Abstract
Objective
The clinical feature of breast cancer is very heterogeneous because of the variable prognostic factors impact its behaviour. The aim of study is to find the prognostic importance of Ki-67 and to analyse the correlation between Ki-67 index and the other conventional prognostic factors in breast cancer patients.
Materials and Methods
Between 2010 and 2017, patients with invasive ductal carcinoma who received radiotherapy after surgery were included in study. A single pathologist re-defined of all cases retrospectively. Ki-67 were established three categories based on Ki-67 levels: low (<10%), intermediate (10–25%) and high (>25%).
Results
A total of 258 patients were included. 46 of 258 (18%) patients were in low, 82 of 258 (32%) patients were in intermediate and 130 of 258 (50%) patients were in high Ki-67 group. There were no correlations between menopausal status, age, and Ki-67 level. Low-pT stages tended to have low Ki-67 expression (p=0.07). Low-pN stages correlated with low Ki-67 values (p=0.007). Patients with ECE (+) were prone to have higher Ki-67 values (p=0.02). The significant correlation was seen between Ki-67 and tumour grading (p=<0.0001). Patients with LVI (+) had higher Ki-67 expression (p=0.007). Luminal A tumours were correlated with low Ki-67 group (p=<0.0001). Ki-67 values had significant effect on DFS (p=0.03) but not OS (p=0.09).
Conclusion
This study showed that high Ki-67 expression is associated with higher pT-stage, higher pN-stage, higher grade, ER/PR negativity, HER2/neu positivity, ECE and LVI positivity. The prognostic impact of Ki-67 was only demonstrated for DFS.
Keywords: Breast cancer, Ki-67, prognostic factors, radiotherapy, survival
Introduction
The most common cancer type in women is breast cancer and the lifetime risk for breast cancer is 12% (1). The clinical feature of breast cancer is very heterogeneous because of the variable prognostic factors impact its behaviour (2). To know prognostic factors may help to estimate the prognosis and to choose the most appropriate treatment modality. Age, histopathologic subtypes, tumour size, tumour grade, lymph node involvement, extracapsular extension (ECE), lymphovascular invasion (LVI), and hormonal receptor status are the most important conventional prognostic factors (3).
In addition to these factors, to know proliferation pattern of tumour is important for the treatment decision. In routine clinical practice, immunohistochemical evaluation of Ki-67 is frequently utilised to assess proliferative features of tumour cells. Except resting phase (G0), Ki-67 is detected in all proliferative phases of the cell cycle (G1, S, G2, and M). Ki-67 existing cells can be immunochemically marked, imaged, counted and showed as a percentage of total cells (4). It has been used for many years for breast cancer; it is currently utilised to distinguish between Luminal A-like and Luminal B-like subtypes in ER+/HER2− breast cancer and physicians frequently use Ki-67 index for making a decision on adjuvant treatment (5–7).
In spite of consistent data about Ki-67 index, the relationship between Ki-67 index and the other prognostic factors remains uncertain. The results of studies evaluating the association between Ki-67 and tumour grade in breast cancer have been varied. Some of the researchers claimed that high grade tumours were correlated with high expression of Ki-67, whereas the others did not find any association (8–11). The relationship between Ki-67 index and steroid hormone receptors (oestrogen hormone receptor (ER) and progesterone hormone receptor (PR)) were investigated in previous studies. Most of the studies showed a negative correlation between steroid hormone receptors and Ki-67 levels (8–10). In regard to human epidermal growth factor receptor 2 (HER2) status, the results are controversial, as some of the researchers have found a positive correlation but the others have not (8, 12, 13). The results of studies which investigated the association between tumour stage and Ki-67 index conflicted with each other. The relationship between nodal status and Ki-67 index is not clear yet (8). The effect of Ki-67 values on survival outcome is also uncertain.
The primary aim of this study was to find the prognostic importance of Ki-67 and to analyse the correlation between Ki-67 index and the other conventional prognostic factors in breast cancer patients who received curative radiotherapy. The secondary end point of this study was to evaluate the other possible prognostic factors that affect overall survival (OS) and progression free survival (PFS).
