Abstract
Background
Overall survival (OS) for patients with uveal melanoma (UM) hepatic metastases is extremely poor. Therefore, stabilization of hepatic metastases is essential to prolonging OS.
Purpose
To assess the safety and effectiveness of radioembolization (RE) for treatment of UM hepatic metastases.
Materials and Methods
Enrollment for this prospective phase II trial began November 2011 and concluded January 2017. Treatment-naïve participants (group A) and participants who progressed after immunoembolization (group B) with hepatic tumor burden less than 50% underwent RE. Participants were followed for 1 month and every 3 months for acute and delayed toxicities, respectively. MRI, CT, and PET were performed every 3 months to evaluate for tumor response and extrahepatic disease. Participants were followed for at least 2 years or until death. Kaplan-Meier method and multivariable Cox proportional hazard models were used for data analysis.
Results
In group A, 24 participants (mean age ± standard deviation, 59 years ± 13; 13 men and 11 women) underwent unilobar (n = 7), fractionated whole-liver (n = 1), or sequential lobar (n = 16) RE. One participant was excluded from the trial. Complete response (n = 0), partial response (n = 9), or stable disease (n = 11) was achieved in 20 of 23 (87.0%; 95% confidence interval [CI]: 66.4%, 97.2%) participants. Median progression-free survival from liver metastasis was 8.1 months (95% CI: 6.4, 11.8; range, 3.3–33.7 months). Median OS was 18.5 months (95% CI: 11.3, 23.5; range, 6.5–73.7 months). In group B, 24 participants (mean age, 58 years ± 10; nine men and 15 women) underwent unilobar (n = 5) or sequential lobar (n = 19) RE. Complete response (n = 0), partial response (n = 8), or stable disease (n = 6) was achieved in 14 of 24 (58.3%; 95% CI: 36.3%, 77.9%) participants. Median progression-free survival from liver metastasis was 5.2 months (95% CI: 3.7, 9.8; range, 2.9–22.0 months). Median OS was 19.2 months (95% CI: 11.5, 24.0; range, 4.8–76.6 months). Grade 3 treatment-related toxicities included transient lymphopenia (group A, n = 1; group B, n = 1), pain (group A, n = 2) and nausea or vomiting (group A, n = 1).
Conclusion
Radioembolization is a promising treatment for patients with uveal melanoma hepatic metastases.
© RSNA, 2019
Summary
The results of a prospective phase II trial suggest that radioembolization is a safe and effective first- or second-line treatment for uveal melanoma hepatic metastases.
Key Results
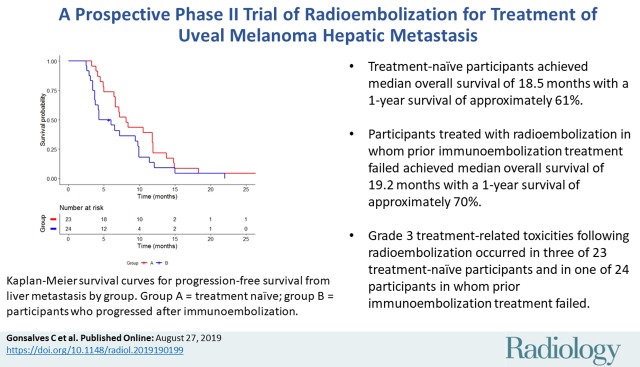
■ In a prospective phase II trial of radioembolization for treatment of uveal melanoma hepatic metastases, treatment-naïve participants (n = 24) achieved median overall survival of 18.5 months with a 1-year survival of approximately 61%.
■ Participants treated with radioembolization in whom prior immunoembolization treatment failed (n = 23) achieved median overall survival of 19.2 months with a 1-year survival of approximately 70%.
■ Grade 3 treatment-related toxicities following radioembolization occurred in three of 23 treatment-naïve participants and in one of 24 participants in whom prior immunoembolization treatment failed.
Introduction
Uveal melanoma (UM) is the most common primary malignant intraocular tumor in adults with an incidence of 5.1 cases per million in the United States (1–3). Up to 50% of patients with UM develop metastatic disease (3). The liver is the primary site of metastasis in greater than 90% of patients, and less than 10% of patients are candidates for surgical resection due to multiplicity of tumors at the time of diagnosis (3). Currently, there are no effective systemic therapies for patients with metastatic UM. In general, without treatment, median overall survival (OS) following detection of hepatic metastases is reportedly less than 6 months, with a 1-year survival of 10%–15% (3). Most commonly, patient death is due to growth of hepatic metastases ultimately leading to hepatic failure. Therefore, stabilization of liver metastases is essential to prolonging OS for patients with metastatic UM. Transarterial catheter–directed therapies used to control the growth of UM hepatic metastases include immunoembolization, chemoembolization, and percutaneous hepatic perfusion (4–15). A few retrospective studies have also reported encouraging results following radioembolization (RE) of UM hepatic metastases (4–7). In 2009, a small multicenter trial reported a 1-year survival of 80% for patients with UM treated with RE (4). In 2011, a median OS of 10.0 months was reported for patients treated with RE following failure of both immunoembolization and chemoembolization (5). In addition, a larger retrospective trial reported a median OS of 12.3 months following RE of 71 patients with UM hepatic metastases (6). The purpose of our study was to evaluate, in a prospective manner, the safety and effectiveness of RE for the treatment of UM hepatic metastases.
Materials and Methods
Our prospective, two-parallel arm, phase II trial (ClinicalTrials.gov identifier: NCT01473004) was conducted under the investigational device exemption (no. G110153) approved by the U.S. Food and Drug Administration. Our study was approved by the institutional review board and was compliant with the Health Insurance Portability and Accountability Act. Written consent was obtained for each participant. Funding for research staff was provided by Sirtex Medical (Woburn, Mass). Insurance coverage was required for imaging and laboratory studies, clinic visits, and procedures. Authors had control of the data and information submitted for publication. Our study protocol is available in Appendix E1 (online).
