Abstract
Selection for production traits with little or no emphasis on health-related traits has the potential to increase susceptibility to disease in food-producing animals. A possible genetic strategy to mitigate such effects is to include both production and health traits in the breeding objective when selecting animals. For this to occur, reliable methodologies are required to assess beneficial health traits, such as the immune capacity of animals. We describe here a methodology to assess the immune competence of beef cattle which is both practical to apply on farm and does not restrict the future sale of tested animals. The methodology also accommodates variation in prior vaccination history of cohorts of animals being tested. In the present study, the immune competence phenotype of 1,100 Angus calves was assessed during yard weaning. Genetic parameters associated with immune competence traits were estimated and associations between immune competence, temperament, and stress-coping ability traits were investigated. Results suggested that immune competence traits, related to an animal’s ability to mount both antibody and cell-mediated immune responses, are moderately heritable (h2 = 0.32 ± 0.09 and 0.27 ± 0.08, respectively) and favorably genetically correlated with the temperament trait, flight time (r = 0.63 ± 0.31 and 0.60 ± 0.29 with antibody and cell-mediated immune responses, respectively). Development of methodologies to assess the immune competence phenotype of beef cattle is a critical first step in the establishment of genetic selection strategies aimed at improving the general disease resistance of beef herds. Strategies aimed at reducing the incidence of disease in beef cattle are expected to significantly improve animal health and welfare, reduce reliance on the use of antibiotics to treat disease, and reduce disease-associated costs incurred by producers.
Keywords: Angus, beef, immune competence, stress, temperament, weaning
Introduction
Strategies aimed at reducing the incidence and severity of disease have the potential to significantly improve the health, welfare, and performance of livestock and will be critical to the sustainable future of the livestock industries. Costs associated with disease monitoring, disease treatment, and disease-related mortalities represent a significant proportion of total livestock production costs. For instance, in the Australian cattle industry, the cumulative cost of disease for pasture based cattle producers has been estimated at approximately $1 billion per annum, with bovine viral diarrhea virus alone costing approximately $114.5 million annually (Lane et al., 2015). In the Australian feedlot sector, costs associated with bovine respiratory disease (BRD) have been estimated to be in excess of $40 million annually, representing around 70% of high-impact disease costs (Sackett et al., 2006). Based on these figures, improving the ability of beef cattle to control disease challenges posed by their production environment could significantly reduce costs associated with disease in beef cattle and could generate flow-on benefits for disease epidemiology realized through reduced environmental pathogen load.
Improved health and welfare outcomes for beef cattle are also expected to provide economic benefits through improved consumer confidence in beef products. There is increased consumer awareness and concern regarding the use of antibiotics in all food-producing animals, both as growth promoters and as prophylactics and therapeutics for the control of disease. It is well accepted that the overuse of antibiotics in the treatment of human disease, combined with the limited number of new antimicrobial compounds available, has led to a continual increase in antibiotic resistant bacteria (Van Wyk, 2015). The contribution of antibiotic use in food-producing animals to this trend is coming under increasing scrutiny with consumers concerned that transmission of antibiotic resistant bacteria from animals to humans is probably via consumption of food (Etienne et al., 2017). Clearly, strategies aimed at reducing the incidence of disease, and, as a consequence, improving animal welfare and reducing reliance on antibiotics to treat disease, will be essential in maintaining consumer confidence in products of the livestock industries into the future (Hine et al., 2014).
Without strategic intervention, the incidence of disease in beef cattle is likely to further increase, in part, due to the unfavorable genetic correlations that are known to exist between production traits and the incidence of many common diseases in livestock (Rauw et al., 1998). These associations suggest that if livestock producers continue to select for enhanced production, with little or no emphasis on maintaining health and fitness, further increases in the incidence of disease are likely. A possible genetic solution is to combine production traits and health related traits into a weighted selection index with the aim of breeding highly productive animals with enhanced general disease resistance, a concept proposed by Gavora and Spencer (1978, 1983). Researchers have had a long-standing interest in assessing immune responses in individual animals both to evaluate the ability of vaccination to stimulate immune responses and to determine the impacts of various factors on immune system function in livestock. Early studies by Rossi et al. (1978) evaluated both cell-mediated and antibody responses to vaccine antigens in calves to evaluate the effectiveness of vaccination, and Watson and Gill (1991) evaluated antibody responses to a range of antigens to assess the impacts of weaning on immune system function. Early evidence that immune responses in cattle are under genetic control was provided by Lie (1977, 1979) who reported moderate to strong heritability estimates for antibody responses to vaccination with human serum albumin in young bulls. Significant sire or major histocompatibility complex type effects on antibody responses to vaccination against Brucella abortus have since been reported in young heifers (Newman et al., 1996). The genetic component of antibody responses to vaccination does vary, however, depending on the nature of the vaccine. In a recent study investigating antibody responses to 4 different viruses associated with BRD in Angus calves, all responses were reported to have a low heritability (Kramer et al., 2019).
Selection for increased resistance to specific diseases has been successful in the livestock industries. Examples include breeding sheep with enhanced resistance to specific internal parasites (Lejambre et al., 1971), dairy cattle with enhanced resistance to mastitis (Heringstad et al., 2000), and beef cattle with increased resistance to brucellosis (Adams and Templeton, 1993) and to cattle ticks (Frisch and O’Neill, 1998). However, based on the knowledge that the host immune system tailors responses to the type of pathogen encountered, it is possible that selection of animals based on their resistance to a specific disease may inadvertently increase their susceptibility to other diseases. Selection of animals based on their resistance to a disease caused by an extracellular pathogen, largely controlled by antibody-mediated immune responses, might inadvertently increase their susceptibility to diseases caused by intracellular pathogens, which are largely controlled by cell-mediated immune responses, and vice versa. In support of this concept, an inverse relationship between antibody production and cell-mediated immune responses in Biozzi mice selected for high and low antibody production has been reported (Biozzi et al., 1972; Hale and Howard, 1981) and also in cattle selected for resistance to Brucella abortus (Price et al., 1990). Furthermore, cell-mediated and antibody-mediated immune responses have been reported to be negatively correlated, genetically, in dairy cattle (Hernandez et al., 2006; Thompson-Crispi et al., 2012b). More research will be required to assess the long-term effects of selection for resistance to a specific disease on susceptibility to other diseases in livestock; however, long-term benefits can be expected from adopting breeding strategies based on enhancing general disease resistance of livestock as an alternative to, or in conjunction with, enhancing resistance to specific diseases of significant economic importance to the livestock industries.
The concept of enhancing general disease resistance by simultaneously selecting animals for their ability to mount both antibody and cell-mediated responses, and as a consequence, enhance general disease resistance was first proposed in pigs by Wilkie and Mallard (Mallard et al., 1992; Wilkie and Mallard, 1999). More recently, research efforts have been focused on developing protocols to assess general immune responsiveness in North American (Heriazon et al., 2009a,b) and Australian (Aleri et al., 2019) dairy cattle, similar to those used initially in pigs (Mallard et al.,1989). Associations between general immune responsiveness phenotypes and the incidence of disease in large-scale commercial dairy farms have been investigated previously with results suggesting that the incidence of infectious and metabolic diseases is reduced in high immune responder vs. low immune responder phenotype animals (Thompson-Crispi et al., 2012a). Favorable associations between immune competence, temperament, and ability to cope with management-induced stress have also been reported in beef cattle, suggesting that host responses to environmental challenges are highly integrated (reviewed by Colditz and Hine, 2016).
We hypothesize that genetic selection strategies aimed at improving immune competence, when used in conjunction with targeted management practices and implementation of effective vaccination protocols, has significant potential to reduce the incidence of disease in beef cattle. We describe here the development of a practical method for on-farm assessment of the ability of Angus beef cattle to mount both antibody- and cell-mediated immune responses and report on the phenotypic variation, which exists in the populations studied. We also provide genetic parameter estimates for immune competence traits and describe the associations that exist between immune competence, stress responsiveness, and temperament in Angus cattle.
