Abstract
Objective:
Studies show that serum levels of 25-hydroxyvitamin D (25(OH)D), a biomarker for vitamin D status, are lower in persons with higher adiposity levels and that police officers have been found to have a high prevalence of obesity. The purpose of this study was to examine relationships between several adiposity measures and 25(OH)D, and also compare those measures to determine the best one that predicts insufficiency of 25(OH)D (<20 ng/mL) among police officers in the Northeast area of the United States.
Methods:
Participants were 281 police officers (71.5% men) from the Buffalo Cardio-Metabolic Occupational Police Stress Study (2011–2016). Associations of body mass index (BMI), abdominal height (AbHt), waist circumference (WC), WC-to-height ratio (WCHtR), percent body fat (PBF), and fat mass index (FMI) with 25(OH)D were obtained using multiple regression models after adjustment for age, race/ethnicity, season, multivitamin supplement use, and high-density lipoprotein cholesterol. The area under the curve (AUC) was used to evaluate the predictive ability of each adiposity measure to identify insufficient 25(OH)D concentrations.
Results:
The prevalence of obesity (BMI ≥ 30) was 50.7% in men and 21.3% in women. Mean levels of 25(OH)D were 32.4 ng/mL in men and 34.4 ng/mL in women. After adjustment for covariates, PBF and FMI among men were inversely associated with 25(OH)D: PBF (β ± SE = −2.40 ± 1.01, P = .018); FMI (−2.21 ± 0.93, .018). Among women, no adiposity measure was associated with 25(OH)D. PBF was the best predictor of insufficient 25(OH)D concentrations regardless of gender (AUC = 0.878).
Conclusion:
Adiposity measures were inversely associated with 25(OH)D, but differed between female and male officers.
1 |. INTRODUCTION
Due to changes in the work environment over the past several decades from predominantly outdoor labor to indoor work (Church et al., 2011; Services USDoHaH, 2014) and increasing concern about reducing risk of skin cancer (Hoel, Berwick, Gruijl, & Holick, 2016; Services USDoHaH, 2014), individuals have been exposed to much lower levels of sunlight, which is the primary method by which vitamin D is obtained (Ganji, Zhang, & Tangpricha, 2012; Services USDoHaH, 2014). According to a recent National Health and Nutrition Examination Survey, 42% of the US adult population is at risk of vitamin D deficiency or insufficiency, and this percentage is increased in the elderly, women, African Americans, those with lower education, and persons with higher levels of adiposity (Forrest and Stuhldreher, 2011; Looker et al., 2011). Hoel et al. (2016) reported that maintaining optimal levels of vitamin D may help to prevent chronic diseases such as various types of cancers (colorectal, prostate, bladder, and breast), heart disease, Alzheimer’s disease and other types of dementia, myopia and other eye disorders, diabetes mellitus, and multiple sclerosis. Ganji et al. (2012) reported that the recent increase in the prevalence of those with vitamin D deficiency or insufficiency in the United States is likely due to the increased prevalence of obesity and lifestyle changes.
Vitamin D is actually a hormone, not a true vitamin (Ross, 2011). Vitamin D is fat soluble and fat tissues absorb vitamin D (Ross, 2011). Vitamin D is hydroxylated in the liver to form 25-hydroxyvitamin D (25(OH)D), the primary circulating form of the vitamin, which reflects upon exposure to sunlight as well as intake of vitamin D from foods and supplements. Because 25(OH)D reflects intake over approximately 3 weeks, it is used as the primary biomarker for vitamin D status (Barragry et al., 1978; Zerwekh, 2008). People who are obese may have lower 25(OH)D levels than those who are not obese because vitamin D is produced in the skin or ingested and then distributed in fat tissue (Ross, 2011; Ross et al., 2011).
There is abundant evidence showing an inverse association between serum 25(OH)D concentration and obesity levels (Cheng et al., 2010; Earthman, Beckman, Masodkar, & Sibley, 2012; Hannemann et al., 2015; Pereira-Santos, Costa, Assis, Santos, & Santos, 2015; Pourshahidi, 2015; Stokic et al., 2015). These studies were conducted in non-occupational samples of obese individuals. A recent study using U.S. National Health Interview Survey data revealed that workers in high-stress occupations, like police officers and correctional security officers, have a high prevalence of obesity (Gu et al., 2014). The study reported that protective service workers had the second highest prevalence of obesity among workers in 41 occupations, and approximately half of the law enforcement workers were obese (Gu et al., 2014). We wanted to know whether there is an association between obesity and 25(OH)D in workers who are regularly exposed to high levels of job stress. Therefore, our main goal was to examine the relationships between several adiposity measures (body mass index [BMI], waist circumference [WC], WC-to-height ratio [WCHtR], abdominal height [AbHt], percent body fat [PBF], fat mass index [FMI]) and serum concentration of 25(OH)D among police officers who worked in the Buffalo Police Department, Buffalo, New York. A secondary goal was to investigate which adiposity measure has the best performance to identify insufficiency (<20 ng/mL) of serum 25(OH)D concentration in this occupational group. We hypothesized that officers with higher levels of each type of adiposity measure would show lower levels of 25(OH)D.
2 |. SUBJECTS, MATERIALS, AND METHODS
2.1 |. Study setting and sample
This study used data collected from police officers in the Buffalo Police Department, New York, who participated in the Buffalo Cardio-Metabolic Occupational Police Stress (BCOPS) study. The BCOPS study has two main examinations: the baseline exam (2004–2009) and the follow-up exam (2011–2014). Additional details about the BCOPS study have been described by Violanti et al. (2006). Four hundred and sixty-four officers voluntarily participated in the baseline exam out of approximately 710 eligible active-duty officers. Two hundred and eighty-one officers, among those who participated in the baseline exam, participated in the follow-up exam. Furthermore, an ancillary study was conducted during 2012–2016 where additional data, for example, serum 25(OH) D, were collected to investigate associations between occupational stress and microvascular outcomes of subclinical cardiovascular disease. All participants (n = 281 officers) completed the anthropometric measurements and provided blood samples at follow-up, except 13 officers who did not complete the dual-energy X-ray absorptiometry (DEXA) exam, which measured PBF. All exams were conducted in the Center for Health Research, School of Public Health and Health Profession, the State University of New York at Buffalo (SUNY-Buffalo). The Institutional Review Boards of the SUNY-Buffalo and the National Institute for Occupational Safety and Health approved the study protocol, and informed consent was obtained from all participants.
