To the Editor:
Idiopathic pulmonary fibrosis (IPF) is a devastating lung disease characterized by physiologic restriction and progressive, ultimately fatal, respiratory failure. These functional changes are caused by fibrotic interstitial remodeling, alveolar destruction, and bronchiolization or “honeycombing” of the distal airspaces. IPF affects almost 0.5% of the U.S. population over the age of 65, and its prevalence is increasing (1). The strongest risk predictor for developing IPF is the minor allele of the polymorphism rs35705950, a promoter variant upstream of the polymeric mucin MUC5B (2, 3). Variant rs35705950 is associated with increased MUC5B expression, suggesting that excessive MUC5B protein may play a role in the pathobiology of IPF. MUC5B is a dominant feature of human honeycomb cysts (HCs) (4, 5), which are composed of a stratified columnar epithelium comprising mucus and ciliated cells over a layer of cytokeratin 5 (KRT5)-expressing basal cells. We hypothesized that MUC5B was involved in HC formation.
Recently it was reported that influenza A infection (H1N1) promotes structural remodeling consistent with HCs in mice (6). Formation of these structures is contingent on mobilization of progenitor “pods” of Krt5-expressing cells (5–7). Using a previously published RNA sequencing Gene Expression Omnibus data set (GSE83467) comparing unstimulated murine progenitors to mobilized Krt5-expressing cells, we found that Muc5b was upregulated in Krt5 cells after H1N1 infection (fold change = 4.59; 95% confidence interval, 4.58–4.60; two biological replicates). We suspected that Muc5b might also be a feature of murine HCs. Given our recent findings that Muc5b modulates fibrosis in bleomycin-treated mice (8), we additionally wanted to determine whether honeycombing could be detected after bleomycin injury, and whether this was dependent on Muc5b.
Mice (8–12 wk old) were exposed to H1N1 or intratracheal bleomycin. Two models were used for bleomycin exposure. In the single-dose model, mice were administered 2.5 U/kg bleomycin intratracheally on day 0, and harvested after 10 weeks. In the repeat bleomycin model, mice were administered 2.5 U/kg, 1.25 U/kg, and 1.25 U/kg intratracheally on days 0, 7, and 14, and harvested at 7 weeks. For H1N1 infection, mice were intranasally administered 104 PFU of lab-adapted influenza A H1N1 PR 8/34 (ATCC) in 20 μl of PBS and harvested after 10 weeks unless specified otherwise. Control studies were performed with UV-irradiated H1N1. Mice who failed to lose >5% body weight after injury in either model were excluded, and those who lost 20% body weight without recovery were killed in accordance with the policies of the University of Colorado Denver Institutional Animal Care and Use Committee. BAL, hydroxyproline (HP), immunohistochemical analysis, and Muc5b dot blot were performed as described previously (9).
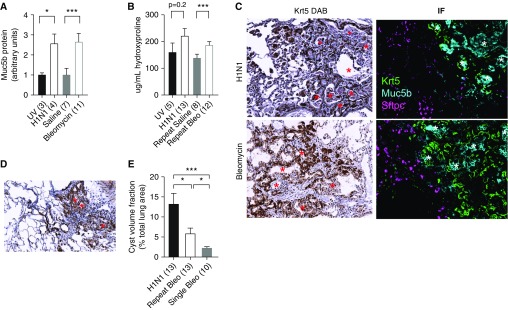
Mice injured with H1N1 demonstrated significant increases in Muc5b protein over baseline 4 weeks after infection (Figure 1A). In preliminary experiments, we were able to identify significant bronchiolization of distal airspaces consistent with HC formation as early as 4 weeks and as late as 30 weeks after H1N1 infection (not shown). At 10 weeks after infection, we noted expansive areas of HCs (Figure 1D, asterisk, top left); however, no significant differences in fibrosis above UV controls were measured by HP (Figure 1B) or second-harmonic two-photon microscopy (not shown). In contrast to human IPF, the HCs were relatively small and uniform; however like IPF they were marked by a close association with Krt5+ basal cells and filled with copious Muc5b (Figure 1C, asterisk, top right). Previous authors have observed mobilization of Krt5 cells after bleomycin exposure, presaging the development of HCs (6). Bleomycin-exposed mice also showed upregulated Muc5b gene expression (9) and elevated airway Muc5b (Figure 1A). We observed that bleomycin-injured mice also had Krt5-associated cystic structures (Figure 1C, asterisk, bottom left). These structures were full of Muc5b, consistent with HCs (Figure 1C, asterisk, bottom right). In contrast to the H1N1 model, however, bleomycin-injured mice developed significant (P < 0.005) interstitial fibrosis based on HP (Figure 1B), consistent with published reports (8, 9).
