Abstract
Biotherapeutic agents must be administered parenterally to obtain therapeutic blood concentrations, lowering patient compliance and complicating care. An oral delivery platform (ODP) was developed to deliver drugs into the small intestinal wall. This proof‐of‐concept study was performed in 17 anesthetized, laparotomized swine. In 8 swine weighing 17.4 ± 1.2 kg (mean ± SEM), 20 IU of recombinant human insulin (RHI) were auto‐injected into the jejunal wall by placing the ODP inside the jejunum via an enterotomy. In 9 control swine weighing 17.0 ± 0.4 kg, 20 IU of RHI were injected subcutaneously. In both groups, under a 60‐80 mg/dL euglycemic glucose clamp, blood glucose was measured with a handheld glucometer and serum insulin was measured using ELISA, at 10‐minute intervals between −20 and +420 minutes after RHI delivery. The peak serum concentration of RHI was 517 ± 109 pmol/L in the ODP and 342 ± 50 pmol/L in the subcutaneous group (ns). The areas under the insulin concentration curves (83 ± 18 and 81 ± 10 nmol/L·min) were also similar in both groups. The mean time to peak serum concentration of insulin was 139 ± 42 minutes in the ODP and 227 ± 24 minutes in the subcutaneous group (ns). In conclusion, (a) The bioactivity of RHI was preserved after its delivery into the jejunal wall, (b) the intrajejunal route delivered insulin as rapidly and physiologically as the subcutaneous route, and (c) these pharmacokinetic and pharmacodynamic characteristics of RHI after intrajejunal delivery suggest that drugs currently administered parenterally, such as basal insulin, could be successfully delivered into the proximal intestinal wall via the ingestible capsule.
Keywords: drug delivery, insulin delivery, oral drug delivery, treatment compliance
Abbreviations
- AUC
area under the curve
- Cmax
peak serum concentration
- IgG
human immunoglobulin G
- ODP
oral delivery platform
- RHI
recombinant human insulin
- Tmax
time to peak serum concentration
1. INTRODUCTION
With the extraordinary growth of the general pharmaceutical armamentarium, protein and peptides in particular, the number of drugs that must be administered via a parenteral route is increasing rapidly.1 The long‐term use of subcutaneous, intramuscular or intravenous injections represents a serious burden, which interferes with the patients’ comfort, quality of life, and compliance with therapy.2, 3 This awareness inspired the development of an oral delivery platform (ODP), which will be contained inside an enteric‐coated capsule shell, deploy after entering the jejunum, and inject its payload into the intestinal wall. The objective of this study was to examine the pharmacokinetics and pharmacodynamics of recombinant human insulin (RHI) delivered by the ODP directly into the jejunal wall of anesthetized swine.
1.1. Rationale for this study
Since the approval of RHI by the US Food and Drug Administration, nearly 40 years ago, therapeutic proteins and peptides have proliferated to become major players in the management of a variety of common and rare diseases.1, 4, 5 One important limitation, however, is the need for many, if not most protein drugs, to be administered parenterally to (a) obviate their destruction by a passage through the acidic gastric environment, (b) promote their access to the bloodstream in high enough concentrations, and (c) preserve their therapeutic activity, factors which all represent an enormous challenge.1 The relationship between the obligation to self‐administer repetitive subcutaneous or intramuscular injections and therapeutic non‐compliance has been well documented by previous studies.2, 3 In a survey of over 500 adult diabetics treated with a mean of 2.7 injections of insulin daily, over 50% of participants admitted to intentionally omit treatment at least occasionally, and 20% reported omitting their injections regularly or frequently.2 Among several correlates of intentional omission of treatment, interference with daily living activities, and pain or embarrassment caused by the injections figured prominently.2 Furthermore, parenteral drug delivery is complicated by a modest incidence of serious adverse events, viral and bacterial infections in particular.6
