Abstract
Background
Periodontal disease and dental caries are highly prevalent oral diseases that can lead to pain and discomfort, oral hygiene and aesthetic problems, and eventually tooth loss, all of which can be costly to treat and are a burden to healthcare systems. Triclosan is an antibacterial agent with low toxicity, which, along with a copolymer for aiding retention, can be added to toothpastes to reduce plaque and gingivitis (inflammation of the gums). It is important that these additional ingredients do not interfere with the anticaries effect of the fluoride present in toothpastes, and that they are safe.
Objectives
To assess the effects of triclosan/copolymer containing fluoride toothpastes, compared with fluoride toothpastes, for the long‐term control of caries, plaque and gingivitis in children and adults.
Search methods
We searched the Cochrane Oral Health's Trials Register (to 19 August 2013), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 7), MEDLINE via OVID (1946 to 19 August 2013), Embase via OVID (1980 to 19 August 2013), and the US National Institutes of Health Trials Register (clinicaltrials.gov) (to 19 August 2013). We applied no restrictions regarding language or date of publication in the searches of the electronic databases.
Selection criteria
We included randomised controlled trials (RCTs) assessing the effects triclosan/copolymer containing toothpastes on oral health.
Data collection and analysis
Two review authors independently assessed the search results against the inclusion criteria for this review, extracted data and carried out risk of bias assessments. We attempted to contact study authors for missing information or clarification when feasible. We combined sufficiently similar studies in meta‐analyses using random‐effects models when there were at least four studies (fixed‐effect models when fewer than four studies), reporting mean differences (MD) for continuous data and risk ratios (RR) for dichotomous data.
Main results
We included 30 studies, analysing 14,835 participants, in this review. We assessed 10 studies (33%) as at low risk of bias, nine (30%) as at high risk of bias and 11 (37%) as unclear.
Plaque
Compared with control, after six to seven months of use, triclosan/copolymer toothpaste reduced plaque by 0.47 on a 0 to 5 scale (MD ‐0.47, 95% confidence interval (CI) ‐0.60 to ‐0.34, 20 studies, 2675 participants, moderate‐quality evidence). The control group mean was 2.17, representing a 22% reduction in plaque. After six to seven months of use, it also reduced the proportion of sites scoring 3 to 5 on a 0 to 5 scale by 0.15 (MD ‐0.15, 95% CI ‐0.20 to ‐0.10, 13 studies, 1850 participants, moderate‐quality evidence). The control group mean was 0.37, representing a 41% reduction in plaque severity.
Gingivitis
After six to nine months of use, triclosan/copolymer toothpaste reduced inflammation by 0.27 on a 0 to 3 scale (MD ‐0.27, 95% CI ‐0.33 to ‐0.21, 20 studies, 2743 participants, moderate‐quality evidence). The control group mean was 1.22, representing a 22% reduction in inflammation. After six to seven months of use, it reduced the proportion of bleeding sites (i.e. scoring 2 or 3 on the 0 to 3 scale) by 0.13 (MD ‐0.13, 95% CI ‐0.17 to ‐0.08, 15 studies, 1998 participants, moderate‐quality evidence). The control group mean was 0.27, representing a 48% reduction in bleeding.
Periodontitis
After 36 months of use, there was no evidence of a difference between triclosan/copolymer toothpaste and control in the development of periodontitis (attachment loss) (RR 0.92, 95% CI 0.67 to 1.27, one study, 480 participants, low‐quality evidence).
Caries
After 24 to 36 months of use, triclosan/copolymer toothpaste slightly reduced coronal caries when using the decayed and filled surfaces (DFS) index (MD ‐0.16, 95% CI ‐0.31 to ‐0.02, four studies, 9692 participants, high‐quality evidence). The control group mean was 3.44, representing a 5% reduction in coronal caries. After 36 months of use, triclosan/copolymer toothpaste probably reduced root caries (MD ‐0.31, 95% CI ‐0.39 to ‐0.23, one study, 1357 participants, moderate‐quality evidence).
Calculus
After six months of use, triclosan/copolymer toothpaste may have reduced the mean total calculus per participant by 2.12 mm (MD ‐2.12 mm, 95% CI ‐3.39 to ‐0.84, two studies, 415 participants, low‐quality evidence). The control group mean was 14.61 mm, representing a 15% reduction in calculus.
Adverse effects
There were no data available for meta‐analysis regarding adverse effects, but 22 studies (73%) reported that there were no adverse effects caused by either the experimental or control toothpaste.
There was considerable heterogeneity present in the meta‐analyses for plaque, gingivitis and calculus. Plaque and gingivitis showed such consistent results that it did not affect our conclusions, but the reader may wish to interpret the results with more caution.
Authors' conclusions
There was moderate‐quality evidence showing that toothpastes containing triclosan/copolymer, in addition to fluoride, reduced plaque, gingival inflammation and gingival bleeding when compared with fluoride toothpastes without triclosan/copolymer. These reductions may or may not be clinically important, and are evident regardless of initial plaque and gingivitis levels, or whether a baseline oral prophylaxis had taken place or not. High‐quality evidence showed that triclosan/copolymer toothpastes lead to a small reduction in coronal caries. There was weaker evidence to show that triclosan/copolymer toothpastes may have reduced root caries and calculus, but insufficient evidence to show whether or not they prevented periodontitis. There do not appear to be any serious safety concerns regarding the use of triclosan/copolymer toothpastes in studies up to three years in duration.
Plain language summary
Triclosan/copolymer containing toothpastes for oral health
Review question
This Cochrane Review has been conducted to assess the effects of using a toothpaste containing triclosan (an antibacterial ingredient) plus copolymer (an ingredient to reduce the amount of triclosan that is washed away by rinsing or saliva) plus fluoride (a mineral that prevents tooth decay) compared with using a fluoride toothpaste (without triclosan/copolymer) for oral health.
Background
Gum disease and dental decay are the main reasons for tooth loss. Unless brushed away, plaque (a sticky film containing bacteria) can build up on the teeth. This can lead to gingivitis (a swelling and redness of the gums that affects most adults), which, if not treated, can then lead to a more serious form of gum disease called periodontitis (which affects up to one out of every five adults aged 35 to 44 years worldwide). Periodontitis can cause pain, eating difficulties, an unpleasant facial appearance and eventually tooth loss. Plaque build‐up can also lead to tooth decay, a problem affecting up to 90% of schoolchildren in industrialised countries, and the majority of adults. Vast healthcare resources are used worldwide to treat gum disease and tooth decay, which are both preventable. Currently there is a lot of ongoing research into possible links between periodontitis and other medical conditions such as diabetes, rheumatoid arthritis, heart disease and also to the premature (too early) birth of underweight babies.
Adding an effective and safe antibacterial ingredient to toothpastes could be an easy and low‐cost answer to these problems. It is thought that triclosan could fight the harmful bacteria in plaque while also reducing the swelling that leads to serious gum disease. It is important that adding triclosan to fluoride toothpastes does not reduce the beneficial effects that fluoride has on preventing tooth decay.
Study characteristics
Authors from the Cochrane Oral Health Group carried out this review of existing studies and the evidence is current up to 19 August 2013. It includes 30 studies published from 1990 to 2012 in which 14,835 participants were randomised to receive a triclosan/copolymer containing fluoride toothpaste or a fluoride toothpaste that did not include triclosan/copolymer. The toothpaste that was used in most of the studies is sold by the manufacturer Colgate.
Key results
The evidence produced shows benefits in using a triclosan/copolymer fluoride toothpaste when compared with a fluoride toothpaste (without triclosan/copolymer). There was a 22% reduction in plaque, a 22% reduction in gingivitis, a 48% reduction in bleeding gums and a 5% reduction in tooth decay. There was insufficient evidence to show a difference between either toothpaste in preventing periodontitis. There was no evidence of any harmful effects associated with the use of triclosan/copolymer toothpastes in studies up to three years in length.
Quality of the evidence
The evidence relating to plaque and gingivitis was considered to be of moderate quality. The evidence on tooth decay was high quality, while the evidence on periodontitis was low quality.
Summary of findings
for the main comparison.
| Triclosan/copolymer/fluoride toothpaste compared with control for oral health | ||||||
|
Patient or population: adults (children in 2 studies) Settings: clinical (schools in 2 studies) Intervention: triclosan/copolymer/fluoride toothpaste Comparison: control toothpaste (no triclosan/copolymer) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Triclosan/copolymer | |||||
|
Plaque at 6 to 7 months (Quigley‐Hein Plaque Index) (0 to 5 on an increasing scale) |
The mean plaque score for the control groups was 2.17 | The mean plaque in the intervention groups was 0.47 lower (0.60 to 0.34 lower) | 2675 (20 studies) | ⊕⊕⊕⊝ moderatea | This evidence was supported by the results using the Plaque Severity Index (proportion of surfaces scoring > 3 on the Quigley‐Hein Plaque Index) at 6 to 7 months The mean plaque severity in the intervention groups was 0.15 lower (0.20 to 0.10 lower) than the control group mean score of 0.37. These results were based on 1850 analysed participants in 13 studies and we assessed the quality of the evidence (GRADE) as: ⊕⊕⊕⊝ moderatea |
|
|
Gingivitis at 6 to 9 months (Löe‐Silness Gingival Index) (0 to 3 on an increasing scale) |
The mean gingivitis score for the control groups was 1.22 | The mean gingivitis in the intervention groups was 0.27 lower (0.33 to 0.21 lower) | 2743 (20 studies) | ⊕⊕⊕⊝ moderatea | This evidence was supported by the results using the Gingivitis Severity Index (proportion of sites bleeding, i.e. 2 or 3 on the Löe‐Silness Gingival Index) at 6 to 7 months The mean gingival bleeding in the intervention groups was 0.13 lower (0.17 to 0.08 lower) than the control group mean score of 0.27. These results were based on 1998 analysed participants in 15 studies and we assessed the quality of the evidence (GRADE) as: ⊕⊕⊕⊝ moderatea |
|
| Periodontitis at 36 months (attachment loss > 0 mm) | 249 per 1000 | 229 per 1000 (167 to 316) |
RR 0.92 (0.67 to 1.27) |
480 (1 study) | ⊕⊕⊝⊝ lowb | |
|
Coronal caries increment at 24 to 36 months (decayed filled surfaces ‐ DFS) (caries increment is the change from baseline to follow‐up) |
The mean DFS score for the control groups was 3.44 | The mean DFS in the intervention groups was 0.16 lower (0.31 to 0.02 lower) | 9692 (4 studies) | ⊕⊕⊕⊕ high | The mean increment of the decayed filled teeth (DFT) index at 30 to 36 months in the intervention groups was 0.06 lower (0.14 lower to 0.02 higher) than the control group mean score of 1.63. These results were based on 6300 analysed participants in 3 studies and we assessed the quality of the evidence (GRADE) as: ⊕⊕⊕⊕ high For root caries, the mean increment of the Katz Root Caries Index at 36 months in the intervention group was 0.31 lower (0.39 to 0.23 lower) than the control group mean score of 0.38. These results were based on 1357 analysed participants in 1 study and we assessed the quality of the evidence (GRADE) as: ⊕⊕⊕⊝ moderatec |
|
| Calculus at 6 months (Volpe‐Manhold Calculus Index in mm ‐ mean total calculus per participant) | The mean calculus score for the control groups was 14.61 | The mean calculus in the intervention groups was 2.12 lower (3.39 to 0.84 lower) | 415 (2 studies) | ⊕⊕⊝⊝ lowd | ||
| Adverse effects | 22 studies reported that there were no adverse effects in either the experimental or control arm of the study. 1 study reported mild adverse effects but not by group/arm. The remaining 7 studies did not report any information on adverse effects | |||||
| CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aThese 4 meta‐analyses all had very high heterogeneity (I2 > 90%), however, we only downgraded by 1 point due to the consistency of the effects favouring triclosan/copolymer. The downgrading for was due to the prediction intervals slightly overlapping 0 (the line of no effect). bSingle study at high risk bias with 95% CI including both an effect favouring the intervention and the control. cSingle study (but with large sample size) at high risk bias. d2 studies (1 at high and 1 at unclear risk of bias) with high heterogeneity (I2 = 91%).
Background
Description of the condition
Periodontal disease and dental caries account for the vast majority of tooth loss (Neely 2005). The primary causative factor for both diseases is the accumulation of dental plaque, a microbial biofilm on the surface of the teeth, which the body reacts to with an inflammatory response (Marsh 1994). Plaque can be present, with its microbial components stable and the gums healthy in a state of microbial homeostasis, but changes in the plaque microflora can affect this equilibrium, leading to a composition that favours disease (Dalwai 2006; Marsh 2006). In gingivitis, a form of gum disease characterised by redness, irritation and inflammation of the gums (Mayo 2010), it has been shown that a significant alteration in plaque composition is that which leads to a reduction in Streptococcus spp, which tends to make up the majority of the microflora in disease‐free individuals, and an increase in Actinomyces spp (Dalwai 2006).
Gingivitis, on the scale of periodontal diseases, is less severe than periodontitis, with most people being unaware of its presence due to lack of pain, leading to underestimation by dental practitioners (Lang 2009). Furthermore, it was discovered as early as 1965 that gingivitis was reversible in a study where participants ceased all oral hygiene measures, which led to gingivitis, and subsequent reinstatement of oral care resulted in a return to gingival health (Löe 1965). However, gingivitis can lead to severe and irreversible periodontal diseases such as periodontitis (Lang 2009), and such diseases can have a significant effect on quality of life, causing eating difficulties, pain, problems with facial aesthetics and tooth loss (Needleman 2005). Studies have suggested there may be an association between periodontitis and a number of systemic diseases such as diabetes, cardiovascular disease, respiratory diseases and also conditions such as preterm birth, leading to underweight babies (Seymour 2007; Simpson 2010).
Studies suggest that 50% to 90% of adults in the UK and USA have gingivitis (NICE 2012), with some studies estimating prevalence to be as high as 94% in the USA (Li 2010), and 98% in China (Zhang 2010). In other less economically developed countries, studies have estimated prevalences of 76% in Jordan (Ababneh 2012), and 96% in Mexico (García‐Conde 2010). The fact that 15% to 20% of adults aged 35 to 44 years have severe periodontal disease demonstrates the burden of this health problem (WHO 2012).
Dental caries (tooth decay) is a localised chemical dissolution of the surface of the tooth due to metabolic events occurring in dental plaque, and the longer the plaque remains on the tooth surface, the more likely the manifestation of caries (Fejerskov 2008; Selwitz 2007). An increase in the consumption of fermentable carbohydrates lowers the pH of plaque, which leads to favourable conditions for acid‐tolerating (and acidogenic) bacteria such as mutans streptococci and lactobacilli, which dominate the microflora thus tipping the balance from a state of equilibrium to demineralisation, potentially resulting in cavities (Marsh 2006). This mechanism is self perpetuating as an increase in these bacteria leads to a faster rate of acid production, and enhancement of the demineralisation process (Marsh 2006).
It is estimated that the prevalence of dental caries ranges from 60% to 90% in schoolchildren of most industrialised countries, and it affects the large majority of adults (Petersen 2003). This is despite the significant decline in the severity and prevalence of caries seen in such countries since the middle of the last century (Blinkhorn 2009; Marthaler 2004; Selwitz 2007).
Description of the intervention
Toothbrushing is the main intervention universally performed in the home in order to remove and control the dental biofilm mechanically and prevent caries and periodontal disease, but for many adults toothbrushing alone is inadequate for this purpose (Alexander 2012; Morris 2001). Standard practice is for toothbrushing to be carried out using a fluoride toothpaste yet, while such treatment has been instrumental in the approximate 50% reduction in caries in the populations of industrialised western countries in the latter half of the twentieth century, it has contributed little to reducing periodontal diseases (Blinkhorn 2009). As such, it has been recommended that adults should incorporate the use of an antiplaque/antigingivitis agent into their routine of oral care (Gunsolley 2006).
Triclosan is a broad‐spectrum antibacterial agent with low toxicity that can be added to toothpastes in order to reach large numbers of the population (Blinkhorn 2009). While chlorhexidine may have a greater antimicrobial effect, triclosan is more compatible with other typical toothpaste ingredients, with the added advantage of not having an unpleasant taste (Blinkhorn 2009). However, there is no evidence of effectiveness for products containing triclosan alone in the control of caries or plaque/gingivitis (Gunsolley 2006), hence it is mostly used in conjunction with a copolymer (e.g. polyvinylmethyl ether maleic acid ‐ PVM/MA), which facilitates uptake and retention of the triclosan to enamel, oral epithelial cells and plaque (Ciancio 2007). There is some evidence to show that this combination might be effective in the control of plaque and gingivitis (Davies 2004; Gunsolley 2006).
How the intervention might work
Triclosan is an antibacterial agent that affects bacterial growth; it is thought to exert this influence via the inhibition of key bacterial metabolic pathways. This action is thought to reduce the bacterial load in the plaque biofilm, which in theory could control caries and gingivitis. However, previous work has suggested that triclosan may go further than simply reducing plaque (Lindhe 1993), and that it reduces gingival inflammation, which is a necessary precursor to the development of more severe periodontal disease (Gunsolley 2006). A possible explanation for this reduction in inflammation is that the cytokine TNFα (tumour necrosis factor alpha), which is involved in systemic inflammation, augments both the expression of messenger ribonucleic acid (mRNA) and protein levels of microsomal prostaglandin E synthase‐1 (mPGES‐1). These are both important in the biosynthesis of prostaglandin E2 (PGE2) in gingival fibroblasts, and it is thought that triclosan inhibits the production of these building blocks of PGE2, thus having an anti‐inflammatory effect (Mustafa 2005).
As caries develop in the dental biofilm, as described above, it may be possible that the antibacterial effect of triclosan, in reducing plaque, disrupts the biofilm and prevents the progression of caries.
Why it is important to do this review
As the prevalence figures above illustrate, periodontal diseases are widespread and, in the USA in 1999, it was estimated that USD 14.4 billion were spent on periodontal and preventive procedures, with USD 4.4 billion of this total being spent on periodontal services alone (Brown 2002). Caries is also a highly prevalent disease and, as it is initially reversible, it has been recommended that the focus of care should be on early preventive action (Pitts 2004). Poor oral health will inevitably affect overall health and well‐being, indeed one study demonstrated that 90% of participants reported feeling that their level of oral health had an impact on their overall quality of life (Needleman 2004). With these negative economic, social and health consequences of caries and periodontal diseases, triclosan, if found to be both effective and safe, may be a low‐cost, simple, non‐invasive and far‐reaching solution globally if added to more fluoride toothpastes.
A systematic review by Davies et al and a meta‐analysis by Gunsolley, both of randomised controlled trials (RCTs), have both shown that triclosan/copolymer toothpastes might be effective against plaque and gingivitis when compared with standard fluoride toothpastes (Davies 2004; Gunsolley 2006). However, it is now seven years since the most recent of these reviews was published, and neither review rigorously assessed the risk of bias of the included studies. Therefore, it is important and timely to conduct a Cochrane Review of triclosan/copolymer toothpastes in order to provide rigorous, up‐to‐date evidence to oral health practitioners and consumers, which takes into account the risk of bias of the studies that have been carried out on the topic. As with all consumer products, it is important to assess the safety of triclosan/copolymer toothpastes.
Objectives
To assess the effects of triclosan/copolymer containing fluoride toothpastes, compared with fluoride toothpastes, for the long‐term control of caries, plaque and gingivitis in children and adults.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) of parallel or cross‐over design, irrespective of language or publication status. Cross‐over studies were eligible but would have required a sufficient washout period to prevent a carry‐over effect, due to the antimicrobial and anti‐inflammatory properties of triclosan, to allow for participants to return to conditions comparable to baseline. We set this period at a minimum of three weeks in accordance with Löe et al's classic experiment (Löe 1965). We only included studies of at least six months' duration (in terms of both use of the toothpaste and follow‐up), as recommended by the US Food and Drug Administration (FDA), in order to represent a person's normal usage more realistically (thus reducing any possible Hawthorne effect (where participants in the studies perform better oral hygiene measures than they normally would due to the knowledge that they are being assessed (McCarney 2007), which may be present in short‐term studies) and to assess long‐term effects (Gunsolley 2006). Therefore, by necessity, cross‐over studies would have to be a minimum of one year (plus washout period) in length. We included studies with and without baseline prophylaxes (scale and polish), but both groups had to have the same treatment, and it must have taken place at the start of both phases in a cross‐over study. We would have included cluster‐RCTs if any such studies existed. It would not be feasible to carry out split‐mouth studies on this topic, therefore, we excluded such designs.
Types of participants
We included RCTs of children or adults (in accordance with other Cochrane reviews, we classified all participants aged 16 years or less as children and those older than 16 years as adults). We excluded any studies including participants with periodontitis at baseline. We excluded studies where participants were selected due to a pre‐existing health condition (e.g. cancer, heart disease, diabetes). We excluded studies where the majority of participants had orthodontic appliances. We also excluded studies where participants were taking another prophylactic regimen for plaque/gingivitis (e.g. chlorhexidine mouthwash), unless this was only in one arm of the study and there was also a triclosan/copolymer/fluoride arm and a fluoride control arm. In this instance, we excluded the chlorhexidine arm and only used data from the eligible arms.
Types of interventions
Experimental intervention: any fluoride toothpaste containing a triclosan/copolymer combination.
Comparator intervention: any fluoride toothpaste without triclosan.
We only included studies where toothbrushing was unsupervised to represent everyday use. We would have excluded any studies assessing caries if the toothpastes in each treatment arm contained a different concentration of fluoride.
Types of outcome measures
We only used outcome data at six months of follow‐up or longer.
Primary outcomes
Plaque levels measured using any appropriate scale.
Gingival health measured using any appropriate scale.
Secondary outcomes
Incidence of periodontitis.
Caries: a) new incidence, and b) caries increment ‐ change in decayed, missing and filled surfaces (DMFS/dmfs) index.
Calculus measured using any appropriate scale.
Adverse effects (e.g. taste disturbance, staining, allergic reaction, etc.).
Participant‐centred outcomes: a) participant‐assessed quality of life scores, and b) participant satisfaction with product.
Search methods for identification of studies
For the identification of studies included or considered for this review, we developed detailed search strategies for each database searched. We based these on the search strategy developed for MEDLINE (Appendix 1) but revised appropriately for each database to take account of differences in controlled vocabulary and syntax rules.
Electronic searches
We searched the following databases:
Cochrane Oral Health's Trials Register (to 19 August 2013) (Appendix 2);
the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 7) (Appendix 3);
MEDLINE via OVID (1946 to 19 August 2013) (Appendix 1);
Embase via OVID (1980 to 19 August 2013) (see Appendix 4).
Searching other resources
We searched the US National Institutes of Health Trials Register for ongoing trials to 4 March 2013 (Appendix 5).
We only included handsearching done as part of the Cochrane Worldwide Handsearching Programme and uploaded to CENTRAL (see the Cochrane Masterlist for details of journals and issues searched to date).
We searched the reference lists of included studies to identify further possibly relevant studies.
We placed no restrictions on the language of publications when searching the electronic databases or reviewing reference lists in identified studies.
Data collection and analysis
Selection of studies
Two review authors screened the titles and abstracts of the list of studies identified by the searching process against the inclusion criteria of the review, independently and in duplicate, to identify eligible and potentially eligible studies. We obtained full‐text copies of all the identified studies, and also of studies with insufficient information in the title/abstract to make a decision on eligibility. Two review authors further assessed the full‐text copies, independently and in duplicate, to ensure they met the inclusion criteria. We contacted study authors for clarification or missing information where necessary and feasible. We linked multiple reports of the same study together under one single study title. We resolved any disagreements on eligibility through discussion but, if this had not been possible, an experienced member of the Cochrane Oral Health Group editorial team would have been consulted to achieve consensus. We recorded any studies failing to meet the inclusion criteria at this stage , along with reasons for exclusion, in the Characteristics of excluded studies table, and summarised in the Main results section under the subheading Description of studies > Excluded studies. We have summarised this process in the 'Study flow diagram' (Figure 1).
1.
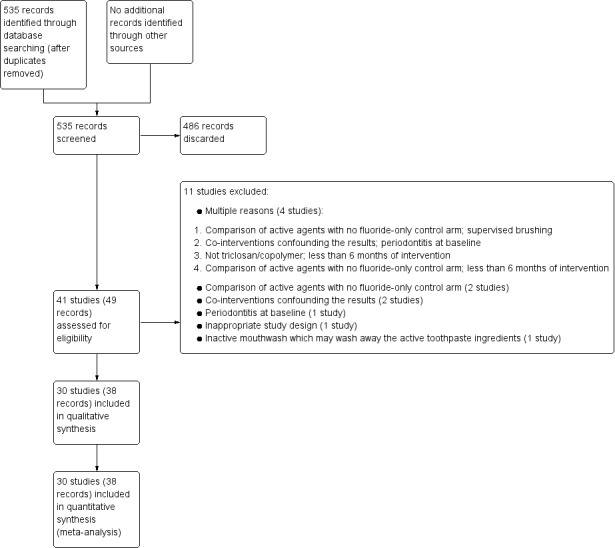
Study flow diagram.
Data extraction and management
Two review authors extracted data from the included studies, independently and in duplicate, using a specially designed data extraction form that was piloted on a small sample of studies. We contacted study authors for clarification or missing information where necessary and feasible. We resolved any disagreements through discussion but, if this had not been possible, an experienced member of the Cochrane Oral Health Group editorial team would have been consulted to achieve consensus. We recorded the extracted data in a spreadsheet, in order to facilitate summarising information in the Main results section under the subheading Description of studies > Included studies.
We recorded the following data for each included study, which was tabulated in the Characteristics of included studies table.
Year of publication, country of origin, study design, number of centres, source of study funding, recruitment period.
Details of the participants including demographic characteristics and criteria for inclusion and exclusion, any relevant information on plaque and gingivitis levels at baseline, numbers randomised to each treatment group, and numbers analysed.
Details of the type of intervention/comparator, timing, dose, and duration, and baseline prophylaxes (scale and polish).
Details of the outcomes reported, including method of assessment, and time(s) assessed.
Sample size calculations.
Assessment of risk of bias in included studies
Two review authors assessed the risk of bias of all included studies, independently and in duplicate, using The Cochrane Collaboration's domain‐based, two‐part tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We contacted study authors for clarification or missing information where necessary and feasible. We resolved any disagreements on risk of bias through discussion but, if this had not been possible, an experienced member of the Cochrane Oral Health Group editorial team would have been consulted to achieve consensus. A 'Risk of bias' table was completed for each included study. For each domain of risk of bias, we first described what was reported to have happened in the study in order to provide a rationale for the second part, which involved assigning a judgement of 'Low risk' of bias, 'High risk' of bias, or 'Unclear risk' of bias.
For each included study, we assessed the following seven domains of risk of bias.
Random sequence generation (selection bias): use of simple randomisation (e.g. random number table, computer‐generated randomisation, central randomisation by a specialised unit), restricted randomisation (e.g. random permuted blocks), stratified randomisation and minimisation were assessed as low risk of bias. Other forms of simple randomisation, such as repeated coin tossing, throwing dice or dealing cards, were also considered as low risk of bias (Schulz 2002). If a study report used the phrase 'randomised' or 'random allocation' but with no further information, we assessed it as unclear for this domain.
Allocation concealment (selection bias): use of centralised/remote allocation, pharmacy‐controlled randomisation (i.e. allocation of sequentially numbered toothpaste containers of identical appearance and weight) and sequentially numbered, sealed, opaque envelopes were assessed as low risk of bias. If a study report did not mention allocation concealment, we assessed it as unclear for this domain.
Blinding of participants (performance bias): as participants performed the intervention, we did not consider personnel blinding. If a study was described as double blind, we assumed that participants and outcome assessors were blinded. If blinding was not mentioned, we assumed that no blinding occurred and we assessed this domain as high risk of bias. It was not possible for a judgement of unclear risk of bias to be assigned for this domain.
Blinding of outcome assessment (detection bias): it should be possible to blind outcome assessors for the main outcomes of this review. If blinding was not mentioned we would have assumed that no blinding occurred and we would have assessed this domain as high risk of bias. It was not possible for a judgement of unclear risk of bias to be assigned for this domain.
