Abstract
Microcystin-LR (MC-LR) is considered the most common hazardous toxin produced during harmful algal blooms. In addition to potential risk of long-term exposure to low concentrations in drinking water, acute toxicity due to MC-LR resulting from algal blooms could result in fatalities in rare cases. Although several methods are currently available to detect MC-LR, development of a low-cost, ultrasensitive measurement method would help limit exposure by enabling early detection and continuous monitoring of MC-LR. Here, we develop a surface-enhanced Raman scattering (SERS) spectroscopic immunosensor for detection and quantification of the hepatotoxic MC-LR toxin in aquatic settings with excellent robustness, selectivity and sensitivity. We have demonstrated that the developed SERS sensor can reach a limit of detection (0.014 μg/L) at least one order of magnitude lower and display a linear dynamic detection range (0.01 μg/L to 100 μg/L) two orders of magnitude wider in comparison to the commercial enzyme-linked immunosorbent assay test. The superior analytical performance of this SERS immunosensor enables monitoring of the dynamic production of MC-LR from a Microcystis aeruginosa culture. We believe that the present method could serve as a useful tool for detection of hepatotoxic microcystin toxins in various aquatic settings such as drinking water, lakes and reservoirs. Further development of this technique could result in single-cell microcystin resolution or real-time monitoring to mitigate the associated toxicity and economic loss.
Keywords: surface-enhanced Raman scattering, microcystin-LR, toxins, biosensors, algal blooms
Harmful algal bloom (HAB) outbreaks fueled by pollution from modern industrial, agricultural and domestic activities exert strong adverse impacts on water safety and public health. Microcystis aeruginosa, a common and wide-spread member of toxic blooms, produces several known toxins, whose accumulation in human body via prolonged exposure is extremely harmful.1–3 Microcystin-LR (MC-LR), a cyclic heptapeptide with seven amino acids, is considered one of the most common and the most dangerous cyanotoxins produced in all cyanobacterial blooms.4 MC-LR is strongly hepatotoxic to mammals and its toxicological mechanism originates from the strong inhibition activities of protein phosphatase type 1 and type 2A in the cytoplasm of liver cells, which not only causes acute liver failure but also promotes liver cancer through long-term exposure even at low doses.5 The World Health Organization (WHO) has proposed a limit of 1 μg/L for total MC-LR in drinking water.6 Microcystin-related events have been occurring globally with expanding frequency, duration and intensity in lakes, reservoirs and river systems over the course of human history. Many factors, such as increasing discharge of industrial and domestic wastewater and global warming resulting from the climate change, are expected to cause an increase in the number of MC-LR associated HAB outbreaks.7,8 As a result, monitoring fresh water sources and water treatment plants for MC-LR contamination is critical to ensure adherence to safety standards.
To date, many analytical methods have been used for detection and quantification of MC-LR, including enzyme-linked immunosorbent assay (ELISA), high-performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS), protein phosphatase inhibition assay (PPIA), and optical or electrochemical techniques.9,10 However, most of these methods are either expensive, time-consuming or require expertise, which renders them unfeasible for implementation in longitudinal monitoring applications. While PPIA and ELISA have emerged as economical methods, they lack high sensitivity, particularly at low concentrations.11 Therefore, there is a pressing need to develop sensitive analytical methods for the reliable detection of MC-LR that can readily be adapted for continuous monitoring and multiplexing applications.
Surface-enhanced Raman scattering (SERS) has emerged as a powerful analytical technique because of its high sensitivity and signal specificity. SERS exploits the localized surface plasmon resonance (LSPR) to amplify the Raman signal of specimen in the vicinity of plasmonic substrates (e.g., Au, Ag) with an enhancement factor of up to 1014.12–15 Due to the advantages such as simplicity, photostability, quantifiability and multiplexing capability, SERS is emerging as an analytical method in applications ranging from biomedical imaging to chemical sensing.12,13,16–19 Few studies have exemplified the success of Raman spectroscopy20 or SERS techniques21 for detection of MC-LR in aquatic settings with superior performance in terms of excellent specificity or high sensitivity. Yet, a SERS-based test for detection of MC-LR has not been translated to the field. To address the need for monitoring MC-LR in fresh water samples, we propose a SERS immunosensor using our previously developed SERS tags, comprising of a plasmonic gold nanostar (GNS) core, Raman reporter molecules and a protective silica shell.22–27 These SERS tags were shown to be excellent alternatives for fluorescent labels widely employed in fluorescence-based sensing and imaging applications.22–28 Due to the presence of dense “hot-spots” around the sharp tips, the GNSs offer an excellent opportunity for achieving significant Raman signal amplification of Raman reporter molecules adsorbed onto their surface.28,29 The protective layer of silica coated onto the Raman molecule modified GNS improves the stability and reproducibility of the SERS immunosensing platform. The MC-LR-specific antibody was covalently attached onto the SERS tag (GNS@Raman reporters@SiO2) to construct the SERS probes (SERS tags with the MC-LR targeting antibody) specific to the MC-LR target that exhibited high SERS intensity, excellent water solubility and biocompatibility. The MC-LR in freshwater samples was then sandwiched between antibody modified quartz substrate and the developed SERS probes for quantitative measurement.
The applicability was further assessed by subjecting the SERS immunosensor to real lake water samples and their derivatives obtained by additional spiking with MC-LR and its structural analogs MC-RR and MC-YR. The observed capabilities of the current assay exceeded the performance of existing comparable rapid methods such as ELISA. Furthermore, to assess the robustness of the developed method for continuous monitoring of changes in MC-LR levels in aquatic settings, we used active cultures of hepatoxic MC-LR producing Microcystis aeruginosa to accurately quantify the production of MC-LR from cells over time. Taken together, our findings highlight the potential of SERS as an ultrasensitive tool for monitoring MC-LR in water samples.