2.2 |. Data measurements of adiposity and 25(OH)D
This study used two major types of adiposity measurements: anthropometric and body composition measures. Anthropometric measurements included physical examinations of height (m), weight (kg), WC (cm), and AbHt (cm). BMI was calculated as weight divided by height squared (kg/m2). WC was measured twice at the midpoint between the lowest rib and the top point of the hip bone, and the average value was used in the analysis. AbHt was measured three times using a sagittometer at the level of the iliac crest in the supine position, and the average value was used. WCHtR was defined as WC divided by height.
The body composition measurements were PBF and FMI derived from a DEXA exam using a Hologic QDR-4500A device (Hologic, Waltham, MA). PBF was calculated by summing body fat mass measurements for all DEXA segments and dividing by total body mass. FMI was calculated as the total PBF divided by height squared, an indicator of how much fat amount individuals have relative to their height.
Venous blood samples were drawn to determine serum 25(OH)D and were analyzed by Heartland Assays, Inc. We used serum 25(OH)D as a continuous variable when assessing associations between adiposity measures and 25(OH)D and as a dichotomous variable (<20 ng/mL for insufficient level and 20 ≥ ng/mL for sufficient level) when assessing the adiposity measure that would be the best predictor to identify vitamin D insufficiency (Ross et al., 2011).
2.3 |. Covariates
Demographic characteristics (age, gender, education, and marital status), lifestyle behaviors (smoking status, alcohol intake, dietary habits, physical activity, and sleep duration), and work characteristics (years of employment and rank) were collected from self-administered questionnaires. “Season” was obtained from the officer’s examination date of blood drawn in Buffalo, NY. “Shift work” was defined as the dominant shift among day, afternoon, or night shift from each participant’s payroll records (Fekedulegn et al., 2016). The time that participants started their shift for regular time work was used to classify each record into one of the following three shifts: day shift if the start time was between 4 am and 11 am; afternoon shift if the start time was between 12 pm and 7 pm; or night shift if the start time was between 8 pm and 3 am. The shift with the highest percentage of hours worked was defined as the dominant shift.
2.4 |. Statistical analysis
Characteristics of all officers are shown in Table 1. Continuous variables were reported as means and SDs, and the Student’s t test was used when gender was compared, whereas categorical variables were reported as frequencies and percentages, and the chi-square test was used. The Pearson’s correlation coefficients were used to assess the relationships between the six adiposity measures.
TABLE 1.
Characteristics of study participants by gender (BCOPS study 2011–2016)
| All (n = 281) | Women (n = 80) | Men (n = 201) | ||
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | P value | |
| Age (years) | 48.2 ± 7.9 | 48.0 ± 6.7 | 48.3 ± 8.4 | .749 |
| Years of service | 21.0 ±7.8 | 19.8 ±6.7 | 21.4 ±8.1 | .095 |
| Physical activity (h/wk) | 7.4 ± 7.7 | 6.4 ± 4.3 | 7.9 ± 8.7 | .070 |
| Alcohol intake (drinks/wk) | 5.4 ± 9.6 | 2.8 ±4.2 | 6.4 ± 10.9 | <.001 |
| Glucose (mg/dL) | 96.7 ±21.3 | 91.0 ± 10.8 | 99.0 ± 23.9 | <.001 |
| Triglyceride (mg/dL) | 120.9 ± 86.0 | 89.3 ± 73.0 | 133.4 ± 87.7 | <.001 |
| Vitamin D intake from foods (mcg) | 6.3 ± 4.8 | 5.9 ± 4.4 | 6.5 ± 4.9 | .383 |
| HDL cholesterol (mg/dL) | 49.3 ± 14.2 | 59.6 ± 16.1 | 45.0 ± 10.8 | <.001 |
| LDL cholesterol (mg/dL) | 124.0 ± 33.0 | 126.3 ± 30.4 | 123.1 ±34.1 | .468 |
| Body mass index (kg/m2) | 29.7 ± 4.9 | 27.4 ±5.1 | 30.6 ±4.3 | <.001 |
| WC (cm) | 97.6 ± 14.1 | 85.9 ± 11.9 | 102.2 ± 12.1 | <.001 |
| WC-to-height ratio | 0.55 ± 0.07 | 0.52 ± 0.07 | 0.57 ± 0.07 | <.001 |
| AbHt (cm) | 21.7 ±3.2 | 19.8 ±3.0 | 22.5 ± 3.0 | <.001 |
| PBF (%) | 27.1 ± 6.6 | 33.1 ±5.9 | 24.6 ±5.1 | <.001 |
| FMI (kg/m2) | 8.1 ± 2.8 | 9.3 ± 3.4 | 7.5 ± 2.4 | <.001 |
| Serum 25(OH)D (ng/mL) | 33.0 ± 11.9 | 34.4 ± 12.7 | 32.4 ± 11.5 | .202 |
| N (%) | N (%) | N (%) | P value | |
| Race/ethnicity | ||||
| White/Hispanic | 226 (80.4) | 55 (68.7) | 171 (85.1) | .001 |
| African American | 55 (19.6) | 5(31.3) | 30 (14.9) | |
| Education | ||||
| ≤HS/GED | 23 (8.2) | 4 (5.0) | 19 (9.5) | .464 |
| <4-year college | 144 (51.2) | 43 (53.7) | 101 (51.2) | |
| ≥4-year college | 114(40.6) | 33 (41.3) | 81 (40.3) | |
| Rank | ||||
| Patrol officer | 152 (54.7) | 49 (61.3) | 103 (52.3) | .393 |
| Sergeant/Lieut./Capt. | 58 (20.9) | 14 (17.5) | 44 (22.3) | |
| Detec./Execu./Others | 67 (24.2) | 17 (21.2) | 50 (25.4) | |
| Cigarette smoking status | .033 | |||
| Current | 27 (9.6) | 9 (11.4) | 18 (9.0) | |
| Former | 88 (31.4) | 33 (41.8) | 55 (27.4) | |
| Never | 165 (58.9) | 37 (46.8) | 128 (63.4) | |
| Season | ||||
| Winter | 62 (22.1) | 21 (26.3) | 41 (20.4) | .641 |
| Spring | 81 (28.8) | 21 (26.2) | 60 (29.9) | |
| Summer | 66 (23.5) | 20 (25.0) | 46 (22.9) | |
| Fall | 72 (25.6) | 18 (22.5) | 54 (26.9) | |
| Shiftwork (entire career) | <.001 | |||
| Day | 131 (47.0) | 58 (73.4) | 73 (36.5) | |
| Afternoon | 94 (33.7) | 14 (17.7) | 80 (40.0) | |
| Night | 54 (19.4) | 7 (8.9) | 47 (23.5) | |
| Vitamin D supplement intake | .030 | |||
| Yes | 79 (28.1) | 27 (33.7) | 52 (25.9) | |
| No | 202 (71.9) | 53 (66.3) | 149 (74.1) | |
| Vitamin D status | .285 | |||
| Insufficient (25(OH)D < 20 ng/mL) | 33 (11.7) | 12 (15.0) | 21 (10.5) | |
| Sufficient (25(OH)D ≥ 20 ng/mL) | 248 (88.3) | 68 (85.0) | 180 (89.5) | |
| BMI (kg/m2) | <.001 | |||
| Normal <25.0) | 38 (13.5) | 27 (33.7) | 11 (5.5) | |
| Overweight (25–29) | 124(44.1) | 36 (45.0) | 88 (43.8) | |
| Obese (≥30) | 119 (42.5) | 17 (21.3) | 102 (50.7) |
Note: P value was obtained from the Student’s t test (for continuous variables) and the chi-square test or Fishers’ exact test (for categorical variables).
