Abstract
Weaning is one of the most stressful periods in the life of a ruminant. Several factors entrenched within typical management practices pose challenges to the calf gastrointestinal health. Weaning is associated with losses in BW and feed intake. In addition, increasing highly fermentable carbohydrates in the diet at the expense of physically effective fiber after weaning predisposes the development of rumen acidosis and increases the concentration of endotoxin in rumen fluid and the permeability of the lower gut to luminal contents. Endotoxin translocation can elicit immune activation, shifting the metabolic priorities toward the immune system, which if sustained over time can hinder animal health and performance. Strategic supplementation of additives with anti-inflammatory capacity could represent a suitable approach to decrease systemic inflammation, restoring barrier function to luminal contents. Bioactive extracts from Olea europaea have anti-inflammatory activity and have been shown to reduce systemic inflammation in other animal models. A generalized randomized block design was used to evaluate the impact of feeding an olive oil bioactive extract (OBE) to newly weaned heifers injected intravenously with sequentially increasing doses of lipopolysaccharide (LPS). A total of 36 heifers, distributed across 3 experimental periods, were randomly assigned to 1 of 4 treatments that consisted of intravenous injection of either saline (CTL−) or with 6 sequentially increasing doses of LPS (0.10, 0.25, 0.50, 0.75, 1.00, and 1.25 µg/kg of BW) over a 10-d period (CTL+), and CTL+ plus dietary supplementation with a low (OBE-L; 0.04% of diet DM) or a high (OBE-H; 0.16% of diet DM) dose of OBE. Feeding OBE reduced some of the negative effects of prolonged immune activation with LPS, such as improved DMI and decreased intravaginal temperature in some, but not all of the days of LPS challenge (P < 0.05). In addition, feeding OBE reduced circulating concentration of inflammatory markers such as IL-6 and haptoglobin (P < 0.05). Heifers supplemented with OBE had reduced cell surface expression of the cluster of differentiation 14 (CD14) in monocyte cells (P < 0.01), a key receptor for LPS recognition, which was correlated with a faster recovery of immune cell counts in plasma. In conclusion, dietary supplementation with OBE was successful in mitigating the negative effects of sustained immune activation in newly weaned heifers.
Keywords: beef cattle, inflammation, olive extract, weaning
Introduction
Weaning is one of the most stressful periods in the life of a ruminant. Particularly in beef cattle, separation from their dams is usually followed by transportation to another operation and commingling with other animals. This is accompanied by a reduction in intake overlapped with a rapid dietary change, often characterized by the occurrence of ruminal acidosis (Penner et al., 2010). Highly fermentable carbohydrates coupled with low ruminal pH favor the development of gram-negative bacteria (GNB), which can liberate large amounts of lipopolysaccharides (LPS; Andersen et al., 1994; Gozho et al., 2006). Nutritional insults not only change the supply of nutrients but also alter luminal pH and osmolarity, which impacts epithelial permeability in the rumen and in more distal regions of the gut (Zhang et al., 2013; Pederzolli et al., 2018). This is accompanied by a proliferation of GNB, which can shed large amounts of LPS and thereby trigger inflammation in the lower gut, causing disruption of barrier function and translocation of LPS into circulation (Emmanuel et al., 2008; Plaizier et al., 2012). Endotoxin translocation into systemic circulation can elicit immune activation shifting the metabolic priorities toward the immune system that, if sustained over time, can hinder animal health and performance (Kvidera et al., 2017a). It is fundamental to find ways to mitigate the negative effects of nutritional and environmental challenges to increase performance, health, and well-being. The use of nutraceuticals with anti-inflammatory capacity could serve to ameliorate the negative effects of systemic inflammation. Bioactive compounds present in olive oil and olive byproducts, such as triterpenes and polyphenols, have been described to have anti-inflammatory and antioxidant bioactivity. Dietary supplementation with an olive oil bioactive extract (OBE) has been shown to decrease the negative effect of subclinical chronic inflammation in piglets (Liehr et al., 2017) and could contribute to reducing the detrimental effect of LPS translocation. We hypothesized that feeding OBE could attenuate the detrimental effects of LPS challenge through modulation of the immune response and reduction of systemic inflammation in beef cattle. The objective of this study was to evaluate the impact of feeding OBE to newly weaned beef heifers injected intravenously with increasing doses of LPS every other day during a 10-d period, on animal performance, immune status, and inflammatory response.
Materials and Methods
All procedures were carried out according to the University of Florida Institutional Animal Care and Use Committee, protocol # 201709971.
Experimental Design, Animals, and Treatments
The experiment was conducted at the University of Florida, North Florida Research and Education Center (NFREC) in Marianna, FL.
A generalized randomized block design was used to evaluate the impact of feeding OBE to newly weaned, Angus-crossbred heifers (210 ± 19 kg of BW) injected intravenously with 6 sequentially increasing doses of LPS (0.10, 0.25, 0.50, 0.75, 1.00, and 1.25 µg/kg of BW). A total of 36 heifers were used, distributed across 3 experimental periods consisting of a minimum of 21 d of adaptation, followed by LPS injections every 48 h for the last 10 d of each period. The LPS used was E. coli 0111: B4 (Sigma–Aldrich, Saint Louis, MO) and was infused into jugular vein using a winged butterfly needle. The protocol for the sequential doses of LPS was based on Fernandes et al. (2019), with some modifications to induce a chronic inflammatory condition.
Heifers were stratified by BW and then randomly assigned to 1 of 4 treatments within BW strata. Treatments were 1) negative control (saline injection, CTL−); 2) positive control (LPS injection, CTL+); and positive control plus a 3) low (0.04% of diet DM, OBE-L) or 4) high (0.16% of diet DM, OBE-H) dose of OBE. The OBE was fed for at least 21 d before the first LPS challenge. The doses of OBE were derived via allometric scaling using as reference the concentration of maslinic acid in the OBE tested by Liehr et al. (2017) in pigs and adapting the equation described by Wojcikowski and Gobe (2014) to supply 1 mg/kg of BW (OBE-L) or 4 mg/kg of BW (OBE-H) of maslinic acid. Based on the predicted DMI and BW of the heifers, OBE doses were converted into percentage of inclusion in the diet DM (0.04% and 0.16% for OBE-L and OBE-H, respectively). The OBE product was standardized to contain 4% oleanolic acid, 10% maslinic acid, and 2% of hydroxytyrosol and was obtained via filtration of pomace oil from Olea europaea, followed by extraction with purified ethanol and quantification with high-performance liquid chromatography (ProNutra Solutions, Madrid, Spain). To facilitate an even distribution in the total mixed ration (TMR), OBE was mixed with the mineral premix before mixing with the rest of ingredients of the TMR. Molasses was included in the diet to minimize small particle separation in the feed bunk. Heifers assigned to the control treatments received the same diet without OBE in the mineral premix (Table 1).
Table 1.
Ingredients and chemical composition of diets fed to beef heifers during the experiment1
| Item2 | CTL− | CTL+ | OBE-L | OBE-H |
|---|---|---|---|---|
| Diet ingredient, % DM basis | ||||
| Corn gluten feed | 61.0 | 61.0 | 61.0 | 61.0 |
| Cottonseed hulls | 32.0 | 32.0 | 32.0 | 32.0 |
| Molasses | 4.0 | 4.0 | 4.0 | 4.0 |
| Premix3 | 3.00 | 3.00 | 2.96 | 2.84 |
| OBE4 | 0.00 | 0.00 | 0.04 | 0.16 |
| Chemical composition | ||||
| DM | 94.3 | 93.9 | 94.0 | 93.6 |
| CP | 16.7 | 16.8 | 17.0 | 16.9 |
| ADF | 31.1 | 30.8 | 26.7 | 30.0 |
| NDF | 51.8 | 51.1 | 49.3 | 50.0 |
| Crude fat | 3.6 | 3.5 | 3.6 | 3.7 |
| TDN | 69.5 | 70.0 | 70.0 | 70.0 |
| NEm, Mcal/kg5 | 1.4 | 1.4 | 1.4 | 1.4 |
| NEg, Mcal/kg5 | 0.8 | 0.8 | 0.8 | 0.8 |
| Ca | 1.3 | 1.3 | 1.5 | 1.2 |
| P | 0.8 | 0.8 | 0.8 | 0.8 |
1CTL− = control diet + saline injections; CTL+ = control diet + LPS injections; OBE-L = control diet + olive oil bioactive extract at 0.04% of the diet + LPS injection; OBE-H = control diet + olive oil bioactive extract at 0.16% of the diet + LPS injection.
2Values other than DM are expressed as a percentage of dietary DM.
3Premix comprised calcium carbonate and was formulated to deliver 0 (CTL− and CTL+), 0.04 (OBE-L), or 0.16% (OBE-H) of OBE in the diet DM.
4OBE = olive oil bioactive extract.
5NEm and NEg were calculated with NASEM (2016).
