Abstract
Rationale: Serial measurements of alveolar macrophage (AM) transcriptional changes in patients with acute respiratory distress syndrome (ARDS) could identify cell-specific biological programs that are associated with clinical outcomes.
Objectives: To determine whether AM transcriptional programs are associated with prolonged mechanical ventilation and 28-day mortality in individuals with ARDS.
Methods: We performed genome-wide transcriptional profiling of AMs purified from BAL fluid collected from 35 subjects with ARDS. Cells were obtained at baseline (Day 1), Day 4, and Day 8 after ARDS onset (N = 68 total samples). We identified biological pathways that were enriched at each time point in subjects alive and extubated within 28 days after ARDS onset (alive/extubatedDay28) versus those dead or persistently supported on mechanical ventilation at Day 28 (dead/intubatedDay28).
Measurements and Main Results: “M1-like” (classically activated) and proinflammatory gene sets such as IL-6/JAK/STAT5 (Janus kinase/signal transducer and activator of transcription 5) signaling were significantly enriched in AMs isolated on Day 1 in alive/extubatedDay28 versus dead/intubatedDay28 subjects. In contrast, by Day 8, many of these same proinflammatory gene sets were enriched in AMs collected from dead/intubatedDay28 compared with alive/extubatedDay28 subjects. Serially sampled alive/extubatedDay28 subjects were characterized by an AM temporal expression pattern of Day 1 enrichment of innate immune programs followed by prompt downregulation on Days 4 and 8. Dead/intubatedDay28 subjects exhibited an opposite pattern, characterized by progressive upregulation of proinflammatory programs over the course of ARDS. The relationship between AM expression profiles and 28-day clinical status was distinct in subjects with direct (pulmonary) versus indirect (extrapulmonary) ARDS.
Conclusions: Clinical outcomes in ARDS are associated with highly distinct AM transcriptional programs.
Keywords: acute respiratory distress syndrome, adult, macrophage, transcriptome
At a Glance Commentary
Scientific Knowledge on the Subject
Alveolar macrophages (AMs) are believed to possess significant phenotypic plasticity and functional heterogeneity and are thus considered to be key orchestrators of the inflammation, injury, and repair in acute respiratory distress syndrome (ARDS). However, human studies characterizing distinct AM phenotypes and functions are limited, and it is unclear whether temporal activation of specific AM programs is associated with meaningful clinical endpoints in ARDS.
What This Study Adds to the Field
This study provides the first genome-wide assessment of serial AM transcriptional activation over the first 8 days of ARDS and associates distinctive transcriptional patterns with clinical outcomes. At the time of ARDS onset, proinflammatory and “M1-like” (classically activated) pathways are enriched in AMs sampled from subjects who ultimately survive and are liberated from mechanical ventilation within 28 days after ARDS onset. This finding is most robust in subjects with direct lung injury. In contrast, proinflammatory and M1-like programs are enriched in AMs sampled on Day 8 after ARDS onset in subjects who die or are persistently supported on mechanical ventilation at Day 28. These results refine emerging models of ARDS pathogenesis that ascribe diverse roles to AMs in lung injury and resolution.
Acute respiratory distress syndrome (ARDS) occurs in three overlapping pathologic phases that have been termed “exudative,” “proliferative,” and “fibrotic” (1). Alveolar macrophages (AMs) are believed to possess significant phenotypic plasticity and functional heterogeneity and are thus increasingly considered to be key orchestrators of the inflammation, injury, and repair programs that determine each of these pathologic phases of ARDS (2, 3). Despite a broad consensus that AM phenotypic states are associated with distinct phases of ARDS, human studies characterizing AM polarization and function in ARDS are extremely limited. Rosseau and colleagues used a seven-marker flow cytometry panel to analyze AMs from BAL fluid (BALF) obtained from patients over the first 3 weeks after the onset of ARDS, and they found that persistent expression of the proinflammatory markers CD11b and MRP8/14 (myeloid-related proteins 8 and 14) on immature AMs/monocytes was associated with decreased survival (4). Previous studies have also shown that cultured AMs collected from subjects with ARDS secrete increased concentrations of proinflammatory cytokines and chemokines such as IL-1β and IL-8 compared with control subjects without ARDS (5, 6). These prior findings suggest that AMs may contribute to alveolar inflammation and tissue injury in ARDS; however, a comprehensive and unbiased assessment of how AM programming is related to clinical outcomes over the course of ARDS has not been performed.
We have recently shown that at the time of ARDS onset, the transcriptional profiles of AMs and peripheral blood monocytes are highly divergent (7). These findings suggest that transcriptomic analyses using whole-blood leukocyte RNA to interrogate lung processes in ARDS may not accurately reflect the transcriptomic response in lung leukocytes such as AMs. In the present study, we examined the site- and cell-specific transcriptome of AMs isolated from subjects over the first 8 days after the onset of ARDS and tested the hypothesis that AM transcriptional programs in subjects who are alive and liberated from mechanical ventilation within 28 days differ from those in subjects who die or are persistently dependent on mechanical ventilation at Day 28. Some of the results of these studies have been reported previously in the form of a research letter (7) and an abstract (8).
Methods
Study Population and Design
We used samples previously collected from patients enrolled in the phase II, randomized, placebo-controlled trial of fish oil for treatment of ARDS conducted between 2006 and 2008 (9). Full inclusion and exclusion criteria are described in the online supplement. All human subject committees and a data and safety monitoring board approved the trial. Enrollment in the trial occurred within 48 hours of ARDS onset, and BALF was obtained at study entry (Day 1), Day 4 ± 1 day (Day 4), and Day 8 ± 1 day (Day 8). BALF was not collected on Day 4 or Day 8 if participants had already been extubated or did not meet safety criteria for bronchoscopy.
Isolation of AM RNA
Isolation of AMs for genome-wide transcriptional profiling was performed in 35 of the 47 patients enrolled in the ARDS fish oil study at the Harborview Medical Center (HMC) site. Negative selection for AMs was achieved by incubating cells collected from the BALF with antibody-conjugated microbeads specific for the following markers: CD3 (T cells), CD15 (neutrophils), CD19 (B cells), CD235a (red blood cells), CD294 (eosinophils, basophils), and CD326 (epithelial cells). RNA extracted from isolated cells was assessed for purity and then hybridized to a HumanRef-8 BeadChip (Illumina) that was inclusive of 18,415 unique genes. Complete microarray data are available in the Gene Expression Omnibus public database (www.ncbi.nlm.nih.gov/geo/; accession no. GSE116560). Complete details on AM isolation, RNA purification, and microarray hybridization can be found in the online supplement.
BALF Measurements
BALF and plasma IL-6, IL-8, and MCP-1 (monocyte chemoattractant protein 1) concentrations were measured using cytometric bead-based immunoassays (R&D Systems). Plasma IL-17A, Ang-1 (angiopoietin-1), Ang-2, and sTNFR-1 (soluble tumor necrosis factor receptor 1) were measured using a chemiluminescence immunoassay (Meso Scale Discovery). Total protein concentration was determined using a bicinchoninic acid protein assay (Thermo Fisher Scientific). BAL polymorphonuclear neutrophil (PMN) and AM percentages were determined by manual inspection of cytospin preparations made from each BALF cell pellet.
