Abstract
Nanodiamonds (NDs) are a carbon nanomaterial with a core diamond crystalline structure and crystal defects, such as graphitic carbon and heteroatoms, on their surface. For electrochemistry, NDs are promising to increase active sites and decrease fouling, but NDs have not been studied for neurotransmitter electrochemistry. Here, we optimized nanodiamond coatings on microelectrodes and found that ND increases the sensitivity for neurotransmitters with fast-scan cyclic voltammetry (FSCV) detection and decreases chemical and biofouling. Different sizes and functionalization of ND were tested, and ND suspensions were drop-casted onto carbon-fiber microelectrodes (CFMEs). Five-nm ND-H and 5 nm ND-COOH formed thick coatings, while the 15 and 60 nm ND-COOH formed more sparse coatings. With electrochemical impedance spectroscopy (EIS), 5 nm ND-H and 5 nm ND-COOH had high charge transfer resistance, while 15 nm and 60 nm ND-COOH had low charge transfer resistance. 15-nm ND-COOH was optimal, with the best electrocatalytic properties and current for dopamine. Sensitivity was enhanced 2.1 ± 0.2 times and the limit of detection for dopamine improved to 3 ± 1 nM. ND coating increased current for other cations such as serotonin, norepinephrine, and epinephrine, but not for the anion ascorbic acid. Moreover, NDs decreased electrochemical fouling from serotonin and 5-hydroxyindole acetic acid (5-HIAA), and also decreased biofouling in brain slice tissue by 50%. The current at biofouled ND-coated electrodes is similar to the signal of pristine, unfouled CFMEs. The carboxylated ND-modified CFMEs are beneficial for neurotransmitter detection because of easy fabrication, improved limit of detection, and antifouling properties.
Keywords: Nanodiamond, Dopamine, Fast-Scan Cyclic Voltammetry, Carbon-Fiber Microelectrode, Antifouling Properties
Graphical Abstract
Nanodiamonds (NDs) or diamond nanoparticles are a carbon nanomaterial which has a diamond crystalline core structure made up from sp3-hybridized carbon atoms, with heteroatoms and defects at their surface.1–3 NDs can be synthesized via several methods, but the most common method is the detonation of trinitrotoluene and hexogen explosives in controlled, oxygen-deficient, high pressure, and high temperature environment;4,5 hence, this ND is called “detonation nanodiamond.” NDs have particle diameters from 1 to 500 nm as synthesized,5–8 but they can aggregate to form a larger particles.9 Although the same sp3 core structure causes bulk diamond to be a perfect electrical insulator, NDs are electrically conductive.6,10 The contrasting electrical behaviors between NDs and bulk diamond are due to the surface defects and terminal groups of NDs which mostly contain sp2-hybridized carbons such as C=C, C=O, and COOH.10 When coated on commonly used electrodes such as gold or glassy carbon, NDs act as an electrocatalyst for redox reactions of electroactive species including [Fe(CN)6]3–/4–, [Ru(NH3)6]3+/2+, and the nitrite ion.10–12 Holt proposed that the energy levels of oxide groups increase the density of state of NDs, facilitating electrical conductivity and electrocatalysis.13 The small size of NDs leads to high specific surface area and also enhances the electrochemical activity.14–17 However, ND has not been widely used as an electrode material and has not been examined in biological applications, such as for neurotransmitter detection.
The neurochemistry community typically utilizes carbon-fiber microelectrodes (CFMEs) as the standard electrochemical sensor for real-time in vivo detection of neurotransmitters.2,18–22 Many carbon nanomaterials including graphene,23,24 carbon nanotubes,25–28 and carbon nanohorns29 have been coated or grown on CFMEs to enhance surface area, electrocatalytic properties, and dopamine adsorption by adding edge plane carbons.2,20,30,31 These properties enhance the faradaic current and signal-to-noise ratio, improving analytical performance for dopamine detection. Carbon nanomaterials that have high number of defects and oxygen-containing surface functional group that increase hydrophilicity also exhibit antifouling properties against biofouling and electrochemical fouling.2,31,32 Biofouling typically occurs when proteins adhere to the electrode surface via electrostatic or hydrophobic interactions, decreasing sensitivity by decreasing the electroactive area.2,33 Electrochemical fouling from redox reactions of other electroactive species such as serotonin (5-HT) and 5-hydroxyindoleacetic acid (5-HIAA) also diminish the electrode sensitivity.34–37 Boron-doped diamond (BDD) electrodes are popularly used to limit biofouling and electrochemical fouling because they have fewer surface functional groups that adsorb fouling products.35,38–44 For example, BDD coated Pt wires are used to measure seorotonin in the harsh conditions of the gut because of their anti-fouling properties. However, BDD does not promote dopamine adsorption and gives less electrochemical activity, especially towards surface sensitive probes. NDs prevent dopamine electrochemical fouling45 and suppress the lithium dendrite growth in rechargeable batteries.8 NDs also have improved biocompatibility compared to nanotube-based materials,46 and promote cell viability because their synthesis does not require metal catalysts, which are sometimes present in CNT and cause cell toxicity.14 These potential antifouling properties, combined with their electrocatalytic and adsorption properties, make NDs a promising electrode material for biological applications.
In this paper, we develop nanodiamond-modified carbon-fiber microelectrodes (ND/CFMEs) and explore their properties for neurochemical detection using fast-scan cyclic voltammetry (FSCV). NDs are produced in various sizes and surface terminal groups, such as hydrogen-terminated or carboxyl-terminated,4 which may affect their surface properties.1,13,47 We optimized a simple drop-casting technique to deposit NDs from aqueous dispersions on the CFME surface and compared 5 nm H-terminated and COOH-functionalized NDs as well as 15 nm and 60 nm COOH-functionalized NDs. Larger, carboxylated (15–60 nm) particles were optimal as they enhanced the dopamine faradaic current, improved the limit of detection for dopamine, and had electrocatalytic properties. ND coatings were antifouling, as the current decayed much less after implantation in brain tissue than CFMEs. ND/CFMEs also resisted electrochemical fouling by serotonin and 5-HIAA. Overall, increased sensitivity and decreased chemical and biofouling make ND-modified electrodes promising for in tissue neurotransmitter sensing.
Experimental Section
Chemicals
Dopamine, epinephrine, norepinephrine, serotonin, 5-hydroxyindoleacetic acid (5-HIAA), ascorbic acid, and potassium ferricyanide were purchased from Acros Organics (Morris Plains, NJ). A 10 mM stock solution of each neurotransmitter was prepared in 0.1 M HClO4. The final working solutions were prepared by diluting the stock solution in a phosphate-buffered saline (PBS) (131.25 mM NaCl, 3.00 mM KCl, 10 mM NaH2PO4, 1.2 mM MgCl2, 2.0 mM Na2SO4, and 1.2 mM CaCl2 with pH adjusted to 7.4) to the desired concentration.
Preparation of ND/CFMEs
Before preparing the modified electrodes, a cylindrical CFME was fabricated by the same procedure as previous work.48 A T-650 carbon fiber (7-μm diameter, Cytec Engineering Materials, West Patterson, NJ) was pulled into a glass capillary (1.28 mm inner diameter × 0.68 mm outer diameter, A-M Systems, Sequim, WA) by an aspirating pump. Then, the capillary was pulled by an electrode puller (model PE-21, Narishige, Tokyo, Japan) to get two electrodes. The extended fiber was cut to a length of about 100 μm. After that, each electrode was epoxied by dipping in an 80°C-solution of 14% m-phenylenediamine hardener (Acros Organics, Morris Plains, NH) in Epon Resin 828 epoxy (Miller-Stephenson, Danbury, CT) for 30 s to seal the fiber with the glass capillary. The electrode was then dipped in acetone for 5 s. Finally, the epoxied electrode was cured overnight at room temperature, cured in an oven at 100°C for 2 h, and 150°C overnight. and the electrode capillary was back filled with 1 M KCl for electrical connection.