Abbreviations: 25(OH)D, 25-Hydroxyvitamin D; Abht, abdominal height; BCOPS, Buffalo Cardio-Metabolic Occupational Police Stress; BMI, body mass index; FMI, fat mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PBF, percent body fat; WC, waist circumference; WCHtR, WC-to-height ratio.
Associations between each adiposity measure and 25(OH)D were tested using linear regression models. The measures of adiposity were standardized using a z-score transformation to ensure that the regression coefficients were directly comparable across models. The adjusted R2 was obtained from the regression model, and it measures how much variation in the dependent variable can be accounted for by the model. The adjusted R2 is the ratio of the sum of squares for the model to the corrected total sum of squares. In general, the larger the value of the adjusted R2, the better the model’s fit. Potential confounders of the adiposity and 25(OH)D association were selected based on whether they were reported as confounders in previous studies, were associated with both the adiposity measures and 25(OH)D in this study (P < .20), or showed a 10% change in estimate in the association between the full model with all potential covariates and the model after removing the covariate from the full model (Greenland, 1989). Our goal was to examine the association between adiposity measures and circulating 25(OH)D after controlling for factors that could explain this association. Tests for effect modification (ie, interaction) by gender, race/ethnicity, season, shiftwork, and vitamin D supplement (yes/no) were performed by including interaction terms in the model (criterion of significance, P < .2). Gender significantly modified the associations, and so we have reported gender-stratified results in Table 5.
TABLE 5.
Comparison of adiposity variables as predictors of insufficient vitamin D status (25(OH)D < 20 ng/mL)
| All (N = 281) | Female (n = 80) | Male (n = 201) | Difference (female-male) | ||||
|---|---|---|---|---|---|---|---|
| AUC | P value for difference | AUC | P value for difference | AUC | P value for difference | P value for difference | |
| BMI | 0.866 | Referent | 0.967 | Referent | 0.839 | Referent | .011 |
| WC | 0.867 | .911 | 0.966 | .793 | 0.842 | .738 | .009 |
| WChtR | 0.867 | .520 | 0.968 | .688 | 0.840 | .805 | .111 |
| Abht | 0.866 | .980 | 0.965 | .528 | 0.844 | .730 | .008 |
| PBF | 0.878 | .123 | 0.968 | .873 | 0.853 | .169 | .018 |
| FMI | 0.874 | .076 | 0.970 | .5280 | 0.847 | .300 | .013 |
Notes: All models were adjusted for age, (gender), race/ethnicity, season, shiftwork, vitamin D supplement intake, and HDL cholesterol.
P value was compared between the AUC of each adiposity and the AUC of BMI.
Abbreviations: 25(OH)D, 25-Hydroxyvitamin D; Abht, abdominal height; AUC, area under the curve; BMI, body mass index; FMI, fat mass index; HDL, high-density lipoprotein; PBF, percent body fat; WC, waist circumference; WCHtR, WC-to-height ratio.
A receiver-operating characteristic (ROC) curve plots the sensitivity (true positive rate) against the 1-specificity (false positive rate) for all possible cutoff values. The area under an ROC curve (AUC) is a popular measure of the accuracy of a diagnostic test. The possible values of AUC range from 0.5 (no diagnostic ability) to 1.0 (perfect diagnostic ability). In general, higher AUC values indicate better test performance. Alternately, the more closely the sensitivity nears 100% (or 1.0 in the true positive rate), the better the diagnostic test. In this study, AUC was used to evaluate the predictive ability of each measure of adiposity to identify insufficient vitamin D status (25(OH)D < 20 ng/mL) for all officers and separately by gender. Logistic regression was used to evaluate the predictive ability, AUC, of each measure of adiposity, and created an ROC curve for comparisons between each measure of adiposity. AUC statistics for the ROC curve were calculated using a nonparametric method by E. R. DeLong, DeLong, and Clarke-Pearson (1988). The higher the AUC of the predictor variable (adiposity measure), the better it predicts insufficient vitamin D status. All statistical analyses were performed using SAS v9.4 (Cary, NC).
3 |. RESULTS
Table 1 shows descriptive statistics for the study subjects. The mean values for all anthropometric measures of adiposity (BMI, WC, WCHtR, and AbHt) were higher in male officers compared to female officers, whereas mean values of the body composition measures (PBF and FMI) were higher in female officers. BMI was significantly associated with gender, where half of the male officers were obese (BMI ≥ 30 kg/m2) while 21% of female officers were obese. As expected, all adiposity measures were significantly correlated with each other (correlation range: 0.688–0.946) (data not shown here).
Table 2 shows the unadjusted associations of the selected covariates with serum 25(OH)D and six adiposity measures. High-density lipoprotein (HDL) cholesterol was significantly correlated with 25(OH)D and all adiposity measures, except FMI. Vitamin D supplement intake was also significantly associated with 25(OH)D (P < .001) and the body composition measures (P = .019 for PBF and P = .031 for FMI).