Differential Gene Expression Analysis
We compared the AM transcriptome in patients with ARDS experiencing divergent ventilator-related outcomes: 1) alive/extubatedDay28, defined as subjects who were alive and liberated from mechanical ventilation 28 days within ARDS onset; or 2) dead/intubatedDay28, defined as subjects who died or were persistently supported on mechanical ventilation at Day 28. We identified genes demonstrating differential expression between groups at each time point using linear models that were fitted for each gene using the Bioconductor limma package. We adjusted for differences in patient age, sex, and treatment arm in our model. A false discovery rate (FDR) less than 0.05 was considered statistically significant.
Pathway Analysis
We identified signaling pathways that were distinct between the two groups by applying gene set enrichment analysis (GSEA) to expression levels for 50 “hallmark” gene sets defined in the Molecular Signature Database (MSigDB) (10). The hallmark gene sets represent highly curated and well-defined biological programs and were generated by consensus clustering of over 8,000 different gene sets from the MSigDB (11). The “M1-like” (classically activated) and “M2-like” (alternatively activated) gene sets were derived from human monocyte–derived macrophages that were linked with IFN-γ and IL-4 stimulation, respectively (12). Leading-edge genes from the enriched gene sets were identified using the GSEA software package (10). We used an FDR threshold of less than 0.05 to identify significantly enriched pathways.
Temporal Expression Analysis
In the subset of patients who had samples obtained at each time point (Days 1, 4, and 8), temporal expression analysis was performed using the Short Time-series Expression Miner (STEM) (13, 14). The genes assigned to each significant temporal gene expression pattern were annotated on the basis of Gene Ontology (GO) (15). Complete details on microarray data processing, differentially expressed gene analysis, GSEA, and STEM analyses can be found in the online supplement.
Statistical Analysis
In the subset of subjects who were serially sampled at all three time points, we used a generalized least squares regression model with repeated measures to test whether the interaction between days and outcome group (alive/extubatedDay28 vs. dead/intubatedDay28) was different for percentage AMs, percentage PMNs, IL-8, IL-6, and total protein. All statistical analyses were performed using R version 3.4.2 software (R Foundation for Statistical Computing).
Results
Study Population
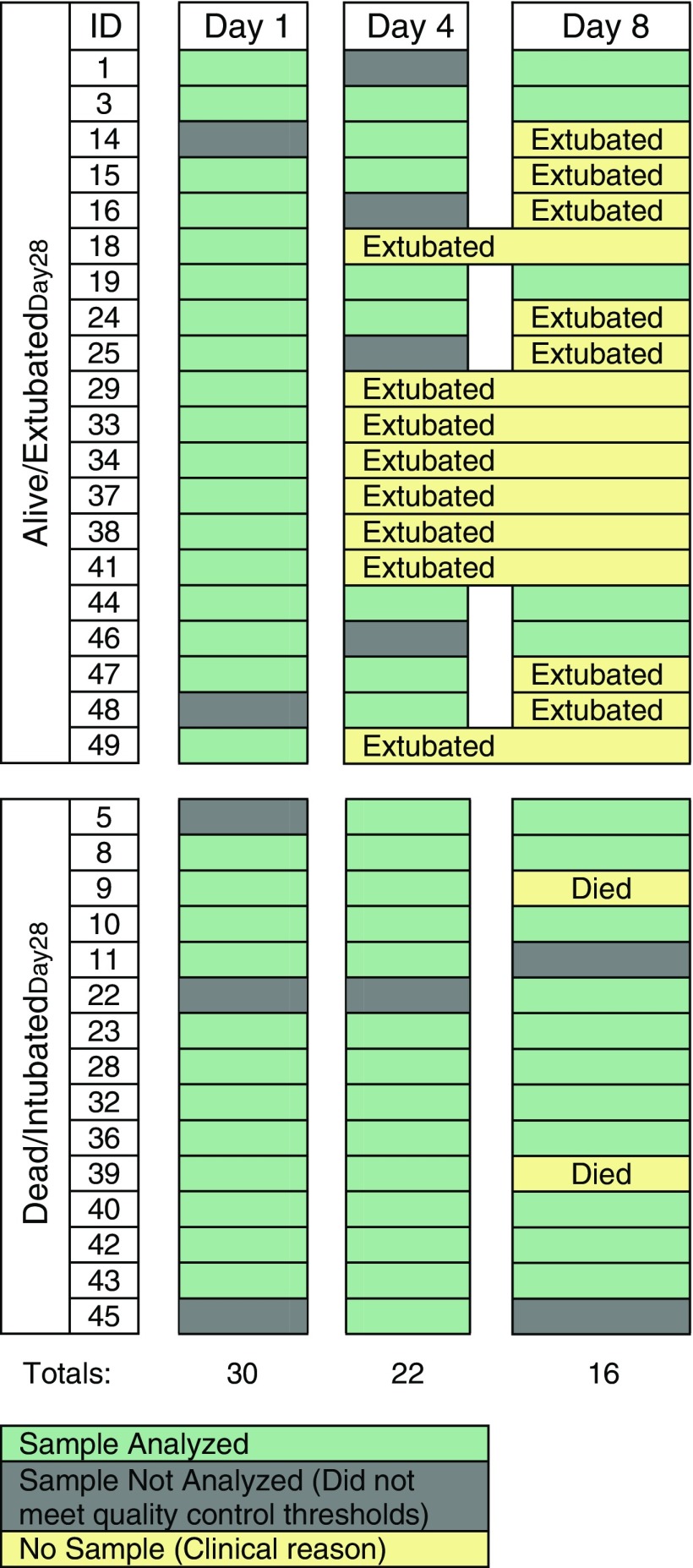
The baseline clinical characteristics, PaO2/FiO2 ratios at each time point, and clinical course of the 35 subjects included in this study are shown in Table 1 and Tables E1 and E2 in the online supplement, respectively. Patients from both the placebo (n = 20) and treatment (n = 15) arms of the trial were included in our study. The baseline characteristics of the subjects in this study were not different from those of the subjects enrolled in the fish oil trial at HMC who were not included in this study, except for prepurification percentage PMNs and percentage AMs (Tables E3 and E4). The number of subjects in whom AMs were isolated decreased over the course of the study owing to liberation from mechanical ventilation or death (Figure 1). As a result, samples analyzed on Day 4 and Day 8 were from subjects who experienced significantly fewer ventilator-free days than subjects who did not have samples analyzed at these later time points (Tables E5 and E6). The baseline plasma inflammatory biomarker concentrations were not significantly different between the dead/intubatedDay28 and alive/extubatedDay28 groups, except for IL-8 (Table E7). All subjects met the revised 2012 Berlin definition for ARDS (16).
Table 1.