Materials and Methods
Animals
All experimental procedures were performed in accordance with methodology outlined in an application approved by the Chiswick, Animal Ethics Committee, Armidale, NSW, Australia (application number 12/31). A total of 1,149 calves were enrolled in the study, comprising 1,062 steers and 87 heifers. Of the steers tested, 978 were progeny of the Australian Angus Sire Benchmarking Program (ASBP), representing years 2 and 3 of the program (ASBP calves). The ASBP is a major initiative of Angus Australia with support from Meat and Livestock Australia (MLA) and industry partners which aims to generate progeny test data on modern Angus bulls, particularly for hard to measure traits such as feed efficiency, abattoir carcass measurements, meat quality attributes, and female reproduction (https://www.angusaustralia.com.au/sire-benchmarking/about/general-information/). Traits measured on the progeny as part of ASBP include birth weight, liveweight at 200, 400, and 600 d of age, live-scan carcass assessment, feed efficiency, and carcass traits. The ASBP calves were tested at cooperator herd farms during the weaning period at various locations across NSW, Australia. The remaining steers (n = 84) and all heifers (n = 87) tested were progeny of the CSIRO, Chiswick herd (CSIRO calves). CSIRO calves were tested at the CSIRO, Armidale research facility. All calves enrolled in the project, both ASBP and CSIRO, were pedigree and performance recorded with Angus Australia. A summary of animals enrolled in the study is provided in Table 1.
Table 1.
Description of animals enrolled in the study
| Herd location | Year of birth | Sex1 | Progeny of program2 | Number of animals tested for immune competence |
|---|---|---|---|---|
| Holbrook, NSW | 2012 | M | ASBP | 171 |
| 2013 | M | ASBP | 278 | |
| Bingara, NSW | 2012 | M | ASBP | 61 |
| 2013 | M | ASBP | 78 | |
| Guyra, NSW | 2012 | M | ASBP | 122 |
| Cassilis, NSW | 2012 | M | ASBP | 164 |
| 2013 | M | ASBP | 104 | |
| Chiswick, Armidale, NSW | 2012 | M | CSIRO | 44 |
| 2014 | M | CSIRO | 40 | |
| 2012 | F | CSIRO | 49 | |
| 2014 | F | CSIRO | 38 | |
| Total | 1,149 |
1M = male (castrated), F = female.
2ASBP = Angus Sire Benchmarking Program, CSIRO = CSIRO, Chiswick Performance Register Herd.
In the ASBP herds, a total of 47 and 40 sires were represented in ASBP calves born in 2012 and 2013, respectively. The majority of sires were used across all cooperator herds within a given year. A total of 5 and 6 sires were represented in CSIRO calves born in 2012 and 2014, respectively, with the majority of these sires used across subsequent years. The sires used in the herd located at Chiswick were only used in that herd, but are linked through pedigree to sires used in the ASBP herds. The pedigree for ASBP and CSIRO calves comprised a total of 4,446 animals.
Experimental Design
Testing to assess immune competence, temperament, and stress responsiveness was undertaken on farm during the weaning period when calves were 5 to 9 mo of age. The timeline of experimental procedures is outlined in Table 2. Timing of blood sample collection (to assess antibody-mediated immune responses) and skin testing (to assess cell-mediated immune responses) post-vaccination was adjusted based on the clostridial vaccination history of calves within each herd prior to testing (Table 2). The vaccination history (number and timing of vaccinations) of calves within a herd testing cohort was identical. The testing cohort in which calves were tested in each herd and in each year will be referred to as herd testing cohort. All animals tested had received either 0, 1, or 2 clostridial vaccinations prior to testing, depending on herd of origin. The timetable of events detailed in Table 2 was used for calves having received a single clostridial vaccination at marking prior to testing. Marking involved castration of males (ring or knife), earmarking, and administration of animal health treatments as per standard on-farm practice. Marking procedures were consistent for all calves of the same gender within each herd. Of calves tested, a total of 61 had received no clostridial vaccinations prior to testing (Bingara 2012 drop steers), 122 had received both a primary and boost clostridial vaccination at marking and 6 wk post-marking (Guyra 2012 drop steers) and all other calves only a primary clostridial vaccination at marking (n = 966). Day 0 of testing coincided with the commencement of yard weaning for all calves with the exception of the Cassilis 2012 drop steers (n = 164) in which day 0 of testing coincided with days 3 (herd testing cohort 1) or 4 (herd testing cohort 2) post the commencement of weaning. In herds where numbers of calves to be tested exceeded 150, calves were tested in 2 herd testing cohorts on consecutive days to minimize the time animals were handled on a given day. All calves assessed for immune competence were yard-weaned for a minimum of 7 d. Yard weaning involved the confinement of calves in an open air cattle handling facility with daily provision of high quality hay and a concentrate supplement. During yard weaning, calves were regularly exposed to human contact during feeding and when handled through facilities. Where calves had to be released from yards due to weather or logistical reasons following this 7-d period and prior to the conclusion of testing, calves were remustered from the paddock to the yards for subsequent testing procedures. Calves were weighed a minimum of twice (start and end of testing period) and maximum of 4 times during the weaning period depending on availability of weighing facilities (Table 2).
Table 2.
Timetable for testing procedures conducted on farm during yard weaning of calves having received a single clostridial vaccination at marking prior to testing
| Day | Operation |
|---|---|
| Day 0 | Wean |
| Bodyweight recording | |
| Crush score | |
| Flight time testing | |
| Vaccinate with Ultravac 7in1 clostridial vaccine | |
| Collect blood sample | |
| Day 3 or 4 (standardized within herd cohort) | Bodyweight recording (where possible) |
| Day 141 (dependent on vaccination history, standardized within herd cohort) | Collect blood sample |
| Conduct skin test | |
| Bodyweight recording (where possible) | |
| Day 162 (dependent on vaccination history, standardized within herd cohort) | Collect blood sample |
| Measure response to skin test | |
| Bodyweight recording |
1For calves having received no clostridial vaccinations or both a primary and boost clostridial vaccination prior to testing, activities listed for day 14 were conducted on day 19 or day 8, respectively.
2For calves having received no clostridial vaccinations or both a primary and boost clostridial vaccination prior to testing, activities listed for day 16 were conducted on day 21 or day 10, respectively.
Immune Competence Testing
Vaccination
Calves received a 7in1 clostridial and leptospira multivalent vaccine (Ultravac 7in1, Zoetis) on day 0 of testing (coinciding with the start of yard weaning) to induce measurable immune responses. According to the manufacturer, the vaccine contained an antigenically balanced mixture of Clostridium perfringens type D (≥5 IU/mL), Clostridium tetani (≥2.5 IU/mL), Clostridium novyi type B (≥3.5 IU/mL), and Clostridium septicum (≥2.5 IU/mL) all as ultrafiltered toxoids, Clostridium chauvoei 0.3 mL/mL, Leptospira borgpetersenii serovar Hardjo type Hardjobovis ≥400 × 106 organisms/mL, and Leptospira interrogans serovar Pomona ≥400 × 106 organisms/mL all as formol cultures. The vaccine also contained an adjuvant consisting of aluminum salts and thiomersal as a preservative. All vaccinations were administered (2.5 mL) subcutaneously high on the neck as per manufacturer’s instructions. Blood samples (20 mL) were collected using jugular venepuncture. Serum was then separated from coagulated blood by centrifugation (700 × g, 20 min, RT) and stored in multiple aliquots at −20 °C (or −80 °C for long-term storage) for subsequent laboratory analysis.
Assessing cell-mediated immune responses
The magnitude of delayed-type hypersensitivity (DTH) reactions to vaccine antigens injected intradermally into the skin was used to assess the ability of calves to mount cell-mediated immune responses (Cell-IR). The DTH responses to the Ultravac 7in1 clostridial and leptospira vaccine were assessed in all animals tested and DTH responses to Ultravac 5in1 clostridial vaccine (Zoetis), containing a subset of vaccine antigens present in the 7in1 vaccine, were also simultaneously assessed in a subset of animals. Subsequent analysis indicated that DTH responses to the Ultravac 7in1 and Ultravac 5in1 were strongly correlated both phenotypically (0.75 ± 0.02) and genetically (1.00 ± 0.07) and therefore only DTH responses to 7in1 were used to assess Cell-IR in the current study. Data describing DTH responses to the 5in1 vaccine are not presented here. To elicit DTH responses, a test or control sample was injected intradermally in the caudal tail fold using an insulin syringe with 30G needle. Prior to injection, injection sites were identified and skin thickness measurements taken with calipers to provide a baseline skin thickness. At 48 h post-injection, changes in skin thickness at the site of injection were assessed again using calipers. All calves received a minimum of 2 intradermal injections as part of the testing procedure including 100 µL of Ultravac 7in1 vaccine on one side of the tail (test reaction) and 100 µL of saline on the other side of the tail (control reaction). A modified DTH test was conducted on a subset of calves (n = 510), which received a total of 3 intradermal injections as part of the testing procedure including 100 µL of Ultravac 5in1 vaccine (test reaction 1) and 100 µL of saline (control reaction) on one side of the tail and 100 µL of Ultravac 7in1 vaccine (test reaction 2) on the other side of the tail. Where test sites and control sites were located on the same side of the tail, injections sites were well separated and the control site located above the test site to avoid interference between reactions. The DTH testing procedure was identical for calves within each herd testing cohort.