TABLE 2.
Associations between the selected characteristics and serum 25(OH)D and adiposity measures
| Outcome | Adiposity measures | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 25(OH)D | BMI | WC | WCHtR | Abht | PBF | FMI | |||
| Characteristics | Corr. | Corr. | Corr. | Corr. | Corr. | Corr. | Corr. | ||
| Age (years) | −0.088 | 0.071 | 0.133 | 0.135 | 0.157 | 0.155 | 0.148 | ||
| P value | .141 | .234 | .026 | .024 | .008 | .011 | .015 | ||
| Years of service | 0.036 | 0.068 | 0.153 | 0.125 | 0.143 | 0.069 | 0.081 | ||
| P value | .543 | .253 | .010 | .036 | 0.017 | .259 | .186 | ||
| Physical activity (h/wk) | 0.038 | −0.041 | −0.044 | −0.065 | −0.084 | −0.158 | −0.117 | ||
| P value | .526 | .496 | .463 | .277 | .163 | .010 | .056 | ||
| Alcohol intake (#/wk) | −0.068 | 0.084 | 0.149 | 0.098 | 0.116 | −0.064 | 0.016 | ||
| P value | .256 | .162 | .013 | .103 | .054 | .296 | .793 | ||
| Glucose (mg/dL) | −0.042 | 0.330 | 0.294 | 0.313 | 0.345 | 0.067 | 0.161 | ||
| P value | .487 | <.001 | <.001 | <.001 | <.001 | .278 | .008 | ||
| Triglyceride (mg/dL) | −0.056 | 0.288 | 0.295 | 0.279 | 0.265 | −0.004 | 0.105 | ||
| P value | .353 | <.001 | <.001 | <.001 | <.001 | .951 | .088 | ||
| HDL cholesterol (mg/dL) | 0.200 | −0.325 | −0.423 | −0.327 | −0.369 | 0.194 | −0.015 | ||
| P value | .001 | <.001 | <.001 | <.001 | <.001 | .001 | .812 | ||
| LDL cholesterol (mg/dL) | −0.106 | 0.080 | 0.010 | 0.061 | 0.014 | 0.102 | 0.102 | ||
| P value | .076 | .180 | .864 | .311 | .819 | .097 | .096 | ||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| Gender | |||||||||
| Female | 34.4 (12.7) | 27.4(5.1) | 85.9 (11.9) | 0.52 (0.07) | 19.8 (3.0) | 33.1 (6.0) | 9.3 (3.4) | ||
| Male | 32.4 (11.5) | 30.6 (4.6) | 102.2(12.1) | 0.57 (0.07) | 22.4 (3.0) | 24.6 (5.1) | 7.5 (2.4) | ||
| P value | .202 | <.001 | <.001 | <.001 | <.001 | <.001 | .003 | ||
| Race/ethnicity | |||||||||
| White/Hispanic | 34.6 (11.2) | 29.5 (4.9) | 97.9 (14.1) | 0.55 (0.07) | 21.6 (3.3) | 26.8 (6.5) | 7.9 (2.7) | ||
| African American | 26.5 (12.3) | 30.5 (5.1) | 96.2 (13.3) | 0.55 (0.07) | 22.2 (2.9) | 28.4 (7.2) | 8.7 (3.2) | ||
| P value | <.001 | .199 | .417 | .992 | .238 | .109 | .056 | ||
| Education | |||||||||
| ≤HS/GED | 35.6 (14.1) | 30.2 (4.4) | 100.8 (14.7) | 0.57 (0.07) | 22.2 (3.5) | 28.2 (6.1) | 8.4 (2.4) | ||
| <4-year college | 32.0 (12.3) | 29.6 (4.7) | 97.2 (14.0) | 0.55 (0.07) | 21.7 (3.2) | 27.3 (5.9) | 8.1 (2.5) | ||
| ≥ 4-year college | 33.7 (10.8) | 29.6 (5.4) | 97.3 (14.1) | 0.55 (0.08) | 21.6 (3.3) | 26.6 (7.6) | 7.9 (3.2) | ||
| P value | .297 | .895 | .525 | .628 | .729 | .527 | .689 | ||
| Rank | |||||||||
| Patrol officer | 32.5 (12.9) | 29.6 (5.0) | 97.0 (14.2) | 0.55 (0.08) | 21.7 (3.2) | 27.2 (6.9) | 8.2 (2.9) | ||
| Sergeant/Lieut./Capt. | 33.7 (9.0) | 29.8 (4.8) | 99.0 (12.9) | 0.56 (0.06) | 21.9 (3.1) | 27.1 (6.7) | 8.2 (3.0) | ||
| Detec./Execu./Others | 33.4 (12.0) | 28.8 (4.7) | 96.1 (13.5) | 0.54 (0.07) | 21.1 (2.9) | 26.7 (5.9) | 7.6 (2.1) | ||
| P value | .763 | .321 | .488 | .474 | .254 | .848 | .313 | ||
| Cigarette smoking status | |||||||||
| Current | 34.9 (13.9) | 30.2 (7.2) | 98.5 (17.6) | 0.56(0.10) | 22.0 (4.5) | 27.8 (6.2) | 8.2 (3.6) | ||
| Former | 34.8 (11.7) | 29.1 (4.6) | 96.1 (13.2) | 0.55 (0.07) | 21.5 (3.1) | 28.2 (7.1) | 8.2 (2.9) | ||
| Never | 31.5 (11.2) | 30.0 (4.7) | 98.3 (13.9) | 0.56 (0.07) | 21.8 (3.0) | 26.4 (6.4) | 7.9 (2.6) | ||
| P value | .066 | .327 | .472 | .571 | .631 | .097 | .695 | ||
| Seasons | |||||||||
| Winter | 31.1 (11.9) | 29.9 (5.2) | 97.4 (14.8) | 0.55 (0.08) | 21.6 (3.4) | 28.0 (7.1) | 8.1 (2.6) | ||
| Spring | 28.8 (10.4) | 29.7 (4.8) | 97.9 (12.6) | 0.55 (0.07) | 21.9 (2.9) | 26.6 (5.7) | 8.0 (2.8) | ||
| Summer | 38.5 (12.1) | 29.9 (4.9) | 97.4 (14.7) | 0.56 (0.08) | 21.7 (3.6) | 27.6 (7.0) | 8.4 (3.0) | ||
| Fall | 34.3 (11.3) | 29.3 (5.0) | 97.5 (14.6) | 0.55 (0.08) | 21.6 (3.1) | 26.4 (6.9) | 7.8 (2.9) | ||
| P value | <.001 | .857 | .996 | .933 | .930 | .505 | .730 | ||
| Shiftwork | |||||||||
| Day | 32.4 (12.9) | 29.0 (4.8) | 95.0 (13.7) | 0.54 (0.07) | 21.3 (3.2) | 28.3 (6.6) | 8.2 (2.7) | ||
| Afternoon | 34.9 (12.1) | 30.5 (5.1) | 100.3 (14.6) | 0.57 (0.08) | 22.1 (3.3) | 26.3 (6.7) | 8.0 (2.8) | ||
| Night | 31.3 (8.4) | 30.0 (5.0) | 99.4 (13.3) | 0.56 (0.07) | 21.9 (3.0) | 25.4 (6.3) | 7.7 (3.0) | ||
| P value | .141 | .060 | .011 | .076 | .137 | .012 | .562 | ||
| Vitamin D supplement intake | |||||||||
| Yes | 31.1 (11.6) | 29.6 (4.8) | 97.1 (13.6) | 0.55 (0.07) | 21.6 (3.2) | 26.5 (6.5) | 7.8 (2.6) | ||
| No | 37.8 (11.4) | 29.8 (5.2) | 98.6 (15.3) | 0.56 (0.08) | 21.9 (3.4) | 28.6 (6.7) | 8.6 (3.2) | ||
| P value | <0.001 | .711 | .435 | .198 | .435 | .019 | .031 | ||
Abbreviations: 25(OH)D, 25-Hydroxyvitamin D; Abht, abdominal height; BMI, body mass index; FMI, fat mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PBF, percent body fat; WC, waist circumference; WCHtR, WC-to-height ratio.