Materials and Methods
Patient population
Between 2010 and 2017, patients with invasive ductal carcinoma who received radiotherapy after surgery were included in this study. Totally, the data of 590 women with breast cancer were retrospectively evaluated. Patients age <18, Karnofsky Performance Status <70, had another concurrent cancer, had an incomplete lymph node dissection, received neoadjuvant chemotherapy, had bilateral tumours, had initially distant metastases, and follow-up period <12 months were excluded. Finally, 258 patients with breast cancer were evaluated.
This research was confirmed by the board of Necmettin Erbakan University Meram School of Medicine and complied with the Declaration of Helsinki. Because of the retrospective nature of study, informed consent was not taken from the patients.
Treatment and follow-up
After surgery, all patients received their radiotherapy, chemotherapy and/or hormonotherapy according to routine treatment procedures. Patients were examined for tumour status in 3-month intervals for two years and in a 6-month interval for three to five years, and annually thereafter.
Histopathological evaluation
A single pathologist (F.S.) re-defined the histologic examples of all cases retrospectively, based on the guideline recommendations of the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP protocols) without information of the patient outcomes (14). The histologic type, tumour grade, tumour dimension, number of metastatic axillary lymph nodes, the existence of ECE and the existence of LVI were re-evaluated using haematoxylin- and eosin-stained, formalin-fixed and paraffin wax-embedded tumour slides. Pathological staging was performed using the 7th American Joint Committee on Cancer (AJCC) TNM staging system. ER and PR were judged as a positive when the nuclei were stained in more than 1% of the cancer cells. HER2 was judged as a positive when strong complete staining in >10% of cancer cells (ie, 3+). Fluorescent in situ hybridization (FISH) or silver-enhanced in-situ hybridization (SISH) was carried out when moderate complete staining in >10 % of cancer cells (i.e., 2+). HER2 was accepted positive when the HER2/CEP17 ratio >2 and gene copy number >4 signal/cell (15).
Immunohistochemically stained sections were used for the assessment of Ki-67. MIB-1 staining for Ki-67 was examined with 4× and 10× object lenses to identify the area of most intense staining (“hot spot”). Scoring Ki-67 was performed by counting at least 500 tumour cells in high-power fields with a 40× object lens. All brown-stained nuclei, regardless of staining intensity, were counted as positive. We did not specify any cut-off value because of there is still no absolute cut-off value was defined for the Ki-67 proliferation index. We established three categories based on Ki-67 level: low (<10%), intermediate (10–25%) and high (>25%) as some authors specified ‘low proliferative activity’ as Ki-67 values <10%, and ‘high proliferation activity’ as Ki-67 values >25%. Ki-67 levels between 10% and 25% were defined as a grey zone interval (16).
Statistical Analysis
All statistical analyses were performed using Statistical Package for Social Sciences software version 22.0 (IBM Corp.; Armonk, NY, USA). Patient, treatment and disease characteristics were evaluated using descriptive statistics. The correlation between Ki-67 groups and other clinicopathologic parameters were evaluated using Pearson’s Chi-square test, and Fisher exact test or Spearman test. The relationship between absolute Ki-67 values and other clinicopathologic parameters were assessed using an ANOVA test. Different groups of continuous variables were compared by Kruskal-Wallis test. The overall survival (OS) was identified as the time from the surgery to the date of the death or last follow-up. The disease-free survival (DFS) was identified as the time from the surgery to the date of demonstrated recurrence/progression or death. Survival analyses were evaluated using Kaplan-Meier test and two-sided log-rank test was performed to make a comparison between subgroups. Hazard ratios and 95% confidence intervals (CIs) were measured using Cox regression analysis. The variables which had statistical significance in univariate analysis (p<0.05) were added in multivariate analysis as covariates. A p value less than 0.05 was accepted statistically significant.
