Abstract
Background
Vitamin D deficiency during pregnancy increases the risk of pre‐eclampsia, gestational diabetes, preterm birth, and low birthweight. In a previous Cochrane Review we found that supplementing pregnant women with vitamin D alone compared to no vitamin D supplementation may reduce the risk of pre‐eclampsia, gestational diabetes, and low birthweight and may increase the risk of preterm births if it is combined with calcium. However the effects of different vitamin D regimens are not yet clear.
Objectives
To assess the effects and safety of different regimens of vitamin D supplementation alone or in combination with calcium or other vitamins, minerals or nutrients during pregnancy, specifically doses of 601 international units per day (IU/d) or more versus 600 IU/d or less; and 4000 IU/d or more versus 3999 IU/d or less.
Search methods
We searched the Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (12 July 2018), and the reference lists of retrieved studies.
Selection criteria
Randomised trials evaluating the effect of different vitamin D regimens (dose, frequency, duration, and time of commencement of supplementation during pregnancy), alone or in combination with other nutrients on pregnancy and neonatal health outcomes. We only included trials that compared 601 IU/d or more versus 600 IU/d or less and 4000 IU/d or more versus 3999 IU/d or less. We did not include in the analysis groups that received no vitamin D, as that comparison is assessed in another Cochrane Review.
Data collection and analysis
Two review authors independently: i) assessed the eligibility of studies against the inclusion criteria; ii) extracted data from included studies, and iii) assessed the risk of bias of the included studies. Our primary maternal outcomes were: pre‐eclampsia, gestational diabetes, and any adverse effects; our primary infant outcomes were preterm birth and low birthweight. Data were checked for accuracy. The certainty of the evidence was assessed using the GRADE approach.
Main results
In this review, we included data from 30 trials involving 7289 women. We excluded 11 trials, identified 16 ongoing/unpublished trials and two trials are awaiting classification. Overall risk of bias for the trials was mixed.
Comparison 1. 601 IU/d or more versus 600 IU/d or less of vitamin D alone or with any other nutrient (19 trials; 5214 participants)
Supplementation with 601 IU/d or more of vitamin D during pregnancy may make little or no difference to the risk of pre‐eclampsia (risk ratio (RR) 0.96, 95% confidence interval (CI) 0.65 to 1.42); 5 trials; 1553 participants,low‐certainty evidence), may reduce the risk of gestational diabetes (RR 0.54, 95% CI 0.34 to 0.86; 5 trials; 1846 participants; moderate‐certainty evidence), may make little or no difference to the risk of preterm birth (RR 1.25, 95% CI 0.92 to 1.69; 4 trials; 2294 participants; low‐certainty evidence); and may make little or no difference to the risk of low birthweight (RR 0.90, 95% CI 0.66 to 1.24; 4 trials; 1550 participants; very low‐certainty evidence) compared to women receiving 600 IU/d or less.
Comparison 2. 4000 IU or more versus 3999 IU or less of vitamin D alone (15 trials; 4763 participants)
Supplementation with 4000 IU/d or more of vitamin D during pregnancy may make little or no difference to the risk of: pre‐eclampsia (RR 0.87, 95% CI 0.62 to 1.22; 4 trials, 1903 participants, low‐certainty evidence); gestational diabetes (RR 0.89, 95% CI 0.56 to 1.42; 5 trials, 2276 participants; low‐certainty evidence); preterm birth (RR 0.85, 95% CI 0.64 to 1.12; 6 trials, 2948 participants, low‐certainty evidence); and low birthweight (RR 0.92, 95% CI 0.49 to 1.70; 2 trials; 1099 participants; low‐certainty evidence) compared to women receiving 3999 IU/d or less.
Adverse events (such as hypercalcaemia, hypocalcaemia, hypercalciuria, and hypovitaminosis D) were reported differently in most trials; however, in general, there was little to no side effects reported or similar cases between groups.
Authors' conclusions
Supplementing pregnant women with more than the current vitamin D recommendation may reduce the risk of gestational diabetes; however, it may make little or no difference to the risk of pre‐eclampsia, preterm birth and low birthweight. Supplementing pregnant women with more than the current upper limit for vitamin D seems not to increase the risk of the outcomes evaluated. In general, the GRADE was considered low certainty for most of the primary outcomes due to serious risk of bias and imprecision of results. With respect to safety, it appears that vitamin D supplementation is a safe intervention during pregnancy, although the parameters used to determine this were either not reported or not consistent between trials. Future trials should be consistent in their reports of adverse events. There are 16 ongoing trials that when published, will increase the body of knowledge.
Plain language summary
Regimens of vitamin D supplementation for women during pregnancy
What is the issue?
This review evaluated if there are beneficial effects of supplementing pregnant women with more than the current vitamin D recommendation (200 international units/day (IU/d) to 600 IU/d) on pregnancy and neonatal health outcomes and to evaluate if there are negative health effects when using more than the current upper limit recommendation (4000 IU/d).
Why is this important?
Vitamin D supplementation in pregnancy compared to no supplementation appears to decrease the risk of pre‐eclampsia, gestational diabetes, low birthweight and may reduce the risk of severe postpartum haemorrhage. However, it is not clear if doses greater than the currently recommended level are needed to observe these health benefits, and if giving more than the upper limit is related to adverse events.
What was studied in the review?
This review included trials evaluating the effect of different vitamin D regimens (doses, frequencies, duration, and times of commencement) to compare the effects of 601 IU/d or more versus 600 IU/d or less and 4000 IU/d or more versus 3999 IU/d or less, of vitamin D alone or with any other nutrient on pregnancy and neonatal health outcomes.
What evidence did we find?
Evidence from 19 trials involving 5214 women suggest that supplementation with 601 IU/d or more of vitamin D during pregnancy may reduce the risk of gestational diabetes but may make little or no difference to the risk of pre‐eclampsia, preterm birth or low birthweight compared to women receiving 600 IU/d or less.
Evidence from 15 trials involving 4763 women suggests that supplementation with 4000 IU/d or more of vitamin D during pregnancy may make little or no difference to the risk of pre‐eclampsia, gestational diabetes, preterm birth or low birthweight compared to women receiving 3999 IU/d or less.
Adverse events were reported differently in most trials; in general, there was little to no side effects reported or similar cases between groups.
What does this mean?
Supplementing pregnant women with more than the current vitamin D recommendation may reduce the risk of gestational diabetes; however, it may make little or no difference in the risk of the other outcomes. Supplementing pregnant women with more than the current upper limit for vitamin D seems not to increase the risk of the outcomes evaluated. Vitamin D supplementation appears to be safe.
Summary of findings
Summary of findings for the main comparison. A dose of vitamin D 601 IU or higher compared to 600 IU or lower alone or with other nutrients for women during pregnancy.
| A dose of vitamin D 601 IU or higher compared to 600 IU or lower alone or with other nutrients for women during pregnancy | ||||||
| Patient or population: women during pregnancy. Setting: trials were carried out between 2004 to 2017 in the following countries: Australia (Yap 2014), Bangladesh (Roth 2013), Iran (Karamali 2015; Mojibian 2015) and the USA (O'Brien 2013; Stephensen 2011; Weiss 2009). Most trials were conducted outside the tropics and in different seasons Intervention: a dose of vitamin D 601 IU or higher, alone or with other nutrients. Comparison: 600 IU or lower, alone or with other nutrients. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with 600 IU or lower alone or with other nutrients | Risk with A dose of vitamin D 601 IU or higher | |||||
| Pre‐eclampsia | Study population | RR 0.96 (0.65 to 1.42) | 1553 (5 RCTs) | ⊕⊕⊝⊝ LOW 1 | Included trials: Karamali 2015; Mojibian 2015; Stephensen 2011; Weiss 2009; Yap 2014 | |
| 63 per 1000 | 60 per 1000 (41 to 89) | |||||
| Gestational diabetes | Study population | RR 0.54 (0.34 to 0.86) | 1846 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | Included trials:Hashemipour 2014; Mojibian 2015; Roth 2013; Stephensen 2011; Yap 2014 Yap 2014 |
|
| 74 per 1000 | 40 per 1000 (25 to 64) | |||||
| Preterm birth | Study population | RR 1.25 (0.92 to 1.69) | 2294 (4 RCTs) | ⊕⊕⊝⊝ LOW 3 | Inluded trials: Karamali 2015; Mojibian 2015; Roth 2013; Weiss 2009. | |
| 68 per 1000 | 85 per 1000 (63 to 115) | |||||
| Low birthweight | Study population | RR 0.90 (0.66 to 1.24) | 1550 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 4 | Included trials: Karamali 2015; Mojibian 2015; O'Brien 2013; Roth 2013. | |
| 120 per 1000 | 108 per 1000 (80 to 149) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded (2) levels for serious limitations in study design due to one trial being assessed as high risk of bias for several domains and for serious limitations in imprecision with wide confidence intervals crossing the line of no effect.
2 We downgraded (1) level for serious limitations in study design due to one trial being assessed as high risk of bias for several domains.
3 We downgraded (2) levels for serious limitations in study design due to one trial being assessed as high risk of bias for several domains and for serious limitations in imprecision with wide confidence intervals crossing the line of no effect.
4 We downgraded (3) levels for very serious limitations in study design due to two trials being assessed as high risk of bias for several domains and for serious limitations in imprecision with wide confidence intervals crossing the line of no effect.
Summary of findings 2. A dose of vitamin D 4000 IU/d or more compared to 3999 IU/d or less alone or with any other nutrient for women during pregnancy.
| A dose of vitamin D 4,000 IU/d or more compared to 3999 IU/d or less alone or with any other nutrient for women during pregnancy | ||||||
| Patient or population: women during pregnancy Setting: trials were carried out between 2004 to 2017 in the following countries: Australia (Yap 2014), Bangladesh (Bacqui 2009; Roth 2013), Iran (Hashemipour 2014; Karamali 2015; Mojibian 2015; Rostami 2017), and the USA (Stephensen 2011; Wagner 2006a; Weiss 2009). Most trials were conducted outside the tropics and in different seasons. Intervention: a dose of vitamin D 4,000 IU/d or more Comparison: 3999 IU/d or less alone or with any other nutrient | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with 3999 IU/d or less alone or with any other nutrient | Risk with A dose of vitamin D 4000 IU/d or more | |||||
| Pre‐eclampsia | Study population | RR 0.87 (0.62 to 1.22) | 1903 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 | Included trials: Karamali 2015; Rostami 2017; Weiss 2009; Yap 2014 | |
| 81 per 1000 | 71 per 1000 (50 to 99) | |||||
| Gestational diabetes | Study population | RR 0.89 (0.56 to 1.42) | 2276 (5 RCTs) | ⊕⊕⊝⊝ LOW 1 | Included trials: Hashemipour 2014; Roth 2013; Wagner 2006a; Yap 2014 | |
| 29 per 1000 | 26 per 1000 (16 to 41) | |||||
| Preterm birth | Study population | RR 0.85 (0.64 to 1.12) | 2948 (6 RCTs) | ⊕⊕⊝⊝ LOW 2 | Included trials: Bacqui 2009; Karamali 2015; Rostami 2017; Roth 2013; Wagner 2006a; Weiss 2009 | |
| 75 per 1000 | 64 per 1000 (48 to 84) | |||||
| Low birthweight | Study population | RR 0.92 (0.49 to 1.70) | 1099 (2 RCTs) | ⊕⊕⊝⊝ LOW 3 | Included trials: Karamali 2015; Roth 2013 | |
| 177 per 1000 | 163 per 1000 (87 to 300) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded (2) levels for serious limitations in study design due to one trial being assessed as high risk of bias for one domain and one study being at unclear risk for allocation concealment and for serious limitations in imprecision with wide confidence intervals crossing the line of no effect.
2 We downgraded (2) levels for serious limitations in study design due to one trial being assessed as high risk of bias for several domains and for serious limitations in imprecision with wide confidence intervals crossing the line of no effect.
3 We downgraded (2) levels for very serious limitations in imprecision as only two trials contributed data to this outcome, with wide confidence intervals crossing the line of no effect and for serious limitations in indirectness as both studies were conducted in Asian women.
Background
Description of the condition
Magnitude of vitamin D deficiency during pregnancy
Vitamin D deficiency during pregnancy is highly prevalent worldwide (Palacios 2014). A systematic review of 17 studies among pregnant and breastfeeding women found a prevalence of vitamin D deficiency (defined as serum 25‐hydroxyvitamin D levels < 25 nmol/L) ranging from 4% in England to 60% in India (Palacios 2014). Another review by World Health Organization (WHO) regions found a vitamin D deficiency prevalence ranging from 9% in the Americas to 79% in the Eastern Mediterranean countries (Saraf 2016). A review of 15 studies (2649 pregnant women) in the Mediterranean region found a prevalence of vitamin D deficiency ranging from 23% to 90% (Karras 2016). Other reports also show a large prevalence of vitamin D deficiency among pregnant women from 18% in Spain (Rodriguez 2016) to 94% in India (Sharma 2016).
The magnitude of vitamin D deficiency varies by skin colour, race/ethnicity, weight status, season, dressing patterns, the use of vitamin D‐containing oral supplements, physical activity, season of gestation and latitude. With respect to skin colour, several studies have observed a greater prevalence of vitamin D deficiency in pregnant women with darker skin colour compared to women with lighter skin colour (Karras 2016; Nassar 2011). Also, those of white race/ethnicity have a lower prevalence of vitamin D deficiency compared to other races/ethnicity (Karras 2016). Obese pregnant women have a higher prevalence of vitamin D deficiency compared to women with adequate weight (Karlsson 2015; Karras 2016; Moon 2015; Pratumvinit 2015). Covered dressing patterns are also important, with greater vitamin D deficiency prevalence among pregnant women using excessive clothing (clothing that covers most of the body) (Karras 2016). Conversely, women engaging in physical activity have lower risk of vitamin D deficiency (Moon 2015; Rodriguez 2016). Vitamin D deficiency during pregnancy is higher in the winter months when there is less sunlight exposure, compared to the summer months (Brembeck 2013; Karlsson 2015; Karras 2016; Moon 2015; Nicolaidou 2006; Pratumvinit 2015; Rodriguez 2016; Sharma 2016). Extreme latitudes (close to the poles) have also been shown to be related to vitamin D deficiency (Brembeck 2013; Karlsson 2015; Rodriguez 2016).
Vitamin D status and its association with maternal and neonatal outcomes
Several meta‐analyses have been conducted in recent years assessing the associations between vitamin D deficiency and low vitamin D concentrations, with a plethora of adverse maternal and neonatal health outcomes being observed. Some of these associations are stronger than others and even though there is a high variability between study designs, most meta‐analyses have shown fairly consistent results.
Five recent meta‐analyses assessed the associations between vitamin D deficiency and risk of pre‐eclampsia from epidemiological studies, including 31 observational studies (Aghajafari 2013), two large‐scale epidemiological studies (Hypönnen 2013), 24 observational studies (Wei 2013), 24 observational studies (Christesen 2012a), and eight observational studies (Tabesh 2013) in pregnant women. All the studies found that vitamin D deficiency significantly increased the risk of pre‐eclampsia, even after adjusting for important confounders. Similarly, four meta‐analyses (including six to 31 observational studies) found a significant increase in the risk of gestational diabetes with vitamin D deficiency (Aghajafari 2013; Christesen 2012a; Poel 2012; Wei 2013), while one meta‐analysis (including two studies) did not find a significant association (Thorne‐Lyman 2012).
With respect to neonatal outcomes, the meta‐analysis by Wei 2013 including 24 observational studies with pregnant women found an increase in the risk of preterm birth with vitamin D deficiency (odds ratio (OR) 1.58, 95% confidence interval (CI) 1.08 to 2.31). However, two meta‐analyses including two and four studies respectively did not find a significant association between vitamin D deficiency and risk of preterm birth (Christesen 2012a; Thorne‐Lyman 2012). In relation to small‐for‐gestational birth, two meta‐analyses including 24 studies (Wei 2013) and 31 studies (Aghajafari 2013) found an increase in the risk of small‐for‐gestational age with vitamin D deficiency. Furthermore, vitamin D deficiency was also found to be associated with a higher risk of lower birthweight in three meta‐analyses (Aghajafari 2013; Christesen 2012b; Harvey 2014).
Vitamin D supplementation effects on maternal and neonatal outcomes
Contrary to the epidemiological evidence, vitamin D supplementation trials in pregnancy have not shown consistent results for improving maternal and neonatal outcomes (Theodoratou 2014). As shown in a recently published Cochrane Review (Palacios 2019), vitamin D supplementation improves maternal vitamin D status during pregnancy. This increase in vitamin D status may have a direct influence on the fetal supply of vitamin D and neonatal levels. This review showed that vitamin D supplementation during pregnancy may reduce the risk of pre‐eclampsia, low birthweight and preterm birth compared to no treatment or placebo, but many of the reported outcomes are based on small numbers of trials and participants. On the other hand, the results also showed that the combination of vitamin D and calcium supplements may increase the risk of preterm birth, as shown in three studies. Most studies evaluated in the aforementioned review were excluded (27 studies in total), mainly because the comparisons were among different doses of vitamin D without including a placebo or no treatment control group. In addition, the authors identified 23 ongoing or unpublished trials; most of which also include different doses of vitamin D supplements.
A few other meta‐analyses of randomised clinical trials using different doses of vitamin D supplementation compared to placebo or to a lower vitamin D dose have been done in recent years. In a meta‐analysis including four studies in pregnant women (Hypönnen 2013), the investigators found a significant reduction in the odds of pre‐eclampsia (OR 0.66, 95% CI 0.52 to 0.83) when vitamin D supplementation was compared with the control group (placebo or lower dose). However, no significant reduction in the odds of pre‐eclampsia was detected in another meta‐analysis (Pérez‐López 2015), which included 13 randomised clinical trials using different doses of vitamin D supplementation compared to placebo or to a lower vitamin D dose (OR 0.88, 95% CI 0.51 to 1.52). In addition, this same meta‐analysis did not find a significant reduction in the odds of gestational diabetes in the vitamin D group compared to the comparison group (OR 1.05, 95% CI 0.60 to 1.84) (Pérez‐López 2015). With respect to neonatal outcomes, a meta‐analysis including five randomised trials showed significant protective effects of vitamin D supplementation on low birthweight but not on small‐for‐gestational age or preterm birth (Thorne‐Lyman 2012). The meta‐analysis by Pérez‐López 2015 found a significant protective effect of vitamin D supplementation for low birthweight and birth length but not for small‐for‐gestational age, preterm birth or caesarean section.
There are several factors that could explain the lack of consistency among trials and meta‐analyses. Studies have used different doses (from 0 international units (IU) to 600,000 IU), regimens (daily, weekly, monthly or single dose), forms (cholecalciferol‐D3 or ergocalciferol‐D2), delivery vehicles (tablets, liquid/syrup, gummies (chewable form of vitamins) and injections) and combinations (alone, with calcium, with other vitamins and minerals and with fish oil). Also, the timing of supplementation has varied considerably between studies, with only a few studies initiating supplementation very early in pregnancy, while most studies have initiated supplementation in the second trimester and a few in the third trimester. In addition, there is large variability in terms of participants' characteristics enrolled between studies, such as with race/ethnicity, skin colour, pre‐pregnancy body mass index (BMI), dressing patterns, baseline vitamin D status, and physical activity levels. Furthermore, there is large variability in geographical characteristics (latitude and season when supplementation or pregnancy started) and in the analytical assays used to assess serum 25‐hydroxyvitamin D concentrations in blood. Other differences between studies include: health outcomes definition and their cut‐off points used and quality of the trial, such as sequence generation, allocation concealment, blinding of participants, staff and outcome assessors, lack of reporting on attrition, missing data and lack of intention‐to‐treat analyses.
Vitamin D toxicity
In animals, vitamin D supplementation studies have suggested a potential for vitamin D‐induced teratogeneses (birth defects) and adverse effects in the offspring, such as growth restriction, delayed ossification, craniofacial hypoplasia (Ariyuki 1987; Chan 1979; Friedman 1969; Ornoy 1968; Ornoy 1969). In humans, very high levels of vitamin D supplementation (> 10,000 IU/d or 250 μg/d) may lead to hypervitaminosis (very high levels of serum vitamin D) and this could lead to hypercalcaemia (serum calcium levels 10.5 mg/dL or higher) and hypercalciuria (urinary calcium levels > 250 mg/day (Heaney 2008). Short‐term studies (< six months) with vitamin D supplementation have shown a potential increase in the risk of renal and kidney stones (Hathcock 207; Heaney 2008; IOM 2011; Vieth 1999). However, there are only a few studies in pregnant women that have assessed the safety of vitamin D supplementation (4000 IU/d or 200,000 IU once), with no adverse effects having been reported from these high doses (Hollis 2011; Yu 2009).
Description of the intervention
Vitamin D recommendations differ among different organisations. The Recommended Nutrient Intakes (RNI) established by the WHO/Food and Agriculture Organization of the United Nations is 200 IU/d (5 µg/d) of vitamin D for pregnant women (WHO 2004). In contrast, the recommended dietary allowance, as established by the Institute of Medicine in the US is 600 IU/d (15 µg/d) of vitamin D for pregnant women (IOM 2011). This was increased from the previous recommended level to maintain serum 25‐hydroxyvitamin D concentrations greater than 50 nmol/L (20 ng/mL) based on the current studies available for musculoskeletal health. In Europe, the vitamin D recommended level varies by country, from 400 IU/d (10 µg/d) in the UK, the Netherlands, Nordic Council of Ministers, Ireland and France and also as established by the European Commission (Spiro 2014); to 600 IU/d (15 µg/d) in Spain; and 800 IU/d (20 µg/d) in Austria, Belgium, Germany, and Switzerland. However, very recently, the European Food Safety Authority (EFSA) issued for the first time vitamin D recommendations for European pregnant women, at a level similar to the US Institute of Medicine (600 IU/d, 15 µg/d) (EFSA 2016).
Several organisations and groups recommend the use of vitamin D supplements during pregnancy to meet the recommendations. The Royal College of Obstetricians and Gynaecologists recommend 400 IU/d (10 µg/d) for all pregnant women (RCOG 2014). For high‐risk women (dark skin, reduced exposure to sunlight, or those who are socially excluded or obese), they recommend at least 1000 IU/d (25 µg/d). In addition, for women at high risk of pre‐eclampsia, they recommend at least 800 IU/d (20 µg/d), combined with calcium. A panel of 30 experts published in 2013 practical guidelines for the supplementation of vitamin D in Central Europe (Pludowski 2013). During pregnancy, they recommend vitamin D supplementation of 1500 to 2000 IU/d (37.5 to 50.0 µg/d). However, the recent US Dietary Guidelines do not explicitly recommend a vitamin D supplement during pregnancy; except for those with limited sunshine exposure or those who use sunscreen (DGA 2015). In addition, the WHO supplementation guidelines in pregnancy also do not recommend vitamin D supplements as part of routine antenatal care (WHO 2012b), mainly due to lack of evidence and only in cases of vitamin D deficiency, which is in alignment with the American Congress of Obstetricians and Gynecologists guidelines (ACOG 2015).
However, some experts ‐ including the Endocrine Society ‐ recommend that serum 25‐hydroxyvitamin D levels should be maintained at higher levels (greater than 75 nmol/L or 30 ng/mL) for optimal health (Dawnson‐Hughes 2005; Holick 2009). To achieve such levels, higher intakes of vitamin D are needed. It has been proposed that doses as high as 1300 IU/d are needed for individuals of light skin during the winter to achieve serum 25‐hydroxyvitamin D levels at or above 75 nmol/L, while individuals of darker skin colour and low sun exposure need 2100 to 3100 IU/d year round (Hall 2010). Such high doses and even higher doses have been used in recent and on‐going supplementation trials among pregnant women for improving health outcomes. However, the safety of such doses has not been proven, in addition to the dose‐response effects of vitamin D supplementation on various maternal and neonatal health outcomes.
How the intervention might work
Vitamin D has several important functions for maternal health and for fetal development from conception to delivery, as the fetus completely relies on the vitamin D supply of the mother. Such biological actions include regulation of calcium homeostasis, cell proliferation and cell differentiation in multiple target tissues (Sato 2000). These actions are exerted through the vitamin D receptor (VDR), a receptor located in the nuclei of target genes.
Vitamin D functions during pregnancy are integrated across maternal, placental and fetal compartments, as suggested by Gernand 2016. In brief, vitamin D specifically promotes or is involved in implantation, vascularisation of the placenta, placental metabolism, modulation of immune function and neurological development. Also, vitamin D promotes cellular differentiation and apoptosis, optimises fetal skeletal growth and may possibly have an effect on fetal programming (Liu 2012). More specifically, vitamin D has been shown to up‐regulate the production of the antimicrobial peptides by macrophages and endothelial cells (Wang 2004), which may inactivate viruses and suppress inflammation (Cantorna 2008), and subsequently reduce the severity of infections.
All these actions are possible in part through the increase in serum levels of 1,25‐dihydroxyvitamin D (the active form of vitamin D) during pregnancy, which increase from early pregnancy until delivery (Moller 2013), but are particularly high during the first and second trimester (Liu 2012). This active form of vitamin D results from the hydroxylation of 25‐hydroxyvitamin D by the enzyme 1α‐hydroxylase (CYP27B1), which occurs in maternal kidneys (Liu 2012). In addition, serum 1,25‐dihydroxyvitamin D can also be synthesised locally by the placenta as both maternal decidual and fetal placental express the enzyme 1α‐hydroxylase (Liu 2012). Therefore, there are two different actions of vitamin D during pregnancy: an endocrine action via the increase in serum 1,25‐dihydroxyvitamin D levels and more localised autocrine or paracrine actions in the placenta (Liu 2012). The VDR is also present in both maternal decidua and fetal placenta, which is further confirmed by its action on fetal development (Liu 2012). In addition, the action of vitamin D in the placenta does not respond to the catabolic enzyme vitamin D 24‐hydroxylase (CYP24A1), which normally converts 25‐hydroxyvitamin D and 1,25‐dihydroxyvitamin D to less potent metabolites, which maximises the synthesis of 1,25‐dihydroxyvitamin D (Liu 2012) and further highlights the importance of vitamin D in the fetal–maternal interface.
As shown in another Cochrane Review, vitamin D supplementation does increase maternal serum 25‐hydroxyvitamin D concentrations during pregnancy (Palacios 2019). The improvement in serum 25‐hydroxyvitamin D levels may have a direct influence on the fetal and neonatal supply of vitamin D and may influence early placental development and thus, the development of pre‐eclampsia through its role in gene regulation and expression. In the aforementioned review, we also showed that supplementing pregnant women with vitamin D alone probably reduces the risk of pre‐eclampsia, gestational diabetes, low birthweight and may reduce the risk of severe postpartum haemorrhage compared to women receiving placebo or no vitamin D supplementation (Palacios 2019).
Supplementing pregnant women with vitamin D and calcium probably reduces the risk of pre‐eclampsia but may increase the risk of preterm births < 37 weeks (these findings warrant further research). Supplementing pregnant women with vitamin D and other nutrients may make little or no difference in the risk of preterm birth < 37 weeks’ gestation or low birthweight (less than 2500 g).
Why it is important to do this review
There is currently a large controversy about the optimal regimen of vitamin D supplementation for women during pregnancy. As stated by the Working Group convened by the Sackler Institute for Nutrition Science at the New York Academy of Sciences and the Bill & Melinda Gates Foundation (in co‐ordination with a scientific organising committee to assess the global prevalence and disease burden of vitamin D deficiency), vitamin D affects pregnancy and birth outcomes, but evidence is conflicting and there is no consensus on the vitamin D dose to maximise maternal and infant benefits (Roth 2018).
This review evaluates the available evidence to try to elucidate the most clinically relevant, yet safe, regimens of vitamin D during pregnancy for improving different pregnancy health outcomes at the population level. Although there are a few other meta‐analyses that have evaluated the effects of vitamin D supplementation during pregnancy on various maternal and neonatal health outcomes, there are several studies that have been recently published or that are in progress. In addition, no previous meta‐analysis has taken into account the regimen effect of vitamin D. Different regimens of vitamin D during pregnancy may have different effects in tissues or systems. No studies have evaluated the possibility of a U‐shaped response, in which there could be an increase in the risk of adverse prenatal and neonatal health outcomes at low dose but also at a high vitamin D dose. Also, it is unknown how safe some of the vitamin D supplementation levels used in pregnancy are, as this has not been systematically evaluated. Therefore, there is enough evidence to test the effect of different regimens of vitamin D supplementation during pregnancy on several maternal and neonatal health outcomes and its safety. Results from this review could contribute to establish practice guidelines at the population level.
Objectives
To assess the effects and safety of different regimens of vitamin D supplementation alone or in combination with calcium or other vitamins, minerals or nutrients during pregnancy, specifically doses of 601 international units per day (IU/d) or more versus 600 IU/d or less; and 4000 IU/d or more versus 3999 IU/d or less.
Methods
Criteria for considering studies for this review
Types of studies
This protocol was published in Prospero in 2018 https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=103763.
Briefly, we included both randomised and quasi‐randomised trials. We planned to include cluster‐randomised trials but none were found. Also, we intended to include studies presented as abstracts, but they did not have enough information for data extraction; therefore, abstracts were categorised as on‐going until the trial is published. We did not include cross‐over trials or other levels of evidence (e.g. cohort or case‐control studies) in this meta‐analysis, but we considered such evidence in the discussion where relevant.
Types of participants
We included trials only among pregnant women of any gestational or chronological age, race/ethnicity, skin colour, and pre‐pregnancy body mass index. Pregnant women with pre‐existing conditions were excluded. In addition, only trials with singleton pregnancy were included, as multiple pregnancies are associated with higher vitamin D deficiency compared to singleton pregnancies (Goswami 2016).
Types of interventions
We included trials on vitamin D supplementation during pregnancy irrespective of the regimen (dose, frequency, duration, or time of commencement of supplementation during pregnancy). However, we only included trials that compared a higher dose to a lower dose, not to placebo, as those trials were already included in the previous Cochrane Review (De Regil 2016), which was recently updated (Palacios 2019). We included trials testing vitamin D in combination with other nutrients as long as the intervention and the control group were treated similarly, except for the dose of vitamin D.
We sought to address the following two questions.
1. Is it better to supplement pregnant women with more than the current vitamin D recommendation (200 IU/d to 600 IU/d) for pregnancy and neonatal health outcomes?
A cut‐off of 600 IU/d was chosen as most countries, organisations, associations recommend vitamin D in a range of 200 IU/d to 600 IU/d during pregnancy. Therefore, this review intended to evaluate whether giving higher doses of vitamin D than usually recommended, results in better maternal and neonatal outcomes when compared to recommended levels. 2. Are there negative health effects of supplementing pregnant women with more than the current upper limit recommendation of vitamin D (4000 IU/d)?
A cut‐off of 4000 IU/d was chosen as this is the upper limit established by the Institute of Medicine in the US (IOM 2011).
To answer these questions, we used the following comparisons.
1. 601 IU or more versus 600 IU or less of vitamin D alone or with any other nutrient
Within this comparison, we also evaluated:
601 IU or more versus 600 IU or less of vitamin D alone;
601 IU or more versus 600 IU or less of vitamin D alone + Ca;
601 IU or more versus 600 IU or less of vitamin D alone + other vitamin/mineral.
2. 4000 IU/d or more versus 3999 IU/d or less of vitamin D alone or with any other nutrient
We included studies using supplements provided by tablets, or given in liquid form, syrup, capsules or injection.
Types of outcome measures
We included maternal antenatal, clinical and laboratory outcomes and infant clinical and laboratory outcomes as described below.
Primary outcomes
Maternal
Pre‐eclampsia (defined as persistent diastolic blood pressure > 90 mm Hg with the occurrence of substantial proteinuria (> 0.3 g of protein in 24 hours) (WHO 2011)
Gestational diabetes (GDM: defined as having one or more of the following criteria: fasting plasma glucose ≥ 7.0 mmol/L (126 mg/dL), two‐hour plasma glucose ≥ 11.1 mmol/L (200 mg/dL) following a 75 g oral glucose load or random plasma glucose ≥ 11.1 mmol/L (200 mg/dL) in the presence of diabetes symptoms (WHO 2013)
Any adverse events (e.g. hypercalcaemia, kidney stones)
Infant
Preterm birth (defined as birth occurring before 37 completed weeks of gestation) (WHO 2012a)
Low birthweight (defined as weight at birth of less than 2500 g) (UNICEF/WHO 2004)
Secondary outcomes
Maternal
Fasting glucose levels (mg/dL)
Caesarean section
Maternal death (death while pregnant or within 42 days of pregnancy termination)
Serum 25‐hydroxyvitamin D concentration at term (in nmol/L)
Gestational hypertension (as defined by trialists)
Infant
Birth length (cm)
Head circumference at birth (cm)
Birthweight (g).
Serum 25‐hydroxyvitamin D concentration in cord blood (in nmol/L)
Admission to special care (including intensive care) during the neonatal period (within 28 days after delivery)
Perinatal death
Stillbirth (as defined by trialists)
Neonatal death (within 28 days after delivery)
Apgar score less than seven at five minutes
Neonatal infection (e.g. respiratory infections within 28 days after delivery)
Very preterm birth (less than 32 weeks' gestation)
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (12 July 2018).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (12 July 2018) using the search terms given in Appendix 1.
Searching other resources
For the identification of ongoing and unpublished studies, we contacted different institutions including the WHO Departments of Reproductive Health and Research, of Nutrition for Health and Development, and of Maternal, newborn, child and adolescent health, the WHO regional offices, WHO Collaborating Centers in Nutrition and Reproductive Health, UNICEF, the Micronutrient Initiative (MI), the Global Alliance for Improved Nutrition (GAIN), the US Centers for Disease Control and Prevention (CDC) and the Vitamin D Workshop (15 May 2018).
We did not apply any language or date restrictions.
Data collection and analysis
The following methods section of this review was based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors (CP and JPPR) independently assessed for inclusion all the potential studies that were identified as a result of the search strategy. A third review author resolved disagreements (MATF).
We created a study flow diagram to map out the number of records identified, included and excluded (Figure 1).
1.
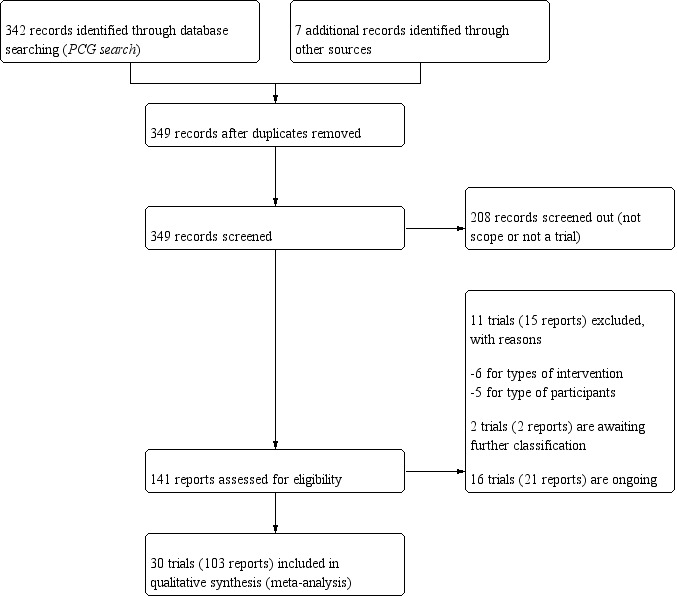
Study flow diagram.
Data extraction and management
We used the extraction form adapted from “Good practice templates” developed by the Cochrane Editorial Resources Committee http://training.cochrane.org/authors/presentations/collecting‐data to extract data. For eligible studies, three review authors extracted the data. We resolved discrepancies through discussion. We entered the data into Review Manager software (RevMan 2014) and checked for accuracy.
We extracted the following.
Methods
Participants
Interventions
Outcomes
Trial funding
Trial dates
Trialists' declarations of interest
Notes
We attempted to contact several authors of the original reports to provide further details. A few responded with additional information on registry of the trial and details about standard deviation and number of participants per group, when missing.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion.
(1) Random sequence generation (checking for possible selection bias)
We described for each included trial the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included trial the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included trial the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classesof outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
We classified blinding as ’high risk of bias’ if the blinding status of a trial was unclear or the trial was open.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included trial the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included trial, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we included missing data in the analyses.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
We considered follow‐up to be ’low risk of bias’ if more than 80% of participants initially randomised in a trial were included in the analysis and any loss was balanced across groups, unclear if the percentage of initially randomised participants included in the analysis was unclear, and ’high risk of bias’ if less than 80% of those initially randomised were included in the analysis or if loss was imbalanced in different treatment groups.
(5) Selective reporting (checking for reporting bias)
We described for each included trial how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included trial any important concerns we hd about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias, as:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
We assessed the quality of the evidence using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes (this was done for the two main comparisons).
Pre‐eclampsia
Gestational diabetes
Any adverse effects
Preterm birth
Low birthweight
We used GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) to create the ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes were produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way in the trials. In future updates, as appropriate, we will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We planned to include cluster‐randomised trials in the analyses along with individually‐randomised trials. No cluster trials were identified for inclusion in this review. In future updates, if cluster trials are included we will adjust the standard errors of the results using the methods described by Higgins 2011 using an estimate of the intra‐cluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study within a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. For cluster‐randomised trials and individually‐randomised trials, we will synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Studies with more than two treatment groups
For studies with more than two intervention groups (multi‐arm studies), we combined groups to create a single pair‐wise comparison (Higgins 2011) and included the disaggregated data in the corresponding subgroup category. When the control (lowest dose) group was shared by two or more trial arms, we divided the control (lowest dose) group (events and total population) over the number of relevant subgroup categories to avoid double counting the participants. The details are described in the Characteristics of included studies tables.
Cross‐over trials
We did not consider cross‐over trials.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses on an intention‐to‐treat basis, i.e. we included all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² is greater than 30% and either a Tau² is greater than zero, or there is a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
We planned to investigate reporting biases (such as publication bias) by using funnel plots for the primary outcomes with 10 or more studies. However, none of the primary outcomes had 10 or more studies.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We intended to use fixed‐effect meta‐analysis for combining data where it would be reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. Since we detected substantial statistical heterogeneity, we used random‐effects meta‐analysis to produce an overall summary of an average treatment effect across trials. We treated the random‐effects summary as the average of the range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. As we used random‐effects analyses, we present the results as the average treatment effect with its 95% confidence interval, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We investigated substantial heterogeneity on the primary outcomes by using subgroup analyses, only if more than four trials reported that outcome. We did this for the two main comparisons, Comparison 1 (601 IU or more versus 600 IU or less of vitamin D alone or with any other nutrient) and Comparison 2 (4000 IU/d or more versus 3999 IU/d or less of vitamin D alone or with any other nutrient), as follows.
1. By time of commencement of supplementation
Less or equal to 20 weeks
Beyond 20 weeks of gestation
Mixed/unknown/other
2. By frequency of supplementation
Daily
Weekly/monthly
Bolus dose
3. By pre‐pregnancy body mass index (kg/m2)
Underweight (lower than 18.5)
Normal weight (18.5 to 24.9)
Overweight (25 or higher)
Unknown/mixed
4. By skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988)
Three or less
Four or more
Mixed/unknown
5. By latitude
Between the Tropics of Cancer and Capricorn
North of the Tropic of Cancer or south of the Tropic of Capricorn
6. By season at the start of pregnancy
Summer
Fall
Winter
Mixed/unknown/unreported
7. By registry in of the international registries
Registered
Not registered
8. By impact factor of the journal (using the scores from the Journal Citation Reports 2017 and the Scientific Journal Rankings (SCImago) 2017). This subgroup analysis was performed due to the rise of predatory journals (journals with high publications fees to authors without checking articles for quality and legitimacy and without providing the other editorial and publishing services associated with legitimate journals). We used journal ranking as a surrogate or indirect way of checking the quality of the journal.
Medium to high (if the journal had a score greater than 2.0 in the Journal Citation Report and greater than 1.0 in the Scientific Journal Rankings)
Low (if below the above mentioned cut‐points)
9. By vitamin D status at baseline (as defined by the trialists)
Low vitamin D status
Not low vitamin D status
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We reported the results of subgroup analyses quoting the Chi2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
Planned sensitivity analysis was restricted to primary outcomes.
We conducted a sensitivity analysis based on the quality of the studies. We considered a study to be of high quality if it was assessed as having low risk of bias in both the randomisation and allocation concealment and additionally a low risk of bias in either blinding or losses to follow‐up. Conversely, we considered a study to be low quality if it was assessed as having high risk of selection bias in both the randomisation and allocation concealment. The studies that were not classified as high or low quality were classified as unclear quality. From all selected studies used for the primary outcomes analysis, only two studies were classified as low quality: Das 2010; O'Brien 2013.
We planned to conduct sensitivity analysis to investigate the effects of the randomisation unit where we combined data from cluster‐randomised controlled trials along with data from the individually‐randomised controlled trials, but no trial with such characteristics was included in the analysis for primary outcomes. This was also the case for the planned sensitivity analysis on the effects of including studies with missing data.
Results
Description of studies
Results of the search
See: Figure 1.
We retrieved 134 records from the search of Cochrane Pregnancy and Childbirth’s Trials Register and seven additional records identified through other sources. From these, we included in this review data from 30 trials (103 reports), involving 7289 participants (Abotorabi 2017; Bacqui 2009; Bhatia 2012; Das 2010; Dawodu 2013; de Menibus 1984; Grant 2010; Hashemipour 2014; Kalra 2012; Karamali 2015; Kiely 2015; Mallet 1986; March 2010; Marya 1981; Mir 2016; Mojibian 2015; Mutlu 2014; O'Brien 2013; Rostami 2017; Roth 2013; Shakiba 2013; Soheilykhah 2011; Stephensen 2011; Thiele 2014; Wagner 2006a; Wagner 2006b; Wagner 2013; Weiss 2009; Yap 2014; Yu 2008). We excluded 11 trials (15 reports) (Ali 2018; Azami 2017; Bisgaard 2009; Hajihashemi 2016; Jamilian 2017; Li 2000; Omotayo 2017; Roth 2016; Sablok 2015; Wheeler 2016; Zhang 2016). We also identified 16 ongoing or unpublished trials (21 reports) (El‐Hajj Fuleihan 2015; Garreto 2016; Hantoshzadeh 2017; Hartman 2010; Hoffman 2017; Judkins 2011; Kachhawa 2014; Lalooha 2012; McCann 2016; McLean 2012; Mosalanejad 2016; Nausheen 2018; Neyestani 2016; Nouripour 2016; Rasmussen 2009; Rich‐Edwards 2015) and two trials are awaiting classification (Gerais 2015; Mobasheri 2016). We identified this study (Mobasheri 2016) that raised concerns with the veracity of the information in relation to the high dose of vitamin D provided daily to participants. We followed the guidelines from the Committee on Publication Ethics (COPE) to investigate the issue with the editors of the journal (Cope 2016) and contacted both the corresponding author of the publication and the editor. There was no response from either and no further reference exists of this publication. For this reason we are placing this reference as “awaiting assessment” in this version and if there is no further clarification will exclude it in a future update, given the implausible dose reported.
Details of these trials are provided in: Characteristics of included studies; Characteristics of excluded studies; Studies awaiting classification; Characteristics of ongoing studies tables.
Included studies
Settings
The trials included in this review were carried in different years; three trials were conducted in 1979‐1983 (de Menibus 1984; Mallet 1986; Marya 1981), but most were done between 2004‐2017. Two trials did not specify when the intervention was implemented (Kalra 2012; Shakiba 2013).
Trials were conducted in Australia (Yap 2014), Bangladesh (Bacqui 2009; Roth 2013), Canada (March 2010), France (de Menibus 1984; Mallet 1986), India (Bhatia 2012; Das 2010; Kalra 2012; Marya 1981; Mir 2016), Iran (Abotorabi 2017; Hashemipour 2014; Karamali 2015; Mojibian 2015; Rostami 2017; Shakiba 2013; Soheilykhah 2011), Ireland (Kiely 2015), New Zealand (Grant 2010), Turkey (Mutlu 2014), United Arab Emirates (Dawodu 2013), United Kingdom (Yu 2008) and United States (O'Brien 2013; Stephensen 2011; Thiele 2014; Wagner 2006a; Wagner 2006b; Wagner 2013, Weiss 2009).
Latitude
All trials were conducted either above or below the Tropics of Cancer and Capricorn.
Seasonality
The seasons varied among trials with some trials occurring all year round (Dawodu 2013; Grant 2010; March 2010Mojibian 2015; O'Brien 2013; Roth 2013; Stephensen 2011; Wagner 2006b), during the fall‐winter (de Menibus 1984; Shakiba 2013), winter (Bacqui 2009; Mallet 1986); spring (Mutlu 2014), spring‐summer period (Thiele 2014), summer (Karamali 2015; Mir 2016); summer‐fall (Rostami 2017), winter and summer (Kiely 2015), and unknown/unreported (Abotorabi 2017; Bhatia 2012; Das 2010; Hashemipour 2014; Kalra 2012; Marya 1981; Soheilykhah 2011; Wagner 2006a; Wagner 2013; Weiss 2009; Yap 2014; Yu 2008).
Participants
The sample size from all the trials ranged between 16 (Thiele 2014) to 1300 pregnant women (Roth 2013).
Pre‐gestational body‐mass index (kg/m2)
Pre‐gestational body mass index (BMI) of the participants was reported only in nine trials (Hashemipour 2014; Karamali 2015; Soheilykhah 2011; Stephensen 2011; Thiele 2014; Wagner 2006a; Wagner 2006b; Wagner 2013; Yap 2014). The remainder of the trials did not report this (Abotorabi 2017; Bacqui 2009; Bhatia 2012; Das 2010; Dawodu 2013; de Menibus 1984; Grant 2010; Kalra 2012; Kiely 2015; Mallet 1986; March 2010; Marya 1981; Mir 2016; Mojibian 2015; Mutlu 2014; O'Brien 2013; Rostami 2017; Roth 2013; Shakiba 2013; Weiss 2009; Yu 2008). Karamali 2015 stratified the intervention groups by BMI (< 25 kg/m2 and ≥ 25 kg/m2).
Skin pigmentation based on Fitzpatrick skin tone chart
None of the trials used the Fitzpatrick skin tone chart; however, several trials reported the ethnicity/race of participants. Most trials were among Middle Eastern (Abotorabi 2017; Bacqui 2009; Bhatia 2012; Dawodu 2013; Karamali 2015; Hashemipour 2014; Mutlu 2014; Rostami 2017; Shakiba 2013; Yu 2008) or South Asian (Das 2010; Kalra 2012; Marya 1981; Mir 2016Roth 2013; Yu 2008) pregnant women. Two trials reported that participants were from mixed ethnicity (Wagner 2006a; Wagner 2006b), Five trials recruited white/European women (de Menibus 1984; Kiely 2015; Mallet 1986; March 2010; Thiele 2014), two were among black women (O'Brien 2013; Wagner 2013), and one was among Pacific, European and Maori women (Grant 2010). Two trials did not report the characteristics of the participants in terms of ethnicity or origin (Stephensen 2011; Weiss 2009).
Methods
All trials started as randomised‐controlled clinical trials. In the case of Das 2010, the authors reported that randomisation was abandoned. However, only 17 trials were reported as double‐blinded (Bhatia 2012; Dawodu 2013; Grant 2010; Hashemipour 2014; Karamali 2015; Kiely 2015; March 2010; O'Brien 2013; Rostami 2017; Roth 2013; Stephensen 2011; Thiele 2014; Wagner 2006a; Wagner 2006b; Wagner 2013; Weiss 2009; Yap 2014). The rest were not blinded (Bacqui 2009; Mallet 1986; Mir 2016; Mojibian 2015; Mutlu 2014; Soheilykhah 2011; Yu 2008) or did not specifically report if blinded (Abotorabi 2017; Das 2010; de Menibus 1984; Marya 1981; Shakiba 2013).
Outcomes
Pre‐eclampsia was reported in Karamali 2015; Mojibian 2015; Rostami 2017; Stephensen 2011; Weiss 2009; Yap 2014.
Gestational diabetes was reported in Hashemipour 2014; Mojibian 2015; Rostami 2017; Roth 2013; Stephensen 2011; Wagner 2006a; Yap 2014.
Adverse effects were reported in Abotorabi 2017, Bhatia 2012; Bacqui 2009; Das 2010; Dawodu 2013; Hashemipour 2014; Kalra 2012; Karamali 2015Kiely 2015; March 2010; Marya 1981; Mojibian 2015; Mutlu 2014; O'Brien 2013; Rostami 2017; Roth 2013; Soheilykhah 2011; Thiele 2014; Stephensen 2011; Wagner 2006a; Wagner 2006b; Weiss 2009; Yap 2014.
Preterm birth was reported in Bacqui 2009; Karamali 2015; Mojibian 2015; Rostami 2017; Roth 2013; Wagner 2006a; Weiss 2009.
Low birthweight was reported in Karamali 2015; Mojibian 2015; O'Brien 2013; Roth 2013.
Fasting glucose levels were reported only by Soheilykhah 2011.
Caesarean section was reported in Bacqui 2009; Rostami 2017; Roth 2013; Stephensen 2011; Wagner 2006a; Wagner 2006b; Weiss 2009; Yap 2014.
Maternal death was reported only by Roth 2013.
Maternal vitamin D concentration at term was reported in Abotorabi 2017; Bacqui 2009; Bhatia 2012; Das 2010; Dawodu 2013; Hashemipour 2014; Karamali 2015; Kiely 2015; March 2010; Mojibian 2015; Mutlu 2014; O'Brien 2013; Rostami 2017; Roth 2013; Soheilykhah 2011; Thiele 2014; Wagner 2006b; Weiss 2009.
Gestational hypertension was reported in Mojibian 2015; Roth 2013; Stephensen 2011; Wagner 2006a; Yap 2014.
Length at birth was reported in Abotorabi 2017; Bhatia 2012; Dawodu 2013; Hashemipour 2014; Kalra 2012; Karamali 2015; Mojibian 2015; Rostami 2017; Roth 2013; Soheilykhah 2011; Thiele 2014; Wagner 2006a; Wagner 2006b; Weiss 2009.
