Abstract
Advances in characterization of molecular and genomic abnormalities specific to lung cancer have made precision therapy the current standard of care for lung cancer treatment. This article will provide a cutting-edge review of imaging of lung cancer in the current era of precision medicine. The focus of the article includes (a) an update on the recent advances in precision therapy for non–small cell lung cancer and their implications on imaging; (b) molecular and genomic biomarkers and pitfalls of image interpretations for lung cancer precision therapy; and (c) review of the current approaches and future directions of precision imaging for lung cancer, emphasizing emerging observations in longitudinal tumor kinetics, radiomics, and molecular and functional imaging. The article is designed to help radiologists to remain up to date in the rapidly evolving world of lung cancer therapy and serve as key members of multidisciplinary teams caring for these patients.
© RSNA, 2019
Summary
The knowledge of the recent advances of precision lung cancer therapy and their impact on image-based diagnosis and treatment monitoring is essential for radiologists to continue to be key members of multidisciplinary lung cancer care.
Essentials
■ The precision medicine approaches to lung cancer continue to rapidly advance in the clinical practice setting.
■ Familiarity with pre- and posttherapy imaging characteristics of lung cancer treated with precision therapy is essential for accurate interpretation of imaging studies.
■ Imaging pitfalls of precision lung cancer therapy, including immune-related tumor response and drug toxicities, should be recognized.
Introduction
Characterization of tumor genomic abnormalities and clinical applications of anticancer agents that can effectively target these abnormalities have transformed treatment approaches to lung cancer in the past decade and have brought precision therapy into the mainstream of lung cancer care. The field of lung cancer therapy continues to rapidly advance, with recent important updates and further breakthroughs that are relevant to radiologists who contribute to diagnosis and treatment monitoring for patients with lung cancer. To provide up-to-date knowledge of precision lung cancer therapy and imaging, this article reviews the recent advances in precision therapy for lung cancer, focusing on strategies for overcoming acquired resistance, discoveries of novel targetable driver mutations, and clinical application of immune-checkpoint inhibitor (ICI) therapy. The latest advances in molecular and genomic biomarkers and their clinical application in lung cancer will then be reviewed, and the imaging pitfalls related to novel therapies will be discussed. Finally, updates and future directions for imaging of precision lung cancer therapy are discussed, featuring longitudinal tumor burden kinetics, radiomics and texture analyses, and molecular and functional imaging. The purpose of the article is to effectively communicate the state-of-the-art knowledge in lung cancer precision therapy and imaging essential for radiologists to continue contributing as key members of the rapidly evolving world of lung cancer.
Updates on the Recent Advances in Precision Lung Cancer Therapy
Background and Overview of Precision Lung Cancer Therapy
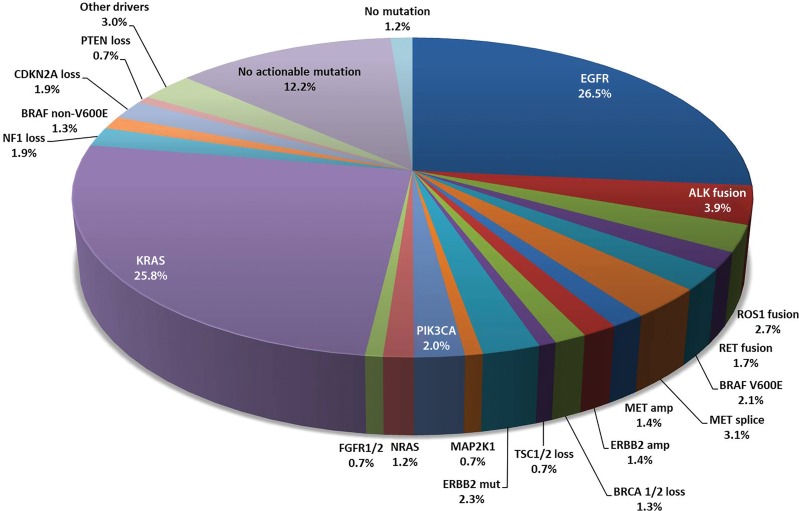
Discovery of oncogenic driver mutations and specific targeted therapy directed to these mutations have brought remarkable progress for treatment approaches in patients with advanced lung cancer in the past decade. The concept of precision medicine is now applied in daily practice, and further efforts are ongoing to identify new genomic targets for the development of novel agents, as well as to overcome acquired resistance to existing therapies (Fig 1) (1) (Table 1).
Figure 1:
Spectrum of oncogenic driver mutations in lung adenocarcinoma and their incidence rates. These oncogenic driver mutations are mostly mutually exclusive, except for rare exceptions. (Modified from reference 1.) ALK = anaplastic lymphoma kinase, Amp = amplification, BRAF = v-Raf murine sarcoma viral oncogene homolog B1, BRCA = breast cancer susceptibility gene, CDKN2A = cyclin-dependent kinase inhibitor 2A, EGFR = epidermal growth factor receptor, ERBB2 = erb-b2 receptor tyrosine kinase 2 (HER2), FGFR = fibroblast growth factor receptor, KRAS = v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog, MAP2K1 = dual specificity mitogen-activated protein kinase kinase 1, MET = mesenchymal-epithelial transition factor, Mut = mutation, NF = neurofibromin, NRAS = neuroblastoma RAS viral (v-ras) oncogene homolog, PIK3CA = phosphatidylinositol 3-kinase, PTEN = phosphatase and tensin homolog, RET = rearranged during transfection, ROS = ROS proto-oncogene 1, TSC = tuberous sclerosis.
Table 1:
Genomic Abnormalities in Lung Cancer and Effective Targeted Therapy Options
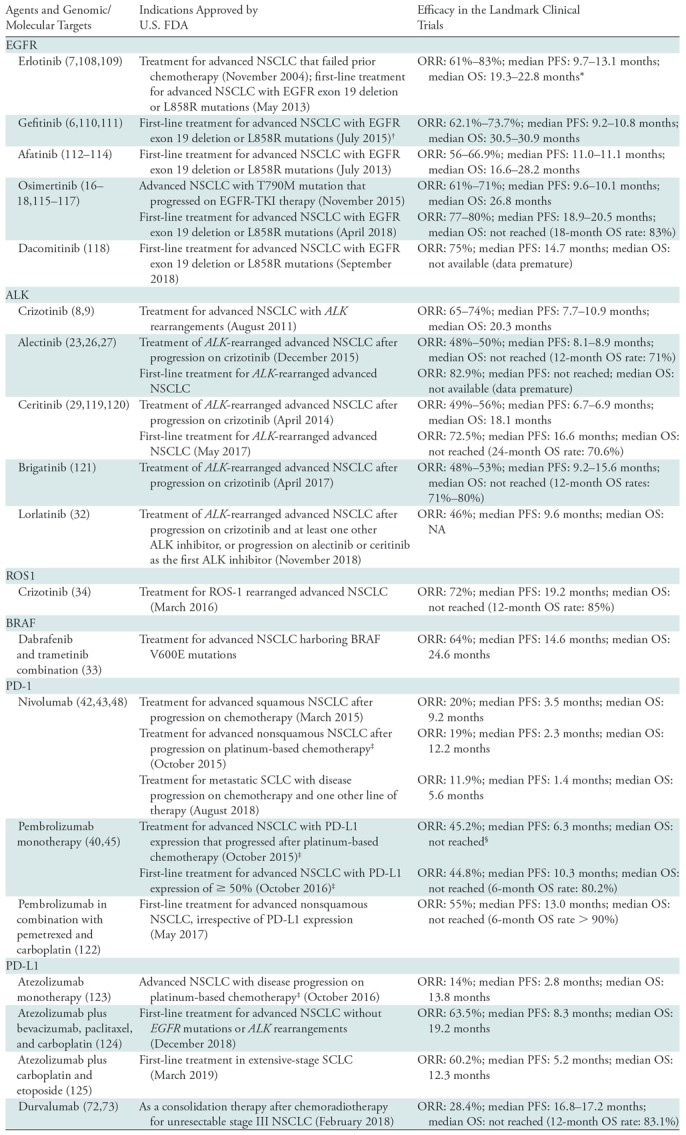
Note.—ALK = anaplastic lymphoma kinase, BRAF = v-Raf murine sarcoma viral oncogene homolog B1, EGFR = epidermal growth factor receptor, FDA = Food and Drug Administration, NSCLC = non–small cell lung cancer, ORR = overall response rate, OS = overall survival, PD-1 = programmed death-1, PD-L1 = programmed death-ligand 1, PFS = progression-free survival, ROS-1 = ROS proto-oncogene 1, SCLC = small cell lung cancer.
*The outcome data are based on trials of first-line erlotinib therapy for EGFR-mutant NSCLC.
†Initially approved in 2003, which was before the discovery of EGFR mutations in NSCLC as a biomarker for the therapy, leading to low effectiveness in patients without EGFR mutations and resulting in retraction of FDA approval in 2005.
‡The outcome data are based on a subcohort of patients with PD-L1 expression of ≥ 50% (40).
§After progression on an appropriate FDA-approved targeted therapy in patients with tumors harboring EGFR or ALK gene abnormalities.
Epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) rearrangements are two well-known oncogenic mutations in non–small cell lung cancer (NSCLC), especially in adenocarcinomas, and are representative examples of successful clinical application of precision lung cancer therapy (Fig 2). EGFR mutations have been reported in approximately 15% of patients in the United States and in 30%–50% of patients in Asia (2–5). The most frequently observed mutations are L858R in exon 21 and in-frame deletion mutations within exon 19. Erlotinib and gefitinib are the conventional tyrosine kinase inhibitors (TKIs) that have been used to effectively treat patients with EGFR-mutant advanced NSCLC (6,7). ALK rearrangements are noted in 3%–7% of patients with NSCLC. The ALK inhibitor crizotinib, which inhibits the marked activity of the ALK fusion proteins (8,9), was the first U.S. Food and Drug Administration (FDA)-approved drug for advanced NSCLC with ALK rearrangements (in 2011). On the basis of the successful application of these agents, precision therapy with genome-based selection of therapeutic agents has become one of the mainstream approaches to advanced lung cancer (especially nonsquamous NSCLC) and has formed a basis for further advances in recent years (10,11) (Table 1).
Figure 2a:
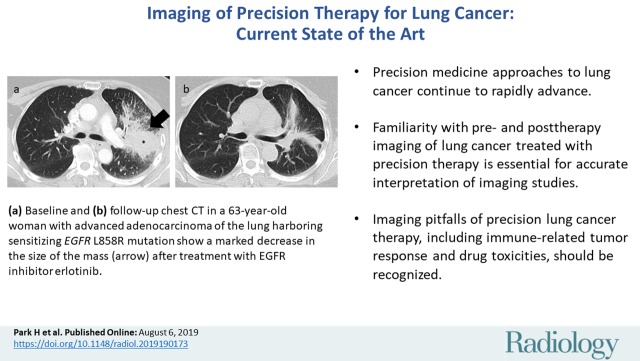
Images in 63-year-old woman with advanced adenocarcinoma of the lung harboring sensitizing epidermal growth factor receptor (EGFR) L858R mutation who was treated with the EGFR inhibitor erlotinib. (a) Baseline chest CT image shows a large irregular mass in the left upper lobe (*) with thickening of the peribronchovascular bundle and interlobular septa, representing a primary tumor with regional lymphangitic spread. (b) Follow-up chest CT image obtained after 2 months of erlotinib therapy shows a marked decrease of the mass with residual opacities, representing response to therapy.
Figure 2b:
Images in 63-year-old woman with advanced adenocarcinoma of the lung harboring sensitizing epidermal growth factor receptor (EGFR) L858R mutation who was treated with the EGFR inhibitor erlotinib. (a) Baseline chest CT image shows a large irregular mass in the left upper lobe (*) with thickening of the peribronchovascular bundle and interlobular septa, representing a primary tumor with regional lymphangitic spread. (b) Follow-up chest CT image obtained after 2 months of erlotinib therapy shows a marked decrease of the mass with residual opacities, representing response to therapy.
Recent Advances in Overcoming Acquired Resistance to Precision Therapy
Although molecular-targeted therapy is initially very effective for subsets of patients with specific tumor genomic mutations, acquired resistance, defined as progression after initial treatment benefit, is inevitable, which is a major limitation (12). The most common mechanism of acquiring resistance for EGFR-mutant NSCLC is the development of EGFR T790M mutation, accounting for up to 50%–60% of acquired resistance cases (13–15). Less commonly, other mechanisms, including MET amplification and ERBB2 (HER2) amplification, as well as transformation to small cell lung cancer (SCLC), have been reported (13–15).
