To the Editor:
Diffuse large B-cell lymphoma (DLBCL) and other aggressive B-cell non-Hodgkin lymphomas (NHL) are curable with front-line chemoimmunotherapy; however, patients with relapsed or refractory (R/R) disease face poor outcomes.1 Patients who were unable to achieve a response to platinum-containing salvage regimens or who relapsed after high-dose therapy and autologous stem cell transplantation had limited options prior to the approval of CD19-specific chimeric antigen receptor T-cell therapy (CART).2–4 Both axicabtagene ciloleucel and tisagenlecleucel have FDA-approval for patients receiving at least two prior lines of therapy for most aggressive B-cell NHL.5,6 Other CD19 CARTs are in late-phase clinical trials, and data to-date suggest durable complete response (CR) rates around 30% to 40%, particularly for individuals with early CR.5–7 Unfortunately, little data exist on the outcomes of the majority of patients who eventually suffer progressive disease (PD) after CD19 CARTs. Herein, we describe outcomes at our institution for individuals with PD following CD19-specific CART.
Study population:
Adults with DLBCL, transformed follicular lymphoma (tFL), primary mediastinal B-cell lymphoma (PMBCL), and high-grade B-cell lymphomas (HGBCL) receiving a CD19-specific CART at the University of Washington/Fred Hutchinson Cancer Research Center and experiencing subsequent PD were included in this analysis. Patients enrolled in a CART clinical trial combined with another agent were excluded. We defined early PD as patients who exhibited disease progression ≤30 days after CART administration and late PD as patients who developed PD > 30 days after CART administration. Bridging therapy was defined as any anti-lymphoma therapy between leukapheresis and CART infusion. Primary endpoint was overall survival (OS) following PD. Secondary endpoints included OS stratified by patient characteristics including use of bridging therapy and type of PD (early vs late). Baseline data were retrieved from the electronic medical record. For original data, please contact vicachow@uw.edu. We identified 61 patients who received any CD19-specific CART between October 2013 and December 2018, then progressed. CART product, dosing and lymphodepleting chemotherapy were administered per study protocols or FDA-approval. Baseline characteristics are listed in Table 1. Histologies included DLBCL (36), HGBCL (13), tFL (9), and PMBCL (3). Median age was 60 years (range 26–75), 41 (67%) were male, median prior regimens was 4 (range 1–8). Twenty-six (43%) patients exhibited early PD, 35 (57%) patients had late PD. The median OS for the entire group landmarked at PD was 5.3 months (Figure 1A). The median OS for early PD and late PD was 3.75 months (95% CI 2.34–11.5) and 9.28 months (3.91-not reached) respectively (P =.042, Figure 1B). A median 27 days (range 1–30) elapsed from CART to early PD vs 63 days (range 34–658) from CART to late PD. Univariate analysis identified a difference in pre-CART lactate dehydrogenase (LDH) levels between the two groups, with a median LDH level of 294 in early PD patients vs 176 in late PD patients (P =.046). These data define early PD as a population with extremely poor outcomes, while those with late PD likely benefited from CART initially and were afforded time to plan for subsequent therapy at progression. This is evidenced by 83% of patients with late PD receiving subsequent therapy, compared to 65% of those with early PD. Still, most late PD patients ultimately succumbed to their disease.
TABLE 1.
