Abstract
Background
Globally, TB remains a serious cause of morbidity and mortality for children. Mozambique is one of 30 high TB and TB/HIV burden countries. This study aimed to assess treatment outcomes of childhood TB in Chókwè District, Mozambique.
Methods
A retrospective cohort study of children <15 years old treated for TB from 2006–2017 was conducted at Carmelo Hospital of Chókwè. Descriptive statistics were used to summarize patient characteristics. Treatment outcomes stratified by HIV status were compared with chi-square. Multivariable logistic regression was used to estimate the odds of a favorable TB treatment outcome. Kaplan-Meier curves were used to estimate cumulative incidence of death.
Results
933 cases of childhood TB were enrolled, 45.9% of which were female and 49.6% were <5 years old. 565 (62%) children were HIV positive. 762 (83.6%) cases had a favorable TB treatment outcome. In comparison to children 0–4 years, the 5–14 age group had a higher odds of a favorable outcome (OR=2.02, 95% CI=1.42–3.05). Being 5–14 years was associated with lower risk of death (HR=0.435; 95% CI=0.299–0.632). Those starting ATT ≤ 3 months after ART initiation had a survival probability of approximately 75% at one year compared with 95% for those who were HIV-negative.
Conclusions
Most children in this cohort had favorable TB treatment outcomes. Worse outcomes were observed for younger children and if ATT started ≤3 months after initiation of ART. Rigorous screening for TB and isoniazid preventative therapy may reduce the burden of TB in this population and lead to better outcomes.
Keywords: Tuberculosis, HIV, pediatrics, Mozambique
INTRODUCTION
Tuberculosis (TB) remains a serious cause of morbidity and mortality for children (<15 years of age) in the developing world. Globally, 10 million people were ill with TB in 2017, of which children accounted for at least 10% of new cases. Overall, 25% of incident TB cases were located in the World Health Organization (WHO) African region.1
National TB prevalence surveys frequently do not include children when estimating the burden of disease. Therefore, the currently reported prevalence of pediatric TB may be underestimating the true dimension of the epidemic in this age group.2–4 In resource limited settings, TB in children can be difficult to diagnose and treat due to the high frequency of atypical forms of the disease; difficulty with obtaining diagnostic specimens; and the severity of presentation when associated with multi-drug resistance (MDR), human immunodeficiency virus (HIV) infection, and/or the presence of malnutrition.1,5,6
Mozambique is classified as one of the 30 high TB and TB/HIV burden countries. In 2016 the rate of new TB cases was 551/100,000 persons.1 National HIV prevalence was 13.2% in adults (2015) and 1.4% in children 0–11 years of age (2009).7,8 HIV infection is the main cause for increased risk of developing TB in Mozambique and leads to an increased likelihood of poor clinical outcomes. It is estimated that nearly 60% of TB patients in Mozambique are co-infected with HIV.9
Mozambique has a well-established National Tuberculosis Control Program (Programa Nacional de Controlo da Tuberculosis, PNCT). The PNCT has made recent progress in addressing TB, with overall treatment successes increasing from 79% in 2005 to 90% in 2017.2,10,11 Further, as of 2015 the national coverage of Bacille Calmette-Guérin (BCG) vaccine was 93% for children 12–23 months of age.7 At the same time, Mozambique has a very low estimated case-detection rate (CDR) of 37%.1,12 For children, this low CDR is further compounded by the fact that disease estimates often assume that the ratio of notified to incident cases is equal for both children and adults. As a result, the TB CDR in Mozambican children is likely even lower, as children are much more likely to be underreported.12
Surveillance data on childhood TB is important for understanding its epidemiology and to gain insights into the predictors of both favorable and unfavorable TB treatment outcomes. There have been a variety of clinical, social, and economic factors that have been reported as important determinants of TB treatment outcomes in children in high TB burden countries. The most frequently reported factors associated with unfavorable TB treatment outcomes include: delayed TB treatment because of diagnostic uncertainty; poor adherence to anti-TB treatment (ATT) regimens; MDR TB; delays in initiating antiretroviral therapy (ART) if TB/HIV co-infected; social determinants of the parents such as alcohol and drug use; poverty; and malnutrition.13–18 However, the volume of literature on this subject is sparse in Mozambique. The objective of this study was to assess treatment outcomes of childhood TB in Chókwè District, Gaza Province, Mozambique.
