Asthma is a chronic, complex pulmonary disease that affects ∼300 million people worldwide. This multifactorial, heterogeneous disorder is characterized by airflow obstruction and airway inflammation, and encompasses various phenotypes (observable characteristics) and endotypes (biological mechanisms of disease). Generally, asthma is classified as eosinophilic or noneosinophilic based on airway or peripheral blood cellular profiles, but marked heterogeneity exists throughout the entire spectrum of the disease and is most pronounced in the subset of severe disease (1). Understanding the complex immune pathways and other disease-modulating factors (i.e., microbiome, metabolome, and genetics) is necessary to refine asthma endotypes and to improve treatment strategies for patients with this disease.
Eosinophilic, atopic asthma is driven by T-helper cell type 2 (Th2) responses (IL-4, IL-5, and IL-3) to inhaled allergens. However, eosinophilic airway inflammation is also present in nonatopic asthma (1). Although allergic asthma and eosinophil-dominant asthma are the most frequent and often effectively managed subgroups, ∼10–15% of individuals with asthma have severe corticosteroid-refractory disease with a noneosinophilic inflammatory response and experience persistent symptoms and frequent exacerbations. Noneosinophilic or type 2 low asthma is diverse and consists of disease with neutrophil-dominant inflammation resulting from type 1 and type 17 cytokines, mixed granulocytic inflammation with concurrent allergic and nonallergic mechanisms, or paucigranulocytic inflammation (2). We have made progress in understanding the heterogeneity of the immunological responses in asthma, but our knowledge of the underlying mechanisms of severe, noneosinophilic asthma is still limited. Experimental models to mimic noneosinophilic or mixed disease phenotypes have emerged and are likely to be essential for developing a better understanding of this heterogeneous disease (3–5).
Calprotectin is a heterodimeric complex of S100A8 (MRP8 [myeloid-related protein 8]) and S100A9 (MRP14) and is associated with a number of inflammatory diseases, including inflammatory bowel disease, arthritis, psoriasis, and pulmonary infection (6). These innate immune proteins are both bacteriostatic and proinflammatory in nature (7). Specifically, S100 proteins, like these, comprise a group of damage-associated molecular pattern molecules that bind to and activate TLR4 (Toll-like receptor 4) and RAGE (receptor for advanced glycation end products), which has been implicated in type 2 allergic airway disease in mice (8, 9). It is known that S100A8 and S100A9 are secreted in a disease-specific manner mainly from neutrophils and macrophages, but few mechanistic studies have focused on defining the role of these proteins during inflammation.
In the lung, both clinical and animal findings have linked calprotectin with asthma. S100A8 and S100A9 are upregulated in individuals with asthma compared with those without asthma and are associated with more severe, uncontrolled disease phenotypes (10–13). Specifically, Lee and colleagues found that S100A9 levels were higher in sputum from patients with severe asthma and neutrophil-dominant inflammation compared than in sputum from eosinophil-dominant and paucigranulocytic groups (12, 13). Furthermore, S100A9 levels significantly correlated with the percentage of neutrophils in the sputum (13). These data suggest that S100A9 may initiate and amplify neutrophilic inflammation in patients with uncontrolled, severe asthma. In experimental animal models of asthma, the role of calprotectin is more ambiguous. Some studies demonstrated that exogenous treatment of S100A8 and S100A9 reduced Th2-mediated responses after ovalbumin-induced allergic airway inflammation (14, 15), whereas others using neutralizing antibodies for S100A8 and S100A9 showed that calprotectin promoted disease in a mixed allergen model (16). Together, these studies show that the role of calprotectin may differ based on the inflammatory context in the asthmatic lung.
In this issue of the Journal, Palmer and colleagues (pp. 459–468) examine the role of S100A9/calprotectin in allergic airway inflammation in mice (17). Although calprotectin is implicated in the pathogenesis of inflammatory diseases by functioning as a ligand for TLR4 and RAGE, this study elucidates an alternative and antiinflammatory mechanism by which S100A9 influences innate and adaptive immune responses (Figure 1). Using the Alternaria alternata model of type 2 high allergic airway inflammation, the authors found that calprotectin-deficient mice (S100A9−/−) had worsened disease as evidenced by increased airway eosinophilia, type 2 helper T cell (Th2) activation, and airway resistance and elastance responses to methacholine challenge. Specifically, calprotectin restricted the number of IL-13/IL-5–producing CD4+ T cells in the lung, but not by altering the amount of group 2 innate lymphoid cells in response to A. alternata. Furthermore, the authors demonstrate that increased allergic airway inflammation in calprotectin-deficient mice results from the inability of T regulatory cells to control Th2 responses, identifying a novel role for S100A9 in regulating CD4+ T-cell responses in the context of asthma. These data also support a central role for antigen-specific Th2 cells in promoting airway hyperresponsiveness. Although their data are consistent with previous findings suggesting that calprotectin protects against allergic airway inflammation (14, 15), their work significantly advances the field by providing mechanistic insight into a physiological and immunological role for calprotectin in asthma.
