Abstract
Remote ischemic preconditioning (RIPC), the phenomenon whereby brief ischemic episodes in distant tissues or organs render the heart resistant to infarction, has been exhaustively demonstrated in preclinical models. Moreover, emerging evidence suggests that exosomes play a requisite role in conveying the cardioprotective signal from remote tissue to the myocardium. However, in cohorts displaying clinically common comorbidities—in particular, type-2 diabetes—the infarct-sparing effect of RIPC may be confounded for as-yet unknown reasons. To investigate this issue, we used an integrated in vivo and in vitro approach to establish whether: (1) the efficacy of RIPC is maintained in the Zucker fatty rat model of type-2 diabetes, (2) the humoral transfer of cardioprotective triggers initiated by RIPC are transported via exosomes, and (3) diabetes is associated with alterations in exosome-mediated communication. We report that a standard RIPC stimulus (four 5-min episodes of hindlimb ischemia) reduced infarct size in normoglycemic Zucker lean rats, but failed to confer protection in diabetic Zucker fatty animals. Moreover, we provide novel evidence, via transfer of serum and serum fractions obtained following RIPC and applied to HL-1 cardiomyocytes subjected to hypoxia-reoxygenation, that diabetes was accompanied by impaired humoral communication of cardioprotective signals. Specifically, our data revealed that serum and exosome-rich serum fractions collected from normoglycemic rats attenuated hypoxia-reoxygenation-induced HL-1 cell death, while, in contrast, exosome-rich samples from Zucker fatty rats did not evoke protection in the HL-1 cell model. Finally, and unexpectedly, we found that exosome-depleted serum from Zucker fatty rats was cytotoxic and exacerbated hypoxia-reoxygenation-induced cardiomyocyte death.
Keywords: Remote ischemic preconditioning, Myocardial ischemia, Myocardial infarction, Infarct size, Cardioprotection, Type-2 diabetes, Exosomes, Extracellular vesicles, Proteomics, Mass spectrometry
Introduction
Remote ischemic preconditioning (RIPC) is the phenomenon whereby brief episodes of ischemia in a distant tissue or organ render the heart resistant to infarction [36, 68, 84]. Infarct size reduction with RIPC has been extensively documented in preclinical models, and is under clinical investigation as a cardioprotective strategy in patients undergoing cardiac surgery or percutaneous coronary intervention [23, 24, 35, 36, 72]. Moreover, progress has been made in elucidating the mechanistic hallmark of RIPC; i.e., the manner by which the cardioprotective signal is conveyed from the site of the RIPC stimulus (typically, one or more limbs) to the heart. While both humoral and neuronal communication have been implicated to play a role [36, 66], recent evidence suggests that exosomes (or, more precisely, extracellular vesicles) serve as the carriers for the humoral component of RIPC and are necessary for RIPC-induced cardioprotection [25].
Despite these advances in our understanding of RIPC, a major gap remains. Preclinical investigations of RIPC— and the attendant mechanisms of the infarct-sparing effects of this phenomenon—have, with rare exceptions [8, 56], been conducted using cohorts of healthy, adult or juvenile animals devoid of clinically relevant comorbidities [20, 34, 67, 86]. Interestingly, recent data have revealed that type-2 diabetes, a well-recognized risk factor for acute myocardial infarction [26, 73, 86], is associated with defects in cardioprotective, exosome-mediated humoral communication [14]. However, the effect of metabolic syndrome and type-2 diabetes on the in vivo efficacy of infarct size reduction with RIPC have not been explored.
Accordingly, in the current study, we used an integrated in vivo and in vitro approach to test two overarching hypotheses. First, we posited, based on the limited insights obtained with other cardioprotective strategies (including conventional ischemic preconditioning and postconditioning [9, 30, 45, 49, 63, 69, 78, 80, 85, 89]), that type-2 diabetes would have a negative, confounding effect on the ability of RIPC to render the myocardium resistant to infarction—a concept that was interrogated in vivo in the well-established Zucker fatty rat model of early-stage type-2 diabetes. Second, using serum and serum fractions harvested from cohorts of diabetic and normoglycemic rats and administered to cultured HL-1 cardiomyocytes prior to hypoxia-reoxygenation, we sought to establish whether: (i) exosomes play a requisite role in the humoral transfer of cardioprotective triggers initiated by RIPC, and (ii) the proposed diabetes-associated loss in efficacy of RIPC was due to a defect in exosome-mediated communication.
Methods
This study was approved by the Institutional Animal Care and Use Committee of Wayne State University and abided by the Guide for the Care and Use of Laboratory Animals from the Institute of Laboratory Animals Resources (NIH Publication Vol. 25 No. 28, revised 1996). Sprague–Dawley retired breeder rats and Zucker rats were purchased from Harlan Laboratories Inc. (Indianapolis, IN, USA). Animals were housed at room temperature on a 12-h light/dark cycle and fed tap water and regular rodent chow, ad libitum. All in vitro experiments were conducted using HL-1 cells [13, 82], generously provided by the late Dr. William Claycomb (Louisiana State University Health Science Center, New Orleans, LA, USA).
Protocol 1: effect of type‑2 diabetes on in vivo cardioprotection with remote ischemic preconditioning
The objective of Protocol 1 was to test the hypothesis that cardioprotection from remote ischemic preconditioning (RIPC) is attenuated or abolished in type-2 diabetes. This concept was investigated using male Zucker fatty rats (ZDF-Leprfa/Crl, 10–12 weeks of age: n = 15), together with age-matched Zucker lean rats (n = 13) as the normoglycemic control cohort, and followed the recommended guidelines for preclinical studies of myocardial ischemia and infarction [54]. Our rationale for enrolling animals within this age range was to investigate the efficacy of RIPC in a model of early-stage diabetes and metabolic syndrome, characterized by modest and physiologically relevant (rather than extreme supra-physiologic [67]) elevations in blood glucose concentrations.
Surgical preparation
Rats were anesthetized with pentobarbital sodium (40 mg/kg intraperitoneal), supplemented as required throughout the protocol to maintain a deep surgical plane of anesthesia. Body temperature was maintained at 37 °C and the ECG was monitored throughout the protocol. A tracheostomy was performed and the rats were ventilated with room air. In addition, the left and right femoral arteries were isolated, and the left or right femoral vein was cannulated. The basal region of the heart was exposed via an intercostal thoracotomy and the left coronary artery was ensnared with 5–0 polypropylene suture by taking an intramyocardial stitch at the distal edge of the left atrial appendage. Additional silk sutures were tied to each arm of the polypropylene suture to facilitate release of the occlusion knot [39, 53, 83].
Study design
Within each cohort, and after surgical preparation was completed, animals were randomly allocated to receive either RIPC or a time-matched control period. For rats in the RIPC groups, four 5-min cycles of bilateral femoral artery occlusion, interspersed with 5 min of reperfusion, were administered (Fig. 1a). After the intervention period, all rats underwent 45 min of coronary artery occlusion, induced by tying a knot in the polypropylene suture. Myocardial ischemia was verified by tissue pallor, changes in the ECG tracing and arrhythmia. Reperfusion was initiated by pulling on the release sutures and verified by the return of tissue blush. At 2 h after relief of ischemia, rats were euthanized under deep pentobarbital anesthesia by intracardiac injection of KCl.
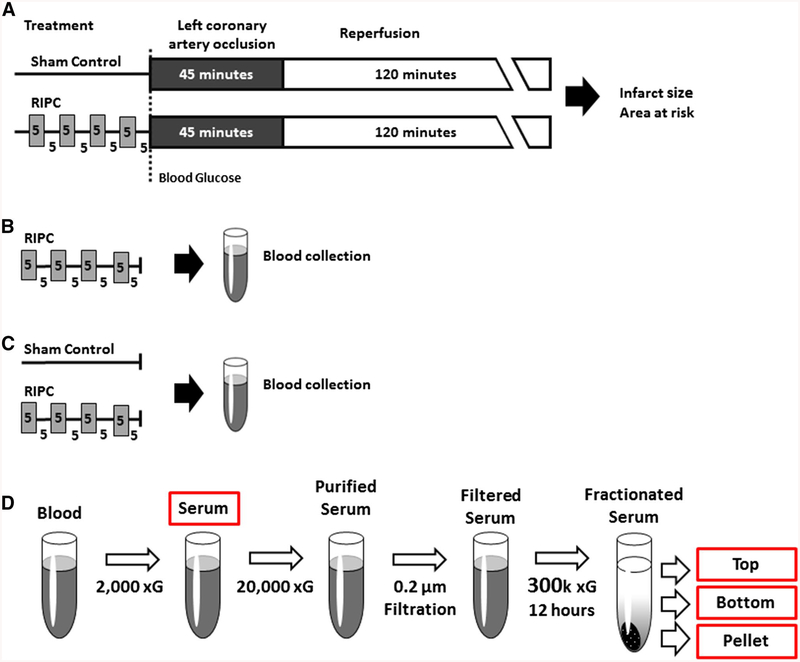
Fig. 1.
a Protocol 1: study design, b Protocols 2 and 3: timing of blood collection, c Protocol 4: timing of blood collection, d Protocols 2, 3 and 4: FRACTIONATION of serum. RIPC remote ischemic preconditioning
Endpoints
Primary endpoints in Protocol 1 were non-fasting blood glucose concentration, area at risk and infarct size. Blood glucose levels were measured immediately prior to the onset of myocardial ischemia using an Accu-Chek© monitor and test strips (F. Hoffmann-La Roche AG). Area at risk was delineated at the end of reperfusion by re-ligation of the coronary artery at the site of previous occlusion and injection of Unisperse Blue pigment (0.5 mL IV) [7, 77]. The hearts were then excised, sectioned transversely and photographed for later analysis. Immediately thereafter, the heart slices were incubated in 2,3,5-triphenyltetrazolium chloride (TTC) for 15 min at 37 °C to differentiate necrotic from viable myocardium and rephotographed [21, 71]. For each heart, area at risk and area of necrosis were quantified by planimetry (SigmaScan Pro SPSS Inc., Chicago, IL) and corrected for tissue weight. All analyses were performed in a blinded manner, without knowledge of the treatment group. Area at risk is reported as a percentage of the left ventricle and infarct size is reported as a percentage of the area at risk.
Protocol 2: role of exosomes in remote preconditioning
Our objective in Protocol 2 was to investigate the hypothesis that exosomes act as a circulating, humoral carrier of protective signaling in RIPC. For initial concept development, and to prepare for testing the hypothesis in the diabetic model (Protocol 3), blood was first collected from adult, normoglycemic Sprague–Dawley rats. After processing (described below), serum and serum fractions were applied to cultured HL-1 cardiomyocytes and evaluated for their ability to confer protection against hypoxia-reoxygenation-induced cell death (Protocol 2A). In addition, to document the efficacy of exosome isolation, serum fractions were probed by immuno-blotting for the presence versus absence of two classic exosome markers, HSP-60 and flotillin-1 [17, 25, 29] (Protocol 2B). Of note, our protocol differs from previous studies [14, 79], in that we investigated the transfer of serum and serum fractions, rather than plasma fractions. Using this approach, exosomes and products released from activated platelets are included in a standardized manner. Accordingly, this choice avoids the confounding effect of inadvertent platelet activation during sample collection (and, thus, variability among samples in the presence of platelet-derived products), and encompasses the reported potential involvement of platelets in the favorable effects of RIPC [55, 61].