Subject Characteristics at Time of Study Enrollment
| Feature | Overall (N = 35) | Alive/ExtubatedDay28 (n = 20) | Dead/IntubatedDay28 (n = 15) |
|---|---|---|---|
| Demographics | |||
| Age, yr, mean ± SD | 45 ± 17 | 42 ± 15 | 50 ± 19 |
| Sex, M/F | 22/13 | 13/7 | 9/6 |
| ARDS risk factors*, n (%) | |||
| Trauma | 19 (54) | 10 (50) | 9 (60) |
| Sepsis | 17 (49) | 9 (45) | 8 (53) |
| Pneumonia | 9 (26) | 5 (25) | 4 (27) |
| Other | 4 (11) | 3 (9) | 1 (7) |
| Physiologic factors, median (IQR) | |||
| Total BALF protein, g/ml | 233 (63–376) | 262 (78–477) | 195 (63–304) |
| PaO2/FiO2 ratio | 186 (158–255) | 211 (170–269) | 178 (144–225) |
| APACHE II score | 20 (17–26) | 19 (14–26) | 24 (18–28) |
| SOFA score | 8 (6–10) | 6 (4–8) | 10 (5–12) |
| Alveolar leukocyte differential, median (IQR) | |||
| % neutrophils | 35 (7–79) | 33 (7–88) | 47 (7–73) |
| % AMs | 56 (21–88) | 57 (12–86) | 53 (27–89) |
| BALF cytokine values, pg/ml, median (IQR) | |||
| IL-8 | 484 (159–1,230) | 323 (61–911) | 949 (168–1,531) |
| IL-6 | 74 (7–263) | 77 (8–318) | 45 (5–195) |
| Outcome | |||
| VFD, median (IQR) | 7 (0–23) | 21.5 (19–24) | 0 (0–0) |
| Mortality, 28 d, n (%) | 5 (14%) | 0 (0%) | 5 (33%) |
Definition of abbreviations: AM = alveolar macrophage; APACHE = Acute Physiology and Chronic Health Evaluation; ARDS = acute respiratory distress syndrome; BALF = BAL fluid; IQR = interquartile range; SOFA = Sequential Organ Failure Assessment (highest score in first 7 d); VFD = ventilator-free days.
Risk factors for ARDS are not mutually exclusive.
Figure 1.
Manifest of analyzed samples.
AM Purity
AMs were purified from BALF by negative immunoselection. We achieved high purity, with manual inspection of cytospin slides after purification demonstrating an average purity of 97% for AMs (Table E8). To verify that the transcriptional signals we measured were predominantly from AMs, we evaluated the probe intensities of alveolar cell markers in our dataset (17). As expected, the probe intensities of AM marker genes such as CD163 and CD71 were very high in the purified samples (Figure E1).
Conversely, the probe intensities of marker genes for other alveolar cell types (CD16, CD19, CD56, CD11c, CD3, SIGLEC8, CD117, and EPCAM [epithelial cellular adhesion molecule]) were at the lower limit of detection, implying that the population of cells we analyzed was not inclusive of these cell types. We did detect weak expression of the plasmacytoid dendritic cell (pDC) marker CD123. However, it is unlikely that these cells are true pDCs, given that pDCs constitute only a very small proportion of human BALF leukocytes (<0.20%) (17) and that CD123 is known to be expressed on a subset of human AMs (17).
AM Differential Gene Expression Analysis
We first tested whether individual genes in AMs were differentially expressed between alive/extubatedDay28 and dead/intubatedDay28 subjects. We compared the genome-wide expression profiles obtained from AMs on Days 1, 4, and 8 between the two groups at each time point. Although there were several genes that demonstrated nominally significant expression differences between the two group (Tables 2 and E9–E11), none remained significant after adjustment for multiple testing.
Table 2.
Differentially Expressed Genes in Alveolar Macrophages on Day 1 of Acute Respiratory Distress Syndrome
| Gene Symbol | Gene Name | FD | Nominal P Value |
|---|---|---|---|
| CTSZ | Cathepsin Z | 3.12 | 9.02 × 10−3 |
| IL18BP | IL-18–binding protein | 1.81 | 2.89 × 10−2 |
| MYOM2 | Myomesin 2 | 1.67 | 1.37 × 10−2 |
| CD274 | PD-L1 | 1.54 | 2.59 × 10−2 |
| CARD16 | Caspase recruitment domain family member 16 | 1.49 | 1.63 × 10−2 |
| MRPL52 | Mitochondrial ribosomal protein L52 | 1.49 | 3.51 × 10−2 |
| IFI27L2 | IFN-α–inducible protein 27–like 2 | 1.43 | 2.26 × 10−2 |
| ZDHHC19 | Zinc finger DHHC-type–containing 19 | 1.43 | 3.97 × 10−2 |
| NR1H3 | Nuclear receptor subfamily 1 group H member 3 | 1.43 | 3.26 × 10−2 |
| CCL22 | C-C motif chemokine ligand 22 | 1.42 | 2.66 × 10−2 |
| MYBPC3 | Myosin-binding protein C, cardiac | 1.41 | 4.11 × 10−2 |
| UNC45A | Unc-45 myosin chaperone A | 1.41 | 1.24 × 10−3 |
| THBD | Thrombomodulin | 1.38 | 3.73 × 10−2 |
| PPP3R1 | Protein phosphatase 3 regulatory subunit B, alpha | 1.37 | 3.07 × 10−2 |
| CLPTM1 | CLPTM1, transmembrane protein | 1.36 | 1.75 × 10−2 |
| RNF146 | Ring finger protein 146 | −1.35 | 4.77 × 10−2 |
| ANKRD57 | Sosondowah ankyrin repeat domain family member C | −1.37 | 4.03 × 10−2 |
| PPHLN1 | Periphilin 1 | −1.38 | 1.99 × 10−2 |
| CYP2R1 | Cytochrome P450 family 2 subfamily R member 1 | −1.38 | 3.27 × 10−3 |
| FAM23B | Transmembrane protein 236 | −1.38 | 4.36 × 10−2 |
| SF1 | Splicing factor 1 | −1.39 | 9.39 × 10−3 |
| PRKCE | Protein kinase C epsilon | −1.41 | 1.06 × 10−2 |
| SCGB1A1 | Secretoglobin family 1A member 1 | −1.43 | 2.13 × 10−2 |
| CUGBP2 | CUGBP Elav-like family member 2 | −1.44 | 4.84 × 10−2 |
| ADORA3 | Adenosine A3 receptor | −1.45 | 9.24 × 10−3 |
| NBPF1 | Neuroblastoma breakpoint family member 1 | −1.47 | 1.79 × 10−2 |
| ALCAM | Activated leukocyte cell adhesion molecule | −1.49 | 8.36 × 10−3 |
| KLF9 | Kruppel-like factor 9 | −1.49 | 4.40 × 10−2 |
| C6ORF48 | Chromosome 6 open reading frame 48 | −1.50 | 3.92 × 10−2 |
| TFCP2L1 | Transcription factor CP2 like 1 | −1.52 | 3.35 × 10−2 |
| DDIT4L | DNA damage inducible transcript 4 like | −1.55 | 1.76 × 10−2 |
| CSNK1G2 | Casein kinase 1 gamma 2 | −1.55 | 2.50 × 10−2 |
| PIK3IP1 | Phosphoinositide-3-kinase–interacting protein 1 | −1.67 | 3.80 × 10−2 |
| TSC22D3 | TSC22 domain family member 3 | −1.69 | 1.36 × 10−2 |
| AMD1 | Adenosylmethionine decarboxylase 1 | −1.69 | 3.01 × 10−2 |
| VSIG4 | V-set and immunoglobulin domain–containing 4 | −1.71 | 9.99 × 10−3 |
| MAFB | MAF BZIP transcription factor B | −1.92 | 2.52 × 10−2 |
| FKBP5 | FK506-binding protein 5 | −1.99 | 3.90 × 10−4 |
| FPR2 | Formyl peptide receptor 2 | −2.23 | 3.16 × 10−2 |
Definition of abbreviation: FD = fold difference.