Head circumference at birth was reported in Abotorabi 2017; Dawodu 2013; Hashemipour 2014; Kalra 2012; Karamali 2015; Mojibian 2015; Rostami 2017; Roth 2013; Thiele 2014; Wagner 2006b; Weiss 2009.
Birthweight was reported in Abotorabi 2017; Bacqui 2009; Bhatia 2012; Dawodu 2013; Hashemipour 2014; Kalra 2012; Karamali 2015; Marya 1981; Mojibian 2015; Mutlu 2014; O'Brien 2013; Rostami 2017; Roth 2013; Stephensen 2011; Thiele 2014; Wagner 2006a; Wagner 2006b; Weiss 2009.
Cord blood vitamin D concentration was reported in Bacqui 2009; Bhatia 2012; Dawodu 2013; Hashemipour 2014; Kiely 2015; March 2010; Mojibian 2015; Rostami 2017; Roth 2013; Weiss 2009; Yap 2014.
Admission to special care unit was reported in Wagner 2006b; Weiss 2009.
Perinatal death was not reported by any of the trials.
Stillbirth was reported in Rostami 2017; Roth 2013; Weiss 2009; Yap 2014.
Neonatal death was reported in Bacqui 2009; Roth 2013; Weiss 2009.
Apgar score less than seven at five minutes was reported only by Stephensen 2011.
Neonatal infection was not reported by any of the trials.
Very preterm birth was reported in Roth 2013; Weiss 2009.
Dose and vitamin D form
The doses of vitamin D and the regimens used varied considerably in the included trials. A total of 24 trials used daily doses (Abotorabi 2017; Bhatia 2012; Dawodu 2013; de Menibus 1984; Grant 2010; Hashemipour 2014; Karamali 2015; Kiely 2015; Mallet 1986; March 2010; Marya 1981; Mir 2016; Mojibian 2015; Mutlu 2014; O'Brien 2013; Soheilykhah 2011; Stephensen 2011; Thiele 2014; Wagner 2006a; Wagner 2006b; Wagner 2013; Weiss 2009; Yap 2014; Yu 2008). These doses ranged for the low‐dose comparison group from 200 IU/d (Soheilykhah 2011), 400 IU (Abotorabi 2017; Bhatia 2012; Dawodu 2013; Hashemipour 2014; Karamali 2015; Kiely 2015; March 2010; Mojibian 2015; Stephensen 2011; Thiele 2014; Wagner 2006b; Weiss 2009; Yap 2014), 600 IU (Mutlu 2014; O'Brien 2013), 800 IU/d (Yu 2008), 1000 IU/d (de Menibus 1984; Grant 2010; Mallet 1986Mir 2016), 1200 IU/d (Marya 1981) to 2000 IU/d (Wagner 2006a; Wagner 2013). The higher doses used as the intervention group were: 800 IU/d (Kiely 2015), 1000‐1200 IU/dt o IU/d (March 2010; Mutlu 2014), 2000 IU/d to 2400 IU/d (Dawodu 2013; Grant 2010; March 2010; Mir 2016; Mutlu 2014; O'Brien 2013; Stephensen 2011; Wagner 2006b), 4000 IU/d to 4999 IU/d (Dawodu 2013; Thiele 2014; Wagner 2006a; Wagner 2006b; Wagner 2013; Weiss 2009), and 5000 IU/d (Yap 2014).
Ten trials used weekly/monthly doses (Abotorabi 2017; Bacqui 2009; Bhatia 2012; Hashemipour 2014;Karamali 2015; Mojibian 2015; Rostami 2017; Roth 2013; Shakiba 2013; Soheilykhah 2011). These doses varied from 4200 IU, 16,800 IU or 28,000 IU per week (Roth 2013), 14,000 IU/week or 35,000 IU/week (Bacqui 2009), 50,000 IU every four weeks (Shakiba 2013; Soheilykhah 2011), 60,000 IU every four or eight weeks (Bhatia 2012), 50,000 IU every two weeks (Karamali 2015; Mojibian 2015; Shakiba 2013; Soheilykhah 2011), or 50,000 IU per week (Abotorabi 2017; Hashemipour 2014). Bacqui 2009 also had a group that received 70,000 IU on day 0 and then 35,000 IU/week, and Shakiba 2013 had a third group that received 50,000 IU per week for four weeks and then 50,000 IU every four weeks.
Six trials used single/bolus dose (Das 2010; de Menibus 1984; Kalra 2012; Mallet 1986; Marya 1981; Yu 2008). Das 2010 provided 60,000 IU in the 5th month of pregnancy or 120,000 IU in the 5th and 7th months of pregnancy. Kalra 2012 provided a single dose of 60,000 IU in the 2nd trimester or a dose of 300,000 IU in the 2nd, and again in the 3rd trimester of pregnancy. de Menibus 1984, Mallet 1986 and Yu 2008 provided 200,000 IU in the 3rd trimester of pregnancy. Marya 1981 provided a dose of 600,000 IU in the 7th month and again in the 8th month of pregnancy.
Rostami 2017 used a combination of weekly, monthly and bolus dose, which varied from 50,000 IU oral D3 weekly for six or 12 weeks and/or 50,000 IU D3 per month until delivery to one or two doses of 300,000 IU D3 intramuscularly and/or 50,000 IU of oral D3 per month until delivery.
In four trials, the initial levels of serum 25(OH)D were taken into account for assigning the intervention (Rostami 2017; Shakiba 2013; Wagner 2006a; Yap 2014). In Rostami 2017, mothers were defined as severely deficient (if serum 25(OH)D levels were < 10 ng/mL), moderately deficient (if serum 25(OH)D levels were 10 ng/mL to 20 ng/mL), and normal status (if serum 25(OH)D were > 20 ng/mL); this latter group served as controls. Among those with moderate deficiency, participants received either 50,000 IU oral D3 weekly for six weeks and/or 50,000 IU D3 per month until delivery or a single dose of 300,000 IU D3 intramuscularly and/or 50,000 IU of oral D3 per month until delivery. Among those with severe deficiency, participants received 50,000 IU of oral D3 weekly for 12 weeks and or 50,000 IU of oral D3 per month until delivery or two doses of 300,000 IU D3 intramuscularly and/or 50,000 IU of oral D3 per month until delivery. In Shakiba 2013, 17 out of the 51 participants had serum 25(OH)D levels < 20 ng/mL; they were allocated to receive 200,000 IU (50,000 IU/week for four weeks), followed by supplementation with 50,000 IU/month. In Wagner 2006a, randomisation to 2000 IU/d or 4000 IU/d of vitamin D3 was stratified using a cut‐off point of 32 ng/mL for the initial 25(OH)D level. In the trial by Yap 2014, only pregnant women with levels < 32 ng/mL (80 nmol/L) were randomly assigned to receive either 5000 IU vitamin D3 daily (HD) or 400 IU daily (LD).
The vitamin D was provided in the form of cholecalciferol‐D3 in most trials (Abotorabi 2017; Bacqui 2009; Bhatia 2012; Das 2010; Dawodu 2013; Grant 2010; Hashemipour 2014; Kalra 2012; Karamali 2015; Kiely 2015; March 2010; Mir 2016; Mojibian 2015; Mutlu 2014; O'Brien 2013; Rostami 2017; Roth 2013; Shakiba 2013; Soheilykhah 2011; Stephensen 2011; Thiele 2014; Wagner 2006a; Wagner 2006b; Wagner 2013; Weiss 2009; Yap 2014), and as ergocalciferol‐D2 in four trials (de Menibus 1984; Mallet 1986; Marya 1981; Yu 2008). Two trials did not report the vitamin D form used (Mir 2016; Soheilykhah 2011).
Start of supplementation
A total of eight trials started supplementation during the first trimester (before week 13) (Dawodu 2013; Mojibian 2015; O'Brien 2013; Rostami 2017; Soheilykhah 2011; Wagner 2006a; Wagner 2006b; Wagner 2013). Eight trials started between week 13 and before week 20, half‐way through pregnancy (Bhatia 2012; Kiely 2015; March 2010; Mir 2016; Mutlu 2014; Stephensen 2011; Weiss 2009; Yap 2014). Roth 2013 started supplementation between week 17 and 24 of gestation. The rest of the trials started supplementation after 20 weeks' gestation (Abotorabi 2017; Bacqui 2009; Das 2010; de Menibus 1984; Grant 2010; Hashemipour 2014; Kalra 2012; Karamali 2015; Mallet 1986; Marya 1981; Shakiba 2013; Thiele 2014; Yu 2008).
Duration of supplementation
Trials varied widely in the duration of supplementation and most did not specify exactly how long it lasted. Approximately, based on the specified gestational week at recruitment and/or randomisation, we calculated that five trials were only six to less than 12 weeks long (Abotorabi 2017; Bacqui 2009; Grant 2010; Hashemipour 2014; Marya 1981). Eight trials were about 12 to less than 20 weeks long (de Menibus 1984; Karamali 2015; Mallet 1986; Mir 2016; O'Brien 2013; Thiele 2014; Stephensen 2011; Yu 2008). Only 13 trials provided supplementation for more than 20 weeks (Bhatia 2012; Dawodu 2013; Kalra 2012; Kiely 2015; March 2010; Mojibian 2015; Shakiba 2013; Soheilykhah 2011; Wagner 2006a; Wagner 2006b; Wagner 2013; Weiss 2009; Yap 2014). Three trials were about eight to 27 weeks long, but it was not clear exactly how long it was as participants were recruited at different stages of pregnancy (Das 2010; Mutlu 2014; Roth 2013). In the case of Rostami 2017, some groups had a duration of six weeks, 12 weeks or more than 20 weeks.
Form of supplementation
Most trials gave vitamin D as a capsule or tablet (Abotorabi 2017; Dawodu 2013; Hashemipour 2014; Kalra 2012; Karamali 2015; Kiely 2015; Mallet 1986; March 2010; Marya 1981; Mir 2016; Mojibian 2015; O'Brien 2013; Roth 2013; Thiele 2014; Wagner 2006a; Wagner 2006b; Wagner 2013; Weiss 2009; Yap 2014). Two trials provided the supplementation through drops or liquid supplement (Bacqui 2009; Mutlu 2014), and one trial used sachets (Bhatia 2012). Rostami 2017 used both capsule/tablet and intramuscular injection. The following trials only specified that vitamin D supplementation was given orally (Das 2010; Grant 2010), and five trials did not specify the form (de Menibus 1984; Shakiba 2013; Soheilykhah 2011; Stephensen 2011; Yu 2008).
Total vitamin D dose provided
We estimated the total amounts of vitamin D given in pregnancy in IU per day based on the level of supplementation stated in each trial, the start of initiation of the supplementation and duration of the supplementation. The approximate lowest dose used as the comparison group were: 200 IU (O'Brien 2013; Soheilykhah 2011), 400 IU (Abotorabi 2017; Bhatia 2012; Das 2010; Dawodu 2013; Hashemipour 2014; Kalra 2012; Karamali 2015; Kiely 2015; March 2010; Mojibian 2015; Stephensen 2011; Thiele 2014; Wagner 2006b; Weiss 2009; Yap 2014), 600 IU (Mutlu 2014; Roth 2013), 800 IU/d (Yu 2008), 1000 IU/d (de Menibus 1984; Grant 2010; Mallet 1986Mir 2016), 1200 IU/d (Marya 1981), 1600 IU/d (Rostami 2017; Shakiba 2013), 2000 IU/d (Bacqui 2009; Wagner 2006a; Wagner 2013), and 3000‐3400 IU/d (Rostami 2017).
The approximate highest dose used as the intervention group were: 800 IU/d (Kiely 2015), 1000 IU/d to 1500 IU/d (Bhatia 2012; March 2010; Mutlu 2014), 1600 IU/d (Das 2010) 2000 IU/d to 2999 IU/d (Bhatia 2012, Dawodu 2013; de Menibus 1984; Grant 2010; Mallet 1986; March 2010; Mir 2016; Mutlu 2014; O'Brien 2013; Roth 2013; Soheilykhah 2011; Stephensen 2011; Wagner 2006b; Yu 2008), 3000 IU/d to 3999 IU/d (Mojibian 2015; Shakiba 2013), IU/d 4000 to 4999 IU/d (Dawodu 2013; Kalra 2012; Karamali 2015; Rostami 2017; Roth 2013; Soheilykhah 2011; Thiele 2014; Wagner 2006a; Wagner 2006b; Wagner 2013; Weiss 2009), 5000 to IU/d 5999 IU/d (Yap 2014), 6000 to 6999 IU/d (Bacqui 2009Marya 1981), and 7000 IU/d to 7999 IU/d (Abotorabi 2017; Hashemipour 2014).
In total, 19 trials were included in the comparison of 601 IU or more compared to 600 IU or less of vitamin D supplementation (Comparison 1: Abotorabi 2017; Bhatia 2012; Das 2010; Dawodu 2013; Hashemipour 2014; Kalra 2012; Karamali 2015; Kiely 2015; March 2010; Mojibian 2015; Mutlu 2014; O'Brien 2013; Roth 2013; Soheilykhah 2011; Stephensen 2011; Thiele 2014; Wagner 2006b; Weiss 2009; Yap 2014).
A total of 15 trials contributed data to the comparison of 4000 IU or more versus 3999 IU or less of vitamin D (Comparison 2: Abotorabi 2017; Bacqui 2009; Dawodu 2013; Hashemipour 2014; Kalra 2012; Karamali 2015; Marya 1981; Rostami 2017; Roth 2013; Soheilykhah 2011; Thiele 2014; Wagner 2006a; Wagner 2006b; Weiss 2009; Yap 2014).
From the trials included in the comparison of 601 IU or more compared to 600 IU or less of vitamin D supplementation, six trials provided vitamin D alone (Kalra 2012;Kiely 2015; Mojibian 2015; Mutlu 2014; Soheilykhah 2011; Yap 2014), 2 trials provided vitamin D plus calcium (Abotorabi 2017; Bhatia 2012), and 11 trials provided vitamin D with other vitamins and/or minerals (Das 2010; Dawodu 2013; Hashemipour 2014; Karamali 2015; March 2010; O'Brien 2013; Roth 2013; Stephensen 2011; Thiele 2014; Wagner 2006b; Weiss 2009).
Six trials did not provide data for any of the established comparisons: de Menibus 1984; Grant 2010; Mallet 1986; Mir 2016; Shakiba 2013; Yu 2008, as the lower dose was not below 600 IU/d or the higher dose was not above 4000 IU/d. Also, the trial by Wagner 2013 has been completed and some results have been reported but none that contribute to this review.
Other nutrients provided
The only two trials that provided vitamin D and calcium alone used 1 g/day of calcium carbonate (Bhatia 2012) or 250 mg/day of calcium (Abotorabi 2017). Ten trials provided a multi‐vitamin, multi‐mineral prenatal supplement (Dawodu 2013; Hashemipour 2014; Karamali 2015; March 2010; O'Brien 2013; Roth 2013; Stephensen 2011; Thiele 2014; Wagner 2006b; Weiss 2009). Das 2010 provided vitamin D and iron. Three trials provided vitamin D, iron and folic acid (Bacqui 2009; Karamali 2015; Roth 2013). For more details on the level of other nutrients provided, see Table 3.
1. Vitamin D, and other vitamins and minerals supplementation profile in included studies.
| Study | Week of gestation when supplementation started | Number of weeks intervention | Type of delivery | Vitamin D | Vitamin D dose (IU/d: daily calculated) | Type of vitamin D (D2 or D3) | Standard prenatal/antenatal supplement (multivitamins + multiminerals) | Calcium (mg) per day | Elemental iron (mg) per day | Folic acid (µg) per day | Vitamin A (IU) per day | Beta‐carotene (IU) per day | Vitamin B1 (thiamine: mg) per day | Vitamin B2 (riboflavine: mg) per day | Vitamin B3 (niacin: mg) per day | Vitamin B6 (IU) per day | Vitamin B12 (µg) per day | Vitamin C (mg) per day | Vitamin E (IU) per day | Vitamin K1 (µg) per day | Zinc (mg) per day | Magnesium (mg) per day | Iodine (mg) per day | Potassium (mg) per day |
| Abotorabi 2017 | 27‐31 weeks | 8 weeks | capsules/pills/tablets | 50,000 IU/week + 400 IU/d | ˜7500 | D3 | Yes | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 27‐31 weeks | 8 weeks | capsules/pills/tablets | 400 IU/d | 400 | D3 | Yes | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| Bacqui 2009 | 27‐31 weeks | ˜12 weeks (till delivery) | liquid supplement 20,000 IU/mL | 35,000 IU/week (+70,000 IU on day 0) | ˜6000 | D3 | not specified | not specified | *60 | 400 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified |
| 27‐31 weeks | ˜12 weeks (till delivery) | liquid supplement 20,000 IU/mL | 14,000 IU/week | 2000 | D3 | not specified | not specified | *60 | 400 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | |
| Bhatia 2012 | 14–20 weeks (randomisation) | 40‐20 =˜20 weeks | sachets | 60,000 IU/4 weeks | ˜2000 | D3 | not specified | 1000 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified |
| 14–20 weeks (randomisation) | 40‐20 =˜20 weeks | sachets | 60,000 IU/8 weeks | ˜1000 | D3 | not specified | 1000 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | |
| 14–20 weeks (randomisation) | 40‐20 =˜20 weeks | sachets | 400 IU/d | 400 | D3 | not specified | 1000 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | |
| Das 2010 | 5th month | 40‐20 =˜20 weeks (2 doses 5th and 7th month) | not specified | 240,000 IU/20 weeks | ˜1700 | D3 | No | 1000 | 60 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5th month | 40‐20 =˜20 weeks (single dose) | not specified | 60,000 IU/20 weeks | ˜400 | D3 | No | 1000 | 60 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Dawodu 2013 | 12‐16 weeks | 40‐16 =˜24 weeks | capsules/pills/tablets | 3600 IU/d/d + 400 IU/d | 4000 | D3 | Yes | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 12‐16 weeks | 40‐16 =˜24 weeks | capsules/pills/tablets | 1600 IU/d/d + 400 IU/d | 2000 | D3 | Yes | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| 12‐16 weeks | 40‐16 =˜24 weeks | capsules/pills/tablets | 400 IU/d | 400 | D3 | Yes | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| de Menibus 1984 | 7th month | 40‐28 =˜12 weeks | not specified | 1000 IU/d1 | 1000 | D2 (Uvesterol) | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified |
| 7th month | 40‐28 =˜12 weeks (single dose) | not specified | 200,000 IU/12 weeks | ˜2400 | D3 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | |
| Grant 2010 | 26‐30 weeks | 40‐28 =˜12 weeks | not specified | 1000 IU/d | 1000 | D3 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified |
| 26‐30 weeks | 40‐28 =˜12 weeks | not specified | 2000 IU/d | 2000 | D3 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | |
| Hashemipour 2014 | 24‐26 weeks | 8 weeks | capsules/pills/tablets | 50,000 IU/week + 400 IU/d | ˜7500 | D3 | yes | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 24‐26 weeks | 40‐25 =˜15 weeks | capsules/pills/tablets | 400 IU/d | 400 | D3 | yes | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| Kalra 2012 | 12‐24 weeks | 40‐20 =˜20 weeks (single dose) | capsules/pills/tablets | 60,000 IU/22 weeks | ˜400 | D3 | not specified | 1000 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified |
| 28 weeks | 40‐28 =˜12 weeks (2 doses) | capsules/pills/tablets | 600,000 IU/12 weeks | ˜7150 | D3 | not specified | 1000 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | |
| Karamali 2015 | 20‐32 weeks | 40‐28 =˜12 weeks (dose every 2 weeks) | capsules/pills/tablets | 50,000 IU/2 weeks + 400 IU/d | ˜3970 | D3 | yes | n/a | 12** | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 20‐32 weeks | capsules/pills/tablets | 400 IU/d | 400 | D3 | yes | n/a | 12** | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | ||
| Kiely 2015 | 15 weeks | 22 weeks | capsules/pills/tablets | 800 IU/d | 800 | D3 | yes2 | 1000 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 15 weeks | 22 weeks | capsules/pills/tablets | 400 IU/d | 400 | D3 | yes2 | 1000 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| Mallet 1986 | 28 weeks | 40‐28 =˜12 weeks | capsules/pills/tablets | 1000 IU/d | 1000 | D2 | not specified | 1000 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified |
| 7th month | 40‐28 =˜12 weeks (single dose) | capsules/pills/tablets | 200,000 IU/12 weeks | ˜2380 | D2 | not specified | 1000 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | |
| March 2010 | 13‐24 weeks | 48‐20 =˜28 weeks | capsules/pills/tablets | 2000 IU/d | 2000 | D3 | yes5 | 250 | 27 | 1000 | 1500 | 1500 | 3 | 3.4 | 20 | 10 | 12 | 100 | 30 | 45 | 25 | 50 | 0.15 | 5 |
| 13‐24 weeks | 48‐20 =˜28 weeks | capsules/pills/tablets | 1000 IU/d | 1000 | D3 | yes5 | 250 | 27 | 1000 | 1500 | 1500 | 3 | 3.4 | 20 | 10 | 12 | 100 | 30 | 45 | 25 | 50 | 0.15 | 5 | |
| 13‐24 weeks | 48‐20 =˜28 weeks | capsules/pills/tablets | 400 IU/d | 400 | D3 | yes5 | 250 | 27 | 1000 | 1500 | 1500 | 3 | 3.4 | 20 | 10 | 12 | 100 | 30 | 45 | 25 | 50 | 0.15 | 5 | |
| Marya 1981 | 7th month | ˜12 weeks (2 doses: 7th and 8th) | capsules/pills/tablets | 1,200,000 IU/12 weeks | ˜14,285 | D2 | No | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 7th month | ˜12 weeks | capsules/pills/tablets | 1200 IU/d | 1200 | D2 | No | 375 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| Mir 2016 | 16 weeks | ˜12 weeks (monthly doses) | capsules/pills/tablets | 60,000 IU/4 weeks | 2000 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified |
| 16 weeks | ˜12 weeks (monthly doses) | capsules/pills/tablets | 30,000 IU/4 weeks | 1000 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | |
| 16 weeks | ˜12 weeks | capsules/pills/tablets | 2000 IU/d | 2000 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | |
| 16 weeks | ˜12 weeks | capsules/pills/tablets | 1000 IU/d | 1000 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | |
| Mojibian 2015 | 12 weeks | 40‐28 =˜12 weeks | capsules/pills/tablets | 50,000 IU/2 weeks | ˜3571 | D3 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified |
| 12 weeks | 40‐28 =˜12 weeks | capsules/pills/tablets | 400 IU/d | 400 | D3 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | |
| Mutlu 2014 | 13‐32 weeks | ˜12 weeks | liquid supplement 20,000 IU/mL | 2000 IU/d | 2000 | D3 | No | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 13‐32 weeks | ˜12 weeks | liquid supplement 20,000 IU/mL | 1200 IU/d | 1200 | D3 | No | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 13‐32 weeks | ˜12 weeks | liquid supplement 20,000 IU/mL | 600 IU/d/d | 600 | D3 | No | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| O'Brien 2013 | 12‐29 weeks | ˜20 weeks | capsules/pills/tablets | 2000 IU/d + 400 IU/d | 2400 | D3 | yes | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 12‐29 weeks | ˜20 weeks | capsules/pills/tablets | 200 IU/week + 400 IU/d | 600 | D3 | yes | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| Rostami 2017 | < 14 weeks | ˜6 weeks | tablets + intramuscular injection | 50,000 IU/week | ˜1650 | D3 | yes2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| < 14 weeks | ˜26 weeks (weekly and monthly doses) | tablets + intramuscular injection | 50,000 IU/wk X 6 weeks + 50,000 IU/mo X 4 mo | ˜2750 | D3 | yes2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| < 14 weeks | ˜26 weeks | tablets + intramuscular injection | 300,000 IU/26 weeks | ˜1650 | D3 | yes2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| < 14 weeks | ˜26 weeks | tablets + intramuscular injection | 300,000 IU/26weeks + 50,000 IU/month X 4 month | ˜2750 | D3 | yes2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| < 14 weeks | ˜12 weeks | tablets + intramuscular injection | 50,000 IU/week | ˜3300 | D3 | yes2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| < 14 weeks | ˜26 weeks (weekly and monthly doses) | tablets + intramuscular injection | 50,000 IU/week X 12 weeks + 50,000 IU/month X 4 mos | ˜4400 | D3 | yes2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| < 14 weeks | ˜26 weeks (2X 6 weeks doses) | tablets + intramuscular injection | 300,000 IU/6 weeks X2 | ˜3300 | D3 | yes2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| < 14 weeks | ˜26 weeks (2X 6 weeks and monthly doses) | tablets + intramuscular injection | 300,000 IU/6 weeks X2 + 50,000 IU/month X 4 month | ˜4400 | D3 | yes2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| Roth 2013 | 17‐24 weeks | 66‐20 =˜46 weeks (˜26 weeks postpartum) | capsules/pills/tablets | 28,000 IU/week | ˜4100 | D3 | not specified | 500 | ***66 | 350 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified |
| 17‐24 weeks | 40‐20 =˜20 weeks | capsules/pills/tablets | 28,800 IU/week | ˜4100 | D3 | not specified | 500 | ***66 | 350 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | |
| 17‐24 weeks | 40‐20 =˜20 weeks | capsules/pills/tablets | 16,800 IU/week | ˜2400 | D3 | not specified | 500 | ***66 | 350 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | |
| 17‐24 weeks | 40‐20 =˜20 weeks | capsules/pills/tablets | 4200 IU/week | ˜600 | D3 | not specified | 500 | ***66 | 350 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | |
| Shakiba 2013 | 12‐14 weeks | 40‐13 =˜27 weeks | not specified | 50,000 IU/2 weeks (Max 100,000 IU per month) | ˜3332 | D3 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified |
| 12‐14 weeks | 40‐13 =˜27 weeks | not specified | 50,000 IU/month | ˜1666 | D3 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | |
| Soheilykhah 2011 | ≤ 12 weeks | 40‐28 =˜12 weeks | not specified | 50,000 IU/2weeks | 4000 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified |
| ≤ 12 weeks | 40‐28 =˜12 weeks | not specified | 50,000 IU/month | 2000 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | |
| ≤ 12 weeks | 40‐28 =˜12 weeks | not specified | 200 IU/d | 200 | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | not specified | |
| Stephensen 2011 | < 20 weeks | 40‐20 =˜20 weeks | capsules/pills/tablets | 2000 IU/d | 2000 | D3 | yes2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| < 20 weeks | 40‐20 =˜20 weeks | capsules/pills/tablets | 400 IU/d | 400 | D3 | yes2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| Thiele 2014 | 24‐28 weeks | 46‐25 =˜21 weeks (˜4‐6 weeks postpartum) | capsules/pills/tablets | 3,800 IU/d | 3800 | D3 | yes2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 24‐28 weeks | 46‐25 =˜21 weeks (˜4‐6 weeks postpartum) | capsules/pills/tablets | 400 IU/d | 400 | D3 | yes2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| Wagner 2006a | 12‐16 weeks | 40‐14 =˜26 weeks | capsules/pills/tablets | 3600 IU/d/d + 400 IU/d | 4000 | D3 | yes6 | 250 | 27 | 1000 | 1000 | 2,500 | 1.4 | 1.4 | 18 | 5 | 2.6 | 120 | 30 | 45 | 7.5 | 50 | 0.22 | 0 |
| 12‐16 weeks | 40‐14 =˜26 weeks | capsules/pills/tablets | 1600 IU/d/d + 400 IU/d | 2000 | D3 | yes6 | 250 | 27 | 1000 | 1000 | 2,500 | 1.4 | 1.4 | 18 | 5 | 2.6 | 120 | 30 | 45 | 7.5 | 50 | 0.22 | 0 | |
| Wagner 2006b | 12‐16 weeks | 40‐13 =˜27 weeks | capsules/pills/tablets | 3600 IU/d/d + 400 IU/d | 4000 | D3 | yes6 | 250 | 27 | 1000 | 1000 | 2,500 | 1.4 | 1.4 | 18 | 5 | 2.6 | 120 | 30 | 45 | 7.5 | 50 | 0.22 | 0 |
| 12‐16 weeks | 40‐13 =˜27 weeks | capsules/pills/tablets | 1600 IU/d/d + 400 IU/d | 2000 | D3 | yes6 | 250 | 27 | 1000 | 1000 | 2,500 | 1.4 | 1.4 | 18 | 5 | 2.6 | 120 | 30 | 45 | 7.5 | 50 | 0.22 | 0 | |
| 12‐16 weeks | 40‐13 =˜27 weeks | capsules/pills/tablets | 0 IU/d + 400 IU/d | 400 | D3 | yes6 | 250 | 27 | 1000 | 1000 | 2,500 | 1.4 | 1.4 | 18 | 5 | 2.6 | 120 | 30 | 45 | 7.5 | 50 | 0.22 | 0 | |
| Wagner 2013 | 12‐16 weeks | 40‐14 =˜26 weeks | capsules/pills/tablets | 3600 IU/d/d + 400 IU/d | 4000 | D3 | yes2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 12‐16 weeks | 40‐14 =˜26 weeks | capsules/pills/tablets | 1600 IU/d/d + 400 IU/d | 2000 | D3 | yes2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| Weiss 2009 | 10‐18 weeks | 40‐14 =˜26 weeks | capsules/pills/tablets | 4000 IU/d + 400 IU/d | 4400 | D3 | yes2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 10‐18 weeks | 40‐14 =˜26 weeks | capsules/pills/tablets | 400 IU/d | 400 | D3 | yes2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| Yap 2014 | ˜14 weeks | 40‐14 =˜26 weeks | capsules/pills/tablets | 5000 IU/d | 5000 | D3 | yes3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| ˜14 weeks | 40‐14 =˜26 weeks | capsules/pills/tablets | 400 IU/d | 400 | D3 | yes3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
| Yu 2008 | 27 weeks (randomisation) | 40‐27 =˜13 weeks (single dose) | not specified | 200,000 IU/13 weeks | ˜2200 | D3 | No | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 27 weeks (randomisation) | 40‐27 =˜13 weeks | not specified | 800 IU/d | 800 | D2 | No | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Missing data were divided in two types as not specified when the corresponding information is necessary but it was not indicated in the study, and as n/a when the corresponding information was either not available or it was not applicable in this specific case.
*Reported as standard supplement, elemental iron not clearly specified.
**60 mg/d ferrous sulphate converted as a rate of 5 to elemental iron.
***Reported as Iron, elemental iron not clearly specified.
1 It states 1000 IU/d of vitamin D or no intake (without specifying).
2 Prenatal multivitamin supplements allowed if not exceed 400 IU/d.
3 Prenatal multivitamin supplements allowed if not exceed 500 IU/d. It is not clear if the excedent is counted in the total daily vitamin D ingestion per day.
4 Prenatal vitamins were Myadec multivitamin‐multimineral (Pfizer®), if women cannot swallow then given Flinstones Complete chewable vitamin (Bayer®).
5 In addition the prenatal supplement contained the following minerals (intake per day): Pantothenic acid 10 mg, Biotin 30 μg, Manganese 1 mg, Copper 2 mg, Chromium 25 μg, Molybdenum 25 μg, Selenium 25 μg.
6 In addition the prenatal supplement contained the following minerals (intake per day): Pantothenic acid 6 mg, Biotin 30 μg, Manganese 2 mg, Copper 9 mg, Chromium 30 μg, Molybdenum 50 μg, Selenium 60 μg.
Abbreviations IU/d: international units per day
Health worker cadre
Most of the trials were conducted in gynaecology and obstetrics care clinics, but also in research centres, directly in the communities or nested within medical surveys. Vitamin D supplements were provided by the researchers’ team, physicians, pharmacists, nurses or community health workers. Clinical, biochemical, anthropometric, or dietary assessment outcome measures were completed, according to their nature, by diverse professionals. Description of the health worker cadre and of the role of the research staff significantly varies across the trials and is detailed in Characteristics of included studies.
Laboratory methodology for the assessment of vitamin D status
Different laboratory methods were used to measure vitamin D status as serum 25‐hydroxyvitamin D concentrations. Seven trials used immunoassay kits to measure vitamin D (Abotorabi 2017; Hashemipour 2014; Karamali 2015; Mojibian 2015; Mutlu 2014; Rostami 2017; Thiele 2014); six trials used radioimmunoassay (Bhatia 2012; Das 2010; Dawodu 2013; Kalra 2012; Wagner 2006a; Wagner 2013); six trials used a chemiluminescent enzyme‐labelled immunometric assay (Bacqui 2009; March 2010; Shakiba 2013; Soheilykhah 2011; Weiss 2009; Yap 2014); five trial used chromatography–tandem mass spectrometry (Grant 2010; Kiely 2015; O'Brien 2013; Roth 2013; Wagner 2006b). One trial used a competitive protein binding assay (Mallet 1986). Two trials did not report the laboratory method used (de Menibus 1984;Yu 2008). The other trials did not report on this outcome (Marya 1981; Mir 2016; Stephensen 2011).
Funding sources
Included trials were financed (solely or in combination) by governmental and institutional research grants, non‐governmental organisations and the private sector. The Department of Biotechnology, a SGPGIMS‐intramural grant and the Indian Council for Medical Research funded Bhatia 2012. Das 2010 was supported by the Government of India‐Department of Biotechnology. The Thrasher Research Fund financed Dawodu 2013. The Regional Direction of Health and Social affairs of Haute‐Normandie supported de Menibus 1984. Grant 2010 was funded by the Health Research Council of New Zealand and by Cure Kids. Hashemipour 2014 was supported by the Metabolic Diseases Research Center‐Qazvin University of Medical Sciences. Kalra 2012 was partially funded by the Indian Council for Medical Research. The Vice‐chancellor for Research, AUMS, and Iran and the Arak University of Medical Sciences supported the Karamali 2015 trial. The European Commission supported Kiely 2015 by funding. March 2010 was supported by the Canadian Institutes for Health Research (CIHR) and by a Frederick Banting and Charles Best Canada Graduate Scholarship from the CIHR; supplements were provided by Natural Factors (Coquitlam, Canada). The research grant for Mir 2016 was provided by the Sher‐i‐Kashmir Institute of Medical Sciences; vitamin D supplementation was provided free of charge by M/S Eris Life sciences and Myer pharmaceuticals. The Shahid Sadoughi University of Medical Sciences funded Mojibian 2015. The US Department of Agriculture, the National Institutes of Health and the Cornell University and the University of Rochester sponsored O'Brien 2013. Rostami 2017 was financially supported by the Research Institute of Endocrine Sciences‐Shahid Beheshti University of Medical Sciences. Roth 2013 was funded by the Bill and Melinda Gates Foundation, the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health and the Career Development Program in Women’s Health Research at Penn State University. The Thrasher Research Fund, the National Center for Research Resources, the National Center for Advancing Translational Sciences of the National Institutes of Health, the Medical University of South Carolina Children’s Hospital Fund and the Division of Neonatology of the Medical University of South Carolina funded both Wagner 2006a and Wagner 2013; Wagner 2006b received financial support from the National Institute of Children’s Health and Human Development and form the South Carolina Clinical & Translational Research Institute. Weiss 2009 was funded by the National Heart, Lung, and Blood Institute (NHLBI) with additional support from the National Centers for Advancing Translational Sciences (NCATS). The Roly Dunlop Scholarship for Neurological Research‐Sydney Medical School Foundation (University of Sydney) funded Yap 2014. Yu 2008 was supported by the Institute of Obstetrics and Gynaecology Trust, Wolfson and Weston Research Centre for Family Health at Imperial College. Thiele 2014 reported no financial support. Abotorabi 2017; Bacqui 2009; Mallet 1986; Marya 1981; Mutlu 2014; Shakiba 2013; Soheilykhah 2011 and Stephensen 2011 did not disclose any financial support statement.
Declarations of interest
Most of the authors reported no conflict of interest. Dawodu 2013 as well as Wagner 2006b and Wagner 2013 reported that Bruce W. Hollis (B.W.H.) served as a consultant for Diasorin, Inc (Stillwater, Minnesota). March 2010 reported that MRL received consulting fees from the Factors Group of Nutritional Companies (Canada’s leading manufacturer of natural health products). The trial by Weiss 2009 reported that Dr. Litonjua received personal fees from UpToDate Inc and Springer Humana Press, that Dr Bacharier reported receiving personal fees from Aerocrine, GlaxoSmithKline, Genentech/Novartis, Merck, Schering, Cephalon, DBV Technologies, Teva, Boehringer Ingelheim, AstraZeneca, WebMD/Medscape, Sanofi, and Vectura, that Dr Zeiger reported receiving grants from AstraZeneca, Aerocrine, MedImmune, Genentech, Merck, and GlaxoSmithKline and personal fees from Genentech, Novartis, GlaxoSmithKline, and TEVA. Abotorabi 2017; Bacqui 2009; Bhatia 2012; Marya 1981; Mutlu 2014; Shakiba 2013; Stephensen 2011 and Yu 2008 did not disclose conflict of interests. All other authors declared no competing interests in their trials.
SeeCharacteristics of included studies for a detailed description of the trials, including vitamin D doses used and regimens compared. This information is also summarised in Table 3.
Excluded studies
We excluded 11 trials (Ali 2018; Azami 2017; Bisgaard 2009; Hajihashemi 2016; Jamilian 2017; Li 2000; Omotayo 2017; Roth 2016; Sablok 2015; Wheeler 2016; Zhang 2016). Four trials were excluded because the treatment groups differed in other nutrients given in the supplements, other than vitamin D (Ali 2018; Azami 2017; Hajihashemi 2016; Li 2000). Omotayo 2017 was excluded because all groups had the same level of vitamin D supplementation. Sablok 2015 was excluded because it only included one dose of vitamin D versus placebo (this trial was included in a separate review), in which placebo was compared with any vitamin D dose (De Regil 2016; Palacios 2019). Bisgaard 2009 was excluded because women had multiple pregnancies. Two trials were carried out in pregnant women with glucose intolerance or with gestational diabetes (Jamilian 2017; Zhang 2016), and two trials were conducted only among postpartum women (Roth 2016; Wheeler 2016). For more detailed descriptions of excluded trials along with the reasons for exclusion, seeCharacteristics of excluded studies.
Risk of bias in included studies
We included figures that summarise our ’Risk of bias’ assessments (Figure 2; Figure 3).
2.
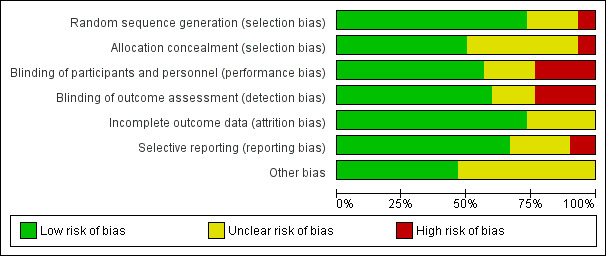
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
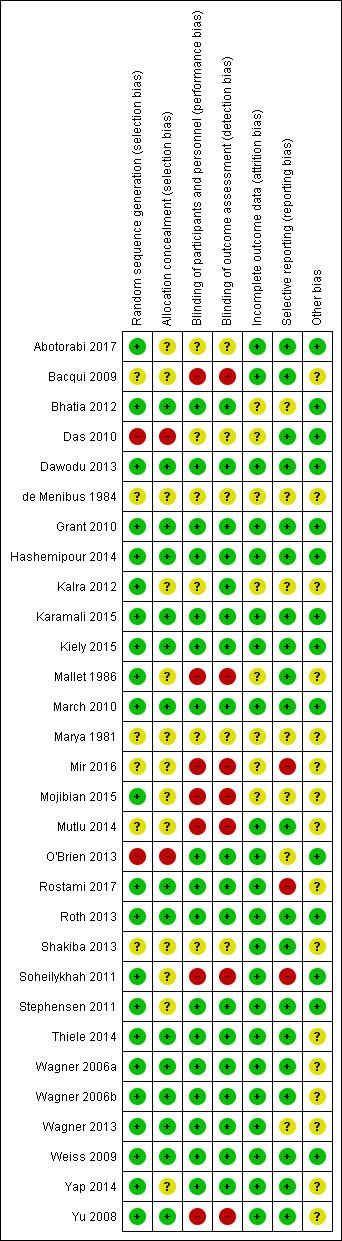
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
We assessed 22 trials as having adequate methods for generating the randomisation sequence. Ten trials used computer‐generated random number sequences (Abotorabi 2017; Hashemipour 2014; Kiely 2015; Mojibian 2015; Rostami 2017; Roth 2013; Soheilykhah 2011; Wagner 2006a; Wagner 2013; Yu 2008), three used computer‐generated block randomisation (Bhatia 2012; Dawodu 2013; Grant 2010); three trials used random numbers tables (Kalra 2012; Karamali 2015; Mallet 1986), one trial used permuted block size of six and sequential assignment (Yap 2014), one trial used single block‐randomised list (Stephensen 2011), two trials used stratified blocked randomisation (March 2010; Wagner 2006b), two trials reported the use of a random sequence generator but did not specify which (Thiele 2014; Weiss 2009). Two trials were judged as high risk for selection: O'Brien 2013 used alternate group assignment to randomise the intervention groups and Das 2010 reported that randomisation was abandoned. The remaining trials reported that the trial was randomised, but the methods used to generate the sequence were not described (Bacqui 2009; de Menibus 1984; Marya 1981; Mir 2016; Mutlu 2014; Shakiba 2013).
Allocation concealment
We judged that 15 trials had adequate methods of allocation concealment (Bhatia 2012; Dawodu 2013; Grant 2010; Hashemipour 2014; Karamali 2015; Kiely 2015; March 2010; Rostami 2017; Roth 2013; Thiele 2014; Wagner 2006a; Wagner 2006b; Wagner 2013; Weiss 2009; Yu 2008). Two trials were judged as high risk for allocation concealment: Das 2010 and O'Brien 2013. The remaining trials did not describe the methods used to conceal the allocation (Abotorabi 2017; Bacqui 2009; de Menibus 1984; Kalra 2012; Mallet 1986; Marya 1981; Mir 2016; Mojibian 2015; Mutlu 2014; Shakiba 2013; Soheilykhah 2011; Stephensen 2011; Yap 2014).
Blinding
Blinding of participants and personnel
A total of 17 trials reported a double‐blinded design by using placebos of similar appearance to active treatment, coded or opaque bottles (Bhatia 2012; Dawodu 2013; Grant 2010; Hashemipour 2014; Karamali 2015; Kiely 2015; March 2010; O'Brien 2013; Rostami 2017; Roth 2013; Stephensen 2011; Thiele 2014; Wagner 2006a; Wagner 2006b; Wagner 2013; Weiss 2009; Yap 2014). Seven trials were not blinded (Bacqui 2009; Mallet 1986; Mir 2016; Mojibian 2015; Mutlu 2014; Soheilykhah 2011; Yu 2008) and six did not report the blinding methods (Abotorabi 2017; Das 2010; de Menibus 1984; Kalra 2012; Marya 1981; Shakiba 2013).
Blinding of outcome assessors
A total of 17 trials reported a double‐blinded design by using placebos of similar appearance to active treatment, coded or opaque bottles (Bhatia 2012; Dawodu 2013; Grant 2010; Hashemipour 2014; Karamali 2015; Kiely 2015; March 2010; O'Brien 2013; Rostami 2017; Roth 2013; Stephensen 2011; Thiele 2014; Wagner 2006a; Wagner 2006b; Wagner 2013; Weiss 2009; Yap 2014). Seven trials were not blinded (Bacqui 2009; Mallet 1986; Mir 2016; Mojibian 2015; Mutlu 2014; Soheilykhah 2011; Yu 2008) and five did not report the blinding methods (Abotorabi 2017; Das 2010; de Menibus 1984; Marya 1981; Shakiba 2013). In the case of Kalra 2012, blinding was specified for infant anthropometry but not for maternal vitamin D concentration; therefore, it was judged as low risk.
Incomplete outcome data
Most trials reported complete data (Abotorabi 2017; Bacqui 2009; Dawodu 2013; Grant 2010; Hashemipour 2014; Karamali 2015; Kiely 2015; March 2010; Mutlu 2014; O'Brien 2013; Roth 2013; Shakiba 2013; Soheilykhah 2011; Stephensen 2011; Thiele 2014; Wagner 2006a; Wagner 2006b; Wagner 2013; Weiss 2009; Yap 2014; Yu 2008). Two trials had missing data (Mallet 1986; Rostami 2017). The others either did not report this or they did not clearly report on attrition, missing data and/or lack of intention‐to treat analyses (Bhatia 2012; Das 2010; de Menibus 1984; Kalra 2012; March 2010; Marya 1981; Mir 2016).
Selective reporting
We did not have access to all study protocols; therefore, we used the manuscript to assess reporting bias. Most trials were judged as low risk of bias: Abotorabi 2017; Bacqui 2009; Das 2010; Dawodu 2013; Grant 2010; Hashemipour 2014; Karamali 2015; Kiely 2015; Mallet 1986; March 2010; Mutlu 2014; Roth 2013; Shakiba 2013; Stephensen 2011; Thiele 2014; Wagner 2006a; Wagner 2006b; Weiss 2009; Yap 2014; Yu 2008. The following trials were judged as high risk of bias for reporting: Mir 2016; Rostami 2017 and Soheilykhah 2011. For the following trials, it was not clear if there was reporting bias: Bhatia 2012; de Menibus 1984; Kalra 2012; Marya 1981; Mojibian 2015; O'Brien 2013; Wagner 2013.
Insufficient trials contributed data to allow us to carry out an exploration of possible publication bias by using funnel plots.
Other potential sources of bias
Other bias was found for Kalra 2012, as the authors reported study limitations and unspecified logistical constraints.
For the following trials, other potential sources of bias were classified as unclear because of the lack of description of the methodology in general, insufficient information which precludes judgment: Bacqui 2009; de Menibus 1984; Mallet 1986; Marya 1981; Mir 2016; Mojibian 2015; Mutlu 2014; Rostami 2017; Shakiba 2013; Thiele 2014; Wagner 2006a; Wagner 2006b; Wagner 2013; Yap 2014; Yu 2008.
Effects of interventions
In this review we included 30 trials assessing a total of 7289 women. We organised the summary results by comparison and by primary and secondary outcomes. For each of the comparisons, we have indicated the number of trials contributing data and the total number of women recruited in these trials.
We contacted the authors of 11 trials to clarify information from their trials and for additional data on the included outcomes that were either missing (based on the protocol information) or aggregated in a way that could not be incorporated into the review. A total of five authors responded with clarification information (Bacqui 2009; Roth 2013; Wagner 2006a; Wagner 2006b; Wagner 2013), three authors provided additional data that were incorporated into the meta‐analysis (Bhatia 2012; Rostami 2017; O'Brien 2013), and three authors did not respond (Mir 2016; Shakiba 2013; Lalooha 2012).
It should be noted that all analyses were carried out using a random‐effects model so the average treatment effect is reported throughout.
SeeData and analyses for detailed results on primary and secondary outcomes.
(1) A dose of vitamin D 601 IU/d or higher versus 600 IU/d or lower alone or with other nutrients
In total, 19 trials involving 5214 women were included in this comparison: Abotorabi 2017; Bhatia 2012; Das 2010; Dawodu 2013; Hashemipour 2014; Kalra 2012; Karamali 2015; Kiely 2015; March 2010; Mojibian 2015; Mutlu 2014; O'Brien 2013; Roth 2013; Soheilykhah 2011; Stephensen 2011; Thiele 2014; Wagner 2006b; Weiss 2009; Yap 2014.
The following trials were judged as having low risk of bias: Dawodu 2013; Hashemipour 2014; Karamali 2015; Kiely 2015; March 2010; Roth 2013; Stephensen 2011; Thiele 2014; Wagner 2006b; Weiss 2009. The following had mixed results, with some components having a high risk, low risk or unclear: Abotorabi 2017; Bhatia 2012; Kalra 2012 and Yap 2014. Five studies were considered high risk of bias: Das 2010; O'Brien 2013; Mojibian 2015; Mutlu 2014; Soheilykhah 2011.
We could not perform subgroup analysis in any of the outcomes for skin pigmentation as none of the studies in this comparison specified this. In addition, we could not perform the subgroup analysis on latitude as all studies were done outside the tropics.
Primary outcomes
Maternal
Pre‐eclampsia
Data from five trials (Karamali 2015; Mojibian 2015; Stephensen 2011; Weiss 2009; Yap 2014) involving 1553 women suggest that there is little or no difference in the risk of pre‐eclampsia for women who received 601 IU/d or more of vitamin D supplements compared to those women receiving 600 IU/d or less (risk ratio (RR) 0.96, 95% confidence interval (CI) 0.65 to 1.42; low‐certainty evidence; Analysis 1.1). Subgroup analysis did not appear to show an effect by time of commencement of supplementation (Analysis 1.2), by frequency of supplementation (Analysis 1.3), by vitamin D status at baseline (Analysis 1.8) or by nutrients included in the supplementation (Analysis 1.9). No effect was apparent when we analysed results by impact factor of the journal (Analysis 1.7). The following subgroup analyses did not have enough trials in each subgroup for meaningful subgroup analysis: pre‐pregnancy BMI (Analysis 1.4); season at the start of the supplementation (Analysis 1.5); or registration of the protocol (Analysis 1.6). In addition, Weiss 2009 reported no eclampsia cases among women receiving either 400 IU/d or 4400 IU/d but they reported two cases of HELLP (Haemolysis, Elevated Liver enzymes, and Low Platelet count) syndrome among those in the 4400 IU/d and one case in those receiving 400 IU/d.
1.1. Analysis.
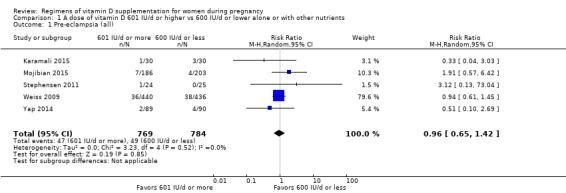
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 1 Pre‐eclampsia (all).