The magnitude of DTH responses was calculated as log [double skinfold thickness (DSFT) at test reaction site/DSFT at control reaction site] at 48 h post-injection (T48). For analysis, log (DSFT at test reaction site/DSFT at control reaction site) at T0 was fitted as a covariate in statistical models. A pilot trial was conducted on a separate group of animals (n = 55), of similar age and breed, using the methodology described above to confirm that the development of skin reactions over time was consistent with a classical DTH skin response. A paired t-test was used to compare the magnitude of skin reactions at consecutive time points.
Assessing antibody-mediated immune responses
Production of anti-tetanus toxoid serum IgG1 antibody, in response to vaccination, was used to assess the ability of calves to mount antibody-mediated immune responses (Ab-IR). Blood samples were collected from individual calves at 3 separate time points to prepare serum for assessment of Ab-IR. Samples were collected on the day of vaccination (baseline sample) and at 2 time points post-vaccination which coincided with DTH testing procedures (test samples). The timing of blood sample collection post-vaccination was modified dependent on the vaccination history of calves being tested but were always collected between days 8 and 21 of testing. Responses for all animals within a herd testing cohort were assessed on the same day post-vaccination. For each herd testing cohort, a pilot study was undertaken on a subset of animals to determine which of the 2 blood samples collected post-vaccination would be analyzed for antibody level. The post-vaccination blood sample collected on the day, which represented the maximal response observed in that subset of animals, was then analyzed to determine antibody levels for all animals in that herd testing cohort. The correlation between primary and secondary responses to tetanus toxoid in calves (n = 40) was also investigated in a previous trial. A significant positive correlation of 0.54 (P < 0.001) was observed (unpublished data), suggesting that primary and secondary responses to tetanus toxoid were well correlated. As the majority of animals enrolled in the study had already received a clostridial vaccination prior to testing at yard weaning, serum collected on day 0 of testing was not assessed for baseline antibody levels in all calves. Baseline antibody levels were assessed in a subset of calves (n = 24 to 30 per herd) from 3 herds during year 1 of the project on day 0 of testing. These calves had received either a primary vaccination (Holbrook and Cassilis herds) or a primary and secondary vaccination (Guyra herd) prior to immune competence testing. Baseline antibody levels in all samples tested were low and close to the detection limit of the assay. Specifically, the mean (± SD) of optical density (OD) values of baseline samples were 0.037 (±0.025), 0.033 (±0.023), and 0.042 (±0.026) vs. 0.291 (±0.232), 0.610 (±0.414), and 0.660 (±0.475) for test samples for the Holbrook, Cassilis, and Guyra herds, respectively. The low baseline antibody values observed on day 0 are likely the consequence of the extended time period between when calves were vaccinated at marking (prior to testing) and immune competence testing commenced at weaning. These low values also suggest that minimal natural exposure to C. tetani occurs on farm. The rationale for not assessing baseline antibody levels in all calves at day 0 of testing was based on several factors. First, baseline antibody-level values for the majority of animals were expected to be low and close to the detectable limit of the assay. Second, should circulating antibody produced in response to previous vaccinations be still detectable in serum of some individual calves at the start of testing, adjusting post-testing antibody-level values for baseline antibody level values was expected to disadvantage those animals that had responded strongly to prior vaccinations. Third, the clostridial vaccination history of calves within each herd testing cohort tested was identical and therefore the response assessed during testing at weaning represented a cumulative response to the vaccination given at day 0 and any vaccinations administered previously. Fourth, as calves were generally between 5 and 9 mo of age at weaning (depending on herd) and the half-life of maternal antibody in the calf estimated to be approximately 10 to 22 d (Cervenak and Kacskovics, 2009), any influence of maternal antibody on responses to vaccination during testing were expected to be minimal.
To assess Ab-IR, total serum IgG1 antibody against tetanus toxoid antigen (Zoetis, Australia) was determined using an in-house developed indirect ELISA method based on the methodology described by Mallard et al. (1997) with minor modifications as described previously by Aleri et al. (2015). All test and control samples were assayed in quadruplicate. The CV of quadruplicate (using all replicates) and all possible combinations of triplicate values (sequentially leaving a single replicate out) were calculated, and the value for the combination with the lowest CV was used in analysis. If a CV value of <10% was not achieved, the sample was reassayed. Pooled pre- and post-vaccination serum samples, prepared by combining equal volumes of serum from multiple individual calves, were used as negative and positive controls, respectively. Mean OD values for replicates were corrected based on the mean OD value of control serum samples assayed on all plates (Aleri et al., 2015). Antigen-specific total IgG1 was detected using affinity purified sheep anti-bovine IgG1 conjugated to alkaline phosphatase (AbD, Serotec, Product No. AAI21AB).
Stress Responsiveness Testing
Average daily weight gain during the yard weaning period (WtGain) was recorded as an indirect measure of responsiveness to management-induced stress. All calves tested were weighed a minimum of twice (start and end of testing period) and a maximum of 4 times during the weaning period, depending on availability of weighing facilities. Timing of bodyweight assessment and number of times bodyweight recorded were consistent within each herd testing cohort. WtGain was calculated as the mean of average daily gain recorded between each weighing event over the weaning period.
Temperament Testing
Both crush score (CS) and flight time (FT) were measured to assess temperament during testing at yard weaning. For CS assessment, calves were placed in the crush/chute (not restrained in head bale) and observed for a period of 30 s with their behavior scored on a scale of 1 (calm, no movement) to 5 (rearing, twisting of the body, or violent struggling) by a trained observer. The same observer scored all animals in a given herd testing cohort. For FT testing, animals were released from the crush/chute following restraint in a head bale for blood collection and their FT over a distance of 1.8 m recorded using electronic equipment as per standard operating procedures (Burrow et al., 1988). Calmer animals are expected to have longer flight times as they exit the crush/chute more slowly. Procedures to assess CS and FT were consistent for all animals tested in each herd testing cohort.
Immune Competence Phenotype Groups
To identify High, Average, and Low immune competence phenotypes for Ab-IR and Cell-IR, calves were ranked on model residual (observed minus predicted) values for antibody and DTH responses, respectively (Hine et al., 2011). Residuals for ranking on Ab-IR and Cell-IR were generated independently using the models described in the Statistical analysis section. Relevant fixed effects, as described in Table 3, were fitted to statistical models when generating residual values. Residual values for each respective trait (Ab-IR and Cell-IR) were then standardized, by dividing each residual value by the SD of all residual values for that trait. Calves with a standardized residual value which was >1.0 were considered High responders for that trait, calves with a standardized residual value <−1.0 were considered Low responders and calves with a standardized residual value ≥−1.0 and ≤1.0 were considered average for that trait.
Table 3.
Description of data transformations, fixed effects, and covariates tested in statistical models
| Trait1 | Transformation | Fixed effects2 | Covariates3 |
|---|---|---|---|
| Cell-IR | Logarithmic | CG4, sex, dam age | Age at measurement4, DSFT at test site/DSFT at control site (at T04) |
| Ab-IR | Square Root | CG 4, sex, dam age | Age at measurement |
| FT | Logarithmic | CG 4, sex 4, dam age | Age at measurement 4 |
| CS | None | CG 4, sex, dam age4 | Age at measurement |
| WtGain | None | CG 4, sex, dam age | Age at measurement 4 , WWT 4 |
1Cell-IR = cell-mediated immune responses, Ab-IR = antibody-mediated immune responses, FT = flight time, CS = crush score, WtGain = average daily weight gain during the yard weaning period.