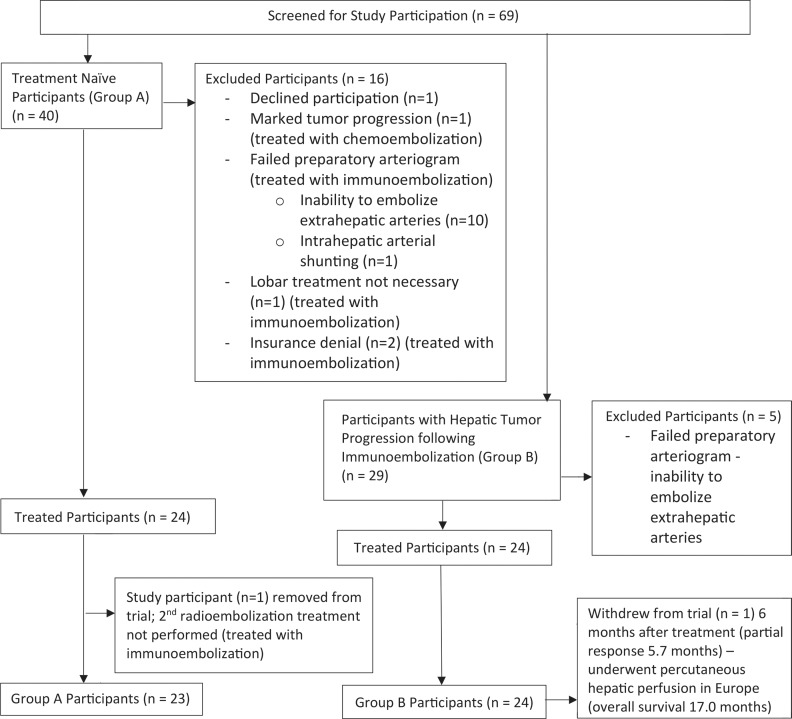
Patients presenting to our multidisciplinary metastatic UM clinic between November 2011 and January 2017 were evaluated for trial eligibility (Fig 1). Participants were enrolled into one of two study groups. Group A included treatment-naïve participants (no prior liver-directed and systemic therapies) and group B included participants who experienced hepatic tumor progression following immunoembolization. Inclusion criteria required histologically confirmed UM hepatic metastases (≥1 cm) and an Eastern Cooperative Oncology Group performance status of less than or equal to 1. Exclusion criteria included extrahepatic metastasis requiring systemic treatment, hepatic tumor burden greater than 50%, bilirubin and/or creatinine level greater than 1.8 mg/dL, uncorrectable arterial shunting to extrahepatic structures, lung shunting greater than 20%, portal vein thrombosis, biliary obstruction, previous radiation therapy to the liver, and severe contrast material allergy refractory to pretreatment steroids.
Figure 1:
Flowchart shows study participants in group A (treatment-naïve participants with no prior liver-directed and systemic therapies) and group B (participants who experienced hepatic tumor progression following immunoembolization).
Participants underwent baseline imaging (PET, CT, MRI) prior to initial RE treatment. Preparatory arteriograms and RE treatments were performed by board-certified interventional radiologists (R.D.A., C.F.G., and D.J.E., with 7 years, 20 years, and 26 years of experience, respectively). Yttrium 90 resin microsphere activity was calculated by a radiation oncologist (P.R.A., with 24 years of experience) by using the body surface area method (16). Activity delivered to normal whole-liver hepatic parenchyma was limited to less than or equal to 35 Gy per participant to prevent radiation-induced liver disease (17,18). The calculated activity was reduced by 20% for participants in group B because of prior immunoembolization treatments. Participants underwent unilobar, fractionated whole-liver, or sequential lobar treatments separated by 3–5 weeks. Procedure-related complications were classified by using the Society of Interventional Radiology classification system of complications by outcome (19). Proton pump inhibitors were used as gastrointestinal prophylaxis for 4 weeks following RE. Oral steroids (4 mg of dexamethasone twice per day for 3 days followed by 4 mg per day for 3 days) were prescribed to reduce inflammation of hepatic parenchyma. Participants were prescribed oral analgesics and antiemetics to use as needed.
Participants were evaluated for 1 month and every 3 months for acute and delayed toxicities, respectively. History and physical examinations were performed by an interventional radiologist (C.F.G., D.J.E., R.D.A.), a medical oncologist (T.S. and M.O., with 39 years and 4 years of experience, respectively) and/or a radiation oncologist (P.R.A.). Laboratory blood tests were performed every week for 1 month, every 2 weeks for 2 months, and every month thereafter.
Chest, abdominal, and pelvic CT were performed 1 month following treatment to help detect extrahepatic disease. CT, PET, and MRI were performed every 3 months for surveillance of extrahepatic metastases and assessment of hepatic tumor response. MRI was performed 6 weeks in between the 3-month imaging protocol to confirm tumor response if concerns were raised by our trial physicians. Best radiographic response of hepatic metastases was determined by using Response Evaluation Criteria in Solid Tumors, version 1.0 (20) (Table E1 [online]). Participants were followed for at least 2 years or until death.
A primary study objective was assessment of clinical response. A clinical benefit rate (complete response + partial response + stable disease) of greater than or equal to 60% was the intended goal for each study group to decrease the probability of concluding treatment ineffectiveness. RE was considered ineffective if the clinical benefit rate was less than or equal to 30%, but considered effective if the clinical benefit rate was greater than 30%. A Simon optimal two-stage design (α = .05, β = .20) was applied separately to each participant group to stop the trial early if the actual clinical benefit rate was less than or equal to 30%. Stage I enrolled eight participants into each study group. If three or fewer participants failed to achieve a clinical benefit in stage I, then enrollment into the study group would stop. If greater than four participants achieved a clinical benefit, then the study would advance to stage II. In stage II, if fewer than or equal to 10 of 24 participants failed to achieve a clinical benefit, then enrollment into the study group would conclude. With this design, there was no more than a 5% chance of concluding effectiveness (clinical benefit rate ≥60%) of RE when the clinical benefit rate was less than or equal to 30%. Similarly, there was no more than a 20% chance of concluding ineffectiveness (clinical benefit rate ≤30%) of RE when the clinical benefit rate was considered effective. If the clinical benefit rate was less than or equal to 30%, then we had a probability of at least 0.81 that the trial would conclude after the first eight participants. If the clinical benefit rate was greater than or equal to 60%, then the probability that the trial would stop during stage I was 0.17. The overall power of our study was 80.6%.
