Abstract
BACKGROUND
Alzheimer’s disease is characterized by the deposition of amyloid-beta (Aβ) plaques in the brain. Aβ is produced from the sequential cleavage of amyloid precursor protein by β-site amyloid precursor protein–cleaving enzyme 1 (BACE-1) followed by y-secretase. Verubecestat is an oral BACE-1 inhibitor that reduces the Aβ level in the cerebrospinal fluid of patients with Alzheimer’s disease.
METHODS
We conducted a randomized, double-blind, placebo-controlled, 78-week trial to evaluate verubecestat at doses of 12 mg and 40 mg per day, as compared with placebo, in patients who had a clinical diagnosis of mild-to-moderate Alzheimer’s disease. The coprimary outcomes were the change from baseline to week 78 in the score on the cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-cog; scores range from 0 to 70, with higher scores indicating worse dementia) and in the score on the Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory scale (ADCS-ADL; scores range from 0 to 78, with lower scores indicating worse function).
RESULTS
A total of 1958 patients underwent randomization; 653 were randomly assigned to receive verubecestat at a dose of 12 mg per day (the 12-mg group), 652 to receive verubecestat at a dose of 40 mg per day (the 40-mg group), and 653 to receive matching placebo. The trial was terminated early for futility 50 months after onset, which was within 5 months before its scheduled completion, and after enrollment of the planned 1958 patients was complete. The estimated mean change from baseline to week 78 in the ADAS-cog score was 7.9 in the 12-mg group, 8.0 in the 40-mg group, and 7.7 in the placebo group (P=0.63 for the comparison between the 12-mg group and the placebo group and P=0.46 for the comparison between the 40-mg group and the placebo group). The estimated mean change from baseline to week 78 in the ADCS-ADL score was −8.4 in the 12-mg group, −8.2 in the 40-mg group, and −8.9 in the placebo group (P=0.49 for the comparison between the 12-mg group and the placebo group and P=0.32 for the comparison between the 40-mg group and the placebo group). Adverse events, including rash, falls and injuries, sleep disturbance, suicidal ideation, weight loss, and hair-color change, were more common in the verubecestat groups than in the placebo group.
CONCLUSIONS
Verubecestat did not reduce cognitive or functional decline in patients with mild-to-moderate Alzheimer’s disease and was associated with treatment-related adverse events.(ClinicalTrials.gov.)
ALZHEIMER’S DISEASE IS CHARACTERized by the deposition of amyloid-beta (Aβ) aggregates and neurofibrillary tangles in the brain.1 The amyloid cascade hypothesis proposes that Aβ aggregates trigger the spreading of tau-related neurofibrillary tangles and subsequent neuronal degeneration.2 Aβ is produced when amyloid precursor protein (APP) is cleaved sequentially by β-site APP–cleaving enzyme 1 (BACE-1; also referred to as β-secretase) and γ-secretase.3 Inhibition of BACE-1 is a potential therapeutic strategy for slowing the progression of Alzheimer’s disease by reducing the production of Aβ. This approach differs from previous approaches in which monoclonal antibodies were used to clear Aβ from the brain; these earlier strategies showed a modest effect on measures of amyloid deposition, resulted in little or no clinical efficacy in patients with symptomatic Alzheimer’s disease, and have been associated with amyloid-related imaging abnormalities.4–6 Other approaches to reducing amyloid burden, such as γ-secretase inhibitors or modulators,7,8 and active immunotherapy9 have also been unsuccessful.
Verubecestat is an oral BACE-1 inhibitor that has been shown to reduce the Aβ level in the cerebrospinal fluid and brain of rodents and nonhuman primates by more than 90%10,11 and in the cerebrospinal fluid of healthy people and of patients with Alzheimer’s disease by more than 75%.11 We conducted a randomized, placebo-controlled, phase 3 trial to determine whether verubecestat at a dose of 12 mg per day or 40 mg per day could slow disease progression in patients with mild-to-moderate Alzheimer’s disease.
METHODS
PATIENT POPULATION
Patients were eligible for enrollment in the trial if they were between 55 and 85 years of age and if they met standard research and clinical criteria for dementia that was probably due to Alzheimer’s disease.12,13 All the patients underwent appropriate medical and neurologic evaluations, including magnetic resonance imaging (MRI) (or computed tomography if MRI was contraindicated), to exclude patients who had alternative causes of dementia. Entry criteria included a score of 15 to 26 on the Mini-Mental State Examination (MMSE), which represented mild or moderate dementia (scores range from 0 to 30, with lower scores indicating poorer cognitive performance).14 Patients could have been receiving an acetylcholinesterase inhibitor, memantine, or both, provided that they were receiving a stable dose for 3 months before screening. The diagnosis of Alzheimer’s disease was confirmed by an independent expert who reviewed the investigator’s written narrative of the patient’s history, including pertinent laboratory data and baseline clinical measures.
TRIAL DESIGN
The trial was conducted at 238 centers in 21 countries from November 2012 through April 2017. A list of investigators is provided in the Supplementary Appendix, available with the full text of this article at NEJM.org. The trial consisted of a randomized, double-blind, placebo-controlled, parallel-group, 78-week trial period (part 1), followed by an optional extension period with a total planned duration of up to 5 years (part 2). Part 1 was designed to include a phase 2 lead-in safety cohort component that was intended to transition to a phase 3 trial if satisfactory safety results were observed. In the phase 2 lead-in safety period, patients were randomly assigned to receive, once daily, one of three oral dose levels of verubecestat (12 mg, 40 mg, or 60 mg) or placebo. These dose levels were selected on the basis of data from phase 1 studies in humans that suggested that doses of 12 mg and 40 mg reduced levels of Aβ−40 and Aβ−42, the major metabolites of BACE-1 cleavage of APP, in cerebrospinal fluid by 60% (12 mg) or 75% (40 mg).11 The 60-mg dose level was included in the phase 2 lead-in safety cohort to explore the safety of this high dose and was prespecified to be dropped for the phase 3 component of the trial. All the assigned trial regimens were administered as identical-appearing tablets.