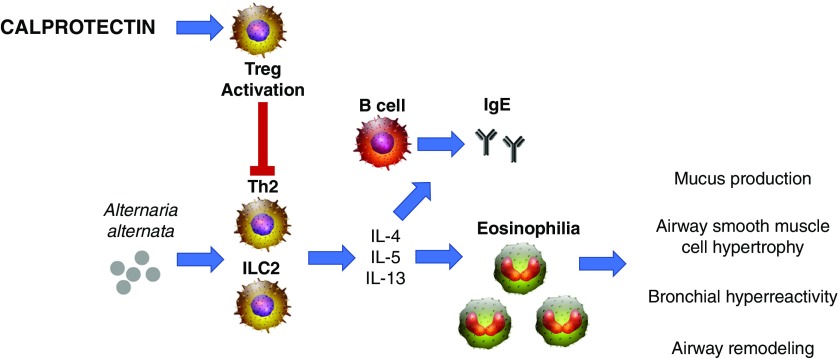
Figure 1.
Effects of calprotectin on Alternaria alternata–induced type 2 allergic airway inflammation. A. alternata challenge results in a robust type 2–driven inflammation (T-helper cell type 2 [Th2] and group 2 innate lymphoid cells [ILC2], type 2–associated cytokines [IL-4, IL-5, and IL-13], and chemokines [eotaxins, such as CCL11 and CCL24]) and recruits eosinophils to the lungs. Type 2 cytokines mediate class switching of B cells to secrete IgE upon exposure to antigen. These type 2 responses contribute to the hallmarks of asthma pathogenesis, including mucus production, subepithelial fibrosis, bronchial remodeling, and airway hyperresponsiveness. Calprotectin significantly limits allergic airway inflammation by limiting the production of IL-13, CCL11, CCL24, serum IgE, eosinophil recruitment, and airway hyperresponsiveness. Furthermore, calprotectin enhances T regulatory cell (Treg) activation, which suppresses Th2-mediated hyperinflammation.
S100A8/S100A9 currently serves as a candidate biomarker and predictive indicator of therapeutic responsiveness in various inflammatory diseases (6). However, the localization and timing of calprotectin induction during disease are still unclear. In the lung, S100A8 was found to be expressed by neutrophils and macrophages and upregulated during acute allergic inflammation (16). Similarly, S100A9 was shown to be localized to neutrophils and bronchial epithelial cells in the airway during neutrophil-dominant allergic airway disease (13). Even though S100A9 is one of the most abundant proteins in the peripheral blood eosinophil proteome (18), eosinophils recruited to the lungs during allergic airway disease have not been shown to express calprotectin. Here, Palmer and colleagues show that S100A9 is not basally present in the respiratory epithelium but is strongly expressed in type 2 pneumocytes. After A. alternata exposure, S100A9 expression is increased in the alveolar and airway epithelium. Together with S100A9’s protective role in allergic airway disease, this observation suggests that proper levels of S100A9/calprotectin may be needed for both immune defense and homeostasis. Although it was demonstrated that calprotectin modulates T regulatory cell activation by directly suppressing Th2 cell function, changes in CCL11 and CCL24 that promote eosinophilia could also indicate direct or indirect effects of calprotectin on the airway epithelium. Similarly, the localization of TLR4 and RAGE within the lung during A. alternata exposure could also influence calprotectin-mediated protection. In addition, previous work demonstrated that S100A8 attenuated airway hyperresponsiveness by suppressing airway smooth muscle cell contractility in an experimental model of type 2 allergic airway disease in rats (19). Given the complexity of the immune system and cross-talk among resident and circulating immune cells, it is likely that multiple cell types are directly or indirectly influenced by calprotectin to confer protection in the lung upon A. alternata challenge. Defining the cellular sources of this protein and its receptors will help to clarify its direct and indirect effects within the lung, and will provide insight into the utility of calprotectin as a personalized therapy for asthma. Furthermore, the studies performed by Palmer and colleagues delineate the role of calprotectin in a type 2–dominant immune setting (17); its biological function in other immunophenotypes of severe asthma is still unknown. Because calprotectin is highly expressed by neutrophils and contributes to severe, uncontrolled, and type 2 low, neutrophil-like asthma (12, 13), further investigations are warranted to extend this important work, focusing on more diverse immune environments and type 2 low or type 17–associated asthma.
