In the United States, chronic obstructive pulmonary disease (COPD) affects 24 million people, and it is projected to become the third leading cause of death worldwide by 2020 (1). The major etiologic risk factor for COPD is exposure to environmental agents, especially cigarette smoke (CS). Inhaling CS or other pollutants stimulates a chronic pulmonary inflammatory response that is characterized by excessive recruitment of innate immune cells and activation of a T-helper cell type 1 (Th1)-type adaptive immune response (2). T-box expressed in T cells (T-bet or Tbx21) is a highly conserved transcription factor that was initially identified as the master regulator of the differentiation of naive T lymphocytes into Th1 cells (3). T-bet also promotes the activation of group I innate lymphoid cells (ILC1), which are crucial mediators of mucosal immunity (4). T-bet promotes the production of IFN-γ and other Th1 cytokines by CD4+ T cells and ILC1 cells in the lungs of patients with COPD (5). T-bet is also expressed by myeloid leukocytes (6, 7), but less is known about its contributions to their function.
T-bet−/− mice spontaneously develop an abnormal phenotype in the lungs characterized by a shift toward Th2 lymphocytes and characteristics of asthma, including bronchial hyperresponsiveness to inhaled acetylcholine and chronic airway remodeling (8). T-bet expression is increased in CD4+ T cells treated with CS in vitro, and in CD4+ T cells and ILC1 cells in the lungs of patients with COPD (9, 10). Based on these observations, one might expect T-bet−/− mice to be protected from CS-induced COPD, but this hypothesis has not been formally tested until now.
In this issue of the Journal, Hayashi and colleagues (pp. 525–536) report that when challenged with porcine pancreatic elastase (PPE) delivered by the intratracheal route, T-bet−/− mice developed exaggerated emphysema compared with wild-type (WT) mice (11). The exaggerated emphysema phenotype in the PPE-treated T-bet−/− mice was associated with enhanced early recruitment of neutrophils and lymphocytes into their lungs. Although the PPE-treated T-bet−/− mice had pulmonary CD8+ T cell counts similar to those obtained in the WT mice, they had higher counts of CD4+ T cells expressing Il-17 and RAR-related orphan receptor gamma (RORγt) (Th17 cells), and increased expression of Il-6 and Il-17 in their lungs. Delivering neutralizing antibodies to Il-6R and Il-17 ameliorated the increased emphysema development in the PPE-treated T-bet−/− mice, indicating that increased Il-6 and Il-17 signaling is required for the exaggerated PPE-induced emphysema in T-bet−/− mice. The authors then localized the sources of Il-6 and Il-17 in the lungs. T-bet−/− macrophages activated ex vivo had increased Il-6 expression compared with activated WT macrophages. Silencing T-bet expression in a murine alveolar macrophage cell line using siRNA increased expression of Il-6, Il-1β, Tnf-α, and transforming growth factor-β (Tgf-β), and plasmid-mediated overexpression of T-bet in these cells reduced the expression of these genes. Il-6 and TGF-β are known to control the development of Il-17–expressing Th17 cells (12). As macrophages can also express (and be activated by) IL-17, the authors performed coculture experiments with WT versus T-bet−/− macrophages and splenic CD4+ T cells to determine the contributions of macrophages and CD4+ T cells to the increased Il-17 levels detected in PPE-treated T-bet−/− lungs. Cocultures containing T-bet−/− CD4+ T cells and either WT or T-bet−/− macrophages had higher Il-17 production than cocultures of WT CD4+ T cells and either WT or T-bet−/− macrophages, indicating that the genotype of CD4+ T cells was crucial for optimal Il-17 responses in these cocultures. However, macrophages (from either genotype) were also required for optimal Il-17 responses in the cocultures, suggesting that macrophage–CD4+ T cell interactions are also needed for optimal Th17 responses.