The first planned interim analysis, which was conducted 3 months after the randomization of 200 patients, informed the decision to progress to phase 3. The data from these first 200 patients were excluded from the primary efficacy and safety analyses. Randomization continued during the 3 months after the 200th patient was enrolled, during which time approximately 200 additional patients were randomly assigned to a trial group, including 53 patients who were assigned to receive a 60-mg dose of verubecestat and whose data were excluded from the primary analyses. The patients who had been assigned to receive the 60-mg dose were switched to the 40-mg dose for the remainder of the trial. Patients who completed the 78-week trial period could enter the extension period, in which patients in the placebo group were switched to the 40-mg dose while patients who had been receiving the 12-mg or the 40-mg dose continued to receive the same dose to which they had been assigned, with preserved masking of doses. The trial design is described in detail in the protocol, available at NEJM.org.
An interactive voice-response system randomly assigned patients according to a computer-generated assignment schedule. Randomization was stratified according to geographic region, baseline severity of disease (mild [MMSE score of 21 to 26] or moderate [MMSE score of 15 to 20]), and use of memantine or anticholinesterase-inhibiting medications.
We performed biomarker substudies to evaluate certain biomarkers in cerebrospinal fluid and to assess amyloid burden with the use of positron-emission tomography (PET). All the patients enrolled in the trial were eligible for participation in the substudy of biomarkers in cerebrospinal fluid (provided that the investigative site where the patient was enrolled was willing to participate in the substudy), but the assessment of amyloid burden (PET amyloid substudy) was conducted only at sites that were near a PET ligand production facility at the start of enrollment and were willing to participate in the substudy.
ASSESSMENTS
Evaluation of clinical efficacy included assessment of cognition according to the 11-item cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-cog; scores range from 0 to 70, with higher scores indicating worse dementia)15 and according to the MMSE,14 assessment of dementia according to the Clinical Dementia Rating Scale-Sum of Boxes (CDR-SB; scores range from 0 to 18, with higher scores indicating worse dementia),16 and assessment of daily function according to the 23-item version of the Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory scale (ADCS-ADL; scores range from 0 to 78, with lower scores indicating worse function).17 Sessions during which trial personnel performed the outcome assessments in patients were recorded, and a subset of these sessions underwent quality review by independent central experts, who provided feedback to the trial personnel for more than 15,000 interviews. Assessment of neuropsychiatric symptoms was performed with the use of the Neuropsychiatric Inventory (NPI; scores range from 1 to 144, with higher scores indicating more severe symptoms).18
Safety assessments included evaluation of adverse events, routine laboratory testing, electrocardiography, and physical examinations. Initially, routine MRI was performed to assess possible instances of amyloid-related imaging abnormalities, but the use of MRI was subsequently discontinued during the trial on the basis of regulatory feedback and feedback from the members of the data and safety monitoring committee, who indicated that it was no longer required. Comprehensive ophthalmologic and dermatologic examinations were also performed at baseline and at selected clinic visits as described in the protocol. Suicidality was assessed at every clinic visit with the use of the Columbia Suicide Severity Rating Scale, a six-question instrument that qualitatively rates the degree of suicidality.19
MRI structural measures of hippocampal volume were assessed by means of an automated segmentation method. The change in hippocampal volume was determined with the use of a tensor-based morphometry algorithm developed by Bioclinica. For the PET amyloid substudy, brain amyloid load was assessed by PET with the use of 18F-flutemetamol. A composite cortical index of amyloid burden was computed as the average of the regional standardized uptake value ratio in the following cortical areas, with a sub-cortical white-matter region used as the reference: frontal, temporal, and parietal lobes; the anterior and posterior cingulate cortex; and the precuneus.20 No partial volume correction was applied. For the substudy of biomarkers in cerebrospinal fluid, concentrations of total tau, phosphorylated tau, Aβ−40, Aβ−42, and sAPPβ were measured in a subgroup of patients.21
OVERSIGHT
The trial was conducted in accordance with International Conference on Harmonisation Good Clinical Practice guidelines and was approved by the relevant institutional review boards. Written informed consent was provided by the patients or their legal representatives. The sponsor (Merck) designed the trial in consultation with the academic authors. Data were collected by the investigators, analyzed by the sponsor, and interpreted by all the authors. The first draft of the manuscript was prepared by a professional medical writer (employed by the sponsor) and the first author. All the authors approved subsequent drafts and agreed to submit the manuscript for publication. The authors had full access to the trial data and vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. The trial was governed by three committees as described in the protocol.
OUTCOMES
The coprimary efficacy outcomes were the change from baseline in the score on the ADAS-cog and the ADCS-ADL at week 78. The secondary outcomes were the change from baseline at week 78 in the CDR-SB score, the total hippocampal volume as assessed by MRI, the concentration of total tau in cerebrospinal fluid, the brain amyloid load as assessed by PET, the MMSE score, and the NPI score. Exploratory outcomes included the change from baseline in other cerebrospinal fluid measures.
STATISTICAL ANALYSIS
The efficacy analyses were performed in the primary population, which included all patients who underwent randomization except the first 200 patients enrolled in the study and the patients who were randomly assigned to receive the 60-mg dose of verubecestat. We performed the efficacy analyses in a subgroup of the primary population — the full-analysis set — using a modified intention-to-treat approach. The full-analysis set included patients who received at least one dose of the trial regimen and who had both a baseline outcome measurement and at least one postrandomization outcome measurement that was obtained within a window of 6 weeks before to 6 weeks after a scheduled assessment visit. When individual subscores were missing, they may have been imputed (subject to restrictions) with the use of the last-observation-carried-forward method to enable the computation of a total score. No imputation was used for total scores. Sensitivity analyses that were planned to explore the results of the efficacy analyses under the assumption that data were not missing at random were not performed given the negative results of the trial.
We used a longitudinal analysis of covariance model to analyze changes in scores, with time considered to be a categorical variable. The model included adjustment for geographic region, trial-group assignment, sex, APOE4 genotype (carrier vs. noncarrier), baseline use of vitamin E (0 to 400 IU per day vs. >400 IU per day), baseline use of medication for Alzheimer’s disease (use vs. no use), trial cohort (safety cohort [patients enrolled before the decision to progress to phase 3] vs. main cohort [patients enrolled after the decision to progress to phase 3]), and the interaction between time and trial-group assignment, with the baseline values of MMSE score and age included as continuous covariates. The baseline value of the dependent variable and the interaction between the baseline value and time were also included. The mean differences between the trial groups (each verubecestat dose group vs. placebo) in the changes from baseline to week 78, as well as the confidence intervals and two-sided P values, were estimated from this model. An unstructured covariance matrix was used to model the correlation among repeated measurements. A Bonferroni correction (which was applied to both dose levels of verubecestat) in conjunction with a closed sequential testing approach was used to control for the type 1 error rate, with testing of primary outcomes and then secondary outcomes, in the order described in the statistical plan in the protocol.