Incomplete outcome data (attrition bias): if 10% or less of randomised participants were excluded from the analysis, we assessed this as low risk of bias. However, when attrition was greater than 10%, assuming the missing participants in one group had a higher mean (e.g. gingivitis score) than those in the other group, as the attrition rate increased, so would the mean difference (MD) between groups, as described in Section 8.13.2.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This situation led to a judgement of high risk of bias if we believed that the attrition was high enough to have resulted in a distortion of the true intervention effect, or if there was considerably greater attrition in one group than another. If attrition was greater than 10%, but with the additional factors of not being reported by group and insufficient reporting of reasons for attrition, this led to a judgement of unclear risk of bias. If it was not clear from the study report how many participants were randomised into each group, we assessed it as unclear risk of bias for this domain.
Selective reporting (reporting bias): if the study either reported outcomes not stated a priori in the methods section (as it is unlikely that the studies have published protocols) or did not report outcomes stated in the methods section, we assessed this as high risk of bias. Furthermore, if the study reported in the methods section that a particular scale would be used, but then a different one was used, we assessed it as high risk of bias; if it was not stated in the methods section, we would have assessed it as unclear risk of bias. If outcomes were reported with insufficient information to allow us to use it in a meta‐analysis (e.g. no information on variance), we assessed it as high risk of bias. Cross‐over studies that did not analyse paired data would have been assessed as high risk of bias. Cluster‐RCTs that did not take clustering effects into account would have been assessed as high risk of bias.
Other bias: any other potential source of bias that may feasibly alter the magnitude of the effect estimate (e.g. possible carry‐over effects in cross‐over studies, only first period data reported in cross‐over studies, incorrect analysis in cross‐over studies, baseline imbalances in potentially important prognostic factors between intervention groups, randomisation by set block size in unblinded studies (or where blinding was broken) as this could enable prediction of future allocation (this is regardless of whether allocation concealment was adequate), and differential diagnostic activity by outcome assessors).
We summarised the risk of bias as follows.
| Risk of bias | Interpretation | In outcome | In included studies |
| Low risk of bias | Plausible bias unlikely to seriously alter the results | Low risk of bias for all key domains | Most information is from studies at low risk of bias |
| Unclear risk of bias | Plausible bias that raises some doubt about the results | Unclear risk of bias for one or more key domains | Most information is from studies at low or unclear risk of bias |
| High risk of bias | Plausible bias that seriously weakens confidence in the results | High risk of bias for one or more key domains | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results |
We present the 'Risk of bias' summary graphically by: a) proportion of studies with each judgement ('Low risk', 'High risk' and 'Unclear risk' of bias) for each risk of bias domain (Figure 2); b) cross‐tabulation of judgements by study and by domain (Figure 3).
2.
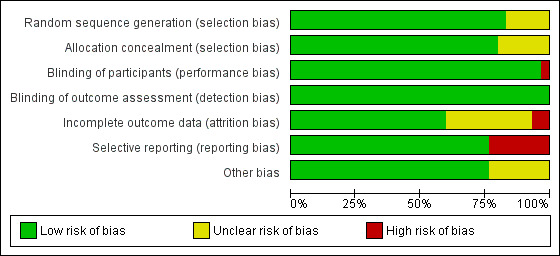
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
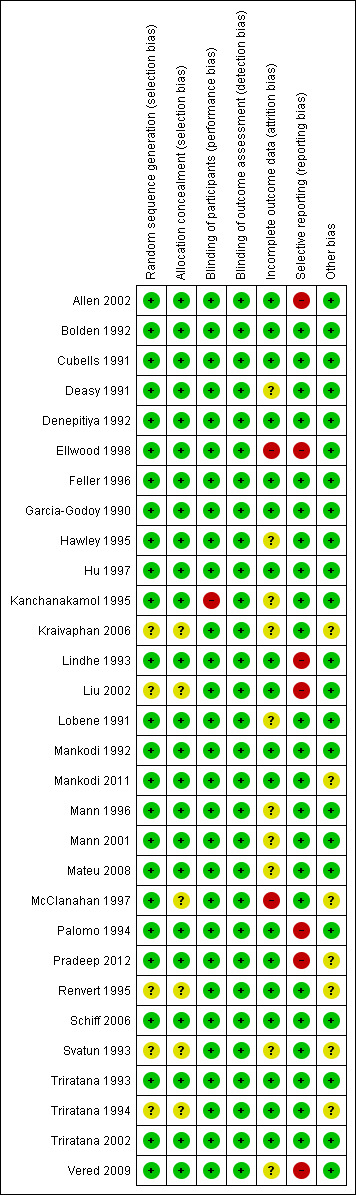
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
For continuous outcomes (e.g. plaque/gingivitis scores), where studies used the same scale, we used the mean values and standard deviations reported in the studies in order to express the estimate of effect of the intervention as MD with 95% confidence interval (CI). Where different scales were used, we would have expressed the treatment effect as standardised mean difference and 95% CI.
For dichotomous outcomes (e.g. attachment loss/no attachment loss), we expressed the estimate of effect of the intervention as a risk ratio (RR) with 95% CI.
For cross‐over studies, we would have extracted appropriate data following the methods outlined by Elbourne et al (Elbourne 2002), and we would have used the generic inverse variance method to enter log RRs or MD/standardised mean difference and standard error into Review Manager 5 (RevMan 2012).
Unit of analysis issues
The participant was the unit of analysis. Cross‐over studies should analyse data using a paired t‐test, or other appropriate statistical test, to take into account the two‐period nature of the data. Cluster‐RCTs should analyse results taking account of the clustering present in the data, otherwise we would have used the methods outlined in Section 16.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions in order to perform an approximately correct analysis (Higgins 2011).
Dealing with missing data
We attempted, where feasible, to contact the author(s) of studies to obtain missing data or for clarification. Where appropriate, we used the methods outlined in Section 7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions in order to estimate missing standard deviations (Higgins 2011). We did not use any further statistical methods or carry out any further imputation to account for missing data.
Assessment of heterogeneity
If meta‐analyses were performed, we assessed the possible presence of heterogeneity visually by inspecting the point estimates and CIs on the forest plots; if the CIs had poor overlap then heterogeneity was considered to be present. We also assessed heterogeneity statistically using a Chi2 test, where a P value < 0.1 indicated statistically significant heterogeneity. Furthermore, we quantified heterogeneity using the I2 statistic. A guide to interpretation of the I2 statistic given in Section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions is as follows (Higgins 2011):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
Assessment of reporting bias within studies has already been described in the section Assessment of risk of bias in included studies.
Reporting biases can occur when reporting (or not reporting) research findings is related to the results of the research (e.g. a study that did not find a statistically significant difference/result may not be published). Reporting bias can also occur if ongoing studies are missed (but that may be published by the time the systematic review is published), or if multiple reports of the same study are published, or if studies are not included in a systematic review due to not being reported in the language of the review authors. If there were more than 10 studies included in a meta‐analysis, we assessed the possible presence of reporting bias by testing for asymmetry in a funnel plot. If present, we would have carried out statistical analysis using the methods described by Egger 1997 for continuous outcomes and Rücker 2008 for dichotomous outcomes. However, we did attempt to limit reporting bias in the first instance by conducting a detailed, sensitive search, including searching for ongoing studies, and any studies not reported in English were translated by a member of The Cochrane Collaboration.
Data synthesis
We only carried out a meta‐analysis where studies of similar comparisons reported the same outcomes. We combined MDs (we would have used standardised mean differences where studies had used different scales) for continuous outcomes, and would have combined RRs for dichotomous outcomes, using a fixed‐effect model if there were only two or three studies, or a random‐effects model if there were four or more studies.
We would have used the generic inverse variance method to include data from cross‐over studies in meta‐analyses as described in Section 16.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Elbourne 2002; Higgins 2011). Where appropriate, we would have combined the results from cross‐over studies with parallel group studies, using the methods described by Elbourne et al (Elbourne 2002). We would have reported the results from studies not suitable for inclusion in a meta‐analysis in an additional table.
Although not stated in the protocol, in order to provide a more complete summary of random‐effects meta‐analyses with high heterogeneity, we calculated 95% prediction intervals where appropriate (Riley 2011).
Subgroup analysis and investigation of heterogeneity
Where there were sufficient studies, we carried out the following subgroup analyses.
Baseline prophylaxes (scale and polish) versus none.
Children versus adults.
Different fluoride concentrations (only for caries outcome).
Initial plaque and inflammation levels.
We would have carried out subgroup analyses according to study design (parallel/cross‐over/cluster‐RCTs).
Sensitivity analysis
In order to ensure our conclusions were robust, we carried out sensitivity analysis (where there were sufficient studies for each outcome) by excluding studies at high and unclear risk of bias.
Presentation of main results
We produced a 'Summary of findings' table for main outcomes of this review using GRADEPro software. We assessed the quality of the body of evidence by considering the overall risk of bias of the included studies, the directness of the evidence, the inconsistency of the results, the precision of the estimates, the risk of publication bias, the magnitude of the effect and whether or not there was evidence of a dose response. We categorised the quality of the body of evidence for each of the primary outcomes as high, moderate, low or very low.
Results
Description of studies
Results of the search
The searches resulted in 535 references following de‐duplication. Two review authors screened the titles and abstracts against the inclusion criteria for this review, independently and in duplicate, discarding 486 references in the process. We obtained full‐text copies of the remaining 49 references and examined them independently and in duplicate, excluding 11 studies at this stage. Eight of the remaining 38 references were abstracts and were subsequently linked to other references. Therefore, 30 studies met the inclusion criteria for this review. This process is presented diagrammatically in Figure 1.
Included studies
Characteristics of the trial designs and settings
Thirty studies met the inclusion criteria for this review and were included (see Characteristics of included studies tables). All studies were of parallel group design, 20 of which had two arms, seven had three arms (Allen 2002; Feller 1996; Liu 2002; Mann 1996; McClanahan 1997; Pradeep 2012; Schiff 2006), and three had four arms (Palomo 1994; Renvert 1995; Svatun 1993). However, two of the three‐arm studies did not report any details regarding the third arm, stating only that it was an experimental toothpaste, the results of which bore no impact on the comparison between the two reported toothpastes (Feller 1996; Mann 1996). Eleven studies were conducted in the USA; five in Thailand; three in Israel; two in Spain; two in the UK; one in each of the Dominican Republic, Guatemala, India, Sweden, and Norway; and two were unclear as the authors were from more than one country and the setting was not explicitly stated (Hu 1997; Lindhe 1993). The setting of the studies was poorly reported, with 17 studies not mentioning the type of setting, seven stating the phrase 'clinical facility' (Allen 2002; Cubells 1991; Mankodi 1992; Mankodi 2011; Mann 1996; Mateu 2008; Palomo 1994), two were conducted in high schools (Ellwood 1998; Hawley 1995), one appeared to be in a university setting (Triratana 2002), one was in a dental college/research institute (Pradeep 2012), one in an antenatal care unit (Kraivaphan 2006) and one was in a dental clinic (Feller 1996).
All studies were single‐centre, with two involving multiple high schools (Ellwood 1998; Hawley 1995), and two involving multiple communities across Israel (Mann 2001; Vered 2009). We report them as single‐centre studies in that they appear to have followed a single study protocol administrated by a single centre/group. Eight studies explicitly stated Colgate Palmolive as a source of support (Hawley 1995; Kanchanakamol 1995; Mankodi 1992; Mankodi 2011; Mateu 2008; Schiff 2006; Triratana 2002; Vered 2009), with a further 15 studies not explicitly stating this, but being clearly associated with Colgate Palmolive through authorship (Allen 2002; Bolden 1992; Cubells 1991; Deasy 1991; Denepitiya 1992; Ellwood 1998; Feller 1996; Garcia‐Godoy 1990; Hu 1997; Lindhe 1993; Lobene 1991; Mann 1996; Mann 2001; Palomo 1994; Triratana 1993). One study explicitly stated Procter & Gamble as a source of support (Liu 2002), with one more study being associated through authorship (McClanahan 1997). One study was associated through authorship to Unilever (Svatun 1993), while one more study stated that LB Aroma provided the toothpastes (Pradeep 2012). Only three studies were potentially truly independent (Kraivaphan 2006; Renvert 1995; Triratana 1994).
Only three studies mentioned sample size calculations. One of these studies achieved the required sample size even after attrition was taken into account (Hawley 1995). Another study performed a sample size calculation but did not report the results of the calculation and it was unclear whether or not the required sample size was achieved (Pradeep 2012). The sample size of the final study was informed by a previous study, stating that approximately 50 participants were required in each of the four arms, yet it was unclear whether this was achieved as the numbers in each arm ranged from 45 to 48 (Svatun 1993).
Characteristics of the participants
A total of 14,835 participants provided data for this review, with the numbers analysed in each study ranging from 54 to 3462. Only two studies were conducted on children (Ellwood 1998; Hawley 1995), both of which had a mean age of 12.7 years, and a range of 11 to 13 years. In the other 28 studies, the age range was 18 to 81 years, with the mean age ranging from 21.5 to 59. All studies had a greater proportion of females than males, except for one study (Schiff 2006). One study was conducted on pregnant women (Kraivaphan 2006).
For the 20 studies that assessed plaque using the Quigley‐Hein Plaque Index, the mean baseline plaque score was 2.52.
For the 20 studies that assessed gingivitis using the Löe‐Silness Gingival Index, the mean baseline gingivitis score was 1.48.
For the four studies that assessed coronal caries, the three conducted on adults had a mean baseline decayed, filled tooth surfaces (DFS) score of 14.54, and the study on children had a mean baseline decayed, missing and filled tooth surfaces (DMFS) score of 5.4. A further study assessed root caries using the Katz Root Caries Index, and the mean baseline score was 0.97.
For the two studies that assessed calculus, using a comparable version of the Volpe‐Manhold Calculus Index, the mean baseline calculus score was 16.85 mm.
Characteristics of the interventions
In 23 studies, the intervention involved brushing the teeth with the assigned toothpaste, twice daily, for one minute each time. Three studies only specified brushing twice daily but did not state a duration of brushing (Ellwood 1998; Mann 2001; Svatun 1993), and another three stated neither frequency nor duration (Hawley 1995; Pradeep 2012; Renvert 1995). One further study only specified brushing twice daily, for one minute each time, in the triclosan/copolymer arm, while the control arm was instructed to follow their "normal oral hygiene procedure" (Kanchanakamol 1995). Eight studies explicitly stated that participants were asked to refrain from all other oral hygiene procedures (Allen 2002; Denepitiya 1992; Hu 1997; Kanchanakamol 1995; Mankodi 2011; Pradeep 2012; Schiff 2006; Triratana 2002), while one study merely stated that the "use of interdental cleaning devices was not advocated" (Lindhe 1993).
All studies had a triclosan/copolymer arm compared with a control arm. The toothpaste that was used in most of the studies is sold by the manufacturer Colgate. In 29 studies, it was clearly stated that the triclosan/copolymer concentration was 0.3% triclosan, 2% copolymer, but one study did not report the concentration of either ingredient (Pradeep 2012).
Twenty‐eight studies stated that the triclosan/copolymer arms also contained sodium fluoride, while one study only stated fluoride (Pradeep 2012), and another study did not clearly report whether or not it contained fluoride in any form (Kraivaphan 2006). The concentration of sodium fluoride in the triclosan/copolymer arms was 0.243% (1100 parts per million (ppm) fluoride), except for one study, which had a concentration of 0.221% (1000 ppm fluoride) (Kanchanakamol 1995), and another of 0.331% (1500 ppm fluoride) (Mann 1996). Twenty‐seven control arms involved brushing with a fluoride‐only toothpaste, while two studies stated placebo (Kraivaphan 2006; Pradeep 2012), and one study stated "normal oral hygiene procedure" (Kanchanakamol 1995). It is possible that the control arm in these three studies contained fluoride‐only toothpastes but, if this was not the case, we did not consider this to be important as the studies were assessing plaque and gingivitis rather than caries. Of the 27 studies that explicitly reported the control arm to be a fluoride‐only toothpaste, two were in the form of sodium monofluorophosphate, one of which was a 0.8% concentration that had an approximate equivalent fluoride content of 0.243% sodium fluoride in the triclosan/copolymer arm (Svatun 1993), while the other study did not state the concentration (Renvert 1995). Twenty‐four of the remaining studies contained 0.243% sodium fluoride, while one study contained 0.331% (Mann 1996).
Twenty studies reported a baseline prophylaxis to remove plaque and thus assess the potential for triclosan/copolymer toothpastes to prevent plaque accumulation and its ability to reduce gingivitis. The remaining 10 studies did not have a baseline prophylaxis. However, of these, five studies were assessing caries (Feller 1996; Hawley 1995; Mann 1996; Mann 2001; Vered 2009), and one was assessing the development of periodontitis (Ellwood 1998). The remaining four studies were thus designed to assess the potential for triclosan/copolymer toothpastes to treat/reduce plaque and gingivitis (Lindhe 1993; Mankodi 2011; Triratana 1993; Triratana 2002).
In 21 studies, the duration of intervention was six months, with two studies having seven months of intervention (Garcia‐Godoy 1990; Svatun 1993), and one study, conducted on pregnant women, having nine months of intervention (including three months postpartum) (Kraivaphan 2006). In the remaining six studies, the duration of intervention was 24 months (Mann 2001), 30 months (Hawley 1995), and 36 months (Ellwood 1998; Feller 1996; Mann 1996; Vered 2009). Five of these six studies assessed caries, while the remaining study assessed periodontitis (Ellwood 1998). In all 30 studies, the final follow‐up assessment was at the end of the intervention phase.
One study had the additional intervention of flossing in both the triclosan/copolymer arm and the control arm (Schiff 2006), while four further studies included an element of oral hygiene instruction (Mann 2001; Pradeep 2012; Renvert 1995; Svatun 1993).
Characteristics of the outcomes
Plaque
Twenty‐one studies included plaque as an outcome, with 20 of these reporting the Turesky et al modification of the Quigley‐Hein Plaque Index, which is a 0 to 5 scale. One of these studies also reported the Löe‐Silness Plaque Index (Renvert 1995), while another study only used the Löe‐Silness Plaque Index (Svatun 1993). Thirteen of the aforementioned 20 studies also reported the Plaque Severity Index, which is a measure of the proportion of higher scores (3 or higher) on the Quigley‐Hein Plaque Index.
Gingivitis
Twenty‐two studies included gingivitis as an outcome, with 20 of these reporting the Löe‐Silness Gingival Index (15 of which specified the Talbot et al modification), which is a 0 to 3 scale. Thirteen of these studies also reported the Gingivitis Severity Index, which is a measure of the proportion of higher scores (2 or 3, i.e. gingival bleeding) on the Löe‐Silness Gingival Index. Two further studies reported gingivitis using the Ainamo‐Bay Bleeding Index, but it was scored in such a way that we believed it equated to the Gingivitis Severity Index (Renvert 1995; Svatun 1993). One of the 20 studies also reported gingival bleeding (2 or 3 on the Löe‐Silness Gingival Index) but as the number of sites rather than a proportion (McClanahan 1997).
Periodontitis
One study included the outcome of periodontitis, which was reported as the dichotomous outcome of attachment loss or no attachment loss (Ellwood 1998).
Caries
Five studies included caries as an outcome. Four of these assessed coronal caries, all reporting the DFS caries increment, which is the change in decayed and filled surfaces (Feller 1996; Hawley 1995; Mann 1996; Mann 2001). Three of the same studies also reported the DFT caries increment, which is the change in decayed and filled teeth (Feller 1996; Hawley 1995; Mann 1996). One study assessed root caries, reporting the Katz Root Caries Index (Vered 2009).
Calculus
Three studies included calculus as an outcome, all stating that they used the Volpe‐Manhold Calculus Index, yet they were reported in different ways. Two of the studies reported the mean total calculus per participant (Liu 2002; Lobene 1991), while the other study reported the mean height of the calculus (Svatun 1993).
Adverse effects
Although 23 studies included adverse effects as an outcome, only one study reported one type of adverse effect (tooth staining using Meckel Stain Scores) in a way amenable to data analysis in this review (McClanahan 1997). However, this was not the fault of the study investigators in most cases, as they simply reported that there were no adverse events/effects, and, therefore, it is not possible to meta‐analyse such data. One study did report adverse events, but not by group or with sufficient details (Liu 2002). The staining in the McClanahan 1997 study was measured on a continuous scale and was not an adverse event as such. The studies investigated local adverse effects such as tooth staining, altered taste and included clinical examination of oral and perioral soft and hard tissues.
Excluded studies
We excluded 11 studies from the review (see Characteristics of excluded studies table). Below is a summary of the reasons for excluding these studies (some studies were excluded for more than one reason).
Four studies compared only active agents with no fluoride‐only control arm (Archila 2004; Boneta 2010; Dóri 1999; Mankodi 2002).
Three studies had co‐interventions confounding the results: powered toothbrushes used in the triclosan/copolymer arm (Bogren 2007; Bogren 2008); interdental cleaning in the control group (Kocher 2000).
Two studies included participants with periodontitis at baseline (Bogren 2008; Cullinan 2003).
Two studies had less than six months of the intervention (de la Rosa 1992; Dóri 1999).
One study involved supervised brushing (Archila 2004).
One study did not include a triclosan/copolymer arm (de la Rosa 1992).
One study had an inactive mouthwash as a co‐intervention and we judged that there was potential for this to wash away the active toothpaste ingredients (Charles 2001).
One study was an inappropriate design whereby participants with fewer than 20 gingival bleeding sites at baseline, accounting for 26% of the study sample, exited the study after three months (Winston 2002). This could undermine the randomisation process and introduce selection bias.
Risk of bias in included studies
We assessed risk of bias based on the information reported in the included studies in the first instance. We attempted to contact study authors for missing information and clarification, and two sources provided additional information for 23 studies (Allen 2002; Bolden 1992; Cubells 1991; Deasy 1991; Denepitiya 1992; Ellwood 1998; Feller 1996; Garcia‐Godoy 1990; Hawley 1995; Hu 1997; Kanchanakamol 1995; Lindhe 1993; Lobene 1991; Mankodi 1992; Mankodi 2011; Mann 1996; Mann 2001; Mateu 2008; Palomo 1994; Schiff 2006; Triratana 1993; Triratana 2002; Vered 2009).
Allocation
Random sequence generation
We assessed 25 studies as at low risk of bias for this domain. Only two of these studies clearly reported the method of random sequence generation, allowing us to make this judgement (McClanahan 1997; Pradeep 2012). We assessed the other 23 studies as at low risk of bias for this domain after email correspondence with study authors, which confirmed that the studies had used appropriate methods. The remaining five studies did not report sufficient information to make a judgement and we assessed them as at unclear risk of bias (Kraivaphan 2006; Liu 2002; Renvert 1995; Svatun 1993; Triratana 1994).
Allocation concealment
We assessed 24 studies as at low risk of bias for this domain, only one of which reported information to allow this judgement (Pradeep 2012). The other 23 studies achieved this judgement after email correspondence. The remaining six studies did not report sufficient information to make a judgement and we assessed them as at unclear risk of bias (Kraivaphan 2006; Liu 2002; McClanahan 1997; Renvert 1995; Svatun 1993; Triratana 1994).
Therefore, the overall risk of selection bias was low in 24 studies and unclear in six studies.
Blinding
Blinding of participants (performance bias)
Twenty‐nine studies made sufficient efforts to ensure that the triclosan/copolymer and the control toothpastes were indistinguishable from each other, and we assessed them as at low risk of bias for this domain. The remaining study assigned participants to either triclosan/copolymer toothpaste or normal oral hygiene procedure (Kanchanakamol 1995). Therefore, the participants were aware of their assignment thus introducing the potential for performance bias, so we assessed this study as at high risk of bias.
Blinding of outcome assessment (detection bias)
We assessed all 30 studies as at low risk of bias for this domain, as they either clearly stated that the outcome assessor(s) was not aware of the participants' assignment or used the phrase 'double blind'.
Incomplete outcome data
We assessed 18 studies as at low risk of bias for this domain, as 17 had 10% or less attrition, and one had 11% attrition but reported attrition by group, which was relatively equal (Hu 1997). We assessed two studies as at high risk of bias, one of which had 25% attrition, which could pose a risk of bias significant enough to have led to a distortion of the true intervention effect (Ellwood 1998), while the other did not report reasons for attrition, which was much higher in the triclosan/copolymer arm than the control arm (McClanahan 1997). We assessed the remaining 10 studies as at unclear risk of attrition bias because seven studies had attrition greater than 10% but with the additional factors of not being reported by group and not reporting reasons (Deasy 1991; Hawley 1995; Kanchanakamol 1995; Kraivaphan 2006; Lobene 1991; Svatun 1993; Vered 2009), while three studies did not report the number of participants initially randomised so it is not possible to calculate overall attrition, and they also did not report reasons for withdrawal/exclusion from the analyses (Mann 1996; Mann 2001; Mateu 2008).
Selective reporting
We assessed 23 studies as at low risk of bias for this domain, as they reported appropriate outcomes in full, as planned in the methods section of each study report. We assessed the remaining seven studies as at high risk of reporting bias. Two of these stated in the methods section that they would assess adverse effects, but did not report any information in the results section (Allen 2002; Pradeep 2012). Two studies assessed additional outcomes that are important to this review at follow‐up points but did not report them: plaque, gingivitis and calculus (Ellwood 1998), and coronal caries (Vered 2009). One of those studies also did not report the main outcome of the study (periodontitis) as stated in the methods section (Ellwood 1998). One study only reported variance of the mean scores visually as 95% confidence interval bars in the graphs, and our interpretation of the graphs gave different means to those reported in the study (Lindhe 1993). One study reported that there had been adverse effects but the data were not reported by group (Liu 2002). The remaining study did not report any information on the variance of the mean scores (Palomo 1994).
Other potential sources of bias
We assessed 23 studies as at low risk of bias for this domain, as no other potential sources of bias were apparent. Ten of these studies clearly reported information suggesting that outcome assessors were adequately trained or calibrated or both, implying that the risk of differential diagnostic activity would have been low (Allen 2002; Cubells 1991; Ellwood 1998; Feller 1996; Hawley 1995; Liu 2002; Mankodi 1992; Mann 1996; Mann 2001; Vered 2009). We judged 13 of the 23 studies to be at low risk of bias after email correspondence with study authors confirmed that the studies followed a protocol whereby all outcome assessors were highly trained in the indices and procedures used, and inter and intra‐examiner calibration occurred where practical (Bolden 1992; Deasy 1991; Denepitiya 1992; Garcia‐Godoy 1990; Hu 1997; Kanchanakamol 1995; Lindhe 1993; Lobene 1991; Mateu 2008; Palomo 1994; Schiff 2006; Triratana 1993; Triratana 2002). We assessed the remaining seven studies as at unclear risk of bias. Six of these studies did not report any methods to minimise differential diagnostic activity (Kraivaphan 2006; McClanahan 1997; Pradeep 2012; Renvert 1995; Svatun 1993; Triratana 1994), and the remaining study reported statistically significant differences between groups at baseline for plaque scores and age, which could indicate a problem with the randomisation process (Mankodi 2011).
Overall risk of bias
We assessed 10 studies as being at low overall risk of bias (Bolden 1992; Cubells 1991; Denepitiya 1992; Feller 1996; Garcia‐Godoy 1990; Hu 1997; Mankodi 1992; Schiff 2006; Triratana 1993; Triratana 2002).
We assessed nine studies as being at high overall risk of bias (Allen 2002; Ellwood 1998; Kanchanakamol 1995; Lindhe 1993; Liu 2002; McClanahan 1997; Palomo 1994; Pradeep 2012; Vered 2009). These studies had at least one domain judged to be at high risk of bias.
We assessed 11 studies as being at unclear overall risk of bias (Deasy 1991; Hawley 1995; Kraivaphan 2006; Lobene 1991; Mankodi 2011; Mann 1996; Mann 2001; Mateu 2008; Renvert 1995; Svatun 1993; Triratana 1994). These studies had at least one domain judged to be at unclear risk of bias, but no domains judged to be at high risk of bias.
The results of the risk of bias assessments are presented graphically in Figure 2 and Figure 3.
