Abstract
Feedlot performance is reduced by heat stress and improved by β adrenergic agonists (βAA). However, the physiological mechanisms underlying these outcomes are not well characterized, and anecdotal reports suggest that βAA may confound the effects of heat stress on wellbeing. Thus, we sought to determine how heat stress and βAA affect growth, metabolic efficiency, and health indicators in lambs on a feedlot diet. Wethers (38.6 ± 1.9 kg) were housed under thermoneutral (controls; n = 25) or heat stress (n = 24) conditions for 21 d. In a 2 × 3 factorial, their diets contained no supplement (unsupplemented), ractopamine (β1AA), or zilpaterol (β2AA). Blood was collected on days −3, 3, 9, and 21. On day 22, lambs were harvested and ex vivo skeletal muscle glucose oxidation was determined to gauge metabolic efficiency. Feet and organ tissue damage was assessed by veterinary pathologists. Heat stress reduced (P < 0.05) feed intake by 21%, final bodyweight (BW) by 2.6 kg, and flexor digitorum superficialis (FDS) muscle mass by 5%. β2AA increased (P < 0.05) FDS mass/BW by 9% and average muscle fiber area by 13% compared with unsupplemented lambs. Blood lymphocytes and monocytes were greater (P < 0.05) in heat-stressed lambs, consistent with systemic inflammation. Plasma insulin was 22% greater (P < 0.05) and glucose/insulin was 16% less (P < 0.05) in heat-stressed lambs than controls. Blood plasma urea nitrogen was increased (P < 0.05) by heat stress on day 3 but reduced (P < 0.05) on days 9 and 21. Plasma lipase and lactate dehydrogenase were reduced (P < 0.05) by heat stress. Glucose oxidation was 17% less (P < 0.05) in muscle from heat-stressed lambs compared with controls and 15% greater (P < 0.05) for β2AA-supplemented compared with unsupplemented lambs. Environment and supplement interacted (P < 0.05) for rectal temperature, which was increased (P < 0.05) by heat stress on all days but more so (P < 0.05) in β2AA-supplemented lambs on days 4, 9, and 16. Heat stress increased (P < 0.05) the frequency of hoof wall overgrowth, but βAA did not produce any pathologies. We conclude that reduced performance in heat-stressed lambs was mediated by reduced feed intake, muscle growth, and metabolic efficiency. β2AA increased muscle growth and improved metabolic efficiency by increasing muscle glucose oxidation, but no such effects were observed with ractopamine. Finally, βAA supplementation was not detrimental to health indicators in this study, nor did it worsen the effects of heat stress.
Keywords: Beta agonist, feedlot performance, glucose metabolism, growth efficiency, muscle growth
Introduction
Heat stress is a well-established barrier to the health and wellbeing, growth efficiency, and performance of livestock (Hahn, 1999; Mader, 2003; Hagenmaier et al., 2016), but the physiological mechanisms that mediate these effects are poorly understood. Feedlot animals are particularly susceptible to environmental heat, as their confinement limits their ability to seek shade, cooler water, or greater air movement as they would on pasture (Renaudeau et al., 2012). Even moderately increased temperature-humidity index (THI) without shade can reduce feed intake and increase stress indicators in feedlot cattle (Brown-Brandl et al., 2003; Brown-Brandl et al., 2017), which in turn reduces average daily gain (ADG), carcass merit, and health (Mitlohner et al., 2002). Texas and Nebraska account for 50% of all US-fed cattle, and a meta-analysis of feedlots estimated that on average heat stress reduced gain by 17.0 and 6.7 kg/head, respectively, and increased death loss by 51 and 15 animals per 10,000 head, respectively, in these states (St-Pierre et al., 2003). Management practices such as cooling pens and adding shade can reduce the impact of heat stress (Sullivan and Mader, 2018) but are not feasible for all feedlots and can produce inconsistent results (Boyd et al., 2015; Hagenmaier et al., 2016).
In addition to stress abatement, feedlot managers must also consider growth-promoting supplements to increase production and maintain economic sustainability. In recent years, producers have benefitted from two FDA-approved β adrenergic agonists: the β1 agonist, ractopamine HCl, and the β2 agonist, zilpaterol HCl (Delmore et al., 2010; Boler et al., 2012). These feed additives increase growth, reduce fat deposition, and improve feed efficiency in cattle supplemented for the last 20 to 45 d on feed (Elam et al., 2009; Montgomery et al., 2009; Buntyn et al., 2016), which allows more meat to be produced from fewer animals. However, anecdotal observations of increased mortality and lameness in β agonist-fed cattle have been reported (Grandin, 2014; Thomson et al., 2015), particularly in cattle experiencing heat stress. A follow-up correlative analysis of three available data sets estimated that β agonist supplementation increased death loss by 2 to 10 animals per 10,000/head (Loneragan et al., 2014), but it was unclear how this compared with other risk factors. Several prospective research studies have been unable to replicate lameness in β agonist-supplemented cattle (Samuelson et al., 2016; Buntyn et al., 2017; Van Bibber-Krueger et al., 2017) or sheep (Avendano-Reyes et al., 2011; Davila-Ramirez et al., 2015). However, only one of these studies assessed the potential interacting effects of β agonists and heat stress (Boyd et al., 2015), and THI in this study was only mildly elevated.
Optimizing growth performance without compromising animal wellbeing is a priority for the livestock industry (Grandin, 2017). As producers face new challenges of climate change, heightened production demands, and greater scrutiny from consumers, understanding the physiological mechanisms that mediate the outcomes of both heat stress and β agonist supplementation will be essential to sustainable livestock production. Our previous findings indicate that skeletal muscle oxidative capacity and myoblast function are improved by β agonists but impaired by inflammatory cytokines and other stress factors (Yates et al., 2016; Cadaret et al., 2017; Yates et al., 2018). Thus, we postulate that muscle-specific glucose oxidative metabolism and hypertrophic growth are 2 mechanisms underlying the negative effects of heat stress and positive effects of β agonist supplementation in feedlot animals. For this study, we used feeder lambs, which are a good model for feedlot cattle because: 1) the major metabolic- and growth-regulating systems are functionally similar between the species, and 2) the differences between sheep and cattle physiology are typically well understood (Sewell et al., 2009; Lundy et al., 2015). The objective of this study was to test this hypothesis by comprehensively assessing the individual and interacting impacts of heat stress and dietary supplementation of ractopamine (β1 agonist) or zilpaterol (β2 agonist) on muscle growth, metabolic efficiency, and health indicators in finishing lambs.
Materials and Methods
Animals and Experimental Design
This study was approved by the Institutional Animal Care and Use Committee at the University of Nebraska – Lincoln (UNL). Studies were performed at the UNL Animal Science Complex, which is accredited by AAALAC International. Rambouillet x Columbia crossbred wether lambs (n = 49) were purchased commercially, stratified by bodyweight (BW), and divided into 2 blocks (block 1, 39.9 ± 1.9 kg; block 2, 37.4 ± 1.9 kg) to accommodate the size limits of the environmental chambers (12 pens). Lambs in block 1 were transitioned to an ad libitum 90% concentrate finishing diet consisting of 49% SweetBran, 37.8% dry-rolled corn, 8.3% chopped alfalfa hay, and 4% mineral supplement with coccidiostat (20 g/ton) over a period of 21 d. Lambs in block 2 were maintained on a diet of 54.8% SweetBran, 41.1% chopped alfalfa hay, and 4% mineral supplement with coccidostat (20 g/ton) at 2% of BW for 41 d until studies on block 1 were complete. They were then transition over 21 d to the 90% concentrate finishing diet. All lambs were fed the 90% concentrate diet for 28 d (days −7 to 21).
Within each block, lambs were stratified by BW and randomly assigned to be housed under thermoneutral conditions (controls; n = 25) or heat stress (n = 24). For heat-stressed lambs, temperatures were gradually increased between days −4 and 0, as illustrated in Figure 1A. In a 2 × 3 factorial, lambs within these groups were also randomly assigned to receive no dietary supplement (unsupplemented), ractopamine HCl (0.03996 g/d), or zilpaterol HCl (0.025 g/d). Supplements were delivered in 200 g ground corn added to the ration, which was fed at 8:00 a.m. each day. Orts were collected and weighed daily to determine average DMI, and daily water intake to the nearest 0.5 liter was also recorded. Lambs were weighed at days 0 and 21, and these values were used to calculate gain-to-feed ratios. Respiratory rates and rectal temperatures were determined daily from days −3 to 21, and blood samples were collected at 3:00 p.m. on days −3, 3, 9, and 21. Weekly carcass ultrasound, bioelectrical impedance assays, and surface temperature assessments not included in this manuscript were also performed on these lambs. On day 22, lambs were transferred to the USDA-inspected Loeffel Meat Lab abattoir at UNL for harvest by captive bolt and exsanguination. Harvest order was randomly assigned and all animals were harvested within 10 h of transport to the facility. Organs were weighed at harvest (rumen weight included digesta) and tissue samples of liver, lung, kidney, and heart along with all feet were assessed for pathologies by the UNL Veterinary Diagnostics Center.
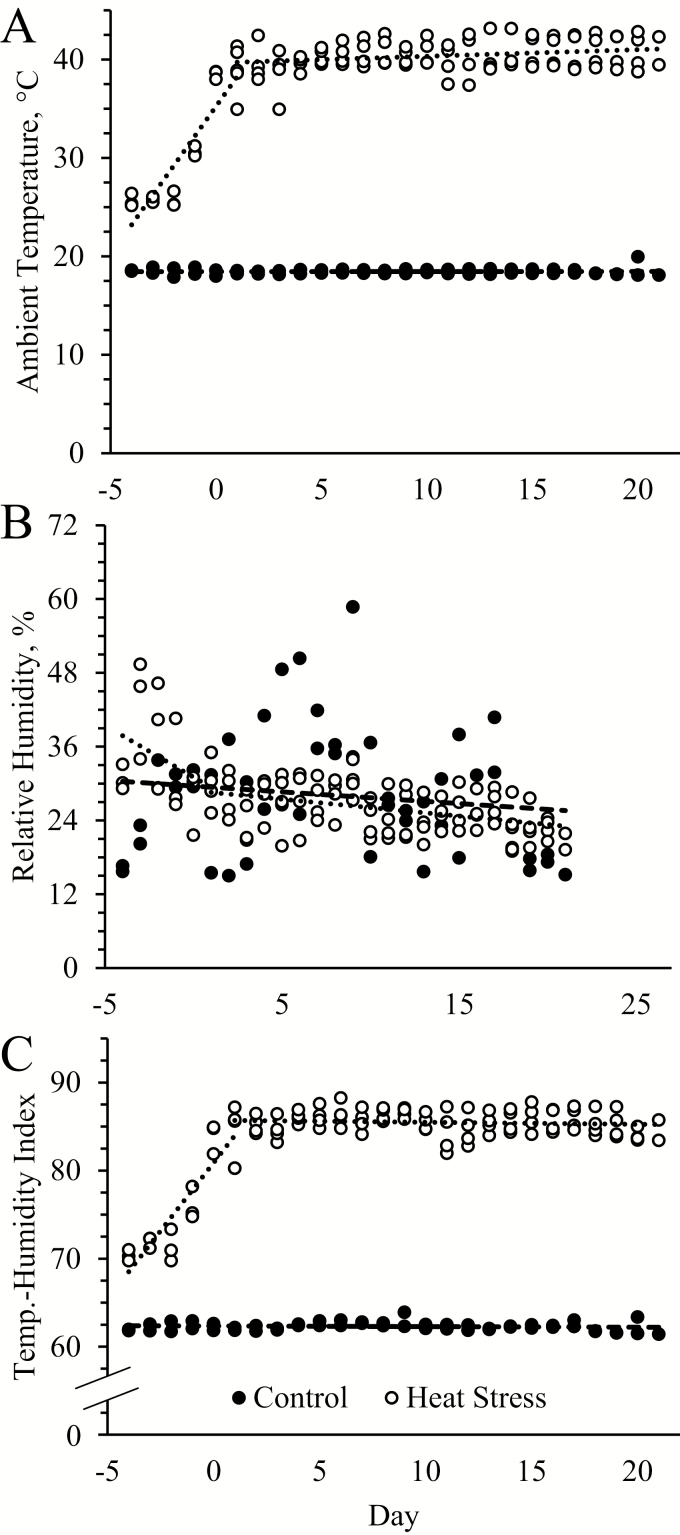
Figure 1.