All patients in the primary population who received at least one dose of verubecestat or placebo were included in the safety analyses. Prespecified adverse events of interest included amyloid-related imaging abnormalities of microhemorrhage, superficial siderosis, or macrohemorrhage and amyloid-related imaging abnormalities of incident vasogenic edema on MRI of the head; delirium; and clinically significant rash. All statistical analyses were performed with the use of SAS software, versions 9.3 and 9.4 (SAS Institute).
We calculated that 570 patients per trial group would be needed to provide the trial with 90% overall power to show a significant difference between at least one of the dose levels of verubecestat and placebo in both coprimary efficacy outcomes. This calculation was based on an anticipated dropout rate of 5.8% every 13 weeks (which would represent a 30% cumulative dropout rate at 78 weeks) and on an assumed drug effect of 35% for both dose levels (which would correspond to a 2-point difference in the ADAS-cog score and a 3.4-point difference in the ADCS-ADL score between patients receiving verubecestat and those receiving placebo, with 18-month rates of disease progression in the placebo group estimated primarily from data published by Schneider and Sano22). Although subgroup analyses that were based on patient characteristics were prespecified, the trial was not adequately powered for such analyses. Interim analyses performed during the trial are described in the protocol and in the Supplementary Appendix.
RESULTS
PATIENT CHARACTERISTICS
A total of 1958 patients were included in the primary population; 653 were randomly assigned to receive verubecestat at a dose of 12 mg per day (the 12-mg group), 652 to receive verubecestat at a dose of 40 mg per day (the 40-mg group), and 653 to receive matching placebo. Of these, 1 patient in the 12-mg group did not receive at least one dose of the trial regimen. The number of patients included in the full-analysis set differed depending on the particular outcome measure assessed (Table 1). A total of 1389 patients (70.2 to 72.1% of the patients in each group) completed part 1 of the trial (Fig. 1), and 1042 of these patients (75.0%) entered part 2 (the optional extension component) (Fig. S1 in the Supplementary Appendix). The decision to stop the trial was made in February 2017 at the recommendation of the independent data and safety monitoring committee on the basis of futility (see the Supplementary Appendix for a description of the stopping rules). At the time of trial termination, enrollment of the planned 1958 patients was complete, and 5 months remained before the scheduled completion of part 1. A total of 39 patients (6.0%) in the 12-mg group, 49 patients (7.5%) in the 40-mg group, and 31 patients (4.7%) in the placebo group withdrew prematurely from the trial because of adverse events. None of the patients in the trial completed part 2 owing to the early termination of the trial (Fig. S1 in the Supplementary Appendix).
Table 1.
Demographic and Baseline Clinical Characteristics.*
| Characteristic | Verubecestat 12-mg Group (N = 652) |
Verubecestat 40-mg Group (N = 652) |
Placebo Group (N = 653) |
|---|---|---|---|
| Demographics | |||
| Age — yr | 71.3±7.4 | 71.8±7.6 | 72.4±7.6 |
| Female sex — no. (%) | 350 (53.7) | 379 (58.1) | 354 (54.2) |
| Race — no.(%)† | |||
| White | 523 (80.2) | 511 (78.4) | 532 (81.5) |
| Asian | 113 (17.3) | 120 (18.4) | 109 (16.7) |
| Other | 11 (1.7) | 14 (2.1) | 10 (1.5) |
| Data not reported | 5 (0.8) | 7 (1.1) | 2 (0.3) |
| Geographic region — no. (%) | |||
| United States or Canada | 269 (41.3) | 271 (41.6) | 270 (41.3) |
| Europe, Australia, or New Zealand | 203 (31.1) | 201 (30.8) | 203 (31.1) |
| Japan | 92 (14.1) | 89 (13.7) | 90 (13.8) |
| Other | 88 (13.5) | 91 (14.0) | 90 (13.8) |
| APOE4 carrier — no. (%) | 424 (65.0) | 400 (61.3) | 415 (63.6) |
| Alzheimer’s disease of mild severity, as evidenced by MMSE score of ≥21 — no. (%)‡ | 318 (48.8) | 305 (46.8) | 314 (48.1) |
| Treatment with acetylcholinesterase inhibitor, memantine, or both for Alzheimer’s disease — no. (%) | 576 (88.3) | 576 (88.3) | 583 (89.3) |
| Educational level below undergraduate degree — no. (%) | 392 (60.1) | 390 (59.8) | 394 (60.3) |
| Positive biomarker test for Alzheimer’s disease — no./total no. (%) | |||
| According to PET§ | 43/47 (91.5) | 23/25 (92.0) | 30/34 (88.2) |
| According to CSF analysis¶ | 54/56 (96.4) | 67/72 (93.1) | 50/55 (90.9) |
| Clinical outcome measures∥ | |||
| ADAS-cog | |||
| No. of patients assessed | 631 | 626 | 644 |
| Score | 21.3±7.5 | 21.4±7.6 | 21.7±7.6 |
| ADCS-ADL | |||
| No. of patients assessed | 627 | 622 | 636 |
| Score | 63.1±9.4 | 62.9±9.9 | 62.1±10.5 |
| CDR-SB | |||
| No. of patients assessed | 611 | 600 | 623 |
| Score | 5.4±2.1 | 5.4±2.1 | 5.6±2.3 |
| MMSE | |||
| No. of patients assessed | 610 | 600 | 628 |
| Score | 20.4±3.3 | 20.2±3.3 | 20.3±3.3 |
| NPI | |||
| No. of patients assessed | 632 | 631 | 639 |
| Score | 8.8±10.6 | 8.2±9.6 | 9.3±11.7 |
| Biomarkers∥ | |||
| Hippocampal volume on MRI — μl | |||
| No. of patients assessed | 308 | 281 | 308 |
| Value | 5875±1217 | 5795±1194 | 5812±1067 |
| CSF concentration of total tau — pg/ml | |||
| No. of patients assessed | 32 | 46 | 33 |
| Value | 211±95 | 243±120 | 254±211 |
| Cortical amyloid load on PET — standardized uptake value ratio | |||
| No. of patients assessed | 20 | 10 | 14 |
| Value | 0.89±0.10 | 0.87±0.11 | 0.88±0.11 |
Plus-minus values are means ±SD. This table summarizes data from patients in the primary population who received at least one dose of the trial regimen. The primary population included all patients who underwent randomization except the first 200 patients enrolled in the study and the patients who were randomly assigned to receive the 60-mg dose of verubecestat. Data from 1 patient in the 12-mg group who did not receive the trial regimen were excluded from this summary. Percentages may not sum to 100 because of rounding.