Immediately after weaning, heifers were allocated to pens (108 m2 per pen; 4 pens of 3 heifers each per period) equipped with 2 GrowSafe (GrowSafe Systems Ltd., Airdrie, AB, Canada) feed bunks at the NFREC Feed Efficiency Facility. Individual DMI was measured daily using the GrowSafe system. An adaption of at least 21 d to facilities and diets preceded the sequential LPS challenge in each period. The diet consisted of 61% gluten feed, 32% cottonseed hulls, 4% molasses, and 3% of a mineral premix (Table 1). The diet was formulated following NASEM (2016) recommendations to attain 600 g of BW gain per day and contain 0.97 Mcal of NEg/kg of diet DM, and 16.7% CP. Before allocation to the different treatments, heifers were weighed and tagged with radiofrequency identification. In addition, heifers were weighted immediately before every LPS challenge to adjust the dose of LPS. Average daily gain was calculated before the challenge onset from day −21 to day −1 and during the challenge from day 0 to day 12.
Feed Sample Collection and Analyses
Representative samples of each treatment TMR were taken before feeding 3 times during each period. All samples were bagged and frozen immediately after collection until drying at 55 °C for 48 h in a forced-air oven and then ground in a Wiley mill (Arthur H. Thomas Co., Philadelphia, PA) using a 1-mm screen. After grinding, samples were composited for analysis on an equal weight basis and subsequently analyzed for DM, CP, TDN, ADF, NDF, calcium, and phosphorus in duplicate by a commercial laboratory (Dairy One Forage Laboratory, Ithaca, NY).
Calf Health Assessment
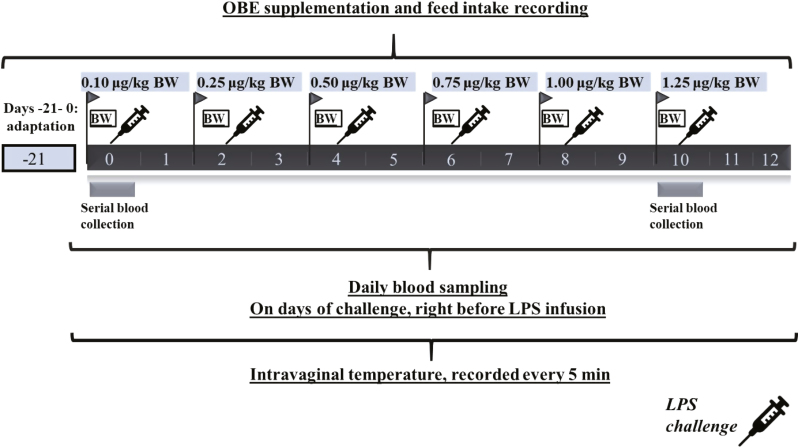
During 8 h after LPS injections, heifers were observed every 2 h for nasal or eye discharge, cough, respiratory score, and fecal consistency. Intravaginal temperature was measured every 5 min during the entire period of repeated LPS injections (days 0 to 12; Fig. 1). To this end, a temperature logging button (iButton, DS1921H-F5#, Whitewater, WI) was placed intravaginally with a blank control internal drug release insert. Temperature recordings were average every 30 min for the first 12 h post-challenge, then every hour up to 24 h and every 12 h until the next challenge. Heifers with a rectal temperature ≥ 39.5 °C were categorized as febrile (Smith and Risco, 2005; Benzaquen et al., 2007).
Figure 1.
Timeline of events at each of the 3 experimental periods.
Sample Collection and Laboratory Analyses
Blood samples from each heifer were collected 1 d before the first LPS challenge and daily from day 0 up to day 12 (Fig. 1). In addition, on the first (day 0) and last day of challenge (day 10), blood samples were serially collected starting immediately before LPS injection and at hours 1, 2, 4, and 8 (Fig. 1). Samples were taken from the jugular vein into a 10-mL evacuated glass vial containing 143 IU of sodium heparin (Vacutainer, Becton Dickinson, Franklin Lakes, NJ), inverted carefully several times, and immediately placed on ice. Within 60 min of collection, chilled samples were centrifuged at 1,500 × g for 15 min at 4 °C (Avanti J-E, Beckman Coulter Inc., Palo Alto, CA), plasma was harvested, and multiple aliquots of 1.5 mL were frozen at −20 °C until analysis.
Plasma concentrations of glucose, NEFA, and haptoglobin were measured in duplicate every day from days 0 to 12. In addition, on day 0 and day 10, glucose, NEFA, and IL-6 were measured in samples taken at 0, 1, 2, 4, and 8 h relative to the LPS injection. Glucose was measured using a quantitative colorimetric kit (G7521-1L; Pointe Scientific Inc., Canton, MI). Intra- and inter-assay CV for glucose were 2.9% and 3.6%, respectively. Interleukin-6 was measured using a commercially available ELISA kit (DuoSet ELISA; R&D Systems, Inc., Minneapolis, MN). A 7-step serially diluted recombinant bovine IL-6 standard curve was used. The inter- and intra-assay CV for IL-6 were 9.7% and 3.4%, respectively. Concentrations of NEFA (NEFA-C kit; Wako Diagnostics Inc., Richmond, VA) were determined using an enzyme assay as modified by Johnson and Peters (1993). Intra- and inter-assay CV for NEFA were 2.1% and 5.7%, respectively. Plasma concentrations of the acute phase protein (APP) haptoglobin were measured using a colorimetric procedure that measures haptoglobin–hemoglobin complexing by estimating differences in peroxidase activity (Makimura and Suzuki, 1982). The absolute absorbance values at 450 nm were multiplied by 100 and used for statistical analysis. Intra- and inter-assay CV were 5.1% and 9.7%, respectively.
Flow Cytometry
On day 0 and day 10, samples of heparinized whole blood were taken at 0, 1, 2, 4, and 8 h relative to the LPS injection. A fraction (200 µL) of the whole blood sample was pipetted into 15-mL conical centrifuge tubes and suspended in 2 mL of cold hypotonic buffer (10.6 mM of Na2HPO4 and 2.7 mM of NaH2PO4; pH 7.2) for 1 min. This was followed by addition of 1 mL of restore solution (10.6 mM of Na2HPO4, 2.7 mM of NaH2PO4, and 462 mM of NaCl; pH 7.2). Tubes were centrifuged at 650 × g for 5 min at 4 °C, and the supernatant was discarded. This process was repeated until erythrocytes were removed. Next, samples were incubated on ice with an antibody cocktail of 0.25 µL phycoerythrin (Abcam Cambridge, MA)-conjugated anti CD62L, 0.25 µL allophycocyanin, conjugated anticluster of differentiation 14 (TüK4, Qdot 800, Thermo Fisher), and 0.25 µL fluorescein isothiocyanate conjugated mouse anti-bovine CD11b (CC126 clone, AbD Serotec; Raleigh, NC) in 25 µL PBS containing 0.5% bovine serum albumin (Fisher Scientific, Fair Lawn, NJ) and 2 mM EDTA (Fisher Scientific). After incubation, 1 mL of PBS was added, vortexed, and centrifuged at 650 × g for 5 min at 4 °C. Supernatant was discarded, and cells resuspended in 200 µL of PBS. Samples were analyzed using an Accuri C6 digital analyzer flow cytometer (Becton Dickinson Biosciences, San Jose, CA). Lymphocytes, monocytes, and neutrophils were analyzed on the basis of their size and granularity in the density cytogram. Data acquisitions of the total amount of cells per sample were analyzed using Flowjo software v10.1 (Single Cell Analysis Software, LLC, OR). Data analyzed were total cell counts of lymphocytes, monocytes, and neutrophils. Likewise, histogram analysis for median fluorescence intensity of cluster of differentiation 14 (CD14), CD62L, and CD11b were used as an indication of the intensity of receptor expression on monocyte and neutrophil populations.
Statistical Analyses
One heifer had to be treated with an anti-inflammatory drug containing fluoxacin due to high body temperature and consequently removed from the experiment. The animal was from the CTL− group and was removed before the first saline injection. Therefore, data from 35 heifers were included in the statistical analysis.
The experiment was analyzed as a generalized randomized block design with period as a blocking factor and heifer nested within treatment was the experimental unit. Data were analyzed using a mixed-effect model with repeated measures over experimental day (SAS version 9.4, SAS Institute Inc., Cary, NC). The model included the fixed effects of treatment, day, and treatment × day interaction, and the random effect of heifer. The following contrasts were used to test treatment effects: 1) effect of LPS (CTL− vs. CTL+), 2) overall effect of OBE (OBE-L + OBE-H vs. CTL+), and 3) effect of OBE dose (OBE-L vs. OBE-H). The covariance structure with the lowest Akaike’s information criterion was selected for each variable. Most analyses used the first-order autoregressive structure for equally spaced measurements or spatial power for unequally spaced measurements. When an interaction of treatment with time was detected as significant, a partitioned analysis of the treatment least square means was performed. For data collected at single time points, the same mixed-effect model without repeated measures was used. The Kenward–Roger method was used to calculate the approximate denominator df for the F tests in the statistical models. Continuous data were examined for normal distribution of residuals after fitting the statistical models using Shapiro-Wilk and homogeneity of variance by plotting residuals against predicted values. Non-normally distributed data were subjected to Box–Cox transformation using the TRANS-REG procedure of SAS to achieve normality before analyses. Least squares means were back-transformed and SEM calculated as outlined by Jørgensen and Pedersen (1998). Statistical significance was considered at P ≤ 0.05, and tendency was considered at 0.05 < P ≤ 0.10.