1.2. Analysis.
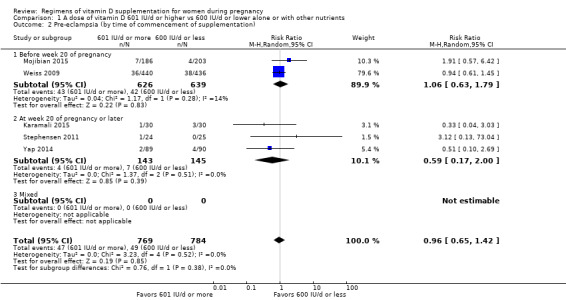
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 2 Pre‐eclampsia (by time of commencement of supplementation).
1.3. Analysis.
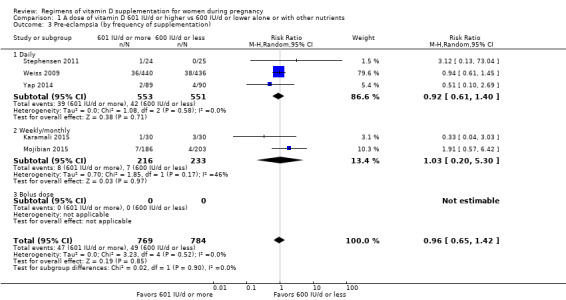
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 3 Pre‐eclampsia (by frequency of supplementation).
1.8. Analysis.
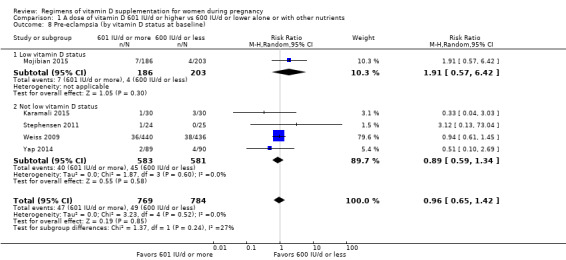
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 8 Pre‐eclampsia (by vitamin D status at baseline).
1.9. Analysis.
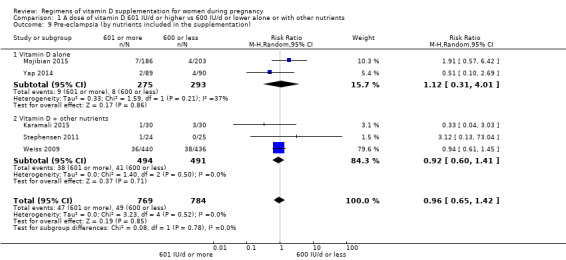
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 9 Pre‐eclampsia (by nutrients included in the supplementation).
1.7. Analysis.
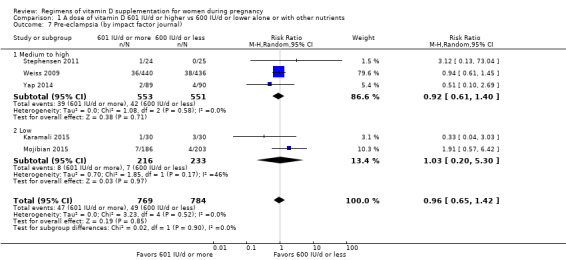
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 7 Pre‐eclampsia (by impact factor journal).
1.4. Analysis.
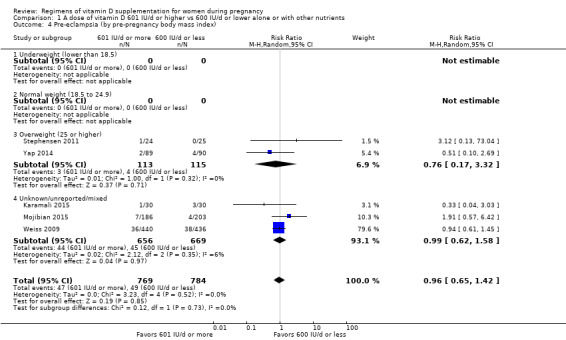
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 4 Pre‐eclampsia (by pre‐pregnancy body mass index).
1.5. Analysis.
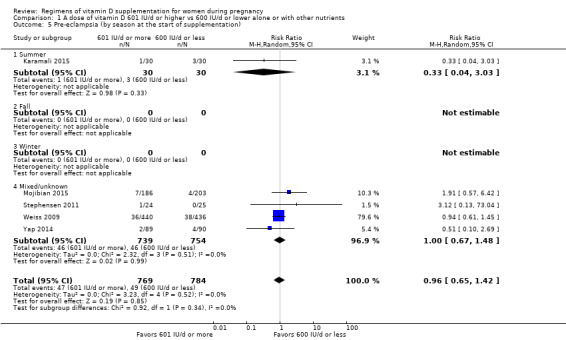
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 5 Pre‐eclampsia (by season at the start of supplementation).
1.6. Analysis.
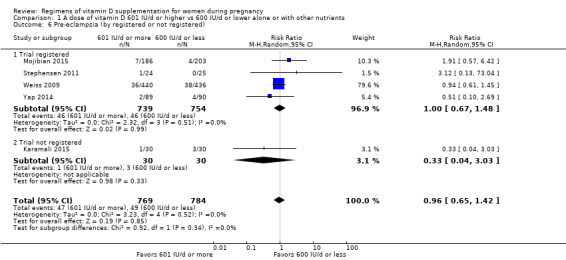
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 6 Pre‐eclampsia (by registered or not registered).
Gestational diabetes
Data from five trials (Hashemipour 2014; Mojibian 2015; Roth 2013; Stephensen 2011; Yap 2014) involving 1846 women suggest that women who received 601 IU/d or more of vitamin D probably have a lower risk of gestational diabetes compared to women receiving 600 IU/d or less (RR 0.54, 95% CI 0.34 to 0.86; moderate‐certainty evidence; Analysis 1.10). Subgroup analysis did not appear to show an effect by time of commencement of supplementation (Analysis 1.11), by impact factor of the journal (Analysis 1.15), by vitamin D status at baseline (Analysis 1.16) or by nutrients included in the supplementation (Analysis 1.17). The following subgroup analyses did not have enough trials in each subgroup for meaningful subgroup analysis: frequency of supplementation (Analysis 1.12); pre‐pregnancy BMI (Analysis 1.13); or registration of the protocol (Analysis 1.14).
1.10. Analysis.
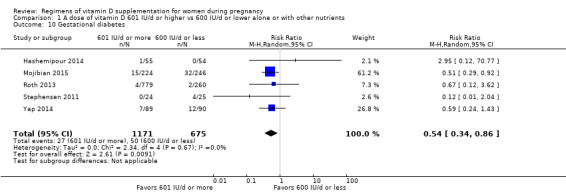
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 10 Gestational diabetes.
1.11. Analysis.
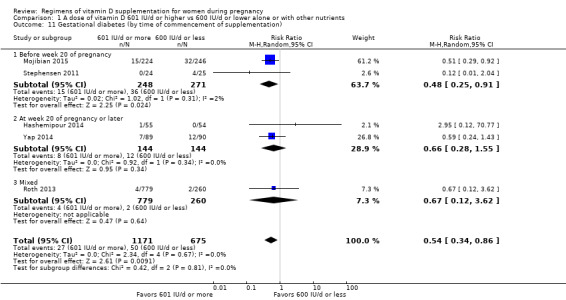
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 11 Gestational diabetes (by time of commencement of supplementation).
1.15. Analysis.
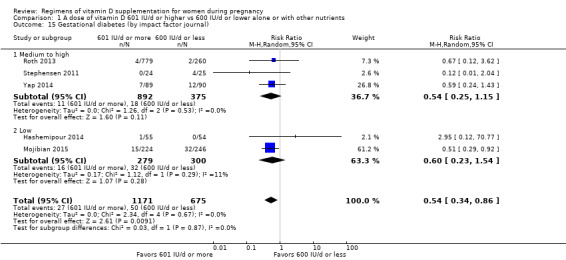
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 15 Gestational diabetes (by impact factor journal).
1.16. Analysis.
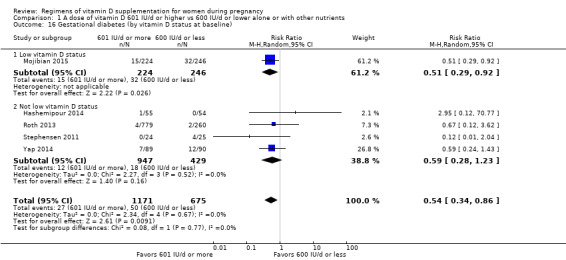
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 16 Gestational diabetes (by vitamin D status at baseline).
1.17. Analysis.
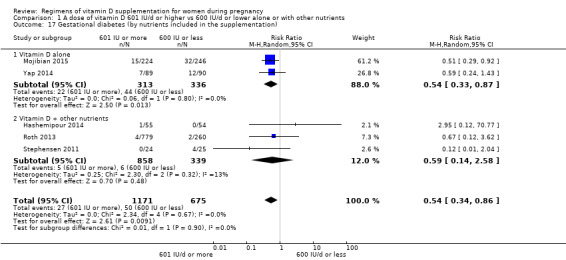
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 17 Gestational diabetes (by nutrients included in the supplementation).
1.12. Analysis.
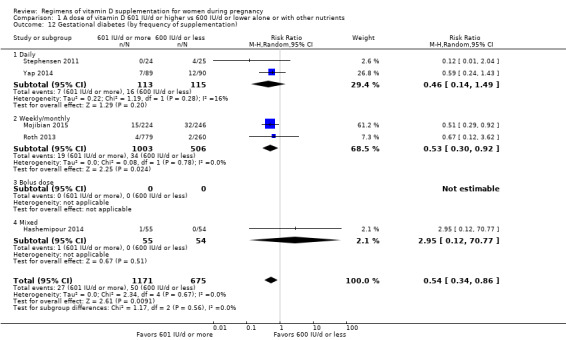
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 12 Gestational diabetes (by frequency of supplementation).
1.13. Analysis.
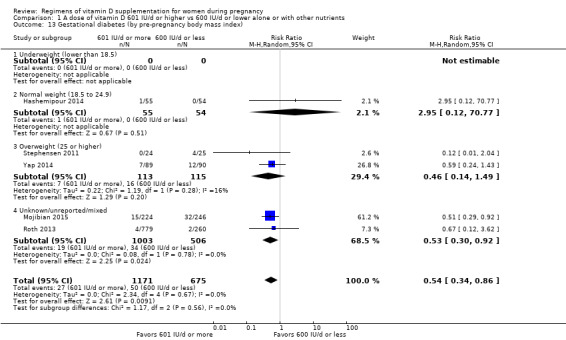
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 13 Gestational diabetes (by pre‐pregnancy body mass index).
1.14. Analysis.
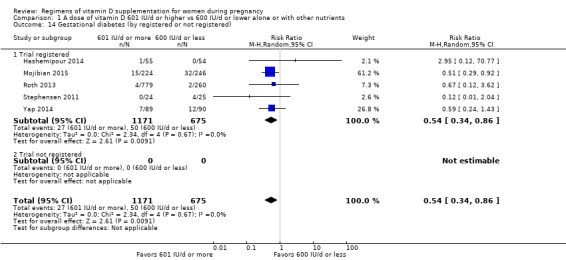
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 14 Gestational diabetes (by registered or not registered).
Adverse effects
Because adverse events were reported differently in most trials, we reported this outcome in a narrative way.
Abotorabi 2017 mentioned that after eight weeks of vitamin D3 supplementation, there were no side effects such as maternal hypercalcaemia. Calcium level was higher in the treatment group (50,000 IU per week) compared to the control group (400 IU/d), but the difference was not significant. They also mentioned that the prevalence of neonatal hypocalcaemia was not different between treatment groups.
Bhatia 2012 mentioned that none of the mothers or infants had hypercalcaemia or hypervitaminosis D (serum 25OHD level > 250 nmol/L).
Das 2010 mentioned that no participant had hypervitaminosis D (serum 25OHD levels > 375 nmol/L).
Dawodu 2013 reported that the quote: “..did not find adverse events attributable to vitamin D".
Hashemipour 2014 reported that no cases of congenital malformations occurred in either group, but did not report on the levels of calcium in blood or urine or any other potential adverse effects.
Kalra 2012 reported that frequency of hypocalcaemia did not differ among groups.
Karamali 2015 reported no difference in neonatal hypocalcaemia between groups.
Kiely 2015 reported that they quote: “detected no hypercalcaemia or intervention‐related adverse events”.
March 2010 reported maternal hypercalcaemia (serum total calcium > 2.7 nmol/L) among 13/113 women in the combined higher dose groups (1000 or 2000 IU/day) and among 5/59 women in the lower‐dose group (400 IU/day).
Mojibian 2015 reported that no hypervitaminosis D (serum 25(OH)D more than 100 ng/mL) was observed in any participant. They also reported two cases of hypoglycaemia in neonates from mothers who had received 50,000 IU of vitamin D every two weeks and eight cases among those from mothers receiving 400 IU/d.
Mutlu 2014 reported that 2.2% had secondary hyperparathyroidism, but did not specify which group of women had this. They also reported that none of the women had hypocalcaemia or hypercalciuria and the serum calcium levels were similar between groups.
O'Brien 2013 reported that serum calcium and phosphorus levels were within normative ranges in all study participants.
Roth 2013 reported that there was a higher risk of maternal hypercalciuria at delivery among the group receiving 28,000 IU per week. Also, they reported that there were two asymptomatic cases of maternal confirmed hypercalciuria, one in mothers randomised to the placebo group and one in mothers randomised to 28,000 IU per week. None of the women with confirmed hypercalcaemia or hypercalciuria had serious adverse events (hospitalisations or deaths) or urinary tract stones. Two of the six infants with confirmed hypercalcaemia were hospitalised but for other reasons. One infant had confirmed hypercalciuria in the 4200 IU per week group.
Soheilykhah 2011 reported that pregnant women consuming 50,000 IU vitamin D every 2 weeks had no adverse effects, such as hypercalcaemia.
Stephensen 2011 reported no maternal hypervitaminosis (serum 25(OH(D levels > 225 nmol/L). They also stated the following: quote: "There were no adverse events related to the supplementation".
Thiele 2014 reported no differences in maternal serum calcium or parathyroid hormones between groups.
Wagner 2006b reported no hypercalciuria, hypercalcaemia or hypervitaminosis D in any of the groups.
Weiss 2009 reported that there were no cases of maternal symptomatic hypercalcaemia in either arm of the trial but reported a maternal death two years post‐birth in the 400 IU/d vitamin D group but no deaths in the 4400 IU/d vitamin D group. There were 44 mothers hospitalised in each group in this study (111 events in these 88 mothers).
Yap 2014 reported no maternal hypervitaminosis D (serum 25(OH)D level > 100 ng/mL) in any of the groups, although they did report one case of mild maternal hypercalcaemia in the high‐vitamin D group (5000 IU/d) and another case in the low‐vitamin D group (400 IU/d). They also reported one neonate in the high‐vitamin D group with high cord 25(OH)D levels (> 102 ng/mL), although this was not associated with hypercalcaemia or other clinical adverse effects. Lastly, they reported similar serum calcium levels in neonates in all groups, although they found isolated cases of infant hypercalcaemia on cord blood samples that were not clinically relevant, as it dissipated within a few days of life.
Infant
Preterm birth
Data from four trials (Karamali 2015; Mojibian 2015; Roth 2013; Weiss 2009) involving 2294 women suggest that there is little or no difference in risk of preterm birth for women who received 601 IU/d or more of vitamin D compared to those consuming 600 IU/d or less of vitamin D (RR 1.25, 95% CI 0.92 to 1.69; low‐certainty evidence; Analysis 1.18). Subgroup analysis did not appear to show an effect by frequency of supplementation (Analysis 1.20), by vitamin D status at baseline (Analysis 1.24), by impact factor of the journal in which the trial was published (Analysis 1.23) or by nutrients included in the supplementation (Analysis 1.25). The following subgroup analyses did not have enough trials in each subgroup for meaningful subgroup analysis: time of commencement (Analysis 1.19); season (Analysis 1.21); or registration of the protocol (Analysis 1.22).
1.18. Analysis.
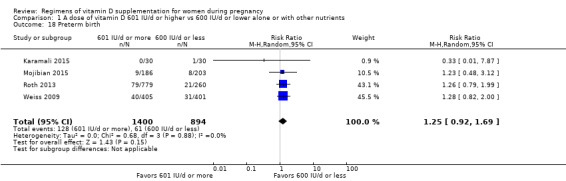
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 18 Preterm birth.
1.20. Analysis.
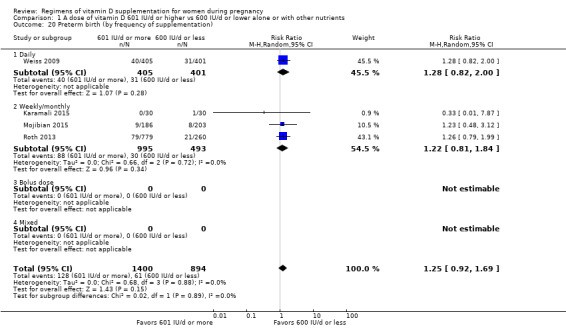
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 20 Preterm birth (by frequency of supplementation).
1.24. Analysis.
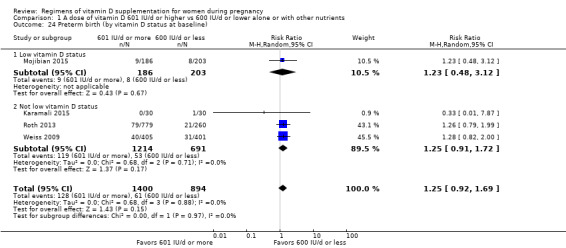
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 24 Preterm birth (by vitamin D status at baseline).
1.23. Analysis.
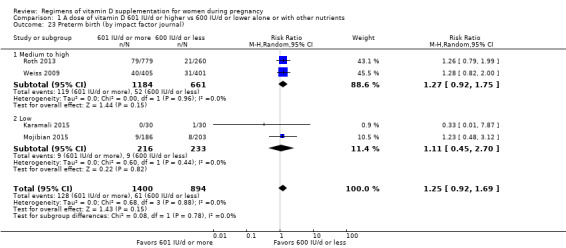
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 23 Preterm birth (by impact factor journal).
1.25. Analysis.
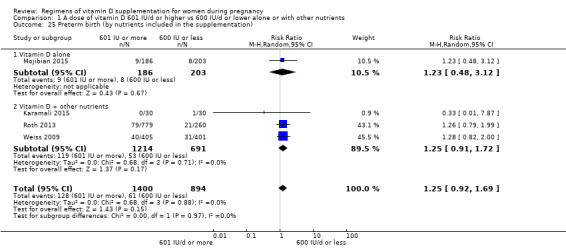
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 25 Preterm birth (by nutrients included in the supplementation).
1.19. Analysis.
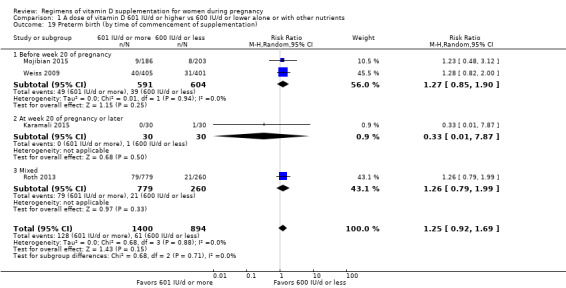
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 19 Preterm birth (by time of commencement of supplementation).
1.21. Analysis.
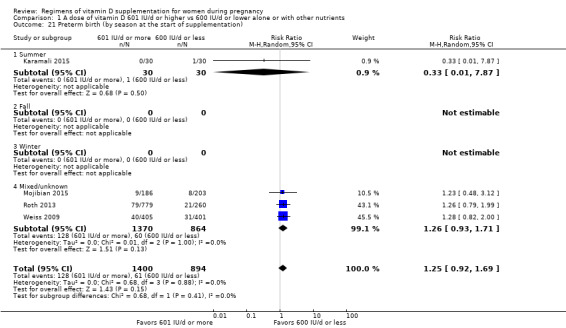
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 21 Preterm birth (by season at the start of supplementation).
1.22. Analysis.
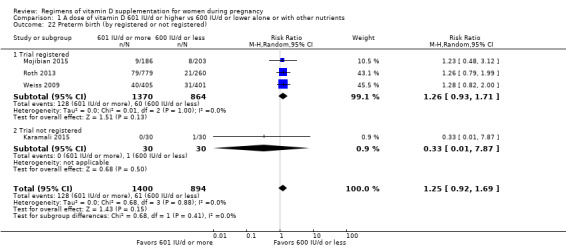
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 22 Preterm birth (by registered or not registered).
Low birthweight
Data from four trials (Karamali 2015; Mojibian 2015; O'Brien 2013; Roth 2013) involving 1550 women appear to suggest a similar risk between those consuming 601 IU/d or more and those consuming 600 IU/d or less of vitamin D (RR 0.90, 95% CI 0.66 to 1.24; very low‐certainty evidence; Analysis 1.26). Subgroup analysis did not appear to show an effect by impact factor of the journal in which the trial was published (Analysis 1.31), or by nutrients included in the supplementation (Analysis 1.33). The following subgroup analyses did not have enough trials in each subgroup for meaningful subgroup analysis: frequency of supplementation: time of commencement (Analysis 1.27); frequency of supplementation (Analysis 1.28); season (Analysis 1.29); registration of the protocol (Analysis 1.30); vitamin D status at baseline (Analysis 1.32). Following the planned sensitivity analysis, after excluding (O'Brien 2013), which was classified as low quality, the effect changed very slightly to RR 0.88, 95% CI 0.67 to 1.15.
1.26. Analysis.
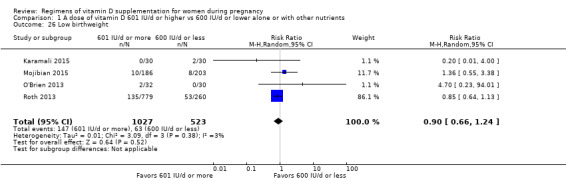
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 26 Low birthweight.
1.31. Analysis.
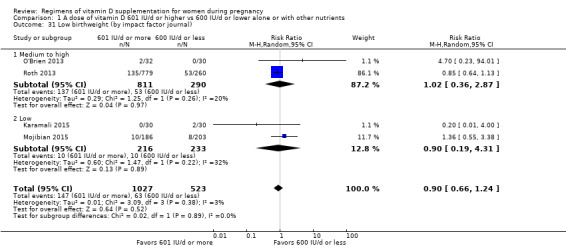
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 31 Low birthweight (by impact factor journal).
1.33. Analysis.
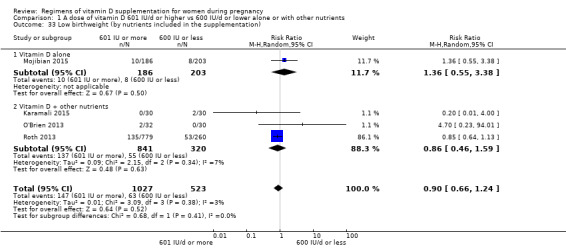
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 33 Low birthweight (by nutrients included in the supplementation).
1.27. Analysis.
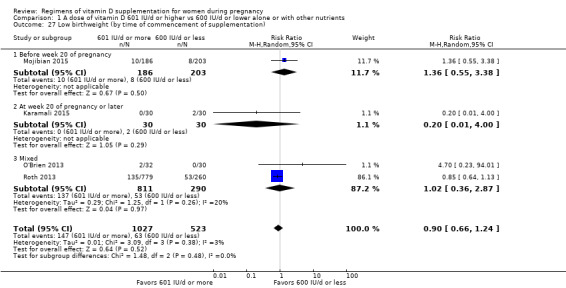
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 27 Low birthweight (by time of commencement of supplementation).
1.28. Analysis.
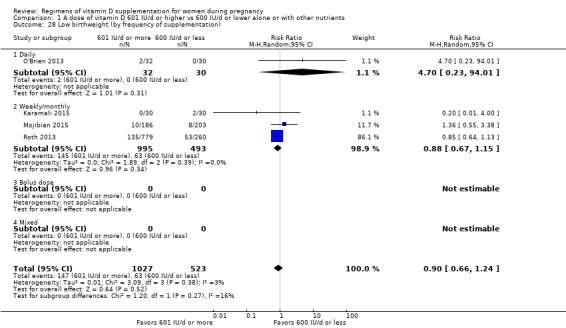
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 28 Low birthweight (by frequency of supplementation).
1.29. Analysis.
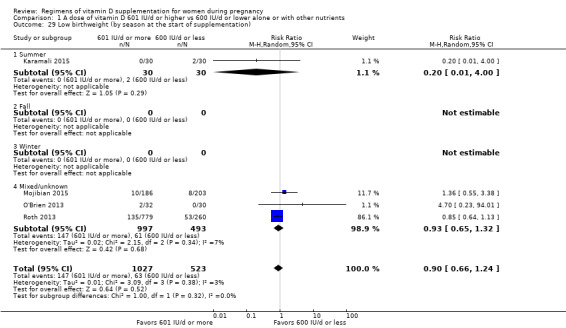
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 29 Low birthweight (by season at the start of supplementation).
1.30. Analysis.
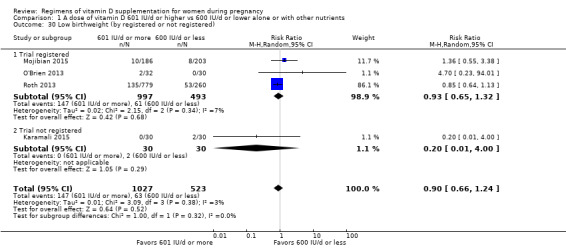
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 30 Low birthweight (by registered or not registered).
1.32. Analysis.
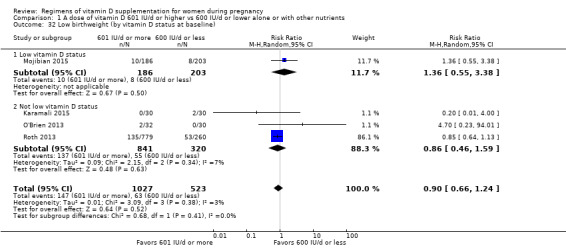
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 32 Low birthweight (by vitamin D status at baseline).
Secondary outcomes
Maternal
Fasting glucose levels
Only one trial reported this outcome (Soheilykhah 2011); therefore, no conclusions can be made.
Caesarean section
Data from five trials including 2419 women (Roth 2013; Stephensen 2011; Wagner 2006b; Weiss 2009; Yap 2014) found there is little or no difference in risk of caesarean section between those consuming 601 IU/d or more and those consuming 600 IU/d or less of vitamin D (RR 0.91, 95% CI 0.78 to 1.07; Analysis 1.35).
1.35. Analysis.
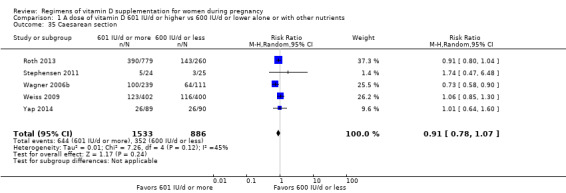
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 35 Caesarean section.
Maternal death
Only one trial reported this outcome (Roth 2013) involving 1039 women; those who received 601 IU/d or more of vitamin D had a similar risk of death compared to those receiving 600 IU/d or less (RR 0.11, 95% CI 0.00 to 2.73; Analysis 1.36).
1.36. Analysis.
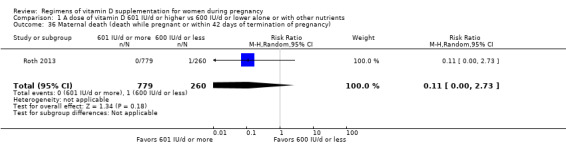
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 36 Maternal death (death while pregnant or within 42 days of termination of pregnancy).
Maternal vitamin D concentration at term (25‐hydroxyvitamin D in nmol/L)
The data from 16 trials (Abotorabi 2017; Bhatia 2012; Das 2010; Dawodu 2013; Hashemipour 2014; Karamali 2015; Kiely 2015; March 2010; Mojibian 2015; Mutlu 2014; O'Brien 2013; Roth 2013; Soheilykhah 2011; Thiele 2014; Wagner 2006b; Weiss 2009) involving 3107 women consistently showed that women consuming 601 IU/d or more of vitamin D supplementation had higher 25‐hydroxyvitamin D concentrations than those women consuming 600 IU/d or less of vitamin D. The average mean difference (MD) between groups was 29.65 nmol 25‐hydroxyvitamin D per litre (95% CI 21.90 to 37.40; Analysis 1.37). The response to supplementation was highly heterogeneous (Tau² = 231.17, I² = 95% and Chi² test for heterogeneity P < 0.00001). Therefore, this result should be interpreted with caution.
1.37. Analysis.
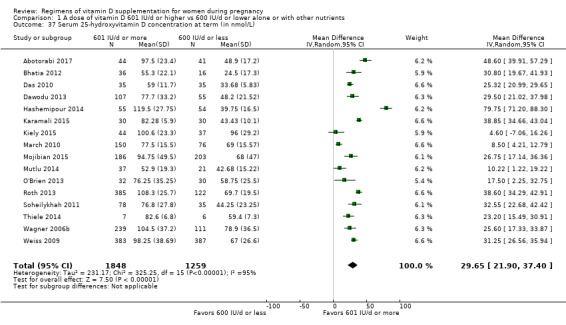
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 37 Serum 25‐hydroxyvitamin D concentration at term (in nmol/L).
Gestational hypertension
Data from four trials (Mojibian 2015; Roth 2013; Stephensen 2011; Yap 2014) involving 1656 women found that there was little or no difference in risk of gestational hypertension between women who received 601 IU/d or more of vitamin D and those receiving 600 IU/d or less (RR 1.10, 95% CI 0.63 to 1.91; Analysis 1.38).
1.38. Analysis.
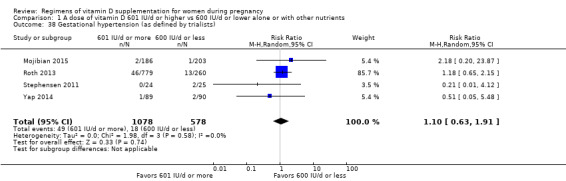
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 38 Gestational hypertension (as defined by trialists).
Infant
Length at birth (cm)
The data from 11 trials (Abotorabi 2017; Bhatia 2012; Dawodu 2013; Hashemipour 2014; Kalra 2012; Karamali 2015; Mojibian 2015; Roth 2013; Soheilykhah 2011; Thiele 2014; Wagner 2006b; Weiss 2009) involving 3058 women suggested a similar birth length among infants from women taking 601 IU/d or more of vitamin D compared to women receiving 600 IU/d or less (MD ‐0.04, 95% CI ‐0.26 to 0.19); Analysis 1.39). No heterogeneity was found.
1.39. Analysis.
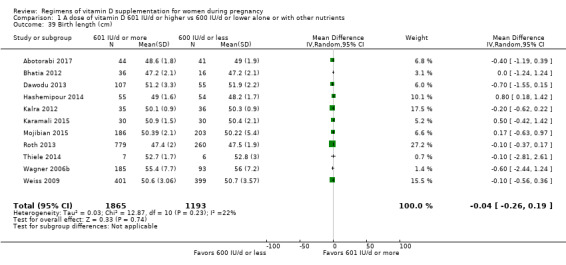
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 39 Birth length (cm).
Head circumference at birth (cm)
The data from 10 trials (Abotorabi 2017; Dawodu 2013; Hashemipour 2014; Kalra 2012; Karamali 2015; Mojibian 2015; Roth 2013; Thiele 2014; Wagner 2006b; Weiss 2009) involving 2998 women suggested little or no difference in head circumference among infants from women taking 601 IU/d or more of vitamin D compared to women receiving 600 IU/d or less (MD 0.08, 95% CI ‐0.09 to 0.25; Analysis 1.40). No heterogeneity was found.
1.40. Analysis.
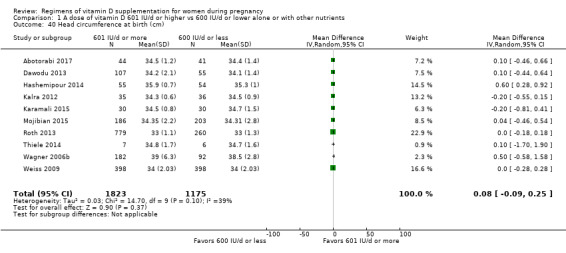
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 40 Head circumference at birth (cm).
Birthweight (g)
The data from 14 trials (Abotorabi 2017; Bhatia 2012; Dawodu 2013; Hashemipour 2014; Kalra 2012; Karamali 2015; Mojibian 2015; Mutlu 2014; O'Brien 2013; Roth 2013; Stephensen 2011; Thiele 2014; Wagner 2006b; Weiss 2009) involving 3300 women suggested a greater birthweight among infants from women taking 601 IU/d or more of vitamin D compared to women receiving 600 IU/d or less (MD 51.57, 95% CI 1.07 to 102.07; Analysis 1.41). The response to supplementation was heterogeneous (Tau² = 3077.36; I² = 42% and Chi² test for heterogeneity P = 0.05). Therefore, this result should be interpreted with caution.
1.41. Analysis.
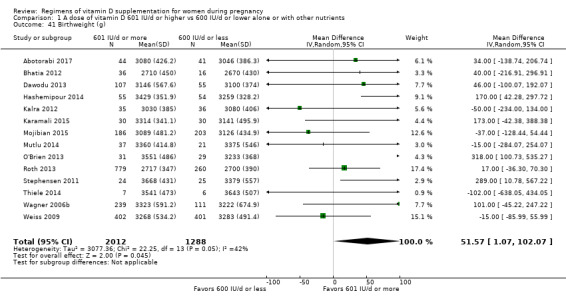
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 41 Birthweight (g).
Cord blood vitamin D concentration (25‐hydroxyvitamin D in nmol/L)
The data from nine trials (Bhatia 2012; Dawodu 2013; Hashemipour 2014; Kiely 2015; March 2010; Mojibian 2015; Roth 2013; Weiss 2009; Yap 2014) involving 2166 women consistently showed that women consuming 601 IU/d or more of vitamin D supplementation had higher cord blood 25‐hydroxyvitamin D concentrations than those women consuming 600 IU/d or less of vitamin D. The average MD between groups was 24.17 nmol 25‐hydroxyvitamin D per litre (95% CI 16.87 to 31.48; Analysis 1.42). The response to supplementation was highly heterogeneous (Tau² = 112, I² = 93% and Chi² test for heterogeneity P < 0.00001). Therefore, this result should be interpreted with caution.
1.42. Analysis.
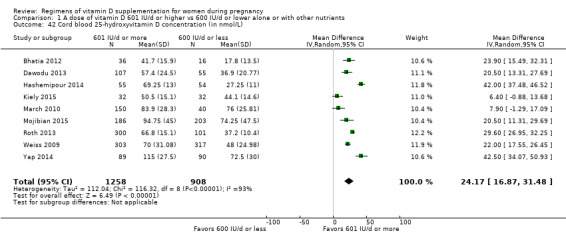
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 42 Cord blood 25‐hydroxyvitamin D concentration (in nmol/L).
Admission to special care unit
Two trials (Wagner 2006b; Weiss 2009) including 1226 women found little or no difference in the risk of admission to special care unit between those consuming 601 IU/d or more and those consuming 600 IU/d or less of vitamin D (RR 1.16, 95% CI 0.79 to 1.70; Analysis 1.43).
1.43. Analysis.
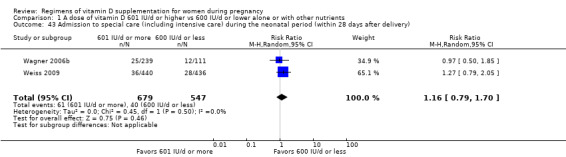
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 43 Admission to special care (including intensive care) during the neonatal period (within 28 days after delivery).
Perinatal death
No trial reported this outcome.
Stillbirth (as defined by trialists)
Three trials (Roth 2013; Weiss 2009; Yap 2014) including 2094 women found little or no difference in the risk between those consuming 601 IU/d or more and those consuming 600 IU/d or less of vitamin D for stillbirth (RR 1.23, 95% CI 0.67 to 2.25; Analysis 1.44).
1.44. Analysis.
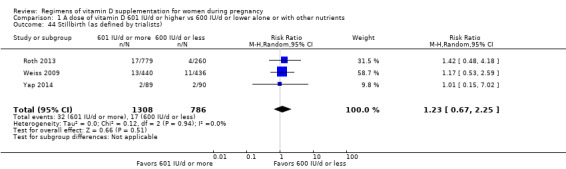
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 44 Stillbirth (as defined by trialists).
Neonatal death (within 28 days after delivery)
Two trials (Roth 2013; Weiss 2009) including 1915 women found little or no difference in the risk of neonatal death between those consuming 601 IU/d or more and those consuming 600 IU/d or less of vitamin D (RR 0.99, 95% CI 0.20 to 4.88; Analysis 1.45).
1.45. Analysis.
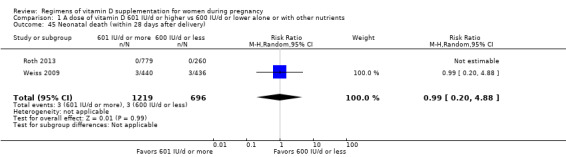
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 45 Neonatal death (within 28 days after delivery).
Apgar score less than seven at five minutes
Only one trial reported this outcome (Stephensen 2011); therefore, no conclusions can be made (Analysis 1.46).
1.46. Analysis.
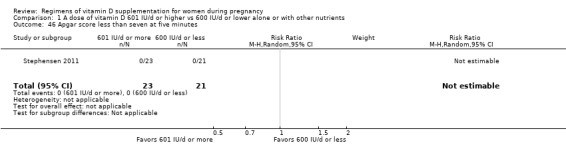
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 46 Apgar score less than seven at five minutes.
Neonatal infection
No trial reported this outcome.
Very preterm birth
Two trials (Roth 2013; Weiss 2009) including 1915 women found little or no difference in the risk of very preterm birth between those consuming 601 IU/d or more and those consuming 600 IU/d or less of vitamin D (RR 0.56, 95% CI 0.18 to 1.72; Analysis 1.47).
1.47. Analysis.
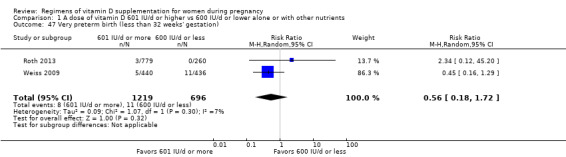
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 47 Very preterm birth (less than 32 weeks' gestation).
(2) A dose of vitamin D 4000 IU/d or more versus 3999 IU/d or less alone or with any other nutrient
A total of 15 trials involving 4763 women were included in this comparison: Abotorabi 2017; Bacqui 2009; Dawodu 2013; Hashemipour 2014; Kalra 2012; Karamali 2015; Marya 1981; Rostami 2017; Roth 2013; Soheilykhah 2011; Thiele 2014; Wagner 2006a; Wagner 2006b; Weiss 2009; Yap 2014.
The following trials were assessed as having low risk of bias: Dawodu 2013; Hashemipour 2014; Karamali 2015; Roth 2013; Wagner 2006a; Wagner 2006b; Weiss 2009. The following had mixed results, with some components having a high risk, low risk or unclear: Abotorabi 2017; Bacqui 2009; Kalra 2012; Marya 1981; Rostami 2017; Thiele 2014; Yap 2014. One study was judged a high risk of bias: Soheilykhah 2011.
We could not perform subgroup analysis in any of the outcomes for skin pigmentation as none of the studies in this comparison specified this. In addition, we could not perform the subgroup analysis on latitude as all studies were done outside the tropics. The data from Rostami 2017 was provided directly by the author as it was not reported by groups in the publication. Also, we did not perform subgroup analysis by nutrients in the supplementation for this comparison.
Primary outcomes
Maternal
Pre‐eclampsia
Data from four trials (Karamali 2015; Rostami 2017; Weiss 2009; Yap 2014) involving 1903 women may make little or no difference in risk of pre‐eclampsia between those women who received 4000 IU/d or more of vitamin D supplements compared to those women receiving 3000 IU/d or less (RR 0.87, 95% CI 0.62 to 1.22; low‐certainty evidence; Analysis 2.1). Subgroup analysis did not appear to show an effect by time of commencement of supplementation (Analysis 2.2), by frequency of supplementation (Analysis 2.3), by season (Analysis 2.4), by registration of protocol (Analysis 2.5), by impact factor of the journal in which the trial was published (Analysis 2.6) or vitamin D status at the start of the trial (Analysis 2.7).
2.1. Analysis.
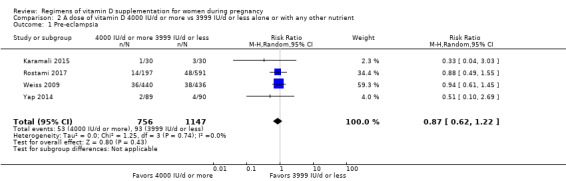
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 1 Pre‐eclampsia.
2.2. Analysis.
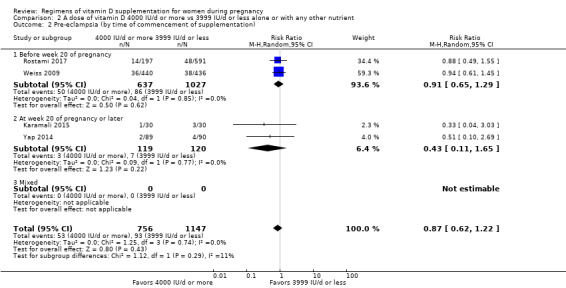
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 2 Pre‐eclampsia (by time of commencement of supplementation).
2.3. Analysis.
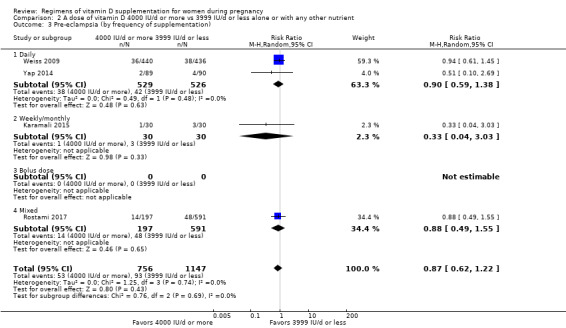
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 3 Pre‐eclampsia (by frequency of supplementation).
2.4. Analysis.
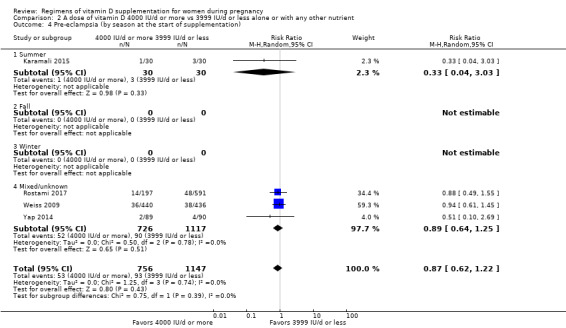
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 4 Pre‐eclampsia (by season at the start of supplementation).
2.5. Analysis.
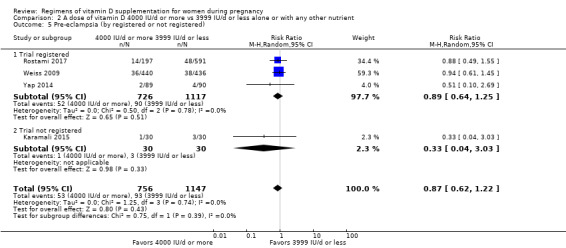
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 5 Pre‐eclampsia (by registered or not registered).
2.6. Analysis.
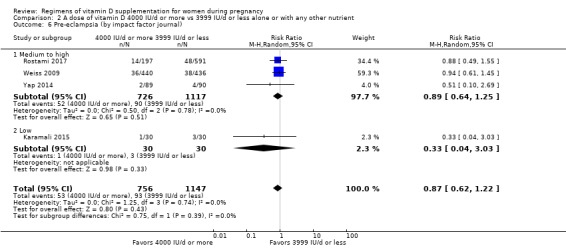
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 6 Pre‐eclampsia (by impact factor journal).
2.7. Analysis.
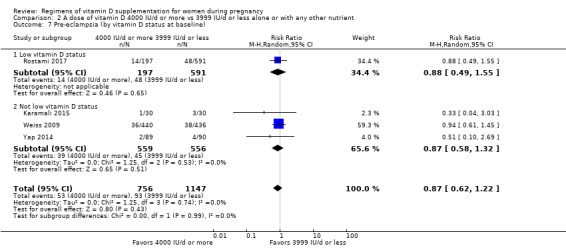
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 7 Pre‐eclampsia (by vitamin D status at baseline).
Gestational diabetes
Data from five trials (Hashemipour 2014; Rostami 2017; Roth 2013; Wagner 2006a; Yap 2014) involving 2276 women suggest little or no difference in risk of gestational diabetes between those women who received 4000 IU/d or more of vitamin D compared to women receiving 3999 IU/d or less (RR 0.89, 95% CI 0.56 to 1.42; low‐certainty evidence; Analysis 2.8). Subgroup analysis did not appear to show an effect by time of commencement of supplementation (Analysis 2.9), by frequency of supplementation (Analysis 2.10) or by impact factor of the journal in which the trial was published (Analysis 2.13) or by vitamin D status at the start of the study (Analysis 2.14). There were not enough trials to make conclusions by pre‐pregnancy BMI (Analysis 2.11).
2.8. Analysis.
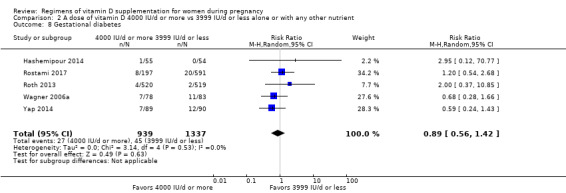
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 8 Gestational diabetes.
2.9. Analysis.
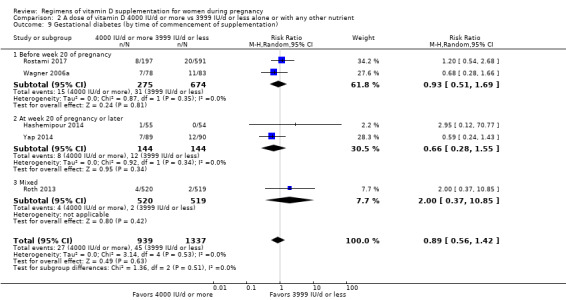
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 9 Gestational diabetes (by time of commencement of supplementation).
2.10. Analysis.
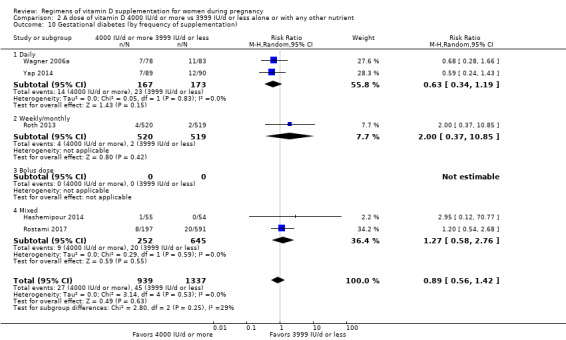
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 10 Gestational diabetes (by frequency of supplementation).
2.13. Analysis.
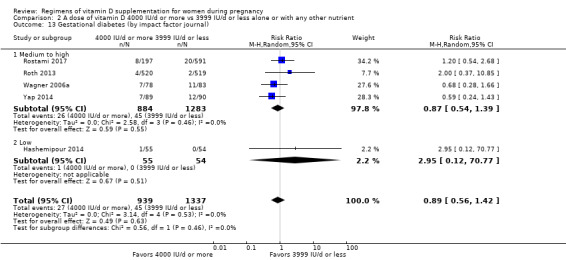
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 13 Gestational diabetes (by impact factor journal).
2.14. Analysis.
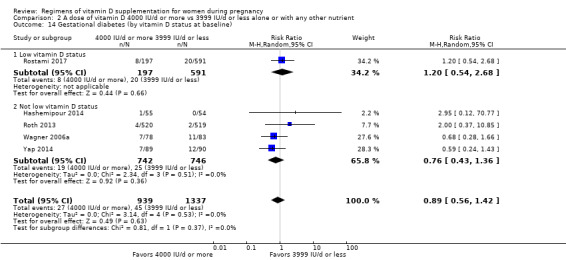
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 14 Gestational diabetes (by vitamin D status at baseline).
2.11. Analysis.
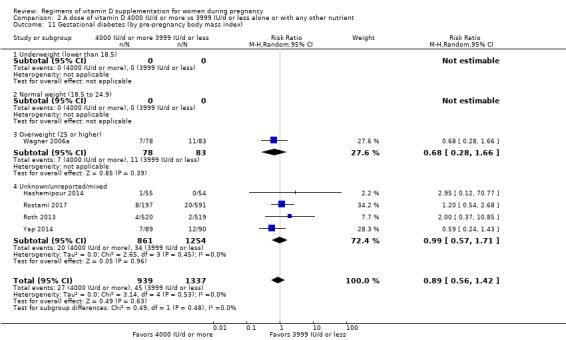
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 11 Gestational diabetes (by pre‐pregnancy body mass index).
Adverse effects
Because adverse events were reported differently in most trials, we reported this outcome in a narrative way.
Abotorabi 2017 mentioned that after eight weeks of vitamin D3 supplementation, there were no side effects such as hypercalcaemia. Calcium level was higher in the treatment group (50,000 IU per week) compared to the control group (400 IU/d) but the difference was not significant. They also mentioned that the prevalence of hypocalcaemia was not different between treatment groups.
Bacqui 2009 reported that there were no known supplement‐related clinical adverse events.
Dawodu 2013 reported that quote: “DMSC did not find adverse events attributable to vitamin D".
Hashemipour 2014 reported that no cases of congenital malformations occurred in either group.
Kalra 2012 reported that frequency of hypocalcaemia did not differ among groups.