Serum collection
Sprague–Dawley rats (total n = 14) were anesthetized with pentobarbital sodium and underwent four 5-min episodes of bilateral femoral artery occlusion as described in Protocol 1. Immediately following the RIPC stimulus, ~ 8 mL of blood was rapidly collected via cardiac puncture, allowed to coagulate, and serum obtained by centrifugation at 2000×g (Fig. 1b).
Preparation of exosome‑rich and exosome-depleted fractions
Exosome-rich and exosome-depleted serum fractions were isolated from the serum using two ultracentrifugation-based methods. In initial experiments (n = 6), we applied a standard protocol for isolation of exosomes from buffers and biologic fluids [25, 76]: serum obtained following RIPC was first purified by low-speed centrifugation at 20,000×g for 45 min and filtered through a 0.2 μm membrane to remove large particles and debris. A 2 mL aliquot of purified serum was then centrifuged at 100,000×g for 2 h [76], with the goal of producing an exosome-rich pellet and exosome-depleted supernatant. However, immunoblot analysis revealed that this standard protocol was sub-optimal (i.e., the supernatant continued to display the presence of exosome markers: data not shown), presumably due to the viscosity of the serum [60]. Accordingly, we developed a modified, enhanced ultra-centrifugation exosome isolation protocol, based on Svedberg kinetics (the forces involved with sedimentation), in which ultracentrifugation was applied at an increased radial force of 300,000×g at 4 °C for a prolonged period of 12 h. This resulted in fractionation of serum into a pellet and a biphasic supernatant (Fig. 1d), and was utilized for all subsequent experiments. All ultracentrifugation was performed using a fixed angle rotor, S110AT-0019 (Hitachi Koki Co., Takeda, Hitachiaka City).
Protocol 2A
HL‑1 cardiomyocyte culture model
Under control conditions, HL-1 cardiomyocytes were maintained at 37 °C in 5% CO2 and 95% room air with 95% humidity in Claycomb medium supplemented with penicillin (100 U/mL) and streptomycin (100 μg/mL), l-glutamine (2 mM) norepinephrine (0.1 mM) and fetal bovine serum (FBS, 10%), as previously described [13, 18]. Hypoxia was achieved by replacing culture medium with hypoxia buffer (mM: 125 NaCl, 8 KCl, 1.2 KH2PO4, 1.25 MgSO4, 1.2 CaCl2, 6.25 NaHCO3, 20 HEPES, 5.5 glucose, 20 2-deoxy-d-glucose, 5 Na-lactate, adjusted to pH 6.6) and sealing the culture dishes in a hermetic chamber with GasPak EZ Gas Generating Sachets (GasPakTM EZ, BD Biosciences, San Jose, CA, USA) for 2.5 h. Cells were reoxygenated for 20 h by removing culture dishes from the hypoxia chamber and replacing the hypoxia buffer with FBS-free Claycomb media [18].
Study design
Five independent experiments were conducted, each using blood collected from one rat following RIPC. For each experiment, HL-1 cells were treated with either serum (concentration of 4% serum/medium by volume; approximately 0.2 mL), serum fractions obtained by enhanced ultracentrifugation (pellet, supernatant-bottom or supernatant-top; see below), or a matched volume of Krebs–Henseleit (KH) buffer. Pellets were resuspended in cold KH buffer (Sigma-Aldrich, Inc., St Louis, MO, USA) at a volume equivalent to the initial volume of purified serum (approximately 2 mL). Supernatants were not diluted, and were applied at a concentration of 4% sample/medium by volume. All treatments were administered at 1 h before the onset of hypoxia, and maintained during hypoxia.
Endpoint
Cell death, was quantified by the Trypan Blue exclusion assay after 20 h of reoxygenation. Briefly, cells were collected from culture dishes, resuspended in Claycomb medium (2–3 million cells per mL) and stained with 0.4% Trypan Blue dye (1:1 ratio of cell suspension:dye) to distinguish viable from dead cells (i.e., cells devoid of blue dye and cells that were stained blue, respectively) [18]. Cells were manually counted (minimum of 250 cells per replicate) and percent cell death was calculated as: cell death (%) = (dead cells/(dead cells + live cells)) × 100.
Protocol 2B
In samples obtained from the remaining three rats, serum fractions obtained by enhanced ultracentrifugation (pellet, supernatant-bottom and supernatant-top) were probed for the presence of the exosome markers HSP-60 and flotillin-1 [17, 29] using standard methods [18]. Briefly, samples were prepared with lysis buffer (8 M urea, 5 mM dithiothreitol, 2% SDS and 25 mM Tris Cl, pH 8) and Laemmli buffer and resolved on 4–15% gradient gels. Samples were loaded onto gels in equal volumes (15–50 μg protein/lane). After electrophoresis, proteins were transferred to nitrocellulose membranes, blocked with 5% non-fat milk, and incubated overnight with primary antibodies (Cell Signaling Technology, Boston, MA; 1:1000 in 5% non-fat milk) at 4 °C. Membranes were labeled with horseradish peroxidase (HRP)-conjugated secondary antibodies (Cell Signaling Technology, Boston MA; 1:10,000 in 5% nonfat milk) for 1 h at room temperature and immunoreactive bands were visualized after incubation with horseradish peroxidase HRP-substrate (SuperSignal West Pico, ThermoFisher Scientific, Waltham, MA).
Protocol 3: efficacy of humoral communication in type‑2 diabetes
In Protocol 3, our objective was to use the in vitro approach, established in Protocol 2, to test the hypothesis that the loss in efficacy of RIPC in type-2 diabetes is associated with a defect in the humoral component of remote preconditioning.
Briefly, Zucker lean and Zucker fatty rats underwent the standard RIPC stimulus (four 5-min episodes of hindlimb ischemia). Serum was collected, and serum or serum fractions (supernatant-bottom and supernatant-top; separated using the enhanced exosome isolation technique) from Zucker lean rats (n = 6: Protocol 3A) and Zucker fatty rats (n = 6; Protocol 3B) were used as treatments for hypoxia-reoxygenation in HL-1 cells, as described in Protocol 2. Based on the outcome of Protocol 2, analysis in Protocols 3A and 3B focused on the supernatant-bottom and supernatant-top fractions; the pellet was not assessed. In separate experiments (Protocol 3C), the effect of the supernatant-top fraction obtained from Zucker lean and Zucker fatty cohorts on HL-1 cell viability was evaluated under normoxic conditions. The primary endpoint was cell death as measured by Trypan Blue viability staining, described in Protocol 2.
Protocol 4: qualitative particle characterization and exploratory proteomics
Our aim in Protocols 4A and 4B was to obtain preliminary, qualitative insight into the contents of the fractions isolated from serum using the methods employed in Protocols 2 and 3.
Protocol 4A
In Protocol 4A, nanoparticle tracking analysis [19] was used to characterize the particle population. Samples from Sprague–Dawley, Zucker lean and Zucker fatty rats, obtained following the standard RIPC stimulus or in time-matched sham-controls (n = 1–3 per cohort; Fig. 1c, d), were analyzed by System Biosciences, Inc. (Palo Alto, CA) using a NanoSight LM10 (NanoSight, Amesbury, UK). Samples from each rat were analyzed in triplicate. Given our focus on exosomes, analysis was limited to particles within the relevant size range (1–200 nm). Particle diameter measurements were tabulated in 1-nm increments and, for each size increment, the particle concentration (number/mL) was quantified.
Protocol 4B
In Protocol 4B, supernatant-bottom and supernatant-top serum fractions from Sprague–Dawley, Zucker lean and Zucker fatty rats, obtained following an RIPC stimulus or from sham-controls (n = 1–3 per cohort; Fig. 1c, d), were analyzed by mass spectrometry (MS) as described previously [51]. Supernatant-bottom samples were depleted of high abundance plasma proteins using the Pierce Albumin/IgG Removal Kit (ThermoFisher Scientific, Waltham, MA) per the manufacturer’s protocol and separated by SDS-PAGE. The gels were stained with GelCode Blue stain reagent (ThermoFisher Scientific, Waltham, MA). Eight gel slices were excised from each lane, washed with 50 mM ammonium bicarbonate, reduced in 10 mM DTT and alkylated with 55 mM iodoacetamide. Proteins were digested overnight at 37 °C with sequencing grade modified trypsin (Promega, Madison, WI). The supernatant-top samples had low protein concentration and were precipitated with cold acetone. Protein pellets were washed, resuspended in 50 mM ammonium bicarbonate, reduced and alkylated before digested with trypsin overnight. The tryptic peptides were separated with reversed-phase chromatography through a C18 column using the Dionex Ultimate™ HPLC system. MS and MS/MS spectra were acquired on an Applied Biosystems QSTAR XL mass analyzer using information dependent acquisition mode. Mascot 2.4.0 (Matrix Science, Inc., Boston, MA) was used to search the National Center for Biotechnology Information non-redundant database for Rattus proteins with carbamidomethyl (C) used as a fixed modification and oxidation (M), N-acetylation (protein N terminus) as variable modifications. The output files were further analyzed and visualized in Scaffold software (Proteome Software, Inc., Portland, OR). For each protein identified, its abundance was estimated by reporting the total spectral count [31].
Statistical analysis
For Protocols 1–3, data were analyzed with GraphPad Prism software (GraphPad Inc., La Jolla, CA) or Stata 12.1 (Stata-Corp LLC, College Station, TX). Endpoints were compared by t test (for comparison of blood glucose concentration values between Zucker lean and Zucker fatty cohorts in Protocol 1), analysis of variance (ANOVA: for comparison of risk region and infarct size among groups in Protocol 1), and repeated measures ANOVA (for comparisons of HL-1 cell viability with serum, serum fraction and KH buffer treatment in Protocols 2 and 3). If significant F values were obtained by ANOVA, subsequent pairwise comparisons were made using Tukey’s test. In addition, in Protocol 1, linear regression and multiple linear regression were used to analyze the relationship between blood glucose and infarct size for the cohorts that underwent remote preconditioning. Results are reported as mean ± SEM.
For Protocol 4, given the small number of independent samples probed in these exploratory experiments, statistical comparisons were not performed. In Protocol 4A, nanoparticle concentration (number/mL) in each serum fraction is reported as a frequency distribution (mean values ± SEM for particles ranging from 0 to 200 nm). For Protocol 4B, qualitative comparisons of protein abundance between groups were made by calculating fold change, defined as [(Value 2 − Value 1)/Value 1] [32]. In cases where Value 1 = 0, fold change = Value 2.
Results
Protocol 1: effect of type‑2 diabetes on cardioprotection with remote ischemic preconditioning
Infarct size
Area at risk did not differ among groups, averaging 18–23% of the total LV weight [p = 0.32 (ns) by ANOVA; data not shown].