Effects of interventions
See: Table 1
Plaque
Quigley‐Hein Plaque Index (six to seven months)
Twenty studies analysing 2675 participants (nine at low risk of bias, six at high risk of bias and five at unclear risk of bias) were combined in a meta‐analysis, which showed a statistically significant reduction in plaque in favour of triclosan/copolymer (mean difference (MD) ‐0.47, 95% confidence interval (CI) ‐0.60 to ‐0.34, P value < 0.00001, I2 = 94%) (Analysis 1.1). The control group mean was 2.17, representing a 22% reduction in plaque. We performed subgroup analyses according to whether or not participants received a baseline prophylaxis and according to whether baseline plaque levels, prior to any baseline prophylaxes, were low or high (we used the median value (2.40) to dichotomise these), and the results are presented in Additional Table 2. All subgroup analyses still showed a statistically significant reduction in plaque in favour of triclosan/copolymer. However, for baseline prophylaxis (MD ‐0.44, 95% CI ‐0.58 to ‐0.30, P value < 0.00001, I2 = 94%), and no baseline prophylaxis (MD ‐0.61, 95% CI ‐0.82 to ‐0.41, P value < 0.00001, I2 = 94%), there was no statistically significant difference between subgroups (Chi2 = 1.92, degrees of freedom (df) = 1, P value = 0.17, I2 = 47.8%). Also, for low baseline plaque (MD ‐0.41, 95% CI ‐0.57 to ‐0.25, P value < 0.00001, I2 = 92%), and high baseline plaque (MD ‐0.54, 95% CI ‐0.72 to ‐0.35, P value < 0.00001, I2 = 95%), there was no statistically significant difference between subgroups (Chi2 = 1.08, df = 1, P value = 0.30, I2 = 7%). As the subgroup analyses could not account for the considerable heterogeneity, it may be assumed that the causes are multiple. The results of this random‐effects meta‐analysis represent the average treatment effect across a range of settings. Therefore, we calculated a 95% prediction interval in order to provide information on the potential effectiveness of the intervention in an individual setting (Riley 2011). This ranged from ‐1.07 to 0.13 indicating that triclosan/copolymer will be beneficial in most settings but, as the interval overlaps zero, there is a small possibility that in some settings it may not be more effective than the control.
1.1. Analysis.
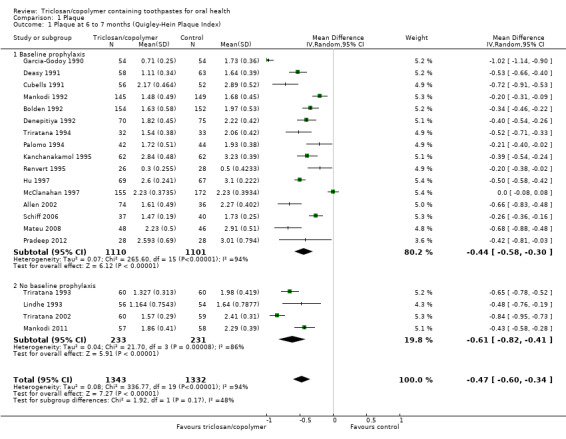
Comparison 1 Plaque, Outcome 1 Plaque at 6 to 7 months (Quigley‐Hein Plaque Index).
1. Subgroup analyses.
| Subgroup factor | Mean difference (95% confidence interval) | Test for subgroup differences | |
| Baseline prophylaxis | Yes | No | |
| QHPI | ‐0.44 (‐0.58 to ‐0.30) | ‐0.61 (‐0.82 to ‐0.41) | Chi2 = 1.92, df = 1, P value = 0.17, I2 = 47.8% |
| PSI | ‐0.13 (‐0.18 to ‐0.08) | ‐0.20 (‐0.26 to ‐0.14) | Chi2 = 3.01, df = 1, P value = 0.08, I2 = 66.7% |
| LSGI | ‐0.26 (‐0.34 to ‐0.18) | ‐0.30 (‐0.39 to ‐0.21) | Chi2 = 0.43, df = 1, P value = 0.51, I2 = 0% |
| GSI | ‐0.12 (‐0.18 to ‐0.07) | ‐0.16 (‐0.27 to ‐0.05) | Chi2 = 0.32, df = 1, P value = 0.57, I2 = 0% |
| Baseline plaque levels |
Low (0.50 to 2.36) |
High (2.45 to 4.40) |
|
| QHPI | ‐0.41 (‐0.57 to ‐0.25) | ‐0.54 (‐0.72 to ‐0.35) | Chi2 = 1.08, df = 1, P value = 0.30, I2 = 7% |
| PSI | ‐0.15 (‐0.18 to ‐0.13) | ‐0.14 (‐0.21 to ‐0.07) | Chi2 = 0.14, df = 1, P value = 0.71, I2 = 0% |
| Baseline gingivitis levels |
Low (0.71 to 1.42) |
High (1.49 to 2.11) |
|
| LSGI | ‐0.21 (‐0.30 to ‐0.13) | ‐0.33 (‐0.36 to ‐0.31) | Chi2 = 7.41, df = 1, P value = 0.006, I2 = 86.5% |
| GSI* | ‐0.13 (‐0.19 to ‐0.07) | ‐0.17 (‐0.22 to ‐0.12) | Chi2 = 0.92, df = 1, P value = 0.34, I2 = 0% |
df: degrees of freedom; GSI: Gingivitis Severity Index (proportion of sites bleeding, i.e. 2 or 3 on the Löe‐Silness Gingival Index); LSGI: Löe‐Silness Gingival Index (0 to 3 on an increasing scale); PSI: Plaque Severity Index (proportion of surfaces scoring > 3 on the Quigley‐Hein Plaque Index); QHPI: Quigley‐Hein Plaque Index (0 to 5 on an increasing scale).
*2 studies not included due to no reporting of baseline LSGI scores (Renvert 1995; Svatun 1993).
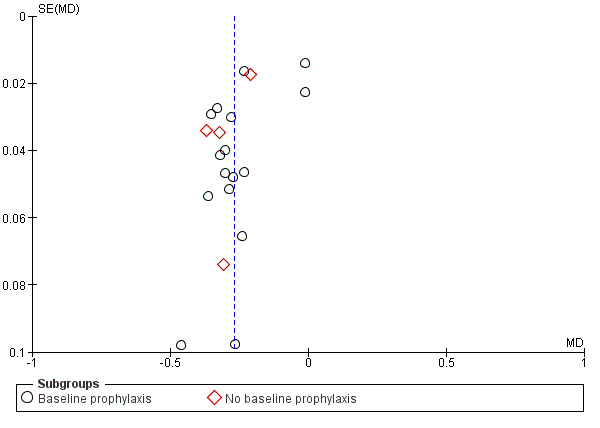
We were unable to detect the presence of any obvious publication bias in the funnel plot analysis (Figure 4).
4.
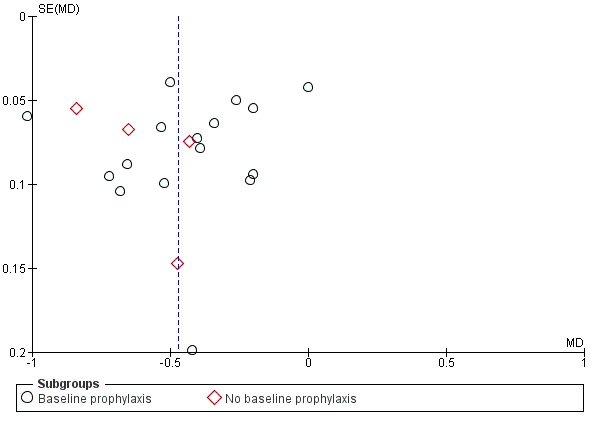
Funnel plot of comparison: 1 Plaque, outcome: 1.1 Plaque at 6 to 7 months (Quigley‐Hein Plaque Index).
A sensitivity analysis, based on restricting the meta‐analysis to the nine studies assessed as being at low risk of bias, produced a similar result to the overall effect estimate in favour of triclosan/copolymer (MD ‐0.55, 95% CI ‐0.73 to ‐0.36, P value < 0.00001, I2 = 96%), indicating that the results are robust.
Plaque Severity Index (six to seven months)
Thirteen studies analysing 1850 participants (seven at low risk of bias, three at high risk of bias and three at unclear risk of bias) were combined in a meta‐analysis, which showed a statistically significant reduction in plaque severity in favour of triclosan/copolymer (MD ‐0.15, 95% CI ‐0.20 to ‐0.10, P value < 0.00001, I2 = 95%) (Analysis 1.2). The control group mean was 0.37, representing a 41% reduction in plaque severity. Subgroup analyses based on baseline prophylaxis/no baseline prophylaxis and low/high baseline plaque scores were carried out and are presented in Additional Table 2. All subgroup analyses still showed a statistically significant reduction in plaque severity in favour of triclosan/copolymer. For baseline prophylaxis (MD ‐0.13, 95% CI ‐0.18 to ‐0.08, P value < 0.00001, I2 = 94%) and no baseline prophylaxis (MD ‐0.20, 95% CI ‐0.26 to ‐0.14, P value < 0.00001, I2 = 77%), there was no statistically significant difference between subgroups (Chi2 = 3.01, df = 1, P value = 0.08, I2 = 66.7%). Also, for low baseline plaque (MD ‐0.15, 95% CI ‐0.18 to ‐0.13, P value < 0.00001, I2 = 34%) and high baseline plaque (MD ‐0.14, 95% CI ‐0.21 to ‐0.07, P value < 0.00001, I2 = 97%), there was no statistically significant difference between subgroups (Chi2 = 0.14, df = 1, P value = 0.71, I2 = 0%). As the subgroup analyses could not account for the considerable heterogeneity, it may be assumed that the causes are multiple. The 95% prediction interval for the average effect ranged from ‐0.34 to 0.05 indicating a beneficial effect in most settings.
1.2. Analysis.
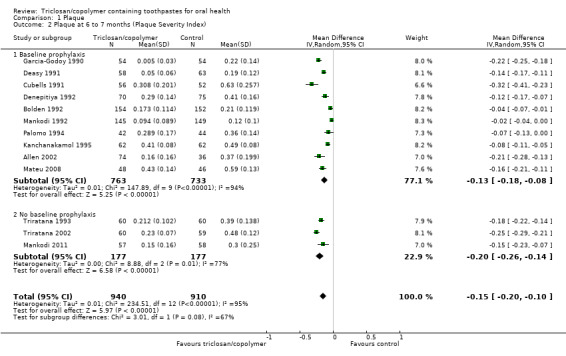
Comparison 1 Plaque, Outcome 2 Plaque at 6 to 7 months (Plaque Severity Index).
A sensitivity analysis, based on restricting the meta‐analysis to the seven studies assessed as being at low risk of bias, produced a similar result to the overall effect estimate in favour of triclosan/copolymer (MD ‐0.16, 95% CI ‐0.24 to ‐0.08, P value < 0.0001, I2 = 97%), indicating that the results are robust.
Löe‐Silness Plaque Index (six to seven months)
Two studies analysing 148 participants (both at unclear risk of bias) were combined in a meta‐analysis which showed a marginally statistically significant reduction in plaque in favour of triclosan/copolymer (MD ‐0.05, 95% CI ‐0.10 to ‐0.01, P value = 0.03, I2 = 8%) (Analysis 1.3).
1.3. Analysis.
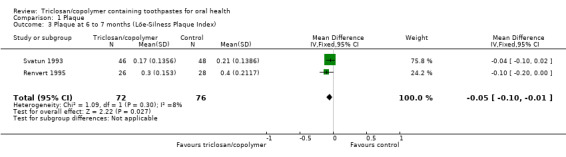
Comparison 1 Plaque, Outcome 3 Plaque at 6 to 7 months (Löe‐Silness Plaque Index).
Gingivitis
Löe‐Silness Gingival Index (six to nine months)
Twenty studies analysing 2743 participants (nine at low risk of bias, six at high risk of bias and five at unclear risk of bias) were combined in a meta‐analysis, which showed a statistically significant reduction in gingivitis in favour of triclosan/copolymer (MD ‐0.27, 95% CI ‐0.33 to ‐0.21, P value < 0.00001, I2 = 95%) (Analysis 2.1). The control group mean was 1.22, representing a 22% reduction in inflammation. We performed subgroup analyses according to whether or not participants received a baseline prophylaxis and according to whether baseline gingivitis (inflammation) levels, prior to any baseline prophylaxes, were low or high (we used the median value (1.455) to dichotomise these), and the results are presented in Additional Table 2. All subgroup analyses still showed a statistically significant reduction in gingivitis in favour of triclosan/copolymer. However, for baseline prophylaxis (MD ‐0.26, 95% CI ‐0.34 to ‐0.18, P value < 0.00001, I2 = 96%) and no baseline prophylaxis (MD ‐0.30, 95% CI ‐0.39 to ‐0.21, P value < 0.00001, I2 = 87%), there was no statistically significant difference between subgroups (Chi2 = 0.43, df = 1, P value = 0.51, I2 = 0%). In contrast, for low baseline gingivitis (MD ‐0.21, 95% CI ‐0.30 to ‐0.13, P value < 0.00001, I2 = 97%) and high baseline gingivitis (MD ‐0.33, 95% CI ‐0.36 to ‐0.31, P value < 0.00001, I2 = 0%), there was a statistically significant difference between subgroups in favour of a larger effect for the high baseline values (Chi2 = 7.41, df = 1, P value = 0.006, I2 = 86.5%). The low baseline gingivitis subgroup still showed considerable heterogeneity while the high baseline subgroup showed no heterogeneity, but the causes of this are unclear and likely to be multiple. The 95% prediction interval for the average effect ranged from ‐0.56 to 0.02 indicating a beneficial effect in most settings.
2.1. Analysis.
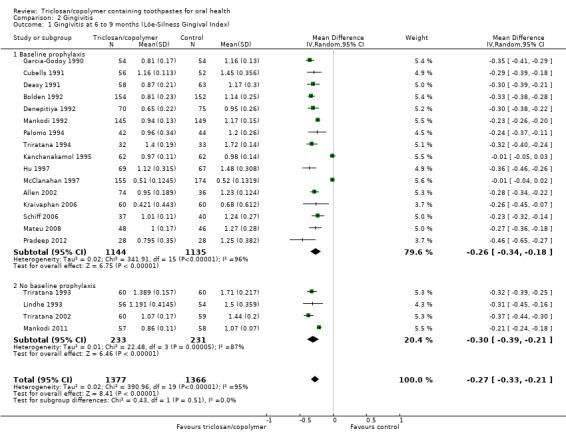
Comparison 2 Gingivitis, Outcome 1 Gingivitis at 6 to 9 months (Löe‐Silness Gingival Index).
We were unable to detect the presence of any obvious publication bias in the funnel plot analysis (Figure 5).
5.
Funnel plot of comparison: 2 Gingivitis, outcome: 2.1 Gingivitis at 6 to 9 months (Löe‐Silness Gingival Index).
A sensitivity analysis, based on restricting the meta‐analysis to the nine studies assessed as being at low risk of bias, produced a similar result to the overall effect estimate in favour of triclosan/copolymer (MD ‐0.31, 95% CI ‐0.35 to ‐0.27, P value < 0.00001, I2 = 73%), indicating that the results are robust.
Gingivitis Severity Index (six to seven months)
Fifteen studies analysing 1998 participants (seven at low risk of bias, three at high risk of bias and five at unclear risk of bias) were combined in a meta‐analysis, which showed a statistically significant reduction in gingivitis severity (gingival bleeding) in favour of triclosan/copolymer (MD ‐0.13, 95% CI ‐0.17 to ‐0.08, P value < 0.00001, I2 = 97%) (Analysis 2.2). The control group mean was 0.27, representing a 48% reduction in bleeding. Subgroup analyses based on baseline prophylaxis/no baseline prophylaxis and low/high baseline gingivitis scores were carried out and are presented in Additional Table 2. All subgroup analyses still showed a statistically significant reduction in gingivitis severity in favour of triclosan/copolymer. For baseline prophylaxis (MD ‐0.12, 95% CI ‐0.18 to ‐0.07, P value < 0.0001, I2 = 97%) and no baseline prophylaxis (MD ‐0.16, 95% CI ‐0.27 to ‐0.05, P value = 0.006, I2 = 97%), there was no statistically significant difference between subgroups (Chi2 = 0.32, df = 1, P value = 0.57, I2 = 0%). Also, for low baseline gingivitis (MD ‐0.13, 95% CI ‐0.19 to ‐0.07, P value < 0.00001, I2 = 97%) and high baseline gingivitis (MD ‐0.17, 95% CI ‐0.22 to ‐0.12, P value < 0.00001, I2 = 86%), there was no statistically significant difference between subgroups (Chi2 = 0.92, df = 1, P value = 0.34, I2 = 0%). As the subgroup analyses could not account for the considerable heterogeneity, it may be assumed that the causes are multiple. The 95% prediction interval for the average effect ranged from ‐0.32 to 0.07 indicating a beneficial effect in most settings.
2.2. Analysis.
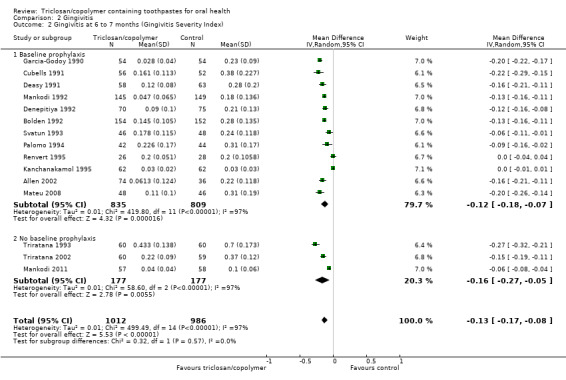
Comparison 2 Gingivitis, Outcome 2 Gingivitis at 6 to 7 months (Gingivitis Severity Index).
A sensitivity analysis, based on restricting the meta‐analysis to the seven studies assessed as being at low risk of bias, produced a similar result to the overall effect estimate in favour of triclosan/copolymer (MD ‐0.17, 95% CI ‐0.20 to ‐0.14, P value < 0.00001, I2 = 84%), indicating that the results are robust.
Number of bleeding sites (six months)
One study at high risk of bias, analysing 329 participants, showed no evidence of a difference between triclosan/copolymer and control (MD 0.14, 95% CI ‐1.11 to 1.39) (Analysis 2.3).
2.3. Analysis.
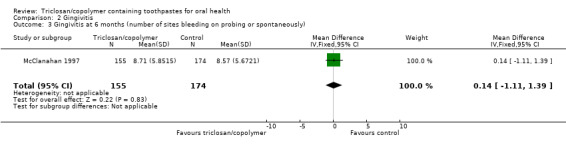
Comparison 2 Gingivitis, Outcome 3 Gingivitis at 6 months (number of sites bleeding on probing or spontaneously).
Periodontitis
Attachment loss (36 months)
One study at high risk of bias, analysing 480 participants, showed no evidence of a difference between triclosan/copolymer and control (risk ratio 0.92, 95% CI 0.67 to 1.27) (Analysis 3.1).
3.1. Analysis.
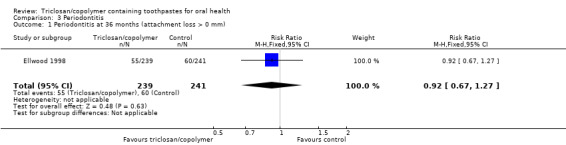
Comparison 3 Periodontitis, Outcome 1 Periodontitis at 36 months (attachment loss > 0 mm).
Caries
Coronal caries
Change in decayed and filled teeth (30 to 36 months)
Three studies analysing 6300 participants (one at low risk of bias and two at unclear risk of bias) were combined in a meta‐analysis, which showed no evidence of a difference between triclosan/copolymer and control (MD ‐0.06, 95% CI ‐0.14 to 0.02, P value = 0.13, I2 = 0%) (Analysis 4.1). There were three subgroups, each consisting of one study, and all of which showed no evidence of a difference. The subgroups were: 1) children (permanent dentition) and 0.243% sodium fluoride/1100 parts per million (ppm) fluoride; 2) adults and 0.243% sodium fluoride/1100 ppm fluoride; and 3) adults and 0.331% sodium fluoride/1500 ppm fluoride. There were no statistically significant differences between the subgroups (Chi2 = 0.20, df = 2, P value = 0.90, I2 = 0%) indicating that it was probably appropriate to pool them in a combined meta‐analysis.
4.1. Analysis.
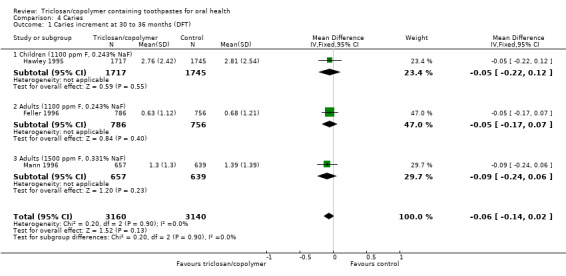
Comparison 4 Caries, Outcome 1 Caries increment at 30 to 36 months (DFT).
Change in decayed and filled surfaces (24 to 36 months)
The same three studies as above, plus one further study (at unclear risk of bias) included in the adults and 0.243% sodium fluoride/1100 ppm fluoride subgroup, were combined in a meta‐analysis of 9692 participants which showed a marginally statistically significant reduction in coronal caries in favour of triclosan/copolymer (MD ‐0.16, 95% CI ‐0.31 to ‐0.02, P value = 0.03, I2 = 0%) (Analysis 4.2). The control group mean was 3.44, representing a 5% reduction in coronal caries. Of the three subgroups, only the adults and 0.243% sodium fluoride/1100 ppm fluoride subgroup showed a statistically significant difference (MD ‐0.21, 95% CI ‐0.40 to ‐0.02, P value = 0.02, I2 = 13%). Again, it was probably appropriate to combine the four studies in a meta‐analysis as there were no statistically significant differences between the subgroups (Chi2 = 1.16, df = 2, P value = 0.56, I2 = 0%).
4.2. Analysis.
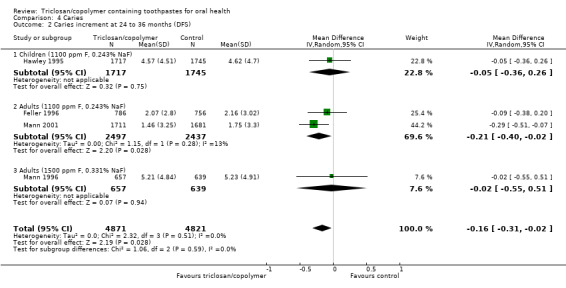
Comparison 4 Caries, Outcome 2 Caries increment at 24 to 36 months (DFS).
Root caries
Katz Root Caries Index (36 months)
One study at high risk of bias, analysing 1357 participants, showed a statistically significant reduction in root caries in favour of triclosan/copolymer (MD ‐0.31, 95% CI ‐0.39 to ‐0.23, P value < 0.00001) (Analysis 4.3).
4.3. Analysis.
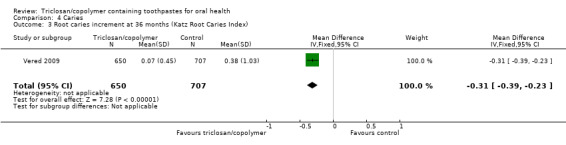
Comparison 4 Caries, Outcome 3 Root caries increment at 36 months (Katz Root Caries Index).
Calculus
Volpe‐Manhold Calculus Index (six months) ‐ mean total calculus per participant in millimetres
Two studies analysing 415 participants (one at high risk of bias and one at unclear risk of bias) were combined in a meta‐analysis, which showed a statistically significant reduction in calculus in favour of triclosan/copolymer (MD ‐2.12, 95% CI ‐3.39 to ‐0.84, P value = 0.001, I2 = 91%) (Analysis 5.1). The control group mean was 14.61 mm, representing a 15% reduction in calculus. We were unable to specify the cause of the considerable heterogeneity present in this meta‐analysis but it is possible that it was related to funding.
5.1. Analysis.
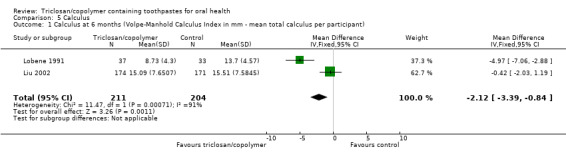
Comparison 5 Calculus, Outcome 1 Calculus at 6 months (Volpe‐Manhold Calculus Index in mm ‐ mean total calculus per participant).
Volpe‐Manhold Calculus index (seven months) ‐ mean height of calculus in millimetres
One study at unclear risk of bias, analysing 78 participants, showed no evidence of a difference between triclosan/copolymer and control (MD ‐0.04, 95% CI ‐0.21 to 0.13, P value = 0.64) (Analysis 5.2).
5.2. Analysis.
Comparison 5 Calculus, Outcome 2 Calculus at 7 months (Volpe‐Manhold Calculus Index in mm ‐ mean height of calculus).
Adverse effects
Tooth staining
Meckel Stain Score (six months)
One study at high risk of bias, analysing 325 participants, showed no evidence of a difference between triclosan/copolymer and control (MD ‐0.15, 95% CI ‐0.60 to 0.30, P value = 0.51) (Analysis 6.1).
6.1. Analysis.

Comparison 6 Adverse effects, Outcome 1 Staining of teeth at 6 months (Meckel Stain Score).
Other adverse effects
Twenty‐two studies reported that there were no adverse effects in either the experimental or control arm of the study. While it is not possible to meta‐analyse such dichotomous data with zero events, it is important information to report in this review. The only study that did report adverse effects, did not provide data amenable to analysis, as adverse events were not reported by group (Liu 2002).
Participant‐centred outcomes
No studies reported any participant‐centred outcomes.
Discussion
Summary of main results
In this review, we have included 30 studies that assessed the effects of brushing teeth with triclosan/copolymer/fluoride toothpastes when compared with a control group of fluoride‐only or placebo toothpastes or usual care on the outcomes of plaque, gingivitis, periodontitis, caries, calculus and adverse effects. We assessed the quality of the body of evidence using GRADE (GRADE 2004), and our assessment is presented in the Table 1.
There was moderate‐quality evidence, from 20 studies analysing 2675 participants, that triclosan/copolymer reduces plaque by 22% compared with control, after six to seven months of use. There was further moderate‐quality evidence, from 13 studies analysing 1850 participants, that triclosan/copolymer reduces more severe plaque levels by 41% compared with control, after six to seven months of use. There was no evidence that undertaking a baseline prophylaxis or that baseline plaque level influences the effect size for either of these outcomes.
There was moderate‐quality evidence, from 20 studies analysing 2743 participants, that triclosan/copolymer reduces gingivitis (inflammation) by 22% compared with control, after six to nine months of use. There was further moderate‐quality evidence, from 15 studies analysing 1998 participants, that triclosan copolymer reduces gingival bleeding by 48% compared with control, after six to seven months of use. There was no evidence that undertaking a baseline prophylaxis influences the effect size for inflammation or bleeding. However, there was some evidence that triclosan/copolymer leads to a greater reduction in inflammation when baseline inflammation levels are high. There was no evidence that baseline inflammation level influences the effect size for bleeding.
There was insufficient evidence, from a single study analysing 480 participants, to show whether or not triclosan/copolymer reduces the incidence of periodontitis, after 36 months of use. The available evidence was rated as low quality.
There was high‐quality evidence, from four studies analysing 9692 participants, that triclosan/copolymer reduces coronal caries by 5% compared with control, after 24 to 36 months of use, when using the decayed and filled surfaces (DFS) index. When using the decayed and filled teeth (DFT) index, high‐quality evidence, from three studies analysing 6300 participants, showed no difference between triclosan/copolymer and control after 30 to 36 months of use. However, despite the high number of participants, it may be that there was a lack of power to detect a small, statistically significant difference. There was weaker evidence, from a single study analysing 1357 participants, showing that triclosan/copolymer reduces root caries, after 36 months of use. This evidence was rated as moderate quality. These results show that adding triclosan/copolymer to toothpaste does not reduce the anticaries effect of fluoride.
There was low‐quality evidence, from two studies analysing 415 participants, that triclosan/copolymer reduces the mean total calculus per participant by 15% compared with control, after six months of use.
The studies did not investigate the possible systemic effects of the toothpastes involved; however, we consider an important finding of the review to be the fact that 22 studies (73%) reported that there were no adverse local effects caused by triclosan/copolymer toothpaste in the short to medium term, although it was not possible to meta‐analyse these data or assess the body of evidence using GRADE.