Race was reported by the patient.
Scores on the Mini–Mental State Examination (MMSE) range from 0 to 30, with lower scores indicating poorer cognitive performance.
The patient’s diagnosis was based on the visual read of a positron-emission tomography (PET) scan with 18F-flutemetamol that was performed according to the product label.
The patient’s diagnosis was based on the cerebrospinal fluid (CSF) concentration of tau or Aβ−42 with a cutoff level of 0.215.21
Results are provided for patients who were included in the full-analysis set. This subgroup consisted of patients who received at least one dose of the trial regimen and who had a baseline measurement and at least one postrandomization outcome measurement that was obtained within a window of ±6 weeks of a scheduled assessment visit. The number of patients analyzed differed depending on the particular outcome measure assessed. Alzheimer’s Disease Assessment Scale (ADAS-cog) scores range from 0 to 70, with higher scores indicating worse dementia. Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory scale (ADCS-ADL) scores range from 0 to 78, with lower scores indicating worse function. Clinical Dementia Rating Scale-Sum of Boxes (CDR-SB) scores range from 0 to 18, with higher scores indicating worse dementia. Neuropsychiatric Inventory (NPI) scores range from 1 to 144, with higher scores indicating more severe symptoms. MRI, CSF measurements, and PET were performed in a subgroup of patients.
Figure 1. Randomization, Trial-Group Assignment, and Follow-up for Part 1 of the Trial.
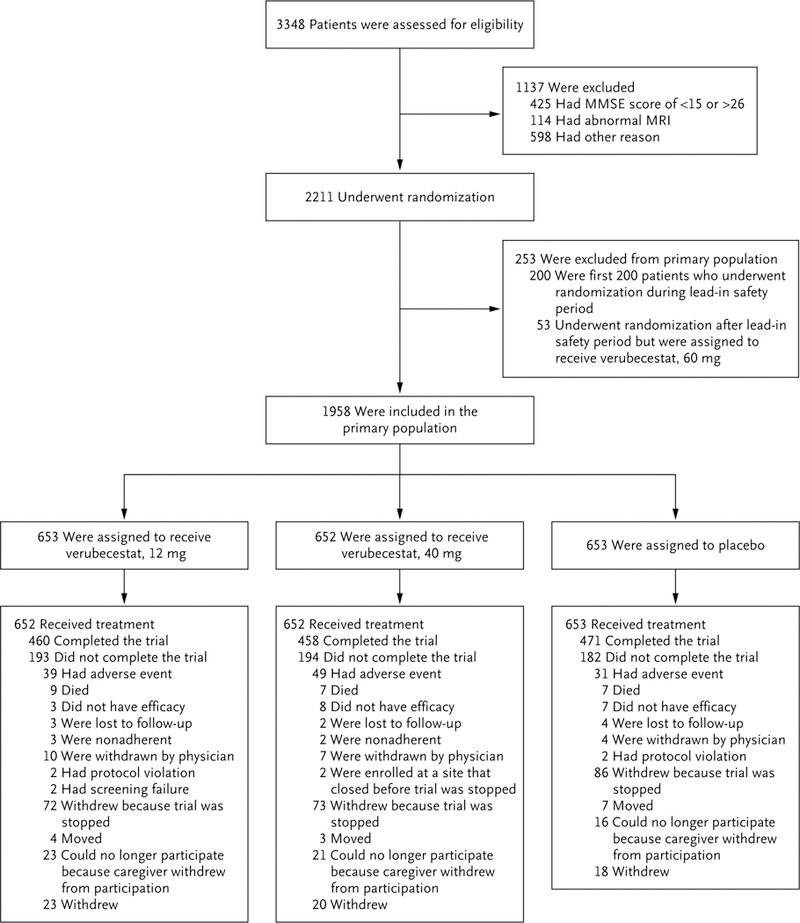
Part 1 of the trial was designed to include a phase 2 lead-in safety cohort component. In the phase 2 lead-in safety period, patients were randomly assigned to one of three dose levels of verubecestat (12 mg, 40 mg, or 60 mg) or placebo. The first planned interim analysis, which was conducted 3 months after the first 200 patients underwent randomization, informed the decision to progress to phase 3. The data from these first 200 patients were excluded from the primary efficacy and safety analyses. Randomization continued during the 3 months after the 200th patient was enrolled, during which time approximately 200 additional patients were randomly assigned to a trial group, including 53 patients who were assigned to receive a 60-mg dose of verubecestat and whose data were excluded from the primary analyses. Scores on the Mini–Mental State Examination (MMSE) range from 0 to 30, with lower scores indicating poorer cognitive performance. MRI denotes magnetic resonance imaging.
Patient characteristics and baseline dementia scores were similar among the three trial groups and reflected mild-to-moderate Alzheimer’s disease (Table 1, and Table S1 in the Supplementary Appendix). The results of the PET amyloid assessment and the assessment of biomarkers in cerebrospinal fluid at baseline showed that approximately 90% of the patients in each group met the criteria for Alzheimer’s disease (Table 1).