To overcome acquired resistance due to T790M mutation, a mutant-selective EGFR-TKI, osimertinib, has been developed (16). Osimertinib is selective for both the sensitizing EGFR mutations and the T790M resistance mutation, whereas it relatively spares wild-type EGFR. In a phase I trial, osimertinib was shown to be effective for patients who experienced disease progression after conventional EGFR-TKI therapy (16) (Table 1). A subsequent phase II trial of osimertinib in patients with T790M-positive disease also demonstrated marked efficacy (17). In 2015, osimertinib received approval for patients with advanced NSCLC harboring T790M mutation who experienced disease progression while being treated with an EGFR-TKI (Fig 3).
Figure 3a:
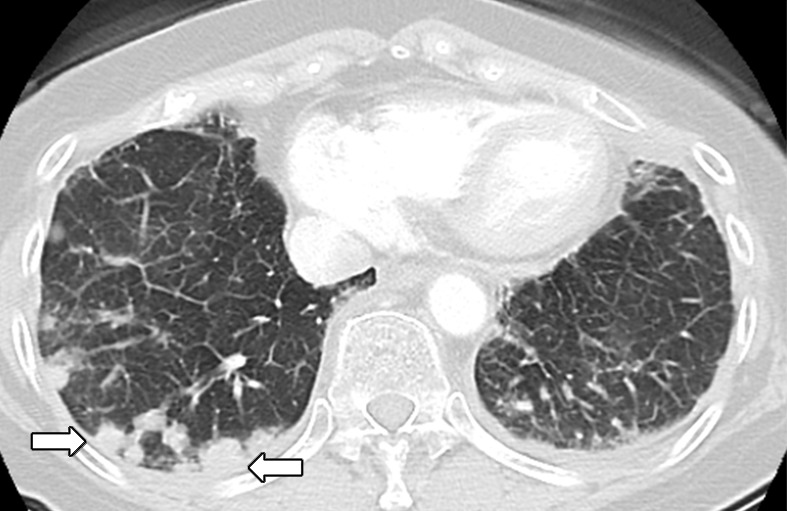
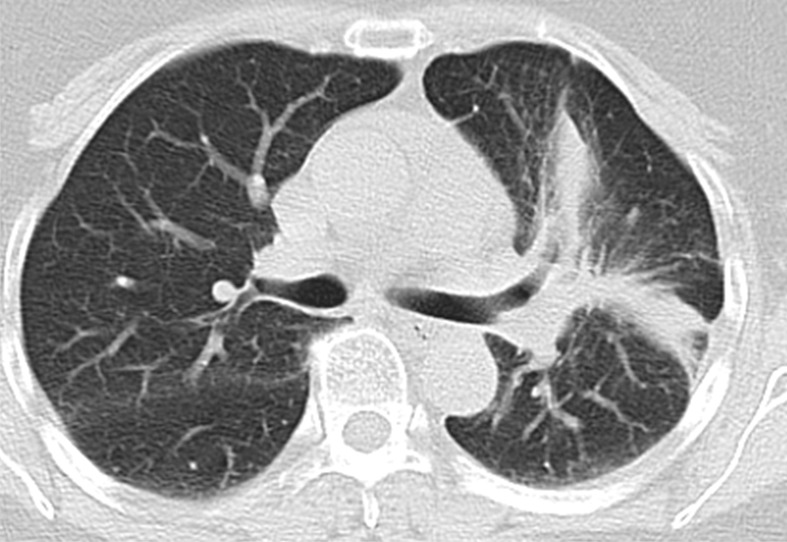
Osimertinib therapy in a 63-year-old woman with advanced adenocarcinoma of the lung that originally manifested with a sensitizing epidermal growth factor receptor (EGFR) L858R mutation and initially responded well to first-line erlotinib therapy. (a) CT image obtained after 15 months of erlotinib therapy shows progression of tumor, with development of multiple new lung nodules in the right lung base (arrows). The patient started osimertinib therapy for acquired resistance to erlotinib. Rebiopsy of the right lower lobe nodule confirmed the presence of a T790M mutation. (b) Follow-up CT image obtained after 2 months of osimertinib therapy shows a near-complete resolution of the nodules, representing a marked response to osimertinib. (c) However, on a CT image obtained at 10 months of osimertinib therapy, a growth of one of the nodules in the right lower lobe (arrow) is noted, indicating progressing tumor despite osimertinib treatment. (d) CT image obtained for further follow-up at 11 months after the initiation of osimertinib shows further growth of the dominant recurrent tumor (arrow), as well as an increase in smaller lung nodules in the right lower lobe, indicating the development of acquired resistance to osimertinib.
Figure 3b:
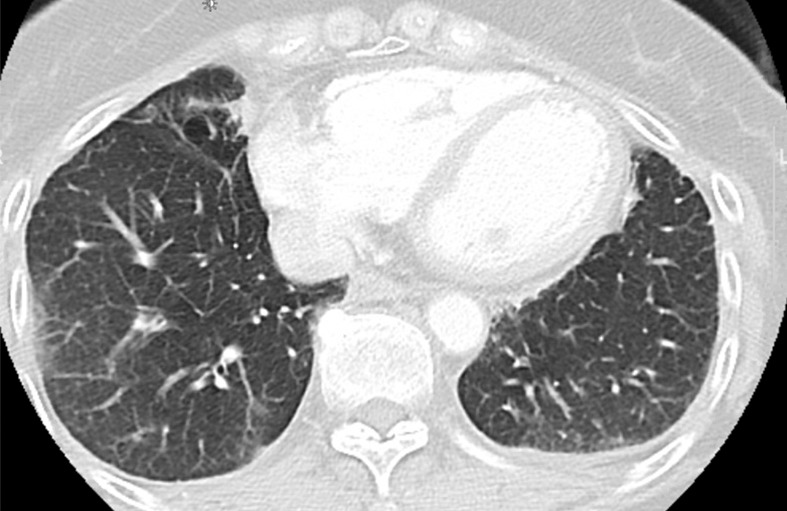
Osimertinib therapy in a 63-year-old woman with advanced adenocarcinoma of the lung that originally manifested with a sensitizing epidermal growth factor receptor (EGFR) L858R mutation and initially responded well to first-line erlotinib therapy. (a) CT image obtained after 15 months of erlotinib therapy shows progression of tumor, with development of multiple new lung nodules in the right lung base (arrows). The patient started osimertinib therapy for acquired resistance to erlotinib. Rebiopsy of the right lower lobe nodule confirmed the presence of a T790M mutation. (b) Follow-up CT image obtained after 2 months of osimertinib therapy shows a near-complete resolution of the nodules, representing a marked response to osimertinib. (c) However, on a CT image obtained at 10 months of osimertinib therapy, a growth of one of the nodules in the right lower lobe (arrow) is noted, indicating progressing tumor despite osimertinib treatment. (d) CT image obtained for further follow-up at 11 months after the initiation of osimertinib shows further growth of the dominant recurrent tumor (arrow), as well as an increase in smaller lung nodules in the right lower lobe, indicating the development of acquired resistance to osimertinib.
Furthermore, a recent trial of osimertinib as the first-line treatment for EGFR-mutant advanced NSCLC demonstrated a longer progression-free survival (PFS) in the osimertinib-treated group than in the group treated with erlotinib or gefitinib (median PFS, 18.9 vs 10.2 months, P < .001) (18). Osimertinib also had a superior efficacy in the central nervous system (CNS), with a CNS response rate of 91%, compared with 68% for erlotinib and gefitinib, reducing the risk of CNS progression (19). Osimertinib was approved for the first-line treatment of EGFR-mutant NSCLC in 2018. The marked efficacy of osimertinib for EGFR-mutant NSCLC in both the TKI-naive and acquired resistance settings represents important progress that has changed the landscape of the treatment approaches for EGFR-mutant advanced NSCLC.
However, acquired resistance is a universal problem in molecular-targeting agents directed to specific mutations, and osimertinib is not an exception. Acquired resistance to osimertinib has been noted after initial response (Fig 3c, 3d). The mechanisms of osimertinib resistance are actively studied and include EGFR C797S mutations, MET amplification, and SCLC transformation (20,21). Ongoing trials use novel TKIs or combination regimens to overcome acquired resistance to osimertinib (22).
Figure 3c:
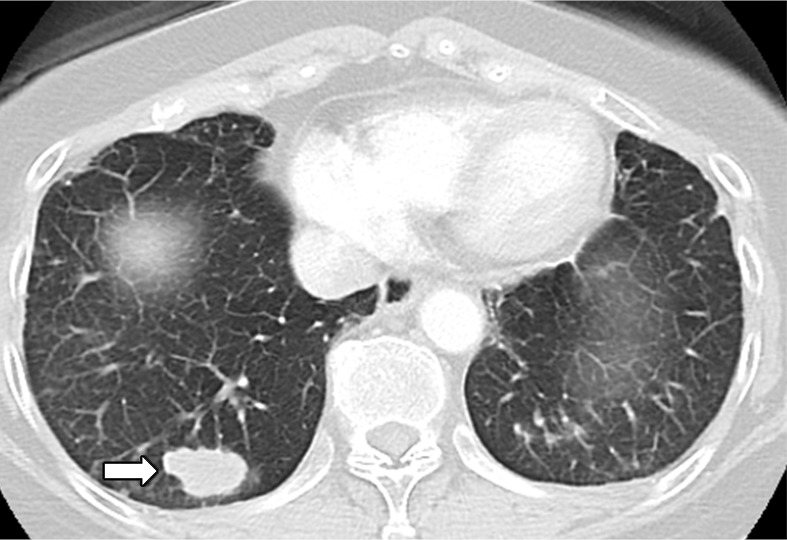
Osimertinib therapy in a 63-year-old woman with advanced adenocarcinoma of the lung that originally manifested with a sensitizing epidermal growth factor receptor (EGFR) L858R mutation and initially responded well to first-line erlotinib therapy. (a) CT image obtained after 15 months of erlotinib therapy shows progression of tumor, with development of multiple new lung nodules in the right lung base (arrows). The patient started osimertinib therapy for acquired resistance to erlotinib. Rebiopsy of the right lower lobe nodule confirmed the presence of a T790M mutation. (b) Follow-up CT image obtained after 2 months of osimertinib therapy shows a near-complete resolution of the nodules, representing a marked response to osimertinib. (c) However, on a CT image obtained at 10 months of osimertinib therapy, a growth of one of the nodules in the right lower lobe (arrow) is noted, indicating progressing tumor despite osimertinib treatment. (d) CT image obtained for further follow-up at 11 months after the initiation of osimertinib shows further growth of the dominant recurrent tumor (arrow), as well as an increase in smaller lung nodules in the right lower lobe, indicating the development of acquired resistance to osimertinib.
Figure 3d:
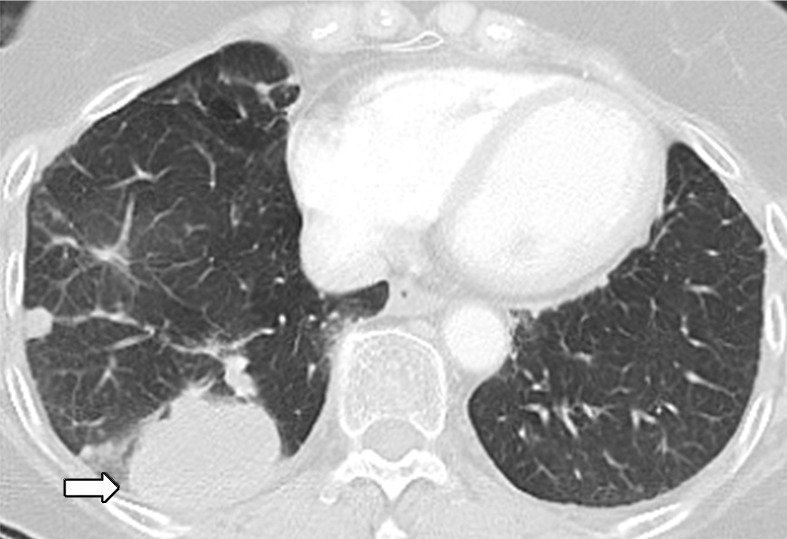
Osimertinib therapy in a 63-year-old woman with advanced adenocarcinoma of the lung that originally manifested with a sensitizing epidermal growth factor receptor (EGFR) L858R mutation and initially responded well to first-line erlotinib therapy. (a) CT image obtained after 15 months of erlotinib therapy shows progression of tumor, with development of multiple new lung nodules in the right lung base (arrows). The patient started osimertinib therapy for acquired resistance to erlotinib. Rebiopsy of the right lower lobe nodule confirmed the presence of a T790M mutation. (b) Follow-up CT image obtained after 2 months of osimertinib therapy shows a near-complete resolution of the nodules, representing a marked response to osimertinib. (c) However, on a CT image obtained at 10 months of osimertinib therapy, a growth of one of the nodules in the right lower lobe (arrow) is noted, indicating progressing tumor despite osimertinib treatment. (d) CT image obtained for further follow-up at 11 months after the initiation of osimertinib shows further growth of the dominant recurrent tumor (arrow), as well as an increase in smaller lung nodules in the right lower lobe, indicating the development of acquired resistance to osimertinib.