Results
Patients, tumour and treatment characteristics
A total of 258 patients were included in the current study with a median follow of 35 (range; 12–133) months from 2010 to 2017. One hundred of 258 patients (39%) were premenopausal, 24 of 258 patients (9%) were perimenopausal and 134 of 258 patients (52%) were postmenopausal. The median age was 52 (range; 27–83 years) years. The detailed patients, tumour, and treatment features are displayed in Table 1.
Table 1.
Patients, tumour and treatment characteristics
| Variables | No. of patients (total: 258) | % |
|---|---|---|
| Age (years) | ||
| Median (range) | 52 (27–83) | |
| Menopausal status | ||
| Premenopausal | 100 | 39 |
| Perimenopausal | 24 | 9 |
| Postmenopausal | 134 | 52 |
| Surgery type | ||
| Modified radical mastectomy | 148 | 57 |
| Breast conserving surgery | 110 | 43 |
| Tumour grade | ||
| Grade 1 | 27 | 11 |
| Grade 2 | 168 | 65 |
| Grade 3 | 63 | 24 |
| Tumour stages | ||
| pT1 | 77 | 30 |
| pT2 | 149 | 58 |
| pT3 | 22 | 8 |
| pT4 | 104 | |
| Lymph node stages | ||
| pN0 | 94 | 36 |
| pN1 | 93 | 36 |
| pN2 | 44 | 17 |
| pN3 | 27 | 11 |
| Hormonal status | ||
| ER (+) PR (+) HER2 (−) | 159 | 62 |
| ER (+) PR (+) HER2 (+) | 53 | 20 |
| ER (−) PR (−) HER2 (+) | 23 | 9 |
| Triple (−) | 23 | 9 |
| ECE | ||
| Yes | 97 | 38 |
| No | 128 | 50 |
| Unknown | 33 | 12 |
| LVI | ||
| Yes | 116 | 45 |
| No | 125 | 48 |
| Unknown | 17 | 7 |
| Ki-67 values | ||
| Low (0–9%) | 46 | 18 |
| Intermediate (10–25%) | 82 | 32 |
| High (>25%) | 130 | 50 |
ER: oestrogen hormone receptor; PR: progesterone hormone receptor; HER2: human epidermal growth factor receptor 2; ECE: extracapsular extension; LVI: lymphovascular invasion
Relationship of Ki-67 status with patient and tumour characteristics
The median Ki-67 value was 27.5% (range: 0 to 95%; mean: 30%). Forty-six of 258 (18%) patients were in low, 82 of 258 (32%) patients were in intermediate and 130 of 258 (50%) patients were in high Ki-67 expression group. There were no correlations between menopausal status, age and Ki-67 groups (p=0.3 and p=0.6, respectively). Concerning the dimension of tumour, low-pT stages tended to have low Ki-67 expression (p=0.07). Ninety-two percent of low expression group had pT1–2 disease, whereas only 8% of the low expression group had pT3–4 disease. Similarly, 87% of the intermediate Ki-67 group had pT1–2 disease, whereas 13% of the intermediate Ki-67 group had pT3–4 disease. Because of low number of pT3–4 cases (32 of 258 patients), to make a conclusion about the high expression group is difficult but 53% of patients with pT3–4 disease were in high expression group. Regarding the nodal status, low-pN stages were correlated with low Ki-67 expression (p=0.007). Eighty-seven percent of low Ki-67 group had pN0–1 disease while 65% of pN2–3 patients had high expression of Ki-67. Patients with ECE (+) were prone to have high Ki-67 values, whereas patients with ECE (−) prone to have low Ki-67 values (p=0.02). Seventy-one percent of patients with ECE (−) were in low expression group, whereas 60% of patients with ECE (+) were in high expression group. The significant association was seen between Ki-67 levels and tumour grading (p=<0.0001). Low-grade tumours were correlated with low Ki-67 expression whereas high-grade tumours were correlated with high Ki-67 expression. Forty-eight percent, 37% and 15% of grade 1 tumours were in low, intermediate and high Ki-67 expression group, respectively. Nine percent, 24% and 67% of grade 3 tumours were in low, intermediate and high Ki-67 expression group, respectively. Patients with LVI (+) had higher expression of Ki-67 than patients with LVI (−) (p=0.007). Eighty percent of patients with LVI (−) were in low