MATERIALS AND METHODS
Study Design and Population
A retrospective cohort study was conducted using routinely collected programmatic data from Carmelo Hospital of Chókwè (CHC). All children (<15 years of age) diagnosed with and treated for TB between January 1, 2006 to December 31, 2017 at CHC were included in the study.
Setting
This study was conducted at CHC, in the southern Chókwè District of Gaza Province, Mozambique (Figure 1). CHC is a government hospital administered by the Daughters of Charity, Saint Vincent de Paul Catholic missionaries since 1993, and it serves as a reference center for the districtś mostly rural catchment area of approximately 200,000 inhabitants. This setting has a high prevalence of both TB and HIV. Per the last national HIV prevalence survey conducted in 2015, Gaza Province had the highest HIV prevalence in the country at 24.4%.7 ATT is offered free of charge to all patients. When possible, bacteriologic confirmation through sputum smear or molecular diagnostics was performed. Molecular diagnostics with GeneXpert MTB/RIF ® (Cepheid; Sunnyvale, California, USA) became available for use as of 2012. Mycobacterial culture was not readily available. For new patients, treatment consists of a 2-month intensive phase with a daily fixed-dose combination of isoniazid (H), rifampin (R), pyrazinamide (Z), and ethambutol (E), followed by a 4-month maintenance phase with H and R. Patients who relapse or who are without clinical improvement at the end of treatment are reevaluated, including a repeat GeneXpert, and subsequent retreatment is based on whether rifampin resistance is detected. If rifampin resistance is detected, samples are sent to the national reference lab for line probe assay, culture, and drug sensitivity testing (DST). While waiting for results, a standard short course (9–12 months) for drug resistant TB is initiated. Based on DST results, in select patients, this may be altered to an extended 18–20 month treatment protocol.19 Pediatric formulations of ATT were available.
Figure 1.
Map of Mozambique with Gaza Province and Chókwè District highlighted
Definitions
Childhood TB is defined by the PNCT as TB occurring in children <15 years old. A child is considered a presumptive TB case if they had signs and symptoms suggestive of TB (cough >14 days, poor growth or weight loss in the last 3 months, or fever >14 days) and a history of contact with a TB case; positive tuberculin skin test >10 mm; and/or chest radiographs findings suggestive of TB.19 Pulmonary TB (PTB) was diagnosed if acid-fast bacilli (AFB) were seen on sputum smear or if there were symptoms suggestive of pulmonary TB in the presence of a positive GeneXpert or chest radiograph findings suggestive of PTB. Extrapulmonary TB (EPTB) was diagnosed clinically and per national guidelines could include disseminated, lymph node, meningeal, osteoarticular, abdominal, pericardial or pleural TB.19
Any diagnosis of new or previously treated active TB enrolled into the PNCT at CHC was considered a unique TB episode. Utilizing WHO definitions, we grouped TB treatment outcomes into favorable and unfavorable categories.20 Documented cure or completion of ATT were considered favorable TB treatment outcomes – the primary outcome of interest. Death, treatment failure, loss to follow-up (LTFU), or unknown outcome (including transfers to other facilities) were considered unfavorable TB treatment outcomes.
Data Collection
Data on age, sex, residence, date of TB diagnosis, clinical presentation, HIV status, ATT regimen, ART status including start date and regimen, and final treatment outcome were collected. Results of sputum AFB smear at treatment start were also recorded. When available, diagnostic chest radiograph or GeneXpert data were recorded.
Data analysis
Descriptive statistics were used to summarize patient characteristics. For continuous variables, median and interquartile ranges (IQR) are reported. Frequency and percentages are reported for categorical variables. Treatment outcomes were compared with Kruskal-Wallis rank sum test for continuous variables and chi-square tests for categorical variables. Multivariable logistic regression was used to estimate the odds ratio (OR) and 95% confidence interval (CI) of a favorable TB treatment outcome. Predictors/covariates included in the multivariable model included: age, sex, distance of residence from CHC (per 10 km), type of TB case, HIV status, and ART status at ATT initiation. Patients with incomplete treatment outcome data were excluded from analysis.
Kaplan-Meier curves were used to estimate the cumulative incidence of death. The associations between patient characteristics with mortality were examined using the Cox proportional hazards model with the starting time point being the date of ATT initiation. Patients were censored at the end of study (365 days after ATT initiation), if they were alive or at their last documented visit for those who did not complete ATT, transferred out, or were LTFU. Analysis was performed in R-version 3.5.2 (www.r-project.org).