RESULTS AND DISCUSSION
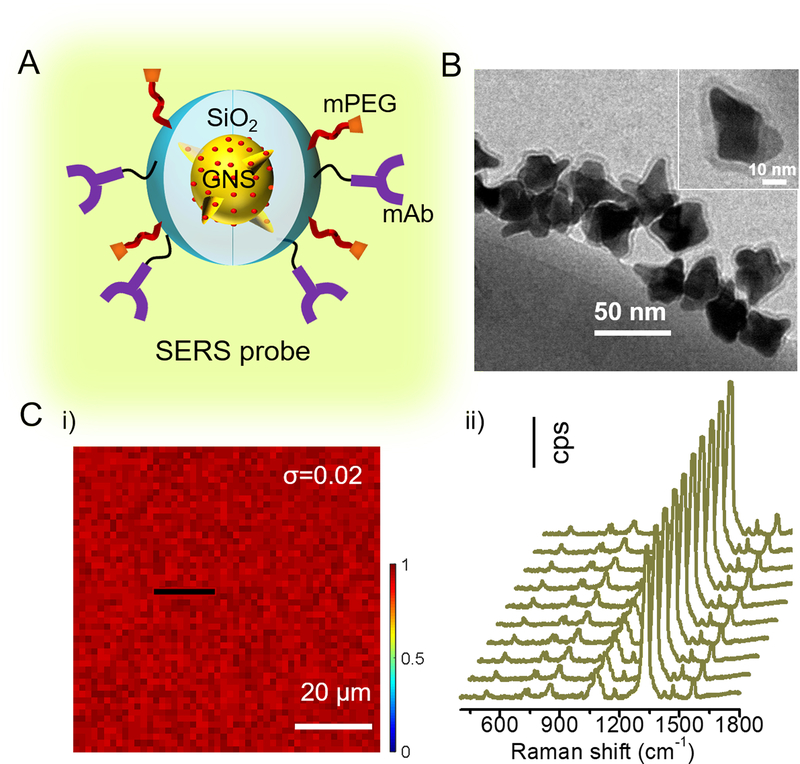
To selectively detect MC-LR, a sandwich assay comprising of the surface functionalized quartz substrate and SERS tags was developed. The SERS tags used in this work were prepared following a three-step process involving – (i) synthesis of GNS nanoparticles, (ii) adsorption of 4-nitrothiophenol (NTP) as Raman reporter molecules on the GNS nanoparticles, and (iii) coating of the nanoparticles with a thin protective silica shell (Figure S1). GNSs were chosen as the plasmonic substrate because of their high SERS enhancement factor, intrinsic high-density “hot spots” and easy tunability of LSPR bands into the near-infrared (NIR) region.28 Here, we tuned the LSPR extinction maximum of the GNSs to lie at 750 nm (Figure S2) in order to achieve an ensemble-averaged SERS enhancement factor of ~2.5×104 under the 785 nm NIR laser excitation.28 The NTP molecules used as the Raman reporter can tightly adsorb and anchor around the GNS surface owing to the strong affinity between the thiol group and the gold surface.30 To contain the Raman reporter tightly around the GNS, a thin silica shell was coated to complete the sandwich structure of SERS tags (Figure 1A). Here, we called the GNS@NTP@SiO2 structure as the SERS tag. Transmission electron microscopic (TEM) measurements were used to confirm the uniform coating of a thin (3–5 nm) dense silica shell around the GNS structure, preventing the NTP molecule desorption from the GNS surface due to their exposure to the harsh environments in practical applications (Figure 1B). The extinction spectra of the SERS tags showed a 36 nm red-shift after coating a silica shell relative to that of GNS nanoparticles (Figure S2), which is attributed to the change in refractive index.31 The surface of the SERS tags was modified using methoxy-poly(ethylene glycol) (mPEG)-silane to improve water solubility and reduce surface fouling during the following operations, and carboxyl-termination was achieved by (3-triethoxysilyl) propylsuccinic anhydride (TEPSA) modification.32 Finally, the MC-LR specific SERS probes (SERS tags modified with the MC-LR targeting monoclonal antibody (mAb)) were obtained by activation of modified SERS tags with N-hydroxysulfosuccinimide (sulfo-NHS) and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC), and addition of the MC-LR mAb (Figures 1A and S1). The high concentration of MC-LR mAb used ensured enough coating on the substrate as well as the probes to facilitate the SERS detection.33
Figure 1. Structure and characterization of SERS probes.
(A) Schematic structure of the SERS probe where a large number of Raman reporter (4-nitrothiophenol, NTP) molecules were tightly sandwiched between the gold nanostar core and the thin silica shell, followed by co-modification with mPEG and mAb. (B) Representative TEM image of the SERS tags, showing a 3–5 nm thick silica shell coated onto the gold nanostar surface. Inset in (B) shows a representative TEM image of a single SERS tag, clearly showing the uniform coating of silica onto the gold nanostar. (C) (i) SERS image of the developed SERS probes, obtained from a pre-defined well with a drop of mPEG and mAb-modified SERS probes is shown; (ii) representative SERS spectra collected from the marked black line in (i). The SERS image was constructed using the stretching vibration Raman band of N-O at 1342 cm−1. The SERS intensity was normalized by the maximum SERS intensity at 1342 cm−1 in the dataset. The uniform distribution of SERS intensity justifies the high quality of SERS probes prepared in this work.
The quality of the synthesized SERS probes was evaluated by drop-coating a monolayer onto pre-defined wells on the assay panel, performing SERS imaging over an area of 80×80 μm2 with a spatial resolution of 1.6 μm in both x and y directions. The SERS image created using the 1342 cm−1 N–O peak intensity shows the uniformity of the SERS signal (standard deviation, σ = 0.02) across the region examined (Figure 1C). Additionally, Figure 1C(ii) shows the representative SERS spectra from the marked black line in Figure 1C(i), to further visualize the spatial and spectral uniformity of the SERS probes deployed in the present work. These unique features present great benefits for constructing sensing and bioimaging platforms.
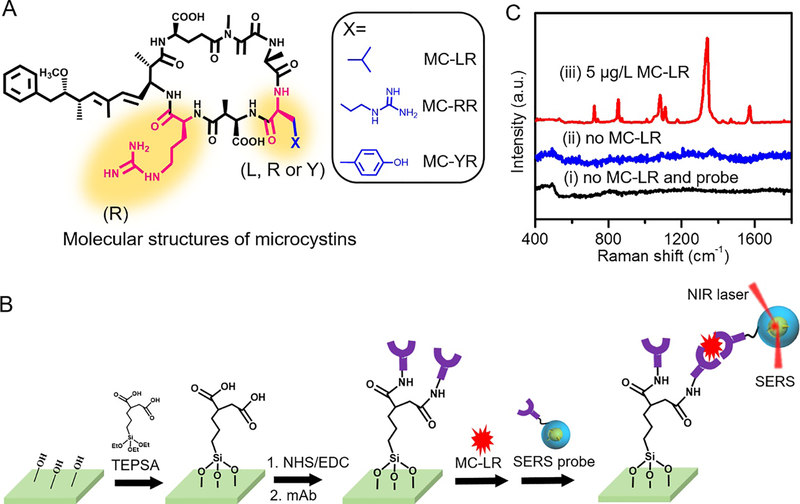
As the most toxic toxin of the microcystin family,4 MC-LR is a small peptide with minor amino acid differences in their sequence in comparison to its two popular analogs – microcystin-RR (MC-RR) and microcystin-YR (MC-YR) (Figure 2A). In this work, we focus on developing the SERS platform for selective detection of MC-LR. The unique molecular structure of MC-LR allows us to develop an immunoassay format using the antibody-antigen type interaction. Thus, our design of the present SERS immunoassay is based on the specific recognition of mAb toward MC-LR. Figure 2B outlines the fabrication process and operation principle of the overall SERS immunoassay platform. Briefly, the quartz chip was carboxyl-terminated by TEPSA modification following treatment by oxygen plasma. The MC-LR mAb was then immobilized onto the pre-defined wells on the quartz chip using the standard amine coupling chemistry. The sequential addition of MC-LR analytes and SERS probes leads to assembly of the sandwich structure, which is then subjected to the SERS measurements.