expression group. ER/PR (+) tumours were correlated with low Ki-67 expression (p=<0.0001). Ninety-eight percent of patient in low Ki-67 expression group had ER/PR (+) disease whereas 28% of patients with ER/PR (+) were in high Ki-67 group. Regarding HER2 status, HER2 (+) tumours were correlated with high expression of Ki-67 (p=<0.0001). In a low Ki-67 group, 80% of patients were HER2 (−) while 68% of HER2 (+) patients were in high Ki-67 group. Parallelly, hormonal receptor status was associated with Ki-67 values (p=<0.0001). Luminal A (ER/PR (+), HER2 (−)) tumours were correlated with the low Ki-67 group. In low expression group, 81% of patients had ER/PR (+), HER2 (−) (Luminal A-like subtype), 17% of patients had ER/PR (+), HER2 (+) (Luminal B-like subtype), 2% of patients had ER/PR (−), HER2/neu (+) and none of the patient had ER/PR (−), HER2 (−) (triple (−). Correlatively, 83%, 17% and 0% of patients with triple (−) disease were in high, intermediate and low Ki-67 expression group, respectively. The relationship between absolute Ki-67 values and clinicopathologic variables were also evaluated and the results were shown in Table 2.
Table 2.
The relationship between absolute Ki-67 values and clinicopathologic variables
| Variables (n=258) | Absolute Ki-67 value (mean) | p |
|---|---|---|
| Menopausal status | ||
| Premenopausal | 30.50 | 0.1 |
| Perimenopausal | 31.50 | |
| Postmenopausal | 29.93 | |
| Total | 30.29 | |
| Tumour grade | ||
| Grade 1 | 11.96 | <0.0001* |
| Grade 2 | 29.05 | |
| Grade 3 | 41.46 | |
| Total | 30.29 | |
| Tumour stages | ||
| pT1–2 | 29.95 | 0.5 |
| pT3–4 | 32.72 | |
| Total | 30.29 | |
| Lymph node stages | ||
| pN0–1 | 27.23 | 0.001* |
| pN2–3 | 38.07 | |
| Total | 30.29 | |
| Hormonal status | ||
| ER (+) PR (+) HER2 (−) | 24.71 | <0.0001* |
| ER (+) PR (+) HER2 (+) | 31.04 | |
| ER (−) PR (−) HER2 (+) | 48.13 | |
| Triple (−) | 49.35 | |
| Total | 30.29 | |
| ECE | ||
| Yes | 27.08 | 0.02* |
| No | 34.07 | |
| Total | 30.09 | |
| LVI | ||
| Yes | 26.93 | 0.02* |
| No | 33.70 | |
| Total | 30.19 | |
| Ki-67 values | ||
| Low (0–9%) | 4.09 | <0.0001* |
| Intermediate (10–25%) | 15.29 | |
| High (>25%) | 49.03 | |
| Total | 30.29 | |
ER: oestrogen hormone receptor; PR: progesterone hormone receptor; HER2: human epidermal growth factor receptor 2; ECE: extracapsular extension; LVI: lymphovascular invasion
Survival Analysis
During a median follow-up of 35 months, 250 of 258 patients (97%) were alive and 16 of 258 patients (4%) had distant metastases. The mean OS and DFS were 127 (range; 123 to 131) and 121 (range; 115 to 126) months, respectively. 5-year OS and DFS rates were 95% and 87%, respectively. The tumour grade (p=0.01), hormonal status (p=0.006), nodal stage (p=0.01), and LVI (p=0.03) were significant prognostic factors for OS in univariate analysis. Regarding Ki-67 values, 6 of 8 died patients (75%) were in high expression group and 2 of 8 died patients 25(%) were in intermediate expression group while there was no died patient in low expression group. However, these differences did not reach significance (Figure 1; p=0.09). In terms of DFS, the tumour grade (p=0.001), hormonal status (p=0.003) and Ki-67 values (p=0.03) were independent prognostic factors for DFS. Twelve of 16 patients (75%) with metastases were in high expression group, 3 of 16 patients with metastases were in intermediate expression group and only 1 patient had metastasis in the low expression group. The disease-free survival outcomes based on Ki-67 values were shown in Figure 2 (p=0.03). According to multivariate analysis, only the hormonal status was independent prognostic factor for both OS (p=0.02; HR=9.98 [1.40–15.41]) and DFS (p=0.03; HR=4.20 [1.14–15.41]).