The 1% nanodiamond dispersions in water were purchased from Adámas Technologies (Raleigh, NC) and were bath sonicated for 15 min before use. Different nanodiamonds were compared: 5-nm hydrogen-terminated nanodiamond (ND-H 5), 5-nm carboxylated nanodiamond (ND-COOH 5), 15-nm carboxylated nanodiamond (ND-COOH 15), and 60-nm carboxylated nanodiamond (ND-COOH 60). ND-H and ND-COOH were reported the zeta potential at pH 7 to be +30 mV and –45 mV, respectively.49 TEM images illustrating the particle sizes, diamond crystalline structure, and sp2 layer of different NDs are shown in Fig. S1, XPS spectra comparing their elemental composition are shown in Fig. S2 and Table S1, and Raman spectra are shown in Fig. S3. These characterizations illustrates the ND features1,50,51. To prepare the ND/CFMEs, 25 μL of the nanodiamond dispersion in water (equivalent to 0.25 mg of ND per drop) was dropped to cover the CFME tip on a glass slide on a hot plate, which accelerated solvent evaporation. The process was repeated to optimize the amount of NDs on CFME via the number of nanodiamond drops. Note that only some of the ND drop was deposited on the carbon fiber, and some was left on the glass slide. All ND/CFMEs were dried overnight at room temperature before characterization.
Surface Characterization
Scanning electron microscopy (SEM) images of the electrodes were taken with Quanta 650 (FEI Company, Hillsboro, OR), at the Nanoscale Materials Characterization Facility, UVa Department of Materials Science and Engineering. The secondary electron images were recorded with an acceleration voltage of 2 kV and a working distance of approximately 10 mm.
Electrochemical Instrumentation
FSCV experiments were conducted using a two-electrode system: CFME or ND/CFME working electrode vs Ag/AgCl reference electrode. The electrodes were connected to a ChemClamp potentiostat and headstage (Dagan, Minneapolis, MN). Unless stated otherwise, the standard FSCV waveform with a holding potential of –0.4 V, switching potential of +1.3 V, scan rate of 400 V/s, and repetition rate of 10 Hz was applied to the electrode. The PBS buffer and test solutions were injected through the flow cell by an automated syringe pump (Harvard Apparatus, Holliston, MA) at a flow rate of 2 mL/min. The FSCV data were collected with HDCV Analysis software (Department of Chemistry, University of North Carolina at Chapel Hill). Electrochemical impedance spectroscopy (EIS) was performed at Gamry Reference 600 (Gamry Instruments, Warminster, PA) in a 10 mM [Fe(CN)6]3– in 1 M KCl using CFME or ND/CFME working electrode, Ag/AgCl reference electrode, and Pt counter electrode. The DC applied potential was +0.24 V, the observed formal potential of [Fe(CN)6]3–, and the AC potential amplitude of 10 mV was applied on the formal potential of [Fe(CN)6]3– with an initial and final frequency of 1 MHz and 1 Hz, respectively. The Nyquist plots were normalized by the electrode surface area.
Statistics
All reported values are given as the mean ± standard error of the mean (SEM) for n number of electrodes. All statistical analyses were performed in GraphPad Prism 7 (GraphPad Software, La Jolla, CA). Statistical significance was defined at p < 0.05.
Results and Discussion
Physical characterization of ND/CFMEs
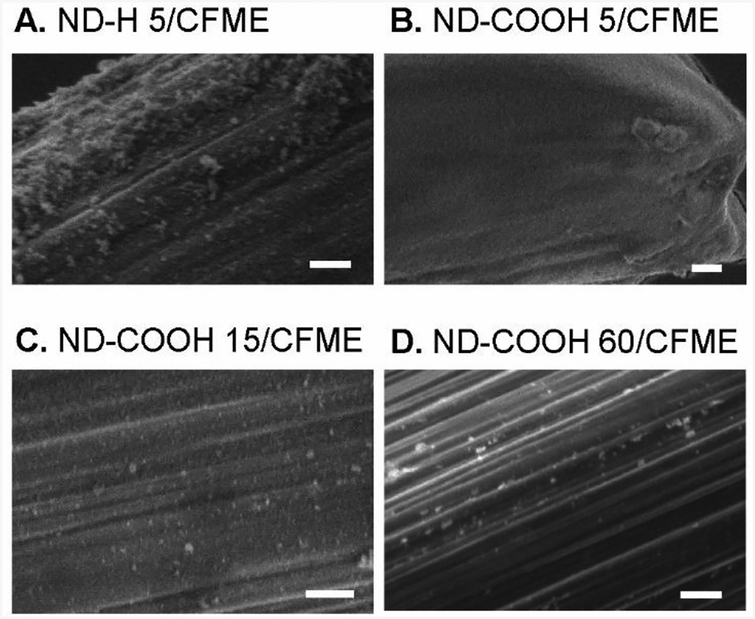
In this work, we examined four types of NDs: 5 nm ND particles with H termination (ND-H 5), 5 nm ND particles with carboxyl termination (ND-COOH 5),15 nm ND particles with carboxyl termination (ND-COOH 15), and 60 nm particles with carboxyl termination (ND-COOH 60). [Add a sentence or two here quickly covering the characterization of the particles. The three supplementary figs should be covered and what the main take aways are: i.e. size, functionalization, graphitic layer on outside.] Microelectrodes were coated by repeated drop casting of 25 μL drops of a 1% aqueous ND dispersion onto a CFME. Fig. 1 shows the SEM images of 10 drop coatings of ND-H 5/CFME (Fig. 1A), ND-COOH 5/CFME (Fig. 1B), ND-COOH 15/CFME (Fig. 1C), and ND-COOH 60/CFME (Fig. 1D). The images illustrate deposited ND particles on the carbon fiber surface, as the unmodified carbon fiber is smooth (SEM image of CFME is shown in Fig. S4). The coatings for the 5 nm particles are denser than the coatings for the larger ND particles, which are more sparse. ND-H 5/CFME had small deposited particles, many in the range of tens of nm. The ND-COOH 5/CFME had a thicker coating consisting mainly of NDs smaller than 10 nm, and the SEM image is noisy because of the charging effects from the insulating, thicker coating. ND-COOH 15/CFME had a sparse coating of ND-COOHs, with larger particles in the tens to hundreds of nm, similar to the particles on the ND-COOH 60/CFME. ND particle size from drop casting was larger than the synthesized size because of aggregation of NDs in the dispersion.9 Future research can investigate direct growth or deposition of ND particles on a conductive substrate to obtain more homogeneous, non-aggregated coatings.
Fig. 1.
Physical characterization of ND/CFMEs. SEM images of (A) ND-H 5/CFME, (B) ND-COOH 5/CFME, (C) ND-COOH 15/CFME, and (D) ND-COOH 60/CFME. The modified electrodes were prepared from drop casting for 10 drops. Scale bar: 500 nm.