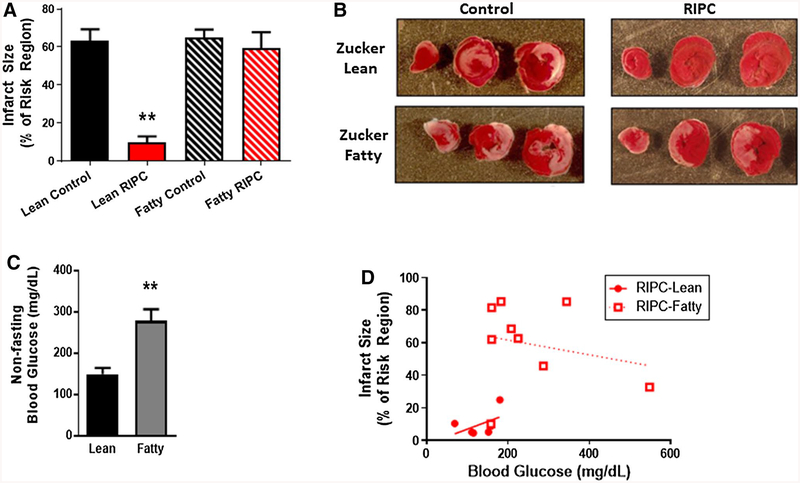
In control cohorts, infarct size, expressed as a percentage of the risk region, was comparable in both strains, averaging 63 ± 6% in the Zucker lean group and 65 ± 4% Zucker fatty rats. As expected, in the Zucker lean cohort, remote ischemic preconditioning was cardioprotective: infarct size was significantly smaller in the RIPC-Lean group versus the Lean-Controls (10 ± 3 versus 63 ± 6%, p < 0.01; Fig. 2a, b). In contrast, RIPC was ineffective in the diabetic strain, with no difference in infarct size between the Fatty-RIPC and the Fatty-Control groups (60 ± 9 versus 65 ± 4%, Fig. 2a, b).
Fig. 2.
a Protocol 1: infarct size, expressed as a % of the myocardium at risk (mean ± SEM), for Zucker lean and Zucker fatty rats randomized to receive remote ischemic preconditioning (RIPC) or a time-matched control period. **p < 0.01 versus the Zucker lean control group, b Protocol 1: images of heart slices obtained from one control and one RIPC-treated rat from the Zucker lean and Zucker fatty cohorts. Heart slices were incubated in triphenyltetrazolium chloride; using this method, viable myocardium stains red while areas of necrosis remain unstained, and thus appear pale, c Protocol 1: non-fasting blood glucose concentration (mg/dL; mean ± SEM) for Zucker lean and Zucker fatty rats. **p < 0.01 versus Zucker lean rats, d Protocol 1: infarct size (expressed as a % of the myocardium) plotted as a function of non-fasting blood glucose concentration (mg/dL) for Zucker lean and Zucker fatty rats that underwent RIPC
Hyperglycemia and cardioprotection
As expected, Zucker fatty rats (including all animals from both Control and RIPC groups) were characterized by a moderate but significant increase in non-fasting blood glucose values when compared with the Zucker lean rats (278 ± 15 versus 150 ± 12 mg/dL, p < 0.01, Fig. 2c). To address the concept raised in some previous studies that hyperglycemia per se may attenuate the efficacy of conditioning-induced cardioprotection [42, 46, 87, 88], infarct size was plotted as a function of blood glucose concentration for all animals (both Zucker fatty and Zucker lean) treated with RIPC. Regression analysis did not support this premise: i.e., there was no association between blood glucose concentration and infarct size (Fig. 2d). In addition, multiple linear regression analysis, including blood glucose levels, risk region and rat strain as covariates, identified strain as the only significant determinant of the extent of necrosis (R2 = 0.63; p values of 0.67, 0.96 and 0.004, respectively).
Protocol 2: role of exosomes in remote preconditioning
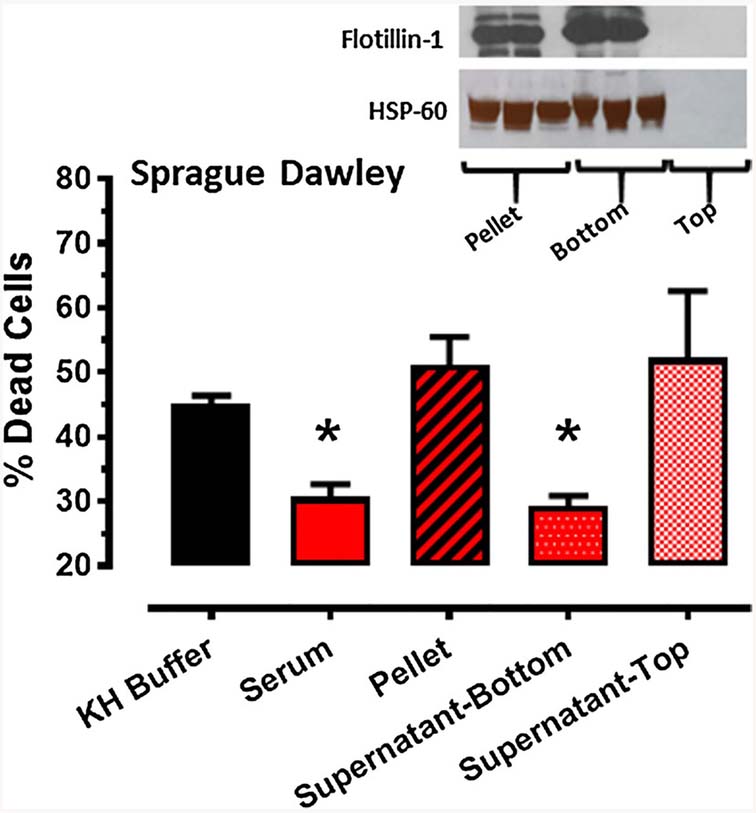
Serum obtained from Sprague–Dawley rats following RIPC and transferred to HL-1 cardiomyocytes rendered the cells resistant to hypoxia-reoxygenation-induced death. Specifically, cell death was 31 ± 2 versus 45 ± 1% in cells treated with RIPC serum versus KH buffer (p < 0.05; Fig. 3).
Fig. 3.
Protocol 2: effect of Krebs–Henseleit (KH) buffer, serum and serum fractions (pellet, supernatant-bottom, supernatant-top), obtained from Sprague–Dawley rats following the standard RIPC stimulus, on % cell death (mean ± SEM) of HL-1 cells subjected to 2.5 h of hypoxia and 24 h of reoxygenation. *p < 0.05 versus KH buffer. Inset: expression of flotillin 1 and HSP-60 in the pellet, supernatant-top and supernatant bottom serum fractions from Sprague–Dawley rats following RIPC
Enhanced ultracentrifugation (300,000×g for 12 h) separated the serum into three fractions: a pellet characterized by the expression of the classic exosome markers HSP-60 and flotillin-1, a bottom phase of the supernatant (directly adjacent to the pellet) in which robust expression of HSP-60 and flotillin-1 was also manifest, and a top phase of the supernatant that was devoid of exosome markers (Fig. 3). Both the exosome-depleted supernatant-top fraction—and, interestingly, the exosome-rich pellet—failed to attenuate HL-1 cell death following hypoxia-reoxygenation. Rather, only the supernatant-bottom fraction conferred protection: HL-1 cell death averaged 29 ± 2%* in cells treated with supernatant-bottom versus 45 ± 1, 51 ± 4 and 52 ± 10% in cells treated with KH buffer, pellet and supernatant-top, respectively (*p < 0.05 versus buffer; Fig. 3).
Protocol 3: efficacy of humoral communication in type‑2 diabetes
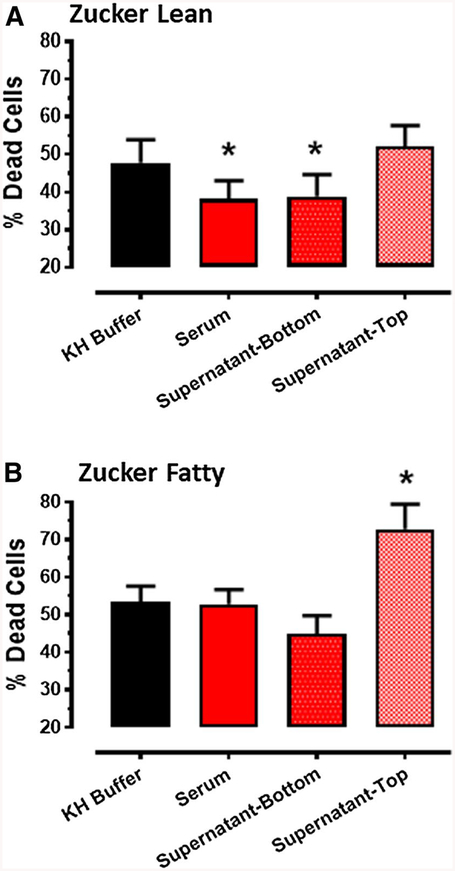
Results obtained with serum and serum fractions collected from Zucker lean rats following RIPC were consistent with (albeit smaller in magnitude than) the findings from Sprague–Dawley rats. Treatment of HL-1 cells with either unfractionated serum, or the supernatant-bottom fraction, attenuated hypoxia-reoxygenation-induced cell death (38–39 versus 48 ± 6% in buffer-treated cells p < 0.05), while the supernatant-top fraction did not confer protection (Fig. 4a).
Fig. 4.
a Protocol 3A: effect of Krebs–Henseleit (KH) buffer, serum and serum fractions (supernatant-bottom, supernatant-top), obtained from Zucker lean rats following the standard RIPC stimulus, on % cell death (mean ± SEM) of HL-1 cells subjected to 2.5 h of hypoxia and 24 h of reoxygenation. *p < 0.05 versus KH buffer. b Protocol 3B: effect of KH buffer, serum and serum fractions (supernatant-bottom, supernatant-top), obtained from Zucker fatty rats following the standard RIPC stimulus, on % cell death (mean ± SEM) of HL-1 cells subjected to 2.5 h of hypoxia and 24 h of reoxygenation. *p < 0.05 versus KH buffer
In contrast, serum obtained from Zucker fatty rats had no effect on HL-1 cell fate following hypoxia-reoxygenation, while treatment with the supernatant-bottom fraction displayed a weak trend toward improved cell viability that did not approach statistical significance versus buffer (q = 2.8 and p > 0.10 by ANOVA: Fig. 4b). Moreover, and contrary to the observations made with samples from the other rat strains, treatment with the supernatant-top fraction exacerbated HL-1 cell death following hypoxia-reoxygenation: cell death was 73 ± 7 versus 53 ± 4% in cells treated with KH buffer (p < 0.05; Fig. 4b). Administration of the supernatant-top fraction from Zucker fatty rats (or from Zucker lean rats) did not, however, evoke significant cytotoxicity when applied to normoxic cells (data not shown).
