Abstract
It has been increasingly recognized lately that aberrant cellular metabolism plays an important role in the pathogenesis of pulmonary fibrosis. In our previous systemic studies, we found that human lung myofibroblasts undergo glutaminolytic reprogramming, which is mediated by an increased expression of glutaminase (Gls) 1. We showed that augmented glutaminolysis critically regulates collagen production by promoting its stabilization in human lung myofibroblasts. Our study indicates that lung fibroblast Gls1 is a promising therapeutic target for this disease. In this investigation, we primarily focused on delineating the in vivo role of fibroblast Gls1 in mouse models of pulmonary fibrosis and determining the efficacy of Gls1 inhibition in treating this pathology. We now show that fibroblast Gls1 is upregulated in fibrotic mouse lungs. We present evidence that mice with ablation of fibroblast Gls1 are protected from bleomycin-induced lung fibrosis. We show that the Gls1 inhibitor, CB-839, is therapeutically efficacious in treating both bleomycin- and transforming growth factor-β1–induced pulmonary fibrosis. Our study has thus established a solid rationale for advancing Gls1 inhibitors, particularly CB-839, to the next stage of testing in the treatment of this disease.
Keywords: glutaminase 1, glutaminolysis, fibroblast, collagen, pulmonary fibrosis
Although notable progress has been made in the past 5 years in the treatment of idiopathic pulmonary fibrosis, it remains a dreadful, debilitating disease (1, 2). It is becoming imperative that we continue to improve our understanding of pulmonary fibrosis pathogenesis and race to find better therapeutics for this disease. Fibroblasts are principal effectors in pulmonary fibrosis in that they are the primary cell type producing scar tissues composed of disorganized collagens and other extracellular matrix proteins (3–7). These cells have long been thought to represent the best therapeutic target for developing effective lung fibrosis treatment (3–6).
Recently, there has been increasing recognition of a major contribution from aberrant metabolism in lung cells to pulmonary fibrosis pathogenesis (8–16). Cellular metabolic programs are intricately intertwined, posing a challenge for precise delineation of their implication in the disease. However, the complexity also presents a plethora of opportunities for identification of novel therapeutic targets. Glutaminolysis is a major metabolic pathway processing intracellular glutamine, which involves conversion of this amino acid to glutamate by glutaminase (Gls) and replenishing the tricarboxylic acid cycle through α-ketoglutarate generation (17–20).
In a previous study, we have shown that human lung myofibroblasts undergo glutaminolytic reprogramming that is attributed to the increased expression of Gls1 in these cells (21). We found that augmented glutaminolysis plays a critical role in the expression of collagens in human lung myofibroblasts by promoting their stabilization and synthesis (21). Our study establishes lung fibroblast Gls1 as a promising therapeutic target for pulmonary fibrosis.
However, despite the fact that we have presented apparently compelling evidence showing that Gls1 is profibrotic in lung fibroblasts, it remains unknown whether lung fibroblast Gls1 is essential to the pathogenesis of pulmonary fibrosis in vivo or whether Gls1 inhibition is beneficial to the treatment of this disease. In our ensuing investigations, our efforts converged to specifically address these two questions. We now demonstrate that fibroblast Gls1 is indeed required for the pathogenesis of pulmonary fibrosis. We show that a Gls1 inhibitor is remarkably efficacious in treating this pathology in mice.
Methods
Reagents
Mouse recombinant transforming growth factor (TGF)-β1 was from R&D Systems, bleomycin (BLM) was from Besse Medical, CB-839 was from Medchemexpress, cycloheximide was from Sigma, and control and mouse Gls1 siRNAs were from Dharmacon.
Mice
C57BL/6 mice, mice with floxed Gls1 alleles (Gls1 fl/fl) (Glstm2.1Sray/J; stock no. 017894), mice with inducible expression of Cre recombinase in fibroblasts (Col1a2 Cre) (Tg(Col1a2-cre/ERT,-ALPP)7Cpd/J; stock no. 029235), and reporter mice with tdTomato fluorescence (B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J; stock no. 007908) were all purchased from The Jackson Laboratory. Mice with fibroblast-specific ablation of Gls1 (fibroblast Gls1−/−) and fibroblast-specific tdTomato reporter mice (fibroblast tdTomato+) were established by cross-breeding Col1a2 Cre line with the Gls1 fl/fl and the tdTomato reporter lines, respectively. To induce Cre recombination, mice were injected intraperitoneally tamoxifen dissolved in corn oil (75 mg/kg body weight) once a day for 5 consecutive days. At 3 days after the last tamoxifen injection, mice were instilled intratracheally with saline or bleomycin.
Mouse Lung Fibroblasts
Primary mouse normal lung fibroblast line MLg (CCL-206) was purchased from the American Type Culture Collection (ATCC). Primary mouse lung fibroblasts isolated in this study were prepared as described subsequently here: lungs were minced and digested in Hank’s balanced salt solution containing 0.1% type I collagenase, 0.1% dispase II, and 0.01% DNase I for 1 hour at 37°C. Single-cell suspensions were prepared by passing the digested lungs through a 40-μm mesh cell strainer. The cells were pelleted and red blood cell lysed. The single-cell suspensions were then incubated with a mixture of biotin-conjugated anti-CD16/32, -CD45, -CD31, and -EpCAM antibodies (BD Biosciences) and streptavidin-conjugated magnetic beads (Promega) to deplete myeloid, endothelial, and epithelial cells. Lung fibroblasts were then isolated from the cell suspensions with biotin-conjugated anti–platelet-derived growth factor receptor-α antibody (BD Biosciences) and streptavidin-conjugated magnetic beads. Primary lung fibroblasts from fibroblast tdTomato+ mice were isolated by fluorescence-activated cell sorting performed on a FACSAria II instrument (BD Biosciences).