The past 30 years have been a period of especially active research directed at the invention and development of new drug delivery systems, with a focus and emphasis on the use of nanoparticles, in hope of targeting cancerous tumors in particular. Despite the abundant resources allocated to that line of research, its fruits have been relatively sparse.7 Besides the harsh, acid, gastric environment, the oral delivery of proteins and peptides is primarily hampered by the physical barrier represented by the intestinal epithelial cells, by the intestinal mucus covering the epithelium, and by various chemical components of the gastrointestinal tract, including bile salts, gastric acids and proteases.8, 9, 10 Alternative methods of proteins and peptides administration, which do not require injections or intravenous access, including the use of permeation enhancers, cell‐penetrating peptides, protease inhibitors, conjugation or enteric coating of the proteins and peptides, and drug delivery using degradable, polymeric or mucoadhesive carriers have all been described and implemented.1, 10, 11, 12 While these methods have been at least partially successful at maintaining the stability and bioactivity of certain drugs, have increased the intestinal permeability, reduced the degradation of proteinic substances, and extended their half‐life in the bloodstream, they may also damage the intestinal epithelium and lower its immune‐protective function, and display considerable variability in diffusion, absorption and localization of the delivery within the gastrointestinal tract, as well as between fasting and postprandial states.1
2. MATERIALS AND METHODS
2.1. Animals preparation and study samples
All study procedures described were approved by the Institutional Animal Care and Use Committee of Biosurg Inc, Winters, CA, and were in compliance with the standard operating procedures of the testing facility. Female domestic swine weighing between 12 and 22 kg were anesthetized by an intramuscular injection of tiletamine and zolazepam (Telazol®), intubated and maintained under anesthesia with a mixture of isoflurane and oxygen delivered under intermittent positive pressure by a mechanical, animal ventilator. The ODP group, in which 0.68 ± 0.1 mg of RHI were delivered into the jejunal wall, included 8 swine weighing 17.4 ± 1.2 kg. The 9 animals in the control group, which received 0.69 ± 0.10 mg of RHI subcutaneously, weighed 17.0 ± 0.4 kg.
2.2. Insulin delivery
2.2.1. By the ingestible device
The ODP (RaniPill™, Rani Therapeutics LLC, San Jose, CA) is an enteric‐coated, capsule‐like, investigational device, which remains intact inside the stomach until it reaches the jejunum, where the higher pH dissolves its coating, exposing a dissolvable needle and a compressed pharmaceutical microtablet, sealed inside a microsyringe attached to a self‐inflating balloon. The uncoated and partially folded ODP is shown in Figure 1A. When folded, the balloon is enclosed inside a 000 hydroxypropyl methylcellulose capsule, shown in Figure 1B. Once sealed, the capsule is enteric‐coated with a polymer, which dissolves at a pH >6.5. The ODP used in this proof‐of‐concept study also contained radiopaque barium sulfate powder and a bismuth‐loaded cover, to track its transit along the gastrointestinal tract. Its final, undeployed, external length and width are 28.0 and 11.0 mm, respectively (Figure 1B). After it has left the stomach and entered the duodenum, the higher environmental pH and intestinal fluid dissolve the capsule coating, enabling the production of carbon dioxide, which inflates the balloon, orients a microsyringe against the intestinal wall and delivers a payload contained inside the ODP. The balloon deflates shortly thereafter and continues its transit along the gastrointestinal tract. For the purpose of this study the payload consisted of an especially manufactured microtablet of 20 IU (approximately 0.69 mg) of RHI (Imgenex, Novus Biologicals, Littleton, CO). A 6‐8 cm long, midline abdominal incision was made just above the umbilicus. A loop of jejunum was isolated and a 1‐cm enterotomy was made on the antimesenteric border to insert the ODP under visual control, directly into the jejunal cavity.
Figure 1.