Patient characteristics
| Characteristic | Total (N = 61) | Early PD (N = 26) | Late PD (N = 35) | P value |
|---|---|---|---|---|
| Gender | .264 | |||
| Female | 20 (32.8%) | 6 (23.1%) | 14 (40.0%) | |
| Male | 41 (67.2%) | 20 (76.9%) | 21 (60.0%) | |
| Median age (range) | 60 (26–75) | 60.5 (29–71) | 60 (26–75) | .767 |
| Histology | .277 | |||
| DLBCL | 36 (59.0%) | 18 (69.2%) | 18 (51.4%) | |
| HGBCL | 13 (21.3%) | 3 (11.5%) | 10 (28.6%) | |
| PMBCL | 3 (4.9%) | 2 (7.7%) | 1 (2.9%) | |
| tFL | 9 (14.8%) | 3 (11.6%) | 6 (17.1%) | |
| Prior lines of treatment (range) | 4 (1–8) | 4 (1–8) | 3 (1–6) | .462 |
| Prior allogeneic transplant | 5 (8.2%) | 3 (11.5%) | 2 (5.7%) | .728 |
| Prior autologous transplant | 24 (39.3%) | 8 (30.1%) | 16 (45.7%) | .761 |
| Cell of origin | .453 | |||
| Germinal center | 27 (44.3%) | 10 (38.5%) | 17 (48.6%) | |
| Non-germinal center | 22 (36.1%) | 9 (34.6%) | 13 (37.1%) | |
| Unknown | 12 (19.6%) | 7 (26.9%) | 5 (14.3%) | |
| Double hit | 9 (14.8%) | 1 (3.8%) | 8 (22.9%) | .088 |
| Triple hit | 2 (3.3%) | 1 (3.8%) | 1 (2.9%) | |
| Baseline bulk | .543 | |||
| <5 cm | 36 (59.0%) | 17 (65.4%) | 19 (54.3%) | |
| ≥5 cm | 25 (41.0%) | 9 (34.6%) | 16 (45.7%) | |
| Extranodal sites | 51 (83.6%) | 24 (92.3%) | 27 (77.1%) | .218 |
| LDH (range) | 210 (107–2339) | 294 (117–2298) | 176 (107–2339) | .046 |
| International Prognostic Index | .094 | |||
| 0–1 | 12 (19.7%) | 2 (7.7%) | 10 (28.6%) | |
| 2–3 | 39 (63.9%) | 18 (69.2%) | 21 (60.0%) | |
| 4–5 | 10 (16.4%) | 6 (23.1%) | 4 (11.4%) | |
| Bridging therapy | 22 (36.1%) | 10 (39.4%) | 12 (28.0%) | .256 |
| Chemotherapy +/− steroids | 10 (45.5%) | 4 (40.0%) | 6 (50.0%) | |
| Intrathecal | 1 (4.5%) | 0 (0.0%) | 1 (8.3%) | |
| Novel/Targeted +/− steroids | 6 (27.3%) | 2 (20.0%) | 4 (33.4%) | |
| Steroids | 5 (22.7%) | 4 (40.0%) | 1 (8.3%) | |
| Additional therapy after progression | 46 (75.4%) | 17 (65.4%) | 29 (82.9%) | .115 |
| Next line of therapy | .207 | |||
| Allogeneic transplant | 1 (2.2%) | 0 (0.0%) | 1 (3.4%) | |
| CART | 14 (30.4%) | 8 (47.1%) | 6 (20.7%) | |
| Chemotherapy | 7 (15.2%) | 4 (23.5%) | 3 (10.4%) | |
| Immunotherapy | 4 (8.7%) | 1 (5.9%) | 3 (10.4%) | |
| Intrathecal | 1 (2.2%) | 0 (0.0%) | 1 (3.4%) | |
| Radiation | 5 (10.9%) | 0 (0.0%) | 5 (17.2%) | |
| Novel/Targeted | 14 (30.4%) | 4 (23.5%) | 10 (34.5%) | |
| Next treatment on clinical trial | 7 (11.5%) | 2 (7.7%) | 5 (14.3%) | .960 |
| Allogeneic transplant after progression | 5 (8.2%) | 1 (3.8%) | 4 (11.4%) | .551 |
Abbreviations: CART, chimeric antigen receptor T-cell therapy; DLBCL, Diffuse large B-cell lymphoma; HGBCL, high-grade B-cell lymphomas; LDH, lactate dehydrogenase; PD, progressive disease; PMBCL, primary mediastinal B-cell lymphoma; tFL, transformed follicular lymphoma.
FIGURE 1.