Another primary study objective was assessment of treatment safety. Study end points included serious treatment-related adverse events and sustained grade 3–4 laboratory abnormalities that precluded or delayed additional treatment. Adverse events were classified by using Common Terminology Criteria for Adverse Events, version 3.0 (21). Secondary study objectives included determination of OS, progression-free survival from liver metastasis (PFS), and progression-free survival from systemic metastasis (Table E2 [online]).
Statistical Analysis
OS was measured from initial RE treatment until death. PFS and progression-free survival from systemic metastasis were measured from initial RE treatment to progression of hepatic and systemic metastasis, respectively. Clinical benefit rates were estimated with corresponding 95% confidence intervals (CIs) separately for each group. The Atkinson and Brown method was used to adjust for the two-stage design (22). Survival outcomes were determined by using the Kaplan-Meier method. Multivariable Cox proportional hazard models were used to evaluate association between survival outcomes and known risk factors, including age (≤60 years vs >60 years), sex, largest primary tumor diameter, and hepatic tumor burden (<20% vs 20% to <50%) (9,10). This model allowed for different hazard rates in each group. The proportional hazards assumption was evaluated by using Schoenfeld residuals. In case of violations of the proportional hazards assumption, the corresponding covariate was incorporated as strata, which corresponds to estimating different baseline hazard functions that are not obligated to be proportional. P < .05 was considered to indicate statistical significance. SAS software (version 9.4; SAS Institute, Cary, NC) was used for data analysis.
Results
Demographics
Group A included 24 treatment-naïve participants (mean age ± standard deviation, 59 years ± 13; 13 men and 11 women) (Table 1). Largest primary eye tumor diameters (median, 15.0 mm; range, 11.0–24.0 mm) were available for 20 of 24 (83.3%) participants. At baseline MRI, hepatic tumor burdens less than 20% and 20% to less than 50% were demonstrated in 18 of 24 (75%) and six of 24 (25%) participants, respectively. Two participants had asymptomatic osseous metastases prior to treatment.
Table 1:
Participant Demographics by Group
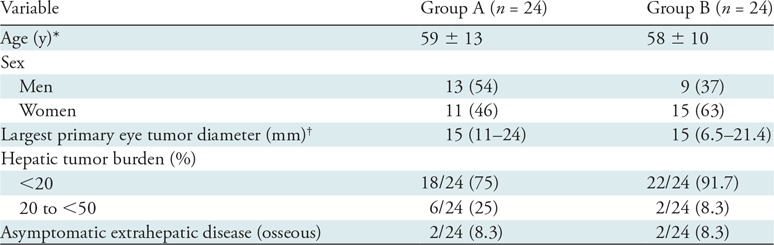
Note.—Unless otherwise specified, data are the number of participants, with percentages in parentheses. Group A includes treatment-naïve participants (no prior liver-directed and systemic therapies) and group B includes participants who experienced hepatic tumor progression following immunoembolization.
* Data are means ± standard deviation.
† Data are medians, with ranges in parentheses.
Group B included 24 participants who progressed following immunoembolization (mean age, 58 years ± 10; nine men and 15 women) (Table 1). Largest primary eye tumor diameters (median, 15.0 mm; range, 6.5–21.4 mm) were available in 22 of 24 (91.7%) participants. At baseline MRI, hepatic tumor burdens less than 20% and 20% to less than 50% were present in 22 of 24 (91.7%) and two of 24 (8.3%) participants, respectively. Two participants had asymptomatic osseous metastases prior to RE.
Excluded Participants
Twenty-one participants were excluded from our trial (Fig 1). In group A, two participants were denied insurance coverage. Eleven participants were deemed inappropriate candidates for RE due to intrahepatic arterial shunting (n = 1) and inability to embolize accessory duodenal (n = 5), right gastric (n = 4), and hepatic falciform (n = 1) arteries. One participant demonstrated marked progression of disease at baseline MRI and was treated with chemoembolization. Another participant demonstrated variant hepatic arterial anatomy and required segmental treatment of the right hepatic lobe. In addition, one participant signed consent but did not pursue treatment at our institution. In group B, the preparatory arteriogram failed in five participants due to inability to embolize accessory duodenal (n = 4) and right gastric (n = 1) arteries.
Radioembolization Treatments
Group A.—Consecutive fractionated whole-liver (n = 1), sequential lobar (n = 16), and unilobar (n = 7) treatments were performed by using a median radioactive microsphere activity of 32.6 mCi (1206.2 MBq) per participant (range, 17.7–56.1 mCi [654.9–2075.7 MBq]). Arterial stasis prevented administration of the prescribed activity in 11 of 41 (26.8%) treatments (right [seven of 11, 63.6%] and left [four of 11, 33.3%] hepatic lobes). Median activity delivered was 90% (range, 66%–96%) of the prescribed dose. Median time interval between baseline MRI and initial RE treatment was 10 days (range, 1–24 days). Median time interval between baseline MRI and treatment of the contralateral lobe, when applicable, was 38 days (range, 22–52 days). One participant was removed from our study at the time of contralateral lobar treatment due to arteriographic findings precluding safe administration of radioactive microspheres. Toxicities associated with this participant’s single lobar treatment were included in our data analysis.
