Abstract
Three experiments were conducted to evaluate the inclusion of crude glycerin (CG) in diets for beef cattle. In Exp. 1, 4 ruminally cannulated steers were fed diets with 0 or 15% CG (DM basis), to evaluate DM disappearance, VFA profiles, and gas production. There was a tendency for an interaction (P = 0.06) between diet fed to donor animals and substrate fed to in vitro system, and digestion was increased when CG was added to cultures with ruminal fluid from CG-fed animals. Total VFA were unaffected by diets or by substrate incubated. The CG increased production of propionate, butyrate, and valerate (P < 0.01) while the gas production was unaffected (P = 0.16). In Exp. 2, 24 crossbred heifers (334.4 ± 0.9 kg BW) were fed the same diets as Exp. 1, for 35 d. Fecal grab samples were collected 3 times daily on day 7, 21, and 35, to evaluate total tract digestibilities of DM, OM, and NDF. The CG improved digestibility of diet OM (P = 0.04), and DM followed a similar trend (P = 0.06), while the NDF digestibility was unaffected (P = 0.29). In Exp. 3, crossbred heifers (n = 374; 375.8 ± 36.1 kg BW) were used to evaluate feedlot performance and carcass traits when fed diets with 0, 7.5, or 15% CG, with or without added 0.3% salt. Heifers were assigned to 25 pens and were harvested on day 125. Removing salt from CG-based diets did not impact performance (P = 0.50). The CG did not influence average daily gain (P = 0.27), but decreased DM intake (P = 0.003), USDA Yield Grade (P = 0.01), and improved feed efficiency (P = 0.03), while tended to decrease USDA prime carcasses (P = 0.10). Carcass weight (P = 0.24), Longissimus muscle area (P = 0.63), and kidney, pelvic, heart fat (P = 0.59) were unaffected by CG. Twelfth-rib fat was less for heifers fed 15% CG without salt compared with the other treatments (P = 0.005), while marbling was less for heifers fed CG diets compared with the control-fed animals (linear, P = 0.004; quadratic, P = 0.02). In conclusion, GC can replace dry-rolled corn in diets for beef heifers when fed at 15% of diet DM, improving OM digestion, increasing ruminal propionate and butyrate without affecting greenhouse gas emissions. Feeding up to 15% CG improves feed efficiency but depresses marbling and tends to decrease Quality Grade. Removing supplemental salt from CG-diets has no impact on performance or carcass traits.
Keywords: by-product, digestion, glycerol, greenhouse gas, NaCl
Introduction
Due to fluctuating corn prices in the United States, the use of alternative energy ingredients, such as crude glycerin (CG), in livestock production has been increasing in recent years. The simultaneous increase in biodiesel production has increased CG availability, which currently exceeds the demand for food and personal care products industries (Yazdani and Gonzalez, 2007).
Typically, about 8.8 kg of CG is generated from every 100 L of biodiesel produced (Cavalheiro et al., 2009). When fed to cattle, glycerol (the main constituent of CG) can be converted to glucose in the liver, providing energy for cellular metabolism (Goff and Horst, 2001). Recent studies have evaluated the nutritive value of CG when fed to nonruminants (Schieck et al, 2010; Jung and Batal, 2011) and ruminants (Chung et al., 2007; Pyatt et al., 2007; Parsons et al., 2009; Gunn et al., 2010a, 2010b; Van Cleef et al., 2014; Ezequiel et al., 2015), demonstrating that this by-product has the potential to replace conventional energy sources, such as corn. However, CG feeding can present some challenges, such as high levels of residual catalyst (methanol) and salt, which could be constraints for use as a livestock feed (Donkin, 2008). Moreover, research found that, depending on the diet, glycerol can negatively impact fiber digestibility, without affecting DM digestibility (Abo El-Nor et al., 2010). Crude glycerin, fed at elevated concentrations, also can reduce production of enteric methane (Van Cleef et al., 2015), due to a reduction in acetate:propionate ratio (Wang et al., 2009). Regarding salt content, CG can contain up to 6.6% sodium chloride (NaCl), according to Kerr et al. (2009), which could depress appetite (Wilson, 1966) and the efficiency of energy use for production (Arieli et al., 1989). The National Research Council (2005) has defined 4.55% as the maximum tolerable level of NaCl in cattle diets. Thus, CG with high salt concentrations may limit feed intake by cattle.
Previous research indicate that rumen microbes can adapt to glycerol, as the disappearance of the by-product from the rumen fluid was more rapid after 7 d of glycerol feeding (Kijora et al., 1998). This observation shows animal adaptation may be necessary to achieve optimal utilization of the byproduct. Therefore, our objective was to evaluate the effects of CG on in vitro fermentation parameters, with or without previous rumen fluid donor adaptation to CG, apparent total tract digestibility, as well as to evaluate performance and carcass characteristics of feedlot finishing heifers fed elevated concentrations of CG, with or without supplemental salt.
Material and Methods
The Kansas State University Institutional Animal Care and Use Committee approved procedures conducted in this trial (#2914).
Experiment 1
Four ruminally cannulated Holstein steers were placed into individual pens, in an indoor barn and fed ad libitum 2 isonitrogenous finishing diets containing 0 (Control, CON) or 15% CG (15GC), on DM basis (Table 1). Analyses performed on CG were as follows: glycerol content by HPLC, according to official method 982.22 (AOAC, 1995); moisture by Karl Fischer, according to official method 966.2 (AOAC, 1995); ash using official method 942.05 (AOAC, 1995); sodium using official method 956.01 (AOAC, 1995), and methanol using official method 973.23 (AOAC, 1995). Crude glycerin used in this trial contained approximately 81.5% glycerol, 13.3% water, 6.3% ash, 2.5% sodium, and less than 0.02% methanol.
Table 1.
Composition of experimental substrates/diets (DM basis, Exp. 1 and 2)
| Item | Substrates/diets1 | |
|---|---|---|
| CON | 15CG | |
| Ingredient, % | ||
| Corn silage | 10.0 | 10.0 |
| Dry-rolled corn | 31.0 | 12.9 |
| Corn gluten feed | 35.0 | 35.0 |
| Soybean hulls | 20.0 | 20.0 |
| Soybean meal | 0.0 | 3.1 |
| Limestone | 1.4 | 1.4 |
| Mineral-vitamin premix2 | 0.1 | 0.1 |
| Feed additive premix3 | 2.2 | 2.2 |
| Crude glycerin4 | 0.0 | 15.0 |
| Salt | 0.3 | 0.3 |
| Nutrient composition, % | ||
| DM | 65.4 | 65.2 |
| CP | 14.3 | 14.2 |
| NDF | 39.9 | 35.4 |
| Na | 0.12 | 0.56 |
1CON = Control diet, without crude glycerin, 15CG = 15% added crude glycerin.