Experimental Pulmonary Fibrosis Model
For bleomycin-induced lung fibrosis, 10-week-old male mice were anesthetized with isoflurane. The mouse tongue was gently pulled forward with forceps and bleomycin (1.5 U/kg body weight dissolved in 50 μl saline) was instilled to the oropharyngeal cavity. The tongue was kept extended until all of the liquid was inhaled into the lung (22). For TGF-β1–induced lung fibrosis, adenovirus that expresses constitutively activated TGF-β1 (6 × 109 pfu/kg body weight) or the control adenovirus was delivered to mouse lung in the same manner as that used for bleomycin instillation (23, 24). The animal protocol was approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
In Situ Immunofluorescence
Mouse lung was inflated with 1 ml Tissue-Tek optimum cutting temperature solution and then embedded in it. Frozen sections (10 μm thickness) were prepared and fixed with cold acetone (10 min, −20°C). Immunofluorescence staining was performed using primary mouse anti-Gls1 (Proteintech) and rabbit anti-RFP (Rockland) antibodies with appropriate fluorescence-conjugated secondary antibodies (ThermoFisher).
Immunofluorescence Microscopy
Immunofluorescence assays were performed as previously described (24). Rabbit anti-Gls1 was from Proteintech, MitoTracker Deep Red FM was from ThermoFisher, and images were captured with an Olympus IX73 fluorescence microscope.
Determination of Lung Hydroxyproline Level
The three right lobes plus the left upper lobe of a mouse lung were homogenized in 3 ml H2O. Homogenates (100 μl) were mixed with 100 μl 12 N HCl and the samples incubated at 120°C for 3 hours. Hydroxyproline levels were then determined with Hydroxyproline Assay Kit (BioVision) (25).
Masson’s Trichrome Staining
Masson’s trichrome staining for collagen deposition was performed as described previously (26).
siRNA Transfection
Mouse lung fibroblasts were transfected with siRNAs using HiperFect (Qiagen) according to the manufacturer’s instructions.
Real-Time PCR
mRNA levels in cells were determined by real-time PCR using SYBR Green Master Mix Kit (Roche). Primer sequences for mouse genes were as follows: tubulin α1: sense, 5′ GGATGCTGCCAATAACTATGCTCGT 3′, antisense, 5′ GCCAAAGCTGTGGAAAACCAAGAAG 3′; fibronectin: sense, 5′ TCTGGGAAATGGAAAAGGGGAATGG 3′, antisense, 5′ CACTGAAGCAGGTTTCCTCGGTTGT 3′; Col1A1: sense, 5′ GGAGGGCGAGTGCTGTGCTTT 3′, antisense, 5′ GGGACCAGGAGGACCAGGAAGT 3′. Col3A1: sense, 5′ CAATCCAGGTCCTCCAGGTTCTCC 3′, antisense, 5′ ATGCCCACTTGTTCCATCTTTTCC 3′; smooth muscle actin-α (SMA-α): sense, 5′ GACGCTGAAGTATCCGATAGAACACG 3′, antisense 5′ CACCATCTCCAGAGTCCAGCACAAT 3′; Gls1: sense, 5′ TCCTGATTTGTGGGGTGTATCTGTCT 3′, antisense, 5′ GAATCTTAATCCACTTGGCTCCTTCC 3′.
Fold change was calculated as 2ΔΔCt.
Western Blotting
Western blotting was performed as previously described (26). Mouse anti-tubulin, mouse anti–SMA-α, and mouse anti-fibronectin antibodies were from Sigma. Rabbit anti–collagen I antibody was from Abcam. Rabbit anti-Gls1 and rabbit anti–collagen III antibodies were from Proteintech. Densitometric analyses of protein bands were performed using ImageJ software (National Institutes of Health).
Statistical Analysis
One-way ANOVA followed by Bonferroni’s test was used for multiple group comparisons. The Student’s t test was used for comparison between two groups. P values less than 0.05 were considered statistically significant.
Results
Gls1 Is Upregulated in Lung Fibroblasts of Mice with Bleomycin-induced Pulmonary Fibrosis
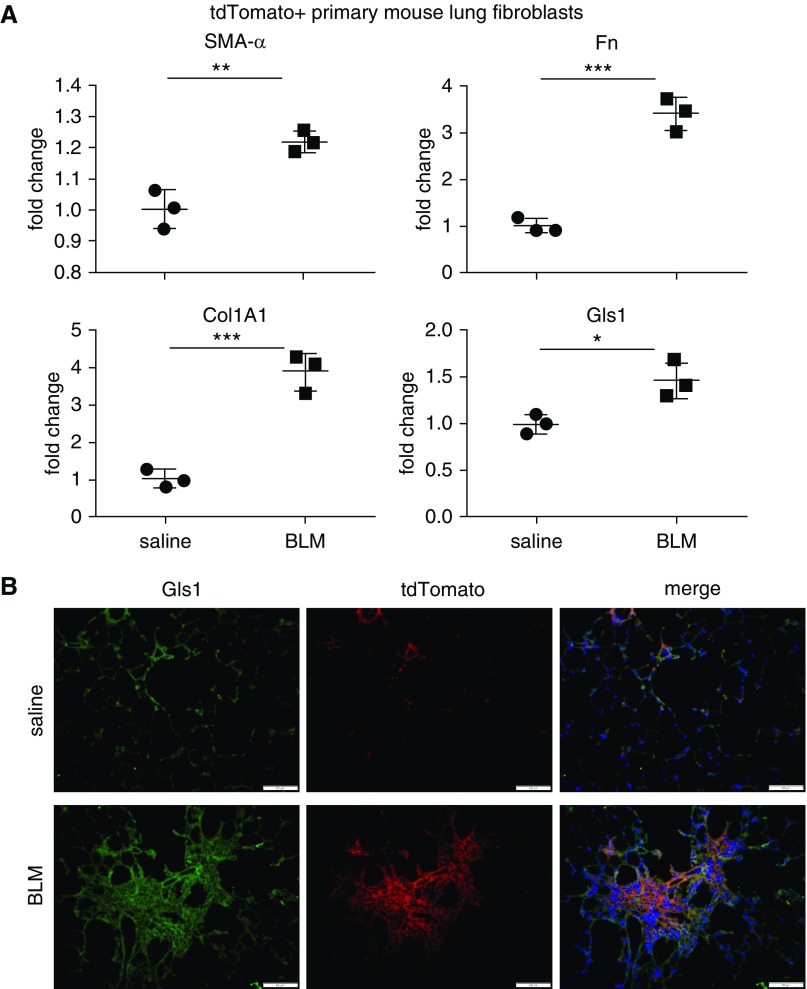
In our previous investigations, we extensively studied the regulation of Gls1 expression in human myofibroblasts (21). We provided compelling evidence that glutaminolysis promotes collagen production in these cells (21). Our findings indicate Gls1 as a potential therapeutic target for treating pulmonary fibrosis. In our following studies, we sought to test the hypothesis in mouse models of this disease. To better characterize Gls1 expression in fibroblasts of mouse lungs, we established a mouse strain with fibroblasts being labeled with tdTomato fluorescence by crossing Col1A2 Cre and Rosa26 tdTomato mice, designated as fibroblast tdTomato+. These mice were then instilled with intratracheal saline or bleomycin. At 3 weeks after the treatment, tdTomato+ lung fibroblasts were harvested by FACS. We confirmed that lung fibroblasts from bleomycin-treated mice demonstrated myofibroblast phenotype, indicated by increased expression of SMA-α, fibronectin, and collagen I (Figure 1A). More importantly, we found increased Gls1 expression in these cells (Figure 1A). The elevation of Gls1 in lung fibroblasts was corroborated by fluorescent microscopy showing colocalization of the increased Gls1 and tdTomato fluorescence (Figure 1B). Taken together, these data suggest that fibroblast Gls1 may play an important role in the pathogenesis of pulmonary fibrosis in vivo.