Figure 2. Detection of MC-LR.
(A) Molecular structures of microcystin-LR, microcystin-RR and microcystin-YR. (B) Schematic illustration of fabrication of the SERS immunoassay platform and its operation principle for microcystin-LR detection. (C) Representative SERS spectra obtained from the developed assay – (i) in the absence of both MC-LR analyte and SERS probes, (ii) in the presence of SERS probes and absence of MC-LR analyte and (iii) in the presence of both 5 μg/L MC-LR analytes and SERS probes.
To test the developed assay for detection of MC-LR, control experiments were first performed at three different conditions – (i) in the absence of both MC-LR and SERS probes, (ii) in the presence of SERS probes and absence of MC-LR, and (iii) in the presence of both 5 μg/L MC-LR and 0.5 nM SERS probes. As illustrated in Figure 2C, the SERS spectra of the NTP Raman reporter molecules present in the SERS tags were very strong in the presence of both MC-LR analytes and SERS probes due to the sandwiching of MC-LR analytes between the capture and detection probes. However, no discernable features were observed in the absence of either the analyte or the probes, indicating no capture of MC-LR analytes. This confirms the inhibition of non-specific adsorption of SERS probes onto the albumin from bovine serum (BSA) modified quartz chip, due to the mPEG coating of SERS probes. Among the major features of the spectra obtained from the sandwich structure assembled in the presence of MC-LR analyte and developed SERS probes, the stretching vibration of N-O in NTP molecules that results in a peak at 1342 cm−1 was chosen for further quantitative analyses in this study. Additionally, the peak at 1574 cm−1 can be assigned to the stretching vibration of phenyl ring, whereas the two peaks observed at 1107 cm−1 and 855 cm−1 stem from the C-H bending and wagging vibrations, respectively. Detailed assignments of the observed SERS peaks can be found in the literature.17,18,22 Not only does this test demonstrate the specificity of the developed SERS probes for MC-LR, but also their suitability for potential applications in sensitive detection of specific analytes of interest.
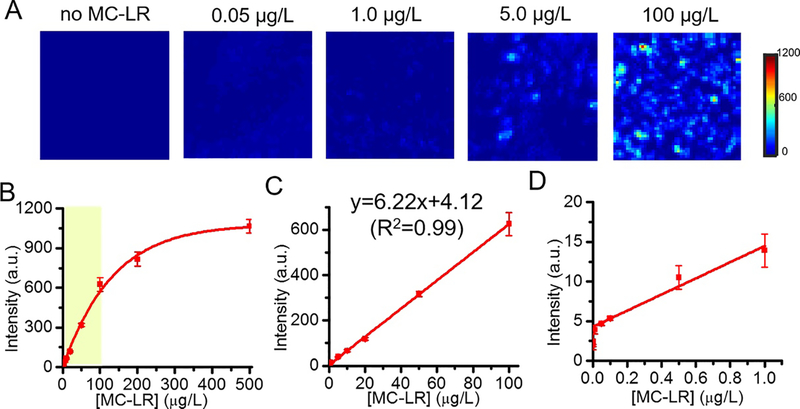
Next, detection sensitivity and linear dynamic range of the reported SERS immunoassay were quantified for determining the feasibility of MC-LR detection at trace levels. To evaluate the sensitivity of the SERS immunoassay, the SERS measurements were performed at a controlled series of concentrations of MC-LR ranging from 0 to 500 μg/L (Figure 3). The spectra were recorded from the wells on the chip as described above and the SERS images of different MC-LR concentrations were constructed using the 1342 cm−1 peak (Figure 3A). It can be noted upon visual inspection of the maps that the SERS intensity increases with increasing MC-LR concentration (Figure S3). To correlate the SERS measurements with MC-LR concentrations, the peak intensity at 1342 cm−1 averaged over the examined area (80×80 μm2) was plotted as a function of the MC-LR concentration for each sample (Figure 3B). The SERS intensity increases first with the increasing MC-LR concentration and subsequently saturates after ca. 500 μg/L. The dynamic range, i.e. the region where the SERS intensity scales linearly with the concentration, extends from ~ 0.01 μg/L to 100 μg/L (Figure 3C,D). Therefore, the dynamic range that spans four orders of magnitude shows significant superiority of the developed SERS immunoassay (two orders of magnitude wider) over the commercial ELISA kit (0.1 μg/L – 2.5 μg/L). The assay exhibited a limit of detection (LOD) of 0.014 μg/L (computed according to the IUPAC definition, LOD=3σ/s),34,35 which is one order of magnitude lower than the commercial ELISA kit (0.1 μg/L). In the linear region, the calibration curve was fitted with a straight line as y=6.22x+4.12 (R2=0.99). The standard deviation (σ) values are shown in the calibration curve of Figure 3, and the relative standard deviation (RSD) values ranged from 4% to 15% over the dynamic range (where RSD is calculated as σ/m×100%, where m is the mean value).36–38 These low RSD values underscore the excellent reproducibility of the SERS immunosensor for detection of MC-LR. These quantitative improvements in detection range and sensitivity establish the developed SERS immunoassay as a superior alternative to the commercial ELISA kit for accurate determination of trace levels of MC-LR in water samples.
Figure 3. Quantification of MC-LR using the developed SERS immunosensor.
(A) Representative SERS images constructed using the 1342 cm−1 peak intensity from the SERS immunoassay (Field of view: 80×80 μm2) at different MC-LR concentrations (0, 0.05, 1.0, 5.0 and 100 μg/L). (B) The plot of the SERS intensity at 1342 cm−1 as a function of the MC-LR concentration. (C,D) The zoom-in versions of the linear regions of concentration-SERS intensity curve in (B). Experiments were performed in triplicates, and the error bars at each concentration of MC-LR represent the standard deviation of the SERS intensity from three independent experiments.