Overall completeness and applicability of evidence
The volume of evidence, and its reasonable quality, has provided clear evidence of the benefits of using a triclosan/copolymer toothpaste. This was further enhanced by the fact that so many studies used the same methods of assessment, which allowed us to confidently combine data. The studies were carried out in at least 10 different countries spanning the socioeconomic gradient and spanning a range of baseline plaque and gingivitis scores, and these factors give rise to a high degree of external validity. Toothbrushing with such a toothpaste is a relatively inexpensive intervention that can be carried out by the vast majority of people in a domestic setting. Furthermore, however modestly the reader interprets the reported effects, they may be translated into worthwhile effects at population level.
The majority of the research on triclosan/copolymer‐containing toothpastes has been directly or indirectly funded by industry. As with all systematic reviews, there is a potential risk of publication bias, whereby studies that report a beneficial effect are more likely to be published than those that do not find a difference or demonstrate harm. This could affect meta‐analysis by overestimating the treatment effect. We were unable to rule out conclusively the possibility of publication bias in this review.
Readers of this review are likely to be interested in the safety of triclosan/copolymer toothpastes; however, it was not possible to assess this in the long term, as randomised controlled trials (RCTs) are not appropriate study designs to assess the possible systemic effects/safety. In the short term, only one study reported mild adverse effects, although it was not clear if these were attributable to triclosan/copolymer or another antiplaque/antigingivitis agent. The large majority of other studies explicitly reported that there were no adverse effects.
It is possible that the effect sizes of the studies were influenced by the Hawthorne effect, whereby participants in the studies perform better oral hygiene measures than they normally would due to the knowledge that they are being assessed (McCarney 2007). The studies generally involved three examinations over six months (including baseline assessment), with regular receipt of new toothbrushes and toothpaste, all of which may have led to a Hawthorne effect.
On the whole, this was a pragmatic review that assessed what is likely to happen in a real‐life situation, over a period of at least six months, rather than within the confines of a short, highly controlled, clinic‐based, supervised explanatory trial. Future versions of this review will consider a broader range of antibacterial agents in other toothpastes.
Quality of the evidence
The studies included in this review were RCTs, which are widely considered the gold standard study design when assessing effectiveness, assuming they are methodologically sound (Petticrew 2003; Schulz 1995). Ten studies (33%) were assessed as at low risk of bias, nine (30%) at high risk, and 11 (37%) at unclear risk. This enabled us to perform sensitivity analyses for all plaque and gingivitis outcomes, restricting the meta‐analyses to low risk of bias studies. In all cases, the results were found to be robust. Indeed, restricting meta‐analyses to low risk of bias studies produced slightly larger reductions in all plaque and gingivitis indices. We were unable to perform such analyses for other outcomes due to insufficient numbers of studies.
There was considerable unexplained heterogeneity in the meta‐analyses for plaque, gingivitis and calculus. However, for plaque and gingivitis, as the results of the individual studies so consistently show a positive effect for triclosan/copolymer, it is reasonable to be confident in the results presented.
Potential biases in the review process
We conducted a sensitive search of multiple databases to identify suitable studies for this review, with no restrictions on language or publication status. We also arranged for several references to be translated to assess their eligibility.
We attempted to contact some study authors for missing information; however, we could not find recent contact details for some studies, and most authors did not respond. Therefore, authors of any included studies are encouraged to contact us to clarify any issues that led to judgements of unclear or high risk of bias. For future updates, we would also welcome any information regarding unpublished or ongoing studies that we may not have identified.
Our assessment of attrition bias in the included studies may have introduced some degree of bias in the review process. This is because we stated an a priori rule in the protocol that only 10% or less attrition would result in a judgement of low risk of bias. While we relaxed this rule for the review, it was difficult to assess attrition bias objectively in included studies ranging from six to 36 months' duration and for different outcomes, as we recognise that longer studies are generally more likely to have higher attrition.
We recognise that some deviations from protocol may have introduced bias in the review process. However, we have clearly reported our reasoning behind our judgements (see Differences between protocol and review) and we have tried to be consistent.
Agreements and disagreements with other studies or reviews
The results of this Cochrane Review are almost identical to those of a systematic review on the same topic conducted almost 10 years ago (Davies 2004). The mean differences for both plaque and gingivitis indices differ by 0.01 at the most. This is despite there being five more studies in our main plaque meta‐analysis, two more in our plaque severity meta‐analysis, six more in our main gingivitis meta‐analysis and two more in our gingivitis meta‐analysis. This adds to the certainty of the results for these outcomes. Another meta‐analysis reported statistically significant benefits in favour of triclosan/copolymer toothpastes for plaque and gingivitis, but it is difficult to compare results as the author reported standardised mean differences (Gunsolley 2006). However, neither of these studies included the outcomes periodontitis, caries, calculus or adverse effects, nor did they conduct a thorough risk of bias assessment enabling sensitivity analyses.
Authors' conclusions
Implications for practice.
This review presents moderate‐quality evidence that toothpastes containing triclosan/copolymer, in addition to fluoride, reduce plaque, gingival inflammation and gingival bleeding, when compared with fluoride toothpastes without triclosan/copolymer, and these reductions may or may not be clinically important. Such reductions are evident regardless of whether or not participants have an oral prophylaxis or not, and regardless of initial plaque and inflammation levels. There is high‐quality evidence that triclosan/copolymer toothpastes also lead to a small reduction in coronal caries. Weaker evidence shows that triclosan/copolymer toothpastes may reduce root caries and calculus, but there was insufficient evidence to show whether or not they prevent periodontitis. Such toothpastes also appear to have no adverse effects in studies up to three years' duration.
Implications for research.
The evidence of the beneficial effects of triclosan/copolymer toothpastes on plaque and gingivitis over six months and coronal caries over two to three years is clear. However, there was only one included study assessing the development of periodontitis, one study looking at reducing root caries and three studies assessing calculus accumulation. None of the included studies investigated participant‐centred outcomes. Therefore, well‐conducted randomised controlled trials are needed to investigate the long‐term (five years) effect of triclosan/copolymer toothpastes on these outcomes.
There were only three studies that appeared to be truly independent, with no involvement from toothpaste manufacturers. Further studies should be led by independent investigators without any direct influence from industry.
Any future studies should be randomised controlled trials and should be planned and carried out according to SPIRIT 2013 guidelines, and reported according to CONSORT 2010 guidelines. Trial protocols should be registered to reduce the risk of publication bias and duplication of effort.
What's new
| Date | Event | Description |
|---|---|---|
| 3 October 2019 | Review declared as stable | This Cochrane Review will not be updated given that as far as we are aware toothpaste containing triclosan is no longer commercially available as of early 2019. |
Notes
This Cochrane Review will not be updated given that as far as we are aware toothpaste containing triclosan is no longer commercially available as of early 2019.
Acknowledgements
We would like to thank: Luisa Fernandez Mauleffinch, Managing Editor of Cochrane Oral Health (COH), and Jo Leese, Editorial Support Co‐ordinator of COH, for managing the editorial process for the review; Anne Littlewood, Information Specialist of COH, for comments on the search strategy; and the editors of COH for their comments on the review. We would also like to thank John C Gunsolley for peer reviewing the protocol, and Derek Richards, Damien Walmsley and Kevin Seymour for peer reviewing the review.
Further thanks to:
Chunjie Li and Andreas Neudecker for translations; and
William Devizio for providing further information on Colgate‐affiliated studies.
Appendices
Appendix 1. MEDLINE (OVID) search strategy
1 exp Dentifrices/ 2 (toothpaste$ or "tooth paste$" or tooth‐paste$).mp. 3 dentifrice$.mp. 4 or/1‐3 5 Triclosan/ 6 triclosan.mp. 7 (Microban or "Colgate Total" or "Janina Diamond" or Irgasan or Biofresh or Lexol‐300 or Ster‐Zac or cloxifenolum).mp. 8 "diphenyl ether".mp. 9 or/5‐8 10 4 and 9
Appendix 2. Cochrane Oral Health's Trials Register search strategy
#1 ((toothpaste* or tooth‐paste* or "tooth paste*"):ti,ab) AND (INREGISTER) #2 (triclosan:ti,ab) AND (INREGISTER) #3 ((Microban or "Colgate Total" or "Janina Diamond" or Irgasan or Biofresh or Lexol‐300 or Ster‐Zac or cloxifenolum):ti,ab) AND (INREGISTER) #4 ("diphenyl ether":ti,ab) AND (INREGISTER) #5 (#2 or #3 or #4) AND (INREGISTER) #6 (#1 AND #5) AND (INREGISTER)
Appendix 3. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 [mh Dentifrices] #2 (toothpaste* or "tooth paste*" or tooth‐paste*) #3 dentifrice* #4 #1 or #2 or #3 #5 [mh ^Triclosan] #6 triclosan #7 (Microban or "Colgate Total" or "Janina Diamond" or Irgasan or Biofresh or Lexol‐300 or Ster‐Zac or cloxifenolum) #8 "diphenyl ether" #9 #5 or #6 or #7 or #8 #10 #4 and #9
Appendix 4. Embase (OVID) search strategy
1. exp Dentifrices/ 2. (toothpaste$ or "tooth paste$" or tooth‐paste$).mp. 3. dentifrice$.mp. 4. or/1‐3 5. Triclosan/ 6. triclosan.mp. 7. (Microban or "Colgate Total" or "Janina Diamond" or Irgasan or Biofresh or Lexol‐300 or Ster‐Zac or cloxifenolum).mp. 8. "diphenyl ether".mp. 9. or/5‐8 10. 4 and 9
Appendix 5. The US National Institutes of Health Trials Register (ClinicalTrials.gov) search strategy
triclosan AND toothpaste
Data and analyses
Comparison 1. Plaque.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Plaque at 6 to 7 months (Quigley‐Hein Plaque Index) | 20 | 2675 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.60, ‐0.34] |
| 1.1 Baseline prophylaxis | 16 | 2211 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.58, ‐0.30] |
| 1.2 No baseline prophylaxis | 4 | 464 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.82, ‐0.41] |
| 2 Plaque at 6 to 7 months (Plaque Severity Index) | 13 | 1850 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.20, ‐0.10] |
| 2.1 Baseline prophylaxis | 10 | 1496 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.18, ‐0.08] |
| 2.2 No baseline prophylaxis | 3 | 354 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.26, ‐0.14] |
| 3 Plaque at 6 to 7 months (Löe‐Silness Plaque Index) | 2 | 148 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.10, ‐0.01] |
Comparison 2. Gingivitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gingivitis at 6 to 9 months (Löe‐Silness Gingival Index) | 20 | 2743 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.33, ‐0.21] |
| 1.1 Baseline prophylaxis | 16 | 2279 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.34, ‐0.18] |
| 1.2 No baseline prophylaxis | 4 | 464 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.39, ‐0.21] |
| 2 Gingivitis at 6 to 7 months (Gingivitis Severity Index) | 15 | 1998 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.17, ‐0.08] |
| 2.1 Baseline prophylaxis | 12 | 1644 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.18, ‐0.07] |
| 2.2 No baseline prophylaxis | 3 | 354 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.27, ‐0.05] |
| 3 Gingivitis at 6 months (number of sites bleeding on probing or spontaneously) | 1 | 329 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐1.11, 1.39] |
Comparison 3. Periodontitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Periodontitis at 36 months (attachment loss > 0 mm) | 1 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.67, 1.27] |
Comparison 4. Caries.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caries increment at 30 to 36 months (DFT) | 3 | 6300 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.14, 0.02] |
| 1.1 Children (1100 ppm F, 0.243% NaF) | 1 | 3462 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.22, 0.12] |
| 1.2 Adults (1100 ppm F, 0.243% NaF) | 1 | 1542 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.17, 0.07] |
| 1.3 Adults (1500 ppm F, 0.331% NaF) | 1 | 1296 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.24, 0.06] |
| 2 Caries increment at 24 to 36 months (DFS) | 4 | 9692 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.31, ‐0.02] |
| 2.1 Children (1100 ppm F, 0.243% NaF) | 1 | 3462 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.36, 0.26] |
| 2.2 Adults (1100 ppm F, 0.243% NaF) | 2 | 4934 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.40, ‐0.02] |
| 2.3 Adults (1500 ppm F, 0.331% NaF) | 1 | 1296 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.55, 0.51] |
| 3 Root caries increment at 36 months (Katz Root Caries Index) | 1 | 1357 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.39, ‐0.23] |
Comparison 5. Calculus.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Calculus at 6 months (Volpe‐Manhold Calculus Index in mm ‐ mean total calculus per participant) | 2 | 415 | Mean Difference (IV, Fixed, 95% CI) | ‐2.12 [‐3.39, ‐0.84] |
| 2 Calculus at 7 months (Volpe‐Manhold Calculus Index in mm ‐ mean height of calculus) | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.21, 0.13] |
Comparison 6. Adverse effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Staining of teeth at 6 months (Meckel Stain Score) | 1 | 325 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.60, 0.30] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allen 2002.
| Methods | Trial design: parallel (3 arms) Location: "clinical facility", New Jersey, USA Number of centres: 1 Recruitment period: not reported Funding source: not reported but some authors associated with Colgate (the manufacturer of the triclosan/copolymer toothpaste) |
|
| Participants | Inclusion criteria: aged 18‐70 years; good general health; minimum 20 scorable teeth; mean baseline modified Quigley‐Hein Plaque Index score of 1.5 or more and mean baseline modified Löe‐Silness Gingival Index score of 1.0 or more Exclusion criteria: wearing orthodontic appliances; wearing removable prostheses; tumours of the soft or hard oral tissues; advanced periodontal disease; use of antibiotics during the 2 weeks before the study began Baseline plaque: (Quigley‐Hein Plaque Index) Gp A: mean 2.13 (SD 0.48); Gp B: mean 2.14 (SD 0.43); (Plaque Severity Index) Gp A: mean 0.34 (SD 0.18); Gp B: mean 0.34 (SD 0.18) Baseline gingivitis: (Löe‐Silness Gingival Index) Gp A: mean 1.38 (SD 0.27); Gp B: mean 1.35 (SD 0.24); (Gingivitis Severity Index) Gp A: mean 0.36 (SD 0.22); Gp B: mean 0.34 (SD 0.20) Baseline caries: not reported Age at baseline (years): Gp A: mean 40; Gp B: mean 43.5 (range not reported) Gender: Gp A: male 16 (22%), female 58 (78%); Gp B: male 6 (17%), female 30 (83%) Any other details of important prognostic factors: not reported Number randomised: 111 (Gp A: 74; Gp B: 37) Number evaluated: 110 (Gp A: 74; Gp B: 36) |
|
| Interventions |
Comparison: triclosan/copolymer/sodium fluoride (1)* versus triclosan/copolymer/sodium fluoride (2)* versus sodium fluoride * We combined (1) and (2) to form Gp A Gp A (n = 74): twice daily brushing for 1 minute with toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride; all participants received thorough baseline oral prophylaxis (removal of all supragingival plaque and calculus deposits), teeth were polished and erythrosin was used to confirm complete plaque removal; asked to refrain from any other oral hygiene procedures during the study period Gp B (n = 37): as above but without triclosan and copolymer Duration of treatment: 6 months |
|
| Outcomes | Plaque (Quigley‐Hein Plaque Index and Plaque Severity Index), gingivitis (Löe‐Silness Gingival Index and Gingivitis Severity Index), adverse effects; assessed at 3 and 6 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...were entered into the study, and stratified..." Comment: insufficient information on the method of sequence generation Additional information from correspondence: simple randomisation using random number tables |
| Allocation concealment (selection bias) | Low risk | Not mentioned Additional information from correspondence: a rigorous allocation procedure was carried out by people not involved in the study and we are satisfied that this was properly concealed from those involved in the study |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "The dentifrices were distributed in plain white tubes to ensure the double‐blind nature of the study" Comment: use of an identical control toothpaste meant that participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The dentifrices were distributed in plain white tubes to ensure the double‐blind nature of the study" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant, from the control group, did not complete the study. The reason for drop‐out was not reported Comment: we do not believe that this could pose a risk of bias |
| Selective reporting (reporting bias) | High risk | Appropriate outcome measures were considered but adverse effects were not reported in the results section |
| Other bias | Low risk | Quote: "The Kappa statistic for intra‐examiner reproducibility...was greater than 0.9, indicating a high level of agreement" Comment: we consider that the risk of differential diagnostic activity is low. We were unable to identify any other potential source of bias |
Bolden 1992.
| Methods | Trial design: parallel (2 arms) Location: Buffalo, New York, USA (type of setting not reported) Number of centres: 1 Recruitment period: not reported Funding source: not reported but some authors associated with Colgate (the manufacturer of the triclosan/copolymer toothpaste) |
|
| Participants | Inclusion criteria: healthy adults; mean baseline modified Quigley‐Hein Plaque Index score of 1.5 or more and mean baseline modified Löe‐Silness Gingival Index score of 1.0 or more Exclusion criteria: periodontitis at baseline (pocket depths more than 4 mm and alveolar bone loss determined by tooth mobility) Baseline plaque: (Quigley‐Hein Plaque Index) Gp A: mean 2.46 (SD 0.49); Gp B: mean 2.45 (SD 0.50); (Plaque Severity Index) Gp A: mean 0.337 (SD 0.130); Gp B: mean 0.346 (SD 0.140) Baseline gingivitis: (Löe‐Silness Gingival Index) Gp A: mean 1.41 (SD 0.22); Gp B: mean 1.43 (SD 0.23); (Gingivitis Severity Index) Gp A: mean 0.429 (SD 0.193); Gp B: mean 0.448 (SD 0.196) Baseline caries: not reported Age at baseline (years): Gp A: mean 32 (range 18‐62); Gp B: mean 32 (range 18‐61) Gender: Gp A: male 57 (37%), female 97 (63%); Gp B: male 65 (43%), female 87 (57%) Any other details of important prognostic factors: not reported Number randomised: 325 (not reported by group) Number evaluated: 306 (Gp A: 154; Gp B: 152) |
|
| Interventions |
Comparison: triclosan/copolymer/sodium fluoride versus sodium fluoride Gp A (n = 154 evaluated): twice daily brushing for 1 minute with toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride; all participants received thorough baseline oral prophylaxis (removal of all supragingival plaque and calculus deposits), teeth were polished and erythrosin was used to confirm complete plaque removal Gp B (n = 152 evaluated): as above but without triclosan and copolymer Duration of treatment: 6 months |
|
| Outcomes | Plaque (Quigley‐Hein Plaque Index and Plaque Severity Index), gingivitis (Löe‐Silness Gingival Index and Gingivitis Severity Index), adverse effects; assessed at 3 and 6 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: none observed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned" Comment: insufficient information on the method of sequence generation Additional information from correspondence: simple randomisation using random number tables |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomly assigned" Comment: not mentioned Additional information from correspondence: a rigorous allocation procedure was carried out by people not involved in the study and we are satisfied that this was properly concealed from those involved in the study |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "double‐blind" and "The dentifrices were distributed to the subjects in identical plain white tubes. The identity of the products remained unknown to the subjects and the dental examiners throughout the course of the study" Comment: use of an identical control toothpaste meant that participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" and "The dentifrices were distributed to the subjects in identical plain white tubes. The identity of the products remained unknown to the subjects and the dental examiners throughout the course of the study" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 6% of randomised participants were not included in the final analysis. Attrition was not reported by group and reasons were not given, but authors stated that reasons were not related to the use of either of the toothpastes Comment: we do not believe that any of the above could pose a risk of bias significant enough to have led to a distortion of the true intervention effect |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome measures were considered and reported in full, as described in the methods section |
| Other bias | Low risk | No mention of calibration of outcome assessors so it is unclear whether or not there was a risk of differential diagnostic activity Additional information from correspondence: this study followed a protocol whereby all outcome assessors were highly trained in the indices and procedures used, and inter‐ and intra‐examiner calibration occur where practical. Therefore, we consider that the risk of differential diagnostic activity is low. We were unable to identify any other potential source of bias |
Cubells 1991.
| Methods | Trial design: parallel (2 arms) Location: "clinical facility", Barcelona, Spain Number of centres: 1 Recruitment period: not reported Funding source: not reported but some authors associated with Colgate (the manufacturer of the triclosan/copolymer toothpaste) |
|
| Participants | Inclusion criteria: healthy adults; mean baseline modified Quigley‐Hein Plaque Index score of 1.5 or more and mean baseline modified Löe‐Silness Gingival Index score of 1.0 or more Exclusion criteria: not reported Baseline plaque: (Quigley‐Hein Plaque Index) Gp A: mean 2.842; Gp B: mean 2.857; (Plaque Severity Index) Gp A: mean 0.617 (SD 0.164); Gp B: mean 0.617 (SD 0.151) Baseline gingivitis: (Löe‐Silness Gingival Index) Gp A: mean 1.406; Gp B: mean 1.405; (Gingivitis Severity Index) Gp A: mean 0.368 (SD 0.172); Gp B: mean 0.373 (SD 0.171) Baseline caries: not reported Age at baseline (years): Gp A: mean 24.3 (range 18‐57); Gp B: mean 22.4 (range 18‐57) Gender: Gp A: male 22 (39%), female 34 (61%); Gp B: male 23 (44%), female 29 (56%) Any other details of important prognostic factors: not reported Number randomised: 120 (not reported by group) Number evaluated: 108 (Gp A: 56; Gp B: 52) |
|
| Interventions |
Comparison: triclosan/copolymer/sodium fluoride versus sodium fluoride Gp A (n = 56 evaluated): twice daily brushing for 1 minute with toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride; all participants received thorough baseline oral prophylaxis (removal of all subgingival and supragingival plaque and calculus deposits), teeth were polished and erythrosin was used to confirm complete plaque removal; participants had to visit the clinical facility every 4 weeks to exchange their used toothpaste tube and toothbrush for a new supply Gp B (n = 52 evaluated): as above but without triclosan and copolymer Duration of treatment: 6 months |
|
| Outcomes | Plaque (Quigley‐Hein Plaque Index and Plaque Severity Index), gingivitis (Löe‐Silness Gingival Index and Gingivitis Severity Index), adverse effects; assessed at 1.5 and 6 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: none observed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned" Comment: insufficient information on the method of sequence generation Additional information from correspondence: simple randomisation using random number tables |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomly assigned" Comment: not mentioned Additional information from correspondence: a rigorous allocation procedure was carried out by people not involved in the study and we are satisfied that this was properly concealed from those involved in the study |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "double‐blind" and "The dentifrices were distributed to the subjects in identical plain white tubes to ensure that neither the subjects nor the dental examiners knew the identity of the products" Comment: use of an identical control toothpaste meant that participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" and "The dentifrices were distributed to the subjects in identical plain white tubes to ensure that neither the subjects nor the dental examiners knew the identity of the products" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10% of randomised participants were not included in the final analysis. Attrition was not reported by group and reasons were not given, but authors stated that reasons were not related to the use of either of the toothpastes Comment: we do not believe that any of the above could pose a risk of bias significant enough to have led to a distortion of the true intervention effect |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome measures were considered and reported in full, as described in the methods section |
| Other bias | Low risk | Quote: "The intrarater reliability coefficient was found to be 0.85" Comment: we consider that the risk of differential diagnostic activity is low. We were unable to identify any other potential source of bias |
Deasy 1991.
| Methods | Trial design: parallel (2 arms) Location: New Jersey, USA (type of setting not reported) Number of centres: 1 Recruitment period: not reported Funding source: not reported but some authors associated with Colgate (the manufacturer of the triclosan/copolymer toothpaste) |
|
| Participants | Inclusion criteria: healthy adults; mean baseline modified Quigley‐Hein Plaque Index score of 1.5 or more and mean baseline modified Löe‐Silness Gingival Index score of 1.0 or more Exclusion criteria: severe periodontitis at baseline (pocket depths more than 5 mm and extensive alveolar bone loss determined by tooth mobility or gingival exudate); extensive dental caries; presence of oral pathology Baseline plaque: (Quigley‐Hein Plaque Index) Gp A: mean 1.79 (SD 0.36); Gp B: mean 1.75 (SD 0.35); (Plaque Severity Index) Gp A: mean 0.21 (SD 0.13); Gp B: mean 0.19 (SD 0.13) Baseline gingivitis: (Löe‐Silness Gingival Index) Gp A: mean 1.16 (SD 0.19); Gp B: mean 1.17 (SD 0.20); (Gingivitis Severity Index) Gp A: mean 0.26 (SD 0.16); Gp B: mean 0.24 (SD 0.14) Baseline caries: not reported Age at baseline (years): Gp A: mean 35.9 (range 18‐64); Gp B: mean 36.6 (range 18‐65) Gender: Gp A: male 11 (19%), female 47 (81%); Gp B: male 15 (24%), female 48 (76%) Any other details of important prognostic factors: not reported Number randomised: 139 (not reported by group) Number evaluated: 121 (Gp A: 58; Gp B: 63) |
|
| Interventions |
Comparison: triclosan/copolymer/sodium fluoride versus sodium fluoride Gp A (n = 58 evaluated): twice daily brushing for 1 minute with toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride; all participants received thorough baseline oral prophylaxis (removal of all subgingival and supragingival plaque and calculus deposits), teeth were polished and erythrosin was used to confirm complete plaque removal Gp B (n = 63 evaluated): as above but without triclosan and copolymer Duration of treatment: 6 months |
|
| Outcomes | Plaque (Quigley‐Hein Plaque Index and Plaque Severity Index), gingivitis (Löe‐Silness Gingival Index and Gingivitis Severity Index), adverse effects; assessed at 3 and 6 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: none observed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The subjects were stratified into two balanced groups" Comment: insufficient information on the method of sequence generation Additional information from correspondence: simple randomisation using random number tables |
| Allocation concealment (selection bias) | Low risk | Not mentioned Additional information from correspondence: a rigorous allocation procedure was carried out by people not involved in the study and we are satisfied that this was properly concealed from those involved in the study |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "double‐blind" and "placebo dentifrice" Comment: use of an identical control toothpaste meant that participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" and "placebo dentifrice" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 13% of randomised participants were not included in the final analysis. Attrition was not reported by group and reasons were not given, but authors stated that reasons were not related to the use of either of the toothpastes. However, if the missing participants had higher mean plaque/gingivitis scores in one group than the other, as the attrition rate increased, so would over/understatement of the mean difference |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome measures were considered and reported in full, as described in the methods section |
| Other bias | Low risk | No mention of calibration of outcome assessor so it is unclear whether or not there was a risk of differential diagnostic activity Additional information from correspondence: this study followed a protocol whereby all outcome assessors were highly trained in the indices and procedures used, and inter‐ and intra‐examiner calibration occur where practical. Therefore, we consider that the risk of differential diagnostic activity is low. We were unable to identify any other potential source of bias |
Denepitiya 1992.