Figure 1.
Glutaminase (Gls) 1 is upregulated in lung fibroblasts of mice with bleomycin (BLM)-induced pulmonary fibrosis. (A) Fibroblast tdTomato+ mice were instilled intratracheally with saline or BLM. At 3 weeks after instillation, lungs were harvested and single–lung cell suspension prepared. tdTomato+ fibroblasts were obtained by FACS sorting and total RNA from these purified cells. The expression of the indicated genes was determined by real-time PCR. n = 3; mean ± SE; *P < 0.05, **P < 0.01, and ***P < 0.001. (B) Fibroblast tdTomato+ mice were treated as in A. Lungs were harvested and frozen tissue sections prepared. In situ immunofluorescence assay was performed with anti-Gls1 and anti-RFP primary antibodies. Nuclei were stained by DAPI. Scale bars: 100 μm. Col1A1 = collagen type I α 1 chain; Fn = fibronectin; SMA = smooth muscle actin.
TGF-β1 Induces Gls1 in Mouse Lung Fibroblasts
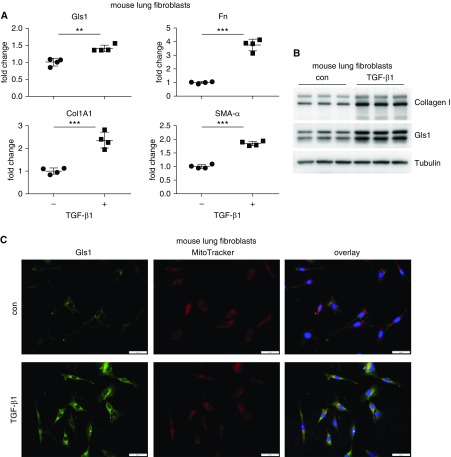
To further characterize the regulation of mouse Gls1 expression, we treated primary mouse lung fibroblasts with TGF-β1, which led to differentiation of these cells into myofibroblasts (Figures 2A and 2B). We found that TGF-β1 increased Gls1 expression at both transcriptional and protein levels in these cells (Figures 2A and 2B). The elevated Gls1 was localized in the mitochondria of TGF-β1–treated mouse lung fibroblasts, suggesting that mitochondrion is the primary subcellular site of activity of this enzyme (Figure 2C). Notably, these findings with mouse cells are similar to our previous observations from human lung fibroblasts (21). Taken together, these data suggest that the regulatory mechanism of Gls1 expression in lung fibroblasts is evolutionarily conserved.
Figure 2.
Transforming growth factor (TGF)-β1 induces Gls1 in mouse lung fibroblasts. (A) Primary normal mouse lung fibroblast line MLg cells were cultured in Eagle’s minimum essential medium (EMEM) supplemented with 1% FBS for 24 hours. The cells were then treated with or without 10 ng/ml mouse TGF-β1 for 24 hours. Total RNA from these cells was purified. The expression of the indicated genes was determined by real-time PCR. n = 4; mean ± SD; **P < 0.01 and ***P < 0.001. (B) MLg cells were treated as in A. The expression of the indicated genes was determined by Western blotting. (C) MLg cells were treated as in A. Gls1 expression was determined by immunofluorescence microscopy. Mitochondria were visualized by MitoTracker Deep Red FM. Nuclei were visualized by DAPI. Scale bars: 50 μm. A representative of two to three independent experiments is shown. Con = control.
Knockdown of Gls1 Inhibits Collagen Expression at the Protein, but Not the mRNA Level, in TGF-β1–treated Mouse Lung Fibroblasts
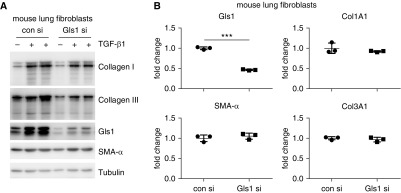
Because we previously demonstrated that Gls1 is required for TGF-β1–induced collagen expression at protein level in human lung fibroblasts (21), we decided to do a similar study on normal mouse lung fibroblasts, intending to provide a rationale for testing the efficacy of Gls1 inhibition in mouse models of pulmonary fibrosis. As shown in Figure 3A and consistent with those observations in human lung fibroblasts, knockdown of Gls1 attenuated TGF-β1–induced expression of collagen I and collagen III, but not SMA-α, in the mouse lung fibroblasts. Furthermore, the decreased expression of collagen proteins was not due to a weakened transcription in Gls1 knockdown mouse cells, which was also in accordance with our findings in human fibroblasts (Figure 3B). Altogether, these data suggest that glutaminolysis regulation of collagen expression shares a common mechanism across species.