2Contemporary group (CG) incorporates property of origin, year born, management group (birth to weaning), and herd testing cohort.
3WWT = weaning weight (kg), DSFT = double skinfold thickness.
4Terms in bold font were retained in the final model (P < 0.05).
Statistical Analysis
Univariate animal models were tested in ASReml (Gilmour et al., 2009) to estimate variance components for immune competence, stress responsiveness, and temperament. Traits were tested for normality using a Shapiro–Wilk test in R (R Core Team, 2013) and data transformed where required to improve normality (Table 3). Contemporary group (CG; with 28 levels) was defined as a combination of property of origin, year drop, management group (birth to weaning), and herd testing cohort. Each CG represented between 1 and 20 sires with 3 to 34 progeny per sire per group. Contemporary group and dam age were tested as fixed effects in models. Sex (steer or heifer) was fitted within cohort for animals from the herd, where both steers and heifers were tested (Chiswick). Covariates fitted and assessed in models included age at measurement, DSFT at test site/DSFT at control site at T0 (for Cell-IR) and weaning weight (for WtGain). Details of fixed effects and covariates assessed and retained in models when analyzing each trait are detailed in Table 3. The main effect of CG, along with relevant covariates, was retained in models regardless of their significance. However, models were reduced by removing other fixed effects which were not significant (P > 0.05). The linear model used to estimate initial variance components for all traits, except the immune competence traits, is presented below.
where y is the vector of observed phenotypic values of the animals, 1n is an n×1 vector of 1s (n is the number of animals with phenotypes), μ is the overall mean, b is a vector of fixed effects, design matrix X relates observations to the corresponding fixed effects, design matrix Z relates observations to random animal genetic effects, a is a vector of polygenic breeding values sampled from N ~ (0, Aσ a2), where σ a2 is additive genetic variance, and A is the additive relationship matrix constructed from the pedigree of the animals and their 3-generation-ancestors; e is a vector of random residual values.
For the immune competence traits (Ab-IR and Cell-IR), the phenotypic values were adjusted in a fixed-effects model and residual values from the model standardized by dividing each residual value by the SD of all residual values for that trait prior to analysis. To estimate the final phenotypic and genetic parameters and heritabilities for this study, a multivariate model including all 5 traits was used.
Least squares means were generated from the linear model for all traits, fitting relevant fixed effects, and the significance of differences between immune competence grouping (low, average, or high) based on Ab-IR and Cell-IR, were analyzed. Package “LSmeans” was used in R to test group differences (R Core Team 2013). Multiple comparisons were evaluated using p-values adjusted using a Bonferroni correction using the R package “LSD.test.”
Results
Delayed-Type Sensitivity Skin Test Pilot Trial
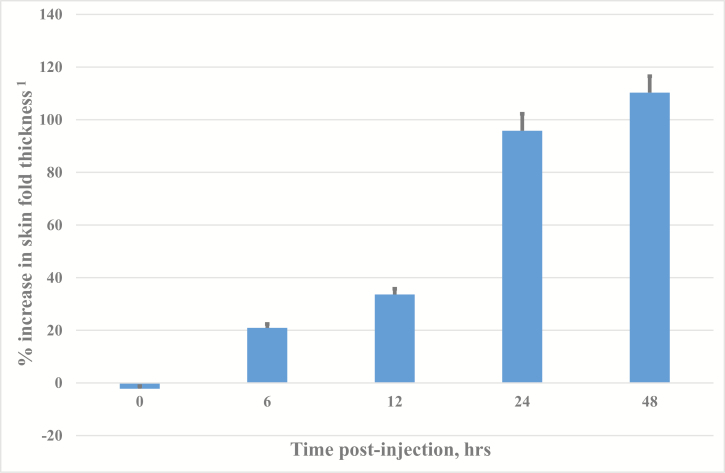
The magnitude of skin reactions increased significantly from 12 to 24 h (P < 0.01) and then again from 24 to 48 h (P < 0.01), consistent with a typical DTH skin reaction in cattle (Heriazon et al., 2009b; Fig. 1). Skin biopsies at injection sites, receiving either saline (control) or vaccine (test, Ultravac 7in1), were also collected at various time points post-injection on a subset of animals (n = 6 per time point). Stained tissue sections were prepared to assess cell types present at injection sites by microscopic examination. There was no evidence of reactions to saline at any time point. Leukocyte infiltration at test sites was evident by 6 h post-injection with neutrophils being the predominant cell type present. Infiltration of leukocytes increased dramatically by 24 h and was maintained to 48 h post-injection. Numbers of neutrophils and mononuclear cells were similar at injection sites at 24 and 48 h post-injection, indicative of a strong cell-mediated immune response to vaccine components (data not shown).
Figure 1.
Time course of cutaneous skin responses to vaccine components injected intradermally. 1Increases relative to control reactions.
Trait Description
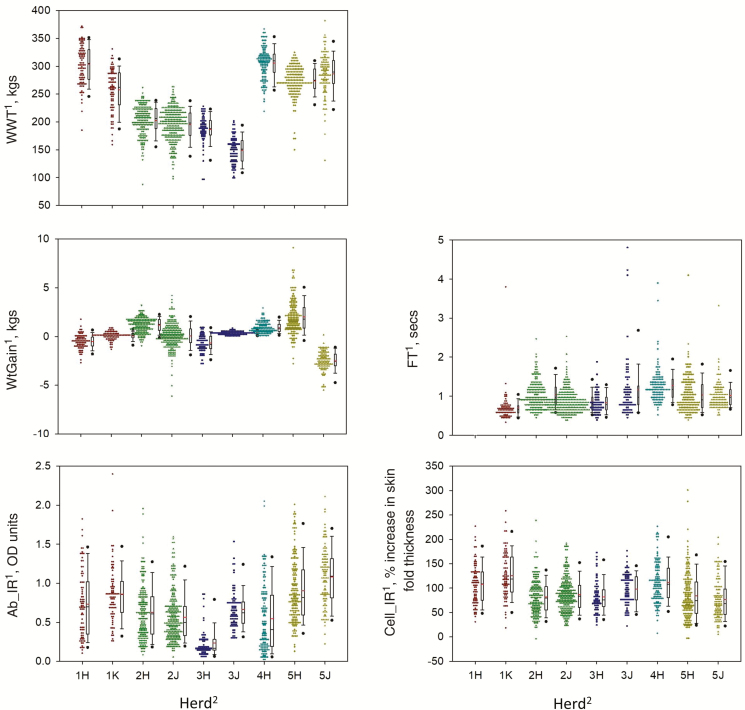
Summary statistics are presented in Table 4. The majority of animals that were immune competence tested were also assessed for stress responsiveness (WtGain) and temperament (FT, CS). For all traits with a continuous distribution, substantial phenotypic variation was observed in the study population (Fig. 2). The distribution of weaning weight is also presented to represent the phenotypic variation observed in a trait under long-term selection pressure in industry. Extreme WtGain values were observed in a small number of animals, possibly due to errors associated with weighing procedures, subclinical illness, or variation in gut fill and hydration levels at the time of weighing. These outlying values were not removed from the data set as their influence on the results of analysis was expected to be negligible considering the number of records analyzed.
Table 4.
Summary of descriptive statistics for traits measured
| Trait1 | Units | n 2 | Mean2 | Min2 | Max2 | SD2 |
|---|---|---|---|---|---|---|
| Cell-IR | Log (increase in skin fold thickness, mm) | 1,101 | 0.29 | −0.02 | 0.64 | 0.10 |
| Ab-IR | Optical density units | 1,118 | 0.70 | 0.02 | 2.40 | 0.41 |
| FT | Time (s) | 1,024 | 0.99 | 0.33 | 4.81 | 0.45 |
| CS | Visual score (1 to 5) | 1,147 | 1.44 | 1.00 | 4.00 | 0.58 |
| WtGain | kg/d | 1,118 | 0.25 | −6.16 | 7.77 | 1.55 |
1Cell-IR = cell-mediated immune responses, Ab-IR = antibody-mediated immune responses, FT = flight time, CS = crush score, WtGain = average daily weight gain during the yard weaning period.