| Methods | Trial design: parallel (2 arms) Location: New York, USA (type of setting not reported) Number of centres: 1 Recruitment period: not reported Funding source: not reported but some authors associated with Colgate (the manufacturer of the triclosan/copolymer toothpaste) |
|
| Participants | Inclusion criteria: healthy adults; minimum 20 natural uncrowned teeth; mean baseline modified Quigley‐Hein Plaque Index score of 1.5 or more and mean baseline modified Löe‐Silness Gingival Index score of 1.0 or more Exclusion criteria: not reported Baseline plaque: (Quigley‐Hein Plaque Index) Gp A: mean 2.25 (SD 0.41); Gp B: mean 2.24 (SD 0.42); (Plaque Severity Index) Gp A: mean 0.38 (SD 0.15); Gp B: mean 0.38 (SD 0.15) Baseline gingivitis: (Löe‐Silness Gingival Index) Gp A: mean 1.60 (SD 0.28); Gp B: mean 1.59 (SD 0.29); (Gingivitis Severity Index) Gp A: mean 0.58 (SD 0.14); Gp B: mean 0.57 (SD 0.14) Baseline caries: not reported Age at baseline (years): Gp A: mean 36 (range 18‐63); Gp B: mean 35 (range 20‐60) Gender: Gp A: male 29 (41%), female 41 (59%); Gp B: male 21 (28%), female 54 (72%) Any other details of important prognostic factors: not reported Number randomised: 159 (not reported by group) Number evaluated: 145 (Gp A: 70; Gp B: 75) |
|
| Interventions |
Comparison: triclosan/copolymer/sodium fluoride versus sodium fluoride Gp A (n = 70 evaluated): twice daily brushing for 1 minute with toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride; all participants received thorough baseline oral prophylaxis (removal of all supragingival plaque and calculus deposits), teeth were polished and erythrosin was used to confirm complete plaque removal; asked to refrain from any other oral hygiene procedures during the study period Gp B (n = 75 evaluated): as above but without triclosan and copolymer Duration of treatment: 6 months |
|
| Outcomes | Plaque (Quigley‐Hein Plaque Index and Plaque Severity Index), gingivitis (Löe‐Silness Gingival Index and Gingivitis Severity Index), adverse effects; assessed at 3 and 6 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: none observed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned" Comment: insufficient information on the method of sequence generation Additional information from correspondence: simple randomisation using random number tables |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomly assigned" Comment: not mentioned Additional information from correspondence: a rigorous allocation procedure was carried out by people not involved in the study and we are satisfied that this was properly concealed from those involved in the study |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "double‐blind" and "The dentifrices were distributed to the subjects in identical plain white tubes to ensure that neither the subjects nor the dental examiner knew the identity of the products" Comment: use of an identical control toothpaste meant that participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" and "The dentifrices were distributed to the subjects in identical plain white tubes to ensure that neither the subjects nor the dental examiner knew the identity of the products" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 9% of randomised participants were not included in the final analysis. Attrition was not reported by group and reasons were not given, but authors stated that reasons were not related to the use of either of the toothpastes Comment: we do not believe that any of the above could pose a risk of bias significant enough to have led to a distortion of the true intervention effect |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome measures were considered and reported in full, as described in the methods section |
| Other bias | Low risk | No mention of calibration of outcome assessor so it is unclear whether or not there was a risk of differential diagnostic activity Additional information from correspondence: this study followed a protocol whereby all outcome assessors were highly trained in the indices and procedures used, and inter‐ and intra‐examiner calibration occur where practical. Therefore, we consider that the risk of differential diagnostic activity is low. We were unable to identify any other potential source of bias |
Ellwood 1998.
| Methods | Trial design: parallel (2 arms) Location: 6 high schools, Manchester, UK Number of centres: 1 Recruitment period: October 1993 to March 1994 Funding source: not reported but some authors associated with Colgate (the manufacturer of the triclosan/copolymer toothpaste) |
|
| Participants | Inclusion criteria: 2nd year high school pupils Exclusion criteria: wearing fixed orthodontic appliances; recent history of systemic disease considered to be a cross‐infection control risk (e.g. tuberculosis) Baseline plaque (no named scale: 0 = no plaque visible; 1 = plaque only visible after drying teeth and wiping with explorer; 2 = plaque visible without drying teeth): Gp A: mean 1.34 (SD 0.55); Gp B: mean 1.34 (SD 0.52) Baseline gingivitis (sites bleeding on probing): Gp A: mean 0.25 (SD 0.19); Gp B: mean 0.25 (SD 0.18) Baseline caries: not reported Age at baseline (years): mean 12.7 (SD 0.33); range 11‐13 Gender: Gp A: male 48%, female 52%; Gp B: male 46%, female 54% Any other details of important prognostic factors: Indian, Pakistani or Bangladeshi ethnicity: Gp A: 36%; Gp B: 36% (overall: 63% European, 36% Asian, 1% African‐Caribbean). Authors stated population was specifically chosen from economically deprived areas in order to ensure a higher percentage of periodontitis‐susceptible participants (Asian and low socioeconomic status adolescents) Number randomised: 641 (Gp A: 328; Gp B: 313) Number evaluated: 480 (Gp A: 239; Gp B: 241) |
|
| Interventions |
Comparison: triclosan/copolymer/sodium fluoride versus sodium fluoride Gp A (n = 328): twice daily brushing with toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride (no baseline prophylaxes) Gp B (n = 313): as above but without triclosan and copolymer Duration of treatment: 36 months |
|
| Outcomes | Periodontitis (attachment loss), adverse effects; assessed at 18 and 36 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: none observed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...subjects were randomly allocated...stratified by school, ethnic group and gender" Comment: insufficient information on the method of sequence generation Additional information from correspondence: "computer generated random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "...subjects were randomly allocated" Comment: not mentioned Additional information from correspondence (with the trial statistician): "no‐one apart from me and independent people labelling the toothpaste knew which groups the participants had been allocated to" |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "double‐blind" and "The control dentifrice was identical apart from the exclusion of triclosan and copolymer" Comment: use of an identical control toothpaste meant that participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 25% of randomised participants were not included in the final analysis (Gp A: 27%; Gp B: 23%). Although reasons for attrition were clearly described and were balanced between groups, if the missing participants had a higher risk of periodontitis in one group than the other, as the attrition rate increased, so would over/understatement of the risk ratio (as periodontitis is reported as a dichotomous outcome in the study report) |
| Selective reporting (reporting bias) | High risk | The authors did not report plaque, gingivitis or calculus levels at follow‐up even though they were measured at baseline, and the report states that they were measured at both follow‐up points. Attachment loss was inadequately reported (i.e. not reported using the 5 categories stated in the 'Methods' section; only reported by percentage of participants with greater than 0 mm attachment loss, and 1 mm or more attachment loss). No results were reported for the 18‐month follow‐up |
| Other bias | Low risk | Quote: "Subjects were examined...by one trained and calibrated examiner" Comment: we consider that the risk of differential diagnostic activity is low. We were unable to identify any other potential source of bias |
Feller 1996.
| Methods | Trial design: parallel (3 arms) but only 2 arms were reported (authors stated that the unreported arm was an experimental toothpaste and that the results bore no impact on the comparison of the reported toothpastes) Location: dental clinic at the Loma Linda Veteran's Administration Hospital, California, USA Number of centres: 1 Recruitment period: not reported Funding source: not reported but some authors associated with Colgate (the manufacturer of the triclosan/copolymer toothpaste) |
|
| Participants | Inclusion criteria: healthy adults; minimum 16 natural permanent teeth; minimum 2 decayed or filled coronal surfaces or 2 areas of gingival recession or both; residing within 50 mile radius of the dental clinic Exclusion criteria: chronic systemic disease; orthodontic appliances involving more than 4 permanent teeth; any condition of the oral soft or hard tissues which the investigator felt would preclude their participation Baseline plaque: not reported Baseline gingivitis: not reported Baseline caries: (DFT) Gp A: mean 8.39 (SD 4.05); Gp B: mean 8.41 (SD 4.18); (DFS) Gp A: mean 15.9 (SD 9.71); Gp B: mean 15.85 (SD 9.61) Age at baseline (years): range 20‐70 Gender: not reported Any other details of important prognostic factors: naturally fluoridated water supply (0.6 ppm) Number randomised: 1636 (not reported by group); this total was not reported in Feller 1996 but in an abstract reporting the 26‐month results (abstract is linked to this study in the reference section) Number evaluated: 1542 (at 36‐month follow‐up) (Gp A: 786; Gp B: 756) |
|
| Interventions |
Comparison: triclosan/copolymer/sodium fluoride versus sodium fluoride versus unspecified Gp A (n = 786 evaluated): twice daily brushing for 1 minute with toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride (no baseline prophylaxes) Gp B (n = 756 evaluated): as above but without triclosan and copolymer Duration of treatment: 36 months |
|
| Outcomes | Caries (DFS and DFT mean increments), adverse effects; assessed at 18, 26 and 36 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: none observed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned" Comment: insufficient information on the method of sequence generation Additional information from correspondence: "random sequence generators were used" |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomly assigned" Comment: not mentioned Additional information from correspondence: a rigorous allocation procedure was carried out by people not involved in the study and we are satisfied that this was properly concealed from those involved in the study |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "double‐blind" and "Study dentifrices were provided to participants in plain, white tubes to preclude product information...neither the study subjects, the investigator, nor the clinical examiner being aware of the dentifrice assigned to each participant" Comment: use of an identical control toothpaste meant that participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" and "Study dentifrices were provided to participants in plain, white tubes to preclude product information...neither the study subjects, the investigator, nor the clinical examiner being aware of the dentifrice assigned to each participant" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 6% of randomised participants were not included in the final analysis. Attrition was not reported by group and the authors stated that reasons were not related to the use of either of the toothpastes, with the predominant reason being relocation away from the study area Comment: we do not believe that any of the above could pose a risk of bias significant enough to have led to a distortion of the true intervention effect |
| Selective reporting (reporting bias) | Low risk | The authors stated that there was a third arm in the study, but the toothpaste is not named or described, and results were not reported. The authors stated that this bore no impact on the comparison of the toothpastes described in this study. However, without any further information it is not possible to confirm this Additional information from correspondence: "the third arm was a non‐antibacterial formula purported to have anti‐caries activity. The product was being considered for commercialization at the time of the publication so the study was published in this manner to protect this intellectual property". We do not believe that this represents a risk of bias |
| Other bias | Low risk | Quote: "the dental examiner was recalibrated at yearly intervals throughout the study to confirm that a consistent and reproducible scoring procedure was being maintained" Comment: we consider that the risk of differential diagnostic activity is low. We were unable to identify any other potential source of bias |
Garcia‐Godoy 1990.
| Methods | Trial design: parallel (2 arms) Location: Santo Domingo, Dominican Republic (type of setting not reported) Number of centres: 1 Recruitment period: not reported Funding source: not reported but some authors associated with Colgate (the manufacturer of the triclosan/copolymer toothpaste) |
|
| Participants | Inclusion criteria: healthy adults; mean baseline modified Quigley‐Hein Plaque Index score of 1.5 or more and mean baseline modified Löe‐Silness Gingival Index score of 1.0 or more Exclusion criteria: not reported Baseline plaque: (Quigley‐Hein Plaque Index) Gp A: mean 2.49 (SD 0.42); Gp B: mean 2.45 (SD 0.39); (Plaque Severity Index) Gp A: mean 0.487 (SD 0.163); Gp B: mean 0.476 (SD 0.15) Baseline gingivitis: (Löe‐Silness Gingival Index) Gp A: mean 1.49 (SD 0.11); Gp B: mean 1.51 (SD 0.19); (Gingivitis Severity Index) Gp A: mean 0.479 (SD 0.108); Gp B: mean 0.485 (SD 0.14) Baseline caries: not reported Age at baseline (years): Gp A: mean 29.3 (range 18‐52); Gp B: mean 27.2 (range 18‐63) Gender: Gp A: male 17 (31%), female 37 (69%); Gp B: male 23 (43%), female 31 (57%) Any other details of important prognostic factors: not reported Number randomised: 120 (not reported by group) Number evaluated: 108 (Gp A: 54; Gp B: 54) |
|
| Interventions |
Comparison: triclosan/copolymer/sodium fluoride versus sodium fluoride Gp A (n = 54 evaluated): twice daily brushing for 1 minute with toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride; all participants received thorough baseline oral prophylaxis (removal of all subgingival and supragingival plaque and calculus deposits), teeth were polished and erythrosin was used to confirm complete plaque removal Gp B (n = 54 evaluated): as above but without triclosan and copolymer Duration of treatment: 7 months |
|
| Outcomes | Plaque (Quigley‐Hein Plaque Index and Plaque Severity Index), gingivitis (Löe‐Silness Gingival Index and Gingivitis Severity Index), adverse effects; assessed at 2.5, 5 and 7 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: none observed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned" Comment: insufficient information on the method of sequence generation Additional information from correspondence: simple randomisation using random number tables |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomly assigned" Comment: not mentioned Additional information from correspondence: a rigorous allocation procedure was carried out by people not involved in the study and we are satisfied that this was properly concealed from those involved in the study |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "double‐blind" and "The dentifrices were distributed to the subjects in identical plain white tubes to ensure that neither the subjects nor the dental examiner knew the identity of the dentifrices" Comment: use of an identical control toothpaste meant that participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" and "The dentifrices were distributed to the subjects in identical plain white tubes to ensure that neither the subjects nor the dental examiner knew the identity of the dentifrices" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10% of randomised participants were not included in the final analysis. Attrition was not reported by group and reasons were not given, but authors stated that reasons were not related to the use of either of the toothpastes Comment: we do not believe that any of the above could pose a risk of bias significant enough to have led to a distortion of the true intervention effect |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome measures were considered and reported in full, as described in the methods section |
| Other bias | Low risk | No mention of calibration of outcome assessor so it is unclear whether or not there was a risk of differential diagnostic activity Additional information from correspondence: this study followed a protocol whereby all outcome assessors were highly trained in the indices and procedures used, and inter‐ and intra‐examiner calibration occur where practical. Therefore, we consider that the risk of differential diagnostic activity is low. We were unable to identify any other potential source of bias |
Hawley 1995.
| Methods | Trial design: parallel (2 arms) Location: 45 high schools, Manchester, UK Number of centres: 1 Recruitment period: May to November 1990 Funding source: "This study was supported by Colgate Palmolive Technology Center, NJ, USA" |
|
| Participants | Inclusion criteria: 2nd year high school pupils Exclusion criteria: not reported Baseline plaque: not reported Baseline gingivitis: not reported Baseline caries: (DMFT) Gp A: mean 3.72 (SD 2.70); Gp B: mean 3.64 (SD 2.56); (DMFS) Gp A: mean 5.48 (SD 4.67); Gp B: mean 5.32 (SD 4.50) Age at baseline (years): mean 12.7 (SD 0.51); range 11‐13 Gender: not reported Any other details of important prognostic factors: low‐fluoride water supply Number randomised: 4060 (not reported by group) Number evaluated: 3462 (at 30‐month follow‐up) (Gp A: 1717; Gp B: 1745) |
|
| Interventions |
Comparison: triclosan/copolymer/sodium fluoride versus sodium fluoride Gp A (n = 1717 evaluated): brushing with toothpaste (frequency not reported, i.e. normal use) containing 0.3% triclosan, 2% copolymer, 0.24% sodium fluoride (no baseline prophylaxes) Gp B (n = 1745 evaluated): as above but without triclosan and copolymer Duration of treatment: 30 months |
|
| Outcomes | Caries (DFS and DFT mean increments), adverse effects; assessed at 15 and 30 months' follow‐up | |
| Notes | Sample size calculation: allowing for 15% attrition over 30 months, it was calculated that 4000 participants were required to have 80% power at a 5% significance level to detect a 10% difference between caries increments of the 2 groups. The required sample size was achieved Adverse effects: none observed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...children were randomly allocated..." Comment: insufficient information on the method of sequence generation Additional information from correspondence: "computer generated random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "...children were randomly allocated..." Comment: not mentioned Additional information from correspondence (with the trial statistician): "no‐one apart from me and independent people labelling the toothpaste knew which groups the participants had been allocated to" |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "double‐blind" Comment: participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 15% of randomised participants were not included in the final analysis (attrition was not reported by group but authors stated that rates were similar). Reasons for attrition were not described. If the missing participants had a higher mean caries increment in one group than the other, as the attrition rate increased, so would over/understatement of the mean difference |
| Selective reporting (reporting bias) | Low risk | For a study looking into anticaries effect, we consider that appropriate outcome measures were considered and reported in full |
| Other bias | Low risk | Quote: "Because of the size of the study sample two examiners...were involved. Training prior to the study and calibration during the examination periods ensured that both achieved and maintained similar levels of caries diagnosis" and "Throughout the study both examiners achieved the required standards for both agreement and reliability" Comment: we consider that the risk of differential diagnostic activity is low. We were unable to identify any other potential source of bias |
Hu 1997.
| Methods | Trial design: parallel (2 arms) Location: China Number of centres: 1 Recruitment period: not reported Funding source: not reported but some authors associated with Colgate (the manufacturer of the triclosan/copolymer toothpaste) |
|
| Participants | Inclusion criteria: not reported Exclusion criteria: not reported Baseline plaque: (Quigley‐Hein Plaque Index) Gp A: mean 3.65 (SD 0.333); Gp B: mean 3.5 (SD 0.314) Baseline gingivitis: (Löe‐Silness Gingival Index) Gp A: mean 1.49 (SD 0.342); Gp B: mean 1.49 (SD 0.321) Baseline caries: not reported Age at baseline (years): Gp A: mean 40.1; Gp B: mean 40.5 Gender: Gp A: male 36 (47%), female 41 (53%); Gp B: male 35 (46%), female 41 (54%) Any other details of important prognostic factors: not reported Number randomised: 153 (Gp A: 77; Gp B: 76) Number evaluated: 136 (Gp A: 69; Gp B: 67) |
|
| Interventions |
Comparison: triclosan/copolymer/sodium fluoride versus sodium fluoride Gp A (n = 77): twice daily brushing for 1 minute with toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride; all participants received thorough baseline oral prophylaxis (tooth scaling); asked to refrain from any other oral hygiene procedures during the study period Gp B (n = 76): as above but without triclosan and copolymer Duration of treatment: 6 months |
|
| Outcomes | Plaque (Quigley‐Hein Plaque Index), gingivitis (Löe‐Silness Gingival Index), adverse effects; assessed at 3 and 6 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: none observed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random" Comment: insufficient information on the method of sequence generation Additional information from correspondence: simple randomisation using random number tables |
| Allocation concealment (selection bias) | Low risk | Quote: "random" Comment: not mentioned Additional information from correspondence: a rigorous allocation procedure was carried out by people not involved in the study and we are satisfied that this was properly concealed from those involved in the study |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "double‐blind" Comment: participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11% of randomised participants were not included in the final analysis (Gp A: 10%; Gp B: 12%). Reasons for attrition were not given, but authors stated that reasons were not related to the use of either of the toothpastes. Comment: as attrition was almost equal between groups, we do not believe that any of the above could pose a risk of bias significant enough to have led to a distortion of the true intervention effect |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome measures were considered and reported in full, as described in the methods section |
| Other bias | Low risk | No mention of calibration of outcome assessor so it is unclear whether or not there was a risk of differential diagnostic activity Additional information from correspondence: this study followed a protocol whereby all outcome assessors were highly trained in the indices and procedures used, and inter‐ and intra‐examiner calibration occur where practical. Therefore, we consider that the risk of differential diagnostic activity is low. We were unable to identify any other potential source of bias |
Kanchanakamol 1995.
| Methods | Trial design: parallel (2 arms) Location: Chiang Mai, Thailand (type of setting not reported) Number of centres: 1 Recruitment period: not reported Funding source: research grant from Colgate Palmolive |
|
| Participants | Inclusion criteria: healthy adults; minimum 20 natural uncrowned teeth; mean baseline modified Quigley‐Hein Plaque Index score of 1.5 or more and mean baseline modified Löe‐Silness Gingival Index score of 1.0 or more Exclusion criteria: orthodontic bands or partial removable dentures; more than 5 carious lesions requiring immediate restorative treatment; advanced periodontitis; use of antibiotics or antiseptics during the month before the study began; pregnant or breastfeeding Baseline plaque: (Quigley‐Hein Plaque Index) Gp A: mean 3.47 (SD 0.5); Gp B: mean 3.55 (SD 0.47); (Plaque Severity Index) Gp A: mean 0.54 (SD 0.1); Gp B: mean 0.53 (SD 0.09) Baseline gingivitis: (Löe‐Silness Gingival Index) Gp A: mean 1.34 (SD 0.21); Gp B: mean 1.34 (SD 0.19); (Gingivitis Severity Index) Gp A: mean 0.14 (SD 0.07); Gp B: mean 0.12 (SD 0.06) Baseline caries: not reported Age at baseline (years): Gp A: mean 35.7 (range 18‐53); Gp B: mean 35.6 (range 18‐55) Gender: Gp A: male 14 (23%), female 48 (77%); Gp B: male 15 (24%), female 47 (76%) Any other details of important prognostic factors: authors stated that the study population had lower educational and socioeconomic status than participants involved in the triclosan/copolymer studies in western countries, possibly had inferior brushing technique, used a limited amount of toothpaste, and in general do not floss Number randomised: 140 (not reported by group) Number evaluated: 124 (Gp A: 62; Gp B: 62) |
|
| Interventions |
Comparison: triclosan/copolymer/sodium fluoride versus usual oral hygiene procedure Gp A (n = 62 evaluated): twice daily brushing for 1 minute with toothpaste containing 0.3% triclosan, 2% copolymer, 0.221% sodium fluoride; all participants received baseline complete dental scaling and prophylaxis of the entire dentition; asked to refrain from using other oral hygiene products during the study period Gp B (n = 62 evaluated): usual oral hygiene procedure; asked to refrain from using toothpaste containing triclosan; same baseline prophylaxes as Gp A Duration of treatment: 6 months |
|
| Outcomes | Plaque (Quigley‐Hein Plaque Index and Plaque Severity Index), gingivitis (Löe‐Silness Gingival Index and Gingivitis Severity Index); assessed at 3 and 6 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned" Comment: insufficient information on the method of sequence generation Additional information from correspondence: simple randomisation using random number tables |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomly assigned" Comment: not mentioned Additional information from correspondence: a rigorous allocation procedure was carried out by people not involved in the study and we are satisfied that this was properly concealed from those involved in the study |
| Blinding of participants (performance bias) All outcomes | High risk | Quote: "single‐blind" Comment: participants were either assigned to a specific toothpaste or asked to continue their usual oral hygiene procedures and, therefore, knew which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "single‐blind" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11% of randomised participants were not included in the final analysis. Attrition was not reported by group and reasons were not given, but authors stated that reasons were not related to the use of either of the toothpastes. However, if the missing participants had higher mean plaque/gingivitis scores in one group than the other, as the attrition rate increased, so would over/understatement of the mean difference |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome measures were considered and reported in full, as described in the methods section |
| Other bias | Low risk | No mention of calibration of outcome assessor so it is unclear whether or not there was a risk of differential diagnostic activity Additional information from correspondence: this study followed a protocol whereby all outcome assessors were highly trained in the indices and procedures used, and inter‐ and intra‐examiner calibration occur where practical. Therefore, we consider that the risk of differential diagnostic activity is low. We were unable to identify any other potential source of bias |
Kraivaphan 2006.
| Methods | Trial design: parallel (2 arms) Location: antenatal care unit, Taksin Hospital, Bangkok, Thailand Number of centres: 1 Recruitment period: not reported Funding source: not reported |
|
| Participants | Inclusion criteria: healthy pregnant women (3 months' gestation); mean baseline modified Löe‐Silness Gingival Index score of 1.0 or more Exclusion criteria: not reported Baseline plaque: not reported Baseline gingivitis: (Löe‐Silness Gingival Index) Gp A: mean 1.778 (SD 0.432); Gp B: mean 1.797 (SD 0.432) Baseline caries: not reported Age at baseline (years): Gp A: mean 27 (range 19‐37); Gp B: mean 26 (range 19‐40) Gender: not applicable Any other details of important prognostic factors: it is important to stress that the main factor differentiating this study population from others included in the review is that they were pregnant women Number randomised: 140 (not reported by group) Number evaluated: 120 (Gp A: 60; Gp B: 60) |
|
| Interventions |
Comparison: triclosan/copolymer (sodium fluoride not stated) versus placebo (sodium fluoride not stated) Gp A (n = 60 evaluated): twice daily brushing for 1 minute with toothpaste containing 0.3% triclosan, 2% copolymer; all participants received thorough baseline oral prophylaxis (removal of all subgingival and supragingival plaque and calculus deposits) Gp B (n = 60 evaluated): as above but with placebo toothpaste Duration of treatment: 9 months (including 3 months' postpartum use) |
|
| Outcomes | Gingivitis (Löe‐Silness Gingival Index); assessed at 3, 5 and 9 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomly assigned" Comment: insufficient information on the method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...randomly assigned" Comment: not mentioned |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "double‐blind" Comment: participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 14% of randomised participants were not included in the final analysis. Attrition was not reported by group and reasons were not given, but authors stated that reasons were not related to the use of either of the toothpastes. However, if the missing participants had higher mean gingivitis scores in one group than the other, as the attrition rate increased, so would over/understatement of the mean difference |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome measure considered and reported in full, as described in the methods section |
| Other bias | Unclear risk | No mention of calibration of outcome assessor so it is unclear whether or not there was a risk of differential diagnostic activity |
Lindhe 1993.
| Methods | Trial design: parallel (2 arms) Location: not reported Number of centres: 1 Recruitment period: not reported Funding source: not reported but some authors associated with Colgate (the manufacturer of the triclosan/copolymer toothpaste) |
|
| Participants | Inclusion criteria: unremarkable medical history; minimum 20 natural permanent teeth; moderate gingivitis and plaque accumulation Exclusion criteria: use of antibiotics during the 6 months before the study began Baseline plaque: (Quigley‐Hein Plaque Index) Gp A: mean 2.1; Gp B: mean 2.2 Baseline gingivitis: (Löe‐Silness Gingival Index) Gp A: mean 1.5; Gp B: mean 1.6 Baseline caries: not reported Age at baseline: not reported Gender: not reported Any other details of important prognostic factors: not reported Number randomised: 120 (Gp A: 60; Gp B: 60) Number evaluated: 110 (Gp A: 56; Gp B: 54) |
|
| Interventions |
Comparison: triclosan/copolymer/sodium fluoride versus sodium fluoride Gp A (n = 60): twice daily brushing for 1 minute with toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride (no baseline prophylaxes); use of interdental cleaning devices was not advocated Gp B (n = 60): as above but without triclosan and copolymer Duration of treatment: 6 months |
|
| Outcomes | Plaque (Quigley‐Hein Plaque Index), gingivitis (Löe‐Silness Gingival Index); assessed at 1.5, 3 and 6 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned" Comment: insufficient information on the method of sequence generation Additional information from correspondence: simple randomisation using random number tables |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomly assigned" Comment: not mentioned Additional information from correspondence: a rigorous allocation procedure was carried out by people not involved in the study and we are satisfied that this was properly concealed from those involved in the study |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "The dentifrices were delivered in identical plain white tubes and cartons as to ensure that neither the examiner nor the subjects were aware of the identity of the product" Comment: participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The dentifrices were delivered in identical plain white tubes and cartons as to ensure that neither the examiner nor the subjects were aware of the identity of the product" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 8% of randomised participants were not included in the final analysis (Gp A: 7%; Gp B: 10%) but reasons for attrition were not given Comment: we do not believe that any of the above could pose a risk of bias significant enough to have led to a distortion of the true intervention effect |
| Selective reporting (reporting bias) | High risk | The mean plaque and gingivitis scores reported in the study text does not accurately match the graphs (figures 1 and 5) and information on the variance of these mean scores was only reported visually as 95% confidence interval bars in the graphs. Therefore, we estimated the mean scores from the graphs along with the 95% confidence intervals. We then used this information to calculate the SDs of the mean scores in order to be able to include the data in the meta‐analyses |
| Other bias | Low risk | No mention of calibration of outcome assessors so it is unclear whether or not there was a risk of differential diagnostic activity Additional information from correspondence: this study followed a protocol whereby all outcome assessors were highly trained in the indices and procedures used, and inter‐ and intra‐examiner calibration occur where practical. Therefore, we consider that the risk of differential diagnostic activity is low. We were unable to identify any other potential source of bias |
Liu 2002.