Protocol 4: qualitative particle characterization and exploratory proteomics
Nanoparticle tracking analysis
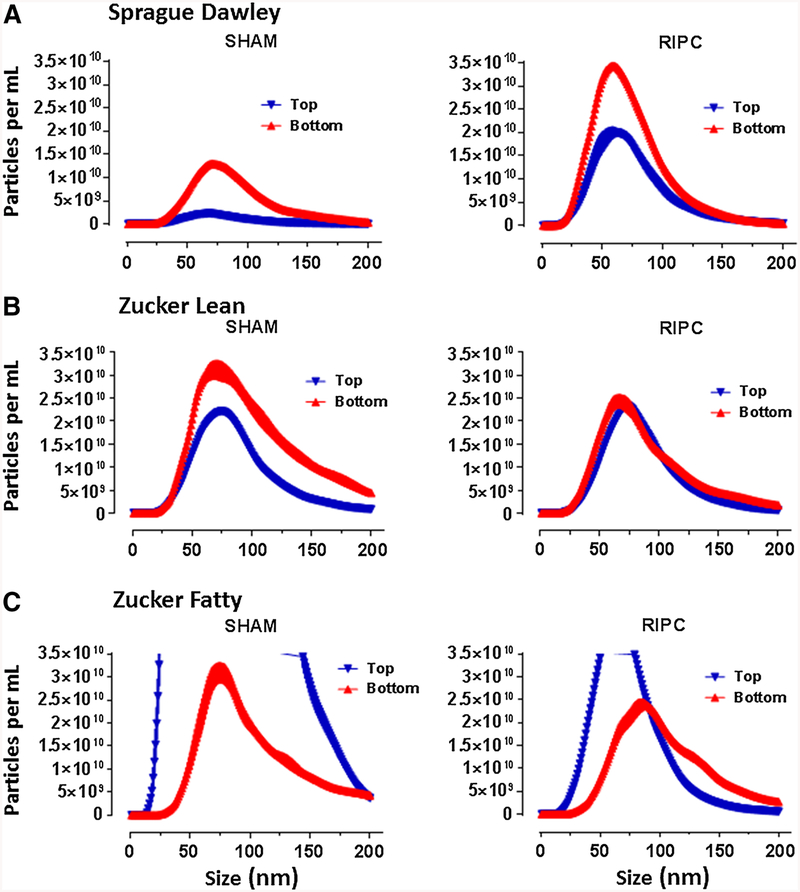
Although prolonged ultracentrifugation may have resulted in the disruption of a proportion of the particles in our samples, nanoparticle tracking analysis revealed that intact nanoparticles within the size range of exosomes (≤ 200 nm) were present in the pellet (data not shown) and supernatant-bottom fractions obtained from all rat strains (Fig. 5). In addition, and despite being devoid of exosome markers, nanoparticles within the size range of exosomes were also present in the supernatant-top fractions (Fig. 5).
Fig. 5.
a Protocol 4A: frequency distribution of nanoparticle concentration (number/mL) in serum fractions (supernatant-bottom, supernatant top) obtained from Sprague–Dawley rats following the standard RIPC stimulus or a time-matched sham-control period. Data are plotted as mean values ± SEM (3 replicates per sample), at 1-nm increments, for particles ranging from 0 to 200 nm. b Protocol 4A: frequency distribution of nanoparticle concentration (number/mL) in serum fractions (supernatant-bottom, supernatant top) obtained from Zucker lean rats following the standard RIPC stimulus or a time-matched sham-control period. Data are plotted as mean values ± SEM (3 replicates per sample), at 1-nm increments, for particles ranging from 0 to 200 nm. c Protocol 4A: frequency distribution of nanoparticle concentration (number/mL) in serum fractions (supernatant-bottom, supernatant top) obtained from Zucker fatty rats following the standard RIPC stimulus or a time-matched sham-control period. Data are plotted as mean values ± SEM (3 replicates per sample), at 1-nm increments, for particles ranging from 1 to 200 nm. Peaks of the frequency distributions for the supernatant-top fractions exceed the range of the common y-axis used for all panels: i.e., mode of 62 nm with a mean concentration of 7.6 × 1011 particles/mL and mode of 65 nm with a mean concentration of 4.5 × 1010 particles/mL for sham-control and RIPC-treated rats, respectively
For Sprague–Dawley rats, RIPC was associated with an apparent increase in nanoparticle concentration in both supernatant fractions when compared with sham-controls (Fig. 5a). This was not, however, a consistent observation among the three rat strains. Rather, samples obtained from sham-control Zucker lean and Zucker fatty rats displayed higher concentrations of nanoparticles versus sham-control Sprague–Dawley rats (Fig. 5, left panels)—a difference that was particularly striking in the Zucker fatty cohort. In addition, in contrast to data obtained in Sprague–Dawley rats, no consistent increases in nanoparticle concentration were seen in either Zucker cohort following RIPC (Fig. 5b, c).
Exploratory proteomics
For Sprague–Dawley rats, serum and supernatant-bottom fractions collected after RIPC both rendered HL-1 cells resistant to hypoxia-reoxygenation-induced death. We reasoned that: (i) our protocol of prolonged, high-speed ultra-centrifugation would potentially facilitate the proteomic analysis because the candidate cardioprotective protein(s), present in the serum, would be enriched in the supernatant-bottom fraction, and (ii) the abundance of these protective protein(s), presumably released in response to an RIPC stimulus, would be higher in the supernatant-bottom fraction obtained following RIPC versus sham-controls. Thus, beginning with the Sprague–Dawley cohort, screening of the proteomic data based on these criteria identified a group of 23 proteins that showed an RIPC-associated increase in abundance (ranging from 0.1- to 3.0-fold) in the supernatant-bottom (Table 1—left) and a ‘cumulative abundance’ of 134 (Table 1—right). Extending the analysis to the Zucker cohorts revealed that, for Zucker lean rats, 14 of the 23 candidate protective protein were expressed in the supernatant-bottom (with a ‘cumulative abundance’ of 52.5), whereas, for Zucker fatty rats, only 9 of the 23 proteins were present (‘cumulative abundance’ of 34.5; Table 1—right).
Table 1.
Proteomic screen: supernatant-bottom fraction from Sprague–Dawley, Zucker lean and Zucker fatty rats
| Identified proteins | Fold change: Sprague-Dawley rats supernatant-bottom RIPC versus Shams | Relative abundance: RIPC samples supernatant-bottom | ||
|---|---|---|---|---|
| Sprague-Dawley | Zucker lean | Zucker fatty | ||
| Leukemia inhibitory factor receptor | 3.0 | 3 | 0 | 0 |
| Complement C4 | 2.0 | 3 | 1.5 | 2 |
| Ig gamma-2B chain C region | 2.0 | 3 | 0 | 0 |
| T-kininogen-2 | 2.0 | 3 | 1 | 0 |
| Actin, cytoplasmic 1 | 2.0 | 2 | 1 | 0 |
| Plasma protease C1 inhibitor | 1.5 | 5 | 0 | 0 |
| Apolipoprotein B-100 | 1.3 | 34 | 0 | 0 |
| Apolipoprotein E | 1.3 | 9 | 4 | 3 |
| Apolipoprotein C-IV | 1.0 | 1 | 0 | 0 |
| Coactosin-like protein | 1.0 | 2 | 0 | 0 |
| C-reactive protein | 1.0 | 2 | 3 | 2 |
| Ig gamma-1 chain C region | 1.0 | 2 | 0.5 | 0 |
| Parvalbumin alpha | 1.0 | 1 | 0 | 0 |
| Protein Z-dependent protease inhibitor | 1.0 | 2 | 1 | 0.5 |
| Secreted phosphoprotein 24 | 1.0 | 1 | 0 | 0 |
| Serum paraoxonase/arylesterase 1 | 1.0 | 2 | 2.5 | 2 |
| Transgelin-2 | 1.0 | 1 | 0.5 | 0 |
| Alpha-1B-glycoprotein | 0.5 | 15 | 0 | 0 |
| Transthyretin | 0.5 | 3 | 5 | 4 |
| Hemoglobin subunit beta-1 | 0.3 | 5 | 4 | 0.5 |
| Ig kappa chain C region, B allele | 0.2 | 6 | 1 | 0 |
| Ceruloplasmin | 0.2 | 21 | 15.5 | 14.5 |
| Carboxylesterase 1C | 0.1 | 8 | 12 | 6 |
| Cumulative abundance | ∑ | 134 | 52.5 | 34.5 |
RIPC remote ischemic preconditioning
Finally, to obtain preliminary insight into proteins that may be involved in the cytotoxic effect of the supernatant-top fraction from Zucker fatty rats, we tabulated the fold-differences in protein abundance in the supernatant-top between the two Zucker strains. A group of 39 candidates emerged, displaying a 0.1- to 9.5-fold greater abundance in samples obtained from Zucker fatty versus Zucker lean rats (Table 2).
Table 2.
Proteomic screen: supernatant-top fraction from Zucker lean and Zucker fatty rats
| Identified proteins | Fold change: supernatant-top | Relative abundance: supernatant-top (RIPC) | |
|---|---|---|---|
| Zucker fatty versus Zucker lean | Zucker lean | Zucker fatty | |
| Protein Z-dependent protease inhibitor | 9.5 | 0.7 | 7 |
| Serotransferrin | 4.0 | 0 | 4 |
| Hemopexin | 3.5 | 0.3 | 1.5 |
| Beta-2 glycoprotein 1 | 2.5 | 0 | 2.5 |
| Apolipoprotein A-I | 2.4 | 2.7 | 9 |
| Alpha-1-acid glycoprotein | 2.0 | 0.3 | 1 |
| Apolipoprotein A-II | 1.6 | 1.3 | 3.5 |
| Alpha-1-inhibitor 3 | 1.5 | 0 | 1.5 |
| Glutathione peroxidase 3 | 1.5 | 1 | 2.5 |
| T-kininogen 1 | 1.5 | 0 | 1.5 |
| Apolipoprotein A-IV | 1.0 | 2.3 | 5 |
| Beta-2-microglobulin | 1.0 | 1 | 2 |
| Serum albumin | 0.9 | 8.3 | 15.5 |
| Anionic trypsin-1 | 0.5 | 0 | 0.5 |
| Ankyrin-3 | 0.5 | 0 | 0.5 |
| Apolipoprotein C-III | 0.5 | 0 | 0.5 |
| Bridging integrator 2 | 0.5 | 1.3 | 2 |
| C4b-binding protein alpha chain | 0.5 | 0 | 0.5 |
| Chromogranin-A | 0.5 | 0.3 | 0.5 |
| Clusterin | 0.5 | 0 | 0.5 |
| Corticosteroid-binding globulin | 0.5 | 0 | 0.5 |
| Gamma-synuclein | 0.5 | 0 | 0.5 |
| Haptoglobin | 0.5 | 0 | 0.5 |
| Isoprenoid synthase domain-containing protein | 0.5 | 0 | 0.5 |
| Keratin, type-II cytoskeletal 6A | 0.5 | 1.3 | 2 |
| Keratin, type II cytoskeletal 73 | 0.5 | 0.3 | 0.5 |
| PHD finger protein 20-like protein 1 | 0.5 | 0 | 0.5 |
| Potassium family subchannel T member 1 | 0.5 | 0 | 0.5 |
| Prenylcysteine oxidase | 0.5 | 0 | 0.5 |
| Reticulon 4 | 0.5 | 0.3 | 0.5 |
| Serine protease inhibitor A3L | 0.5 | 1.7 | 2.5 |
| Serum paraoxonase/arylesterase 1 | 0.5 | 1 | 1.5 |
| Sterol regulating element-binding protein cleavage-activating protein | 0.5 | 0 | 0.5 |
| Vitamin K-dependent protein C | 0.5 | 0.3 | 0.5 |
| Prothrombin | 0.5 | 4 | 5.5 |
| Apolipoprotein M | 0.3 | 2.7 | 3.5 |
| Apolipoprotein E | 0.3 | 18 | 23 |
| Apolipoprotein C-IV | 0.3 | 6 | 7.5 |
| Complement C4 | 0.1 | 1.3 | 1.5 |
RIPC remote ischemic preconditioning
Discussion
In this study, we make the novel observation that RIPC, administered in a manner that is well-documented to reduce myocardial infarct size in healthy cohorts, is ineffective in the Zucker fatty rat model of early-stage type-2 diabetes. We further report that this failure of RIPC to limit infarct size may be explained, at least in part, by a defect in an exosome-associated humoral component of cardioprotective signaling. This concept is supported by evidence that: (1) serum and the exosome-rich serum supernatant fraction, collected from normoglycemic rats following the RIPC stimulus and applied to cultured HL-1 cardiomyocytes, attenuated hypoxia-reoxygenation-induced cell death while, in contrast (2) transfer of serum and exosome-rich serum supernatant from Zucker fatty rats did not evoke protection in the HL-1 cell model. Finally, and unexpectedly, we found that the exosome-depleted supernatant fraction harvested from Zucker fatty rats was cytotoxic and exacerbated HL-1 cell death following hypoxia-reoxygenation.