A similar issue of acquired resistance is noted in patients with ALK-rearranged NSCLC. Although crizotinib is initially effective, most patients eventually experience relapse, commonly within 12 months, because of the development of acquired resistance to crizotinib (23) (Fig 4). The mechanisms of acquired resistance include mutation within the ALK tyrosine kinase domain or amplification of the ALK fusion gene (24). Additionally, because of poor CNS penetration of crizotinib, the CNS is the most common site of progression in patients treated with crizotinib (25) (Fig 5). To overcome these limitations, newer ALK inhibitors have been developed and tested in trials. Alectinib, a second-generation ALK inhibitor, has demonstrated promising activity in patients (26,27) (Table 1). Alectinib was initially approved by the FDA in 2015 for treatment of patients with ALK rearrangement who experienced disease progression with crizotinib. Subsequently, alectinib also demonstrated marked efficacy as the first-line treatment for ALK-rearranged NSCLC, with a superior efficacy compared with crizotinib (23). Alectinib has better blood-brain barrier penetration, and 12% of patients treated with alectinib had an event of CNS progression, compared with 45% of patients treated with crizotinib (23) (Fig 5). On the basis of these results, alectinib is now approved as the first-line therapy for ALK-rearranged NSCLC (23,28). Other ALK inhibitors—ceritinib, brigatinib, and lorlatinib—have also shown activity in patients with ALK-rearranged NSCLC and have been approved (29–32) (Table 1). Availability of these newer ALK-directed agents in the first-line setting as well as after progression on the prior ALK inhibitor therapy has brought further advances in the approach to ALK-rearranged NSCLC.
Figure 4a:
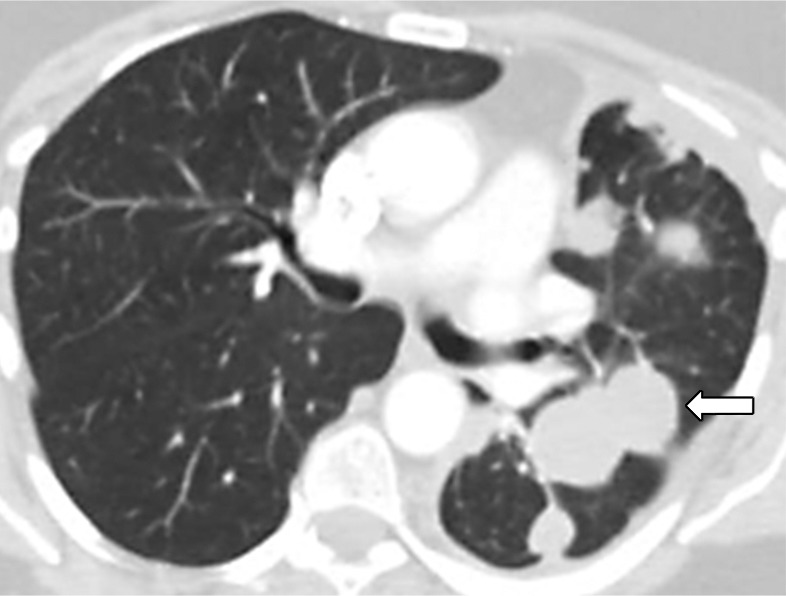
Images in 77-year-old woman with anaplastic lymphoma kinase (ALK)-rearranged advanced non–small cell lung cancer who developed acquired resistance to crizotinib and subsequently responded to alectinib. (a) Baseline CT image obtained prior to crizotinib therapy shows a dominant mass in the left lower lobe (arrow) and multiple lung nodules. (b) Follow-up CT image obtained after 5 months of crizotinib therapy shows marked response to therapy, with a clinically significant reduction of the dominant lung mass (arrow) and lung nodules. However, the mass (arrow) started to grow back over the course of treatment, as noted on (c) a follow-up CT image obtained after 17 months of crizotinib therapy, indicating the development of acquired resistance to crizotinib. Crizotinib therapy was stopped, and the patient was treated with alectinib. On (d) follow-up CT image obtained after 2 months of alectinib therapy, the recurrent tumor responded to therapy (arrow).
Figure 5a:
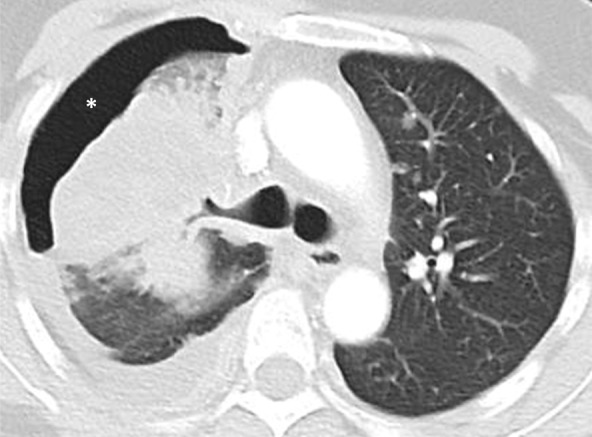
Images show central nervous system (CNS) progression on crizotinib and subsequent response to alectinib in a patient with anaplastic lymphoma kinase (ALK)-rearranged advanced non–small cell lung cancer. (a) Baseline chest CT image shows dominant consolidative opacity in the right upper lobe and left lung nodules. Note right hydropneumothorax (*) due to prior thoracentesis. (b, c) The tumor responded very well to crizotinib therapy, with a small residual band-like opacity in the lung (b) after 2 years of crizotinib therapy. However, a new enhancing lesion in the right cerebellum (arrow) was noted at brain MRI, suggesting CNS progression. The patient switched therapy from crizotinib to alectinib, and the cerebellar lesion is seen to have resolved on (d) image from initial follow-up brain MRI 1.5 months after the initiation of alectinib, demonstrating higher effectiveness of alectinib for CNS lesions because of better blood-brain barrier penetration.
Figure 4b:
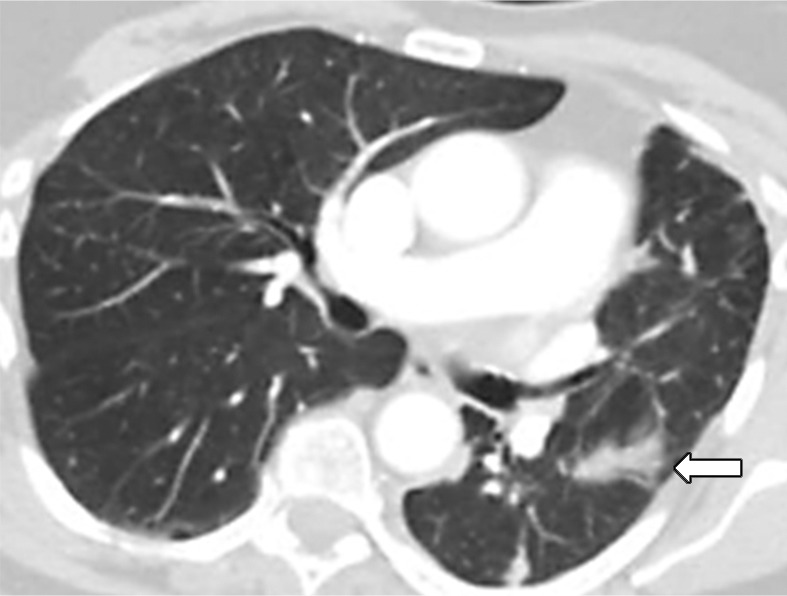
Images in 77-year-old woman with anaplastic lymphoma kinase (ALK)-rearranged advanced non–small cell lung cancer who developed acquired resistance to crizotinib and subsequently responded to alectinib. (a) Baseline CT image obtained prior to crizotinib therapy shows a dominant mass in the left lower lobe (arrow) and multiple lung nodules. (b) Follow-up CT image obtained after 5 months of crizotinib therapy shows marked response to therapy, with a clinically significant reduction of the dominant lung mass (arrow) and lung nodules. However, the mass (arrow) started to grow back over the course of treatment, as noted on (c) a follow-up CT image obtained after 17 months of crizotinib therapy, indicating the development of acquired resistance to crizotinib. Crizotinib therapy was stopped, and the patient was treated with alectinib. On (d) follow-up CT image obtained after 2 months of alectinib therapy, the recurrent tumor responded to therapy (arrow).
Figure 4c:
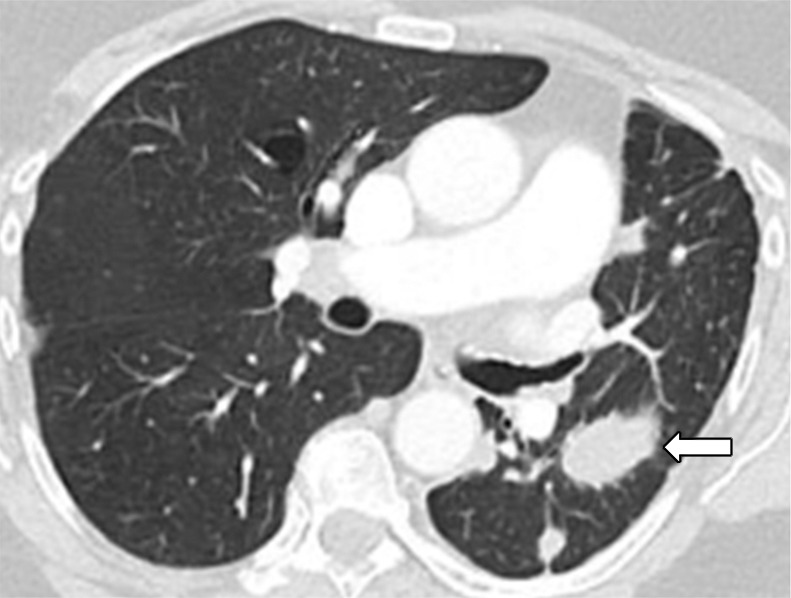
Images in 77-year-old woman with anaplastic lymphoma kinase (ALK)-rearranged advanced non–small cell lung cancer who developed acquired resistance to crizotinib and subsequently responded to alectinib. (a) Baseline CT image obtained prior to crizotinib therapy shows a dominant mass in the left lower lobe (arrow) and multiple lung nodules. (b) Follow-up CT image obtained after 5 months of crizotinib therapy shows marked response to therapy, with a clinically significant reduction of the dominant lung mass (arrow) and lung nodules. However, the mass (arrow) started to grow back over the course of treatment, as noted on (c) a follow-up CT image obtained after 17 months of crizotinib therapy, indicating the development of acquired resistance to crizotinib. Crizotinib therapy was stopped, and the patient was treated with alectinib. On (d) follow-up CT image obtained after 2 months of alectinib therapy, the recurrent tumor responded to therapy (arrow).
Figure 4d:
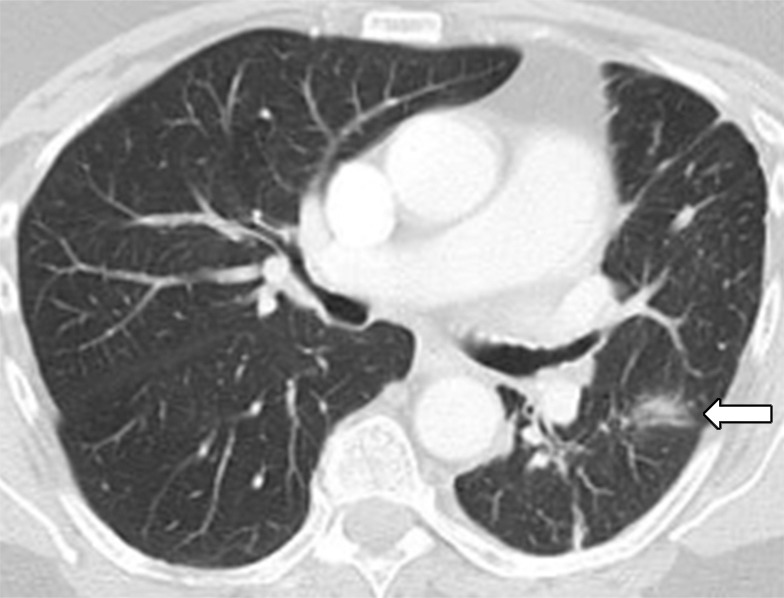
Images in 77-year-old woman with anaplastic lymphoma kinase (ALK)-rearranged advanced non–small cell lung cancer who developed acquired resistance to crizotinib and subsequently responded to alectinib. (a) Baseline CT image obtained prior to crizotinib therapy shows a dominant mass in the left lower lobe (arrow) and multiple lung nodules. (b) Follow-up CT image obtained after 5 months of crizotinib therapy shows marked response to therapy, with a clinically significant reduction of the dominant lung mass (arrow) and lung nodules. However, the mass (arrow) started to grow back over the course of treatment, as noted on (c) a follow-up CT image obtained after 17 months of crizotinib therapy, indicating the development of acquired resistance to crizotinib. Crizotinib therapy was stopped, and the patient was treated with alectinib. On (d) follow-up CT image obtained after 2 months of alectinib therapy, the recurrent tumor responded to therapy (arrow).