Results
DMI and ADG
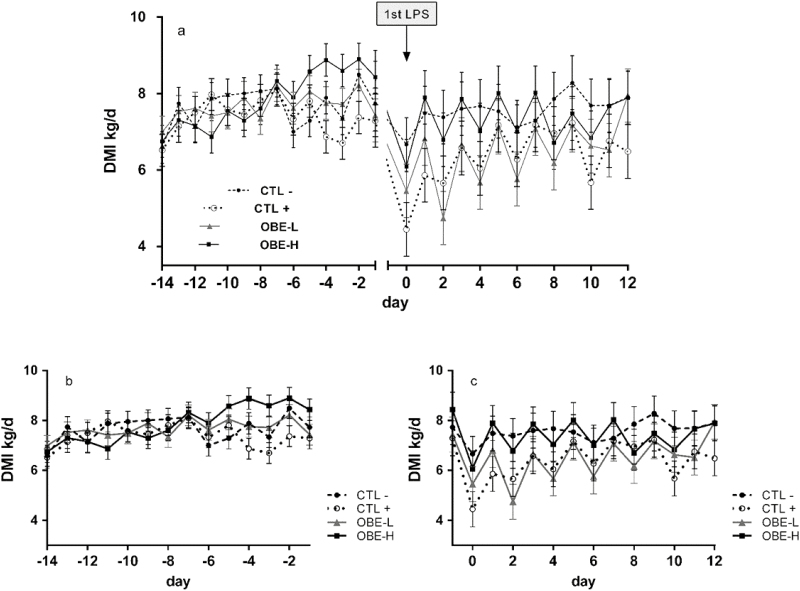
Overall, no treatment differences were observed prior to the LPS challenge (DMI pre-challenge; P = 0.43; Table 2), but the sequentially increasing doses of LPS tended to have a hypophagic effect when compared with saline injections (CTL+ vs. CTL−; 6.4 kg/d vs. 7.6 kg/d; P = 0.07; Table 2). Furthermore, during the challenge period, a treatment × time interaction was observed (P = 0.05; Fig. 2c). Specifically, heifers in the CTL+ treatment had reduced intakes on days 0, 1, 2, and 4 when compared with CTL− heifers (P < 0.05; Fig. 2c). This effect was attenuated on days 0 and 1 by feeding OBE-H (OBE-H compared with CTL+; 6.1 and 7.9 kg/d vs. 4.4 and 5.9 kg/d; P < 0.05; Fig. 2c) and tended to be counteracted on day 12 (OBE-H compared with CTL+; 7.9 vs. 6.5 kg/d; P = 0.07; Fig. 2c). On day 12, heifers supplemented with OBE-L had a tendency for greater intake compared with CTL+ (8.0 vs. 6.5 kg/d; P = 0.07; Fig. 2c).
Table 2.
Performance of newly weaned injected repeatedly with saline (CTL−), increasing doses of LPS over a 10-d period (CTL+), or CTL+ plus fed the diet supplemented with a low (OBE-L; 0.04% of diet DM) or a high (OBE-H; 0.16% of diet DM) dose of an olive oil bioactive extract (OBE)
| Treatments1 | Contrasts and P-values | |||||||
|---|---|---|---|---|---|---|---|---|
| Item2 | CTL− | CTL+ | OBE-L | OBE-H | SEM | CTL+ vs. OBE | OBE-L vs. OBE-H | CTL− vs. CTL+ |
| DMI pre-challenge, kg/d | 7.7 | 7.4 | 7.7 | 7.9 | 0.42 | 0.43 | 0.71 | 0.62 |
| DMI during challenge, kg/d | 7.6 | 6.4 | 6.5 | 7.4 | 0.70 | 0.29 | 0.15 | 0.07 |
| ADG pre-challenge, kg | 0.48 | 0.52 | 0.73 | 0.81 | 0.34 | 0.21 | 0.47 | 0.93 |
| ADG during challenge, kg | 0.36 | −0.11 | −0.02 | 0.28 | 0.24 | 0.30 | 0.26 | 0.09 |
1CTL− = control diet + saline injections; CTL+ = control diet + LPS injections; OBE-L = control diet + olive oil bioactive extract at 0.04% of the diet + LPS injection; OBE-H = control diet + olive oil bioactive extract at 0.16% of the diet + LPS injection.
2Results are expressed as least square means from 12 heifers per treatment.
Figure 2.
Dry matter intake of newly weaned heifers injected repeatedly with saline (CTL−), increasing doses of LPS on days 0, 2, 4, 6, 8, and 10 (CTL+), or CTL+ plus fed the diet supplemented with a low (OBE-L; 0.04% of diet DM) or a high (OBE-H; 0.16% of diet DM) dose of an olive oil bioactive extract (OBE). (a) Dry matter intake pre-challenge, from day −14 until day −1, and during the 12-d LPS challenge, from day 0 until day 12. (b) Dry matter intake pre-challenge, from day −14 until day −1. Effect of treatment (P = 0.43). (c) Dry matter intake during the 12-d LPS challenge, from day 0 until day 12. Effect of treatment × time (P = 0.05). Error bars denote SEM.
As expected, repeated LPS injections tended to decrease ADG (CTL− compared with CTL+; 0.4 vs. −0.1 kg/d; P = 0.09; Table 2). Even though the LPS-induced loss in BW was numerically alleviated by the addition of OBE-H into the diet (across feeding rate), the OBE vs. CTL + contrast was not statistically significant (−0.1 vs. 0.3 kg/d; P = 0.30; Table 2).
Intravaginal Temperature
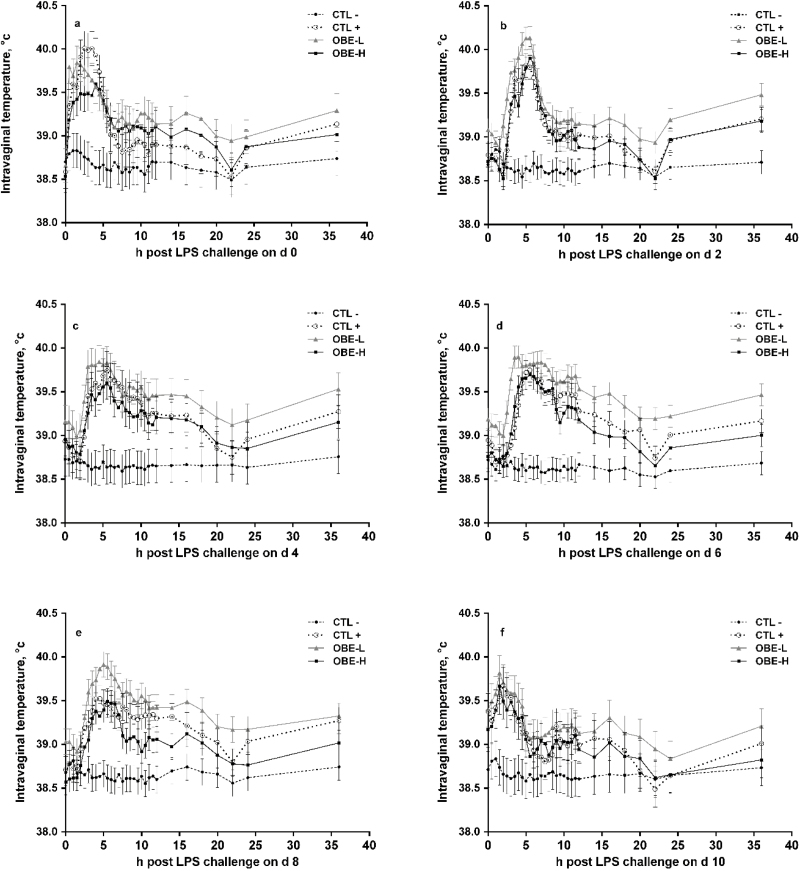
Repeated exposure to LPS increased mean (CTL+ compared with CTL−; 39.0 vs. 38.6 °C; P < 0.01; Table 3) and maximal (CTL+ compared with CTL−; 39.6 °C vs. 38.8 °C; P < 0.01; Table 3) intravaginal temperature, and tended to increase the time heifers remained above 39.5 °C (P = 0.07; Table 3). Furthermore, LPS injections elevated intravaginal temperature for at least 6 h post-challenge on every day of challenge (P < 0.05; Fig. 3a–f). In contrast, CTL− heifers maintained relatively constant intravaginal temperature (Fig. 3a–f).
Table 3.