Karamali 2015 reported no difference in neonatal hypocalcaemia.
Marya 1981 did not report on adverse effects.
Rostami 2017 reported that there were no adverse outcome or complaints of clinical features attributable to supplementation.
Roth 2013 reported that there was a higher risk of maternal hypercalciuria at delivery among the group receiving 28,000 IU per week. Also, they reported that there were two asymptomatic cases of maternal confirmed hypercalciuria, one in mothers randomised to the placebo group and one in mothers randomised to 28,000 IU per week. None of the women with confirmed hypercalcaemia or hypercalciuria had serious adverse events (hospitalisations or deaths) or urinary tract stones. Two of the six infants with confirmed hypercalcaemia were hospitalised but for other reasons. One infant had confirmed hypercalciuria in the 4200 IU per week group.
Soheilykhah 2011 reported that pregnant women consuming 50,000 IU vitamin D every two weeks had no adverse effects, such as hypercalcaemia.
Thiele 2014 reported no differences in maternal serum calcium or parathyroid hormones between groups.
Wagner 2006a reported that there were no supplement‐related adverse events.
Wagner 2006b reported that quote: “Review of adverse events by the DSMC showed that not a single adverse event in this trial was attributed to vitamin D supplementation”.
Weiss 2009 reported that there were no cases of maternal symptomatic hypercalcaemia in either arm of the trial but reported a maternal death two years post‐birth in the 400 IU/day vitamin D group but no deaths in the 4400 IU/day vitamin D group. There were 44 mothers hospitalised in each group in this study (111 events in these 88 mothers).
Yap 2014 reported no maternal hypervitaminosis D (serum 25(OH)D level > 100 ng/mL) in any of the groups, although they did report one case of mild maternal hypercalcaemia in the high‐vitamin D group (5000 IU/d) and another case in the low‐vitamin D group (400 IU/d). They also reported one neonate in the high‐vitamin D group with high cord 25(OH)D levels (> 102 ng/mL), although this was not associated with hypercalcaemia or other clinical adverse effects. Lastly, they reported similar serum calcium levels in neonates in all groups, although they found cases isolated cases of infant hypercalcaemia on cord blood samples that were not clinically relevant, as it dissipated within a few days of life.
Infant
Preterm birth
Data from six trials (Bacqui 2009; Karamali 2015; Rostami 2017; Roth 2013; Wagner 2006a; Weiss 2009) involving 2948 women suggest little or no difference in risk of preterm birth between those women consuming 4000 IU/d or more and those consuming 3999 IU/d or less of vitamin D (RR 0.85, 95% CI 0.64 to 1.12; low‐certainty evidence; Analysis 2.15). Subgroup analysis did not appear to show an effect by time of commencement of supplementation (Analysis 2.16), by frequency of supplementation (Analysis 2.17), by vitamin D status at baseline (Analysis 2.22) or by impact factor of the journal in which the trial was published (Analysis 2.21). The other subgroup analyses did not have enough trials in each subgroup to make conclusions: Analysis 2.17; Analysis 2.18; Analysis 2.19; Analysis 2.20.
2.15. Analysis.
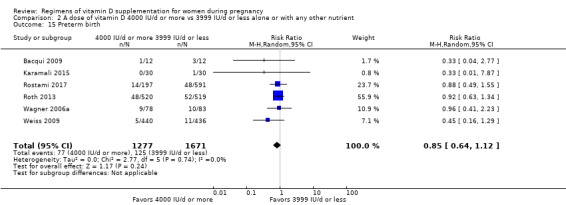
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 15 Preterm birth.
2.16. Analysis.
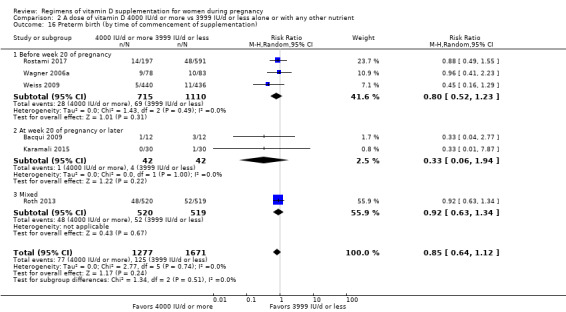
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 16 Preterm birth (by time of commencement of supplementation).
2.17. Analysis.
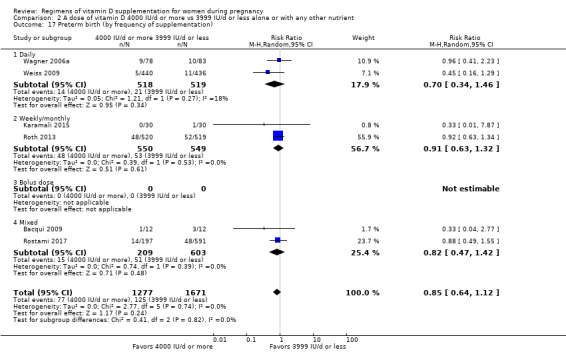
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 17 Preterm birth (by frequency of supplementation).
2.22. Analysis.
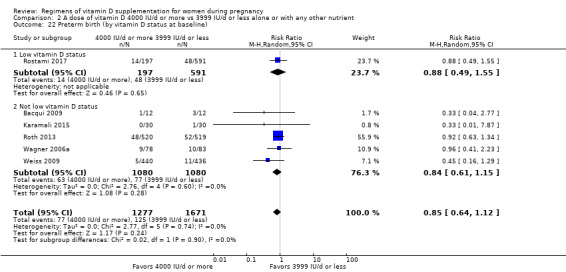
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 22 Preterm birth (by vitamin D status at baseline).
2.21. Analysis.
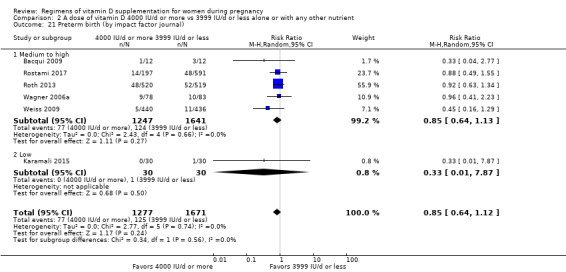
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 21 Preterm birth (by impact factor journal).
2.18. Analysis.
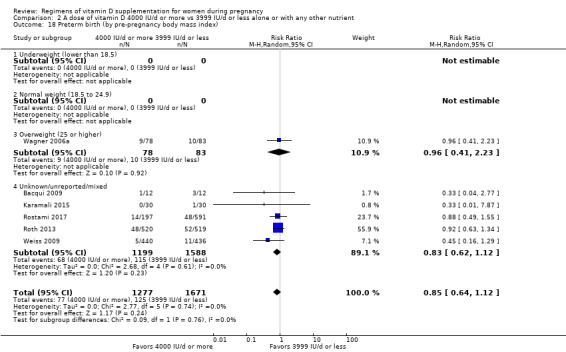
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 18 Preterm birth (by pre‐pregnancy body mass index).
2.19. Analysis.
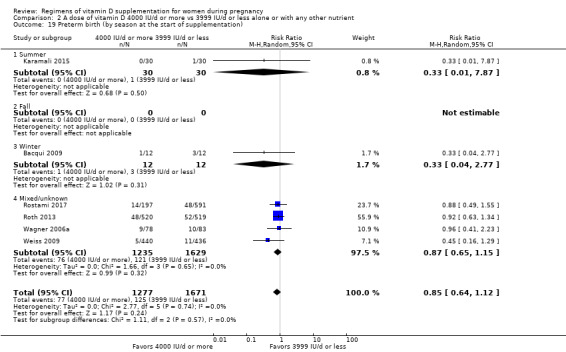
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 19 Preterm birth (by season at the start of supplementation).
2.20. Analysis.
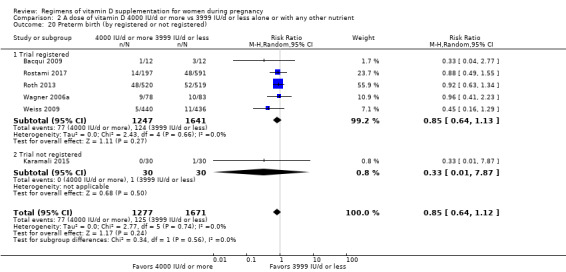
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 20 Preterm birth (by registered or not registered).
Low birthweight
Data from two trials (Karamali 2015; Roth 2013) involving 1099 women may suggest little or no difference in low birthweight between those consuming 4000 IU/d or more and those consuming 3999 IU/d or less of vitamin D (RR 0.92, 95% CI 0.49 to 1.70); low‐certainty evidence; Analysis 2.23). Subgroup analyses were not conducted due to the low number of trials.
2.23. Analysis.
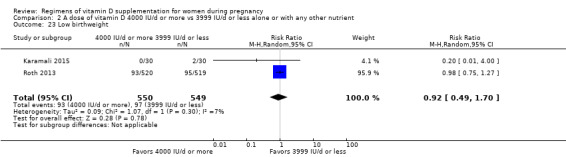
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 23 Low birthweight.
Secondary outcomes
Maternal
Fasting glucose levels
Only one trial reported this outcome (Soheilykhah 2011); therefore, no conclusions can be made (Analysis 2.24).
2.24. Analysis.
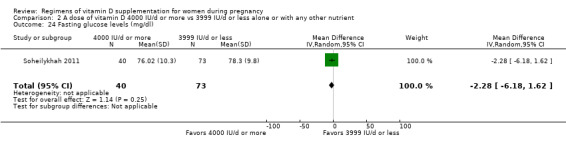
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 24 Fasting glucose levels (mg/dl).
Caesarean section
Data from seven trials including 3343 women (Bacqui 2009; Rostami 2017; Roth 2013; Wagner 2006a; Wagner 2006b; Weiss 2009; Yap 2014) suggest little or no difference in risk of caesarean section between those women who consuming 4000 IU/d or more and those consuming 3999 IU/d or less of vitamin D (RR 1.06, 95% CI 0.93 to 1.20; Analysis 2.25).
2.25. Analysis.
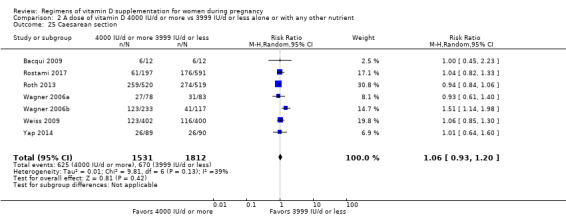
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 25 Caesarean section.
Maternal death
Only one trial reported this outcome (Roth 2013) involving 1039 women; those who received 601 IU/d or more of vitamin D had a similar risk of death compared to those receiving 600 IU/d or less (RR 0.33, 95% CI 0.01 to 8.15; Analysis 2.26).
2.26. Analysis.
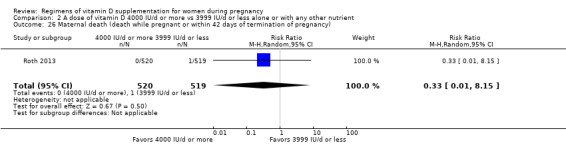
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 26 Maternal death (death while pregnant or within 42 days of termination of pregnancy).
Maternal vitamin D concentration at term (25‐hydroxyvitamin D in nmol/L)
The data from 11 trials (Abotorabi 2017; Bacqui 2009; Dawodu 2013; Hashemipour 2014; Karamali 2015; Rostami 2017; Roth 2013; Soheilykhah 2011; Thiele 2014; Wagner 2006b; Weiss 2009) involving 2981 women consistently showed that women consuming 4000 IU/d or more of vitamin D supplementation had higher 25‐hydroxyvitamin D concentrations than those women consuming 3999 IU or less of vitamin D. The average MD between groups was 31.61 nmol 25‐hydroxyvitamin D per litre higher (95% CI 20.83 to 42.38; Analysis 2.27). The response to supplementation was highly heterogeneous (Tau² = 319.40, I² = 98% and Chi² test for heterogeneity P < 0.00001). Therefore, this result should be interpreted with caution.
2.27. Analysis.
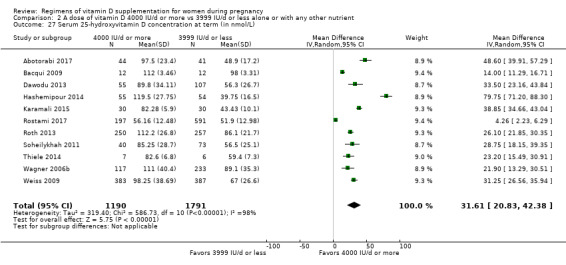
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 27 Serum 25‐hydroxyvitamin D concentration at term (in nmol/L).
Gestational hypertension
Data from three trials (Roth 2013; Wagner 2006a; Yap 2014) involving 1379 women found little or no difference in risk of gestational hypertension between those women who received 4000 IU/d or more of vitamin D and those women receiving 3999 IU or less (RR 1.08, 95% CI 0.67 to 1.74; Analysis 2.28).
2.28. Analysis.
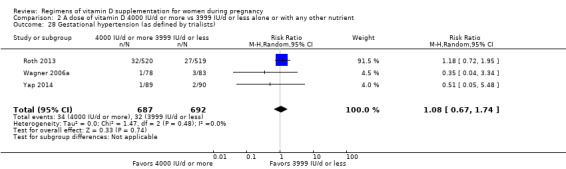
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 28 Gestational hypertension (as defined by trialists).
Infant
Length at birth (cm)
The data from 10 trials (Abotorabi 2017; Dawodu 2013; Hashemipour 2014; Kalra 2012; Karamali 2015; Rostami 2017; Roth 2013; Thiele 2014; Wagner 2006a; Weiss 2009) involving 3288 women suggest little or no difference in length at birth among infants from women in both groups (MD 0.05, 95% CI ‐0.26 to 0.36; Analysis 2.29). The response to supplementation was highly heterogeneous (Tau² = 0.12, I² = 64% and Chi² test for heterogeneity P < 0.003). Therefore, this result should be interpreted with caution.
2.29. Analysis.
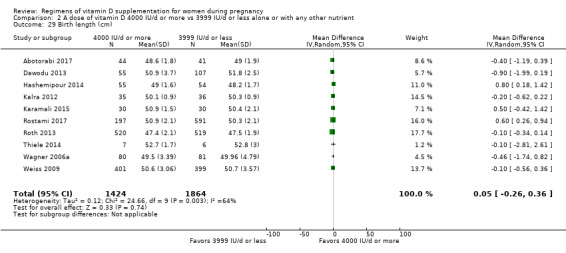
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 29 Birth length (cm).
Head circumference at birth (cm)
The data from 10 trials (Abotorabi 2017; Dawodu 2013; Hashemipour 2014; Kalra 2012; Karamali 2015; Rostami 2017; Roth 2013; Thiele 2014; Wagner 2006a; Weiss 2009) involving 3278 women suggest a similar head circumference among infants from women in both groups (MD 0.10, 95% CI ‐0.09 to 0.29; Analysis 2.30). The response to supplementation was highly heterogeneous (Tau² = 0.05, I² = 66% and Chi² test for heterogeneity P = 0.001). Therefore, this result should be interpreted with caution.
2.30. Analysis.
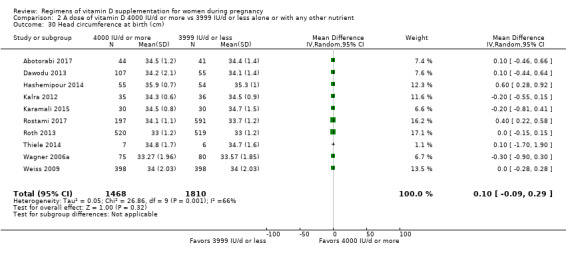
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 30 Head circumference at birth (cm).
Birthweight (g)
The data from 13 trials (Abotorabi 2017; Bacqui 2009; Dawodu 2013; Hashemipour 2014; Kalra 2012; Karamali 2015; Marya 1981; Rostami 2017; Roth 2013; Thiele 2014; Wagner 2006a; Wagner 2006b; Weiss 2009) involving 3710 women suggest little or no difference in birthweight among infants from women in both groups (MD 46.00, 95% CI ‐8.99 to 101.00; Analysis 2.31). The response to supplementation was highly heterogeneous (Tau² = 4217.58, I² = 56% and Chi² test for heterogeneity P = 0.008). Therefore, this result should be interpreted with caution.
2.31. Analysis.
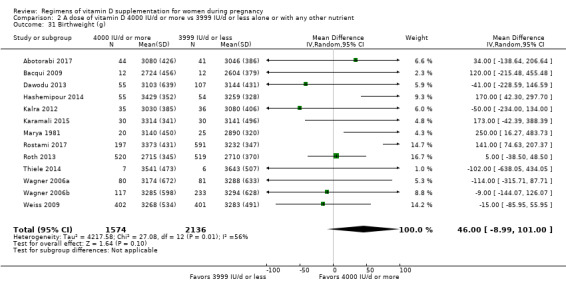
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 31 Birthweight (g).
Cord blood vitamin D concentration (25‐hydroxyvitamin D in nmol/L)
The data from seven trials (Bacqui 2009; Dawodu 2013; Hashemipour 2014; Rostami 2017; Roth 2013; Weiss 2009; Yap 2014) involving 2283 women consistently showed that women consuming 4000 IU/d or more of vitamin D supplementation had higher cord blood 25‐hydroxyvitamin D concentrations than those women consuming 3999 IU or less of vitamin D. The average MD between groups was 23.84 nmol 25‐hydroxyvitamin D per litre (95% CI 13.55 to 34.13; Analysis 2.32). The response to supplementation was highly heterogeneous (Tau² = 186.28, I² = 98% and Chi² test for heterogeneity P < 0.00001); therefore, this result should be interpreted with caution.
2.32. Analysis.
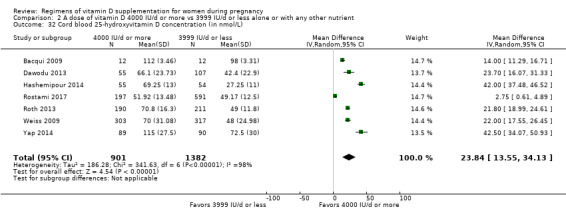
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 32 Cord blood 25‐hydroxyvitamin D concentration (in nmol/L).
Admission to special care unit
Only one trial reported this outcome (Wagner 2006b); therefore, no conclusions can be made (Analysis 2.33).
2.33. Analysis.
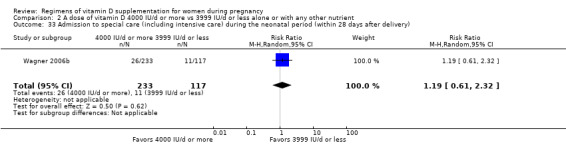
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 33 Admission to special care (including intensive care) during the neonatal period (within 28 days after delivery).
Perinatal death
No trial reported this outcome.
Stillbirth (as defined by trialists)
Four trials (Rostami 2017; Roth 2013; Weiss 2009; Yap 2014) including 2882 women found little or no difference between those consuming 4000 IU/d or more and those consuming 3999 IU or less of vitamin D for stillbirth (RR 1.37, 95% CI 0.75 to 2.51; Analysis 2.34).
2.34. Analysis.
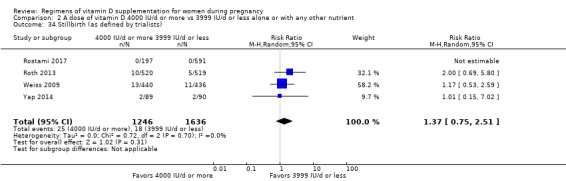
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 34 Stillbirth (as defined by trialists).
Neonatal death (within 28 days after delivery)
Three trials (Bacqui 2009; Roth 2013; Weiss 2009) followed 1939 women to report on this data but no death was reported; therefore, the RR could not be estimated (Analysis 2.35).
2.35. Analysis.
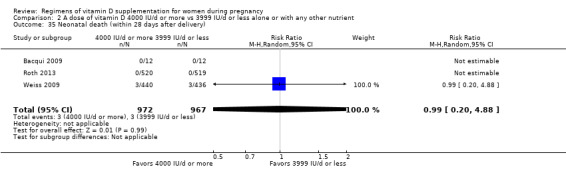
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 35 Neonatal death (within 28 days after delivery).
Apgar score less than seven at five minutes
No trial reported this outcome.
Neonatal infection
No trial reported this outcome.
Very preterm birth
No trial reported this outcome.
Discussion
Summary of main results
This review evaluated if there are beneficial effects of supplementing pregnant women with more than the current vitamin D recommendation (200 IU/d‐ to ‐600 IU/d), alone or in combination with other nutrients, on pregnancy and neonatal health outcomes. We also evaluated if there are negative effects of supplementing pregnant women with more than the current upper limit recommendation of vitamin D (4000 IU/d) on these outcomes.
We included 30 trials involving 7289 women, 19 of which compared a dose of vitamin D 601 IU/d or higher versus 600 IU/d or lower alone or with other nutrients and 15 trials compared a dose of vitamin D 4000 IU/d or more versus 3999 IU/d or less alone or with any other nutrient. From the trials included in the comparison of 601 IU/d or more versus 600 IU/d or less of vitamin D supplementation, six trials provided vitamin D alone, two trials provided vitamin D plus calcium, and 11 trials provided vitamin D with other vitamins and/or minerals.
Supplementation with 601 IU/d or more of vitamin D (+ any other nutrient) compared to 600 IU/d or less of vitamin D (+ any other nutrient)
May make little or no difference to the risk of pre‐eclampsia (five 5 trials); preterm birth (four trials) or low birthweight (four trials).
May reduce the risk of gestational diabetes (five trials).
Seems to be safe, as most trials reported little to no side effects (such as hypercalcaemia, hypercalciuria, hypocalcaemia, and hypervitaminosis D) with the intervention.
Probably increases the levels of 25‐hydroxyvitamin D at term in the mother (16 trials) and levels of cord blood 25‐hydroxyvitamin D (nine trials). However, the response to supplementation was highly heterogeneous.
Supplementation with 4000 IU/d or more of vitamin D (+any other nutrient) compared to a dose of 3999 IU/d or less of vitamin D (+ any other nutrient)
May make little or no difference to the risk of pre‐eclampsia (four trials), gestational diabetes (five trials), preterm birth (six trials), or low birthweight (two trials).
Seems to be safe, as most trials reported little to no side effects with the intervention.
Probably increases the levels of 25‐hydroxyvitamin D at term in the mother (11 trials) and levels of cord blood 25‐hydroxyvitamin D (seven trials). However, the response to supplementation was highly heterogeneous.
It is important to note that heterogeneity was detected for maternal and cord blood 25‐hydroxyvitamin D. These inconsistencies could be related to the different doses used in the included trials, different treatment regimens, different populations, the quality of the trials, and also in the difference in methods to assess serum 25‐hydroxyvitamin D. This biomarker is difficult and complex, with high variability in results depending on the methods used (Holick 2008). Also, heterogeneity was detected for birthweight. Therefore, results should be interpreted with caution. In addition, most trials used vitamin D supplementation in the form of cholecalciferol‐D3, which may have potential implications for defining guidelines on vitamin D supplementation.
With respect to safety, although it appears that vitamin D supplementation is a safe intervention during pregnancy as reported in the trials included, the ranges used to define hypercalcaemia, hypercalciuria, hypocalcaemia and hypervitaminosis D were either not included or varied widely between trials. Also, most trials did not show the results in the tables but rather say or report that no adverse events were observed. Therefore, future trials should be consistent in their reports of adverse events.
Overall completeness and applicability of evidence
Vitamin D supplementation during pregnancy aims to improve maternal and neonatal outcomes. In this review we aimed to compare different doses. In particular, we compared 601 IU/d or more versus 600 IU/d or less as most countries/associations/organisations recommend doses of 200 IU/d to 600 IU/d during pregnancy. We also compared 4000 IU/d or more versus 3999 IU/d or less, as 4000 IU/d is the upper limit established by the Institute of Medicine in the USA (IOM 2011). We did not include data from trials comparing results with placebo (no vitamin D supplementation) as that was recently reported by another review (De Regil 2016) and recently updated (Palacios 2019). Although in this review we included 30 trials, most of the secondary outcomes were only reported in a few trials. The exception was maternal levels of vitamin D at term and cord blood vitamin D levels, which were reported in many trials. In addition, adverse events, such as hypercalcaemia, hypercalciuria, hypocalcaemia, and hypervitaminosis D were either not clearly defined or the range to define these differed greatly between studies.
To the best of our knowledge, there are currently 16 ongoing trials that, once published, will further increase the body of evidence. After their publication and overall assessment, conclusions on the effects of this intervention may be updated. However, many of these randomised controlled trials will probably never be published as some either completed recruitment several years ago and no results have been published, or, never even started.
Quality of the evidence
Among the studies contributing to data, risk of bias was low in 11 studies and high in six studies. Also, there were nine trials with three or more components that were unclear or had a combination of unclear and high risk for one to two components. The main risk was related to risk of bias for blinding and allocation concealment (seeRisk of bias in included studies).
We evaluated the certainty of the body of evidence for the primary outcomes with the GRADE methodology. In comparison 1 (a dose of vitamin D 601 IU/d or more versus 600 IU/d or lower alone or with other nutrient; Table 1), we considered the risk of bias and the imprecision resulted in: evidence of moderate certainty for gestational diabetes, evidence of low certainty for pre‐eclampsia, preterm, and very low certainty for low birthweight.
In comparison 2 (a dose of vitamin D 4000 IU/d or more versus 3999 IU/d or less alone or with any other nutrient; Table 2), we considered the risk of bias, the imprecision and some indirectness resulted in: evidence of low certainty for pre‐eclampsia, gestational diabetes, preterm and low birthweight.
We also evaluated data in terms of trials being registered in one of the available registries, such as Clinicaltrials.gov, International Standard Randomised Controlled Trials Number (ISRCTN), Australian New Zealand Clinical Trials Registry (ANZCTR), Clinical Trials Registry – India (CTRI) and Iranian Registry of Clinical Trials (IRCT). Almost all trials were registered at one of these registries. Furthermore, we also evaluated the quality of the data indirectly by evaluating the impact factor of the journal in which the trial was published. However, this did not seem to have an effect, although there was a trend for a larger variability of the size of effects on trials published in low‐impact factor journals.
Potential biases in the review process
We identified several potential biases in the review process. They were minimised in two ways: (1) eligibility for inclusion and data extraction were assessed independently by two review authors and (2) assessments of risk of bias and data entry were also assessed independently by two review authors. However, this type of review requires that we make a number of subjective judgements and others may have reached different decisions regarding assessments of eligibility and risk of bias. We would encourage readers to examine the Characteristics of included studies tables to assist in the interpretation of results.
Agreements and disagreements with other studies or reviews
This review had somewhat different results compared to the updated Cochrane Review conducted by our group on vitamin D supplementation in pregnancy (Palacios 2019). In that review, only trials comparing vitamin D supplementation with placebo (no vitamin D) were included. Also, trials were carried out between the 1980s and 2015, in different seasons and in countries outside the tropics with populations of different ethnicity and cultures (Bangladesh, India, Iran, New Zealand and the UK). In the present review, trials were carried out more recently (2004 to 2017), in different seasons, and also in countries outside the tropics with populations of different ethnicity and cultures (Australia, Bangladesh, Iran and the USA). The Palacios 2019 review found that supplementing pregnant women with vitamin D alone compared to placebo or no vitamin D probably reduces the risk of pre‐eclampsia, gestational diabetes, low birthweight and may make little or no difference in the risk of having a preterm birth. However, in the present review, we only found that supplementation with 601 IU/d or more of vitamin D may reduce the risk of gestational diabetes. Therefore, it seems that any dose of vitamin D supplementation (200 IU/d to 600 IU/d or greater) may be enough to positively affect these maternal and neonatal health outcomes, but this needs to be confirmed in future trials.
We also compared our results to other systematic reviews and meta‐analysis published so far. One of these included 13 trials involving 2299 women and compared different doses of vitamin D supplementation during pregnancy, alone or with calcium, to a group that received 400 IU/d (control group) (Pérez‐López 2015). Similar to our review, they also found that a higher dose of vitamin D significantly increased serum 25‐hydroxyvitamin D at term, with a mean difference (MD) of 66.5 nmol/L (95% confidence interval (CI) 66.2 to 66.7; 10 trials; 1468 women) compared to the control group. Also, similar to ours, vitamin D supplementation makes little or no difference to the risk of pre‐eclampsia (three trials; 654 participants), low birthweight (four trials; 496 participants) and preterm birth (three trials; 384 participants). However, Pérez‐López 2015 found a higher birthweight among infants from mothers supplemented with vitamin D (10 trials; 1489 participants), which was similar to our results. However, contrary to our results, they did not find effects of vitamin D on the risk of gestational diabetes (three trials; 384 participants), but they found a greater birth length (six trials; 866 participants) with vitamin D supplementation. In another meta‐analysis of four trials involving 5871 women, vitamin D supplementation significantly reduced the risk of pre‐eclampsia compared with the control group (0 IU/d to 400 IU/d) (OR 0.66; 95% CI 0.52 to 0.83) (Hypönnen 2013). The meta‐analysis by Thorne‐Lyman 2012 found a 60% lower risk of low birthweight in women supplemented with vitamin D during pregnancy (three trials; 507 participants) compared to the control group (400 IU/d), similar to our results. Most recently, Roth 2017 published a comprehensive meta‐analysis of 43 trials involving 8406 women comparing any dose of vitamin D supplementation to a group that received equal or less than 600 IU/d (which included 0 IU/d). Similar to our review, vitamin D supplementation in pregnancy increased maternal and cord serum levels of 25‐hydroxyvitamin D. They also found that vitamin D increased mean birthweight (MD of 58.33 g; 95% CI 18.88 to 97.78; 37 trials; 5273 participants), with no effect on the risk of preterm birth or gestational diabetes. Similar to our findings, most outcomes were not reported and only 19% of the trials were assessed as low risk of bias.
With respect to safety, the trials reporting on maternal and infant safety‐related outcomes suggest that vitamin D supplementation in doses higher than 600 IU/d or doses of 4000 IU/d or greater appear to be safe during pregnancy. However, adverse events were rarely clearly identified or defined in the trials and they were reported differently, therefore, more trials with clearly defined adverse events are needed. Also, several of the secondary outcomes defined in this review (fasting glucose, maternal death, neonatal admission to intensive care unit, perinatal death, Apgar score less than seven at five minutes, neonatal infection or very preterm birth) were reported by either one trial or none.
It is important to note that studies have reported that vitamin D is needed early in pregnancy to detect clinical significant effects, as evidenced by Rostami 2017, one of the largest trials of vitamin D supplementation in pregnant women. In that trial, vitamin D supplementation in vitamin D deficient women early in pregnancy (before 14 weeks of pregnancy) resulted in significant reductions in the risk of pre‐eclampsia, gestational diabetes and preterm delivery compared to the control group. In fact, in vivo trials have demonstrated that the enzyme 1‐alpha‐hydroxylase, which catalyses the synthesis of 1,25 dihydroxy vitamin D3, has the highest level of expression in the first trimester, which is much less pronounced in the third trimester, highlighting its possible role as an autocrine/paracrine activator of vitamin D early in pregnancy (Zhender 2012). However, most trials on vitamin D supplementation start later in pregnancy, which may explain the lack of significant effects on most clinical outcomes.
Authors' conclusions
Implications for practice.
Supplementing pregnant women with more than the current vitamin D recommendation may reduce the risk of gestational diabetes; however, it may make little or no difference to the risk of pre‐eclampsia, preterm birth and low birthweight. Supplementing pregnant women with more than the current upper limit for vitamin D may make little or no difference to the risk of pre‐eclampsia, gestational diabetes, preterm birth or low birthweight. In general, the quality of the evidence was considered as "low certainty" for most of the primary outcomes. This grade was given due to the serious risk of bias of some of the trials contributing data and imprecision of results.
With respect to safety, it appears that vitamin D supplementation is a safe intervention during pregnancy as most studies reported no or few cases of adverse events such as hypercalcaemia, hypercalciuria, hypocalcaemia, and hypervitaminosis D. However, the parameters used to determine these events were either not reported or not consistent between trials. Future trials should be consistent in their reports of adverse events and specify the parameters used for each of these adverse events. There are 16 ongoing trials that when published, may increase the body of knowledge.
Implications for research.
Additional rigorous high quality and larger randomised trials are required to evaluate different vitamin D supplementation regimens in pregnancy. Future research could evaluate if in the same population different doses (low, medium and high), different frequencies (daily, weekly, monthly and bolus), different forms (tablets, liquids and injections), different types (D2 and D3), and different commencement periods (first trimester, second trimester and third trimester) in women with different degrees of body mass index, skin pigmentation and settings. Also, the effects of vitamin D supplementation in women with increased risk of gestational diabetes or pre‐eclampsia should be assessed.
The need to establish the dose‐dependent effects of vitamin D supplementation on maternal and infant outcomes was also suggested by the Working Group convened by the Sackler Institute for Nutrition Science and the Bill & Melinda Gates Foundation D deficiency (Roth 2018). This information is needed to inform policy‐making before vitamin D supplementation can be established as routine antenatal care.
Acknowledgements
The World Health Organization and Cristina Palacios, Paul Lips, James A Salisi, Jessica C John, Lucero Lopez‐Perez, Ricardo X Martinez and Maria Angelica Trak‐Fellermeier retain copyright and all other rights in their respective contributions to the manuscript of this review as submitted for publication.
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), two members of Cochrane Pregnancy and Childbirth's international panel of consumers and the Group's Statistical Adviser. The authors are grateful to the following peer reviewer for her time and comments: Dr Alice Rumbold, The University of Adelaide, Australia.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Departmentof Health.
This project was supported by the Evidence and Programme Guidance, Department of Nutrition for Healthand Development, World Health Organization, Switzerland and also the Bill & Melinda Gates Foundation, USA.
Appendices
Appendix 1. Search terms for ClinicalTrials.gov and ICTRP
ICTRP
vitamin D AND pregnancy
vitamin D AND pregnant
vitamin D AND antenatal
vitamin D AND prenatal
ClinicalTrials.gov
Advanced search
pregnancy | Interventional Studies | Vitamin D
pregnant | Interventional Studies | Vitamin D
Data and analyses
Comparison 1. A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pre‐eclampsia (all) | 5 | 1553 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.65, 1.42] |
| 2 Pre‐eclampsia (by time of commencement of supplementation) | 5 | 1553 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.65, 1.42] |
| 2.1 Before week 20 of pregnancy | 2 | 1265 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.63, 1.79] |
| 2.2 At week 20 of pregnancy or later | 3 | 288 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.17, 2.00] |
| 2.3 Mixed | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Pre‐eclampsia (by frequency of supplementation) | 5 | 1553 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.65, 1.42] |
| 3.1 Daily | 3 | 1104 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.61, 1.40] |
| 3.2 Weekly/monthly | 2 | 449 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.20, 5.30] |
| 3.3 Bolus dose | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Pre‐eclampsia (by pre‐pregnancy body mass index) | 5 | 1553 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.65, 1.42] |
| 4.1 Underweight (lower than 18.5) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Normal weight (18.5 to 24.9) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Overweight (25 or higher) | 2 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.17, 3.32] |
| 4.4 Unknown/unreported/mixed | 3 | 1325 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.62, 1.58] |
| 5 Pre‐eclampsia (by season at the start of supplementation) | 5 | 1553 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.65, 1.42] |
| 5.1 Summer | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 3.03] |
| 5.2 Fall | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Winter | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Mixed/unknown | 4 | 1493 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.67, 1.48] |
| 6 Pre‐eclampsia (by registered or not registered) | 5 | 1553 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.65, 1.42] |
| 6.1 Trial registered | 4 | 1493 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.67, 1.48] |
| 6.2 Trial not registered | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 3.03] |
| 7 Pre‐eclampsia (by impact factor journal) | 5 | 1553 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.65, 1.42] |
| 7.1 Medium to high | 3 | 1104 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.61, 1.40] |
| 7.2 Low | 2 | 449 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.20, 5.30] |
| 8 Pre‐eclampsia (by vitamin D status at baseline) | 5 | 1553 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.65, 1.42] |
| 8.1 Low vitamin D status | 1 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.57, 6.42] |
| 8.2 Not low vitamin D status | 4 | 1164 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.59, 1.34] |
| 9 Pre‐eclampsia (by nutrients included in the supplementation) | 5 | 1553 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.65, 1.42] |
| 9.1 Vitamin D alone | 2 | 568 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.31, 4.01] |
| 9.2 Vitamin D + other nutrients | 3 | 985 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.60, 1.41] |
| 10 Gestational diabetes | 5 | 1846 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.34, 0.86] |
| 11 Gestational diabetes (by time of commencement of supplementation) | 5 | 1846 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.34, 0.86] |
| 11.1 Before week 20 of pregnancy | 2 | 519 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.25, 0.91] |
| 11.2 At week 20 of pregnancy or later | 2 | 288 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.28, 1.55] |
| 11.3 Mixed | 1 | 1039 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.12, 3.62] |
| 12 Gestational diabetes (by frequency of supplementation) | 5 | 1846 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.34, 0.86] |
| 12.1 Daily | 2 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.14, 1.49] |
| 12.2 Weekly/monthly | 2 | 1509 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.30, 0.92] |
| 12.3 Bolus dose | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.4 Mixed | 1 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 2.95 [0.12, 70.77] |
| 13 Gestational diabetes (by pre‐pregnancy body mass index) | 5 | 1846 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.34, 0.86] |
| 13.1 Underweight (lower than 18.5) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Normal weight (18.5 to 24.9) | 1 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 2.95 [0.12, 70.77] |
| 13.3 Overweight (25 or higher) | 2 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.14, 1.49] |
| 13.4 Unknown/unreported/mixed | 2 | 1509 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.30, 0.92] |
| 14 Gestational diabetes (by registered or not registered) | 5 | 1846 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.34, 0.86] |
| 14.1 Trial registered | 5 | 1846 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.34, 0.86] |
| 14.2 Trial not registered | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Gestational diabetes (by impact factor journal) | 5 | 1846 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.34, 0.86] |
| 15.1 Medium to high | 3 | 1267 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.25, 1.15] |
| 15.2 Low | 2 | 579 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.23, 1.54] |
| 16 Gestational diabetes (by vitamin D status at baseline) | 5 | 1846 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.34, 0.86] |
| 16.1 Low vitamin D status | 1 | 470 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.29, 0.92] |
| 16.2 Not low vitamin D status | 4 | 1376 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.28, 1.23] |
| 17 Gestational diabetes (by nutrients included in the supplementation) | 5 | 1846 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.34, 0.86] |
| 17.1 Vitamin D alone | 2 | 649 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.33, 0.87] |
| 17.2 Vitamin D + other nutrients | 3 | 1197 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.14, 2.58] |
| 18 Preterm birth | 4 | 2294 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.92, 1.69] |
| 19 Preterm birth (by time of commencement of supplementation) | 4 | 2294 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.92, 1.69] |
| 19.1 Before week 20 of pregnancy | 2 | 1195 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.85, 1.90] |
| 19.2 At week 20 of pregnancy or later | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.87] |
| 19.3 Mixed | 1 | 1039 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.79, 1.99] |
| 20 Preterm birth (by frequency of supplementation) | 4 | 2294 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.92, 1.69] |
| 20.1 Daily | 1 | 806 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.82, 2.00] |
| 20.2 Weekly/monthly | 3 | 1488 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.81, 1.84] |
| 20.3 Bolus dose | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.4 Mixed | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Preterm birth (by season at the start of supplementation) | 4 | 2294 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.92, 1.69] |
| 21.1 Summer | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.87] |
| 21.2 Fall | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Winter | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.4 Mixed/unknown | 3 | 2234 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.93, 1.71] |
| 22 Preterm birth (by registered or not registered) | 4 | 2294 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.92, 1.69] |
| 22.1 Trial registered | 3 | 2234 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.93, 1.71] |
| 22.2 Trial not registered | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.87] |
| 23 Preterm birth (by impact factor journal) | 4 | 2294 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.92, 1.69] |
| 23.1 Medium to high | 2 | 1845 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.92, 1.75] |
| 23.2 Low | 2 | 449 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.45, 2.70] |
| 24 Preterm birth (by vitamin D status at baseline) | 4 | 2294 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.92, 1.69] |
| 24.1 Low vitamin D status | 1 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.48, 3.12] |
| 24.2 Not low vitamin D status | 3 | 1905 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.91, 1.72] |
| 25 Preterm birth (by nutrients included in the supplementation) | 4 | 2294 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.92, 1.69] |
| 25.1 Vitamin D alone | 1 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.48, 3.12] |
| 25.2 Vitamin D + other nutrients | 3 | 1905 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.91, 1.72] |
| 26 Low birthweight | 4 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.66, 1.24] |
| 27 Low birthweight (by time of commencement of supplementation) | 4 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.66, 1.24] |
| 27.1 Before week 20 of pregnancy | 1 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.55, 3.38] |
| 27.2 At week 20 of pregnancy or later | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.01, 4.00] |
| 27.3 Mixed | 2 | 1101 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.36, 2.87] |
| 28 Low birthweight (by frequency of supplementation) | 4 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.66, 1.24] |
| 28.1 Daily | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 4.70 [0.23, 94.01] |
| 28.2 Weekly/monthly | 3 | 1488 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.67, 1.15] |
| 28.3 Bolus dose | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.4 Mixed | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Low birthweight (by season at the start of supplementation) | 4 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.66, 1.24] |
| 29.1 Summer | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.01, 4.00] |
| 29.2 Fall | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.3 Winter | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.4 Mixed/unknown | 3 | 1490 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.65, 1.32] |
| 30 Low birthweight (by registered or not registered) | 4 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.66, 1.24] |
| 30.1 Trial registered | 3 | 1490 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.65, 1.32] |
| 30.2 Trial not registered | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.01, 4.00] |
| 31 Low birthweight (by impact factor journal) | 4 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.66, 1.24] |
| 31.1 Medium to high | 2 | 1101 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.36, 2.87] |
| 31.2 Low | 2 | 449 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.19, 4.31] |
| 32 Low birthweight (by vitamin D status at baseline) | 4 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.66, 1.24] |
| 32.1 Low vitamin D status | 1 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.55, 3.38] |
| 32.2 Not low vitamin D status | 3 | 1161 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.46, 1.59] |
| 33 Low birthweight (by nutrients included in the supplementation) | 4 | 1550 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.66, 1.24] |
| 33.1 Vitamin D alone | 1 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.55, 3.38] |
| 33.2 Vitamin D + other nutrients | 3 | 1161 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.46, 1.59] |
| 34 Fasting glucose levels (mg/dl) | 1 | 113 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐4.12, 3.92] |
| 35 Caesarean section | 5 | 2419 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.07] |
| 36 Maternal death (death while pregnant or within 42 days of termination of pregnancy) | 1 | 1039 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.00, 2.73] |
| 37 Serum 25‐hydroxyvitamin D concentration at term (in nmol/L) | 16 | 3107 | Mean Difference (IV, Random, 95% CI) | 29.65 [21.90, 37.40] |
| 38 Gestational hypertension (as defined by trialists) | 4 | 1656 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.63, 1.91] |
| 39 Birth length (cm) | 11 | 3058 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.26, 0.19] |
| 40 Head circumference at birth (cm) | 10 | 2998 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.09, 0.25] |
| 41 Birthweight (g) | 14 | 3300 | Mean Difference (IV, Random, 95% CI) | 51.57 [1.07, 102.07] |
| 42 Cord blood 25‐hydroxyvitamin D concentration (in nmol/L) | 9 | 2166 | Mean Difference (IV, Random, 95% CI) | 24.17 [16.87, 31.48] |
| 43 Admission to special care (including intensive care) during the neonatal period (within 28 days after delivery) | 2 | 1226 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.79, 1.70] |
| 44 Stillbirth (as defined by trialists) | 3 | 2094 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.67, 2.25] |
| 45 Neonatal death (within 28 days after delivery) | 2 | 1915 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.20, 4.88] |
| 46 Apgar score less than seven at five minutes | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 47 Very preterm birth (less than 32 weeks' gestation) | 2 | 1915 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.18, 1.72] |
1.34. Analysis.
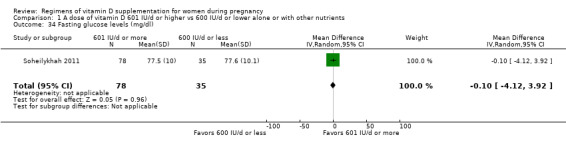
Comparison 1 A dose of vitamin D 601 IU/d or higher vs 600 IU/d or lower alone or with other nutrients, Outcome 34 Fasting glucose levels (mg/dl).