CLINICAL OUTCOMES
In all three trial groups, the scores for the coprimary outcomes of cognition (ADAS-cog) and function (ADCS-ADL) worsened over time (Table 2 and Fig. 2, and Table S2 in the Supplementary Appendix). The declines in the verubecestat groups did not differ significantly from those in the placebo group at week 78 for either of the coprimary outcomes. The mean change from baseline to week 78 in the ADAS-cog score was 7.9 in the 12-mg group, 8.0 in the 40-mg group, and 7.7 in the placebo group (P=0.63 for the comparison between the 12-mg group and the placebo group and P=0.46 for the comparison between the 40-mg group and the placebo group). The mean change from baseline to week 78 in the ADCS-ADL score was −8.4 in the 12-mg group, −8.2 in the 40-mg group, and −8.9 in the placebo group (P=0.49 for the comparison between the 12-mg group and the placebo group and P = 0.32 for the comparison between the 40-mg group and the placebo group). There was no treatment benefit with verubecestat as compared with placebo at time points earlier than 78 weeks. Scores for the secondary clinical outcomes of CDR-SB, MMSE, and NPI worsened over time in all three trial groups, and there were no significant differences between verubecestat and placebo for any of these outcomes (Table 2, and Table S2 and Figs. S2, S6, and S7 in the Supplementary Appendix). In an exploratory subgroup analysis of the coprimary outcomes (ADAS-cog and ADCS-ADL), no significant differences between the placebo group and either verubecestat group were noted either among patients with mild Alzheimer’s disease (as defined by the score on the MMSE) or among patients who tested positive for APOE4 (Table S3 in the Supplementary Appendix).
Table 2.
Change in Primary and Secondary Outcomes from Baseline to Week 78.*
| Outcome | Change from Baseline | Difference between Verubecestat Groups and Placebo Group in Mean Change† |
|||||
|---|---|---|---|---|---|---|---|
| Verubecestat 12-mg Group |
Verubecestat 40-mg Group |
Placebo Group |
Verubecestat 12-mg Group vs. Placebo Group (97.51% CI or 95% CI)‡ |
P Value§ | Verubecestat 40-mg Group vs. Placebo Group (97.51% CI or 95% CI)‡ |
P Value§ | |
| Primary | |||||||
| ADAS-cog score | 7.9±0.3 | 8.0±0.4 | 7.7±0.3 | 0.2 (−0.9 to 1.3) | 0.63 | 0.4 (−0.8 to 1.5) | 0.46 |
| ADCS-ADL score | −8.4±0.5 | −8.2±0.5 | −8.9±0.5 | 0.5 (−1.1 to 2.1) | 0.49 | 0.7 (−0.9 to 2.3) | 0.32 |
| Secondary | |||||||
| CDR-SB score | 2.1±0.1 | 2.1±0.1 | 2.1±0.1 | 0.0 (−0.4 to 0.3) | 0.0 (−0.3 to 0.4) | ||
| Hippocampal volume on MRI | |||||||
| No. of patients assessed | 308 | 281 | 308 | ||||
| % change | −5.6±0.1 | −5.7±0.1 | −5.0±0.1 | −0.6 (−1.0 to−0.2) | −0.7 (−1.1 to-0.3) | ||
| CSF concentration of total tau † | |||||||
| No. of patients assessed | 32 | 46 | 33 | ||||
| Magnitude of change | 1.02±0.03 | 1.04±0.02 | 1.07±0.03 | 0.95 (0.87 to 1.04) | 0.97 (0.90 to 1.05) | ||
| Cortical amyloid load on PET | |||||||
| No. of patients assessed | 20 | 10 | 14 | ||||
| Standardized uptake value ratio | −0.02±0.01 | −0.04±0.01 | 0.00±0.01 | −0.03 (−0.05 to 0.00) | −0.04 (−0.06 to −0.02) | ||
| MMSE score | −3.9±0.2 | −3.6±0.2 | −4.1±0.2 | 0.2 (−0.3 to 0.7) | 0.5 (0.0 to 1.0) | ||
| NPI score | 3.4±0.5 | 3.8±0.5 | 2.7±0.5 | 0.7 (−0.6 to 2.1) | 1.1 (−0.4 to 2.6) | ||
Plus-minus values are model-based least-squares means ±SE. This table summarizes data from patients in the primary population who received at least one dose of the trial regimen. For ADAS-cog (with scores ranging from 0 to 70), CDR-SB (with scores ranging from 0 to 18), and NPI (with scores ranging from 1 to 144), a higher positive mean change score corresponds to greater decline relative to baseline and a positive treatment difference favors placebo. For ADCS-ADL (with scores ranging from 0 to 78) and MMSE (with scores ranging from 0 to 30), a higher negative mean change score corresponds to greater decline relative to baseline and a positive treatment difference favors verubecestat. For biomarkers other than the CSF concentration of total tau, a negative mean change corresponds to a reduction in the biomarker value relative to baseline and a negative difference corresponds to a greater reduction with verubecestat than with placebo.
For the CSF concentration of total tau only, the change refers to the magnitude of the change and the difference refers to the ratio of the magnitude of the change with verubecestat to the magnitude of the change with placebo. The standard error is presented on the log scale. A magnitude of change greater than 1 indicates an increase in total tau relative to baseline, and a ratio of change in magnitude of less than 1 indicates a smaller increase with verubecestat than with placebo.
According to the strategy of adjustment for multiple testing, 97.51% confidence intervals are presented for all outcomes except for MMSE and NPI, for which 95% confidence intervals are presented.
According to the strategy of adjustment for multiple testing, formal hypothesis testing ceased within each dose level after the first hypothesis (which was based on the results of the ADAS-cog analysis) failed to be rejected.
Figure 2. Mean Change from Baseline in the ADAS-cog and ADCS-ADL Scores over 78 Weeks (Part 1 of the Trial).