Figure 5b:
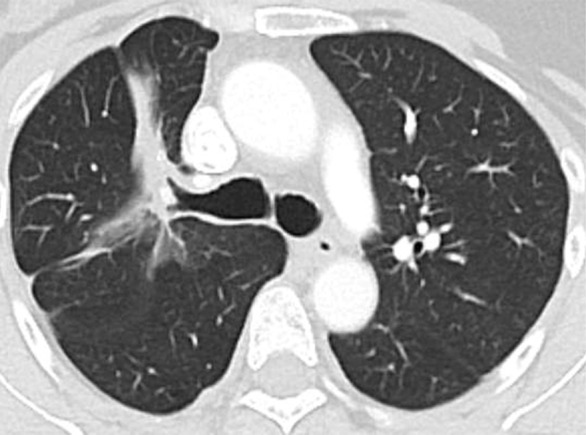
Images show central nervous system (CNS) progression on crizotinib and subsequent response to alectinib in a patient with anaplastic lymphoma kinase (ALK)-rearranged advanced non–small cell lung cancer. (a) Baseline chest CT image shows dominant consolidative opacity in the right upper lobe and left lung nodules. Note right hydropneumothorax (*) due to prior thoracentesis. (b, c) The tumor responded very well to crizotinib therapy, with a small residual band-like opacity in the lung (b) after 2 years of crizotinib therapy. However, a new enhancing lesion in the right cerebellum (arrow) was noted at brain MRI, suggesting CNS progression. The patient switched therapy from crizotinib to alectinib, and the cerebellar lesion is seen to have resolved on (d) image from initial follow-up brain MRI 1.5 months after the initiation of alectinib, demonstrating higher effectiveness of alectinib for CNS lesions because of better blood-brain barrier penetration.
Figure 5c:
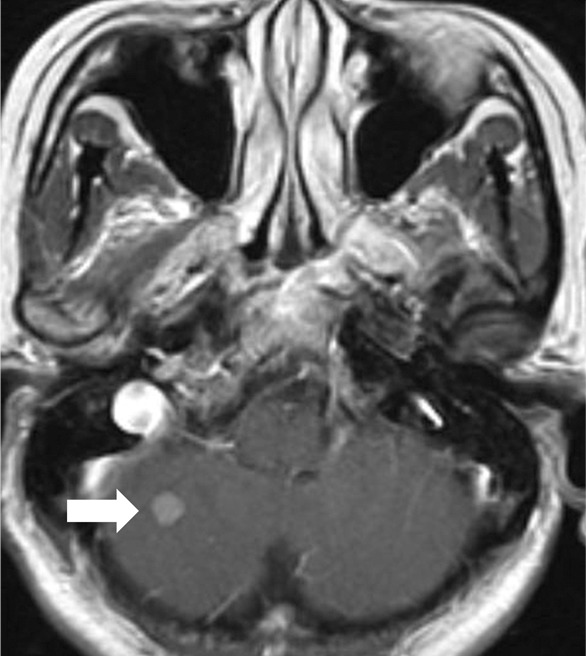
Images show central nervous system (CNS) progression on crizotinib and subsequent response to alectinib in a patient with anaplastic lymphoma kinase (ALK)-rearranged advanced non–small cell lung cancer. (a) Baseline chest CT image shows dominant consolidative opacity in the right upper lobe and left lung nodules. Note right hydropneumothorax (*) due to prior thoracentesis. (b, c) The tumor responded very well to crizotinib therapy, with a small residual band-like opacity in the lung (b) after 2 years of crizotinib therapy. However, a new enhancing lesion in the right cerebellum (arrow) was noted at brain MRI, suggesting CNS progression. The patient switched therapy from crizotinib to alectinib, and the cerebellar lesion is seen to have resolved on (d) image from initial follow-up brain MRI 1.5 months after the initiation of alectinib, demonstrating higher effectiveness of alectinib for CNS lesions because of better blood-brain barrier penetration.
Figure 5d:
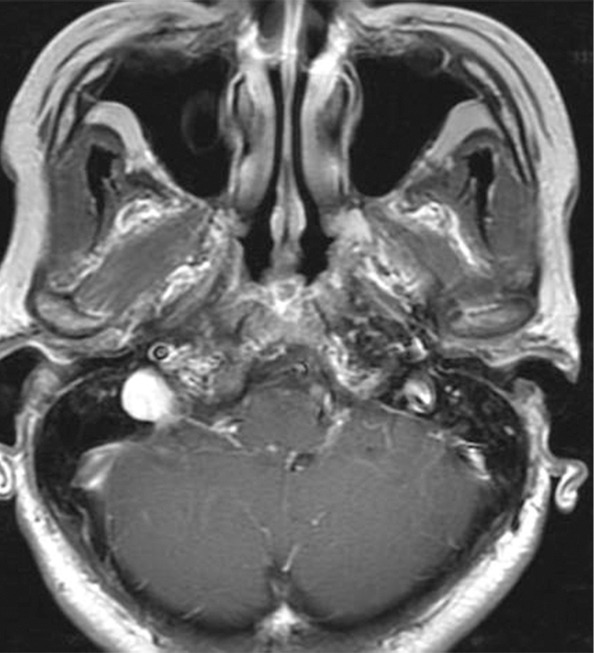
Images show central nervous system (CNS) progression on crizotinib and subsequent response to alectinib in a patient with anaplastic lymphoma kinase (ALK)-rearranged advanced non–small cell lung cancer. (a) Baseline chest CT image shows dominant consolidative opacity in the right upper lobe and left lung nodules. Note right hydropneumothorax (*) due to prior thoracentesis. (b, c) The tumor responded very well to crizotinib therapy, with a small residual band-like opacity in the lung (b) after 2 years of crizotinib therapy. However, a new enhancing lesion in the right cerebellum (arrow) was noted at brain MRI, suggesting CNS progression. The patient switched therapy from crizotinib to alectinib, and the cerebellar lesion is seen to have resolved on (d) image from initial follow-up brain MRI 1.5 months after the initiation of alectinib, demonstrating higher effectiveness of alectinib for CNS lesions because of better blood-brain barrier penetration.
Other oncogenic driver mutations beyond EGFR and ALK have been actively investigated, leading to recent approvals of dabrafenib (a BRAF inhibitor) and trametinib (a MEK inhibitor) combination therapy for BRAF V600E mutation-positive metastatic NSCLC and crizotinib for ROS1-rearranged NSCLC (33,34) (Table 1) (Fig E1 [online]). Discoveries of these newer genomic abnormalities and development of specific targeting agents continue to advance precision lung cancer therapy and overcome acquired resistance. Imaging plays an important role by objectively characterizing tumor response and progression. Awareness of the rapidly advancing landscape of genome-based selection for precision lung cancer therapy is essential for radiologists who contribute as key members of state-of-the-art lung cancer care, as these approved agents are increasingly used in clinical practice settings.
ICI Therapy for Lung Cancer
Cancer immunotherapy using immune-checkpoint blockade has brought another paradigm shift in advanced cancer treatment, as recognized by the Nobel Prize in Physiology or Medicine in 2018, awarded to James Allison and Tasuku Honjo “for their discovery of cancer therapy by inhibition of negative immune regulation” (35). ICIs block immune inhibition by tumors and thereby activate cellular immune response against tumors. Their clinical application has added another important dimension to the precision medicine approach to lung cancer (36,37).
Among a variety of immune-checkpoint molecules that can be targeted in immunotherapy, programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors are two major groups of agents that are actively used in advanced NSCLC (36,37). Currently, four PD-1/PD-L1 inhibitors have been approved for treatment of NSCLC (Table 1). Before the introduction of ICIs, cytotoxic chemotherapy was the mainstay of treatment for patients with advanced NSCLC who do not have targetable mutations, without significant improvement in outcomes (38). However, successful clinical application of PD-1/PD-L1 inhibitors has revolutionized the treatment approaches for these patients without targetable mutations and has enabled marked and durable responses and prolonged clinical benefits in some patients (39–47) (Fig 6).
Figure 6a:
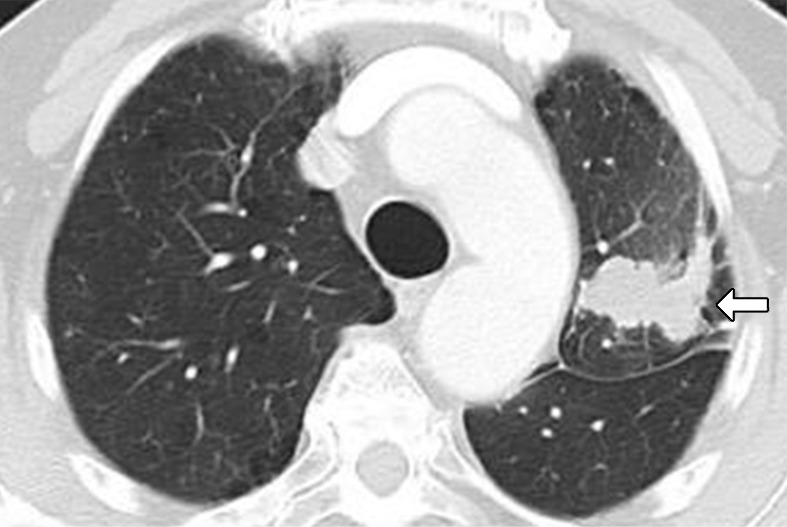
Images in 75-year-old man with advanced non–small cell lung cancer with 90% programmed death-ligand 1 (PD-L1) expression at immunohistochemistry. (a) Baseline CT image shows lobulated mass in left upper lobe, consistent with primary lung cancer (arrow). (b) Follow-up CT image obtained after 2 months of nivolumab therapy shows marked tumor shrinkage, representing response to PD-1 blockade in this high PD-L1–expressing tumor (arrow).
Figure 6b:
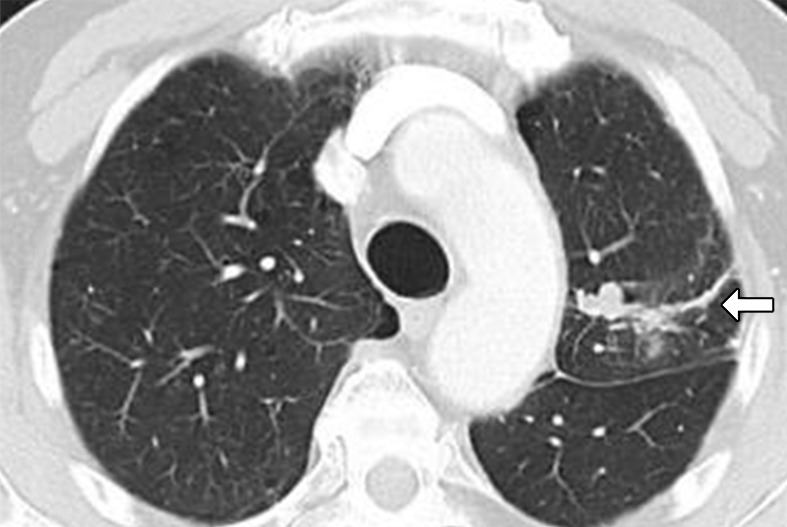
Images in 75-year-old man with advanced non–small cell lung cancer with 90% programmed death-ligand 1 (PD-L1) expression at immunohistochemistry. (a) Baseline CT image shows lobulated mass in left upper lobe, consistent with primary lung cancer (arrow). (b) Follow-up CT image obtained after 2 months of nivolumab therapy shows marked tumor shrinkage, representing response to PD-1 blockade in this high PD-L1–expressing tumor (arrow).
ICI therapy has also made contributions to the progress of treatment for SCLC, which is also an area in need of more effective therapies. Nivolumab showed durable response in a subset of patients with SCLC whose disease progressed after chemotherapy (48), and it was granted an accelerated approval for the treatment of patients with SCLC with disease progression after platinum-based chemotherapy and one other line of therapy in 2018 (Table 1).
Molecular and Genomic Biomarkers and Pitfalls of Image Interpretations for Lung Cancer Precision Therapy
Advances in Molecular and Genomic Biomarkers for Lung Cancer
Given the advances of precision therapy for lung cancer, identification of targetable mutations at the time of diagnosis has become critically important to determine the most optimal treatment strategies. Massively parallel sequencing is a high-throughput method that allows comprehensive tumor genomic profiling with a high identification rate of “actionable” mutations (49) (Figs E2, E3 [online]). Another emerging strategy is plasma genotyping with “liquid biopsy,” which identifies driver mutations in circulating cell-free tumor DNA from peripheral blood samples and is increasingly used in evaluation of EGFR mutations in NSCLC (50,51).