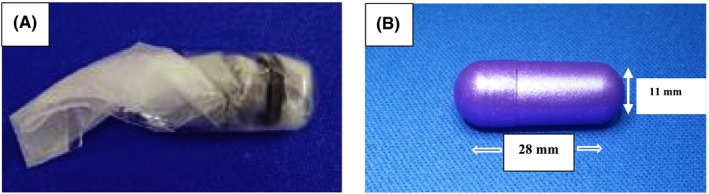
(A) Deflated, partially folded ODP. (B) Folded ODP, enclosed inside the coated capsule. See text for more details
2.2.2. Subcutaneously
In the control group, a sham abdominal incision was made, similar to that in the ODP group, though without enterotomy, and a 1‐cm stab incision was made over the biceps femoris to allow the subcutaneous insertion of similarly manufactured RHI microtablets.
2.3. Euglycemic clamp
The euglycemic clamp method13, 14 was used to keep the animals’ blood glucose concentration between 60 and 80 mg/dL by titrating a 50% dextrose solution infused through a peripheral venous cannula while monitoring the arterial concentration at 10‐minute intervals, using a handheld OneTouch Ultra® 2 glucometer (LifeScan, Inc. ‐ a Johnson & Johnson Company).
2.4. Blood sampling and processing and data management
Blood was collected at −20, −10, and 0 minute before the intrajejunal wall or subcutaneous injection of RHI, and every 10 minutes thereafter, for 420 minutes. The samples were allowed to clot for 30 minutes at room temperature before their centrifugation at 2053 g for 10‐15 minutes at 4°C. Serum aliquots were then processed for measurements of RHI concentration, using a Human Insulin ELISA Kit and standard operating procedure recommended by the manufacturer (Alpha Diagnostic International Inc, San Antonio, TX). The detection of the assay ranged between 6.25 and 100 μIU/mL.
The following measurements were made and compared in both study groups: (a) the serum concentrations and areas under the serum concentration‐time curve (AUClast), between RHI delivery (time zero) and 420 minutes later, the time of last measured concentrations of insulin and glucose, (b) the peak serum concentrations (C max) of RHI, and (c) the mean time to peak serum concentration (T max) of RHI.
2.5. Statistical analysis
The measurements are presented as means ± SEM. C max and T max were calculated using standard non‐compartmental methods from the serum concentration vs time in each subject, and AUClast was determined by the linear trapezoidal rule, between the first and last measured serum concentrations. Data were analyzed by one‐way analysis of variance, using the Microsoft® Office Excel software (Microsoft Corporation, Redmond, WA). Between‐groups differences were considered significant when P was < 0.05.
3. RESULTS
3.1. Pharmacokinetics of recombinant human insulin
The mean serum concentrations of RHI, measured between its delivery and 7 hour of follow‐up in the ODP and the control groups, are shown in Figure 2A and B, respectively, and at selected time points in Table 1A and B, respectively. The respective AUClast, were 2126 ± 80 and 2178 ± 49 pmol/L·min (ns; Tables 2 & 3). The Cmax of RHI in the jejunal wall (6.04 ± 0.25 pmol/L) and subcutaneous (5.74 ± 0.17 pmol/L) groups were, likewise, similar (Tables 2 & 3). Finally, T max of RHI (139 ± 39 vs 240 ± 31 minutes) was shorter in the ODP than in the subcutaneous group, though the difference was not statistically significant (Tables 2 & 3).
Figure 2.
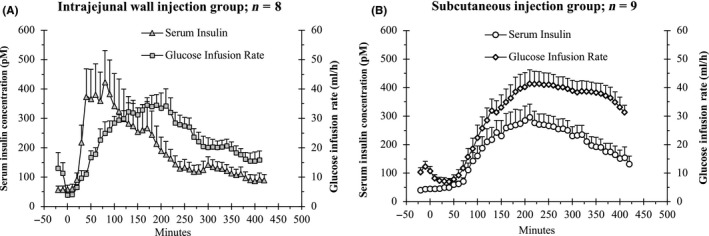
Interactions, over the 7 hour of experiments, between mean serum concentrations of RHI and mean glucose infusion rates, after ODP (A) vs subcutaneous (B) delivery of RHI
Table 1.