Average intravaginal temperature and average maximum temperature on °C, and hours experienced fever of newly weaned heifers injected repeatedly with saline (CTL−), increasing doses of LPS over a 10-d period (CTL+), or CTL+ plus fed the diet supplemented with a low (OBE-L; 0.04% of diet DM) or a high (OBE-H; 0.16% of diet DM) dose of an olive oil bioactive extract (OBE)
| Treatments1 | Contrasts and P-values | |||||||
|---|---|---|---|---|---|---|---|---|
| Item2 | CTL− | CTL+ | OBE-L | OBE-H | SEM | CTL+ vs. OBE | OBE-L vs. OBE-H | CTL− vs. CTL+ |
| Average | 38.6 | 39.0 | 39.2 | 39.0 | 0.16 | 0.49 | 0.02 | <0.01 |
| Max | 38.8 | 39.6 | 39.8 | 39.5 | 0.17 | 0.84 | 0.02 | <0.01 |
| Time with fever3 | 0.0 | 5.8 | 9.9 | 5.0 | 3.41 | 0.56 | 0.12 | 0.07 |
1CTL− = control diet + saline injections; CTL+ = control diet + LPS injections; OBE-L = control diet + olive oil bioactive extract at 0.04% of the diet + LPS injection; OBE-H = control diet + olive oil bioactive extract at 0.16% of the diet + LPS injection.
2Results are expressed as least square means from 12 heifers per treatment.
3Rectal temperature ≥ 39.5 °C was considered febrile.
Figure 3.
Intravaginal temperature of newly weaned heifers injected repeatedly with saline (CTL−), increasing doses of LPS on days 0, 2, 4, 6, 8, and 10 (CTL+), or CTL+ plus fed the diet supplemented with a low (OBE-L; 0.04% of diet DM) or a high (OBE-H; 0.16% of diet DM) dose of an olive oil bioactive extract (OBE). (a–f) Temperature during 36 h post-challenge on days 0, 2, 4, 6, 8, and 10, respectively. Effects of treatment × time (P < 0.05). Error bars denote SEM.
Compared with CTL+, dietary supplementation with OBE-H minimized the increase in intravaginal temperature the first day of challenge (day 0) between hours 2 and 4 (P < 0.05; Fig. 3a) and tended to do the same on day 8 between hours 7.5 and 14 (P ≤ 0.10; Fig. 3e). Furthermore, OBE-H resulted in decreased maximum temperature on day 9 (39.2 vs. 39.6 °C; P = 0.03) and tended to cause the same response on day 0 (39.9 vs. 40.2 °C; P = 0.08) when compared with CTL+.
Haptoglobin and IL-6
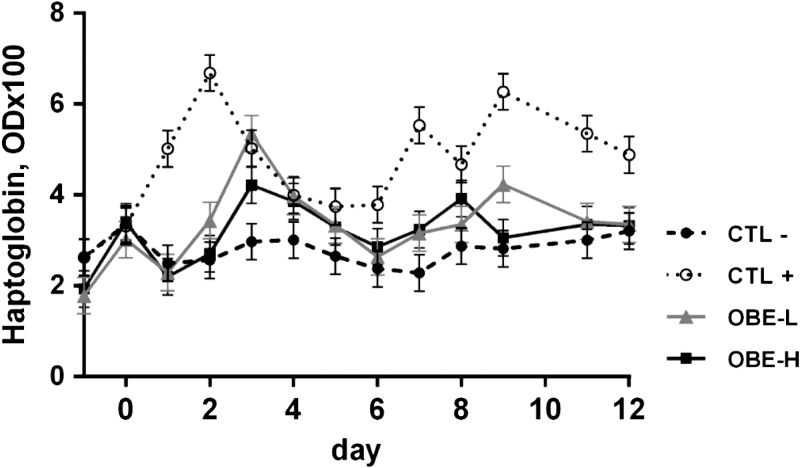
On average, the CTL+ group had greater mean plasma concentrations of haptoglobin than CTL− treatment (4.7 vs. 2.8 OD × 100; P < 0.01; Table 4). In addition, dietary supplementation with OBE reduced mean circulating concentrations of haptoglobin compared with CTL+ (3.3 vs. 4.7 OD × 100; P < 0.01; Table 4), but no difference was observed between the 2 doses of OBE (P = 0.72; Table 4). Furthermore, in CTL+ heifers this acute phase protein showed a biphasic behavior over time (treatment × day; P < 0.01; Fig. 4), reaching maximal concentrations on days 2 and 10. Compared with CTL+, feeding OBE at both inclusion rates resulted in a similar time-dependent response but with reduced peaks (P < 0.03; Fig. 4).
Table 4.
Responses of blood plasma metabolites of newly weaned heifers injected repeatedly with saline (CTL−), increasing doses of LPS over a 10-d period (CTL+), or CTL+ plus fed the diet supplemented with a low (OBE-L; 0.04% of diet DM) or a high (OBE-H; 0.16% of diet DM) dose of an olive oil bioactive extract (OBE)
| Treatments1 | Contrasts and P-values | |||||||
|---|---|---|---|---|---|---|---|---|
| Item2 | CTL− | CTL+ | OBE-L | OBE-H | SEM | CTL+ vs. OBE | OBE-L vs. OBE-H | CTL− vs. CTL+ |
| Glucose, mg/dL | 69.4 | 75.2 | 75.6 | 80.2 | 1.66 | 0.19 | 0.05 | 0.02 |
| NEFA, µEq/L | 1.2 | 1.3 | 1.4 | 1.3 | 0.05 | 0.48 | 0.37 | 0.13 |
| Haptoglobin OD3 × 100 | 2.8 | 4.7 | 3.3 | 3.2 | 0.40 | <0.01 | 0.72 | <0.01 |
1CTL− = control diet + saline injections; CTL+ = Control diet + LPS injections; OBE-L = control diet + olive oil bioactive extract at 0.04% of the diet + LPS injection; OBE-H = control diet + olive oil bioactive extract at 0.16% of the diet + LPS injection.
2Results are expressed as least square means from 12 heifers per treatment.
3OD = optical density.
Figure 4.
Plasma haptoglobin concentrations of newly weaned heifers injected repeatedly with saline (CTL−), increasing doses of LPS on days 0, 2, 4, 6, 8, and 10 (CTL+), or CTL+ plus fed the diet supplemented with a low (OBE-L; 0.04% of diet DM) or a high (OBE-H; 0.16% of diet DM) dose of an olive oil bioactive extract (OBE). Effect of treatment × time (P < 0.01). Error bars denote SEM.
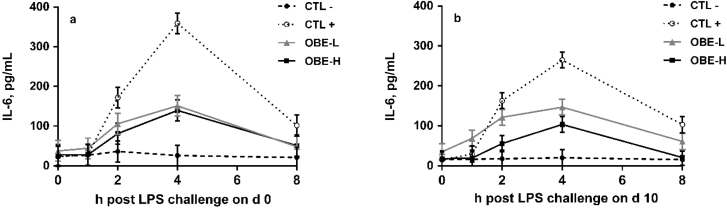
A treatment × hour interaction was observed for IL-6 concentration on days 0 and 10 (P < 0.01; Fig. 5a and b). In response to LPS administration, on day 0 IL-6 concentration in CTL+ registered a 16-fold increase at hour 4 when compared with baseline at hour 0 (P < 0.01; Fig. 5a). Similarly, IL-6 concentration in CTL+ increased 17-fold from hour 0 to hour 4 on day 10 (P < 0.01; Fig. 5b). In contrast, IL-6 concentration in CTL− heifers remained unchanged throughout the sampling period (P > 0.80; Fig. 5a and b). Compared with CTL+, dietary supplementation with OBE alleviated the increase in plasma IL-6 during both sampling days (P < 0.05; Fig. 5a and b) irrespective of the dose.
Figure 5.
Plasma IL-6 concentrations of newly weaned heifers injected repeatedly with saline (CTL−), increasing doses of LPS (CTL+), or CTL+ plus fed the diet supplemented with a low (OBE-L; 0.04% of diet DM) or a high (OBE-H; 0.16% of diet DM) dose of an olive oil bioactive extract (OBE). (a) Plasma IL-6 concentrations immediately before LPS challenge on hour 0, and at hours 1, 2, 4, and 8 post-challenge on day 0. Effect of treatment × time (P < 0.01). (b) Plasma IL-6 concentrations immediately before LPS challenge on hour 0, and at hours 1, 2, 4, and 8 post-challenge on day 10. Effect of treatment × time (P < 0.01). Error bars denote SEM.