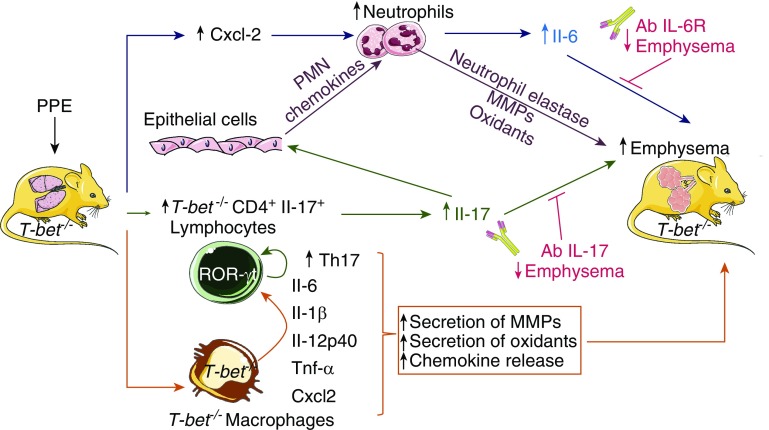
Taken together, these findings indicate that T-bet deficiency has unexpected deleterious effects during PPE-induced emphysema development by generating an aberrant inflammatory response in the lung that is dependent on increased pulmonary Il-6 and Il-17 levels (Figure 1). It is likely that T-bet deficiency in both macrophages and CD4+ T cells contributes to the phenotype of the mice. T-bet deficiency in CD4+ T cells increases Il-17 production in the lungs by polarizing the T cells toward a Th17 phenotype, consistent with the known activity of T-bet in suppressing the development of Th17 cells by inhibiting the transcription of Rorc, which encodes the transcription factor RORγt, which drives Th17 differentiation (13), However, interactions between CD4+ T cells and macrophages may also contribute to Th17 polarization, as T-bet deficiency in macrophages increases the production Il-6 and Tgf-β, which increase the differentiation of Th17 cells (Figure 1). This process increases the levels of Il-17 in the lungs, which induces the release of chemokines from lung epithelial cells and macrophages that recruit polymorphonuclear neutrophils (PMNs) and monocytes into the lungs. Proteinases and oxidants released by the recruited myeloid leukocytes contribute to the destruction of the alveolar walls (14). T-bet deficiency in macrophages may also promote an exaggerated innate immune response by increasing macrophage Cxcl-2 expression, which stimulates PMN recruitment and Tnf-α expression, which activates macrophages to release proteinases and oxidants (14) (Figure 1).
Figure 1.
T-bet (T-box expressed in T cells) deficiency leads to exaggerated emphysema development in porcine pancreatic elastase (PPE)-treated mice. When challenged with PPE delivered by the intratracheal route, T-bet−/− mice developed exaggerated emphysema compared with wild-type mice. The exaggerated emphysema phenotype in the PPE-treated T-bet−/− mice was associated with enhanced early recruitment of neutrophils and lymphocytes into their lungs. PPE-treated T-bet−/− mice had higher IL-17– and RAR-related orphan receptor gamma (RORγt)-expressing CD4+ T cells, and increased expression of Il-6 and Il-17 in their lungs. Delivering neutralizing antibodies to Il-6R and Il-17 ameliorated the increased emphysema development in PPE-treated T-bet−/− mice, indicating that increased Il-6 and Il-17 signaling is required for the exaggerated PPE-induced emphysema in T-bet−/− mice. It is likely that T-bet deficiency in both macrophages and CD4+ T cells contributes to the phenotype of the mice. T-bet deficiency in CD4+ T cells increases Il-17 production in the lungs by polarizing the T cells toward a T-helper cell type 17 (Th17) phenotype, consistent with the known activity of T-bet in suppressing the development of Th17 cells by inhibiting transcription of Rorc, which encodes the transcription factor RORγt, which drives Th17 differentiation. Direct interactions between CD4+ T cells and macrophages may also contribute to Th17 polarization, as T-bet deficiency in macrophages increases the production of Il-6 and Tgf-β, which increase the differentiation of Th17 cells, and also the release of other proinflammatory mediators. The increased levels of Il-17 in the lung activate epithelial cells to release chemokines for polymorphonuclear neutrophils (PMNs) and monocytes, thereby increasing the recruitment of PMNs and monocytes into the lung, and their release of oxidants, serine proteinases, and matrix metalloproteinases (MMPs) that injure the alveolar walls. T-bet deficiency in macrophages may also promote an exaggerated innate immune response by increasing macrophage expression of Cxcl-2, which stimulates PMN recruitment, and Tnf-α, which activates macrophages to release MMPs and oxidants that also contribute to emphysema development. Ab = antibody; Tgf = transforming growth factor.