FD in alive/extubatedDay28 (n = 18) compared with dead/intubatedDay28 (n = 12). The nominal P value is gene specific and not adjusted for genome-wide multiple hypothesis testing. Only genes with 1.3 FD and a nominal P value less than 0.05 are displayed. The model was adjusted for age, sex, and treatment group.
Of note, among the genes showing nominal significance (P < 0.05), several have previously been implicated in ARDS biology. For example, CD274 (PD-L1 [programmed death-ligand 1]) was the fourth most upregulated transcript in alive/extubatedDay28 versus dead/intubatedDay28 subjects on Day 1 (Table 2). AM cell surface expression of PD-L1 has been shown to be significantly decreased in subjects with ARDS compared with healthy control subjects (18). FPR2 (ALX/FPR2 [N-formyl peptide receptor 2 or lipoxin A4 receptor]) was the most upregulated transcript in dead/intubatedDay28 versus alive/extubatedDay28 subjects on Days 1 and 4 (Tables 2 and E9). The ALX/FPR2 receptor binds to many classes of lipid mediators (including aspirin-triggered resolvin D1 and lipoxin A4) that have been associated with the pathogenesis of acute lung injury (ALI) (19–21).
AM Transcriptional Pathway Analysis
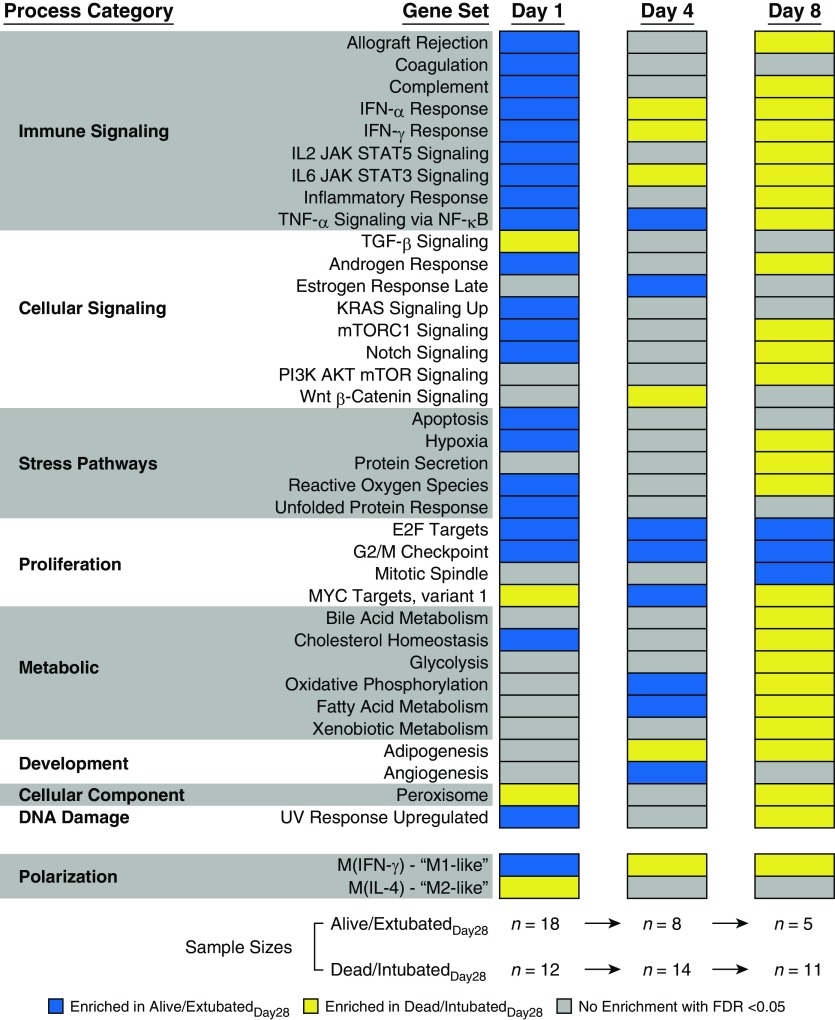
It is rare for individual genes to drive disease progression in complex disorders such as ARDS (22). Therefore, we next applied GSEA to our transcriptomic data from AMs to identify biological pathways that were differentially expressed in the two groups. We found that proinflammatory and M1-like pathways were strongly enriched in AMs from alive/extubatedDay28 compared with dead/intubatedDay28 subjects at the time of ARDS onset (Day 1) (Figure 2). We observed a similar overall pattern of AM pathway enrichment when we used 28-day mortality as an outcome (Figure E2). In contrast, there was a progressive shift in this enrichment profile between the two patient groups at later stages of ARDS. In subjects who remained alive and intubated to at least Day 4, several proinflammatory gene sets, such as IL6/JAK/STAT (Janus kinase/signal transducer and activator of transcription) signaling, were enriched in dead/intubatedDay28 compared with alive/extubatedDay28 subjects. The association between innate immune activation and worse ventilator-related outcomes was even more robust in subjects who remained alive and intubated to at least Day 8. Multiple “immune-signaling” gene sets and the M1-like gene set were enriched in dead/intubatedDay28 compared with alive/extubatedDay28 subjects on Day 8. Enrichment of M2-like genes on Day 4 or Day 8 was associated with 28-day survival (Figure E2). Although treatment with fish oil could theoretically influence AM expression profiles, our primary GSEA results did not significantly change when we excluded subjects who received fish oil (Figure E3).
Figure 2.
Alveolar macrophage transcriptional programs are associated with ventilator-related outcomes in acute respiratory distress syndrome. Gene set enrichment analysis demonstrates enrichment of hallmark pathways in alive/extubatedDay28 (blue) versus dead/intubatedDay28 (yellow) subjects on Days 1, 4, and 8 (false discovery rate [FDR] < 0.05). Gene sets with no enrichment in either group are shown in gray. We evaluated the entire hallmark gene set collection; however, only gene sets that were enriched in either alive/extubatedDay28 or dead/intubatedDay28 at one of the three time points with an FDR less than 0.05 are displayed. Our FDR cutoff accounted for multiple hypothesis testing of all 50 hallmark gene sets. M1-like = classically activated; M2-like = alternatively activated; UV = ultraviolet.