2Formulated to provide 0.1 mg/kg Co; 10 mg/kg Cu; 0.6 mg/kg I; 60 mg/kg Mn; 0.25 mg/kg Se; 60 mg/kg Zn; 2,200 IU/kg of vitamin A; and 22 IU/kg vitamin E in the total diet DM.
3Formulated to provide 300 mg monensin (Rumensin, Elanco Animal Health, Greenfield, IN), 90 mg tylosin (Tylan, Elanco Animal Health, Greenfield, IN), and 0.4 mg melengestrol acetate (MGA, Pfizer Animal Health, Exton, PA) per heifer daily.
4Crude glycerin composition: 81.5% glycerol, 13.3% water, 6.3% ash, 2.5% Na, and <0.02% methanol.
Animals were fed once daily and CG replaced dry-rolled corn. The animals were adapted to their respective diets and pens for 14 d. Ruminal content from these animals were collected, strained through eight layers of cheesecloth, placed into preheated thermos (39 °C), and transported to Kansas State Pre-Harvest Food Safety Laboratory. In a 37 °C room, the strained ruminal fluid was placed into separatory funnels, gassed with oxygen-free gas, and kept for 30 to 40 min to allow the stratification of the mat, fluid, and protozoa fractions. The protozoa-rich fraction was voided, and the clarified liquid layer was mixed with McDougall’s buffer in a ratio of 1:2. Substrates consisted of the same total mixed finishing diets with or without 15% CG as provided to rumen fluid donors. The diets were ground through a 1-mm mesh screen, and 1.5 g were used in each flask. Ten fermentation flasks of each substrate received 100 mL McDougall’s buffer and 50 mL clarified rumen fluid. Half of flasks received rumen fluid from animals fed the control diet and half received rumen fluid from animals fed 15% CG diets. The flasks were capped with modules equipped with pressure sensitive membranes, and RF transmitters that record production of fermentative gasses at 5-min intervals (ANKOMRF Gas Production System; Ankom Technology, Macedon, NY). Four blank flasks containing inoculum without substrate were also incubated at the same conditions. The flasks were purged with oxygen-free gas and immediately placed in a 39 °C prewarmed shaking water bath equipped with metal claws to hold bottles during the shaking procedure. Samples of fermentative gas were collected from the flasks at the moment of incubation and 24 h later stored in sealed 50-mL vials filled with nitrogen gas and refrigerated. The concentrations of CO2 and CH4 were obtained by gas chromatography (Model 5890A, Hewlett-Packard, Palo Alto, CA; 2-m × 2-mm column; Carbopack BDA 80/120 4% CW 20M column packing, Supelco, Bellefonte, PA), with He as the carrier gas, a flow rate of 24 mL/min, and a column temperature of 175 °C. At the end of the 24-h fermentation, contents of the flasks were chilled in an ice bath to cease microbial activity. The contents were centrifuged (3,000 × g for 10 min), and a 4-mL sample of supernatant were combined with 1 mL of meta-phosphoric acid (25%) and used to characterize concentrations of VFA.
To evaluate substrate DM disappearance, feed samples from both diets (CON and 15CG) were dried at 55 °C oven for 48 h and ground through 1-mm mesh screen. Approximately 0.5 g (DM basis) of feed samples were weighed into duplicate preweighed 50-mL centrifuge plastic tubes, totaling 24 tubes. Prewarmed McDougall’s buffer (20 mL) were mixed with 10 mL rumen fluid to obtain a 1:2 ruminal fluid to buffer ratio. Half of the tubes received rumen fluid from animals fed CON diet and half received rumen fluid from animals fed 15CG diet. Fermentation tubes were purged with nitrogen, capped with a rubber cap equipped with Bunsen-type valves and then placed in a 39 °C incubator for 24 h. The tubes were gently swirled every 3 h during incubation time. After incubation, the tubes were placed in ice water bath to cease microbial activity and centrifuged at 30,000 × g for 20 min. The supernatant was voided, and centrifuged tubes containing pellets were dried at 105 °C oven overnight. Centrifuge tubes and residues were weighed and in vitro DM digestibility (IVDMD) was calculated. All the procedures adopted in this trial were repeated for 2 d.
Experiment 2
Twenty-four crossbred heifers (334.4 ± 0.9 kg initial BW) were randomly paired, distributed in 12 shaded pens and received either the control (CON) or 15% CG (15CG) diet ad libitum once daily (Table 1). Animals were selected at processing day by weight, phenotype, and temperament. Heifers were housed in an indoor metabolism barn and adapted for 14 d. After adaptation to diets and facilities, animals were fed chromic oxide (0.1% of diet DM basis). Fecal grab samples were collected 3 times daily (8, 16, and 24 h after feeding) at day 7, 21, and 35, composited by animal, dried, and ground through a 1-mm screen. Chromic oxide contents of feces and diets were determined by atomic absorption spectroscopy (Model 3110, Perkin Elmer, Waltham, MA), and it was used to calculate apparent total tract digestibilities of DM, OM, and NDF, using the formula: Digestibility of nutrient (%) = 100 – [100 × (% marker in feed/% marker in feces) × (% nutrient in feces/% nutrients in feed)]. Weights of feed DM delivered and refused were recorded daily to calculate DMI.
To determine DM content, samples were dried in a forced-air oven set to 55 °C for 48 h, ground using a Willey-type mill (1 mm) and dried at 105 °C for 24 h. Crucibles with dried samples were placed in a muffle oven for 8 h at 450 °C to determine ash content. The N contents were multiplied by 6.25 to estimate crude protein contents. Analyses of NDF were performed using an ANKOM fiber analyzer (ANKOM Technology Corp., Fairport, NY).
Experiment 3
Three hundred seventy-four crossbred heifers (375.8 ± 36.1 kg initial BW) were used to evaluate the impact of removing supplemental NaCl from finishing diets containing 7.5 or 15% CG.
On arrival, all cattle were offered ad libitum access to ground alfalfa hay and water before processing. Twenty-four hours after arrival, heifers were identified with uniquely numbered ear tags, vaccinated (Vista 3 and Vision, Merck Animal Health, Summit, NJ) and dewormed (Safe Guard, Merck Animal Health, Summit, NJ). Pregnancy status was assessed by rectal palpation, and pregnant animals received a prostaglandin F2α analog (Lutalyse, Pfizer Animal Health, NewYork, NY). All heifers received implants (Revalor-200, Intervet, Millsboro, DE) and 4 step-up diets (5 d each) for adaptation to a final diet containing 90% concentrate, using 10% corn silage as roughage.
Cattle were stratified by initial BW and randomly assigned, within strata, to each of the 5 treatments. Five BW strata were used, with 14 to 15 animals per pen and 5 pens per treatment (total of 25 pens).