Type‑2 diabetes, remote conditioning and infarct size
Among the small number of experiments conducted using rodent models of type-2 diabetes (including Zucker fatty rats, Goto Kakizaki rats, db/db mice, ob/ob mice and WOWK rats), there is a consensus that the infarct-sparing effect of ischemic pre- and postconditioning is either attenuated or eliminated [9, 30, 45, 49, 63, 69, 78, 80, 85, 89]. Our study is the first to focus on remote conditioning, and the in vivo data obtained in Protocol 1 are consistent with these observations: RIPC did not reduce infarct size in Zucker fatty rats displaying modest elevations in blood glucose levels. Interestingly, these data are also in agreement with clinical findings, in which RIPC was reportedly ineffective in attenuating peri-operative myocardial injury, assessed by quantifying release of cardiac troponin I, in diabetic subjects [48].
The mechanisms responsible for the loss of conditioning-induced cardioprotection in the setting of type-2 diabetes are unresolved. Clinical evidence suggests that the diabetic myocardium may be sensitized to ischemia–reperfusion [3, 57], causing larger infarcts and potentially affecting the efficacy of ischemic conditioning [59]. Alternatively, even brief exposure of normal myocardium to hyperglycemic conditions, achieved in non-diabetic models by acute administration of high concentrations of dextrose or glucose, has been reported to abrogate or mitigate the favorable effects of ischemic conditioning [5, 27, 46, 87, 88], implying that hyperglycemia per se may be responsible for the resistance to cardioprotection associated with diabetes. Neither of these paradigms is supported by our in vivo results obtained in Protocol 1: i.e., infarct sizes in control animals were not exacerbated in the Zucker fatty versus Zucker lean cohorts (suggesting that hearts from Zucker fatty rats were not innately sensitized to ischemia–reperfusion), and regression analysis revealed that blood glucose concentration was not a determinant of infarct size in the RIPC-treated groups. Moreover, the clinical observation that RIPC was not cardio-protective in diabetic patients, despite treatment with sulphonylurea agents for the management of hyperglycemia, provides an additional argument against the latter concept [48].
Exosomes and the humoral component of RIPC: first evidence
There is general agreement that, at the level of the cardiomyocyte, all conditioning strategies (including RIPC, preconditioning and postconditioning) render the myocardium resistant to ischemia–reperfusion injury and reduce infarct size via complex and redundant, receptor-mediated upregulation of three cardiac signal transduction pathways [the reperfusion injury salvage kinase (RISK) pathway, the survivor activating factor enhancement (SAFE) pathway, and/or the endothelial nitric oxide synthase-protein kinase G pathway] and subsequent stabilization of mitochondria [36, 47]. Among these three cardioprotective paradigms, the unique and distinguishing feature of RIPC is the as-yet poorly understood communication of the protective signal from the site of the remote ischemic stimulus to the target organ (in our in vivo experiments, the hindlimbs and heart, respectively). The current hypothesis is that the protective trigger is conveyed by neuronal stimulation and transmission, by release and circulation of one or more humoral factors, or, in some models, by a combination of both mechanisms [36, 66].
A nascent theory, first posited by Giricz and colleagues [25], is that the protective humoral factors involved in RIPC are transported via exosomes—or, more precisely, extracellular vesicles, formed by endocytosis and secreted from cells by exocytosis [2, 6, 15]. Exosomes are characterized by a lipid bilayer membrane that encapsulates a diverse cargo (including proteins, lipids and nucleic acids), the precise composition of which is determined in part by both the cellular origin of the exosomes and the cellular milieu. Moreover, exosomes are increasingly recognized to play a pivotal role in cell–cell communication and targeted delivery of bio-active materials under both physiologic and pathologic conditions [70], including the transport of paracrine factors that purportedly contribute to cardiac regeneration and improved post-infarct healing with stem cell and cardiac progenitor cell therapy [6, 15, 16, 22, 41].
Using isolated buffer-perfused rat hearts, Giricz et al. collected coronary effluent from hearts subjected to brief preconditioning ischemia or normoxic perfusion and applied standard ultracentrifugation (100,000×g for 90 min) to yield an exosome-rich pellet and a remaining exosome-depleted perfusate [25]. There was an increase in expression of the exosome marker HSP-60, assessed by immunoblotting, in the exosome-rich pellet obtained from preconditioned hearts versus controls, thereby suggesting that brief preconditioning ischemia was accompanied by an augmented release of exosomes from the heart. Moreover, transfer of unfractionated coronary effluent from preconditioned hearts rendered naïve acceptor hearts resistant to infarction caused by a subsequent 30 min ischemic insult, while, in contrast, transfer of exosome-depleted perfusate (confirmed to be devoid of HSP-60 expression) did not reduce infarct size. Based on these data, the authors concluded that extracellular vesicles are responsible for communication of the cardioprotective signal associated with RIPC, and are necessary for RIPC-induced protection [25].
Subsequent studies from other groups have not, however, fully corroborated this concept. First, Li and coworkers found no increase in circulating exosomes following RIPC in the in vivo mouse model, and concluded that the specific cardioprotective candidate of interest in their study, miR-144, was not transported in its intact form in exosomes [52]. In contrast, Vicencio and colleagues provided robust evidence that plasma concentrations of circulating exosomes are, indeed, increased following a brief RIPC stimulus, and that exosomes are cardioprotective and can attenuate lethal ischemia–reperfusion injury in cultured cardiomyocytes and intact hearts [79]. However, an intriguing observation—and an issue not addressed in the study by Giricz [25]—was that exosomes from both RIPC and control groups conferred protection, with no added benefit provided by RIPC [79]. This finding that exosomes, isolated from rat and human plasma under control conditions (in the absence of RIPC) and transferred to cardiomyocytes in culture, confer significant cardioprotection was recently confirmed by Davidson et al. [14]. Moreover, and of particular relevance to the current study, Davidson and colleagues further reported that exosome-rich plasma harvested under control conditions (in the absence of RIPC) from diabetic cohorts failed to limit hypoxia-reoxygenation-induced death of cultured cardiomyocytes [14]. Our results obtained in Protocol 3B share a common theme and corroborate the concept that type-2 diabetes has a confounding effect on exosome-associated humoral communication. There are, however, fundamental differences between the two studies: in contrast to our protocols, Davidson et al. did not incorporate RIPC into any aspect of the experimental design, and focused exclusively on the transfer of exosome-rich plasma (no experiments were conducted using unfractionated plasma or exosome-depleted plasma fractions) [14].
Exosomes may contribute to, but are not sufficient for, transfer of a cardioprotective stimulus
In our initial experiments conducted using Sprague–Dawley rats, we found that, as expected [43, 74, 75], serum collected following an in vivo RIPC stimulus and transferred to cultured HL-1 cells significantly attenuated hypoxia-reoxygenation-induced cell death. However, our goal of interrogating the role of exosomes was confounded by the failure of standard ultracentrifugation to yield a supernatant that was devoid of exosome markers—an observation that is consistent with previous reports showing that standard ultra-centrifugation does not completely sediment exosomes from viscous biofluids [4, 60]. Despite the lack of a valid negative control, the pellet and supernatant obtained by standard ultracentrifugation were evaluated in our HL-1 cell model. We found that treatment with the supernatant (displaying residual expression of HSP-60) significantly reduced cell death; however, treatment with the exosome-rich pellet was, unexpectedly, not protective.
By increasing the radial force and duration of ultracentrifugation, serum was fractionated into three distinct phases: the pellet and a biphasic supernatant. In addition, although nanoparticle tacking analysis revealed that all three fractions contained a high abundance of particles within the size range of exosomes (total concentrations on the order of 1012 particles/mL), we were successful in obtaining a fraction, the supernatant-top, in which expression of the classic exosome markers HSP-60 and flotillin-1 was not detected by immunoblotting (Fig. 3). These results suggest that: (1) even a prolonged, 12 h period of ultracentrifugation does not sediment all particles from serum, and (2) the remaining nanoparticles present in the supernatant-top are presumably not exosomes, or, alternatively, are a population of exosomes that do not express HSP-60 or flotillin-1. Most importantly, transfer of the supernatant-top fraction, devoid of exosome markers, to cultured HL-1 cells was ineffective in attenuating hypoxia-reoxygenation-induced cell death.
These latter data, obtained with the ostensibly exosome-depleted fraction, are consistent with the paradigm proposed by Giricz et al. Interestingly, however, the ‘exosome hypothesis’ is based exclusively on this observation; the complementary experiments, involving isolation of an exosome-rich fraction of the buffer perfusate and evaluation of this fraction for cardioprotective efficacy, was not included in their study design [25]. In this regard, we found that the presence of exosomes per se was not sufficient to evoke protection: both the pellet and the supernatant-bottom fraction displayed robust expression of HSP-60 and flotillin-1, yet only the supernatant-bottom (and not the reconstituted pellet), applied to HL-1 cells, evoked a significant reduction in cell death. A potential explanation for this disparate outcome is that distinct and less dense subpopulation(s) of exosomes (or non-exosome nanoparticles of a similar size), present in the supernatant-bottom fraction but not precipitated in the pellet, may contribute to the transfer of a cardioprotective signal with RIPC. Alternatively, the protective factor(s) may be associated with and co-localized in the exosome-rich supernatant-bottom, but may not be transported by exosomes per se.
Finally, in all experiments discussed to this point, serum (and serum fractions) were collected from Sprague–Dawley rats following an in vivo RIPC stimulus. An unanswered question, prompted by the observations made by Vicencio el al. [79] and Davidson et al. [14], is whether equivalent protection would be achieved by transfer of serum from nonischemic control cohorts. To obtain preliminary insight into this issue, ancillary post hoc experiments were performed in which serum was obtained from time-matched sham-operated rats (left and right femoral arteries exposed, but not ligated; n = 5) and applied to HL-1 cells as described in Protocol 2. An attenuation in hypoxia-reoxygenation-induced HL-1 cell death was seen with administration of serum from sham-controls (39 ± 3 versus 45 ± 6% in concurrent KH buffer-treated cultures); however, this difference was not significant (p = 0.14), and was not comparable in magnitude to the reduction in cell death achieved with transfer of serum obtained following RIPC (Fig. 3). This trend may reflect a modest level of innate cardioprotection conferred by control serum, or, as these were sham-operated animals, may reflect a component of protection evoked by surgical trauma [44].
