Abstract
Objective:
To assess the regional variability of corneal endothelial cell density (ECD) between guttae and non-guttae areas in subjects with Fuchs endothelial corneal dystrophy (FECD) using non-contact specular microscopy and confocal microscopy.
Design:
Retrospective chart review from 2009 to 2014 at the Massachusetts Eye and Ear Infirmary.
Participants:
One hundred fifteen eyes of 73 subjects with FECD.
Methods:
Subjects with FECD underwent same day specular and confocal microscopy in the same eye. Clinical stage of disease was documented on the day of image acquisition. Regional variability of ECD associated with guttae and non-guttae areas was assessed. Manual endothelial cell counts were performed.
Results:
Thirty-two percent of subjects had high quality endothelial images by both specular and confocal microscopy. Of these subjects, 83% were classified clinically as early-stage FECD. There was a significant association between stage of disease and the ability to obtain high quality specular images (χ2; p=0.0012). There was no difference in mean ECD derived from specular (1363 ± 594 cells/mm2) or confocal (1391 ± 493 cells/mm2; p=0.75) images. There was a statistically significant decrease of 31.8 ± 21.7% in mean ECD in areas surrounding guttae (1296 ± 560 cells/mm2) compared to non-guttae areas (1926 ± 674 cells/mm2; p<0.0001) as determined by confocal microscopy.
Conclusion:
These findings support confocal microscopy as an alternative to specular microscopy for evaluating the corneal endothelium of patients with FECD, especially those with advanced disease. Confocal microscopy also revealed regional differences in ECD in guttae and non-guttae areas in patients with FECD.
Keywords: Fuchs, confocal, specular, regional variability, guttae, endothelium
Introduction:
Fuchs endothelial corneal dystrophy (FECD) is characterized by the slow, progressive degeneration of the corneal endothelium, resulting in corneal edema and vision loss.1 FECD most commonly manifests as an age-related disorder that progresses through well-documented clinical stages.1 In early stage disease, there are non-confluent central guttae without significant corneal opacification and edema. As the disease progresses, there are morphological changes in the endothelial cells’ hexagonal shape and size, as well as the concomitant formation of extracellular deposits called guttae.2 Guttae originate in the central cornea and radiate out toward the periphery, leading to reduced endothelial cell density, loss of normal cell morphology, and endothelial cell death.3 Guttae are thought to be excrescences of abnormal collagen deposited by the corneal endothelium, and the accumulation of guttae is the first clinical sign of FECD.4 In advanced FECD there is a decrease in endothelial cell density and guttae become confluent with ensuing corneal edema and opacification.1
Microscopy is a non-invasive technique used to assess the structure of the corneal endothelium.5 In 1978, Maurice introduced microscopy to study the corneal endothelium of enucleated eyes.6 His techniques were further developed into the specular microscope, which is now the most widely used imaging modality to study FECD.6 However, the corneal endothelium can also be analyzed by a variety of imaging techniques such as confocal microscopy. Compared to standard specular microscopy, confocal microscopy allows for greater resolution of corneal endothelium, provides images of all layers of the cornea, and offers the ability to analyze structures through corneal opacities and corneal edema.7 In addition, confocal microscopy can consistently image the central, paracentral and peripheral areas of the cornea. In vivo confocal microscopy (IVCM) provides a clear picture of the endothelial mosaic with discernible cell borders that allows for identification and visualization of corneal guttae.8 While previous studies have used IVCM to study the corneal endothelium in FECD patients, no studies have used IVCM to assess the regional variability in ECD in guttae and non-guttae areas in Fuchs patients.9,8,10, 11 The purpose of this study was to assess the regional variability of ECD between guttae and non-guttae areas in patients with early and late stage FECD using specular microscopy and confocal microscopy.
Materials and Methods:
Study Participants
This study adhered to the tenets of the Declaration of Helsinki and was approved by the Massachusetts Eye and Ear Institutional Review Board. A retrospective chart review was performed at the Massachusetts Eye and Ear Infirmary from 2009 to 2014 and subjects were included based on the following criteria: a diagnosis of Fuchs endothelial corneal dystrophy (FECD), having both a non-contact specular (Konan Noncon Robo Specular Microscope NSP-7700, Nishinomiya, Japan) and a confocal microscopy (Heidelberg Retina Tomograph Rostock Cornea Module [HRT3], Heidelberg Engineering, Heidelberg, Germany) scan on the same day in the same eye. A total of 115 eyes from 73 subjects met the inclusion criteria.
