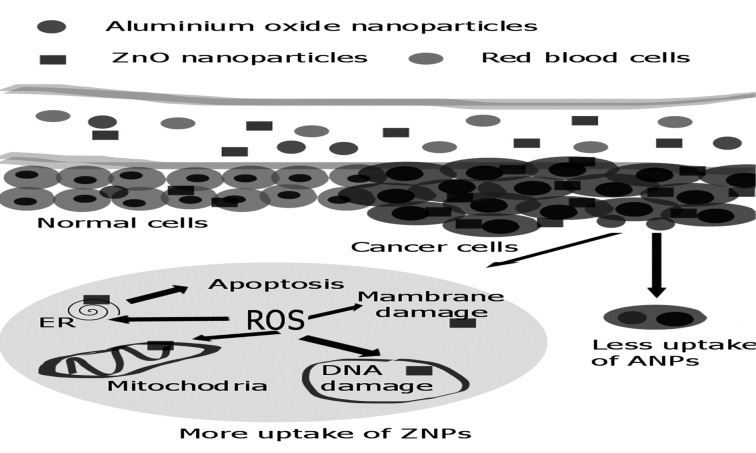
Abstract
Background and aim of the study:
Use of commercial products containing nanoparticles formulated from zinc oxide (ZnO) and aluminium oxide (Al2O-3) has increased significantly. These nanoparticles are widely used as ingredient in cosmetics, and also in food packaging industry although their toxicity status is yet to be studied. Here, we aimed to explore the effect of zinc oxide nanoparticles (ZnO-NPs) and aluminium oxide nanoparticles (ANPs) in human HT29 colon cancer cell line.
Methods:
In this study, ZnO-NPs were synthesized by chemical method and ANPs synthesized by sol-gel method and were characterized using UV-Vis spectroscopy, X ray diffraction and Transmittance electron microscopy. The effects of ZnO-NPs and ANPs was determined by cell viability, membrane integrity and colony formation potentials.
Results:
ZnO-NPs and ANPs inhibit HT29, colon cancer cell proliferation in a dose dependent manner, and affect the membrane potentials and also prevent the colony formation. Conclusions: The results suggest that ZnO NPs are found to be more effective than ANPs in reducing colon cancer cell proliferation. (www.actabiomedica.it)
Keywords: cell proliferation, nanoparticles, anti proliferative, toxicity, colon cancer and cytotoxicity
Introduction
Nanoparticles have high volume to surface area and results in enhanced potentials. The use of ZnO and as well as other metal oxide nanoparticles in biomedical and cancer applications are acquiring importance due to the physical and chemical properties of these nanomaterials. Nanoparticles are similar in size as biological molecules so it can penetrate through the cells and interact with the biological system. Metal oxide nanoparticles, including ZnO-NPs and ANPs, has application in sunscreens, food packaging and as components of various cosmetics (1). Among five zinc compounds, ZnO is the one which is currently recognized as safe for the nutrients by the U.S. Food and Drug Administration (21CFR182.8991) (2). Nanoparticles endowed with targeting abilities offer a novel approach for site-specific delivery of chemotherapeutic agents (3). But, after treatment with nanoformulation, toxicity status are not yet studied.
Some studies have been conducted to investigate the cytotoxicity of ZnO-NPs in various cell lines, human hepatocytes and found that oxidative stress and lipid peroxidation play important role in cell disruption provoked by ZNO-NPs (4). Studies conducted in human lung epithelial cells (L-132) (5) and human alveolar adenocarcinoma cell line A549 (6), reveal that ZnO NPs can selectively penetrate the tumor cells and thereby interact with the cancerous cells and destroy them. In the case of ANPs, are found to inhibit the cell division in a dose dependent manner on CHO-K1 cells and UMR106 cells (7-8). The A549 cells after exposure to various nanoparticles exhibited that ANPs induces minor cytotoxic effects, compared to nanometric titanium dioxide or carbon nanotubes (9). Despite the already existing studies on the toxicity of ANPs, the underlying mechanism of toxicity remains unclear. The genotoxicity effect of NPs is of specific concern because of the alteration in the genetic material that have potential for tissue malfunction, development of cancer and cell death (10). Recent in-vitro studies reveal metal oxide nanoparticles to selectively destruct cancer cells with relatively less toxicity against normal cells (11-12).