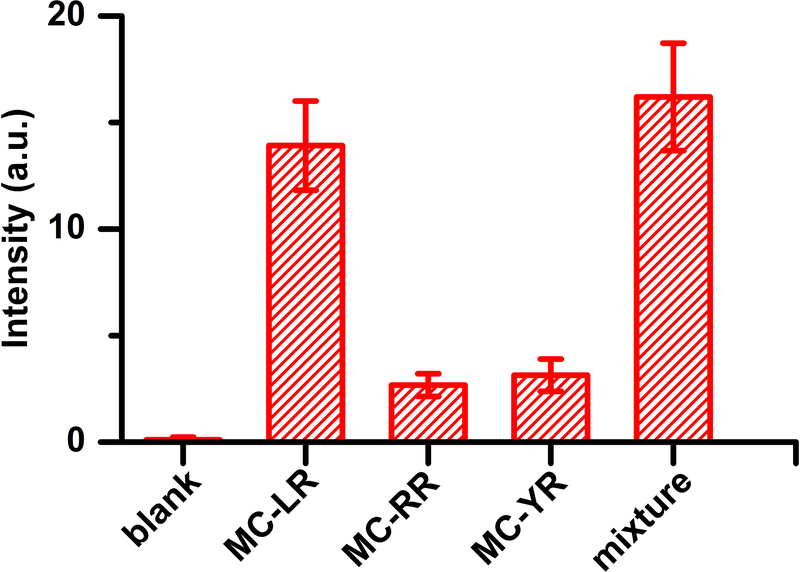
The specificity of the SERS immunoassay to MC-LR was further examined by performing the SERS measurement in the presence of other closely related analogs – MC-RR and MC-YR (the most popular analogs of MC-LR). As shown in Figures 4 and S4, the SERS intensity is significantly higher at 1 μg/L MC-LR than that at 10 μg/L MC-RR or MC-YR. To test the cross-reactivity, we also examined the SERS response from a mixture containing 1 μg/L MC-LR, 10 μg/L MC-RR and 10 μg/L MC-YR. It can be seen that the mixture shows SERS signal similar to that of 1 μg/L MC-LR alone. Therefore, it can be inferred that there is negligible interference introduced in the MC-LR detection by the simultaneous abundant presence of MC-RR and MC-YR. The excellent selectivity of our present method toward MC-LR over its analogs, MC-RR and MC-YR, can be attributed to the specific interaction between MC-LR and the used mAb and the clearance of unbound SERS probes from the assay during formation of the sandwich structure.
Figure 4. Specificity of SERS immunoassay toward MC-LR.
SERS intensity at 1342 cm−1 in the absence of (blank) or the presence of 1 μg/L MC-LR, 10 μg/L MC-RR, 10 μg/L MC-YR or their mixture containing 1 μg/L MC-LR, 10 μg/L MC-RR and 10 μg/L MC-YR. Experiments were performed in triplicates, and the error bars represent the standard deviation from three independent experiments.
To evaluate the suitability of the developed SERS immunoassay for the detection of MC-LR in real samples, lake samples that were collected from the Upper Mystic Lake in Massachusetts were analyzed by both the developed SERS immunoassay method and the commercial ELISA kit (Table 1). The original water sample (“lake+sample 0”) was spiked with the stock solution of MC-LR to obtain the remaining samples (“lake+sample 1, lake+sample 2, lake+sample 3”) in Table 1. We found that the concentration estimates obtained by using the SERS immunoassay were comparable to those determined by ELISA for samples whose concentrations were above its LOD (0.1 μg/L). However, the SERS immunoassay could detect 0.03 μg/L of MC-LR in the original sample that had no detectable MC-LR presence (according to the ELISA test). This sensitive measurement was made possible due to the lower LOD (0.01 μg/L) of the SERS immunoassay in comparison to that of ELISA (0.1 μg/L). We also calculated the recovery percentage, which is equal to (C2-C0)/C1×100, where C0, C1 and C2 are the MC-LR concentration (0.03 μg/L) in the Mystic Lake water samples, the nominal spiked MC-LR concentration, and the determined MC-LR concentration by the present SERS immunoassay of samples with spiked MC-LR, respectively.36–39 We observe an expected monotonic decrease in RSD with increasing MC-LR concentration. Together, these results demonstrate that the developed sensor is sensitive to heterogeneous contaminants in the lake water samples, and offers great promise for the detection and quantification of MC-LR in real samples.
Table 1.
MC-LR levels of original lake water sample and its MC-LR spiked variants tested by the present SERS method and the commercial ELISA kit.
| Sample | Spiked [MC-LR] μg/L | ELISA μg/L | SERS immunoassay μg/L | RSD (%) | Recovery percentage |
|---|---|---|---|---|---|
| Lake+sample 0 | 0 | undetectable | 0.03 | 17 | NA |
| Lake+sample 1 | 0.21 | 0.23 | 0.22 | 14 | 105% |
| Lake+sample 2 | 0.76 | 0.73 | 0.81 | 8.0 | 107% |
| Lake+sample 3 | 6.10 | 6.40 | 6.12 | 1.8 | 100% |
Note: Lake+sample 0 is the Mystic Lake surface water collected from the Upper Mystic Lake in Massachusetts without the addition of MC-LR; Lake+samples 1, 2 and 3 were generated by spiking with the stock solution of MC-LR into the Mystic Lake water sample; RSD is the relative standard deviation.
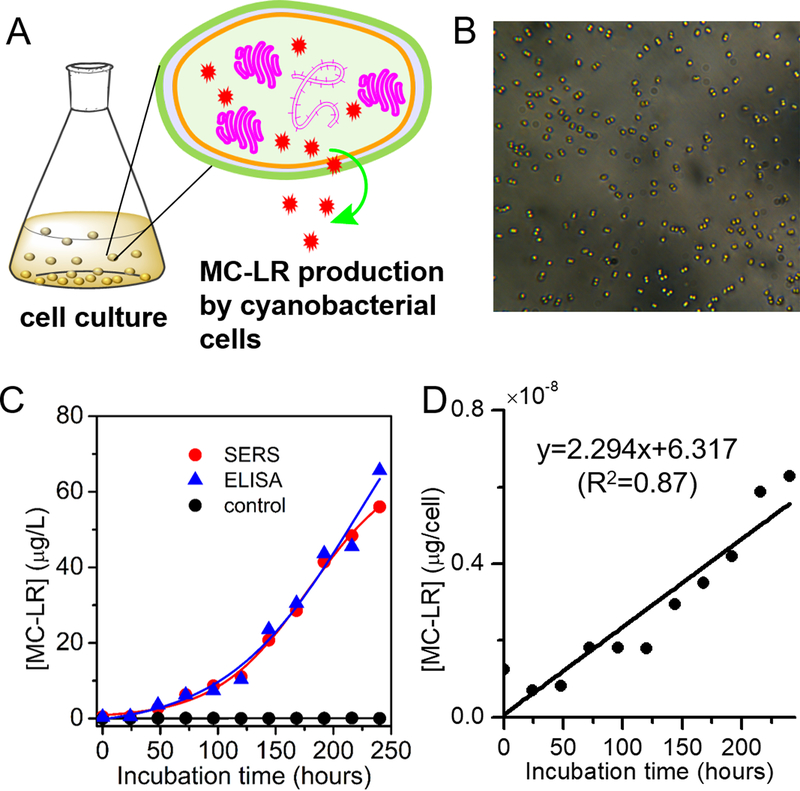
The successful implementation of any biosensor requires the ability to use it for dynamic monitoring of the analytes over a long period of time. The ability to monitor the changes in MC-LR over time is critical to limiting human exposure. To test the feasibility of using this method for monitoring purposes, we monitored the production of MC-LR from cells in culture. Here, the developed SERS immunoassay was deployed to investigate the production of MC-LR from a toxin producer – Microcystis aeruginosa UTEX LB 2385 in comparison to Microcystis aeruginosa UTEX LB 2386, a non-toxin producer control (Figure 5). As shown in Figure 5C, the MC-LR level increases monotonically with the increasing incubation time for Microcystis aeruginosa UTEX LB 2385 and the growth curves obtained using both SERS immunoassay and ELISA kit were similar to each other. As expected, the non-toxin producer Microcystis aeruginosa UTEX 2386 did not show any detectable MC-LR production upon incubation.