Environmental conditions for control and heat-stressed lambs over the 21-d feeding period. Maximum daily values and trend lines are shown for ambient temperature (A), relative humidity (B), and temperature–humidity index (C).
Blood Analyses
Blood samples were collected via jugular venipuncture into EDTA vacutainer tubes (~6 mL) and heparinized syringes (~0.4 mL). Additional blood (~6 mL) for transcriptomics not included in this manuscript was also collected. Differential white blood cell (WBC) counts as well as well as red blood cells (RBC), hematocrit, hemoglobin, and platelet concentrations were determined with a HemaTrue Veterinary Chemistry Analyzer (Heska, Loveland, CO) from a 125-μL aliquot of whole blood collected into EDTA tubes. Plasma was isolated from the remaining whole blood in the EDTA tubes by centrifugation (14,000 × g, 5 min). Plasma insulin concentrations were determined in duplicate from 50-μL aliquots of plasma with a commercial ELISA kit (Ovine Insulin, Alpco Diagnostics, Windham, NH) as described previously (Yates et al., 2012). Intra-assay and inter-assay coefficients of variance were less than 10%. Blood concentrations of glucose, lactate, pH, HCO3, Na+, K+, and Ca++ as well as partial pressures of O2 and CO2 (pO2, pCO2) were determined with an ABL90 FLEX blood gas analyzer (Radiometer, Brea, CA) from 90-μL aliquots of whole blood collected in heparinized syringes. Concentrations of blood plasma urea nitrogen (BUN), cholesterol, high-density lipoprotein cholesterol (HDLC), triglycerides, total protein, γ-glutamyltransferase, aspartate aminotransferase, alanine aminotransferase, creatine kinase, lipase, lactate dehydrogenase, and alkaline phosphatase were determined with a Vitros-250 Chemistry Analyzer (Ortho Clinical Diagnostics, Linden, NJ) by the University of Nebraska Biomedical Obesity Research Core.
Skeletal Muscle Fiber Phenotypes
Immunohistochemistry
A 1-cm section from the mid-portion of the right flexor digitorum superficialis (FDS) muscle was fixed in 4% paraformaldehyde in phosphate buffered saline (PBS), embedded in optimal cutting temperature (OCT) Compound, and frozen at −80 °C. Eight-micrometer cross sections were then mounted on Fisherbrand Superfrost Plus microscope slides (Thermo Fisher, Waltham, MA), and immunostaining was performed as described previously (Yates et al., 2016). Briefly, tissues were washed in PBS containing 0.1% Triton-X-100 (Sigma–Aldrich, St. Louis, MO) and then boiled in 10 mM citric acid buffer (pH 6, Sigma–Aldrich) for antigen retrieval. Nonspecific binding was blocked by incubation with 0.5% NEN blocking buffer (Perkin-Elmer, Waltham, MA) for 1 h at room temperature, and primary antiserum diluted in PBS + 1% bovine serum albumin (Gibco Life, Grand Island, NY) was applied overnight at 4 °C. Fiber types were determined with mouse antiserum raised against myosin heavy chain (MyHC)-I (BA-D5, 1:20; DSHB, University of Iowa, Iowa City, IA) and MyHC-II (F18, 1:10; DSHB). Fibers were counterstained with rabbit antiserum raised against desmin (1:200; Sigma–Aldrich). Negative-staining images were produced from sections incubated in PBS + 1% bovine serum albumin without primary antiserum. Immunocomplexes were detected using immunoglobulin antiserum conjugated with either Alexa Fluor 488 (1:1,000; Cell Signaling, Danvers, MA) or Alexa Fluor 594 (1:2,000; Invitrogen Life Technologies, Carlsbad, CA) and visualized on an Olympus IX73 microscope. Images were captured with an Olympus CB5S camera and analyzed with CellSens software (Olympus, Center Valley, PA) to determine fiber cross-sectional areas. Images were encoded to blind the evaluator of animal and experimental group to prevent bias.
Myosin heavy-chain electrophoresis
Protein isolated from the right FDS muscle was used to determine proportions of MyHC-I and MyHC-II in these muscles as described previously (Yates et al., 2016). A protein amount of 15 μg was combined with BioRad 4x Laemmli Sample Buffer to make a 1X solution, which was incubated for 10 min at room temperature, heated to 70 °C for 10 min, and then loaded into a gel at 15 µg/well. The MyHC isoforms were separated by SDS-PAGE. We previously reported the respective compositions of stacking and separating gels (Yates et al., 2016). Electrophoresis was performed on a Mini-PROTEAN Tetra Cell (BioRad Laboratories, Hercules, CA) at 4 °C for 30 h at a constant 100 V. Gels were stained overnight with Gel-Code Blue (Thermo Fisher), destained in distilled water, and imaged on an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE). MyHC-I and collective MyHC-II bands were measured by densitometry (Image Studio Lite Ver 5.2; LI-COR).
Ex Vivo Skeletal Muscle Glucose Metabolism
Flexor digitorum superficialis muscle isolation
At harvest, the left FDS muscle was collected tendon-to-tendon and separated into intact longitudinal strips (51.0 ± 2.7 mg) to measure glucose oxidation as described previously (Cadaret et al., 2017). The strips were washed in ice-cold PBS and then preincubated for 1 h at 37 °C in gassed (95% O2, 5% CO2) Krebs-Henseleit bicarbonate buffer (KHB) containing 0.1% bovine serum albumin, 5 mM D-glucose (Millipore Sigma), and either 0 (basal) or 5 mU/mL insulin (Humulin-R; Ely Lilly, Indianapolis, IN). Muscle strips were then washed in basal or insulin-spiked KHB with no glucose for 20 min at 37 °C.
Glucose oxidation
Skeletal muscle glucose oxidation rates were determined by ex vivo oxidation of radiolabeled glucose as previously described (Cadaret et al., 2017) with some modifications. Muscle strips were placed in one side of sealed dual-well chambers and incubated for 2 h at 37 °C in basal or insulin-spiked KHB with 5 mM [14C-U]-D-glucose (0.25 µCi/mmol; Perkin-Elmer). The adjacent side of the chamber contained 2M NaOH to capture the CO2 produced by the muscle strip. Following the 2-h incubation, chambers were cooled at −20 °C for 2 min, and 2M HCl was injected into the media through the rubber seal to release media-bound CO2. The chambers were incubated for 1 h at 4 °C, and then muscle strips were removed and weighed. The NaOH was collected and mixed with UltimaGold scintillation fluid (Perkin-Elmer) to determine specific activity of 14CO2 via liquid scintillation with a BC 1900 TA LC counter (Beckman-Coulter, Brea, CA). Glucose oxidation in pmol was calculated from dpm counts for 14CO2 using the specific activity of the media, which was determined from the average of six 10-µL aliquots of unused media mixed with 500-µL distilled water and scintillation fluid. Rates were calculated by normalizing the amount of glucose oxidized to the mass of the muscle strip and time in incubation.
Akt phosphorylation
Insulin-signaling responsiveness in muscle strips was estimated from the proportion of phosphorylated Akt to total Akt as described previously (Cadaret et al., 2017). Muscle strips were incubated in either basal or insulin-spiked KHB media for 20 min at 37 °C and then snap frozen in liquid nitrogen and stored at −80 °C. Frozen muscle strips were homogenized in RIPA buffer containing manufacturer-recommended concentrations of protease and phosphatase inhibitors (Thermo Fisher), sonicated for 15 s, and centrifuged (14,000 × g, 5 min, 4 °C). Total protein concentration was determined from the supernatant using a Pierce BCA Protein Assay Kit (Thermo Fisher). A protein amount of 30 μg was combined with BioRad 4X Laemmli Sample Buffer to make a 1X solution, heated for 5 min at 95 °C, and then separated by SDS-PAGE. Gels were transferred to poly-vinylidene fluoride low-fluorescent membranes (BioRad), incubated in Odyssey block buffer (LI-COR) for 1 h at room temperature, and washed with 1X TBS-T (20 mM Tris-HCl + 150 mM NaCl + 0.01% Tween 20). Membranes were incubated with rabbit antiserum raised against Akt (1:1,000; Cell Signaling) or phosphorylated Akt (Ser473) (1:2,000; Cell Signaling) diluted in Odyssey block buffer + 0.05% Tween-20 at 4 °C for 1 h (Akt) or overnight (phosphorylated Akt). The following day, membranes were incubated with goat anti-rabbit IR800 IgG secondary antiserum (LI-COR) diluted in Odyssey block buffer containing 0.05% Tween-20 and 0.01% SDS at room temperature for 1 h. Blots were then scanned with the Odyssey Infrared Imaging System and analyzed with Image Studio Lite Software.
Statistical Analysis
All data except histopathology data were analyzed using the Mixed procedure of SAS 9.4 (SAS Institute, Cary, NC) to determine the effects of environmental condition (n = 24), supplement (n = 16), and their interactions (n = 8) in a 2 × 3 factorial. Lamb was considered the experimental unit, and repeated measures (day) were used for serial measurements, including blood components. Ex vivo data were analyzed with environmental condition and supplement as main effects and type of incubation media as a repeated measure. Glucose oxidation was measured in 6 technical reps/media condition for each lamb, which were then averaged. The average cross-sectional area for fibers of each type in the FDS muscle was determined from a minimum of 250 fibers across 3 nonoverlapping fields of view per lamb. Histopathological data were analyzed for differences due to environmental conditions or dietary supplement by Chi-squared test using the frequency procedure of SAS. Fisher’s exact test was used for frequency analysis in which more than 25% of cells contained expected frequencies of less than 5. These data are presented as frequency of occurrence (%). All other data are presented as means ± standard error. The threshold for significance was α = 0.05.