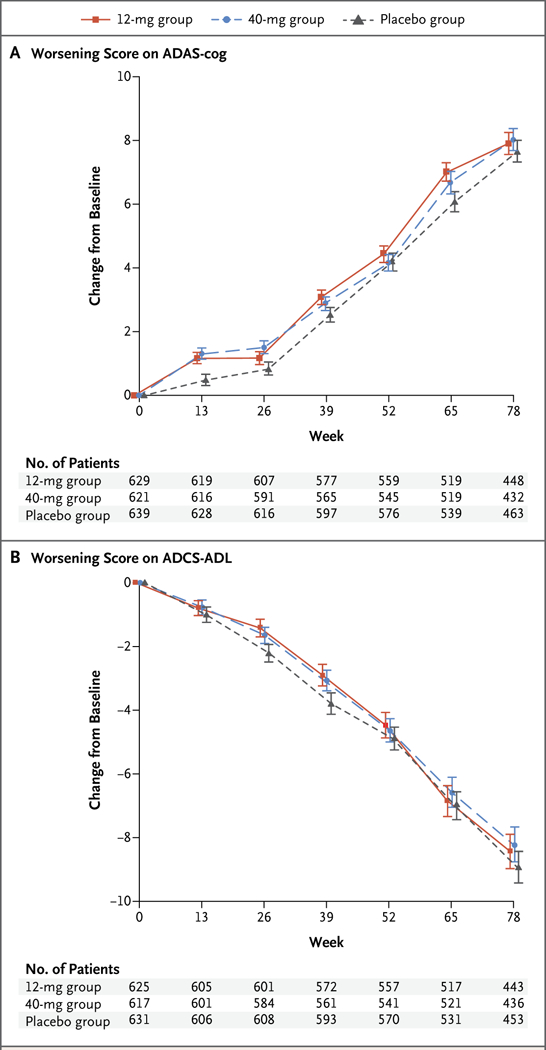
Panel A shows the mean change from baseline in the score on the cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-cog); scores range from 0 to 70, with higher scores indicating worse dementia. Panel B shows the mean change from baseline in the score on the Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory scale (ADCS-ADL); scores range from 0 to 78, with lower scores indicating worse function. I bars indicate standard errors.
BIOMARKERS
The hippocampal volume, as assessed by MRI, was lower at week 78 than at baseline in all three trial groups, but the reductions in the verubecestat groups were numerically greater than the reduction in the placebo group (Table 2, and Fig. S3 in the Supplementary Appendix).
In the PET amyloid substudy, no change from baseline in the brain amyloid load was observed in the placebo group at week 78; in contrast, both verubecestat groups showed a reduction from baseline (Table 2, and Fig. S5 in the Supplementary Appendix). In the 40-mg group, the mean (±SD) standardized uptake value ratio changed from 0.87±0.11 at baseline to 0.83±0.10 at week 78.
In the substudy of biomarkers in cerebrospinal fluid, the concentration of total tau increased by 7.5% from baseline to week 78 in the placebo group, but there were no marked differences between the verubecestat groups and the placebo group in either the change in total tau or the change in phosphorylated tau from baseline to week 78 (Table 2, and Table S4 and Fig. S4 in the Supplementary Appendix). The changes from baseline to week 78 in the concentrations of Aβ−40, Aβ−42, and sAPPβ in cerebrospinal fluid were less than 10% in the placebo group, whereas verubecestat was associated with reductions of 71.1 to 80.6% in Aβ−40, reductions of 62.7 to 76.4% in Aβ−42, and reductions of 76.6 to 86.1% in sAPPβ (Table S4 in the Supplementary Appendix).
SAFETY
Adverse events were more common with verubecestat than with placebo, and the percentage of patients who had an adverse event that led to discontinuation of the trial regimen was 9.4% in the 40-mg group, 8.3% in the 12-mg group, and 5.8% in the placebo group (Table 3). Serious adverse events that were reported in more than 1% of the patients in at least one trial group were syncope (7 of 652 patients [1.1%] in the 12-mg group, 8 of 652 patients [1.2%] in the 40-mg group, and 5 of 653 patients [0.8%] in the placebo group) and basal-cell carcinoma (8 of 652 patients [1.2%] in the 12-mg group, 9 of 652 patients [1.4%] in the 40-mg group, and 15 of 653 patients [2.3%] in the placebo group). There were 9 deaths in the 12-mg group, 12 deaths in the 40-mg group, and 5 deaths in the placebo group (Table 3, and Table S5 in the Supplementary Appendix).
Table 3.
Adverse Events That Occurred within 14 Days after the Last Dose over the Course of 78 Weeks.*
| Event | Verubecestat 12-mg Group (N = 652) |
Verubecestat 40-mg Group (N = 652) |
Placebo Group (N = 653) |
Absolute Difference between 12-mg Group and Placebo Group |
Absolute Difference between 40-mg Group and Placebo Group |
|---|---|---|---|---|---|
| number of patients (percent) | percentage points (95% CI) | ||||
| Any adverse event | 582 (89.3) | 601 (92.2) | 533 (81.6) | 7.64 (3.84 to 11.48) | 10.55 (6.96 to 14.23) |
| Any serious adverse event | 128 (19.6) | 148 (22.7) | 117 (17.9) | 1.71 (−2.53 to 5.96) | 4.78 (0.42 to 9.15) |
| Adverse event that resulted in discontinuation of assigned trial regimen | 54 (8.3) | 61 (9.4) | 38 (5.8) | 2.46 (−0.32 to 5.31) | 3.54 (0.67 to 6.48) |
| Death | 9 (1.4) | 12 (1.8) | 5 (0.8) | 0.61 (−0.57 to 1.93) | 1.07 (−0.17 to 2.51) |
| Prespecified adverse events of clinical interest | |||||
| Amyloid-related imaging abnormalities of microhemorrhage, superficial siderosis, or macrohemorrhage | 8/312 (2.6) | 14/341 (4.1) | 13/331 (3.9) | −1.36 (−4.36 to 1.52) | 0.18 (−2.97 to 3.31) |
| Amyloid-related imaging abnormalities of incident vasogenic edema | 0/312 | 0/341 | 1/331 (0.3) | −0.30 (−1.69 to 0.92) | 0.30 (−1.69 to 0.82) |
| Delirium | 5 (0.8) | 12 (1.8) | 6 (0.9) | −0.15 (−1.32 to 0.98) | 0.92 (−0.38 to 2.38) |
| Rash† | 30 (4.6) | 28 (4.3) | 8 (1.2) | 3.38 (1.63 to 5.39) | 3.07 (1.36 to 5.04) |
| Specific adverse events with incidence >5.0% in a verubecestat group and with greater incidence in a verubecestat group than in the placebo group‡ | |||||
| Falls and injuries§ | 132 (20.2) | 151 (23.2) | 103 (15.8) | 4.47 (0.30 to 8.65) | 7.39 (3.10 to 11.68) |
| Rash, dermatitis, or urticaria§ | 79 (12.1) | 66 (10.1) | 38 (5.8) | 6.30 (3.24 to 9.47) | 4.30 (1.38 to 7.32) |
| Sleep disturbance§ | 67 (10.3) | 55 (8.4) | 31 (4.7) | 5.53 (2.71 to 8.48) | 3.69 (1.01 to 6.47) |
| Diarrhea | 53 (8.1) | 57 (8.7) | 38 (5.8) | 2.31 (−0.46 to 5.14) | 2.92 (0.10 to 5.81) |
| Weight decrease | 42 (6.4) | 42 (6.4) | 20 (3.1) | 3.38 (1.10 to 5.81) | 3.38 (1.10 to 5.81) |
| Suicidal ideation | 39 (6.0) | 38 (5.8) | 21 (3.2) | 2.77 (0.51 to 5.15) | 2.61 (0.37 to 4.98) |
| Anxiety | 39 (6.0) | 46 (7.1) | 24 (3.7) | 2.31 (−0.02 to 4.73) | 3.38 (0.96 to 5.93) |
| Dizziness | 31 (4.8) | 53 (8.1) | 32 (4.9) | −0.15 (−2.53 to 2.23) | 3.23 (0.56 to 5.99) |
| Psychotic symptoms§ | 30 (4.6) | 36 (5.5) | 20 (3.1) | 1.54 (−0.56 to 3.73) | 2.46 (0.27 to 4.77) |
| Other adverse events of interest¶ | |||||
| Hair-color change | 12 (1.8) | 16 (2.5) | 0 | 1.84 (1.06 to 3.19) | 2.45 (1.52 to 3.95) |
| Syncope-like events§ | 26 (4.0) | 27 (4.1) | 17 (2.6) | 1.38 (−0.58 to 3.44) | 1.54 (−0.44 to 3.62) |
This table summarizes data from patients in the primary population who received at least one dose of the trial regimen. Denominators are given for data that apply to fewer than the total number of patients in a group.