Figure 5. Dynamic production of MC-LR from Microcystis aeruginosa.
(A) Schematic illustration of production of MC-LR by cells in Microcystis aeruginosa culture. (B) Optical image of cyanobacterial cells. (C) MC-LR levels produced by toxic cyanobacteria (Microcystis aeruginosa UTEX LB 2385) and the control, non-toxic cyanobacteria (Microcystis aeruginosa UTEX LB 2386) over the course of incubation (0 to 240 hours) tested the SERS immunoassay and compared to the measurements obtained by the commercial ELISA kit. Samples with MC-LR concentrations > 2.0 μg/L were diluted prior to the ELISA measurement, taking the narrow dynamic range of the ELISA kit into account. (D) MC-LR production rate by the toxic cyanobacteria from single cyanobacterial cells, which was measured by dividing the total MC-LR level by the calculated abundance of Microcystis aeruginosa.
Multiple factors play important roles in the production of MC-LR40–42 and monitoring cell specific production rate will aid in understanding which factors are most important. To decouple the increase in MC-LR from increase in the number of cells producing microcystin over time, real-time quantitative polymerase chain reaction (qPCR) assay was used to determine the abundance of Microcystis aeruginosa population through quantification of 16S rRNA gene.43 Measuring 16S rRNA gene copy number as a proxy for cellular abundances enabled us to quantify the MC-LR production per cell as a function of time. As shown in Figure 5D, the MC-LR produced per cell increases almost linearly as a function of incubation time. The feasibility of employing the developed SERS immunoassay for monitoring applications, together with its high specificity and low LOD, makes it a promising tool not only for quantifying accumulation of MC-LR due to biosynthesis but also for monitoring of natural or treated water samples where concentrations are routinely below the LODs of other methods, such as ELISA.
CONCLUSIONS
In summary, we have developed a very robust, ultrasensitive and selective SERS immunosensor for quantitative detection and monitoring of MC-LR produced from cyanobacteria in aquatic settings using the unique SERS probe-based platform. The combination of plasmonic GNSs, tightly packed Raman reporter molecules, and protective silica layer offers high sensitivity and excellent biocompatibility for detection of trace contaminants like MC-LR. The realization of a sandwich assay using the developed SERS probes and MC-LR mAb allowed extraordinary selectivity of the sensor to hepatotoxic MC-LR over its structural analogs. We demonstrated that the developed SERS sensor can reach a LOD as low as 0.014 μg/L and displays a wide linear dynamic range from 0.01 μg/L to 100 μg/L, which are at least one order of magnitude lower and two orders of magnitude wider, respectively, than those of the commercial ELISA method. This sensitivity is necessary to detect MC-LR from natural water samples, where the concentration is routinely below the LOD for other methods. Furthermore, we used the developed SERS sensor to monitor the dynamic production of MC-LR from Microcystis aeruginosa strains, which can aid in the identification of factors affecting MC-LR production. Due to non-overlapping nature of narrow Raman bands, the developed SERS immunosensor can be easily adapted for applications requiring simultaneous monitoring of multiple contaminants in water, e.g. multiple analogs of microcystin and their relative abundance. While we have used the confocal Raman microscope to perform the Raman imaging measurement in this proof-of-concept study, our ongoing efforts are focused on making the same set of measurements using a portable Raman system developed in our laboratory.44 If successful, we will further harness commercially available handheld Raman systems to fully exploit the promise of this approach. Our overarching goal is to focus on engineering nanostructured probes with maximum signal enhancement such that less expensive and handheld Raman systems can be used for field detection of MC-LR.
MATERIALS AND METHODS
Materials.
Sodium silicate (Na2O(SiO2)x•xH2O, reagent grade), 4-nitrothiophenol (NTP, technical grade 80%), sodium hydroxide (NaOH, 99.99%), chloroauric acid (HAuCl4•xH2O, 99.999% trace metals basis), trisodium citrate dihydrate (HOC(COONa)(CH2COONa)2•2H2O, ≥99%), poly(vinylpyrrolidone) (PVP, (C6H9NO)n, molecular weight-10 kg/mol), sodium borohydride (≥99%), (3-aminopropyl) trimethoxysilane (APTMS, 97%), albumin from bovine serum (BSA, >98%), N,N-dimethyformamide (DMF, anhydrous 99.8%), N-hydroxysulfosuccinimide (sulfo-NHS, ≥98% (HPLC)), 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC, ≥97.0%), cyanobacteria BG-11 freshwater solution, microcystin-LR (MC-LR), microcystin-RR (MC-RR) and microcystin-YR (MC-YR) were purchased from Sigma-Aldrich (St. Louis, MO). (3-triethoxysilyl) propylsuccinic anhydride (TEPSA, C13H25O6Si, >95%) was purchased from Gelest (Morrisville, PA). Methoxy-poly(ethylene glycol)-silane (mPEG-silane, molecular weight-2 kg/mol) was obtained from Laysan Bio (Arab, AL). Microcystin-LR monoclonal antibody (MC-LR mAb) was obtained from MyBioSource (San Diego, CA). Phosphate buffered saline (1×PBS, 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4) solution was purchased from Quality Biology (Gaithersburg, MD). Quartz coverslips from Alfa Aesar (Ward Hill, MA). Both Microcystis aeruginosa UTEX LB 2385 and UTEX LB 2386 were obtained from the University of Texas at Austin (Austin, TX). The microcystin tube ELISA kit was purchased from Beacon Analytical System (Saco, ME). All other reagents and solvents were of analytical grade and were used as received.
Synthesis of SERS Tags.