Figure 1.
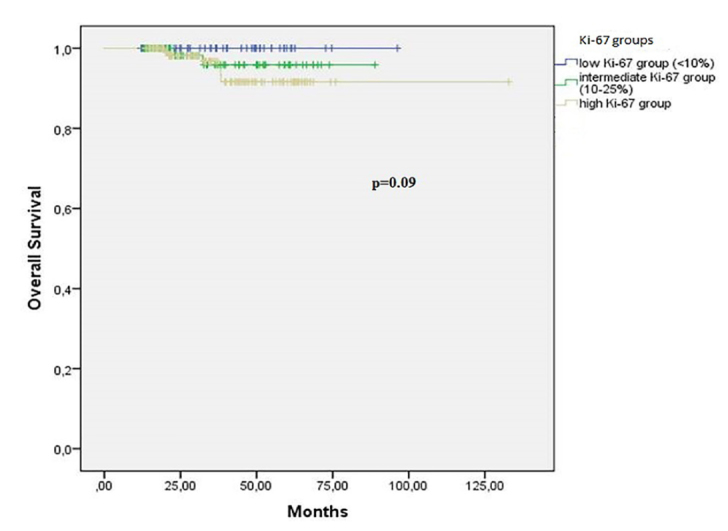
Overall survival based on Ki-67 values
Figure 2.
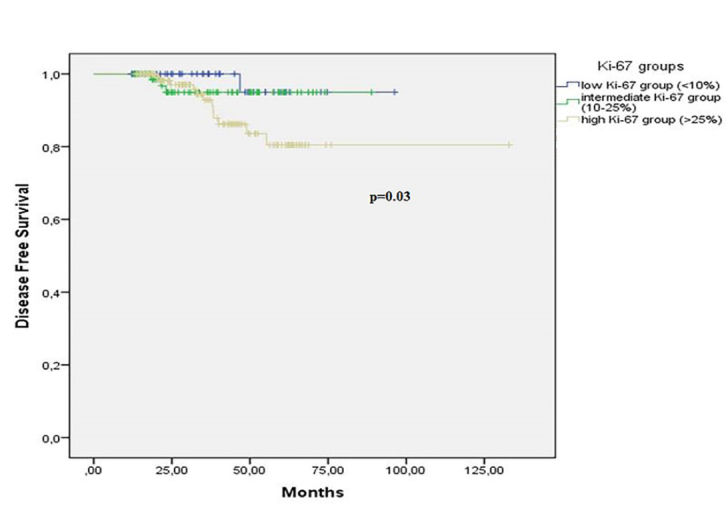
Disease free survival based on Ki-67 values
Discussion and Conclusion
The primary aim of this study was to find the prognostic importance of Ki-67 index and to analyse the correlation between Ki-67 index and other conventional prognostic factors in breast cancer patients who received curative radiotherapy.
Despite the variability in the cut-off points (5% to 34% or more) and the lack of standardized procedure for Ki-67 assessment, to find its predictive and prognostic value has been frequently attractive for researchers. The 2009 St. Gallen consensus divided three subgroups according to Ki-67 levels: low (≤15%), intermediate (16% to 30%), and high (≥30%); the 2011 St. Gallen recommended a Ki-67 cut-off point of 14% for distinguishing between Luminal A-like and Luminal B-like tumours; the 2013 St. Gallen changed the cut-off point to 20%, the 2015 St. Gallen advised the Ki-67 values between 20% and 29% was used to distinguish luminal B-like disease (5, 6, 16).