Supplementary Material
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Carr TF, Zeki AA, Kraft M. Eosinophilic and noneosinophilic asthma. Am J Respir Crit Care Med. 2018;197:22–37. doi: 10.1164/rccm.201611-2232PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391:783–800. doi: 10.1016/S0140-6736(17)33311-1. [DOI] [PubMed] [Google Scholar]
- 3.Manni ML, Trudeau JB, Scheller EV, Mandalapu S, Elloso MM, Kolls JK, et al. The complex relationship between inflammation and lung function in severe asthma. Mucosal Immunol. 2014;7:1186–1198. doi: 10.1038/mi.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manni ML, Mandalapu S, McHugh KJ, Elloso MM, Dudas PL, Alcorn JF. Molecular mechanisms of airway hyperresponsiveness in a murine model of steroid-resistant airway inflammation. J Immunol. 2016;196:963–977. doi: 10.4049/jimmunol.1501531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raundhal M, Morse C, Khare A, Oriss TB, Milosevic J, Trudeau J, et al. High IFN-γ and low SLPI mark severe asthma in mice and humans. J Clin Invest. 2015;125:3037–3050. doi: 10.1172/JCI80911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J. S100A8/A9 in inflammation. Front Immunol. 2018;9:1298. doi: 10.3389/fimmu.2018.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007;81:28–37. doi: 10.1189/jlb.0306170. [DOI] [PubMed] [Google Scholar]
- 8.Oczypok EA, Milutinovic PS, Alcorn JF, Khare A, Crum LT, Manni ML, et al. Pulmonary receptor for advanced glycation end-products promotes asthma pathogenesis through IL-33 and accumulation of group 2 innate lymphoid cells J Allergy Clin Immunol 2015136747–756, e4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aoki T, Matsumoto Y, Hirata K, Ochiai K, Okada M, Ichikawa K, et al. Expression profiling of genes related to asthma exacerbations. Clin Exp Allergy. 2009;39:213–221. doi: 10.1111/j.1365-2222.2008.03186.x. [DOI] [PubMed] [Google Scholar]
- 11.Gray RD, MacGregor G, Noble D, Imrie M, Dewar M, Boyd AC, et al. Sputum proteomics in inflammatory and suppurative respiratory diseases. Am J Respir Crit Care Med. 2008;178:444–452. doi: 10.1164/rccm.200703-409OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee TH, Jang AS, Park JS, Kim TH, Choi YS, Shin HR, et al. Elevation of S100 calcium binding protein A9 in sputum of neutrophilic inflammation in severe uncontrolled asthma. Ann Allergy Asthma Immunol. 2013;111:268–275, e1. doi: 10.1016/j.anai.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 13.Lee TH, Chang HS, Bae DJ, Song HJ, Kim MS, Park JS, et al. Role of S100A9 in the development of neutrophilic inflammation in asthmatics and in a murine model. Clin Immunol. 2017;183:158–166. doi: 10.1016/j.clim.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Endoh I, Hsu K, Tedla N, Endoh Y, Geczy CL. S100A8 modulates mast cell function and suppresses eosinophil migration in acute asthma. Antioxid Redox Signal. 2011;14:1589–1600. doi: 10.1089/ars.2010.3583. [DOI] [PubMed] [Google Scholar]
- 15.Yin LM, Li HY, Zhang QH, Xu YD, Wang Y, Jiang YL, et al. Effects of S100A9 in a rat model of asthma and in isolated tracheal spirals. Biochem Biophys Res Commun. 2010;398:547–552. doi: 10.1016/j.bbrc.2010.06.116. [DOI] [PubMed] [Google Scholar]
- 16.Greenlee KJ, Corry DB, Engler DA, Matsunami RK, Tessier P, Cook RG, et al. Proteomic identification of in vivo substrates for matrix metalloproteinases 2 and 9 reveals a mechanism for resolution of inflammation. J Immunol. 2006;177:7312–7321. doi: 10.4049/jimmunol.177.10.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer LD, Maloney KN, Boyd KL, Goleniewska AK, Toki S, Maxwell CN, et al. The innate immune protein S100A9 protects from T-helper cell type 2–mediated allergic airway inflammation. Am J Respir Cell Mol Biol. 2019;61:459–468. doi: 10.1165/rcmb.2018-0217OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkerson EM, Johansson MW, Hebert AS, Westphall MS, Mathur SK, Jarjour NN, et al. The peripheral blood eosinophil proteome. J Proteome Res. 2016;15:1524–1533. doi: 10.1021/acs.jproteome.6b00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu YD, Wang Y, Yin LM, Park GH, Ulloa L, Yang YQ. S100A8 protein attenuates airway hyperresponsiveness by suppressing the contraction of airway smooth muscle. Biochem Biophys Res Commun. 2017;484:184–188. doi: 10.1016/j.bbrc.2017.01.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.