Some of the inorganic NPs, such as iron oxide NPs, titanium dioxide NPs, ZnO NPs, copper oxide NPs, silica NPs, show anti-cancer activity so that they can be used in anti-cancer therapy (8-9). Basically, nanoparticles have some unique features, which make them novel and efficient anticancer agent. Even these ZnO NPs based microfluidic immunosensor coupled with the laser instigated fluorescence is used for the detection of epithelial cell adhesion molecule (13). Studies have shown that ZnO NPs can very selectively instigate apoptosis in the cancer cells, which is mediated much likely by the reactive oxygen species through p53 pathway (14), although extensive studies were needed for assessment of anti-proliferative effect of ZnO NPs on cancer cells.
In this study, we have synthesized and characterized ZnO-NPs and ANPs, and investigated their anti-proliferative potentials in HT29 cells, a colon cancer cell line.
Graphical Abstract
Methods
Synthesis of ZnO NP and ANPs
In this study, ZnO NPs were synthesized by the bottom-up approach. The atomic mixing of the constituents yields a final product of near stoichiometric perfection without high-temperature treatments. In the typical process, 1 g of ZnCl2.6H2O was dissolved in 100 ml of double distilled water. It was neutralized with 20 ml of NaOH (1 M) solution under constant stirring at room temperature (pH.8). It led to the anionic and cationic interaction, followed by the nucleation and formation of crystallites together with the by-products of NH4NO3. In order to remove the by-product, the product was then washed with the distilled water for several times, and then air dried in room temperature for few days and grounded well to obtain an amorphous powder. To obtain different crystallite size, the prepared amorphous sample was calcined at 200±2°C for 30 mins.
Aluminum oxide nanoparticles were successfully synthesized by sol-gel method using aluminium chloride, ethanol, and Ammonia. 0.1M aluminium chloride solution was prepared in ethanol and under a stirring condition, 28% of ammonia was added dropwise to aluminium chloride solution. A white colored gel was formed and it was kept at room temperature for 30 h and then the nano gel was dried at 200°C for 10 min in the box furnace.
Characterization of the ZnO NPs and ANPs
The synthesized ZnO-NPs and ANPs were characterized using UV-visible spectroscopy, X-ray diffraction (XRD), and transmission electron microscopy (TEM), which are the widely being used techniques to examine the optical, structural and microstructural properties, of ANPsand ZnO NPs. The synthesized samples were characterized by XRD using focused monochromatized Cu Kα1 source to determine the structure and average crystallite size of the calcined Al2O3 and ZnO. The detailed morphology studied under Transmission Electron Microscope (TEM) JEOL 3010). TEM images were taken on a JEOL 1200 EXII (IIT Madras) at 100 kV. Samples were prepared by evaporating single drop of ethanolic nanoparticle solution on a carbon-coated copper grid.
Cell lines and cell culture conditions
Colon cancer cell line (HT29) was obtained from National Centre for Cell Sciences, Pune, India. The cells were cultured in standard DMEM supplemented with 10% FBS and 1% antibiotic and incubated at 37°C with 5% CO2. The cells were trypsinized using 0.025% trypsin and 1x105 cells were re-plated on each well in a 96 well plate containing standard DMEM, incubated at 37°C with 5% CO2 and allowed to adhere for 24 h before treatment.
In Vitro Cell viability assay
The anti-proliferative effect of ZnO NPs and ANPs in HT 29 was determined using MTT assay. MTT assay was performed by following the procedure of Mossman(1983) (15). Cells (1x105) were seeded in 96-well plates and were cultured in standard DMEM. The cells were allowed to grow until 80 % confluency was reached and cells were treated with different concentration of nanoparticles (0, 10, 25, 50, 100 and 250 μg/ml) in dose dependent manner, and incubated for 48 h. After incubation, the spent medium from each well was collected and were stored for further studies (LDH assay). The MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) was added to each of the well and incubated for around 4 h. After incubation with MTT, resulting formazan crystals were solubilized by the addition of dimethyl sulphoxide and were quantified by measuring absorbance at 570 nm in a multiwall plate reader (Biorad).
Assessment of cell morphology
Colon cancer cells (HT29) were plated in 6-well culture plates (5000 cells/well) and cultured in DMEM supplemented with 10% FBS for 48 h and treated with Zn NPs and ANPs at a 100 μg/ml. After incubation, nanoparticle induced morphological changes were assessed using inverted phase contrast microscope (Olympus CKX41) connected with Optika B5 digital camera.