Ethical Considerations
Both the Mozambican National Bioethics Committee for Health (Comité Nacional de Bioética para Saúde, 25/CNBS/2019) and the Institutional Review Board of Vanderbilt University Medical Center (IRB# 190523) approved this analysis.
RESULTS
A total of 933 cases of childhood TB were enrolled at CHC between January 1, 2006 to December 31, 2017, representing 8% of the 11,153 total TB cases enrolled during the study period. Twenty-two childhood TB cases (2%) were excluded due to incomplete data (21 for missing treatment dates and 1 for missing treatment outcome). Of the 911 childhood TB cases included in our analysis, 418 (45.9%) were female and 452 (49.6%) were less than 5 years of age. All children in this analysis were first identified through a household contact and subsequently tested/diagnosed clinically. The vast majority of cases were classified as new TB cases (863; 94.7%) (Table 1).
Table 1.
Characteristics of pediatric tuberculosis (TB) patients (≤15 years old) enrolled at Carmelo Hospital of Chókwè between January 1, 2006 and December 31, 2017 by TB outcome status
| Characteristic | Unfavorable | Favorable | |
|---|---|---|---|
| N=149 | N=762 | p-value | |
| Sex (%) | |||
| Male | 86 (57.7) | 407 (53.4) | 0.382 |
| Female | 63 (42.3) | 355 (46.6) | |
| Age Group (%) | |||
| 0–4 | 98 (65.8) | 354 (46.5) | <0.001 |
| 5–14 | 51 (34.2) | 408 (53.5) | |
| TB Case (%) | |||
| New Case | 141 (94.6) | 722 (94.8) | 1.000 |
| All Others | 8 (5.4) | 40 (5.2) | |
| Type of TB (%) | |||
| Pulmonary | 119 (79.9) | 616 (80.8) | 0.871 |
| Extrapulmonary | 30 (20.1) | 146 (19.2) | |
| ART Status (%) | |||
| HIV(−) | 43 (28.9) | 303 (39.8) | 0.004 |
| HIV (+) | 106 (71.1) | 459 (60.2) | |
| ATT before ART initiation | 76 (51.0) | 324 (42.5) | |
| ATT ≤90 days after ART initiation | 18 (12.1) | 47 (6.2) | |
| ATT >90 days after ART initiation | 12 (8.1) | 88 (11.5) | |
| TB Treatment Initiation Year (%) | |||
| 2006 | 6 (4.0) | 30 (3.9) | 0.001 |
| 2007 | 7 (4.7) | 46 (6.0) | |
| 2008 | 10 (6.7) | 63 (8.3) | |
| 2009 | 13 (8.7) | 74 (9.7) | |
| 2010 | 23 (15.4) | 63 (8.3) | |
| 2011 | 17 (11.4) | 58 (7.6) | |
| 2012 | 19 (12.8) | 38 (5.0) | |
| 2013 | 7 (4.7) | 47 (6.2) | |
| 2014 | 7 (4.7) | 87 (11.4) | |
| 2015 | 7 (4.7) | 84 (11.0) | |
| 2016 | 17 (11.4) | 68 (8.9) | |
| 2017 | 16 (10.7) | 102 (13.4) | |
| Distance to HF (km) (median [IQR]) | 10 [1.0, 23.0] | 4 [1.01, 20.0] | 0.285 |
ATT= anti-tuberculosis treatment; ART= antiretroviral therapy; HF=health facility
Favorable Outcome defined as “completed treatment” or “cured”
Unfavorable Outcome defined as “death, treatment failure, loss to follow-up (LTFU), or unknown outcome”
Kruskal-Wallis rank sum test for comparison of continuous variables/Chi-square test for categorical variables
Among the 733 (80.4%) cases classified as PTB, 49 (6.7%) were diagnosed by smear microscopy, 74 (10.0%) were diagnosed by chest radiograph, and 18 (2.5%) were diagnosed by GeneXpert. The remaining 592 (80.8%) PTB cases and all 178 cases classified as EPTB were clinically diagnosed. Among those with EPTB, the most common sites were lymph nodes (39.8%), pleura (6.7%), abdomen (5.6%), and bone/joint (5%). Forty-one percent of those classified as EPTB had no data recorded for site of disease and no cases of meningeal TB were documented. Five hundred sixty-five (62%) children in this pediatric TB cohort were HIV-positive.
