlation coefficient. Analysis was performed using GraphPad Prism version 7.03 for Windows (GraphPad Software) and SAS 9.4.
Results
Clinical Outcomes
Forty-nine subjects with suspected AERD underwent aspirin challenge and attempted aspirin desensitization. Forty-seven subjects demonstrated a positive clinical reaction to aspirin and 42 went on to tolerate high-dose aspirin therapy for 8 weeks (Figures 1A and 1B). Twenty-three ATA subjects were screened and 15 completed an observed single-dose aspirin challenge and returned on high-dose aspirin therapy after 8 weeks (Figure 1B). Subjects with AERD were older, had higher baseline peripheral blood eosinophil counts, and better asthma control as measured by ACQ-7 compared with ATAs (Table 1). Each group used similar amounts of inhaled corticosteroids, whereas subjects with AERD were more likely to be treated with a long-acting β-agonist. There was no difference in sex, race, ethnicity, body mass index, baseline lung function, or TNSS between groups.
Table 1.
Patient Demographics
| All AERD (n = 47) | ATA (n = 16) | P Value | |
|---|---|---|---|
| Age, yr | 47.0 ± 9.2 | 34.4 ± 15.3 | <0.0001 |
| Sex, F | 27 (57) | 12 (75) | NS |
| Race | |||
| White | 43 (92) | 12 (75) | NS |
| Ethnicity | |||
| Hispanic | 3 (6) | 2 (13) | NS |
| Body mass index | 28.6 ± 6.0 | 26.8 ± 4.7 | NS |
| FEV1, L | 3.06 (0.82) | 2.95 (0.56) | NS |
| FEV1% predicted | 91.2 ± 12.5 | 86.7 ± 10.9 | NS |
| Fraction of exhaled nitric oxide, ppb | 46.1 ± 26.8 | DNC | |
| Peripheral eosinophil count, /μl | 0.40 ± 0.33 | 0.23 ± 0.11 | <0.05 |
| Total IgE, ng/ml | 795 ± 1,370 | 1,062 ± 1,370 | NS |
| ACQ-7 | 0.68 ± 0.59 | 1.21 ± 0.36 | <0.01 |
| TNSS | 4.3 ± 4.6 | 2.2 ± 2.9 | 0.12 |
| Low ICS dose (≤200 μg*) | 17 (36) | 5 (33) | |
| Medium ICS dose (201–500 μg*) | 19 (40) | 5 (33) | |
| High ICS dose (>500 μg*) | 4 (9) | 1 (7) | |
| Oral glucocorticoid use | 2 (4) | 0 | |
| Long-acting β-agonist use | 30 (64) | 4 (27) | <0.05 |
Definition of abbreviations: ACQ = asthma control questionnaire; AERD = aspirin-exacerbated respiratory disease; ATA = aspirin-tolerant asthma; DNC = data not collected; ICS = inhaled corticosteroid; NS = not significant; TNSS = total nasal symptoms score.
Values are n (%) or means ± SD.
Fluticasone proprionate dry powder equivalent.
On high-dose aspirin, TNSS decreased in subjects with AERD (4.3 ± 0.7 to 2.2 ± 0.6; P < 0.05), whereas no change was noted in ATAs, despite similar baseline TNSS scores in the ATA subjects. There was no change in FEV1% predicted or ACQ-7 in either group. FeNO increased significantly on high-dose aspirin (from 46.2 ± 26.8 to 64.2 ± 42.6; P < 0.0) in subjects with AERD (Table 2). FeNO was not assessed in ATA.
Table 2.
Change in Clinical Parameters on High-Dose Aspirin
| AERD | ATA | |
|---|---|---|
| FEV1, % | −0.76 ± 1.78 | 1.41 ± 1.73 |
| FeNO, ppb | 15.8 ± 5.8* | DNC |
| ACQ-7 | −0.11 ± 0.11 | −0.17 ± 0.19 |
| TNSS | −1.63 ± 0.73† | −0.79 ± 0.97 |
Definition of abbreviations: ACQ = asthma control questionnaire; AERD = aspirin-exacerbated respiratory disease; ATA = aspirin-tolerant asthma; DNC = data not collected; FeNO = fractional exhaled nitric oxide; TNSS = total nasal symptoms score.