Endothelial imaging and cell count analysis
Endothelial cells were evaluated by NAVIS software (NAVIS; Nidek Technologies). A 400 μm magnification lens was used for examination (mag ×400/Achroplan ×63w/NA 0.95/AA 2.00 mm 670 nm/Zeiss), which resulted in a scan-captured area of 400×400 μm (384×384 pixels) per image, with a transverse optical resolution of 2 μm and longitudinal optical resolution of 4 μm (Heidelberg Engineering, supplied information). The central confocal images that were chosen to analyze regional variability and mean ECD for each subject had to be high resolution and have countable (clear endothelial cells throughout a scan captured area of 400×400 μm per image) areas of endothelial cells. A region of interest (ROI) was defined on the original onscreen image and the number of cells within the ROI was counted. For regional variability, a manual fixed-frame cell counting technique was employed using a mouse-based cursor, and selecting a ROI of equal size on the same image for both guttae (≥ 1 guttae) (n=115) and non-guttae areas (n=115). ROIs between eyes were variable and were determined by the ability to select ROI of equal size on the same image for both guttae and non-guttae areas. To account for the area of guttae in the selected ROI, a smaller ROI surrounding each guttae in the image was selected and the area was measured. To determine the endothelial cell density for the ROI containing the guttae, the total number of endothelial cells counted was divided by the total area ROI minus the total guttae area ROI. Total number of guttae per ROI and mean guttae area were recorded. For mean ECD of confocal images, a manual fixed-frame technique was also used, taking the largest possible ROI (using an image on the same date as the images selected for regional variability and specular analyses), and counting the endothelial cells. Manual fixed-frame counts can have their limitations in that non-whole cells fall within the ROI and decisions must be made to achieve the most accurate ECD.23 To address this, we defined two of the four sides of the ROI as areas where partial (non-whole endothelial cells) cells would be counted for each image that was analyzed. This methodology was constant across all imaging types. After marking each cell and non-whole cell within the ROI with a ‘dot’ using a point and click method, ECD was calculated by NAVIS imaging software as cells/mm2. For mean specular ECD, images were analyzed by similar methodology to mean confocal ECD as described above. A high quality specular image was defined as an image that allowed for clearly discernible cell borders and cell morphology within the endothelial mosaic throughout the entire image. One image per eye was used for both specular and confocal imaging ECD analysis. Manual fixed-frame counts (including partial cells) were performed by two experienced technicians trained in both specular and confocal imaging modalities. Central corneal thickness (CCT) was measured using ultrasound pachymetry (RKI Surgical Products, Ultrasonic Pachymeter RK-500, Easton, Pennsylvania) by technicians on the date the images were acquired. Early stage FECD was defined as non-confluent guttae and/or 1 to 2+ guttae and no edema. Late stage FECD was defined as confluent guttae and/or 3+ guttae with edema. Early and late stage FECD was determined by attending ophthalmologists on the date the images were acquired using the modified Krachmer scale.12
Statistical Analyses
Data was collected and analyzed in Microsoft Excel and significance was determined using paired student’s t-test analysis. Averages are presented as mean ± standard deviation. The agreement between methods was assessed by Bland-Altman analysis.13 The mean difference and limits of agreement were calculated. Difference between data sets was further analyzed in relation to the mean by linear regression analysis. The association of disease stage and image quality was assessed by the McNemar test. Correlation between CCT, stage of FECD disease and ECD as determined by either specular or confocal microscopy was analyzed with a Pearson correlation analysis.