Comparison 2. A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pre‐eclampsia | 4 | 1903 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.62, 1.22] |
| 2 Pre‐eclampsia (by time of commencement of supplementation) | 4 | 1903 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.62, 1.22] |
| 2.1 Before week 20 of pregnancy | 2 | 1664 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.29] |
| 2.2 At week 20 of pregnancy or later | 2 | 239 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.11, 1.65] |
| 2.3 Mixed | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Pre‐eclampsia (by frequency of supplementation) | 4 | 1903 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.62, 1.22] |
| 3.1 Daily | 2 | 1055 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.59, 1.38] |
| 3.2 Weekly/monthly | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 3.03] |
| 3.3 Bolus dose | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Mixed | 1 | 788 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.49, 1.55] |
| 4 Pre‐eclampsia (by season at the start of supplementation) | 4 | 1903 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.62, 1.22] |
| 4.1 Summer | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 3.03] |
| 4.2 Fall | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Winter | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Mixed/unknown | 3 | 1843 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.64, 1.25] |
| 5 Pre‐eclampsia (by registered or not registered) | 4 | 1903 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.62, 1.22] |
| 5.1 Trial registered | 3 | 1843 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.64, 1.25] |
| 5.2 Trial not registered | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 3.03] |
| 6 Pre‐eclampsia (by impact factor journal) | 4 | 1903 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.62, 1.22] |
| 6.1 Medium to high | 3 | 1843 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.64, 1.25] |
| 6.2 Low | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 3.03] |
| 7 Pre‐eclampsia (by vitamin D status at baseline) | 4 | 1903 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.62, 1.22] |
| 7.1 Low vitamin D status | 1 | 788 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.49, 1.55] |
| 7.2 Not low vitamin D status | 3 | 1115 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.58, 1.32] |
| 8 Gestational diabetes | 5 | 2276 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.56, 1.42] |
| 9 Gestational diabetes (by time of commencement of supplementation) | 5 | 2276 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.56, 1.42] |
| 9.1 Before week 20 of pregnancy | 2 | 949 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.51, 1.69] |
| 9.2 At week 20 of pregnancy or later | 2 | 288 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.28, 1.55] |
| 9.3 Mixed | 1 | 1039 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.37, 10.85] |
| 10 Gestational diabetes (by frequency of supplementation) | 5 | 2276 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.56, 1.42] |
| 10.1 Daily | 2 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.34, 1.19] |
| 10.2 Weekly/monthly | 1 | 1039 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.37, 10.85] |
| 10.3 Bolus dose | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 Mixed | 2 | 897 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.58, 2.76] |
| 11 Gestational diabetes (by pre‐pregnancy body mass index) | 5 | 2276 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.56, 1.42] |
| 11.1 Underweight (lower than 18.5) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Normal weight (18.5 to 24.9) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Overweight (25 or higher) | 1 | 161 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.28, 1.66] |
| 11.4 Unknown/unreported/mixed | 4 | 2115 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.57, 1.71] |
| 12 Gestational diabetes (by registered or not registered)n) | 5 | 2276 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.56, 1.42] |
| 12.1 Trial registered | 5 | 2276 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.56, 1.42] |
| 12.2 Trial not registered | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Gestational diabetes (by impact factor journal) | 5 | 2276 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.56, 1.42] |
| 13.1 Medium to high | 4 | 2167 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.54, 1.39] |
| 13.2 Low | 1 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 2.95 [0.12, 70.77] |
| 14 Gestational diabetes (by vitamin D status at baseline) | 5 | 2276 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.56, 1.42] |
| 14.1 Low vitamin D status | 1 | 788 | Risk Ratio (M‐H, Random, 95% CI) | 1.2 [0.54, 2.68] |
| 14.2 Not low vitamin D status | 4 | 1488 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.43, 1.36] |
| 15 Preterm birth | 6 | 2948 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.12] |
| 16 Preterm birth (by time of commencement of supplementation) | 6 | 2948 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.12] |
| 16.1 Before week 20 of pregnancy | 3 | 1825 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.52, 1.23] |
| 16.2 At week 20 of pregnancy or later | 2 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.06, 1.94] |
| 16.3 Mixed | 1 | 1039 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.63, 1.34] |
| 17 Preterm birth (by frequency of supplementation) | 6 | 2948 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.12] |
| 17.1 Daily | 2 | 1037 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.34, 1.46] |
| 17.2 Weekly/monthly | 2 | 1099 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.63, 1.32] |
| 17.3 Bolus dose | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.4 Mixed | 2 | 812 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.47, 1.42] |
| 18 Preterm birth (by pre‐pregnancy body mass index) | 6 | 2948 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.12] |
| 18.1 Underweight (lower than 18.5) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Normal weight (18.5 to 24.9) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Overweight (25 or higher) | 1 | 161 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.41, 2.23] |
| 18.4 Unknown/unreported/mixed | 5 | 2787 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.62, 1.12] |
| 19 Preterm birth (by season at the start of supplementation) | 6 | 2948 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.12] |
| 19.1 Summer | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.87] |
| 19.2 Fall | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Winter | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 2.77] |
| 19.4 Mixed/unknown | 4 | 2864 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.65, 1.15] |
| 20 Preterm birth (by registered or not registered) | 6 | 2948 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.12] |
| 20.1 Trial registered | 5 | 2888 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.13] |
| 20.2 Trial not registered | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.87] |
| 21 Preterm birth (by impact factor journal) | 6 | 2948 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.12] |
| 21.1 Medium to high | 5 | 2888 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.13] |
| 21.2 Low | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.87] |
| 22 Preterm birth (by vitamin D status at baseline) | 6 | 2948 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.12] |
| 22.1 Low vitamin D status | 1 | 788 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.49, 1.55] |
| 22.2 Not low vitamin D status | 5 | 2160 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.61, 1.15] |
| 23 Low birthweight | 2 | 1099 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.49, 1.70] |
| 24 Fasting glucose levels (mg/dl) | 1 | 113 | Mean Difference (IV, Random, 95% CI) | ‐2.28 [‐6.18, 1.62] |
| 25 Caesarean section | 7 | 3343 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.93, 1.20] |
| 26 Maternal death (death while pregnant or within 42 days of termination of pregnancy) | 1 | 1039 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.15] |
| 27 Serum 25‐hydroxyvitamin D concentration at term (in nmol/L) | 11 | 2981 | Mean Difference (IV, Random, 95% CI) | 31.61 [20.83, 42.38] |
| 28 Gestational hypertension (as defined by trialists) | 3 | 1379 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.67, 1.74] |
| 29 Birth length (cm) | 10 | 3288 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.26, 0.36] |
| 30 Head circumference at birth (cm) | 10 | 3278 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.09, 0.29] |
| 31 Birthweight (g) | 13 | 3710 | Mean Difference (IV, Random, 95% CI) | 46.00 [‐8.99, 101.00] |
| 32 Cord blood 25‐hydroxyvitamin D concentration (in nmol/L) | 7 | 2283 | Mean Difference (IV, Random, 95% CI) | 23.84 [13.55, 34.13] |
| 33 Admission to special care (including intensive care) during the neonatal period (within 28 days after delivery) | 1 | 350 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.61, 2.32] |
| 34 Stillbirth (as defined by trialists) | 4 | 2882 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.75, 2.51] |
| 35 Neonatal death (within 28 days after delivery) | 3 | 1939 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.20, 4.88] |
2.12. Analysis.
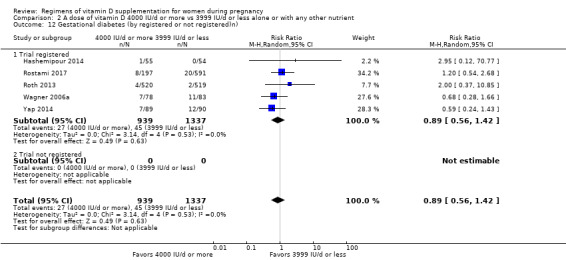
Comparison 2 A dose of vitamin D 4000 IU/d or more vs 3999 IU/d or less alone or with any other nutrient, Outcome 12 Gestational diabetes (by registered or not registered)n).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abotorabi 2017.
| Methods | Randomised controlled trial | |
| Participants | Pregnant women, between 22‐26 weeks' gestation,with simultaneous mild hypocalcaemia (8 < serum calcium < 8.5 mg/dL) and vitamin D deficiency (25 (OH) D < 75 nmol/L), seeking antenatal care to a private gynaecology and obstetrics clinic in Quazvin, Iran. Those presenting with PROM, preterm labour, history of parathyroid disorders, renal or liver disease, osteomalacia, malnutrition or epilepsy were excluded. |
|
| Interventions | Participants were randomised to 1 of 2 groups. ‐ Group 1 (n = 55): 50,000 U vitamin D/week for 8 weeks plus a prenatal capsule/day until delivery (400 Units (U) vitamin D + 250 mg elemental calcium from single company until delivery); ‐ Group 2 (n = 55): women received only a prenatal capsule/day (400 Units (U) vitamin D + 250 mg elemental calcium from single company until delivery). Health worker cadre: trial was conducted in a private gynaecology and obstetrics clinic in Quazvin, Iran (north of tropics). Data were collected through a questionnaire including demographics variables, GA, and BMI. All anthropometric indices were measured by a gynaecologist. The height was measured in barefoot standing position using a wall mounted stadiometer Seca nearest 1 mm. The weight was also measured using Seca scale (Vogel and Halke, Hamburg, Germany), nearest 100 g. At delivery, a 5 mL venous blood sample was taken to measure calcium and 25 (OH) D in mothers. After delivery, weight, height, and head circumference of the neonate were measured in labour room and were recorded in the birth documents. The values recorded in birth documents were applied in the present trial. No details about who dispensed the intervention pills, applied the questionnaires or conducted the blood draw were given. |
|
| Outcomes |
Maternal Primary
Secondary
Infant Primary
Secondary
Laboratory method used for assessment of vitamin D concentrations: vitamin D was measured by ELISA method using MAN Co kit. Inter‐assay and intra‐assay coefficient of variations (CVs) were 1.9% and 1.1%, respectively. Calcium was measured by calorimetric method using Pars Azmoon kit. Inter‐assay and intra‐assay CVs were 2.7% and 1.4%, respectively. |
|
| Notes | • Start of supplementation: less than 26 weeks of pregnancy • Pre‐gestational BMI (kg/m2): unknown/mixed • Supplementation scheme/regimen: single dose followed by daily doses until delivery • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): unknown • Latitude: above Cancer Tropic • Season at the start of pregnancy: not available Setting/country: Iran Source of funding: not reported Starting date of the trial: January 2014 Declarations of interest among primary researchers: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based method (random number generator). |
| Allocation concealment (selection bias) | Unclear risk | Concealment method is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding aspects are not described . |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding aspects are not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 55 pregnant women were evaluated in every study groups (intervention and control groups). Eleven women (20%) in the intervention group (3 (5.45%) due to Premature rupture of membranes (PROM), and 8 (14.55%) due to delivery in other places) and 14 women (25.45%) in the control group (2 (3.63%) due to PROM and 12 (21.82%) due to delivery in other places) were excluded. |
| Selective reporting (reporting bias) | Low risk | All in the methods prespecified outcomes were reported. |
| Other bias | Low risk | There is no any evidence of other bias. |
Bacqui 2009.
| Methods | Randomised controlled trial with 3 allocation groups. | |
| Participants | Included in this trial are women attending a maternal health clinic in inner‐city Dhaka, Bangladesh (between 23°N and 24°N), just north of Tropics, aged 18 to < 35 years; at 27 to < 31 completed weeks of gestation (based on their reported first day of their LMP) who held permanent residence in Dhaka, Bangladesh, at a fixed address and who planned to stay in Dhaka for at least 4 months. Exclusion criteria: pre‐existing medical condition; current vitamin D supplement use; anti‐convulsant or anti‐mycobacterial medications; severe anaemia (haemoglobin concentration < 70 g/L); hypertension at enrolment (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg on at least 2 measurements); major risk factors for preterm delivery or pregnancy complications; or previous delivery of an infant with a congenital anomaly or perinatal death. |
|
| Interventions | Women were randomly assigned to 1 of 3 groups. ‐ Group 1 (n = 12): single dose 70,000 IU D3 on day 0 plus 35,000 IU/week. D3 from day 7 until delivery (“PH”; pregnant, higher dose) for a total dose of 420,000 IU (about 6000 IU/d) ‐ Group 2 (n = 12): 14,000 IU/wk. D3 on day 0 until delivery (“PL”; pregnant, lower dose) to achieve a total dose of 140,000 IU (about 2000 IU/d) ‐ Group 3 (excluded from our analyses): non‐pregnant cohort, single dose 70,000 IU D3 on day 0 plus 35,000 IU/week. D3 until day 63 (total of 10 doses). Only those assigned to groups 1 and 2 (pregnant participants) were provided with standard prenatal supplemental iron (60 mg/day) and folic acid (400 mcg/day), Health worker cadre: during the intervention women with abnormal urinalyses, hypertension, reported severe symptoms, or persistence of any mild symptomatic complaints were referred to the study physician for further evaluation. Participants were referred to an antenatal care physician at the maternity clinic for treatment of urinary tract infections, hypertension, or other medical problems. |
|
| Outcomes |
Maternal Primary
Secondary
Infant Primary
Secondary (infant):
Laboratory method used for assessment of vitamin D concentrations: Diasorin Liaison Total assay in the laboratory of Dr. Reinhold Vieth (Mount Sinai Hospital, Toronto). |
|
| Notes | *Authors state that only one participant developed gestational hypertension; no details are given as to which group she belonged to. • Start of supplementation: less than 31 weeks of pregnancy • Pre‐gestational BMI (kg/m2): unknown/mixed • Supplementation scheme/regimen: single dose followed by weekly doses until delivery • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): unknown • Latitude: outside tropics • Season at the start of pregnancy: mid‐winter Source of funding: not reported Dates of the study: July 2009 ‐ June 2010 Setting/country: Shimantik Maternity Centre, Dhaka, Bangladesh Declarations of interest among primary researchers: not reported by the trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding or incomplete blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding or incomplete blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant flow over the course of the study (screening, enrolment, exclusions, and withdrawal) is described in detail. Total of 28 pregnant women were randomised; G1:14 (2 moved away, 14%) and G2:14 (2 moved away, 14%) |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods section are reported. |
| Other bias | Unclear risk | There is insufficient information to allow judgement. |
Bhatia 2012.
| Methods | Double‐blind, randomised, placebo‐controlled, multi‐arm parallel trial | |
| Participants | Women over 18 years of age with a singleton pregnancy, with a GA of less than 20 weeks. Exclusion criteria: metabolic disease, complicated pregnancy (e.g. renal or liver disease), medication use for conditions such as tuberculosis or epilepsy and vitamin D supplementation in the previous 3 months (prior to the trial). |
|
| Interventions | Participants were allocated to either 1 of the following groups. ‐ Group 1* (n = 100): oral cholecalciferol 60,000 units (in sachets) every 4 weeks ‐ Group 2* (n = 100): 60,000 units (in sachets) every 8 week ‐ Group 3* (n = 100): placebo sachets until delivery *All 3 groups had been provided 1 g elemental calcium daily (groups 1 and 2 without added vitamin D and group 3 with 400 units vitamin D). Health worker cadre: the trial was conducted at the King George’s Medical University, Lucknow, Utter Pradesh, India; tertiary care centre which provide services to people of all socioeconomic strata. Under observation, oral cholecalciferol 60,000 units (in sachets) was administered every 4 weeks (group 1) or every 8 weeks (group 2) or placebo sachets were administered (group 3) until delivery. Calcium tablets were provided for a month at a time. The participants were asked to bring back the empty blisters to check the compliance. All the medications were dispensed in sequentially‐numbered, identical, opaque, sealed packs (carrying the name of the participant) by a research assistant, who was blinded to intervention. The allocation remained concealed for the participants, researcher enrolling and assessing mothers and the one performing data analysis. All the women received standard antenatal care. Maternal serum was measured at recruitment and at term and in cord blood. It is unclear who performed the blood draw or conducted the lab test. |
|
| Outcomes |
Maternal Secondary
Infant Secondary
Laboratory method used for assessment of vitamin D concentrations: maternal serum at term and cord blood were collected for 25OHD concentrations, measured by radio‐immunoassay (Diasorin, Stillwater, USA). This assay measured all forms of vitamin D2 and D3. The analytical sensitivity of the assay was 3.75 nmol/L. The intra‐assay and inter‐assay coefficient of variation was 8.6% to 12.5 % and 8.2% to 11 % at different concentrations of 25OHD. |
|
| Notes | • Start of supplementation: unknown • Pre‐gestational BMI (kg/m2): indifferent • Supplementation scheme/regimen • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988):not mentioned • Latitude: north of Tropic of Cancer • Season at the start of pregnancy: not available Source of funding: grant support from Department of Biotechnology (BT/PR/13985/SPD/11/1297/2010) to V. Bhatia, intramural grant to S. K. Sahoo and V. Bhatia from SGPGIMS,Indian Council for Medical Research grant (manpower development scheme) to S. K. Sahoo. Dates of the study: enrolment started in February 2010 Declaration of interests: not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done by a computer‐generated sequence in randomly permuted blocks of hundred. |
| Allocation concealment (selection bias) | Low risk | All the medications were dispensed in sequentially‐numbered, identical, opaque, sealed packs (carrying the name of the participant) by a research assistant, who was blinded to intervention. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The allocation sequence was also concealed from all participants; the researcher enrolling and assessing mothers, the researcher assessing offspring and DXA scan images, and performing data analysis. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The allocation sequence was also concealed from all participants; the researcher enrolling and assessing mothers, the researcher assessing offspring and DXA scan images, and performing data analysis. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data are balanced across intervention groups. Main reason for refusal: DXA, Dual‐energy X‐ray. More relevant outcomes/AEs may be available. e.g.: hypercalcaemia.G1: 100 pregnant women, 54 delivered in the institution, 2 unable to contact, 29 refused DXA; G2: 100 pregnant women; 52 delivered in the institution, 5 unable to contact, 34 refused scan,. |
| Selective reporting (reporting bias) | Unclear risk | Relevant outcomes results may be available, but are not reported. |
| Other bias | Low risk | No other bias detected. |
Das 2010.
| Methods | Quasi‐randomised controlled trial alternate allocation | |
| Participants | Women (from 6 rural villages) in their second trimester of pregnancy attending the antenatal clinic at Queen Mary Hospital in CSMMU, Lucknow, Utter Pradesh, India | |
| Interventions | ‐ Group A: no dose of vitamin D (this group was not used in this review). ‐ Group B (n = 35): 60,000 IU cholecalciferol in the fifth month + (calcium carbonate (1 g elemental calcium/d) + ferrous sulphate (60 mg elemental iron/d)) and sun exposure. ‐ Group C (n = 35): 120,000 IU cholecalciferol each in the 5th and 7th months of pregnancy + (calcium carbonate (1 g elemental calcium/d) + ferrous sulphate (60 mg elemental iron/day)) and sun exposure. Health worker cadre: study was conducted in 6 villages of a poor socio‐economic region in district Barabanki (latitude 26.81N, Uttar Pradesh, India, north of tropics). Each woman was provided with calcium carbonate in the dosage of 1 g elemental calcium per day, to be taken in 2 divided doses with meals, and ferrous sulphate as 60 mg elemental iron per day, to be had while fasting. Investigators chose to give cholecalciferol doses to coincide with the routine antenatal visits of the community health worker. A food frequency questionnaire was used for the calculation of dietary calcium intake. The duration of daily sun exposure between 1000 hours and 1600 hours was recorded for summer and winter. Detailed roles of the investigators and research staff, prenatal care characteristics, blood drawing and sample handling, etc. are not described. |
|
| Outcomes |
Maternal Secondary
Infant
Laboratory method used for assessment of vitamin D concentrations: Serum 25OHD was measured by radioimmunoassay (Diasorin, Stillwater, OK, USA). |
|
| Notes | • Start of supplementation • Pre‐gestational BMI (kg/m2): unknown/mixed • Supplementation scheme/regimen • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988) • Latitude • Season at the start of pregnancy Source of funding: Grant support (BT/PR 3552/SPD/11/349/2002) to V Bhatia. BT/PR 3552/SPD/11/349/2002 to V Bhatia, from the Department of Biotechnology, Government of India Dates of the study: not specified Declarations of interest among primary researchers: the authors declare no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation was abandoned subsequently (because of rampant Vitamin. D deficiency) and 2 comparison groups were followed up, alternate women receiving either 60,000 U in the 5th month or 120,000 U each in the 5th and 7th months of pregnancy (Group C). |
| Allocation concealment (selection bias) | High risk | The randomisation of vitamin D supplementation groups started at the onset of the trial could not be maintained throughout the trial and that alternate rather than random numbers were used to allocate women to groups B and C. Alternate allocation introduces selection bias. Alternate numbers were used to allocate women. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information related to blinding was provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Maternal 25OHD at delivery (nmol/L): there is insufficient information to allow judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A total of 84 women consented for blood sampling after delivery, 14 in group A and 35 each in groups B and C (the equal number in final follow‐up at delivery in groups B and C was purely by chance, resulting from the greater drop out rate in group B than in group C). Reasons for high attrition rate in group B were not described. |
| Selective reporting (reporting bias) | Low risk | Reported outcomes are aligned with the methods. |
| Other bias | Low risk | There is no any evidence of other bias. |
Dawodu 2013.
| Methods | Randomised, controlled,double‐blind trial | |
| Participants | Arab pregnant women, of any age, with a GA 12–16 weeks (after LMP or by ultrasound assessment) with a singleton pregnancy, who planned to receive prenatal and delivery care in Tawan Hospital, Al Ain, United Arab Emirates were included. Those with pre‐existing calcium and parathyroid conditions, thyroid disease, liver or kidney disease or Type 1 diabetes, which are likely to affect vitamin D and calcium status, were deemed ineligible. |
|
| Interventions | ‐ Group 1 (n = 63): 3600 IU/d vitamin D3 (12 weeks GA ‐ delivery) + prenatal vitamins containing 400 IU vitamin D (1d/until delivery); ‐ Group 2 (n = 65): 1600 IU/day vitamin D3 tablets (12 weeks GA ‐ delivery) + prenatal vitamins containing 400 IU D vitamin (1d/delivery). Health worker cadre: the trial was conducted at primary healthcare clinics affiliated with Tawam Hospital (UAE University Teaching Hospital) in Al Ain (UAE 24°11′30″ N, 55°45′38″ E. Above Cancer Tropic). Sociodemographic, health status, and pregnancy information were completed by questionnaires (not clear who administered the questionnaires). Food frequency questionnaire appropriate for Middle Eastern culture were completed by the mothers to calculate vitamin D and calcium intake (it was not stated who reviewed or entered the information). Maternal weight and height were recorded (personnel not specified). Baseline maternal blood was drawn by venepuncture and urine samples were collected (personnel not specified). The study vitamin D tablets, 1600 IU/d and 3600 IU/d vitamin D3, and placebo with a similar colour and taste were manufactured and supplied by Tischon Corp (Salisbury, Maryland); the vitamin D3 concentration in the study tablets were verified by the same company at the end of the study. Each participant received a 40‐day supply of vitamin D3 study tablets at 1 of 3 dosing regimens determined by randomisation and a 90‐day supply of prenatal vitamins containing 400 IU vitamin D3 per tablet (total of 400 IU vitamin D3 (existing recommended intake), 2000 IU vitamin D3 (existing upper safe intake), or 4000 IU vitamin D3 (estimated intake to achieve mean 25(OH)D concentration of 32 ng/mL considered vitamin D sufficiency at the time of the study). participants were seen monthly from enrolment until delivery by the research nurse. The monthly visits coincided with the routine prenatal visits of each participant, and the research nurse completed a questionnaire on interval maternal health and medication history as well as any hospital admissions and the medical diagnosis. The mode of delivery, complications during delivery, infant’s health, weight (grams), head circumference (centimetres), and crown‐heel length (centimetres) were recorded at delivery (recorder role not specified). The number of pills taken during the interval between the visits divided by the number that should have been taken was used to calculate compliance that served as her adherence to medication between study visits. The research co‐ordinator made phone calls a day or 2 prior to a scheduled visit to remind the participant of her upcoming visit. If the participant then missed her appointment, a follow‐up call was made, and every effort was made to reschedule the participant. Maternal serum 25(OH)D was measured at enrolment and 40 weeks or the time of delivery and in the cord blood at Cincinnati Children’s Hospital. Maternal serum and cord blood calcium concentrations were measured in Tawam Hospital to assess calcium homeostasis and safety. A non fasting midmorning urine sample at monthly visits was used to measure urine calcium and creatinine for calculating urine calcium (Ca) to creatinine (Cr) ratio as early indicators of hypervitaminosis D. In general, limited description of specific roles and responsibilities of the research team was made available. |
|
| Outcomes |
Maternal Primary
Secondary
Infant Primary
Secondary
Laboratory method used for assessment of vitamin D concentrations: serum 25(OH)D was measured using a RIA (DiaSorin, Stillwater, Minnesota). The intra‐ and inter assay coefficients of variation were 4% and 11%, respectively. |
|
| Notes | • Start of supplementation:12‐16 weeks GA • Pre‐gestational BMI (kg/m2): unknown/mixed • Supplementation scheme/regimen: daily • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not described • Latitude: north of Tropic of Cancer • Season at the start of pregnancy: all year long, but classified as: hot season (April through September) and the cool season (October through March) Source of funding: this work was supported by Thrasher Research Fund Award 0286‐4. (P2345) Dates of the study: May 2008‐December 2011 Declarations of interest among primary researchers: B.W.H. serves as a consultant for Diasorin, Inc (Stillwater, Minnesota). All other authors have no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random assignment was a stratified block design so that each month an approximately equal number of participants were randomly assigned to achieve a seasonally balanced study population. The randomisation list was computer‐generated by the statistician. |
| Allocation concealment (selection bias) | Low risk | A secretary not involved in the project allocated and kept a list of the randomisation code of the enrolled patients. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The investigators, patients, healthcare providers, and the laboratory staff performing the biochemical tests were blinded to the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Performed at Cincinnati Children’s Hospital and Tawan Hospital (respectively) by blinded laboratory staff. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After allocation, 30 patients (15%) discontinued participation without specific reasons or due to the husband’s refusal and 162 were followed up to delivery. The women who exited the study before delivery had similar baseline characteristics as those who were followed. up to delivery except for lower vitamin intake. G1: n = 63 (discontinued the study n = 8), G2; n = 65 (discontinued the study n = 13). |
| Selective reporting (reporting bias) | Low risk | All in the methods predefined outcomes were reported. |
| Other bias | Low risk | No other bias detected |
de Menibus 1984.
| Methods | Clinical trial | |
| Participants | White females regardless of their GA | |
| Interventions | Vitamin D supplementation during the last 3 months of pregnancy. ‐ Group 1: received 1000 IU from months 7th to 9th (n = 21) ‐ Group 2: received a single dose of 200,000 IU at 7th months or controls (n = 27) Health worker cadre: not reported. |
|
| Outcomes |
Maternal Secondary
Infant Secondary
|
|
| Notes | • Start of supplementation: month 7 • Pre‐gestational BMI (kg/m2): unknown/mixed • Supplementation scheme/regimen • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not available • Latitude • Season at the start of pregnancy: fall‐winter Setting/country: Normandy, France Source of funding: Regional Direction of Health and Social affairs of Haute‐Normandie Dates of the study: not specified, estimated around 1983 Declarations of interest among primary researchers: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There is insufficient information to allow judgement. |
| Allocation concealment (selection bias) | Unclear risk | Not enough information to make a judgment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not enough information to make a judgment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not enough information to make a judgment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not enough information to make a judgment. |
| Selective reporting (reporting bias) | Unclear risk | Not enough information to make a judgment. |
| Other bias | Unclear risk | There is insufficient information to allow judgement. |
Grant 2010.
| Methods | Randomised, double‐blind, placebo‐controlled multi‐arm parallel study | |
| Participants | Women with a singleton pregnancy and a GA between 26 to 30 weeks. Those taking vitamin D supplementation > 200 IU per day, with history of renal stones or hypercalcaemia or with any serious pregnancy complication at enrolment were excluded from participation. |
|
| Interventions | Participants were randomly assigned to 1 of 2 groups. ‐ Intervention group (n = 85): received 2000 IU of vitamin D3; ‐ Comparison group (n = 87) received 1000 IU of vitamin D3. Excluded from participation were women taking vitamin D supplementation (> 200 IU per day), history of renal stones or hypercalcaemia and who had any serious pregnancy complication at enrolment. Health worker cadre: the study was conducted in a community‐based primary care maternity clinic in Auckland, New Zeeland (Latitude 36°S: South of Capricorn Tropic). The study statistician randomly allocated a treatment to each participant and labelled identical study medicine bottles. Study medicine bottles were sequentially numbered with an identical numbering code used for each mother‐infant pair. Bottles of study medicine (study medicine bottles for the 3 groups being identical in colour, shape, and volume and the study medicine identical in colour, consistency, and taste) were prepared by the Ddrops Company (Woodbridge, Ontario, Canada).Face‐to‐face interviews were completed with women at enrolment; at 36 weeks’ gestation; and postpartum. Data collected described demographics, adherence, supplement use, and infant feeding. Mothers were phoned at 2‐weekly intervals to check adherence. Venous (women and umbilical cord) and capillary (infant) blood samples were collected. Serum calcium concentration was measured and then samples were stored at –80°C until study completion. Research staff roles were not specified (unclear who delivered the treatment or perform the assessments). |
|
| Outcomes |
Maternal Primary
Secondary
Infant Primary
Secondary
Laboratory method used for assessment of vitamin D concentrations: Serum 25(OH)D concentration was measured using isotope‐dilution LC–tandem mass spectrometry in a Vitamin D External Quality Assurance Scheme–certified laboratory. |
|
| Notes | • Start of supplementation: from enrolment (26‐30 weeks GA) • Pre‐gestational BMI (kg/m2): unknown/mixed • Supplementation scheme/regimen: daily • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not available • Latitude: south of Capricorn Tropic • Season at the start of pregnancy: all Source of funding: the study was funded by the Health Research Council of New Zealand, grant number 09/215R. Dr. Mitchell is supported by Cure Kids. The donor played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Setting/country: South Auckland Maternity Care Limited, Auckland, New Zealand Dates of the study: April 2010 to July 2011 Declarations of interest among primary researchers: the authors have indicated they have no potential conflicts of interest to disclose |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation to the 3 study arms was by restricted randomisation within blocks of variable size using a computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | The allocation sequence was concealed from research staff involved in recruitment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study statistician randomly allocated a treatment to each participant and labelled identical study medicine bottles such that study staff and participants were unaware of the treatment status. Study medicine bottles were sequentially numbered with an identical numbering code used for each mother‐infant pair. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study statistician randomly allocated a treatment to each participant and labelled identical study medicine bottles such that study staff and participants were unaware of the treatment status. Study medicine bottles were sequentially numbered with an identical numbering code used for each mother‐infant pair. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For all outcomes attrition was reported and comparable. Intervention group: allocated (n = 86) (received intervention (n = 84); took vitamin D supplements during pregnancy (excluded) n = 2, withdrew (n = 2), moved out (n = 1)). Control group: allocated (n = 87) (received intervention (n = 87); withdrew (n = 2), moved out (n = 1)). |
| Selective reporting (reporting bias) | Low risk | All predefined measures described in the methods were reported. |
| Other bias | Low risk | There is no any evidence of other bias. |
Hashemipour 2014.
| Methods | Open‐label randomised clinical trial | |
| Participants | Women with a GA of 24–26 weeks, singleton pregnancy and BMI of 19–26 kg/m2. Exclusion criteria: women with diabetes before pregnancy, chronic hypertension, history of repeated abortion, rheumatoid arthritis, parathyroid disorders, hepatic or renal diseases, use of aspirin, anticonvulsive and immunosuppressive drugs were excluded from the study. |
|
| Interventions | Women were randomised to 2 groups. ‐G1:50,000 IU oral D3/week for 8 weeks (from 26 to 28 weeks of pregnancy) + multivitamin with 400 IU D3 + 200 mg elemental calcium each day until delivery; ‐G2: multivitamin with 400 IU D3 + 200 mg elemental calcium each day until delivery. Health worker cadre: the study was conducted among women attending Obstetric clinic for antenatal care. Qazvin, Iran (above Cancer Tropic); participants were visited once every 2 weeks during the second trimester and once a week in the third trimester; parameters such as weight, blood pressure, uterine fundal length, and use of vitamin D supplement and multivitamin were measured or checked. Following delivery, maternal and cord blood samples (5 mL) were taken after clamping and sent to the hospital laboratory to be centrifuged and kept frozen until use. On admission for labour, maternal weight, neonatal weight, neonatal length and neonatal head circumference were measured. Neonatal weight and length were measured using a calibrated instrument (Seca Medical Measuring Systems). Head circumference (largest occipitofrontal circumference) was measured to the nearest 1 mm using an un‐stretchable tape measure. Anthropometric measurements were taken by a nurse who was blinded to the patient’s group. It is unclear who conducted the visits, the blood draw and the questionnaires. |
|
| Outcomes |
Maternal Primary
Secondary
Infant Primary
Secondary
Laboratory method used for assessment of vitamin D concentrations: Serum vitamin D was determined using a commercial enzyme‐linked immunosorbent assay kit (Euroimmun, Lubeck, Germany). The intra‐assay and inter assay coefficients of variation for 25(OH)D were 3.3% and 6.7%, respectively. |
|
| Notes | • Start of supplementation: 24‐26 GA • Pre‐gestational BMI (kg/m2): unknown/mixed;19‐26 kg/m2 • Supplementation scheme/regimen: weekly and daily • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not available • Latitude: above Cancer Tropic • Season at the start of pregnancy: not available Source of funding: this study was supported by a grant from the Metabolic Diseases Research Center affiliated to Qazvin University of Medical Sciences Setting/country: Qazvin, Iran Dates of the study: Dec 2011‐Mar2012 Declarations of interest among primary researchers: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed. Assignment into groups was performed by an obstetrician responsible for antenatal care. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators taking measurements were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Maternal and neonatal 25(OH)D concentrations: unclear whether there is blinding for this outcome assessment, but the lack of blinding is unlikely to influence outcome. Anthropometric outcome measurements were taken by a nurse who was blinded to the patient’s group. Blood samples were sent to the hospital lab (independent third party). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for attrition were included. G1: n = 65 (loss of 10 participants (15.4%): 1 PROM and 9 delivered in places with difficult access); G2: n = 65 (loss of 11 participants (16.9%): 1 GDM and 10 delivered in places with difficult access). |
| Selective reporting (reporting bias) | Low risk | Results for all the outcomes pre‐specified in the methodology were reported. |
| Other bias | Low risk | There is no any evidence of other bias. |
Kalra 2012.
| Methods | Randomised clinical trial | |
| Participants | Pregnant women attending the antenatal clinic between 12 and 24 weeks of gestation Participants were excluded from the study if they were already on Ca or vitamin D supplementation, anticonvulsants, antitubercular treatment or had any medical condition that affected Ca and vitamin D metabolism (including renal and hepatic disease). |
|
| Interventions | Participants were randomly assigned to: ‐ Group 1: 2 oral doses of 3000 mcg (7500 mcg/300,000 IU) vitamin D3 in the 2nd and again in the 3rd trimester (600,000 IU in total) (n = 48); ‐ Group 2 (comparison): 1 dose of 1500 mcg (60,000 IU) vitamin D (n = 48). Health worker cadre: unclear who did what but it seems that the study team at the antenatal clinic in Queen Mary Hospital, Chhatrapati Sahuji Maharaj (formerly King George’s) Medical University, Lucknow, India (between tropics) took detailed history of and examined the participants at induction and subsequent visits. Detailed anthropometry of the newborn, including weight, length, head circumference and longest diameter of the anterior fontanelle, was measured at birth and subsequently at 3, 6 and 9 months of age. At each visit, history suggestive of lower respiratory tract infections was examined. Investigators who measured anthropometry of infants and followed them were blinded to the mothers’ treatment category. |
|
| Outcomes |
Maternal
Infant
Laboratory method used for assessment of vitamin D concentrations: 10 mL of maternal blood collected at induction into the study and again at delivery were immediately transported on ice. Serum or plasma was stored at ‐70oC for future analysis of serum 25(OH)D by RIA/immunoradiometric assay (Diasorin). |
|
| Notes | • Start of supplementation: second trimester • Pre‐gestational BMI (kg/m2): unknown/mixed • Supplementation scheme/regimen: 2 oral single doses, 2 oral doses in the 2nd and again in the 3rd trimester (600,000 IU in total) • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not available • Latitude: between Tropics • Season at the start of pregnancy: not specified Setting/country: Queen Mary Hospital, Chhatrapati Sahuji Maharaj Medical University, Lucknow, Utter Pradesh, India Source of funding: this study was partially funded by an Indian Council for Medical Research grant (3/2/2006/PG‐MPD‐7) to P. K Dates of the study: not specified Declarations of interest among primary researchers: none of the authors reported a conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using random number tables. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to allow judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information whether the participants and study team were blinded during the time when the supplements were being given. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | For the outcome serum 25(OH)D levels, it is unclear if it was not blinded but the lack of blinding is unlikely to influence outcome. For the outcome anthropometry at birth, it states that investigators who measured this were blinded to the mothers’ treatment category. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for high attrition was explained however whether they were similar across the interventions is unclear. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allow judgment. |
| Other bias | Unclear risk | The authors reported study limitations, also with the control group (which is not part of this review) and unspecified logistical constraints. Quote "The control group was not a result of the randomisation process, but was a group of women attending the same hospital, whom we could not recruit early enough to give the second trimester medication. Although there was no statistical difference between the groups in a number of biological and biochemical variables, either at registration or at delivery, this is an important limitation to the interpretation of the present results,especially since there was an unexpected trend towards higher median maternal serum 25(OH)D concentration in the usual‐care group compared with group 1. Third, we were unable to test for maternal hypercalcaemia due to logistical constraints." |
Karamali 2015.
| Methods | Randomised, double‐blind placebo‐controlled clinical trial with 2 arms | |
| Participants | Primigravida women, attending a maternity clinic in Arak, Iran, aged 18–40 years old and at risk for pre‐eclampsia. Women were identified as “at‐risk” by abnormal uterine artery Doppler waveform (18–20 weeks’ gestation, mean resistance index > 0.67 or pulsatility index > 1.65 with or without the presence of unilateral or bilateral diastolic notches) | |
| Interventions | Women were randomly divided into 2 groups to receive: ‐ Group 1: 50,000 IU vitamin D supplements (n = 30) ‐ Group 2 (placebo) (n = 30) every 2 weeks from 20 to 32 weeks of gestation. All pregnant women were also taking 400 μg/d folic acid from the start of pregnancy, 60 mg/d ferrous sulphate from the second trimester, and a multivitamin mineral capsule (containing 400 IU vitamin D) from the second half of pregnancy. Health worker cadre: a trained midwife at maternity clinic (Arak,Iran, outside the tropics) did the randomised allocation sequence with a computer random number generator. An investigator with no clinical involvement in the study packed cholecalciferol and placebos in numbered bottles based on the random list. Participants were requested not to alter their regular physical activity or normal dietary intakes throughout the study and not to take any supplements other than the ones provided by the investigators. All pregnant women were also taking 400 μg/d folic acid from the start of pregnancy, 60 mg/d ferrous sulphate from the second trimester, and a multivitamin mineral capsule (containing 400 IU vitamin D) from the second half of pregnancy. Information on pre‐pregnancy weight and BMI were obtained from clinical records. A trained midwife at maternity clinic conducted the anthropometric measurements at the beginning of the study and the end of the intervention. |
|
| Outcomes |
Maternal Primary
Secondary
Infant Primary
Secondary
Laboratory method used for assessment of vitamin D concentrations: Serum 25‐hydroxyvitamin D concentrations was assayed by a commercial ELISA kit (IDS, Boldon, UK). The inter‐ and intra‐assay CVs for serum 25‐hydroxyvitamin D assays ranged from 4.9 to 7.2% |
|
| Notes | • Start of supplementation: from 20 weeks of pregnancy • Pre‐gestational BMI (kg/m2): the Intervention was stratified by BMI (< 25 and ≥ 25 kg/m2) • Supplementation scheme/regimen: UI every 2 weeks • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not applicable • Latitude: outside the Tropics • Season at the start of pregnancy: summer? Source of funding: this trial was supported by a grant from the Vice‐chancellor for Research, AUMS, and Iran. The study was supported by a grant (no. 92–12–161) from Arak University of Medical Sciences Dates of the study: July 2014 and October 2014 Declarations of interest among primary researchers: the authors declare no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised allocation sequence by a computer random number generator. |
| Allocation concealment (selection bias) | Low risk | An investigator with no clinical involvement in the study packed cholecalciferol and placebos in numbered bottles based on the random list. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomisation and allocation were hidden from the researchers and pregnant women until the statistical analysis was completed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Randomisation and allocation were hidden from the researchers and pregnant women until the statistical analysis was completed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study (G1: n = 30, G2: 30); data for both groups were reported. |
| Selective reporting (reporting bias) | Low risk | According to the methodology, all outcomes data were reported. |
| Other bias | Low risk | No other bias source was identified. |
Kiely 2015.
| Methods | Randomised controlled trial | |
| Participants | White‐skinned adults > 18 years of age, with gravidae no more than 18 week of gestation, in good general health, with a low risk pregnancy and not consuming > 10 mcg/d vitamin D from supplements | |
| Interventions | Group 1: once daily, 50 nmol/L or 20 mcg (800 IU) dose of vitamin D3 taken from baseline visit (approximately 15 weeks' gestation) until endpoint (delivery) (n = 48); Group 2 (comparison): once daily, 25 nmol/L or 10mcg (400 IU) dose of vitamin D3 taken from baseline visit (approximately 15 weeks' gestation) until endpoint (delivery)(n = 48). The placebo group is not included in this review. Health worker cadre: all study participants received a container of 90 tablets at both the baseline and midpoint visits at the Human Nutrition Studies Unit at the Cork Center for Vitamin D and Nutrition Research, University College Cork, Ireland (north of tropics). Compliance was monitored by a tablet count at each visit. It is unclear who provided the supplements. An interviewer collected information on general health, lifestyle, socio‐demographic characteristics at baseline. An interviewer administered quantitative food‐frequency questionnaire for vitamin D and calcium at baseline, and antennal supplement use was re‐assessed at the second and third study visit. Height and weight were taken at baseline using standard scales (Leicester height measure; CMS Weighing Equipment Ltd.; digital weighing scales; Seca Ltd.), and body weight measurements were repeated at the second and third visits. A research nurse collected non fasting blood sample at each visit. Venous umbilical cord blood sampled was collected at delivery. |
|
| Outcomes |
Maternal Primary
Secondary
Infant Secondary
Laboratory method used for assessment of vitamin D concentrations: circulating serum 25‐hydroxyvitamin D3 3‐epimer of 25‐hydroxyvitamin D3 (3‐epi‐25(OH)D3), 24,25‐ dihydroxyvitamin D3 (24,25(OH)2D3), and 25‐hydroxyvitamin D2 (25(OH)D2) were analysed at the Cork Center for Vitamin and Nutrition Research using a CDC‐certified LC‐MS/MS method. The instrument used was a Waters Acquity UPLC system coupled to an Acquity Triple Quadrupole (TQD) mass spectrometer detector (Waters, Santry, Dublin 9, Ireland). Concentrations of 25(OH)D3 and 25(OH)D2 were quantified separately and summed to generate total 25(OH)D. Chromatographic separation and quantification of 3‐epi‐25(OH)D3 were also achieved. 4 levels of serum‐based National Institute of Standards and Technology (NIST)–certified quality‐assurance material (SRM 972) were used for method validation, whereas quality‐control materials assayed in parallel to all samples were purchased fromChrom‐ systems. NIST calibrators (SRM 2972) were used throughout the analysis. |
|
| Notes | • Start of supplementation: 14‐week mean GA • Pre‐gestational BMI (kg/m2): indifferent • Supplementation scheme/regimen: daily • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not applicable • Latitude: north of Tropics • Season at the start of pregnancy: summer and winter Source of funding: supported by funding to MEK and KDC from the European Commission under grant agreement 613977 for the ODIN Integrated Project (Food‐based solutions for optimal vitamin D nutrition and health throughout the life cycle; http://www.odin‐vitd.eu/) Dates of the study: actual study start date was November 1, 2014, actual primary completion date April 1, 2017 (final data collection date for primary outcome measure) Declarations of interest among primary researchers: the authors had no conflicts of interest to report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Group codes were randomly assigned by a senior scientist not involved in the implementation or analysis of the study to a computer‐generated list of random numbers, which were assigned to consecutive participant identification number. |
| Allocation concealment (selection bias) | Low risk | Participants were allocated an identification number consecutively, the group allocation of which was done by a senior scientist not part of the implementation or analysis of the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants–blinded, tablets were packaged and coded into identical, white, plastic containers in a food sensory facility in the research facility. Study team–blinded, tablets were packaged and coded into identical, white, plastic containers in a food sensory facility in the research facility. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study team and participants were not likely to be aware of the allocation of participants; both were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were similar across groups. Quote: “Any participant who did not provide a blood sample at baseline was included in the descriptive and biochemical analysis at later time points but excluded from the dose‐response analysis, whereas women missing a midpoint sample only were included in both the dose‐response analysis and the analysis at endpoint, if a blood sample was collected at this time point. The numbers of women who provided both a baseline and ≥ 1 follow‐up sample (midpoint or endpoint) were 43, 42, and 43 for the placebo and 10‐ and 20‐µg groups, respectively, which left a final number of 128 for the dose‐response analysis.” Of the 23 participants who did not provide an endpoint sample, 8 were due to a pregnancy‐associated adverse event (Placebo : n = 3,G2:n = 5), 5 withdrew for personal reasons (Placebo group: n = 2, G1:n = 2, G2:n = 3), 5 were lost to follow‐up (Placebo group: n = 2, G1:n = 0, G2:n = 3), 2 began consuming vitamin D supplements containing > 10 µg/d and were excluded (group not mentioned), and 3 delivered their infants before the final visit was conducted (Placebo group: n = 2, G2:n = 1). |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the protocol are reported in the study results. |
| Other bias | Low risk | No other bias source was identified |
Mallet 1986.
| Methods | Randomised controlled trial; 3‐arm design with individual randomisation | |
| Participants | White pregnant women 18‐36 years of age in the last trimester of pregnancy. Health worker cadre: the study was conducted by the research team at the maternity of Balvedere, Rouen, France but the roles are not described. It is unclear who provided the supplements and measured the outcomes. |
|
| Interventions | Participants were randomly assigned to 1 of 3 groups: ‐ Group 1: women received daily 1000 IU of vitamin D (ergocalciferol‐D2) for the last 3 months of pregnancy (estimated total dose throughout pregnancy: 90,000 IU) (n = 21); ‐ Group 2: women received a single dose of 200,000 IU (5 mg) vitamin D at the 7th month of pregnancy (n = 27); ‐ Group 3: women received no supplement and served as controls (n = 29). This group was not used in the present analysis. Health worker cadre: this study is based on a medical survey conducted in an industrial town in the Northwest of France. Medical surveys, and biologic determination were performed by blind staff (roles were not specified). |
|
| Outcomes |
Maternal Secondary
Infant Secondary
Laboratory method used for assessment of vitamin D concentrations: for 25‐hydroxyvitamin D and 1,25‐dihydroxyvitamin D determinations the following techniques were used: extraction with chloroform‐methanol‐water according to Pre‐ece, double step purification, first on a Sephadex LH 20 column with chloroform hexane 45 to 55 vol/vol as solvent, then on a high‐pressure liquid pression system according to Shepard. Plasma metabolites were measured by competitive assay using rat protein for 25 OHD and chicken intestine cytosol for 1,25 (OH)2 D according to Jongen. Assay sensitivity for 1, 25 (OH)2 D was 5 pmol/tube and for 25 OHD was 25 pmol/tube. |
|
| Notes | • Start of supplementation: 20 weeks of pregnancy or more • Pre‐gestational BMI (kg/m2): unknown/mixed • Supplementation scheme/regimen: single/daily • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): unknown/mixed • Latitude: north of the Tropic of Cancer • Season at the start of pregnancy: winter pregnancy. Infants born during February and March Source of funding: unknown/unreported Dates of the study: January 1979 to December 1982 Declarations of interest among primary researchers: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed by random numbers table. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different interventions were used: daily dose or single dose or no supplement; therefore, it is assumed that there was no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Different interventions were used: daily dose or single dose or no supplement; therefore, it is assumed that there was no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear if there was attrition, but given the uneven number of participants reported it is likely that there were losses to follow‐up. Quote: "Groups did not differ in terms of maternal age, parity, calcium intake, or frequency of outings." |
| Selective reporting (reporting bias) | Low risk | All intended outcomes (methods) were reported. |
| Other bias | Unclear risk | Insufficient information to allow judgement. |
March 2010.
| Methods | Double‐blind randomised controlled trial (blocked by ethnicity as either European or non‐European) | |
| Participants | 226 healthy pregnant women age of 18–45 years from Greater Vancouver, British Columbia, Canada, from 13 to 24 weeks of gestation (based on LMP). Women taking vitamin D supplements > 10 ug/d, with any metabolic, inflammatory or genetic problems (e.g. diabetes, TB, cardiac or renal disease, HIV/AIDS, chronic hypertension, inflammatory bowel disease, autoimmune disease, liver disease, or epilepsy) or with digestive and intestinal problems that may affect vitamin D absorption (e.g. coeliac disease or gastric bypass) were excluded from the study. Additionally, those with history of adverse pregnancy outcome (e.g. preterm delivery < 37 weeks of gestation; stillbirth; haemolytic anaemia, elevated liver enzymes and low platelet count syndrome; severe pre‐eclampsia or eclampsia) were excluded. | |
| Interventions | Within each block, women were randomly allocated to 1 of the 3 vitamin D doses: ‐ 10 mcg/d (n = 76); ‐ 25 mcg/d (n = 76); ‐ 50 mcg/d (n = 74). Health worker cadre: women attended the study clinics at BC Women’s Hospital in Canada (north of tropics). Although not explicitly mentioned, study staff facilitated the self‐administration of questionnaires; measured height and weight at each visit according to standardise procedures with a calibrated standing weight scale and stadiometer after enrolment, at 36 weeks of gestation, and 8 weeks postpartum; collected maternal non‐fasting venous blood and urine at each time point, and cord blood at birth and 8 weeks after birth; and dispensed and counted the supplements at different time points. |
|
| Outcomes |
Maternal
Infant
Laboratory method used for assessment of vitamin D concentrations:Serum 25(OH)D concentrations were determined using a LIAISON 25‐OH Vitamin D Vitamin D TOTAL assay (DiaSorin), a competitive chemiluminescence immunoassay that equally detects 25‐OHD2 and 25‐OHD metabolites. |
|
| Notes | • Start of supplementation: Started at 13‐24 weeks of gestation • Pre‐gestational BMI (kg/m2): mostly healthy weight (62% to 78%) and the rest OW/OB • Supplementation scheme/regimen: daily • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988):No skin pigmentation assessed • Latitude: north of Tropics • Season at the start of pregnancy: varied Source of funding: supported by the Canadian Institutes for Health Research (CIHR) and Frederick Banting and Charles Best Canada Graduate Scholarship from the CIHR (KMM). Supplements were provided by Natural Factors (Coquitlam, Canada). Natural Factors had no role in the study design, implementation, or interpretation of the study findings. Dates of the study: June 2010 and March 2013, included 3 calendar years with 4 full seasons each year: summer,fall, winter, and spring. Declarations of interest among primary researchers: MRL receives consulting fees from the Factors Group of Nutritional Companies (Canada’s leading manufacturer of natural health products). All other authors declared no conflicts of interest related to this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were blocked by ethnicity as either European or non‐European; then, within each block, they were randomly allocated to 1 of the 3 vitamin D doses. |
| Allocation concealment (selection bias) | Low risk | The supplements were coded by the manufacturer to ensure blinding of all study participants. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The supplements were coded by the manufacturer to ensure blinding of all study staff; tablets all identical in size and colour but containing 10, 25, or 50 mg vitamin D3/d. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not mentioned but it is unlikely that the staff and the participants knew of their treatment group during periodic assessments and laboratory testing. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Across the treatment groups, the dropout rate was similar: 7.5%(n = 17) in the 10 mg/d group, 8.4% (n = 19) in the 25 mg/d group, and 8.0% (n = 18) in the 50 mg/d group. In the 10 mg/d group, 13 women were lost to follow‐up, and 4 withdrew from the study for personal reasons. In the 25 mg/d group, 17 women were lost to follow‐up, and 2 withdrew from the study for personal reasons. In the 50 mg/d group, 16 women were lost to follow‐up, and 2 withdrew from the study for personal reasons. |
| Selective reporting (reporting bias) | Low risk | All outcomes included in the methods were reported. |
| Other bias | Low risk | No other source of bias were identified |
Marya 1981.
| Methods | Randomised controlled trial | |
| Participants | Hindu women | |
| Interventions | Women were randomised throughout the 3rd trimester pregnancy to 1 of the following groups: ‐ Group 1: 600,000 IU vitamin D2 orally in both the 7th and 8th months of pregnancy (n = 20); ‐ Group 2: 1200 IU vitamin D (with 375 mg calcium, as tablets) per day (n = 25). Type of setting was not specified; India, exact location was not specified. Health worker cadre was not described. |
|
| Outcomes |
Infant
Laboratory method used for assessment of vitamin D concentrations: not available |
|
| Notes | • Start of supplementation: during the third trimester (both 7th and 8th months of pregnancy) • Pre‐gestational BMI (kg/m2): unknown/mixed • Supplementation scheme/regimen: single doses in both the 7th and 8th months of pregnancy versus daily doses (comparison) • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not specified • Latitude: India (location not specified) • Season at the start of pregnancy: not specified Source of funding: not declared Dates of the study: not declared Declarations of interest among primary researchers: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about how randomisation was done. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to determine whether allocation concealment was done and how. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to allow judgment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit. judgment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit. judgment. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgment. |
| Other bias | Unclear risk | Insufficient information to permit judgment. |
Mir 2016.