All diets were formulated (NRC, 1996) to provide 300 mg/kg monensin (Rumensin, Elanco Animal Health, Greenfield, IN), 90 mg/kg tylosin (Tylan, Elanco Animal Health, Greenfield, IN), and 0.4 mg of melengesterol acetate (MGA, Pfizer Animal Health, Exton, PA) per heifer daily. Dietary treatments (Table 2) consisted of: 1) Control, with no CG and 0.3% added salt (CON); 2) 7.5% CG with 0.3% added salt (LCG); 3) 7.5% CG with no added salt (LCGNS); 4) 15% CG with 0.3% salt (HCG); and 5) 15% CG with no added salt (HCGNS).
Table 2.
Diet formulation (DM basis) and dietary nutrients of experimental diets containing 0, 7.5, and 15% crude glycerin, with or without 0.3% salt fed to finishing heifers (Exp. 3)
| Item | Treatments1 | ||||
|---|---|---|---|---|---|
| CON | LCG | LCGNS | HCG | HCGNS | |
| Ingredient, % | |||||
| Corn silage | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Dry-rolled corn | 31.0 | 21.9 | 22.3 | 12.9 | 13.3 |
| Corn gluten feed | 35.0 | 35.0 | 35.0 | 35.0 | 35.0 |
| Soybean hulls | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Soybean meal | 0 | 1.6 | 1.5 | 3.1 | 3.0 |
| Limestone | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 |
| Mineral-vitamin premix2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Feed additive premix3,4 | 2.2 | 2.2 | 2.2 | 2.2 | 2.2 |
| Crude glycerin5 | 0 | 7.5 | 7.5 | 15.0 | 15.0 |
| Salt | 0.3 | 0.3 | 0.0 | 0.3 | 0.0 |
| Nutrient composition, % | |||||
| DM | 65.4 | 65.1 | 64.9 | 65.2 | 65.0 |
| CP | 14.3 | 14.4 | 14.4 | 14.2 | 14.1 |
| NDF | 39.9 | 36.2 | 36.6 | 35.4 | 35.9 |
| Na | 0.12 | 0.37 | 0.23 | 0.56 | 0.41 |
1CON = Control diet, without crude glycerin, LCG = 7.5% added crude glycerin and 0.3% added salt (DM basis), LCGNS = 7.5% added crude glycerin and no salt (DM basis), HCG = 15% added crude glycerin and 0.3% added salt (DM basis), HCGNS = 15% added crude glycerin and no salt (DM basis).
2Formulated to provide 0.1 mg/kg Co; 10 mg/kg Cu; 0.6 mg/kg I; 60 mg/kg Mn; 0.25 mg/kg Se; 60 mg/kg Zn; 2,200 IU/kg of vitamin A; and 22 IU/kg vitamin E in the total diet DM.
3Formulated to provide 300 mg monensin (Rumensin, Elanco Animal Health, Greenfield, IN), 90 mg tylosin (Tylan, Elanco Animal Health, Greenfield, IN), and 0.4 mg melengestrol acetate (Pfizer Animal Health, Exton, PA) per heifer daily.
4Contained Zilpaterol hydrochloride (Merk Animal Health, Summit, NJ) to provide 90 mg per heifer daily, followed by a 3-d withdrawal before harvest.
5Crude glycerin composition: 81.5% glycerol, 13.3% water, 6.3% ash, 2.5% Na, and <0.02% methanol.
Dry matter content of each ingredient was verified every time that a new batch of that ingredient was used. To determine DM content, diets were dried in a forced-air oven set to 55 °C for 48 h, ground using a Willey-type mill (1 mm) and dried at 105 °C for 24 h. Crucibles with dried samples were placed in a muffle oven for 8 h at 450 °C to determine ash content. Nitrogen contents were determined using a Leco N analyzer (Leco Corporation, St. Joseph, MI; AOAC, 1995). The N contents were multiplied by 6.25 to estimate crude protein contents. Analyses of NDF were performed using an ANKOM fiber analyzer (ANKOM Technology Corp., Fairport, NY).
Animals were fed their respective experimental diets once daily for 125 d. Starting 23 d prior to harvest, zilpaterol hydrochloride (Zilmax, Merck Animal Health, Summit, NJ) was fed at 8.3 mg/kg of diet DM basis, followed by a 3-d withdrawal before harvest. At the end of the experimental period, heifers were weighed, loaded onto trucks, and then transported approximately 450 km to a commercial abattoir, where carcass data were collected. Hot carcass weight and incidence and severity of liver abscesses were obtained on the day of harvest and after 24 h of refrigeration the following information was obtained from each carcass: subcutaneous fat thickness over the 12th-rib; longissimus muscle (LM) area, and kidney, pelvic, heart fat (KPH); marbling score; USDA Quality Grade, and USDA Yield Grade.
Statistical Analysis
Experiment 1 was arranged as a 2 × 2 factorial, with factor 1 being the diet to which steers were fed (CON or 15CG), and factor 2 being type of substrate added to microbial cultures (CON or 15CG). Data from Exp. 2 were analyzed as a completely randomized design with repeated measures with diet, time, diet × time as fixed effects, and animal within diet × time as a random effect. Experimental unit in this study was the pen. Experiment 3 was conducted as a randomized complete block design where treatment was included in the model as fixed effect, weight strata was the random effect, and the experimental unit was the pen. Orthogonal contrasts were performed to test the linear and quadratic effects of CG, the effect of CG inclusion (0% CG vs. CG treatments), and the effect of addition of salt to CG-containing diets. The Mixed procedure of SAS was used in all experiments (SAS version 9.2, SAS Inst. Inc., Cary, NC). Treatment means were computed with the LSMEANS option and significance was considered at P < 0.05 and tendency at 0.05 ≤ P ≤ 0.10.