Results
Growth Metrics and Clinical Indicators
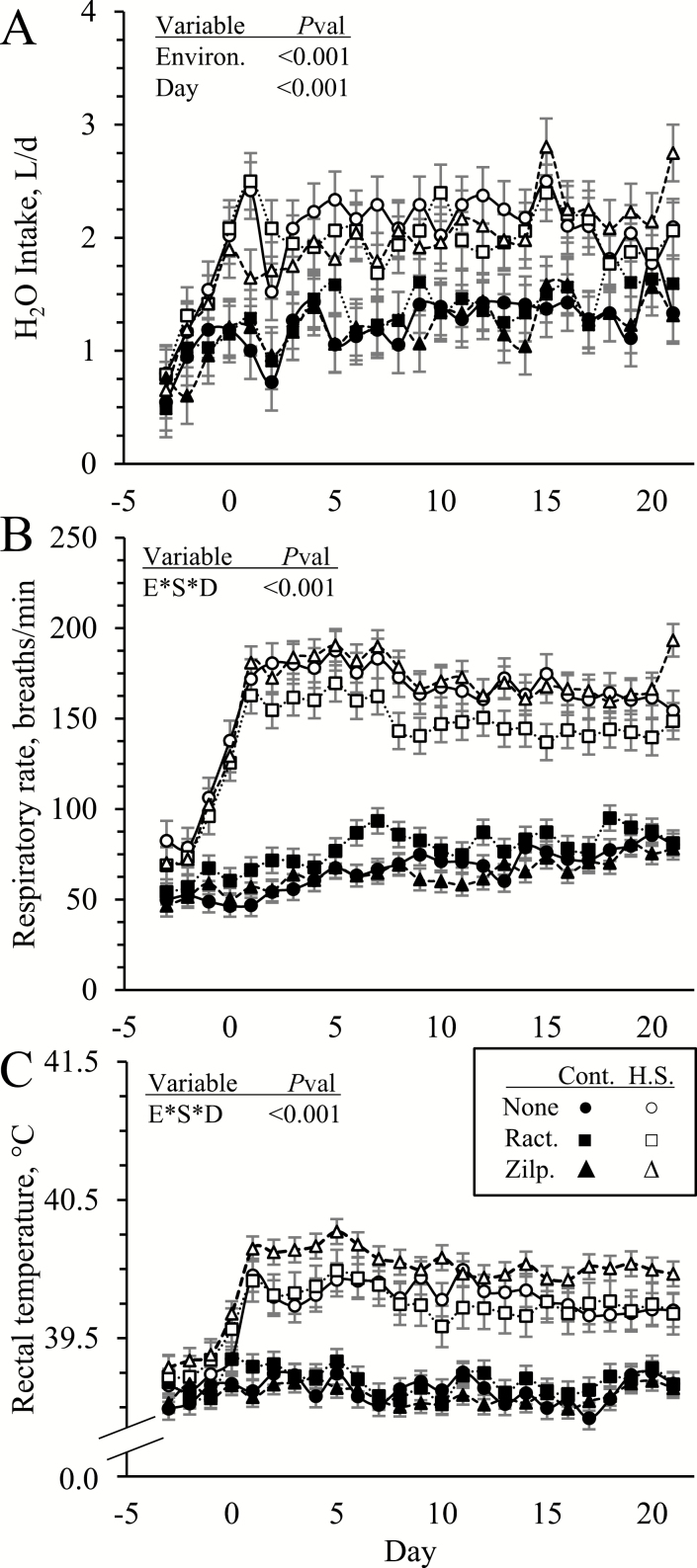
No environmental condition x dietary supplement interactions were observed for initial or final BW, gain-to-feed ratios, or any organ weights (Table 1). Initial BW (49.3 ± 1.8 kg) did not differ between environmental conditions or among supplements but final BW was less (P < 0.05) in heat-stressed lambs than controls (51.8 ± 1.7 vs. 54.4 ± 1.7 kg). Environmental condition x dietary supplement x day interactions were not observed for DMI or H2O intake (Figure 2A). Daily DMI (1.10 ± 0.06 vs. 1.39 ± 0.06 kg/d) was less (P < 0.05) and daily H2O intake (2.02 ± 0.24 vs. 1.23 ± 0.24 L/d) was greater (P < 0.05) in heat-stressed lambs than in controls, but neither differed among supplements. Gain-to-feed ratios for the entire feeding period were less (P < 0.05) in heat-stressed lambs than in controls (0.14 ± 0.01 vs. 0.20 ± .01 kg/kg) but were not affected by supplement. Daily H2O intake also increased (P < 0.05) over time, but DMI did not. Environmental condition x dietary supplement x day interactions were observed (P < 0.05) for respiratory rates and rectal temperatures (Figure 2B and C, respectively). Respiratory rates and rectal temperatures were similar among all lambs initially, as expected, but environmental condition x dietary supplement interactions were observed (P < 0.05) for each day after day 0. In general, heat stress increased (P < 0.05) respiratory rates on all of these days, but to a lesser (P < 0.05) degree in lambs supplemented with ractopamine on most days. Conversely, respiratory rates were greatest (P < 0.05) in control lambs when supplemented with ractopamine. Zilpaterol supplementation did not affect respiratory rates in controls or heat-stressed lambs. Heat stress also increased (P < 0.05) rectal temperatures on all days after day 0, but on most days it did so to a greater (P < 0.05) extent in lambs supplemented with zilpaterol. In controls, rectal temperatures did not differ among supplement groups.
Table 1.
Bodyweights and organ weights in heat-stressed wethers fed concentrate diets supplemented with ractopamine HCl or zilpaterol HCl for 21 d1
| Mass | No Suppl. | Ractopamine | Zilpaterol | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Heat stress | Control | Heat stress | Control | Heat stress | Envir. | Suppl. | E*S | |
| d0 BW, kg | 49.1 ± 1.8 | 47.7 ± 1.8 | 49.8 ± 1.8 | 49.2 ± 1.8 | 50.4 ± 1.8 | 49.5 ± 1.8 | NS | NS | NS |
| d21 BW, kg | 54.3 ± 1.7 | 51.7 ± 1.7 | 54.3 ± 1.7 | 51.0 ± 1.7 | 54.8 ± 1.7 | 52.6 ± 1.7 | 0.005 | NS | NS |
| Heart, g | 265 ± 10 | 264 ± 8 | 260 ± 9 | 259 ± 14 | 264 ± 11 | 241 ± 8 | NS | NS | NS |
| Kidney, g | 139 ± 4 | 133 ± 3 | 126 ± 8 | 134 ± 8 | 139 ± 2 | 138 ± 6 | NS | NS | NS |
| Spleen, g | 101 ± 7 | 91 ± 5 | 112 ± 8 | 113 ± 14 | 106 ± 8 | 97 ± 8 | NS | NS | NS |
| Liver, g | 907 ± 50 | 826 ± 22 | 871 ± 36 | 807 ± 35 | 891 ± 41 | 746 ± 39 | 0.003 | NS | NS |
| Lung, g | 638 ± 50 | 581 ± 25 | 649 ± 46 | 587 ± 19 | 658 ± 30 | 612 ± 42 | 0.07 | NS | NS |
| Rumen, kg | 5.2 ± 0.3 | 5.1 ± 0.3 | 5.6 ± 0.3 | 4.6 ± 0.2 | 5.3 ± 0.3 | 5.6 ± 0.4 | NS | NS | NS |
| GI, kg | 11.5 ± 0.5 | 11.0 ± 0.4 | 11.9 ± 0.5 | 10.0 ± 0.4 | 11.5 ± 0.4 | 11.3 ± 0.4 | 0.01 | NS | NS |
| FDS, g | 57 ± 3 | 54 ± 1 | 56 ± 2 | 55 ± 3 | 62 ± 2 | 55 ± 2 | 0.04 | NS | NS |
| FDS/BW | 1.01 ± 0.04 | 1.05 ± 0.03 | 1.01 ± 0.04 | 1.04 ± 0.04 | 1.18 ± 0.05 | 1.05 ± 0.03 | NS | 0.04 | 0.06 |
1BW = bodyweight; FDS = flexor digitorum superficialis; GI = gastrointestinal tract; NS = not significant.
Figure 2.
Average daily H2O intake (A), respiratory rates (B), and rectal temperatures (C) in heat-stressed lambs fed concentrate diets supplemented with ractopamine or zilpaterol for 21 d. Lambs were fed in a 2 × 3 factorial: Cont./None, unsupplemented controls (n = 9); Cont./Ract., controls supplemented with 0.03996 g/d ractopamine HCl (n = 8); Cont./Zilp., controls supplemented with 0.025 g/d zilpaterol HCl (n = 8); H.S./None, unsupplemented heat-stressed lambs (n = 8); H.S./Ract., heat-stressed lambs supplemented with ractopamine HCl (n = 8); H.S./Zilp., heat-stressed lambs supplemented with zilpaterol (n = 8). Effects of environmental condition (Environ.), dietary supplement (Suppl.), day, and the interaction (E*S*D) are noted when significant (P < 0.05).
Heart, kidney, spleen, lung, and rumen mass at necropsy did not differ between environments or among supplements, but liver mass (793 ± 19 vs. 890 ± 25 g) and total gastrointestinal tract mass (10.8 ± 0.2 vs. 11.6 ± 0.3 kg) were less (P < 0.05) in heat-stressed lambs than controls. Flexor digitorum superficialis muscle mass (55 ± 1 vs. 58 ± 1 g) was less (P < 0.05) in heat-stressed lambs compared with controls, and FDS mass/BW was greater (P < 0.05) in zilpaterol-supplemented lambs than in ractopamine-supplemented or unsupplemented lambs (1.12 ± 0.03, 1.02 ± 0.03, and 1.03 ±0.02 g/kg, respectively). No differences among groups were observed for any organ weights as a fraction of BW.
Histopathological assessment of feet showed that wall overgrowth was present in a greater (P < 0.05) number of heat-stressed lambs than controls (Table 2), but the frequency of occurrence did not differ among dietary supplements. The presence of vacuolated keratinocytes and/or keratohyaline granules in the transition zone of the coronary band did not differ among environmental or supplemental groups. The frequency of pathologies in lung, liver, kidney, and cardiac tissues also did not differ among any groups.
Table 2.