Electrocatalytic Properties of ND/CFMEs
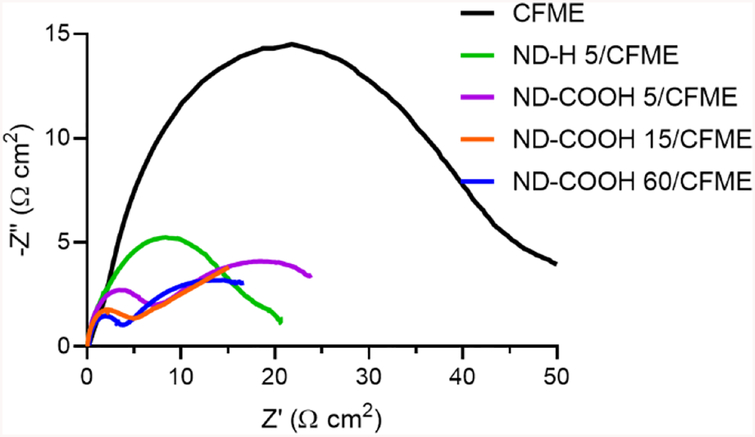
We explored the electrocatalytic properties of all four types of NDs toward surface-sensitive redox probes. Fig. 2 shows the Nyquist plot from electrochemical impedance spectroscopy of 10 mM [Fe(CN)6]3–. The EIS spectra did not resemble a perfect semicircle in the high frequency range and a line of unity slope in the low frequency range because diffusion profiles at microelectrodes are different from those of larger electrodes.52 The size of the “semicircle” was qualitatively compared and fit to Randles equivalent circuit (Fig. S5) to investigate charge-transfer resistance (Rct). The semicircle features of ND/CFMEs were all smaller than CFMEs, indicating a lower Rct. The order of Rct was ND-COOH 60 (5.4 ± 1.7 Ω cm2) ≤ ND-COOH 15 (5.2 ± 1.8 Ω cm2) < ND-COOH 5 (6.3 ± 0.8 Ω cm2) < ND-H 5 (21 ± 6 Ω cm2) ≤ CFME (35 ± 4 Ω cm2) (n = 4). There was a big difference between the hydrogenated and carboxylated 5 nm particles with carboxylated particles better for electron transfer to ferricyanide. Interestingly, the more sparsely coated 15 and 60 nm ND particles had smaller Rct values than 5-nm ND-COOH. This result was counterintuitive because usually smaller nanoparticles display better electrocatalytic enhancement.53 The SEM image of larger ND-COOHs showed sparse coatings, which effectively adds active sites but does not destroy conductivity. However, the thick, film-like coatings of the smaller particles will worsen the charge transfer kinetics if the coating is resistant to electron transfer due to the sp3 insulating nature of NDs, compared to graphitic carbon in the CFME.30
Fig. 2.
Nyquist plot from EIS of 10 mM [Fe(CN)6]3– in 1 M KCl using unmodified CFME (black), ND-H 5/CFME (green), ND-COOH 5/CFME (purple), ND-COOH 15/CFME (orange), and ND-COOH 60/CFME (blue). Both impedance axes were normalized by electrode surface area.
FSCV of Dopamine at ND/CFMEs
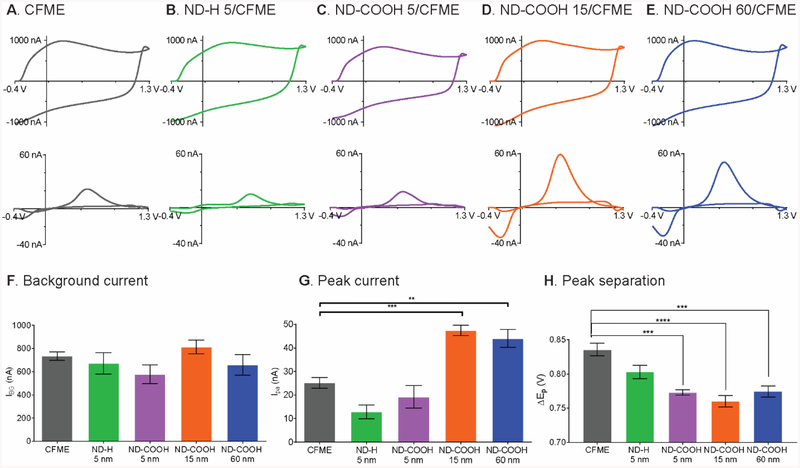
All ND/CFMEs were investigated for their FSCV responses (Fig. 3). Example background charging currents are shown in Fig. 3A–E and they are all about the same size, around 700 nA. The average background currents (Fig. 3F) were not significantly different from each other (p = 0.1839, one-way ANOVA with Bonferroni post-test, n = 4–6). The background current is proportional to specific capacitance of the electrode material and electroactive surface area.54 Although the sp2 surface groups can increase the capacitance of boron-doped diamond,55,56 the 15 and 60 nm ND particles do not fully coat the surface, so they might not add much material for charging. The 5 nm particles actually show slightly decreased capacitance despite the fact they formed thicker coatings, implying the sp3 hybridized nanodiamond exhibits a slightly lower capacitance that CFMEs.
Fig. 3.
FSCV responses: 1 μM dopamine (upper) and background current (lower) of (A) CFME, (B) ND-H 5/CFME, (C) ND-COOH 5/CFME, (D) ND-COOH 15/CFME, and (E) ND-COOH 60/CFME in PBS pH 7.4. The modified electrode was prepared from 10 drop castings. The average data includes (F) background current, (G), 1 μM dopamine peak current, and (H) peak separation. (**p < 0.01, ***p < 0.001, ****p < 0.0001, one-way ANOVA with Bonferroni post-test, n = 4–6 electrodes).
The background-subtracted CVs of 1 μM dopamine were also compared (Fig. 3A–E). The example CVs show that dopamine current for the two 5 nm ND samples are slightly lower than CFMEs, while the current for the 15 and 60 nm ND are twice as large as CFMEs. There was a significant main effect of ND type on the peak current (Fig. 3G, p < 0.0001, one-way ANOVA with Bonferroni post-test, n = 4–6). Bonferroni post-tests indicate ND-COOH 15 and ND-COOH 60 significantly increased the dopamine peak current (p < 0.001 and p < 0.01, respectively) while ND-H 5 and ND-COOH 5 did not change the current (p = 0.0808 and p = 0.9517, respectively). The highest dopamine peak current was obtained at the ND-COOH 15/CFME treated with 10 rounds of drop-casting; the peak current was enhanced by 1.9 ± 0.1 times. Fig. S6 shows optimization of the number of drops for drop casting and 10 drops were used for all further experiments.
Another interesting feature of the dopamine CV is the separation between anodic and cathodic peak (ΔEp), as smaller ΔEp values are correlated with faster electron transfer kinetics. Fig. 3H shows that there was a significant main effect of ND type on the ΔEp (p < 0.0001, one-way ANOVA with Bonferroni post-test, n = 4–6). Bonferroni post-tests indicated that all three ND-COOHs significantly decreased the ΔEp (p < 0.001 for ND-COOH 5, p < 0.0001 for ND-COOH 15, and p < 0.001 for ND-COOH 60). However, ND-H 5 did not significantly affect the ΔEp (p = 0.0970). Because smaller peak separation indicates better electrocatalytic properties,54 ND-COOH catalyzed dopamine redox reaction at CFME but ND-H did not, consistent with the EIS experiment, which found higher Rct values for ND-H than ND-COOH.
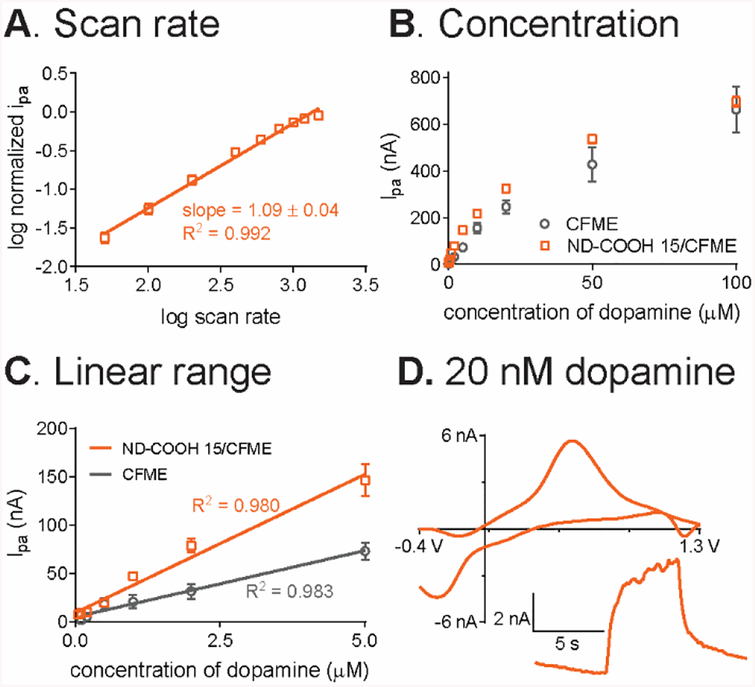
The optimized ND-COOH 15/CFME was further tested to look at adsorption dependence by varying the scan rate from 50 V/s to 1000 V/s. The log-log plot of normalized dopamine anodic peak current vs scan rate (Fig. 4A) had a slope of 1.09 ± 0.04 (n = 3) which is close to 1. The slight deviation might due to the heterogeneous coating on CFME. The slope indicates that anodic current was proportional to the scan rate, so the dopamine oxidation at ND-COOH 15/CFMEs is adsorption-controlled.54
Fig. 4.