Figure 3.
Knockdown of Gls1 inhibits collagen expression at the protein, but not the mRNA, level in TGF-β1–treated mouse lung fibroblasts. (A) Primary normal mouse lung fibroblast MLg cells were transfected with 20 nM control siRNA or Gls1 siRNA, followed by treatment with or without 10 ng/ml TGF-β1 for 48 hours. Levels of the indicated proteins were determined by Western blotting. (B) MLg cells were transfected as in A, followed by treatment with TGF-β1. The expression of the indicated genes was determined by real-time PCR. n = 3; mean ± SD; ***P < 0.001. A representative of two independent experiments is shown. si = siRNA.
Pharmaceutical Inhibition of Gls1 Diminishes TGF-β1–induced Collagen Production in Mouse Lung Fibroblasts
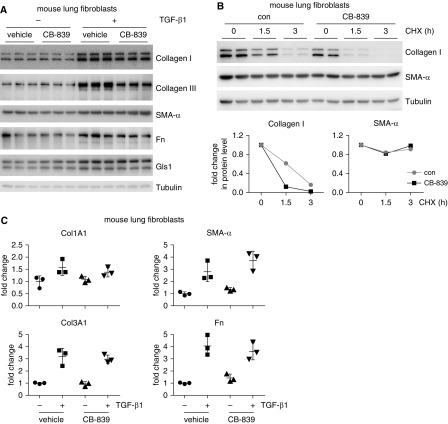
Elevated glutaminolysis is a metabolic hallmark of many types of malignancies (17). Specific Gls1 inhibitors, such as CB-839 and BPTES, have been gaining special interest due to their remarkable efficacy in cancer preclinical trials (27, 28). Those studies with the Gls1 inhibitors in the cancer field shall surely facilitate testing of them in treating lung fibrosis, which has not yet been explored. To determine if pharmaceutical Gls1 inhibition is efficacious in treating pulmonary fibrosis in vivo, we first examined the effect of CB-839 on TGF-β1–induced profibrotic mediators in mouse lung fibroblasts in vitro. Consistent with the findings with Gls1 knockdown, the Gls1 inhibitor, CB-839, selectively inhibited the protein levels of collagen I and collagen III, but not SMA-α or fibronectin, in TGF-β1–treated mouse lung fibroblasts (Figure 4A). The decrease in collagen levels caused by CB-839 in TGF-β1–treated mouse lung fibroblasts likely resulted from their reduced stabilization upon Gls1 inhibition, as suggested by the accelerated collagen degradation displayed in CB-839–treated cells (Figure 4B). Also in accordance with Gls1 knockdown, CB-839 had no effect on the transcription of these profibrotic mediators in TGF-β1–treated cells (Figure 4C). Taken together, these data further support our previous findings with human lung fibroblasts that glutaminolysis is required for collagen protein expression, partially by promoting collagen stabilization (21).
Figure 4.
Pharmaceutical inhibition of Gls1 diminishes TGF-β1–induced collagen production in mouse lung fibroblasts. (A) Primary normal mouse lung fibroblast MLg cells were cultured in EMEM supplemented with 1% FBS for 24 hours. The cells were then pretreated with or without 5 μM CB-839 for 1 hour, followed by treatment with or without 10 ng/ml TGF-β1 for 48 hours. Levels of the indicated proteins were determined by Western blotting. (B) MLg cells were treated with TGF-β1 for 24 hours, washed, and treated again with or without 5 μM CB-839 for 24 hours. The cells were then treated with 5 μg/ml cycloheximide (CHX) for 0, 1.5, and 3 hours. Levels of the indicated proteins were determined by Western blotting and densitometric analyses performed using ImageJ. (C) MLg cells were treated as in A. The expression of the indicated genes was determined by real-time PCR. n = 3; mean ± SD. A representative of two to three independent experiments is shown.