Lactate dehydrogenase assay
Cells treated with ZnO NPs and ANPs were incubated for 48 h in the CO2 incubator, and after the incubation medium was collected as lysis solution. In a 96 well plate, 10 μl of lysis solution was added to LDH control wells, 10 μl of test compounds to the experimental wells, 10 μl of PBS to untreated control wells and 10 μl of solvent was added to the vehicle wells respectively. The plate was incubated in a CO2 incubator at room temperature for 40-45 min. 50 μl of supernatant from each well was taken in another 96 well plate and to each well, the LDH reagent was added, followed by 20-30 mins of incubation at room temperature. Finally, absorbance was measured at 490 nm which was used as the main wavelength and 600 nm as a reference wavelength.
Lipid peroxidation
The colorimetric assay of thiobarbituric acid (TBA) was performed based on Buege and Aust (1978) method with minor modifications (16). 1 ml of TBA–TCA–HCl reagent was mixed with 1 ml of cell lysate and the mixture was heated in boiling water bath for 15 min. The reaction mixture was centrifuged at 4000 rpm for 10 mins and the absorbance of the supernatant was measured at about 535 nm against blank that contained all the reagents except cell lysate. The MDA concentration was calculated using molar extinction coefficient of 1.56×105 M−1 cm−1 and was reported in nmol/mg protein.
Colony formation assay
Clonogenic assays serve as a very useful tool to test whether the given cancer therapy can reduce the clonogenic survival of the tumor cells. This method is used to determine the cells reproductive health after treatment with nanoparticles. 