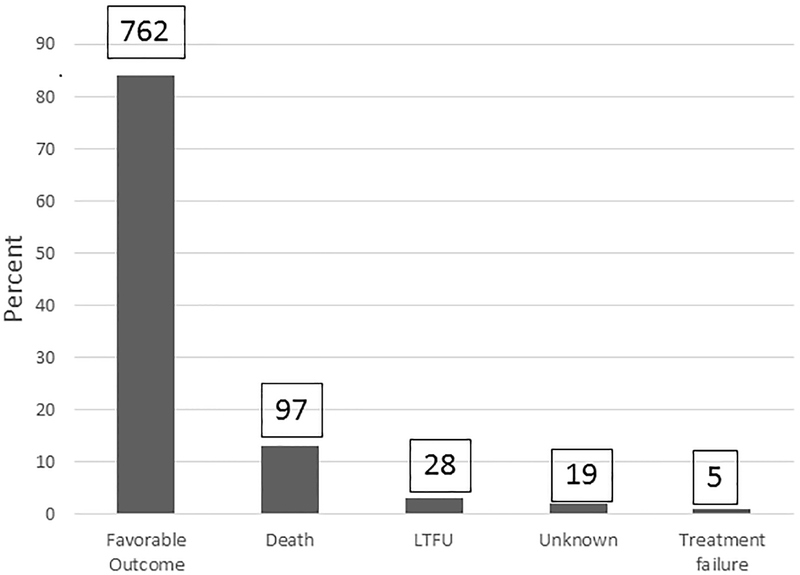
Seven hundred sixty-two (83.6%) childhood TB cases had a favorable treatment outcome (cured [44, 5.7%] or completed treatment [717, 94%]). Of the 149 (16.3%) cases with an unfavorable treatment outcome, 97 (65.1%) died, 28 (18.8%) were lost to follow-up, 19 (12.8%) were listed as unknown (including transferred to another facility), and 5 (3.3%) had treatment failure (Figure 2). The proportion of unfavorable treatment outcomes was significantly higher among children 0–4 years of age (65.8% vs. 34.2% for those 5–14 years of age; p<0.001; Table 1). Younger age was also identified as an independent risk factor for unfavorable TB treatment outcomes in our multivariable logistic regression model. Children 5–14 years of age had a more than two-fold higher likelihood of a favorable TB treatment outcome compared with younger children (OR 2.08; 95% CI 1.42–3.05; p <0.0001). Gender, ART status, type of TB case, year of ATT initiation, and the distance one lives from the health facility were not significantly associated with TB treatment outcome (Table 2).
Figure 2.
Tuberculosis treatment outcomes among 911 children at Carmelo Hospital of Chókwè (2006–2017)
Table 2.
Logistic Regression Model: Predictors of a favorable tuberculosis (TB) treatment outcome (defined as cured or treatment completed) among childhood TB cases at Carmelo Hospital of Chókwè (2006–2017)
| n= 476 | OR (95% CI) | p-value |
|---|---|---|
| Gender | ||
| Female | ref | |
| Male | 0.89 (0.61, 1.28) | 0.514 |
| Age (years) | ||
| 0–4 | ref | |
| 5–14 | 2.08 (1.42, 3.05) | <0.001 |
| ART status | ||
| ATT before ART initiation | ref | |
| ATT ≤90 days after ART initiation | 0.62 (0.34, 1.13) | 0.118 |
| ATT >90 days after ART initiation | 1.40 (0.70, 2.79) | 0.340 |
| HIV (−) not on ART | 1.49 (0.97, 2.27) | 0.065 |
| TB case definition | ||
| New case (PTB; bacteriologically confirmed) | ref | |
| New case (PTB; not bacteriologically confirmed) | 0.77 (0.26, 2.28) | 0.638 |
| New case (EPTB) | 0.70 (0.22, 2.17) | 0.532 |
| Other | 0.63 (0.17, 2.38) | 0.496 |
| TB treatment initiation (per 1-year increase) | 1.03 (0.97, 1.03) | 0.364 |
| Distance to health facility (per 10km increase) | 0.96 (0.89, 1.03) | 0.287 |
ATT= anti-tuberculosis treatment; ART= antiretroviral therapy; EPTB= extrapulmonary TB; PTB= pulmonary TB
Among the 97 children who died, 58 (59.8%) were males and 88 (90.1%) were aged 0–4 years old. In the multivariable Cox-proportional hazards model, being 5–14 years of age was associated with lower risk of death as compared with children 0–4 years of age (HR=0.435; 95% CI=0.299–0.632). Mortality decreased over time; every one-year increase in the year of enrollment in the TB program was associated with a lower risk of death (HR=0.922; 95% CI=0.875–0.972). Gender, type of TB case, and the distance one lives from the health facility were not significantly associated with mortality (Table 3).