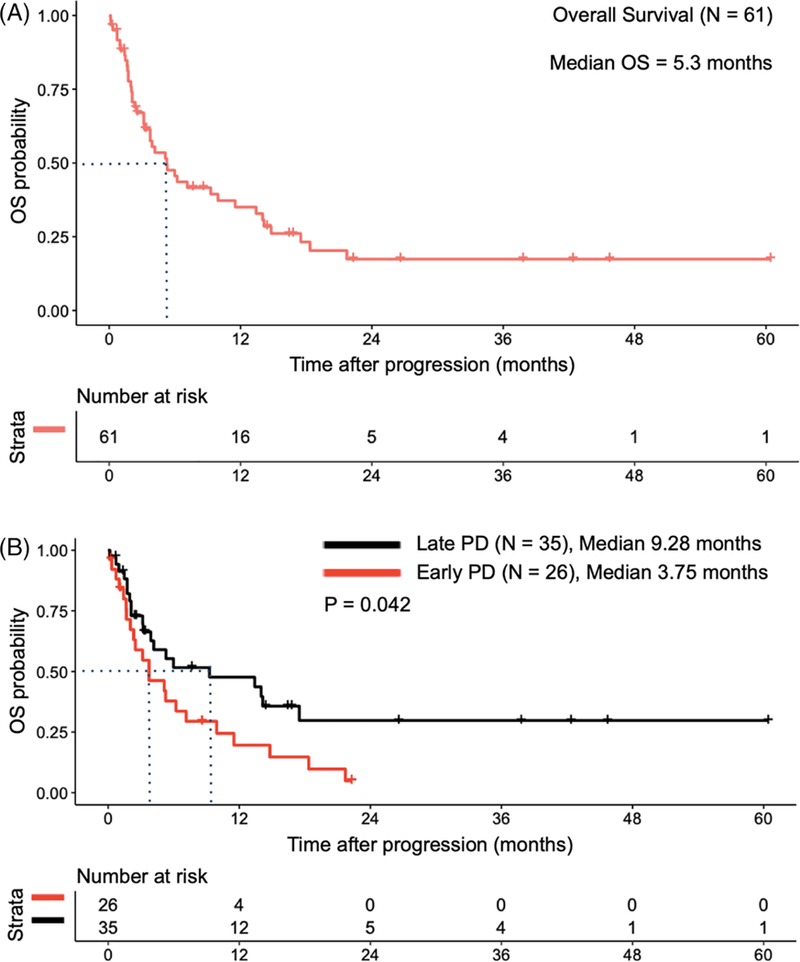
OS results analyzed using Kaplan-Meier methodology and are shown for the overall population A, and by timing of progression (B, Early PD vs Late PD). OS, overall survival; PD, progressive disease
Twenty-two (36%) individuals displayed kinetically active disease, warranting bridging therapy for disease stabilization prior to CART administration. These therapies included: chemoimmunotherapy +/− steroids (10), steroids (5), novel/targeted therapy (including rituximab) +/− steroids (6), and intrathecal therapy (1). There was no statistical difference in OS after PD between those receiving and not receiving bridging therapy (3.16 vs 7.14 months, P =.26), although patients with no bridging therapy and late PD (N = 23) survived the longest, with a median OS of 13.42 months. While bridging therapy did not appear to impact outcomes, the numbers are small, and it may still be a surrogate for more aggressive disease. Alternatively, CART may have abrogated the potential inferior outcomes in patients requiring the use of bridging therapy, rendering no difference in OS in this population. The role of bridging therapy should ideally be examined outside of clinical trials, as eligibility criteria may exclude higher risk individuals.
Forty-six (75%) patients received ≥1 subsequent therapy after PD. Initial therapies included: second CART of same construct (14), novel/targeted therapy (14), chemotherapy +/− rituximab (7), radiotherapy (5), PD1-inhibitors (4), intrathecal chemotherapy (1), and allogeneic HSCT (1). Fifteen (25%) patients received no further therapy after PD. Those who received therapy for PD had a lower risk of death (HR 0.257, 95% CI 0.115–0.572, P =.0009) compared to those who did not receive subsequent therapy. No single approach appeared to confer a survival advantage, though numbers in each group were small. We identified nine patients alive and in remission for ≥12 months after progression. Last line of therapy included radiotherapy (2), allogeneic HSCT (2), ibrutinib (2), subsequent CD19-specific CART (1), nivolumab (1), and lenalidomide (1). We are mindful that the pursuit of additional therapy may reflect patient and disease-specific characteristics, including better functional status or less aggressive disease. Therefore, we cannot draw conclusions regarding the efficacy of therapy after PD based on these results. As most patients received additional therapy after CART failure in our study, prospective clinical trials should be designed to quickly transition patients to subsequent therapy at time of progression.
Overall, seven (11%) patients enrolled onto a separate clinical trial utilizing novel agents after PD, and five (8%) patients eventually received an allogeneic HSCT after PD, two of whom are still alive. We could not identify specific reasons for low accrual onto clinical trials but hypotheses include patient choice, poor performance status, a transition to hospice, and travel logistics. At time of progression, 16% (N = 10) and 26% (N = 16) of patients in our population were noted to have grade ≥3 neutropenia and thrombocytopenia per Common Terminology Criteria for Adverse Events criteria, respectively.8 Therefore, cytopenias may play a limited role in excluding individuals from clinical trials, and enrolling patients onto trials at time of PD should be highly encouraged. As a whole, these data suggest that planning for the potential of post-CART disease progression before it occurs should figure into the treatment algorithm. Approaches may include human leukocyte antigen typing, pre-screening for clinical trials, or obtaining insurance approval for off-label use of novel agents.