SERS tags were synthesized according to our previously reported protocols.22–26 Gold nanostars (GNSs) were first synthesized by the seed-mediated growth method.28,45 The LSPR band of GNSs could be tailored by the gold seed/AuCl4– ratio to meet the demand of applications as reported in the literature.28 To make the SERS tags, the as-synthesized GNSs were dispersed into deionized water with a concentration of 1.7 pM, and then an aqueous solution of Raman reporter molecules (NTP, 10 μM) was added under rapid magnetic stirring. Following 30 min stirring, a freshly prepared APTMS ethanolic solution was added to reach a final concentration of 33 μM. After stirring for an additional 30 min, the pH value was adjusted to ca. 9–10 by the addition of NaOH aqueous solution. Then, 200 μL of freshly prepared 0.54 wt% sodium silicate solution was added dropwise and stirred for one day, followed by addition of 5 mL anhydrous ethanol to generate a condensed silica layer. The reaction solution was kept standing for an additional day prior to centrifugation and subjected to washing with anhydrous ethanol and deionized water, respectively. Finally, the pellet was dispersed into 1×PBS buffer solution to obtain a concentration of 0.5 nM.
Functionalization with MC-LR mAb.
Antibody-SERS tag conjugates.
The MC-LR specific mAb was covalently attached to the SERS tags following the well-established protocols detailed in the literature.24,27 To introduce surface functionality and mitigate nanoparticle-induced toxicity, SERS tags were first co-modified by TEPSA and mPEG-silane with a 2:1 molar ratio. Typically, 11.3 mM TEPSA and 5.65 mM mPEG-silane were added to an ethanolic solution of 50 pM SERS tags. After the reaction continued for 12 hours under magnetic stirring, the solution was centrifuged and washed with ethanol and 1×PBS, respectively, to remove excess reagents. The pellets were re-dispersed into 1 mL 1×PBS buffer solution for further use. The carboxyl group modified SERS tags were activated via incubation in a 1×PBS solution of 50 mM sulfo-NHS and 200 mM EDC for 2 hours at room temperature. Subsequently, a solution of 20 μg/mL MC-LR mAb was added to the activated SERS tags, reacted overnight under magnetic stirring at room temperature and purified by successive washing with 1×PBS buffer solution. The mAb was linked to the SERS tags to produce MC-LR specific SERS probes (SERS tags modified with the MC-LR mAb targeting moiety), which were finally dissolved in a 1×PBS buffer solution to reach a concentration of 0.5 nM and stored at 4 °C until use.
Antibody modified quartz chip.
The quartz chip was treated by oxygen plasma, bonded with the Parafilm, and then pre-defined with the punched wells (ϕ: 0.75 in) for sensing regions.24 The pre-defined wells were rinsed with ethanol. The mAb was immobilized onto the wells through the standard amine coupling chemistry. First, all wells were incubated overnight in an ethanolic solution of 100 mM TEPSA, and then sequentially washed at least three times with ethanol and 1×PBS buffer solution to remove excess TEPSA, resulting in a carboxyl group modified surface. Following this, the carboxyl groups on the wells were activated with a PBS buffer solution of 50 mM sulfo-NHS and 200 mM EDC similar to that for antibody-SERS tag conjugates described above. After washing with 1×PBS solution to remove free sulfo-NHS and EDC, the corresponding wells were incubated overnight in the buffer solution of 20 μg/mL MC-LR mAb. Unbound mAb molecules were removed by washing with 1×PBS buffer solution. To achieve high specificity, we used BSA as the surface blocking reagent because of its excellent stability and biocompatibility.24,46 The mAb-modified wells were incubated for 2 hours in a 1×PBS buffer solution of 1 mg/mL BSA, followed by washing with 1×PBS buffer solution.
Microcystis aeruginosa Culture.
Microcystis aeruginosa strains UTEX LB 2385 and UTEX LB 2386 from the UTEX culture collection were used in this study. Microcystis aeruginosa UTEX LB 2385 is a microcystin-producing strain. Microcystis aeruginosa UTEX LB 2386 is not a toxin producer and was used as the control. Microcystis aeruginosa strains were cultured in 50 mL of sterile cyanobacteria BG11 freshwater liquid media. The cyanobacteria cultures were maintained at 23 °C and 12:12 hour light-dark photoperiod illuminated under 32 W Daylight Deluxe lamps (Philips) at an intensity of 2 mW/cm2. Culture extracts (1 mL) were collected every 24 hours for monitoring the growth of cyanobacteria and the production of microcystin toxins. All extracts in the cyanobacteria BG11 freshwater liquid media were kept at −20 °C until used and the supernatant was tested in triplicates after centrifugation at 3000 rpm to remove cells.
Quantitative PCR Measurement.
The 16S rRNA gene was amplified and quantified by quantitative PCR (qPCR). Briefly, 2 μL of each sample was combined with SsoAdvanced Universal SYBR Green Supermix (BioRad, final concentration 1×) and universal primers 515F (5’-GTG CCA GCM GCC GCG GTA A-3’) and 786R (5’-GGA CTA CHV GGG TWT CTA AT-3’) at a final concentration of 0.3 μM each. Reactions were incubated at 98 °C for 10 min, followed by 40 cycles of the following: 98 °C for 10 seconds, 52 °C for 10 seconds and 72 °C for 20 seconds. Cellular abundances were estimated by comparing sample amplification to control samples whose cellular abundances were determined by direct count microscopy.
Lake Water.
Lake water was sampled at the surface of Upper Mystic Lake on 7/17/13 as previously described.47 Briefly, water was collected through a peristaltic pump into 50 mL conical tubes and placed onto dry ice immediately until storage at −80 °C for 3 years before processing. Unfiltered samples were thawed and used for detection of microcystins. Original lake samples without amendment were tested for MC-LR using both SERS and ELISA methods. Additionally, lake samples were spiked with a 5.0 μg/L MC-LR standard solution to achieve final concentrations of 0.3, 1.0, and 4.7 μg/L and then tested by both SERS and ELISA methods.
Detection of Microcystins.
The present SERS platform for detection of microcystins is based on the sandwich immunoassay through the specific antibody-antigen type interaction. First, the mAb-modified wells were incubated for 1 hour with the MC-LR solution (50 μL) of various concentrations (0, 0.001, 0.01, 0.05, 0.1, 0.5, 1.0, 5.0, 10.0, 100, 200 and 500 μg/L), which were prepared by spiking with various amounts of MC-LR into deionized water. Then, it was rinsed at least three times with 1×PBS to remove free MC-LR, followed by addition of 2 μL mAb-SERS probes (0.5 nM). After incubation for 1 hour, the wells were washed at least three times with 1×PBS buffer solution. The resulting wells were subjected to the SERS measurement. For samples taken from the Microcystis aeruginosa culture, the samples were centrifuged for 3 min at 3000 rpm, and the supernatant was used for the test. Culture samples with initial ELISA results > 2.0 μg/L were diluted to ~0.5 μg/L with sterile water and re-analyzed. MC-LR concentrations were determined by multiplying the MC-LR concentration by the dilution factor.
SERS Measurements and Instrumentation.
SERS measurements were conducted using a home-built, inverted high-speed Raman confocal microscope (Figure S5) equipped with a compact LM series volume holographic grating-stabilized 785 nm near-infrared diode laser, a 60× oil immersion objective lens with a numerical aperture of 0.65–1.25 (RMS60X-PFOD, Olympus), a HoloSpec f/1.8 spectrograph and an iDus CCD Camera. The detailed information related to the set-up can be found in our previous work.24–27 The SERS measurements were acquired at room temperature with 100 ms integration time and a laser power of 5 mW at the sample, unless otherwise mentioned.