Group B.—Unilobar (n = 5) or sequential lobar (n = 19) treatments were performed with a median radioactive microsphere activity of 35.0 mCi (1295 MBq) per participant (range, 19.2–50.8 mCi [710.4–1879.5 MBq]). Arterial stasis prevented administration of the prescribed activity in four of 43 (9.3%) treatments (right [two of four, 50%] and left [two of four, 50%] hepatic lobes). Median activity delivered was 80% (range, 73%–98%) of the prescribed dose. Median time interval between baseline MRI and first RE treatment was 13 days (range, 1–26 days). If applicable, median time interval between baseline MRI and treatment of the contralateral lobe was 42 days (range, 24–54 days).
Survival Outcome and Treatment Response
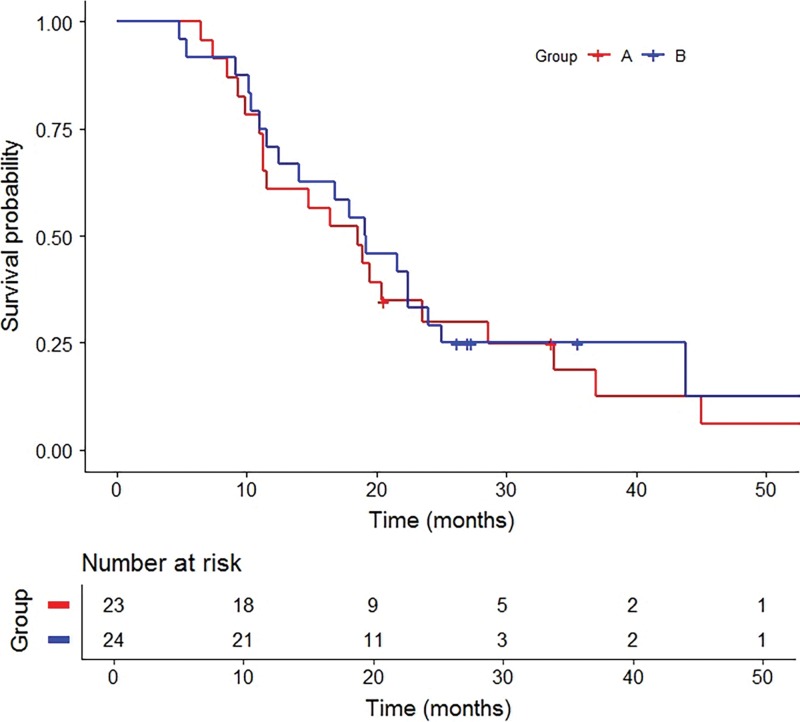
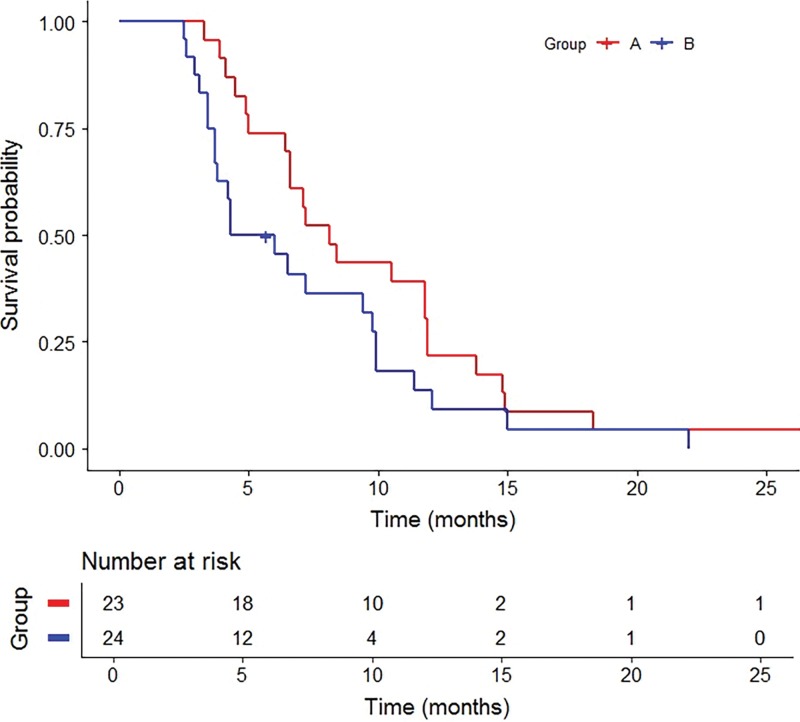
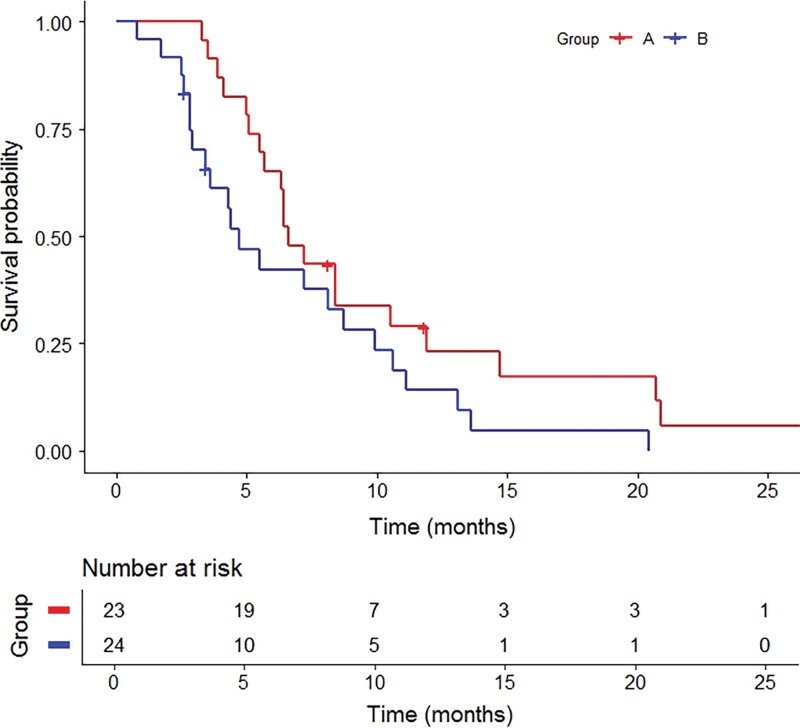
Group A.—Best radiographic response included partial response (nine of 23, 39.1%), stable disease (11 of 23, 47.8%), and progression of disease (three of 23, 13.0%) (Table 2). No participants achieved a complete response. The clinical benefit rate was 87.0% (95% CI: 66.4%, 97.2%). Median OS following RE was 18.5 months (95% CI: 11.3, 23.5; range, 6.5–73.7 months) with three surviving participants (range, 20.6–73.7 months) (Fig 2). Survivals at 1 year, 2 years, and 3 years were 60.9% (14 of 23), 26.1% (six of 23), and 13.0% (three of 23), respectively. Median PFS was 8.1 months (95% CI: 6.4, 11.8; range, 3.3–33.7 months) (Fig 3). Progression of nontarget and target and nontarget lesions occurred in 21 of 23 (91.3%) and two of 23 (8.7%) participants, respectively. New hepatic tumors were detected in all group A participants when progression of disease was ultimately diagnosed. Extrahepatic disease developed or progressed in 21 of 23 (91.3%) participants at a median of 6.6 months (95% CI: 5.5, 10.5; range, 3.3–33.7 months) (Fig 4).