Results:
Comparison of corneal endothelial cell density between specular microscopy and confocal microscopy
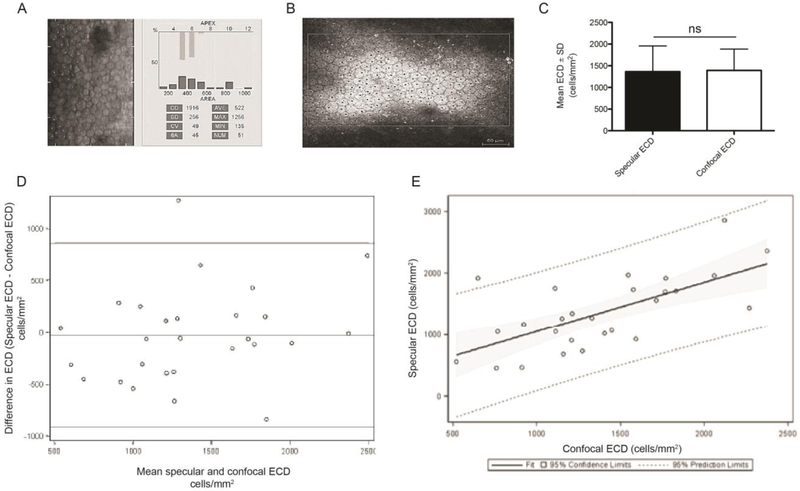
To investigate the abilities of specular microscopy and confocal microscopy to measure corneal endothelial cell density, we compared specular and confocal images acquired from the central cornea from the same subject eye on the same day. Subject demographics are presented in Table 1. High quality specular (Figure. 1A) and confocal (Figure.1B) images were used to obtain endothelial cell counts for the same FECD subject. Twenty-three subjects (27 eyes) out of 73 subjects (115 eyes) had countable cells using both of specular and confocal microscopy. There was no significant difference between the mean ECDs obtained by specular microscopy compared to confocal microscopy (1363 ± 594 cells/mm2 versus 1391 ± 493 cells/mm2; p=0.75) (Fig. 1C). A Bland-Altman analysis was performed to evaluate the agreement among specular and confocal microscopy in measuring mean ECD from the same subject (Fig. 1D). The mean difference between the two methods was 27.74 cells/mm2, which represents a 2% difference in ECD measurement. This analysis supports that the two imaging modalities are highly correlated. To further investigate the association between specular and confocal microscopy, a linear regression analysis of ECD counts by each microscope was performed (Fig. 1E). The data yielded a positive fixed line with only one data point outside the 95% prediction limits, with most clustering within the 95% confidence limit along the fit line. This indicated a high level of association between the data obtained from each microscope.
Table 1.
Subject demographics.
| Characteristics | |
|---|---|
| Total subjects | 73 |
| Right eye only | 21 |
| Left eye only | 10 |
| Both eyes | 42 |
| Mean age (years ± SD) | 68.9 ± 10.6 |
| Mean CCT (μm ± SD) | 602 ± 50 |
| Early stage Fuchs, n (%) | 40 (54.8) |
| Late stage Fuchs, n (%) | 33 (45.2) |
n=number of subjects or eyes, SD= standard deviation, CCT= central corneal thickness, %=percentage.
Figure 1. Non-contact specular microscopy and confocal microscopy to image the corneal endothelium.
Manual cell counts using point and click method to achieve mean endothelial cell density (ECD) on (A) specular and (B) confocal images. Specular NAVIS computer software (A) used to calculate ECD on the specular microscope. The outside margin (B) denotes the region of interest (ROI), with dots marking each whole cell that was counted. (C) The bar graph represents mean ECD (cells/mm2) obtained by specular and confocal imaging techniques of subjects with both specular and confocal high quality images (n=23 subjects). Paired two-tailed Student’s t=test; ns= no significance. (D) Bland Altman and (E) fit plot analyses for mean specular and confocal ECD measurements (n=23 subjects). Data are expressed as mean ± SEM. The upper and lower horizontal lines (862.59, −918.07) represented 95% confidence intervals, where 95% of the differences between the two methods fell. The upper and lower horizontal lines were ± 1.96 standard deviations from the mean. The middle horizontal line represented the mean difference between the two methods of measurement (the ‘bias’). SD=standard deviation.
To explore the relationship between central corneal thickness, stage of disease and corneal endothelial density, a correlation analysis was performed on data from all 115 eyes from the 73 subjects. No significant correlation between CCT and stage of disease (early or late stage) was found (Pearson correlation, r=0.15, 95% CI, −0.04 to 0.32; p=0.12) or between CCT and ECD as determined by specular microcopy (Pearson correlation, r=−0.02, 95% CI, −0.39 to 0.36; p=0.92). There was a weak but statistically significant negative correlation between CCT and ECD as determined by confocal microscopy (Pearson correlation, r=−0.23, 95% CI, −0.39 to −0.05; p=0.01).