Histopathology of feet and organ tissues from heat-stressed wethers fed concentrate diets supplemented with ractopamine HCl or zilpaterol HCl for 21 d
| Pathology1 | Environment | P | Dietary supplement | P | |||
|---|---|---|---|---|---|---|---|
| Control | Heat stress | No Suppl. | Ractopamine | Zilpaterol | |||
| Foot | |||||||
| Wall overgrowth | 0% | 92% | <0.001 | 27% | 36% | 36% | NS |
| Vacuolated keratinocytes in coronary band | 56% | 42% | NS | 41% | 56% | 50% | NS |
| Lung | |||||||
| Lesions | 4% | 13% | NS | 12% | 6% | 6% | NS |
| Liver | |||||||
| Lesions | 12% | 8% | NS | 6% | 19% | 6% | NS |
| Kidney | |||||||
| Mineralization | 80% | 75% | NS | 82% | 81% | 69% | NS |
| Nephritis | 12% | 8% | NS | 6% | 25% | 0% | NS |
| Cardiac | |||||||
| Sacrocysts | 68% | 75% | NS | 64% | 68% | 81% | NS |
| Lymphocytic aggregates | 48% | 38% | NS | 41% | 38% | 50% | NS |
1Values are % of lambs in each group presenting the pathology. NS = not significant.
Hematology
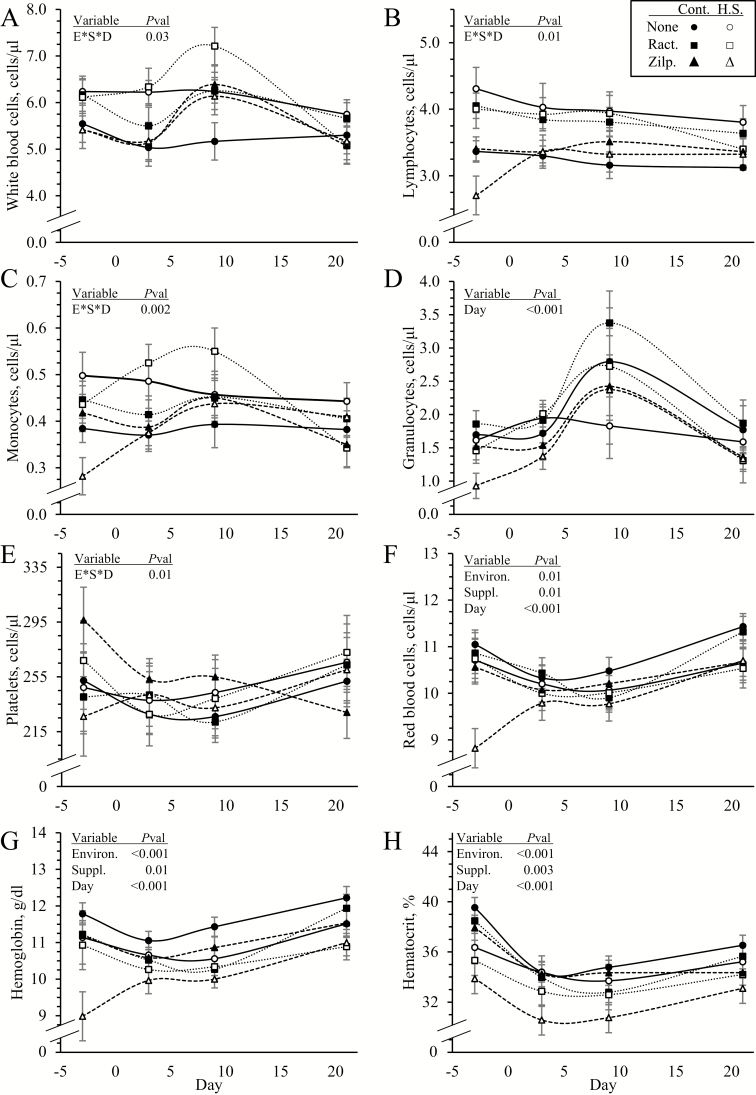
Environmental condition x dietary supplement x day interactions were observed (P < 0.05) for blood concentrations of total WBC but not for any other components of hematology. Circulating WBC concentrations were similar among all groups except on day 3, when they were greater (P < 0.05) in heat-stressed lambs (Figure 3A). Regardless of environmental conditions, WBC concentrations increased (P < 0.05) on day 9 in lambs supplemented with ractopamine or zilpaterol compared with unsupplemented lambs, but returned to normal by day 21. Blood lymphocytes were greater (P < 0.05) in heat-stressed lambs than controls (3.68 ± 0.08 vs. 3.47 ± 0.06 cells/μl) but did not differ among supplements and did not change over time (Figure 3B). Blood monocytes were greater (P < 0.05) in heat-stressed lambs than controls (0.44 ± 0.01 vs. 0.39 ± 0.01 cells/μL) and were highest (P < 0.05) on day 9 in all lambs (Figure 3C). Blood granulocytes did not differ between environmental conditions or among supplements but were highest (P < 0.05) on day 9 in all lambs (Figure 3D). Blood platelets did not differ among any groups but were reduced (P < 0.05) on day 3 in all lambs (Figure 3E). Concentrations of RBC (9.7 ± 0.1 vs. 10.2 ±0.1 cells/μL), hemoglobin (10.6 ± 0.1 vs. 11.3 ± 0.1 g/dL), and hematocrit (33.6 ± 0.4 vs. 35.6 ± 0.3 %) were less (P < 0.05) in heat-stressed lambs than in controls (Figure 3F, G, and H, respectively). Hemoglobin (10.8 ± 0.1 and 10.7 ± 0.1 vs. 11.4 ± 0.1 g/dL) and hematocrit (34.4 ± 0.4 and 33.7 ± 0.4 vs. 35.6 ± 0.4 %) were also reduced (P < 0.05) in ractopamine-supplemented and zilpaterol-supplemented lambs compared with unsupplemented lambs.
Figure 3.
Hematology for heat-stressed lambs fed concentrate diets supplemented with ractopamine or zilpaterol for 21 d. Data are shown for whole blood concentration of total white blood cells (A), lymphocytes (B), monocytes (C), granulocytes (D), platelets (E), red blood cells (F), hemoglobin (G), and hematocrit (H). Lambs were fed in a 2 × 3 factorial: Cont./None, unsupplemented controls (n = 9); Cont./Ract., controls supplemented with 0.03996 g/d ractopamine HCl (n = 8); Cont./Zilp., controls supplemented with 0.025 g/d zilpaterol HCl (n = 8); H.S./None, unsupplemented heat-stressed lambs (n = 8); H.S./Ract., heat-stressed lambs supplemented with ractopamine HCl (n = 8); H.S./Zilp., heat-stressed lambs supplemented with zilpaterol (n = 8). Effects of environmental condition (Environ.), dietary supplement (Suppl.), day, and the interaction (E*S*D) are noted when significant (P < 0.05).
Blood Insulin, Metabolites, and Gases
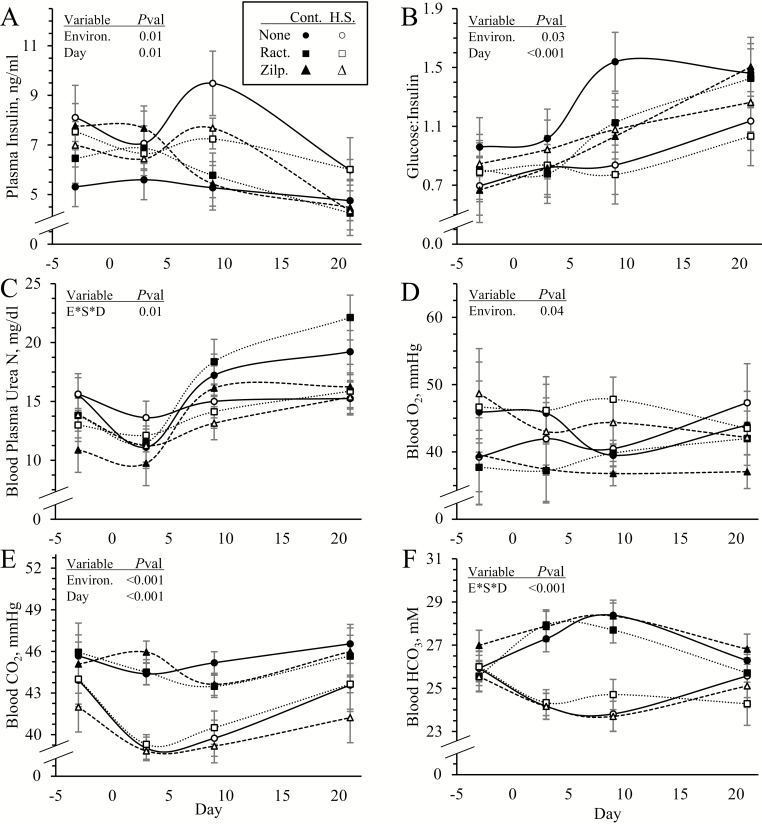
An environmental condition x dietary supplement x day interaction was observed (P < 0.05) for BUN and HCO3 but not for plasma insulin, blood glucose, glucose-to-insulin ratio, lactate, or blood gases. Plasma insulin (6.46 ± 0.37 vs. 5.30 ± 0.27 ng/mL) was greater (P < 0.05) and glucose-to-insulin ratios (0.92 ±0.06 vs. 1.09 ± 0.06) were less (P < 0.05) in heat-stressed lambs than controls but did not differ among supplements (Figure 4A and B, respectively). Blood glucose (4.5 ± 0.1 mM), lactate (1.22 ± 0.14 mM), and pH (7.396 ± 0.005) did not differ among any groups. BUN concentrations were greater (P < 0.05) in heat-stressed lambs on day 3 and less (P < 0.05) on days 9 and 21 than controls (Figure 4C). Blood cholesterol (57.5 ± 2.4 mg/dL), HDLC (35.3 ± 1.1 mg/dL), triglyceride (16.6 ± 0.68 mg/dL), and total protein (7.61 ± 0.13 g/dL) concentrations did not differ among groups. Blood pO2 (44.3 ± 1.6 vs. 40.2 ±1.1 mmHg) was greater (P < 0.05) and CO2 (41.2 ± 0.5 vs. 45.2 ± 0.4 mmHg) was less (P < 0.05) in heat-stressed lambs than controls, regardless of supplement or day (Figure 4D and E, respectively). Blood HCO3 did not differ among groups on day −3 but was less (P < 0.05) in heat-stressed lambs than controls on days 3, 9, and 21, regardless of supplement (Figure 4F).
Figure 4.
Circulating insulin and metabolite concentrations in heat-stressed lambs fed concentrate diets supplemented with ractopamine or zilpaterol for 21 d. Data are shown for blood plasma insulin concentrations (A), glucose-to-insulin ratios (B), blood plasma urea nitrogen (C), whole blood O2 partial pressures (D), whole blood CO2 partial pressures (E), and whole blood HCO3 concentrations (F). Lambs were fed in a 2 × 3 factorial: Cont./None, unsupplemented controls (n = 9); Cont./Ract., controls supplemented with 0.03996 g/d ractopamine HCl (n = 8); Cont./Zilp., controls supplemented with 0.025 g/d zilpaterol HCl (n = 8); H.S./None, unsupplemented heat-stressed lambs (n = 8); H.S./Ract., heat-stressed lambs supplemented with ractopamine HCl (n = 8); H.S./Zilp., heat-stressed lambs supplemented with zilpaterol (n = 8). Effects of environmental condition (Environ.), dietary supplement (Suppl.), day, and the interaction (E*S*D) are noted when significant (P < 0.05).