Figure 1.
Wild-type mice develop Muc5b-filled honeycomb cysts (HCs) in response to lung injury. Antibodies used for immunohistochemistry included goat anti-mouse Muc5b (11765, 1:1000; Everest), rabbit anti-mouse pro-SPC (ab90716, 1:100; Abcam), and chicken anti-mouse Krt5 (Poly9059, 1:1000; BioLegend). Appropriate fluorescent secondary antibodies were obtained from Jackson ImmunoResearch, reconstituted at 1 mg/ml, and used at 1:200. (A) Muc5b protein is increased in response to productive influenza infection (28 d postinfection) or repeated bleomycin treatment (*P < 0.05 and ***P < 0.005). (B) Influenza A H1N1 (H1N1) infection does not lead to significant fibrosis compared with UV-irradiated control virus as measured by hydroxyproline, whereas repeated bleomycin injury leads to significant increases in hydroxyproline compared with saline-treated control (***P < 0.005). (C) H1N1 generates considerable airspace remodeling resembling honeycombing (Krt5 stained DAB) 75 days after infection (top left). Similar to what is observed in humans, H1N1 HCs are characterized by Krt5-basal cells surrounding dense mucociliary structures filled with Muc5b+ colloid and generally exclude type II pneumocytes (top right). HCs also develop in mice subjected to repeated bleomycin injury (bottom left). Repeat bleomycin injury–derived HCs are generally indistinguishable from H1N1-derived HCs (bottom right). Honeycomb structures are highlighted by red (DAB panels) and white (IF panels) asterisks. (D) Cysts in mice subjected to single-dose bleomycin persist in the absence of significant fibrosis. (E) In general, resolution of H1N1 injury involves more robust honeycombing than repeat or single-dose bleomycin.
Single-dose bleomycin injury leads to fibrosis that peaks at 21 days and then is believed to resolve. To investigate whether this resolution extends to HCs, we injured mice once with bleomycin and quantified cysts after 10 weeks, at which point total lung collagen content resolves to baseline and is indistinguishable from saline-treated controls (HP data not shown). We found that instead of resolving in a manner consistent with fibrotic lung injury, single-dose bleomycin-injured mice retained a minimal (but detectable) fraction of HCs (Figures 1D and 1E). We applied an unbiased point-counting approach to estimate the volume fraction of cystic regions from thin sections of injured mouse lung. These measurements were also used to calculate the total lung volume using the Cavalieri method (9). We then selected points overlying cystic areas at random, overlaid an additional grid, and quantified the number of cysts and number of points per cyst. The total HC burdens of all models of lung injury are compared in Figure 1E.
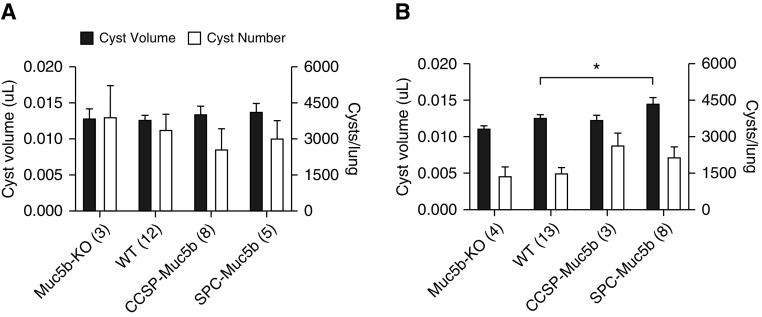
To address the role of Muc5b expression in HC development, we determined the functional consequences of altering Muc5b levels on HC formation in transgenic mice. Muc5b-overexpressing mice (SFTPC-Muc5b [referred to as SPC-Muc5b] and Scgb1a1-Muc5b [referred to as CCSP-Muc5b]) and Muc5b-deficient mice (Muc5b-KO) were used (9, 10). Using the volume estimate and cystic density estimate, we were able to calculate the number and volume of cysts per lung. Measurements of fibrosis by HP did not differ significantly among H1N1-exposed transgenic mice (not shown). Surprisingly, after H1N1 exposure, we found that Muc5b was not required for HC formation (Figure 2A). Although transgenic mice exposed to H1N1 demonstrated comparable cyst volume fractions, sizes, and numbers across genotypes, SPC-Muc5b–overexpressing mice demonstrated significantly larger cysts when exposed to repeated bleomycin (Figure 2B). These data support the possibility that the observed increase in HC may be an epiphenomenon of increased type II alveolar epithelial susceptibility to bleomycin due to ectopic Muc5b, rather than Muc5b expression per se. However, we note that ectopic MUC5B expression is also a feature of human IPF (11). This extends our recent report showing worsened bleomycin-induced fibrosis in SPC-Muc5b mice (8), and suggests that Muc5b may play a role in HC formation in lung fibrosis, or may sensitize cells to genotoxic stress, worsening injury.