Table 3 shows the associations between adiposity and 25(OH)D using regression analysis. Most adiposity measures (BMI, WCHtR, PBF, and FMI) were significantly and inversely associated with 25(OH)D after adjusting for age, gender, race/ethnicity, season of the year, shiftwork status, vitamin D supplement intake, and HDL cholesterol. Among all of the adiposity measures, PBF and FMI had the largest decrease in 25(OH)D for 1-SD increase in the adiposity measure (β ± SE, P value: −2.94 ± 0.80, <.001 and −2.51 ± 0.68, <.001 respectively) after adjusting for all covariates, and PBF and FMI were the best predictors of 25(OH)D (R2 for both PBF and FMI = 0.277).
TABLE 3.
Associations between six adiposity variables and 25(OH)D among all officers
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Adiposity | βa ± SE | P value | Adj R2 | βa±SE | P value | Adj R2 | βa ± SE | P value | Adj R2 |
| BMI | −2.32 ± 0.70 | .001 | 0.035 | −1.90 ± 0.68 | .006 | 0.189 | −1.92 ± 0.68 | .005 | 0.249 |
| WC | −1.34 ±0.71 | .058 | 0.009 | −1.19 ±0.77 | .122 | 0.173 | −1.19 ±0.77 | .124 | 0.234 |
| WCHtR | −1.43 ± 0.71 | .043 | 0.011 | −1.37 ± 0.69 | .048 | 0.178 | −1.48 ± 0.69 | .032 | 0.240 |
| Abht | −1.68 ± 0.70 | .018 | 0.016 | −1.27 ±0.71 | .077 | 0.175 | −1.24 ± 0.72 | .084 | 0.235 |
| PBF | −0.77 ± 0.73 | .295 | 0.001 | −2.64 ± 0.82 | .001 | 0.212 | −2.94 ± 0.80 | .003 | 0.277 |
| FMI | −1.62 ± 0.73 | .026 | 0.015 | −2.27 ± 0.69 | .001 | 0.214 | −2.51 ± 0.68 | .003 | 0.277 |
Notes: Model 1: unadjusted. Model 2: adjusted for age, gender, race/ethnicity, and season. Model 3: adjusted for model 2 + (shiftwork, vitamin D supplement intake, HDL cholesterol). Adj R2: adjusted R2 values.
Abbreviations: 25(OH)D, 25-Hydroxyvitamin D; Abht, abdominal height; BMI, body mass index; FMI, fat mass index; HDL, high-density lipoprotein; PBF, percent body fat; WC, waist circumference; WCHtR, WC-to-height ratio.
Standardized β coefficient. The coefficient represents change in 25(OH)D for 1 SD increase in adiposity.
After stratification by gender (Table 4), all associations were statistically significant in the unadjusted model (Model 1) for women, but the associations in the model adjusted for age, race/ethnicity, and season (Model 2) and in the fully-adjusted model (Model 3) were attenuated. Among men, most associations were not significant in Models 1 and 2, but the associations of PBF and FMI with 25(OH)D were significant in Model 3, the fully adjusted model (β ± SE: −2.40 ± 1.01, P = .018, and −2.21 ± 0.93, P = .018, respectively).
TABLE 4.