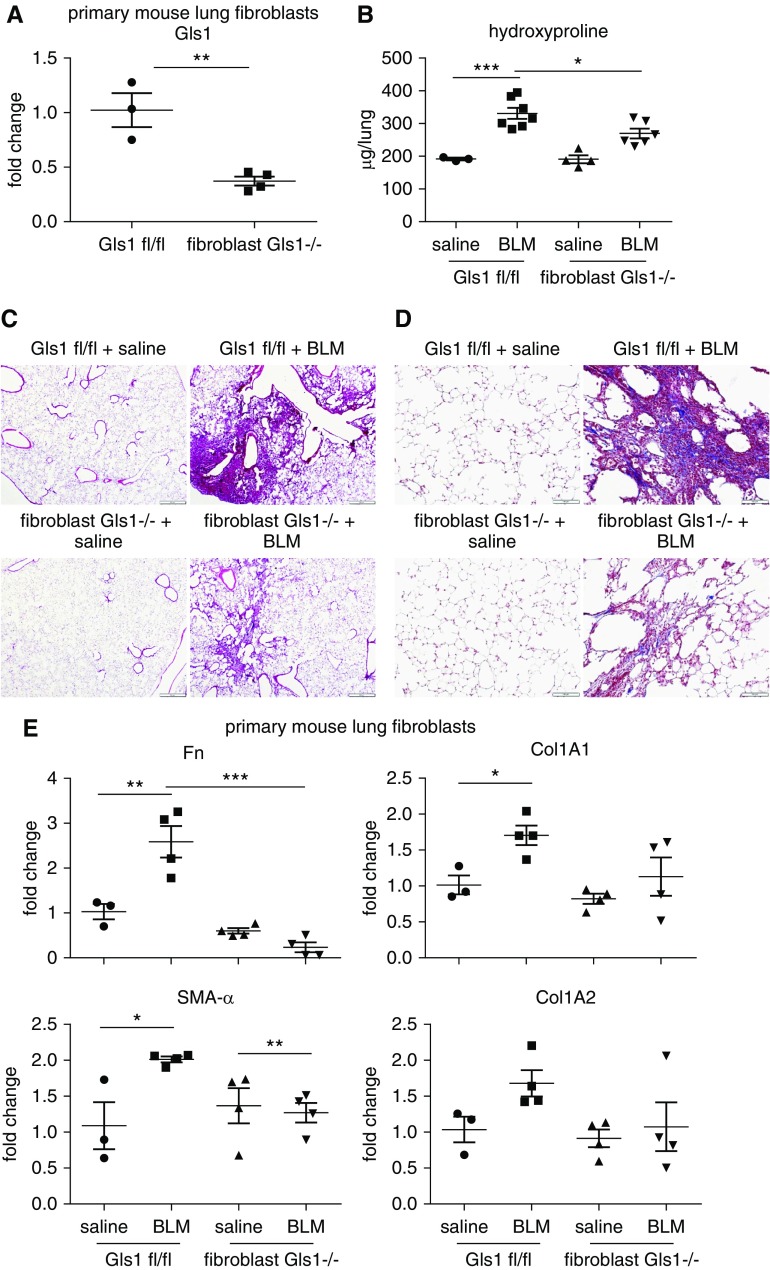
Conditional Ablation of Gls1 in Fibroblasts Attenuates Bleomycin-induced Pulmonary Fibrosis
We have shown that lung fibroblast Gls1 is upregulated in mice with bleomycin-induced pulmonary fibrosis (Figure 1). We have also demonstrated that Gls1 is required for collagen expression in lung fibroblasts (Figures 3 and 4). These findings prompted us to determine the role of fibroblast Gls1 in the pathogenesis of pulmonary fibrosis, which is characterized by excessive, scar-forming collagen production. We generated a mouse line with conditional knockout of Gls1 in fibroblasts, designated as fibroblast Gls1−/−, by crossing Col1A2 Cre and Gls1 fl/fl mice. We confirmed that Gls1 expression was significantly reduced in lung fibroblasts from fibroblast Gls1−/− mice compared with that in the control Gls1 fl/fl animals (Figure 5A). More importantly, we found that bleomycin-induced pulmonary fibrosis was markedly attenuated in fibroblast Gls1−/− mice, reflected by the decreased level of lung hydroxyproline, abated destruction of normal lung architecture, and attenuated deposition of lung collagens (Figures 5B–5D) in these animals. Consistent with the improved lung fibrosis in bleomycin-treated fibroblast Gls1−/− mice, lung fibroblasts in these animals also demonstrated diminished profibrotic activities, as evidenced by reduced transcription of fibronectin, SMA-α, and collagens in these cells (Figure 5E). Of note, these findings seemingly differ from Gls1 regulation of these profibrotic mediators in TGF-β1–treated lung fibroblasts in vitro. The apparent discrepancy likely resulted from continuous phenotypic transformation of lung fibroblasts due to the improving pulmonary microenvironment in the bleomycin-treated fibroblast Gls1−/− mice
Figure 5.
Conditional ablation of Gls1 in fibroblasts attenuates BLM-induced pulmonary fibrosis. (A) Primary mouse lung fibroblasts were isolated from Gls1 fl/fl and fibroblast Gls1−/− mice. Total RNA was purified and the expression of Gls1 determined by real-time PCR. n = 3 and 4; mean ± SE; **P < 0.01. (B) Gls1 fl/fl and fibroblast Gls1−/− mice were instilled intratracheally with saline or BLM. At 3 weeks after instillation, lungs were harvested and hydroxyproline levels in the lungs determined. n = 3, 7, 4, 6 for groups Gls1 fl/fl saline, Gls1 fl/fl BLM, fibroblast Gls1−/− saline and fibroblast Gls1−/− BLM, respectively; mean ± SE; *P < 0.05 and ***P < 0.001. (C and D) Gls1 fl/fl and fibroblast Gls1−/− mice were treated as in B. Lungs were harvested and tissue sections prepared. (C) Hematoxylin and eosin and (D) Masson’s trichrome stainings were performed. Scale bars: 500 μm in C and 100 μm in D. (E) Gls1 fl/fl and fibroblast Gls1−/− mice were treated as in B. Primary mouse lung fibroblasts were isolated and the expression of the indicated genes determined by real-time PCR. n = 3, 4, 4, 4 for groups Gls1 fl/fl saline, Gls1 fl/fl BLM, fibroblast Gls1−/− saline and fibroblast Gls1−/− BLM, respectively; mean ± SE; *P < 0.05, **P < 0.01, and ***P < 0.001.