Glucose and NEFA
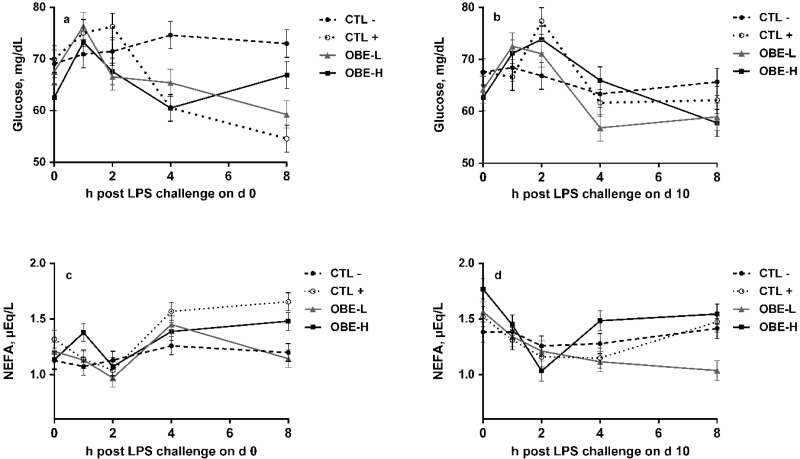
Heifers exposed to repeated LPS injections had increased mean plasma glucose concentration during the experimental period (CTL+ compared with CTL−; 75.2 mg/dL vs. 69.4 mg/dL; P = 0.02; Table 4). There was no difference in glucose concentration between heifers supplemented with OBE and nonsupplemented heifers (P = 0.19; Table 4). Heifers supplemented with OBE-H had greater plasma glucose concentration than those in OBE-L (P = 0.05, Table 4). There was no difference in plasma NEFA concentration between treatments (P > 0.13; Table 4).
There was a treatment × hour interaction on day 0 for glucose plasma concentration (P = 0.02; Fig. 6a). Heifers that received only saline (CTL−) maintained a steady concentration of glucose across the 8 h period (P > 0.28; Fig. 6a). On the contrary, in CTL+ heifers plasma glucose tended to peak by hour 2 compared with baseline concentration at hour 0 (P < 0.10). Afterwards, glucose concentration progressively decreased until the end of the sampling period (time effect; P < 0.01; Fig. 6a). In heifers supplemented with OBE, glucose peaked 1 h before CTL+ (P < 0.05; Fig. 6a) and OBE-H heifers were able to recover baseline plasma glucose concentrations by hour 8 (Fig. 6a). On day 10, no differences among treatments were observed (P = 0.28; Fig. 6b). There was a treatment × hour interaction for plasma NEFA concentrations on both day 0 and day 10 (P ≤ 0.01; Fig. 6c and d). Heifers that only received saline (CTL−) did not experience changes in plasma NEFA concentration during the 8 h post-challenge on both days (P > 0.24; Fig. 6c and d). On the contrary, heifers treated with LPS had increased plasma NEFA concentration on hours 4 and 8 in day 0 (P < 0.05; Fig. 6c). Compared with CTL+, heifers supplemented with OBE-L had lower plasma NEFA on hour 8 (P < 0.03; Fig. 6c and d) on both sampling days.
Figure 6.
Plasma glucose and NEFA concentrations of newly weaned heifers injected repeatedly with saline (CTL−), increasing doses of LPS (CTL+), or CTL+ plus fed the diet supplemented with a low (OBE-L; 0.04% of diet DM) or a high (OBE-H; 0.16% of diet DM) dose of an olive oil bioactive extract (OBE). (a) Plasma glucose concentrations immediately before LPS challenge on hour 0, and at hours 1, 2, 4, and 8 post-challenge on day 0. Effect of treatment × time (P = 0.02). (b) Plasma glucose concentrations immediately before LPS challenge on hour 0, and at hours 1, 2, 4, and 8 post-challenge on day 10. Effect of treatment × time (P = 0.28). (c) Plasma NEFA concentrations immediately before LPS challenge on hour 0, and at hours 1, 2, 4, and 8 post-challenge on day 0. Effect of treatment × time (P < 0.01). (d) Plasma NEFA concentrations immediately before LPS challenge on hour 0, and at hours 1, 2, 4, and 8 post-challenge on day 10. Effect of treatment × time (P = 0.01). Error bars denote SEM.
Monocytes and Neutrophil Cell Counts
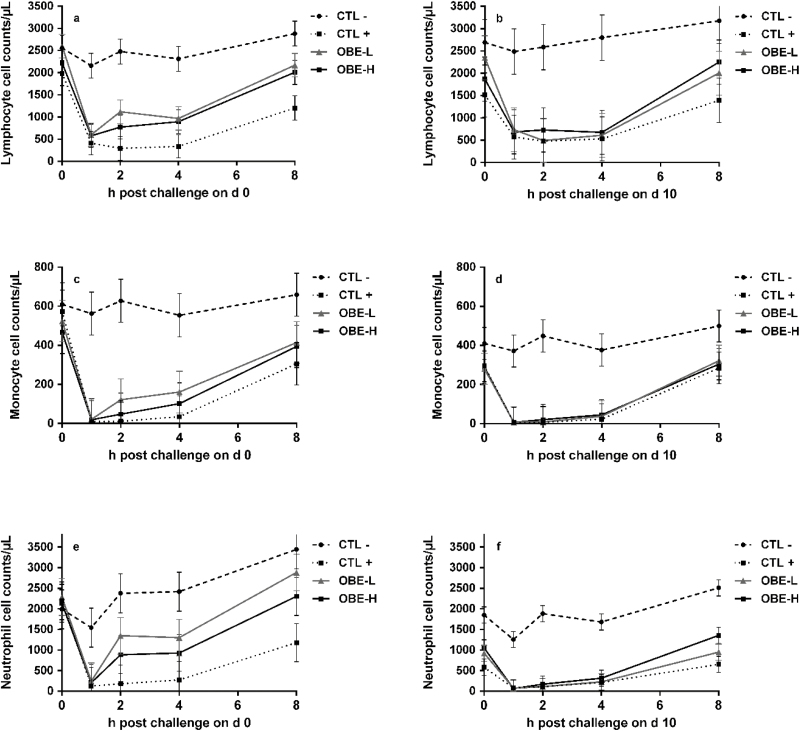
On day 0, there was a treatment by hour interaction for lymphocytes, monocytes, and neutrophils (P < 0.04; Fig. 7a, c, and e). At hour 0, no treatment differed from each other (Fig. 7a, c, and e). Following the LPS injection in the CTL+ group, lymphocytes, monocytes, and neutrophils decreased within the first hour and all counted cells remained below their initial values until hour 8 compared with CTL− (P < 0.01; Fig. 7a, c, and e). On day 0, OBE-L tended to rescue lymphocyte and neutrophil counts compared with CTL+ at hours 2, 4, and 8 (P < 0.10; Fig. 7a and e), whereas OBE-H did the same but only at hour 8 (P < 0.08; Fig. 7a and e). On day 10, there was a treatment by time interaction for lymphocytes (P < 0.01; Fig. 7b) were CTL+ heifers had lower counts compared with CTL− for the entire 8-h sampling period and even before LPS injections at hour 0 (P < 0.02; Fig. 7b). There was only a treatment effect for monocyte and neutrophil cell counts on day 10 (P < 0.01; Fig. 7d and f), when LPS injections decreased their numbers (CTL+ vs. CTL−; P < 0.01; Fig. 7d and e).
Figure 7.
White blood cell counts in newly weaned heifers injected repeatedly with saline (CTL−), increasing doses of LPS (CTL+), or CTL+ plus fed the diet supplemented with a low (OBE-L; 0.04% of diet DM) or a high (OBE-H; 0.16% of diet DM) dose of an olive oil bioactive extract (OBE). (a and b) Cell counts of lymphocytes immediately before LPS challenge on hour 0, and at hours 1, 2, 4, and 8 post-challenge on day 0 (a) and day 10 (b). Effect of treatment × time on day 0 and day 10 (a, b; P < 0.01). (c and d) Cell counts of monocytes immediately before LPS challenge on hour 0, and at hours 1, 2, 4, and 8 post-challenge on day 0 (c) and day 10 (d). Effect of treatment × time on day 0 (c; P < 0.01). Effect of treatment × time on day 10 (d; P = 0.27). (e and f) Cell counts of neutrophils immediately before LPS challenge on hour 0, and at hours 1, 2, 4, and 8 post-challenge on day 0 (e) and day 10 (f). Effect of treatment × time on day 0 (e; P = 0.04). Effect of treatment × time on day 10 (f; P = 0.07). Error bars denote SEM.