Direct (e.g., pneumonia and aspiration) and indirect (e.g., nonpulmonary sepsis) ARDS may possess unique molecular characteristics that differentially affect clinical outcomes (23, 24). Therefore, we stratified our Day 1 primary comparison between alive/extubatedDay28 versus dead/intubatedDay28 subjects on the basis of each subject’s risk factor for ARDS. In subjects with direct ARDS, M1-like and immune-signaling gene sets were significantly enriched in alive/extubatedDay28 versus dead/intubatedDay28 subjects at the time of ARDS onset (Figure E4). In subjects with indirect ARDS, M1-like, M2-like, and several important inflammatory response gene sets were not significantly different between alive/extubatedDay28 and dead/intubatedDay28 subjects. GSEA for subjects with trauma-associated ARDS is shown in Figure E5.
Leading-Edge Analysis
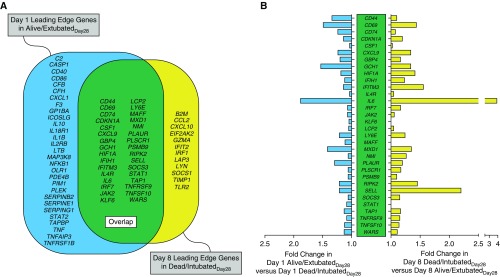
We performed a leading-edge analysis of the significantly enriched immune-signaling gene sets on Day 1 and Day 8 to identify the subset of genes that were the core drivers of the gene set enrichment displayed in Figure 2. There were 61 leading-edge genes that were conserved across more than three immune-signaling gene sets in Day 1 alive/extubatedDay28 subjects (Figure 3A, blue), and there were 44 leading-edge genes that were conserved across more than three immune-signaling gene sets in Day 8 dead/intubatedDay28 subjects (Figure 3A, yellow). Notably, 32 of the leading-edge genes from Day 1 alive/extubatedDay28 subjects were also leading-edge genes for Day 8 dead/intubatedDay28 subjects (Figure 3A, green). In other words, these 32 genes were enriched in alive/extubatedDay28 subjects at ARDS onset, but they were also enriched in dead/intubatedDay28 subjects on Day 8. These findings reinforce the idea that the relationship between AM immune function and ARDS clinical outcomes changes over the course of ARDS and that coordinated shifts in AM polarization may play a role in patient outcomes.
Figure 3.
Leading-edge analysis of significantly enriched immune-signaling gene sets in subjects with acute respiratory distress syndrome. (A) Venn diagram showing the Day 1 alive/extubatedDay28 leading-edge genes (blue), the Day 8 dead/intubatedDay28 leading-edge genes (yellow), and the leading-edge genes that overlapped between the two groups (green). Only the genes that were identified as leading-edge genes in at least three of the significantly enriched immune-signaling gene sets in Figure 2 are displayed. (B) Fold change in gene expression at Days 1 and 8 of the leading-edge genes that overlapped between Day 1 alive/extubatedDay28 and Day 8 dead/intubatedDay28.
AM Time-Series Analysis
Our GSEA directly compared AM genome-wide expression between alive/extubatedDay28 and dead/intubatedDay28 subjects at each specific time point. We next sought to better capture the dynamics of gene expression during ARDS in a subset of patients who were sampled at all three time points. To identify clusters of genes whose expression was significantly associated with specific temporal trajectory patterns over the first 8 days of ARDS, we performed time-course clustering in alive/extubatedDay28 and dead/intubatedDay28 subjects separately. We then performed GO functional enrichment analysis of these gene clusters to identify which biological processes were the most dynamically regulated in each patient group.
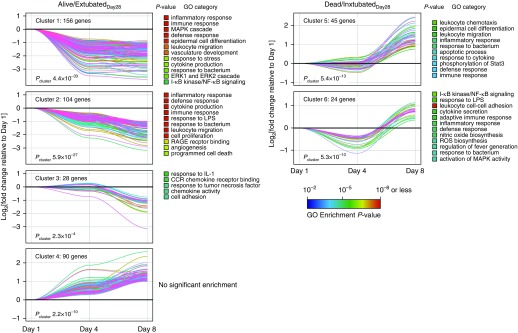
We identified four AM gene clusters that significantly changed over the first 8 days in alive/extubatedDay28 subjects, three of which displayed a downregulated expression trajectory over course of ARDS (Figure 4, left panels). GO analysis of these gene clusters revealed that these downregulated profiles were highly overrepresented in proimmunoinflammatory processes (Table E12). In contrast, we identified two AM gene clusters that significantly changed over the first 8 days in dead/intubatedDay28 subjects, each of which was linked to an upregulated temporal expression trajectory (Figure 4, right panels). The gene clusters associated with these upregulated profiles were also overrepresented in proinflammatory GO categories (Table E12). Taken together, this orthogonal analysis is consistent with our primary GSEA finding (Figure 2) and demonstrates how the dynamic activation of transcriptional programs in AMs during ARDS might be related to distinct clinical outcomes.
Figure 4.
Alveolar macrophage temporal expression profiles over the course of acute respiratory distress syndrome. Time-series analysis of subjects who had samples obtained at each of the time points (Days 1, 4, and 8). Microarray data from either alive/extubatedDay28 (n = 3) or dead/intubatedDay28 (n = 9) subjects at each time point were analyzed by Short Time-series Expression Miner as described in the Methods section. Temporal changes in gene expression on Days 4 and 8 are depicted relative to their Day 1 values. Four statistically significant gene clusters were identified in alive/extubatedDay28, and two clusters of genes were identified in dead/intubatedDay28 subjects (Bonferroni-adjusted Pcluster < 0.05). Gene Ontology (GO) analysis of each of the respective clusters is shown to the right of each panel with a list of overrepresented categories (adjusted enrichment P < 0.05). A complete list of all genes in each cluster is shown in Table E12. CCR = C-C chemokine receptor; ERK = extracellular signal–regulated kinase; I-κB = inhibitor of nuclear factor-κB; MAPK = mitogen-activated protein kinase; NF-κB = nuclear factor-κB; RAGE = receptor for advanced glycation endproducts; ROS = reactive oxygen species; Stat3 = signal transducer and activator of transcription 3.