Results and Discussion
Experiment 1
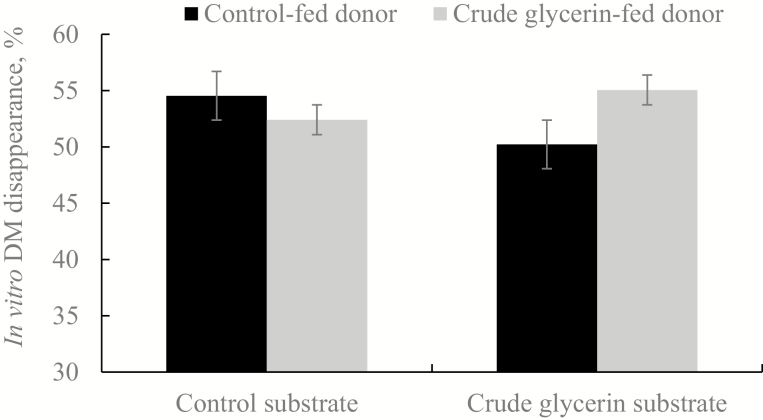
The effects of donor animal diet and in vitro substrate on DM disappearance are illustrated in Fig. 1. There was a tendency for an interaction (P = 0.06) of diet of the donor animals and substrate used in the in vitro system. When CG was added to in vitro microbial cultures containing ruminal fluid from CON-fed animals, disappearance was depressed; however, digestion was increased when CG was added to cultures containing ruminal fluid from 15CG-fed animals. This result supports the hypothesis that prior adaptation is needed to optimize CG utilization and is consistent with findings of previous studies that used pure glycerin. Kijora et al. (1998) fed twice daily 200 g of glycerol (corresponding to 10% of diet DM) to bulls for 6 d and observed a greater glycerol disappearance at the last day of experiment indicating the adaptation of rumen microbes to glycerol utilization. Moreover, Krehbiel (2008) reported that microorganisms may rapidly adapt to dietary glycerol, as greater disappearance rates of glycerol were observed with increased days of glycerol feeding.
Figure 1.
In vitro dry matter disappearance after 24-h incubation of experimental diets, with rumen fluid from control-fed and crude glycerin-fed donors (Effects; Diet, P = 0.48; Substrate, P = 0.86; D×S, P = 0.06).
Total production of volatile fatty acids (Table 3) were unaffected by diet (P = 0.47) of the donor animals nor by substrate (diet; P = 0.20) used in the in vitro system. Crude glycerin increased production of propionate, butyrate, and valerate (P < 0.01), but diet of donor animals had no effect (P = 0.21) on the VFA. Increases in propionate with glycerol supplementation are consistent with observations from previous studies in which beef bulls, goats, or dairy cows were fed cracked corn or ground corn (Wang et al., 2009;Chanjula et al., 2014; Van Cleef et al., 2015).
Table 3.
Production of volatile fatty acids by in vitro cultures containing ruminal contents from control-fed and crude glycerin-fed steers, when cultures were fed substrates consisting of diets containing 0 or 15% crude glycerin (Exp. 1)
| Item, mM | Control-fed rumen fluid | Crude glycerin-fed rumen fluid | P-value3 | ||||
|---|---|---|---|---|---|---|---|
| CON1 | 15CG2 | CON | 15CG | Diet | Substrate | D×S | |
| Total VFA | 24.2 | 25.2 | 12.4 | 27.9 | 0.47 | 0.20 | 0.26 |
| Acetate | 18.3 | 12.4 | 6.9 | 13.3 | 0.29 | 0.95 | 0.22 |
| Propionate | 4.2 | 7.7 | 3.5 | 9.8 | 0.54 | <0.01 | 0.23 |
| Butyrate | 1.6 | 4.1 | 1.8 | 3.7 | 0.91 | <0.01 | 0.70 |
| Isobutyrate | 0.03 | 0.14 | 0.16 | 0.18 | 0.41 | <0.01 | 0.99 |
| Valerate | 0.22 | 0.66 | 0.24 | 0.64 | 0.98 | <0.01 | 0.87 |
| Isovalerate | 0.04 | 0.17 | 0.19 | 0.17 | 0.88 | 0.01 | 0.83 |
1CON = Control diet, without crude glycerin.
215CG = 15% added crude glycerin.
3Diet = Finishing diet fed to donor animals, Substrate = Diet that was included in the in vitro fermentations, D×S = Interaction of diet of the donor animal and substrate fed to the in vitro culture.
Increases in butyrate were also reported by Carvalho et al. (2011), who fed dairy cows up to 11.5% glycerol in high-moisture corn diet, and by Van Cleef et al. (2015), who fed Nellore bulls up to 30% CG in cracked corn diet. The increased butyrate concentration is beneficial because this fatty acid is the main energy substrate used by ruminal epithelial cells (Baldwin and Jesse, 1992).
The CG increased the production of isobutyrate (P < 0.01) and isovalerate (P = 0.01). These findings are relevant because these branched-chain volatile fatty acids are essential nutrients for many cellulolytic rumen bacteria (Bryant, 1973) and could negate the harmful effect of CG in fiber fraction degradation.
Production of fermentative gas (Table 4), including CH4 (P = 0.11, P = 0.61) and CO2 (P = 0.24, P = 0.98), was unaffected by diets of donor animals or substrate used in the in vitro fermentations, which agrees with Avila et al. (2011) findings but disagrees with most previously reported results (Lee et al., 2011, Van Cleef et al., 2015). A reduction was expected in the production of CH4, due to the expected increase in propionate (especially in CG-fed donors) and decreased acetate:propionate ratio. This situation leads to a decrease in H2 concentration available in the rumen to form CH4 (Janssen, 2010).
Table 4.
Production and composition of fermentative gasses produced by in vitro cultures containing ruminal contents from control-fed and crude glycerin-fed steers, when cultures were fed substrates consisting of diets with 0 or 15% crude glycerin (Exp. 1)
| Item, mL | Control-fed rumen fluid | Crude glycerin-fed rumen fluid | P-value3 | ||||
|---|---|---|---|---|---|---|---|
| CON1 | 15CG2 | CON | 15CG | Diet | Substrate | D×S | |
| Total gas | 107.7 | 108.3 | 83.4 | 88.9 | 0.16 | 0.84 | 0.87 |
| Methane | 5.0 | 4.7 | 5.9 | 7.3 | 0.11 | 0.61 | 0.45 |
| Carbon dioxide | 29.3 | 25.9 | 18.0 | 21.2 | 0.24 | 0.98 | 0.61 |
1CON = Control diet, without crude glycerin.
215CG = 15% added crude glycerin.
3Diet = Finishing diet fed to donor animals, Substrate = Diet that was included in the in vitro fermentations, D×S = Interaction of diet of the donor animal and substrate fed to the in vitro culture.
Experiment 2
There was no interaction of treatment and day of sampling (period) for DMI, DM digestibility, OM digestibility, and NDF digestibility (P = 0.37, P = 0.81, P = 0.74, P = 0.44, respectively; Table 5), suggesting no adaptive response to dietary CG in the 35-d trial. Dry matter intake was similar among treatments (10.93 and 10.92 kg/d for CON and 15CG diets, respectively; P = 0.99). The lack of effect in DMI during this trial agrees with recent studies with inclusions of up to 10% CG in steam-flaked corn diet for beef steers (Weiss et al., 2017), 21% CG in ground corn diets for goats (Chanjula et al., 2016), or 30% of CG in cracked corn diets for beef bulls (Van Cleef et al., 2014).
Table 5.