Exosomes and the humoral component of RIPC: loss of efficacy in type‑2 diabetes
In Protocol 3, we investigated the effect of serum and serum fractions (supernatant-bottom and supernatant-top), obtained following RIPC from Zucker lean and Zucker fatty rats, on the viability of HL-1 cells subjected to hypoxia-reoxygenation. Data obtained using the normoglycemic Zucker lean strain were similar to those seen with Sprague–Dawley rats: significant reductions in cell death were evoked with transfer of both the serum and supernatant-bottom fraction, while treatment with the supernatant-top was not protective. There were, however, apparent qualitative differences between the two rat strains in terms of nanoparticle tracking analysis: nanoparticle concentrations in serum fractions obtained from sham-control animals were higher in Zucker lean versus Sprague–Dawley rats, with no additional increase seen with RIPC (Fig. 5). This may suggest that the composition of the nanoparticles, rather than absolute numbers of particles, may be the primary determinant in communicating a protective signal.
In contrast, in samples obtained from Zucker fatty rats, communication of the protective signal was impaired: serum obtained following RIPC and transferred to HL-1 cells failed to render the cells resistant to lethal hypoxia-reoxygenation-induced injury. These data appear to differ from the outcome of the one previously published study that investigated the efficacy of the humoral component of RIPC in diabetic subjects [43]. Using a model in which plasma was harvested from human subjects and administered to buffer-perfused rabbit hearts subsequently subjected to global ischemia–reperfusion, Jensen and colleagues concluded that type-2 diabetes did affect the humoral component of RIPC. However, a defect in the efficacy of transferred cardioprotection (i.e., an inability of the transferred plasma to reduce infarct size in rabbit hearts) was only observed in the subset of diabetic subjects with peripheral neuropathy. Accordingly, the authors posited that neuropathy (rather than diabetes per se) was responsible for the failed humoral communication, thereby implicating neuronal mechanisms in the RIPC-induced release of circulating cardioprotective factors [43]. While Zucker fatty rats also develop peripheral neuropathy [10], this is in all likelihood not the sole explanation for the results obtained in our model: the onset of neuronal dysfunction occurs at > 12 weeks of age [62], and our animals were enrolled at an age range of 10–12 weeks.
Serum from Zucker fatty rats contains a toxic component
There is evidence from some (but not all [14]) studies that metabolic disorders, including type-2 diabetes, are associated with increased concentrations of circulating exosomes, reportedly originating from platelets, endothelial cells and neutrophils [1, 58]. In this regard, we observed that total nanoparticle concentration in the supernatant-top fraction of serum from Zucker fatty rats was orders of magnitude greater than concentrations in the Zucker lean and Sprague–Dawley cohorts (4 × 1013 versus 1.5 × 1012 and 1.5 × 1011 particles/mL, respectively; Fig. 5). Moreover, by designing our study to include the evaluation of a ‘negative control’ (i.e., a fraction devoid of exosome markers, an approach that, as noted above, was not used in previous reports [14, 25, 79]), we made the novel and unanticipated observation that the supernatant-top fraction from Zucker fatty rats significantly augmented HL-1 cell death caused by hypoxia-reoxygenation. Indeed, it could be argued that the impaired humoral communication of a protective signal seen with the transfer of serum from Zucker fatty rats may not be explained exclusively by a loss in efficacy of the supernatant-bottom fraction; rather, the remaining weak trend toward protection achieved by factors in the supernatant-bottom may be offset and overwhelmed by the toxic effect of the supernatant-top. Interestingly, however, this cytotoxic effect was only manifest in the setting of hypoxia-reoxygenation; the supernatant-top fraction from the Zucker fatty cohort had no significant effect on cell viability when applied to normoxic cells.
Identities of the cardioprotective and cytotoxic factors?
An obvious but as-yet unanswered question is: what is the exosome-associated, humoral protective factor(s) responsible for the infarct-sparing effect of RIPC? Among published studies that have utilized proteomic approaches to detect relative changes in protein abundance in blood sampled after versus before an RIPC stimulus [31–33, 37, 38, 50, 64], approximately 65 candidates have been identified that are either up- or downregulated (summarized in [31]). However, as emphasized by Helgeland et al., no common or consistent biomarker(s) of RIPC have been reproducibly identified, possibly due to both technical pitfalls in the proteomic analyses and physiologic variations among protocols (i.e., blood obtained from human subjects versus rodents, after one versus multiple episodes of brief ischemia applied to one or more limbs) [31].
Despite the exploratory nature of our analysis, we reasoned that our study design—in which we capitalized on the enrichment of protective proteins by prolonged ultracentrifugation in the supernatant bottom, identified a potential pool of candidates in Sprague–Dawley rats based on increased protein abundance in samples obtained following RIPC, and compared the abundance of these proteins in samples from Zucker lean and Zucker fatty rats—may facilitate the identification of putative humoral protective factor(s). Interestingly, there is apparent overlap among our 23 candidates and the ~ 65 proteins that have been implicated in previous studies [31] (including Apolipoprotein B-100, Complement C4, hemoglobin beta and immunoglobulins). In addition, at least two candidates (ceruloplasmin and Apolipoprotein-E) have been shown to attenuate ischemia–reperfusion injury in neuronal models and have been associated with exosomes [28, 40, 81]. However, we cannot speculate on which factor (or, in all likelihood, combination of factors [31]), contribute to the cardioprotection achieved with RIPC in the normoglycemic cohorts, and, conversely, the relative deficit of which may underlie the inability of the supernatant-bottom from diabetic Zucker fatty rats to confer significant protection.
A second question, unique to our study, is the identity of the toxic factor(s) present in the supernatant-top fraction of serum harvested from diabetic Zucker fatty rats. Comparative analysis revealed a total of 39 proteins that displayed an increase in abundance in the supernatant-top samples obtained from Zucker fatty versus Zucker lean rats, including lipoproteins that have been implicated to exacerbate cell injury caused by ischemia [65] and hypoxia [11]. Moreover, four candidate proteins were unexpectedly identified as potentially contributing to cardioprotection (Table 1) and cardiotoxicity (Table 2: i.e., Complement C4, Apolipoprotein E, Apolipoprotein C-IV, Protein Z-dependent pro-tease inhibitor). It must, therefore, be emphasized that this analysis is preliminary and suffers from the limitations of protein analysis by mass spectrometry [12]. For example, despite utilizing the standard technique of depleting high-abundance proteins and the sensitivity of mass spectrometry, quantification of lower-abundance proteins in our samples may nonetheless have been compromised, particularly at the isoelectric point of these proteins [12, 31]. In addition, the relative abundance and ‘fold change’ in proteins does not, in itself, provide definitive insight regarding their possible relevance to RIPC. Accordingly, our analysis may, at best, serve to identify a potential pool of diabetes-associated toxic factors to be pursued and validated in future studies.
Summary and future directions
In summary, we demonstrate that the infarct-sparing effect of remote ischemic preconditioning is ineffective in the Zucker fatty rat model of early-stage type-2 diabetes. This failure to evoke protection is due, at least in part, to: (1) a diabetes-associated defect in the exosome-associated humoral component of cardioprotective signaling, and/or (2) the presence of one or more toxic factors in exosome-depleted supernatant-top fraction of serum isolated by prolonged high-speed ultra-centrifugation. The precise role of exosomes in the humoral communication initiated by an RIPC stimulus, as well as the identities of the factors that mediate cardioprotection and toxicity in the normoglycemic and diabetic cohorts, respectively, remain unresolved.
Acknowledgements
JW was supported in part by NIH T32 HL120822 (Detroit Cardiovascular Training Program).
Footnotes
Presented in part at the 2017 Scientific Sessions of the American Heart Association, Anaheim, CA, USA, November 2017.
Conflict of interest On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Agouni A, Lagrue-Lak-Hal AH, Ducluzeau PH, Mostefai HA, Draunet-Busson C, Leftheriotis G, Heymes C, Martinez MC, Andriantsitohaina R (2008) Endothelial dysfunction caused by circulating microparticles from patients with metabolic syndrome. Am J Pathol 173:1210–1219. 10.2353/ajpath.2008.080228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ailawadi S, Wang X, Gu H, Fan GC (2015) Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochim Biophys Acta 1852:1–11. 10.1016/j.bbadis.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alegria JR, Miller TD, Gibbons RJ, Yi QL, Yusuf S, Collaborative Organization of RheothRx Evaluation Trial I (2007) Infarct size, ejection fraction, and mortality in diabetic patients with acute myocardial infarction treated with thrombolytic therapy. Am Heart J 154:743–750. 10.1016/j.ahj.2007.06.020 [DOI] [PubMed] [Google Scholar]
- 4.Baranyai T, Herczeg K, Onodi Z, Voszka I, Modos K, Marton N, Nagy G, Mager I, Wood MJ, El Andaloussi S, Palinkas Z, Kumar V, Nagy P, Kittel A, Buzas EI, Ferdinandy P, Giricz Z (2015) Isolation of exosomes from blood plasma: qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS ONE 10:e0145686 10.1371/journal.pone.0145686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baranyai T, Nagy CT, Koncsos G, Onodi Z, Karolyi-Szabo M, Makkos A, Varga ZV, Ferdinandy P, Giricz Z (2015) Acute hyper-glycemia abolishes cardioprotection by remote ischemic perconditioning. Cardiovasc Diabetol 14:151 10.1186/s12933-015-0313-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barile L, Moccetti T, Marban E, Vassalli G (2017) Roles of exosomes in cardioprotection. Eur Heart J 38:1372–1379. 10.1093/eurheartj/ehw304 [DOI] [PubMed] [Google Scholar]
- 7.Bauer B, Simkhovich BZ, Kloner RA, Przyklenk K (1999) Preconditioning-induced cardioprotection and release of the second messenger inositol (1,4,5)-trisphosphate are both abolished by neomycin in rabbit heart. Basic Res Cardiol 94:31–40 [DOI] [PubMed] [Google Scholar]