UV-vis extinction spectra were collected on an Aviv Model 14DS UV-vis spectrophotometer (Aviv Biomedical, Lakewood, NJ). qPCR was performed on a CFX96 Touch real-time PCR detection system (Bio-Rad, Hercules, CA). Transmission electron microscopic (TEM) images were taken with an FEI Tecnai T12 microscope (FEI, Hillsboro, OR) at an accelerating voltage of 120 kV. The air-dried samples were prepared through deposition of a drop of samples onto the TEM 200-mesh copper grid (Ted Pella, Redding, CA).
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by JHU Water Institute Seed Grant. This research was also partially supported by the JHU Whiting School of Engineering Seed Funds. I. B. acknowledges the support from the National Institute of Biomedical Imaging and Bioengineering (2-P41-EB015871–31) and National Institute of General Medical Sciences (DP2GM128198). M. L. acknowledges financial support by the National Thousand Young Talents Program of China, National Natural Science Foundation of China (No. 51871246), Innovation-Driven Project of Central South University (No. 2018CX002) and Hunan Provincial Science & Technology Program (No. 2017XK2027).
Footnotes
Supporting Information. The Supporting Information is available free of charge on the ACS Publications website.
Schematic illustration of preparation of NTP-encoded SERS tags and SERS probes; Normalized extinction spectra of GNSs and SERS tags; SERS spectra of the present SERS immunoassay; Schematic of the setup of the Raman measurement
The authors declare no competing financial interest.
REFERENCES
- (1).de Figueiredo DR; Azeiteiro UM; Esteves SM; Gonçalves FJ; Pereira MJ Microcystin-producing blooms – a serious global public health issue. Ecotoxicol Environ Saf. 2004, 59, 151–163. [DOI] [PubMed] [Google Scholar]
- (2).Amyadi A; Choo F; Newcombe G; Stuetz R; Henderson RK A review of monitoring technologies for real-time management of cyanobacteria: recent advances and future direction. Trends Anal. Chem 2016, 85, 83–96. [Google Scholar]
- (3).Michalak AM; Anderson EJ; Beletsky D; Boland S; Bosch NS; Bridgeman TB; Chaffin JD; Cho K; Confesor R; Daloğlu I; DePinto JV Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc. Natl. Acad. Sci. U.S.A 2013, 110, 6448–6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Dawson RM The toxicology of microcystins. Toxicon 1998, 36, 953–962. [DOI] [PubMed] [Google Scholar]
- (5).Campos A; Vasconcelos V Molecular mechanisms of microcystin toxicity in animal cells. Int. J. Mol. Sci 2010, 11, 268–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).World Health Organization (WHO), Cyanobacterial toxins: microcystin-LR in drinking-water. background document for development of WHO guidelines for drinking-water quality. Geneva, Switzerland: World Health Organization, 2nd ed. Geneva. 2003. [Google Scholar]
- (7).American water works association, Cyanotoxins in US drinking water: occurrence, case studies and state approaches to regulation. September 2016. https://www.awwa.org/Portals/0/files/resources/water%20knowledge/rc%20cyanotoxins/201609_Cyanotoxin_Occurrence_States_Approach.pdf
- (8).Paul VJ Global warming and cyanobacterial harmful algal blooms In cyanobacterial harmful algal blooms: state of the science and research needs. Springer, New York, NY, 2008. [Google Scholar]
- (9).Li R; Xia Q; Li Z; Sun X; Liu J Electrochemical immunosensor for ultrasensitive detection of microcystin-LR based on graphene–gold nanocomposite/functional conducting polymer/gold nanoparticle/ionic liquid composite film with electrodeposition. Biosens. Bioelectron 2013, 44, 235–240. [DOI] [PubMed] [Google Scholar]
- (10).Chen K; Liu M; Zhao G; Shi H; Fan L; Zhao S Fabrication of a novel and simple microcystin-LR photoelectrochemical sensor with high sensitivity and selectivity. Environ. Sci. Technol 2012, 46, 11955–11961. [DOI] [PubMed] [Google Scholar]
- (11).Hawkins PR; Novic S; Cox P; Neilan BA; Burns BP; Shaw G; Wickramasinghe W; Peerapornpisal Y; Ruangyuttikarn W; Itayama T; Saitou T A review of analytical methods for assessing the public health risk from microcystin in the aquatic environment. J. Water Supply Res. Technol. AQUA, 2005, 54, 509–518. [Google Scholar]
- (12).Li M; Cushing SK; Wu N Plasmon-enhanced optical sensors: a review. Analyst 2015, 140, 386–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Porter MD; Lipert RJ; Siperko LM; Wang G; Narayanan R, SERS as a bioassay platform: fundamentals, design, and applications. Chem. Soc. Rev 2008, 37, 1001–1011. [DOI] [PubMed] [Google Scholar]
- (14).Kneipp K; Wang Y; Kneipp H; Perelman LT; Itzkan I; Dasari RR; Feld MS Single molecule detection using surface-enhanced Raman scattering (SERS). Phys. Rev. Lett 1997, 78, 667. [DOI] [PubMed] [Google Scholar]
- (15).Nie S; Emory SR Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [DOI] [PubMed] [Google Scholar]
- (16).Lane LA; Qian X; Nie S SERS nanoparticles in medicine: from label-free detection to spectroscopic tagging. Chem. Rev 2015, 115, 10489–10529. [DOI] [PubMed] [Google Scholar]
- (17).Wang Y; Yan B; Chen L SERS tags: novel optical nanoprobes for bioanalysis. Chem. Rev 2012, 113, 1391–1428. [DOI] [PubMed] [Google Scholar]
- (18).Zong C; Xu M; Xu LJ; Wei T; Ma X; Zheng XS; Hu R; Ren B Surface-enhanced Raman spectroscopy for bioanalysis: reliability and challenges. Chem. Rev 2018, 118, 4946–4980. [DOI] [PubMed] [Google Scholar]
- (19).Schlücker S Surface-enhanced Raman spectroscopy: concepts and chemical applications. Angew. Chem. Int. Ed 2014, 53, 4756–4795. [DOI] [PubMed] [Google Scholar]
- (20).Halvorson RA; Vikesland PJ Drop coating deposition Raman (DCDR) for microcystin-LR identification and quantitation. Environ. Sci. Technol 2011, 45, 5644–5651. [DOI] [PubMed] [Google Scholar]
- (21).Zhu Y; Kuang H; Xu L; Ma W; Peng C; Hua Y; Wang L; Xu C Gold nanorod assembly based approach to toxin detection by SERS. J. Mater. Chem 2012, 22, 2387–2391. [Google Scholar]