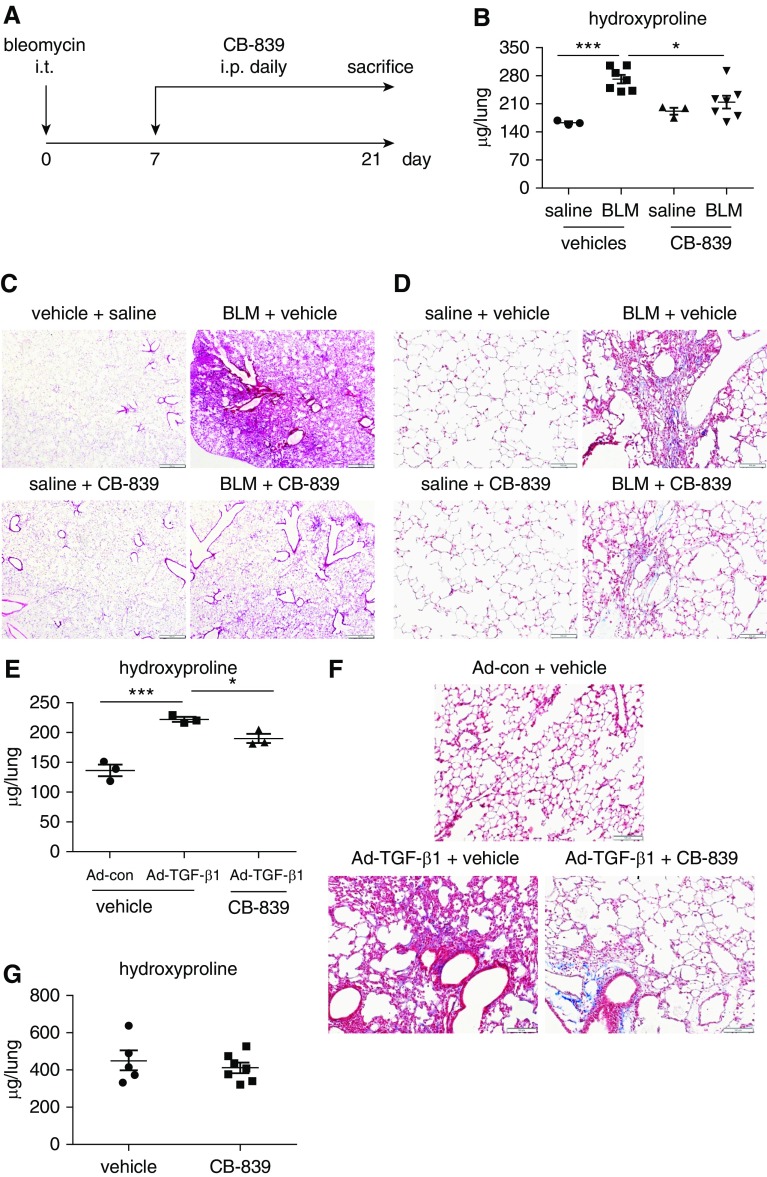
Gls1 Inhibitor Is Therapeutically Effective in Mice with Bleomycin or Active TGF-β1–induced Pulmonary Fibrosis
We have shown that conditional ablation of fibroblast Gls1 protects mice from bleomycin-induced lung fibrosis. We then asked if Gls1 is an effective novel therapeutic target for lung fibrosis in vivo. To test this hypothesis, we first administered intratracheal bleomycin into mouse lungs. Starting at 1 week after bleomycin instillation, a time when acute pulmonary inflammation gives way to the most active fibrogenesis (29), we treated these mice with vehicle or the Gls1 inhibitor, CB-839, once a day for 2 weeks (Figure 6A). We found that CB-839 demonstrated outstanding therapeutic efficacy in abating bleomycin-induced pulmonary fibrosis, as evidenced by markedly diminished lung hydroxyproline levels in mice treated with this compound (Figure 6B). Histological examination and Masson’s trichrome staining also revealed attenuated disease severity and reduced collagen deposition in CB-839–treated animals (Figures 6C and 6D). To determine whether Gls1 inhibition is therapeutically effective in other pulmonary fibrosis models, we tested CB-839 in mice treated intratracheally with adenovirus that expresses active TGF-β1. As shown in Figures 6E and 6F and consistent with previous studies, treatment with TGF-β1–expressing adenoviral particles led to lung fibrosis in mice compared with those treated with control virus. More importantly, TGF-β1–induced lung fibrosis was attenuated by CB-839, although the efficacy is relatively modest compared with that demonstrated in bleomycin-induced lung fibrosis (Figures 6E and 6F). Taken together, these data suggest that Gls1 inhibition is a promising therapeutic strategy to treat pulmonary fibrosis.
Figure 6.
Gls1 inhibitor is therapeutically effective in mice with BLM or active TGF-β1–induced pulmonary fibrosis. (A) Schematic illustration of the experimental design. C57BL/6 mice were instilled intratracheally with saline or BLM. At 7 days after the instillation, the mice were injected intraperitoneally with CB-839 (28 mg/kg body weight dissolved in 30 μl DMSO) or the vehicle DMSO once a day for 2 weeks. At the end of the treatment, mice were killed and lungs harvested. (B) Lung hydroxyproline levels were determined. n = 3, 7, 3, 7 for groups vehicles saline, vehicles BLM, CB-839 saline and CB-839 BLM, respectively; mean ± SE; *P < 0.05 and ***P < 0.001. (C and D) Experiments were performed as in A. Lungs were harvested and tissue sections prepared. (C) Hematoxylin and eosin and (D) Masson’s trichrome stainings were performed. Scale bars: 500 μm in C and 100 μm in D. (E) C57BL/6 mice were instilled intratracheally with control adenovirus (ad-con) or adenovirus expressing active TGF-β1 (Ad-TGF-β1) (6 × 109 pfu/kg body weight). At 7 days after the instillation, the mice were injected intraperitoneally with CB-839 or the vehicle DMSO once a day for 2 weeks. Mice were then killed and lung hydroxyproline levels determined. n = 3 each; mean ± SE; *P < 0.05 and ***P < 0.001. (F) Experiments were performed as in E. Lung tissue sections were prepared and Masson’s trichrome staining performed. Scale bar: 100 μm. (G) Fibroblast Gls1−/− mice were instilled intratracheally with BLM. At 7 days after the instillation, the mice were injected intraperitoneally with CB-839 or the vehicle DMSO once a day for 2 weeks. Mice were then killed and lung hydroxyproline levels determined. n = 5 and 7; mean ± SE. i.p. = intraperitoneal; i.t. = intratracheal.
To determine if systemic application of CB-839 takes effect through inhibiting lung fibroblast Gls1, we tested this compound in fibroblast Gls1−/− mice, and found that CB-839 virtually lost its efficacy in treating bleomycin-induced pulmonary fibrosis (Figure 6G). Together with the finding that mice with ablation of fibroblast Gls1 are protected from pulmonary fibrosis, these data suggest that Gls1 in lung fibroblasts plays the central role in the onset and progression of pulmonary fibrosis.