| Methods | Trial design: parallel (3 arms) Location: USA Number of centres: 1 Recruitment period: not reported Funding source: "This research was supported by The Procter & Gamble Company" (the manufacturer of the sodium hexametaphosphate toothpaste) |
|
| Participants | Inclusion criteria: healthy adults; minimum 16 natural teeth (including minimum of 5 of the 6 lower anterior teeth) Exclusion criteria: wearing fixed orthodontic appliances; using chlorhexidine or anything else that might affect the ability to measure calculus accumulation Baseline plaque: not reported Baseline gingivitis: not reported Baseline caries: not reported Age at baseline (years): Gp A: mean 46.3 (range 18‐81); Gp B: mean 46.3 (range 20‐77) Gender: Gp A: male 71 (39%), female 113 (61%); Gp B: male 70 (38%), female 112 (62%) Any other details of important prognostic factors: baseline calculus (Volpe‐Manhold Calculus Index ‐ mean total calculus per participant): Gp A: mean 19.33 mm; Gp B: mean 18.92 mm Number randomised: 366 (Gp A: 184; Gp B: 182) Number evaluated: 345 (Gp A: 174; Gp B: 171) |
|
| Interventions |
Comparison: sodium hexametaphosphate* versus triclosan/copolymer/sodium fluoride versus sodium fluoride *We excluded this arm from our data extraction, risk of bias assessment and analyses Gp A (n = 184): twice daily brushing for 1 minute with toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride; all participants received a baseline "dental prophylaxis" Gp B (n = 182): as above but without triclosan and copolymer Duration of treatment: 6 months |
|
| Outcomes | Calculus (Volpe‐Manhold Calculus Index), adverse effects (oral soft tissue tolerance); assessed at 3 and 6 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: in all 3 arms of the study there were 162 reported oral soft tissue adverse events (involving 133 participants). Only 59 (36%) of these events were considered as potentially related to product use. All events were classified as mild apart from 1 event in the sodium hexametaphosphate arm. The authors stated that none of the events were related to attrition rates |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomized to one of the treatment groups" Comment: insufficient information on the method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...all study dentifrices were over‐packaged in identical test kits...Both the test products and test kits were uniquely labelled to preclude identification of either treatment assignment or study group" Comment: it appears that the authors consider allocation of the random sequence to be concealed, but it is not clear if anybody involved in the study controlled this process, or if it was done remotely. Also, the sodium hexametaphosphate kit was heavier, although it is unclear if this was detectable |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "examiner blind" and "The control dentifrices were supplied in identical white foil laminate 6.4 ounce tubes and the experimental dentifrice in 5.2 ounce pumps. To assure blinding, all study dentifrices were over‐packaged in identical test kits...Both the test products and test kits were uniquely labelled to preclude identification of either treatment assignment or study group" Comment: as we have excluded the sodium hexametaphosphate arm, the use of an identical toothpaste in the remaining arms (the 2 included in this review) meant that participants did not know which group they were assigned to, and this study can be considered double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "examiner blind" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 6% of randomised participants were not included in the final analysis (Gp A: 5%; Gp B: 6%). Reasons for attrition were not given, but authors stated that reasons were not related to the use of any of the toothpastes Comment: we do not believe that any of the above could pose a risk of bias significant enough to have led to a distortion of the true intervention effect |
| Selective reporting (reporting bias) | High risk | For a study looking into anticalculus effect, we consider that an appropriate outcome measure was considered and reported in full. However, adverse effects were observed yet they were not reported in a way that would allow us to include the data in a meta‐analysis |
| Other bias | Low risk | Quote: "Calculus measurement repeatability for the examiner had been established in a previous study, wherein triplicate examinations of 26 subjects yielded an intraclass correlation estimate of 0.98" Comment: we consider that the risk of differential diagnostic activity was low. We were unable to identify any other potential source of bias |
Lobene 1991.
| Methods | Trial design: parallel (2 arms) Location: New Jersey, USA (type of setting not reported) Number of centres: 1 Recruitment period: not reported Funding source: not reported but some authors associated with Colgate (the manufacturer of the triclosan/copolymer toothpaste) |
|
| Participants | Inclusion criteria: history of supragingival calculus formation (identified by participation in a pretest study) Exclusion criteria: not reported Baseline plaque: not reported Baseline gingivitis: not reported Baseline caries: not reported Age at baseline (years): Gp A: mean 48.3 (range 37‐63); Gp B: mean 43.9 (range 22‐65) Gender: Gp A: male 9 (24%), female 28 (76%); Gp B: male 7 (21%), female 26 (79%) Any other details of important prognostic factors: baseline calculus (Volpe‐Manhold Calculus Index ‐ mean total calculus per participant): Gp A: mean 14.67 mm; Gp B: mean 13.45 mm Number randomised: 84 (not reported by group) Number evaluated: 70 (Gp A: 37; Gp B: 33) |
|
| Interventions |
Comparison: triclosan/copolymer/sodium fluoride versus sodium fluoride Gp A (n = 37 evaluated): twice daily brushing for 1 minute with toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride; all participants received a baseline "oral prophylaxis" Gp B (n = 33 evaluated): as above but without triclosan and copolymer Duration of treatment: 6 months |
|
| Outcomes | Calculus (Volpe‐Manhold Calculus Index), adverse effects; assessed at 3 and 6 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: none observed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The subjects were stratified into two balanced groups" Comment: insufficient information on the method of sequence generation Additional information from correspondence: simple randomisation using random number tables |
| Allocation concealment (selection bias) | Low risk | Not mentioned Additional information from correspondence: a rigorous allocation procedure was carried out by people not involved in the study and we are satisfied that this was properly concealed from those involved in the study |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "double‐blind" and "The dentifrices were packaged in identical plain white tubes so that neither the subjects nor the dental examiner knew the identity of the dentifrices throughout the study" Comment: use of an identical control toothpaste meant that participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" and "The dentifrices were packaged in identical plain white tubes so that neither the subjects nor the dental examiner knew the identity of the dentifrices throughout the study" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 17% of randomised participants were not included in the final analysis. Attrition was not reported by group and reasons were not given, but authors stated that reasons were not related to the use of either of the toothpastes. However, if the missing participants had higher mean calculus scores in one group than the other, as the attrition rate increased, so would over/understatement of the mean difference |
| Selective reporting (reporting bias) | Low risk | For a study looking into anticalculus effect, we consider that an appropriate outcome measure was considered and reported in full |
| Other bias | Low risk | No mention of calibration of outcome assessor so it is unclear whether or not there was a risk of differential diagnostic activity Additional information from correspondence: this study followed a protocol whereby all outcome assessors were highly trained in the indices and procedures used, and inter‐ and intra‐examiner calibration occur where practical. Therefore, we consider that the risk of differential diagnostic activity is low. We were unable to identify any other potential source of bias |
Mankodi 1992.
| Methods | Trial design: parallel (2 arms) Location: Besselaar Clinical Research Unit, West Palm Beach, Florida, USA Number of centres: 1 Recruitment period: not reported Funding source: "...with support from the Colgate Palmolive Company" |
|
| Participants | Inclusion criteria: healthy subjects; mean baseline modified Quigley‐Hein Plaque Index score of 1.5 or more and mean baseline modified Löe‐Silness Gingival Index score of 1.0 or more Exclusion criteria: not reported Baseline plaque: (Quigley‐Hein Plaque Index) Gp A: mean 2.46 (SD 0.39); Gp B: mean 2.43 (SD 0.35); (Plaque Severity Index) Gp A: mean 0.254 (SD 0.146); Gp B: mean 0.243 (SD 0.136) Baseline gingivitis: (Löe‐Silness Gingival Index) Gp A: mean 1.29 (SD 0.18); Gp B: mean 1.29 (SD 0.16); (Gingivitis Severity Index) Gp A: mean 0.295 (SD 0.179); Gp B: mean 0.296 (SD 0.157) Baseline caries: not reported Age at baseline (years): Gp A: mean 36 (range 18‐64); Gp B: mean 37 (range 18‐63) Gender: Gp A: male 46 (32%), female 99 (68%); Gp B: male 39 (26%), female 110 (74%) Any other details of important prognostic factors: not reported Number randomised: 318 (not reported by group) Number evaluated: 294 (Gp A: 145; Gp B: 149) |
|
| Interventions |
Comparison: triclosan/copolymer/sodium fluoride versus sodium fluoride Gp A (n = 145 evaluated): twice daily brushing for 1 minute with toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride; all participants received thorough baseline oral prophylaxis (removal of all supragingival plaque and calculus deposits), teeth were polished and erythrosin was used to confirm complete plaque removal Gp B (n = 149 evaluated): as above but without triclosan and copolymer Duration of treatment: 6 months |
|
| Outcomes | Plaque (Quigley‐Hein Plaque Index and Plaque Severity Index), gingivitis (Löe‐Silness Gingival Index and Gingivitis Severity Index), adverse effects; assessed at 3 and 6 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: none observed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...were entered into the study and stratified...into two balanced treatment groups" Comment: insufficient information on the method of sequence generation Additional information from correspondence: simple randomisation using random number tables |
| Allocation concealment (selection bias) | Low risk | Not mentioned Additional information from correspondence: a rigorous allocation procedure was carried out by people not involved in the study and we are satisfied that this was properly concealed from those involved in the study |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "double‐blind" and "The two treatment dentifrices were distributed in identical plain white tubes to ensure that neither the subject nor the examiner knew the identity of the treatment" Comment: use of an identical control toothpaste meant that participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" and "The two treatment dentifrices were distributed in identical plain white tubes to ensure that neither the subject nor the examiner knew the identity of the treatment" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 8% of randomised participants were not included in the final analysis. Attrition was not reported by group and reasons were not given, but authors stated that reasons were not related to the use of either of the toothpastes Comment: we do not believe that any of the above could pose a risk of bias significant enough to have led to a distortion of the true intervention effect |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome measures were considered and reported in full, as described in the methods section |
| Other bias | Low risk | Quote: "...evaluated by calibrated dental examiners" Comment: we consider that the risk of differential diagnostic activity was low. We were unable to identify any other potential source of bias |
Mankodi 2011.
| Methods | Trial design: parallel (2 arms) Location: "clinical facility" (Dental Products Testing), West Palm Beach, Florida, USA Number of centres: 1 Recruitment period: not reported Funding source: "The study was supported by the Colgate‐Palmolive Company" |
|
| Participants | Inclusion criteria: healthy adults; minimum 20 uncrowned permanent natural teeth (excluding third molars); mean baseline modified Quigley‐Hein Plaque Index score of 1.5 or more and mean baseline Löe‐Silness Gingival Index score of 1.0 or more Exclusion criteria: wearing orthodontic appliances; wearing partial removable prostheses; tumours of the oral soft or hard tissues; advanced periodontal disease (purulent exudates, tooth mobility, extensive periodontal attachment loss or alveolar bone loss, or a combination of these); 5 or more carious lesions requiring immediate restorative treatment; history of allergy to personal care/consumer products or their ingredients; any medical condition precluding participants from not eating and drinking for periods up to 4 hours; use of any prescription medication that might interfere with the study outcomes; pregnant or lactating women; use of antibiotics during the 1 month before the study began; participation in any other clinical study or test panel during the 1 month before the study began; received a dental prophylaxis during the 2 weeks before the study began Baseline plaque: (Quigley‐Hein Plaque Index) Gp A: mean 2.46 (SD 0.44); Gp B: mean 2.26 (SD 0.46); (Plaque Severity Index) Gp A: mean 0.37 (SD 0.28); Gp B: mean 0.29 (SD 0.26) Baseline gingivitis: (Löe‐Silness Gingival Index) Gp A: mean 1.1 (SD 0.09); Gp B: mean 1.1 (SD 0.09); (Gingivitis Severity Index) Gp A: mean 0.12 (SD 0.1); Gp B: mean 0.12 (SD 0.09) Baseline caries: not reported Age at baseline (years): Gp A: mean 38.9 (range 20‐60); Gp B: mean 43.6 (range 19‐68) Gender: Gp A: male 17 (30%), female 40 (70%); Gp B: male 17 (29%), female 41 (71%) Any other details of important prognostic factors: not reported Number randomised: 125 (not reported by group) Number evaluated: 115 (Gp A: 57; Gp B: 58) |
|
| Interventions |
Comparison: triclosan/copolymer/sodium fluoride versus sodium fluoride Gp A (n = 57 evaluated): twice daily brushing for 1 minute with toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride (no baseline prophylaxes); patients asked to refrain from all oral hygiene procedures for at least 12 hours and from eating, drinking or smoking for 4 hours before their baseline examination); asked to refrain from any other oral hygiene procedures during the study period Gp B (n = 58 evaluated): as above but without triclosan and copolymer Duration of treatment: 6 months |
|
| Outcomes | Plaque (Quigley‐Hein Plaque Index and Plaque Severity Index), gingivitis (Löe‐Silness Gingival Index and Gingivitis Severity Index), adverse effects; assessed at 3 and 6 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: none observed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned" Comment: insufficient information on the method of sequence generation Additional information from correspondence: simple randomisation using random number tables |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomly assigned" Comment: not mentioned Additional information from correspondence: a rigorous allocation procedure was carried out by people not involved in the study and we are satisfied that this was properly concealed from those involved in the study |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "double‐blind" and "Both of the dentifrice products were supplied in their original packaging and over‐wrapped with a white label to mask the product's identity" Comment: participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 8% of randomised participants were not included in the final analysis. Attrition was not reported by group and reasons were not given, but authors stated that reasons were not related to the use of either of the toothpastes Comment: we do not believe that any of the above could pose a risk of bias significant enough to have led to a distortion of the true intervention effect |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome measures were considered and reported in full, as described in the methods section |
| Other bias | Unclear risk | There were statistically significant differences between groups at baseline for both mean Quigley‐Hein Plaque Index score (in favour of the control group, i.e. a lower score) and mean age. This could indicate that there was a problem with the randomisation process and may have led to a bias towards the null (in terms of the mean difference in Quigley‐Hein Plaque Index score at 6 months' follow‐up, which was statistically significant in favour of the test group) |
Mann 1996.
| Methods | Trial design: parallel (3 arms) but only 2 arms were reported (authors stated that the unreported arm was an experimental toothpaste and that the results bore no impact on the comparison of the reported toothpastes) Location: "clinical dental facility", Kiryat Gat, Israel Number of centres: 1 Recruitment period: not reported Funding source: not reported but some authors associated with Colgate (the manufacturer of the triclosan/copolymer toothpaste) |
|
| Participants | Inclusion criteria: healthy adults; minimum 16 natural permanent teeth; minimum 2 decayed or filled coronal surfaces; residing within 50 mile radius of the dental clinic Exclusion criteria: chronic systemic disease; orthodontic appliances involving more than 4 permanent teeth; any condition of the oral soft or hard tissues that the investigator felt would preclude their participation Baseline plaque: not reported Baseline gingivitis: not reported Baseline caries: (DFT) Gp A: mean 6.95 (SD 4.15); Gp B: mean 7.03 (SD 3.95); (DFS) Gp A: mean 12.22 (SD 9.40); Gp B: mean 12.26 (SD 8.96) Age at baseline (years): range 20‐70 Gender: not reported Any other details of important prognostic factors: authors stated population was specifically chosen partly due to high caries prevalence; suboptimally fluoridated water supply (less than 0.3 ppm) Number randomised: not reported Number evaluated: 1296 (Gp A: 657; Gp B: 639) |
|
| Interventions |
Comparison: triclosan/copolymer/sodium fluoride versus sodium fluoride versus unspecified Gp A (n = 657 evaluated): twice daily brushing for 1 minute with toothpaste containing 0.3% triclosan, 2% copolymer, 0.331% sodium fluoride (no baseline prophylaxes) Gp B (n = 639 evaluated): as above but without triclosan and copolymer Duration of treatment: 36 months |
|
| Outcomes | Caries (DFS and DFT mean increments), adverse effects; assessed at 18, 26 and 36 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: none observed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned" Comment: insufficient information on the method of sequence generation Additional information from correspondence: "random sequence generators were used" |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomly assigned" Comment: not mentioned Additional information from correspondence: a rigorous allocation procedure was carried out by people not involved in the study and we are satisfied that this was properly concealed from those involved in the study |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "Study dentifrices were provided to participants in plain white tubes to preclude product identification. Thus, the study was conducted in a double‐blind manner, with neither the study subjects, the investigator, nor the clinical examiner being aware of the dentifrice assigned to each participant" Comment: use of an identical control toothpaste meant that participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Study dentifrices were provided to participants in plain white tubes to preclude product identification. Thus, the study was conducted in a double‐blind manner, with neither the study subjects, the investigator, nor the clinical examiner being aware of the dentifrice assigned to each participant" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors did not report the initial number of participants randomised; they only reported the number analysed. Attrition was not reported by group and the authors stated that reasons were not related to the use of either of the toothpastes, with the predominant reason being relocation away from the study area |
| Selective reporting (reporting bias) | Low risk | The authors stated that there was a third arm in the study, but the toothpaste is not named or described, and results were not reported. The authors stated that this bore no impact on the comparison of the toothpastes described in this study. However, without any further information it is not possible to confirm this Additional information from correspondence: "the third arm was a non‐antibacterial formula purported to have anti‐caries activity. The product was being considered for commercialization at the time of the publication so the study was published in this manner to protect this intellectual property". We do not believe that this represents a risk of bias |
| Other bias | Low risk | Quote: "the dental examiner was recalibrated at yearly intervals throughout the study to confirm that a consistent and reproducible scoring procedure was being maintained" Comment: we consider that the risk of differential diagnostic activity was low. We were unable to identify any other potential source of bias |
Mann 2001.
| Methods | Trial design: parallel (2 arms) Location: 38 settlement communities throughout Israel (type of setting not reported) Number of centres: not reported (but presumably multicentre) Recruitment period: not reported Funding source: not reported but some authors associated with Colgate (the manufacturer of the triclosan/copolymer toothpaste); "sponsor" is mentioned in the 'Materials and Methods' section but with no further information |
|
| Participants | Inclusion criteria: minimum 5 decayed or filled coronal surfaces; minimum 14 natural uncrowned teeth (excluding third molars) Exclusion criteria: orthodontic appliances involving more than 4 permanent teeth; participation in any other clinical study or test panel during the 3 months before the study began; any condition that the investigator felt would preclude their participation Baseline plaque: not reported Baseline gingivitis: not reported Baseline caries: (DFS) Gp A: mean 21.96 (SD 11.50); Gp B: mean 21.49 (SD 11.15) Age at baseline (years): Gp A: mean 45.37 (range 20‐70); Gp B: mean 45.67 (range 21‐70) Gender: Gp A: male 733 (43%), female 978 (57%); Gp B: male 754 (45%), female 927 (55%) Any other details of important prognostic factors: not reported Number randomised: not reported Number evaluated: 3392 (Gp A: 1711; Gp B: 1681) |
|
| Interventions |
Comparison: triclosan/copolymer/sodium fluoride versus sodium fluoride Gp A (n = 1711 evaluated): twice daily brushing with toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride (no baseline prophylaxes); all participants received instruction in good oral hygiene procedures (brushing technique) from dental professionals, plus pamphlets supplied by the sponsor, plus annual mailings emphasising good oral hygiene and the importance of compliance with the study Gp B (n = 1681 evaluated): as above but without triclosan and copolymer Duration of treatment: 24 months |
|
| Outcomes | Caries (DFS mean increments), adverse effects; assessed at 12 and 24 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: none observed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned" Comment: insufficient information on the method of sequence generation Additional information from correspondence: "random sequence generators were used" |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomly assigned" Comment: not mentioned Additional information from correspondence: a rigorous allocation procedure was carried out by people not involved in the study and we are satisfied that this was properly concealed from those involved in the study |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "double‐blind" and "Dentifrice tubes were covered with white overwrap to mask the identity of the product. When new tubes of the dentifrice were delivered, subjects returned their previous tubes so that compliance with dentifrice use could be monitored" Comment: participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors did not report the initial number of participants randomised; they only reported the number analysed. Attrition (if there was any) was not reported by group and reasons were not given |
| Selective reporting (reporting bias) | Low risk | For a study looking into anticaries effect, we believe that appropriate outcome measures were considered and reported in full |
| Other bias | Low risk | Quote: "Dental caries was scored by two trained and calibrated examiners" and "The Kappa Statistic for inter‐ and intra‐examiner reproducibility of caries scores was greater than 0.9, indicating a high level of agreement within and between the two examiners" Comment: we consider that the risk of differential diagnostic activity was low. We were unable to identify any other potential source of bias |
Mateu 2008.
| Methods | Trial design: parallel (2 arms) Location: "clinical facility", Barcelona, Spain Number of centres: 1 Recruitment period: not reported Funding source: "This study was supported by the Colgate‐Palmolive Company" |
|
| Participants | Inclusion criteria: healthy adults; minimum 20 uncrowned permanent natural teeth (excluding third molars); mean baseline modified Quigley‐Hein Plaque Index score of 1.5 or more and mean baseline Löe‐Silness Gingival Index score of 1.0 or more Exclusion criteria: wearing orthodontic appliances; wearing partial removable prostheses; tumours of the oral soft or hard tissues; advanced periodontal disease (purulent exudates, tooth mobility, extensive periodontal attachment loss or alveolar bone loss, or a combination of these); 5 or more carious lesions requiring immediate restorative treatment; history of allergy to personal care/consumer products or their ingredients; any medical condition precluding participants from not eating and drinking for periods up to 4 hours; use of any prescription medication that might interfere with the study outcomes; pregnant or lactating women; use of antibiotics during the 1 month before the study began; participation in any other clinical study or test panel during the 1 month before the study began; received a dental prophylaxis during the 2 weeks before the study began Baseline plaque: (Quigley‐Hein Plaque Index) Gp A: mean 3.19 (SD 0.52); Gp B: mean 3.23 (SD 0.53); (Plaque Severity Index) Gp A: mean 0.66 (SD 0.12); Gp B: mean 0.67 (SD 0.11) Baseline gingivitis: (Löe‐Silness Gingival Index) Gp A: mean 1.39 (SD 0.27); Gp B: mean 1.39 (SD 0.23); (Gingivitis Severity Index) Gp A: mean 0.37 (SD 0.21); Gp B: mean 0.38 (SD 0.18) Baseline caries: not reported Age at baseline (years): Gp A: mean 35.9 (range 22‐58); Gp B: mean 37.2 (range 21‐72) Gender: Gp A: male 13 (27%), female 35 (73%); Gp B: male 15 (33%), female 31 (67%) Any other details of important prognostic factors: not reported Number randomised: not reported Number evaluated: 94 (Gp A: 48; Gp B: 46) |
|
| Interventions |
Comparison: triclosan/copolymer/sodium fluoride versus sodium fluoride Gp A (n = 48 evaluated): twice daily brushing for 1 minute with toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride; all participants received thorough baseline oral prophylaxis; asked to refrain from all oral hygiene procedures for at least 12 hours and from eating, drinking or smoking for 4 hours before their baseline and follow‐up examinations Gp B (n = 46 evaluated): as above but without triclosan and copolymer Duration of treatment: 6 months |
|
| Outcomes | Plaque (Quigley‐Hein Plaque Index and Plaque Severity Index), gingivitis (Löe‐Silness Gingival Index and Gingivitis Severity Index), adverse effects; assessed at 3 and 6 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: none observed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomized into two treatment groups" Comment: insufficient information on the method of sequence generation Additional information from correspondence: simple randomisation using random number tables |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomized into two treatment groups" Comment: not mentioned Additional information from correspondence: a rigorous allocation procedure was carried out by people not involved in the study and we are satisfied that this was properly concealed from those involved in the study |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "double‐blind" and "All dentifrices were over‐wrapped in their original package" Comment: participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors did not report the initial number of participants randomised; they only reported the number analysed. Attrition (if there was any) was not reported by group and reasons were not given |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome measures were considered and reported in full, as described in the methods section |
| Other bias | Low risk | No mention of calibration of outcome assessor so it is unclear whether or not there was a risk of differential diagnostic activity Additional information from correspondence: this study followed a protocol whereby all outcome assessors were highly trained in the indices and procedures used, and inter‐ and intra‐examiner calibration occur where practical. Therefore, we consider that the risk of differential diagnostic activity is low. We were unable to identify any other potential source of bias |
McClanahan 1997.
| Methods | Trial design: parallel (3 arms) Location: Indianapolis, USA (type of setting not reported) Number of centres: 1 Recruitment period: not reported Funding source: not reported but some authors associated with Procter and Gamble (the manufacturer of the stannous fluoride toothpaste) |
|
| Participants | Inclusion criteria: healthy adults; minimum 5 gingival bleeding sites; minimum 16 natural teeth (including 4 molars) Exclusion criteria: "rampant" caries; advanced periodontal disease; chronic dental neglect; serious medical condition Baseline plaque: (Quigley‐Hein Plaque Index) Gp A: mean 1.88 (SE 0.04); Gp B: mean 1.9 (SE 0.04) Baseline gingivitis: (Löe‐Silness Gingival Index) Gp A: mean 0.7 (SE 0.02); Gp B: mean 0.71 (SE 0.02); (gingival bleeding on probing or spontaneously ‐ number of sites) Gp A: mean 15.46 (SE 0.92); Gp B: mean 16.4 (SE 1.03) Baseline caries: not reported Age at baseline (years): Gp A: mean 35.5 (range 19‐71); Gp B: mean 36.5 (range 19‐70) Gender: Gp A: male 52 (34%), female 103 (66%); Gp B: male 60 (34%), female 114 (66%) Any other details of important prognostic factors: baseline staining (Meckel Stain Score): Gp A: mean 1.16 (SE 0.18); Gp B: mean 1.14 (SE 0.19) Number randomised: 378 (Gp A: 187; Gp B: 191) Number evaluated: 329 (Gp A: 155; Gp B: 174) |
|
| Interventions |
Comparison: stannous fluoride* versus triclosan/copolymer/sodium fluoride versus sodium fluoride *We excluded this arm from our data extraction, risk of bias assessment and analyses Gp A (n = 187): twice daily brushing for 1 minute with toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride; all participants received thorough baseline oral prophylaxis Gp B (n = 191): as above but without triclosan and copolymer Duration of treatment: 6 months |
|
| Outcomes | Plaque (Quigley‐Hein Plaque Index), gingivitis (Löe‐Silness Gingival Index and Gingival bleeding on probing or spontaneously), adverse effects (Meckel Stain Scores and oral soft tissue status); assessed at 3 and 6 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: none observed; staining was reported as continuous data but no adverse events were reported as such |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned...Subjects were separated by gender and by intervals of initial gingivitis scores. Within strata, subjects were assigned to treatment groups by random permutations of five" Comment: sufficient description of the method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...randomly assigned...Subjects were separated by gender and by intervals of initial gingivitis scores. Within strata, subjects were assigned to treatment groups by random permutations of five" Comment: unclear whether remote/central randomisation and no variation of block size |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "Each subject received four 4.6 ounce uniquely labelled plain white tubes containing one of the following dentifrices...The study was conducted in a double‐blind fashion so neither the examiners nor subjects knew the identity of the dentifrices throughout the course of the trial" Comment: use of an identical control toothpaste meant that participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Each subject received four 4.6 ounce uniquely labelled plain white tubes containing one of the following dentifrices...The study was conducted in a double‐blind fashion so neither the examiners nor subjects knew the identity of the dentifrices throughout the course of the trial" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13% of randomised participants were not included in the final analysis (Gp A: 17%; Gp B: 9%). Reasons for attrition were not given but the rate was much higher in the triclosan/copolymer group than the control group. Also, if the missing participants had higher mean plaque/gingivitis scores in one group than the other, as the attrition rate increased, so would over/understatement of the mean difference |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome measures were considered and reported in full, as described in the methods section |
| Other bias | Unclear risk | No mention of calibration of outcome assessor so it is unclear whether or not there was a risk of differential diagnostic activity |
Palomo 1994.