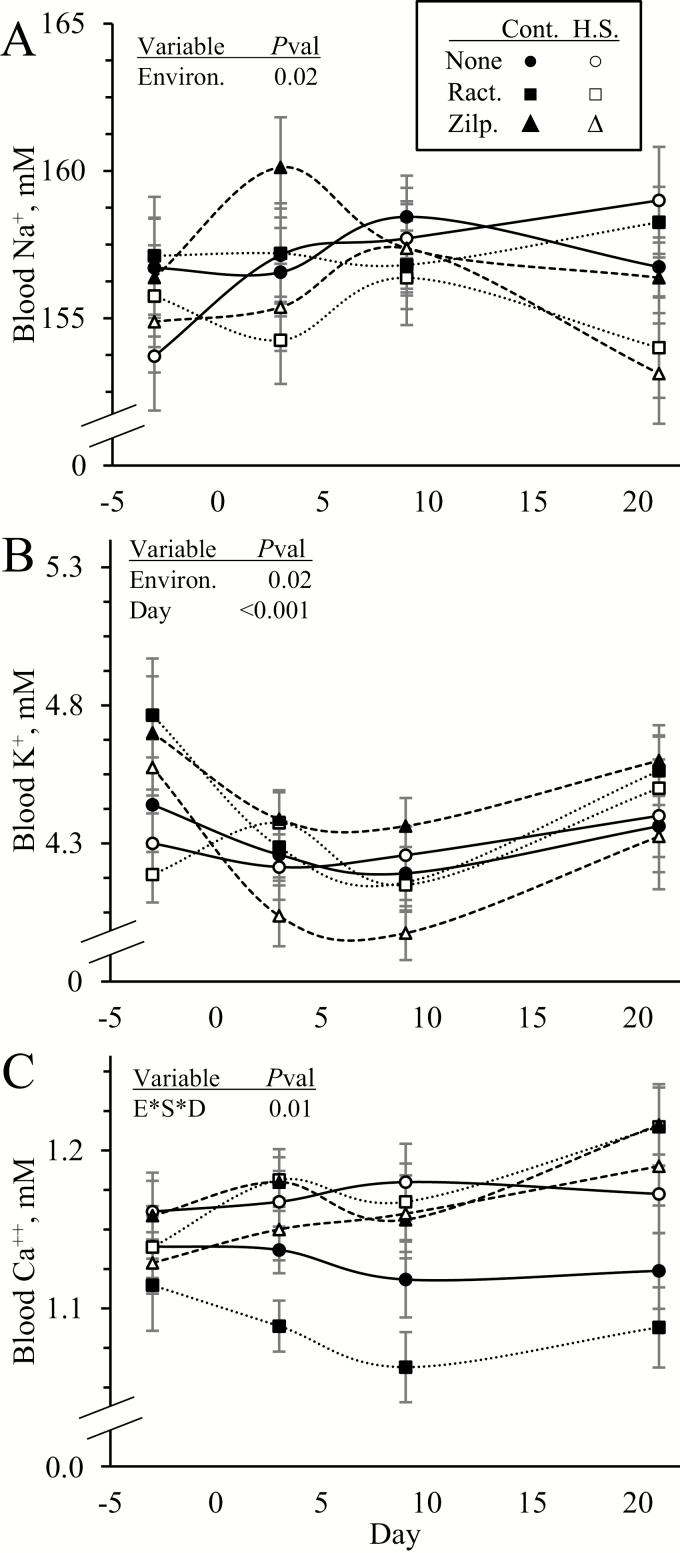
An environmental condition x dietary supplement x day interaction was observed (P < 0.05) for blood Ca++ concentration but not for blood Na+ or K+. Blood Na+ and K+ concentrations were less (P < 0.05) in heat-stressed lambs than controls but did not differ among supplements (Figure 5A and B, respectively). Blood Ca++ concentrations were greater (P < 0.05) in heat-stressed lambs than controls on day 9 regardless of supplement (Figure 5C). On day 21, heat stress increased (P < 0.05) blood Ca++ in unsupplemented lambs, ractopamine supplementation increased (P < 0.05) it in heat-stressed lambs only, and zilpaterol supplementation increased (P < 0.05) it in controls only.
Figure 5.
Circulating electrolyte concentrations in heat-stressed lambs fed concentrate diets supplemented with ractopamine or zilpaterol for 21 d. Data are shown for whole blood concentrations of Na+ (A), K+ (B), and Ca++ (C). Lambs were fed in a 2 × 3 factorial: Cont./None, unsupplemented controls (n = 9); Cont./Ract., controls supplemented with 0.03996 g/d ractopamine HCl (n = 8); Cont./Zilp., controls supplemented with 0.025 g/d zilpaterol HCl (n = 8); H.S./None, unsupplemented heat-stressed lambs (n = 8); H.S./Ract., heat-stressed lambs supplemented with ractopamine HCl (n = 8); H.S./Zilp., heat-stressed lambs supplemented with zilpaterol (n = 8). Effects of environmental condition (Environ.), dietary supplement (Suppl.), day, and the interaction (E*S*D) are noted when significant (P < 0.05).
Blood Enzymes
No environmental condition x dietary supplement x day interactions were observed for any blood enzymes. Plasma γ-glutamyltransferase (127.4 ± 5.2 IU/L), aspartate aminotransferase (26.3 ± 1.2 IU/L), alanine aminotransferase (34.4 ± 0.8 IU/L), creatine kinase (131.6 ± 16.3 IU/L), and alkaline phosphatase (16.8 ± 0.9 IU/L) concentrations did not differ among groups. Plasma lipase (164 ± 8 vs. 198 ± 11 IU/L) and blood lactate dehydrogenase (625 ± 13 vs. 673 ± 14 IU/L) were less (P < 0.05) in heat-stressed lambs than controls regardless of dietary supplement or day.
Skeletal Muscle Fiber Profile and Metabolism
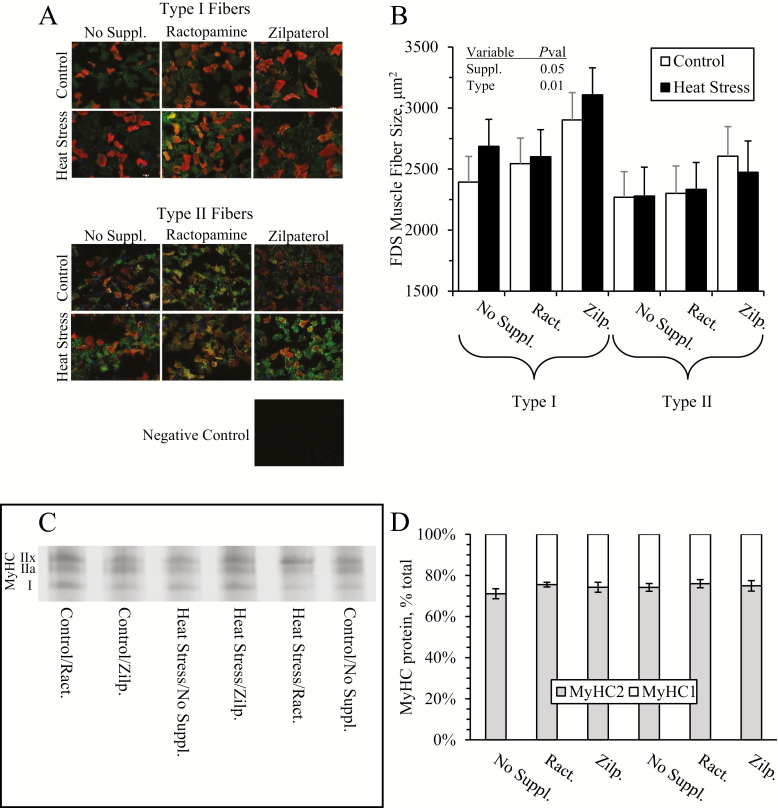
Representative micrographs for immunohistochemistry of FDS fibers are shown in Figure 6A. Cross-sectional fiber areas were greater (P < 0.05) in zilpaterol-supplemented lambs than in ractopamine-supplemented or unsupplemented lambs (2980 ± 130 vs. 2472 ± 230 and 2627 ± 120 μm2, respectively), regardless of environmental condition or fiber type (Figure 6B). Representative gels for SDS-PAGE MyHC separation are shown in Figure 6C. The number of Type I and Type II fibers and the proportion of MyHC-I to MyHC-II protein in the FDS muscle did not differ among groups (Figure 6D).
Figure 6.
Skeletal muscle fiber profiles in heat-stressed lambs fed concentrate diets supplemented with ractopamine or zilpaterol for 21 d. Representative micrographs are shown for type I and type II fibers of the flexor digitorum superficialis (FDS) muscle (A), and data are shown for average fiber area (B). Representative electrophoresis gels are shown for myosin heavy-chain (MyHC) types (C), and data are shown for % of total MyHC (D). Lambs were fed in a 2 × 3 factorial: Cont./No Suppl., unsupplemented controls (n = 9); Cont./Ract., controls supplemented with 0.03996 g/d ractopamine HCl (n = 8); Cont./Zilp., controls supplemented with 0.025 g/d zilpaterol HCl (n = 8); H.S./No Suppl., unsupplemented heat-stressed lambs (n = 8); H.S./Ract., heat-stressed lambs supplemented with ractopamine HCl (n = 8); H.S./Zilp., heat-stressed lambs supplemented with zilpaterol (n = 8). Effects of environmental condition (Environ.), dietary supplement (Suppl.), fiber type, and the interaction are noted when significant (P < 0.05).