| Methods | Open‐label, parallel group, prospective, randomised, and controlled trial | |
| Participants | Pregnant women who fulfilled the inclusion criteria (which are not clear in the article) | |
| Interventions | Study participants were assigned to 4 treatment groups: Group 1 (n = 26), 1000 IU of vitamin D daily; Group 2 (n = 21), 30,000 IU of vitamin D monthly; Group 3 (n = 27), 2000 IU of vitamin D daily; Group 4 (n = 26), 60,000 IU vitamin D monthly. Group 1 and 2 were further analysed together as Group 1K (1000 IU daily and 30,000 IU monthly), and Group 3 and 4 as Group 2K (2000 IU daily and 60,000 IU monthly). Health worker cadre: although not explicitly stated in the article the specific health staff involved, the study was conducted in the out‐patient department of Obstetrics and Gynaecology, Sheri‐i‐Kashmir Institute of Medical Sciences, and in a maternity hospital in Lal Ded, Srinagar, India (north of Tropics). The study team conducted the following: administration of questionnaire, anthropometric measurements, drawing of blood for laboratory testing, distribution of vitamin D supplementation tablets, monitoring during follow up visits during which the monthly pill was taken in front of doctor, and extraction of blood samples at delivery. |
|
| Outcomes |
Maternal Primary
Secondary outcomes
* mentioned in the results section but no actual numbers or categorisation were given Infant Secondary outcomes
Laboratory method used for assessment of vitamin D concentrations: Levels were measured by a radioimmunoassay (RIA) technique using the commercially available kit as per the manufacturers’ instructions. The DiaSorin 25(OH)D assay consists of a 2 step procedure. The first procedure involves a rapid extraction of 25(OH) D and other hydroxylated metabolites from serum or plasma with acetonitrile. Following extraction, the treated sample is then assayed using an equilibrium RIA procedure. |
|
| Notes | • Start of supplementation: not clear • Pre‐gestational BMI (kg/m2): mixed • Supplementation scheme/regimen: daily or monthly • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not available • Latitude: north of Tropics • Season at the start of pregnancy: summer Source of funding: research grant provided by Sher‐i‐Kashmir Institute of Medical Sciences, Srinagar Jammu Nad Kashmir, India. vitamin D supplementation was provided free of cost to participants by M/S Eris Life sciences and Myer pharmaceuticals. Dates of the study: not reported. Declarations of interest among primary researchers: there are no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough information to make a judgment. |
| Allocation concealment (selection bias) | Unclear risk | Not enough information to make a judgment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an open‐label trial – participants and study team were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This was an open‐label trial – participants and study team were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to allow judgment of low risk or high risk, no reason for missing data provided. |
| Selective reporting (reporting bias) | High risk | Not all of the study’s pre‐specified primary outcomes have been reported sufficiently. Specified outcome measures in the materials and methods were not sufficiently or comprehensively reported in the results section (e.g. pregnancy outcomes and fetal cord vitamin D) |
| Other bias | Unclear risk | Insufficient information to allow judgment. |
Mojibian 2015.
| Methods | Unblinded randomised clinical trial | |
| Participants | Pregnant women with GA 12‐16 weeks who had serum 25(OH) D less than 30 ng/mL. Women with a history of diabetes or participants who consumed vitamin D supplements during the previous 6 months and women with thyroid or parathyroid disorders were deemed not eligible. | |
| Interventions | Women randomised to: Group 1 (n = 250): 50,000 IU vitamin D every 2 weeks orally; Group 2 (n = 250): 400 IU vitamin D daily. Health worker cadre: women were recruited from 2 prenatal clinics (Shahid and Mojibian hospitals) in Yazd, Iran (north of tropics). The study was conducted in 2 prenatal clinics; researchers obtained general information, including maternal age, height, prepregnancy weight, level of education, reproductive and medical histories, and prepregnancy BMI. Assessment of primary outcome, such as gestational diabetes was done by 100 gr oral glucose tolerance test between 24‐28 weeks. The participants delivered at the prenatal clinics (Shahid Sadoughi and Mojibian hospitals). The researchers assessed other outcomes such as serum levels of 25 (OH) D at the time of delivery from mother and cord, neonatal weight, length, head circumference and Apgar of 1 and 5 minutes, as well as other neonatal complications such as macrosomia, respiratory distress and hypoglycaemia. |
|
| Outcomes |
Maternal Primary
Secondary
Infant Primary
Secondary
Laboratory method used for assessment of vitamin D concentrations: 25 (OH) D was analysed by Eliza (Euroimmun Kit, Nima Pooyesh Teb Company, Tehran, Iran) with an inter‐assay coefficient of variation of 7.8% and an intra assay coefficient of variation of 3.2% |
|
| Notes | • Start of supplementation: supplementation was started in the 12th week of pregnancy • Pre‐gestational BMI (kg/m2): unknown/mixed • Supplementation scheme/regimen: daily • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not available • Latitude: north of Tropics • Season at the start of pregnancy: all year round regardless of season Source of funding: study was supported by Shahid Sadoughi University of Medical Sciences Dates of the study: between 2010‐2012 Declarations of interest among primary researchers: authors reported no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number lists were drawn by an independent researcher. |
| Allocation concealment (selection bias) | Unclear risk | insufficient information to allow judgment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and researchers were not blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants and researchers were not blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for missing outcome data unlikely to be related to true outcome. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available, insufficient information to allow judgment |
| Other bias | Unclear risk | Protocol not available, insufficient information to allow judgment. |
Mutlu 2014.
| Methods | Randomised clinical trial | |
| Participants | At least 16 years old and up to 42 years old (traditional dressing style), with a singleton pregnancy, having no previously known Cancer or untreated thyroidal disorders | |
| Interventions | Women randomised to: ‐ Group 1: 1200 IU/d of vitamin D3; ‐ Group 2: 2000 IU/d form of vitamin D3 (50,000 units of cholecalciferol/15 mL). Health worker cadre: recruitment for the study was done by an obstetrician and paediatric endocrinologist in the Kocaeli Maternity and Children Hospital outpatient obstetric clinics (Turkey, north of tropics). It was not stated who administered the supplements nor who measured the outcomes. |
|
| Outcomes |
Maternal Primary
Infant Secondary
Laboratory method used for assessment of vitamin D concentrations: Enzyme immunoassay method (IDS ‐ Immunodiagnostic Systems) |
|
| Notes | • Start of supplementation:earliest at 13 weeks, latest at 32 • Pre‐gestational BMI (kg/m2): unknown/mixed; • Supplementation scheme/regimen: daily • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not available • Latitude: north of Tropics • Season at the start of pregnancy: spring Source of funding: not available Dates of the study: April 2011 – April 2012 Declarations of interest among primary researchers: this information is not reported by the trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were randomised using a simple randomisation method. Details on simple randomisation method used not provided. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an unblinded study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This was an unblinded study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data seems to have been reported for all participants who completed the study. G1:n = 28 (7 lost to follow‐up, 3 withdrawals, 4 could not be contacted); G2:n = 31 (7 lost to follow‐up, 4 withdrawals, 3 could not be contacted); G3:n = 32 (9 lost to follow‐up, 7 withdrawals, 2 could not be contacted). |
| Selective reporting (reporting bias) | Low risk | All intended outcomes (methods) were reported |
| Other bias | Unclear risk | There is insufficient information to allow judgement. |
O'Brien 2013.
| Methods | Parallel quasi‐randomised clinical trial | |
| Participants | Adolescents 13–18 years of age, carrying a singleton pregnancy, and between 12 and 29 weeks of gestation at enrolment. Exclusion criteria: use of tobacco, steroids, or medications that influence vitamin D or calcium metabolism as well as HIV infection, malabsorption disease, diabetes, history of an eating disorder, history of drug abuse, or medical history of elevated blood lead. |
|
| Interventions | Adolescents randomised to: ‐ Group 1 (n = 42): 2000 IU of vitamin D3 daily (pills) plus prenatal supplement containing 400 IU of vitamin D3; ‐ Group 2 (n = 41): 200 IU of vitamin D3 daily plus prescribed prenatal supplement containing 400 IU of vitamin D3. Health worker cadre: participants received prenatal care at the Rochester Adolescent Maternity Program (a program that provides specialised prenatal care for pregnant adolescents) USA, Rochester, New York (latitude 43°N). North of Tropics. A study co‐ordinator allocated participants. Women received a 6‐week supply of vitamin D3 supplements (Tishcon Corporation, Westbury, New York) at enrolment. At each monthly prenatal visit, a new supplement bottle was supplied and the previous bottle along with any remaining pills was collected and subsequently pills were counted. Participants were remunerated for each bottle that was returned regardless of adherence. The total number of pills consumed by each participant was based on the number of bottles and pills returned. If a bottle was not returned, then it was determined that the participant consumed 0 pills from that bottle; the cumulative intake of vitamin D3 from the intervention was calculated by multiplying the number of pills consumed by either 200 or 2000 IU. To aid interpretation, cumulative intake was rescaled to an estimate of daily intake by dividing it by the overall mean number of days in the study. Intervention adherence was defined as the number of pills consumed divided by the number of pills dispensed. The vitamin D3 content of a randomly selected supplement from each study arm was assessed annually by HPLC at an external lab (Heartland Laboratories, Ames, IA). The article does not specify who provided the treatment, conducted the questionnaires, drew blood or assessed dietary intake. Study assistants entered 24‐h recalls into the Nutrition Data System for Research 2014 (University of Minnesota, Minneapolis, MN) in duplicate. To encourage participation, study personnel sent weekly text messages and provided educational handouts at prenatal visits.Blood samples were analysed at the Department of Laboratory Medicine at the University of Washington (Seattle, WA). |
|
| Outcomes |
Maternal Primary
Secondary
Infant Primary
Secondary
Laboratory method used for assessment of vitamin D concentrations: vitamin D metabolites (25‐hydroxyergocalciferol (25(OH)D2), 25‐hydroxycholecalciferol (25(OH)D3), 1,25‐dihydroxyergocalciferol, 1,25‐dihydroxycholecalciferol, and 24,25‐dihydroxycholecalciferol (24,25(OH)2D3)) in pristine serum were analysed simultaneously by LC with tandem MS. |
|
| Notes | • Start of supplementation: at enrolment between 12 and 29 weeks of gestation • Pre‐gestational BMI (kg/m2): all included. • Supplementation scheme/regimen: daily • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not available • Latitude: north of Tropic • Season at the start of pregnancy: all Source of funding: supported by United States Department of Agriculture (USDA)‐ award 2011–03424 and NIH award T32‐DK007158. Cornell University, University of Rochester Dates of the study: from October 2012 until August 2015 Declarations of interest among primary researchers: CMB, EKP, RAQ, EC, FV, and KOO disclose no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate group assignment method used. |
| Allocation concealment (selection bias) | High risk | Quote: "A study coordinator allocated participants by alternate assignment to 2 parallel groups that received either 200 IU or 2000 IU of vitamin D3 daily in addition to their prescribed prenatal supplement containing 400 IU of vitamin D3." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, providers, and study personnel were blinded to supplement group identity. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, providers, and study personnel were blinded to supplement group identity. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants that completed the visit was similar between groups.G1: n = 42 (delivery blood sample collected n = 32, sample missed n = 1, miscarried pregnancy n = 2, became ineligible n = 3, withdrew n = 1, lost to follow‐up n = 3); G2; n = 41 (withdrew n = 2) delivery blood sample collected n = 30, sample missed = 1, miscarried pregnancy n = 2, became ineligible n = 1, withdrew n = 2, lost to follow‐up n = 3) |
| Selective reporting (reporting bias) | Unclear risk | Quote: “The original data analysis plan was to assess change in the serum markers as a function of assignment to cholecalciferol (vitamin D3) supplement group. However, because of low adherence to the intervention, we had to treat the study as an observational cohort study. We identified predictors of the change in 25(OH)D across pregnancy and tested whether GA and maternal 25(OH)D status interacted to affect serum 1,25(OH)2D, 24,25(OH)2D, or PTH concentration.” AE were not reported. |
| Other bias | Low risk | No other bias detected. |
Rostami 2017.
| Methods | Eight‐arm blind randomised clinical trial | |
| Participants | Women with a GA < 14 weeks based on lLMP or obstetrical estimation, with a singleton pregnancy, and who had planned to receive ongoing prenatal and delivery in the Masjed‐Soleyman. Participants were excluded if they consumed multivitamins containing more than 400 international units (IU) per day of vitamin D3; used anticonvulsants; and had history of chronic diseases like diabetes, hypertension, renal dysfunction, liver diseases, and complicated medical or obstetrical history |
|
| Interventions | Women randomised by levels of vitamin D in serum Participants with moderate deficiency
Participants with severe deficiency
Health worker cadre: the study was conducted at prenatal care centres of urban areas of Masjed‐Soleyman and Shushtar, Iran; 2 cities with similar cultural, geographic, nutritional habits, and sun exposure conditions; 1 of these cities (Masjed‐Soleyman) was assigned to intervention. Masjed‐Soleyman County is in the northeast of Khuzestan province. Its area is 9/6327 km2 with a population of 103,369 people with Persian ethnicity. This is a sunny region with a hot and humid climate. Its altitude is 260 metres above sea level. In terms of geographical location, it is between 31°59′ E longitude and 49°17′ N latitude. Shushtar County is in the north of Khuzestan. Its area is 2436 km2 with a population of 192,361 people with Persian ethnicity. The climate is similar to Masjed‐Soleyman. Its altitude is 150 metres above sea level. In terms of geographical location, it is between 48°20′ E longitude and 32°30′ N latitude. Outside of Tropics
|
|
| Outcomes |
Maternal Primary
Secondary
Infant Primary
Secondary (infant)
Laboratory method used for assessment of vitamin D concentrations: circulating 25(OH) levels were measured using enzyme‐linked immunosorbent assay (ELISA) method and a kit of Immunodiagnostics Systems Ltd (IDS Ltd) by Auto Analyzer (Human Corporation, Germany). This 25(OH)D assay is FDA‐cleared for clinical use in the USA. The inter‐ and intra‐assay coefficient of variations were 3.891% and 3.37%, respectively (sensitivity of 5nmol/L). Calibration of the instruments was done as per the manufacturer's instructions and validation studies were done prior to the test. Samples were analysed by a single technician using the same equipment throughout the study in a reference laboratory and were measured according to the standard operating procedures. |
|
| Notes | • Start of supplementation: July 1‐September 31, 2014 • Pre‐gestational BMI (kg/m2): unknown/mixed • Supplementation scheme/regimen: sIngle, weekly, monthly • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not available • Latitude: outside of Tropics • Season at the start of pregnancy: summer/autumn Source of funding: this work was financially supported by the Research Institute of Endocrine Sciences (grant number; 493). Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences http://dx.doi.org/10.13039/501100007427, 493, Fahimeh Ramezani Tehrani. Dates of the study: July 1‐September 31, 2014. Declarations of interest among primary researchers: none reported. The authors provided additional data by email which were incorporated into the meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants in each group of severe or moderate deficiencies were randomly divided into 4 subgroups using a computer‐generated list/permuted block randomisation by a biostatistician to achieve balance across treatment groups. The number of participants per block was 8. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes were assigned to each participant by a research assistant not associated in the trial. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Physicians, who participated in various phases of the study, were blinded to grouping of women; only the midwife, who did not participate in any phase of the study, was aware of the group that each patient was in. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Physicians, who participated in various phases of the study, were blinded to grouping of women; only the midwife, who did not participate in any phase of the study, was aware of the group that each patient was in. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up was low. Moderate deficiency group: n = 400; I1: n = 100, 2 miscarriages, I2: n = 100, 1 miscarriage, I3 n = 100, 1 miscarriage, 2 discontinued participation), I4:n = 100. Severe deficiency group: I5: n = 100, 1 miscarriage, 1 discontinued participation, I6:n = 100, 2 miscarriages, I7:n = 100, 1 miscarriage, I8:n = 100, 1 miscarriage. |
| Selective reporting (reporting bias) | High risk | Outcomes not reported as pre‐specified or expected. |
| Other bias | Unclear risk | Insufficient information to allow judgement. |
Roth 2013.
| Methods | Individually‐randomised, double‐blind, placebo‐controlled trial, with 5 arms | |
| Participants | Women aged 18 years and above; with 17 to 24 completed weeks of gestation (i.e. 17 weeks + 0 days to 24 weeks + 0 days, inclusive) based on recalled LMP and/or ultrasound; intending to reside in the trial area (for at least 18 months). Exclusion criteria listed below applied: history of any medical condition or medications that may predispose to vitamin D sensitivity, altered vitamin D metabolism, and/or hypercalcaemia, including active tuberculosis or current therapy for tuberculosis, sarcoidosis, renal/ureteral stones, PTH disease, renal/liver failure, use of anti‐convulsants; high‐risk pregnancy based on 1 or more of the following findings by point‐of‐care testing: severe anaemia: haemoglobin < 70 g/L assessed by Hemocue; moderate‐severe proteinuria: ≥ 300 mg/dL (3+ or 4+) based on urine dipstick, hypertension: ≥ 1 systolic blood pressure reading ≥ 140 mm hg and/or ≥ 1 diastolic blood pressure reading ≥ 90 mm Hg, in repeat measurements taken at least 1 minute apart; high‐risk pregnancy based on 1 or more of the following findings by maternal history and/or ultrasound: multiple gestation, major congenital anomaly, severe oligohydramnios; unwillingness to stop taking non‐study vitamin D or calcium supplements or a multivitamin containing calcium and/or vitamin D; use of vitamin D supplements as part of a physician's treatment plan for vitamin D deficiency; previous enrolment in the trial during a previous pregnancy. |
|
| Interventions | Experimental intervention (1300 randomised): · Group prenatal 4200: 4200 IU per week (prenatal 4200 group) during pregnancy and no postpartum vitamin D; · Group prenatal 16,800: 16,800 IU per week during pregnancy – no postpartum vitamin D; · Group prenatal 28,000: 28,000 IU per week during pregnancy and no postpartum vitamin D; · Group (prenatal and postpartum 28,000: 28,000 IU per week during pregnancy and 28,000 IU per week in the postpartum (26 weeks). All participants received daily supplementation with 500 mg calcium (as calcium carbonate), 66 mg elemental iron, 350 μg (0.35 mg) folic acid throughout the intervention phase. Health worker cadre: participants attending antenatal care the Maternal and Child Health Training Institute,a public hospital in Dhaka, Bangladesh (north of Cancer Tropic). Trial personnel contacted participants weekly from enrolment until 26 weeks postpartum, and infants were further assessed at 9 months and 12 months of age. Visits were conducted in the home or at a clinic and included the use of standardised questionnaires, point‐of‐care tests, anthropometric measurements, and specimen collection, the detailed medical screening by a study physician to assess inclusion criteria. Once a participant, study personnel maintain and store all tablet supplies in locked study offices and directly observe tablet ingestion during home or clinic visits. Trained personnel collect participant data using questionnaires, point of care clinical tests, abstraction of prenatal ultrasound reports, anthropometric measurements, and specimen collection throughout the interventional and observational phases of the study. Trained phlebotomists collect maternal blood, paternal blood, cord venous and arterial blood, and infant blood specimens according to standard sampling procedures. Recording of the umbilical cord clamping and cutting was determined by the attending physician or birthing attendant. Participants were provided with free medical care and encouraged to seek medical attention from trial physicians and to notify trial personnel of concerns about their health. |
|
| Outcomes |
Maternal
Infant
Laboratory method used for assessment of vitamin D concentrations: Maternal (baseline, delivery, 3 months postpartum, 6 months postpartum), venous cord, and infant (3 months, 6 months, 12 months) 25‐hydroxyvitamin D (25(OH)D) concentrations were measured using high‐performance liquid chromatography‐ tandem mass spectrometry (LC‐MS/MS). |
|
| Notes | • Start of supplementation: 17 to 24 weeks of gestation • Pre‐gestational BMI (kg/m2): unknown/mixed • Supplementation scheme/regimen: weekly • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not available • Latitude: north of the Tropic of Cancer • Season at the start of pregnancy: unknown Source of funding: the trial is funded by the Bill and Melinda Gates Foundation (BMGF, OPP1066764). ADG’s research is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH) under BIRCWH award number K12HD055882, Career Development Program in Women’s Health Research at Penn State. Setting/country: Dhaka, Bangladesh Dates of the study: March 18, 2014. Conflicts of interest: the authors declare that they have no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A computer‐generated, simple randomisation scheme was created independently by the trial statistician.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Concealment of trial‐group assignments was ensured with the use of pre‐labeled and sequentially numbered but otherwise identical supplement vials, which were provided to participants in accordance with the assignment sequence.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Outcome group: All Quote:“The master list linking participant identifiers to supplementation groups was held by the supplement manufacturer and not accessed by any trial personnel until final group assignments were revealed.” Quote:”Tablets with different doses were identical in appearance and taste.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:"The master list linking participant identifiers to supplementation groups was held by the supplement manufacturer and not accessed by any trial personnel until final group assignments were revealed” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 5% participants withdrawn or excluded after randomisation until birth. There were not much variation among the groups. Group prenatal 4200: 4200 IU per week (prenatal 4200 group) during pregnancy and no postpartum vitamin D; n = 260 (6 Exclusions/withdrawals during pregnancy: 1 maternal death, 0 Protocol violation, 1 voluntary withdrawal, 0 lost to follow‐up, 4 intrauterine death/stillbirth) Group prenatal 16,800: 16,800 IU per week during pregnancy – no postpartum vitamin D; 8 Exclusions/withdrawals during pregnancy: 0 maternal death, 1 protocol violation, 2 voluntary withdrawal, 3 lost to follow‐up, 2 intrauterine death/stillbirth Group prenatal 28,000: 28,000 IU per week during pregnancy and no postpartum vitamin D; 8 Exclusions/withdrawals during pregnancy: 0 maternal death, 0 protocol violation, 1 voluntary withdrawal, 0 lost to follow‐up, 7 intrauterine death/stillbirth Group (prenatal and postpartum 28,000: 28,000 IU per week during pregnancy and 28,000 IU per week in the postpartum (26 weeks). 11 Exclusions/withdrawals during pregnancy:0 maternal death, 0 protocol violation, 1 voluntary withdrawal, 2 lost to follow‐up, 8 intrauterine death/stillbirth |
| Selective reporting (reporting bias) | Low risk | The manuscript and the appendix supplementary material correspond to the planned protocol. The protocol available along with the manuscript includes a section summarizing changes from the original protocol, and to the original statistical analysis. The study was registered at clinicaltrials.gov |
| Other bias | Low risk | There is no any evidence of other bias. |
Shakiba 2013.
| Methods | Randomised clinical trial | |
| Participants | Healthy, pregnant women from the beginning of their second trimester of pregnancy. Exclusion criteria were not explicitly stated. |
|
| Interventions | Group A (n = 17): 50,000 IU/month. Group B (n = 17): 100,000 IU/month (50,000 IU every 2 weeks). Group C (n = 17): vitamin D deficient women (25(OH)D levels < 20 ng/mL) were treated with a total of 200,000 IU (50,000 IU/week for 4 weeks), followed by supplementation with 50,000 IU/month. Health worker cadre: the women were randomly recruited from 2 primary care clinics, a locality known to have a high prevalence of vitamin D deficiency, in Yazd (31°53’50”N/54°22’04”E), Iran (north of tropics), where > 90% of the days are sunny. Obstetricians and midwives conducted monthly visits to ensure that the participants adhered to the recommended dosage of vitamin D3. A paediatrician examined the neonate for possible anomalies and recorded the anthropometric measurements at the time of delivery. Other health workers not mentioned. |
|
| Outcomes |
Maternal Secondary
Infant Primary
Secondary (infant)
Laboratory method used for assessment of vitamin D concentrations: Chemiluminescence immunoassays (DiaSorin, spA, Via Crescentino, Vercelli, Italy) |
|
| Notes | • Start of supplementation: second trimester • Pre‐gestational BMI (kg/m2): unknown/mixed; • Supplementation scheme/regimen: weekly/monthly • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not available • Latitude: north of Tropics • Season at the start of pregnancy: autumn and winter of 2009 Source of funding: not reported. Dates of the study: not described. Declarations of interest among primary researchers: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation. Of the 51 participants, 34 were randomly classified into 2 groups (Groups A and B) and the remaining 17 women, were allocated to Group C based on their serum 25(OH)D levels |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to allow judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not reported in the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding was not reported in the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all mentioned outcomes (methods), including those that were pre‐specified. |
| Other bias | Unclear risk | Insufficient information to allow judgement. |
Soheilykhah 2011.
| Methods | Randomised controlled trial | |
| Participants | Pregnant women with GA less than 12 weeks without gestational diabetes, history of PCO, BMI less than 30 kg/m2 before pregnancy, no vitamin D supplementation in the past 6 months. Exclusion criteria: women with diabetes or gestational diabetes, treated with insulin, women with thyroid or parathyroid disorders, polycystic ovary disease before pregnancy, pre‐pregnancy BMI > 30 kg/m2, and, women who received vitamin D supplementation during the prior 6 months. |
|
| Interventions | Group B: 50,000 IU monthly (2000 IU daily) Group C: 50,000 IU every 2 weeks (4000 IU daily). Health worker cadre: the study was conducted in 2 prenatal clinics: Mojibian Hospital and Shahid Sadoughi Hospital) in Yazd, Iran. Latitude: 31.89 north of Tropics. Pregnant women were followed up every month during pregnancy and were evaluated regarding adverse effects of vitamin D. A blood sample for measurement of FBS, insulin, vitamin D and calcium was taken at the end of pregnancy for each participant. Specific research staff roles were not described. |
|
| Outcomes |
Maternal Primary
Secondary
Laboratory method used for assessment of vitamin D concentrations: vitamin D was analysed by chemiluminescence assay with an inter‐assay coefficient of variation (CV) of 2.7% and an intra assay CV of 3.2%. |
|
| Notes | • Start of supplementation: supplementation began in the 12th week of pregnancy • Pre‐gestational BMI (kg/m2): < 30 kg/m2 • Supplementation scheme/regimen: biweekly and monthly • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not available • Latitude: north of Tropics • Season at the start of pregnancy: not stated Source of funding: none disclosed. Dates of the study: 2009‐2011. Declarations of interest among primary researchers: the authors report no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were divided into 3 groups randomly. Computer‐generated random number lists were drawn up by an independent researcher. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to allow judgment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Pregnant women and researchers were not blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Pregnant women and researchers were not blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 40 participants from group C, 38 cases from group B and 35 pregnant women from group A completed participation. Reasons for attrition were not mentioned, but group characteristics remained similar. |
| Selective reporting (reporting bias) | High risk | Authors reported that supplementation with 50,000 IU vitamin D every 2 weeks resulted in no adverse effects, such as hypercalcaemia, in pregnant women. Incidents of AE not reported for women in other intervention groups. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Stephensen 2011.
| Methods | Double‐blind randomised study | |
| Participants | Participants aged > 18 years and with a singleton pregnancy of < 20 weeks at enrolment were included in this study. Exclusion criteria included regular and recent use of vitamin D supplements (> 600 IU/d); a recent history of tanning bed use; regular and midday sun exposure > 90 min/d; a history of hypertensive, digestive, or endocrinologic diseases, autoimmune disease, or type 1 diabetes; use of anticonvulsant therapy or other medications known to affect vitamin D or calcium metabolism; and previously diagnosed digestive or absorptive problems. |
|
| Interventions | Participants were randomised to: ‐ Intervention group: 2000 IU/d of vitamin D3 (daily prenatal multivitamin and multi‐mineral that contained 400 IU vitamin D3 plus the daily study supplement with 1600 IU/d cholecalciferol). ‐ Comparison: 400 IU/d vitamin D3 (daily prenatal multivitamin and multi‐mineral that contained 400 IU vitamin D3 plus a daily study supplement with no vitamin D (containing rice flour). Health worker cadre: participants were recruited from the obstetrics and gynaecology clinics at the UC Davis Medical Center (UCDMC) and the Davis, California, USA; study visits at the USDA Western Human Nutrition Research Center (WHNRC). Latitude 38.5, north of tropics. A UCDMC Investigational Drug Service pharmacist generated a single block‐randomised list and distributed the study supplements to participants in sequential order as they were enrolled.and trained blinded research staff conducted the study. |
|
| Outcomes |
Maternal Primary
Secondary
Infant Secondary
Laboratory method used for assessment of vitamin D concentrations: not available. |
|
| Notes | • Start of supplementation: week 20 until delivery • Pre‐gestational BMI (kg/m2): unknown/mixed • Supplementation scheme/regimen: daily • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not available • Latitude: north of Tropics • Season at the start of pregnancy: summer or fall 57.9% of participants; Winter or spring 42.1% of participants Source of funding: not available. Dates of the study: August 2010 and June 2013. Declarations of interest among primary researchers: not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A UCDMC Investigational Drug Service pharmacist generated a single block‐randomised list (block size of 4). |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to allow judgment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study staff and participants were blinded to the treatment group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study staff and participants were blinded to the treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data observed. Intervention group: n = 28; lost to follow‐up:(n = 3), excited the study due to pregnancy loss (n = 2); discontinued due to medical DQ (n = 1). Comparison group: group: n = 28; lost to follow‐up:(n = 3), excited the study due to pregnancy loss (n = 1);discontinued due to medical DQ (n = 2). |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Thiele 2014.
| Methods | Double‐blind, randomised controlled trial | |
| Participants | Inclusion criteria were pregnancy between 24 and 28 weeks, history of breastfeeding for at least 4 weeks with a prior infant, intent to breastfeed for at least 4 to 6 weeks, and maternal age 18 years or greater. Those with pre‐existing type 1 or type 2 diabetes, pre‐existing hypertension, parathyroid disease, uncontrolled thyroid disease, and use of vitamin D supplements beyond a prenatal vitamin in the last 6 months were excluded from study participation. |
|
| Interventions | Women were randomised to (16 randomised): ‐ Intervention arm: 3800 IU/d (prenatal vitamin containing 400 IU vitamin D3 plus a vitamin D capsule containing 3400 IU). ‐ Placebo arm: 400 IU/d (prenatal multivitamin and multi‐mineral containing 400 IU vitamin D3 plus a placebo capsule containing rice flour). Health worker cadre: the study was conducted in the Upper Midwestern United States, in a hospital‐based obstetric practice. 47º north latitude (north of tropics). The clinic nurse identified potential study participants, who were approached by the research team members and were given a description of the study that included a review of inclusion and exclusion criteria. The placebo capsule and the 3,400‐IU vitamin D3 (intervention arm) capsule were compounded using a vegetable cellulose base, were visually identical, and were packaged in identical pill bottles. The vitamin D 3 and placebo were sealed in packets numbered to correspond with the coded random group assignment; this assignment was made by an unblinded member of the research team who was not involved in determining eligibility or recruiting participants. Blinding of the intervention was maintained at the level for participants, the data collector, and the data analyst. A research team member met with each participant every 30 days to assess intervention fidelity and address any concerns. At the monthly meeting, participants returned any unused capsules and received a new 30‐day supply of the study capsules. Phone or e‐mail contacts were established in monthly intervals, allowing for further evaluation of intervention fidelity and maintaining participant interest in the study. Blood samples at enrolment were collected by a laboratory technician, concurrent with regularly scheduled obstetric blood draws. Blood samples at the time of birth were collected concurrently with blood sampling for other obstetric and neonatal care needs. A research team member was present for blood collection and transported samples to the laboratory for processing. |
|
| Outcomes |
Maternal Secondary
Infant Secondary
Laboratory method used for assessment of vitamin D concentrations: the 25(OH)D was measured by enzyme immunoassay (Immunodiagnostic Systems Ltd., Gaithersburg, MD). Blood samples were processed using established protocol with consistent equipment at a single location. |
|
| Notes | • Start of supplementation: 24 to 28 weeks' gestation • Pre‐gestational BMI (kg/m2): unknown/mixed • Supplementation scheme/regimen: daily • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not available • Latitude • Season at the start of pregnancy: women were recruited in summer months, gave birth in the fall, and completed the study by winter Source of funding: not reported. Dates of the study: July 2012 and January 2013. Declarations of interest among primary researchers: the authors reported no conflict of interest. No relevant financial relationships were reported. No commercial support was received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A random sequence generator was used for group assignment corresponding to the participant numbers in a 1:1 ratio. This random sequence was generated independently from the research team.” |
| Allocation concealment (selection bias) | Low risk | Quote:“The vitamin D3 and placebo were sealed in packets numbered to correspond with the coded random group assignment; this assignment was made by an unblinded member of the research team who was not involved in determining eligibility or recruiting participants.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote:“Blinding of the intervention was maintained at the level of participants, the data collector, and the data analyst until completion of all data collection.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study staff was blinded until completion. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition during the study was reported. Intervention arm: n = 8; consent withdrawal (n = 1,12.5%); control arm: n = 8; consent withdrawal (n = 1,12.5%); meet exclusion criteria (n = 1, 12.5%). |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes. |
| Other bias | Unclear risk | Insufficient information to allow judgment. |
Wagner 2006a.
| Methods | Two‐centre, randomised, double‐blinded study of vitamin D supplementation | |
| Participants | Women with maternal age ≥ 16 years; confirmed singleton pregnancy of < 16 completed weeks of gestation at the time of enrolment; and intention to receive ongoing prenatal care at the community health centre where consent was obtained. Mothers with pre‐existing calcium or parathyroid conditions or who required chronic diuretic or cardiac medication therapy, including calcium channel blockers, were not eligible for enrolment into the study. Mothers with active thyroid disease (e.g. Graves, Hashimoto, or thyroiditis) also were not eligible to participate in the study; however, mothers on thyroid supplement with normal serological parameters could participate in the study if they were without any other endocrine dysfunction. |
|
| Interventions | Recruited pregnant women were randomised to: ‐ Group 1 (n = 127): 4000 IU/d (vitamin D tablets of 3600 IU/d plus a prenatal multivitamin‐multi mineral tablet containing 400 IU vitamin D3). ‐ Group 2 (n = 130): 2000 IU/d (vitamin D tablets of 1600 IU/d) plus a prenatal multivitamin‐multi mineral tablet containing 400 IU vitamin D3). Those mothers unable to swallow a prenatal vitamin were given a Flintstones Complete chewable vitamin (Bayer Healthcare, Morristown, NJ). Health worker cadre: not specified. |
|
| Outcomes |
Maternal Primary
Secondary
Infant Primary
Secondary
Laboratory method used for assessment of vitamin D concentrations: a rapid, direct RIA developed in an author laboratory (B.W.H.) and manufactured by Diasorin Corp (Stillwater, MN) was used to measure total circulating 25(OH)D concentration in serum samples). |
|
| Notes | • Start of supplementation: not before 12 weeks GA • Pre‐gestational BMI (kg/m2): unknown/mixed • Supplementation scheme/regimen: daily • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not available • Latitude: north of Tropics • Season at the start of pregnancy: all, however the season that each blood sample was drawn was defined Source of funding: funded by the Thrasher Research Fund; grant numbers NIH RR01070 from the National Center for Research Resources, and UL1 RR029882 from the National Center for Advancing Translational Sciences, National Institutes of Health; and Medical University of South Carolina Children’s Hospital Fund and the Division of Neonatology, Medical University of South Carolina, Charleston, SC. Setting/country: Eau Claire, South Carolina, USA Dates of the study: Nov. 21, 2006. Declarations of interest among primary researchers: not reported by the trial authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:“Randomization lists were generated by computer prior to the start of the study.” |
| Allocation concealment (selection bias) | Low risk | Quote:“Dose groups were identified for logistical purposes using 6 letters (3 per dose group) as an additional measure against inadvertent unblinding.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomisation assignment was blinded to all participants and to the investigators except for the study biostatistician. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Randomisation assignment was blinded to all participants and to the investigators except for the study biostatistician. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was a large number of participants that were lost to follow‐up but it was similar between groups. Group 1:n = 127 (exited before starting the intervention n = 1, lost to follow‐up n = 47), Group 2:n = 130 (exited before starting the intervention n = 2, lost to follow‐up n = 50) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | Insufficient information to allow judgment. |
Wagner 2006b.
| Methods | Single‐centre, randomised, controlled, double blind study | |
| Participants | Women with an age range from 17 to 44 years, with a confirmed singleton pregnancy of fewer than 16 completed weeks of gestation at the time of consent and who planned to receive ongoing prenatal care in the Charleston, South Carolina, USA area. Women with a pregnancy at greater than 16 weeks of gestation as calculated by their LMP were not eligible to participate. Pregnant women with pre‐existing calcium or parathyroid conditions or who required chronic diuretic or cardiac medication therapy, including calcium channel blockers, or who suffered chronic hypertension were not eligible for enrolment in the study. Pregnant women with active thyroid disease (e.g. Graves disease, Hashimoto disease, or thyroiditis) also were excluded, but mothers on thyroid supplement with normal serologic parameters could participate in the study if they were without any other endocrine dysfunction. |
|
| Interventions | Recruited women were randomised into 3 groups of vitamin D3 (2 pills/d): ‐ Group 1 (n = 167): 2000 IU/d (tablet with 1600 IU/d D3 + prenatal multivitamin vitamin with 400 IU D3); ‐ Group 2 (n = 168): 4000 IU/d (tablet with 3600 IU/d D3 + prenatal multivitamin vitamin with 400 IU D3); ‐ Group 3 (comparison; n = 166): 400 IU/d (tablet with 0 IU/d + prenatal multivitamin vitamin containing 400 IU D3). Multivitamin‐Multi‐mineral Supplement (distributed by Pfizer Consumer Healthcare, Morris Plains, NJ, USA). Mothers who were unable to swallow a prenatal vitamin were given Flintstones Complete chewable vitamin (Bayer Healthcare, Morristown, NJ, USA), which provided 400 IU of vitamin D3 per tablet. vitamin D tablets were manufactured by Tishcon Corporation (Westbury, NY, USA), a Good‐Manufacturing‐Practice (GMP) facility. The cholecalciferol contained in the vitamin D tablet was supplied to Tishcon Corporation by Hoffman‐La Roche, Ltd. (Basel, Switzerland). Health worker cadre: the study was conducted at the Medical University of South Carolina’s (MUSC) facilities. If a woman received her obstetrical care at a facility separate from MUSC, then she came to MUSC’s Clinical and Translational Research Center (CTRC) outpatient research facility for each of the study visits. Characteristics of each mother’s health status and complications during pregnancy, labour, and delivery were recorded and reviewed by an obstetrician (DDJ, blinded to treatment). If the mother required hospitalisation, a copy of the hospital record was obtained after the mother had signed a release of medical information form. Any acute illnesses, hospitalisations, or development of pregnancy‐related conditions that were not pre‐existing also were recorded.Maternal blood and urine samples were collected at each visit. Cord blood was obtained at delivery. If the cord blood sample could not be obtained, a neonatal blood sample was drawn within 2 weeks of delivery. Maternal serum total calcium, creatinine, and inorganic phosphorus levels were measured by MUSC’s Clinical Chemistry Laboratory using standard methodology and laboratory normative data. Results were reported to the clinical principal investigator (PI; CLW) and downloaded to the research database from the clinical chemistry registry. All results were reviewed by the clinical principal investigator of the study on a weekly basis for any abnormal values and reported to the DSMC. Detailed description of the research team is lacking. |
|
| Outcomes |
Maternal Primary
Secondary
Infant Secondary
Laboratory method used for assessment of vitamin D concentrations: circulating vitamin D2 and D3 were measured in serum using direct ultraviolet detection preceded by organic extraction and HPLC. An RIA manufactured by Diasorin Corporation and developed in the Hollis laboratory was used to measure total circulating 1,25‐dihydroxyvitamine D3. |
|
| Notes | • Start of supplementation:12 to 16 weeks’ gestation • Pre‐gestational BMI (kg/m2): pre‐pregnancy BMI classified by intervention arm and as > 30 and ≤ 30 kg/m2 • Supplementation scheme/regimen: daily • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not available • Latitude: north of Tropics • Season at the start of pregnancy: April–September and October–March Source of funding: funded by the National Institute of Children’s Health and Human Development #R01 HD47511, NIH #RR01070 and by the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, NIH/NCRR Grant number UL1 RR029882. Dates of the study: 2004‐2010. Declarations of interest among primary researchers: Bruce W. Hollis, Ph.D. serves as a consultant for Diasorin Inc., Stillwater, MN. All other authors (DDJ, TCH, ME, and CLW) state that they have no conflicts of interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Our study used stratified blocked randomisation to balance by ethnicity and also to balance by enrolment (as a cautionary measure against a potential temporal or seasonal bias). A randomisation scheme was developed separately for each of the 3 ethnic groups (i.e., the strata). Within each stratum, the treatments were assigned within blocks. Because there were 3 treatment groups, the block size had to be divisible by 3; the data team selected a block size of 6, which was unknown to the investigators or the pharmacists. In this way, at the end of each block (i.e., enrolment of 6 participants), each ethnic group was balanced in the number randomly assigned to the 400‐, 2000‐, and 4000‐IU treatment groups.” |
| Allocation concealment (selection bias) | Low risk | The data team selected a block size of 6, which was unknown to the investigators or the pharmacists. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Each completed Food Frequency form was sent to the processing centre (Berkeley, CA, USA), and these data were reviewed later for accuracy by a registered dietician who was blinded to subject treatment group assignment.” Quote: “…the data team selected a block size of 6, which was unknown to the investigators or the pharmacists.” Quote: “Characteristics of each mother’s health status and complications during pregnancy, labor, and delivery were recorded and reviewed by an obstetrician (DDJ, blinded to treatment).” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | This study was a single‐centre, randomised, controlled, double blind study of vitamin D supplementation stratified by race |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Group 1:n = 167 (exited before starting the intervention n = 1, lost to follow‐up n = 44), Group 2:n = 169 (exited before starting the intervention n = 2, lost to follow‐up n = 50), Group 3: n = 166 (exited before starting the intervention n = 2, lost to follow‐up n = 53), |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Insufficient information to allow judgement |
Wagner 2013.
| Methods | Single‐centre, randomised, double‐blinded study of vitamin D supplementation | |
| Participants | This study was part of a randomised, placebo‐controlled clinical trial (NCT 01932788) in which enrolled mothers were 18–45 years of age who presented at 8‐14 weeks’ gestation with a singleton pregnancy. Participants were excluded in they had pre‐existing calcium or uncontrolled thyroid/parathyroid diseases and other similar conditions. Mothers were randomised to receive placebo or 4000 IU/d vitamin D3. All participants received the standard prenatal vitamin (containing 400 IU vitamin D3). Mothers were followed monthly through delivery, which coincided with a total of 6 to 7 visits prior to delivery. | |
| Interventions | Participants were randomised to 1 of 2 groups: (1) Group A (n = 173): 400 IU vitamin D3/d—Standard dose treatment of placebo (0 IU vitamin D3) plus prenatal vitamin (400 IU/d); or (2) Group B (n = 169): 4400 IU/d (4000 IU/2 gummies/d + 400 IU/d in prenatal). Health worker cadre: not specified as only preliminary findings have been reported. |
|
| Outcomes |
Maternal Primary
Secondary
Infant Primary
Secondary
Laboratory method used for assessment of vitamin D concentrations: A rapid, direct radioimmunoassay developed in an author laboratory (B.W.H.) and manufactured by Diasorin Corp (Stillwater,MN) was used to measure total circulating 25(OH)D concentration in serum samples). |
|
| Notes | • Start of supplementation: Irrespective of enrolment GA, vitamin D supplementation did not begin before the 12th week of gestation (12 and 0/7 weeks) • Pre‐gestational BMI (kg/m2): unknown/mixed • Supplementation scheme/regimen: daily • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not available • Latitude: north of Tropics • season at the start of pregnancy Setting/country: Charleston, South Carolina, USA Source of funding: W.K. Kellogg Foundation. Dates of the study: 2013‐2018. Declarations of interest among primary researchers: B.W.H. served as a scientific consultant for Diasorin Inc, Stillwater, MN, during the study period. The remaining authors report no potential conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization lists were generated by computer prior to the start of the study.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Dose groups were identified for logistical purposes using 6 letters (3 per dose group) as an additional measure against inadvertent unblinding.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Randomization assignment was blinded to all participants and to the investigators except for the study biostatistician.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Randomization assignment was blinded to all participants and to the investigators except for the study biostatistician.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | G1: n = 173 (Protocol violation n = 18, lost to follow‐up n = 18, lack of efficacy n = 2; G2: n = 169 (lost to follow‐up = 46, withdrawal by participant n = 5). |
| Selective reporting (reporting bias) | Unclear risk | The full results have not been published yet, therefore, we cannot judge this. |
| Other bias | Unclear risk | Insufficient information to allow judgment. |
Weiss 2009.
| Methods | Randomised, double‐blind, placebo‐controlled study | |
| Participants | Pregnant women were include if they met the following inclusion criteria: ‐ Maternal personal history of or biological father history of asthma ‐ GA between 10 and 18 weeks at the time of randomisation ‐ Maternal age between 18 and 39 years ‐ Not a current smoker (defined as not having smoked for at least 1 month prior to enrolment) and not a user of other nicotine products (e.g. nicotine patch) for at least 1 month prior to enrolment ‐ English‐ or Spanish‐speaking ‐ Intent to participate for the full 4 years (through pregnancy and then until the 3rd birthday of the child) Women were excluded if: ‐ GA > 18 weeks ‐ Presence of chronic medical conditions: (i) hypertension on medications, (ii) diabetes mellitus, (iii) parathyroid disease, (iv) uncontrolled thyroid disease, (v) kidney stones, and (vi) sarcoidosis ‐ Intake of vitamin D supplements containing 2000 IU/d of vitamin D3 ‐ Multiple gestation pregnancy ‐ Pregnancy achieved by assisted reproduction techniques (e.g. IUI, IVF) ‐ Current use of illicit drugs (defined as any use in the past 6 months prior to enrolment) ‐ Previously enrolled in VDAART for a prior pregnancy ‐ Any major fetal anomalies detected prior to delivery ‐ Patient health questionnaire (PHQ‐9) (41) depression scale ≥ 15 ‐ Any condition, in the opinion of the Clinical Center Principal Investigator, that would inhibit compliance with the study medications or prohibit long‐term participation in the trial |
|
| Interventions | Group 1 (n = 405): 4400 IU/d (4000 IU/d from vitamin D capsules + a multivitamin containing 400 IU/d); Gropu 2 (n = 401): 400 IU/d (0 IU/d from vitamin D capsules+ a multivitamin containing 400 IU/d). Health worker cadre: research staff reviewed prenatal schedules for potential participants. The research staff described the study, presented the potential participant with a written description of the study, and reviewed the eligibility criteria via a screening questionnaire and a study admissions criteria questionnaire. At the enrolment visit, research staff reviewed study procedures and the consent form. After enrolment, the research staff noted the participant's scheduled obstetrical visits and made sure that urine samples were collected at each of these scheduled monthly clinical prenatal visit. Additionally, the research staff conducted monthly reviews of electronic medical records to check for pregnancy complications. At 32–38 weeks' gestation, in addition to the monthly routine, a blood draw, skin pigmentation determination, and a number of the questionnaires that were administered at the enrolment visit were repeated. At delivery, cord blood was collected and the research staff collected information regarding the type of delivery, birthweight, and other anthropometric measures. After delivery, the research staff made telephone calls every 3 months and inquired about the health and symptoms of the infant, medication use, the type and frequency of feeding of the child, and supplement use. |
|
| Outcomes |
Maternal Primary
Secondary
Infant Primary
Secondary
Laboratory method used for assessment of vitamin D concentrations: Circulating 25(OH)D was determined using the DiaSorin Liaison® machine, which uses a chemiluminescence immunoassay (CLIA) (59), to determine plasma concentrations of 25(OH)D. For quality control, the laboratory uses US National Institute of Standards and Technology (NIST) level 1 SRM (Standard Reference Material) 972 vitamin D in Human Serum, in each run. |
|
| Notes | • Start of supplementation: supplementation started at 14 week age of gestation • Pre‐gestational BMI (kg/m2): unknown/mixed • Supplementation scheme/regimen: daily • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not available • Latitude: north of Tropics • Season at the start of pregnancy: not specified Source of funding: VDAART was supported by grant U01HL091528 from the NHLBI. Additional support was provided by grant U54TR001012 from the National Centers for Advancing Translational Sciences (NCATS) for participant visits at the Boston Medical Center. Dates of the study: Enrollment began in October 2009 and follow‐up was completed in January 2015. Declarations of interest among primary researchers: Dr Litonjua reported receiving personal fees from UpToDate Inc and Springer Humana Press. Dr McElrath reported receiving grants from the National Institutes of Health (NIH). Dr O’Connor reported receiving grants from the NIH. Dr Bacharier reported receiving grants from the NIH and National Heart, Lung, and Blood Institute (NHBLI), and personal fees from Aerocrine, GlaxoSmithKline, Genentech/Novartis, Merck, Schering, Cephalon, DBV Technologies, Teva, Boehringer Ingelheim, AstraZeneca,WebMD/Medscape, Sanofi, and Vectura. Dr Zeiger reported receiving grants from the NHBLI, AstraZeneca, Aerocrine, MedImmune, Genentech, Merck, and GlaxoSmithKline and personal fees from Genentech, Novartis, GlaxoSmithKline, and TEVA. Dr Hornsby reported receiving an NIH ancillary grant. Dr Hawrylowicz reported receiving an NIH ancillary grant, a fellowship grant fromWellcome Trust Clinical Training Research Fellowship, grant G100758 from the Medical Research Council Centre, and grants from Asthma UK, the Lord Leonard and Lady Estelle Wolfson Foundation, and the Alpha 1 Foundation. No other disclosures were reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by the DCC, Data Coordinating Center, using a system that automates the random assignment of treatment groups to Study ID numbers. The randomisation scheme employed stratified permuted blocks with randomly varied block sizes of 4 and 6, and 1 block allocation list per stratum (study site and racial/ethnic group). |
| Allocation concealment (selection bias) | Low risk | Assignment to treatment arms was done centrally by the study data co‐ordinating centre. Participants received a study ID number generated and pre‐assigned a randomisation number by the centre. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Outcome group: Study participants and personnel were both blinded – Quote: “Clinical Center investigators and staff were blinded to the treatment code.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Until end of the trial, all investigators, clinical staff and participants were masked to trial outcome data, with the exception of trial statisticians, the data manager, and the data, Safety and Monitoring Committee.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome were sufficiently explained, and missing outcome appears balanced between the groups.Group 1: n = 440 (54 maternal blood samples missing:13 Fetal or neonatal deaths, 19 unable to contact or lost to follow‐up, 22 sample not available), Group 2:n = 436 (45 maternal blood samples missing, 13 Fetal or neonatal deaths, 18 unable to contact or lost to follow‐up 14 sample not available). |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods section are reported. |
| Other bias | Low risk | No other risk of bias was identified. |
Yap 2014.