These adverse events of rash were determined by the investigator to be clinically significant.
A greater incidence in a verubecestat group than in the placebo group was defined as a case in which the lower boundary of the 95% confidence interval for the difference between the verubecestat group and the placebo group was greater than 0.
Several specific adverse event terms that appeared to be related were combined in a post hoc and unblinded fashion in the composite items shown; psychotic symptoms included adverse event terms of paranoia, delusion, and hallucination.
The adverse events in this category were based on preclinical or phase 1 data.
Among the prespecified adverse events of clinical interest, verubecestat was associated with the occurrence of rash but not of delirium or amyloid-related imaging abnormalities. Common adverse events (those that occurred in more than 5% of the patients in either verubecestat group) that were reported in a numerically higher percentage of patients in both verubecestat groups than in the placebo group included falls and injuries, rash-related events, sleep disturbance, decreased weight, and suicidal ideation (Table 3). Adverse events of suicidal ideation were judged by the investigator to be mild in severity; 73.6% of the patients in whom suicidal ideation was reported had a history of psychiatric disorders, as compared with 46.6% of the patients in the overall trial population. Verubecestat was associated with changes in hair color. Verubecestat was associated with a mean weight change of −1.4±4.7 kg in the 12-mg group and −1.7±5.0 kg in the 40-mg group as compared with 0.1±4.2 in the placebo group. Adverse events reported in part 2 of the trial are summarized in Table S6 in the Supplementary Appendix.
Drug exposure levels were similar to those reported in phase 1 studies.11 Results of the pharmacokinetic analysis are provided in the Supplementary Appendix.
DISCUSSION
In patients with clinically diagnosed mild or moderate dementia due to Alzheimer’s disease, the BACE-1 inhibitor verubecestat did not reduce the decline in cognition or in overall function as compared with placebo. The baseline characteristics of the patients were similar to those of patients enrolled in other trials that evaluated the ability of agents to reduce brain amyloid burden,5–7 and the placebo group in the current trial showed rates of cognitive decline over the course of 78 weeks that were typical of Alzheimer’s disease.
A limitation of our trial was the lack of requirement for the presence of an amyloid biomarker at screening, thereby potentially allowing patients who had dementia owing to nonamyloid-related disease to be included. Some previous trials of Alzheimer’s disease have shown that amyloid was not observed on PET scans in up to 25% of the patients.5,23 However, in the PET amyloid and cerebrospinal fluid biomarker substudies in our trial, approximately 90% of the patients had findings that were consistent with Alzheimer’s disease. Bias may have been introduced in the uncontrolled selection of patients for the biomarker substudies, but the demographic characteristics of these patients were similar to those of the overall population of the trial. Approximately 63% of the patients in our trial were carriers of APOE4 and therefore had a higher likelihood of Aβ-related disease than noncarriers.5,24 The finding of no treatment benefit occurred both among carriers of APOE4 and among noncarriers. Taken together, these observations suggest that the proportion of patients without amyloid-related disease who were included in the trial was probably small and unlikely to account for the negative results.
A consideration in interpreting the negative trial results is whether an appropriate dose of verubecestat was evaluated. Treatment with verubecestat reduced the concentration of Aβ−40 and Aβ−42 in cerebrospinal fluid by 63 to 81%, which confirms that the drug had the intended action of reducing Aβ production. In the PET amyloid substudy, treatment with verubecestat reduced total brain amyloid load by a modest amount; the mean standardized uptake value ratio was reduced from 0.87 at baseline to 0.83 at week 78 in the 40-mg group. These results suggest that lowering Aβ in the cerebrospinal fluid is associated with some reduction in brain amyloid. On the basis of pharmacokinetic and pharmacodynamic modeling in phase 1 studies, a 60-mg dose of verubecestat was not substantially more effective than a 40-mg dose in reducing the Aβ concentration in cerebrospinal fluid.11 The reduction in hippocampal volume at week 78 was greater in the verubecestat groups than in the placebo group. Similar reductions have been reported with other antiamyloid therapies, and the clinical significance of this finding is unknown.25,26
Adverse events were more common with verubecestat than with placebo, and these events tended to be more common with the 40-mg dose of verubecestat. However, verubecestat was not found to be associated with the amyloid-related imaging abnormalities that have been observed with some other antiamyloid therapies.4–6 Verubecestat was associated with rash-related events and hair-color changes, findings consistent with those from preclinical and phase 1 studies. The hair-color changes may be related to BACE-2 inhibition.11 The percentages of patients who had falls, injuries, weight loss, and neuropsychiatric symptoms, such as sleep disruption and suicidal ideation, were numerically higher in the verubecestat groups than in the placebo group.