Expression of Clusters of Differentiation on Leukocyte Cell Surface
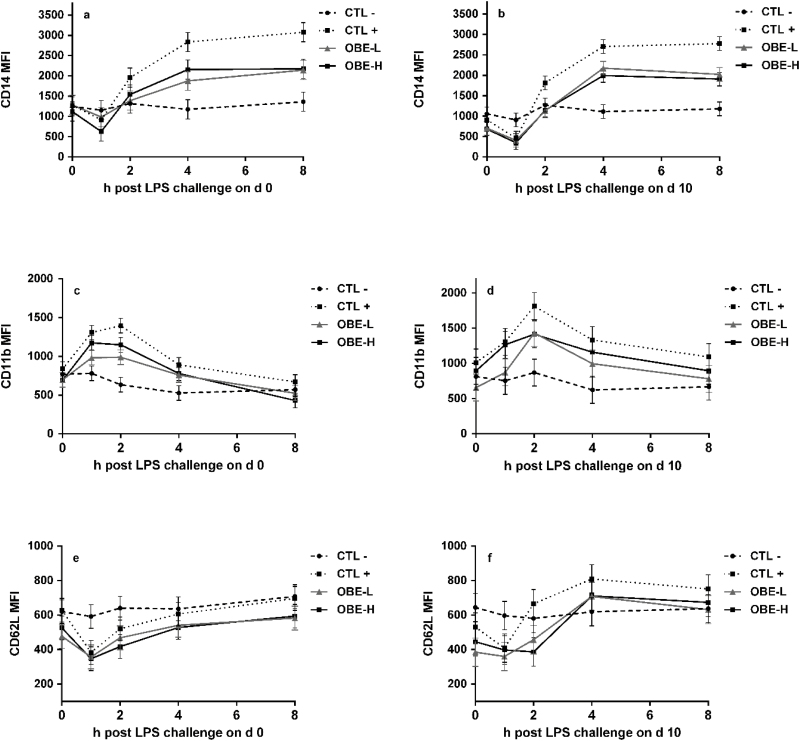
There was a treatment by hour interaction both on days 0 and 10 for the expression of CD14 in monocyte populations (P ≤ 0.01; Fig. 8a and b). Injection with LPS led to sizable increase in the LPS receptor CD14 after 2 h of the injections and remained higher for the rest of the sampling period on day 0 (CTL− vs. CTL+; P ≤ 0.04; Fig. 8a), whereas on day 10 was significantly higher between hours 4 and 8 (CTL− vs. CTL+; P ≤ 0.01; Fig. 8b). Likewise, there was a treatment by h interaction in the expression of CD11b in monocytes on day 0 (P ≤ 0.01; Fig. 8c), increasing after 1 h of LPS injection in CTL+ heifers compared with CTL− (P ≤ 0.02; Fig. 8c) and continued to be higher for the remainder of the sampling period. On day 10, LPS injections increased the expression of CD11b in monocytes (CTL+ vs. CTL−; P ≤ 0.01). The expression of CD62L in monocytes was not different between treatments on day 0 (P = 0.18; Fig. 8e). However, LPS injection increased its expression at hour 1 on day 10 (CTL− vs. CTL+; P = 0.04; Fig. 8f).
Figure 8.
Median fluorescence intensity (MFI) of cluster of differentiation 14 (CD14), CD11b, and CD62L, used as an indication of the intensity of receptor expression on monocytes populations in newly weaned heifers injected repeatedly with saline (CTL−), increasing doses of LPS on (CTL+), or CTL+ plus fed the diet supplemented with a low (OBE-L; 0.04% of diet DM) or a high (OBE-H; 0.16% of diet DM) dose of an olive oil bioactive extract (OBE). (a and b) MFI of receptor CD14 on monocytes immediately before LPS challenge on hour 0, and at hours 1, 2, 4, and 8 post-challenge on day 0 (a) and day 10 (b). Effect of treatment × time on day 0 and day 10 (a, b; P < 0.01). (c and d) MFI of receptor CD11b on monocytes immediately before LPS challenge on hour 0 and at hours 1, 2, 4, and 8 post-challenge on day 0 (c) and day 10 (d). Effect of treatment × time on day 0 (c; P < 0.01) and on day 10 (d; P = 0.17). (e and f) MFI of receptor CD62L on monocytes immediately before LPS challenge on hour 0 and at hours 1, 2, 4, and 8 post-challenge on day 0 (e) and day 10 (f). Effect of treatment × time on day 0 (e; P = 0.24) and day 10 (f; P < 0.01).
Supplementation with both OBE-L and OBE-H ameliorated the increase in expression of CD14 in monocytes on day 0 between hours 4 and 8 when compared with CTL+ (P ≤ 0.01; Fig. 8a). On day 10, OBE-L tended to have lower CD14 expression in monocytes from hours 2 to 8 (P ≤ 0.07; Fig. 8b) and OBE-H from hours 2 to 4 (P ≤ 0.03; Fig. 8b). Furthermore, compared with CTL+, OBE-L reduced the expression of CD11b in monocytes from hours 1 to 2 on day 0 (P ≤ 0.03; Fig. 8c). On day 10, feeding OBE reduced the expression of CD11b (OBE vs. CTL+; P < 0.01; Fig. 8d). The expression of CD11b in neutrophils tended to differ only on day 0 (effect of treatment; P = 0.07; Table 5) and feeding OBE reduced the expression of this receptor (OBE vs. CTL+; P = 0.03; Table 5). The expression of CD62L in neutrophil populations only differed between treatments on day 10 (P = 0.04; Table 5), when OBE-H had greater expression compared with OBE-L (P = 0.05; Table 5).
Table 5.
Mean fluorescence intensity (MFI) of expression of cluster of differentiation (CD) 11b, and 62L, by peripheral blood neutrophils of newly weaned heifers injected repeatedly with saline (CTL−), increasing doses of LPS over a 10-d period (CTL+), or CTL+ plus fed the diet supplemented with a low (OBE-L; 0.04% of diet DM) or a high (OBE-H; 0.16% of diet DM) dose of an olive oil bioactive extract (OBE)
| Treatments1 | Contrasts P-value | |||||||
|---|---|---|---|---|---|---|---|---|
| Item2 | CTL− | CTL+ | OBE-L | OBE-H | SEM | CTL+ vs. OBE | OBE-L vs. OBE-H | CTL− vs. CTL+ |
| Day 0 | ||||||||
| Neutrophil | ||||||||
| CD11b (MFI × 1,000) | 1.9 | 2.1 | 1.8 | 1.5 | 0.18 | 0.03 | 0.14 | 0.33 |
| CD62L (MFI × 1,000) | 0.63 | 0.57 | 0.72 | 0.60 | 0.08 | 0.38 | 0.28 | 0.63 |
| Day 10 | ||||||||
| Neutrophil | ||||||||
| CD11b (MFI × 1,000) | 1.7 | 2.2 | 2.0 | 2.0 | 0.43 | 0.30 | 0.70 | 0.03 |
| CD62L (MFI × 1,000) | 0.53 | 0.62 | 0.60 | 0.74 | 0.11 | 0.38 | 0.05 | 0.22 |
1CTL− = control diet + saline injections; CTL+ = control diet + LPS injections; OBE-L = control diet + olive oil bioactive extract at 0.04% of the diet + LPS injection; OBE-H = control diet + olive oil bioactive extract at 0.16% of the diet + LPS injection.
2Results are expressed as least square means from 12 heifers per treatment.
Discussion
This study shows that dietary supplementation with OBE was able to ameliorate some of the detrimental effects triggered by repeated LPS injections on the immune and metabolic response of newly weaned heifers. Thus, as hypothesized, feeding OBE led to a reduction in systemic inflammation as evidenced by a reduction in inflammatory markers and intravaginal temperature. These findings also confirm that the model was successful in inducing sustained systemic inflammation.
It has been previously shown that repeated injections of LPS at low doses represent a suitable approach to generate a phenotype exhibiting typical characteristics of low-grade chronic inflammation in piglets (Rakhshandeh and de Lange, 2012; Liehr et al., 2017) and heifers (Fernandes et al., 2019). These characteristics include partial suppression of feed intake, reduction of BW gain, and changes in the concentration of inflammatory markers in peripheral circulation, including cytokines and APP (Rakhshandeh and de Lange, 2012; Liehr et al., 2017). In addition, subacute inflammation contributes to chronic and progressive changes in tissue function (Bradford et al., 2015). Compared with acute episodes of induced inflammation, the subacute inflammatory response is less abrupt but more persistent over time (Carroll et al., 2009). In agreement with this notion, we found that repeated injections of LPS at increasing doses (from 0.10 to 1.25 µg/kg of BW) over a 10-d period decreased feed intake but only tended to impair weight gain. The magnitude of the LPS-induced decrease in DMI was greatest the first day of challenge, becoming less pronounced in subsequent days despite the fact that LPS doses doubled at every new injection. Dietary supplementation with OBE counteracted the impact of LPS on feed intake in some but not all days of challenge (Fig. 2c). The predicted ADG of a 6-mo-old heifer fed the same diet used in this study (Table 1) was modeled using the 2016 Beef Cattle Nutrient Requirements Model software (NASEM, 2016). Based on that modeling exercise, and using the observed difference in diet DMI between CTL+ and CTL− heifers, we were able to calculate the difference in ADG that is explained by the decreased intake. The total ADG difference between CTL+ and CTL− treatments was of 470 g. When this response was modeled, based on observed feed intake and diet composition, a 1-kg reduction in diet DMI between CTL+ and CTL− resulted in a decrease in ADG of only 143 g. Thus, the remainder 327 g of ADG reduction not explained by the model might be due to an increase in protein and energy requirements due to immune activation.