Analytical performance of ND-COOH 15/CFME for dopamine detection. (A) log-log relationship between anodic peak current of 1 μM dopamine and scan rate (n = 3). (B) Concentration dependence from the anodic peak current of 20 nM to 100 μM dopamine in PBS pH 7.4 (n = 4), compared to unmodified CFME. (C) Linear range at both electrodes. (D) Example CV of 20 nM dopamine in PBS pH 7.4 with current-time trace inset for 5-s bolus injection.
Both size and functional groups of NDs affect electrochemistry
The FSCV and EIS data revealed that carboxylated NDs had better electrocatalytic properties and peak currents than hydrogen terminated NDs. For electrocatalytic properties, we consider the density of electronic states (DOS), and C=C and C=O ND surface groups can fill the bandgap, increasing the DOS and accelerating electron transfer kinetics.13 In contrast, ND-H 5 lacks functional groups to increase the DOS and electrocatalytic effects were not observed.13 Surface oxide groups such as hydroxyl, carbonyl, and carboxyl groups also enhance adsorption of dopamine (pKa 8.9)57 carbon electrodes, leading to higher peak oxidation currents.13,58 Indeed, the 15 and 60 nm ND-COOH electrodes exhibited increased anodic peak currents for dopamine FSCV as they added adsorption sites for dopamine.59 On the other hand, hydrogenation of ND reduced adsorption by reducing active surface groups and the dopamine currents were slightly lower than at CFMEs, which are naturally partially oxygen-terminated. Hydrogenated NDs also exhibited less electrocatalytic properties, showing that the oxygen groups also facilitate electron transfer of surface sensitive species, as has been previously shown by Holt et al. as well10 Therefore, ND-COOH is a better electrode material for neurotransmitter detection than ND-H, a findings consistent with previous studies finding surface functional groups of NDs affect their physical and electrochemical properties.8,10,13,14,45
ND particle size affected the dopamine sensitivity for the COOH functionalized particles. The unanticipated result is that ND-COOH 5 did not increase the dopamine peak current, unusual because smaller nanoparticles have more concentrated surface functional groups and thus often exhibit higher current enhancement.53 The SEM image of ND-COOH 5/CFME (Fig. 1B) showed a thick, film-like morphology coating on the carbon fiber surface, which could lead to slower mass transport to the electrode and lower dopamine peak current. Active adsorption sites that are too close together will not lead to larger current if the dopamine cannot access all of them. The ND-COOH 15 and ND-COOH 60 had similar currents and similar morphologies, with occasional particles adhered to the fiber surface, but not a full monolayer of coverage. While typically denser coverage might be better, the ND might also impede charge transfer and the smaller ND particles had higher Rct, so the more sparse coatings of the 15 and 60 nm particles were more ideal.
Analytical Performance of ND electrodes
ND-COOH 15/CFMEs were used to characterize the analytical performance of nanodiamond electrodes compared to bare CFMEs. Fig. 4B shows current was linear with concentration from 20 nM to 5 μM (Fig. 4C) with R2 = 0.980 for ND-COOH 15/CFME and R2 = 0.983 for CFME. The sensitivity for dopamine detection was calculated from the slope of the calibration curve and ND-COOH 15/CFME were twice as sensitive, with a sensitivity of 29 ± 2 nA/μM compared to 14 ± 1 nA/μM for CFME (p < 0.01, unpaired t-test, n = 4). At higher concentrations, the adsorption sites were all occupied, and the response becomes more diffusion-controlled, as detailed in previous studies.59
The limit of detection (LOD) of ND/CFMEs was calculated from the signal-to-noise (S/N) ratio obtained from peak current of 100 nM dopamine, and LOD was defined at S/N = 3. From Table 1, the LOD of ND-COOH 15/CFME is 2.6 ± 1.0 nM, which is significantly better than the unmodified CFME (p < 0.05, unpaired t-test, n = 4). Noise is proportional to background current and since the Faradaic current increased, but the background did not, the S/N ratio and LOD improved.60 The FSCV of 20 nM dopamine (Fig. 4D) shows how ND electrodes can detect very low concentrations of dopamine with the usual dopamine CV shape, and a current-time trace with a signal much larger than the baseline noise. Table 1 also shows that the rise times (defined as time from 10% to 90% of the maximum current) were not changed for ND electrodes (p = 0.628, unpaired t-test, n = 4). The ND/CFMEs electrodes were stable when FSCV waveform was continuously applied to the electrode for 4 h, and the 1 μM dopamine peak current was measured every hour (Fig. S7). The normalized peak current remained the same with the relative standard deviation (RSD) of 3.4 ± 0.6% (n = 4), so the ND particles were attached well to the CFME surface.
Table 1:
Analytical characterization of ND electrodes.
| Sensitivity (nA/mM) | LOD (nM) | ΔEp (mV) | rise time (s) | |
|---|---|---|---|---|
| CFME | 14 ± 1 | 7 ± 1 | 835 ± 9 | 1.9 ± 0.1 |
| ND-COOH 15/CFME | 29 ± 2** | 3 ± 1* | 760 ± 8*** | 1.9 ± 0.1 |
p < 0.05,
p < 0.01,
p < 0.001 by unpaired t-test. n=4–5
Response to Other Neurochemicals
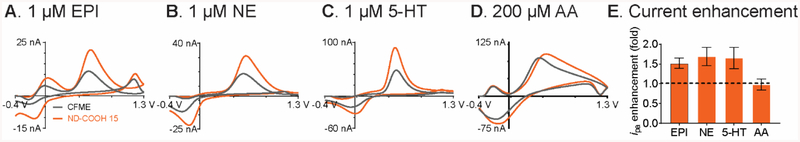
The ND-COOH 15/CFME was also investigated for detection of other neurochemicals, including epinephrine, norepinephrine, serotonin, and ascorbic acid (Fig. 5). The oxidation currents were larger at the ND-coated electrodes for the cations epinephrine, norepinephrine, and serotonin than for the anion ascorbic acid. For epinephrine, there was an additional anodic peak around 0.0 V, which corresponds to the oxidative cyclization making leucoaminochrome.61 The cyclization kinetics of epinephrine are faster than that of dopamine and norepinephrine, where the cyclization peak is not observed.62 Serotonin is an important neurotransmitter for regulation of mood34 and has an oxidation peak at 0.6 V, similar to dopamine, but a reduction peak at 0 V, which is more positive than dopamine. The peak current enhancements for cationic neurotransmitters were similar to dopamine: 1.5 ± 0.1 times increase for epinephrine (Fig. 5A), 1.7 ± 0.2 times for norepinephrine (Fig. 5B), and 1.7 ± 0.3 times for serotonin (Fig. 5C) (n = 4). In contrast, ascorbic acid, in the form of anionic ascorbate at physiological pH, exhibited no current enhancement at ND-COOH 15/CFME: 1.0 ± 0.1 times (n = 4) (Fig. 5D). The carboxylate and surface oxide groups of ND-COOHs selectively adsorbed cationic molecules and repelled anionic molecules. Also, NDs did not increase electrode surface area, thus the peak current of ascorbic acid was not increased. Therefore, ND-COOHs provided selectivity toward cationic neurotransmitter detection and limited the anionic interferents from high concentration of anionic ascorbate in vivo.63
Fig. 5.