Discussion
In the last 5 years, there has been rapidly growing evidence that compellingly establishes cellular metabolic aberration as a key mechanism implicated in pulmonary fibrosis (8, 9). Most of those findings are derived from fibroblasts in diseased lungs, because this type of cell is the direct effector that produces the majority of the scar-forming collagens and other extracellular matrix molecules (3–6). In our systemic effort to investigate the interaction between cellular metabolic dysregulation and pulmonary fibrosis, we have demonstrated the regulation of collagen production by Gls1-mediated glutaminolysis in human lung myofibroblasts in vitro (21). Our data suggested that Gls1 is a potential therapeutic target for this disease. In ensuing studies, our first priority was to definitively characterize the role of Gls1 in the pathogenesis of pulmonary fibrosis in vivo. Another pressing goal was to thoroughly test the therapeutic efficacy of Gls1 inhibition in preclinical models of pulmonary fibrosis. Here, we show that we have accomplished these two objectives by demonstrating that fibroblast Gls1 has a central role in the pathogenesis of lung fibrosis and that inhibition of Gls1 is indeed efficacious in treating this disease in vivo. Our conclusion is based on the evidence that ablation of fibroblast Gls1 protects mice from bleomycin-induced pulmonary fibrosis and that the Gls1 inhibitor, CB-839, improves the disease induced by either bleomycin or active TGF-β.
Increased Gls1 expression and the resulting augmented glutaminolysis have been frequently found in many types of cancers (30). Boosted glutamine consumption bestows advantages of cancer cells for rapid proliferation and survival from nutrient restriction (17). This feature of metabolic aberration in cancer cells has made Gls1 a valuable therapeutic target for cancer treatment (17, 30). There are several pipelines to develop specific Gls1 inhibitors in both academia and industry (31). Among those, CB-839 has received great interest, and is currently being evaluated in phase 1 and 2 clinical trials for treating various cancers (31). Although it seems compelling from the previous studies by us and others that the enhanced glutaminolysis has an important role in lung fibrosis (21, 32), almost all those investigations were performed on cells in in vitro settings. In the present study, we, for the first time, demonstrated that the Gls1 inhibitor, CB-839, is strikingly efficacious in treating lung fibrosis in mice. Despite the mechanistic differences that may exist between the actions of CB-839 in treating cancer and tissue fibrosis, the pharmacokinetics and pharmacodynamics of this compound derived from those ongoing cancer trials will certainly be of great value for expediting the testing of CB-839 in lung fibrosis treatment.
We showed that the Gls1 inhibitor, CB-839, has little effect on bleomycin-induced pulmonary fibrosis in mice with ablation of fibroblast Gls1. However, we want to be cautious not to assert that only fibroblast Gls1, but not the enzyme in all other types of lung cells, is involved in the pathogenesis of pulmonary fibrosis. A possible cause for the lack of effect of CB-839 in fibroblast Gls1−/− mice is that Gls1 in lung fibroblasts is not sufficient, but essential to the onset and progression of pulmonary fibrosis. Therefore, the augmented fibroblast Gls1 might still act in concert with the enzyme in other lung cells that also likely undergo glutaminolytic reprogramming to promote this pathology. However, such a hypothetical scenario could only possibly be tested in transgenic animals that have Gls1 overexpression in fibroblasts. Regardless, this question, although mechanistically interesting, is not critically important from a therapeutic standpoint, because CB-839 has already demonstrated remarkable efficacy in two preclinical pulmonary fibrosis models.
One should also be reminded that Gls1 inhibition or deficiency does not affect the normal collagen expression in mouse lungs, as evidenced by the fact that there were no alterations in the basal levels of lung hydroxyproline in either fibroblast Gls1−/− mice or mice treated with CB-839. These data suggest that Gls1 is not essential to homeostatic collagen synthesis in the lung. This is medicinally significant, because normal collagen production is crucial to the maintenance of physiological functions of many tissues. A compromise of this process, even a moderate one, may lead to intolerable side effects, thus rendering Gls1 an unfavorable therapeutic target for lung fibrosis treatment.
In all, the present investigation fills the knowledge gap between the well-established profibrotic activity of Gls1 in vitro and the prospect of a novel therapeutic strategy in Gls1 inhibition for lung fibrosis. More significantly, our study has built a solid foundation with strong evidence in two preclinical mouse models for future clinical trials of Gls1 inhibitors, particularly CB-839, to treat this disease.
Supplementary Material
Footnotes
Supported by National Institutes of Health grant HL135830 (G.L.).
Author Contributions: H.C., N.X., and D.J. contributed equally to this work; G.L. designed the study; H.C., N.X., D.J., S.B., J.G., Y.Y.S., and G.L. performed the experiments and/or analyzed the data; H.C. and G.L. wrote the manuscript.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0051OC on April 3, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074. doi: 10.1038/nrdp.2017.74. [DOI] [PubMed] [Google Scholar]
- 2.Kolb M, Bonella F, Wollin L. Therapeutic targets in idiopathic pulmonary fibrosis. Respir Med. 2017;131:49–57. doi: 10.1016/j.rmed.2017.07.062. [DOI] [PubMed] [Google Scholar]
- 3.Kis K, Liu X, Hagood JS. Myofibroblast differentiation and survival in fibrotic disease. Expert Rev Mol Med. 2011;13:e27. doi: 10.1017/S1462399411001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest. 2012;122:2756–2762. doi: 10.1172/JCI60323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eickelberg O, Laurent GJ. The quest for the initial lesion in idiopathic pulmonary fibrosis: gene expression differences in IPF fibroblasts. Am J Respir Cell Mol Biol. 2010;42:1–2. doi: 10.1165/rcmb.2009-0245ED. [DOI] [PubMed] [Google Scholar]