Data are expressed as means ± SEM.
P < 0.01.
P < 0.05.
Urinary Eicosanoids
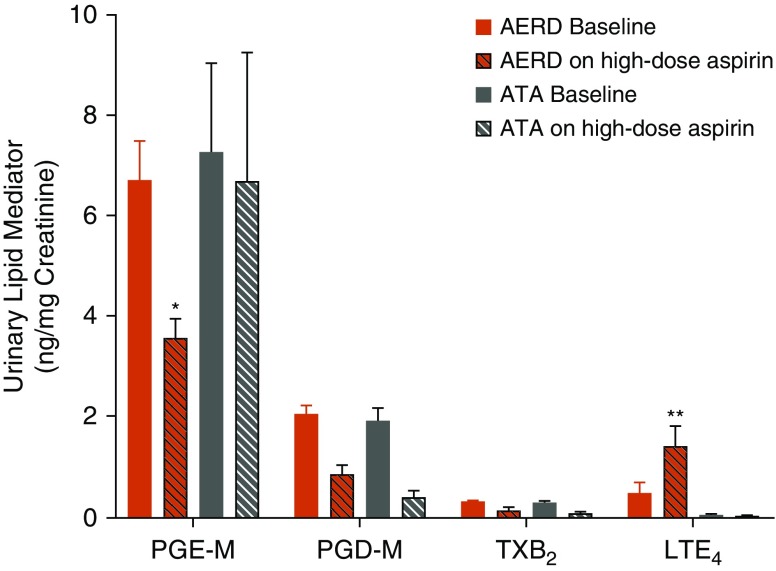
Eicosanoid levels were assessed in the urine at baseline and after 8 weeks of high-dose aspirin in both subjects with AERD and ATA. High-dose aspirin decreased urinary (u) PGE-M and increased uLTE4, the stable end metabolite of the cysLTs, in subjects with AERD but not ATA, while suppressing uPGD-M and uTXB2 similarly in both groups (Figure 2). Change in uPGD-M, uPGE-M, and uLTE4 from baseline did not correlate with change in TNSS, ACQ-7, FEV1% predicted, or FeNO (see Table E1 in the online supplement).
Figure 2.
High-dose aspirin suppresses prostaglandin E2 metabolite and increases cysteinyl leukotrienes in subjects with aspirin-exacerbated respiratory disease (AERD). Urinary eicosanoids were assessed at baseline and after 8 weeks of high-dose aspirin in subjects with AERD and aspirin-tolerant asthma (ATA). P values reflect a difference in change from baseline between the AERD and ATA groups. LTE4 = leukotriene E4 (AERD n = 40, ATA n = 13); PGD-M = prostaglandin D2 metabolite (AERD n = 43, ATA n = 15); PGE-M = prostaglandin E2 metabolite (AERD n = 43, ATA n = 15); TXB2 = thromboxane B2 (AERD n = 41, ATA n = 15). Data are expressed as means + SEM. *P < 0.05 and **P < 0.01.