Alveolar Inflammation over the Course of the Study
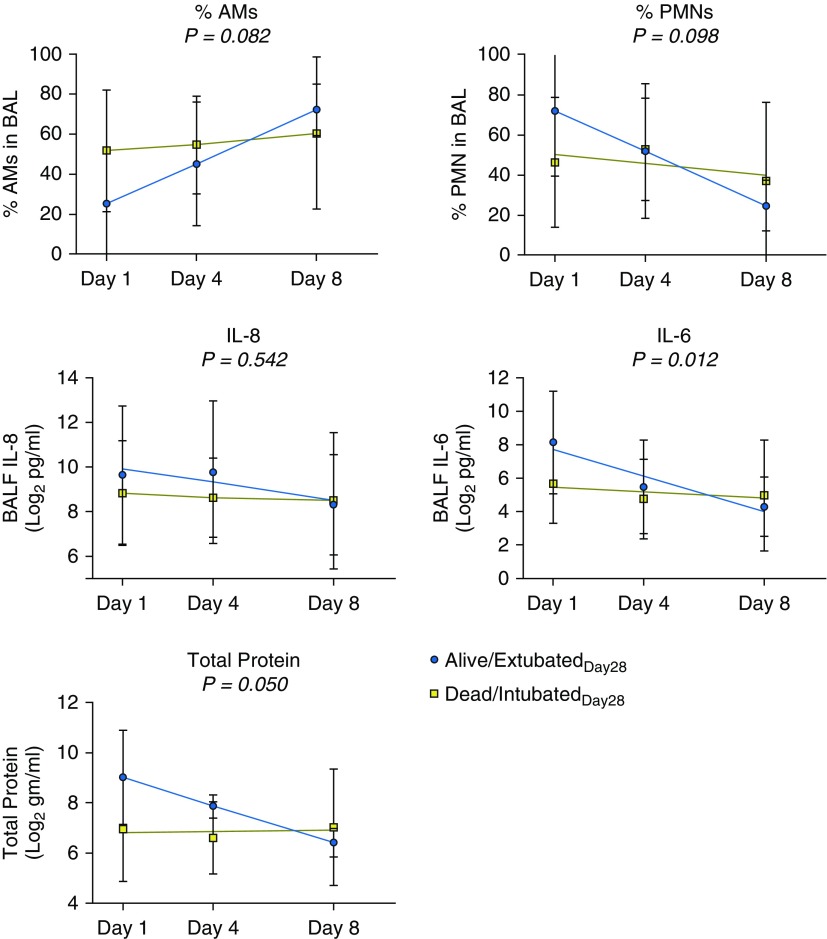
We next compared the temporal pattern of alveolar inflammation in alive/extubatedDay28 versus dead/intubatedDay28 subjects. In the subset of subjects with samples at all time points, we found that the change in BALF IL-6 and total protein concentrations over the course of the study was different in alive/extubatedDay28 versus dead/intubatedDay28 subjects (Figure 5). These findings are consistent with our pathway-based analysis demonstrating distinct enrichment of IL-6–associated genes between the two groups at different time points (Figure 2).
Figure 5.
Measures of alveolar inflammation over the course of acute respiratory distress syndrome (ARDS) in serially sampled subjects. BAL fluid (BALF) was collected and analyzed for percentage polymorphonuclear neutrophils (% PMNs), percentage alveolar macrophages (% AMs), IL-8, IL-6, and total protein concentration on Days 1, 4, and 8 after ARDS onset in subjects who were sampled at all three time points. Shown are the mean, SD, and best-fit linear regression line for each analyte at each time point in alive/extubatedDay28 versus dead/intubatedDay28 subjects. P values were determined using a generalized least squares regression model with repeated measures to test whether there was an interaction between days and outcome group (alive/extubatedDay28/dead/intubatedDay28) on each measure of alveolar inflammation.
Discussion
In this study, we report the first transcriptomic analysis of AMs serially collected from subjects with ARDS. We found that cell-specific AM proinflammatory and M1-like transcriptional signatures at the time of ARDS onset were associated with better clinical outcomes (alive/extubatedDay28). However, in subjects who remained on mechanical ventilation for at least 8 days after ARDS onset, an AM temporal expression pattern characterized by persistence and progression of a proinflammatory profile was associated with worse clinical outcomes (dead/intubatedDay28) (Figures 2 and 4). Our results demonstrate, for the first time, to our knowledge, that distinct AM transcriptional programs might be associated with 28-day clinical outcomes and suggest that AM polarization may play an important role in the initiation and resolution of inflammation in ARDS.
Our finding that AMs are characterized by dynamic changes in their gene expression over the first 8 days of ARDS is consistent with animal studies that have identified the presence of multiple AM transcriptional and phenotypic subtypes during ALI (25–27). Prior studies by two different groups have shown that the natural history of recruited and resident AM gene expression in nonlethal murine models of ALI is marked by early enrichment of M1-like genes followed by a gradual return to baseline gene expression (25, 26). In separate studies, mice treated with IL-4 (an M2 stimulus) 2–4 days after lung injury (28) or an inhibitor of MEK1/2 (mitogen-activated protein kinase 1/2, which dampens M1 and promotes M2 AM activity) (29) have enhanced resolution of ALI. Our finding that the Day 1 association between increased AM proinflammatory gene expression and alive/extubatedDay28 is reversed by Day 8 provides the first direct evidence that the overall transcriptomic landscape of AMs is variable in humans with ARDS.
These results may also explain, in part, some of the failures that have been seen in prior clinical trials testing therapies that influence macrophage polarization. Granulocyte–macrophage colony–stimulating factor has a proinflammatory effect on macrophage polarization (30), whereas statins have an antiinflammatory effect on macrophage polarization (31). A phase II trial comparing intravenous granulocyte–macrophage colony–stimulating factor with placebo administered 3–7 days after ARDS onset failed to demonstrate improvement in the primary outcome (32). Likewise, multiple trials evaluating statins administered within 48 hours after ARDS onset have not demonstrated improved clinical outcomes compared with placebo (33, 34). On the basis of our finding that the relationship between AM proinflammatory transcriptional signatures and clinical outcomes shifts over the first 8 days after ARDS onset, it is tempting to speculate that administration of each of these agents at different time points after ARDS onset may have resulted in different findings. Future ARDS interventional trials will need to take into consideration the dynamic nature of AM function, even within the first 8 days after ARDS onset, which has previously been considered to fall within the “exudative phase” of ARDS (1, 3).
Our results showing that an early proinflammatory AM transcriptional response is associated with improved outcomes in ARDS are more evident in subjects with direct ARDS than in those with indirect ARDS (Figure E4). This finding echoes prior studies demonstrating that direct and indirect ARDS possess distinct molecular and clinical features (23, 24). We found that in subjects with direct ARDS, enrichment of proinflammatory and M1-like pathways in AMs at the time of ARDS onset was associated with improved outcomes (Figure E4). The M1-like and M2-like gene sets were not enriched in either the alive/extubatedDay28 or dead/intubatedDay28 subjects who had indirect ARDS. Our results provide molecular targets for future studies seeking mechanistic distinctions between direct and indirect ARDS that explain differential clinical outcomes.