Selecting the subgroup of patients who would benefit from ICIs becomes important because the efficacy of treatment varies across individual patients and among different tumor types. The level of PD-L1 expression in tumor cells at immunohistochemistry helps identify patients who may benefit from ICIs (36). Several previous studies, including phase III trials of pembrolizumab in patients with advanced NSCLC, reported a higher response rate and longer PFS and overall survival (OS) in patients whose tumors had high PD-L1 expression (45). Pembrolizumab was approved for first-line treatment for patients with NSCLC with 50% or greater PD-L1 staining in tumor cells, which consists of approximately 30% of stage IV NSCLCs with no sensitizing EGFR mutations or ALK rearrangements (45) (Fig E4 [online]). Combining pembrolizumab with chemotherapy has also shown improvements in PFS and OS, regardless of PD-L1 expression levels, compared with chemotherapy alone, leading to the approval of this regimen for both nonsquamous (47) and squamous (52) NSCLC (Table 1). Though some controversies still remain, PD-L1 immunohistochemistry as a biomarker for ICI therapy has expanded the subset of patients with NSCLC who can benefit from precision medicine approaches (45). Interestingly, NSCLCs harboring EGFR mutations or ALK rearrangements are associated with low response rates to PD-1/PD-L1 inhibitors and are thus excluded from some of the indications for ICI therapy (Table 1) (53).
Microsatellite instability/mismatch repair deficiency, though uncommon in lung cancer, has also become a biomarker for ICI therapy for all solid tumors, representing the first ever tumor-agnostic biomarker. Additional biomarkers for ICI therapy, including tumor mutation burden, tumor-infiltrating lymphocytes (especially CD8+ T cells), and cytokine profiles, are being actively investigated (36,46).
Pitfalls of Image Interpretations for Lung Cancer Precision Therapy
Immune-related tumor response and progression.—ICI therapy may lead to unique patterns of tumor response and progression at imaging in subsets of patients. The most well-described pattern is pseudoprogression, which includes (a) response after an initial increase in tumor burden, and (b) respose during or after the appearance of new lesions (36,37,54–58). Although pseudoprogression is becoming increasingly known, it is important to recognize the low incidence of pseudoprogression, especially in patients with NSCLC treated with PD-1/PD-L1 inhibitors, with the frequency ranging from 0.6% to 5% (41,57,59). While imaging strategies that help reliably differentiate pseudoprogression from true progression in the early course of therapy remain to be established, accumulating evidence of the low incidence of pseudoprogression in patients with NSCLCs indicates that an increase in tumor burden is more likely to reflect true progression than pseudoprogression in most patients (36).
Although ICIs can lead to durable tumor responses in some patients, a small subset of patients may experience accelerated tumor growth after starting ICI compared with the period before starting therapy (60) (Fig 7). This phenomenon is termed hyperprogressive disease, and is recognized as one of the patterns of tumor dynamics during ICI therapy (60). In the first report of this phenomenon, hyperprogressive disease was defined as progression according to Response Evaluation Criteria in Solid Tumors (RECIST) at the first evaluation with a twofold or greater increase in the tumor growth rate during PD-1/PD-L1 therapy compared with the pretherapy period, and was noted in 9% of advanced solid cancers (60). In a more recent study focusing on patients with advanced NSCLC, hyperprogressive disease was noted in 13.8% of patients with NSCLC and was associated with shorter survival (61). Attention to this pattern of rapid tumor growth is important when evaluating imaging studies in patients receiving ICI therapy. Further studies are ongoing to identify predictive markers for the phenomenon.
Figure 7a:
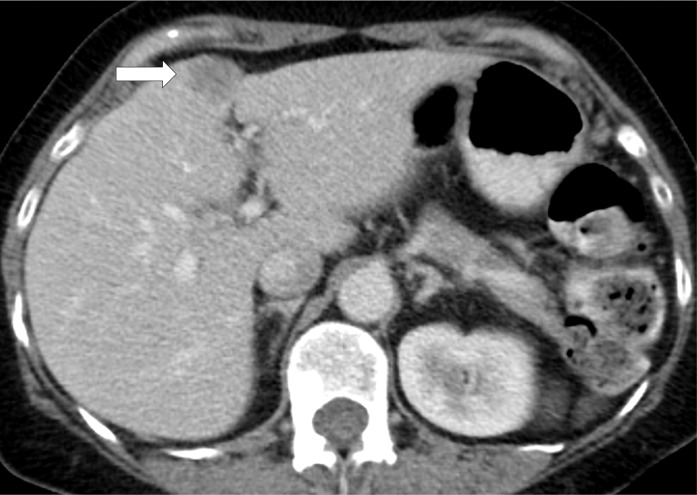
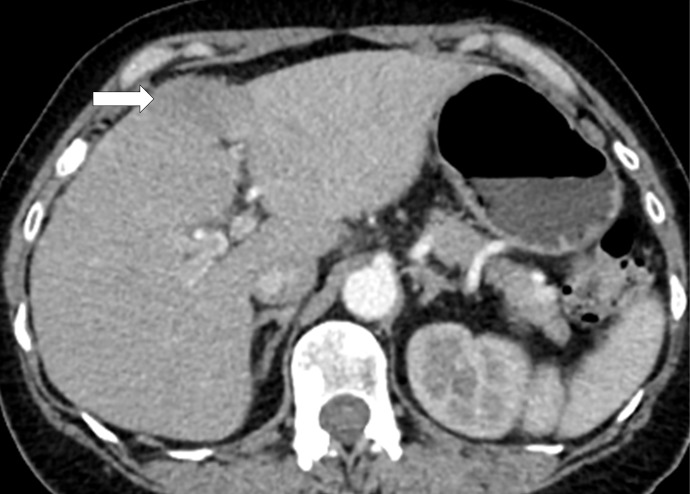
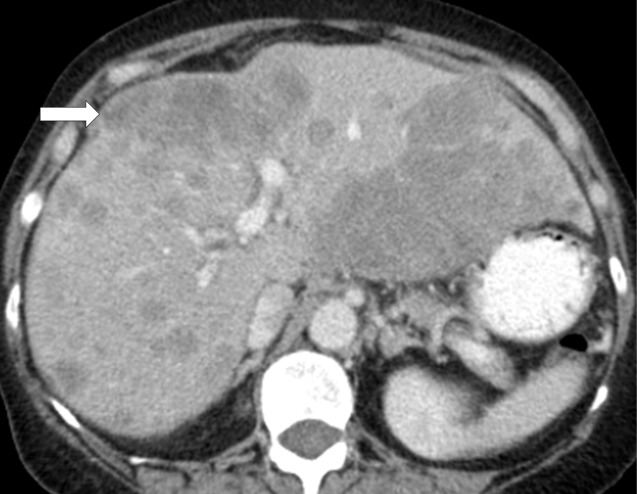
Hyperprogressive disease in a 64-year-old woman with stage IV non–small cell lung cancer treated with nivolumab. (a) CT image of the abdomen obtained 2.5 months before the initiation of nivolumab therapy shows metastatic liver lesions (arrow). (b) Baseline CT image obtained immediately before the initiation of nivolumab therapy shows a moderate increase in the liver lesion (arrow) compared with a. (c) Initial follow-up CT image obtained after 2 months of nivolumab therapy shows a rapid and marked increase in the existing liver mestastasis (arrow), as well as the appearance of immunerable new liver lesions occupying majority of liver parenchyma in both lobes, indicating hyperprogressive disease on nivolumab therapy.
Figure 7b:
Hyperprogressive disease in a 64-year-old woman with stage IV non–small cell lung cancer treated with nivolumab. (a) CT image of the abdomen obtained 2.5 months before the initiation of nivolumab therapy shows metastatic liver lesions (arrow). (b) Baseline CT image obtained immediately before the initiation of nivolumab therapy shows a moderate increase in the liver lesion (arrow) compared with a. (c) Initial follow-up CT image obtained after 2 months of nivolumab therapy shows a rapid and marked increase in the existing liver mestastasis (arrow), as well as the appearance of immunerable new liver lesions occupying majority of liver parenchyma in both lobes, indicating hyperprogressive disease on nivolumab therapy.
Figure 7c:
Hyperprogressive disease in a 64-year-old woman with stage IV non–small cell lung cancer treated with nivolumab. (a) CT image of the abdomen obtained 2.5 months before the initiation of nivolumab therapy shows metastatic liver lesions (arrow). (b) Baseline CT image obtained immediately before the initiation of nivolumab therapy shows a moderate increase in the liver lesion (arrow) compared with a. (c) Initial follow-up CT image obtained after 2 months of nivolumab therapy shows a rapid and marked increase in the existing liver mestastasis (arrow), as well as the appearance of immunerable new liver lesions occupying majority of liver parenchyma in both lobes, indicating hyperprogressive disease on nivolumab therapy.
Drug-related toxicities of precision lung cancer therapy.—Drug toxicities related to novel precision therapy agents can be noted at imaging, often with characteristic imaging features, which should be recognized as an imaging pitfall. Immune-related adverse events (irAEs) in patients treated with ICIs can involve literally any of the organs from head to toe, and many organ-specific irAEs have characteristic radiologic manifestations that call for radiologists’ attention, as described in detail in a recent review article in Radiology focusing on the topic and in other publications (37,62–65). Among various irAEs, pneumonitis is relatively uncommon but has clinically serious toxicity and has been shown to be the leading cause of PD-1/PD-L1 inhibitor–related deaths (66). A meta-analysis of ICI-related pneumonitis reported a higher incidence of pneumonitis in NSCLC, indicating a need for increased awareness for this toxicity among patients with NSCLC (Table 2) (67). A wide spectrum of imaging manifestations of pneumonitis was noted, which were described using the radiographic patterns according to the classification of interstitial pneumonias (37,68–71) (Table 2). Additionally, durvalumab (a PD-L1 inhibitor) consolidation therapy after chemotherapy and radiation therapy is a recently approved treatment for unresectable stage III NSCLC with OS benefit in a phase III trial (72,73). Pneumonitis, including radiation pneumonitis, was noted in 33.9% of the durvalumab group, which was higher than the 24.8% in the placebo group. The results suggest combined effects of radiation and ICI therapy in the development of pneumonitis, which requires further investigation (74).
Table 2:
Pneumonitis as a Class-Effect Toxicity of Precision Therapy for NSCLC
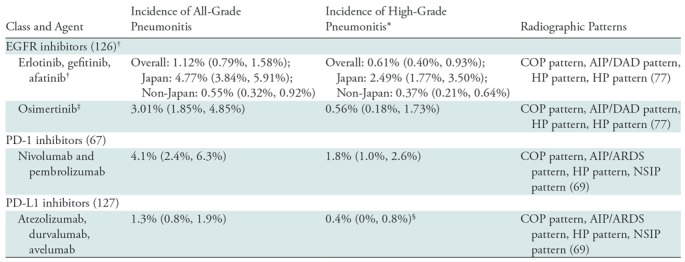
Note.— The table includes the incidence rates of pneumonitis for these agents used as single-agent therapy for non–small cell lung cancer (NSCLC) and not combined with other agents or radiation therapy on the basis of findings of recent meta-analyses studies (67,126,127). Data in parentheses are 95% confidence intervals. AIP = acute interstitial pneumonia, ARDS = acute respiratory distress syndrome, COP = cryptogenic organizing pneumonia, DAD = diffuse alveolar damage, EGFR = epidermal growth factor receptor, HP = hypersensitivity pneumonitis, NSIP = nonspecific interstitial pneumonia, PD-1 = programmed death-1, PD-L1 = programmed death-ligand 1.
*High-grade pneumonitis was considered to be that with a Common Terminology Criteria for Adverse Events grade of 3 or above.
†The incidence is among patients treated with EGFR inhibitors without prior exposure to EGFR-directed therapy. The incidence was significantly higher in studies from Japan compared with studies of non-Japan origin, for all-grade (P < .001) and for high-grade (P < .001) pneumonitis.
‡The data include patients who received osimertinib after previous treatment with conventional EGFR inhibitors. The overall incidence of pneumonitis was 4% in a recent phase III trial of first-line treatment of osimertinib for EGFR-mutant NSCLC (18).
§The study addressed only grade 3–4 pneumonitis.
Pneumonitis is also recognized as a class-effect toxicity of other agents used in precision therapy (68,75,76). Among the agents for lung cancer, EGFR inhibitors are known to be associated with pneumonitis, although the incidence is very low, except in the Japanese population (Table 2) (77). A spectrum of radiographic patterns similar to ICI-related pneumonitis has also been described. More recently, the combination of EGFR inhibitors and ICIs has been reported to lead to a higher incidence of pneumonitis, resulting in several lethal cases (78–80). The knowledge on drug-related toxicities is also evolving with the advances of novel lung cancer therapy, requiring radiologists to continue to remain up to date regarding the clinical and imaging manifestations of emerging toxicities (37,68).