Limitations of this study include our single-center experience, the inclusion of all CD19-specific CART products/doses whether commercially or under clinical trial, univariate analyses, and the varying practices regarding bridging and subsequent therapy. To broadly highlight the challenges we face when managing a patient in clinic who progresses after CD19-specific CART, we did not include data regarding the CART product, cell dose, inflammatory cytokines/cytokine release syndrome, neurotoxicity, or potential mechanisms of relapse in our final analysis. Future studies should incorporate this information when analyzing datasets of uniformly treated patients.
In summary, outcomes after CD19-specific CART progression are poor, particularly among those suffering from early PD. To our knowledge this is the first manuscript to detail outcomes in patients with PD following CD19-specific CART. These data can be used to inform novel interventions to both prevent and treat PD following CD19-specific CART.
ACKNOWLEDGMENTS
This work was supported by 5K24CA184039, T32CA009515, donations from Frank and Betty Vandermeer and Sonya and Tom Campion.
V.A.C. has no disclosures. A.K.G. reports grants and nonfinancial support from Teva, Bristol-Myers Squibb, Merck, Takeda, TG Therapeutics, and Effector; grants, personal fees, and nonfinancial support from Seattle Genetics, Pfizer, Janssen, Gilead, Spectrum, Amgen and Incyte; personal fees from Aptevo, BRIM Bio, Seattle Genetics and Sanofi. D.J.M. reports honoraria from Celgene, Gilead Sciences, Kite Pharma, Roche and research funding from Juno Therapeutics. C.J.T. receives research funding from Juno Therapeutics/Celgene and Nektar Therapeutics, has patents licensed to Juno Therapeutics/Celgene, has served on advisory boards and has options in Precision Biosciences, Eureka Therapeutics, and Caribou Biosciences, and has served on advisory boards for Nektar Therapeutics, Aptevo, Kite/Gilead, Novartis, and Juno Therapeutics/Celgene. S.D.S. reports funding from Astra Zeneca, Acerta Pharma, Genentech Research, Incyte Corporation, Merck Sharp and Dohme Corporation, Pharmacyclics, Portola Pharmaceuticals, Seattle Genetics and provides consultancy for Astra Zeneca and Merck Sharp and Dohme Corporation. C.S.U. has no disclosures. M.S. provides consultancy for Abbvie, Genentech, Sound Biologics, is on the advisory board for Abbvie, Genentech, Vera-stem, ADC Therapeutics and has research funding from Mustang Biopharma, Celgene, Pharmacyclics, Gilead, Genentech, TG therapeutics, Bigene, Acerta Pharma, Emergent, Merck. R.D.C. has received research funding from Amgen, Kite/Gilead, Merck, and Pfizer, and has served as a consultant/advisor to Adaptive Biotechnologies, Amgen, Pfizer, and Jazz Pharmaceuticals. B.G.T. reports patents/royalties and research funding from Mustang Bio. Y.D.T. has no disclosures. E.H.W. has no disclosures. A.R.S. has no disclosures. M.P.M. has no disclosures. S.K. has no disclosures. U.H.A. received research support from JUNO Therapeutics and provides consultancy for Celgene, Teva, Kite. E.M. has no disclosures. L.M.H. has no disclosures. J.M.V. has no disclosures. T.G. has no disclosures. R.C.L. reports research funding from Incyte, Juno Therapeutics, Rhizen Pharmaceuticals, Takeda, TG Therapeutics.
Footnotes
CONFLICT OF INTERESTS
REFERENCES
- 1.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Den Neste E, Schmitz N, Mounier N, et al. Outcomes of diffuse large B-cell lymphoma patients relapsing after autologous stem cell transplantation: an analysis of patients included in the CORAL study. Bone Marrow Transplant. 2017;52:216–221. [DOI] [PubMed] [Google Scholar]
- 3.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Onc. 2010;28:4184–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow VA, Shadman M, Gopal AK. Translating anti-CD19 CAR T-cell therapy into clinical practice for relapsed/refractory diffuse large B-cell lymphoma. Blood. 2018;132(8):777–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017; 377:2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. [DOI] [PubMed] [Google Scholar]
- 7.Abramson JS, Palomba LM, Gordon LI, et al. High durable CR rates in R/R aggressive B-NHL treated with JCAR017 (lisocabtagene maraleucel; liso-cel) (TRANSCEND NHL 001): defined composition CD19-directed CAR T cell product allows for dose finding and definition of pivotal cohort. American Society of Hematology Annual Meeting; 2017; Abstract #581. [Google Scholar]
- 8.Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0. U.S. Department of Health and Human Services; November 27, 2017; National Institutes of Health, National Cancer Institute. [Google Scholar]