Our findings demonstrate novel evidence linking AM transcriptional programs to outcomes in ARDS, but it is unclear how these programs might lead to differences in alveolar damage. Soluble biomarker measurements in plasma from the same subjects showed that proinflammatory biomarkers were not significantly increased in subjects with worse clinical outcomes, except for IL-8 (Table E7). In exploratory analyses, we applied latent class analysis (LCA) to the plasma biomarker and clinical variables documented at enrollment. Recent studies have used LCA to identify ARDS subphenotypes (35, 36). A subphenotype is a subset of a disease phenotype that exhibits more uniform characteristics. The purpose of classifying subphenotypes is to identify patients who share similar underlying pathophysiology and respond in a similar fashion to specific therapies. However, LCA did not converge on any multiclass model (Table E13). Additional work with larger sample sizes will be necessary to determine the links between AM transcriptional programs and the previously reported ARDS subphenotypes.
Despite finding multiple links between AM gene set enrichment and clinical outcomes, we did not observe any differentially expressed genes in AMs between alive/extubatedDay28 and dead/intubatedDay28 subjects at any time point that met our genome-wide threshold for statistical significance. Prior gene expression studies of whole blood (37–39) in larger sample sizes than ours have identified a limited number of differentially expressed genes between subjects at risk for or with ARDS that achieved genome-wide significance. This suggests that our study’s modest sample size had insufficient power to overcome patient heterogeneity and technical noise. Furthermore, the precision of our studies may also have been hampered by limits of microarray-based measurements. Future work will likely employ RNA sequencing, which has a greater dynamic range and can discern the breadth of RNA species (40).
Our study has multiple strengths. First, we used very well–characterized cases of ARDS for which we had serially obtained BALF samples. Second, although smaller prior studies have compared transcriptomes of AMs between healthy subjects and subjects with ARDS (41), our study is the first to link genome-wide AM transcriptional programs to clinical outcomes in ARDS. Third, our use of negative immunoselection facilitated the purification of AMs in a manner that minimizes transcriptional noise from postpurification activation by receptor cross-linking that can occur in positive immunoselection (42–44). Finally, our use of genome-wide gene set enrichment as opposed to single gene–based comparisons or individual biomarkers/cytokines allows for a more comprehensive and unbiased assessment of the complexities of AM functional states in ARDS.
Our study also has several limitations. First, our findings are derived from a single cohort and will need to be verified in future ARDS studies that employ alveolar sampling. Second, our ARDS cohort had a higher percentage of patients with trauma than most ARDS cohorts. This is due to the high number of severely injured patients with trauma referred to HMC, the only level 1 trauma center in a five-state region. Thus, our findings in this cohort may not be generalizable to other critically ill populations. Third, our microarray approach measured transcript levels averaged across all AM populations; therefore, we cannot determine the extent to which changes in the proportion of AM subtypes (e.g., resident or monocyte derived) might have accounted for the transcriptional differences observed (18). Fourth, the sample sizes were modest, particularly for our time-series analysis (Figure 4). Despite the limited sample sizes, the differences in gene set enrichment between alive/extubatedDay28 and dead/intubatedDay28 subjects at each time point were large enough to detect many significant differences using a strict FDR threshold. Finally, our findings on Days 4 and 8 in our primary analysis (Figure 2) could have been biased by dropout (owing to extubation because of improved status or death), the presence of secondary/persistent infections, or pharmacologic interventions such as corticosteroid administration. However, our analyses limited to subjects with the full complement of serial samples (Figures 4 and 5) showed findings that were largely consistent with our primary analysis, supporting the robustness of our findings.
In conclusion, our study provides direct evidence in humans that AM gene expression patterns are highly dynamic in ARDS and that distinct AM transcriptional programs are associated with meaningful endpoints. These findings suggest that therapeutic modulation of AM function to promote a transition from proinflammatory to reparative might be beneficial in ARDS, but that efficacy of such therapeutic interventions could be restricted to specific time periods after ARDS onset.
Supplementary Material
Footnotes
Supported by NIH grants T32 HL007287, F32 HL138746, P50 HL073996, and AI137111.
Author Contributions: M.M.W. and S.A.G. contributed to the conception and design of the work. E.D.M., P.K.B., C.R.M., F.R., A.M.M., R.D.S., M.M.W., and S.A.G. contributed to the acquisition, analysis, and interpretation of the data for the work. E.D.M., M.M.W., and S.A.G. drafted and revised the manuscript for important intellectual content. E.D.M., P.K.B., C.R.M., F.R., A.M.M., R.D.S., M.M.W., and S.A.G. significantly contributed to and approved the final version of the manuscript for publication. E.D.M., P.K.B., C.R.M., F.R., A.M.M., R.D.S., M.M.W., and S.A.G. agree to be accountable for all aspects of the work.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201807-1381OC on April 16, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Thille AW, Esteban A, Fernández-Segoviano P, Rodriguez JM, Aramburu JA, Vargas-Errázuriz P, et al. Chronology of histological lesions in acute respiratory distress syndrome with diffuse alveolar damage: a prospective cohort study of clinical autopsies. Lancet Respir Med. 2013;1:395–401. doi: 10.1016/S2213-2600(13)70053-5. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal NR, King LS, D’Alessio FR. Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol. 2014;306:L709–L725. doi: 10.1152/ajplung.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 4.Rosseau S, Hammerl P, Maus U, Walmrath HD, Schütte H, Grimminger F, et al. Phenotypic characterization of alveolar monocyte recruitment in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2000;279:L25–L35. doi: 10.1152/ajplung.2000.279.1.L25. [DOI] [PubMed] [Google Scholar]
- 5.Donnelly SC, Strieter RM, Kunkel SL, Walz A, Robertson CR, Carter DC, et al. Interleukin-8 and development of adult respiratory distress syndrome in at-risk patient groups. Lancet. 1993;341:643–647. doi: 10.1016/0140-6736(93)90416-e. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs RF, Tabor DR, Burks AW, Campbell GD. Elevated interleukin-1 release by human alveolar macrophages during the adult respiratory distress syndrome. Am Rev Respir Dis. 1989;140:1686–1692. doi: 10.1164/ajrccm/140.6.1686. [DOI] [PubMed] [Google Scholar]
- 7.Morrell ED, Radella F, II, Manicone AM, Mikacenic C, Stapleton RD, Gharib SA, et al. Peripheral and alveolar cell transcriptional programs are distinct in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2018;197:528–532. doi: 10.1164/rccm.201703-0614LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrell ED, Gharib SA, Radella F, Stapleton RD, Wurfel MM.An early M1-like transcriptional program in alveolar macrophages is associated with good outcomes in acute respiratory distress syndrome [abstract] Am J Respir Crit Care Med 2017;195:A5224