| Methods | Double‐blind randomised controlled trial of low‐dose (LD) versus high‐dose (HD) vitamin D3 supplementation | |
| Participants | Women with singleton pregnancies who were age 18 years or older and at a GA of < 20 weeks at study entry were eligible to participate. This cutoff was to ensure at least 6 weeks of treatment prior to evaluation of the primary outcome (glucose levels on oral glucose tolerance test (OGTT) at 26–28 weeks). Women were excluded if they had a history of diabetes (type 1 or type 2 diabetes or glucose intolerance already diagnosed in this pregnancy), calcium or vitamin D metabolism disorders, hypercalcaemia (serum corrected calcium > 10.4 mg/dL (> 2.6 mmol/L)), or significant renal impairment (serum creatinine > 1.7 mg/dL (> 150 μmol/L)) or were taking vitamin D supplements of ≥ 1000 IU daily. Women who had a fasting blood glucose level (BGL) > 126 mg/dL (7.0 mmol/L) or HbA1c > 6.5% (48 mmol/mol) at baseline received an early OGTT to exclude undiagnosed diabetes. |
|
| Interventions | Group 1: (n = 89): 5000 IU/d of vitamin D3 (capsule) Group 2: (n = 90): 400 IU/d of vitamin D3 (capsule). vitamin D3 capsules were provided by Blackmores Pty Ltd. Participants were instructed to take 1 capsule daily until delivery of their baby. Health worker cadre: study was conducted in a single institution in Australia (Women's Health Centre and Birth Unit, Westmead Hospital, Westmead, New South Wales, Australia), Latitude 31.25 south of tropics. A safety officer oversaw the trial. Capsules appeared identical. |
|
| Outcomes |
Maternal Primary
Secondary
Infant Secondary
Laboratory method used for assessment of vitamin D concentrations: 25OHD was measured in plasma using the DiaSorin LIAISON chemiluminescent immunoassay, which has a concordance correlation coefficient. |
|
| Notes | • Start of supplementation: supplementation started at 14 week age of gestation • Pre‐gestational BMI (kg/m2): unknown/mixed • Supplementation scheme/regimen: daily • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not available • Season at the start of pregnancy: not specified Source of funding: Roly Dunlop Scholaraship for Neurologlcal Research,Sydney Medical School Foundation, University of Sydney, Sydney, New South Wales, Australia, for financial support in her postgraduate study. The authors thank Blackmores Pty Ltd. for supplying the vitamin D supplements. Blackmores Pty Ltd. had no role ln the study design; data collection, analysis, or Interpretation; or preparation, review, or approval of the manuscript and provided no other funding, Dates of the study: February 2010 and November 2011. Declarations of interest among primary researchers: no potential conflicts of interest relevant to this article were reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “participants with plasma 25OHD of < 32 ng/mL (80 nmol/L) were randomly assigned to receive either 5,000 IU vitamin D3 daily (HD) or 400 IU daily (LD)…." Randomization was in a 1:1 ratio, with a permuted block size of 6 and sequential assignment. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Treatment allocation was made after measurement of baseline plasma 25OHD…” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Outcome group: Quote: “…study investigators and participants were blinded to the intervention allocated.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome measurement is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Group 1:n = 89 (10 withdrew consent, 1 excluded from the analysis due to incomplete OGTT), Group 2: n = 90 (10 discontinued:6 withdrew consent, 3 miscarriage 1 preterm). |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported. |
| Other bias | Unclear risk | There is insufficient information to allow judgement. |
Yu 2008.
| Methods | Prospective randomised study | |
| Participants | Included were pregnant women from the following ethnic populations; 45 Indian Asians, 45 Middle Eastern, 45 Black and 45 Caucasian women. Women who did not speak English were only included if a health advocate was able to interpret and a leaflet was provided in their language. Women with pre‐existing sarcoidosis, osteomalacia, renal dysfunction and tuberculosis were excluded from the study |
|
| Interventions | Women were randomised within each ethnic group to 3 arms from 27 weeks until delivery: ‐ Group 1 (n = 60) a daily dose of vitamin D (ergocalciferol) at 800 IU; ‐ Group 2 (n = 60) a 1 dose of 200,000 IU of (calciferol); ‐ Group 3 (n = 59) no treatment (not used in the present analysis). Health worker cadre: the study setting was an antenatal unit at St Mary’s Hospital London, United Kingdom. Latitude 51.5º north of tropics. All study personnel and participants were not blinded to treatment assignment. The person seeing the pregnant women allocated the next available number on entry to the trial, and each woman collected her tablets directly from the hospital pharmacy department or her local pharmacy. Study staff roles were not specified. |
|
| Outcomes |
Maternal Secondary
Infant Secondary
Laboratory method used for assessment of vitamin D concentrations: not available |
|
| Notes | • Start of supplementation:27 weeks GA • Pre‐gestational BMI (kg/m2): unknown/mixed • Supplementation scheme/regimen: daily, stat or none • Skin pigmentation based on Fitzpatrick skin tone chart (Fitzpatrick 1988): not available • Latitude: north of Tropics • Season at the start of pregnancy: not available Source of funding: this study was supported by the Institute of Obstetrics and Gynaecology Trust, Wolfson and Weston Research Centre for Family Health, Imperial College, Du Cane Road, Hammersmith Hospital, London W12 0NN, UK. Dates of the study: April 2007 and November 2007. Declarations of interest among primary researchers: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Computer generated random number lists were drawn up by an independent researcher, with randomisation in blocks of 15.” |
| Allocation concealment (selection bias) | Low risk | Quote: “The person seeing the pregnant women allocated the next available number on entry to the trial, and each woman collected her tablets directly from the hospital pharmacy department or her local pharmacy.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study personnel and participants were not blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study personnel and participants were not blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Group 1:n = 60; discontinued the intervention, changed their minds (n = 3), delivered elsewhere (n = 4), declined blood at delivery (n = 1). Group 2:delivered elsewhere (n = 1), declined blood at delivery (n = 1) |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported. |
| Other bias | Unclear risk | There is insufficient information to allow judgement. |
25(OH)D: 25‐hydroxyvitamin D AE: Adverse events AUMS: Amrita Vishwa Vidyapeetham Management System ?? BMI: body mass index Ca: calcium CSMMU: Chhatrapati Shahuji Maharaj Medical University DQ: disqualified (medical DQ: disqualified from the study due to medical reasons) DSMC: Data Safety and Monitoring Committee DNA: deoxyribonucleic acid DXA: dual‐emission X‐ray absorptiometry ELISA: enzyme‐linked immunosorbent assay FBS: fasting blood sugar FDA: Food and Drug Administration, USA G: Group GA: gestational age GDM: gestational diabetes mellitus HELLP syndrome: pregnancy complication characterised by Haemolysis, Elevated Liver enzymes, and Low Platelet count HPLC: high‐performance liquid chromatography ICU: intensive care unit IU: international unit IU/d: international unit per day IUI: intrauterine insemination IVF: in‐vitro fertilisation LC: liquid chromatography LMP: last menstrual period
OW/OB: overweight/obese mcg: microgram MS: mass spectrometryNIH: National Institutes of Health (US) MD: Medical Doctor PCO: polycystic ovaries (syndrome) PI: Principal Investigator PROM: premature rupture of membrane PTH: parathyroid hormone RIA: radioimmunoassay UAE: United Arab Emirates USDA: United States Department of Agriculture VDAART: Vitamin D Antenatal Asthma Reduction Trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ali 2018 | This open‐labelled randomised controlled trial conducted in Riyadh, Saudi Arabia, recruited women pregnant women age of 20 to 40 years, with a confirmed singleton pregnancy of less than 13 completed weeks of gestation at the time of consent. Women were assigned to 1 of 2 study arms: G1: “Materna” Multivitamin‐Multimineral Supplement (distributed by Wyeth) containing 400 IU vitamin D3/tablet once daily or G2: 4000 IU vitamin D3 (40 drops daily) “Vidrop” by Medical Union Pharmaceuticals (MUP) One intervention regimen did not contain minerals and vitamins, hence regimens were not comparable. |
| Azami 2017 | 90 pregnant women, with least 1 of the risk factors for PE (including chronic vascular disease, hydatidiform mole, multiparity, diabetes mellitus, thyroid disease, chronic hypertension, nulliparity, history of pre‐eclampsia, maternal age > 35 years, kidney disease, collagen vascular disease, antiphospholipid antibody syndrome, family history of pre‐eclampsia, history of thrombophilia, and obesity (BMI > 25)) older than 20 weeks of gestational age who have received ferrous sulphate according to prenatal care program and who were referred to Ilam Educational Center of Obstetrics and Gynecology, Iran in 2014, were divided into 3 groups (n = 30). Participants were randomly divided into 3 groups according to randomised selection: Group A received 1 ferrous sulphate tablet (Rooz daru©, Iran) + 1 Claci‐care multi mineral‐vitamin D tablet ((VitanePharma©, Germany) contained 800 mg Ca, 200 mg Mg, 8 mg Zn and 400 IU vitamin D3)) per day; Group B received 1 Ferrous sulphate tablet (Rooz daru©, Iran,) + 250 mg vitamin C and 55 mg vitamin E, and control group only received Ferrous sulphate daily. This study aimed to investigate the effect of multi mineral‐vitamin D supplements (calcium, magnesium, zinc and vitamin D) and vitamins (C+ E) in the prevention of pre‐eclampsia, hence, groups differed in other nutrients, not only in vitamin D, hence the scope of the study was not appropriate for this revision. |
| Bisgaard 2009 | The aim of this randomised double‐blinded Danish study (N = 600) was to prevent asthma symptoms (recurrent wheeze) in childhood by supplementation with high‐dose vitamin D to the mother during pregnancy. Mothers older than 18 years of age, were recruited during pregnancy (22‐26 weeks of gestation) for the Asthma Begins in Childhood study (ABC). Women were supplemented with 2400 IU of vitamin D3/day (2 tablets of 1200 UI cholecalciferol/day) or placebo (2 tablets containing no active substance) from week 24 of gestation until 1 week after delivery. In addition all mothers were advised to continue taking the recommended dose of 400 IU vitamin D daily. The mothers in ABC simultaneously participated in an interventional trial with fish oil supplementation, and the vitamin D randomisation was stratified by fish oil treatment group. Infants were followed‐up from birth until 3 years of age to assess recurrent wheeze and other allergy related outcomes. The study only had 1 regimen of vitamin D supplementation, hence it is outside of the scope of this review. |
| Hajihashemi 2016 | This randomised clinical trial was conducted in pregnant women with single fetus, who were referred to obstetrics and gynaecology department of Al Zahra and Shahid Beheshti hospitals (Iran), with a diagnosis of vitamin D deficiency. Women were assigned to 1 of 2 groups: Group A received 4000 IU vitamin D per day for 10 weeks and Group B was exposed to sunlight. The study was designed to compare 1 regimen of vitamin D supplementation with sun exposure, hence it is outside of the scope of this review. |
| Jamilian 2017 | The 6‐week prospective randomised double‐blind placebo‐controlled clinical trial aimed to assess the effects of vitamin D and omega‐3 supplementation on glycaemic control and lipid concentrations in women with gestational diabetes. This study was conducted among 140 Iranian women referred to Kosar Clinic in Arak (Iran), aged 18‐40 years without prior diabetes, who have been diagnosed with GDM by “one‐step” 2‐hour 75‐g oral glucose tolerance test (OGTT) at 24‐28 weeks' GA. The intervention groups received (G1) 1000 mg omega‐3 fatty acids containing 360 mg eicosapentaenoic acid (EPA) and 240 mg docosahexaenoic acid (DHA) twice a day and 1 vitamin D placebo (n 5 35); (G2) 50,000 IU vitamin D every 2 weeks and 1 omega‐3 fatty acids placebo (n 5 35); and (G3) 50,000 IU vitamin D every 2 weeks 1 and 1000 mg omega‐3 fatty acids twice a day (n 5 35) for 6 weeks. Study participants had GDM at baseline, which is an exclusion criterion according to our protocol. |
| Li 2000 | In this clinical controlled trial with 3 arms,.88 pregnant women with a predisposition to pregnancy‐induced hypertension, at 20‐24 weeks’ gestation, a BMI index of lower than 24, and an arterial pressure of < 11.3 kPa attending an outpatient clinic and labour ward of the First Afilliated Hospital of Xi’an, Medical University, Xi’an, China Participants were divided into 3 groups: group 1 (n = 29) received a daily dose of a tablet containing 600 mg of calcium and 200 IU of vitamin D (Caltrate‐D) daily from 20‐24 weeks until delivery; group 2 (n = 29) received 1200 mg of calcium and 400 IU vitamin D (Caltrate‐D) daily from 20‐24 weeks until deliver; group 3 (n = 30) received no intervention from 20‐24 weeks until delivery The doses of calcium differed between groups, this type of comparison is not included. |
| Omotayo 2017 | The aim of this parallel, cluster‐randomised,non‐inferiority trial was to compare supplement consumption and adherence to different dosing regimens for antenatal calcium and iron‐folic acid supplementation to prevent pre‐eclampsia and anaemia in women between 16 and 30 weeks of gestation (N = 990) in 16 primary healthcare facilities in rural Kenya. Women received either 1500 mg elemental Ca/day (as calcium carbonate) in 3 pill‐taking events (500 mg Ca with 200 IU cholecalciferol/pill) and 1 IFA pill (60 mg Fe with 400 mg folic acid) or 1000 mg elemental Ca/day (as calcium carbonate) in 2 pill‐taking events (500 mg Ca with 200 IU cholecalciferol/pill) and 1 IFA (60 mg Fe with 400 mg folic acid) In this study, groups did not differ by vitamin D intake. Outcome measures did not include the ones under consideration in our protocol. |
| Roth 2016 | Healthy exclusively breastfeeding mother–infant pairs (≥ 35 weeks' gestation) were enrolled at 4–6 weeks postpartum and randomised to 1 of 3 groups of vitamin D supplementation: (1) maternal 400 IU/day and infant 400 IU/day; (2) maternal 2400 IU/day and infant 0 IU/day; (3) maternal 6400 IU/day and infant 0 IU/day. Main outcomes were maternal and infant serum 25(OH)D at 4 or 7 months postnatal age, and the proportion of mothers or infants in each group who attained 25(OH)D ≥ 50 nmol/L. This postpartum study is outside the scope of our review. |
| Sablok 2015 | Randomised controlled trial with 2 arms, with randomisation at the individual level from years 2010 to 2012 180 primigravidae women with singleton pregnancy at 14‐20 weeks in the Department of Obstetrics and Gynaecology in Safdarjung Hospital, New Delhi, India. Participants were randomly assigned to 1 of 2 groups: group 1 (n = 60) women did not receive any supplementation of vitamin D; group 2 (n = 120) women received vitamin D (cholecalciferol‐D3) supplementation in dosages depending upon the level of serum 25 (OH)‐D levels estimated at entry into the study. Participants from this second group with sufficient levels of vitaminD(serum25(OH)‐D levels > 50 nmol/L), received only 1 dose of 60,000 IU vitamin D (cholecalciferol‐D3) at 20 weeks; participants with insufficient levels of vitamin D (serum 25(OH)‐D levels 25‐50 nmol/L) received 2 doses of 120,000 IU vitamin D (cholecalciferol‐D3) at 20 weeks and 24 weeks; and participants with deficient levels of vitamin D status (serum 25(OH)‐D levels < 25 nmol/L) received 4 doses of 120,000 IU vitamin D cholecalciferol‐D3) at 20, 24, 28 and 32 weeks This study was excluded from the analysis since the non intervention group did not receive any vitamin D supplementation. |
| Wheeler 2016 | The objective of the study was to determine the effect of 2 different monthly maternal doses of cholecalciferol on maternal and infant 25‐hydroxyvitamin D (25(OH)D) status during the first 5 months of breastfeeding. In this randomised, double‐blind, placebo‐controlled design, women recruited through the Queen Mary Maternity Centre, Dunedin Hospital, Dunedin, New Zealand and who were planning to exclusively breastfeed for 6 months (n = 90; mean age: 32.1 years; 71% exclusively breastfeeding at week 20) were randomly assigned to receive either cholecalciferol (50,000 or 100,000 IU) or a placebo monthly from week 4 to week 20 postpartum. This postpartum study is outside the scope of our review. |
| Zhang 2016 | In this randomised, double‐blind,controlled clinical trial, 283 pregnant women were recruited from the Obstetrics and Gynecology Hospital of Fudan University (Shanghai, China). The main inclusion criteria for this trial was a GDM diagnosis before 12 weeks of pregnancy.Women were randomly divided into 4 groups: the control group (n = 20) received a placebo (sucrose; 1 granule/day), the low‐dosage group (n = 38) received the daily recommended intake of 200 IU vitamin D (calciferol) daily, the medium dosage group (n = 38) received 50,000 IU monthly (2000 IU daily for 25 days) and the high‐dosage group (n = 37) received 50,000 IU every 2 weeks (4000 IU daily for 12.5 days). The type of participants is outside the scope of this review (GDM at baseline is an exclusion criteria). |
25(OH)D: 25‐hydroxyvitamin D BMI: body mass index GDM: gestational diabetes mellitus IU: international units PE: pre‐eclampsia
Characteristics of studies awaiting assessment [ordered by study ID]
Gerais 2015.
| Methods | Prospective randomised study |
| Participants | Women recruited at different GA |
| Interventions | single daily dose 1000 IU/6weeks * single daily dose 2000 IU/6weeks * *according to the level of deficiency |
| Outcomes |
Maternal Secondary
Infants Secondary
|
| Notes | Funding: NA Declarations of interest among primary researchers: NA City/Country: Khartoum, Sudan |
Mobasheri 2016.
| Methods | Randomised clinical trial |
| Participants | Pregnant women with a GA between 12‐16 weeks (confirmed by first‐trimester ultrasound or exact last menstrual period), nulliparous presenting a singleton pregnancy and vitamin D deficiency. |
| Interventions | The intervention group (Group B; n = 45) received 50,000 IU/day vitamin D supplement orally and was compared to participants in Group A (n = 45), who received 200 IU/day oral vitamin D. Both groups received the supplements for an 8‐week period. |
| Outcomes |
Maternal Primary
Secondary
Infant
|
| Notes | Source of funding: the study was financially supported by the Department of Research and Technology of Golestan University of Medical Sciences (Grant number 35/298377). Dates of the study: between 2010 to 2012. Declarations of interest among primary researchers: authors declare no competing interests. |
AE: adverse event GA: gestational age GDM: gestational diabetes mellitus IU: international units IUGR: intrauterine growth restriction
Characteristics of ongoing studies [ordered by study ID]
El‐Hajj Fuleihan 2015.
| Trial name or title | Effect of vitamin D replacement on maternal and neonatal outcomes: a randomised controlled trial in pregnant women with hypovitaminosis D |
| Methods | Randomised clinical trial |
| Participants | Pregnant Middle Eastern women GA < 14 weeks at screening visit. (Middle East countries defined by WHO: Bahrain, Egypt, Iran, Iraq, Palestine, Jordan, Kuwait, Lebanon, Oman, Qatar, Saudi Arabia, Syria, United Arab Emirates, Yemen) ‐ Age > 18‐50 years ‐ Presenting hypovitaminosis D: 25(OH)D level between 10 ng/mL and 30 ng/mL |
| Interventions | Group 1: vitamin D3 Euro D 10,000 IU (1 tablet) plus Euro D placebo (1 tablet) weekly, alternating with Euro D placebo (2 tablets) weekly, starting at the second trimester and continued until delivery. Group 2: vitamin D3 Euro D 10,000 IU (2 tablets, equivalent to 20,000 IU) weekly, starting at the second trimester and continued until delivery. |
| Outcomes |
Maternal Primary
Secondary
Infants
Primary
Secondary
|
| Starting date | July 2015 |
| Contact information | Ghada El hajj Fuleihan, PI, Professor of Medicine 961‐1‐737868 gf01@aub.edu.lb Anwar Nassar, Professor, Co‐Investigator an21@aub.edu.lb |
| Notes | Funging: AUBMC‐GE‐HF‐2, AUBMC‐IM‐GE‐HF‐22 (Other Grant/Funding Number: American University of Beirut) Sponsored by: American University of Beirut Medical Center, University of Southampton, Bahman Hospital Beirut Lebanon Declarations of interest among primary researchers: NA City/Country: Hamra, Lebanon |
Garreto 2016.
| Trial name or title | A randomised control trial of vitamin D prophylaxis in the prevention of hypertensive disorders of pregnancy |
| Methods | Randomised open‐label clinical trial (parallel assignment) |
| Participants | Women 18 years of age and older, with a confirmed intrauterine pregnancy, less than 16 weeks' gestation and carrying a singleton gestation. Those taking vitamin D supplementation outside of prenatal vitamins are excluded from study participation. Additional exclusion criteria were: known disorder that will affect vitamin D levels (i.e. hyperparathyroidism, mal‐absorption disorder, history of gastric bypass surgery, immunocompromised state, maternal use of immune‐modulators etc.), carrying a fetus with known aneuploidy or anomaly, fetal demise, chronic use of diuretic or cardiac medication therapy including calcium channel blockers. |
| Interventions | G1: vitamin D prophylaxis: participants will be provided vitamin D 3000 IU daily or vitamin D 4000 IU daily with and without concurrent use of prenatal vitamins, respectively. G2: participants will not receive additional vitamin D in the pregnancy (but prenatal vitamins?) |
| Outcomes |
Maternal Primary
Secondary
Infant Primary
Secondary
|
| Starting date | October 2016 |
| Contact information | Diana J Garretto, MD; (631) 444‐7650; diana.garretto@stonybrookmedicine.edu Malini D Persad, MD MPH; (631) 444‐7650; malini.persad@stonybrookmedicine.edu |
| Notes | Funding: Stony Brook University Declarations of interest among primary researchers: NA City/country: New York, US |
Hantoshzadeh 2017.
| Trial name or title | Comparison of 2 therapeutic regimens vitamin D3 deficiency in the first trimester of pregnancy on the level of vitamin D3 in the second trimester of pregnancy |
| Methods | Single‐blinded randomised controlled trial |
| Participants | Pregnant women with: ‐ Single pregnancy ‐ Serum vitamin D level is less than 30 ng/mL ‐ Gestational age < 14 weeks ‐ Age 18‐42 years ‐ Not chronic disease, impaired absorption and metabolism of food ‐ Not receiving any supplement except multivitamins to 400 units vitamin D3, folic acid, iron · and calcium with 400 units vitamin D3 |
| Interventions | 50,000 units of vitamin D3 once a week. * Control group: 1000 unit per day. * *Both groups received these doses from week 14 of pregnancy to 10 weeks. |
| Outcomes |
Maternal Secondary
|
| Starting date | 20 July 2016 |
| Contact information | Dr. Sedigheh Hantoshzadeh farahnaz.rostami@modares.ac.ir |
| Notes | Funding: Tarbiat Modares of Medical Science Declarations of interest among primary researchers: NA City/country: Tehran, Iran |
Hartman 2010.
| Trial name or title | Vitamin D supplementation during pregnancy and bone status in children at birth and at 1 year of age |
| Methods | Prospective randomised controlled study. Parallel assignment |
| Participants | Pregnant women 20 to 40 years, of any parity status (recruited at 27 weeks' gestation) |
| Interventions | G1: 400 IU/day vitamin D supplementation from 27 weeks until delivery. G2: 2000 IU/day vitamin D supplementation from 27 weeks until delivery. |
| Outcomes |
Maternal Primary
Secondary
Infants Secondary
|
| Starting date | November 2010 |
| Contact information | Contact: Corina Hartman, MD (PI); 972‐3‐9253674: corinah@clalit.org.il Raanan Shamir, Professor; 972‐3‐9253673: raanans@clalit.org.il |
| Notes | Funding: sponsored by: Rabin Medical Center Declarations of interest among primary researchers: NA City/country: Petah Tikva, Israel, 49202 |
Hoffman 2017.
| Trial name or title | A randomised single‐centre study of the effects of high‐dose cholecalciferol to reduce the incidence of gestational diabetes in high‐risk pregnant women |
| Methods | Randomised, open‐label, single‐centre study with a target group of 300 women to determine the effect of increased vitamin D supplementation (4000 IU vitamin D3 + prenatal vitamin) on the incidence of gestational diabetes compared to a standard prenatal vitamin among pregnant women at high risk for gestational diabetes, and explore the effect of increased vitamin D supplementation (4000 IU daily + prenatal vitamin), compared to a standard prenatal vitamin, on glycaemic control, need for oral hypoglycaemic agents and/or insulin, and delivery outcomes among the subset of women who develop gestational diabetes. Women will be followed from enrolment through the remained of their pregnancy. |
| Participants |
|
| Interventions | 4000 IU vitamin D3 + prenatal vitamin compared to a standard prenatal vitamin |
| Outcomes |
Primary
Incidence of gestational diabetes Secondary
|
| Starting date | July 2017 |
| Contact information | Samantha Hoffman, MD Tel. No: 612‐626‐3111 Email: kehoe018@umn.edu University of Minnesota Medical Center Minneapolis, Minnesota, United States, 55455 |
| Notes | Funding: sponsored by University of Minnesota – Clinical and Translational Science Institute Declarations of interest among primary researchers: NA |
Judkins 2011.
| Trial name or title | Vitamin D deficiency in pregnancy – a comparison of 2 treatments |
| Methods | Pregnant women in trial will be randomly allocated to receive vitamin D oral supplementation from 20 weeks' gestation until birth of baby. |
| Participants | Pregnant women seeking maternity care with midwifery services involved in the study. |
| Interventions | 50,000 IU vitamin D oral tablet supplementation monthly versus 50,000 IU vitamin D oral tablet supplementation twice a month |
| Outcomes | Newborn vitamin D sufficiency |
| Starting date | 1 June 2011 |
| Contact information | Dr Annie Judkins Newton Union Health Service 14 Hall Ave, Newton, Wellington 6021 New Zealand 644 3802020 annie.judkins@nuhs.org.nz Dr Jeremy Krebs Capital and Coast Health Riddiford street, Newton, Wellington 6021 New Zealand 644 3855999 jeremy.krebs@ccdhb.org,nz |
| Notes | Funding: Royal New Zealand College of GP’s Declarations of interest among primary researchers: NA |
Kachhawa 2014.
| Trial name or title | A randomised controlled trial to investigate the effects of vitamin D supplementation on maternal and new‐born baby’s vitamin D status in Asian‐Indian participants |
| Methods | Open‐label randomised clinical trial to investigate the efficacy of 3 doses of vitamin D supplementation on vitamin D status of mother and newborn at the time of delivery, in comparison to control group who will receive 600 IU/day. Participants will be randomised into 1 of the 4 groups (3 intervention groups and 1 control group) in the ratio of 1:1. participants in intervention group will receive vitamin D (cholecalciferol) in increasing supplemental doses, similar nutritional and lifestyle advices as part of standard management of pregnancy. After 6‐7 months of supplementation, all groups will be compared for primary and secondary outcomes of the study at the time of delivery. |
| Participants | Pregnant women between 12‐16 weeks of gestation Age between 18‐35 years |
| Interventions | Group 1‐ 100 units/day; group 2 – 2000 units/day; group 3‐ 4000 units per day) while control group will receive 600 units of vitamin D per day. In addition, all study participants (intervention as well as control group) will also receive 1000 mg of elemental calcium (in 2 divided doses) |
| Outcomes |
Primary
Secondary
|
| Starting date | 1 February 2014 |
| Contact information | Garima Kachhawa Assistant Professor All India Institute of Medical Sciences Department of Obstetrics and Gynecology AIIMS, New Delhi‐29, Delhi 110029 India 09868398231 Garimakacchawa2012@gmail.com |
| Notes | Funding: Indian Council of Medical Research (ICMR). Declarations of interest among primary researchers: NA |
Lalooha 2012.
| Trial name or title | The effects of vitamin D supplementation during pregnancy on newborn’s anthropometric index |
| Methods | A randomised controlled trial to assess the effect of vitamin D supplementation during pregnancy on newborns anthropometric indexes. |
| Participants | Pregnant women from 15‐40 years old; between 24‐28 weeks of gestation; BMI 19‐26; vitamin D < 75 nmol/L Exclusion criteria: History of liver, renal, parathyroid, bone, metabolic diseases or epilepsy or malabsorption; medications that influence the metabolism of vitamin d and calcium; recurrent abortion; diabetes or gestational diabetes, hypertension or pre‐eclampsia; fetus with anomalies or poli hydramnios or oligohydramnios or intrauterine growth retardation. |
| Interventions | The intervention group will receive vitamin D capsule 50,000 u weekly for 8 weeks from 28 gestational age and multivitamin tab including 400 u vitamin D daily until termination. The control group will receive multivitamin tab including 400 u vitamin D until termination. |
| Outcomes |
Primary
Secondary
|
| Starting date | Actual start date information not available |
| Contact information | Dr. Fatemeh Lalooha Assistant Professor Gynecology Department of Kosar Hospital Qazvin, Iran Phone +98 28 1223 6374 Fax +98 28 1224 2661 rramezaninezhad@qums.ac.ir |
| Notes | Funding: Qhazvin University of Medical Sciences Declarations of interest among primary researchers: NA |
McCann 2016.
| Trial name or title | Impact of maternal body weight on vitamin D status during pregnancy. |
| Methods | A randomised supplementation where pregnant women from the Western Health and Social Care Trust will be invited to participate. Blood samples (20 mL) will be taken at 12, 28 and 36 weeks' gestation. A sample of blood will be taken from the cord after delivery. Vitamin D status is the main outcome measurement. All blood samples will be analysed for vitamin D and other associated metabolites. Data will be collected on health and lifestyle, supplementation use and food intake. Body composition measurements will be recorded at each appointment and infant anthropometric measurements will be taken from the maternal notes after delivery. Findings from this research will be used to inform nutrition policy on appropriate vitamin D supplementation levels in pregnancy which may be dependent upon pre pregnancy BMI. |
| Participants | Inclusion criteria: pregnant women; age ≥ 18 years; BMI > 18.5 kg/m²; without current pregnancy‐related complications; at least 12 weeks' gestation; having a singleton pregnancy (as confirmed at first scan) · Pregnant women who are currently taking vitamin D and have had a sun holiday will be included in this study. All participants will agree to discontinue any current supplementation and will be provided with a multivitamin for the duration of pregnancy. Exclusion criteria: aged < 18 years; pregnancy BMI < 18.5 kg/m²; multiple pregnancy; currently involved in another research study; history of gastrointestinal, hepatic, renal, vascular or haematological disorders; have had in vitro fertilisation (IVF) treatment; history of NTD affected pregnancies; pregnant women with active thyroid disease (e.g. Graves, Hashimoto or thyroiditis); planned home births |
| Interventions | Participants will be randomised to receive either 0 μg (placebo) plus a multivitamin or 10 μg vitamin D plus a multivitamin from 12 weeks' gestation until delivery. The multivitamin already contains 10 μg vitamin D; Therefore participants will be randomised to receive a total of 10 μg or 20 μg vitamin D. |
| Outcomes |
Primary
Secondary
|
| Starting date | November 2015 |
| Contact information | Mary T McCann +4428 70 123969 mt.mccann@ulster.ac.uk |
| Notes | Funding: University of Ulster and Western Health And Social Care Trust Declarations of interest among primary researchers: NA |
McLean 2012.
| Trial name or title | Does vitamin D supplementation in pregnancy improve maternal glucose metabolism or prevent gestational diabetes? |
| Methods | Randomised controlled trial where pregnant women will be allocated to take high‐dose vitamin D supplementation (5000 IU/day) or standard dose pregnancy vitamin supplementation (400 IU vitamin D daily), administered as an oral capsule, from the time of the first antennal clinic visit (around 12 weeks' gestation) until delivery. Patients will be recruited at their first antenatal clinic visit. Baseline tests will exclude pre‐existing diabetes, hypercalcaemia or vitamin D toxicity. Treatment will be stratified according to baseline serum vitamin D levels and randomised by the trial pharmacist using opaque envelopes containing a treatment allocation. Patient, treating clinician and researchers will remain blinded to treatment group. The trial will be conducted in Australia. |
| Participants | Inclusion criteria: pregnancy, less than 20 weeks' gestation at recruitment, 18 years and above Exclusion criteria: known diabetes, calcium metabolic disorder, multiple pregnancy |
| Interventions | Pregnant women will be randomly allocated to take high‐dose vitamin D supplementation (5000IU/day) or standard dose pregnancy vitamin supplementation (400 IU vitamin D daily), administered as an oral capsule, from the time of the first antenatal clinic visit (around 12 weeks gestation) until delivery. Women in the control group will receive a standard dose of vitamin D supplementation (as in commonly used in pregnancy multivitamin preparations). |
| Outcomes |
Maternal Primary:
Infant Primary:
|
| Starting date | June 2017 |
| Contact information | Samantha Hoffman, MD Tel. No: 612‐626‐3111 Email: kehoe018@umn.edu University of Minnesota Medical Center Minneapolis, Minnesota, United States, 55455 |
| Notes | Funding: sponsored by University of Minnesota – Clinical and Translational Science Institute Declarations of interest among primary researchers: NA |
Mosalanejad 2016.
| Trial name or title | Compare the effect of vitamin D and calcium plus vitamin D on pregnancy outcomes in pregnant women |
| Methods | Randomised single‐blind controlled clinical trial |
| Participants | Pregnant women (from 20 years old to 40 years old). Gestational age of less than 10 weeks; with no history of diabetes; hypertension; a history of polycystic ovary syndrome; lack of family history of diabetes in first‐degree relatives; no family history of high blood pressure in first‐degree relatives; BMI between 19‐26; lack of vitamin D during the last 6 months; singleton pregnancy. |
| Interventions | Intervention group: vitamin D3 1000 units oral/daily starting at 16 weeks GA until the end of pregnancy. Routine prenatal care multi vitamin containing 400 units vitamin ca‐D was also simultaneously prescribed. The control group received a multivitamin that had 400 unit vitamin ca‐D daily from 16 weeks GA until the end of pregnancy. |
| Outcomes |
Maternal Primary
Secondary
Infants Primary
Secondary
|
| Starting date | 21 March 2015 (expected) |
| Contact information | Dr Najmehsadat Mosalanejad Phone: +98 76 3333 7192 Email address: mosalanejad@hums.ac.ir |
| Notes | Funding: study funded by the Hormozgan University of Medical Sciences Declarations of interest among primary researchers: NA City/country: Bandarabas/Iran |
Nausheen 2018.
| Trial name or title | Assessment of dose effectiveness of vitamin D supplementation during pregnancy A dose comparison clinical trial |
| Methods | Randomised double‐blinded hospital‐based (Aga Khan Hospital Kharadar, Pakistan) trial of vitamin D supplementation to pregnant women, 15 years to 45 years. Pregnant women were individually randomised to 3 groups receiving a dose of 400, 2000 and 4000 IU/day till the time of delivery. The group which received 400 IUs was treated as control group. A blood sample was also collected from the participant at the time of recruitment/before the starting of the supplementation for the assessment of Calcium, Phosphorus, Alkaline Phosphatase and vitamin D levels. A second blood sample for vitamin D level to assess vitamin D status was done after completion of the supplementation phase at the time of delivery (till 48 hours of delivery). Cord blood also taken. The samples were sent to Aga Khan University laboratory. |
| Participants | Women with singleton pregnancies from 12 to 16 weeks |
| Interventions | Control: 400 IU/day vitamin D3 Intervention group 1: 2000 IU/day vitamin D3 Intervention group 2: 4000 IU vitamin D3/day |
| Outcomes |
Primary outcome
Secondary outcome
|
| Starting date | June 2013 |
| Contact information | Dr Sidrah Nausheen, Aga Khan University, Karachi, Sindh, Pakistan, 75300 |
| Notes | Funding: NA Declarations of interest among primary researchers: NA Clinicaltrials.gov NCT02215213 |
Neyestani 2016.
| Trial name or title | Evaluation and comparison of the efficacy of 1000 and 2000 IU/day vitamin D supplementation during pregnancy on maternal and newborn vitamin D status and pregnancy outcomes |
| Methods | In this randomised controlled trial, 84 pregnant women will be divided into 2 groups: intervention group 1: multivitamin supplementation during pregnancy (iron, folic acid, multivitamin) + 1000 IU/day vitamin D and intervention group 2: multivitamin supplementation during pregnancy (iron, folic acid, multivitamin) + 2000 IU/day vitamin D. Demographic, socioeconomic and lifestyle data as well as blood samples and urine samples will be collected at baseline and in the last month of pregnancy. Moreover the cord blood will be collected at birth. |
| Participants | 84 pregnant women aged at 18‐40 years with gestational age of < 12 weeks |
| Interventions | Intervention group 1: multivitamin supplementation during pregnancy (iron, folic acid, multivitamin) + 1000 IU/d vitamin D Intervention group 2: multivitamin supplementation during pregnancy (iron, folic acid, multivitamin) + 2000 IU/d vitamin D |
| Outcomes |
Maternal Primary
Secondary
Infant Primary
Secondary
Spontaneous abortion also a secondary outcome |
| Starting date | January 2017 |
| Contact information | Tirang R. Neyestani, Ph.D., National Nutrition and Food Technology Institute Ph: 00989123507663 Email: neytr@yahoo.com |
| Notes | Funding: NA Declarations of interest among primary researchers: NA Registry: NCT03308487 and IRCT2016090329675N1 |
Nouripour 2016.
| Trial name or title | Clinical trial on the evaluation of calcium and vitamin D in the cord serum of neonates, whose mothers were under vitamin D treatment during their pregnancy |
| Methods | 2 groups of 90 pregnant mothers with vitamin D deficiency,were selected and named intervention and control groups. Inclusion criteria: pregnant mothers with vitamin D deficiency; age of 16‐35 years; singleton pregnancy; gestational age of among 8 to 12 weeks which is confirmed by LMP or sonography; BMI lower than 30 at the first prenatal visit; no history of gestational diabetes; not suffering from glycosuria, fasting blood sugar less than 92; no precedent of polyhydramnios; no history of macrosomia (weight of 4 kg) in children; with no history of stillbirth or a baby with malformations; non‐smoking participants. Exclusion criteria: patients with diabetes type 1 or 2; those with hypertension; patients with parathyroid disease and other metabolic diseases; women with untreated thyroid disease and liver or kidney deficiency; patients taking anti‐epileptic drugs and corticosteroids; and women with a particular disease. Also, those who refused consent for participating in this study, were excluded. Intervention group: 8 doses of vitamin D pearls every 2 weeks; each dose contained 50,000 units, plus multi prenatal tablet including 400 units of vitamin D, every day. Control group: only received the multi prenatal tablet, every day. The methods which were used in collecting data were a demographic questionnaire and recording data check‐lists which were filled with laboratory assessments. Additionally, this is a single‐blinded study that include just research groups. In order to collect data, 3 mL of cord blood in related tubes were collected, at the time of childbirth. These were then were transferred to the laboratory of Semnan Amir al‐mo'menin Hospital and the levels of serum 25‐OH vitamin D and calcium were evaluated with England Euroimmun and Semnan Rezatec kits using ELISA method, respectively. |
| Participants | The population of this study includes all neonates of pregnant women who were aged 16 to 35 years and between 14 to 16 weeks of pregnancy referred to Amiral‐mo'menin Hospital in 1393 for prenatal care |
| Interventions | Group 1 –offspring of mothers who received injection of 50,000 units of vitamin D, 8 doses every 2 weeks Group 2 – (control group) offspring of mothers who received multi prenatal tab including 400 units of vitamin D every day |
| Outcomes |
Primary outcome measure
|
| Starting date | 21 March 2015 |
| Contact information | Shamsollah Nooripour, Amiralmomenin Hospital, Iran Email: amir.hospital@semums.ac.ir Phone +98 23 3346 0077 |
| Notes | Funding: Vice chancellor for research, Semnan University of Medical Science Declarations of interest among primary researchers: NA |
Rasmussen 2009.
| Trial name or title | Effects of high dose vitamin D supplementation on bone metabolism in pregnant women with hypovitaminosis D – a randomised controlled trial * |
| Methods | Investigator‐initiated double‐blind, randomised, placebo‐controlled, parallel‐group trial. Women, aged 20‐40 years, with P‐25OHD < 50 nmol/L all planning pregnancy (N = 193), were randomised to a daily supplementation with 70 μg (2800 IU), 35 μg (1400 IU) vitamin D3 (VitD3), or placebo. Supplementation was initiated before conception and continued until 16 weeks postpartum. |
| Participants | Females 20 years to 35 years, in good general health |
| Interventions | Control: placebo Intervention group 1: 70 μg (2800 IU) vitamin D3 Intervention group 2: 35 μg (1400 IU) vitamin D3 |
| Outcomes |
Maternal Secondary
Infant Secondary
|
| Starting date | 2009 |
| Contact information | Contact: Gitte Bloch Rasmussen, MD, University of Aarhus, Aarhus University hospital, Denmark Ph:+45 89 4976 81 Email: gittebr@ki.au.dk Contact: Lars Rejnmark, MD, PhD, DrMed Email: rejnmark@post6.tele.dk |
| Notes | Funding: NA Declarations of interest among primary researchers: NA Registry: NCT01038453 |
Rich‐Edwards 2015.
| Trial name or title | Trial of vitamin D supplements to raise Calcidiol levels of pregnant women in Mongolia ClinicalTrials.gov Identifier: NCT02395081 |
| Methods | A double‐blind randomised, placebo‐controlled trial. 120 women will be randomised to each of the 3 doses of vitamin D (600, 2000, 4000 IU) included in a standard prenatal vitamin, which will be taken from 12‐16 weeks' gestation and continue throughout pregnancy. |
| Participants | Women age 18 or older, 12‐16 weeks pregnant receiving prenatal care at Zuun Kharaa Hospital, Mongolia and planning to deliver at Zuun Kharaa Hospital, Mongolia |
| Interventions | Placebo: 600 IU/day Intervention group 1: 2000 IU Intervention group 2: 4000 IU |
| Outcomes |
Maternal Primary
Secondary
Infant Primary
|
| Starting date | February 2015 |
| Contact information | Janet Rich‐Edwards, Brigham and Women's Hospital |
| Notes | Funding: NA Declarations of interest among primary researchers: NA |
25(OH)D: 25‐hydroxyvitamin D BMI: body mass index CNM: Certified Nurse Midwife DO: Doctor of Osteopathic Medicine ELISA: enzyme‐linked immunosorbent assay GA: gestational age GDM: gestational diabetes HELLP syndrome: pregnancy complication characterised by Haemolysis, Elevated Liver enzymes and Low Platelet count IU: international unit LMP: last menstrual period NTD: neural tube defects MD: Medical Doctor PI: Principal Investigator WHO: World Health Organization
Differences between protocol and review
The protocol for this Cochrane Review was published in PROSPERO, not the Cochrane Library. We sought to conduct the following subgroup comparisons: 600 IU/d or less versus 601 IU/d to 1000 IU/d, 600 IU/d or less versus 1,001 IU/d to 3,999 IU/d and 600 IU/d or less versus 4000 IU/d or more; however, some of these comparisons were not possible due to lack of studies to include in all subgroups. Also, we did not specify in the protocol that women with pre‐existing conditions were excluded from the analysis; this was added in the methods section of this review.
Contributions of authors
Cristina Palacios prepared the protocol and the review as a consultant of the Evidence and Programme Guidance of the Department of Nutrition for Health and Development in the World Health Organization. Cristina Palacios and Juan Pablo Peña‐Rosas assessed eligibility of the new trials. Any differences were discussed and resolved with Maria Angelica Trak‐Fellermeier. James A Salisi, Jessica C John and Maria Angelica Trak‐Fellermeier extracted the data. Maria Angelica Trak‐Fellermeier and Ricardo X Martinez prepared the tables of the review. Ricardo X Martinez and Lucero Lopez‐Perez provided guidance in the statistical issues. Paul Lips provided extensive comments on the protocol and the review. All authors commented and provided extensive feedback and discussed the document and provided edits and references.
Sources of support
Internal sources
-
Evidence and Programme Guidance, Department of Nutrition for Health and Development, World Health Organization, Switzerland.
Dr Juan Pablo Peña‐Rosas is full time staff of the World Health Organization.
External sources
-
Evidence and Programme Guidance, Department of Nutrition for Health and Development, World Health Organization, Switzerland.
Cristina Palacios, Maria Angelica A Trak‐Fellermeier and Ricardo X Martinez received partial funding for this work.
-
The Bill & Melinda Gates Foundation, USA.
WHO thanks the Bill & Melinda Gates Foundation for their financial support to the Department of Nutrition for Health and Development for conducting systematic reviews on nutrition‐specific and nutrition‐sensitive interventions.
Declarations of interest
Cristina Palacios received payment from the World Health Organization for preparing this protocol and subsequent review, and to cover the travel expenses to meet with co‐authors for the preparation of the protocol.
Maria Angelica Trak‐Fellermeier: none known.
Ricardo X Martinez received payment from the World Health Organization for preparing this protocol and subsequent review, and to cover the travel expenses to meet with co‐authors for the preparation of this protocol. He has also received payment from WHO for consultancy relating to other topics. Ricardo holds Growth UBS options (government options not causing conflict with this review).
Lucero Lopez‐Perez: none known.
James A Salisi: none known.
Jessica C John: none known.
Paul Lips provided expert testimony to Friesland Campina in 2015, and received a lecture fee from Abiogen in 2017, both unrelated to the present work. He is a member of the Programme Advisory Committee of the Vitamin D Workshop.
Juan Pablo Peña‐Rosas: The World Health Organization gratefully acknowledges the financial contribution of the Bill & Melinda Gates Foundation (2016‐2019) for this work. Donors do not fund specific guidelines and do not participate in any decision related to the guideline development process including the composition of policy questions, membership of the guideline groups, the conduct and interpretation of systematic reviews, or the formulation of recommendations.
New
References
References to studies included in this review
Abotorabi 2017 {published data only}
- Abotorabi S, Hashemi poor S, Esmailzadehha N, Ziaee A, Khoeiniha MH. Effect of treatment with Vitamin D on maternal and neonatal indices in pregnant women with hypocalcemia: a randomized controlled trial. International Journal of Pediatrics 2017;5(9):5733‐9. [Google Scholar]
Bacqui 2009 {published data only}
- Bacqui A, NCT00938600. Antenatal vitamin d supplementation to improve neonatal health outcomes in Dhaka, Bangladesh: preliminary dose‐finding and safety study. clinicaltrials.gov/ct2/show/NCT00938600 (first received 14 July 2009).
- Roth DE, Al A, Raqib R, Akhtar E, Black RE, Baqui AH. Pharmacokinetics of high‐dose weekly oral vitamin D3 supplementation during the third trimester of pregnancy in Dhaka, Bangladesh. Nutrients 2013;5(3):788‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth DE, Al‐Mahmud A, El S, Raqib R, Black RE, Baqui AH. Randomized open‐label trial of two weekly oral vitamin D3 supplementation regimens during the third trimester of pregnancy in Bangladeshi women: Effects on maternal vitamin D status and safety. FASEB Journal 2011;25:236.6. [Google Scholar]
Bhatia 2012 {published data only}
- Bhatia V, CTRI/2012/02/002395. Vitamin D supplementation in pregnancy: regimens and long term effects on offspring. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=4150 (first received 1 February 2012).
- Bhatia V, Katam K, Agarwal A, Das V, Ramesh V. Vitamin D supplementation in pregnancy and its effect on cord blood 25 hydroxycholecalciferol and anthropometry of the newborn. Hormone Research in Paediatrics 2013;80(Suppl 1):220. [Google Scholar]
- Sahoo SK, Katam KK, Das V, Agarwal A, Bhatia V. Maternal vitamin d supplementation in pregnancy and offspring outcomes: a double‐blind randomized placebo‐controlled trial. Journal of Bone and Mineral Metabolism 2017; Vol. 35, issue 4:464‐71. [DOI] [PubMed]
Das 2010 {published data only}
- Das V. Vitamin D and calcium nutrition in pregnancy ‐ evaluation of optimal supplementation dose of vitamin D during antenatal period. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=1553 (first received 2 June 2010). [CTRI/2010/091/000352]
- Das V, Agarwal A, Bhatia V, Pandey A, Agarwal S, Saxena P, et al. Evaluation of Vit D status and need for supplementation in pregnant women of a rural area of North India. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S151. [CTRI/2010/091/000352] [Google Scholar]
- Sahu M, Das V, Aggarwal A, Rawat V, Saxena P, Bhatia V. Vitamin D replacement in pregnant women in rural north India: a pilot study. European Journal of Clinical Nutrition 2009;63(9):1157‐9. [DOI] [PubMed] [Google Scholar]
Dawodu 2013 {published data only}
- Dawodu 2013. High prevalence of rickets and subclinical maternal and childhood vitamin d deficiency in the middle east: a randomized controlled trial of prenatal vitamin d supplementation to prevent vitamin d deficiency in mothers and their infants. clinicaltrials.gov/ct2/show/NCT00610688 (first received 7 June 2013). [NCT00610688]
- Dawodu A, Saadi HF, Bekdache G, Javed Y, Altaye M, Hollis BW. Randomized controlled trial (RCT) of vitamin D supplementation in pregnancy in a population with endemic vitamin D deficiency. Journal of Clinical Endocrinology & Metabolism 2013;98(6):2337‐46. [DOI] [PubMed] [Google Scholar]
de Menibus 1984 {published data only}
- Menibus CH, Mallet E, Henocq A, Lemeur H. [Should vitamin d supplements be given to pregnant women?]. Bulletin De L'academie Nationale De Medecine 1984;168(7‐8):909‐16. [PubMed] [Google Scholar]
Grant 2010 {published data only}
- Grant C. Randomised placebo controlled study of vitamin D during pregnancy and infancy. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12610000483055 (first received 1 April 2010). [ACTRN12610000483055]
- Grant C, Milne T, Knight J, Sinclair J, Camargo C. Vitamin D supplementation during pregnancy and infancy reduces food allergen sensitisation and parental‐reported food allergy: a randomised controlled trial. European Journal of Pediatrics 2016;175(11):1438. [DOI] [PubMed] [Google Scholar]
- Grant C, Stewart A, Scragg R, Milne T, Rowden J, Ekeroma A, et al. What dose of vitamin D supplementation is required in pregnancy and infancy to increase infants' serum 25‐hydroxyvitamin D concentration > 20ng/ml?. Pediatric Academic Societies Annual Meeting; 2013 May 4‐7; Washington DC, USA. 2013.