Mean serum concentrations of RHI and glucose infusion rates measured at selected time points in the ODP (A) and the subcutaneous (B) study groups
| Study groups | ||||
|---|---|---|---|---|
| Insulin delivery | ||||
| A. ODP | B. Subcutaneous | |||
| n = 8 | n = 9 | |||
| Time of measurement (min) | Serum insulin (pmol/L) | Glucose infusion rate (mL/h) | Serum insulin (pmol/L) | Glucose infusion rate (mL/h) |
| 10 | 60.0 ± 23.2 | 5.4 ± 1.3 | 45.5 ± 4.5 | 8.2 ± 1.3 |
| 30 | 217.4 ± 95.9 | 9.4 ± 1.9 | 50.4 ± 5.6 | 7.2 ± 1.2 |
| 60 | 379.3 ± 98.8 | 18.4 ± 1.5 | 63.0 ± 9.4 | 9.1 ± 2.0 |
| 120 | 297.1 ± 92.8 | 28.3 ± 3.3 | 210.8 ± 45.2 | 28.6 ± 4.3 |
| 180 | 244.0 ± 60.5 | 30.9 ± 2.7 | 269.5 ± 54.5 | 38.6 ± 5.1 |
| 240 | 128.4 ± 28.0 | 29.0 ± 2.1 | 270.2 ± 37.6 | 41.0 ± 4.2 |
| 300 | 141.0 ± 25.1 | 24.9 ± 3.2 | 231.0 ± 32.2 | 38.9 ± 3.7 |
| 360 | 106.8 ± 24.3 | 23.4 ± 3.1 | 190.6 ± 29.2 | 37.9 ± 3.6 |
| 410 | 91.7 ± 20.0 | 21.0 ± 3.2 | 154.2 ± 37.9 | 31.3 ± 2.5 |
Values are means ± SEM.
Table 2.
Pharmacokinetics of RHI delivered via the ODP vs subcutaneously on natural logarithmic scale
| Intrajejunal Wall | Subcutaneous | |
|---|---|---|
| Dose of RHI, IU | 19.9 ± 0.5 | 19.8 ± 0.3 |
| AUClast, pmol/L·min | ||
| Non‐transformed | 82 562 ± 18 054 | 81 413 ± 9 512 |
| Log‐transformed | 2126 ± 80 | 2178 ± 49 |
| C max, pmol/L | ||
| Non‐transformed | 517 ± 109 | 342 ± 50 |
| Log‐transformed | 6.04 ± 0.25 | 5.74 ± 0.17 |
| T max, min | 139 ± 42 | 227 ± 24 |
Values are means ± SEM. All between‐groups differences are statistically non‐significant.
Relative bioavailability with ODP compared with subcutaneous delivery: 2126/2178 = 98%.
Abbreviations: AUC, area under the curve; RHI, recombinant human insulin.
Table 3.
Details of analysis of variance of RHI pharmacokinetics after its ODP vs subcutaneous delivery
| Sum of Squares | df | Mean Square | F | P | ||
|---|---|---|---|---|---|---|
| C max, pmol/L | Between Groups | 0.39 | 1 | 0.39 | 0.99 | 0.34 |
| Within Groups | 5.95 | 15 | 0.40 | |||
| Total | 6.34 | 16 | ||||
| AUClast, pmol/L·min | Between Groups | 11 348 | 1 | 11 348 | 0.29 | 0.60 |
| Within Groups | 580 066 | 15 | 38 671 | |||
| Total | 591 414 | 16 | ||||
| T max, min | Between Groups | 32 736 | 1 | 32 736 | 3.45 | 0.08 |
| Within Groups | 142 288 | 15 | 9486 | |||
| Total | 175 024 | 16 |
3.2. Pharmacodynamic analysis
The biological activity of RHI delivered by the ODP was confirmed by the increased demand for glucose delivery to maintain the euglycemic clamp following insertion of the device (Figure 2A and B). The mean glucose infusion rates measured at selected time points in the ODP and the control groups are shown in Table 1A and B, respectively. The mean AUClast of glucose infusion rates was 85 ± 4 g/min2 in the ODP vs 106 ± 10 g/min2 in the subcutaneous delivery group (ns). The interactions during the 7‐h experiments between the serum insulin concentrations and the glucose infusion rates in the ODP and the control groups are shown in Figure 2A and B, respectively.