5x103 cells were seeded into 6-well plate and were cultured in DMEM with 10% FBS. After incubation, the cells were harvested and treated with ZnO NPs and ANPs (100 μg/ ml) followed by incubating for one week in the CO2 incubator. The spent medium was removed and cells were rinsed with PBS. It was followed by the addition of 2-3 ml of fixation solution [Acetic acid/MeOH 1:7] and left at room temperature for 5 min. 0.5% Crystal violet solution was added after removing fixation solution and incubated at the room temperature for 30 min. Crystal violet was removed carefully by immersing the 6 well plates in tap water. The plates were allowed to air dry and the numbers of colonies were counted.
Results and discussion
The size of the nanoparticles plays a significant role in altering the properties of materials in entirety. UV-visible spectroscopy is widely utilized technique to examine the optical absorbance of NPs. The absorbance spectra of ZnO NPs and ANPs exhibit a strong absorption band at about 367 nm and 260 nm respectively. It is also evident that the significant sharp absorption of these NPs indicates the monodispersed nature of the NP distribution. The UV-visible spectra are shown in Figure 1.
Figure 1.
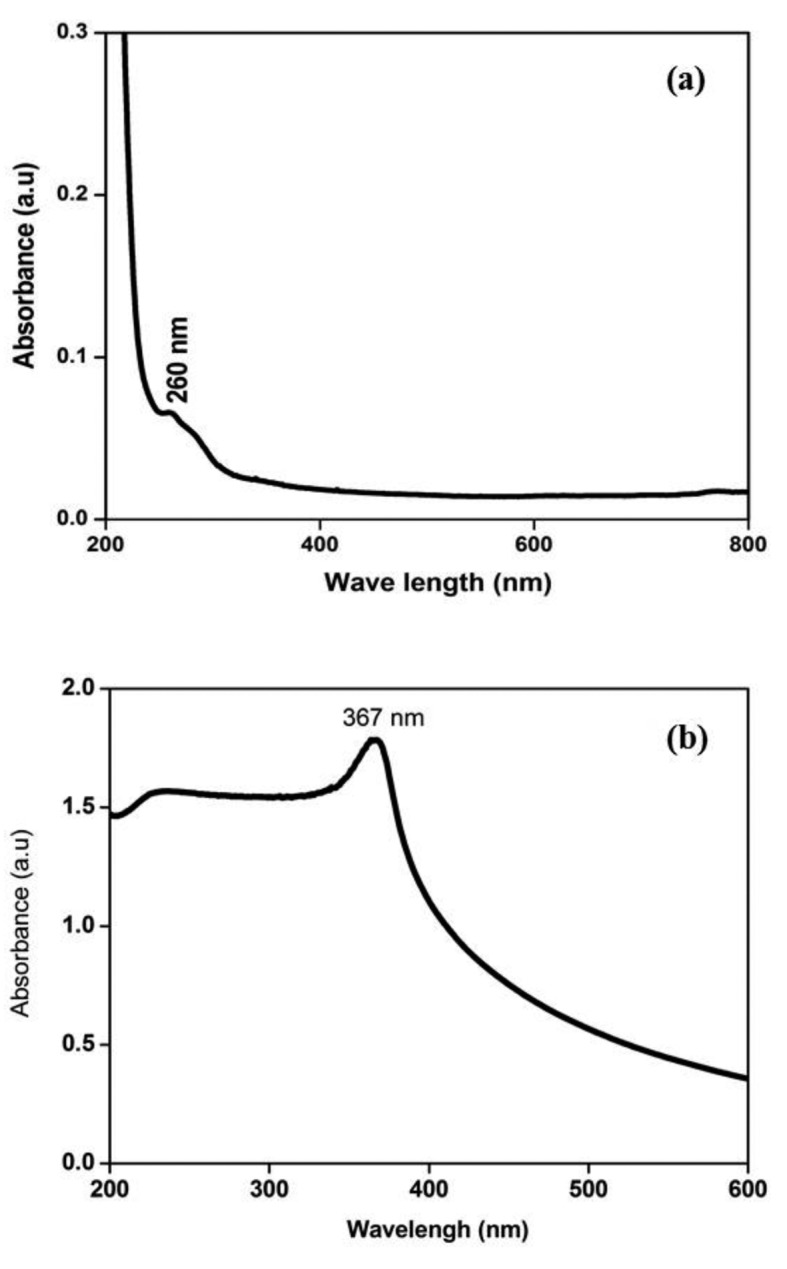
UV vis spectrum of nanoparticles (a) UV vis spectrum of ANPs (b) ZnO NPs
Diffraction pattern of ANPs were shown in Figure 2a. The obtained pattern was compared with JCPDS standard files of (46-1212) aluminium oxide which indicates hexagonal structure of ANPs. The sharp peak pattern indicates the nanoscale size with high purity. The peaks were indexed as planes (012), (104), (113), (024), (116) and (300) of ANPS. The estimated average crystalline size is 50.89 nm. Diffraction pattern of ZnO NPs were shown in Figure 2b. The obtained pattern was compared with JCPDS standard files of (36-1451) zinc oxide which indicates hexagonal structure. The sharp peak patterns indicate the nanoscale size with high purity. The peaks were indexed as planes (101), (002), (101), (102), (110), (103), (112), (201), (004) and (202) of ZnO. Diffraction pattern corresponding to impurities was found to be absent. The estimated average crystalline size is 32.11 nm.
Figure 2.
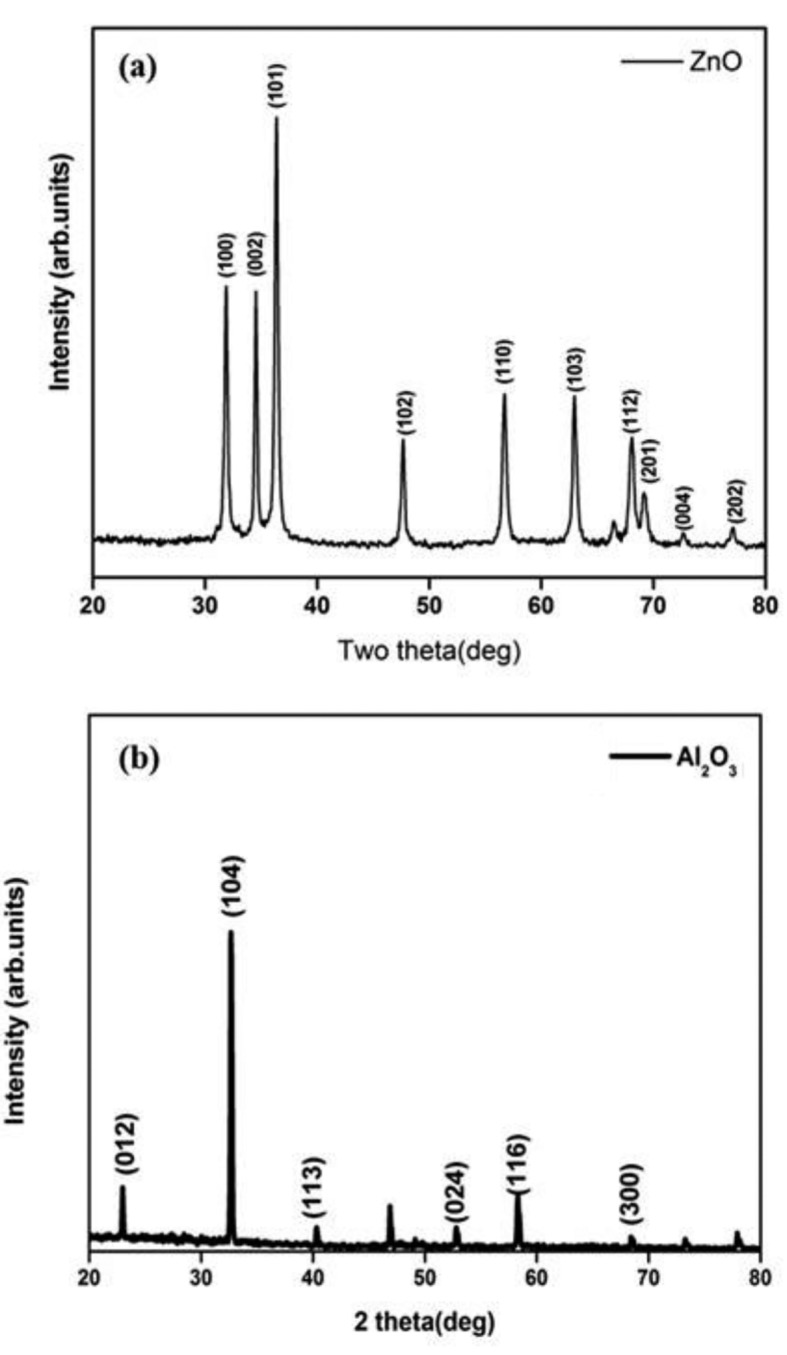
X- ray diffraction pattern of nanoparticles (a) ZnO NPs (b) ANPs
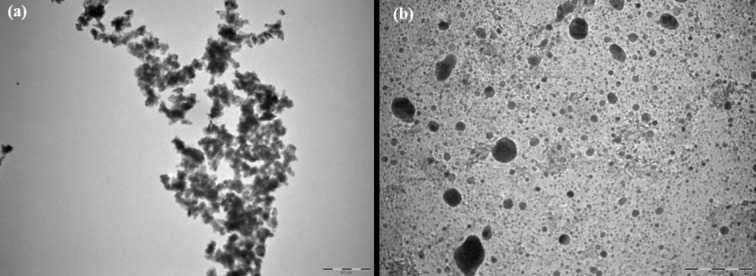
The detailed morphology of ANPs and ZnO NPs were analyzed using HRTEM. The average crystal size of ANPs and ZnO NPs was matched to the microscopic images. TEM images of ANPs and ZnO NPs are given in Figure 3. These images showed high homogeneity on the surface of the samples. The morphology of the particles was viewed under Transmission Electron Microscope (TEM) and found to be spherical and less agglomerate. TEM analysis results were used to find the actual size of the particles and distribution of the crystallites.
Figure 3.
Transmission electron microscopic images (a) ZnO NPs (b) ANPs
The in vitro cytotoxic effect of ZnO NPs and ANPs on colon cancer cells was explored by treatment of the colon cancer cells with different concentrations of nanoparticles. The results are presented in Figure 4. The anti-proliferative effect was determined using MTT assay. The results showed that ZnO-NPs significantly inhibit cell proliferation in a dose dependent manner. ZnO NPs at concentration of 50, 100 μg/ml showed only 49% and 33% of cell viability respectively in colon cancer cells, The toxic effect to colon cancer cells by ANPs showed 65% and 41% of cell viability respectively at concentration of 50, 100 μg/ml .
Figure 4.