Table 2:
Summary of Treatment Response and Survival: Results of Univariable Analysis
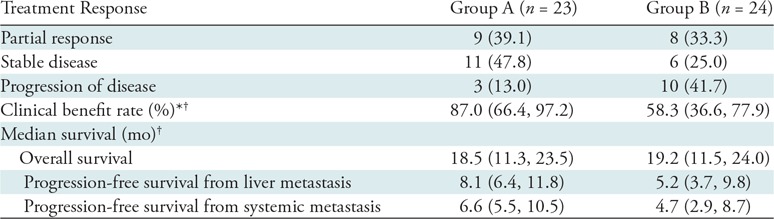
Note.—Unless otherwise specified, data are the number of participants, with percentages in parentheses. Group A includes treatment-naïve participants (no prior liver-directed and systemic therapies) and group B includes participants who experienced hepatic tumor progression following immunoembolization.
* Rate was calculated as follows: complete response + partial response + stable disease.
† Data in parentheses are 95% confidence intervals.
Figure 2:
Graph shows Kaplan-Meier curves for overall survival by group. Group A includes treatment-naïve participants with no prior liver-directed and systemic therapies and group B includes participants who experienced hepatic tumor progression following immunoembolization.
Figure 3:
Graph shows Kaplan-Meier survival curves for progression-free survival from liver metastasis by group. Group A includes treatment-naïve participants with no prior liver-directed and systemic therapies and group B includes participants who experienced hepatic tumor progression following immunoembolization.
Figure 4:
Graph shows Kaplan-Meier survival curves for progression-free survival from extrahepatic metastasis by group. Group A includes treatment-naïve participants with no prior liver-directed and systemic therapies and group B includes participants who experienced hepatic tumor progression following immunoembolization.
Group B.—Best radiographic response included partial response (eight of 24, 33.3%), stable disease (six of 24, 25.0%), and progression of disease (10 of 24, 41.7%) (Table 2). No participants achieved a complete response. The clinical benefit rate was 58.3% (95% CI: 36.3%, 77.9%). One participant withdrew from our trial to pursue percutaneous hepatic perfusion in Europe. Median OS following RE was 19.2 months (95% CI: 11.5, 24.0; range, 4.8–76.6 months) with five surviving participants (range, 26.2–76.6 months) (Fig 2). Survivals at 1 year, 2 years, and 3 years were 69.6% (16 of 24), 30.4% (seven of 24), and 8.7% (two of 24), respectively. Median OS from initial immunoembolization treatment was 24.7 months (range, 11.5–90.5 months). Median PFS was 5.2 months (95% CI: 3.7, 9.8; range, 2.9–22.0 months) (Fig 3). Progression of nontarget, target and nontarget, and target lesions occurred in 18 of 24 (78.3%), three of 24 (13.0%), and two of 24 (8.7%) participants, respectively. Most participants (21 of 24, 91.3%) developed new hepatic tumors when progression of disease was diagnosed. Extrahepatic disease developed in 22 of 24 (91.7%) participants (median, 4.7 months; 95% CI: 2.9, 8.7; range 0.8–20.4 months) (Fig 4). The participant who withdrew from our trial developed extrahepatic disease 1 month following treatment and was included in this analysis.
Treatment-related Toxicities and Adverse Events
Treatment-related toxicities are outlined in Table 3. No major procedure-related complications occurred in either group.
Table 3:
Cumulative Highest Grade of Toxicity
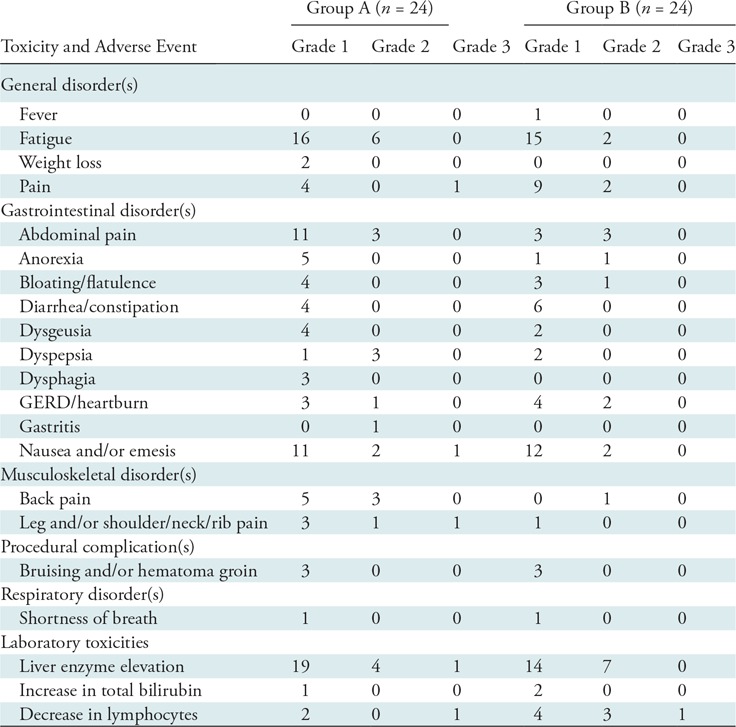
Note.—No grade 4 toxicities occurred. Group A includes treatment-naïve participants (no prior liver-directed and systemic therapies) and group B includes participants who experienced hepatic tumor progression following immunoembolization. GERD = gastroesophageal reflux disease.