Comparison of corneal endothelial cell density between specular microscopy and confocal microscopy in early versus late stage FECD
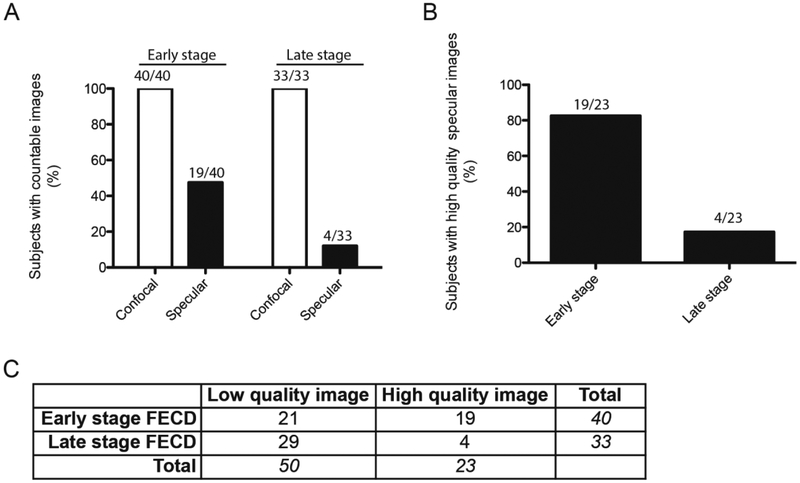
To investigate the relationship between the stage of FECD and the ability to acquire a high quality image of the central corneal endothelium with either specular microscopy or confocal microscopy, each image was classified according to its associated clinical stage of early or late stage FECD. Images were also categorized into unclear (low quality) and clear (high quality) specular images. Forty subjects were classified as having early stage FECD, and 33 subjects were classified as having late stage FECD. Twenty-three out of 73 subjects had high quality specular images, which 19 were classified as having early stage FECD disease and 4 were classified as having late stage FECD disease (Figure 2A, B). A significant relationship was found between the disease stage and the ability to obtain a high quality specular image (McNemar test, χ2; p=0.0012) (Figure 2C). Furthermore, subjects with high quality specular images had an 83% likelihood of having early stage disease (Figure 2B). In contrast to specular microscopy, the confocal microscope was able to acquire high quality images of the corneal endothelium in all 73 subjects, irrespective of disease state (Figure 2A).
Figure 2. Association between clinical stage of FECD and quality of image.
(A) Bar graph showing percentage of subjects with countable images broken down by stage of disease and imaging technique. (B) Bar graph representation of subjects with high quality specular images. (C) Table showing number of low and high quality specular images by stage of FECD. McNemar Test (χ2; p=0.0012). FECD= Fuchs endothelial corneal dystrophy.
Comparison of regional corneal endothelial cell density between guttae and non-guttae areas
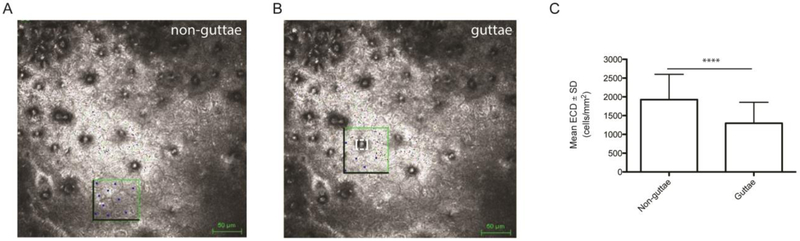
To investigate the relationship between corneal endothelial cell density and corneal guttae, endothelial cells were counted within equal area ROIs in non-guttae (Figure 3A) and guttae areas (Figure 3B) on the same confocal image. The guttae area within the ROI was measured and subtracted from the total ROI to account for the area taken up by the guttae when determining endothelial cell density (Figure 3B). Since confocal images were acquired in all 73 subjects (115 eyes) with varying stages of FECD, we analyzed ECD counts in guttae and non-guttae areas on confocal images only. The mean number of endothelial cells counted in guttae area images was 6.5 ± 3.3 cells. The mean area ROI selected was 5550 ± 2060 μm2 with a mean number of guttae of 1.4 ± 0.6 per area ROI. The mean guttae area was 256 ± 257 μm2 and accounted for 7.0 ±6.1% of the total ROI. Eighty (69.6%) of the 115 ROIs contained 1 guttae, 31 (27.0%) contained 2 guttae, 2 (1.7%) contained 3 guttae and 2 (1.7%) contained 4 guttae. We found a statistically significant decrease of 31.8 ± 21.7 % in mean ECD in areas surrounding guttae compared to non-guttae areas (1296 ± 560 cells/mm2 versus 1926 ± 675 cells/mm2; p<0.0001) (Figure 3C).