Emerging Strategies of Precision Imaging for Lung Cancer
In response to the recent advances of precision lung cancer therapy, imaging strategies of treatment monitoring have also been evolving. The areas of focus include image-based evaluation of tumor growth rates and tumor burden kinetics, radiomics, and molecular and functional imaging. The observations are mostly in investigational settings, with ongoing efforts for translation into clinical practice.
Beyond RECIST: Tumor Volume and Tumor Growth Rates
RECIST has been most widely used as a standard method to evaluate tumor response to therapy in solid tumors, including lung cancer (81,82). Although they are simple and practical, the limitations of RECIST being based only on unidimensional measurements are increasingly acknowledged, especially in patients treated with precision therapy (10,11).
To complement some of the limitations of RECIST, tumor volume has been investigated as an alternate or additional method in lung cancer. With advances in multi–detector row CT technology and the increased availability of image-processing software, tumor volume burden can be segmented and quantified for more comprehensive assessment of tumor burden (10,83). Many prior studies have also shown that tumor volume is more reproducible than tumor size and can thus more accurately characterize small changes in tumor burden during therapy (10,83,84). Tumor volume analysis has also been investigated in the setting of precision lung cancer therapy as a marker for clinical outcome (Table 3). In patients with EGFR-mutant advanced NSCLC treated with erlotinib or gefitinib, the percentage tumor volume decrease at 8 weeks of therapy was reproducibly shown to be associated with OS, proposing an early imaging marker for survival (56,85,86). Similar observations have also been noted in patients with ALK rearrangement treated with crizotinib, indicating a wider applicability of tumor volume analyses in the setting of precision lung cancer therapy (87).
Table 3:
Studies of Tumor Size and Volume for Lung Cancer Outcome
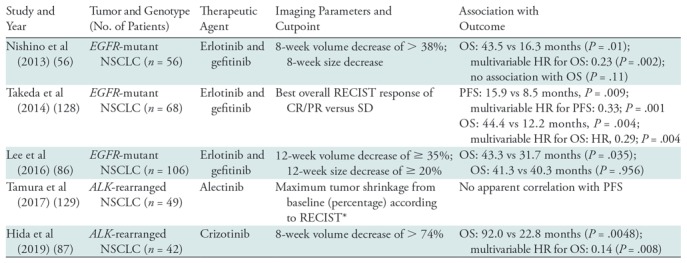
Note.—ALK = anaplastic lymphoma kinase, CR = complete response, EGFR = epidermal growth factor receptor, HR = hazard ratio, NSCLC = non–small cell lung cancer, OS = overall survival, PFS = progression-free survival, PR = partial response, RECIST = Response Evaluation Criteria in Solid Tumors, SD = Stable Disease.
*As a continuous variable without cutpoint; correlation with PFS assessed by the scatter plot.
Taking advantage of the higher reproducibility of tumor volume measurements, volumetric tumor growth rate can also be evaluated, which can be particularly useful in patients whose tumors are slowly growing back after initial response to effective precision therapy. As opposed to the simple percentage changes per RECIST without consideration of the length of time of therapy, tumor growth rate estimates the changes in tumor volume over time (88,89). In patients with EGFR-mutant advanced NSCLC treated with erlotinib or gefitinib, the tumor growth rate after initial response was 0.12 per month for the logarithm of the volume (originally measured in cubic millimeters), proposing a reference value to define slow progression in this subpopulation with NSCLC (85,89). Further efforts are ongoing to translate these investigational approaches into the clinical setting (90).
Radiomics for Precision Therapy in NSCLC
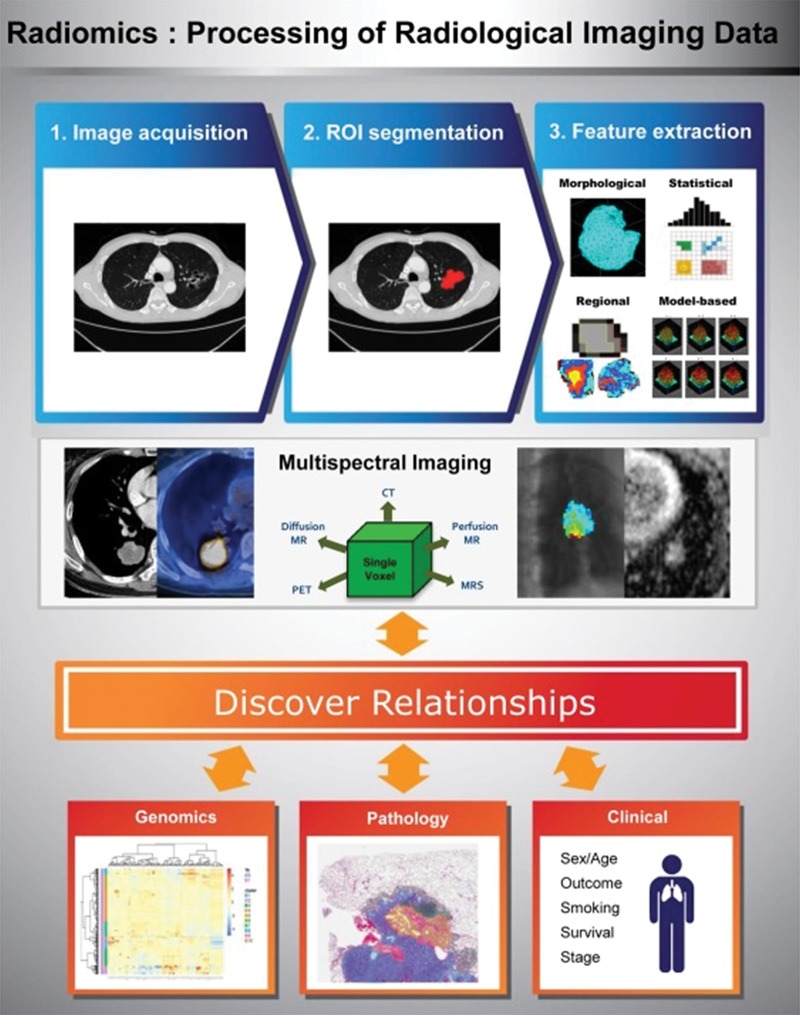
Radiomics refers to the extraction of multiple quantitative features from radiologic images and correlation with clinical or genetic features (91). The combination of radiomics features and genomic, histologic, and clinical information can be useful in predicting biologic features of the tumors, as well as tumor response to therapy and prognosis (Fig 8) (91–93).
Figure 8:
Overview of radiomics, the processing of radiologic imaging data. (Reprinted, with permission, from reference 91.) Regions of interest (ROIs) are segmented for the whole tumor, and multiple quantitative features are extracted. Combining information from multiple imaging modalities provides a multispectral view of the tumor and allows improved tumor characterization. Discovering relationships among the radiomic features and genomic, pathologic, and clinical data is a challenging but important step. MRS = MR spectroscopy.
CT radiomic features are studied for their relationship with the specific driver mutational status of NSCLC. Evaluation of chest CT characteristics of 285 patients with NSCLC with known EGFR mutation and/or ALK rearrangement and/or v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation status demonstrated that air bronchogram, pleural retraction, and small tumor size were related to EGFR mutation; pleural effusion and younger age were related to ALK mutation; and round lesion shape and nodules in nontumor lobes were related to KRAS mutation (94). In 298 patients with surgically resected peripheral lung adenocarcinomas (137 EGFR-mutant and 161 wild type) (95), EGFR mutation status could be predicted by a set of five radiomic features from three broad groups, including CT attenuation energy, tumor main direction, and texture defined according to wavelets and Laws (area under the receiver operating characteristic curve [AUC]: 0.647). Adding radiomic features to a clinical model resulted in a significant improvement in predicting power, with an increase in AUC from 0.667 to 0.709 (P < .0001) (95). However, in the current status, definitive treatment decisions for molecular-targeted therapy require tumor genomic testing from tissue samples.
Texture features were also studied as predictors of treatment response and prognosis. In 35 patients with advanced NSCLC treated with nab-paclitaxel, carboplatin, and bevacizumab, CT texture parameters, including the mean value of positive pixels (MPP) (the mean value of all the pixels with positive values) and entropy (a measure of irregularity) were evaluated at pretherapy CT. High MPP and low entropy from CT texture analysis were significantly associated with longer PFS and OS (96). In a study of patients with stage I–III NSCLC treated with chemotherapy and radiation therapy, parameters derived from neighborhood gray-tone difference matrices at pretherapy fluorodeoxyglucose (FDG) PET/CT were evaluated, including coarseness, contrast, busyness, and complexity, which describe the local tumor texture based on differences between each voxel and the neighboring voxels in adjacent image planes (97). RECIST responders at 12 weeks of therapy had lower coarseness and higher contrast and busyness compared with nonresponders. High primary tumor coarseness was associated with shorter OS and PFS and was an independent predictor of OS (97).
Texture analyses can also be applied to modalities beyond CT. Cook et al (98) performed texture analyses at FDG PET/CT in 47 patients with advanced NSCLC treated with erlotinib. Larger reductions in maximum standardized uptake value (SUVmax), standard deviation, and first-order entropy, as well as larger increases in first-order uniformity at 6-week PET, were associated with a higher chance of RECIST response. High-order contrast at 6 weeks and percentage change in first-order entropy were independently associated with survival. A one-unit increase in contrast was associated with an 80% increased risk of death, while a 10% increase in entropy was associated with a 14% increased risk (98). The study indicated the utility of texture analyses in patients with advanced NSCLC treated with precision therapy.
Radiomic approaches are also used in predicting the immune phenotype of tumors. A recent multicohort retrospective study (99) of advanced tumors, including lung cancer, revealed that radiomic signature may serve as a biomarker for immune-profiles and response to immune-checkpoint inhibition. In this study, first, the CT images and genomic data from RNA sequencing in 135 patients with advanced solid malignancies were evaluated to identify a radiomic signature that indicated the abundance of tumor-infiltrating CD8 cells. The genomic data were based on the CD8B gene, which encodes a part of the CD8 antigen (a cell surface glycoprotein found on most cytotoxic T lymphocytes) and were used to estimate the abundance of CD8 cells in the tumor biopsy samples. The data were then aligned with the images to generate the radiomic signatures, obtained from the segmented lesion corresponding to biopsy site. From 84 input variables (78 radiomic features, five location variables, and peak kilovoltage as the one technical variable), a radiomics-based predictor of the CD8 cell expression signature was built by using machine learning with an elastic-net regularized regression method (99). The ability of the radiomic scores to classify high versus low abundance of CD8 infiltrate had an AUC of 0.74 in this training set of 135 patients and was validated in an independent cohort of 119 patients, with an AUC of 0.67 (99). Cancers in a third cohort of 100 patients were classified into immune-inflamed or immune-desert tumor immune phenotypes, defined somewhat anecdotally on the basis of the known sensitivity to immunotherapy and propensity to involve lymph nodes of the tumor types. The radiomic score was able to classify immune-inflamed or immune-desert phenotypes with an AUC of 0.76 (99). Finally, an immunotherapy-treated cohort consisting of 137 patients with advanced solid tumors treated in phase I trials of PD-1/PD-L1 monotherapy was divided into high versus low radiomic score groups by using the median score of the cohort. The radiomic score at baseline CT was higher in RECIST responders at 3 and at 6 months, compared with patients with progressive disease or stable disease. OS was longer in the high radiomic score group (hazard ratio = 0.58, P = .0081) than in the low radiomic score group (median OS: 24.3 vs 11.5 months). Radiomic score was the strongest independent prognostic factor in multivariate analysis (hazard ratio: 0.52, P = .0022). The study provided encouraging initial results, which need to be further pursued for the outcome prediction of individual tumor types and specific therapies (99).
In parallel to the ongoing efforts to develop robust radiomic markers for treatment benefit and outcome for precision lung cancer therapy, standardization of the methodologic approaches for radiomics and texture data extraction, analyses, and interpretation are needed before the method is widely used as an additional approach that complements the limitations of RECIST.