In the PACS01 study, the authors showed that, with using a cut-off point as 14%, the risk of misclassification was 37% when Ki-67 value was between 10–25%, and it was 11% when Ki-67 value was <10% or ≥25% (17). In this study, there was not any cut-off point defined and Ki-67 was established as three categories based on levels: low (<10%), intermediate (10–25%) and high (>25%).
In the current study, we did not show any correlation between patient age, menopausal status, and Ki-67 index but most of well-known conventional prognostic factors significantly associated with Ki-67 values. Our data indicated that low-pT stages tended to have low expression of Ki-67. These findings were in accordance with the outcomes of Fausto et al. (18) and Inwald et al. (19). In accordance with the current research, Alco et al. (20) reported the results of largest study from Turkey in 2015 and revealed that the Ki-67 index was positively correlated with an increasing tumour size. Low-pN stages were also correlated with low Ki-67 expression. Our findings were consistent with the results of previous studies (19–21). In the current study, the significant association was seen between Ki-67 levels and tumour grading. Low-grade tumours were correlated with low Ki-67 expression whereas high-grade tumours were correlated with high Ki-67 expression. This correlation was demonstrated in many previous studies (8–10, 19–22). The other powerful correlation was shown in steroid hormone receptor status and expression of Ki-67 in former research (8–10, 19–22). These studies showed a remarkable association between higher Ki-67 expression and ER/PR negativity. Our results were consistent with the literature. Regarding HER2 status, the results were inconsistent. Some of the studies showed a positive association between higher Ki-67 expression and HER2 negativity, but most of the studies displayed a positive correlation between higher Ki-67 expression and HER2 positivity (8, 12, 13, 19–22). In the current study, high Ki-67 expression was correlated with HER2 positivity. In addition, with these results, we found that Luminal A (ER/PR(+), HER2(−)) tumours tended to have low Ki-67 expression and triple (−) tumours tended to have high Ki-67 expression. In accordance with our results, Alco et al. (20) showed that the Ki-67 index was negatively correlated with HR positivity, and positively correlated with HER2 positivity.
We did not get any data which investigate the correlation between ECE and Ki-67 index in literature but according to our results, patients with ECE (+) prone to had higher Ki-67 values. There are very limited data analysing the correlation between LVI and Ki-67. In the present study, patients with LVI had high expression levels of Ki-67 similar to the results of Alco et al. (20).
The results of the studies investigating the effect of Ki-67 on survival outcomes were conflicting with each other. Although some of the researchers showed prognostic effects of Ki-67 expression on survival outcomes, the others did not demonstrate any correlation (8, 19, 21, 23). We found a significant relationship between high expression of Ki-67 and poor DFS. In spite of most of deaths (75%) were in high expression group we did not find any correlation between Ki-67 expression and OS. This may be because of a relatively small number of patients and short follow-up time.
Currently, Ki-67 assessment is used for prediction of prognosis, to distinguish between Luminal A-like and Luminal B-like subtypes in ER+/HER2− and to help with decision making on adjuvant chemotherapy (5–7, 16). Although, its routine use in clinical practice is still not recommended due to the lack of a standardized procedure of Ki-67 evaluation, interpretation, scoring, and definition of cut-off value; it has been suggested that each pathology department should specify their own assessment methodology of Ki-67 (24).
We are aware of that there are some limitations of the study, including limited sample size, relatively short follow-up time, and its retrospective nature. The retrospective design of the study did not negatively affect the association of the Ki-67 index with the other patient and tumour characteristics, but the survival outcomes might be affected by this situation.
In conclusion, this single institution study showed that high expression of Ki-67 is associated with higher pT-stage, higher pN-stage, higher grade, ER/PR negativity, HER2/neu positivity, ECE and LVI positivity. The prognostic impact of Ki-67 was only demonstrated for DFS and longer follow-up time may be required to see its effect on OS.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Necmettin Erbakan University Meram School of Medicine.