Figure 3. Regional variability of ECD between non-guttae and guttae areas.
Confocal endothelial cell density (ECD) counts within equal region of interests (ROIs) in (A) non-guttae and (B) guttae areas on the same image. Darkened borders represent edges where partial cells were counted. Box with dashed white line represents guttae area ROI. (C) Bar graph of ECD in non-guttae and guttae areas. Data are expressed as mean ± SD (n=115 eyes, ****P<0.0001). SD=standard deviation.
Discussion:
FECD is a disease that first manifests as central corneal guttae followed by peripheral corneal involvement.3 It has been shown through an advanced CEM-530 specular microscope that there are regional differences between the central, paracentral and peripheral zones in patients with FECD, whereby the central zone is damaged more than peripheral zones.14 As corneal endothelial cells undergo apoptosis in patients with FECD, corneal thickness increases and eventually leads to corneal edema.1 In FECD patients with significant corneal edema, confocal microscopy has been shown to be superior to non-contact specular microscopy for imaging the corneal endothelium.8,10 In addition to providing higher quality images of the endothelial mosaic compared to specular microscopy, confocal microscopy is capable of imaging both the central and peripheral cornea.11, 14 In our study, we found the mean ECD in 115 eyes of 73 FECD subjects to be 1391 ± 493 cells/mm2. This finding is similar to previously reported mean ECDs in FECD patients acquired through confocal microscopy.9,8,15 However, any differences in mean ECDs can be explained by the inclusion of FECD subjects at multiple stages of the disease.
Regional differences in ECD in patients with FECD has previously been reported.11, 14 Our finding that ECD is decreased in areas adjacent to guttae compared to non-guttae areas further supports regional variability in ECD in patients with FECD. These regional differences in ECD associated with guttae, where central guttae occur before peripheral guttae, could explain the regional zone differences seen in FECD.3, 14 It has been previously shown that endothelial cells in the spaces between guttae have a lower density than the normal endothelial mosaic.16 Previous studies have also shown that in FECD, corneal endothelial cells around guttae form rosettes with clear acellular centers and residual cells surrounding them.17,18,19 The cytoplasm can be thinned or absent in endothelial cells that lie directly over guttae, and the cell density and cell shape are both altered.2,20,21 The etiology of the decrease in cell density surrounding guttae has been shown to involve apoptotic cell death of endothelial cells.22,23 This is consistent with the finding that large guttae induce TUNEL-positive apoptosis on endothelial cells in a rosette pattern, similar to ex vivo FECD specimens.24 Our findings that ECD is reduced adjacent to guttae in FECD patients further support the association between guttae and endothelial apoptosis.
Central corneal thickness (CCT) measured by an ultrasonic pachymeter is a commonly used parameter to help determine clinical grade and treatment options for FECD patients.25 While the accepted clinical grade for thickened cornea is greater than 640 microns, normal corneas in healthy patients may rarely be that thick. Interestingly, patients with significant FECD may present with corneas far thinner than 640 microns even in later stages of disease.26,27 Other methodologies for determining clinical severity of FECD have been proposed including correlating central and paracentral corneal thickness with the percentage of abnormal endothelial areas, and using the ratio between CCT and peripheral corneal thickness (PCT).11, 14, 28 We found no significant correlation between CCT, stage of disease or mean ECD as determined by specular microscopy in our FECD subjects. However, we found a weak but statistically significant negative correlation between CCT and mean ECD as determined by confocal microscopy. This suggests that as mean ECD decreases, there is an increase in CCT. These differences in correlation may attributed to a selection bias, since we were able to acquire ECD images in all our 115 FECD subjects with confocal microscopy compared to only 28 FECD subjects with specular microscopy. Therefore, with specular microscopy, there was a selection of early stage FECD subjects that made specular microscopy possible. Future studies could investigate the use confocal microscopy to assess regional ECD in the context of longitudinal CCT or PCT changes.