2 n = number of animals tested, mean = arithmetic mean, min = minimum, max = maximum.
Figure 2.
Phenotypic variation observed in measured traits. In plots, central rectangle spans the 25th to 75th percentile, red horizontal line in rectangle represents the median and the whiskers above and below the box describe the 10th and 90th percentile, respectively. Black dots represent outliers. 1WWt = weaning weight, WtGain = average daily weight gain during weaning period, FT = flight time, Ab-IR = antibody-mediated immune response as assessed by measuring the production of serum antibody in response to vaccination, Cell-IR = cell-mediated immune response as assessed by measuring magnitude of delayed-type hypersensitivity skin reactions to vaccine components (relative to control reactions). 2Herd = number refers to property and letter refers to year drop of animals tested.
Genetic and Phenotypic Parameters
Heritability estimates for immune competence (Ab-IR and Cell-IR), stress responsiveness (WtGain), and temperament (FT and CS) traits, along with genetic and phenotypic correlations between traits, are presented in Table 5. Both immune competence traits were heritable (h2 ~ 0.3) and were positively genetically correlated (r = 0.48). Heritability estimates for the stress responsiveness trait, WtGain and for the temperament traits, FT and CS, were between 0.10 and 0.13. Phenotypic correlations between immune competence traits and stress responsiveness and temperament traits were low to negligible. The majority of genetic correlations between immune competence traits and stress responsiveness and temperament traits were favorable, with positive correlations observed between both Cell-IR and Ab-IR and FT (r = 0.60 and 0.63, respectively) and negative correlations observed between both Cell-IR and Ab-IR with CS (r = −0.40 and −0.09, respectively). The immune competence trait, Ab-IR, was favorably genetically correlated with WtGain (r = 0.16). However, the estimate had a large associated error. A negative genetic correlation was observed between Cell-IR and WtGain (r = −0.10). All genetic correlation estimates had large standard errors.
Table 5.
Genetic and phenotypic parameters for immune competence traits1
| Ab-IR2 | Cell-IR2 | FT2 | CS2 | WtGain2 | |
|---|---|---|---|---|---|
| Vp3 | 4.42 | 0.66 | 11.22 | 0.32 | 1.25 |
| Vg3 | 1.47 | 0.18 | 1.38 | 0.03 | 0.16 |
| Ab-IR | 0.33 ± 0.09 | 0.15 ± 0.03 | −0.01 ± 0.03 | 0.05 ± 0.03 | −0.01 ± 0.04 |
| Cell-IR | 0.48 ± 0.19 | 0.27 ± 0.08 | 0.08 ± 0.03 | −0.05 ± 0.03 | 0.11 ± 0.03 |
| FT | 0.63 ± 0.31 | 0.60 ± 0.29 | 0.12 ± 0.07 | −0.22 ± 0.03 | 0.07 ± 0.03 |
| CS | −0.09 ± 0.32 | −0.40 ± 0.33 | −0.45 ± 0.39 | 0.10 ± 0.06 | −0.04 ± 0.03 |
| WtGain | 0.16 ± 0.29 | −0.10 ± 0.31 | 0.28 ± 0.40 | −0.20 ± 0.44 | 0.13 ± 0.07 |
1Heritabilities are shown in bold, phenotypic correlations above the diagonal and genetic correlations below the diagonal.
2Cell-IR = cell-mediated immune responses, Ab-IR = antibody-mediated immune responses, FT = flight time, CS = crush score, WtGain = average daily weight gain during the yard weaning period.
3Vp = total phenotypic variance, Vg = genetic variance.
Association Between Immune Competence Phenotype, Temperament, and Stress Responsiveness
As described earlier, calves were categorized as low (Low), average (Avg), or high (High) responders for Ab-IR and Cell-IR as described above. Numbers of animals in each immune competence phenotype grouping are shown in Table 6. The classification of high, average, and low provide sufficient numbers of animals in each category to infer statistical differences, with about 15% of animals in each of the high and low categories. The influence of immune competence phenotype on stress responsiveness and temperament was assessed by comparing the least-squares means of each immune competence phenotype group. Least square means for traits are presented in Table 6.
Table 6.
Associations between immune competence phenotype and stress responsiveness (WtGain) and temperament (FT, CS) traits1
| WtGain2 | logFT2 | CS2 | |
|---|---|---|---|
| Ab-IR3 | P < 0.01 | ns | ns |
| High (n = 170) | 0.13 (0.10)a | −0.18 (0.05) | 1.50 (0.05) |
| Avg (n = 768) | 0.39 (0.05)b | −0.17 (0.04) | 1.44 (0.04) |
| Low (n = 180) | 0.36 (0.05)b | −0.14 (0.05) | 1.41 (0.04) |
| Cell-IR3 | P < 0.01 | ns | ns |
| High (n = 169) | 0.56 (0.09)a | −0.13 (0.05) | 1.39 (0.05) |
| Avg (n = 755) | 0.34 (0.05)b | −0.17 (0.04) | 1.44 (0.03) |
| Low (n = 177) | 0.16 (0.09)c | −0.13 (0.05) | 1.50 (0.04) |
1Values in table are least squares means for traits in calves classified as high, average, or low responders for Ab-IR and Cell-IR.
2WtGain = average daily weight gain during the yard weaning period, FT = flight time, CS = crush score. Where the group effect was significant, least squares means with different superscripts are significantly different.
3Cell-IR = cell-mediated immune responses, Ab-IR = antibody-mediated immune responses. Significance of group effect shown in table row, ns = nonsignificant. n = number of animals in phenotype grouping.
Discussion
In this article, we have described a methodology to assess the immune competence of Angus beef cattle, which is practical to apply on farm. The methodology is based on the use of a registered and commercially available multivalent vaccine that induces measurable immune responses. It is therefore suitable for use in commercial beef herds where restrictions on the future sale of animals for human consumption is an important consideration. Specific test antigens have been used previously to assess immune competence in dairy cattle (Heriazon et al., 2009a,b). However, these antigens could not be used in the present study as the use of these biological agents in Australian beef cattle is prohibited without prior registration with the Australian Pesticides and Veterinary Medicines Authority (https://apvma.gov.au). In the present study, although antibody responses to only the tetanus toxoid component of the multivalent vaccine was used to assess Ab-IR, DTH skin responses to the multiple antigens the vaccine were used to assess Cell-IR. As animals are simultaneously exposed to multiple pathogens in their production environment, we hypothesize there may be potential benefits in using a multivalent vaccine to induce measurable immune responses when assessing immune competence.
The methodology described here, also differs from previously described methods, as it specifically aims to assess the immune competence of animals while exposed to management-induced stress. The potential for stressors to compromise immune functions is well recognized (Dantzer and Kelley, 1989). Growing evidence also suggests that the “best” immune system is not the strongest one, but rather, the one that “maximizes fitness in light of constraints” (Martin and Coon, 2010). To achieve a constraint in the current study, immune competence testing of calves was conducted over the weaning period when they are exposed to a variety of potential stressors including separation anxiety, social mixing, change of diet, interaction with humans, and exposure to unfamiliar spatial environments (Enriquez et al., 2011). Overlaying immune competence testing on the weaning period allows individual animals to be identified, which have an enhanced ability to mount immune responses while under stress. It is well documented that the incidence of disease in livestock increases significantly during periods of heightened management-induced stress. For example, the additive effects of stressors encountered at feedlot induction significantly increase the risk of cattle contracting BRD during the early stages of feedlot finishing (Taylor et al., 2010).