Response to other neurochemicals for unmodified CFME (black) and ND-COOH 15/CFME (orange) in PBS, pH 7.4. (A) 1 μM epinephrine (EPI), (B) 1 μM norepinephrine (NE), (C) 1 μM serotonin (5-HT), (D) 200 μM ascorbic acid (AA), and (E) anodic peak current enhancement (n = 4).
Antifouling Properties
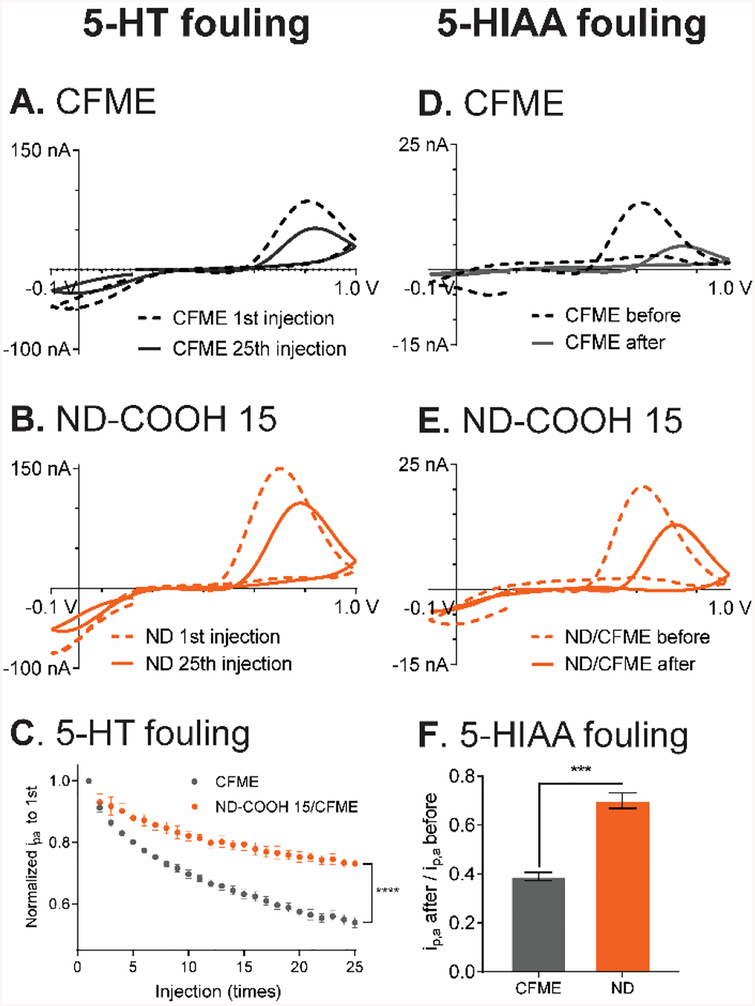
Electrode fouling is a major concern in biological experiments as the response can decrease due to either the production of electroactive species that polymerizes on the electrode or adhesion of large biomolecules in biological environments.32 ND-modified electrodes have previously been show to resist some forms of fouling35,38–44 so we evaluated the ND-COOH 15/CFME for antifouling properties. First, electrochemical fouling of serotonin was tested by repeatedly exposing the electrode to serotonin, as serotonin produces a radical species after oxidation that can polymerize and reduce the electroactive surface area.35 FSCV was performed with the Jackson waveform, specific for serotonin (0.2 V holding potential, ramped up to +1.0 V, back to −0.1 V, then to 0.2 V at a scan rate of 1000 V/s), which has fast scan rates to “outrun” the polymerization and reduce the fouling.34 Fig. 6A and 6B compare the CVs of serotonin from 1st and 25th repeated exposure to serotonin. At the CFME, the anodic peak current decreased by almost half, from 90 nA to 50 nA, but the ND-COOH 15/CFME decreased by only a quarter, from 150 to 110 nA. The peak current from 25th injection of serotonin at ND-COOH 15/CFME (i.e. after fouling) was higher than the peak current from 1st injection of serotonin at the unmodified CFME. Fig. 6C shows the normalized currents over the repeated injections, which show less fouling and less decay at ND-COOH 15/CFMEs (one-way ANOVA, p < 0.0001). The normalized current from 25th injection at ND-COOH 15/CFME (73 ± 1%) was significantly higher than CFME (54 ± 2%, p < 0.0001, one-way ANOVA, n = 5). We also tested electrodes coated with fewer drops of ND, and they alleviated fouling, but to a lesser extent, suggesting that fouling is related to surface coverage of ND(Fig. S8).
Fig. 6.
Electrochemical fouling by serotonin (5-HT) and 5-HIAA. CV of 1 μM serotonin from 1st (dashed) and 25th (solid) 3-s injection at (A) CFME and (B) ND-COOH 15/CFME. (C) Normalized peak current for repeated injections of 5-HT. The currents are significantly different by the 25th injection (****p < 0.0001, one-way ANOVA, n = 5). (D) CFME and (E) ND-COOH 15/CFME CVs of 1 μM 5-HIAA before (dashed) and after (solid) electrodes were bathed in 1 μM 5-HIAA solution with the continuous waveform application for 1 hour. (F) 1 μM 5-HIAA normalized peak current after 1 hour of 5-HIAA fouling (***p < 0.001, unpaired t-test, n = 4). All electrochemical fouling experiment were performed using the Jackson serotonin waveform in PBS pH 7.4.
Electrochemical fouling was also tested for 5-hydroxyindoleacetic acid (5-HIAA), the serotonin metabolite found at high concentrations in vivo, which severely fouls the electrode via electropolymerization.34 Despite the similar structure and oxidation between serotonin and 5-HIAA, the peak current of 5-HIAA was much lower because of the electrostatic repulsion between negative functional groups and anionic nature of 5-HIAA at pH 7.4. The electrode was tested for response to 5-HIAA, then bathed in the 1 μM 5-HIAA solution for 1 h with the Jackson waveform applied, and then the response to an injection of 1 μM 5-HIAA tested again. At CFMEs, the 5-HIAA anodic peak current decreased from 14 nA to 4 nA (Fig. 6D), while at ND electrodes, the decrease after 5-HIAA was only from 20 nA to 14 nA (Fig. 6E). As with serotonin, the current of the ND electrode after 5-HIAA fouling was similar to the bare CFME before fouling, proving that the ND modified electrode has bigger signals, even after fouling. Fig. 6F shows the average data, where the ND-COOH 15/CFME had 70 ± 3% of the original signal after fouling while the CFME had only 39 ± 2% (p < 0.001, unpaired t-test, n = 4). Therefore, ND-COOHs decreased electrochemical fouling from serotonin and 5-HIAA by about half.