Peripheral Blood Cells
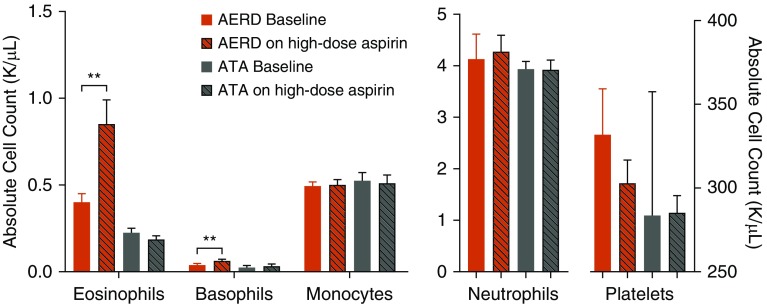
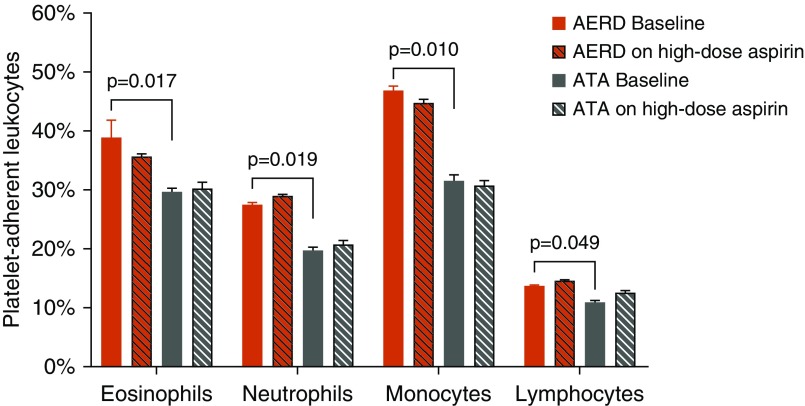
At baseline, the subjects with AERD demonstrated a trend toward higher absolute peripheral blood eosinophil counts as compared with ATA (Figure 3), which further increased on high-dose aspirin as previously reported (8). In this larger cohort of subjects, basophils also increased, whereas neutrophils, monocytes, and platelets remained unchanged (Figure 3). Aspirin therapy had no effect on peripheral blood leukocyte counts in ATA. Although we confirmed subjects with AERD had greater numbers of platelet–leukocyte aggregates as compared with ATA at baseline (Figure 4) (4, 5), there was no change in numbers of platelet–leukocyte aggregates on high-dose aspirin in either group (Figure 4), or in platelet activation as assessed by CD62P (data not shown). Eosinophil counts on high-dose aspirin in subjects with AERD correlated weakly with FeNO levels (Pearson correlation coefficient rp = 0.314; P = 0.07; data not shown).
Figure 3.
High-dose aspirin increases CRTH2+ peripheral blood cells in aspirin-exacerbated respiratory disease (AERD). Peripheral blood leukocyte counts at baseline and at 8 weeks on high-dose aspirin in subjects with AERD and aspirin-tolerant asthma (ATA). No difference in baseline eosinophil, basophil, monocyte, neutrophil, or platelet counts between ATA and AERD were observed. High-dose aspirin resulted in marked elevation of peripheral blood eosinophil (415–879/μl; P < 0.01) and basophil (43–66/μl; P < 0.01) counts, whereas no change was noted in neutrophils, monocytes, or platelets. Data are expressed as means + SEM. **P < 0.01.
Figure 4.
Platelet–leukocyte aggregates are increased in the blood of patients with aspirin-exacerbated respiratory disease (AERD) at baseline and do not change with 8 weeks of high-dose aspirin therapy. Percentages of leukocytes with adherent platelets (as determined by staining with CD61) in blood of aspirin-tolerant asthma control subjects (n = 15) and subjects with AERD (n = 36). Data are expressed as means + SEM. ATA = aspirin-tolerant asthma.
Mast Cells
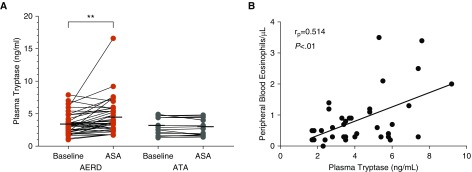
No difference in baseline plasma tryptase was observed between subjects with ATA and AERD. Plasma total tryptase increased and correlated with peripheral blood eosinophil counts (Pearson r = 0.514; P < 0.01) (Figures 5A and 5B) but not with uLTE4, uPGD-M, or uPGE-M (see Figures E1A and E1B) in subjects with AERD on high-dose aspirin. Mature tryptase, reflecting processing associated with mast cell degranulation (18), was not detected at baseline or on high-dose aspirin in either group (data not shown). Aspirin had no effect on total tryptase levels in ATA subjects (Figure 5A).
Figure 5.
Mast cell activation increases on high-dose aspirin and correlates with peripheral blood eosinophilia observed in aspirin-exacerbated respiratory disease (AERD). (A) Plasma total tryptase at baseline and on 8 weeks of high-dose aspirin in subjects with AERD and aspirin-tolerant asthma. Horizontal bars reflect group mean. **P < 0.01. (B) Plasma tryptase correlation (Pearson) with peripheral blood eosinophil counts on high-dose aspirin in subjects with AERD. ASA = aspirin; ATA = aspirin-tolerant asthma.