Group A.—Most common toxicities included fatigue (22 of 24, 91.7%), abdominal pain (14 of 24, 58.3%), and nausea or vomiting (14 of 24, 58.3%). Three participants developed grade 3 treatment-related toxicities (nausea or vomiting [n = 1], pain [n = 2]), which did not delay or prevent subsequent treatments. Transient laboratory abnormalities occurred in a majority of participants including grade 1 (19 of 24, 79.2%) and grade 2 (four of 24, 16.7%) liver enzyme elevation. One participant developed delayed grade 3 liver enzyme elevation due to excessive alcohol use, which normalized following behavior modification. A transient grade 3 lymphopenia occurred in one participant following treatment.
Group B.—Most common toxicities included fatigue (17 of 24, 70.8%) and nausea or vomiting (14 of 24, 58.3%). Grade 1 (14 of 24, 58.3%) and grade 2 (seven of 24, 29.2%) liver enzyme elevation occurred in a majority of participants. A transient grade 3 lymphopenia occurred in one participant.
Multivariable Analysis
Results from multivariable Cox proportional hazard models are shown in Table 4. For consistency, all Cox models included predictors for at least one survival outcome such as study group, diameter of largest primary eye tumor, age, and tumor burden. The hazard of death was not different between the study groups (P = .91), but was 11% greater with every 1-mm increase in diameter of primary eye tumor (hazard ratio, or HR, 1.1; 95% CI: 1.0, 1.2; P = .047), and 3.9 times (HR, 3.9; 95% CI: 1.5, 10.6; P = .01) greater for hepatic tumor burden 20% to less than 50% compared with hepatic tumor burden less than 20%. The hazard of liver metastasis progression was not different based on age (≤60 years vs >60 years; P = .81), but was 17% greater with every 1-mm increase in diameter of primary eye tumor (HR, 1.2; 95% CI: 1.1, 1.3; P = .002), and 7.2 times (HR, 7.2; 95% CI: 2.3, 22.1; P = .001) greater for hepatic tumor burden 20% to less than 50% compared with hepatic tumor burden less than 20%. The hazard of systemic progression was not associated with diameter of largest primary eye tumor (P = .08), but was 2.6 times (HR, 2.6; 95% CI: 1.2, 5.2; P = .01) greater for patients older than 60 years and 7.8 times (HR, 7.8; 95% CI: 2.6, 23.1; P < .001) greater for hepatic tumor burden 20% to less than 50% compared with hepatic tumor burden less than 20%. In addition, group A had a 73% lower hazard of systemic disease progression (HR, 0.3; 95% CI: 0.1, 0.6; P = .001) compared with group B.
Table 4:
Results of Multivariable Cox Proportional Hazard Models
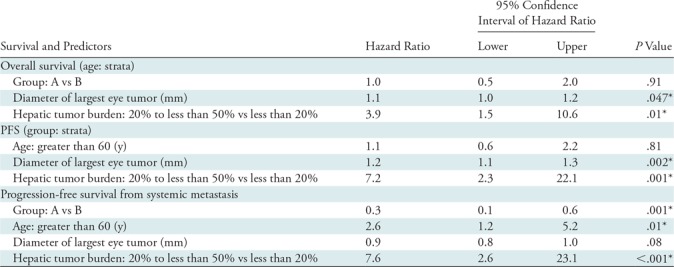
Note.— Group A includes treatment-naïve participants (no prior liver-directed and systemic therapies) and group B includes participants who experienced hepatic tumor progression following immunoembolization. PFS = progression-free survival from liver metastasis.
*Significant P value < .05.
Discussion
Overall survival (OS) for patients with metastatic uveal melanoma (UM) is largely dependent on the ability to control the progression of hepatic tumors. Currently, there are no effective systemic treatments for metastatic UM. Therefore, transarterial catheter–directed therapies have been used to stabilize hepatic metastases, thereby prolonging OS for patients with this aggressive disease (Table 5). Compared with historic control participants, our study participants experienced prolonged OS following radioembolization of UM hepatic metastases. A median OS of 18.5 months and 19.2 months with a 1-year survival of 60.9% and 69.6% was achieved in treatment-naïve participants and participants in whom prior immunoembolization treatment failed, respectively. These survival outcomes can be attributed to the stabilization of hepatic tumors in 87.0% and 58.3% of participants in groups A and B, respectively. Furthermore, progression-free survival from liver metastases (PFS) was 8.1 months and 5.2 months for group A and B participants, respectively. However, despite stabilization of hepatic tumors in a majority of our participants, greater than 90% of participants developed new hepatic and extrahepatic metastases following treatment. In addition, radioembolization was well tolerated by our study participants. There were no instances of radiation-induced liver disease and clinically significant treatment-related toxicities were transient and uncommon.
Table 5:
Summary of Overall Survival and PFS following Chemoembolization, Immunoembolization, Radioembolization, and Percutaneous Hepatic Perfusion

Note.—BCNU = 1,3-bis (2-chloroethyl)-1-nitrosourea, N/A = not available, PFS = progression-free survival from liver metastases.