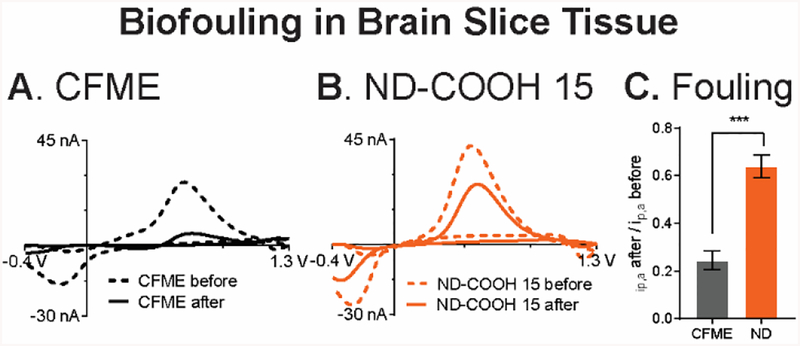
Finally, the ND-COOH 15/CFME were investigated for their biofouling in rat brain slices, a popular ex vivo sample for neuroscience experiments.64,65 Electrode fouling in biological samples is caused by not only electropolymerization of neurochemicals but also adsorption of proteins or other biomolecules.2,20 To evaluate the fouling, the electrodes were inserted in brain slice tissue, and the standard FSCV waveform was applied to the electrode continuously for 1 h. CVs of 1 μM dopamine CV were compared before and after being inserted to the brain. At CFMEs, the ΔEp for dopamine is larger (about 150 mV) after the biofouling, which demonstrates sluggish kinetics caused by protein adsorption (Fig. 7A). The peak current also decreased from 28 nA to 5 nA at the CFME. In contrast, the ND-COOH 15/CFME had a smaller increase in ΔEp (only about 30 mV), and the peak current decreased less, from 44 nA to 26 nA (Fig. 7B). On average, Fig. 7C shows that the normalized dopamine peak current after brain slice fouling for ND-CCOH 15/CFME (64 ± 5%) was significantly higher than CFME (24 ± 4%) (p < 0.001, unpaired t-test, n = 4). Again, the dopamine peak current at the partially fouled ND-COOH 15/CFME was approximately the same as that from the unfouled CFME.
Fig. 7.
Biofouling in brain slice tissue. CV of 1 μM dopamine in PBS pH 7.4 obtained before (dashed) and after (thick) the electrodes were implanted in the brain slice tissue for 1 hour with the dopamine waveform applied. (A) CFME and (B) ND-COOH 15/CFME. (C) Normalized peak current of 1 μM dopamine after to before brain slice implantation (***p < 0.001, unpaired t-test, n = 4).
These experiments demonstrate that ND-modified CFMEs exhibit antifouling properties and signals after electrochemical or tissue fouling were greater than bare CFMEs that had no fouling. A recent study found that CNT yarns prevent biofouling by proteins but not by serotonin electropolymerization, but this study shows that ND is beneficial for reducing fouling by both serotonin and proteins.66 Contact angle measurements were performed on a flat, screen-printed carbon electrode (SPCE)for ND-COOH 15 and ND-H 5 drop casting (Fig. S9). The contact angle of ND-COOH 15(30 ± 2°) was significantly lower than bare SPCE (69 ± 2°, p < 0.0001, unpaired t-test, n = 4). Hence, NDs with carboxylic acid functionalization reduced severe fouling of CFMEs because they increased electrode surface hydrophilicity4,6,45 and minimized the adsorption of hydrophobic biomolecules and polymers generated from electropolymerization of serotonin and 5-HIAA. NDs are an improvement on the antifouling properties of hydrogen-terminated BDD electrodes, which show resistance to electrode fouling and a wide potential range, but have less sensitivity and electrocatalytic properties for dopamine.35,40,41,67 Indeed, other polymer-based strategies for combatting fouling also suffer from reducing the overall sensitivity of the electrode.68,69 Hence, this work establishes NDs as a candidate electrode coating that actually increases the signal as well as inhibits fouling. Peltola et al also show that NDs enhance the biocompatibility of the electrode,14 so these advantages of NDs may facilitate the long-term in vivo monitoring of neurotransmitters. More surface functional group, such as zwitterionic groups, could be also tested to better inhibit biofouling.2 Future research could explore the direct fabrication of NDs70,71 on CFME or other microelectrodes to obtain more complete surface coverage of the larger particles, to enhance sensitivity and antifouling properties.
Conclusions
In summary, we optimized a simple drop-casting fabrication of NDs on CFMEs. The surface functional groups and size affected the electrochemical properties of the modified electrodes, with 15 or 60 nm COOH functionalized particles giving the best properties. The ND/CFMEs exhibited electrocatalytic properties toward surface-sensitive redox species and low charge transfer resistance. ND modification was beneficial for FSCV detection of dopamine as it doubled the dopamine faradaic current without changing the background current, improving the limit of detection to 3 nM. The modified electrodes also increased the selectivity from cationic neurotransmitters and against anionic interferents such as ascorbic acid. ND coating also alleviated electrochemical fouling and biofouling, and the signals after fouling were larger or the same as CFMEs that had not been fouled. Overall, NDs are a promising nanomaterial to reduce electrode fouling while maintaining enhanced electrochemical signal.
Supplementary Material
Acknowledgments
This research was supported by the National Institute of Health (NIH) grant R01 EB026497. Electrode characterization was conducted at the Center of Nanophase Materials Sciences, ORNL, which is a DOE Office of Science User Facility (User grant CNMS2017–076 and CNMS2019–034). Travel aid to ORNL was supported by ORNL-UVA Travel Award (University of Virginia). We thank Yuanyu Chang for preparing the brain slice tissue.
Footnotes
Supporting Information
Supplemental figures include Raman spectra of different NDs, SEM image of CFME, EIS equivalent circuit, and optimization of ND amount from drop casting.
References
- (1).Mochalin VN; Shenderova O; Ho D; Gogotsi Y The Properties and Applications of Nanodiamonds. Nat. Nanotechnol 2012, 7, 11–23. [DOI] [PubMed] [Google Scholar]
- (2).Cao Q; Puthongkham P; Venton BJ Review: New Insights into Optimizing Chemical and 3D Surface Structures of Carbon Electrodes for Neurotransmitter Detection. Anal. Methods 2019, 11, 247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Georgakilas V; Perman JA; Tucek J; Zboril R Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev 2015, 115, 4744–4822. [DOI] [PubMed] [Google Scholar]
- (4).Dolmatov VY Detonation Synthesis Ultradispersed Diamonds: Properties and Applications. Russ. Chem. Rev 2001, 70, 607–626. [Google Scholar]
- (5).Iakoubovskii K; Baidakova MV; Wouters BH; Stesmans A; Adriaenssens GJ; Vul’ AY; Grobet PJ Structure and Defects of Detonation Synthesis Nanodiamond. Diam. Relat. Mater 2000, 9 (3), 861–865. [Google Scholar]
- (6).Schrand AM; Hens SAC; Shenderova OA Nanodiamond Particles: Properties and Perspectives for Bioapplications. Crit. Rev. Solid State Mater. Sci 2009, 34 (1–2), 18–74. [Google Scholar]
- (7).Mchedlov-Petrossyan NO; Kamneva NN; Marynin AI; Kryshtal AP; Ōsawa E Colloidal Properties and Behaviors of 3 Nm Primary Particles of Detonation Nanodiamonds in Aqueous Media. Phys. Chem. Chem. Phys 2015, 17 (24), 16186–16203. [DOI] [PubMed] [Google Scholar]
- (8).Cheng X-B; Zhao M-Q; Chen C; Pentecost A; Maleski K; Mathis T; Zhang X-Q; Zhang Q; Jiang J; Gogotsi Y Nanodiamonds Suppress the Growth of Lithium Dendrites. Nat. Commun 2017, 8 (1), 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Krüger A; Kataoka F; Ozawa M; Fujino T; Suzuki Y; Aleksenskii AE; Vul’ AY; Osawa E Unusually Tight Aggregation in Detonation Nanodiamond: Identification and Disintegration. Carbon 2005, 43 (8), 1722–1730. [Google Scholar]