The immune system first recognizes LPS through specific pathogen sensing receptors, such as CD14, which transport LPS to the receptor MD-2 to form a protein complex with the toll-like receptor 4 (TLR4). This event triggers the dimerization and activation of TLR4, which engages its intracellular portion (TIR) to initiate a downstream signaling cascade (Kim et al., 2007; Steimle et al., 2016). This result in the phosphorylation of the repressor subunit of the transcription factor NF-κB, allowing the transcription factor to translocate from the cytosol into the nucleus and bind to its promoter region in target genes. This, in turn, leads to the transcription of an array of genes involved in the inflammatory response, including proinflammatory cytokines such as TNF-α and IL-6, and adhesion molecules such as L-selectin and CD11b (Zebeli and Metzler-Zebeli, 2012; Guo et al., 2017). In line with this knowledge, challenging heifers with LPS increased CD14 expression in monocytes, and the circulating concentration of IL-6 by up to 16-fold. The upregulation of CD14 is key to recognize LPS and stimulate the production of proinflammatory cytokines. Proinflammatory cytokines, such as IL-6, activate leukocytes and promote the production of eicosanoids by endothelial cells, which eventually act on neurons of the preoptic area of the brain to increase core body temperature (Nakamura, 2011; Bradford et al., 2015). In our study, LPS injection increased intravaginal temperature within the first h of the challenge, which reached a maximum 5 h later coinciding with the peak in plasma IL-6, and with the highest expression of CD14 in monocyte cell surface. The feeding of OBE reduced the LPS-induced increase in CD14, suggesting that OBE downregulated the expression of this pathogen-sensing receptor in immune cells. In addition, OBE reduced plasma concentration of IL-6 demonstrating the anti-inflammatory capacity of this extract. The impact on body temperature, however, appeared to depend on the dose of OBE as only OBE-H tended to have an antifebrile action in some days of the challenge period.
Interleukin-6 also promotes AA uptake by hepatocytes and synthesis of APP supported by muscle catabolism (Johnson, 1997). Haptoglobin is one of the most prominent APP in ruminants and widely used as biomarker of inflammation. In the present study, plasma concentration of haptoglobin from heifers exposed to LPS remained elevated throughout the entire challenge period, indicating the development of chronic inflammation and further proving that the model succeed in inducing a sustained inflammatory condition. Across doses, the inclusion of OBE into the diet mitigated the increase in plasma haptoglobin in response to LPS injections, most likely due to the decrease in circulating IL-6.
On the first and last day of challenge, and 1 h after LPS injections, lymphopenia and neutropenia were observed. After immune cells recognize LPS and become activated, a rapid cell migration occurs in an attempt to localize and clear the infection (Griel et al., 1975; Bieniek et al., 1998). This is further corroborated by the increased expression of the adhesion molecule CD11b in leucocytes and neutrophils from heifers injected with LPS because adhesion molecules mediate immune cell transvasation from peripheral circulation into the site of infection (Pierce et al., 1996). Even though heifers challenged with LPS and fed OBE suffered a similar drop in neutrophil and lymphocyte populations than CTL+ heifers, they were able to recover cell-count levels similar to the nonchallenged heifers (CTL−) faster than their control (CTL+) counterparts (Fig. 7). Provided that the expression of adhesion molecules in immune cells is reduced by anti-inflammatory treatments (Pierce et al., 1996), this effect might have resulted from a diminished ability of immune cells to migrate out of circulation due to the OBE-induced reduction in CD11b and L-Selectin (CD62L). In addition, the decrease in CD14 expression and IL-6 concentration in plasma probably reduced the capacity of leukocytes to recognize LPS and activate immune cells.
During an infection, energy expenditure and glucose utilization by the host is largely increased to support the demand of rapidly dividing immune cells that become obligated glucose consumers (McGuinness, 2005; Burdick Sanchez et al., 2013; Kvidera et al., 2017b). In our study, heifers exposed to LPS entered into a hyperglycemic phase 1 h after immunostimulation followed by hypoglycemia that lasted for the remaining 8 h of the sampling period. This is in agreement with Kvidera et al. (2017b) who reported that injecting lactating cows with LPS increased glucose concentration in plasma during the first hour post-challenge but decreased it afterwards. Endotoxemia stimulates glycogenolysis and gluconeogenesis in the liver and muscle, which increases plasma glucose concentrations to support the immune system (Burdick Sanchez et al., 2013; Kvidera et al., 2017b). The hyperglycemic state is only possible because is coupled with insulin resistance in peripheral tissue to reprioritize and spare fuels for utilization by immune cells. The hypoglycemic state is the outcome of the inability of the system to keep providing glucose at the same rate as needed by the immune system. In addition, in cattle, glycogen stores are very limited; hence, the capacity to mobilize glucose is very limited (Burdick Sanchez et al., 2013). Noticeably, heifers challenged with LPS and supplemented with OBE-H recovered normal glycemia faster than CTL+ heifers. A reduction in the synthesis of inflammatory mediators and the development of a more moderate immune response triggered by OBE probably resulted in a lower demand of glucose for supporting the metabolism of immune cells, therefore returning to baseline values faster than the CTL+ group. Plasma concentrations of NEFA followed an inverse relationship with glucose during the 8 h post-challenge. Probably the high concentrations of glucose in plasma immediately after the challenge inhibited lipolysis and, as a consequence, NEFA concentration decreased. When plasma glucose concentration started to drop, fat mobilization might have initiated to provide a source of energy during the hypoglycemic state. This relationship has been previously shown by Burdick Sanchez et al. (2013) in an endotoxin challenge model with Brahman bulls. Furthermore, elevated concentrations of NEFA can impair glycogenolysis (McMahon et al., 1988) further compromising glucose availability.
To be able to regulate the magnitude of the inflammatory response, the host relies on several mechanisms to prevent an excessive immune response and endure a sustained presence of endotoxin in circulation, generating tolerance over time. Acyloxyacyl hydrolase is an enzyme that removes acyl chains from the lipid A disaccharide backbone of the LPS molecule. This enzyme is present in antigen-presenting cells and neutrophils (Munford and Hall, 1986) and can decrease the immunogenicity of the LPS molecule. In our model, we accounted for the development of endotoxin tolerance by increasing the dose at every new administration of LPS. Yet, the largest responses in intake, temperature, and inflammatory marker concentrations were developed by the heifers during the first 2 administrations of LPS. Heifers in the CTL+ group experienced a 26% reduction in the maximal IL-6 concentration between the first (day 0) and last day of challenge (day 10) and haptoglobin attained the greatest concentration by day 2. In addition, CTL+ heifers registered the greatest drop in intake the first day of LPS administration and the highest intravaginal temperatures post-LPS administration were achieved during the first 2 d of challenge. Thus, even though the dose of LPS increased between the first and last injection more than a 10-fold, the greatest immune responses were achieved during the first 2 d of challenge.
This study shows that dietary supplementation with OBE contributed to ameliorate some of the negative effects of sustained immune activation in newly weaned heifers. The immunomodulatory effect of OBE appears to be associated with a reduction in the expression of the LPS receptor CD14, which could have partially blocked the downstream signaling cascade and thereby decrease the synthesis of proinflammatory cytokines. The OBE-mediated reduction in body temperature, circulating APP, and the recovery of DMI can be explained by the attenuated presence of inflammatory mediators in circulation. Our findings raise the question whether the immunomodulatory action of OBE can translate into improved animal performance, efficiency of feed conversion, and a shift toward a more contained immune response. Further research is needed to understand whether the model used in this study accurately represents the natural challenges experienced by weaned beef calves and if dietary supplementation with OBE can improve their performance in a commercial setting of beef production.
Footnotes
The authors gratefully acknowledge ProNutra Solutions S.L. for donating the extract from Olea europaea used in this study.