OS outcomes achieved in our study compare favorably to those reported following immunoembolization, chemoembolization, and percutaneous hepatic perfusion of UM hepatic metastases. In a prospective phase I immunoembolization trial using granulocyte-macrophage colony-stimulating factor, Sato et al (8) reported a median OS of 14.4 months and a 1-year survival of 62.0%. In addition, a median OS of 21.5 months was reported by Valsecchi et al (10) in a subsequent phase II immunoembolization trial. Huppert et al (11) and Vogl et al (12) reported a median OS of 11.5 months and 19.5 months, respectively, following chemoembolization of UM hepatic metastases. In addition, a retrospective multicenter study by Karydis et al (15) reported a median OS of 15.3 months and a 1-year survival of 64.6% following percutaneous hepatic perfusion. To determine the most effective transarterial catheter–directed therapy for the treatment of UM hepatic metastases, a multicenter, prospective, randomized trial would be required.
PFS in our study participants were also comparable to those reported for other transarterial catheter–directed therapies (7–15). Following immunoembolization of UM hepatic metastases, Sato et al (8) and Valsecchi et al (10) reported median PFS of 4.8 months and 3.9 months, respectively. Furthermore, following chemoembolization, a median PFS of 8.5 months was reported by Huppert et al (11), and Karydis et al (15) reported a median PFS of 9.1 months following percutaneous hepatic perfusion. However, assessment of PFS may be imprecise for transarterial catheter–directed therapies requiring sequential lobar treatments, unlike percutaneous hepatic perfusion that allows whole-liver single-session treatments. In our trial, imaging studies used to assess PFS were performed a median of 38 days (group A) and 42 days (group B) prior to treatment of the contralateral hepatic lobe. Therefore, for aggressive tumors such as UM, this treatment delay may be responsible for the development of new tumors and growth of existing tumors in the untreated lobe, negatively impacting the accuracy of PFS. Ideally, contemporaneous imaging should be performed prior to each lobar treatment for better assessment of PFS.
Treatment-related toxicities experienced by our study participants were similar to toxicities previously reported for immunoembolization and chemoembolization (8–13). However, in our study, radioembolization had fewer treatment-related adverse events compared with percutaneous hepatic perfusion (14,15). Karydis et al (15) reported grade 3–4 hematologic (58.8%) and nonhematologic (37.5%) adverse events following percutaneous hepatic perfusion, which is not unexpected given the complexity of this particular treatment.
A majority of our study participants developed new hepatic and extrahepatic metastases regardless of treatment response. Studies have detected UM micrometastases in the bloodstream after successful treatment of the primary eye tumor (23,24). Therefore, the development of new metastases is not unexpected following radioembolization. Unfortunately, systemic immunotherapies such as immune checkpoint inhibitors, known for their effectiveness in metastatic cutaneous melanoma, have been studied in small retrospective and prospective metastatic UM trials with poor response rates and no survival benefit (25). A few retrospective studies have reported their results using transarterial catheter–directed therapies in combination with immune checkpoint inhibitors. Zheng et al (26) reported a median OS of 17.0 months for 11 patients who received various immune checkpoint inhibitors either before or after radioembolization of UM hepatic metastases. In addition, Karydis et al (15) combined percutaneous hepatic perfusion and immune checkpoint inhibitors in 60.7% (31 of 51) of their patients, which may have positively affected survival outcomes reported in their study. However, prospective randomized trials are necessary to determine the safety and effectiveness of combination local-regional and systemic immunotherapies for the treatment of metastatic UM.
A limitation of our trial included its small sample size. In addition, our study was not powered to compare potential differences between the two study groups. We also limited normal whole-liver parenchymal activity to a maximum of 35 Gy per participant based on reports of radiation-induced liver disease occurring in patients receiving greater than 35 Gy of external radiation to normal liver parenchyma (17,18). Although this likely prevented serious treatment-related adverse events, this may also have negatively affected our survival and tumor response outcomes. However, in our opinion, this cautious approach is essential to ensuring the safety of radioembolization. Furthermore, it is our assumption that the inherent imaging delay between lobar treatments negatively affected PFS outcomes in our study participants.
In conclusion, our study found radioembolization to be a safe and effective first- or second-line treatment for uveal melanoma (UM) hepatic metastases. However, additional research is necessary to identify treatments that may improve hepatic tumor response and prevent the development of extrahepatic disease. Currently, there is an ongoing multicenter trial using radioembolization (ClinicalTrials.gov identifier: NCT02913417) and a single institutional trial using immunoembolization (ClinicalTrials.gov identifier: NCT03472586), in combination with ipilimumab and nivolumab, studying the effectiveness of concomitant treatments on survival outcomes for patients with metastatic UM. Furthermore, a bispecific antibody known as IMCgp100 is a promising systemic treatment for metastatic UM. IMCgp100 strategically targets the melanocyte-associated antigen GP100 on UM surface membranes and simultaneously activates CD3-positive T-cells to destroy cancer cells (27). Initial phase I study results reported a 1-year survival of 73% (27). Future clinical trials combining transarterial catheter–directed therapy and IMCgp100 should be considered with the intent of improving survival outcomes for patients with metastatic UM.
APPENDIX
Supported by Sirtex Medical.
Disclosures of Conflicts of Interest: C.F.G. disclosed no relevant relationships. D.J.E. disclosed no relevant relationships. R.D.A. disclosed no relevant relationships. P.R.A. disclosed no relevant relationships. M.M.O. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Bristol-Myers Squibb and Immunocore; has grants/grants pending with Melanoma Research Alliance; received payment for lectures including service on speakers bureaus from Bristol-Myers Squibb; received payment for development of educational presentations from Bristol-Myers Squibb and Immunocore; institution received research funding from Bristol-Myers Squibb. Other relationships: disclosed no relevant relationships. M.T. disclosed no relevant relationships. A.N.H. disclosed no relevant relationships. M.Y. Activities related to the present article: institution received a grant from the National Institutes of Health. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. I.C. Activities related to the present article: institution received a grant from the National Institutes of Health. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. T.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a board member of IDEAYA Biosciences and Immunocore; is employed by Thomas Jefferson University; received payment for lectures including service on speakers bureaus from Immunocore. Other relationships: disclosed no relevant relationships.