Figure 2.
Durable murine honeycombing varies with expression of Muc5b in the bleomycin, but not H1N1, models. HC were morphometrically quantified via unbiased stereology; initially a 350 micron grid was used to quantify the volume fraction of the cysts. Cystic areas were then randomly sampled using a 100 micron grid to determine cyst size. Lung volume was calculated using the Cavalieri method. (A) No significant differences were observed in cyst volume or number based on genotype among mice infected with H1N1. (B) Bleomycin-injured mice expressing Muc5b under control of the SPC promoter had a significant increase in cyst volume (P < 0.05). The overall number of cysts is independent of genotype (*P < 0.05, **P < 0.01, and ***P < 0.005). KO = knockout; WT = wild-type (see text for additional strain details).
Here, we describe murine HCs characterized by pseudostratified columnar epithelial structures in close association with Krt5-expressing cells. We show that, as in humans, murine HCs are filled with Muc5b, and that HCs can be enhanced by Muc5b overexpression in the bleomycin model. However, Muc5b does not appear to be necessary for HC changes in response to H1N1 or bleomycin. Moreover, whereas the degree of fibrosis and honeycombing correlate based on Muc5b expression and extent of injury in the bleomycin model, in the H1N1 model fibrosis is insignificant and HCs are relatively uniform independent of genotype. Differences in targets of injury, inflammation, mitogens, mucin production, susceptibility to genotoxic stress, or glycoprotein biology could account for these findings.
Supplementary Material
Footnotes
Supported by National Heart, Lung, and Blood Institute grants F32 HL134243-01A1 (J.S.K.), R01 HL080396 (C.M.E.), and R33 HL120770 and UH3 HL123442 (D.A.S., I.V.Y., E.D., M.I.S, T.O., C.D.C., J.H., L.A.H., A.E., and C.E.H.).
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Raghu G, Chen SY, Yeh WS, Maroni B, Li Q, Lee YC, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med. 2014;2:566–572. doi: 10.1016/S2213-2600(14)70101-8. [DOI] [PubMed] [Google Scholar]
- 2.Fingerlin TE, Murphy E, Zhang W, Peljto AL, Brown KK, Steele MP, et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45:613–620. doi: 10.1038/ng.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakano Y, Yang IV, Walts AD, Watson AM, Helling BA, Fletcher AA, et al. Muc5b promoter variant rs35705950 affects muc5b expression in the distal airways in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;193:464–466. doi: 10.1164/rccm.201509-1872LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang IV, Coldren CD, Leach SM, Seibold MA, Murphy E, Lin J, et al. Expression of cilium-associated genes defines novel molecular subtypes of idiopathic pulmonary fibrosis. Thorax. 2013;68:1114–1121. doi: 10.1136/thoraxjnl-2012-202943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seibold MA, Smith RW, Urbanek C, Groshong SD, Cosgrove GP, Brown KK, et al. The idiopathic pulmonary fibrosis honeycomb cyst contains a mucocilary pseudostratified epithelium. PLoS One. 2013;8:e58658. doi: 10.1371/journal.pone.0058658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517:621–625. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xi Y, Kim T, Brumwell AN, Driver IH, Wei Y, Tan V, et al. Local lung hypoxia determines epithelial fate decisions during alveolar regeneration. Nat Cell Biol. 2017;19:904–914. doi: 10.1038/ncb3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hancock LA, Hennessy CE, Solomon GM, Dobrinskikh E, Estrella A, Hara N, et al. Muc5b overexpression causes mucociliary dysfunction and enhances lung fibrosis in mice. Nat Commun. 2018;9:5363. doi: 10.1038/s41467-018-07768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okamoto T, Mathai SK, Hennessy CE, Hancock LA, Walts AD, Stefanski AL, et al. The relationship between complement C3 expression and the MUC5B genotype in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2018;315:L1–L10. doi: 10.1152/ajplung.00395.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, et al. Muc5b is required for airway defence. Nature. 2014;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y, Mizuno T, Sridharan A, Du Y, Guo M, Tang J, et al. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight. 2016;1:e90558. doi: 10.1172/jci.insight.90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.