Molecular and Functional Images for Precision Therapy in NSCLC
Molecular and functional imaging can be an attractive option in the evaluation of patients with lung cancer undergoing precision therapy because of its potential to noninvasively visualize molecular and genomic characteristics of the tumors, and it may be useful in predicting response and clinical outcome. To this end, novel PET tracers have been introduced and studied. Fluorine 18 (18F) N-(3-chloro-4-fluorophenyl)-7-(2-(2-(2-(2-18F-fluoroethoxy)ethoxy)ethoxy)ethoxy)-6-methoxyquinazolin-4-amine (MPG) is one of such novel PET tracers with high specificity to activating EGFR mutant kinase. In NSCLC mice models, 18F-MPG could reveal EGFR-activating mutations with high sensitivity and specificity (100). In 75 patients with NSCLC, patients with EGFR mutations had significantly higher 18F-MPG uptake than did those with wild-type EGFR (100). The concordance between the detection of EGFR mutation at 18F-MPG PET/CT and tissue biopsy reached 84.29%. Although this is not surprising given the high concordance between EGFR mutation status and 18F-MPG uptake, in 38 patients with 18F-MPG SUVmax of 2.23 or greater who were treated with EGFR-TKI, 31 achieved objective responses, and four showed stable disease (100), indicating that 18F-MPG may help to noninvasively identify patients who benefit from EGFR-TKI (100).
Recent studies have shown that radiolabeling an anticancer agent itself enables visualization and quantification of the agent in vivo. Carbon 11–erlotinib can accumulate in lung tumors or lymph nodes, including lesions that were not visible at FDG PET/CT in patients with NSCLC awaiting erlotinib therapy (101). Key immune-checkpoint molecules such as PD-1/PD-L1 can also be radiolabeled (37,102–105). In a recent first-in-humans study of zirconium 89 (89Zr)-labeled atezolizumab, 22 patients with NSCLC (n = 9), bladder cancer (n = 9), or breast cancer (n = 4) were imaged with 89Zr-atezolizumab PET before atezolizumab therapy (105). Uptake of 89Zr-atezolizumab corresponded to PD-L1 expression at sites of inflammation and in lymphoid tissues. Tumoral uptake was generally high but heterogeneous among lesions, patients, and tumor types. Importantly, the lesions at all main metastatic sites were visualized at 89Zr-atezolizumab PET. Furthermore, higher 89Zr-atezolizumab tumor uptake at baseline was associated with better RECIST response categories and larger tumor size decrease, indicating its role as a predictor of response to atezolizumab (105). A newer approach using a high-affinity competitive nonantibody antagonist of PD-L1 has also been investigated, demonstrating superior tumor penetration and high and persistent strong uptake in PD-L1–positive tumors (106). A preclinical study showed that copper 64–labeled antimouse antibody PD-1 showed higher uptake in the lymphoid organs and tumors, suggesting potential to assess the value of PD-1 in immunotherapy (107). Further studies are ongoing to translate these novel methods and approaches into the clinical setting of oncologic imaging to effectively inform treatment monitoring of precision lung cancer therapy.
Conclusion
The precision medicine approaches to lung cancer continue to rapidly advance, enabling translation of novel therapies and genomic analyses methods in clinical practice. The knowledge of these new therapies and how they relate to pre- and posttreatment imaging findings are essential for radiologists, who play a role as key members of multidisciplinary lung cancer care. The imaging assessment of lung cancer for precision therapy will continue to evolve in parallel with the advances in lung cancer treatment.
SUPPLEMENTAL FIGURES
Supported by the National Cancer Institute (R01CA203636).
Disclosures of Conflicts of Interest: H.P. disclosed no relevant relationships. L.M.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Foghorn Therapeutics, LOXO Oncology, and AstraZeneca Pharmaceuticals; has received travel expenses from Bristol Myers Squibb Pharmaceuticals. Other relationships: disclosed no relevant relationships. H.H. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Mitsubishi Chemical; institution has grants or grants pending from Canon Medical and Konica Minolta. Other relationships: disclosed no relevant relationships. M.M.A. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Bristol-Myers Squibb, Syndax, Blueprint Medicine, AstraZeneca, Genentech/Roche, Nektar, Ariad, and Merck; institution has grants or grants pending from Bristol-Myers Squibb, AstraZeneca/Medimmune, Lilly, and Genentech/Roche. Other relationships: disclosed no relevant relationships. M.N. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for WorldCare Clinical, Toshiba Medical Systems, and Daiichi Sankyo; institution has grants or grants pending from Merck, Canon Medical Systems, and AstraZeneca; has received honoraria from Bayer and Roche. Other relationships: disclosed no relevant relationships.
Abbreviations:
- ALK
- anaplastic lymphoma kinase
- AUC
- area under the receiver operating characteristic curve
- CNS
- central nervous system
- EGFR
- epidermal growth factor receptor
- FDA
- Food and Drug Administration
- FDG
- fluorodeoxyglucose
- ICI
- immune-checkpoint inhibitor
- NSCLC
- non–small cell lung cancer
- OS
- overall survival
- PD-1
- programmed death-1
- PD-L1
- programmed death-ligand 1
- PFS
- progression-free survival
- RECIST
- Response Evaluation Criteria in Solid Tumors
- SCLC
- small cell lung cancer
- SUVmax
- maximum standardized uptake value
- TKI
- tyrosine kinase inhibitor
References
- 1.Jordan EJ, Kim HR, Arcila ME, et al. Prospective Comprehensive Molecular Characterization of Lung Adenocarcinomas for Efficient Patient Matching to Approved and Emerging Therapies. Cancer Discov 2017;7(6):596–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gazdar AF. Personalized medicine and inhibition of EGFR signaling in lung cancer. N Engl J Med 2009;361(10):1018–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350(21):2129–2139. [DOI] [PubMed] [Google Scholar]
- 4.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004;101(36):13306–13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304(5676):1497–1500. [DOI] [PubMed] [Google Scholar]
- 6.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361(10):947–957. [DOI] [PubMed] [Google Scholar]
- 7.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12(8):735–742. [DOI] [PubMed] [Google Scholar]
- 8.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371(23):2167–2177. [DOI] [PubMed] [Google Scholar]
- 9.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368(25):2385–2394. [DOI] [PubMed] [Google Scholar]
- 10.Nishino M, Jackman DM, Hatabu H, Jänne PA, Johnson BE, Van den Abbeele AD. Imaging of lung cancer in the era of molecular medicine. Acad Radiol 2011;18(4):424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishino M, Hatabu H, Johnson BE, McLoud TC. State of the art: Response assessment in lung cancer in the era of genomic medicine. Radiology 2014;271(1):6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol 2014;11(8):473–481. [DOI] [PubMed] [Google Scholar]
- 13.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3(75):75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol 2018;29(suppl_1):i10–i19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005;2(3):e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372(18):1689–1699. [DOI] [PubMed] [Google Scholar]
- 17.Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2016;17(12):1643–1652. [DOI] [PubMed] [Google Scholar]
- 18.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378(2):113–125. [DOI] [PubMed] [Google Scholar]
- 19.Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018 Aug 28:JCO2018783118 [Epub ahead of print] 10.1200/JCO.2018.78.3118. [DOI] [PubMed] [Google Scholar]
- 20.Ortiz-Cuaran S, Scheffler M, Plenker D, et al. Heterogeneous Mechanisms of Primary and Acquired Resistance to Third-Generation EGFR Inhibitors. Clin Cancer Res 2016;22(19):4837–4847. [DOI] [PubMed] [Google Scholar]
- 21.Oxnard GR, Hu Y, Mileham KF, et al. Assessment of Resistance Mechanisms and Clinical Implications in Patients With EGFR T790M-Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncol 2018;4(11):1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uchibori K, Inase N, Araki M, et al. Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nat Commun 2017;8:14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377(9):829–838. [DOI] [PubMed] [Google Scholar]
- 24.Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med 2012;4(120):120ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costa DB, Shaw AT, Ou SH, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol 2015;33(17):1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ou SH, Ahn JS, De Petris L, et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 2016;34(7):661–668. [DOI] [PubMed] [Google Scholar]
- 27.Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 2016;17(2):234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camidge DR, Peters S, Mok T, et al. Updated efficacy and safety data from the global phase III ALEX study of alectinib (ALC) vs crizotinib (CZ) in untreated advanced ALK+ NSCLC. J Clin Oncol 2018;36(15_suppl):9043. [Google Scholar]
- 29.Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370(13):1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gettinger SN, Bazhenova LA, Langer CJ, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol 2016;17(12):1683–1696. [DOI] [PubMed] [Google Scholar]
- 31.Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 2018;19(12):1654–1667. [DOI] [PubMed] [Google Scholar]
- 32.Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol 2017;18(12):1590–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 2017;18(10):1307–1316. [DOI] [PubMed] [Google Scholar]
- 34.Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371(21):1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The Nobel Prize . The Nobel Prize in Physiology or Medicine. https://www.nobelprize.org/prizes/medicine/2018/summary. Published 2018. Accessed December 23, 2018.
- 36.Nishino M, Ramaiya NH, Hatabu H, Hodi FS. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol 2017;14(11):655–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishino M, Hatabu H, Hodi FS. Imaging of Cancer Immunotherapy: Current Approaches and Future Directions. Radiology 2019;290(1):9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breathnach OS, Freidlin B, Conley B, et al. Twenty-two years of phase III trials for patients with advanced non-small-cell lung cancer: sobering results. J Clin Oncol 2001;19(6):1734–1742. [DOI] [PubMed] [Google Scholar]
- 39.Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16(3):257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372(21):2018–2028. [DOI] [PubMed] [Google Scholar]
- 41.Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33(18):2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373(2):123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373(17):1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387(10027):1540–1550. [DOI] [PubMed] [Google Scholar]
- 45.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375(19):1823–1833. [DOI] [PubMed] [Google Scholar]
- 46.Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med 2018;378(22):2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378(22):2078–2092. [DOI] [PubMed] [Google Scholar]
- 48.Ready N, Farago AF, de Braud F, et al. Third-Line Nivolumab Monotherapy in Recurrent SCLC: CheckMate 032. J Thorac Oncol 2019;14(2):237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sholl LM, Do K, Shivdasani P, et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI Insight 2016;1(19):e87062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rolfo C, Mack PC, Scagliotti GV, et al. Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC. J Thorac Oncol 2018;13(9):1248–1268. [DOI] [PubMed] [Google Scholar]
- 51.Thress KS, Markovets A, Barrett JC, et al. Complete clearance of plasma EGFR mutations as a predictor of outcome on osimertinib in the AURA trial. J Clin Oncol 2017;35(15_suppl):9018. [Google Scholar]
- 52.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379(21):2040–2051. [DOI] [PubMed] [Google Scholar]
- 53.Gainor JF, Shaw AT, Sequist LV, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res 2016;22(18):4585–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15(23):7412–7420. [DOI] [PubMed] [Google Scholar]
- 55.Nishino M. Tumor Response Assessment for Precision Cancer Therapy: Response Evaluation Criteria in Solid Tumors and Beyond. Am Soc Clin Oncol Educ Book 2018;38(38):1019–1029. [DOI] [PubMed] [Google Scholar]
- 56.Nishino M, Dahlberg SE, Cardarella S, et al. Tumor volume decrease at 8 weeks is associated with longer survival in EGFR-mutant advanced non-small-cell lung cancer patients treated with EGFR TKI. J Thorac Oncol 2013;8(8):1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishino M, Dahlberg SE, Adeni AE, et al. Tumor Response Dynamics of Advanced Non-small Cell Lung Cancer Patients Treated with PD-1 Inhibitors: Imaging Markers for Treatment Outcome. Clin Cancer Res 2017;23(19):5737–5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishino M. Immune-related response evaluations during immune-checkpoint inhibitor therapy: establishing a “common language” for the new arena of cancer treatment. J Immunother Cancer 2016;4(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fujimoto D, Yoshioka H, Kataoka Y, et al. Pseudoprogression in Previously Treated Patients with Non-Small Cell Lung Cancer Who Received Nivolumab Monotherapy. J Thorac Oncol 2019;14(3):468–474. [DOI] [PubMed] [Google Scholar]