- (10).Holt KB; Ziegler C; Caruana DJ; Zang J; Millán-Barrios EJ; Hu J; Foord JS Redox Properties of Undoped 5 Nm Diamond Nanoparticles. Phys. Chem. Chem. Phys 2008, 10 (2), 303–310. [DOI] [PubMed] [Google Scholar]
- (11).Holt KB; Caruana DJ; Millán-Barrios EJ Electrochemistry of Undoped Diamond Nanoparticles: Accessing Surface Redox States. J. Am. Chem. Soc 2009, 131 (32), 11272–11273. [DOI] [PubMed] [Google Scholar]
- (12).Chen LH; Zang JB; Wang YH; Bian LY Electrochemical Oxidation of Nitrite on Nanodiamond Powder Electrode. Electrochim. Acta 2008, 53 (8), 3442–3445. [Google Scholar]
- (13).Holt KB Undoped Diamond Nanoparticles: Origins of Surface Redox Chemistry. Phys. Chem. Chem. Phys 2010, 12, 2048. [DOI] [PubMed] [Google Scholar]
- (14).Peltola E; Wester N; Holt KB; Johansson LS; Koskinen J; Myllymäki V; Laurila T Nanodiamonds on Tetrahedral Amorphous Carbon Significantly Enhance Dopamine Detection and Cell Viability. Biosens. Bioelectron 2017, 88, 273–282. [DOI] [PubMed] [Google Scholar]
- (15).Dai W; Li M; Gao S; Li H; Li C; Xu S; Wu X; Yang B Fabrication of Nickel/Nanodiamond/Boron-Doped Diamond Electrode for Non-Enzymatic Glucose Biosensor. Electrochim. Acta 2016, 187, 413–421. [Google Scholar]
- (16).Chen TW; Palanisamy S; Chen SM; Velusamy V; Liu YH; Tseng TW; Yu MC; Lee SY; Chang WH; Liu X Sensitive and Low-Potential Electrochemical Detection of Hydroquinone Using a Nanodiamond Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci 2017, 12 (9), 8021–8032. [Google Scholar]
- (17).Simioni NB; Silva TA; Oliveira GG; Fatibello-filho O A Nanodiamond-Based Electrochemical Sensor for the Determination of Pyrazinamide Antibiotic. Sens. Actuators, B 2017, 250, 315–323. [Google Scholar]
- (18).Huffman ML; Venton BJ Carbon-Fiber Microelectrodes for in Vivo Applications. Analyst 2009, 134, 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Shin M; Copeland JM; Venton BJ Drosophila as a Model System for Neurotransmitter Measurements. ACS Chem. Neurosci 2018, 9 (8), 1872–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Ganesana M; Lee ST; Wang Y; Venton BJ Analytical Techniques in Neuroscience: Recent Advances in Imaging, Separation, and Electrochemical Methods. Anal. Chem 2017, 89, 314–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Roberts JG; Sombers LA Fast-Scan Cyclic Voltammetry: Chemical Sensing in the Brain and Beyond. Anal. Chem 2018, 90 (1), 490–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Hersey M; Berger SN; Holmes J; West A; Hashemi P Recent Developments in Carbon Sensors for At-Source Electroanalysis. Anal. Chem 2019, 91 (1), 27–43. [DOI] [PubMed] [Google Scholar]
- (23).Taylor IM; Robbins EM; Catt KA; Cody PA; Happe CL; Cui XT Enhanced Dopamine Detection Sensitivity by PEDOT/Graphene Oxide Coating on in Vivo Carbon Fiber Electrodes. Biosens. Bioelectron 2017, 89, 400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Zestos AG; Yang C; Jacobs CB; Hensley D; Venton BJ Carbon Nanospikes Grown on Metal Wires as Microelectrode Sensors for Dopamine. Analyst 2015, 140, 7283–7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Xiao N; Venton BJ Rapid, Sensitive Detection of Neurotransmitters at Microelectrodes Modified with Self-Assembled SWCNT Forests. Anal. Chem 2012, 84, 7816–7822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Ross AE; Venton BJ Nafion-CNT Coated Carbon-Fiber Microelectrodes for Enhanced Detection of Adenosine. Analyst 2012, 137 (13), 3045–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Yang C; Jacobs CB; Nguyen MD; Ganesana M; Zestos AG; Ivanov IN; Puretzky AA; Rouleau CM; Geohegan DB; Venton BJ Carbon Nanotubes Grown on Metal Microelectrodes for the Detection of Dopamine. Anal. Chem 2016, 88, 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Swamy BEK; Venton BJ Carbon Nanotube-Modified Microelectrodes for Simultaneous Detection of Dopamine and Serotonin in Vivo. Analyst 2007, 132 (9), 876–884. [DOI] [PubMed] [Google Scholar]
- (29).Puthongkham P; Yang C; Venton BJ Carbon Nanohorn-Modified Carbon Fiber Microelectrodes for Dopamine Detection. Electroanalysis 2018, 30, 1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).McCreery RL Advanced Carbon Electrode Materials for Molecular Electrochemistry. Chem. Rev 2008, 108, 2646–2687. [DOI] [PubMed] [Google Scholar]
- (31).Yang C; Denno ME; Pyakurel P; Venton BJ Recent Trends in Carbon Nanomaterial-Based Electrochemical Sensors for Biomolecules: A Review. Anal. Chim. Acta 2015, 887, 17–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Yang C; Wang Y; Jacobs CB; Ivanov I; Venton BJ O2 Plasma Etching and Anti-Static Gun Surface Modifications for CNT Yarn Microelectrode Improve Sensitivity and Anti-Fouling Properties. Anal. Chem 2017, 5605–5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Xiao T; Wu F; Hao J; Zhang M; Yu P; Mao L In Vivo Analysis with Electrochemical Sensors and Biosensors. Anal. Chem 2016, 89, 300–313. [DOI] [PubMed] [Google Scholar]
- (34).Hashemi P; Dankoski EC; Petrovic J; Keithley RB; Wightman RM Voltammetric Detection of 5-Hydroxytryptamine Release in the Rat Brain. Anal. Chem 2009, 81 (22), 9462–9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Patel AN; Unwin PR; MacPherson JV Investigation of Film Formation Properties during Electrochemical Oxidation of Serotonin (5-HT) at Polycrystalline Boron Doped Diamond. Phys. Chem. Chem. Phys 2013, 15 (41), 18085–18092. [DOI] [PubMed] [Google Scholar]
- (36).Fang H; Pajski ML; Ross AE; Venton BJ Quantitation of Dopamine, Serotonin and Adenosine Content in a Tissue Punch from a Brain Slice Using Capillary Electrophoresis with Fast-Scan Cyclic Voltammetry Detection. Anal. Methods 2013, 5 (11), 2704–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Zestos AG; Jacobs CB; Trikantzopoulos E; Ross AE; Venton BJ Polyethylenimine Carbon Nanotube Fiber Electrodes for Enhanced Detection of Neurotransmitters. Anal. Chem 2014, 86 (17), 8568–8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Sarada BV; Rao TN; Tryk DA; Fujishima A Electrochemical Oxidation of Histamine and Serotonin at Highly Boron-Doped Diamond Electrodes. Anal. Chem 2000, 72 (7), 1632–1638. [DOI] [PubMed] [Google Scholar]
- (39).Bennet KE; Tomshine JR; Min H-K; Manciu FS; Marsh MP; Paek SB; Settell ML; Nicolai EN; Blaha CD; Kouzani AZ; et al. A Diamond-Based Electrode for Detection of Neurochemicals in the Human Brain. Front. Hum. Neurosci 2016, 10 (March), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Patel BA; Bian X; Quaiserová-Mocko V; Galligan JJ; Swain GM In Vitro Continuous Amperometric Monitoring of 5-Hydroxytryptamine Release from Enterochromaffin Cells of the Guinea Pig Ileum. Analyst 2007, 132 (1), 41–47. [DOI] [PubMed] [Google Scholar]