Plasma Cytokines
No change in cytokine levels was observed in either AERD or ATA subjects on daily aspirin. Of the assays performed, levels of IL-5, IL-6, IL-8, IL-9, monocyte chemoattractant protein 1, MIP1α, MIP1β, and tumor necrosis factor-α were consistently detected in most samples, with no trends toward differences across time points. Levels of granulocyte-macrophage colony–stimulating factor, IL-10, IL-1β, IL-12p10, IL-13, IL-7A, and INF-γ were detected in none or only a few of the samples. Total IgE levels remained unchanged on high-dose aspirin in both groups (data not shown).
Peripheral Blood Granulocyte RNA Sequencing
Because aspirin is a known acetylator that could influence gene expression (20), we analyzed RNA-sequencing data from peripheral blood granulocytes between subjects with AERD (n = 25) and ATA (n = 10) to look for changes in gene expression attributable to disease, adjusting for age, sex, and race (see Table E2). Forty-five transcripts were differentially expressed at an adjusted P less than 0.05. However, only one transcript, EFHC1, remained statistically significant (adjusted P < 0.05) after correcting for peripheral blood eosinophil counts. No differential expression was observed when the analysis was restricted to the 29 subjects (19 with AERD, 10 with ATA) with normal eosinophil counts (<500 cells/μl). We then evaluated the effect of high-dose aspirin in AERD by analyzing the subset of 15 subjects who had analyzable RNA-sequencing data at baseline and following aspirin therapy. In a model that accounted for the paired data and treatment group, we found 379 transcripts that were differentially expressed following aspirin treatment, including CYSLTR2, RNASE3, and ORMDL3 (see Table E3). After adjustment for eosinophil counts, there were no differentially expressed genes, suggesting that the bulk of the observed signal likely reflects treatment-induced changes in granulocyte composition.
Discussion
High-dose aspirin therapy is currently the only disease-specific therapeutic that modifies the progression of nasal polyposis, decreases topical and systemic steroid requirements, and improves quality of life for patients with AERD. These benefits are observed in 67–87% of patients with AERD treated with high-dose aspirin for 6–12 months (1), but high-dose aspirin provides no therapeutic benefit to patients with ATA (3). The mechanism of the benefit of daily aspirin therapy in patients with AERD remains poorly understood with no available surrogate biomarker of response. Baseline impairments in PGE2 production and/or receptor signaling are well described in multiple cell types, across multiple studies in subjects with AERD (21–24).
In 42 carefully phenotyped subjects with AERD, we observed that 650 mg of aspirin twice-daily for 8 weeks failed to correct any of the known baseline derangements in PGE2 production, cysLT overproduction (Figure 2), and mast cell activation (Figure 4A). In fact, the high-dose aspirin-induced reduction in PGE-M levels (reflecting the anticipated pharmacologic effect of aspirin on COX function) was associated with greater systemic markers of type 2 inflammation: increased total mast cell activation (Figure 4B), a further increase in urinary LTE4 (Figure 2), the stable end metabolite of the cysLTs, and a doubling of peripheral blood eosinophil counts (Figure 3). The sustained urinary PGE-M levels observed after 8 weeks of high-dose aspirin therapy in subjects with ATA, although surprising, are in agreement with some studies of long-term aspirin use in animals and humans, and potentially reflect increased COX-2 expression induced by aspirin in some cell types (25–28). These observations validate PGE2 as an important regulator of mast cell activation and 5-lipoxygenase activity, and demonstrate that the failure to induce COX-2, and subsequently sustain PGE2, is a central defect in AERD. However, they also reveal that the therapeutic benefit of high-dose aspirin is independent of a global change in the PGE2 pathway.