Associations between six adiposity variables and 25(OH)D stratified by gender
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| βa ± SE | P value | Adj R2 | βa ± SE | P value | Adj R2 | βa ± SE | P value | Adj R2 | |
| Female (n = 80) | |||||||||
| BMI | −4.27 ± 1.30 | .002 | 0.110 | −3.28 ± 1.29 | .013 | 0.268 | −2.40 ± 1.21 | .050 | 0.425 |
| WC | −3.80 ± 1.65 | .024 | 0.051 | −2.73 ± 1.67 | .106 | 0.231 | −2.02 ± 1.53 | .191 | 0.406 |
| WCHtR | −3.56 ± 1.38 | .012 | 0.067 | −2.78 ± 1.36 | .045 | 0.246 | −2.01 ± 1.25 | .112 | 0.414 |
| AbHt | −3.52 ± 1.49 | .020 | 0.055 | −2.26 ± 1.54 | .148 | 0.225 | −1.58 ± 1.44 | .278 | 0.402 |
| PBF | −3.25 ± 1.57 | .042 | 0.040 | −3.21 ± 1.56 | .044 | 0.248 | −2.68 ± 1.43 | .066 | 0.420 |
| FMI | −3.11 ± 1.17 | .010 | 0.072 | −2.65 ± 1.16 | .025 | 0.258 | −1.97 ± 1.08 | .073 | 0.419 |
| Male (n = 201) | |||||||||
| BMI | −1.23 ± 0.88 | .164 | 0.005 | −1.27 ±0.81 | .122 | 0.156 | −1.41 ±0.83 | 0.090 | 0.190 |
| WC | −0.21 ± 0.95 | .827 | −0.005 | −0.66 ± 0.88 | .458 | 0.148 | −0.73 ± 0.90 | 0.416 | 0.181 |
| WCHtR | −0.23 ± 0.88 | .798 | −0.005 | −0.79 ± 0.81 | .336 | 0.149 | −1.03 ± 0.83 | 0.215 | 0.184 |
| AbHt | −0.76 ± 0.88 | .385 | 0.001 | −0.93 ± 0.82 | .255 | 0.151 | −0.92 ± 0.83 | 0.273 | 0.183 |
| PBF | −1.35 ± 1.09 | .216 | 0.003 | −2.07 ± 1.01 | .041 | 0.183 | −2.40 ± 1.01 | 0.018 | 0.212 |
| FMI | −1.19 ±0.99 | .233 | 0.002 | −1.80 ±0.91 | .051 | 0.182 | −2.21 ± 0.93 | 0.018 | 0.212 |
Notes: Model 1: unadjusted. Model 2: adjusted for age, race/ethnicity, and season. Model 3: Model 2 + (shiftwork, vitamin D supplement intake, and HDL cholesterol). Adj R2: adjusted R2 values.
Gender interaction term P = .0323.
Abbreviations: 25(OH)D, 25-Hydroxyvitamin D; Abht, abdominal height; BMI, body mass index; FMI, fat mass index; HDL, high-density lipoprotein; PBF, percent body fat; WC, waist circumference; WCHtR, WC-to-height ratio.
Standardized β coefficient. The coefficient represents change in 25(OH)D for 1 SD increase in adiposity.
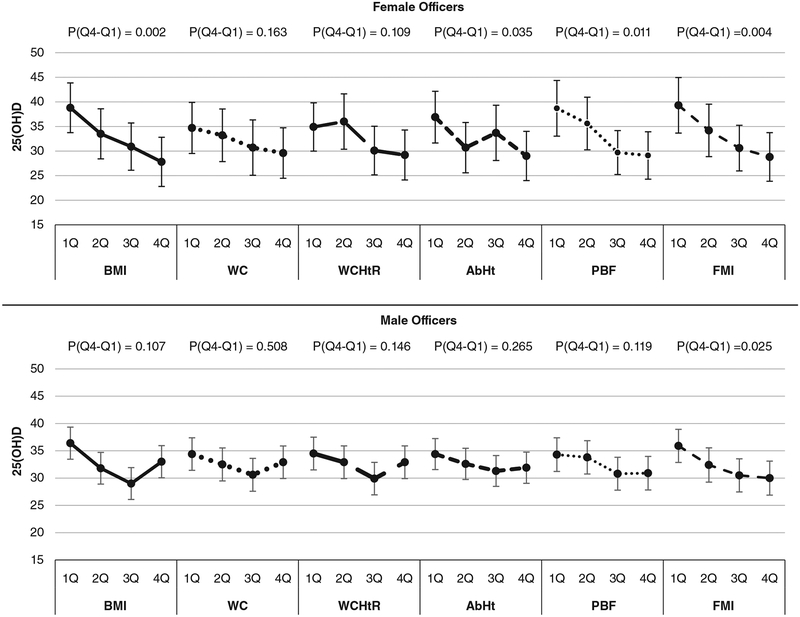
Figure 1 shows the mean level of 25(OH)D across quartiles of each adiposity measure and P values for a comparison between the 1st and 4th quartiles. The significant mean difference between the 1st and 4th quartiles of 25(OH)D was found with BMI, AbHt, PBF, and FMI among female officers (P value for mean difference = .002, .035, .011, and .004, respectively). Among male officers, there was no statistical significance in the mean difference between the 1st and 4th quartiles of the adiposity measures with 25(OH)D, except in the case of FMI (P = .025).
FIGURE 1.
Mean levels of 25(OH)D across quartiles of each adiposity variable stratified by gender. P(Q4-Q1): P value for comparison between 4th and 1st quartiles. All models were adjusted for age, race/ethnicity, season, shiftwork, vitamin D supplement intake, and HDL cholesterol. 25(OH)D, 25-Hydroxyvitamin D; HDL, high-density lipoprotein
Table 5 shows the AUC of the ROC for identifying insufficient vitamin D status (25(OH)D < 20 ng/mL) by adiposity. All six measures showed moderately high accuracy for the identification of insufficient vitamin D status, ranging from 0.866 to 0.878 for all officers. The AUCs among females (0.965–0.970) were significantly higher than among males (0.839–0.853). PBF had the highest AUC (0.878) among six adiposity measures, which means that PBF was the most appropriate to identify insufficient vitamin D status regardless of gender. Compared to BMI, the lowest AUC (0.866), PBF exhibit higher performance for identification of insufficient vitamin D, but not significantly (P value for PBF vs BMI = .076). Thus, PBF would not identify insufficient vitamin D status any better than BMI.
4 |. DISCUSSION
This study investigated associations between several measures of adiposity and serum vitamin 25(OH)D in police officers from Buffalo, New York. The overall findings indicate that the adiposity measures were significantly and inversely associated with 25(OH)D even after adjustment for potential confounding variables. Our results were consistent with and support previous studies of non-occupational samples (Arunabh, Pollack, Yeh, & Aloia, 2003; Earthman et al., 2012; Pourshahidi, 2015; Vanlint, 2013). We also observed that the associations between the body composition measures (PBF, FMI) and 25(OH)D were stronger than those between the anthropometric measures (BMI, WC, WCHtR, and AbHt) and 25(OH)D. This finding is biologically plausible because 25(OH)D is fat soluble, and the body fat components would be more directly affected by the sequestration of 25(OH)D in adipose tissue than would the anthropometric measures. In addition, BMI and the other anthropometric measures are not able to distinguish between fat mass and lean mass and capture the anatomical distribution of adipose tissue (Mooney, Baecker, & Rundle, 2013).