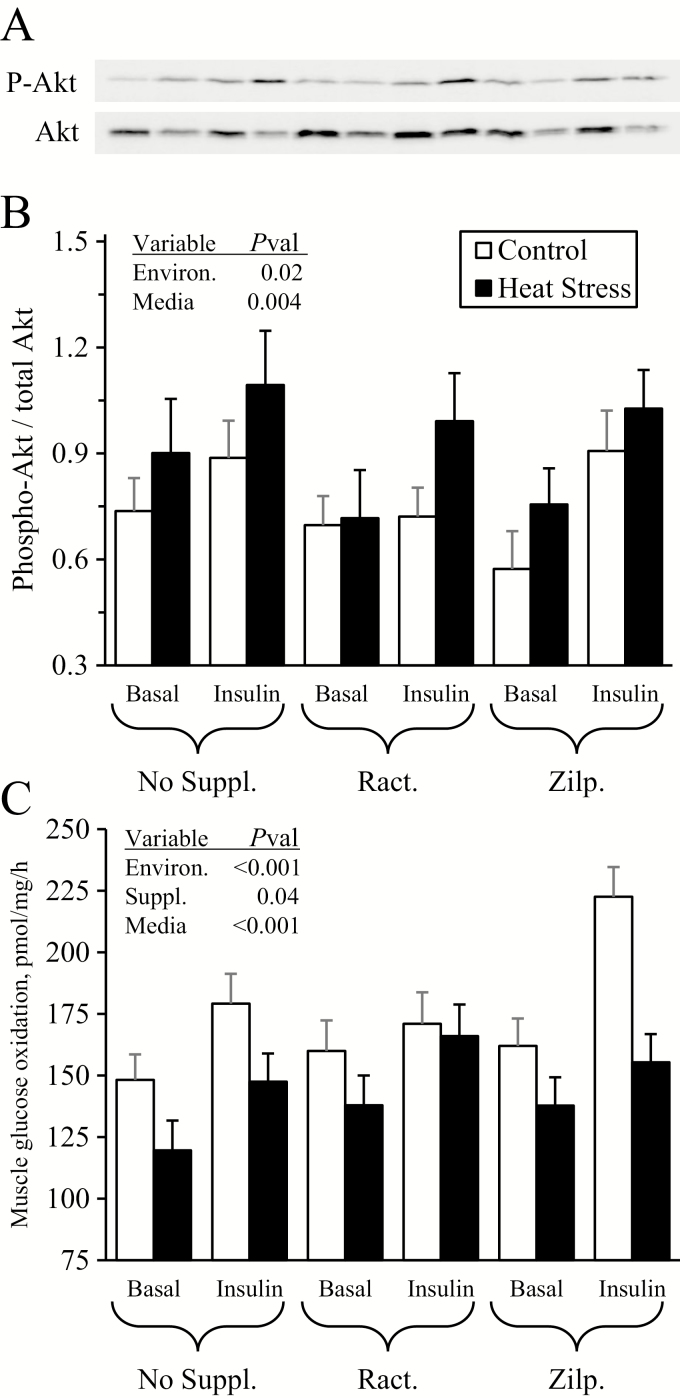
No environmental condition x dietary supplement x media interactions were observed for Akt phosphorylation or for glucose oxidation rates. Representative gels for phosphorylated and total Akt are shown in Figure 7A. Incubation of FDS muscle strips in media spiked with insulin increased (P < 0.05) phosphorylated Akt/total Akt ratios by ~29% over incubation in basal media (0.94 ± 0.05 vs. 0.73 ± 0.05), regardless of environment or supplement (Figure 7B). Phosphorylated Akt/total Akt ratios were ~18% greater (P < 0.05) in muscle from heat-stressed lambs than from controls (0.91 ± 0.05 vs. 0.75 ± 0.04), regardless of supplement or incubation media. Incubation of muscle in media spiked with insulin increased (P < 0.05) glucose oxidation rates by ~21% over muscle incubated in basal media (174 ± 5 vs. 144 ± 5 pmol/mg/h), regardless of experimental groups (Figure 7C). Glucose oxidation rates were ~17% less (P < 0.05) in muscle from heat-stressed lambs compared with controls (144 ± 4.3 vs. 174 ± 5.3 pmol/mg/h) and ~15% greater (P < 0.05) in muscle from zilpaterol-supplemented lambs than from unsupplemented lambs (170 ± 6 vs. 149 ± 6 pmol/mg/h, ractopamine-supplemented = 159 ± 6 pmol/mg/h).
Figure 7.
Ex vivo skeletal muscle metabolic function for heat-stressed lambs fed concentrate diets supplemented with ractopamine or zilpaterol for 21 d. Representative micrographs are shown for total and phosphorylated Akt in flexor digitorum superficialis (FDS) muscle incubated with insulin (A), and data are shown for the ratio of phosphorylated Akt/total Akt (C) and for glucose oxidation rates (D). Lambs were fed in a 2 × 3 factorial: Cont./None, unsupplemented controls (n = 9); Cont./Ract., controls supplemented with 0.03996 g/d ractopamine HCl (n = 8); Cont./Zilp., controls supplemented with 0.025 g/d zilpaterol HCl (n = 8); H.S./None, unsupplemented heat-stressed lambs (n = 8); H.S./Ract., heat-stressed lambs supplemented with ractopamine HCl (n = 8); H.S./Zilp., heat-stressed lambs supplemented with zilpaterol (n = 8). Effects of environmental condition (Environ.), dietary supplement (Suppl.), culture media, and the interaction are noted when significant (P < 0.05).
Discussion
In this study, we found that sustained heat stress diminished muscle growth and metabolic efficiency and increased stress indicators in feedlot lambs. Over the course of 3 wk, heat-stressed lambs consumed less, grew slower, and exhibited dysregulated insulin/glucose homeostasis. They also presented greater body temperatures, respiratory rates, and circulating leukocyte concentrations along with diminished red blood cell, hemoglobin, and hematocrit concentrations. By the end of the 3-wk period, heat-stressed lambs not only weighed less but produced lighter FDS muscles. They also exhibited disruptions in muscle-specific insulin signaling and glucose oxidative metabolism. We observed minimal interacting or cumulative effects of heat stress and β agonist supplements, as outcomes of heat stress were generally similar whether lambs were fed ractopamine, zilpaterol, or no supplement. Muscle growth and metabolic efficiency were improved by supplementation with zilpaterol but not ractopamine, and neither β agonist increased the prevalence of diagnosed pathologies in feet or organ tissue specimens. Together, these findings indicate that the detrimental impact of heat stress on performance and wellbeing in feedlot lambs was not confounded by the concurrent supplementation of ractopamine or zilpaterol. Likewise, the benefit of zilpaterol on muscle growth and metabolic efficiency was not diminished by the presence of heat stress.
Reduced weight gain in heat-stressed lambs after 21 d on feed can be attributed in part to suppressed feed intake. The ~21% reduction in DMI for lambs housed at an average THI of 84 (~40 °C, 35% RH) compared with those housed at a THI of 63 (~19 °C, 35% RH) aligned well with the 19.9% reduction predicted by Terry Mader’s DMI/THI algorithms for confined beef cattle (Mader, 2003). Additionally, direct effects of heat stress on metabolic function appeared to further contribute to the 36% reduction in growth. Greater circulating insulin concentrations relative to glycemic levels throughout the heat stress period were indicative of reduced insulin sensitivity for glucose clearance, which we previously observed during acute inflammatory responses in sheep (Yates et al., 2011). However, the paradoxical increases in basal and insulin-stimulated Akt phosphorylation in muscle from heat-stressed lambs show that this was not due to disruption of the canonical insulin/Akt signaling pathway at this major hub. In fact, greater skeletal muscle insulin sensitivity for Akt phosphorylation in this study and a previous study of heat-stressed dairy cows (Xie et al., 2016) may reflect compensatory responses to disruptions downstream from Akt, especially since short-term heat stress reduced phosphorylated Akt in pigs (Ganesan et al., 2018), although the outputs of the present study limit this to speculation. Despite greater insulin/Akt coupling, glucose oxidation rates were reduced by almost 18% in muscle isolated from heat-stressed lambs at harvest. Impaired skeletal muscle glucose oxidation reveals a key mechanism by which heat stress reduces metabolic efficiency in feedlot animals that, to the best of our knowledge, has not been previously demonstrated. This cannot be attributed to a proportional reduction in oxidative muscle fibers, as fiber type population did not change with heat stress. Rather, the circulating leukocyte profiles observed in this study lead us to speculate that the effects of heat stress on glucose metabolism may have been mediated by inflammation. Ganesan et al. (2016) found that exposure to heat stress increased NFκB-mediated inflammatory activity in skeletal muscle of pigs, and we recently found that acute stimulation by inflammatory cytokines impaired glucose oxidation in primary rat muscle (Cadaret et al., 2017). Cytokine concentrations were not measured in the present study, but greater circulating leukocytes, particularly monocytes, implicate systemic inflammation as a potential mechanism for impaired glucose oxidation in heat-stressed lambs. Although on a much shorter timeframe and in pigs, Montilla et al. (2014) showed evidence that heat stress effects on muscle may also be mediated by oxidative stress.
Greater BUN concentrations on day 3 of heat stress indicated that our lambs’ initial response included a spike in protein catabolism, presumably to support energy homeostasis as observed in heat-stressed dairy cows (Gao et al., 2017). By day 9, however, BUN concentrations were no longer increased and were in fact decreased in our heat-stressed lambs, which could represent differential responses to acute and chronic heat stress. Greater apparent protein catabolism in heat-stressed lambs was not surprising, especially considering their marked reduction in DMI, but the transient nature was unexpected. Moreover, it is unclear what if any mechanisms for energy production replaced it, as we did not observe evidence of greater lipid utilization or an increase in DMI. It is perhaps possible that glucose metabolism was more severely impaired in the first few days of the heat stress period than at harvest, necessitating protein catabolism to meet energy needs early on but not later in the study. We cannot discount this as a possibility, as we only assessed glucose metabolism at the conclusion of the 3-wk period. Nevertheless, even transient protein catabolism helps us to explain the disproportionate effect of heat stress on FDS muscle mass and liver mass, which accounted for a greater proportion of the disparity in BW between heat-stressed and control lambs than other organs.
Dietary supplementation of zilpaterol for 3 wk increased both basal and insulin-stimulated glucose oxidation in skeletal muscle isolated at harvest by ~21%. This was consistent with our previous findings showing that acute incubation of skeletal muscle with zilpaterol in vitro stimulated glucose oxidation rates (Cadaret et al., 2017). It also demonstrates that improved metabolic efficiency is one mechanism by which the β2 agonist increases feedlot performance. Zilpaterol-supplemented lambs also exhibited larger FDS muscles relative to their final BW, although final BW itself was not different. This recapitulates a recent study’s observation of greater longissimus muscle growth in lambs supplemented with zilpaterol for 33 d (Rivera-Villegas et al., 2019), despite our much shorter supplementation period. Furthermore, the greater cross-sectional size of FDS muscle fibers in our zilpaterol-supplemented lambs shows that stimulating fiber hypertrophy is another mechanism by which the β2 agonist improves feedlot performance. It is also worth noting that the benefits of zilpaterol on muscle growth and glucose oxidation were observed in lambs fed under thermoneutral or heat-stress conditions, despite the independent effects of heat stress on these outcomes. Ractopamine, on the other hand, failed to improve muscle growth or glucose oxidation under either environmental condition, which is consistent with our previous observations during acute stimulation (Cadaret et al., 2017). It also indicates that the β1 agonist improves performance via an alternative mechanism not identified in this study. Neither β agonist affected any indicators of lipid metabolism measured in this study, which was somewhat surprising since zilpaterol in particular has been shown to reduce fat mass in lambs and cattle (Judy Walter et al., 2018; Rivera-Villegas et al., 2019).