- 8.Behmenburg F, Heinen A, Bruch LV, Hollmann MW, Huhn R (2017) Cardioprotection by remote ischemic preconditioning is blocked in the aged rat heart in vivo. J Cardiothorac Vasc Anesth 31:1223–1226. 10.1053/j.jvca.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 9.Bouhidel O, Pons S, Souktani R, Zini R, Berdeaux A, Ghaleh B (2008) Myocardial ischemic postconditioning against ischemia–reperfusion is impaired in ob/ob mice. Am J Physiol Heart Circ Physiol 295:H1580–H1586. 10.1152/ajpheart.00379.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brussee V, Guo G, Dong Y, Cheng C, Martinez JA, Smith D, Glazner GW, Fernyhough P, Zochodne DW (2008) Distal degenerative sensory neuropathy in a long-term type 2 diabetes rat model. Diabetes 57:1664–1673. 10.2337/db07-1737 [DOI] [PubMed] [Google Scholar]
- 11.Castellano J, Farre J, Fernandes J, Bayes-Genis A, Cinca J, Badimon L, Hove-Madsen L, Llorente-Cortes V (2011) Hypoxia exacerbates Ca(2+)-handling disturbances induced by very low density lipoproteins (VLDL) in neonatal rat cardiomyocytes. J Mol Cell Cardiol 50:894–902. 10.1016/j.yjmcc.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 12.Chandramouli K, Qian PY (2009) Proteomics: challenges, techniques and possibilities to overcome biological sample complexity. Hum Genom Proteom. 10.4061/2009/239204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Claycomb WC, Lanson NA Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ Jr (1998) HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA 95:2979–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson SM, Riquelme JA, Takov K, Vicencio JM, Boi-Doku C, Khoo V, Doreth C, Radenkovic D, Lavandero S, Yellon DM (2018) Cardioprotection mediated by exosomes is impaired in the setting of type II diabetes but can be rescued by the use of non-diabetic exosomes in vitro. J Cell Mol Med 22:141–151. 10.1111/jcmm.13302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson SM, Takov K, Yellon DM (2017) Exosomes and cardiovascular protection. Cardiovasc Drugs Ther 31:77–86. 10.1007/s10557-016-6698-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Couto G, Liu W, Tseliou E, Sun B, Makkar N, Kanazawa H, Arditi M, Marban E (2015) Macrophages mediate cardioprotective cellular postconditioning in acute myocardial infarction. J Clin Invest 125:3147–3162. 10.1172/jci81321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Gassart A, Geminard C, Fevrier B, Raposo G, Vidal M (2003) Lipid raft-associated protein sorting in exosomes. Blood 102:4336–4344. 10.1182/blood-2003-03-0871 [DOI] [PubMed] [Google Scholar]
- 18.Dong Y, Undyala VVR, Przyklenk K (2016) Inhibition of mitochondrial fission as a molecular target for cardioprotection: critical importance of the timing of treatment. Basic Res Cardiol 111:59 10.1007/s00395-016-0578-x [DOI] [PubMed] [Google Scholar]
- 19.Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJ, Hole P, Carr B, Redman CW, Harris AL, Dobson PJ, Harrison P, Sargent IL (2011) Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomedicine 7:780–788. 10.1016/j.nano.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R (2014) Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev 66:1142–1174. 10.1124/pr.113.008300 [DOI] [PubMed] [Google Scholar]
- 21.Fishbein MC, Meerbaum S, Rit J, Lando U, Kanmatsuse K, Mercier JC, Corday E, Ganz W (1981) Early phase acute myocar-dial infarct size quantification: validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. Am Heart J 101:593–600 [DOI] [PubMed] [Google Scholar]
- 22.Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R, Tseliou E, Luthringer D, Kreke M, Smith RR, Marban L, Ghaleh B, Marban E (2016) Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J. 10.1093/eurheartj/ehw240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garratt KN, Whittaker P, Przyklenk K (2016) Remote ischemic conditioning and the long road to clinical translation: lessons learned from ERICCA and RIPHeart. Circ Res 118:1052–1054. 10.1161/circresaha.115.308102 [DOI] [PubMed] [Google Scholar]
- 24.Giblett JP, Hoole SP (2017) Remote ischemic conditioning in elective PCI? J Cardiovasc Pharmacol Ther 22:310–315. 10.1177/1074248417702479 [DOI] [PubMed] [Google Scholar]
- 25.Giricz Z, Varga ZV, Baranyai T, Sipos P, Paloczi K, Kittel A, Buzas EI, Ferdinandy P (2014) Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extra-cellular vesicles. J Mol Cell Cardiol 68:75–78. 10.1016/j.yjmcc.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 26.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB (2014) Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 129:e28–e292. 10.1161/01.cir.0000441139.02102.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu W, Kehl F, Krolikowski JG, Pagel PS, Warltier DC, Kersten JR (2008) Simvastatin restores ischemic preconditioning in the presence of hyperglycemia through a nitric oxide-mediated mechanism. Anesthesiology 108:634–642. 10.1097/aln.0b013e3181672590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guitart K, Loers G, Buck F, Bork U, Schachner M, Kleene R (2016) Improvement of neuronal cell survival by astrocyte-derived exosomes under hypoxic and ischemic conditions depends on prion protein. Glia 64:896–910. 10.1002/glia.22963 [DOI] [PubMed] [Google Scholar]
- 29.Gupta S, Knowlton AA (2007) HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Physiol Heart Circ Physiol 292:H3052–H3056. 10.1152/ajpheart.01355.2006 [DOI] [PubMed] [Google Scholar]
- 30.Hausenloy DJ, Wynne AM, Mocanu MM, Yellon DM (2013) Glimepiride treatment facilitates ischemic preconditioning in the diabetic heart. J Cardiovasc Pharmacol Ther 18:263–269. 10.1177/1074248412468945 [DOI] [PubMed] [Google Scholar]
- 31.Helgeland E, Breivik LE, Vaudel M, Svendsen OS, Garberg H, Nordrehaug JE, Berven FS, Jonassen AK (2014) Exploring the human plasma proteome for humoral mediators of remote ischemic preconditioning—a word of caution. PLoS ONE 9:e109279 10.1371/journal.pone.0109279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hepponstall M, Ignjatovic V, Binos S, Attard C, Karlaftis V, d’Udekem Y, Monagle P, Konstantinov IE (2015) Remote ischemic preconditioning (RIPC) modifies the plasma proteome in children undergoing repair of tetralogy of fallot: a randomized controlled trial. PLoS ONE 10:e0122778 10.1371/journal.pone.0122778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hepponstall M, Ignjatovic V, Binos S, Monagle P, Jones B, Cheung MH, d’Udekem Y, Konstantinov IE (2012) Remote ischemic preconditioning (RIPC) modifies plasma proteome in humans. PLoS ONE 7:e48284 10.1371/journal.pone.0048284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heusch G (2017) Critical issues for the translation of cardioprotection. Circ Res 120:1477–1486. 10.1161/circresaha.117.310820 [DOI] [PubMed] [Google Scholar]
- 35.Heusch G (2017) Remote ischemic conditioning in cardiovascular surgery. J Cardiovasc Pharmacol Ther 22:297–301. 10.1177/1074248416687874 [DOI] [PubMed] [Google Scholar]
- 36.Heusch G, Botker HE, Przyklenk K, Redington A, Yellon D (2015) Remote ischemic conditioning. J Am Coll Cardiol 65:177–195. 10.1016/j.jacc.2014.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hibert P, Prunier-Mirebeau D, Beseme O, Chwastyniak M, Tamareille S, Lamon D, Furber A, Pinet F, Prunier F (2013) Apolipoprotein a-I is a potential mediator of remote ischemic preconditioning. PLoS ONE 8:e77211 10.1371/journal.pone.0077211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hibert P, Prunier-Mirebeau D, Beseme O, Chwastyniak M, Tamareille S, Pinet F, Prunier F (2014) Modifications in rat plasma proteome after remote ischemic preconditioning (RIPC) stimulus: identification by a SELDI–TOF–MS approach. PLoS ONE 9:e85669 10.1371/journal.pone.0085669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Himori N, Matsuura A (1989) A simple technique for occlusion and reperfusion of coronary artery in conscious rats. Am J Physiol 256:H1719–H1725 [DOI] [PubMed] [Google Scholar]
- 40.Horsburgh K, Kelly S, McCulloch J, Higgins GA, Roses AD, Nicoll JA (1999) Increased neuronal damage in apolipoprotein E-deficient mice following global ischaemia. NeuroReport 10:837–841 [DOI] [PubMed] [Google Scholar]
- 41.Ibrahim AG, Cheng K, Marban E (2014) Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep 2:606–619. 10.1016/j.stemcr.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishihara M, Inoue I, Kawagoe T, Shimatani Y, Kurisu S, Nishioka K, Umemura T, Nakamura S, Yoshida M (2003) Effect of acute hyperglycemia on the ischemic preconditioning effect of prodromal angina pectoris in patients with a first anterior wall acute myocardial infarction. Am J Cardiol 92:288–291 [DOI] [PubMed] [Google Scholar]
- 43.Jensen RV, Stottrup NB, Kristiansen SB, Botker HE (2012) Release of a humoral circulating cardioprotective factor by remote ischemic preconditioning is dependent on preserved neural pathways in diabetic patients. Basic Res Cardiol 107:285 10.1007/s00395-012-0285-1 [DOI] [PubMed] [Google Scholar]
- 44.Jones WK, Fan GC, Liao S, Zhang JM, Wang Y, Weintraub NL, Kranias EG, Schultz JE, Lorenz J, Ren X (2009) Peripheral nociception associated with surgical incision elicits remote nonischemic cardioprotection via neurogenic activation of protein kinase C signaling. Circulation 120:S1–S9. 10.1161/circulationaha.108.843938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katakam PV, Jordan JE, Snipes JA, Tulbert CD, Miller AW, Busija DW (2007) Myocardial preconditioning against ischemia–reperfusion injury is abolished in Zucker obese rats with insulin resistance. Am J Physiol Regul Integr Comp Physiol 292:R920–R926. 10.1152/ajpregu.00520.2006 [DOI] [PubMed] [Google Scholar]
- 46.Kersten JR, Schmeling TJ, Orth KG, Pagel PS, Warltier DC (1998) Acute hyperglycemia abolishes ischemic preconditioning in vivo. Am J Physiol 275:H721–H725 [DOI] [PubMed] [Google Scholar]
- 47.Kleinbongard P, Skyschally A, Heusch G (2017) Cardioprotection by remote ischemic conditioning and its signal transduction. Pflugers Arch 469:159–181. 10.1007/s00424-016-1922-6 [DOI] [PubMed] [Google Scholar]
- 48.Kottenberg E, Thielmann M, Kleinbongard P, Frey UH, Heine T, Jakob H, Heusch G, Peters J (2014) Myocardial protection by remote ischaemic pre-conditioning is abolished in sulphonylurea-treated diabetics undergoing coronary revascularisation. Acta Anaesthesiol Scand 58:453–462. 10.1111/aas.12278 [DOI] [PubMed] [Google Scholar]
- 49.Kristiansen SB, Lofgren B, Stottrup NB, Khatir D, Nielsen-Kudsk JE, Nielsen TT, Botker HE, Flyvbjerg A (2004) Ischaemic preconditioning does not protect the heart in obese and lean animal models of type 2 diabetes. Diabetologia 47:1716–1721. 10.1007/s00125-004-1514-4 [DOI] [PubMed] [Google Scholar]
- 50.Lang SC, Elsasser A, Scheler C, Vetter S, Tiefenbacher CP, Kubler W, Katus HA, Vogt AM (2006) Myocardial preconditioning and remote renal preconditioning—identifying a protective factor using proteomic methods? Basic Res Cardiol 101:149–158. 10.1007/s00395-005-0565-0 [DOI] [PubMed] [Google Scholar]