- 9.Stapleton RD, Martin TR, Weiss NS, Crowley JJ, Gundel SJ, Nathens AB, et al. A phase II randomized placebo-controlled trial of omega-3 fatty acids for the treatment of acute lung injury. Crit Care Med. 2011;39:1655–1662. doi: 10.1097/CCM.0b013e318218669d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–425. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40:274–288. doi: 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ernst J, Bar-Joseph Z. STEM: a tool for the analysis of short time series gene expression data. BMC Bioinformatics. 2006;7:191. doi: 10.1186/1471-2105-7-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ernst J, Nau GJ, Bar-Joseph Z. Clustering short time series gene expression data. Bioinformatics. 2005;21(Suppl 1):i159–i168. doi: 10.1093/bioinformatics/bti1022. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Vasaikar S, Shi Z, Greer M, Zhang B. WebGestalt 2017: a more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Res. 2017;45:W130–W137. doi: 10.1093/nar/gkx356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 17.Yu YRA, Hotten DF, Malakhau Y, Volker E, Ghio AJ, Noble PW, et al. Flow cytometric analysis of myeloid cells in human blood, bronchoalveolar lavage, and lung tissues. Am J Respir Cell Mol Biol. 2016;54:13–24. doi: 10.1165/rcmb.2015-0146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrell ED, Wiedeman A, Long SA, Gharib SA, West TE, Skerrett SJ, et al. Cytometry TOF identifies alveolar macrophage subtypes in acute respiratory distress syndrome. JCI Insight. 2018;3:99281. doi: 10.1172/jci.insight.99281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eickmeier O, Seki H, Haworth O, Hilberath JN, Gao F, Uddin M, et al. Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal Immunol. 2013;6:256–266. doi: 10.1038/mi.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao Z, Dong J, Wu W, Yang T, Wang T, Guo L, et al. Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARγ/NF-κB pathway. Respir Res. 2012;13:110. doi: 10.1186/1465-9921-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang X, Abbott J, Cheng L, Colby JK, Lee JW, Levy BD, et al. Human mesenchymal stem (stromal) cells promote the resolution of acute lung injury in part through lipoxin A4. J Immunol. 2015;195:875–881. doi: 10.4049/jimmunol.1500244. [DOI] [PubMed] [Google Scholar]
- 22.Schadt EE. Molecular networks as sensors and drivers of common human diseases. Nature. 2009;461:218–223. doi: 10.1038/nature08454. [DOI] [PubMed] [Google Scholar]
- 23.Calfee CS, Janz DR, Bernard GR, May AK, Kangelaris KN, Matthay MA, et al. NIH NHLBI ARDS Network. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest. 2015;147:1539–1548. doi: 10.1378/chest.14-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo L, Shaver CM, Zhao Z, Koyama T, Calfee CS, Bastarache JA, et al. Clinical predictors of hospital mortality differ between direct and indirect ARDS. Chest. 2017;151:755–763. doi: 10.1016/j.chest.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnston LK, Rims CR, Gill SE, McGuire JK, Manicone AM. Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am J Respir Cell Mol Biol. 2012;47:417–426. doi: 10.1165/rcmb.2012-0090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mould KJ, Barthel L, Mohning MP, Thomas SM, McCubbrey AL, Danhorn T, et al. Cell origin dictates programming of resident versus recruited macrophages during acute lung injury. Am J Respir Cell Mol Biol. 2017;57:294–306. doi: 10.1165/rcmb.2017-0061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srivastava M, Jung S, Wilhelm J, Fink L, Bühling F, Welte T, et al. The inflammatory versus constitutive trafficking of mononuclear phagocytes into the alveolar space of mice is associated with drastic changes in their gene expression profiles. J Immunol. 2005;175:1884–1893. doi: 10.4049/jimmunol.175.3.1884. [DOI] [PubMed] [Google Scholar]
- 28.D’Alessio FR, Craig JM, Singer BD, Files DC, Mock JR, Garibaldi BT, et al. Enhanced resolution of experimental ARDS through IL-4-mediated lung macrophage reprogramming. Am J Physiol Lung Cell Mol Physiol. 2016;310:L733–L746. doi: 10.1152/ajplung.00419.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long ME, Eddy WE, Gong KQ, Lovelace-Macon LL, McMahan RS, Charron J, et al. MEK1/2 inhibition promotes macrophage reparative properties. J Immunol. 2017;198:862–872. doi: 10.4049/jimmunol.1601059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuomisto TT, Lumivuori H, Kansanen E, Häkkinen S-K, Turunen MP, van Thienen JV, et al. Simvastatin has an anti-inflammatory effect on macrophages via upregulation of an atheroprotective transcription factor, Kruppel-like factor 2. Cardiovasc Res. 2008;78:175–184. doi: 10.1093/cvr/cvn007. [DOI] [PubMed] [Google Scholar]
- 32.Paine R, III, Standiford TJ, Dechert RE, Moss M, Martin GS, Rosenberg AL, et al. A randomized trial of recombinant human granulocyte-macrophage colony stimulating factor for patients with acute lung injury. Crit Care Med. 2012;40:90–97. doi: 10.1097/CCM.0b013e31822d7bf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McAuley DF, Laffey JG, O’Kane CM, Perkins GD, Mullan B, Trinder TJ, et al. HARP-2 Investigators Irish Critical Care Trials Group Simvastatin in the acute respiratory distress syndrome N Engl J Med 20143711695–1703.[Published erratum appears in N Engl J Med 375:2010.] [DOI] [PubMed] [Google Scholar]
- 34.National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med. 2014;370:2191–2200. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA NHLBI ARDS Network. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bos LD, Schouten LR, van Vught LA, Wiewel MA, Ong DSY, Cremer O, et al. MARS Consortium. Identification and validation of distinct biological phenotypes in patients with acute respiratory distress syndrome by cluster analysis. Thorax. 2017;72:876–883. doi: 10.1136/thoraxjnl-2016-209719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kangelaris KN, Prakash A, Liu KD, Aouizerat B, Woodruff PG, Erle DJ, et al. Increased expression of neutrophil-related genes in patients with early sepsis-induced ARDS. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1102–L1113. doi: 10.1152/ajplung.00380.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med. 2012;185:1225–1234. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z, Beach D, Su L, Zhai R, Christiani DC. A genome-wide expression analysis in blood identifies pre-elafin as a biomarker in ARDS. Am J Respir Cell Mol Biol. 2008;38:724–732. doi: 10.1165/rcmb.2007-0354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kovach MA, Stringer KA, Bunting R, Wu X, San Mateo L, Newstead MW, et al. Microarray analysis identifies IL-1 receptor type 2 as a novel candidate biomarker in patients with acute respiratory distress syndrome. Respir Res. 2015;16:29. doi: 10.1186/s12931-015-0190-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beliakova-Bethell N, Massanella M, White C, Lada S, Du P, Vaida F, et al. The effect of cell subset isolation method on gene expression in leukocytes. Cytometry A. 2014;85:94–104. doi: 10.1002/cyto.a.22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elkord E, Williams PE, Kynaston H, Rowbottom AW. Human monocyte isolation methods influence cytokine production from in vitro generated dendritic cells. Immunology. 2005;114:204–212. doi: 10.1111/j.1365-2567.2004.02076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fanning SL, George TC, Feng D, Feldman SB, Megjugorac NJ, Izaguirre AG, et al. Receptor cross-linking on human plasmacytoid dendritic cells leads to the regulation of IFN-γ production. J Immunol. 2006;177:5829–5839. doi: 10.4049/jimmunol.177.9.5829. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.