Heat stress imposed extensive changes in stress indicators in feedlot lambs as expected, but the stress responses to β agonist supplementation were minimal and typically independent of heat stress. In fact, the only 2 variables for which the effects of heat stress and β agonist supplementation interacted were body temperature and respiratory rates. Core body (rectal) temperatures were increased by heat stress in all supplement groups, but the increase was ~0.3 °C greater in magnitude in those receiving zilpaterol. This was somewhat surprising, as zilpaterol supplementation actually reduced average and maximum body temperatures in feedlot steers under mild heat stress or thermoneutral conditions (Boyd et al., 2015). In fact, body temperature in heifers fed zilpaterol under thermoneutral conditions remained ~0.1 °C lower even 4 d after withdrawing the supplement (Buntyn et al., 2016). The reason for the differences between the present and previous studies is not clear, but we speculate that it could be due to general differences in fat content and relative ages/maturity between feedlot sheep and cattle. Respiratory rates were increased by heat stress in all supplement groups, but to a lesser extent in those receiving ractopamine, which reflected ractopamine’s effects on respiration in feedlot cattle in the absence of heat stress (Hagenmaier et al., 2017). Hyperventilation can affect wellbeing by reducing blood CO2 concentrations, which if low enough can in turn increase blood pH (Entin et al., 1998). Although hypocapnia was observed in our heat-stressed lambs, it was not severe enough to elicit blood alkalosis. Hyperventilation by heat-stressed lambs also increased blood O2 partial pressure by ~9% throughout the 3-wk period, which likely contributed to reductions in RBC and hemoglobin concentrations. In addition, thermoregulation in sheep involves sweating (Godfrey et al., 2017), which in humans is a major mechanism for H2O and electrolyte loss (Baker, 2017). The almost 70% increase in H2O intake by our heat-stressed sheep appeared to compensate for dehydration, which is reflected in their slightly lower hematocrit values, but did not replenish blood Na+ or K+ concentrations.
Heat stress resulted in greater circulating concentrations of agranulocytic leukocytes but not granulocytes or platelets, which was consistent with observations in cattle exposed to long-term heat stress (Wegner et al., 1976; Brown-Brandl et al., 2017) but not short-term heat stress (Pandey et al., 2017). The increased monocyte concentrations observed in our study would help us to explain previously reported increases in circulating inflammatory cytokine concentrations in heat-stressed cattle (Zhang et al., 2014). However, blood lymphocytes were also increased, which leads us to speculate that agranulocyte populations may have been increased to facilitate phagocytosis of damaged cells and debris from heat stress-damaged tissues. Indeed, leukocytes isolated from sheep exposed to 10-d heat stress were previously found to exhibit greater phagocytic activity (Saker et al., 2004). Although circulating concentrations of blood enzymes and professional histopathological assessments did not indicate widespread tissue damage, increases in circulating lipase and LDH observed in our heat-stressed lambs have been associated with both systemic inflammation and damage of pancreatic tissues (Manjuck et al., 2005; Ismail and Bhayana, 2017; Yu et al., 2017).
We conclude from this study that heat stress reduces feedlot performance in large part by impairing hypertrophic muscle growth and by reducing skeletal muscle glucose oxidation, an important factor in metabolic efficiency. Zilpaterol supplementation over the same 3-wk period improved both muscle hypertrophy and glucose oxidation, and these benefits occurred independently of the impact of heat stress. The lack of interacting effects on muscle was unexpected considering that both heat stress and β agonists stimulate β adrenergic pathways. This leads us to speculate that inflammation plays a larger role in heat stress deficits than previously recognized, although we cannot ignore potential roles for the less-prevalent β3 or α1D adrenergic pathways or for other stress-mediating pathways. Perhaps most importantly, our comprehensive assessment of health and wellbeing indicators, which included evaluations of feet and organ tissues by board-certified veterinary pathologists, showed no evidence that zilpaterol or ractopamine supplementation for 3 wk increased the risk for organ/tissue injury or foot lameness, nor did either supplement increase the impact of heat stress on indicators of health and wellbeing. Together, these findings demonstrate important mechanisms by which heat stress impedes feedlot performance, as well as how β2 agonists improve performance. Future studies will allow us to more comprehensively determine the extent to which inflammation mediates the effects of heat stress and to identify additional potential mechanisms that may provide targets for improving outcomes in heat-stressed animals.
Conflict of interest statement
None declared.
Literature Cited
- Avendaño-Reyes L., Macías-Cruz U., Alvarez-Valenzuela F. D., Aguila-Tepato E., Torrentera-Olivera N. G., and Soto-Navarro S. A.. . 2011. Effects of zilpaterol hydrochloride on growth performance, carcass characteristics, and wholesale cut yield of hair-breed ewe lambs consuming feedlot diets under moderate environmental conditions. J. Anim. Sci. 89:4188–4194. doi: 10.2527/jas.2011-3904 [DOI] [PubMed] [Google Scholar]
- Baker L. B. 2017. Sweating rate and sweat sodium concentration in athletes: a review of methodology and intra/interindividual variability. Sports Med. 47(Suppl 1):111–128. doi: 10.1007/s40279-017-0691-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boler D. D., Shreck A. L., Faulkner D. B., Killefer J., McKeith F. K., Homm J. W., and Scanga J. A.. . 2012. Effect of ractopamine hydrochloride (optaflexx) dose on live animal performance, carcass characteristics and tenderness in early weaned beef steers. Meat Sci. 92:458–463. doi: 10.1016/j.meatsci.2012.05.011 [DOI] [PubMed] [Google Scholar]
- Boyd B. M., Shackelford S. D., Hales K. E., Brown-Brandl T. M., Bremer M. L., Spangler M. L., Wheeler T. L., King D. A., and Erickson G. E.. . 2015. Effects of shade and feeding zilpaterol hydrochloride to finishing steers on performance, carcass quality, heat stress, mobility, and body temperature. J. Anim. Sci. 93:5801–5811. doi: 10.2527/jas.2015-9613 [DOI] [PubMed] [Google Scholar]
- Brown-Brandl T. M., Chitko-McKown C. G., Eigenberg R. A., Mayer J. J., Welsh T. H., Davis J. D., and Purswell J. L.. . 2017. Physiological responses of feedlot heifers provided access to different levels of shade. Animal 11:1344–1353. doi: 10.1017/S1751731116002664 [DOI] [PubMed] [Google Scholar]
- Brown-Brandl T.M., Nienaber J.A., Eigenberg R.A., Hahn G.L., and Freetly H.. . 2003. Thermoregulatory responses of feeder cattle. J. Ther. Biol. 28:149–57. 10.1016/S0306-4565(02)00052-9 [DOI] [PubMed] [Google Scholar]
- Buntyn J. O., Burdick Sanchez N. C., Schmidt T. B., Erickson G. E., Sieren S. E., Jones S. J., and Carroll J. A.. . 2016. The metabolic, stress axis, and hematology response of zilpaterol hydrochloride supplemented beef heifers when exposed to a dual corticotropin-releasing hormone and vasopressin challenge. J. Anim. Sci. 94:2798–2810. doi: 10.2527/jas.2015-0192 [DOI] [PubMed] [Google Scholar]
- Buntyn J. O., Steffen D., Sanchez N. C. B., Sieren S. E., Jones S. J., Erickson G. E., Carroll J. A., and Schmidt T. B.. . 2017. Serum blood metabolite response and evaluation of select organ weight, histology, and cardiac morphology of beef heifers exposed to a dual corticotropin-releasing hormone and vasopressin challenge following supplementation of zilpaterol hydrochloride. J. Anim. Sci. 95:5327–5338. doi: 10.2527/jas2017.1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadaret C. N., Beede K. A., Riley H. E., and Yates D. T.. . 2017. Acute exposure of primary rat soleus muscle to zilpaterol hcl (β2 adrenergic agonist), tnfα, or IL-6 in culture increases glucose oxidation rates independent of the impact on insulin signaling or glucose uptake. Cytokine. 96:107–113. doi: 10.1016/j.cyto.2017.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dávila-Ramírez J. L., Macías-Cruz U., Torrentera-Olivera N. G., González-Ríos H., Peña-Ramos E. A., Soto-Navarro S. A., and Avendaño-Reyes L.. . 2015. Feedlot performance and carcass traits of hairbreed ewe lambs in response to zilpaterol hydrochloride and soybean oil supplementation. J. Anim. Sci. 93:3189–3196. doi: 10.2527/jas.2014-8723 [DOI] [PubMed] [Google Scholar]
- Delmore R.J., Hodgen J.M., and Johnson B.J.. . 2010. Perspectives on the application of zilpaterol hydrochloride in the United States beef industry. J. Anim. Sci. 88:2825–8. doi:10.2527/jas.2009–2473 [DOI] [PubMed] [Google Scholar]
- Elam N. A., Vasconcelos J. T., Hilton G., VanOverbeke D. L., Lawrence T. E., Montgomery T. H., Nichols W. T., Streeter M. N., Hutcheson J. P., Yates D. A., . et al. 2009. Effect of zilpaterol hydrochloride duration of feeding on performance and carcass characteristics of feedlot cattle. J. Anim. Sci. 87:2133–2141. doi: 10.2527/jas.2008-1563 [DOI] [PubMed] [Google Scholar]
- Entin P.L., Robertshaw D., and Rawson R.E.. . 1998. Thermal drive contributes to hyperventilation during exercise in sheep. J. Appl. Physiol. (1985). 85:318–25. 10.1152/jappl.1998.85.1.318 [DOI] [PubMed] [Google Scholar]
- Ganesan S., Reynolds C., Hollinger K., Pearce S. C., Gabler N. K., Baumgard L. H., Rhoads R. P., and Selsby J. T.. . 2016. Twelve hours of heat stress induces inflammatory signaling in porcine skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310:R1288–R1296. doi: 10.1152/ajpregu.00494.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan S., Summers C. M., Pearce S. C., Gabler N. K., Valentine R. J., Baumgard L. H., Rhoads R. P., and Selsby J. T.. . 2018. Short-term heat stress altered metabolism and insulin signaling in skeletal muscle. J. Anim. Sci. 96:154–167. doi: 10.1093/jas/skx083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S. T., Guo J., Quan S. Y., Nan X. M., Fernandez M. V. S., Baumgard L. H., and Bu D. P.. . 2017. The effects of heat stress on protein metabolism in lactating holstein cows. J. Dairy Sci. 100:5040–5049. doi: 10.3168/jds.2016-11913 [DOI] [PubMed] [Google Scholar]