Informed Consent: Informed consent was not received due to the retrospective nature of the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - G.K., S.F.; Design - G.K., B.B.Y., M.A.; Supervision - G.K., M.U.; Resources - G.K.; Materials - G.K., B.B.Y., M.A., M.K., S.F.; Data Collection and/or Processing - G.K., M.U., S.F.; Analysis and/or Interpretation - G.K., M.U.; Literature Search - G.K.; Writing Manuscript - G.K.; Critical Review - G.K., B.B.Y., M.A. M.K., M.U., S.F.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Ferlay J, Soerjomataram I, Erwik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012 v1.0. Available from: URL: https://publications.iarc.fr/Databases/Iarc-Cancerbases/GLOBOCAN-2012-Estimated-Cancer-Incidence-Mortality-And-Prevalence-Worldwide-In-2012-V1.0-2012. (cited 2018 June 27).
- 2.Koseoglu RD, Markoc F, Muslehiddinoglu A, Ileri AB, Deresoy FA, Etikan I. HER-2/Neu and Hormon Receptor Analysis in Breast Carcinomas and Their Association with Clinicopathologic Parameters. Eur J Breast Health. 2019;15:43–50. doi: 10.5152/ejbh.2018.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haque R, Ahmed SA, Inzhakova G, Shi J, Avila C, Polikoff J, Bernstein L, Enger SM, Press MF. Impact of breast cancer subtypes and treatment on survival: an analysis spanning two decades. Cancer Epidemiol Biomarkers Prev. 2012;21:1848–1855. doi: 10.1158/1055-9965.EPI-12-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanyilmaz G, Onder H, Aktan M, Koc M, Bora H, Karahacioglu E, Erkal SK, Yirmibesoglu Erkal E. Prognostic Importance of Ki-67 Labelling Index in Grade II Glial Tumors. Turk J Oncol. 2018;33:48–53. doi: 10.5505/tjo.2018.1752. [DOI] [Google Scholar]
- 5.Penault-Llorca F, Radosevic-Robin N. Ki67 assessment in breast cancer: an update. Pathology. 2017;49:166–171. doi: 10.1016/j.pathol.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, Thürlimann B, Senn H. Tailoring therapies improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26:1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan AC, Li BT, Nahar K, Danieletto S, Fong ES, Currer T, Parasyn A, Middleton P, Wong H, Smart D, Rutovitz JJ, McCloud P, Hughes TM, Marx GM. Correlating Ki67 and other prognostic markers with Oncotype DX recurrence score in early estrogen receptor-positive breast cancer. Asia Pas J Clin Oncol. 2018;14:161–166. doi: 10.1111/ajco.12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiesner FG, Magener A, Fasching PA, Wesse J, Bani MR, Rauh C, Jud S, Schrauder M, Loehberg CR, Beckmann MW, Hartmann A, Lux MP. Ki-67 as a prognostic molecular marker in routine clinical use in breast cancer patients. Breast. 2009;18:135–141. doi: 10.1016/j.breast.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Liu S, Edgerton SM, Moore DH, 2nd, Thor AD. Measures of cell turnover (proliferation and apoptosis) and their association with survival in breast cancer. Clin Cancer Res. 2001;7:1716–1723. [PubMed] [Google Scholar]
- 10.Spyratos F, Ferrero-Poüs M, Trassard M, Hacène K, Phillips E, Tubiana-Hulin M, Le Doussal V. Correlation between MIB-1 and other proliferation markers: clinical implication and other proliferation markers: clinical implication of the MIB-1 cut off value. Cancer. 2002;94:2151–2159. doi: 10.1002/cncr.10458. [DOI] [PubMed] [Google Scholar]
- 11.Tanei T, Shimomura A, Shimazu K, Nakayama T, Kim SJ, Iwamoto T, Tamaki Y, Noguchi S. Prognostic significance of Ki-67 index after neoadjuvant chemotherapy in breast cancer. Eur J Surg Oncol. 2011;37:155–161. doi: 10.1016/j.ejso.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Bottini A, Berruti A, Bersiga A, Brizzi MP, Bruzzi P, Aguggini S, Brunelli A, Bolsi G, Allevi G, Generali D, Betri E, Bertoli G, Alquati P, Dogliotti L. Relationship between tumour shrinkage and reduction in Ki67 expression after primary chemotherapy in human breast cancer. Br J Cancer. 2001;85:1106–1112. doi: 10.1054/bjoc.2001.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shokouh TZ, Ezatollah A, Barand P. Interrelationship Between Ki67, HER2/neu, p53, ER, and PR Status and Their Associations with Tumor Grade and Lymph Node Involvement in Breast Carcinoma Subtypes. Medicine (Baltimore) 2015;94:1359–1364. doi: 10.1097/MD.0000000000001359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.College of American Pathologist. [Accessed 2018 July 24]. Available from: URL: http://www.cap.org.