- 6.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burman A, Tanjore H, Blackwell TS. Endoplasmic reticulum stress in pulmonary fibrosis. Matrix Biol. 2018;68–69:355–365. doi: 10.1016/j.matbio.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu G, Summer R. Cellular metabolism in lung health and disease. Annu Rev Physiol. 2019;81:403–428. doi: 10.1146/annurev-physiol-020518-114640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao H, Dennery PA, Yao H. Metabolic reprogramming in the pathogenesis of chronic lung diseases, including BPD, COPD, and pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2018;314:L544–L554. doi: 10.1152/ajplung.00521.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nigdelioglu R, Hamanaka RB, Meliton AY, O’Leary E, Witt LJ, Cho T, et al. Transforming growth factor (TGF)-β promotes de novo serine synthesis for collagen production. J Biol Chem. 2016;291:27239–27251. doi: 10.1074/jbc.M116.756247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamanaka RB, Nigdelioglu R, Meliton AY, Tian Y, Witt LJ, O’Leary E, et al. Inhibition of phosphoglycerate dehydrogenase attenuates bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2018;58:585–593. doi: 10.1165/rcmb.2017-0186OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodwin J, Choi H, Hsieh MH, Neugent ML, Ahn JM, Hayenga HN, et al. Targeting hypoxia-inducible factor-1α/pyruvate dehydrogenase kinase 1 axis by dichloroacetate suppresses bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2018;58:216–231. doi: 10.1165/rcmb.2016-0186OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie N, Cui H, Ge J, Banerjee S, Guo S, Dubey S, et al. Metabolic characterization and RNA profiling reveal glycolytic dependence of profibrotic phenotype of alveolar macrophages in lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2017;313:L834–L844. doi: 10.1152/ajplung.00235.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryu C, Sun H, Gulati M, Herazo-Maya JD, Chen Y, Osafo-Addo A, et al. Extracellular mitochondrial DNA is generated by fibroblasts and predicts death in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2017;196:1571–1581. doi: 10.1164/rccm.201612-2480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho SJ, Moon JS, Lee CM, Choi AM, Stout-Delgado HW. Glucose transporter 1–dependent glycolysis is increased during aging-related lung fibrosis, and phloretin inhibits lung fibrosis. Am J Respir Cell Mol Biol. 2017;56:521–531. doi: 10.1165/rcmb.2016-0225OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang YP, Lee SB, Lee JM, Kim HM, Hong JY, Lee WJ, et al. Metabolic profiling regarding pathogenesis of idiopathic pulmonary fibrosis. J Proteome Res. 2016;15:1717–1724. doi: 10.1021/acs.jproteome.6b00156. [DOI] [PubMed] [Google Scholar]
- 17.Jin L, Alesi GN, Kang S. Glutaminolysis as a target for cancer therapy. Oncogene. 2016;35:3619–3625. doi: 10.1038/onc.2015.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang C, Ko B, Hensley CT, Jiang L, Wasti AT, Kim J, et al. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol Cell. 2014;56:414–424. doi: 10.1016/j.molcel.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka K, Sasayama T, Irino Y, Takata K, Nagashima H, Satoh N, et al. Compensatory glutamine metabolism promotes glioblastoma resistance to mTOR inhibitor treatment. J Clin Invest. 2015;125:1591–1602. doi: 10.1172/JCI78239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durán RV, Oppliger W, Robitaille AM, Heiserich L, Skendaj R, Gottlieb E, et al. Glutaminolysis activates Rag–mTORC1 signaling. Mol Cell. 2012;47:349–358. doi: 10.1016/j.molcel.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 21.Ge J, Cui H, Xie N, Banerjee S, Guo S, Dubey S, et al. Glutaminolysis promotes collagen translation and stability via α-ketoglutarate–mediated mTOR activation and proline hydroxylation. Am J Respir Cell Mol Biol. 2018;58:378–390. doi: 10.1165/rcmb.2017-0238OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie N, Tan Z, Banerjee S, Cui H, Ge J, Liu RM, et al. Glycolytic reprogramming in myofibroblast differentiation and lung fibrosis. Am J Respir Crit Care Med. 2015;192:1462–1474. doi: 10.1164/rccm.201504-0780OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu RM, Vayalil PK, Ballinger C, Dickinson DA, Huang WT, Wang S, et al. Transforming growth factor β suppresses glutamate-cysteine ligase gene expression and induces oxidative stress in a lung fibrosis model. Free Radic Biol Med. 2012;53:554–563. doi: 10.1016/j.freeradbiomed.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang S, Cui H, Xie N, Icyuz M, Banerjee S, Antony VB, et al. miR-145 regulates myofibroblast differentiation and lung fibrosis. FASEB J. 2013;27:2382–2391. doi: 10.1096/fj.12-219493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui H, Banerjee S, Guo S, Xie N, Ge J, Jiang D, et al. Long noncoding RNA Malat1 regulates differential activation of macrophages and response to lung injury. JCI Insight. 2019;4:124522. doi: 10.1172/jci.insight.124522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang S, Banerjee S, de Freitas A, Sanders YY, Ding Q, Matalon S, et al. Participation of miR-200 in pulmonary fibrosis. Am J Pathol. 2012;180:484–493. doi: 10.1016/j.ajpath.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiang Y, Stine ZE, Xia J, Lu Y, O’Connor RS, Altman BJ, et al. Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis. J Clin Invest. 2015;125:2293–2306. doi: 10.1172/JCI75836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Momcilovic M, Bailey ST, Lee JT, Fishbein MC, Braas D, Go J, et al. The gsk3 signaling axis regulates adaptive glutamine metabolism in lung squamous cell carcinoma. Cancer Cell. 2018;33:905–921.e5. doi: 10.1016/j.ccell.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294:L152–L160. doi: 10.1152/ajplung.00313.2007. [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Venneti S, Nagrath D. Glutaminolysis: a hallmark of cancer metabolism. Annu Rev Biomed Eng. 2017;19:163–194. doi: 10.1146/annurev-bioeng-071516-044546. [DOI] [PubMed] [Google Scholar]
- 31.Song M, Kim SH, Im CY, Hwang HJ. Recent development of small molecule glutaminase inhibitors. Curr Top Med Chem. 2018;18:432–443. doi: 10.2174/1568026618666180525100830. [DOI] [PubMed] [Google Scholar]
- 32.Bernard K, Logsdon NJ, Benavides GA, Sanders Y, Zhang J, Darley-Usmar VM, et al. Glutaminolysis is required for transforming growth factor-β1–induced myofibroblast differentiation and activation. J Biol Chem. 2018;293:1218–1228. doi: 10.1074/jbc.RA117.000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.