- Godfrey R. W., Preston W. D., Joseph S. R., LaPlace L., Hillman P. E., Gebremedhin K. G., Lee C. N., and Collier R. J.. . 2017. Evaluating the impact of breed, pregnancy, and hair coat on body temperature and sweating rate of hair sheep ewes in the tropics. J. Anim. Sci. 95:2936–2942. doi: 10.2527/jas.2016.1125 [DOI] [PubMed] [Google Scholar]
- Grandin T. 2014. Animal welfare and society concerns finding the missing link. Meat Sci. 98:461–9. 10.1016/j.meatsci.2014.05.011 [DOI] [PubMed] [Google Scholar]
- Grandin T. 2017. On-farm conditions that compromise animal welfare that can be monitored at the slaughter plant. Meat Sci. 132:52–58. doi: 10.1016/j.meatsci.2017.05.004 [DOI] [PubMed] [Google Scholar]
- Hagenmaier J. A., Reinhardt C. D., Bartle S. J., and Thomson D. U.. . 2016. Effect of shade on animal welfare, growth performance, and carcass characteristics in large pens of beef cattle fed a beta agonist in a commercial feedlot. J. Anim. Sci. 94:5064–5076. doi: 10.2527/jas.2016-0935 [DOI] [PubMed] [Google Scholar]
- Hagenmaier J.A., Reinhardt C.D., Ritter M.J., Calvo-Lorenzo M.S., Vogel G.J., Guthrie C.A., Siemens M.G., Lechtenberg K.F., Rezac D.J., and Thomson D.U.. . 2017. Effects of ractopamine hydrochloride on growth performance, carcass characteristics, and physiological response to different handling techniques. J. Anim. Sci. 95:1977–92. 10.2527/jas.2016.0936 [DOI] [PubMed] [Google Scholar]
- Hahn G. L. 1999. Dynamic responses of cattle to thermal heat loads. J. Anim. Sci. 77(Suppl 2):10–20. doi: 10.2527/1997.77suppl_210x [DOI] [PubMed] [Google Scholar]
- Ismail O.Z., and Bhayana V.. . 2017. Lipase or amylase for the diagnosis of acute pancreatitis? Clin. Biochem. 50:1275–80. 10.1016/j.clinbiochem.2017.07.003 [DOI] [PubMed] [Google Scholar]
- Judy Walter L. A., Schmitz A. N., Nichols W. T., Hutcheson J. P., and Lawrence T. E.. . 2018. Live growth performance, carcass grading characteristics, and harvest yields of beef steers supplemented zilpaterol hydrochloride and offered ad libitum or maintenance energy intake. J. Anim. Sci. 96:1688–1703. doi: 10.1093/jas/sky105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loneragan G. H., Thomson D. U., and Scott H. M.. . 2014. Increased mortality in groups of cattle administered the β-adrenergic agonists ractopamine hydrochloride and zilpaterol hydrochloride. PLoS ONE 9:e91177. doi: 10.1371/journal.pone.0091177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy E. L., Loy D. D., and Hansen S. L.. . 2015. Influence of distillers grains resulting from a cellulosic ethanol process utilizing corn kernel fiber on nutrient digestibility of lambs and steer feedlot performance. J. Anim. Sci. 93:2265–2274. doi: 10.2527/jas.2014-8572 [DOI] [PubMed] [Google Scholar]
- Mader T.L. 2003. Environmental stress in confined beef cattle1. J. Anim. Sci. 81:E110–E9. 10.2527/2003.8114_suppl_2E110x [DOI] [Google Scholar]
- Manjuck J., Zein J., Carpati C., and Astiz M.. . 2005. Clinical significance of increased lipase levels on admission to the ICU. Chest. 127:246–250. doi: 10.1378/chest.127.1.246 [DOI] [PubMed] [Google Scholar]
- Mitlöhner F. M., Galyean M. L., and McGlone J. J.. . 2002. Shade effects on performance, carcass traits, physiology, and behavior of heat-stressed feedlot heifers. J. Anim. Sci. 80:2043–2050. doi: 10.2527/2002.8082043x [DOI] [PubMed] [Google Scholar]
- Montgomery J.L., Krehbiel C.R., Cranston J.J., Yates D.A., Hutcheson J.P., Nichols W.T., Streeter M.N., Bechtol D.T., Johnson E., TerHune T., . et al. 2009. Dietary zilpaterol hydrochloride. I. Feedlot performance and carcass traits of steers and heifers. J Anim Sci. 87:1374–83. doi: 10.2527/jas.2008-1162 [DOI] [PubMed] [Google Scholar]
- Montilla S. I., Johnson T. P., Pearce S. C., Gardan-Salmon D., Gabler N. K., Ross J. W., Rhoads R. P., Baumgard L. H., Lonergan S. M., and Selsby J. T.. . 2014. Heat stress causes oxidative stress but not inflammatory signaling in porcine skeletal muscle. Temperature (Austin). 1:42–50. doi: 10.4161/temp.28844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey P., Hooda O. K., and Kumar S.. . 2017. Impact of heat stress and hypercapnia on physiological, hematological, and behavioral profile of tharparkar and karan fries heifers. Vet. World. 10:1146–1155. doi: 10.14202/vetworld.2017.1146-1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudeau D., Collin A., Yahav S., de Basilio V., Gourdine J. L., and Collier R. J.. . 2012. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 6:707–728. doi: 10.1017/S1751731111002448 [DOI] [PubMed] [Google Scholar]
- Rivera-Villegas A., Estrada-Angulo A., Castro-Pérez B. I., Urías-Estrada J. D., Ríos-Rincón F. G., Rodríguez-Cordero D., Barreras A., Plascencia A., González-Vizcarra V. M., Sosa-Gordillo J. F., . et al. 2019. Comparative evaluation of supplemental zilpaterol hydrochloride sources on growth performance, dietary energetics and carcass characteristics of finishing lambs. Asian-Australas. J. Anim. Sci. 32:209–216. doi: 10.5713/ajas.18.0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saker K. E., Fike J. H., Veit H., and Ward D. L.. . 2004. Brown seaweed- (tasco) treated conserved forage enhances antioxidant status and immune function in heat-stressed wether lambs. J. Anim. Physiol. Anim. Nutr. (Berl). 88:122–130. doi: 10.1111/j.1439-0396.2003.00468.x [DOI] [PubMed] [Google Scholar]
- Samuelson K. L., Hubbert M. E., and Löest C. A.. . 2016. Effects of dietary urea concentration and zilpaterol hydrochloride on performance and carcass characteristics of finishing steers. J. Anim. Sci. 94:5350–5358. doi: 10.2527/jas.2016-0875 [DOI] [PubMed] [Google Scholar]
- Sewell J. R., Berger L. L., Nash T. G., Cecava M. J., Doane P. H., Dunn J. L., Dyer M. K., and Pyatt N. A.. . 2009. Nutrient digestion and performance by lambs and steers fed thermochemically treated crop residues. J. Anim. Sci. 87:1024–1033. doi: 10.2527/jas.2008-0974 [DOI] [PubMed] [Google Scholar]
- St-Pierre N.R., Cobanov B., and Schnitkey G.. . 2003. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 86:E52–E77. doi:10.3168/jds.S0022-0302(03)74040–5 [Google Scholar]
- Sullivan K. F., and Mader T. L.. . 2018. Managing heat stress episodes in confined cattle. Vet. Clin. North Am. Food Anim. Pract. 34:325–339. doi: 10.1016/j.cvfa.2018.05.001 [DOI] [PubMed] [Google Scholar]
- Thomson D. U., Loneragan G. H., Henningson J. N., Ensley S., and Bawa B.. . 2015. Description of a novel fatigue syndrome of finished feedlot cattle following transportation. J. Am. Vet. Med. Assoc. 247:66–72. doi: 10.2460/javma.247.1.66 [DOI] [PubMed] [Google Scholar]
- Van Bibber-Krueger C. L., Miller K. A., Amachawadi R. G., Scott H. M., Gonzalez J. M., and Drouillard J. S.. . 2017. Interaction between supplemental zinc oxide and zilpaterol hydrochloride on growth performance, carcass traits, and blood metabolites in feedlot steers. J. Anim. Sci. 95:5573–5583. doi: 10.2527/jas2017.1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner T. N., Schuh J. D., Nelson F. E., and Stott G. H.. . 1976. Effect of stress on blood leucocyte and milk somatic cell counts in dairy cows. J. Dairy Sci. 59:949–956. [DOI] [PubMed] [Google Scholar]
- Xie G., Cole L. C., Zhao L. D., Skrzypek M. V., Sanders S. R., Rhoads M. L., Baumgard L. H., and Rhoads R. P.. . 2016. Skeletal muscle and hepatic insulin signaling is maintained in heat-stressed lactating holstein cows. J. Dairy Sci. 99:4032–4042. doi: 10.3168/jds.2015-10464 [DOI] [PubMed] [Google Scholar]
- Yates D. T., Cadaret C. N., Beede K. A., Riley H. E., Macko A. R., Anderson M. J., Camacho L. E., and Limesand S. W.. . 2016. Intrauterine growth-restricted sheep fetuses exhibit smaller hindlimb muscle fibers and lower proportions of insulin-sensitive type I fibers near term. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310:R1020–R1029. doi: 10.1152/ajpregu.00528.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates D. T., Löest C. A., Ross T. T., Hallford D. M., Carter B. H., and Limesand S. W.. . 2011. Effects of bacterial lipopolysaccharide injection on white blood cell counts, hematological variables, and serum glucose, insulin, and cortisol concentrations in ewes fed low- or high-protein diets. J. Anim. Sci. 89:4286–4293. doi: 10.2527/jas.2011-3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates D. T., Macko A. R., Chen X., Green A. S., Kelly A. C., Anderson M. J., Fowden A. L., and Limesand S. W.. . 2012. Hypoxaemia-induced catecholamine secretion from adrenal chromaffin cells inhibits glucose-stimulated hyperinsulinaemia in fetal sheep. J. Physiol. 590:5439–5447. doi: 10.1113/jphysiol.2012.237347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates D. T., Petersen J. L., Schmidt T. B., Cadaret C. N., Barnes T. L., Posont R. J., and Beede K. A.. . 2018. ASAS-SSR triennnial reproduction symposium: looking back and moving forward-how reproductive physiology has evolved: fetal origins of impaired muscle growth and metabolic dysfunction: lessons from the heat-stressed pregnant ewe. J. Anim. Sci. 96:2987–3002. doi: 10.1093/jas/sky164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S. L., Xu L. T., Qi Q., Geng Y. W., Chen H., Meng Z. Q., Wang P., and Chen Z.. . 2017. Serum lactate dehydrogenase predicts prognosis and correlates with systemic inflammatory response in patients with advanced pancreatic cancer after gemcitabine-based chemotherapy. Sci. Rep. 7:45194. doi: 10.1038/srep45194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F. J., Weng X. G., Wang J. F., Zhou D., Zhang W., Zhai C. C., Hou Y. X., and Zhu Y. H.. . 2014. Effects of temperature-humidity index and chromium supplementation on antioxidant capacity, heat shock protein 72, and cytokine responses of lactating cows. J. Anim. Sci. 92:3026–3034. doi: 10.2527/jas.2013-6932 [DOI] [PubMed] [Google Scholar]