| Methods | Trial design: parallel (4 arms) Location: "clinical facility", San Pedro La Laguna, Guatemala Number of centres: 1 Recruitment period: not reported Funding source: not reported but some authors associated with Colgate (the manufacturer of the triclosan/copolymer toothpaste) |
|
| Participants | Inclusion criteria: healthy adults; mean baseline modified Quigley‐Hein Plaque Index score of 1.5 or more and mean baseline Löe‐Silness Gingival Index score of 1.0 or more Exclusion criteria: not reported Baseline plaque: (Quigley‐Hein Plaque Index) Gp A: mean 2.995; Gp B: mean 2.997; (Plaque Severity Index) Gp A: mean 0.623; Gp B: mean 0.623 Baseline gingivitis: (Löe‐Silness Gingival Index) Gp A: mean 2.095; Gp B: mean 2.119; (Gingivitis Severity Index) Gp A: mean 0.754; Gp B: 0.776 Baseline caries: not reported Age at baseline (years): Gp A: median 29 (range 18‐63); Gp B: median 31 (range 18‐52) Gender: Gp A: male 14 (33%), female 28 (67%); Gp B: male 9 (20%), female 35 (80%) Any other details of important prognostic factors: not reported Number randomised: 95 (Gp A: 47; Gp B: 48) Number evaluated: 86 (Gp A: 42; Gp B: 44) |
|
| Interventions |
Comparison: triclosan/pyrophosphate* versus triclosan/zinc citrate* versus triclosan/copolymer/sodium fluoride versus sodium fluoride *We excluded these arms from our data extraction, risk of bias assessment and analyses Gp A (n = 47): twice daily brushing for 1 minute with toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride; all participants received thorough baseline oral prophylaxis (removal of all supragingival plaque and calculus deposits), teeth were polished and erythrosin was used to confirm complete plaque removal; participants had to visit the clinical facility every 4 weeks to exchange their used toothpaste tube and toothbrush for a new supply (such visits were also used to reinforce instructions regarding the required duration and frequency of brushing) Gp B (n = 48): as above but without triclosan and copolymer Duration of treatment: 6 months |
|
| Outcomes | Plaque (Quigley‐Hein Plaque Index and Plaque Severity Index), gingivitis (Löe‐Silness Gingival Index and Gingivitis Severity Index), adverse effects; assessed at 1.5, 3 and 6 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: none observed Although information on variance was not reported in the study, we used SDs reported in another published systematic review to enable us to include this study in the meta‐analyses (Davies 2004) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned" Comment: insufficient information on the method of sequence generation Additional information from correspondence: simple randomisation using random number tables |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomly assigned" Comment: not mentioned Additional information from correspondence: a rigorous allocation procedure was carried out by people not involved in the study and we are satisfied that this was properly concealed from those involved in the study |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "double‐blind" and "The dentifrices were distributed to the subjects in identical plain white tubes to ensure that neither the subjects nor the dental examiner knew the identity of the products" Comment: use of an identical control toothpaste meant that participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" and "The dentifrices were distributed to the subjects in identical plain white tubes to ensure that neither the subjects nor the dental examiner knew the identity of the products" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 9% of randomised participants were not included in the final analysis (Gp A: 11%; Gp B: 8%). Reasons for attrition were not given, but authors stated that reasons were not related to the use of any of the toothpastes Comment: we do not believe that any of the above could pose a risk of bias significant enough to have led to a distortion of the true intervention effect |
| Selective reporting (reporting bias) | High risk | There is no information reported on the variance of the mean plaque and gingivitis scores (see 'Notes' section in above table) |
| Other bias | Low risk | No mention of calibration of outcome assessor so it is unclear whether or not there was a risk of differential diagnostic activity Additional information from correspondence: this study followed a protocol whereby all outcome assessors were highly trained in the indices and procedures used, and inter‐ and intra‐examiner calibration occur where practical. Therefore, we consider that the risk of differential diagnostic activity is low. We were unable to identify any other potential source of bias |
Pradeep 2012.
| Methods | Trial design: parallel (3 arms) Location: Department of Periodontics, Government Dental College and Research Institute, Bangalore, India Number of centres: 1 Recruitment period: not reported Funding source: not reported but the toothpastes were provided by LB Aroma and Health Care, Mumbai, India |
|
| Participants | Inclusion criteria: diagnosis of chronic generalised gingivitis; minimum 20 natural teeth; bleeding on gentle probing at more than 30% of sites examined and mean baseline Löe‐Silness Gingival Index score of 1.0 or more at more than 60% of sites examined; pocket probing depth of 3 mm or less; no clinical attachment loss; mean baseline modified Quigley‐Hein Plaque Index score of more than 2.0; no evidence of radiographic bone loss Exclusion criteria: received periodontal therapy or used antibiotics or anti‐inflammatory medication during the 6 months before the study began; known allergy to any of the toothpaste ingredients; haematological disorders or other systemic illness; pregnant or lactating women; receiving orthodontic treatment; smokers Baseline plaque: (Quigley‐Hein Plaque Index) Gp A: mean 4.369 (SD 0.595); Gp B: mean 4.436 (SD 0.704) Baseline gingivitis: (Löe‐Silness Gingival Index) Gp A: mean 1.963 (SD 0.4); Gp B: mean 1.934 (SD 0.368) Baseline caries: not reported Age at baseline (years): Gp A: mean 29.4; Gp B: mean 30.4 (range not reported) Gender: Gp A: male 13 (46%), female 15 (54%); Gp B: male 14 (50%), female 14 (50%) Any other details of important prognostic factors: not reported Number randomised: 60 (Gp A: 30; Gp B: 30) Number evaluated: 56 (Gp A: 28; Gp B: 28) |
|
| Interventions |
Comparison: aloe vera* versus triclosan/copolymer/fluoride versus placebo (sodium fluoride not stated) *We excluded this arm from our data extraction, risk of bias assessment and analyses Gp A (n = 30): brushing with toothpaste (frequency not reported, i.e. normal use) containing triclosan, copolymer, fluoride (concentrations not stated); all participants received thorough baseline oral prophylaxis (removal of all supragingival plaque and calculus deposits) plus instruction/demonstration of the modified Bass method of brushing; asked to refrain from all oral hygiene procedures (including chewing gum) for at least 8 hours before their baseline and follow‐up examinations; asked to refrain from any other oral hygiene procedures during the study period Gp B (n = 30): as above but with placebo toothpaste Duration of treatment: 6 months |
|
| Outcomes | Plaque (Quigley‐Hein Plaque Index), gingivitis (Löe‐Silness Gingival Index), microbial counts, adverse effects; assessed at 1.5, 3 and 6 months' follow‐up | |
| Notes | Sample size calculation: sample size was decided by power analysis with 90% power at a 5% significance level but it is not clear if the required sample size was achieved after attrition Adverse effects: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...participants were assigned randomly by a computer‐generated numbering sequence" Comment: this is the ideal way to generate a random sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "The dentifrices were dispensed to patients by a dental assistant not involved in the study" Comment: this is similar to remote/centralised allocation and the study investigators would not be able to influence the allocation sequence |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "double‐masked" and "All tubes had a plain white covering labelled only with lot numbers to ensure proper masking of the product from the patients and examiner" Comment: participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐masked" and "All tubes had a plain white covering labelled only with lot numbers to ensure proper masking of the product from the patients and examiner" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 7% of randomised participants were not included in the final analysis (Gp A: 7%; Gp B: 7%). Reasons for attrition were discussed Comment: we do not believe that any of the above could pose a risk of bias significant enough to have led to a distortion of the true intervention effect |
| Selective reporting (reporting bias) | High risk | Appropriate outcome measures were considered but adverse effects were not reported in the results section |
| Other bias | Unclear risk | No mention of calibration of outcome assessor so it is unclear whether or not there was a risk of differential diagnostic activity |
Renvert 1995.
| Methods | Trial design: parallel (4 arms) Location: Kristianstad, Sweden (type of setting not reported) Number of centres: 1 Recruitment period: not reported Funding source: not reported |
|
| Participants | Inclusion criteria: clinical signs of gingivitis Exclusion criteria: 4 or more periodontal pockets at 5 mm or more; bone loss as revealed by radiograph; pregnancy, diabetes or immunosuppressive disease Baseline plaque: (Quigley‐Hein Plaque Index) Gp A: mean 0.5 (SE 0.07); Gp B: mean 0.5 (SE 0.07); (Löe‐Silness Plaque Index) Gp A: mean 0.5 (SE 0.05); Gp B: mean 0.5 (SE 0.03) Baseline gingivitis: (Ainamo‐Bay Bleeding Index but with a similar scoring method to plaque so as to calculate mean bleedings = Gingivitis Severity Index) Gp A: mean 0.3 (SE 0.02); Gp B: mean 0.3 (SE 0.02) Baseline caries: not reported Age at baseline (years): mean 21.5; range 18‐33 Gender: not reported Any other details of important prognostic factors: not reported Number randomised: 60 (Gp A: 30; Gp B: 30) Number evaluated: 54 (Gp A: 26; Gp B: 28) |
|
| Interventions |
Comparison: triclosan/pyrophosphate* versus triclosan/zinc citrate* versus triclosan/copolymer/sodium fluoride versus sodium monofluorophosphate *We excluded these arms from our data extraction, risk of bias assessment and analyses Gp A (n = 30): brushing with toothpaste (frequency not reported, i.e. normal use) containing 0.3% triclosan, 2% copolymer, 0.24% sodium fluoride; all participants received scale and polish at the start of a pre‐experimental period 1 month before the study began; all participants received thorough instructions on how to use their toothpaste before the start of the pre‐experimental period, at baseline and at 3 months' follow‐up Gp B (n = 30): as above but without triclosan and copolymer. Also, the fluoride content was in a different form (sodium monofluorophosphate), but it is not clear if this was equivalent to 1100 ppm fluoride as the concentration was not reported (however, we do not consider this to be a problem as this study is concerned with plaque and gingivitis, rather than caries Duration of treatment: 6 months |
|
| Outcomes | Plaque (Quigley‐Hein Plaque Index and Löe‐Silness Plaque Index), gingivitis (Ainamo‐Bay Bleeding Index/Gingivitis Severity Index), microbial counts; assessed at 3 and 6 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The subjects were allocated to 4 groups" Comment: insufficient information on the method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "double‐blind" and "The dentifrices were distributed in identical packages so that neither the patient nor the examiner knew the identity of the products. The code was not broken until the study had been completed and the data analyzed statistically" Comment: use of an identical control toothpaste meant that participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" and "The dentifrices were distributed in identical packages so that neither the patient nor the examiner knew the identity of the products. The code was not broken until the study had been completed and the data analyzed statistically" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 10% of randomised participants were not included in the final analysis (Gp A: 13%; Gp B: 7%) but reasons for attrition were not given Comment: we do not believe that any of the above could pose a risk of bias significant enough to have led to a distortion of the true intervention effect |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome measures were considered and reported in full, as described in the methods section |
| Other bias | Unclear risk | No mention of calibration of outcome assessor so it is unclear whether or not there was a risk of differential diagnostic activity |
Schiff 2006.
| Methods | Trial design: parallel (3 arms) Location: San Francisco, USA (type of setting not reported) Number of centres: 1 Recruitment period: not reported Funding source: "This study was supported by the Colgate‐Palmolive Company" |
|
| Participants | Inclusion criteria: healthy adults; minimum 20 uncrowned permanent natural teeth (excluding third molars); mean baseline modified Quigley‐Hein Plaque Index score of 1.5 or more and mean baseline Löe‐Silness Gingival Index score of 1.0 or more Exclusion criteria: wearing orthodontic appliances; wearing removable prostheses; tumours of the oral soft or hard tissues; advanced periodontal disease; 5 or more carious lesions requiring immediate restorative treatment; history of allergy to personal care/consumer products or their ingredients; use of any prescription medication that might interfere with the study outcomes; pregnant or lactating women; use of antibiotics during the 1 month before the study began; participation in any other clinical study or test panel during the 1 month before the study began Baseline plaque: (Quigley‐Hein Plaque Index) Gp A: mean 2.02 (SD 0.25); Gp B: mean 1.98 (SD 0.24) Baseline gingivitis: (Löe‐Silness Gingival Index) Gp A: mean 1.02 (SD 0.05); Gp B: mean 1.1 (SD 0.26) Baseline caries: not reported Age at baseline (years): Gp A: mean 28.3 (range 22‐46); Gp B: mean 27.3 (range 20‐50) Gender: Gp A: male 20 (54%), female 17 (46%); Gp B: male 22 (55%), female 18 (45%) Any other details of important prognostic factors: not reported Number randomised: not reported (120 across 3 arms with 5% attrition overall) Number evaluated: 77 (Gp A: 37; Gp B: 40) |
|
| Interventions |
Comparison: triclosan/copolymer/sodium fluoride plus flossing versus triclosan/copolymer/sodium fluoride without flossing* versus sodium fluoride plus flossing *We excluded this arm from our data extraction, risk of bias assessment and analyses Gp A (n = 37 evaluated): twice daily brushing for 1 minute with toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride, plus flossing once daily after brushing; all participants received thorough baseline oral prophylaxis and a red disclosing solution was used to confirm complete plaque removal; asked to refrain from using any other oral hygiene products and routine (non‐emergency) dental treatment during the study period Gp B (n = 40 evaluated): as above but without triclosan and copolymer Duration of treatment: 6 months |
|
| Outcomes | Plaque (Quigley‐Hein Plaque Index), gingivitis (Löe‐Silness Gingival Index), adverse effects; assessed at 3 and 6 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: none observed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned" Comment: insufficient information on the method of sequence generation Additional information from correspondence: simple randomisation using random number tables |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomly assigned" Comment: not mentioned Additional information from correspondence: a rigorous allocation procedure was carried out by people not involved in the study and we are satisfied that this was properly concealed from those involved in the study |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "examiner blind" and "All dentifrice products were packaged in their original tubes, but over‐wrapped with a white label to ensure that neither the subject nor the examiner would be aware of the identity of the product" Comment: as we have excluded the arm without flossing, the use of an identical control toothpaste plus flossing in the remaining arms meant that participants did not know which group they were assigned to, and this study can be considered double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "examiner blind" and "All dentifrice products were packaged in their original tubes, but over‐wrapped with a white label to ensure that neither the subject nor the examiner would be aware of the identity of the product" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 5% of randomised participants were not included in the final analysis, when considering all 3 arms. Attrition was not reported by group and reasons were not given, but authors stated that reasons were not related to any of the treatment regimens Comment: we do not believe that any of the above could pose a risk of bias significant enough to have led to a distortion of the true intervention effect |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome measures were considered and reported in full, as described in the methods section |
| Other bias | Low risk | No mention of calibration of outcome assessor so it is unclear whether or not there was a risk of differential diagnostic activity Additional information from correspondence: this study followed a protocol whereby all outcome assessors were highly trained in the indices and procedures used, and inter‐ and intra‐examiner calibration occur where practical. Therefore, we consider that the risk of differential diagnostic activity is low. We were unable to identify any other potential source of bias |
Svatun 1993.
| Methods | Trial design: parallel (4 arms) Location: Oslo, Norway (type of setting not reported) Number of centres: 1 Recruitment period: not reported Funding source: not reported but some authors associated with Unilever Dental Research (the manufacturer of the triclosan/zinc citrate toothpaste) |
|
| Participants | Inclusion criteria: healthy adults; mild to moderate gingivitis Exclusion criteria: periodontitis at baseline (pocket depths more than 4 mm); untreated caries Baseline plaque: (Löe‐Silness Plaque Index) Gp A: mean 0.28 (SE 0.03); Gp B: mean 0.29 (SE 0.03) Baseline gingivitis: (Ainamo‐Bay Bleeding Index ‐ equates to the Gingivitis Severity Index when presented as a proportion) Gp A: mean 27.4 (SE 1.9); Gp B: mean 27.3 (SE 1.4) Baseline caries: not reported Age at baseline (years): Gp A: mean 26 (range 21‐44); Gp B: mean 24 (range 19‐39) Gender: Gp A: male 15 (33%), female 31 (67%); Gp B: male 11 (23%), female 37 (77%) Any other details of important prognostic factors: baseline calculus (Volpe‐Manhold Calculus Index ‐ mean height of calculus ‐ measured in a different way to Liu 2002 and Lobene 1991 and not able to combine in meta‐analysis): Gp A: mean 0.48 mm (SE 0.08); Gp B: mean 0.48 mm (SE 0.08) Number randomised: not reported (220 across 4 arms with 16% attrition overall) Number evaluated: 94 (Gp A: 46; Gp B: 48) (for calculus, only subjects exhibiting calculus at baseline were included in the analysis: Gp A: 39; Gp B: 39) |
|
| Interventions |
Comparison: triclosan/pyrophosphate* versus triclosan/zinc citrate* versus triclosan/copolymer/sodium fluoride versus sodium monofluorophosphate *We excluded these arms from our data extraction, risk of bias assessment and analyses Gp A (n = 46 evaluated): twice daily brushing with toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride (1100 ppm fluoride); all participants received thorough baseline oral prophylaxis (removal of all subgingival and supragingival plaque and calculus deposits) plus a short period of oral hygiene instruction Gp B (n = 48 evaluated): as above but without triclosan and copolymer, and with 0.8% sodium monofluorophosphate (approximately equivalent ppm fluoride to the sodium fluoride in Gp A) Duration of treatment: 7 months |
|
| Outcomes | Plaque (Löe‐Silness Plaque Index), gingivitis (Ainamo‐Bay Bleeding Index/Gingivitis Severity Index), calculus (Volpe‐Manhold Calculus Index), adverse effects; assessed at 1, 4 and 7 months' follow‐up | |
| Notes | Sample size calculation: sample size was informed by a previous study, with approximately 50 participants in each of the 4 arms required to have 80% power (significance level not stated) to detect a 25% difference in gingival bleeding. It is not clear whether or not this was achieved Adverse effects: none observed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...random allocation" Comment: insufficient information on the method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...random allocation" Comment: not mentioned |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "...retubed into 50 ml white laminate tubes to Good Manufacturing Practice standard to maintain double blindness in the study" Comment: use of an identical control toothpaste meant that participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...retubed into 50 ml white laminate tubes to Good Manufacturing Practice standard to maintain double blindness in the study" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 16% of randomised participants were not included in the final analysis, when considering all 4 arms. Reasons for attrition were only partially given, but authors stated that reasons were not related to any of the toothpastes. As attrition was not reported by group, it is not possible to state whether or not the 2 arms included in this review had 10% or less attrition, or if attrition was equivalent in each group |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome measures were considered and reported in full, as described in the methods section |
| Other bias | Unclear risk | No mention of calibration of outcome assessor so it is unclear whether or not there was a risk of differential diagnostic activity |
Triratana 1993.
| Methods | Trial design: parallel (2 arms) Location: Chiangmai Province, Thailand (type of setting not reported) Number of centres: 1 Recruitment period: not reported Funding source: not reported but some authors associated with Colgate (the manufacturer of the triclosan/copolymer toothpaste) |
|
| Participants | Inclusion criteria: healthy adults; mean baseline modified Quigley‐Hein Plaque Index score of 1.5 or more and mean baseline modified Löe‐Silness Gingival Index score of 1.0 or more Exclusion criteria: not reported Baseline plaque: (Quigley‐Hein Plaque Index) Gp A: mean 2.14 (SD 0.49); Gp B: mean 2.10 (SD 0.45); (Plaque Severity Index) Gp A: mean 0.41 (SD 0.19); Gp B: mean 0.39 (SD 0.15) Baseline gingivitis: (Löe‐Silness Gingival Index) Gp A: mean 1.80 (SD 0.17); Gp B: mean 1.82 (SD 0.17); (Gingivitis Severity Index) Gp A: mean 0.80 (SD 0.13); Gp B: mean 0.82 (SD 0.12) Baseline caries: not reported Age at baseline (years): Gp A: mean 31.6 (range 21‐46); Gp B: mean 30.5 (range 22‐40) Gender: Gp A: male 6 (10%), female 54 (90%); Gp B: male 5 (8%), female 55 (92%) Any other details of important prognostic factors: not reported Number randomised: 120 (Gp A: 60; Gp B: 60) Number evaluated: 120 (Gp A: 60; Gp B: 60) |
|
| Interventions |
Comparison: triclosan/copolymer/sodium fluoride versus sodium fluoride Gp A (n = 60): twice daily brushing for 1 minute with liquid toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride (no baseline prophylaxes) Gp B (n = 60): as above but without triclosan and copolymer Duration of treatment: 6 months |
|
| Outcomes | Plaque (Quigley‐Hein Plaque Index and Plaque Severity Index), gingivitis (Löe‐Silness Gingival Index and Gingivitis Severity Index), adverse effects; assessed at 1.5 and 6 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: none observed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned" Comment: insufficient information on the method of sequence generation Additional information from correspondence: simple randomisation using random number tables |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomly assigned" Comment: not mentioned Additional information from correspondence: a rigorous allocation procedure was carried out by people not involved in the study and we are satisfied that this was properly concealed from those involved in the study |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "double‐blind" and "The dentifrices were distributed to the subjects in identical plain white tubes to ensure that neither the subject nor the dental examiner knew the identity of the dentifrices" Comment: use of an identical control toothpaste meant that participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" and "The dentifrices were distributed to the subjects in identical plain white tubes to ensure that neither the subject nor the dental examiner knew the identity of the dentifrices" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome measures were considered and reported in full, as described in the methods section |
| Other bias | Low risk | No mention of calibration of outcome assessor so it is unclear whether or not there was a risk of differential diagnostic activity Additional information from correspondence: this study followed a protocol whereby all outcome assessors were highly trained in the indices and procedures used, and inter‐ and intra‐examiner calibration occur where practical. Therefore, we consider that the risk of differential diagnostic activity is low. We were unable to identify any other potential source of bias |
Triratana 1994.
| Methods | Trial design: parallel (2 arms) Location: Chiangmai Province, Thailand (type of setting not reported) Number of centres: 1 Recruitment period: not reported Funding source: not reported |
|
| Participants | Inclusion criteria: healthy adults; mean baseline modified Quigley‐Hein Plaque Index score of 1.5 or more and mean baseline modified Löe‐Silness Gingival Index score of 1.0 or more Exclusion criteria: not reported Baseline plaque: (Quigley‐Hein Plaque Index) Gp A: mean 2.38 (SD 0.42); Gp B: mean 2.25 (SD 0.44) Baseline gingivitis: (Löe‐Silness Gingival Index) Gp A: mean 1.80 (SD 0.19); Gp B: mean 1.79 (SD 0.15) Baseline caries: not reported Age at baseline (years): Gp A: mean 38.4 (range 24‐71); Gp B: mean 34.8 (range 23‐52) Gender: Gp A: male 16 (50%), female 16 (50%); Gp B: male 12 (36%), female 21 (64%) Any other details of important prognostic factors: not reported Number randomised: 65 (Gp A: 32; Gp B: 33) Number evaluated: 65 (Gp A: 32; Gp B: 33) |
|
| Interventions |
Comparison: triclosan/copolymer/sodium fluoride versus sodium fluoride Gp A (n = 32): twice daily brushing for 1 minute with liquid toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride; all participants received thorough baseline oral prophylaxis Gp B (n = 33): as above but without triclosan and copolymer Duration of treatment: 6 months |
|
| Outcomes | Plaque (Quigley‐Hein Plaque Index), gingivitis (Löe‐Silness Gingival Index), microbial counts; assessed at 6 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomly assigned" Comment: insufficient information on the method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...randomly assigned" Comment: not mentioned |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "double‐blind" and "The dentifrices were distributed to the subjects in identical plain white tubes to ensure that neither the subject nor the dental examiner knew the identity of the dentifrices" Comment: use of an identical control toothpaste meant that participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" and "The dentifrices were distributed to the subjects in identical plain white tubes to ensure that neither the subject nor the dental examiner knew the identity of the dentifrices" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome measures were considered and reported in full, as described in the methods section |
| Other bias | Unclear risk | No mention of calibration of outcome assessor so it is unclear whether or not there was a risk of differential diagnostic activity |
Triratana 2002.
| Methods | Trial design: parallel (2 arms) Location: Faculty of Dentistry, Mahidol University, Bangkok, Thailand Number of centres: 1 Recruitment period: not reported Funding source: "...Colgate‐Palmolive paid for the study to be conducted" |
|
| Participants | Inclusion criteria: minimum 20 scorable teeth; mean baseline modified Quigley‐Hein Plaque Index score of 1.5 or more and mean baseline modified Löe‐Silness Gingival Index score of 1.0 or more Exclusion criteria: wearing orthodontic appliances; wearing removable prostheses; tumours; advanced periodontal disease; use of antibiotics during the 2 weeks before the study began Baseline plaque: (Quigley‐Hein Plaque Index) Gp A: mean 2.95 (SD 0.21); Gp B: mean 2.96 (SD 0.29); (Plaque Severity Index) Gp A: mean 0.63 (SD 0.06); Gp B: mean 0.62 (SD 0.07) Baseline gingivitis: (Löe‐Silness Gingival Index) Gp A: mean 1.70 (SD 0.19); Gp B: mean 1.72 (SD 0.20); (Gingivitis Severity Index) Gp A: mean 0.57 (SD 0.04); Gp B: mean 0.58 (SD 0.03) Baseline caries: not reported Age at baseline (years): Gp A: mean 38 (range 20‐60); Gp B: mean 38 (range 20‐60) Gender: Gp A: male 42%, female 58%; Gp B: male 42%, female 58% Any other details of important prognostic factors: not reported Number randomised: 124 (not reported by group) Number evaluated: 119 (Gp A: 60; Gp B: 59) |
|
| Interventions |
Comparison: triclosan/copolymer/sodium fluoride versus sodium fluoride Gp A (n = 60 evaluated): twice daily brushing for 1 minute with liquid toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride (no baseline prophylaxes); asked to refrain from any other oral hygiene procedures during the study period Gp B (n = 59 evaluated): as above but without triclosan and copolymer Duration of treatment: 6 months |
|
| Outcomes | Plaque (Quigley‐Hein Plaque Index and Plaque Severity Index), gingivitis (Löe‐Silness Gingival Index and Gingivitis Severity Index), adverse effects; assessed at 3 and 6 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: none observed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned" Comment: insufficient information on the method of sequence generation Additional information from correspondence: simple randomisation using random number tables |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomly assigned" Comment: not mentioned Additional information from correspondence: a rigorous allocation procedure was carried out by people not involved in the study and we are satisfied that this was properly concealed from those involved in the study |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "The dentifrices were distributed in plain white wrappers to ensure the double‐blind nature of the study" Comment: use of an identical control toothpaste meant that participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 4% of randomised participants were not included in the final analysis. Attrition was not reported by group and reasons were not given, but authors stated that reasons were not related to the use of either of the toothpastes Comment: we do not believe that any of the above could pose a risk of bias significant enough to have led to a distortion of the true intervention effect |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome measures were considered and reported in full, as described in the methods section |
| Other bias | Low risk | Quote: "Baseline examinations and subsequent examinations were performed by Drs. Terdphong Triratana and Titikan Fongsmut" Comment: no mention of calibration of outcome assessors so it is unclear whether or not there was a risk of differential diagnostic activity Additional information from correspondence: this study followed a protocol whereby all outcome assessors were highly trained in the indices and procedures used, and inter‐ and intra‐examiner calibration occur where practical. Therefore, we consider that the risk of differential diagnostic activity is low. We were unable to identify any other potential source of bias |
Vered 2009.
| Methods | Trial design: parallel (2 arms) Location: 25 settlement communities throughout Israel (type of setting not reported) Number of centres: not reported (but presumably multicentre) Recruitment period: 2003‐2004 Funding source: "This study was supported by the Colgate‐Palmolive Company" |
|
| Participants | Inclusion criteria: adults over 25 years old; minimum 1 intact crown (fixed dental prosthetic treatment) Exclusion criteria: orthodontic appliances involving more than 4 permanent teeth; periodontal disease (mobility of at least 4 teeth and with a potential of losing those teeth during the study); participation in any other clinical study during the 3 months before the study began; any condition which the investigator felt would preclude their participation Baseline plaque: not reported Baseline gingivitis: not reported Baseline caries: (Katz Root Caries Index) Gp A: mean 1.07 (SD 1.72); Gp B: mean 0.87 (SD 1.57) Age at baseline (years): Gp A: mean 58.8 (SD 8.8); Gp B: mean 58.2 (SD 8.3) Gender: Gp A: male 43%, female 57%; Gp B: male 44%, female 56% Any other details of important prognostic factors: not reported Number randomised: 1547 (not reported by group) Number evaluated: 1357 (Gp A: 650; Gp B: 707) |
|
| Interventions |
Comparison: triclosan/copolymer/sodium fluoride versus sodium fluoride Gp A (n = 650 evaluated): twice daily brushing for 1 minute with toothpaste containing 0.3% triclosan, 2% copolymer, 0.243% sodium fluoride (no baseline prophylaxes) Gp B (n = 707 evaluated): as above but without triclosan and copolymer Duration of treatment: 36 months |
|
| Outcomes | Root caries (Katz Root Caries Index), dental crown failure, adverse effects; assessed at 36 months' follow‐up | |
| Notes | Sample size calculation: not reported Adverse effects: none observed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned" Comment: insufficient information on the method of sequence generation Additional information from correspondence: "random sequence generators were used" |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomly assigned" Comment: not mentioned Additional information from correspondence: a rigorous allocation procedure was carried out by people not involved in the study and we are satisfied that this was properly concealed from those involved in the study |
| Blinding of participants (performance bias) All outcomes | Low risk | Quote: "double‐blind" and "Dentifrice tubes were covered with white overwrap to mask the identity of the product. When new tubes of the dentifrice were delivered, subjects returned their previous tubes so that compliance could be monitored" Comment: use of an identical control toothpaste meant that participants did not know which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" and "Dentifrice tubes were covered with white overwrap to mask the identity of the product. When new tubes of the dentifrice were delivered, subjects returned their previous tubes so that compliance could be monitored" Comment: the examiner did not know which group the participants they were assessing had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 12% of randomised participants were not included in the final analysis. Attrition was not reported by group and reasons were not described. If the missing participants had a higher mean root caries increment in one group than the other, as the attrition rate increased, so would over/understatement of the mean difference |
| Selective reporting (reporting bias) | High risk | The authors stated that coronal caries was assessed at 12 and 24 months but no results were reported |
| Other bias | Low risk | Quote: "A subset of 20 subjects...were examined by both potential examiners...results produced a kappa of 0.87. In addition, an intra‐examiner calibration was conducted by the two examiners in the following days, and results produced a kappa of 0.88" Comment: we consider that the risk of differential diagnostic activity is low. We were unable to identify any other potential source of bias |
Dentifrice = toothpaste; DFS: decayed filled surfaces; DFT: decayed filled teeth; DMFS: decayed missing filled surfaces; DMFT: decayed missing filled teeth; Gp: group (group A is the test group; group B is the control group); ppm: parts per million; SD: standard deviation; SE: standard error.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Archila 2004 | Comparison of 2 active agents (stannous fluoride/sodium hexametaphosphate versus triclosan/copolymer/fluoride) with no fluoride‐only control arm. Toothbrushing was supervised |
| Bogren 2007 | Triclosan/copolymer arm also used powered toothbrushes while control arm used manual toothbrushes |
| Bogren 2008 | Triclosan/copolymer arm also used powered toothbrushes while control arm used manual toothbrushes. Participants had periodontitis at baseline |
| Boneta 2010 | Comparison of 2 active agents (stannous fluoride/sodium hexametaphosphate versus triclosan/copolymer/fluoride) with no fluoride‐only control arm |
| Charles 2001 | Even though it would be possible to use 2 of the 3 arms (triclosan/copolymer/fluoride toothpaste plus inactive mouthrinse versus fluoride‐only control toothpaste plus inactive mouthrinse), we consider that any mouthrinse could wash away the active toothpaste ingredients |
| Cullinan 2003 | Participants had periodontitis at baseline |
| de la Rosa 1992 | Triclosan and pyrophosphate, not triclosan/copolymer. Only 9 weeks of intervention |
| Dóri 1999 | From translator: "3 weeks" and "triclosan toothpaste in all three arms" |
| Kocher 2000 | Additional intervention of interdental cleaning in control group only |
| Mankodi 2002 | Comparison of 2 active agents (stannous fluoride versus triclosan/copolymer/fluoride) with no fluoride‐only control arm |
| Winston 2002 | Participants with fewer than 20 gingival bleeding sites at baseline exited the study after 3 months (26%). This could have ruined the effect of the randomisation process, thus introducing selection bias |
Differences between protocol and review
On consideration, we decided that the cut‐off rule (greater than 10%) for the risk of bias domain 'incomplete outcome data' (attrition bias) as stated in the protocol was too restrictive and we decided to relax this rule and judge each study on its individual circumstances.