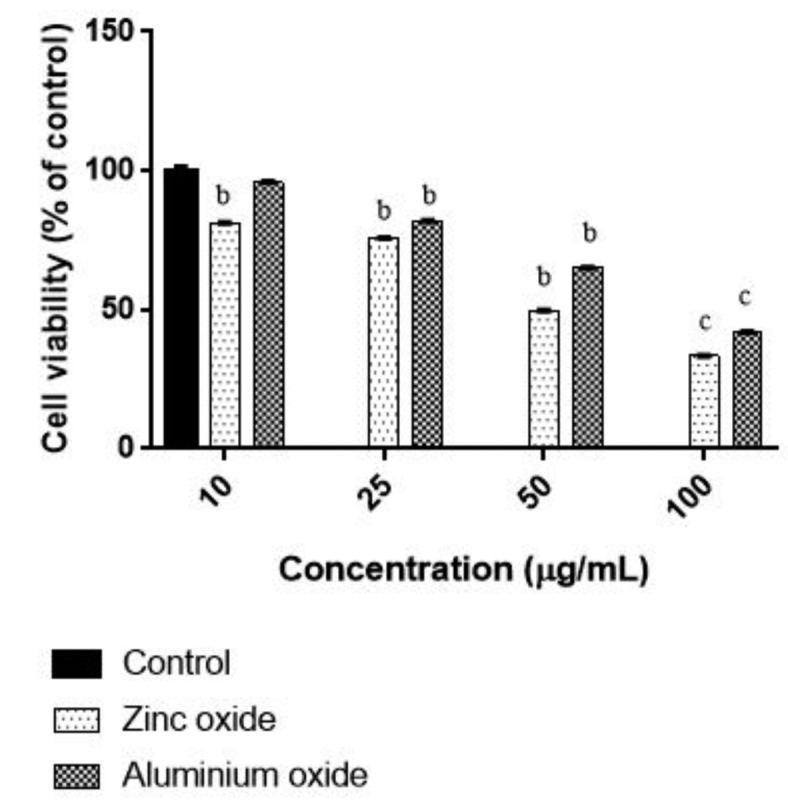
Concentration dependent Cytotoxicity of ZnO NPs and ANPs in HT 29 cells. Cells were exposed to different concentrations of ZnO NPs and ANPs for 48 h and the viability was determined by MTT assay. Unexposed cells as a control and run it in parallel to the exposed groups. Values were the mean±SD from three independent experiments. Significance was indicated by bp<0.001 and cp<0.01 verus control
ROS generation contribute to cell killing mechanism of ZnO NPs and ANPs in cancer cells. However, the induced morphological changes by treatment with ANPs were not observed in the present study. To explore the effect of ZnO-NPs and ANPs on membrane integrity, LDH leakage assay was performed. Normally, even minor damage to the plasma membrane can lead to the release of LDH enzyme throughout the cytosol. For LDH leakage study, colon carcinoma cells incubated with ZnO-NPs and ANPs and the results are represented in Figure 5. The highest LDH release was detected from colon carcinoma cells treated with ZnO-NPs (50 and 100 μg/ml) for 48 h. The LDH release caused by ZnO-NPs was found to be in a dose dependent manner of nanoparticles treatment. These results reveal that ZnO-NP is highly effective to kill colon cancer cells when its compared to ANPs treatment.
Figure 5.
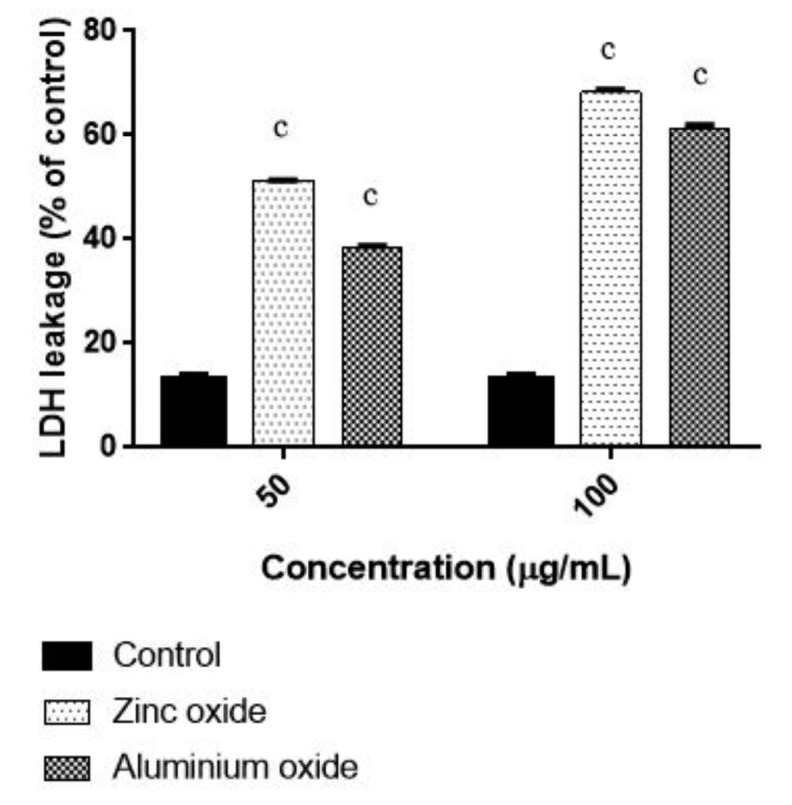
Effect of ZnO NPs and ANPs on LDH leakage in HT 29 cells. Cells were exposed to 50 and 100 μg/mL for 48 h. Control cells cultured in nanoparticles free medium run in parallel to the exposed groups. Values were the mean±SD from three independent experiments. Significance was indicated by cp<0.01 verus control