When assessing immune competence, it is important to consider the “immunological history” of the animals being tested. It is well known that adaptive immune responses have a memory component, which influence how the immune system responds to pathogens on subsequent exposure. There is also growing evidence, which suggests that the innate immune system, once thought to have no memory component, can also be trained (Netea and van der Meer, 2017). Therefore, prior exposure to pathogens influence how the immune system responds to a subsequent challenge from the same pathogen. More generally, exposure to pathogens early in life, can polarize the immune system and result in the preferential induction of antibody- or cell-mediated type immune responses to unrelated pathogens later in life. This concept is well described in humans and forms the basis of the commonly known “hygiene hypothesis” (Yazdanbakhsh et al., 2002). The methodology to assess immune competence described here is based on the use of a commercial colostridial vaccine to induce measurable immune responses. As clostridial vaccines are routinely used in the beef cattle industry to protect against disease, it is important that the clostridial (and other) vaccination history of animals being tested is known. To model the immune competence phenotype of individual animals in the present study, animals were assigned to a cohort, which was fitted as a fixed effect when analyzing immune competence data (see Table 3). Animals in a given cohort were born in the same year and on the same property, were raised in the same contemporary management group from birth until weaning (when they were immune competence tested), and had an identical vaccination history in regard to the type, number, and timing of vaccinations. Although the effects of varied prior pathogen and vaccine exposure on immune responses measured is confounded with contemporary group, fitting contemporary group as a fixed effect, when analyzing immune competence data is expected to largely remove the differential effects of prior exposure. A similar paradigm of prior antigen exposure of the immune system was encountered when responses to the ubiquitous environmental antigen, Candida albicans, were used to rank dairy cattle on their ability to mount cell-mediated immune responses (Heriazon et al., 2009a,b).
The ability to assess the immune competence phenotype of beef cattle on farm provides a critical first step toward the development of genetic strategies aimed at reducing the incidence of disease in beef cattle through selection of animals with enhanced immune competence. Results from the current study suggest that significant phenotypic and genetic variance exists in the Australian Angus cattle population in their ability to mount an immune response when under stress. Significant variation was even observed between animals born in the same year on the same property (Fig. 2). There is evidence that selection for production, with little or no emphasis on health and fitness traits, leads to an increase in disease susceptibility in farm animals (reviewed by Rauw et al., 1998). As genetic selection of Angus cattle in Australia has traditionally focused on improvements in production and other economically important traits, with limited emphasis on improving health and fitness, it could be speculated that susceptibility to disease has increased in the population as a consequence of indirect selection pressure. Although the relationship between the immune competence phenotype of individual animals and susceptibility to disease is yet to be confirmed in the study population, the substantial variation in immune competence traits observed was somewhat unexpected when the potential effects of indirect selection for productivity traits on disease susceptibility are considered. It is noteworthy, however that even when substantial variation in immune competence traits were observed in the current study, the average immune competence of animals may still be suboptimal. Regardless, this variation, combined with heritability estimates of 0.32 (±0.09) and 0.27 (± 0.08) for the immune competence traits Ab-IR and Cell-IR, respectively, suggests that there is considerable scope for improving the immune competence of Australian Angus beef cattle through genetic selection. The genetic gains, which could be expected when placing selection pressure on immune competence traits, need to be investigated in a selection index context, as has been described previously using hypothetical parameters (Dominik and Hine, 2016).
In the present study, a positive phenotypic (r = 0.15 ± 0.03) and genetic correlation (r = 0.48 ± 0.19) was observed between Ab-IR and Cell-IR. This is in contrast to findings in North American dairy cattle, where reported genetic correlations have ranged from r = −0.13 ± 0.37 to −0.45 ± 0.32 (depending on timing of antibody-mediated response assessment), between antibody- and cell-mediated immune responses (Thompson-Crispi et al., 2012b). However, these correlations had high standard errors. In another study in North American dairy cattle, Hernandez et al. (2006) reported a positive genetic correlation (r = 0.31) between antibody- and cell-mediated responses when using one combination of antigens to induce responses and a negative genetic correlation when inducing responses with a different combination of antigens (r = −0.30). Regardless, results from the current study suggest that based on their strong positive genetic correlation, selecting for Ab-IR in beef cattle will also have a desirable response on Cell-IR and vice versa. Based on this finding it is tempting to suggest that assessing either Ab-IR or Cell-IR, but not both, is all that is required to improve the immune competence of beef herds. However, it is important to consider that even when Ab-IR and Cell-IR are strongly positively genetically correlated, when selection is based on only Ab-IR or Cell-IR that a proportion of animals will be low responders for the other trait. Elimination of intracellular pathogens by the immune system generally requires that infected cells be destroyed. This job is carried out by specialized phagocytic and cytotoxic cells, the actions of which can be described as “cell-mediated immune responses.” In contrast, extracellular pathogens and soluble antigens are more effectively controlled by “antibody-mediated immune responses.” Antibodies bind to pathogens and soluble antigens in the extracellular environment, preventing them from damaging or entering cells and tagging them for destruction. Therefore, resistance to diseases caused by intracellular pathogens, such as brucellosis in cattle, is largely reliant on cell-mediated immune responses (Skendros and Boura, 2013). Whereas, resistance to diseases caused by extracellular pathogens, such as parasitic liver fluke in cattle, is largely reliant on antibody-mediated immune responses (Brown et al., 1994).
As the immune system is constantly challenged by both intracellular and extracellular pathogens, it is critical that selection strategies aimed at improving general disease resistance are based on selecting individuals which have a balanced ability to mount both antibody-mediated and cell-mediated immune responses. Therefore, we suggest to combine the immune competence traits, Ab-IR and Cell-IR, to evaluate the immune competence of individual animals. This combined trait could then be used as a breeding objective trait to develop selection strategies aimed at improving the overall immune competence and general disease resistance of beef cattle, as has been previously proposed in dairy cattle (Wilkie and Mallard, 1999). A general immune responsiveness trait, combining measures of antibody- and cell-mediated immune responses, has been reported previously in dairy cattle and associations between general immune responsiveness, productivity, and disease incidence investigated (Thompson-Crispi et al., 2012a; Aleri et al., 2015). To calculate the combined immune responsiveness trait in these previous studies, residual (observed minus predicted) values from statistical models analyzing antibody and DTH responses independently, were calculated for individual animals (Hine et al., 2011). Residual values were then standardized by dividing values by the SD of all residual values for that trait, to ensure equal weighting was given to both traits, and summed together to generate a general immune response trait (Hine et al., 2011; Thompson-Crispi et al., 2012a; Aleri et al., 2015). Calves with standardized residual values for both antibody- and cell-mediated traits that were above a set threshold were considered high immune responders. Those with standardized residual values for both traits that were lower than a set threshold were considered low immune responders. Although all other animals were considered average immune responders. In these previous studies, threshold levels were arbitrarily set to allow for sufficient animals to be categorized into high, average, and low general immune response groupings to enable statistical differences to be inferred. General immune competence values for individual animals were not calculated in the current study as we continue to explore the most appropriate method to combine individual trait values, when aiming to develop selection strategies which target genetic gains for component traits at similar and optimal rates.
Two alternative strategies could be employed in an attempt to improve immune competence in beef cattle. One strategy being to target selection of animals with enhanced immune competence to increase the numbers of “high immune competence” animals in the herd. An alternative strategy is to avoid selection of animals with suboptimal immune competence to reduce the numbers of “low immune competence” animals in the herd. Although both approaches have merit and would ultimately lead to an increase in the average immune competence of the herd, we suggest that reducing the numbers of “low immune competent” animals in the herd may be a preferred approach. This suggestion is based on the hypotheses that 1) both high and average immune competent animals are expected to be reasonably well equipped to handle disease challenges faced in their production environments; 2) a disproportionately high incidence of disease is expected in low immune component animals which may influence disease dynamics within a herd; and 3) low immune competence animals are expected to have reduced responses to vaccines (Magnusson et al., 1997), therefore eliminating these animals from the herd may improve the efficacy of vaccinations routinely used in industry to prevent economically important diseases of beef cattle. Regardless of the approach employed, future generation of estimated breeding values for immune competence in beef cattle will be required to allow producers to place selection emphasis on the trait.