Our trial showed that near-maximal reduction of Aβ in cerebrospinal fluid and a modest reduction in brain amyloid load by means of BACE-1 inhibition for 78 weeks was not effective in slowing the clinical progression of mild-to-moderate Alzheimer’s disease. This suggests that once dementia is present, disease progression may be independent of Aβ production or, alternatively, that the amyloid hypothesis of Alzheimer’s disease may not be correct. Because Aβ deposition takes place years before clinical symptoms become apparent, it has been proposed that treatments targeting amyloid should be implemented early in the disease process, before the onset of clinical symptoms.27,28
Supplementary Material
Acknowledgments
Supported by Merck.
We thank the patients, caregivers, and families who participated in the trial; the site investigators and their staff; the members of the trial committees; Julie Stone and Marissa Dockendorf (both from Merck) and David Jaworowicz and Rebecca Humphrey (both from Cognigen) for the pharmacokinetic analysis; Li Lin, Amy Locco, Erin Paradis, Christopher J. Jones, Arpan Patel, Regina Gottwald, Ingrid Banks, Julie Stromswold, Karen E. Ramsey, Agnieszka Napiórkowska-Palka, Kate Civello, Kathleen Bruschi, and Deborah Matzura-Wolfe for their administrative support; Christopher Randolph of MedAvante for his assistance with the central rater review process; Christopher Lines of Merck for his assistance with the preparation of an earlier version of the manuscript; and Sheila Erespe of Merck for her assistance with the submission of the manuscript.
Funded by Merck
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL Alzheimer’s disease. Nat Rev Dis Primers 2015;1:15056. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 2016;8:595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan R, Vassar R. Targeting the β secretase BACE1 for Alzheimer’s disease therapy. Lancet Neurol 2014;13:319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sevigny J, Chiao P, Bussiere T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016;537:50–6. [DOI] [PubMed] [Google Scholar]
- 5.Salloway S, Sperling R, Fox NC, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med 2014;370:322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doody RS, Thomas RG, Farlow M, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med 2014;370:311–21. [DOI] [PubMed] [Google Scholar]
- 7.Doody RS, Raman R, Farlow M, et al. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N Engl J Med 2013;369:341–50. [DOI] [PubMed] [Google Scholar]
- 8.Coric V, van Dyck CH, Salloway S, et al. Safety and tolerability of the γ-secretase inhibitor avagacestat in a phase 2 study of mild to moderate Alzheimer disease. Arch Neurol 2012;69:1430–40. [DOI] [PubMed] [Google Scholar]
- 9.Gilman S, Koller M, Black RS, et al. Clinical effects of Aβ immunization (AN1792) in patients with AD in an interrupted trial. Neurology 2005;64:1553–62. [DOI] [PubMed] [Google Scholar]
- 10.Scott JD, Li SW, Brunskill AP, et al. Discovery of the 3-imino-1,2,4-thiadiazinane 1,1-dioxide derivative verubecestat (MK-8931)-a β-site amyloid precursor protein cleaving enzyme 1 inhibitor for the treatment of Alzheimer’s disease. J Med Chem 2016;59:10435–50. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy ME, Stamford AW, Chen X, et al. The BACE1 inhibitor verubecestat (MK-8931) reduces CNS β-amyloid in animal models and in Alzheimer’s disease patients. Sci Transl Med 2016;8:363ra150. [DOI] [PubMed] [Google Scholar]
- 12.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34:939–44. [DOI] [PubMed] [Google Scholar]
- 13.Diagnostic and statistical manual of mental disorders, 4th ed., text revision: DSM-IV-TR. Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12: 189–98. [DOI] [PubMed] [Google Scholar]
- 15.Rosen WG, Mohs RC, Davis KL A new rating scale for Alzheimer’s disease. Am J Psychiatry 1984;141:1356–64. [DOI] [PubMed] [Google Scholar]
- 16.Morris JC The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–4. [DOI] [PubMed] [Google Scholar]
- 17.Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease: the Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997;11:Suppl 2:S33–S39. [PubMed] [Google Scholar]
- 18.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44: 2308–14. [DOI] [PubMed] [Google Scholar]
- 19.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 2011;168:1266–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landau SM, Fero A, Baker SL, et al. Measurement of longitudinal β-amyloid change with 18F-florbetapir PET and standardized uptake value ratios. J Nucl Med 2015;56:567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mo Y, Stromswold J, Wilson K, et al. A multinational study distinguishing Alzheimer’s and healthy patients using cerebrospinal fluid tau/Aβ42 cutoff with conordance to amyloid positron emission tomography imaging. Alzheimers Dement (Amst) 2017;6:201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider LS, Sano M. Current Alzheimer’s disease clinical trials: methods and placebo outcomes. Alzheimers Dement 2009;5:388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siemers ER, Sundell KL, Carlson C, et al. Phase 3 solanezumab trials: secondary outcomes in mild Alzheimer’s disease patients. Alzheimers Dement 2016;12: 110–20. [DOI] [PubMed] [Google Scholar]
- 24.Ba M, Kong M, Li X, Ng KP, Rosa-Neto P, Gauthier S. Is ApoE s 4 a good biomarker for amyloid pathology in late onset Alzheimer’s disease? Transl Neuro-degener 2016;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox NC, Black RS, Gilman S, et al. Effects of Aβ immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology 2005;64:1563–72. [DOI] [PubMed] [Google Scholar]
- 26.Novak G, Fox N, Clegg S, et al. Changes in brain volume with bapineuzumab in mild to moderate Alzheimer’s disease. J Alzheimers Dis 2016;49:1123–34. [DOI] [PubMed] [Google Scholar]
- 27.Sperling R, Mormino E, Johnson K. The evolution of preclinical Alzheimer’s disease: implications for prevention trials. Neuron 2014;84:608–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jack CR Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013;12:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