- 51.Lee JS, Wu Y, Schnepp P, Fang J, Zhang X, Karnovsky A, Woods J, Stemmer PM, Liu M, Zhang K, Chen X (2015) Proteomics analysis of rough endoplasmic reticulum in pancreatic beta cells. Proteomics 15:1508–1511. 10.1002/pmic.201400345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li J, Rohailla S, Gelber N, Rutka J, Sabah N, Gladstone RA, Wei C, Hu P, Kharbanda RK, Redington AN (2014) Micro-RNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res Cardiol 109:423 10.1007/s00395-014-0423-z [DOI] [PubMed] [Google Scholar]
- 53.Li YW, Whittaker P, Kloner RA (1992) The transient nature of the effect of ischemic preconditioning on myocardial infarct size and ventricular arrhythmia. Am Heart J 123:346–353 [DOI] [PubMed] [Google Scholar]
- 54.Lindsey ML, Bolli R, Canty JM, Du XJ, Frangogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner R, Lefer DJ, Liao R, Murphy E, Ping P, Przyklenk K, Recchia FA, Schwartz Longacre L, Ripplinger CM, Van Eyk JE, Heusch G (2018) Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol. 10.1152/ajpheart.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma F, Liu H, Shen Y, Zhang Y, Pan S (2015) Platelet-derived microvesicles are involved in cardio-protective effects of remote preconditioning. Int J Clin Exp Pathol 8:10832–10839 [PMC free article] [PubMed] [Google Scholar]
- 56.Ma LL, Kong FJ, Guo JJ, Zhu JB, Shi HT, Li Y, Sun RH, Ge JB (2017) Hypercholesterolemia abrogates remote ischemic preconditioning-induced cardioprotection: role of reperfusion injury salvage kinase signals. Shock 47:363–369. 10.1097/shk.0000000000000737 [DOI] [PubMed] [Google Scholar]
- 57.Marso SP, Miller T, Rutherford BD, Gibbons RJ, Qureshi M, Kalynych A, Turco M, Schultheiss HP, Mehran R, Krucoff MW, Lansky AJ, Stone GW (2007) Comparison of myocardial reperfusion in patients undergoing percutaneous coronary intervention in ST-segment elevation acute myocardial infarction with versus without diabetes mellitus (from the EMERALD Trial). Am J Cardiol 100:206–210. 10.1016/j.amjcard.2007.02.080 [DOI] [PubMed] [Google Scholar]
- 58.Martinez MC, Andriantsitohaina R (2017) Extracellular vesicles in metabolic syndrome. Circ Res 120:1674–1686. 10.1161/circresaha.117.309419 [DOI] [PubMed] [Google Scholar]
- 59.Miki T, Itoh T, Sunaga D, Miura T (2012) Effects of diabetes on myocardial infarct size and cardioprotection by preconditioning and postconditioning. Cardiovasc Diabetol 11:67 10.1186/1475-2840-11-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Momen-Heravi F, Balaj L, Alian S, Trachtenberg AJ, Hochberg FH, Skog J, Kuo WP (2012) Impact of biofluid viscosity on size and sedimentation efficiency of the isolated microvesicles. Front Physiol 3:162 10.3389/fphys.2012.00162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oberkofler CE, Limani P, Jang JH, Rickenbacher A, Lehmann K, Raptis DA, Ungethuem U, Tian Y, Grabliauskaite K, Humar R, Graf R, Humar B, Clavien PA (2014) Systemic protection through remote ischemic preconditioning is spread by platelet-dependent signaling in mice. Hepatology 60:1409–1417. 10.1002/hep.27089 [DOI] [PubMed] [Google Scholar]
- 62.Oltman CL, Coppey LJ, Gellett JS, Davidson EP, Lund DD, Yorek MA (2005) Progression of vascular and neural dysfunction in sciatic nerves of Zucker diabetic fatty and Zucker rats. Am J Physiol Endocrinol Metab 289:E113–E122. 10.1152/ajpendo.00594.2004 [DOI] [PubMed] [Google Scholar]
- 63.Oosterlinck W, Dresselaers T, Geldhof V, Nevelsteen I, Janssens S, Himmelreich U, Herijgers P (2013) Diabetes mellitus and the metabolic syndrome do not abolish, but might reduce, the cardioprotective effect of ischemic postconditioning. J Thorac Cardiovasc Surg 145:1595–1602. 10.1016/j.jtcvs.2013.02.016 [DOI] [PubMed] [Google Scholar]
- 64.Pang T, Zhao Y, Zhang NR, Jin SQ, Pan SQ (2013) Transient limb ischemia alters serum protein expression in healthy volunteers: complement C3 and vitronectin may be involved in organ protection induced by remote ischemic preconditioning. Oxid Med Cell Longev 2013:859056 10.1155/2013/859056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perman JC, Bostrom P, Lindbom M, Lidberg U, StAhlman M, Hagg D, Lindskog H, Scharin Tang M, Omerovic E, Mattsson Hulten L, Jeppsson A, Petursson P, Herlitz J, Olivecrona G, Strickland DK, Ekroos K, Olofsson SO, Boren J (2011) The VLDL receptor promotes lipotoxicity and increases mortality in mice following an acute myocardial infarction. J Clin Investig 121:2625–2640. 10.1172/jci43068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pickard JM, Botker HE, Crimi G, Davidson B, Davidson SM, Dutka D, Ferdinandy P, Ganske R, Garcia-Dorado D, Giricz Z, Gourine AV, Heusch G, Kharbanda R, Kleinbongard P, MacAl-lister R, McIntyre C, Meybohm P, Prunier F, Redington A, Robertson NJ, Suleiman MS, Vanezis A, Walsh S, Yellon DM, Hausenloy DJ (2015) Remote ischemic conditioning: from experimental observation to clinical application: report from the 8th Biennial Hatter Cardiovascular Institute Workshop. Basic Res Cardiol 110:453 10.1007/s00395-014-0453-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Przyklenk K (2015) Ischaemic conditioning: pitfalls on the path to clinical translation. Br J Pharmacol 172:1961–1973. 10.1111/bph.13064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P (1993) Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 87:893–899 [DOI] [PubMed] [Google Scholar]
- 69.Przyklenk K, Maynard M, Greiner DL, Whittaker P (2011) Cardioprotection with postconditioning: loss of efficacy in murine models of type-2 and type-1 diabetes. Antioxid Redox Signal 14:781–790. 10.1089/ars.2010.3343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rana S, Yue S, Stadel D, Zoller M (2012) Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol 44:1574–1584. 10.1016/j.biocel.2012.06.018 [DOI] [PubMed] [Google Scholar]
- 71.Riess ML, Rhodes SS, Stowe DF, Aldakkak M, Camara AK (2009) Comparison of cumulative planimetry versus manual dissection to assess experimental infarct size in isolated hearts. J Pharmacol Toxicol Methods 60:275–280. 10.1016/j.vascn.2009.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmidt MR, Rasmussen ME, Botker HE (2017) Remote ischemic conditioning for patients with STEMI. J Cardiovasc Pharmacol Ther 22:302–309. 10.1177/1074248417702481 [DOI] [PubMed] [Google Scholar]
- 73.Selvin E, Parrinello CM, Sacks DB, Coresh J (2014) Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med 160:517–525. 10.7326/m13-2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shimizu M, Tropak M, Diaz RJ, Suto F, Surendra H, Kuzmin E, Li J, Gross G, Wilson GJ, Callahan J, Redington AN (2009) Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci (Lond) 117:191–200. 10.1042/cs20080523 [DOI] [PubMed] [Google Scholar]
- 75.Skyschally A, Gent S, Amanakis G, Schulte C, Kleinbongard P, Heusch G (2015) Across-species transfer of protection by remote ischemic preconditioning with species-specific myocardial signal transduction by reperfusion injury salvage kinase and survival activating factor enhancement pathways. Circ Res 117:279–288. 10.1161/circresaha.117.306878 [DOI] [PubMed] [Google Scholar]
- 76.Thery C, Amigorena S, Raposo G, Clayton A (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 3:22 10.1002/0471143030.cb0322s30 [DOI] [PubMed] [Google Scholar]
- 77.Toombs CF, McGee DS, Johnston WE, Vinten-Johansen J (1993) Protection from ischaemic–reperfusion injury with adenosine pre-treatment is reversed by inhibition of ATP sensitive potassium channels. Cardiovasc Res 27:623–629 [DOI] [PubMed] [Google Scholar]
- 78.Tsang A, Hausenloy DJ, Mocanu MM, Carr RD, Yellon DM (2005) Preconditioning the diabetic heart: the importance of Akt phosphorylation. Diabetes 54:2360–2364 [DOI] [PubMed] [Google Scholar]
- 79.Vicencio JM, Yellon DM, Sivaraman V, Das D, Boi-Doku C, Arjun S, Zheng Y, Riquelme JA, Kearney J, Sharma V, Multhoff G, Hall AR, Davidson SM (2015) Plasma exosomes protect the myocardium from ischemia–reperfusion injury. J Am Coll Cardiol 65:1525–1536. 10.1016/j.jacc.2015.02.026 [DOI] [PubMed] [Google Scholar]
- 80.Wagner C, Kloeting I, Strasser RH, Weinbrenner C (2008) Cardioprotection by postconditioning is lost in WOKW rats with metabolic syndrome: role of glycogen synthase kinase 3beta. J Cardiovasc Pharmacol 52:430–437. 10.1097/fjc.0b013e31818c12a7 [DOI] [PubMed] [Google Scholar]
- 81.Wang H, Anderson LG, Lascola CD, James ML, Venkatraman TN, Bennett ER, Acheson SK, Vitek MP, Laskowitz DT (2013) ApolipoproteinE mimetic peptides improve outcome after focal ischemia. Exp Neurol 241:67–74. 10.1016/j.expneurol.2012.11.027 [DOI] [PubMed] [Google Scholar]
- 82.White SM, Constantin PE, Claycomb WC (2004) Cardiac physiology at the cellular level: use of cultured HL-1 cardiomyocytes for studies of cardiac muscle cell structure and function. Am J Physiol Heart Circ Physiol 286:H823–H829. 10.1152/ajpheart.00986.2003 [DOI] [PubMed] [Google Scholar]
- 83.Whittaker P, Kloner RA, Przyklenk K (1996) Intramyocardial injections and protection against myocardial ischemia. An attempt to examine the cardioprotective actions of adenosine. Circulation 93:2043–2057 [DOI] [PubMed] [Google Scholar]
- 84.Whittaker P, Przyklenk K (1994) Reduction of infarct size in vivo with ischemic preconditioning: mathematical evidence for protection via non-ischemic tissue. Basic Res Cardiol 89:6–15 [DOI] [PubMed] [Google Scholar]
- 85.Whittington HJ, Harding I, Stephenson CI, Bell R, Hausenloy DJ, Mocanu MM, Yellon DM (2013) Cardioprotection in the aging, diabetic heart: the loss of protective Akt signalling. Cardiovasc Res 99:694–704. 10.1093/cvr/cvt140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wider J, Przyklenk K (2014) Ischemic conditioning: the challenge of protecting the diabetic heart. Cardiovasc Diagn Ther 4:383–396. 10.3978/j.issn.2223-3652.2014.10.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang Z, Tian Y, Liu Y, Hennessy S, Kron IL, French BA (2013) Acute hyperglycemia abolishes ischemic preconditioning by inhibiting Akt phosphorylation: normalizing blood glucose before ischemia restores ischemic preconditioning. Oxid Med Cell Longev 2013:329183 10.1155/2013/329183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zalesak M, Blazicek P, Pancza D, Ledvenyiova V, Bartekova M, Nemcekova M, Carnicka S, Ziegelhoffer A, Ravingerova T (2014) Severity of lethal ischemia/reperfusion injury in rat hearts subjected to ischemic preconditioning is increased under conditions of simulated hyperglycemia. Physiol Res 63:577–585 [DOI] [PubMed] [Google Scholar]
- 89.Zhu SG, Xi L, Kukreja RC (2012) Type 2 diabetic obese db/db mice are refractory to myocardial ischaemic post-conditioning in vivo: potential role for Hsp 20, F1-ATPase delta and Echs1. J Cell Mol Med 16:950–958. 10.1111/j.1582-4934.2011.01376.x [DOI] [PMC free article] [PubMed] [Google Scholar]