Livestock face a variety of environmental challenges during their productive life, including exposure to pathogens that cause disease, climatic extremes, management-induced stress, and social stressors as a result of herd hierarchy and mixing with unfamiliar animals. Animals with the ability to cope with such challenges and quickly return to pre-challenge production levels are considered “resilient.” Animals respond to environmental challenges through a variety of host-defense reactions involving immunological, behavioral, and physiological responses. These responses are highly integrated and in combination determine an animal’s resilience (Colditz and Hine, 2016). Although the immune competence of an animal is expected to make an important contribution to their overall resilience, we believe it is important to consider the relationships, which may exist between immune competence and other resilience traits when developing genetic selection strategies. Therefore, we investigated associations between immune competence (Ab-IR and Cell-IR), stress responsiveness (WtGain), and temperament (FT and CS) in the present study. No significant associations between immune competence phenotype group and temperament traits were observed. Both FT and CS values were similar between high, average and low Ab-IR and Cell-IR phenotype animals. Although no significant effects of Ab-IR and Cell-IR phenotype on temperament were observed at the phenotypic level, results suggested that immune competence traits are favorably genetically correlated with temperament in Angus beef cattle. A strong favorable genetic correlation was observed between the immune competence traits, Ab-IR and Cell-IR, with FT, whereas the genetic correlation between immune competence traits and CS was low, but favorable. Significant differences in the stress responsiveness trait, WtGain, were observed between immune competence phenotype groups. High Cell-IR phenotype animals had the highest WtGain (0.56 ± 0.09 kg/d) followed by average Cell-IR animals (0.34 ± 0.05 kg/d) and then low Cell-IR animals (0.16 ± 0.09 kg/d). In contrast, average and low Ab-IR phenotype animals had higher WtGain (0.39 ± 0.05 and 0.36 ± 0.05 kg/d, respectively) than did high Ab-IR animals (0.13 ± 0.10 kg/d). These contrasting phenotypic relationships between immune competence traits and WtGain warrant further investigation. A weak favorable genetic correlation was also observed between Ab-IR and WtGain. However, no correlation was observed between Cell-IR and WtGain. Favorable associations between immune competence and other resilience traits support the concept that animals respond to challenges in their production environment through a series of highly integrated host-defense reactions. Furthermore, these results suggest that selection for immune competence in Angus beef cattle may result in additional indirect benefits for beef producers, which are realized through improvements in temperament and stress-coping ability, and which, when combined, are expected to enhance the “general resilience” of animals in their herds.
Associations between immune function, temperament, and stress responsiveness in cattle have been reported previously. Oliphint (2006) reported that calmer cattle tended to have higher primary and secondary antibody responses to clostridial vaccination. In another study, no differences in various immune parameters, with the exception of serum IgM levels, were observed between calm and nervous cattle. However, nervous cattle had a higher incidence of disease during feedlot finishing (Fell et al., 1999). Another study investigated the influence of temperament on innate immune responses in cattle (Hulbert et al., 2011). Results indicated that neutrophil phagocytosis, oxidative burst, and cell adhesion molecule expression were heightened in calm vs. temperamental bulls post-transportation, suggesting that calm animals would be better equipped to fight off a microbial pathogen challenge at that time. These researchers have also shown that temperament also influences rectal temperature, sickness behavior and concentrations of epinephrine in the blood in responses to endotoxin challenge (Burdick et al., 2011). Studies have also reported links between temperament and stress responsiveness in beef cattle with favorable associations between temperament and cortisol stress responses induced by handling stress reported in both Bos indicus and Bos taurus cattle (Fell et al., 1999; King et al., 2006; Cafe et al., 2011). Favorable genetic correlations between tick count used as a measure of tick resistance, and rectal temperature used as a measure of heat tolerance, have also been reported in a composite breed of tropically adapted beef cattle (Burrow, 2001). Several methods of assessing temperament in cattle have been developed. These include chute/crush scoring (Tulloh, 1961) and flight speed testing (Burrow et al., 1988). For a comprehensive review of the various methods of assessing the temperament of cattle, see Burrow and Dillon (1997) and Haskell et al. (2014). The temperament traits assessed in the present study, FT and CS, were favorably correlated both phenotypically (r = −0.22 ± 0.03) and genetically (r = −0.45 ± 0.39). Several studies have reported favorable associations between temperament and performance in both pasture-fed and feedlot cattle, with calmer animals showing improved growth during finishing and improved meat quality at slaughter (Fordyce et al., 1988; Voisinet et al., 1997; Fell et al., 1999; Petherick et al., 2002; Kadel et al., 2006; Porto-Neto et al., 2014). The benefits of improving temperament extend beyond productivity gains, with calmer animals more easily and efficiently handled in yards, reducing chances of injury for both the operator and animal and reducing labor costs associated with routine husbandry procedures. A significant increase in the incidence of bruising in excitable vs. calm cattle when handled in yards has also been reported previously (Fordyce et al., 1988).
Development of a method to assess the immune competence phenotype of beef cattle is a critical first step in the establishment of genetic selection strategies aimed at improving the general disease resistance of beef herds. Gaining knowledge about the associations that exist between immune competence traits, health, and production traits in beef cattle during pasture backgrounding and feedlot finishing are the critical next steps in this work. Future studies will aim to investigate whether enhanced immune competence incurs a production cost as a consequence of nutrients being redirected from production to support immune function and will also investigate the influence of immune competence phenotype on disease incidence in commercial feedlot environments. For this to occur, detailed recording of disease incidence data, including treatments administered, recovery times, and health outcomes, along with performance data, including average daily gain and carcass assessment at slaughter will be required. Future studies will also work toward developing genomically enhanced estimated breeding values for immune competence to inform breeding decisions in industry.
Strategies aimed at reducing the incidence of disease in beef cattle are expected to significantly improve animal health and welfare, reduce reliance on the use of antibiotics to treat disease, and reduce disease-associated costs incurred by producers.
Literature Cited
- Adams L. G., and Templeton J. W.. . 1993. Selection experiments to alter disease resistance traits in domestic animals. Vet. Immunol. Immunopathol. 38:387–394. doi: 10.1016/0165-2427(93)90096-m [DOI] [PubMed] [Google Scholar]
- Aleri J. W., Hine B. C., Pyman M. F., Mansell P. D., Wales W. J., Mallard B., and Fisher A. D.. . 2015. Assessing adaptive immune response phenotypes in Australian Holstein-Friesian heifers in a pasture-based production system. J. Anim. Sci. 93:3713–3721. doi: 10.2527/jas.2015-9078 [DOI] [PubMed] [Google Scholar]
- Aleri J. W., Hine B. C., Pyman M. F., Mansell P. D., Wales W. J., Mallard B., Stevenson M. A., and Fisher A. D.. . 2019. Associations between immune competence, stress responsiveness, and production in Holstein-Friesian and Holstein-Friesian × Jersey heifers reared in a pasture-based production system in Australia. J. Dairy Sci. 102:3282–3294. doi: 10.3168/jds.2018-14578 [DOI] [PubMed] [Google Scholar]