Systemic mast cell activation, as assessed by plasma tryptase, increases in the peripheral blood of subjects with AERD who are on high-dose aspirin (Figure 5A), but not in subjects with ATA. Previous studies have observed an increase in sputum tryptase in subjects with AERD treated with daily aspirin (11). Our data suggest that increases in both mast cell activation and/or burden and cysLT generation occur, whereas total body COX products (PGE-M, PGD-M, and TXB2) are suppressed on high-dose aspirin. Furthermore, the increase in plasma tryptase levels we demonstrate correlates with a rise in PGD2-responsive CRTH2+ eosinophils and basophils in the peripheral blood (Figure 5B), despite the suppression in urinary PGD2 metabolites (Figure 2). We suspect that although the depletion of PGE2 may allow for increasingly leaky mast cells (reflected in the increases in tryptase), the high-dose aspirin-induced suppression of PGD2 leads to a loss of the chemotactic gradient that drives eosinophils and basophils into the respiratory tissues. Notably, FeNO (commonly used as a surrogate of eosinophilic inflammation) significantly increased on high-dose aspirin (Table 2). Given that inhalation challenges with PGE2 and PGF2α decrease exhaled FeNO in healthy humans and subjects with asthma (29), it seems likely that the effect of aspirin on FeNO reflects a direct effect of endogenous COX products on epithelial cells or other cells that express inducible nitric oxide synthase.
High-dose aspirin therapy increases cysLT production while having no effect on the levels of platelet–leukocyte aggregation or platelet activation seen in the blood of subjects with AERD. Any residual effects of prasugrel exposure 8–14 weeks before the assessments on 8 weeks of daily aspirin in subjects with AERD, although unlikely given the drug half-life of approximately 7 hours, cannot be ruled out. Although mast cells may be a primary source of increased cysLTs in patients with AERD on high-dose aspirin, a global loss of the PGE2 needed to control 5-lipoxygenase function could also increase the throughput of LTA4 into other granulocytes and platelets, thereby providing the existing platelet–leukocyte aggregates more substrate to convert to cysLTs. A reduction in end-organ responsiveness (10), or desensitization to excessive cysLT production, has been offered as an explanation for the mechanism of desensitization and tolerance of daily aspirin therapy and has been suggested separately in smaller cohorts of patients. Our current study, with a much larger cohort and direct comparison with ATA subjects, supports and strengthens the previous findings.
Clinical experience has shown that patients who fail to tolerate daily aspirin therapy because of side effects of gastrointestinal symptoms, rash, or worsening lung function typically present with these side effects within the first few weeks of therapy. The increase in mast cell activation and elevation of cysLTs observed in this study may not be tolerated by all patients, which may explain why some patients are unable to continue daily aspirin therapy. The modest clinical benefit, a reduction in nasal symptoms, we observed after 8 weeks of daily aspirin therapy is consistent with our clinical experience and prior publications. The full benefit from daily aspirin therapy can take 6–12 months (1) to be readily clinically evident and is primarily evident as a reduction in nasal polyp regrowth, glucocorticoid use, sinusitis, and/or asthma exacerbations. This study was inadequately powered to report on these late clinical endpoints.
We have found that further and/or persistent reductions in systemic measurements of PGE2 levels during daily aspirin therapy are associated with increased markers of type 2 inflammation characterized by mast cell activation, cysLT generation, and peripheral blood eosinophilia. These effects of high-dose aspirin are unique to the patient population with AERD and are not seen in patients with ATA. We conclude that high-dose aspirin therapy does not correct the impaired eicosanoid and mast cell homeostasis that characterize the chronic baseline and acute COX-1 inhibitor–induced reaction disease states in AERD. Future studies need to look beyond the known biomarkers of AERD and focus on the local respiratory tract microenvironment to solve this enigma.
Supplementary Material
Footnotes
Supported by the NIH (grants K23HL111113, K23AI118804, R01HL128241, U19 AI095219, R01AI136041, R01HL136209, RO1 AI078908, R37AI052353, and T32AI007306-32) and by generous contributions from the Kaye and Vinik families.