Abbreviations:
- CI
- confidence interval
- OS
- overall survival
- PFS
- progression-free survival from liver metastasis
- RE
- radioembolization
- UM
- uveal melanoma
References
- 1.Singh AD, Topham A. Survival rates with uveal melanoma in the United States: 1973-1997. Ophthalmology 2003;110(5):962–965. [DOI] [PubMed] [Google Scholar]
- 2.Aronow ME, Topham AK, Singh AD. Uveal Melanoma: 5-Year Update on Incidence, Treatment, and Survival (SEER 1973-2013). Ocul Oncol Pathol 2018;4(3):145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh M, Durairaj P, Yeung J. Uveal melanoma: A review of the literature. Oncol Ther 2018;6(1):87–104 [Published correction appears in Oncol Ther 2019;7(1):93.] 10.1007/s40487-018-0056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy AS, Nutting C, Jakobs T, et al. A first report of radioembolization for hepatic metastases from ocular melanoma. Cancer Invest 2009;27(6):682–690. [DOI] [PubMed] [Google Scholar]
- 5.Gonsalves CF, Eschelman DJ, Sullivan KL, Anne PR, Doyle L, Sato T. Radioembolization as salvage therapy for hepatic metastasis of uveal melanoma: a single-institution experience. AJR Am J Roentgenol 2011;196(2):468–473. [DOI] [PubMed] [Google Scholar]
- 6.Eldredge-Hindy H, Ohri N, Anne PR, et al. Yttrium-90 Microsphere Brachytherapy for Liver Metastases From Uveal Melanoma: Clinical Outcomes and the Predictive Value of Fluorodeoxyglucose Positron Emission Tomography. Am J Clin Oncol 2016;39(2):189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klingenstein A, Haug AR, Zech CJ, Schaller UC. Radioembolization as locoregional therapy of hepatic metastases in uveal melanoma patients. Cardiovasc Intervent Radiol 2013;36(1):158–165. [DOI] [PubMed] [Google Scholar]
- 8.Sato T, Eschelman DJ, Gonsalves CF, et al. Immunoembolization of malignant liver tumors, including uveal melanoma, using granulocyte-macrophage colony-stimulating factor. J Clin Oncol 2008;26(33):5436–5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto A, Chervoneva I, Sullivan KL, et al. High-dose immunoembolization: survival benefit in patients with hepatic metastases from uveal melanoma. Radiology 2009;252(1):290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valsecchi ME, Terai M, Eschelman DJ, et al. Double-blinded, randomized phase II study using embolization with or without granulocyte-macrophage colony-stimulating factor in uveal melanoma with hepatic metastases. J Vasc Interv Radiol 2015;26(4):523–532.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huppert PE, Fierlbeck G, Pereira P, et al. Transarterial chemoembolization of liver metastases in patients with uveal melanoma. Eur J Radiol 2010;74(3):e38–e44. [DOI] [PubMed] [Google Scholar]
- 12.Vogl T, Eichler K, Zangos S, et al. Preliminary experience with transarterial chemoembolization (TACE) in liver metastases of uveal malignant melanoma: local tumor control and survival. J Cancer Res Clin Oncol 2007;133(3):177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bedikian AY, Legha SS, Mavligit G, et al. Treatment of uveal melanoma metastatic to the liver: a review of the M. D. Anderson Cancer Center experience and prognostic factors. Cancer 1995;76(9):1665–1670. [DOI] [PubMed] [Google Scholar]
- 14.Vogl TJ, Koch SA, Lotz G, et al. Percutaneous Isolated Hepatic Perfusion as a Treatment for Isolated Hepatic Metastases of Uveal Melanoma: Patient Outcome and Safety in a Multi-centre Study. Cardiovasc Intervent Radiol 2017;40(6):864–872. [DOI] [PubMed] [Google Scholar]
- 15.Karydis I, Gangi A, Wheater MJ, et al. Percutaneous hepatic perfusion with melphalan in uveal melanoma: A safe and effective treatment modality in an orphan disease. J Surg Oncol 2018;117(6):1170–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol 2006;17(8):1251–1278. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Jung Y. Radiation-induced liver disease: current understanding and future perspectives. Exp Mol Med 2017;49(7):e359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tam HH, Al-Nahhas A. Treatment of liver tumors with yttrium radioembolization. Clin Transl Imaging 2014;2(2):165–182. [Google Scholar]
- 19.Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 2003;14(9 Pt 2):S199–S202. [DOI] [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92(3):205–216. [DOI] [PubMed] [Google Scholar]
- 21.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13(3):176–181. [DOI] [PubMed] [Google Scholar]
- 22.Atkinson EN, Brown BW. Confidence limits for probability of response in multistage phase II clinical trials. Biometrics 1985;41(3):741–744. [PubMed] [Google Scholar]
- 23.Terai M, Mu Z, Eschelman DJ, et al. Arterial Blood, Rather Than Venous Blood, is a Better Source for Circulating Melanoma Cells. EBioMedicine 2015;2(11):1821–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bidard FC, Madic J, Mariani P, et al. Detection rate and prognostic value of circulating tumor cells and circulating tumor DNA in metastatic uveal melanoma. Int J Cancer 2014;134(5):1207–1213. [DOI] [PubMed] [Google Scholar]
- 25.Komatsubara KM, Carvajal RD. Immunotherapy for the treatment of uveal melanoma: current status and emerging therapies. Curr Oncol Rep 2017;19(7):45. [DOI] [PubMed] [Google Scholar]
- 26.Zheng J, Irani Z, Lawrence D, Flaherty K, Arellano RS. Combined Effects of Yttrium-90 Transarterial Radioembolization around Immunotherapy for Hepatic Metastases from Uveal Melanoma: A Preliminary Retrospective Case Series. J Vasc Interv Radiol 2018;29(10):1369–1375. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Manson DK, Marr BP, Carvajal RD. Treatment of uveal melanoma: where are we now? Ther Adv Med Oncol 2018 Feb 21;10:1758834018757175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.