- (41).Granger MC; Witek M; Xu J; Wang J; Hupert M; Hanks A; Koppang MD; Butler JE; Lucazeau G; Mermoux M; et al. Standard Electrochemical Behavior of High-Quality, Boron-Doped Polycrystalline Diamond Thin-Film Electrodes. Anal. Chem 2000, 72 (16), 3793–3804. [DOI] [PubMed] [Google Scholar]
- (42).Siddiqui S; Dutta G; Tan C; Arumugam PU Nanocrystalline Diamond Electrodes: Enabling Electrochemical Microsensing Applications with High Reliability and Stability. IEEE Nanotechnol. Mag 2016, 10 (3), 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Park J; Quaiserová-Mocko V; Pecková K; Galligan JJ; Fink GD; Swain GM Fabrication, Characterization, and Application of a Diamond Microelectrode for Electrochemical Measurement of Norepinephrine Release from the Sympathetic Nervous System. Diam. Relat. Mater 2006, 15 (4–8), 761–772. [Google Scholar]
- (44).Chang AY; Dutta G; Siddiqui S; Arumugam PU Surface Fouling of Ultrananocrystalline Diamond Microelectrodes during Dopamine Detection: Improving Lifetime via Electrochemical Cycling. ACS Chem. Neurosci 2018, 10, 313–322. [DOI] [PubMed] [Google Scholar]
- (45).Peltola E; Sainio S; Holt KB; Palomäki T; Koskinen J; Laurila T Electrochemical Fouling of Dopamine and Recovery of Carbon Electrodes. Anal. Chem 2017, acs.analchem.7b04793. [DOI] [PubMed] [Google Scholar]
- (46).Schrand AM; Dai L; Schlager JJ; Hussain SM; Osawa E Differential Biocompatibility of Carbon Nanotubes and Nanodiamonds. Diam. Relat. Mater 2007, 16 (12), 2118–2123. [Google Scholar]
- (47).Zang J; Wang Y; Bian L; Zhang J; Meng F; Zhao Y; Ren S; Qu X Surface Modification and Electrochemical Behaviour of Undoped Nanodiamonds. Electrochim. Acta 2012, 72, 68–73. [Google Scholar]
- (48).Huffman ML; Venton BJ Electrochemical Properties of Different Carbon-Fiber Microelectrodes Using Fast-Scan Cyclic Voltammetry. Electroanalysis 2008, 20, 2422–2428. [Google Scholar]
- (49).Nunn N; Shenderova O Toward a Golden Standard in Single Digit Detonation Nanodiamond. Phys. Status Solidi Appl. Mater. Sci 2016, 213 (8), 2138–2145. [Google Scholar]
- (50).Prawer S; Nugent KW; Jamieson DN; Orwa JO; Bursill LA; Peng JL The Raman Spectrum of Nanocrystalline Diamond. Chem. Phys. Lett 2000, 332, 93–97. [Google Scholar]
- (51).Korepanov VI; Hamaguchi H. o.; Osawa E; Ermolenkov V; Lednev IK; Etzold BJM; Levinson O; Zousman B; Epperla CP; Chang HC Carbon Structure in Nanodiamonds Elucidated from Raman Spectroscopy. Carbon 2017, 121, 322–329. [Google Scholar]
- (52).Gabrielli C; Keddam M; Portail N; Rousseau P; Takenouti H; Vivier V Electrochemical Impedance Spectroscopy Investigations of a Microelectrode Behavior in a Thin-Layer Cell: Experimental and Theoretical Studies. J. Phys. Chem. B 2006, 110 (41), 20478–20485. [DOI] [PubMed] [Google Scholar]
- (53).Varley TS; Hirani M; Harrison G; Holt KB Nanodiamond Surface Redox Chemistry: Influence of Physicochemical Properties on Catalytic Processes. Faraday Discuss. 2014, 172 (0), 349–364. [DOI] [PubMed] [Google Scholar]
- (54).Bard AJ; Faulkner LR Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley and Sons: New York, 2001. [Google Scholar]
- (55).Takagi K; Natsui K; Watanabe T; Einaga Y Increasing the Electric Double-Layer Capacitance in Boron-Doped Diamond Electrodes. ChemElectroChem 2019, 1683–1687. [Google Scholar]
- (56).Watanabe T; Shimizu TK; Tateyama Y; Kim Y; Kawai M; Einaga Y Giant Electric Double-Layer Capacitance of Heavily Boron-Doped Diamond Electrode. Diam. Relat. Mater 2010, 19 (7–9), 772–777. [Google Scholar]
- (57).Lv X; Hu B; Wang Z; Peng J; Weng J Two-Electron Oxidation of Dopamine Controlled by Surface Modification of Few-Layer Graphene. Electrochim. Acta 2015, 180, 43–52. [Google Scholar]
- (58).Mochalin V; Osswald S; Gogotsi Y Contribution of Functional Groups to the Raman Spectrum of Nanodiamond Powders. Chem. Mater 2009, 128, 273–279. [Google Scholar]
- (59).Bath BD; Michael DJ; Trafton BJ; Joseph JD; Runnels PL; Wightman RM Subsecond Adsorption and Desorption of Dopamine at Carbon-Fiber Microelectrodes. Anal. Chem 2000, 72, 5994–6002. [DOI] [PubMed] [Google Scholar]
- (60).Morgan DM; Weber SG Noise and Signal-to-Noise Ratio in Electrochemical Detectors. Anal. Chem 1984, 56 (13), 2560–2567. [DOI] [PubMed] [Google Scholar]
- (61).Chen SM; Chen JY; Vasantha VS Electrochemical Preparation of Epinephrine/Nafion Chemically Modified Electrodes and Their Electrocatalytic Oxidation of Ascorbic Acid and Dopamine. Electrochim. Acta 2006, 52 (2), 455–465. [Google Scholar]
- (62).Hu M; Fritsch I Application of Electrochemical Redox Cycling: Toward Differentiation of Dopamine and Norepinephrine. Anal. Chem 2016, 88 (11), 5574–5578. [DOI] [PubMed] [Google Scholar]
- (63).Robinson DL; Venton BJ; Heien MLAV; Wightman RM Detecting Subsecond Dopamine Release with Fast-Scan Cyclic Voltammetry in Vivo. Clin. Chem 2003, 49 (10), 1763–1773. [DOI] [PubMed] [Google Scholar]
- (64).Pajski ML; Venton BJ Adenosine Release Evoked by Short Electrical Stimulations in Striatal Brain Slices Is Primarily Activity Dependent. ACS Chem. Neurosci 2010, 1 (12), 775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Lee ST; Venton BJ Regional Variations of Spontaneous, Transient Adenosine Release in Brain Slices. ACS Chem. Neurosci 2018, 9 (3), 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Weese ME; Krevh RA; Li Y; Alvarez NT; Ross AE Defect Sites Modulate Fouling Resistance on Carbon-Nanotube Fiber Electrodes. ACS Sensors 2019, 4, 1001–1007. [DOI] [PubMed] [Google Scholar]
- (67).Güell AG; Meadows KE; Unwin PR; MacPherson JV Trace Voltammetric Detection of Serotonin at Carbon Electrodes: Comparison of Glassy Carbon, Boron Doped Diamond and Carbon Nanotube Network Electrodes. Phys. Chem. Chem. Phys 2010, 12 (34), 10108–10114. [DOI] [PubMed] [Google Scholar]
- (68).Liu X; Xiao T; Wu F; Shen M-Y; Zhang M; Yu H; Mao L Ultrathin Cell-Membrane-Mimic Phosphorylcholine Polymer Film Coating Enables Large Improvements for In Vivo Electrochemical Detection. Angew. Chem., Int. Ed 2017, 56 (39), 11802–11806. [DOI] [PubMed] [Google Scholar]
- (69).Singh YS; Sawarynski LE; Dabiri PD; Choi WR; Andrews AM Head-to-Head Comparisons of Carbon Fiber Microelectrode Coatings for Sensitive and Selective Neurotransmitter Detection by Voltammetry. Anal. Chem 2011, 83 (17), 6658–6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Wang X; Shen X; Gao J; Sun F Consecutive Deposition of Amorphous SiO2 Interlayer and Diamond Film on Graphite by Chemical Vapor Deposition. Carbon 2017, 117, 126–136. [Google Scholar]
- (71).Antonin O; Schoeppner R; Gabureac M; Pethö L; Michler J; Raynaud P; Nelis T Nano Crystalline Diamond MicroWave Chemical Vapor Deposition Growth on Three Dimension Structured Silicon Substrates at Low Temperature. Diam. Relat. Mater 2018, 83 (November 2017), 67–74. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.