- (22).Li M; Zhang J; Suri S; Sooter LJ; Ma D; Wu N Detection of adenosine triphosphate with an aptamer biosensor based on surface-enhanced Raman scattering. Anal. Chem 2012, 84, 2837–2842. [DOI] [PubMed] [Google Scholar]
- (23).Li M; Cushing SK; Zhang J; Lankford J; Aguilar ZP; Ma D; Wu N Shape-dependent surface-enhanced Raman scattering in gold–Raman-probe–silica sandwiched nanoparticles for biocompatible applications. Nanotechnology 2012, 23, 115501. [DOI] [PubMed] [Google Scholar]
- (24).Li M; Kang JW; Sukumar S; Dasari RR; Barman I Multiplexed detection of serological cancer markers with plasmon-enhanced Raman spectro-immunoassay. Chem. Sci 2015, 6, 3906–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Li M; Banerjee SR; Zheng C; Pomper MG; Barman I Ultrahigh affinity Raman probe for targeted live cell imaging of prostate cancer. Chem. Sci 2016, 7, 6779–6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Li M; Cushing SK; Zhang J; Suri S; Evans R; Petros WP; Gibson LF; Ma D; Liu Y; Wu N Three-dimensional hierarchical plasmonic nano-architecture enhanced surface-enhanced Raman scattering immunosensor for cancer biomarker detection in blood plasma. ACS Nano 2013, 7, 4967–4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Shan B; Pu Y; Chen Y; Liao M; Li M Novel SERS labels: rational design, functional integration and biomedical applications. Coord. Chem. Rev 2018. 371, 11–37. [Google Scholar]
- (28).Li M; Kang JW; Dasari RR; Barman I Shedding light on the extinction-enhancement duality in gold nanostar-enhanced raman spectroscopy. Angew. Chem. Int. Ed 2014, 53, 14115–14119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Jimenez de Aberasturi D; Serrano-Montes AB; Langer J; Henriksen-Lacey M; Parak WJ; Liz-Marzán LM SERS-encoded gold nanostars for multiplexed cell discrimination. Chem. Mater 2016, 28, 6779–6790. [Google Scholar]
- (30).Zhang P; Sham TK X-ray studies of the structure and electronic behavior of alkanethiolate-capped gold nanoparticles: the interplay of size and surface effects. Phys. Rev. Lett 2003, 90, 245502. [DOI] [PubMed] [Google Scholar]
- (31).Pastoriza-Santos I; Pérez-Juste J; Liz-Marzán LM Silica-coating and hydrophobation of CTAB-stabilized gold nanorods. Chem. Mater 2006, 18, 2465–2467. [Google Scholar]
- (32).Nie FQ; Xu ZK; Huang XJ; Ye P; Wu J Acrylonitrile-based copolymer membranes containing reactive groups: surface modification by the immobilization of poly (ethylene glycol) for improving antifouling property and biocompatibility. Langmuir 2003, 19, 9889–9895. [Google Scholar]
- (33).Zhang JJ; Kang TF; Hao YC; Lu LP and Cheng SY Electrochemiluminescent immunosensor based on CdS quantum dots for ultrasensitive detection of microcystin-LR. Sens. Actuator B-Chem 2015, 214, 117–123. [Google Scholar]
- (34).Currie LA Nomenclature in evaluation of analytical methods including detection and quantification capabilities (IUPAC Recommendations 1995). Pure Appl. Chem 1995, 67, 1699–1723. [Google Scholar]
- (35).Mocak J; Bond AM; Mitchell S; Scollary G A statistical overview of standard (IUPAC and ACS) and new procedures for determining the limits of detection and quantification: application to voltammetric and stripping techniques (technical report). Pure Appl. Chem 1997, 69, 297–328. [Google Scholar]
- (36).He S; Xie W; Fang S; Zhou D; Djebbi K; Zhang Z; Du J; Du C; Wang D Label-free identification of trace microcystin-LR with surface-enhanced Raman scattering spectra. Talanta 2019, 195, 401–406. [DOI] [PubMed] [Google Scholar]
- (37).Eissa S; Zourob M Competitive voltammetric morphine immunosensor using a gold nanoparticle decorated graphene electrode. Microchim. Acta 2017, 184(7), 2281–2289. [Google Scholar]
- (38).Zhang CH; Liu LW; Liang P; Tang LJ; Yu RQ; Jiang JH Plasmon coupling enhanced raman scattering nanobeacon for single-step, ultrasensitive detection of cholera toxin. Anal. Chem 2016, 88(15), 7447–7452. [DOI] [PubMed] [Google Scholar]
- (39).Loyprasert S; Thavarungkul P; Asawatreratanakul P; Wongkittisuksa B; Limsakul C; Kanatharana P Label-free capacitive immunosensor for microcystin-LR using self-assembled thiourea monolayer incorporated with Ag nanoparticles on gold electrode. Biosens. Bioelectron 2008, 24(1), 78–86. [DOI] [PubMed] [Google Scholar]
- (40).Utkilen H; Gjølme N Toxin production by Microcystis aeruginosa as a function of light in continuous cultures and its ecological significance. Appl. Environ. Microbiol 1992, 58, 1321–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Sivonen K Cyanobacterial toxins and toxin production. Phycologia 1996, 35, 12–24. [Google Scholar]
- (42).Wicks RJ; Thiel PG Environmental factors affecting the production of peptide toxins in floating scums of the cyanobacterium Microcystis aeruginosa in a hypertrophic African reservoir. Environ. Sci. Technol 1990, 24, 1413–1418. [Google Scholar]
- (43).Yu L; Kong F; Zhang M; Yang Z; Shi X; Du M The dynamics of Microcystis genotypes and microcystin production and associations with environmental factors during blooms in Lake Chaohu, China. Toxins 2014, 6, 3238–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Paidi SK; Siddhanta S; Strouse R; McGivney JB; Larkin C; Barman I Rapid identification of biotherapeutics with label-free Raman spectroscopy. Anal. Chem 2016, 88, 4361–4268. [DOI] [PubMed] [Google Scholar]
- (45).Pu Y; Zhao Y; Zheng P; Li M Elucidating the growth mechanism of plasmonic gold nanostars with tunable optical and photothermal properties. Inorg. Chem 2018, 57, 8599–8607. [DOI] [PubMed] [Google Scholar]
- (46).Papavassiligy AG; and Bohmann D Optimization of the signal-to-noise ratio in south-western assays by using lipid-free BSA as blocking reagent. Nucleic Acids Res. 1992, 20, 4365–4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Preheim SP; Olesen SW; Spencer SJ; Materna A; Varadharajan C; Blackburn M; Friedman J; Rodríguez J; Hemond H; Alm EJ Surveys, simulation and single-cell assays relate function and phylogeny in a lake ecosystem. Nat. Microbiol 2016, 1, 16130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.