Author Contributions: K.N.C., B.A.R., E.I., J.A.B., and T.M.L. designed the study. K.N.C. and T.M.L. executed the study design. K.N.C., J.C., P.K., K.M., B.A.R., J.S., and T.M.L. acquired and analyzed the data. K.N.C., J.C., P.K., B.A.R., E.I., J.A.B., and T.M.L. interpreted the data. K.N.C., J.C., P.K., J.A.B., and T.M.L. wrote the manuscript draft, which was reviewed and revised by all authors. All authors attest to the accuracy of the work submitted.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201809-1755OC on April 12, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Berges-Gimeno MP, Simon RA, Stevenson DD. Long-term treatment with aspirin desensitization in asthmatic patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2003;111:180–186. doi: 10.1067/mai.2003.7. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson DD, Hankammer MA, Mathison DA, Christiansen SC, Simon RA. Aspirin desensitization treatment of aspirin-sensitive patients with rhinosinusitis-asthma: long-term outcomes. J Allergy Clin Immunol. 1996;98:751–758. doi: 10.1016/s0091-6749(96)70123-9. [DOI] [PubMed] [Google Scholar]
- 3.Świerczyńska-Krępa M, Sanak M, Bochenek G, Stręk P, Ćmiel A, Gielicz A, et al. Aspirin desensitization in patients with aspirin-induced and aspirin-tolerant asthma: a double-blind study. J Allergy Clin Immunol. 2014;134:883–890. doi: 10.1016/j.jaci.2014.02.041. [DOI] [PubMed] [Google Scholar]
- 4.Laidlaw TM, Kidder MS, Bhattacharyya N, Xing W, Shen S, Milne GL, et al. Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood. 2012;119:3790–3798. doi: 10.1182/blood-2011-10-384826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitsui C, Kajiwara K, Hayashi H, Ito J, Mita H, Ono E, et al. Platelet activation markers overexpressed specifically in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016;137:400–411. doi: 10.1016/j.jaci.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 6.Bosso JV, Schwartz LB, Stevenson DD. Tryptase and histamine release during aspirin-induced respiratory reactions. J Allergy Clin Immunol. 1991;88:830–837. doi: 10.1016/0091-6749(91)90238-j. [DOI] [PubMed] [Google Scholar]
- 7.Fischer AR, Rosenberg MA, Lilly CM, Callery JC, Rubin P, Cohn J, et al. Direct evidence for a role of the mast cell in the nasal response to aspirin in aspirin-sensitive asthma. J Allergy Clin Immunol. 1994;94:1046–1056. doi: 10.1016/0091-6749(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 8.Cahill KN, Bensko JC, Boyce JA, Laidlaw TM. Prostaglandin D2: a dominant mediator of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2015;135:245–252. doi: 10.1016/j.jaci.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eastman JJ, Cavagnero KJ, Deconde AS, Kim AS, Karta MR, Broide DH, et al. Group 2 innate lymphoid cells are recruited to the nasal mucosa in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2017;140:101–108, e3. doi: 10.1016/j.jaci.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sousa AR, Parikh A, Scadding G, Corrigan CJ, Lee TH. Leukotriene-receptor expression on nasal mucosal inflammatory cells in aspirin-sensitive rhinosinusitis. N Engl J Med. 2002;347:1493–1499. doi: 10.1056/NEJMoa013508. [DOI] [PubMed] [Google Scholar]
- 11.Katial RK, Strand M, Prasertsuntarasai T, Leung R, Zheng W, Alam R. The effect of aspirin desensitization on novel biomarkers in aspirin-exacerbated respiratory diseases. J Allergy Clin Immunol. 2010;126:738–744. doi: 10.1016/j.jaci.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 12.Katial RK, Martucci M, Burnett T, Faino A, Finkas L, Liu S, et al. Nonsteroidal anti-inflammatory-induced inhibition of signal transducer and activator of transcription 6 (STAT-6) phosphorylation in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016;138:579–585. doi: 10.1016/j.jaci.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 13.Laidlaw TM, Cahill KN, Cardet JC, Murphy K, Cui J, Dioneda B, et al. A trial of type 12 purinergic (P2Y12) receptor inhibition with prasugrel identifies a potentially distinct endotype of patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2019;143:316–324, e7. doi: 10.1016/j.jaci.