- Grant CC, Crane J, Mitchell EA, Sinclair J, Stewart A, Milne T, et al. Vitamin D supplementation during pregnancy and infancy reduces aeroallergen sensitisation: a randomised controlled trial. Allergy 2016;71(9):1325‐34. [DOI] [PubMed] [Google Scholar]
- Grant CC, Crane J, Mitchell EA, Sinclair J, Stewart A, Milne T, et al. Vitamin D supplementation during pregnancy and infancy reduces aeroallergen sensitization: A randomized controlled trial. Pediatric Academic Societies Annual Meeting; 2016 April 30‐May 3; Baltimore, USA. 2016:1650.8. [DOI] [PubMed]
- Grant CC, Kaur S, Waymouth E, Mitchell EA, Scragg R, Ekeroma A, et al. Reduced primary care respiratory infection visits following pregnancy and infancy vitamin D supplementation: a randomised‐controlled trial. Acta Paediatrica 2015;104(4):396‐404. [DOI] [PubMed] [Google Scholar]
- Grant CC, Stewart AW, Scragg R, Milne T, Rowden J, Ekeroma A, et al. Vitamin D during pregnancy and infancy and infant serum 25‐hydroxyvitamin D concentration. Pediatrics 2014;133(1):e143‐53. [DOI] [PubMed] [Google Scholar]
- Wall CR, Stewart AW, Camargo CA J, Scragg R, Mitchell EA, Ekeroma A, et al. Vitamin D activity of breast milk in women randomly assigned to vitamin D3 supplementation during pregnancy. American Journal of Clinical Nutrition 2016;103:382‐8. [DOI] [PubMed] [Google Scholar]
Hashemipour 2014 {published data only}
- Hashemipour S. Effect of treatment of vitamin D deficiency during pregnancy on hypocalcemia. clinicaltrials.gov/ct2/show/NCT02021864 (first received 27 December 2013). [NCT02021864]
- Hashemipour S, Ziaee A, Javadi A, Movahed F, Elmizadeh K, Javadi EH, et al. Effect of treatment of vitamin D deficiency and insufficiency during pregnancy on fetal growth indices and maternal weight gain: a randomized clinical trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 2014;172:15‐9. [DOI] [PubMed] [Google Scholar]
Kalra 2012 {published data only}
- Kalra P, Das V, Agarwal A, Kumar M, Ramesh V, Bhatia E, et al. Effect of vitamin D supplementation during pregnancy on neonatal mineral homeostasis and anthropometry of the newborn and infant. British Journal of Nutrition 2012;108(6):1052‐8. [DOI] [PubMed] [Google Scholar]
Karamali 2015 {published data only}
- Karamali M, Beihaghi E, Mohammadi AA, Asemi Z. Effects of high‐dose vitamin D supplementation on metabolic status and pregnancy outcomes in pregnant women at risk for pre‐eclampsia. Hormone and Metabolic Research 2015;47(12):867‐82. [DOI] [PubMed] [Google Scholar]
Kiely 2015 {published data only}
- Kiely M. Randomized controlled trial to determine the nutritional requirement for vitamin d for prevention of deficiency during pregnancy and in the early neonatal period (D‐MAT). clinicaltrials.gov/ct2/show/NCT02506439 (first received first received 23 July 2015). [NCT02506439]
- O'Callaghan K, Hennessy A, Dowling KG, Hull G, Kenny LC, Cashman KD, et al. Estimation of the dietary requirement for vitamin D in pregnancy: a dose‐response, double‐blind, randomized placebo‐controlled trial. Annals of Nutrition and Metabolism 2017;71(Suppl 2):624. [Google Scholar]
- O'Callaghan K, Hennessy A, Kiely M, Kenny LC. Habitual calcium and vitamin D intakes in pregnant Irish women; preliminary data from the DMAT randomised controlled trial. Proceedings of the Nutrition Society 2016;75(OCE3):E191. [Google Scholar]
- O'Callaghan KM, Hennessy A, Hull GL, Healy K, Ritz C, Kenny LC, et al. Estimation of the maternal vitamin D intake that maintains circulating 25‐hydroxyvitamin D in late gestation at a concentration sufficient to keep umbilical cord sera >/=25‐30 nmol/L: a dose‐response, double‐blind, randomized placebo‐controlled trial in pregnant women at northern latitude. American Journal of Clinical Nutrition 2018;108(1):77‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mallet 1986 {published data only}
- Mallet E, Gugi B, Brunelle P, Henocq A, Basuyau JP, Lemeur H. Vitamin D supplementation in pregnancy: a controlled trial of two methods. Obstetrics & Gynecology 1986;68:300‐4. [DOI] [PubMed] [Google Scholar]
March 2010 {published data only}
- Anon. Erratum for march et al. Maternal vitamin d3 supplementation at 50 microg/d protects against low serum 25‐hydroxyvitamin d in infants at 8 wk of age: a randomized controlled trial of 3 doses of vitamin d beginning in gestation and continued in lactation. American Journal of Clinical Nutrition 2015;102:402‐10. [DOI] [PubMed] [Google Scholar]
- Chen NN, March K, Innis SM, Shand A, Dadelszen P, Lyon P, et al. The effect of vitamin D supplementation during pregnancy and lactation on maternal and infant 25‐hydroxyvitamin D (25OHD) concentration. FASEB Journal 2013; Vol. 27, issue Suppl 1.
- March KM. Vitamin D dose‐response study throughout pregnancy and lactation. clinicaltrials.gov/ct2/show/NCT01112891 (first received 29 April 2010). [NCT01112891]
- March KM, Chen NN, Karakochuk CD, Shand AW, Innis SM, Dadelszen P, et al. Maternal vitamin D3 supplementation at 50 ug/d protects against low serum 25‐hydroxyvitamin D in infants at 8 wk of age: a randomized controlled trial of 3 doses of vitamin D beginning in gestation and continued in lactation. American Journal of Clinical Nutrition 2015;102(2):402‐10. [DOI] [PubMed] [Google Scholar]
Marya 1981 {published data only}
- Marya RK, Rathee S, Lata V, Mudgil S. Effects of vitamin D supplementation in pregnancy. Gynecologic and Obstetric Investigation 1981;12:155‐61. [DOI] [PubMed] [Google Scholar]
Mir 2016 {published data only}
- Mir SA, Masoodi SR, Shafi S, Hameed I, Dar MA, Bashir MI, et al. Efficacy and safety of Vitamin D supplementation during pregnancy: A randomized trial of two different levels of dosing on maternal and neonatal Vitamin D outcome. Indian Journal of Endocrinology and Metabolism 2016;20(3):337‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mojibian 2015 {published data only}
- Mojibian M. The effect of 50000 IU Vitamin D supplement administered two weekly on neonatal and pregnant women outcome. http://en.irct.ir/trial/7525 (first received 27 February 2012).
- Mojibian M, Soheilykhah S, Fallah Zadeh MA, Jannati Moghadam M. The effects of vitamin D supplementation on maternal and neonatal outcome: a randomized clinical trial. Iranian Journal of Reproductive Medicine 2015;13(11):687‐96. [PMC free article] [PubMed] [Google Scholar]
Mutlu 2014 {published data only}
- Mutlu GY, Ozsu E, Kalaca S, Yuksel A, Cizmecioglu FM, Hatun S. The evaluation of vitamin D supplementation dose during pregnancy in a high‐risk population. Hormone Research in Paediatrics 2013;80(Suppl 1):92. [DOI] [PubMed] [Google Scholar]
- Mutlu GY, Ozsu E, Kalaca S, Yuksel A, Pehlevan Y, Cizmecioglu F, et al. Evaluation of vitamin D supplementation doses during pregnancy in a population at high risk for deficiency. Hormone Research in Paediatrics 2014;81(6):402‐8. [DOI] [PubMed] [Google Scholar]
O'Brien 2013 {published data only}
- Best CM, Pressman EK, Queenan RA, Cooper E, Vermeylen F, O'Brien KO. Gestational age and maternal serum 25‐hydroxyvitamin d concentration interact to affect the 24,25‐dihydroxyvitamin d concentration in pregnant adolescents. Journal of Nutrition 2018; Vol. 148, issue 6:868‐75. [DOI] [PMC free article] [PubMed]
- O'Brien K. Vitamin D status impacts inflammation and risk of infections during pregnancy. clinicaltrials.gov/ct2/show/NCT01815047 (first received 20 March 2013). [NCT01815047]
Rostami 2017 {published and unpublished data}
- Rostami M, Ramezani Tehrani F, Simbar M, Bidhendi Yarandi R, Minooee S, Hollis BW, et al. Effectiveness of prenatal vitamin D deficiency screening and treatment program: a stratified randomized field trial. Journal of Clinical Endocrinology and Metabolism 2018; Vol. 103, issue 8:2936–48. [DOI] [PubMed]
- Rostami M, Ramezani Tehrani F, Simbar M, Hosseinpanah F, Alavi Majd H. Rationale and design of Khuzestan vitamin D deficiency screening program in pregnancy: a stratified randomized vitamin D supplementation controlled trial. JMIR Research Protocols 2017;6(4):e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roth 2013 {published data only}
- Morris SK, Pell LG, Rahman MZ, Dimitris MC, Mahmud A, Islam MM, et al. Maternal vitamin D supplementation during pregnancy and lactation to prevent acute respiratory infections in infancy in Dhaka, Bangladesh (MDARI trial): protocol for a prospective cohort study nested within a randomized controlled trial. BMC Pregnancy and Childbirth 2016;16(1):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth DE. Vitamin D supplementation in pregnant and non‐pregnant women in Dhaka, Bangladesh: Pharmacokinetic and safety studies [thesis]. John Hopkins University, 2011. [Google Scholar]
- Roth DE. Maternal vitamin D for infant growth (MDIG) trial. clinicaltrials.gov/ct2/show/https://clinicaltrials.gov/ct2/show/NCT01924013 (first received 16 August 2013). [NCT01924013]
- Roth DE, Gernand AD, Morris SK, Pezzack B, Islam MM, Dimitris MC, et al. Maternal vitamin D supplementation during pregnancy and lactation to promote infant growth in Dhaka, Bangladesh (MDIG trial): study protocol for a randomized controlled trial. Trials 2015;16:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth DE, Mahmud AA, Morris S, Zlotkin S, Gernand A, Tahmeed A, et al. Maternal vitamin D supplementation during pregnancy and lactation to promote infant growth in Dhaka, Bangladesh (MDIG trial): a randomized controlled trial. Annals of Nutrition and Metabolism 2017;71(Suppl 2):571. [Google Scholar]
- Roth DE, Morris SK, Zlotkin S, Gernand AD, Ahmed T, Shanta SS, et al. Vitamin D supplementation in pregnancy and lactation and infant growth. New England Journal of Medicine 2018;379(6):535‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shakiba 2013 {published data only}
- Shakiba M, Iranmanesh MR. Vitamin D requirement in pregnancy to prevent deficiency in neonates: A randomised trial. Singapore Medical Journal 2013;54(5):285‐8. [DOI] [PubMed] [Google Scholar]
Soheilykhah 2011 {published data only}
- Soheilykhah S. Effect of different doses of vitamin D on insulin resistance in pregnant women attending in Shahid Sadoughi and Mojibian prenatal clinics. en.irct.ir/trial/3385 (first received 23 May 2011). [IRCT138811203312N1]
- Soheilykhah S, Mojibian M, Moghadam MJ, Shojaoddiny‐Ardekani A. The effect of different doses of vitamin D supplementation on insulin resistance during pregnancy. Gynecological Endocrinology 2013;29(4):396‐9. [DOI] [PubMed] [Google Scholar]
Stephensen 2011 {published data only}
- Stephensen CB, NCT01417351. Effects of vitamin D supplementation during pregnancy on clinical outcomes and immune function. clinicaltrials.gov/ct2/show/NCT01417351 (first received 6 August 2011).
- Zerofsky M, Jacoby B, Pedersen TL, Stephensen CB. Effects of a randomized, controlled trial of daily vitamin D3 supplementation during pregnancy on regulatory immunity and inflammation. FASEB Journal 2016;30(Suppl 1):296.7. [DOI] [PubMed] [Google Scholar]
- Zerofsky MS, Jacoby BN, Pedersen TL, Stephensen CB. Daily cholecalciferol supplementation during pregnancy alters markers of regulatory immunity, inflammation, and clinical outcomes in a randomized controlled trial. Journal of Nutrition 2016;146(11):2388‐97. [DOI] [PubMed] [Google Scholar]
Thiele 2014 {published data only}
- Anderson CM, Gillespie SL, Thiele DK, Ralph JL, Ohm JE. Effects of maternal vitamin d supplementation on the maternal and infant epigenome. Breastfeeding Medicine 2018;13(5):371‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Thiele DK, Ralph JL, Perley D, OHM JE. Vitamin D supplementation and DNA methylation patterns during pregnancy in and lactation in mothers and infants. FASEB Journal 2016;30(Suppl 1):Abstract no: 1028.3. [Google Scholar]
- Keesling Thiele D. The Impact Of Continuous Prenatal and Early Postpartum Maternal Vitamin D Supplementation on The Vitamin D Status of Exclusively Breastfed Infants [thesis]. University of North Dakota, 2013. [Google Scholar]
- Thiele D, Anderson CM. CS_018. Maternal vitamin D supplementation increases infant vitamin D status at birth but the impact diminishes during breastfeeding. 17th Conference of the International Society for Research in Human Milk and Lactation (ISRHML); 2014 Oct 23‐27; Kiawah Island, South Carolina, USA. 2014:122.
- Thiele DK, Ralph J, El‐Masri M, Anderson CM. Vitamin d3 supplementation during pregnancy and lactation improves vitamin d status of the mother‐infant dyad. Journal of Obstetric, Gynecologic, and Neonatal Nursing : Jognn 2017;46:135‐47. [DOI] [PubMed] [Google Scholar]
Wagner 2006a {published data only}
- Hamilton SA, McNeil R, Hollis BW, Davis DJ, Winkler J, Cook C, et al. Profound vitamin d deficiency in a diverse group of women during pregnancy living in a sun‐rich environment at latitude 32°N. International Journal of Endocrinology 2010;2010:917428. [DOI: 10.1155/2010/917428] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CL, McNeil R, Hamilton SA, Davis DJ, Prudgen C, Winkler J, et al. Vitamin D (vitD) supplementation during pregnancy: Thrasher Research Fund RCT in SC community center networks. Pediatric Academic Societies' 2010 Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010.
- Wagner CL, McNeil R, Hamilton SA, Winkler J, Rodriguez Cook C, Warner G, et al. A randomized trial of vitamin D supplementation in 2 community health center networks in South Carolina. American Journal of Obstetrics and Gynecology 2013;208(2):137.e1‐137.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CL, NCT00412087. Vitamin D status of pregnant women and their children in Eau Claire, South Carolina: a prevalence and supplementation model for community health care centers in the U.S. clinicaltrials.gov/ct2/show/NCT00412087 (first received 15 December 2006).
Wagner 2006b {published and unpublished data}
- Appelgren KE, Nietert PJ, Hulsey TC, Hollis BW, Wagner CL. Analyzing adherence to prenatal supplement: does pill count measure up?. International Journal of Endocrinology 2010;2010:Article ID 631971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke B, Shary J, Ebeling M, Dezsi K, Wagner C. CS_033. Vitamin D protects bone mineral density in lactating women and its effect varies by race/ethnicity. 17th Conference of the International Society for Research in Human Milk and Lactation (ISRHML); 2014 Oct 23‐27; Kiawah Island, South Carolina, USA. 2014:167.
- Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: Double‐blind, randomized clinical trial of safety and effectiveness. Journal of Bone and Mineral Research 2011;26(10):2341‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RS, Mulligan JK, Harmon H, Hollis BW, Wagner CL. Immune mediators and vitamin D status in the development of comorbidities of pregnancy. Pediatric Academic Societies Annual Meeting; 2016 April 30‐May 3; Baltimore, USA. 2016:4109.85.
- Powell A, Shary J, Ramakrishnan V, Eckard A, Wagner C. Impact of vitamin d supplementation on bacterial vaginosis in pregnancy. American Journal of Obstetrics and Gynecology 2017;217(6):723‐4. [Google Scholar]
- Wagner CL, Johnson D, Hulsey TC, Ebeling M, Shary J, Smith PG, et al. Vitamin D supplementation during pregnancy Part I NICHD/CTSA randomized clinical trial (RCT): safety consideration. Pediatric Academic Societies Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010.
- Wagner CL, Johnson D, Hulsey TC, Ebeling M, Shary J, Smith PG, et al. Vitamin D supplementation during pregnancy part 2 NICHD/CTSA randomized clinical trial (RCT): outcomes. Pediatric Academic Societies' 2010 Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010.
- Wagner CL, McNeil RB, Johnson DD, Hulsey TC, Ebeling M, Robinson C, et al. Health characteristics and outcomes of two randomized vitamin D supplementation trials during pregnancy: A combined analysis. Journal of Steroid Biochemistry & Molecular Biology 2013;136:313‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CL, Sen S, Ebeling M, Hollis BW. BMI and vitamin D status are inversely related in breastfeeding mothers through 7 months. Breastfeeding Medicine 2016;11(2):A‐75, Abstract no:P‐121. [Google Scholar]
- Wagner, NCT00292591. Evaluation of vitamin D requirements during pregnancy. https://clinicaltrials.gov/ct2/show/NCT00292591 (first received 16 February 2006).
- Wei W, Shary JR, Garrett‐Mayer E, Anderson B, Forestieri NE, Hollis BW, et al. Bone mineral density during pregnancy in women participating in a randomized controlled trial of vitamin D supplementation. American Journal of Clinical Nutrition 2017;106(6):1422‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wagner 2013 {published and unpublished data}
- Schulz EV, Cruze L, Wei W, Garrett Mayer E, Gehris J, Wagner C. Effects of maternal vitamin D supplementation on placental gene expression. Pediatric Academic Societies Annual Meeting; 2016 April 30‐May 3; Baltimore, USA. 2016:3455.2.
- Schulz EV, Wagner CL, Cruze L, Wei W, Gehris J. Maternal vitamin d sufficiency and reduced placental gene expression in angiogenic biomarkers related to comorbidities of pregnancy. Journal of Steroid Biochemistry and Molecular Biology 2017;173:273‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CL, NCT01932788. Preventing health disparities during pregnancy through vitamin D supplementation. https://clinicaltrials.gov/ct2/show/NCT01932788 (first received 30 August 2013).
Weiss 2009 {published data only}
- Al‐Garawi A, Carey VJ, Chhabra D, Mirzakhani H, Morrow J, Lasky‐Su J, et al. The role of vitamin D in the transcriptional program of human pregnancy. Plos One 2016;11(10):e0163832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Garawi A, Carey VJ, Chhabra D, Morrow J, Lasky‐Su J, Koh A, et al. Differentially expressed genes during the course of pregnancy and their correlation with maternal vitamin d levels. American Journal of Respiratory and Critical Care Medicine 2015;191:A5994. [Google Scholar]
- Al‐Garawi A, Carey VJ, Qiu W, Mirzakhani H, Litonjua AA, Weiss ST. Cord blood vitamin D levels and gene expression profiles at birth are associated with wheezing in the first year of life: results from the vitamin D antenatal asthma reduction trial (vdaart). American Journal of Respiratory and Critical Care Medicine 2016;193:A3168. [Google Scholar]
- Bhaskaran K, Smeeth L, Evans S. Prenatal vitamin d and offspring wheezing. JAMA 2016;315(24):2730. [DOI] [PubMed] [Google Scholar]
- Blighe K, Chawes BL, Kelly RS, Mirzakhani H, McGeachie M, Litonjua AA, et al. Vitamin d prenatal programming of childhood metabolomics profiles at age 3 y. American Journal of Clinical Nutrition 2017;106(4):1092‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotegut CA. Prevention of preeclampsia. Journal of Clinical Investigation 2016;126(12):4396‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsby E, Pfeffer PE, Laranjo N, Cruikshank W, Tuzova M, Litonjua AA, et al. Vitamin D supplementation during pregnancy: effect on the neonatal immune system in a randomized controlled trial. Journal of Allergy and Clinical Immunology 2018;141(1):269‐78.e1. [DOI] [PubMed] [Google Scholar]
- Litonjua A, Weiss T. Prenatal vitamin d and offspring wheezing‐‐reply. JAMA 2016;315(24):2731. [DOI] [PubMed] [Google Scholar]
- Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O'Connor GT, et al. Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 years: the VDAART randomized clinical trial. JAMA 2016;315(4):362‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litonjua AA, Lange NE, Carey VJ, Brown S, Laranjo N, O'Connor GT, et al. The Vitamin D Antenatal Asthma Reduction Trial (VDAART): Rationale, design, and methods of a randomized, controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children. Contemporary Clinical Trials 2014;38:37‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merenstein D, D'Amico F. Prenatal vitamin d and offspring wheezing. JAMA 2016;315(24):2731. [DOI] [PubMed] [Google Scholar]
- Mirzakhani H, Litonjua AA, McElrath TF, O'Connor G, Lee‐Parritz A, Iverson R, et al. Early pregnancy vitamin D status and risk of preeclampsia. Journal of Clinical Investigation 2016;126(12):4702‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzakhani H, O'Connor GT, Bacharier L, Zeiger RS, Schatz MX, Weiss S, et al. Asthma control status during pregnancy and its relationship with vitamin D level: an observation from the vitamin D antenatal asthma reduction trial (VDAART). American Journal of Respiratory and Critical Care Medicine 2017;195:A3182. [Google Scholar]
- Sordillo JE, McGeachie MJ, Ziniti J, Lange N, Laranjo N, Carey V, et al. Factors influencing the infant gut microbiome at age 3‐6 months: findings from the ethnically diverse Vitamin D Antenatal Asthma Reduction Trial (VDAART). Journal of Allergy and Clinical Immunology 2017;139(2):482‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss ST, NCT00920621. Randomized trial: maternal vitamin d supplementation to prevent childhood asthma (vdaart). https://clinicaltrials.gov/ct2/show/NCT00920621 (first received 15 June 2009). [NCT00920621]
- Wolsk HM, Chawes BL, Litonjua AA, Hollis BW, Waage J, Stokholm J, et al. Prenatal vitamin D supplementation reduces risk of asthma/recurrent wheeze in early childhood: a combined analysis of two randomized controlled trials. PLOS One 2017; Vol. 12, issue 10:e0186657. [DOI] [PMC free article] [PubMed]
- Wolsk HM, Harshfield BJ, Laranjo N, Carey VJ, O'Connor GT, Sandel M, et al. Vitamin d supplementation in pregnant women of different races and the risk of asthma/recurrent wheeze in the child: findings from the vitamin d antenatal asthma reduction trial (vdaart). American Journal of Respiratory and Critical Care Medicine 2016;193:A3169. [Google Scholar]
- Wolsk HM, Harshfield BJ, Laranjo N, Carey VJ, Weiss ST, Litonjua AA, et al. Vitamin D supplementation in pregnancy, prenatal 25(oh)d levels, race, and subsequent asthma or recurrent wheeze in offspring: secondary analyses from the vitamin D antenatal asthma reduction trial. Journal of Allergy and Clinical Immunology 2017; Vol. 140, issue 5:1423. [DOI] [PubMed]
Yap 2014 {published data only}
- Yap C, Cheung NW, Gunton JE, Athayde N, Munns CF, Duke A, et al. Vitamin D supplementation and the effects on glucose metabolism during pregnancy: a randomized controlled trial. Diabetes Care 2014;37(7):1837‐44. [DOI] [PubMed] [Google Scholar]
Yu 2008 {published data only}
- Goldring ST, Griffiths CJ, Martineau AR, Robinson S, Yu C, Poulton S, et al. Prenatal vitamin D supplementation and child respiratory health: a randomised controlled trial. PLOS One 2013;8(6):e66627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring, ISRCTN68645785. Effects of prenatal vitamin D supplementation on respiratory and allergic phenotypes and bone density in the first three years of life. http://www.isrctn.com/ISRCTN68645785 (first received 24 June 2010).
- Yu C, Newton L, Robinson S, Teoh TG, Sethi M. Vitamin D deficiency and supplementation in pregnant women of four ethnic groups. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(Suppl 1):Fa68. [Google Scholar]
- Yu CK, Sykes L, Sethi M, Teoh TG, Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clinical Endocrinology 2009;70(5):685‐90. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ali 2018 {published data only}
- Ali A. Effect of antenatal Vitamin D3 supplementation on risk of pre‐eclampsia. clinicaltrials.gov/ct2/show/NCT03101150 (first received 4 April 2017). [NCT03101150]
- Ali AM, Alobaid A, Malhis TN, Khattab AF. Effect of vitamin d3 supplementation in pregnancy on risk of pre‐eclampsia ‐ randomized controlled trial. Clinical Nutrition (Edinburgh, Scotland) 2019;38(2):557‐63. [DOI] [PubMed] [Google Scholar]
Azami 2017 {published data only}
- Azami M, Azadi T, Farhang S, Rahmati S, Pourtaghi K. The effects of multi mineral‐vitamin D and vitamins (C+E) supplementation in the prevention of preeclampsia: An RCT. International Journal of Reproductive Biomedicine (Yazd, Iran) 2017;15(5):273‐8. [PMC free article] [PubMed] [Google Scholar]
Bisgaard 2009 {published and unpublished data}
- Bisgaard H. Vitamin D supplementation during pregnancy for prevention of asthma in childhood (ABCvitaminD). ClinicalTrials.gov (http://clinicaltrials.gov) (accessed 31 July 2009) (first received 6 March 2009).
- Chawes B, Bønnelykke K, Stokholm J, Heickendorff L, Brix S, Rasmussen M, et al. Effect of vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: a randomised clinical trial. Clinical and Translational Allergy 2016;6:6 [OP10]. [DOI] [PubMed] [Google Scholar]
- Chawes BL, Bonnelykke K, Stokholm J, Vissing NH, Bjarnadottir E, Schoos AM, et al. Effect of vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: a randomized clinical trial. JAMA 2016;315(4):353‐61. [DOI] [PubMed] [Google Scholar]
Hajihashemi 2016 {published data only}
- Hajhashemi M, Khorsandi A, Haghollahi F. Comparison of sun exposure versus vitamin D supplementation for pregnant women with vitamin D deficiency. Journal of Maternal‐fetal & Neonatal Medicine 2017;32(8):1347‐52. [DOI] [PubMed] [Google Scholar]
- Hajihashemi M, IRCT2016101227998N2. Comparison of sun exposure versus vitamin D supplementation for pregnant women with vitamin D deficiency. en.irct.ir/trial/22811 (first received 15 December 2016).
Jamilian 2017 {published data only}
- Jamilian M, Samimi M, Ebrahimi FA, Hashemi T, Taghizadeh M, Razavi M, et al. The effects of vitamin D and omega‐3 fatty acid co‐supplementation on glycemic control and lipid concentrations in patients with gestational diabetes. Journal of Clinical Lipidology 2017;11(2):459‐68. [DOI] [PubMed] [Google Scholar]
Li 2000 {published data only}
- Li X, Gou W. Study on prevention of pregnancy induced hypertension and effect of platelet intracellular free ca˜(2+) by calcium supplementation. Journal of Xi'an Medical University 2000; Vol. 21, issue 1:46‐8.
Omotayo 2017 {published data only}
- Omotayo MO, Dickin KL, Pelletier DL, Mwanga EO, Kung'u JK, Stoltzfus RJ. A simplified regimen compared with WHO guidelines decreases antenatal calcium supplement intake for prevention of preeclampsia in a cluster‐randomized noninferiority trial in rural Kenya. Journal of Nutrition 2017;147:1986‐91. [DOI] [PubMed] [Google Scholar]
Roth 2016 {published data only}
- Roth DE. Maternal postpartum high‐dose vitamin D3 supplementation (6400 IU/day) or conventional infant vitamin D3 supplementation (400 IU/day) lead to similar vitamin D status of healthy exclusively/fully breastfeeding infants by 7 months of age. BMJ Evidence‐Based Medicine 2016;21(2):75. [DOI] [PubMed] [Google Scholar]
Sablok 2015 {published data only}
- Sablok A, Batra A, Thariani K, Batra A, Bharti R, Aggarwal AR, et al. Supplementation of vitamin D in pregnancy and its correlation with feto‐maternal outcome. Clinical Endocrinology 2015;83:536‐41. [DOI] [PubMed] [Google Scholar]
Wheeler 2016 {published data only}
- Wheeler BJ, Taylor BJ, Herbison P, Haszard JJ, Mikhail A, Jones S, et al. High‐dose monthly maternal cholecalciferol supplementation during breastfeeding affects maternal and infant vitamin D status at 5 months postpartum: a randomized controlled trial. Journal of Nutrition 2016;146(10):1999‐2006. [DOI] [PubMed] [Google Scholar]
Zhang 2016 {published data only}
- Zhang Q, Cheng Y, He M, Li T, Ma Z, Cheng H. Effect of various doses of vitamin D supplementation on pregnant women with gestational diabetes mellitus: a randomized controlled trial. Experimental and Therapeutic Medicine 2016;12(3):1889‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Gerais 2015 {published data only}
- Gerais AS, Hussein S. Vitamin D deficiency in pregnancy and pregnancy outcome. International Journal of Gynecology and Obstetrics 2015;131(Suppl 5):E352. [Google Scholar]
Mobasheri 2016 {published data only}
- Azadehrah M, Mobasheri E, Behnampour N, Azadehrah M. Effect of maternal supplementation with 200 and 50,000 IU vitamin D on serum 25 (OH)D levels of pregnant women. Pharmacophore 2017;8(6S):e‐117368. [Google Scholar]
- Mobasheri E, IRCT2014121120276N1. Comparing the effect of therapeutic doses of 200 and 50000 units of vitamin d on serum 25(oh)d levels in pregnant women referred to shahid sayyad shirazi hospital of gorgan between 2013‐14. en.irct.ir/trial/18015 (first received 24 Serptember 2016).
References to ongoing studies
El‐Hajj Fuleihan 2015 {published data only}
- Chakhtoura M, Nassar A, Arabi A, Cooper C, Harvey N, Mahfoud Z, et al. Effect of vitamin D replacement on maternal and neonatal outcomes: A randomised controlled trial in pregnant women with hypovitaminosis D. A protocol. BMJ Open 2016;6:e010818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Hajj Fuleihan G, NCT02434380. Effect of vitamin d replacement on maternal and neonatal outcomes: a randomized controlled trial in pregnant women with hypovitaminosis d. clinicaltrials.gov/ct2/show/NCT02434380 (first received 5 May 2015). [DOI] [PMC free article] [PubMed]
Garreto 2016 {published and unpublished data}
- Garreto DJ, Persad MD. A randomized control trial of vitamin D prophylaxis in the prevention of hypertensive disorders of pregnancy. https://clinicaltrials.gov/ct2/show/NCT02920593 (first received 20 September 2016). [NCT02920593]
Hantoshzadeh 2017 {published data only}
- Hantoshzadeh S. Comparison of two therapeutic regimens Vitamin D3 deficiency in the first trimester of pregnancy on the level of vitamin D3 in the second trimester of pregnancy. http://en.irct.ir/trial/22353 (first received 10 July 2017).
Hartman 2010 {published data only}
- Hartman C, NCT01060735. The effect of different doses of vitamin d supplementation during pregnancy on bone and vitamin d status in children at birth and at one year age. clinicaltrials.gov/ct2/show/NCT01060735 (first received 2 February 2010).
Hoffman 2017 {published data only}
- Hoffman S, NCT03037593. A randomized single‐center study of the effects of high‐dose cholecalciferol to reduce the incidence of gestational diabetes in high‐risk pregnant women. clinicaltrials.gov/ct2/show/NCT03037593 (first received 31 January 2017).
Judkins 2011 {published data only}
- Judkins A, Krebs J, ACTRN12610001044011. Vitamin D deficiency in pregnancy ‐ a comparison of two treatments. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12610001044011 (first received 23 November 2010).
Kachhawa 2014 {published data only}
- Gupta T, Kachhawa G, Sharma H, Bajpai J, Kulshreshtha V, Khadgawat R, et al. A randomized double blind controlled trial to investigate the effects of vitamin D supplementation on maternal and new‐born baby's vitamin D status in Asian‐Indian subjects. Indian Journal of Endocrinology and Metabolism 2017;21(8 Suppl 2):S44‐5. [Google Scholar]
- Kachhawa G, CTRI/2014/06/004670. A randomized controlled trial to investigate the effects of vitamin D supplementation on maternal and new‐born baby’s vitamin D status in Asian‐Indian subjects. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=8544 (first received 16 June 2014).
Lalooha 2012 {published data only}
- Lalooha F, IRCT201205119491N2. The effect of vitamin D supplementation during pregnancy on newborns antropometric index. en.irct.ir/trial/10079 (first received 16 August 2012).
McCann 2016 {published data only}
- McCann M, NCT02713009. Investigation of the impact of maternal body weight on Vitamin D status during pregnancy: a randomised supplementation study. clinicaltrials.gov/ct2/show/NCT02713009 (first received 18 March 2016).
McLean 2012 {published data only}
- McLean M, ACTRN12612001145897. Does vitamin D supplementation in pregnancy improve maternal glucose metabolism or prevent gestational diabetes?. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12612001145897 (first received 30 October 2012).
Mosalanejad 2016 {published data only}
- Mosalanejad N, IRCT2016121430612N2. Compare the effect of vitamin D and calcium plus vitamin D on pregnancy outcomes in pregnant women. en.search.irct.ir/view/34642 (first received 26 December 2016).
Nausheen 2018 {published data only}
- Nausheen S. Assessment of dose effectiveness of vitamin D supplementation during pregnancy. clinicaltrials.gov/ct2/show/NCT02215213 (1first received 3 August 2014).
- Nausheen S, Soofi S. Assessment of dose effectiveness of vitamin D supplementation during pregnancy A dose comparison clinical trial. BJOG: an international journal of obstetrics and gynaecology 2018;125(Suppl 1):15. [Google Scholar]
Neyestani 2016 {published and unpublished data}
- Neyestani T, IRCT2016090329675N1. Evaluation the efficacy of vitamin D supplementation during pregnancy on maternal and newborn vitamin D status and pregnancy outcomes compared with control group. //en.irct.ir/trial/23794 (first received 25 September 2016).
- Neyestani TR, NCT03308487. Evaluation and comparison of the efficacy of 1000 and 2000 iu/d vitamin d supplementation during pregnancy on maternal and newborn vitamin d status and pregnancy outcomes. clinicaltrials.gov/ct2/show/NCT03308487 (first received 12 October 2017).
Nouripour 2016 {published data only}
- Nouripour S, IRCT2015041321736N1. Clinical trial on the evaluation of calcium and vitamin d in the cord serum of neonates, whose mothers were under vitamin d treatment during their pregnancy. en.irct.ir/trial/18965 (first received 25 January 2016).
Rasmussen 2009 {published data only}
- Mosekilde L, Sikjaer T, Vestergaard P, Heickendorff L, Uldbjerg N, Langdahl B, et al. Effects of high dose vitamin D supplementation on bone metabolism in pregnant women with hypovitaminosis D ‐ A randomized controlled trial. Journal of Bone and Mineral Research. 2015; Vol. 30, issue Suppl 1.
- Rasmussen GB, NCT01038453. Effects of vitamin D supplement before and during pregnancy on birth weight (gravita). clinicaltrials.gov/ct2/show/NCT01038453 (24 December 2009).
Rich‐Edwards 2015 {published data only}
- Rich‐Edwards J, NCT02395081. Trial of Vitamin D supplements to raise calcidiol levels of pregnant women in Mongolia. clinicaltrials.gov/ct2/show/NCT02395081 (first received 20 March 2015).
Additional references
ACOG 2015
- American College of Obstetricians and Gynecologists. Vitamin D: screening and supplementation during pregnancy. Committee Opinion No. 495. Obstetrics and Gynecology 2011 (Reaffirmed 2015);118:197–8. [DOI] [PubMed] [Google Scholar]
Aghajafari 2013
- Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O’Beirne M, Rabi DM. Association between maternal serum 25‐hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta‐analysis of observational studies. British Medical Journal 2013;346:f1169. [DOI] [PubMed] [Google Scholar]
Ariyuki 1987
- Ariyuki F. Growth retardation induced in rat fetuses by maternal fasting and massive doses of ergocalciferol. Journal of Nutrition 1987;117(2):342–8. [DOI] [PubMed] [Google Scholar]
Brembeck 2013
- Brembeck P, Winkvist A, Olausson H. Determinants of vitamin D status in pregnant fair‐skinned women in Sweden. British Journal of Nutrition 2013;110(5):856‐64. [DOI] [PubMed] [Google Scholar]
Cantorna 2008
- Cantorna MT, Yu S, Bruce D. The paradoxical effects of vitamin D on type 1 mediated immunity. Molecular Aspects of Medicine 2008;29:369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 1979
- Chan GM, Buchino JJ, Mehlhorn D, Bove KE, SteichenJJ, Tsang RC. Effect of vitamin D on pregnant rabbits and their offspring. Pediatric Research 1979;13(2):121–6. [DOI] [PubMed] [Google Scholar]
Christesen 2012a
- Christesen HT, Falkenberg T, Lamont RF, Jorgensen JS. The impact of vitamin D on pregnancy: a systematic review. Acta Obstetricia et Gynecologica Scandinavica 2012;91:1357‐67. [DOI] [PubMed] [Google Scholar]
Christesen 2012b
- Christesen HT, Elvander C, Lamont RF, Jorgensen JS. The impact of vitamin D in pregnancy on extraskeletal health in children: a systematic review. Acta Obstetricia et Gynecologica Scandinavica 2012;91:1368‐80. [DOI] [PubMed] [Google Scholar]
Cope 2016
- Committee on Publication Ethics (COPE). What to do if you suspect plagiarism flowchart. https://publicationethics.org/files/Full%20set%20of%20English%20flowcharts_9Nov2016.pdf 2016.
Dawnson‐Hughes 2005
- Dawson‐Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporosis International 2005;16:713–6. [DOI] [PubMed] [Google Scholar]
De Regil 2016
- De‐Regil LM, Palacios C, Lombardo LK, Peña‐Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD008873.pub3] [DOI] [PubMed] [Google Scholar]
DGA 2015
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015 – 2020 Dietary Guidelines for Americans. http://health.gov/dietaryguidelines/2015/guidelines (accessed 2 June 2016) 2015; Vol. 8th edition.
EFSA 2016
- European Food Safety Authority (EFSA). Scientific Opinion on Dietary Reference Values for vitamin D. European Food Safety Authority Journal 2016. [DOI] [PMC free article] [PubMed]
Fitzpatrick 1988
- Fitzpatrick TB. The validity and practicality of sun‐reactive skin types I through VI. Archives of Dermatology 1988;124(6):869‐73. [DOI] [PubMed] [Google Scholar]
Friedman 1969
- Friedman WF, Mills LF. The relationship between vitamin D and the craniofacial and dental anomalies of the supravalvular aortic stenosis syndrome. Pediatrics 1969;43(1):12‐8. [PubMed] [Google Scholar]
Gernand 2016
- Gernand AD, Schulze KJ, Stewart CP, West KP Jr, Christian P. Micronutrient deficiencies in pregnancy worldwide: health effects and prevention. Nature Reviews: Endocrinology 2016;12(5):274‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goswami 2016
- Goswami D, Rani R, Saxena A, Arora MS, Batra S, Sreenivas V. Maternal and neonatal vitamin‐D status in twin versus singleton pregnancies. Journal of Obstetrics and Gynaecology Research 2016;42(10):1250‐57. [DOI] [PubMed] [Google Scholar]
Hall 2010
- Hall LM, Kimlin MG, Aronov PA, Hammock BD, Slusser JR, Woodhouse LR, et al. Vitamin D intake needed to maintain target serum 25‐hydroxyvitamin D concentrations in participants with low sun exposure and dark skin pigmentation is substantially higher than current recommendations. Journal of Nutrition 2010;140(3):542‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harvey 2014
- Harvey NC, Holroyd C, Ntani G, Javaid K, Cooper P, Moon R, et al. Vitamin D supplementation in pregnancy:a systematic review. Health Technology Assessment 2014;18(45):1–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hathcock 207
- Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. American Journal of Clinical Nutrition 2007;85(1):6‐18. [DOI] [PubMed] [Google Scholar]
Heaney 2008
- Heaney RP. Vitamin D: criteria for safety and efficacy. Nutrition Reviews 2008;66(10 Suppl 2):S178–S181. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holick 2008
- Holick MF. Vitamin D deficiency: a worldwide problem with health consequences. American Journal of Clinical Nutrition 2008;87(4):1080S‐1086S. [DOI] [PubMed] [Google Scholar]
Holick 2009
- Holick MF. Vitamin D status: measurement, interpretation and clinical application. Annals of Epidemiology 2009;19:73‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hollis 2011
- Hollis BW, Wagner CL. Vitamin D requirements and supplementation during pregnancy. Current Opinion in Endocrinology, Diabetes, and Obesity 2011;18(6):371‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hypönnen 2013
- Hyppönen E, Cavadino A, Williams D. Vitamin D and pre‐eclampsia: original data, systematic review and meta‐analysis. Annals of Nutrition Metabolism 2013;63:331‐40. [DOI] [PubMed] [Google Scholar]
IOM 2011
- IOM (Institute of Medicine). Dietary Reference Intakes for Calcium and Vitamin D. 2nd Edition. Washington, DC: The National Academies Press, 2011. [PubMed] [Google Scholar]
Karlsson 2015
- Karlsson T, Andersson L, Hussain A, Bosaeus M, Jansson N, Osmancevic A, et al. Lower vitamin D status in obese compared with normal‐weight women despite higher vitamin D intake in early pregnancy. Clinical Nutrition 2015;34(5):892‐8. [DOI] [PubMed] [Google Scholar]
Karras 2016
Liu 2012
- Liu NQ, Hewison M. Vitamin D, the placenta and pregnancy. Archives of Biochemestry and Biophysics 2012;523(1):37‐47. [DOI] [PubMed] [Google Scholar]
Moller 2013
- Møller UK, Streym S, Mosekilde L, Heickendorff L, Flyvbjerg A, Frystyk J, et al. Changes in calcitropic hormones, bone markers and insulin‐like growth factor I (IGF‐I) during pregnancy and postpartum: a controlled cohort study. Osteoporosis International 2013;24(4):1307–20. [DOI] [PubMed] [Google Scholar]
Moon 2015
- Moon RJ, Crozier SR, Dennison EM, Davies JH, Robinson SM, Inskip HM, et al. Tracking of 25‐hydroxyvitamin D status during pregnancy: the importance of vitamin D supplementation. American Journal of Clinical Nutrition 2015;102(5):1081‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nassar 2011
- Nassar N, Halligan GH, Roberts CL, Morris JM, Ashton AW. Systematic review of first‐trimester vitamin D normative levels and outcomes of pregnancy. American Journal of Obstetrics and Gynecology 2011;205(208):e1‐e7. [DOI] [PubMed] [Google Scholar]
Nicolaidou 2006
- Nicolaidou P, Hatzistamatiou Z, Papadopoulou A, Kaleyias J, Floropoulou E, Lagona E. Low vitamin D status in mother‐newborn pairs in Greece. Calcified Tissue International 2006;78(6):337–42. [DOI] [PubMed] [Google Scholar]
Ornoy 1968
- Ornoy A, Menczel J, Nebel L. Alterations in the mineral composition and metabolism of rat fetuses and their placentas induced by maternal hypervitaminosis D2. Israel Journal of Medical Sciences 1968;4(4):827–32. [PubMed] [Google Scholar]
Ornoy 1969
- Ornoy A, Nebel L, Menczel Y. Impaired osteogenesis of fetal long bones.Induced by maternal hypervitaminosis D2. Archives of Pathology 1969;87(6):563–71. [PubMed] [Google Scholar]
Palacios 2014
- Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem?. Journal of Steroid Biochemistry and Molecular Biology 2014;144:138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palacios 2019
- Palacios C, Kostiuk LK, Peña‐Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2019, Issue 7. [DOI: 10.1002/14651858.CD008873.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pludowski 2013
- Płudowski P, Karczmarewicz E, Bayer M, Carter G, Chlebna‐Sokół D, Czech‐Kowalska J, et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe — recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynologia Polska 2013;64(4):319‐27. [DOI] [PubMed] [Google Scholar]
Poel 2012
- Poel YHM, Hummel P, Lips P, Stam F, Ploeg T, Simsek S. Vitamin D and gestational diabetes:a systematic review and meta‐analysis. European Journal of Internal Medicine 2012;23:465‐69. [DOI] [PubMed] [Google Scholar]
Pratumvinit 2015
- Pratumvinit B, Wongkrajang P, Wataganara T, Hanyongyuth S, Nimmannit A, Chatsiricharoenkul S, et al. Maternal vitamin D status and its related factors in pregnant women in Bangkok, Thailand. PLoS One 2015;10(7):e0131126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pérez‐López 2015
- Pérez‐López FR, Pasupuleti V, Mezones‐Holguin E, Benites‐Zapata VA, Thota P, Deshpande A, et al. Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: a systematic review and meta‐analysis of randomized controlled trials. Fertility and Sterility 2015;103(5):1278‐88.e4. [DOI] [PubMed] [Google Scholar]
RCOG 2014
- Royal College of Obstetricians and Gynaecologists. Vitamin D in Pregnancy. Scientific Impact Paper No. 43 2014;https://www.rcog.org.uk/globalassets/documents/guidelines/scientific‐impact‐papers/vitamin_d_sip43_june14.pdf (accessed 2 June 2016):1‐11. [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rodriguez 2016
- Rodriguez A, Santa Marina L, Jimenez AM, Esplugues A, Ballester F, Espada M, et al. Vitamin D status in pregnancy and determinants in a Southern European cohort study. Paediatric and Perinatal Epidemiology 2016;30(3):207‐28. [DOI] [PubMed] [Google Scholar]
Roth 2017
- Roth DE, Leung M, Mesfin E, Qamar H, Watterworth J, Papp E. Vitamin D supplementation during pregnancy: state of the evidence from a systematic review of randomised trials. BMJ 2017;359(j5237):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roth 2018
- Roth DE, Abrams SA, Aloia J, Bergeron G, Bourassa MW, Brown KH, et al. Global prevalence and disease burden of vitamin D deficiency: a roadmap for action in low‐ and middle‐income countries. Ann N Y Acad Sci 2018;1430(1):44‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saraf 2016
- Saraf R, Morton SM, Camargo CA Jr, Grant CC. Global summary of maternal and newborn vitamin D status ‐ a systematic review. Maternal and Child Nutrition 2016;12(4):647‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sato 2000
- Sato S. The function of vitamin D receptor in vitamin D action. Journal of Biochemistry 2000;127(5):717‐22. [DOI] [PubMed] [Google Scholar]
Sharma 2016
- Sharma S, Kumar A, Prasad S, Sharma S. Current scenario of vitamin D status during pregnancy in North Indian population. Journal of Obstetric and Gynaecology of India 2016;66(2):93‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spiro 2014
- Spiro A, Buttriss JL. Vitamin D: An overview of vitamin D status and intake in Europe. Nutrition Bulletin 2014;39(4):322‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tabesh 2013
- Tabesh M, Salehi‐Abargouei A, Tabesh M, Esmaillzadeh A. Maternal vitamin D status and risk of pre‐eclampsia: a systematic review and meta‐analysis. Journal of Clinical Endocrinology and Metabolism 2013;98:3165‐73. [DOI] [PubMed] [Google Scholar]
Theodoratou 2014
- Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta‐analyses of observational studies and randomised trials. BMJ 2014;348:g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorne‐Lyman 2012
- Thorne‐Lyman A, Fawzi WW. Vitamin D during pregnancy and maternal, neonatal and infant health incomes: a systematic review and meta‐analysis. Peadiatric and Perinatal Epidemiology 2012;26(Suppl 1):75‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
UNICEF/WHO 2004
- The United Nations Children’s Fund and World Health Organization. Low Birthweight: Country, Regional and Global Estimates. UNICEF, 2004. [Google Scholar]
Vieth 1999
- Vieth R. Vitamin D supplementation, 25‐hydroxyvitaminD concentrations, and safety. American Journal of Clinical Nutrition 1999;69(5):842–56. [DOI] [PubMed] [Google Scholar]
Wang 2004
- Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25‐dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. Journal of Immunology 2004;173:2909–12. [DOI] [PubMed] [Google Scholar]
Wei 2013
- Wei SQ, Qi HP, Luo ZC, Fraser WD. Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta‐analysis. Journal of Maternal‐Fetal and Neonatal Medicine 2013;26:889‐99. [DOI] [PubMed] [Google Scholar]
WHO 2004
- World Health Organization and Food and Agriculture Organization. Vitamin and Mineral Requirements in Human Nutrition. 2nd Edition. Geneva: WHO, 2004. [Google Scholar]
WHO 2011
- World Health Organization. WHO Recommendations for Prevention and Treatment of Pre‐eclampsia and Eclampsia. Geneva: WHO, 2011. [PubMed] [Google Scholar]
WHO 2012a
- World Health Organization. The WHO Application of ICD‐10 to Deaths During Pregnancy, Childbirth and the Puerperium: ICD‐MM. Geneva: WHO, 2012. [Google Scholar]
WHO 2012b
- World Health Organization. Guideline: Vitamin D Supplementation in Pregnant Women. Geneva: WHO, 2012. [PubMed] [Google Scholar]
WHO 2013
- World Health Organization. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. Geneva: WHO, 2013. [PubMed] [Google Scholar]
Yu 2009
- Yu CK, Sykes L, Sethi M, Teoh TG, Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clinical Endocrinology 2009;70(5):685‐90. [DOI] [PubMed] [Google Scholar]
Zhender 2012
- Zehnder D, Evans KN, Kilby MD, Bulmer JN, Innes BA, Stewart PM, et al. The ontogeny of 25‐hydroxyvitamin D(3) 1alpha‐hydroxylase expression in human placenta and decidua. American Journal of Pathology 2002;161(1):105‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
PROSPERO
- Palacios C, Lips P, Martinez RX, Lopez‐Perez L, Peña‐Rosas JP, Trak‐Fellermeier MA. Regimens of vitamin D supplementation for women during pregnancy. http://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=103763. [DOI] [PMC free article] [PubMed]