Table 3.
Cox Proportional Hazards Model for Mortality among Childhood Tuberculosis (TB) cases at Carmelo Hospital of Chókwè (2006–2017)
| Exposure | Hazard Ratio | Lower CI | Upper CI |
|---|---|---|---|
| Age Group | |||
| 0–4 | -- | -- | -- |
| 5–14 | 0.435 | 0.299 | 0.632 |
| Gender | |||
| Female | -- | -- | -- |
| Male | 0.986 | 0.698 | 1.395 |
| ART status | |||
| ATT before ART initiation | -- | -- | -- |
| ATT ≤90 days after ART initiation | 1.332 | 0.800 | 2.218 |
| ATT >90 days after ART initiation | 0.666 | 0.349 | 1.270 |
| HIV (−) not on ART | 0.397 | 0.237 | 0.666 |
| TB case | |||
| New case (PTB; bacteriologically confirmed) | -- | -- | -- |
| New case (PTB; not bacteriologically confirmed) | 0.679 | 0.239 | 1.931 |
| New case (EPTB) | 0.941 | 0.318 | 2.789 |
| Other | 0.609 | 0.157 | 2.363 |
| Year of TB treatment Initiation | 0.922 | 0.875 | 0.972 |
| Distance from health facility (per 10km increase) | 0.962 | 0.873 | 1.060 |
ATT= anti-tuberculosis treatment; ART= antiretroviral therapy; EPTB= extrapulmonary TB; PTB= pulmonary TB
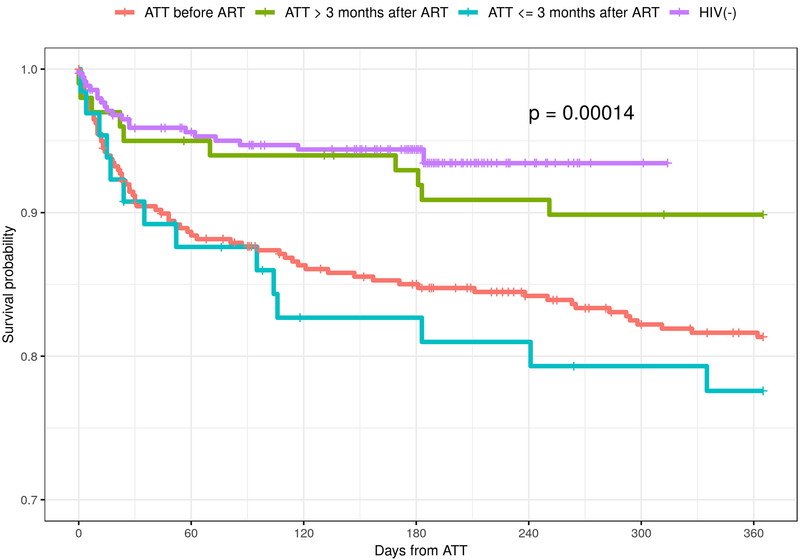
Kaplan-Meier estimates for the probability of one-year survival by ATT initiation in relation to ART treatment initiation are presented in Figure 3. Those who received ATT and were HIV negative had the highest probability of survival at one-year following ATT initiation, while those starting ATT ≤3 months after ART was initiated had a survival probability of approximately 75% at one year.
Figure 3.