ZnO-NPs and ANPs (50 and 100 μg/ml) induced oxidative stress, was assessed by determining the lipid peroxidation level. Figure 6 shows induced by increased level of lipid peroxidation in colon cancer cells. Alarifi et al also reported the similar result with heptocarcinoma cells (14). The long-term cytotoxicity of ZnO-NPs and ANPs was studied by clonogenic assay, which was employed to determine the capability of a single cell to grow into a colony. ZnO-NPs treatment affected the cell survival of colon cancer cells. The colony formation was reduced about 60% in the concentration range of 100 μg/ml. The difference in ROS production was found to be statistically significant between the treatments which contribute the cell killing of ZnO NP in cancer cells.
Figure 6.
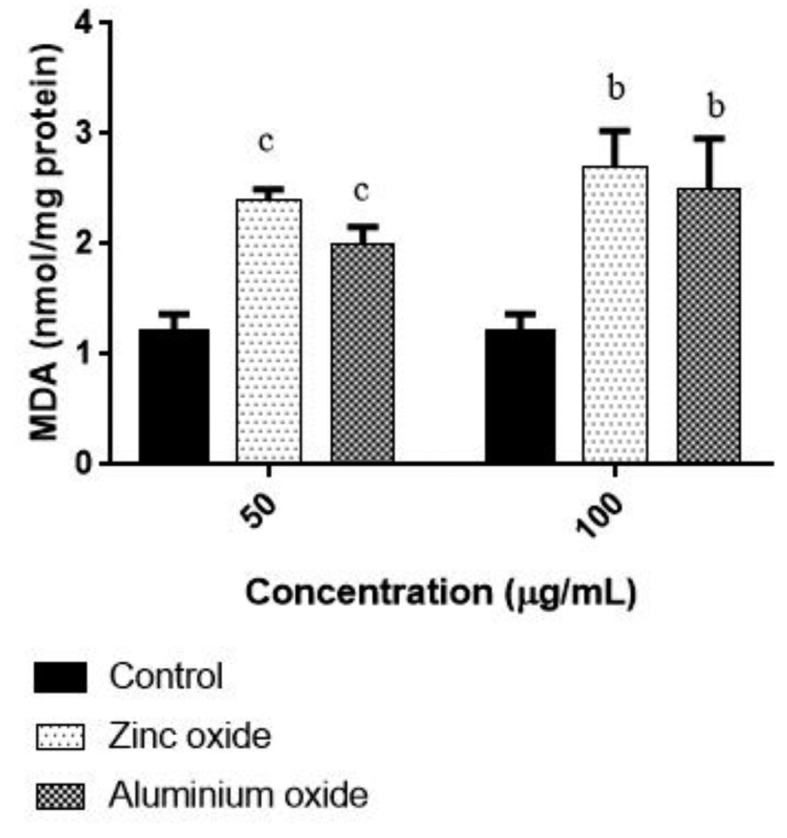
Effect of ZnO NPs and ANPs on MDA level in HT 29 cells. Cells were exposed to 50 and 100 μg/mL for 48 h. Control cells cultured in nanoparticles free medium run in parallel to the exposed groups. Values were the mean±SD from three independent experiments. Significance was indicated by bp<0.001 and cp<0.01 verus control
Conclusions
Anti cancer effect of ZnO NPs and ANPs was evaluated in human colon carcinoma cells. Both ZnO NPs and ANPs showed reduced cell proliferation. LDH leakage and colony formation were assessed and the results also supports the anti-proliferative effects of ZnO NPs and ANPs. However, as compared to ANPs, ZnO NPs is a potential anti-proliferative agent in case of reducing cellular growth in colon carcinoma cells. Although, mechanistic studies of the action of ZnO NPs on more human colon carcinoma cells, may throw more light on its potential anti cancer activity.