4. DISCUSSION
4.1. Main findings of the study
This is, to the best of our knowledge, the first study of the bioavailability and bioactivity of a pharmaceutical product auto‐injected into the intestinal wall. The uptake of RHI delivered by the ODP was as rapid as when delivered subcutaneously, and its relative bioavailability was nearly 100% compared to the subcutaneous group, suggesting that this new means of delivering biotherapeutic agents might be able to replace the currently used parenteral route.
4.2. Significance and anticipated impact of our observations
In its current design, the ODP is able to accommodate and protect a variety of drugs until the device reaches the proximal small intestine, where it self‐deploys and delivers its payload into the jejunal wall. The observations made in this pilot study indicate that the site chosen to deliver RHI, ie the jejunal wall, (a) preserved its bioactivity, (b) distributed insulin to the peripheral circulation expeditiously, and (c) should be suitable for the delivery of multiple biotherapeutic agents currently administered parenterally. Other pharmaceuticals which have been delivered successfully in preclinical studies include octreotide, teriparatide, and immunoglobulin G (IgG).15 The first human application of the ODP has recently begun, with the delivery of octreotide to healthy volunteers.16 Several issues will need to be examined and resolved as the clinical testing of the device progresses, including its long‐term tolerability, safety and ability to reliably deliver a wide variety of candidate molecules. Other important questions to be answered are the pharmacokinetic and pharmacodynamic consequences of delivering the drugs in the portal instead of the systemic circulation, and the effects of various gastrointestinal or systemic disorders on device function.
4.3. Limitations of this study
The results reported here were obtained in a series of technically successful experiments, during which the prototype device self‐deployed flawlessly, delivering the drug precisely into the jejunal wall. In addition, the device was manually inserted through an enterotomy under visual control, instead of being swallowed as planned with the final product. The purpose of this study, therefore, was neither to report the performance nor to study the safety of the ODP, but to examine the pharmacokinetic and pharmacodynamic profiles of RHI when administered via the jejunal wall route. Furthermore, while these experiments were performed in an animal model whose anatomy and functional characteristics of the digestive tract resemble those of humans, unsuspected differences among species may become apparent upon further testing of the device. It is noteworthy, however, that in pilot experiments performed in conscious dogs, the oral delivery of human IgG by the ODP was associated with a 60% bioavailability of the protein, vs 50% after subcutaneous injections. In addition, T max was shorter and C max was higher after oral than after subcutaneous administration of IgG.17 Finally, the observations made in this pilot study followed the delivery of single doses of RHI, and their long‐term reproducibility remains to be confirmed.
5. CONCLUSION
The delivery of RHI into the jejunal wall of anesthetized, juvenile swine was associated with a high bioavailability of insulin. These observations need to be confirmed in humans.
DISCLOSURE
All authors are paid employees of RANI Therapeutics, LLC.
AUTHORS’ CONTRIBUTIONS
Mir Hashim made substantial contributions to the conception and design of the study.
Radhika Korupolu made substantial contributions to the acquisition, analysis and interpretation of the data.
Baber Syed made major contributions to the design and development of the ODP.
Kyle Horlen made substantial contributions to the acquisition of the data.
Simret Beraki made major contributions to the conception and design of the study.
Padma Karamchedu made substantial contributions to the acquisition of the data.
Arvinder K. Dhalla made substantial contributions to the conception and design of the study, and to the analysis and interpretation of the data.
Rodolphe Ruffy made major contributions to the interpretation of the data and to the composition of the manuscript.
Mir Imran is the inventor of the ODP and made major contributions to the conception and design of the study.