Kaplan- Meier plot for TB infected Children enrolled at Carmelo Hospital of Chókwè (2006–2017) by ART treatment status in relation to TB treatment
DISCUSSION
This study investigated the treatment outcomes of children enrolled for TB treatment at a rural, district hospital in Gaza Province, Mozambique. Overall, children accounted for 8% of the total TB patients registered for treatment at CHC, a proportion that is consistent with the 6–15% reported by other high burden TB countries.15–17,21 Misdiagnosis and missed diagnosis of childhood TB are common and can lead to inaccurate reporting of childhood TB cases.12,22 In our population, the possibility of misdiagnosis was quite high, since 84.5% of all cases were diagnosed based on clinical signs and symptoms alone (i.e., without bacteriologic confirmation) and 41% of EPTB cases did not document a site of disease.
This fact highlights an urgent need to improve approaches to TB diagnosis among children in Mozambique. Barriers to accurate diagnosis of childhood TB include the paucibacillary nature of TB disease in children and the fact that it is difficult to obtain diagnostic sputum specimens from younger children.23,24 While CHC does have the capacity to conduct early morning gastric aspirates in children, this requires hospitalization and is not likely a sustainable option for a hospital that manages and treats such a large number of TB patients. Bacterial and mycobacterial culture is not currently available at CHC. GeneXpert was introduced for diagnosis at CHC in 2012, yet this was the diagnostic modality in only 18 of 451 (4%) children enrolled at CHC during the 5-year period from 2013–2017. CHC has recently begun using GeneXpert with stool samples as described elsewhere,25 and anecdotally is showing promising results, which going forward, may lead to improved diagnosis for those children unable to produce sputum. Ancillary radiology is limited to chest radiograph and while lumbar puncture is available, further exploration is needed to understand clinician comfort in its use in the pediatric population or other potential reasons for the lack of meningeal TB cases being reported, an unlikely result in a population with such a high HIV co-infection rate.
The risk of disease progression and unfavorable TB treatment outcomes is most alarming among young children, especially in those less than two-years of age and who may be immunocompromised.23 TB/HIV co-infection in our pediatric cohort was 62% overall and 51% in the age group 0–4 years. This is higher than the 44% TB/HIV co-infection estimate for children in a similar age group in Mozambique and much higher than the TB/HIV co-infection rates reported in similar studies.12,15–17 The high prevalence of TB/HIV co-infection in our cohort may be due to the fact that CHC is a reference hospital to which all district satellite clinics refer their complicated cases, thus over-representing the true prevalence of childhood TB/HIV co-infection in the population. Additionally, in the Mozambican context, women frequently serve as the inpatient caregiver to sick relatives that are hospitalized and could potentially represent a population at increased risk of nosocomial transmission of TB. As they are also the main caregiver to their children, it is speculated that subsequent transmission to their household contacts, may represent a previously unrecognized means of exposure for their children, though further research is needed. BCG coverage nationally is quite high at 93% of children and is typically offered before discharge following birth or at the initial post-partum visit. This high coverage likely contributes to our lack of documented meningeal TB cases. It is further speculated that many cases of meningeal TB could have died before arrival at hospital, though again this does not completely explain the lack of any meningeal TB cases being documented.
A large proportion of this pediatric cohort had favorable TB treatment outcomes, with higher age (5–14 years) at enrollment into TB treatment being associated with both a favorable TB treatment outcome and a lower risk of death. However, it should be noted that most of these favorable outcomes were attributable to completion of ATT rather than documented cure, further highlighting the need for improved diagnostic accuracy for both confirmation of disease and treatment success. Nineteen percent of our TB/HIV co-infected cohort had an unfavorable TB treatment outcome, which is similar to other study results.13 Those who initiated ATT more than 3 months after starting ART had a higher probability of survival at one-year compared with those who started ATT ≤3 months after initiating ART. While initiating ART has been shown to decrease the risk of TB infection in children, this benefit tends to be at its maximum one to two years following initiation of ART, coinciding with improved immunologic function. TB infection remains common in the first three months after starting ART, especially in those children who present with advanced clinical and/or immunologic stages of HIV/AIDS.26–28 While we were unable to definitively determine what, if any, influence immune reconstitution inflammatory syndrome (IRIS) had on children with poor outcomes, IRIS likely contributed to the observed higher proportion of unfavorable outcomes experienced by those who initiated ATT ≤3 months after initiating ART.29–31 These facts underscore the importance of TB screening before initiating ART and the use of isoniazid preventive therapy (IPT) once active TB is excluded.