The strengths of this study are the novel and unexpected findings that T-bet deficiency leads to exaggerated emphysema by increasing innate immune responses and polarizing the adaptive immune response toward a Th17 phenotype. The authors used a murine model and complementary mechanistic cell culture systems using T-bet silencing and overexpression approaches. However, the study has several limitations. The PPE emphysema model has limitations as a model of COPD because the inciting agent is not clinically relevant, and the model lacks other key pathologies that occur in COPD lungs, including small airway disease. The phenotype of T-bet−/− mice should be evaluated in the chronic CS exposure model (15). Studies of cell-specific T-bet−/− mice in models of COPD would help to elucidate the nature of the cross-talk between innate and adaptive immune cells. Studies are needed to understand the mechanisms by which T-bet modulates macrophage function, and to evaluate the contributions of T-bet deficiency in other cells, including ILCs and dendritic cells. Translational studies of T-bet expression in lung cells from patients with COPD are needed to determine whether T-bet expression in different cell types is related to COPD disease severity. Although the studies of Hayashi and colleagues are intriguing, more research into the contributions of T-bet to the progression of COPD is required before strategies to activate T-bet can be considered as an approach to limit the progression of COPD by restraining aberrant innate and adaptive immunity responses in the lungs.
Supplementary Material
Footnotes
Supported by Public Health Service, National Institute of Allergy and Infectious Disease grant AI111475-01, Flight Attendants Medical Research Institute grant CIA123046, and Department of Defense (Congressionally Directed Medical Research Programs) grant PR152060.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 2.Grumelli S, Corry DB, Song LZ, Song L, Green L, Huh J, et al. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med. 2004;1:e8. doi: 10.1371/journal.pmed.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lazarevic V, Glimcher LH, Lord GM. T-bet: a bridge between innate and adaptive immunity. Nat Rev Immunol. 2013;13:777–789. doi: 10.1038/nri3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng SL. The T-box transcription factor T-bet in immunity and autoimmunity. Cell Mol Immunol. 2006;3:87–95. [PubMed] [Google Scholar]
- 5.Murillo MM, del Castillo G, Sánchez A, Fernández M, Fabregat I. Involvement of EGF receptor and c-Src in the survival signals induced by TGF-beta1 in hepatocytes. Oncogene. 2005;24:4580–4587. doi: 10.1038/sj.onc.1208664. [DOI] [PubMed] [Google Scholar]
- 6.Alcaide P, Jones TG, Lord GM, Glimcher LH, Hallgren J, Arinobu Y, et al. Dendritic cell expression of the transcription factor T-bet regulates mast cell progenitor homing to mucosal tissue. J Exp Med. 2007;204:431–439. doi: 10.1084/jem.20060626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yi W, Zhang P, Liang Y, Zhou Y, Shen H, Fan C, et al. T-bet-mediated Tim-3 expression dampens monocyte function during chronic hepatitis C virus infection. Immunology. 2017;150:301–311. doi: 10.1111/imm.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finotto S, Neurath MF, Glickman JN, Qin S, Lehr HA, Green FH, et al. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science. 2002;295:336–338. doi: 10.1126/science.1065544. [DOI] [PubMed] [Google Scholar]
- 9.Silver JS, Kearley J, Copenhaver AM, Sanden C, Mori M, Yu L, et al. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat Immunol. 2016;17:626–635. doi: 10.1038/ni.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman CM, McCubbrey AL, Crudgington S, Nelson J, Martinez FJ, Han MK, et al. Basal gene expression by lung CD4+ T cells in chronic obstructive pulmonary disease identifies independent molecular correlates of airflow obstruction and emphysema extent. PLoS One. 2014;9:e96421. doi: 10.1371/journal.pone.0096421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi S, Matsuno Y, Tsunoda Y, Sakurai H, Kiwamoto T, Morishima Y, et al. Transcription factor T-bet attenuates the development of elastase-induced emphysema in mice. Am J Respir Cell Mol Biol. 2019;61:525–536. doi: 10.1165/rcmb.2018-0109OC. [DOI] [PubMed] [Google Scholar]
- 12.Nalbant A, Eskier D. Genes associated with T helper 17 cell differentiation and function. Front Biosci (Elite Ed) 2016;8:427–435. doi: 10.2741/E777. [DOI] [PubMed] [Google Scholar]
- 13.Lazarevic V, Chen X, Shim JH, Hwang ES, Jang E, Bolm AN, et al. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORγt. Nat Immunol. 2011;12:96–104. doi: 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Owen CA. Proteinases and oxidants as targets in the treatment of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:373–385. doi: 10.1513/pats.200504-029SR. [Discussion, pp. 394–395.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Polverino F, Rojas-Quintero J, Zhang D, Sánchez J, Yambayev I, et al. A disintegrin and A metalloproteinase-9 (ADAM9): a novel proteinase culprit with multifarious contributions to COPD. Am J Respir Crit Care Med. doi: 10.1164/rccm.201711-2300OC. [online ahead of print] 4 Jun 2018; DOI: 10.1164/rccm.201711-2300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.