Dry matter intake and digestibility of diets with or without 15% crude glycerin (Exp. 2)
| Item | Treatments1 | SEM | P-value | |||
|---|---|---|---|---|---|---|
| CON | 15CG | Treatment | Period | T×P2 | ||
| DMI, kg/d | 10.9 | 10.9 | 0.67 | 0.99 | 0.78 | 0.37 |
| Digestibility, % | ||||||
| DM | 58.8 | 64.1 | 1.93 | 0.06 | 0.004 | 0.81 |
| OM | 61.7 | 67.4 | 1.82 | 0.04 | 0.009 | 0.74 |
| NDF | 51.6 | 54.7 | 2.06 | 0.29 | 0.002 | 0.44 |
1CON = Control diet, without crude glycerin, 15CG = 15% added crude glycerin (DM basis).
2T×P = Interaction of diets and periods.
Organic matter digestibility of 15CG diet was greater than that of the Control diet (P = 0.04), and DM digestibility followed a similar trend (P = 0.06). In studies where up to 25% glycerol was added, most of the glycerol disappeared within 6 h (Bergner et al., 1995), evidencing the improvement in diet DM and OM digestibility. Similarly, Del Bianco et al. (2016) fed finishing beef bulls with 15% of CG by partial replacement of corn found an increase of DM and OM digestibility when CG was added. An explanation for a higher digestibility could be that the rapid disappearance of glycerol allows the rest of DM to remain longer in the rumen.
The digestibility of NDF was unaffected by CG addition (P = 0.29), although many studies using CG in beef cattle diets reported reductions of fiber fraction digestibility, due to CG deleterious effects on cellulolytic activity of ruminal bacteria (Roger et al., 1992).
However, results of several studies are contradictory, with some studies finding that CG affects DM or nutrients digestibility while others do not find these effects. This could be explained by the differences in treatments (i.e., ingredients, CG levels, animals) in the various experiments (Del Bianco et al., 2016).
Experiment 3
Average daily DMI was not affected by the addition of salt to the diets, but feeding CG caused linear (P = 0.003) reductions in DMI compared with control heifers (Table 6). Parsons et al. (2009) reported decreases in DMI when feedlot heifers were fed up to 16% CG. Likewise, Del Benedeti et al. (2016) observed decreased DMI when feeding up to 15% CG to 3,640 Nellore bulls on ground corn diet. In contrast, Mach et al. (2009) feeding high concentrate, corn meal diets containing up to 12% CG to Holstein bulls, and Van Cleef et al. (2014) feeding up to 30% CG to Nellore bulls on cracked corn diets, did not observe any change in DMI. Moreover, the difference observed between DMI from Exp. 2 and 3 was due to the contrasts in the experiments’ duration and housing arrangements, which was 35 d and covered pens for 2 individuals in Exp. 2 and 125 d and open collective pens for 15 individuals in Exp. 3.
Table 6.
Effect of diets containing 0, 7.5, and 15% crude glycerin, with or without 0.3% added salt on heifer’s feedlot performance (Exp. 3)
| Item | Treatments1 | SEM | P-value2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CON | LCG | LCGNS | HCG | HCGNS | L | Q | S | CON vs. CG | ||
| Heifers, n | 73 | 73 | 75 | 71 | 73 | - | - | - | - | - |
| Days on feed | 125 | 125 | 125 | 125 | 125 | - | - | - | - | - |
| Initial weight, kg | 376 | 378 | 376 | 375 | 374 | 17.7 | 0.53 | 0.37 | 0.61 | 0.90 |
| Shrunk final BW3, kg | 581 | 569 | 582 | 576 | 570 | 19.2 | 0.30 | 0.84 | 0.63 | 0.89 |
| Final weight, kg | 605 | 593 | 606 | 600 | 593 | 20.0 | 0.30 | 0.84 | 0.63 | 0.35 |
| Carcass-adjusted FBW4, kg | 582 | 574 | 583 | 574 | 570 | 19.8 | 0.11 | 0.76 | 0.21 | 0.24 |
| ADG, kg | 1.8 | 1.7 | 1.8 | 1.8 | 1.8 | 0.04 | 0.30 | 0.53 | 0.42 | 0.27 |
| DMI, kg/d | 12.4 | 11.8 | 11.8 | 11.4 | 11.3 | 0.3 | 0.003 | 0.83 | 0.71 | 0.009 |
| G:F5, kg/kg | 0.15 | 0.15 | 0.16 | 0.16 | 0.16 | 0.004 | 0.03 | 0.62 | 0.25 | 0.12 |
1CON = Control diet, without crude glycerin, LCG = 7.5% added crude glycerin and 0.3% added salt (DM basis), LCGNS = 7.5% added crude glycerin and no salt (DM basis), HCG = 15% added crude glycerin and 0.3% added salt (DM basis), HCGNS = 15% added crude glycerin and no salt (DM basis).
2L = Linear effect of crude glycerin, Q = Quadratic effect of crude glycerin, S = Compares 0 and 0.3% salt in diets that contain crude glycerin, CON vs. CG = Contrast control vs. crude glycerin treatments.
3Calculated by multiplying gross final BW by 96% (i.e., 4% pencil shrink).
4Calculated by dividing hot carcass height by a common dressing percentage of 63.5%.
5G:F = weight gain per feed consumed.
No difference was observed between ADG of control and CG treatments (P = 0.27). Heifers fed CON, LCG, HCG, LCGNS, and HCGNS treatments had ADG of 1.83, 1.72, 1.84, 1.81, and 1.75 kg, respectively. Supporting the current study, Gunn et al. (2010a) fed finishing wethers with diets containing up to 20% CG and dry-rolled corn and observed no change in ADG. Several cattle studies have shown a decrease in ADG when CG was included at more than 10% of total diet DM (Versemann et al., 2008; Mach et al., 2009; Parsons et al., 2009), regardless the energy source replaced by CG.
As a result of decreasing DMI and no changes in ADG, a linear improvement in feed efficiency was observed with increasing concentrations of CG (P = 0.03). The improvement in feed efficiency is related to the increase in propionic acid concentration in the rumen (observed in Exp. 2), as also reported by Gunun et al. (2013). Removing salt from diets with CG had no discernible impact on efficiency (P = 0.25). Pyatt et al. (2007) reported an improvement in G:F of beef steers with addition of CG to diets of feedlot steers. Likewise, Parsons et al. (2009) observed improvements in feed efficiency of 10.8, 10.0, 7.2, and 3.1%, respectively, when CG was included in finishing diets of heifers at 2, 4, 8, or 12% of diet DM. When CG was fed at 16% of diet DM, the authors noted a decrease in efficiency of cattle compared to those fed the control diet without CG. Similarly, Gunn et al. (2010a) fed finishing lambs diets containing 0, 5, 10, 15, or 20% CG, and observed no impact on G:F. However, Gunn et al. (2010b) fed finishing lambs diets containing 15, 30, or 45% CG in dry-rolled corn diets and observed a linear decrease in G:F compared to lambs fed a control diet without CG.