Literature Cited
- Andersen P. H., Bergelin B., and Christensen K. A.. 1994. Effect of feeding regimen on concentration of free endotoxin in ruminal fluid of cattle. J. Anim. Sci. 72:487–491. doi: 10.2527/1994.722487x [DOI] [PubMed] [Google Scholar]
- Benzaquen M. E., Risco C. A., Archbald L. F., Melendez P., Thatcher M. J., and Thatcher W. W.. 2007. Rectal temperature, calving-related factors, and the incidence of puerperal metritis in postpartum dairy cows. J. Dairy Sci. 90:2804–2814. doi: 10.3168/jds.2006-482 [DOI] [PubMed] [Google Scholar]
- Bieniek K., Szuster-Ciesielska A., Kamińska T., Kondracki M., Witek M., and Kandefer-Szerszeń M.. 1998. Tumor necrosis factor and interferon activity in the circulation of calves after repeated injection of low doses of lipopolysaccharide. Vet. Immunol. Immunopathol. 62:297–307. doi: 10.1016/S0165-2427(98)00102-0 [DOI] [PubMed] [Google Scholar]
- Bradford B. J., Yuan K., Farney J. K., Mamedova L. K., and Carpenter A. J.. 2015. Invited review: Inflammation during the transition to lactation: New adventures with an old flame. J. Dairy Sci. 98:6631–6650. doi: 10.3168/jds.2015-9683 [DOI] [PubMed] [Google Scholar]
- Burdick Sanchez N. C., Carroll J. A., Randel R. D., Vann R. C., and Welsh T. H.. 2013. Associations between endotoxin-induced metabolic changes and temperament in Brahman bulls. J. Anim. Physiol. Anim. Nutr. (Berl). 98:178–190. doi: 10.1111/jpn.12074 [DOI] [PubMed] [Google Scholar]
- Carroll J. A., Arthington J. D., and Chase C. C. Jr. 2009. Early weaning alters the acute-phase reaction to an endotoxin challenge in beef calves. J. Anim. Sci. 87:4167–4172. doi: 10.2527/jas.2009-2016 [DOI] [PubMed] [Google Scholar]
- Emmanuel D. G., Dunn S. M., and Ametaj B. N.. 2008. Feeding high proportions of barley grain stimulates an inflammatory response in dairy cows. J. Dairy Sci. 91:606–614. doi: 10.3168/jds.2007-0256 [DOI] [PubMed] [Google Scholar]
- Fernandes A. C. C., Davoodi S., Kaur M., Veira D., Melo L. E. H., and Cerri R. L. A.. 2019. Effect of repeated intravenous lipopolysaccharide infusions on systemic inflammatory response and endometrium gene expression in Holstein heifers. J. Dairy Sci. 102:3531–3543. doi: 10.3168/jds.2018-14616 [DOI] [PubMed] [Google Scholar]
- Gozho G. N., Krause D. O., and Plaizier J. C.. 2006. Rumen lipopolysaccharide and inflammation during grain adaptation and subacute ruminal acidosis in steers. J. Dairy Sci. 89:4404–4413. doi: 10.3168/jds.S0022-0302(06)72487-0 [DOI] [PubMed] [Google Scholar]
- Griel L. C. Jr, Zarkower A., and Eberhart R. J.. 1975. Clinical and clinico-pathological effects of Escherichia coli endotoxin in mature cattle. Can. J. Comp. Med. 39:1–6. PMID: 1089461; PMCID: PMC1277407. [PMC free article] [PubMed] [Google Scholar]
- Guo J., Chang G., Zhang K., Xu L., Jin D., Bilal M. S., and Shen X.. 2017. Rumen-derived lipopolysaccharide provoked inflammatory injury in the liver of dairy cows fed a high-concentrate diet. Oncotarget 8:46769–46780. doi: 10.18632/oncotarget.18151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. M., and J. P. Peters. 1993. Technical note: An improved method to quantify nonesterified fatty acids in bovine plasma. J. Anim. Sci. 71:753–756. doi: 10.2527/1993.713753x [DOI] [PubMed] [Google Scholar]
- Johnson R. W. 1997. Inhibition of growth by pro-inflammatory cytokines: An integrated view. J. Anim. Sci. 75:1244–1255. doi: 10.2527/1997.7551244x [DOI] [PubMed] [Google Scholar]
- Jørgensen E., and Pedersen A. R.. 1998. How to obtain those nasty standard errors from transformed data and why they should not be used. Biometry Res. Unit, Int. Rep. 7. Danish Inst. Agric. Sci., Aarhus, Denmark. [Google Scholar]
- Kim H. M., Park B. S., Kim J. I., Kim S. E., Lee J., Oh S. C., Enkhbayar P., Matsushima N., Lee H., Yoo O. J., . et al. 2007. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 130:906–917. doi: 10.1016/j.cell.2007.08.002 [DOI] [PubMed] [Google Scholar]
- Kvidera S. K., Dickson M. J., Abuajamieh M., Snider D. B., Fernandez M. V. S., Johnson J. S., Keating A. F., Gorden P. J., Green H. B., Schoenberg K. M., and Baumgard L. H.. 2017a. Intentionally induced intestinal barrier dysfunction causes inflammation, affects metabolism, and reduces productivity in lactating Holstein cows. J. Dairy Sci. 100:4113–4127. doi: 10.3168/jds.2016-12349 [DOI] [PubMed] [Google Scholar]
- Kvidera S. K., Horst E. A., Abuajamieh M., Mayorga E. J., Sanz Fernandez M. V., and Baumgard L. H.. 2017b. Glucose requirements of an activated immune system in lactating Holstein cows. J. Dairy Sci. 100:1–15. doi: 10.2527/jas2016-0765 [DOI] [PubMed] [Google Scholar]
- Liehr M., Mereu A., Pastor J. J., Quintela J. C., Staats S., Rimbach G., and Ipharraguerre I. R.. 2017. Olive oil bioactives protect pigs against experimentally-induced chronic inflammation independently of alterations in gut microbiota. PLoS One 12:e0174239. doi: 10.1371/journal.pone.0174239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makimura S., and Suzuki N.. 1982. Quantitative determination of bovine serum haptoglobin and its elevation in some inflammatory diseases. Nihon Juigaku Zasshi. 44:15–21. doi:10.1292/jvms1939.44.15 [DOI] [PubMed] [Google Scholar]
- McGuinness O. P. 2005. Defective glucose homeostasis during infection. Annu. Rev. Nutr. 25:9–35. doi: 10.1146/annurev.nutr.24.012003.132159 [DOI] [PubMed] [Google Scholar]
- McMahon M., Gerich J., and Rizza R.. 1988. Effects of glucocorticoids on carbohydrate metabolism. Diabetes Metab. Rev. 4:17–30. [DOI] [PubMed] [Google Scholar]
- Munford R. S., and Hall C. L.. 1986. Detoxification of bacterial lipopolysaccharides (endotoxins) by a human neutrophil enzyme. Am. Assoc. Adv. Sci. 234:203–205. doi:10.1126/science.3529396 [DOI] [PubMed] [Google Scholar]
- Nakamura K. 2011. Central circuitries for body temperature regulation and fever. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301:R1207–R1228. doi: 10.1152/ajpregu.00109.2011 [DOI] [PubMed] [Google Scholar]
- NASEM 2016. Nutrient requirements of beef cattle. 8th rev. ed The National Academies Press, Washington, DC. [Google Scholar]
- Pederzolli R. L. A., Van Kessel A. G., Campbell J., Hendrick S., Wood K. M., and Penner G. B.. 2018. Effect of ruminal acidosis and short-term low feed intake on indicators of gastrointestinal barrier function in Holstein steers. J. Anim. Sci. 96:108–125. doi: 10.1093/jas/skx049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner G. B., Oba M., Gäbel G., and Aschenbach J. R.. 2010. A single mild episode of subacute ruminal acidosis does not affect ruminal barrier function in the short term. J. Dairy Sci. 93:4838–4845. doi: 10.3168/jds.2010-3406 [DOI] [PubMed] [Google Scholar]
- Pierce J. W., Read M. A., Ding H., Luscinskas F. W., and Collins T.. 1996. Salicylates inhibit I kappa B-alpha phosphorylation, endothelial-leukocyte adhesion molecule expression, and neutrophil transmigration. J. Immunol. 156:3961–3969. [PubMed] [Google Scholar]
- Plaizier J. C., Khafipour E., Li S., Gozho G. N., and Krause D. O.. 2012. Subacute ruminal acidosis (SARA), endotoxins and health consequences. Anim. Feed Sci. Technol. 172:9–21. doi: 10.1016/j.anifeedsci.2011.12.004 [DOI] [Google Scholar]
- Rakhshandeh A., and de Lange C. F.. 2012. Evaluation of chronic immune system stimulation models in growing pigs. Animal 6:305–310. doi: 10.1017/S1751731111001522 [DOI] [PubMed] [Google Scholar]
- Smith B. I., and Risco C. A.. 2005. Management of periparturient disorders in dairy cattle. Vet. Clin. North Am. Food Anim. Pract. 21:503–521. doi: 10.1016/j.cvfa.2005.02.007 [DOI] [PubMed] [Google Scholar]
- Steimle A., Autenrieth I. B., and Frick J. S.. 2016. Structure and function: Lipid A modifications in commensals and pathogens. Int. J. Med. Microbiol. 306:290–301. doi: 10.1016/j.ijmm.2016.03.001 [DOI] [PubMed] [Google Scholar]
- Wojcikowski K., and Gobe G.. 2014. Animal studies on medicinal herbs: Predictability, dose conversion and potential value. Phytother. Res. 28:22–27. doi: 10.1002/ptr.4966 [DOI] [PubMed] [Google Scholar]
- Zebeli Q., and Metzler-Zebeli B. U.. 2012. Interplay between rumen digestive disorders and diet-induced inflammation in dairy cattle. Res. Vet. Sci. 93:1099–1108. doi: 10.1016/j.rvsc.2012.02.004 [DOI] [PubMed] [Google Scholar]
- Zhang S., Albornoz R. I., Aschenbach J. R., Barreda D. R., and Penner G. B.. 2013. Short-term feed restriction impairs the absorptive function of the reticulo-rumen and total tract barrier function in beef cattle. J. Anim. Sci. 91:1685–1695. doi: 10.2527/jas.2012-5669 [DOI] [PubMed] [Google Scholar]