One limitation of our study was that we were unable to compare the ECD in subjects with very advanced disease, since the quality of specular microscopy was often poor in these subjects. Therefore while we found a high correlation between mean ECD acquired by specular microscopy and confocal microscopy in FECD subjects with early disease, we were unable to analyze this correlation in FECD subjects with advanced disease. Another limitation of our study was that researchers were not masked to disease status within the subject cohort, and the selection of ROI’s could be a source of bias in our study. A third limitation was that we selected small ROIs surrounding the guttae to determine ECD, and this resulted in a small number of endothelial cells being counted for the analysis. The large standard deviations for mean ECD associated with guttae and non-guttae areas reflect this limitation. A fourth limitation was that we approximated guttae area with a best-fit rectangular ROI since we were unable to manually trace each guttae with the software. We do not believe that the ability to manually trace each guttae would have impacted our conclusion since mean guttae area occupied approximately 7% of the ROI.
In summary, we have demonstrated that central corneal endothelial cell density as determined by either non-contact specular microscopy or confocal microscopy is highly correlated in subjects with early stage FECD. In cases of late FECD, where corneal edema prevents adequate specular imaging, confocal resulted in a larger percentage of high quality images of the endothelium. We provide evidence through confocal microscopy that there are regional differences in ECD in guttae and non-guttae areas in subjects with FECD. These findings support confocal microscopy as an alternative to non-contact specular microscopy for evaluating the corneal endothelium of patients with FECD, especially those with advanced disease.
Acknowledgments
Funding: This work was supported by RO EY020581 and by Cornea Donor Research Funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: None
References:
- 1.Hamill CE, Schmedt T, Jurkunas U. Fuchs endothelial cornea dystrophy: a review of the genetics behind disease development. Semin Ophthalmol 2013;28:281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamis AP, Filatov V, Tripathi BJ, Tripathi RC. Fuchs' endothelial dystrophy of the cornea. Surv Ophthalmol 1993;38:149–68. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Patel DV. The pathophysiology of Fuchs' endothelial dystrophy--a review of molecular and cellular insights. Exp Eye Res 2015;130:97–105. [DOI] [PubMed] [Google Scholar]
- 4.Arffa RC. Clinical applications of corneal topographic analysis. Semin Ophthalmol 1991;6:122–32. [DOI] [PubMed] [Google Scholar]
- 5.McLaren JW, Bachman LA, Kane KM, Patel SV. Objective assessment of the corneal endothelium in Fuchs' endothelial dystrophy. Invest Ophthalmol Vis Sci 2014;55:1184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maurice DM. Cellular membrane activity in the corneal endothelium of the intact eye. Experientia 1968;24:1094–5. [DOI] [PubMed] [Google Scholar]
- 7.Labbe A, Khammari C, Dupas B, et al. Contribution of in vivo confocal microscopy to the diagnosis and management of infectious keratitis. Ocul Surf 2009;7:41–52. [DOI] [PubMed] [Google Scholar]
- 8.Hara M, Morishige N, Chikama T, Nishida T. Comparison of confocal biomicroscopy and noncontact specular microscopy for evaluation of the corneal endothelium. Cornea 2003;22:512–5. [DOI] [PubMed] [Google Scholar]
- 9.Mustonen RK, McDonald MB, Srivannaboon S, Tan AL, Doubrava MW, Kim CK. In vivo confocal microscopy of Fuchs' endothelial dystrophy. Cornea 1998;17:493–503. [DOI] [PubMed] [Google Scholar]