The effects of CG and salt addition on carcass characteristics of finishing heifers are presented in Table 7. Dietary CG did not affect HCW (average = 366 kg, P = 0.24) or dressing percentage (average = 63.6%, P = 0.99). Mach et al. (2009), observed no difference in these traits when Holstein bulls were fed up to 12% CG. Gunn et al. (2010b) also reported no changes in HCW of lambs fed up to 45% CG. However, Parsons et al. (2009), feeding finishing heifers up to 16% CG, reported increases in HCW of 8.1, 5.1, and 3.2 kg, when the animals received 2, 4, or 8%, respectively, but HCW decreased by 1.2, and 9.1 kg compared to animals fed a control diet without CG, when heifers were fed diets with 12 or 16% CG, respectively.
Table 7.
Carcass characteristics and liver abscess score of heifers fed 0, 7.5, and 15% crude glycerin with or without 0.3% added salt (Exp. 3)
| Item | Treatments1 | SEM | P-value2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CON | LCG | LCGNS | HCG | HCGNS | L | Q | S | CON vs. CG | ||
| HCW3, kg | 369 | 364 | 370 | 364 | 362 | 12.6 | 0.11 | 0.76 | 0.21 | 0.24 |
| Dressed yield, % | 63.6 | 64.0 | 63.7 | 63.2 | 63.6 | 0.55 | 0.76 | 0.51 | 0.96 | 0.99 |
| LM area4, cm2 | 93.5 | 93.8 | 93.6 | 93.6 | 95.5 | 1.90 | 0.46 | 0.76 | 0.47 | 0.63 |
| KPH5, % | 3.2 | 3.2 | 3.2 | 3.3 | 3.1 | 0.05 | 0.46 | 0.88 | 0.55 | 0.59 |
| Subcutaneous fat, cm | 2.2 | 2.1 | 2.1 | 2.1 | 1.8 | 0.08 | 0.005 | 0.48 | 0.02 | 0.04 |
| Liver abscesses, % | 10.7 | 17.3 | 13.2 | 16.0 | 17.6 | 4.10 | 0.23 | 0.70 | 0.71 | 0.25 |
| USDA Yield Grade | 2.6 | 2.5 | 2.6 | 2.4 | 2.4 | 0.11 | 0.01 | 0.64 | 0.04 | 0.06 |
| Marbling score6 | 512 | 485 | 458 | 476 | 474 | 12.9 | 0.004 | 0.02 | 0.73 | <0.001 |
| USDA quality grades | ||||||||||
| Prime, % | 2.7 | 4.0 | 0.0 | 0.0 | 0.0 | 1.32 | 0.10 | 0.59 | 0.13 | 0.26 |
| Choice, % | 82.7 | 82.7 | 79.0 | 88.0 | 87.8 | 4.26 | 0.31 | 0.26 | 0.09 | 0.72 |
| Select, % | 14.6 | 13.3 | 19.7 | 12.0 | 10.8 | 4.03 | 0.51 | 0.35 | 0.20 | 0.88 |
| Ungraded, % | 0.0 | 0.0 | 1.3 | 0.0 | 1.4 | 0.80 | 0.51 | 0.68 | 0.98 | 0.48 |
1CON = Control diet, without crude glycerin, LCG = 7.5% added crude glycerin and 0.3% added salt (DM basis), LCGNS = 7.5% added crude glycerin and no salt (DM basis), HCG = 15% added crude glycerin and 0.3% added salt (DM basis), HCGNS = 15% added crude glycerin and no salt (DM basis).
2L = Linear effect of crude glycerin, Q = Quadratic effect of crude glycerin, S = Compares 0 and 0.3% salt in diets that contain crude glycerin, CON vs. CG = Contrast control vs. crude glycerin treatments.
3HCW = Hot carcass weight.
4LM area = Longissimus muscle area.
5KPH = Kidney, pelvic and heart fat.
6Marbling scores determined by USDA graders; slight = 300–399, small = 400–499, and modest = 500–599.
No differences among treatments were observed for LM area (linear, P = 0.46; quadratic, P = 0.76; salt, P = 0.47) or percentage KPH (linear, P = 0.46; quadratic, P = 0.88; salt, P = 0.55). Feeding CG and additional salt to finishing heifers decreased subcutaneous fat (linear, P = 0.005; salt, P = 0.02). The negative effect of CG on fat deposition seems plausible, as glycerol is noted for decreasing ruminal production of acetate and acetate:propionate ratio due to CG conversion to propionate (Remond et al., 1993). Acetate has been observed to contribute as much as 70 to 80% of the acetyl units required for lipogenesis in subcutaneous adipose tissue (Smith and Crouse, 1984); so, the observed effects of CG on subcutaneous fat deposition were not unexpected. These changes in subcutaneous fat thickness contributed to changes in USDA yield grade as well. Crude glycerin, or CG plus salt, decreased USDA yield grades (linear, P = 0.01; salt, P = 0.05). Glycerol is a glucogenic substance, thus, the inclusion of this by-product in the diets might be theorized to increase glucose availability, which provides 50 to 75% of acetyl units to adipose tissue in the intramuscular depot (Smith and Crouse, 1984). Contrary to this, however, marbling scores were actually decreased when the animals were fed CG (linear, P = 0.004; quadratic, P = 0.02) compared to animals fed CON. Similarly, Parsons et al. (2009) reported linear reduction on marbling scores of heifers fed 0, 2, 4, 8, 12, or 16% CG.
Feeding CG tended to decrease the proportion of carcasses that qualified for Prime (linear, P = 0.10), however, no differences were observed among treatments for Choice and Select grades. Parsons et al. (2009) reported a similar tendency for lesser USDA Quality Grades when heifers were fed up to 16% CG, reducing the percentage of carcasses grading Choice and increasing carcasses grading Select. No differences were observed among treatments for percentage or severity of liver abscesses (P = 0.25). The presence of liver abscesses, depending on the severity, can be associated with decreased feed intake, weight gain, feed efficiency, and carcass dressing percentage (Nagaraja and Lechtenberg, 2007). The lack of effect of CG on liver abscess occurrence was also found by Parsons et al. (2009) and Weiss et al. (2017).
In conclusion, CG can effectively replace dry-rolled corn in diets for beef heifers when fed at 15% of diet DM, improving OM digestion without adversely affecting NDF digestibility. Crude glycerin also increases ruminal propionate and butyrate concentrations, but in this study had no effect on greenhouse gas emissions. Feeding up to 15% CG to crossbred heifers improves feed efficiency by decreasing feed intake, but also depresses marbling score and tends to decrease quality grade. Removing supplemental salt from finishing diets containing CG has no impact on performance or carcass characteristics. Overall, CG added up to 15% has a positive impact in feedlot feeding, decreasing the need of corn with no negative effects on feedlot performance or carcass characteristics.