Acknowledgements
The authors are thankful to Chettinad Academy of Research and Education (CARE), Chennai, India for the research facilities and support.
Funding:
This study was partially financially supported by grants from Chettinad Hospital & Research Institute (CHRI), Chettinad Academy of Research and Education (CARE), Kelambakkam, Chennai-603103, India.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Serpone N, Dondi D, Albini A. Inorganic and organic UV filters: Their role and efficacy in sunscreens and suncare products. Inorg. Chim Acta. 2007;360:794–802. [Google Scholar]
- 2.Palanikumar L, Ramasamy S, Hariharan G, Balachandran C. Influence of particle size of nano zinc oxide on the controlled delivery of Amoxicillin. Appl Nanosci. 2013;3(5):441–451. [Google Scholar]
- 3.Banerjee A, Pathak S, Vimala DS, Vasan D, Murugesan R, Verma RS. Strategies for targeted drug delivery in treatment of colon cancer: current trends and future perspectives. Drug Discovery Today. 2017;22:1224–1232. doi: 10.1016/j.drudis.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Guan R, Kang T, Lu F, Zhang Z, Shen H, Liu M. Cytotoxicity, oxidative stress, and genotoxicity in human hepatocyte and embryonic kidney cells exposed to ZnO nanoparticles. Nanoscale res Let. 2012;7(1):602–609. doi: 10.1186/1556-276X-7-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahu D, Kannan GM, Tailang M, Vijayaraghavan R. In Vitro Cytotoxicity of Nanoparticles: A Comparison between Particle Size and Cell Type. J Nanosci 2016. 2016 [Google Scholar]
- 6.Ahamed M, Akhtar MJ, Raja M, et al. ZnO nanorod-induced apoptosis in human alveolar adenocarcinoma cells via p53, survivin and bax/bcl-2 pathways: role of oxidative stress. Nanomed Nanotechnol Biol Med. 2011;7(6):904–913. doi: 10.1016/j.nano.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Wahab R, Dwivedi S, Umar A, et al. ZnO nanoparticles induce oxidative stress in Cloudman S91 melanoma cancer cells. J biomed Nanotech. 2013;9(3):441–449. doi: 10.1166/jbn.2013.1593. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharyya S, Kudgus RA, Bhattacharya R, Mukherjee P. Inorganic nanoparticles in cancer therapy. Pharma Res. 2011;28(2):237–259. doi: 10.1007/s11095-010-0318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinardell MP, Mitjans M. Antitumor activities of metal oxide nanoparticles. Nanomat. 2015;5(2):1004–1021. doi: 10.3390/nano5021004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alarifi, Saud, Ali D, Alkahtani S. Nanoalumina induces apoptosis by impairing antioxidant enzyme systems in human hepatocarcinoma cells. Int J nanomed. 2015;10:3751. doi: 10.2147/IJN.S82050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanley C, Layne J, Punnoose A, et al. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotech. 2008;19(29):295103–295114. doi: 10.1088/0957-4484/19/29/295103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Wingett D, Engelhard MH, et al. Fluorescent dye encapsulated ZnO particles with cell-specific toxicity for potential use in biomedical applications. J material sci mater med. 2009;20(1):1409–1420. doi: 10.1007/s10856-008-3541-z. [DOI] [PubMed] [Google Scholar]
- 13.Baldo F, Martín A, Ortega FG, et al. Nanostructured platform integrated into a microfluidic immunosensor coupled to laser-induced fluorescence for the epithelial cancer biomarker determination. Microchem J. 2016;128:18–25. [Google Scholar]
- 14.Akhtar MJ, Ahamed M, Kumar S, et al. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int J Nanomedicine. 2012;7:845–857. doi: 10.2147/IJN.S29129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of immunological methods. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 16.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods in enzymology. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]