We stated in the protocol that studies had to report the primary outcomes of this review (plaque and gingivitis) in order to be judged as low risk of bias for the domain 'selective reporting' (reporting bias). We later decided that this rule was too restrictive and that it was acceptable for a study to only assess caries, periodontitis, calculus or any other outcome of interest.
In the protocol, we stated that we would only include periodontitis data if they were measured by probing depth, as we thought that this would be the most accurate measure. On consideration, we decided to include any measure of periodontitis (e.g. attachment loss) if this meant we would be able to report useful data to healthcare professionals, patients and decision makers.
We relaxed the rule on the control arm having fluoride‐only toothpaste, that is some trials were not clear whether the control arm had fluoride only or no active ingredients. However, this would not be important in studies assessing plaque and gingivitis as fluoride is not aimed at reducing them.
We decided to run extra analyses to calculate prediction intervals for random‐effects meta‐analyses with high heterogeneity.
Contributions of authors
Philip Riley and Thomas Lamont developed the protocol and carried out all screening of search results, data extraction and risk of bias assessment, data analysis, interpreted the results and wrote up the review.
Sources of support
Internal sources
-
MAHSC, UK.
Cochrane Oral Health is supported by the Manchester Academic Health Sciences Centre (MAHSC) and the NIHR Manchester Biomedical Research Centre.
-
The University of Manchester, UK.
Cochrane Oral Health is part of the Division of Dentistry.
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed are those of the authors and not necessarily those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been the American Association of Public Health Dentistry, USA; AS‐Akademie, Germany; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland, UK; and the Swiss Society for Endodontology, Switzerland.
Declarations of interest
Philip Riley: no interests to declare. Thomas Lamont: no interests to declare.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Allen 2002 {published and unpublished data}
- Allen DR, Battista GW, Petrone DM, Petrone ME, Chaknis P, DeVizio W, et al. The clinical efficacy of Colgate Total Plus Whitening Toothpaste containing a special grade of silica and Colgate Total Fresh Stripe Toothpaste in the control of plaque and gingivitis: a six‐month clinical study. Journal of Clinical Dentistry 2002; Vol. 13, issue 2:59‐64. [PubMed]
Bolden 1992 {published and unpublished data}
- Bolden TE, Zambon JJ, Sowinski J, Ayad F, McCool JJ, Volpe AR, et al. The clinical effect of a dentifrice containing triclosan and a copolymer in a sodium fluoride/silica base on plaque formation and gingivitis: a six‐month clinical study. Journal of Clinical Dentistry 1992; Vol. 3, issue 4:125‐31. [PubMed]
Cubells 1991 {published and unpublished data}
- Cubells AB, Dalmau LB, Petrone ME, Chaknis P, Volpe AR. The effect of a triclosan/copolymer/fluoride dentifrice on plaque formation and gingivitis: a six‐month clinical study. Journal of Clinical Dentistry 1991; Vol. 2, issue 3:63‐9. [PubMed]
Deasy 1991 {published and unpublished data}
- Deasy MJ, Singh SM, Rustogi KN, Petrone DM, Battista G, Petrone ME, et al. Effect of a dentifrice containing triclosan and a copolymer on plaque formation and gingivitis. Clinical Preventive Dentistry 1991; Vol. 13, issue 6:12‐9. [PubMed]
Denepitiya 1992 {published and unpublished data}
- Denepitiya JL, Fine D, Singh S, DeVizio W, Volpe AR, Person P. Effect upon plaque formation and gingivitis of a triclosan/copolymer/fluoride dentifrice: a 6‐month clinical study. American Journal of Dentistry 1992; Vol. 5, issue 6:307‐11. [PubMed]
Ellwood 1998 {published and unpublished data}
- Ellwood RP, Worthington HV, Blinkhorn AS, Volpe AR, Davies RM. Effect of a triclosan/copolymer dentifrice (Colgate Total) on the incidence of periodontal attachment loss in adolescents. International Dental Journal 1999;49(5):294. [Google Scholar]
- Ellwood RP, Worthington HV, Blinkhorn AS, Volpe AR, Davies RM. Effect of a triclosan/copolymer dentifrice on the incidence of periodontal attachment loss in adolescents. Journal of Clinical Periodontology 1998; Vol. 25, issue 5:363‐7. [DOI] [PubMed]
Feller 1996 {published and unpublished data}
- Feller R, Kiger R, Triol C, Volpe A, Garcia L. Anticaries efficacy of a triclosan/copolymer dentifrice [abstract]. Journal of Dental Research 1993;72(Spec Iss):248 (Abstract No 1159). [Google Scholar]
- Feller RP, Kiger RD, Triol CW, Sintes JL, Garcia L, Petrone ME, et al. Comparison of the clinical anticaries efficacy of an 1100 NaF silica‐based dentifrice containing triclosan and a copolymer to an 1100 NaF silica‐based dentifrice without those additional agents: a study on adults in California. Journal of Clinical Dentistry 1996;7(4):85‐9. [PubMed] [Google Scholar]
Garcia‐Godoy 1990 {published and unpublished data}
- Garcia‐Godoy F, DeVizio W, Volpe AR, Ferlauto RJ, Miller JM. Effect of a triclosan/copolymer/fluoride dentifrice on plaque formation and gingivitis: a 7‐month clinical study. [Erratum appears in American Journal of Dentistry 1991;4(2):102]. American Journal of Dentistry 1990; Vol. 3 Spec No:S15‐26. [PubMed]
Hawley 1995 {published and unpublished data}
- Hawley GM, Hamilton FA, Worthington HV, Blinkhorn A, Davies RM. The clinical anti‐caries efficacy of a triclosan/copolymer/NaF dentifrice. Journal of Dental Research 1994;73(Spec Iss):240 (Abstract No 1106). [Google Scholar]
- Hawley GM, Hamilton FA, Worthington HV, Davies RM, Holloway PJ, Davies TG, et al. A 30‐month study investigating the effect of adding triclosan/copolymer to a fluoride dentifrice. Caries Research 1995; Vol. 29, issue 3:163‐7. [DOI] [PubMed]
Hu 1997 {published and unpublished data}
- Hu D, Zhang J, Wan H, Fan X. Efficacy of a triclosan/copolymer dentifrice: a six month study in China. Journal of Dental Research 1998;77(Spec Iss B):897 (Abstract No 2122). [Google Scholar]
- Hu D, Zhang J, Wan H, Zhang Y, Volpe AR, Petrone ME. Efficacy of a triclosan/copolymer dentifrice in the control of plaque and gingivitis: a six‐month study in China. Hua Xi Kou Qiang Yi Xue Za Zhi [West China Journal of Stomatology] 1997;15(4):333‐5. [PubMed] [Google Scholar]
Kanchanakamol 1995 {published and unpublished data}
- Kanchanakamol U, Umpriwan R, Jotikasthira N, Srisilapanan P, Tuongratanaphan S, Sholitkul W, et al. Reduction of plaque formation and gingivitis by a dentifrice containing triclosan and copolymer. Journal of Periodontology 1995; Vol. 66, issue 2:109‐12. [DOI] [PubMed]
- Kanchanakamol U, Umpriwan R, Jottkasthira N, Srisilapanan P. Reduction of plaque formation and gingivitis by a dentifrice containing triclosan/copolymer [abstract]. Journal of Dental Research 1995;74(Spec Iss):590 (Abstract No 1514). [DOI] [PubMed] [Google Scholar]
Kraivaphan 2006 {published data only}
- Kraivaphan P, Amornchat C, Triratana T, Leethochawalit U. Clinical effect of a triclosan containing dentifrice on gingivitis during pregnancy and post‐partum. Southeast Asian Journal of Tropical Medicine and Public Health 2006; Vol. 37, issue 4:820‐5. [PubMed]
Lindhe 1993 {published and unpublished data}
Liu 2002 {published data only}
- Liu H, Segreto VA, Baker RA, Vastola KA, Ramsey LL, Gerlach RW. Anticalculus efficacy and safety of a novel whitening dentifrice containing sodium hexametaphosphate: a controlled six‐month clinical trial. Journal of Clinical Dentistry 2002; Vol. 13, issue 1:25‐8. [PubMed]
Lobene 1991 {published and unpublished data}
- Lobene RR, Battista GW, Petrone DM, Volpe AR, Petrone ME. Clinical efficacy of an anticalculus fluoride dentifrice containing triclosan and a copolymer: a 6‐month study. American Journal of Dentistry 1991; Vol. 4, issue 2:83‐5. [PubMed]
Mankodi 1992 {published and unpublished data}
- Mankodi S, Walker C, Conforti N, DeVizio W, McCool JJ, Volpe AR. Clinical effect of a triclosan‐containing dentifrice on plaque and gingivitis: a six‐month study. Clinical Preventive Dentistry 1992; Vol. 14, issue 6:4‐10. [PubMed]
Mankodi 2011 {published and unpublished data}
- Mankodi S, Chaknis P, Panagakos FS, DeVizio W, Proskin HM. Comparative investigation of a dentifrice containing triclosan/copolymer/sodium fluoride and specially‐designed silica and a dentifrice containing 0.243% sodium fluoride in a silica base for the control of established supra‐gingival plaque and gingivitis: a 6‐month clinical study. American Journal of Dentistry 2011; Vol. 24 Spec No A, issue Spec No A:21A‐7A. [PubMed]
Mann 1996 {published and unpublished data}
- Mann J, Karniel C, Triol CW, Sintes JL, Garcia L, Petrone ME, et al. Comparison of the clinical anticaries efficacy of a 1500 NaF silica‐based dentifrice containing triclosan and a copolymer to a 1500 NaF silica‐ based dentifrice without those additional agents: a study on adults in Israel. Journal of Clinical Dentistry 1996;7(4):90‐5. [PubMed] [Google Scholar]
- Mann JKCTCVAGLMJ, Mann J, Karniel C, Triol C, Volpe A, Garcia L, et al. Clinical caries study of a triclosan/copolymer dentifrice. Journal of Dental Research 1993;72(Special Issue IADR Abstracts):248 (Abstract No 1158). [Google Scholar]
Mann 2001 {published and unpublished data}
- Mann J, Vered Y, Babayof I, Sintes J, Petrone ME, Volpe AR, et al. The comparative anticaries efficacy of a dentifrice containing 0.3% triclosan and 2.0% copolymer in a 0.243% sodium fluoride/silica base and a dentifrice containing 0.243% sodium fluoride/silica base: a two‐year coronal caries clinical trial on adults in Israel. Journal of Clinical Dentistry 2001; Vol. 12, issue 3:71‐6. [PubMed]
Mateu 2008 {published and unpublished data}
- Mateu FA, Boneta AE, DeVizio W, Stewart B, Proskin HM. A clinical investigation of the efficacy of two dentifrices for controlling established supragingival plaque and gingivitis. Journal of Clinical Dentistry 2008; Vol. 19, issue 3:85‐94. [PubMed]
McClanahan 1997 {published data only}
- McClanahan SF, Beiswanger BB, Bartizek RD, Lanzalaco AC, Bacca L, White DJ. A comparison of stabilized stannous fluoride dentifrice and triclosan/copolymer dentifrice for efficacy in the reduction of gingivitis and gingival bleeding: six‐month clinical results. Journal of Clinical Dentistry 1997;8(2 Spec No):39‐45. [PubMed] [Google Scholar]
Palomo 1994 {published and unpublished data}
- Palomo F, Wantland L, Sanchez A, DeVizio W, Petrone M, Volpe A, et al. Plaque/gingivitis efficacy of triclosan dentifrices. Journal of Dental Research 1993;72(Special Issue IADR Abstracts):334 (Abstract No 1849). [Google Scholar]
- Palomo F, Wantland L, Sanchez A, Volpe AR, McCool J, DeVizio W. The effect of three commercially available dentifrices containing triclosan on supragingival plaque formation and gingivitis: a six month clinical study. International Dental Journal 1994; Vol. 44, issue 1 Suppl 1:75‐81. [PubMed]
Pradeep 2012 {published data only}
Renvert 1995 {published data only}
Schiff 2006 {published and unpublished data}
- Schiff T, Proskin HM, Zhang YP, Petrone M, DeVizio W. A clinical investigation of the efficacy of three different treatment regimens for the control of plaque and gingivitis. Journal of Clinical Dentistry 2006; Vol. 17, issue 5:138‐44. [PubMed]
Svatun 1993 {published data only}
- Svatun B, Sadxton CA, Huntington E, Cummins D. The effects of three silica dentifrices containing Triclosan on supragingival plaque and calculus formation and on gingivitis. International Dental Journal 1993; Vol. 43, issue 4 Suppl 1:441‐52. [PubMed]
Triratana 1993 {published and unpublished data}
- Triartana T, Tuongratanaphan S, Rustogi K, Petrone M, Volpe A, Petrone D. Plaque/gingivitis effect of a triclosan/copolymer dentifrice. Journal of Dental Research 1993;72(Special Issue IADR Abstracts):334 (Abstract No 1850). [Google Scholar]
- Triratana T, Tuongratanaphan S, Kraivaphan P, Rustogi KN, Volpe AR. The effect on established plaque formation and gingivitis of a Triclosan/copolymer/fluoride dentifrice: a six month clinical study. Journal of the Dental Association of Thailand 1993;43(1):19‐28. [Google Scholar]
Triratana 1994 {published data only}
- Triratana T, Amornchat C, Kraivaphan P, Tandhachoon K. Clinical study of a triclosan/copolymer dentifrice on plaque formation, gingivitis and streptococci mutans level in saliva. Journal of the Dental Association of Thailand 1994; Vol. 44, issue 1:27‐31.
Triratana 2002 {published and unpublished data}
Vered 2009 {published and unpublished data}
- Vered Y, Zini A, Mann J, DeVizio W, Stewart B, Zhang YP, et al. Comparison of a dentifrice containing 0.243% sodium fluoride, 0.3% triclosan, and 2.0% copolymer in a silica base, and a dentifrice containing 0.243% sodium fluoride in a silica base: a three‐year clinical trial of root caries and dental crowns among adults. Journal of Clinical Dentistry 2009; Vol. 20, issue 2:62‐5. [PubMed]
References to studies excluded from this review
Archila 2004 {published data only}
- Archila L, Bartizek RD, Winston JL, Biesbrock AR, McClanahan SF, He T. The comparative efficacy of stabilized stannous fluoride/sodium hexametaphosphate dentifrice and sodium fluoride/triclosan/copolymer dentifrice for the control of gingivitis: a 6‐month randomized clinical study. Journal of Periodontology 2004; Vol. 75, issue 12:1592‐9. [DOI] [PubMed]
Bogren 2007 {published data only}
- Bogren A, Teles RP, Torresyap G, Haffajee AD, Socransky SS, Wennström JL. Clinical and microbiologic changes associated with the combined use of a powered toothbrush and a triclosan/copolymer dentifrice: a 3‐year prospective study. Journal of Periodontology 2007; Vol. 78, issue 9:1708‐17. [DOI] [PubMed]
Bogren 2008 {published data only}
Boneta 2010 {published data only}
- Boneta AE, Aguilar MM, Romeu FL, Stewart B, DeVizio W, Proskin HM. Comparative investigation of the efficacy of triclosan/copolymer/sodium fluoride and stannous fluoride/sodium hexametaphosphate/zinc‐lactate‐ dentifrices‐for‐the‐control of established supragingival plaque and gingivitis in a six‐month clinical study. Journal of Clinical Dentistry 2010; Vol. 21, issue 4:117‐23. [PubMed]
Charles 2001 {published data only}
- Charles CH, Sharma NC, Galustians HJ, Qaqish J, McGuire JA, Vincent JW. Comparative efficacy of an antiseptic mouthrinse and an antiplaque/antigingivitis dentifrice. A six‐month clinical trial. Journal of the American Dental Association 2001;132(5):670‐5. [DOI] [PubMed] [Google Scholar]
Cullinan 2003 {published data only}
de la Rosa 1992 {published data only}
- Rosa M, Schoroeder KL. Effects of a triclosan dentifrice on plaque and gingivitis in adolescents. Journal of Dental Research 1992;72(Spec Iss):583 (Abstract No 544). [Google Scholar]
Dóri 1999 {published data only}
- Dóri F, Gera I, Szabó G, Keglevich T, Nagy E. Laboratory and clinical investigation of multifunctional dentifrices. Clinical study of a new triclosan‐containing toothpaste. Fogorvosi Szemle 1999;92(3):67‐78. [PubMed] [Google Scholar]
Kocher 2000 {published data only}
Mankodi 2002 {published data only}
- Mankodi S, Lopez M, Smith I, Petrone DM, Petrone ME, Chaknis P, et al. Comparison of two dentifrices with respect to efficacy for the control of plaque and gingivitis, and with respect to extrinsic tooth staining: a six‐month clinical study on adults. Journal of Clinical Dentistry 2002; Vol. 13, issue 6:228‐33. [PubMed]
Winston 2002 {published data only}
- Winston JL, Bartizek RD, McClanahan SF, Mau MS, Beiswanger BB. A clinical methods study of the effects of triclosan dentifrices on gingivitis over six months. Journal of Clinical Dentistry 2002; Vol. 13, issue 6:240‐8. [PubMed]
Additional references
Ababneh 2012
- Ababneh KT, Abu Hwaij ZM, Khader YS. Prevalence and risk indicators of gingivitis and periodontitis in a multi‐centre study in North Jordan: a cross sectional study. BMC Oral Health 2012;12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alexander 2012
- Alexander DC. Selecting the right toothbrush for optimal patient care. Compendium of Continuing Education in Dentistry 2012;33(7):548‐52. [PubMed] [Google Scholar]
Blinkhorn 2009
- Blinkhorn A, Bartold PM, Cullinan MP, Madden TE, Marshall RI, Raphael SL, et al. Is there a role for triclosan/copolymer toothpaste in the management of periodontal disease?. British Dental Journal 2009;207(3):117‐25. [DOI] [PubMed] [Google Scholar]
Brown 2002
- Brown LJ, Johns BA, Wall TP. The economics of periodontal diseases. Periodontology 2000 2002;29:223‐34. [DOI] [PubMed] [Google Scholar]
Ciancio 2007
- Ciancio SG. Improving our patients' oral health: the role of a triclosan/copolymer/fluoride dentifrice. Compendium of Continuing Education in Dentistry 2007;28(4):178‐83. [PubMed] [Google Scholar]
CONSORT 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. International Journal of Surgery 2011;9(8):672‐7. [DOI] [PubMed] [Google Scholar]
Dalwai 2006
- Dalwai F, Spratt DA, Pratten J. Modeling shifts in microbial populations associated with health or disease. Applied and Environmental Microbiology 2006;72(5):3678‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 2004
- Davies RM, Ellwood RP, Davies GM. The effectiveness of a toothpaste containing triclosan and polyvinyl‐methyl ether maleic acid copolymer in improving plaque control and gingival health: a systematic review. Journal of Clinical Periodontology 2004;31(12):1029‐33. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schnerder M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Fejerskov 2008
- Fejerskov O, Kidd EAM, Nyvad B, Baelum V. Defining the disease: an introduction. In: Fejerskov O, Kidd E editor(s). Dental Caries: The Disease and its Clinical Management. 2nd Edition. Oxford: Blackwell Munksgaard, 2008:3‐6. [Google Scholar]
García‐Conde 2010
- García‐Conde GG, Santillana IA, Martínez‐Arroniz F, Huerta‐Herrera N, Islas‐Márquez AJ, Medina‐Solís CE. Periodontal treatment needs in adults from Mixteca rural area in Puebla State, Mexico. Revista de Salud Pública 2010;12(4):647‐57. [DOI] [PubMed] [Google Scholar]
GRADE 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gunsolley 2006
- Gunsolley JC. A meta‐analysis of six‐month studies of antiplaque and antigingivitis agents. Journal of the American Dental Association 2006;137(12):1649‐57. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lang 2009
- Lang NP, Schätzle MA, Löe H. Gingivitis as a risk factor in periodontal disease. Journal of Clinical Periodontology 2009;36 Suppl 10:3‐8. [DOI] [PubMed] [Google Scholar]
Li 2010
- Li Y, Lee S, Hujoel P, Su M, Zhang W, Kim J, et al. Prevalence and severity of gingivitis in American adults. American Journal of Dentistry 2010;23(1):9‐13. [PubMed] [Google Scholar]
Löe 1965
- Löe H, Theilade E, Jensen SB. Experimental gingivitis in man. The Journal of Periodontology 1965;36:177‐87. [DOI] [PubMed] [Google Scholar]
Marsh 1994
- Marsh PD. Microbial ecology of dental plaque and its significance in health and disease. Advances in Dental Research 1994;8(2):263‐71. [DOI] [PubMed] [Google Scholar]
Marsh 2006
- Marsh PD. Dental plaque as a biofilm and a microbial community ‐ implications for health and disease. BMC Oral Health 2006;6(Suppl 1):S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marthaler 2004
- Marthaler TM. Changes in dental caries 1953‐2003. Caries Research 2004;38(3):173‐81. [DOI] [PubMed] [Google Scholar]
Mayo 2010
- Mayo Clinic. Gingivitis, 2010. www.mayoclinic.com/health/gingivitis/DS00363 (accessed 19 November 2013).
McCarney 2007
- McCarney R, Warner J, Iliffe S, Haselen R, Griffin M, Fisher P. The Hawthorne effect: a randomised, controlled trial. BMC Medical Research Methodology 2007;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 2001
- Morris AJ, Steele J, White DA. The oral cleanliness and periodontal health of UK adults in 1998. British Dental Journal 2001;191(4):186‐92. [DOI] [PubMed] [Google Scholar]
Mustafa 2005
- Mustafa M, Wondimu B, Yucel‐Lindberg T, Kats‐Hallström AT, Jonsson AS, Modéer T. Triclosan reduces microsomal prostaglandin E synthase‐1 expression in human gingival fibroblasts. Journal of Clinical Periodontology 2005;32(1):6‐11. [DOI] [PubMed] [Google Scholar]
Needleman 2004
- Needleman I, McGrath C, Floyd P, Biddle A. Impact of oral health on the life quality of periodontal patients. Journal of Clinical Periodontology 2004;31(6):454‐7. [DOI] [PubMed] [Google Scholar]
Needleman 2005
- Needleman I, Suvan J, Moles DR, Pimlott J. A systematic review of professional mechanical plaque removal for prevention of periodontal diseases. Journal of Clinical Periodontology 2005;32 Suppl 6:229‐82. [DOI] [PubMed] [Google Scholar]
Neely 2005
- Neely AL, Holford TR, Löe H, Anerud A, Boysen H. The natural history of periodontal disease in humans: risk factors for tooth loss in caries‐free subjects receiving no oral health care. Journal of Clinical Periodontology 2005;32(9):984‐93. [DOI] [PubMed] [Google Scholar]
NICE 2012
- National Institute for Health and Care Excellence (NICE). NICE Clinical Knowledge Summaries. Gingivitis and Periodontitis, 2012. http://cks.nice.org.uk/gingivitis‐and‐periodontitis#!backgroundsub:10 (accessed 24 January 2013).
Petersen 2003
- Petersen PE. The World Oral Health Report 2003: continuous improvement of oral health in the 21st century ‐ the approach of the WHO Global Oral Health Programme. Community Dentistry and Oral Epidemiology 2003;31 Suppl 1:3‐23. [DOI] [PubMed] [Google Scholar]
Petticrew 2003
- Petticrew M, Roberts H. Evidence, hierarchies, and typologies: horses for courses. Journal of Epidemiology and Community Health 2003;57(7):527‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pitts 2004
- Pitts NB. Are we ready to move from operative to non‐operative/preventive treatment of dental caries in clinical practice?. Caries Research 2004;38(3):294‐304. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:549. [DOI] [PubMed] [Google Scholar]
Rücker 2008
- Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta‐analyses with binary outcomes. Statistics in Medicine 2008;27(5):746‐63. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Schulz 2002
- Schulz KF, Grimes DA. Generation of allocation sequences in randomised trials: chance, not choice. Lancet 2002;359(9305):515‐9. [DOI] [PubMed] [Google Scholar]
Selwitz 2007
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet 2007;369(9555):51‐9. [DOI] [PubMed] [Google Scholar]
Seymour 2007
- Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clinical Microbiology and Infection 2007;13 Suppl 4:3‐10. [DOI] [PubMed] [Google Scholar]
Simpson 2010
- Simpson TC, Needleman I, Wild SH, Moles DR, Mills EJ. Treatment of periodontal disease for glycaemic control in people with diabetes. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD004714.pub2] [DOI] [PubMed] [Google Scholar]
SPIRIT 2013
- Chan A‐W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža‐Jerić K, et al. SPIRIT 2013 Statement: Defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158(3):200‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2012
- World Health Organization (WHO). Oral Health: Key Facts, 2012. www.who.int/mediacentre/factsheets/fs318/en/index.html (accessed 25 January 2013).
Zhang 2010
- Zhang J, Xuan D, Fan W, Zhang X, Dibart S, Vizio W, et al. Severity and prevalence of plaque‐induced gingivitis in the Chinese population. Compendium of Continuing Education in Dentistry 2010;31(8):624‐9. [PubMed] [Google Scholar]