It is encouraging that mortality significantly decreased over the 12-year study period. However, it seems that this improvement was despite underutilization of improved access to more sensitive diagnostic modalities (i.e., GeneXpert). Rather, this improvement likely corresponds to the relatively better outcomes for those who were HIV-negative, or who were HIV-positive but benefitting from sustained ART before being diagnosed with and treated for TB.
The strengths of this study include its large sample size, long study period, and that data were retrieved from detailed registers. Additional study limitations include first, its retrospective design and an inability to directly connect the National TB Program register data to CHĆs more comprehensive electronic medical record utilized in its HIV care and treatment program. This limited our ability to go back to individual patients to further explore or gather additional information related to questionable documentation in the TB registers or to address missing data. Second, all pediatric cases in this cohort were first identified through contact tracing of an index TB case, usually an adult patient. Greater effort needs to be made to identify pediatric cases presenting with signs and symptoms suspicious for TB in the pediatric wards and clinics, in the absence of a contact, as this may underestimate the true TB prevalence.
In conclusion, a large proportion of this pediatric cohort had a favorable outcome to their TB treatment course. Outcomes were worse among those who were TB/HIV co-infected, and especially for those who started ATT ≤3 months after initiation of ART. Patients also had worse outcomes if they were diagnosed with and treated for TB at a younger age. These findings emphasize the importance of rigorous screening for TB in HIV care and treatment clinics, as well as initiation of IPT to prevent of TB among the youngest and most vulnerable segments of the population. With improved approaches to prevention, diagnosis, and treatment of TB and HIV, we will be able to significantly reduce the burden of these diseases and ensure optimal clinical outcomes.
Financial support:
Research reported in this publication was supported by the Fogarty International Center of the National Institutes of Health (NIH) under award number D43 TW009745. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: The authors have no conflicts of interest to disclose
REFERENCES
- 1.World Health Organization. Global Tuberculosis Report 2018. Available at: https://www.who.int/tb/publications/global_report/en/. Accessed June 6, 2019.
- 2.Global Plan to End TB, The Paradigm Shift: 2016–2020. Available at: http://www.stoptb.org/assets/documents/global/plan/GlobalPlanToEndTB_TheParadigmShift_2016-2020_StopTBPartnership.pdf. Accessed June 6, 2019.
- 3.García-Basteiro AL, Schaaf HS, Diel R, et al. Migliori GB. Adolescents and young adults: a neglected population group for tuberculosis surveillance. Eur Respir J. 2018;51(2):1800176. [DOI] [PubMed] [Google Scholar]
- 4.López-Varela E, Augusto OJ, Guerra L, et al. Low paediatric tuberculosis case detection rate in Southern Mozambique. Eur Respir J. 2016;47(3):1003–1005. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Velez CM, Marais BJ. Tuberculosis in Children. N Engl J Med. 2012;367(4):348–361. [DOI] [PubMed] [Google Scholar]
- 6.No More Crying, No More Dying. Towards Zero TB Deaths in Children. Available at: http://www.stoptb.org/assets/documents/news/ChildhoodTB_report_singles.pdf. Accessed June 6, 2019.
- 7.Survey of indicators on immunizations, malaria, and HIV/AIDS (IMASIDA). Available at: https://dhsprogram.com/pubs/pdf/AIS12/AIS12.pdf. Accessed June 6, 2019.
- 8.National survey of prevalence, risk behaviors, and information about HIV and AIDS in Mozambique (INSIDA 2009). https://dhsprogram.com/pubs/pdf/ais8/ais8.pdf. Accessed June 6, 2019.
- 9.García-Basteiro AL, Respeito D, Augusto OJ, et al. Poor tuberculosis treatment outcomes in Southern Mozambique (2011–2012). BMC Infect Dis. 2016;16:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mozambique Strategic Operation Plan for Tuberculosis 2014–2018. Available at: http://gard-cplp.ihmt.unl.pt/Documentos/Paises/Mocambique/Plano_Estrategico_Operacional_Tuberculose_Mocambique_2014-2018.pdf. Accessed June 6, 2019.