All authors have approved the final version of the manuscript, have participated sufficiently to the work to take public responsibility for appropriate portions of the content, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ETHIC STATEMENT
All procedures used for this study were approved by the Institutional Animal Care and Use Committee of Biosurg Inc, Winters, CA, and were in compliance with the standard operating procedures of the testing facility.
ACKNOWLEDGEMENT
None.
Hashim M, Korupolu R, Syed B, et al. Jejunal wall delivery of insulin via an ingestible capsule in anesthetized swine—A pharmacokinetic and pharmacodynamic study. Pharmacol Res Perspect. 2019;e00522 10.1002/prp2.522
Funding information
This work was funded by RANI Therapeutics, LLC.
REFERENCES
- 1. Wagner AM, Gran MP, Peppas NA. Designing the new generation of intelligent biocompatible carriers for protein and peptide delivery. Acta Pharm Sin B. 2018;8:147‐164. 10.1016/j.apsb.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peyrot M, Rubin RR, Kruger DF, Travis LB. Correlates of insulin injection omission. Diabetes Care. 2010;33:240‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fu AZ, Qiu Y, Radican L. Impact of fear of insulin or fear of injection on treatment outcomes of patients with diabetes. Curr Med Res Opin. 2009;25:1413‐1420. [DOI] [PubMed] [Google Scholar]
- 4. Leader B, Baca QJ, Golan DE. Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov. 2008;7:21‐39. [DOI] [PubMed] [Google Scholar]
- 5. Usmani SS, Bedi G, Samuel JS, et al. THPdb: database of FDA‐approved peptide and protein therapeutics. PLoS ONE. 2017;12:e0181748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frid AH, Kreugel G, Grassi G, et al. New insulin delivery recommendations. Mayo Clin Proc. 2016;91:1231‐1255. [DOI] [PubMed] [Google Scholar]
- 7. Park K. The drug delivery field at the inflection point: time to fight its way out of the egg. J Control Release. 2017;267:2‐14. [DOI] [PubMed] [Google Scholar]
- 8. Gedawy A, Martinez J, Al‐Salami H, Dass CR. Oral insulin delivery: existing barriers and current counter‐strategies. J Pharm Pharmacol. 2018;70:197‐213. [DOI] [PubMed] [Google Scholar]
- 9. Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv. Drug Delivery Rev. 2012;64:557‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tyagi P, Pechenov S, Anand SJ. Oral peptide delivery: translational challenges due to physiological effects. J Control Release. 2018;287:167‐176. [DOI] [PubMed] [Google Scholar]
- 11. Khafagy E‐S, Morishita M. Oral biodrug delivery using cell‐penetrating peptide. Adv Drug Deliv Rev. 2012;64:531‐539. [DOI] [PubMed] [Google Scholar]
- 12. Goldberg M, Gomez‐Orellana I. Challenges for the oral delivery of macromolecules. Nat. Rev. Drug Discov. 2003;289–295. [DOI] [PubMed] [Google Scholar]
- 13. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214‐E223. [DOI] [PubMed] [Google Scholar]
- 14. Bergman RN, Hope ID, Yang YJ, et al. Assessment of insulin sensitivity in vivo: a critical review. Diabetes Metab Rev. 1989;5:411‐429. [DOI] [PubMed] [Google Scholar]
- 15. Ruffy R, Hashim M, Dhalla AK, et al.An Ingestible Capsule to Deliver Parenteral Pharmaceuticals into the Jejunal Wall. Abstract. 2019; American Diabetes Association Annual Meeting. https://ada.scientificposters.com/epsAbstractADA.cfm?xml:id=1. Accessed August 19, 2019.
- 16. https://www.clinicaltrials.gov/ct2/show/NCT03798912?term=ranipill&rank=1. Accessed August 19, 2019.
- 17. Hashim M, Beraki S, Korupolu R, et al.Pharmacokinetics of an Orally Delivered Antibody in Awake Dogs. Abstract presented at the 2019 Annual Meeting of the American Society for Clinical Pharmacology and Therapeutics. Washington DC. 2019.