- 15.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologist Clinical Practice Guideline Update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 16.Andre F, Arnedos M, Goubar A, Ghouadni A, Delaloge S. Ki-67-no evidence for its use in node positive breast cancer. Nat Rev Clin Oncol. 2015;12:296–301. doi: 10.1038/nrclinonc.2015.46. [DOI] [PubMed] [Google Scholar]
- 17.Penault-Llorca F. Interpathologists discrepancies in Ki-67 assessment in the PACS01 trial: an independent prognostic factor (abstract) J Clin Oncol. 2012;30:543. [Google Scholar]
- 18.Fausto P, Viale G, Cabiddu M, Barni S. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat. 2015;153:477–491. doi: 10.1007/s10549-015-3559-0. [DOI] [PubMed] [Google Scholar]
- 19.Inwald EC, Klinkhammer-Schalke M, Hofstädter F, Zeman F, Koller M, Gerstenhauer M, Ortmann O. Ki-67 is a prognostic parameter in breast cancer patients: results of large population-based cohort of a cancer registry. Breast Cancer Res Treat. 2013;139:539–552. doi: 10.1007/s10549-013-2560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alco G, Bozdogan A, Selamoglu D, Pilanci KN, Tuzlali S, Ordu C, Igdem S, Okkan S, Dincer M, Demir G, Ozmen V. Clinical and histopathological factors associated with Ki-67 expression in breast cancer patients. Oncol Lett. 2015;9:1046–1054. doi: 10.3892/ol.2015.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilickap S, Kaya Y, Yucel B, Tuncer E, Babacan Akgul N, Elagoz S. Higher Ki-67 expression associates with unfavorable prognostic factors and shorter survival in breast cancer. Asian Pac J Cancer Prev. 2014;15:1381–1385. doi: 10.7314/APJCP.2014.15.3.1381. [DOI] [PubMed] [Google Scholar]
- 22.Soliman NA, Yussif SM. Ki-67 as a prognostic marker according to breast molecular subtype. Cancer Biol Med. 2016;13:496–504. doi: 10.20892/j.issn.2095-3941.2016.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zenzola V, Cabezas-Quintario MA, Arguelles M, Perez-Fernandez E, Izarzugaza Y, Correa A, Garcia-Foncillas J. Prognostic value of Ki-67 according to age in patients with triple-negative breast cancer. Clin Transl Oncol. 2018;20:1448–1454. doi: 10.1007/s12094-018-1877-5. [DOI] [PubMed] [Google Scholar]
- 24.Colomer R, Aranda-López I, Albanell J, García-Caballero T, Ciruelos E, López-García MÁ, Cortés J, Rojo F, Martín M, Palacios-Calvo J. Biomarkers in breast cancer: A consensus statement by the Spanish Society of Medical Oncology and the Spanish Society of Pathology. Clin Transl Oncol. 2018;20:815–826. doi: 10.1007/s12094-017-1800-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