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax. 1992;47:76–83. doi: 10.1136/thx.47.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific quality of life questionnaire. J Clin Epidemiol. 1994;47:81–87. doi: 10.1016/0895-4356(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 16.Morrow JD, Minton TA. Improved assay for the quantification of 11-dehydrothromboxane B2 by gas chromatography-mass spectrometry. J Chromatogr A. 1993;612:179–185. doi: 10.1016/0378-4347(93)80161-v. [DOI] [PubMed] [Google Scholar]
- 17.Morrow JD, Guzzo C, Lazarus G, Oates JA, Roberts LJ., II Improved diagnosis of mastocytosis by measurement of the major urinary metabolite of prostaglandin D2. J Invest Dermatol. 1995;104:937–940. doi: 10.1111/1523-1747.ep12606209. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz LB, Bradford TR, Rouse C, Irani AM, Rasp G, Van der Zwan JK, et al. Development of a new, more sensitive immunoassay for human tryptase: use in systemic anaphylaxis. J Clin Immunol. 1994;14:190–204. doi: 10.1007/BF01533368. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz LB. Tryptase, a mediator of human mast cells. J Allergy Clin Immunol. 1990;86:594–598. doi: 10.1016/s0091-6749(05)80222-2. [DOI] [PubMed] [Google Scholar]
- 20.Alfonso L, Ai G, Spitale RC, Bhat GJ. Molecular targets of aspirin and cancer prevention. Br J Cancer. 2014;111:61–67. doi: 10.1038/bjc.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roca-Ferrer J, Garcia-Garcia FJ, Pereda J, Perez-Gonzalez M, Pujols L, Alobid I, et al. Reduced expression of COXs and production of prostaglandin E(2) in patients with nasal polyps with or without aspirin-intolerant asthma. J Allergy Clin Immunol. 2011;128:66–72, e1. doi: 10.1016/j.jaci.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 22.Cahill KN, Raby BA, Zhou X, Guo F, Thibault D, Baccarelli A, et al. Impaired E Prostanoid2 expression and resistance to prostaglandin E2 in nasal polyp fibroblasts from subjects with aspirin-exacerbated respiratory disease. Am J Respir Cell Mol Biol. 2016;54:34–40. doi: 10.1165/rcmb.2014-0486OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laidlaw TM, Cutler AJ, Kidder MS, Liu T, Cardet JC, Chhay H, et al. Prostaglandin E2 resistance in granulocytes from patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2014;133:1692–1701, e3. doi: 10.1016/j.jaci.2013.12.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corrigan CJ, Napoli RL, Meng Q, Fang C, Wu H, Tochiki K, et al. Reduced expression of the prostaglandin E2 receptor E-prostanoid 2 on bronchial mucosal leukocytes in patients with aspirin-sensitive asthma. J Allergy Clin Immunol. 2012;129:1636–1646. doi: 10.1016/j.jaci.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Quilley CP, McGiff JC, Quilley J. Failure of chronic aspirin treatment to inhibit urinary prostaglandin excretion in spontaneously hypertensive rats: comparison with indomethacin and flurbiprofen. J Pharmacol Exp Ther. 1987;240:916–921. [PubMed] [Google Scholar]
- 26.Collier JG, Flower RJ. Effect of aspirin on human seminal prostaglandins. Lancet. 1971;2:852–853. doi: 10.1016/s0140-6736(71)90225-x. [DOI] [PubMed] [Google Scholar]
- 27.Horton EW, Jones RL, Marr CG. Effects of aspirin on prostaglandin and fructose levels in human semen. J Reprod Fertil. 1973;33:385–392. doi: 10.1530/jrf.0.0330385. [DOI] [PubMed] [Google Scholar]
- 28.Mifflin RC, Saada JI, Di Mari JF, Valentich JD, Adegboyega PA, Powell DW. Aspirin-mediated COX-2 transcript stabilization via sustained p38 activation in human intestinal myofibroblasts. Mol Pharmacol. 2004;65:470–478. doi: 10.1124/mol.65.2.470. [DOI] [PubMed] [Google Scholar]
- 29.Kharitonov SA, Sapienza MA, Barnes PJ, Chung KF. Prostaglandins E2 and F2alpha reduce exhaled nitric oxide in normal and asthmatic subjects irrespective of airway caliber changes. Am J Respir Crit Care Med. 1998;158:1374–1378. doi: 10.1164/ajrccm.158.5.9707076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.