Among male officers, none of the anthropometric measures (BMI, WC, WCHtR, and AbHt) were significantly associated with 25(OH)D, whereas the body composition measures (PBF and FMI) were significantly associated with 25(OH)D. Men in this cohort had a greater prevalence of being overweight and obese than women, and we observed stronger associations between body composition and 25(OH)D in men than in women. Peltz, Aguirre, Sanderson, and Fadden (2010) reported that the body composition measure (FMI) more accurately assessed obesity than the anthropometric measure (BMI) in a study of Mexican Americans, who had a much higher prevalence of obesity than other ethnic groups. Previous studies showed that AbHt and WC, which are highly correlated with visceral and subcutaneous fat, respectively (Chen et al., 2014; Gletsu-Miller et al., 2013; Goodwin et al., 2013), were strongly associated with 25(OH)D (Cheng et al., 2010; Hannemann et al., 2015). However, our study did not find that the abdominal fat measures were strongly related with 25(OH)D.
Our comparison of the adiposity measures to predict vita-min D insufficiency (25(OH)D ≤ 20 ng/mL) identified the strongest predictor as the measure having the largest area under the ROC curve (AUC). The body composition measures of obesity (PBF and FMI) were better predictors of vitamin D insufficiency than the conventional measures (BMI, WC, WCHtR, and AbHt). After gender stratification, the AUC statistics for the body composition and anthropometric measures were not different. Therefore, we cannot say with confidence that the body composition measures were superior to the anthropometric measures in predicting vitamin D insufficiency. Even though PBF was found to be the best predictor for insufficient 25(OH)D, the differences between PBF and the other adiposity measures were slight. Therefore, the use of BMI as a predictor of 25(OH)D insufficiency is acceptable since it is easy and inexpensive to obtain.
Another finding of this study is that the prevalence of vitamin D insufficiency in police officers was much lower than that of the US adult population (12% vs 42%) (Forrest & Stuhldreher, 2011). One possible explanation is that the lower prevalence of vitamin D insufficiency in police officers may be reflective of the “healthy worker effect,” which Finkelstein (1998) described in his research of Ontario police officers in Canada. The other explanation is that police officers spent more time in outdoor physical activities than other occupational groups. Gu et al. (2016) reported that the prevalence of sufficient leisure-time physical activity in Protective Service (67% in 2013) was one of the highest among 21 US occupational groups (average 55%). It may be that police officers have more sun exposure than the general population.
This study has several limitations and strengths. Because this study is cross-sectional, the findings do not indicate causality or temporality, whether adiposity levels preceded the observed measures of vitamin D status, or vice versa. However, the possible mechanisms of vitamin D and obesity have been well-described in review papers, in vitro and in vivo studies (Blum et al., 2008; Ekwaru, Zwicker, Holick, Giovannucci, & Veugelers PJ, 2014; Mutt, Hypponen, Saarnio, Jarvelin, & Herzig, 2014; Vanlint, 2013). Ekwaru et al. (2014) reported that vitamin D supplementation was two or three times higher for those who were obese and one and a half times higher for those who were overweight compared to those with normal weight. Those who were obese were less willing to have sun exposure and would have lower serum vitamin D concentration than non-obese persons (Kull, Kallikorm, & Lember, 2009).
Visceral or subcutaneous adipose tissues measured directly by using magnetic resonance imaging (MRI) or computed tomography (CT) are the objective assessments for abdominal adiposity. Two studies using these methods revealed that visceral and subcutaneous adipose tissues were strongly and inversely associated with serum 25(OH)D concentrations, especially visceral adiposity (Cheng et al., 2010; Hannemann et al., 2015). Unfortunately, we could not assess associations using these adipose tissue measures because DEXA does not accurately distinguish between visceral and subcutaneous fat.
One of the strengths of this study is that our sample comprises a healthy and physically active group of officers with low comorbidities and little medication usage, which means there is less chance of interactions due to medication usage (Robien, Oppeneer, Kelly, & Hamilton-Reeves, 2013). Additionally, we observed a robust association between adiposity and serum 25(OH)D concentration as set up in an optimal model, considering all potential confounders, including lifestyle, vitamin D supplement use, biomarkers of metabolic syndrome or heart disease, season, and occupational factors (eg, length of employment, rank, and shiftwork). There are numerous methods for assessing obesity. The strength of this study is the use of both the anthropometric and body composition measures for obesity. The anthropometric measures (BM, WC, AbHt, etc.) are traditional and broadly used in large epidemiological studies because they are simple and noninvasive methods of measurement. The body composition measures that were assessed by DEXA, such as PBF or FMI, are scientific and accurate, but they are limited in large epidemiological studies because they require large specialized equipment and are expensive to measure.
In conclusion, several measures of adiposity were negatively associated with 25(OH)D concentrations among police officers. Our findings are consistent with those of the published literature.
ACKNOWLEDGMENTS
This work was supported by the National Institute for Occupational Safety and Health (NIOSH), Contract No. 200-2003-01580 and Grant No. 1R01OH 009640–01A1. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
Funding information
Luenda Charles, Grant/Award Number: 1R01OH 009640–01A1; National Institute for Occupational Safety and Health (NIOSH), Grant/Award Number: 200-2003-01580
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
All participants signed written informed consent. The Institutional Review Boards at the University at Buffalo and the National Institute for Occupational Safety and Health approved the studies.