Footnotes
This is contribution number 15-313-J from the Kansas Agricultural Experiment Station.
Literature Cited
- Abo El-Nor S., AbuGhazaleh A. A., Potu R. B., Hastings D., and Khattab M. S. A.. . 2010. Effects of differing levels of glycerol on rumen fermentation and bacteria. Anim. Feed Sci. Technol. 162:99–105. doi: 10.1016/j.anifeedsci.2010.09.012 [DOI] [Google Scholar]
- AOAC 1995. Official methods of analysis. 15th ed Assoc. Off. Anal. Chem., Arlington, VA. [Google Scholar]
- Arieli A., Naim E., Benjamin R. W., and Pasternak D.. 1989. The effect of feeding saltbush and sodium chloride on energy metabolism in sheep. Anim. Prod. 49:451–457. doi: 10.1017/S0003356100032657 [DOI] [Google Scholar]
- Avila J. S., Chaves A. V., Hernandez-Calva M., Beauchemin K. A., McGinn S. M., Wang Y., Harstad O. M., and McAllister T. A.. . 2011. Effects of replacing barley grain in feedlot diets with increasing levels of glycerol on in vitro fermentation and methane production. Anim. Feed Sci. Technol. 166–167:265–268. doi: 10.1016/j.anifeedsci.2011.04.016 [DOI] [Google Scholar]
- Baldwin R. L. VI, and Jesse B. W.. 1992. Developmental changes in glucose and butyrate metabolism by isolated sheep ruminal cells. J. Nutr. 122:1149–1153. doi: 10.1093/jn/122.5.1149 [DOI] [PubMed] [Google Scholar]
- Bergner H., Kijora C., Ceresnakova Z., and Szakacs J.. . 1995. In vitro studies on glycerol transformation by rumen microorganisms. Arch Tierernahr. 48:245–256. doi: 10.1080/17450399509381845 [DOI] [PubMed] [Google Scholar]
- Bryant M. P. 1973. Nutritional requirements of the predominant rumen cellulolytic bacteria. Fed. Proc. 32:1809–1813. [PubMed] [Google Scholar]
- Carvalho E. R., Schmelz-Roberts N. S., White H. M., P. H. Doane, and Donkin S. S.. . 2011. Replacing corn with glycerol in diets for transitioning dairy cows. J. Dairy Sci. 94:908–916. doi: 10.3168/jds.2010-3581 [DOI] [PubMed] [Google Scholar]
- Cavalheiro J. M. B. T., de Almeida M. C. M. D., Grandfils C., and da Fonseca M. M. R.. . 2009. Poly(3-hydroxybutyrate) production by Cupriavidus necator using waste glycerol. Process Biochem. 44:509–515. doi: 10.1016/j.procbio.2009.01.008 [DOI] [PubMed] [Google Scholar]
- Chanjula P., Pakdeechanuan P., and Wattanasit S.. . 2014. Effects of dietary crude glycerin supplementation on nutrient digestibility, ruminal fermentation, blood metabolites, and nitrogen balance of goats. Asian-Australas. J. Anim. Sci. 27:365–374. doi: 10.5713/ajas.2013.13494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanjula P., Raungprim T., Yimmongkol S., Poonko S., Majarune S., and Maitreejet W.. . 2016. Effects of elevated crude glycerin concentrations on feedlot performance and carcass characteristics in finishing steers. Asian-Australas. J. Anim. Sci. 29:80–88. doi: 10.5713/ajas.15.0219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y. H., Rico D. E., Martinez C. M., Cassidy T. W., Noirot V., Ames A., and Varga G. A.. . 2007. Effects of feeding dry glycerin to early postpartum Holstein dairy cows on lactational performance and metabolic profiles. J. Dairy Sci. 90:5682–5691. doi: 10.3168/jds.2007-0426 [DOI] [PubMed] [Google Scholar]
- Del Benedeti P. B., Paulino P. V. R., Marcondes M. I., Maciel I. F. S., da Silva M. C., and Faciola A. P.. . 2016. Partial replacement of ground corn with glycerol in beef cattle diets: Intake, digestibility, performance, and carcass characteristics. PLoS ONE. 11:e0148224. doi: 10.1371/journal.pone.0148224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bianco P., Veiga P., Inácio M., Franca I., Custódio M., and Pinheiro A.. . 2016. Partial replacement of ground corn with glycerol in beef cattle diets: Intake, digestibility, performance, and carcass characteristics. PLoS One. 11:e01148224. doi: 10.1371/journal.pone.0148224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkin S. S. 2008. Glycerol from biodiesel production: The new corn for dairy cattle. Rev. Bras. Zootecn. 37:280–286. doi: 10.1590/S1516-35982008001300032 [DOI] [Google Scholar]
- Ezequiel J. M. B., Sancanari J. B. D., Machado Neto O. R., Silva Z. F., Almeida M. T. C., Silva D. A. V., Van Cleef F. O. S., and Van Cleef E. H. C. B.. . 2015. Effects of high concentrations of dietary crude glycerin on dairy cow productivity and milk quality. J. Dairy Sci. 98:8009–8017. doi: 10.3168/jds.2015-9448 [DOI] [PubMed] [Google Scholar]
- Goff J. P., and Horst R. L.. . 2001. Oral glycerol as an aid in treatment of ketosis/fatty liver complex. J. Dairy Sci. 84 (Suppl. 1):153. (Abstr.) [Google Scholar]
- Gunn P. J., Neary M. K., Lemenager R. P., and Lake S. L.. . 2010a. Effects of crude glycerin on performance and carcass characteristics of finishing wether lambs. J. Anim. Sci. 88:1771–1776. doi: 10.2527/jas.2009-2325 [DOI] [PubMed] [Google Scholar]
- Gunn P. J., Schultz A. F., Van Emon M. L., Neary M. K., Lemenager R. P., Rusk C. P., and Lake S. L.. . 2010b. Effects of elevated crude glycerin concentrations on feedlot performance, carcass characteristics, and serum metabolite and hormone concentrations in finishing ewe and wether lambs. Prof. Anim. Sci. 26:298–306. doi: 10.15232/S1080-7446(15)30597-0 [DOI] [Google Scholar]