- 60.Champiat S, Dercle L, Ammari S, et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin Cancer Res 2017;23(8):1920–1928. [DOI] [PubMed] [Google Scholar]
- 61.Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive Disease in Patients With Advanced Non-Small Cell Lung Cancer Treated With PD-1/PD-L1 Inhibitors or With Single-Agent Chemotherapy. JAMA Oncol 2018;4(11):1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tirumani SH, Ramaiya NH, Keraliya A, et al. Radiographic Profiling of Immune-Related Adverse Events in Advanced Melanoma Patients Treated with Ipilimumab. Cancer Immunol Res 2015;3(10):1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nishino M, Tirumani SH, Ramaiya NH, Hodi FS. Cancer immunotherapy and immune-related response assessment: The role of radiologists in the new arena of cancer treatment. Eur J Radiol 2015;84(7):1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alessandrino F, Sahu S, Nishino M, et al. Frequency and imaging features of abdominal immune-related adverse events in metastatic lung cancer patients treated with PD-1 inhibitor. Abdom Radiol (NY) 2019 Feb 21 [Epub ahead of print] 10.1007/s00261-019-01935-2. [DOI] [PubMed] [Google Scholar]
- 65.Bronstein Y, Ng CS, Hwu P, Hwu WJ. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. AJR Am J Roentgenol 2011;197(6):W992–W1000. [DOI] [PubMed] [Google Scholar]
- 66.Wang DY, Salem JE, Cohen JV, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4(12):1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2016;2(12):1607–1616. [DOI] [PubMed] [Google Scholar]
- 68.Nishino M, Hatabu H, Sholl LM, Ramaiya NH. Thoracic Complications of Precision Cancer Therapies: A Practical Guide for Radiologists in the New Era of Cancer Care. RadioGraphics 2017;37(5):1371–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nishino M, Ramaiya NH, Awad MM, et al. PD-1 Inhibitor-Related Pneumonitis in Advanced Cancer Patients: Radiographic Patterns and Clinical Course. Clin Cancer Res 2016;22(24):6051–6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nishino M, Chambers ES, Chong CR, et al. Anti-PD-1 Inhibitor-Related Pneumonitis in Non-Small Cell Lung Cancer. Cancer Immunol Res 2016;4(4):289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nishino M, Sholl LM, Hodi FS, Hatabu H, Ramaiya NH. Anti-PD-1-Related Pneumonitis during Cancer Immunotherapy. N Engl J Med 2015;373(3):288–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Antonia SJ, Özgüroğlu M. Durvalumab in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2018;378(9):869–870. [DOI] [PubMed] [Google Scholar]
- 73.Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379(24):2342–2350. [DOI] [PubMed] [Google Scholar]
- 74.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377(20):1919–1929. [DOI] [PubMed] [Google Scholar]
- 75.Nishino M, Boswell EN, Hatabu H, Ghobrial IM, Ramaiya NH. Drug-Related Pneumonitis During Mammalian Target of Rapamycin Inhibitor Therapy: Radiographic Pattern-Based Approach in Waldenström Macroglobulinemia as a Paradigm. Oncologist 2015;20(9):1077–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nishino M, Brais LK, Brooks NV, Hatabu H, Kulke MH, Ramaiya NH. Drug-related pneumonitis during mammalian target of rapamycin inhibitor therapy in patients with neuroendocrine tumors: a radiographic pattern-based approach. Eur J Cancer 2016;53:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Min JH, Lee HY, Lim H, et al. Drug-induced interstitial lung disease in tyrosine kinase inhibitor therapy for non-small cell lung cancer: a review on current insight. Cancer Chemother Pharmacol 2011;68(5):1099–1109. [DOI] [PubMed] [Google Scholar]
- 78.Ministry of Health, Labour and Welfare (Japan) . Precautions relating to interstitial lung disease during administration of epidermal growth factor receptor tyrosine kinase inhibitors. Pharmaceuticals and Medical Devices Safety Information. 2016;336:4. http://www.pmda.go.jp/files/000214024.pdf#page=4.pdf. Published September 2016. Accessed July 15, 2019. [Google Scholar]
- 79.Ahn MJ, Yang J, Yu H, et al. 136O: Osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: Results from the TATTON phase Ib trial. J Thorac Oncol 2016;11(4 Suppl):S115. [Google Scholar]
- 80.Kotake M, Murakami H, Kenmotsu H, Naito T, Takahashi T. High incidence of interstitial lung disease following practical use of osimertinib in patients who had undergone immediate prior nivolumab therapy. Ann Oncol 2017;28(3):669–670. [DOI] [PubMed] [Google Scholar]
- 81.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 82.Nishino M, Jagannathan JP, Ramaiya NH, Van den Abbeele AD. Revised RECIST guideline version 1.1: What oncologists want to know and what radiologists need to know. AJR Am J Roentgenol 2010;195(2):281–289. [DOI] [PubMed] [Google Scholar]
- 83.Zhao B, James LP, Moskowitz CS, et al. Evaluating variability in tumor measurements from same-day repeat CT scans of patients with non-small cell lung cancer. Radiology 2009;252(1):263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jennings SG, Winer-Muram HT, Tarver RD, Farber MO. Lung tumor growth: assessment with CT--comparison of diameter and cross-sectional area with volume measurements. Radiology 2004;231(3):866–871. [DOI] [PubMed] [Google Scholar]
- 85.Nishino M, Dahlberg SE, Fulton LE, et al. Volumetric Tumor Response and Progression in EGFR-mutant NSCLC Patients Treated with Erlotinib or Gefitinib. Acad Radiol 2016;23(3):329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee JH, Lee HY, Ahn MJ, et al. Volume-based growth tumor kinetics as a prognostic biomarker for patients with EGFR mutant lung adenocarcinoma undergoing EGFR tyrosine kinase inhibitor therapy: a case control study. Cancer Imaging 2016;16(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hida T, Dahlberg SE, Lydon CA, et al. Tumor Volume Analysis as a Predictive Marker for Prolonged Survival in Anaplastic Lymphoma Kinase-rearranged Advanced Non-Small Cell Lung Cancer Patients Treated With Crizotinib. J Thorac Imaging 2019 Apr 12 [Epub ahead of print] 10.1097/RTI.0000000000000413. [DOI] [PubMed] [Google Scholar]
- 88.Gomez-Roca C, Koscielny S, Ribrag V, et al. Tumour growth rates and RECIST criteria in early drug development. Eur J Cancer 2011;47(17):2512–2516. [DOI] [PubMed] [Google Scholar]
- 89.Nishino M, Dahlberg SE, Cardarella S, et al. Volumetric tumor growth in advanced non-small cell lung cancer patients with EGFR mutations during EGFR-tyrosine kinase inhibitor therapy: developing criteria to continue therapy beyond RECIST progression. Cancer 2013;119(21):3761–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nishino M, Wakai S, Hida T, et al. Automated image analysis tool for tumor volume growth rate to guide precision cancer therapy: EGFR-mutant non-small-cell lung cancer as a paradigm. Eur J Radiol 2018;109:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee G, Lee HY, Park H, et al. Radiomics and its emerging role in lung cancer research, imaging biomarkers and clinical management: State of the art. Eur J Radiol 2017;86:297–307. [DOI] [PubMed] [Google Scholar]
- 92.Lambin P, Leijenaar RTH, Deist TM, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 2017;14(12):749–762. [DOI] [PubMed] [Google Scholar]
- 93.Constanzo J, Wei L, Tseng HH, El Naqa I. Radiomics in precision medicine for lung cancer. Transl Lung Cancer Res 2017;6(6):635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rizzo S, Petrella F, Buscarino V, et al. CT Radiogenomic Characterization of EGFR, K-RAS, and ALK Mutations in Non-Small Cell Lung Cancer. Eur Radiol 2016;26(1):32–42. [DOI] [PubMed] [Google Scholar]
- 95.Liu Y, Kim J, Balagurunathan Y, et al. Radiomic Features Are Associated With EGFR Mutation Status in Lung Adenocarcinomas. Clin Lung Cancer 2016;17(5):441–448.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hayano K, Kulkarni NM, Duda DG, Heist RS, Sahani DV. Exploration of Imaging Biomarkers for Predicting Survival of Patients With Advanced Non-Small Cell Lung Cancer Treated With Antiangiogenic Chemotherapy. AJR Am J Roentgenol 2016;206(5):987–993. [DOI] [PubMed] [Google Scholar]
- 97.Cook GJ, Yip C, Siddique M, et al. Are pretreatment 18F-FDG PET tumor textural features in non-small cell lung cancer associated with response and survival after chemoradiotherapy? J Nucl Med 2013;54(1):19–26. [DOI] [PubMed] [Google Scholar]
- 98.Cook GJ, O’Brien ME, Siddique M, et al. Non-Small Cell Lung Cancer Treated with Erlotinib: Heterogeneity of (18)F-FDG Uptake at PET-Association with Treatment Response and Prognosis. Radiology 2015;276(3):883–893. [DOI] [PubMed] [Google Scholar]
- 99.Sun R, Limkin EJ, Vakalopoulou M, et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol 2018;19(9):1180–1191. [DOI] [PubMed] [Google Scholar]
- 100.Sun X, Xiao Z, Chen G, et al. A PET imaging approach for determining EGFR mutation status for improved lung cancer patient management. Sci Transl Med 2018;10(431):eaan8840. [DOI] [PubMed] [Google Scholar]
- 101.Petrulli J, Contessa J, Morris E. Texture analysis based stratification of non-small cell lung cancer type using 11C-erlotinib PET. J Nucl Med 2015;56(Suppl 3):125. [Google Scholar]
- 102.Natarajan A, Mayer AT, Reeves RE, Nagamine CM, Gambhir SS. Development of Novel ImmunoPET Tracers to Image Human PD-1 Checkpoint Expression on Tumor-Infiltrating Lymphocytes in a Humanized Mouse Model. Mol Imaging Biol 2017;19(6):903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mayer AT, Natarajan A, Gordon SR, et al. Practical Immuno-PET Radiotracer Design Considerations for Human Immune Checkpoint Imaging. J Nucl Med 2017;58(4):538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Heskamp S, Hobo W, Molkenboer-Kuenen JD, et al. Noninvasive Imaging of Tumor PD-L1 Expression Using Radiolabeled Anti-PD-L1 Antibodies. Cancer Res 2015;75(14):2928–2936. [DOI] [PubMed] [Google Scholar]
- 105.Bensch F, van der Veen EL, Lub-de Hooge MN, et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med 2018;24(12):1852–1858. [DOI] [PubMed] [Google Scholar]
- 106.Maute RL, Gordon SR, Mayer AT, et al. Engineering high-affinity PD-1 variants for optimized immunotherapy and immuno-PET imaging. Proc Natl Acad Sci U S A 2015;112(47):E6506–E6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Natarajan A, Mayer AT, Xu L, Reeves RE, Gano J, Gambhir SS. Novel Radiotracer for ImmunoPET Imaging of PD-1 Checkpoint Expression on Tumor Infiltrating Lymphocytes. Bioconjug Chem 2015;26(10):2062–2069. [DOI] [PubMed] [Google Scholar]
- 108.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13(3):239–246. [DOI] [PubMed] [Google Scholar]
- 109.Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol 2015;26(9):1877–1883. [DOI] [PubMed] [Google Scholar]
- 110.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362(25):2380–2388. [DOI] [PubMed] [Google Scholar]
- 111.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11(2):121–128. [DOI] [PubMed] [Google Scholar]
- 112.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31(27):3327–3334. [DOI] [PubMed] [Google Scholar]
- 113.Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15(2):213–222. [DOI] [PubMed] [Google Scholar]
- 114.Yang JC, Shih JY, Su WC, et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): a phase 2 trial. Lancet Oncol 2012;13(5):539–548. [DOI] [PubMed] [Google Scholar]
- 115.Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376(7):629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ahn MJ, Tsai CM, Shepherd FA, et al. Osimertinib in patients with T790M mutation-positive, advanced non-small cell lung cancer: Long-term follow-up from a pooled analysis of 2 phase 2 studies. Cancer 2019;125(6):892–901. [DOI] [PubMed] [Google Scholar]
- 117.Ramalingam SS, Yang JC, Lee CK, et al. Osimertinib As First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36(9):841–849. [DOI] [PubMed] [Google Scholar]
- 118.Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017 Nov;18(11):1454–1466. [DOI] [PubMed] [Google Scholar]
- 119.Shaw AT, Kim TM, Crinò L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2017;18(7):874–886. [DOI] [PubMed] [Google Scholar]
- 120.Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389(10072):917–929. [DOI] [PubMed] [Google Scholar]
- 121.Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol 2017;35(22):2490–2498. [DOI] [PubMed] [Google Scholar]
- 122.Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17(11):1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389(10066):255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378(24):2288–2301. [DOI] [PubMed] [Google Scholar]
- 125.Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379(23):2220–2229. [DOI] [PubMed] [Google Scholar]
- 126.Suh CH, Park HS, Kim KW, Pyo J, Hatabu H, Nishino M. Pneumonitis in advanced non-small-cell lung cancer patients treated with EGFR tyrosine kinase inhibitor: Meta-analysis of 153 cohorts with 15,713 patients: Meta-analysis of incidence and risk factors of EGFR-TKI pneumonitis in NSCLC. Lung Cancer 2018;123:60–69. [DOI] [PubMed] [Google Scholar]
- 127.Khunger M, Rakshit S, Pasupuleti V, et al. Incidence of Pneumonitis With Use of Programmed Death 1 and Programmed Death-Ligand 1 Inhibitors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Trials. Chest 2017;152(2):271–281. [DOI] [PubMed] [Google Scholar]
- 128.Takeda M, Okamoto I, Nakagawa K. Survival outcome assessed according to tumor response and shrinkage pattern in patients with EGFR mutation-positive non-small-cell lung cancer treated with gefitinib or erlotinib. J Thorac Oncol 2014;9(2):200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tamura T, Kiura K, Seto T, et al. Three-Year Follow-Up of an Alectinib Phase I/II Study in ALK-Positive Non-Small-Cell Lung Cancer: AF-001JP. J Clin Oncol 2017;35(14):1515–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.