REFERENCES
- Arunabh S, Pollack S, Yeh J, & Aloia JF (2003). Body fat content and 25-hydroxyvitamin D levels in healthy women. The Journal of Clinical Endocrinology and Metabolism, 88(1), 157–161. [DOI] [PubMed] [Google Scholar]
- Barragry JM, France MW, Corless D, Gupta SP, Switala S, Boucher BJ, & Cohen RD (1978). Intestinal cholecalciferol absorption in the elderly and in younger adults. Clinical Science and Molecular Medicine, 55(2), 213–220. [DOI] [PubMed] [Google Scholar]
- Blum M, Dolnikowski G, Seyoum E, Harris SS, Booth SL, Peterson J, … Dawson-Hughes B (2008). Vitamin D(3) in fat tissue. Endocrine, 33(1), 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Chen YY, Chuang CL, Chiang LM, Chiao SM, & Hsieh KC (2014). The study of anthropometric estimates in the visceral fat of healthy individuals. Nutrition Journal, 13, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, et al. (2010). Adiposity, cardiometabolic risk, and vitamin D status: The Framingham heart study. Diabetes, 59(1), 242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church TS, Thomas DM, Tudor-Locke C, Katzmarzyk PT, Earnest CP, Rodarte RQ, … Bouchard C (2011). Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS One, 6(5), e19657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong ER, DeLong DM, & Clarke-Pearson DL (1988). Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics, 44(3), 837–845. [PubMed] [Google Scholar]
- Earthman CP, Beckman LM, Masodkar K, & Sibley SD (2012). The link between obesity and low circulating 25-hydroxyvitamin D concentrations: Considerations and implications. International Journal of Obesity, 36(3), 387–396. [DOI] [PubMed] [Google Scholar]
- Ekwaru JP, Zwicker JD, Holick MF, Giovannucci E, & Veugelers PJ (2014). The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLoS One, 9(11), e111265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekedulegn D, Burchfiel CM, Charles LE, Hartley TA, Andrew ME, & Violanti JM (2016). Shift work and sleep quality among urban police officers: The BCOPS study. Journal of Occupational and Environmental Medicine, 58(3), e66–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein MM (1998). Cancer incidence among Ontario police officers. American Journal of Industrial Medicine, 34(2), 157–162. [DOI] [PubMed] [Google Scholar]
- Forrest KY, & Stuhldreher WL (2011). Prevalence and correlates of vitamin D deficiency in US adults. Nutrition Research, 31(1), 48–54. [DOI] [PubMed] [Google Scholar]
- Ganji V, Zhang X, & Tangpricha V (2012). Serum 25-hydroxyvitamin D concentrations and prevalence estimates of hypovitaminosis D in the U.S. population based on assay-adjusted data. The Journal of Nutrition, 142(3), 498–507. [DOI] [PubMed] [Google Scholar]
- Gletsu-Miller N, Kahn HS, Gasevic D, Liang Z, Frediani JK, Torres WE, … Lin E (2013). Sagittal abdominal diameter and visceral adiposity: Correlates of beta-cell function and dysglycemia in severely obese women. Obesity Surgery, 23(7), 874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin K, Syme C, Abrahamowicz M, Leonard GT, Richer L, Perron M, … Pausova Z (2013). Routine clinical measures of adiposity as predictors of visceral fat in adolescence: A population-based magnetic resonance imaging study. PLoS One, 8(11), e79896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S (1989). Modeling and variable selection in epidemiologic analysis. American Journal of Public Health, 79(3), 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JK, Charles LE, Bang KM, Ma CC, Andrew ME, Violanti JM, & Burchfiel CM (2014). Prevalence of obesity by occupation among US workers: The National Health Interview Survey 2004–2011. Journal of Occupational and Environmental Medicine, 56(5), 516–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JK, Charles LE, Ma CC, Andrew ME, Fekedulegn D, Hartley TA, … Burchfiel CM (2016). Prevalence and trends of leisure-time physical activity by occupation and industry in U.S. workers: The National Health Interview Survey 2004–2014. Annals of Epidemiology, 26(10), 685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannemann A, Thuesen BH, Friedrich N, Volzke H, Steveling A, Ittermann T, … Wallaschofski H (2015). Adiposity measures and vitamin D concentrations in Northeast Germany and Denmark. Nutrition & Metabolism (London), 12, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoel DG, Berwick M, de Gruijl FR, & Holick MF (2016). The risks and benefits of sun exposure 2016. Dermatoendocrinol, 8 (1), e1248325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kull M, Kallikorm R, & Lember M (2009). Body mass index determines sunbathing habits: Implications on vitamin D levels. Internal Medicine Journal, 39(4), 256–258. [DOI] [PubMed] [Google Scholar]
- Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, & Sempos CT (2011). Vitamin D status: United States, 2001–2006. NCHS Data Brief, (59), 1–8. [PubMed] [Google Scholar]
- Mooney SJ, Baecker A, & Rundle AG (2013). Comparison of anthropometric and body composition measures as predictors of components of the metabolic syndrome in a clinical setting. Obesity Research & Clinical Practice, 7(1), e55–e66. [DOI] [PubMed] [Google Scholar]
- Mutt SJ, Hypponen E, Saarnio J, Jarvelin MR, & Herzig KH (2014). Vitamin D and adipose tissue-more than storage. Frontiers in Physiology, 5, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltz G, Aguirre MT, Sanderson M, & Fadden MK (2010). The role of fat mass index in determining obesity. American Journal of Human Biology, 22(5), 639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira-Santos M, Costa PR, Assis AM, Santos CA, & Santos DB (2015). Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obesity Reviews, 16(4), 341–349. [DOI] [PubMed] [Google Scholar]
- Pourshahidi LK (2015). Vitamin D and obesity: Current perspectives and future directions. The Proceedings of the Nutrition Society, 74 (2), 115–124. [DOI] [PubMed] [Google Scholar]
- Robien K, Oppeneer SJ, Kelly JA, & Hamilton-Reeves JM (2013). Drug-vitamin D interactions: A systematic review of the literature. Nutrition in Clinical Practice, 28(2), 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AC (2011). The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutrition, 14(5), 938–939. [DOI] [PubMed] [Google Scholar]
- Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. (2011). The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. The Journal of Clinical Endocrinology and Metabolism, 96(1), 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Services USDoHaH. (2014). The surgeon general’s call to action to prevent skin cancer. Washington (DC): Washington, DC: U.S: Dept of Health and Human Services, Office of the Surgeon General. [Google Scholar]
- Stokic E, Kupusinac A, Tomic-Naglic D, Zavisic BK, Mitrovic M, Smiljenic D, … Isenovic E (2015). Obesity and vitamin D deficiency: Trends to promote a more proatherogenic cardiometabolic risk profile. Angiology, 66(3), 237–243. [DOI] [PubMed] [Google Scholar]
- Vanlint S (2013). Vitamin D and obesity. Nutrients, 5(3), 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violanti JM, Burchfiel CM, Miller DB, Andrew ME, Dorn J, Wactawski-Wende J, … Trevisan M (2006). The Buffalo cardio-metabolic occupational police stress (BCOPS) pilot study: Methods and participant characteristics. Annals of Epidemiology, 16(2), 148–156. [DOI] [PubMed] [Google Scholar]
- Zerwekh JE (2008). Blood biomarkers of vitamin D status. The American Journal of Clinical Nutrition, 87(4), 1087S–1091S. [DOI] [PubMed] [Google Scholar]