- Gunun P., Wanapat M., and Anantasook N.. . 2013. Effects of physical form and urea treatment of rice straw on rumen fermentation, mi-crobial protein synthesis and nutrient digestibility in dairy steers. Asian-australas. J. Anim. Sci. 26:1689–1697. doi: 10.5713/ajas.2013.13190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen P. H. 2010. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim. Feed Sci. Technol. 160:1–22. doi: 10.1016/j.anifeedsci.2010.07.002 [DOI] [Google Scholar]
- Jung B., and Batal A. B.. . 2011. Nutritional and feeding value of crude glycerin for poultry. 1. Nutritional value of crude glycerin. J. Appl. Poult. Res. 20:162–167. doi: 10.3382/japr.2011-00338 [DOI] [Google Scholar]
- Kerr B. J., Weber T. E., Dozier W. A. III, and Kidd M. T.. . 2009. Digestible and metabolizable energy content of crude glycerin originating from different sources in nursery pigs. J. Anim. Sci. 87:4042–4049. doi: 10.2527/jas.2008-1676 [DOI] [PubMed] [Google Scholar]
- Kijora C., Bergner H., Gotz K.P., Barttelt J., Szakács J., and Sommer A.. . 1998. Research note: Investigation on the metabolism of glycerol in the rumen of bulls. Arch Tierernahr. 51:341–348. doi: 10.1080/17450399809381931 [DOI] [PubMed] [Google Scholar]
- Krehbiel C. R. 2008. Ruminal and physiological metabolism of glycerin. J. Anim. Sci. 86(E-Suppl.):392. (Abstr.) [Google Scholar]
- Lee S.-Y., Lee S.-M., Cho Y.-B., Kam D.-K., Lee S.-C., Kim C.-H., and Seo S.. . 2011. Glycerol as a feed supplement for ruminants: In vitro fermentation characteristics and methane production. Anim. Feed Sci. Technol. 166–167:269–274. doi: 10.1016/j.anifeedsci.2011.04.070 [DOI] [Google Scholar]
- Mach N., Bach A., and Devant M.. . 2009. Effects of crude glycerin supplementation on performance and meat quality of Holstein bulls fed high-concentrate diets. J. Anim. Sci. 87:632–638. doi: 10.2527/jas.2008-0987 [DOI] [PubMed] [Google Scholar]
- Nagaraja T. G., and Lechtenberg K. F.. . 2007. Liver abscesses in feedlot cattle. Vet. Clin. North Am. Food Anim. Pract. 23:351–369. doi: 10.1016/j.cvfa.2007.05.002 [DOI] [PubMed] [Google Scholar]
- NRC 1996. Nutrient requirements of beef cattle. 7th ed Natl. Acad. Press, Washington, DC. [Google Scholar]
- NRC 2005. Mineral tolerance of animals. 2nd rev. ed Natl. Acad. Press, Washington, DC. [Google Scholar]
- Parsons G. L., Shelor M. K., and Drouillard J. S.. . 2009. Performance and carcass traits of finishing heifers fed crude glycerin. J. Anim. Sci. 87:653–657. doi: 10.2527/jas.2008-1053 [DOI] [PubMed] [Google Scholar]
- Pyatt N. A., Doane P. H., and Cecava M. J.. . 2007. Effect of crude glycerin in finishing cattle diets. J. Anim. Sci. 85(Suppl. 1):530. (Abstr.) [Google Scholar]
- Remond B., Souday E., and Jouany J. P.. . 1993. In vitro and in vivo fermentation of glycerol by rumen microbes. Anim. Feed Sci. Technol. 41:121–132. doi: 10.1016/0377-8401(93)90118-4 [DOI] [Google Scholar]
- Roger V., Fonty G., Andre C., and Gouet P.. . 1992. Effects of glycerol on the growth, adhesion, and cellulolytic activity of ruminal cellulolytic bacteria and anaerobic fungi. Curr Microbiol. 25:197–201. doi: 10.1007/BF01570719 [DOI] [PubMed] [Google Scholar]
- Schieck S. J., Shurson G. C., Kerr B. J., and Johnston L. J.. . 2010. Evaluation of glycerol, a biodiesel coproduct, in grow-finish pig diets to support growth and pork quality. J. Anim. Sci. 88:3927–3935. doi: 10.2527/jas.2010-2858 [DOI] [PubMed] [Google Scholar]
- Smith S. B., and Crouse J. D.. . 1984. Relative contributions of acetate, lactate and glucose to lipogenesis in bovine intramuscular and subcutaneous adipose tissue. J. Nutr. 114:792–800. doi: 10.1093/jn/114.4.792 [DOI] [PubMed] [Google Scholar]
- Van Cleef E. H. C. B., Almeida M. T. C., Perez H. L., Van Cleef F. O. S., Silva D. A. V., and Ezequiel J. M. B.. . 2015. Crude glycerin changes ruminal parameters, in vitro greenhouse gas profile, and bacterial fractions of beef cattle. Livest. Sci. 178:158–164. doi: 10.1016/j.livsci.2015.06.016 [DOI] [Google Scholar]
- Van Cleef E. H. C. B., Ezequiel J. M. B., D’Aurea A. P., Fávaro V. R., and Sancanari J. B. D.. . 2014. Crude glycerin in diets for feedlot Nellore cattle. Rev. Bras. Zootec. 43:86–91. doi: 10.1590/S1516-35982014000200006 [DOI] [Google Scholar]
- Versemann B. A., Wiegand B. R., Kerley M. S., Porter J. H., Roberts K. S., and Evans H. L.. . 2008. Dietary inclusion of crude glycerol changes beef steer growth performance and intramuscular fat deposition. J. Anim. Sci. 86 (E-Suppl. 2):478. (Abstr.) [Google Scholar]
- Wang C., Liu Q., Huo W. J., Yang W. Z., K. H. Dong, Y. X. Huang, and Guo G.. . 2009. Effects of glycerol on ruminal fermentation, urinary excretion of purine derivatives and feed digestibility in steers. Livest. Sci. 121:15–20. doi: 10.1016/j.livsci.2008.05.010 [DOI] [Google Scholar]
- Weiss C. P., Gentry W. W., Cole N. A., McCollum F. T., and Jennings J. S.. . 2017. Effects of feeding condensed distiller’s solubles and crude glycerin alone or in combination on finishing beef cattle performance, carcass characteristics, and in vitro fermentation. J. Anim. Sci. 95:922–929. doi: 10.2527/jas.2016.0941 [DOI] [PubMed] [Google Scholar]
- Wilson A. D. 1966. The tolerance of sheep to sodium chloride in food or drinking water. Aust. J. Agric. Res. 17:503–514. doi: 10.1071/AR9660503 [DOI] [Google Scholar]
- Yazdani S. S., and Gonzalez R.. . 2007. Anaerobic fermentation of glycerol: A path to economic viability for the biofuel industry. Curr. Opin. Biotechnol. 18:213–219. doi: 10.1016/j.copbio.2007.05.002 [DOI] [PubMed] [Google Scholar]