- 11.Report of activities developed during 2017 (March 2018). National Program for the Control of Tuberculosis, Ministry of Health, Republic of Mozambique. [Google Scholar]
- 12.López-Varela E, Augusto OJ, Gondo K, et al. Incidence of Tuberculosis Among Young Children in Rural Mozambique. Pediatr Infect Dis J. 2015;34(7):686–692. [DOI] [PubMed] [Google Scholar]
- 13.Carlucci JG, Blevins Peratikos M, Kipp AM, et al. Tuberculosis Treatment Outcomes Among HIV/TB-Coinfected Children in the International Epidemiology Databases to Evaluate AIDS (IeDEA) Network. J Acquir Immune Defic Syndr. 1999. 2017;75(2):156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Zyl S, Marais BJ, Hesseling AC, et al. Adherence to anti-tuberculosis chemoprophylaxis and treatment in children. Int J Tuberc Lung Dis. 2006;10(1):13–8. [PubMed] [Google Scholar]
- 15.Oeltmann JE, Chengeta B, Mboya JJ, et al. Reported childhood tuberculosis treatment outcomes, Gaborone and Francistown, Botswana, 1998–2002. Int J Tuberc Lung Dis. 2008;12(2):186–92. [PubMed] [Google Scholar]
- 16.Adejumo OA, Daniel OJ, Adebayo BI, et al. Treatment Outcomes of Childhood TB in Lagos, Nigeria. J Trop Pediatr. 2016;62(2):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hailu D, Abegaz WE, Belay M. Childhood tuberculosis and its treatment outcomes in Addis Ababa: a 5-years retrospective study. BMC Pediatr. 2014;14:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hicks RM, Padayatchi N, Shah NS, et al. Malnutrition associated with unfavorable outcome and death among South African MDR-TB and HIV co-infected children. Int J Tuberc Lung Dis. 2014;18(9):1074–1083. [DOI] [PubMed] [Google Scholar]
- 19.Evaluation and management of patients with TB: national protocols Version 1 2018. National Program for the Control of Tuberculosis, Ministry of Health, Republic of Mozambique. [Google Scholar]
- 20.Definitions and reporting framework for tuberculosis- 2013 revision (updated December 2014). Available at: http://apps.who.int/iris/bitstream/handle/10665/79199/9789241505345_eng.pdf.;jsessionid=08C4768326845B68BF67CBA21B9FAB00?sequence=1. Accessed June 6, 2019. [PubMed]
- 21.Epidemiology of childhood TB (Module 1). Available at: https://www.who.int/tb/challenges/ChildhhoodTB_section1.pdf?ua=1. Accessed June 6, 2019.
- 22.Safdar N, Hinderaker SG, Baloch NA, et al. Diagnosis and outcome of childhood tuberculosis: implementing public health policy in three districts of Pakistan. Int J Tuberc Lung Dis. 2010;14(7):872–877. [PubMed] [Google Scholar]
- 23.Newton SM, Brent AJ, Anderson S, et al. Paediatric tuberculosis. Lancet Infect Dis. 2008;8(8):498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Detjen AK, DiNardo AR, Leyden J, et al. Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(6):451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicol MP, Spiers K, Workman L, et al. Xpert MTB/RIF Testing of Stool Samples for the Diagnosis of Pulmonary Tuberculosis in Children. Clin Infect Dis Off. 2013;57(3):e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frigati L, Archary M, Rabie H, et al. Priorities for Decreasing Morbidity and Mortality in Children with Advanced HIV Disease. Clin Infect Dis. 2018;66(Suppl 2):S147–S151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abuogi LL, Mwachari C, Leslie HH, et al. Impact of expanded antiretroviral use on incidence and prevalence of tuberculosis in children with HIV in Kenya. Int J Tuberc Lung Dis. 2013;17(10):1291–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crook AM, Turkova A, Musiime V, et al. Tuberculosis incidence is high in HIV-infected African children but is reduced by co-trimoxazole and time on antiretroviral therapy. BMC Med. 2016;14:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Link-Gelles R, Moultrie H, Sawry S, et al. Tuberculosis Immune Reconstitution Inflammatory Syndrome in Children Initiating Antiretroviral Therapy for HIV Infection. Pediatr Infect Dis J. 2014;33(5):499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Battegay M, Nüesch R, Hirschel B, et al. Immunological recovery and antiretroviral therapy in HIV-1 infection. Lancet Infect Dis. 2006;6(5):280–287. [DOI] [PubMed] [Google Scholar]
- 31.Kilborn T, Zampoli M. Immune reconstitution inflammatory syndrome after initiating highly active antiretroviral therapy in HIV-infected children. Pediatr Radiol. 2009;39(6):569–574. [DOI] [PubMed] [Google Scholar]