- 10.Chiou AG, Kaufman SC, Beuerman RW, Ohta T, Soliman H, Kaufman HE. Confocal microscopy in cornea guttata and Fuchs' endothelial dystrophy. Br J Ophthalmol 1999;83:185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Syed ZA, Tran JA, Jurkunas UV. Peripheral Endothelial Cell Count Is a Predictor of Disease Severity in Advanced Fuchs Endothelial Corneal Dystrophy. Cornea 2017;36:1166–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louttit MD, Kopplin LJ, Igo RP Jr., et al. A multicenter study to map genes for Fuchs endothelial corneal dystrophy: baseline characteristics and heritability. Cornea 2012;31:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bland JM, Altman dG. Measuring agreement in method comparison studies. Stat Methods Med Res 1999;8:135–60. [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto H, Maeda N, Soma T, et al. Quantitative regional differences in corneal endothelial abnormalities in the central and peripheral zones in Fuchs' endothelial corneal dystrophy. Invest Ophthalmol Vis Sci 2014;55:5090–8. [DOI] [PubMed] [Google Scholar]
- 15.Schrems-Hoesl LM, Schrems WA, Cruzat A, et al. Cellular and subbasal nerve alterations in early stage Fuchs' endothelial corneal dystrophy: an in vivo confocal microscopy study. Eye (Lond) 2013;27:42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks AM, Grant G, Gillies WE. A comparison of corneal endothelial morphology in cornea guttata, Fuchs' dystrophy and bullous keratopathy. Aust N Z J Ophthalmol 1988;16:93–100. [DOI] [PubMed] [Google Scholar]
- 17.Tuberville AW, Wood TO, McLaughlin BJ. Cytochrome oxidase activity of Fuchs' endothelial dystrophy. Curr Eye Res 1986;5:939–47. [DOI] [PubMed] [Google Scholar]
- 18.Jurkunas Uv, Bitar MS, Rawe I, Harris DL, Colby K, Joyce NC. Increased clusterin expression in Fuchs' endothelial dystrophy. Invest Ophthalmol Vis Sci 2008;49:2946–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jurkunas UV, Bitar M, Rawe I. Colocalization of increased transforming growth factor-beta-induced protein (TGFBIp) and Clusterin in Fuchs endothelial corneal dystrophy. Invest Ophthalmol Vis Sci 2009;50:1129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergmanson JP, Sheldon TM, Goosey JD. Fuchs' endothelial dystrophy: a fresh look at an aging disease. Ophthalmic Physiol Opt 1999;19:210–22. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues MM, Krachmer JH, Hackett J, Gaskins R, Halkias A. Fuchs' corneal dystrophy. A clinicopathologic study of the variation in corneal edema. Ophthalmology 1986;93:789–96. [PubMed] [Google Scholar]
- 22.Jurkunas UV, Bitar MS, Funaki T, Azizi B. Evidence of oxidative stress in the pathogenesis of fuchs endothelial corneal dystrophy. Am J Pathol 2010;177:2278–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azizi B, Ziaei A, Fuchsluger T, Schmedt T, Chen Y, Jurkunas UV. p53-regulated increase in oxidative-stress--induced apoptosis in Fuchs endothelial corneal dystrophy: a native tissue model. Invest Ophthalmol Vis Sci 2011;52:9291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kocaba V, Katikireddy KR, Gipson I, Price MO, Price FW, Jurkunas UV. Association of the Gutta-Induced Microenvironment With Corneal Endothelial Cell Behavior and Demise in Fuchs Endothelial Corneal Dystrophy. JAMA Ophthalmol, 2018:886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seitzman GD, Gottsch JD, Stark WJ. Cataract surgery in patients with Fuchs' corneal dystrophy: expanding recommendations for cataract surgery without simultaneous keratoplasty. Ophthalmology 2005;112:441–6. [DOI] [PubMed] [Google Scholar]
- 26.Kopplin LJ, Przepyszny K, Schmotzer B, et al. Relationship of Fuchs endothelial corneal dystrophy severity to central corneal thickness. Arch Ophthalmol 2012;130:433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed kA, McLaren JW, Baratz KH, Maguire LJ, Kittleson KM, Patel SV. Host and graft thickness after Descemet stripping endothelial keratoplasty for Fuchs endothelial dystrophy. Am J Ophthalmol 2010;150:490–497 e2. [DOI] [PubMed] [Google Scholar]
- 28.Repp DJ, Hodge DO, Baratz KH, McLaren JW, Patel SV. Fuchs' endothelial corneal dystrophy: subjective grading versus objective grading based on the central-to-peripheral thickness ratio. Ophthalmology 2013;120:687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]