- Biozzi G., Stiffel C., Mouton D., Bouthillier Y., and Decreusefond C.. . 1972. Cytodynamics of the immune response in two lines of mice genetically selected for “high” and “low” antibody synthesis. J. Exp. Med. 135:1071–1094. doi: 10.1084/jem.135.5.1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. C., Davis W. C., Dobbelaere D. A., and Rice-Ficht A. C.. . 1994. CD4+ T-cell clones obtained from cattle chronically infected with Fasciola hepatica and specific for adult worm antigen express both unrestricted and Th2 cytokine profiles. Infect. Immun. 62:818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick N. C., Carroll J. A., Hulbert L. E., Dailey J. W., Ballou M. A., Randel R. D., Willard S. T., Vann R. C., and Welsh T. H. Jr. 2011. Temperament influences endotoxin-induced changes in rectal temperature, sickness behavior, and plasma epinephrine concentrations in bulls. Innate Immun. 17:355–364. doi: 10.1177/1753425910379144 [DOI] [PubMed] [Google Scholar]
- Burrow H. M. 2001. Variances and covariances between productive and adaptive traits and temperament in a composite breed of tropical beef cattle. Liv. Prod. Sci. 70:213–233. doi: 10.1016/S0301-6226(01)00178-6 [DOI] [Google Scholar]
- Burrow H. M., and Dillon R. D.. . 1997. Relationships between temperament and growth in a feedlot and commercial carcass traits of Bos indicus crossbreds. Aust. J. Exp. Agric. 37: 407–411. doi: 10.1071/EA96148 [DOI] [Google Scholar]
- Burrow H. M., Seifert G. W., and Corbet N. J.. . 1988. A new technique for measuring temperament in cattle. Proc. Aust. Soc. Anim. Prod. 17:154–157. [Google Scholar]
- Cafe L. M., Robinson D. L., Ferguson D. M., Geesink G. H., and Greenwood P. L.. . 2011. Temperament and hypothalamic-pituitary-adrenal axis function are related and combine to affect growth, efficiency, carcass, and meat quality traits in Brahman steers. Domest. Anim. Endocrinol. 40:230–240. doi: 10.1016/j.domaniend.2011.01.005 [DOI] [PubMed] [Google Scholar]
- Cervenak J., and Kacskovics I.. . 2009. The neonatal Fc receptor plays a crucial role in the metabolism of IgG in livestock animals. Vet. Immunol. Immunopathol. 128:171–177. doi: 10.1016/j.vetimm.2008.10.300 [DOI] [PubMed] [Google Scholar]
- Colditz I. G., and Hine B. C.. . 2016. Resilience in farm animals: Biology, management, breeding and implications for animal welfare. Anim. Prod. Sci. 56:1961–1983. doi: 10.1071/AN15297 [DOI] [Google Scholar]
- Dantzer R., and Kelley K. W.. . 1989. Stress and immunity: An integrated view of relationships between the brain and the immune system. Life Sci. 44:1995–2008. doi: 10.1016/0024-3205(89)90345-7 [DOI] [PubMed] [Google Scholar]
- Dominik S., and Hine B.. . 2016. Selection for immune competence in beef breeding programs modelled on potential reductions in the incidence of bovine respiratory disease. In: Hermesch S., Dominik S., editors, Breeding focus 2016 – Animal welfare. Animal Genetics and Breeding Unit, Univ. of New England, Armidale, NSW, Australia: p. 45–57. [Google Scholar]
- Enriquez D., Hötzel M. J., and Ungerfeld R.. . 2011. Minimising the stress of weaning of beef calves: A review. Acta Vet. Scand. 53:28. doi: 10.1186/1751-0147-53-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne J., Chirico S., Gunabalasingham T., Dautzenberg S., and Gysen S.. . 2017. EU-Insights – Perceptions on the human health impact of antimicrobial resistance (AMR) and antibiotics use in animals across the EU. EFSA supporting publication. 14:EN-1183. doi: 10.2903/sp.efsa.2017.EN-1183 [DOI]
- Fell L. R., Colditz I. G., Walker K. H., and Watson D. L.. . 1999. Associations between temperament, performance and immune function in cattle entering a commercial feedlot. Aust. J. Exp. Agric. 39:795–802. doi: 10.1071/EA99027 [DOI] [Google Scholar]
- Fordyce G., Dodt R. M., and Wythes J. R.. . 1988. Cattle temperaments in extensive beef herds in northern Queensland. 1. Factors affecting temperament. Aust. J. Exp. Agric. 28:683–687. doi: 10.1071/EA9880683 [DOI] [Google Scholar]
- Frisch J. E., and O’Neill C. J.. . 1998. Comparative evaluation of beef cattle breeds of African, European and Indian origins. 2. Resistance to cattle ticks and gastrointestinal nematodes. Anim. Sci. 67:39–48. doi: 10.1017/S1357729800009772 [DOI] [Google Scholar]
- Gavora J. S., and Spencer J. L.. . 1978. Breeding for genetic resistance to disease: Specific or general? Worlds Poult. Sci. J. 34:137–148. doi: 10.1079/WPS19960034 [DOI] [Google Scholar]
- Gavora J. S., and Spencer J. L.. . 1983. Breeding for immune responsiveness and disease resistance. Anim. Blood Groups Biochem. Genet. 14:159–180. [DOI] [PubMed] [Google Scholar]
- Gilmour A. R., Gogel B. J., Cullis B. R., and Thompson R.. . 2009. ASReml user guide release 3.0. ed. VSN Int. Ltd., Hemel Hempstead, UK. [Google Scholar]
- Hale C., and Howard J. G.. . 1981. Immunological regulation of experimental cutaneous leishmaniasis. 2. Studies with Biozzi high and low responder lines of mice. Paras. Immunol. 3:45–55. [DOI] [PubMed] [Google Scholar]
- Haskell M. J., Simm G., and Turner S. P.. . 2014. Genetic selection for temperament traits in dairy and beef cattle. Front. Genet. 21:368. doi: 10.3389/fgene.2014.00368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heriazon A., Thompson K. A., Wilkie B. N., Mathes-Sears W., Quinton M., and Mallard B. A.. . 2009a. Antibody to ovalbumin and delayed-type hypersensitivity to Candida albicans and mycobacteria in lactating Holstein cows using Quil A or Freund’s complete adjuvant. Vet. Immunol. Immunopathol. 127:220–227. doi: 10.1016/j.vetimm.2008.10.002 [DOI] [PubMed] [Google Scholar]
- Heriazon A., Yager J. A., Sears W., and Mallard B. A.. . 2009b. Induction of delayed-type hypersensitivity and interferon-gamma to Candida albicans and anti-hen-egg white lysozyme antibody as phenotypic markers of enhanced bovine immune response. Vet. Immunol. Immunopathol. 129:93–100. doi: 10.1016/j.vetimm.2008.12.019 [DOI] [PubMed] [Google Scholar]
- Heringstad B., Klemetsdal G., and Ruane J.. . 2000. Selection for mastitis resistance in dairy cattle: A review with focus on the situation in the Nordic countries. Liv. Prod. Sci. 64:95–106. doi: 10.1016/S0301-6226(99)00128-1 [DOI] [Google Scholar]
- Hernandez A., Quinton M., Miglior F., and Mallard B. A.. . 2006. Genetic parameters of dairy cattle immune response traits. In: Proc. 8th World Congress Genet. App. Liv. Prod. p. 15–18. [Google Scholar]
- Hine B. C., Cartwright S. L., and Mallard B. A.. . 2011. Effect of age and pregnancy status on adaptive immune responses of Canadian Holstein replacement heifers. J. Dairy Sci. 94:981–991. doi: 10.3168/jds.2010-3329 [DOI] [PubMed] [Google Scholar]
- Hine B. C., Mallard B. A., Ingham A. B., and Colditz I. G.. . 2014. In: Hermesch S., Dominik S., editors, Breeding focus 2014 – Resilience. Animal Genetics and Breeding Unit, University of New England, Armidale, NSW, Australia: p. 49–64. [Google Scholar]
- Hulbert L. E., Carroll J. A., Burdick N. C., Randel R. D., Brown M. S., and Ballou M. A.. . 2011. Innate immune responses of temperamental and calm cattle after transportation. Vet. Immunol. Immunopathol. 143:66–74. doi: 10.1016/j.vetimm.2011.06.025. [DOI] [PubMed] [Google Scholar]
- Kadel M. J., Johnston D. J., Burrow H. M., Graser H. U., and Ferguson D. M.. . 2006. Genetics of flight time and other measures of temperament and their value as selection criteria for improving meat quality traits in tropically adapted breeds of beef cattle. Aust. J. Agric. Res. 57:1029–1035. doi: 10.1071/AR05082 [DOI] [Google Scholar]
- King D. A., Schuehle Pfeiffer C. E., Randel R. D., Welsh T. H. Jr, Oliphint R. A., Baird B. E., Curley K. O. Jr, Vann R. C., Hale D. S., and Savell J. W.. . 2006. Influence of animal temperament and stress responsiveness on the carcass quality and beef tenderness of feedlot cattle. Meat Sci. 74:546–556. doi: 10.1016/j.meatsci.2006.05.004 [DOI] [PubMed] [Google Scholar]
- Kramer L. M., Mayes M. S., Downey E. D., Tait R. G. Jr, Woolums A., Chase C., and Reecy J. M.. . 2019. Genome-wide association study for response to vaccination in Angus calves. BMC Genet. 20:6. doi: 10.1186/s12863-018-0709-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane J., Jubb T., Shephard R., Webb-Ware J., and Fordyce G.. . 2015. Priority list of endemic diseases for the red meat industries. MLA Project B.AHE.0010. Meat & Livestock Australia; https://www.mla.com.au/research-and-development/search-rd-reports/final-report-details/Animal-Health-and-Biosecurity/Priority-list-of-endemic-diseases-for-the-red-meat-industries/2895. [Google Scholar]
- Lejambre L. F., Ractliffe L. H., Uhazy L. S., and Whitlock J. H.. . 1971. Fecal egg output of lambs in relationship to Haemonchus contortus burden. Int. J. Parasitol. 1:157–160. doi: 10.1016/0020-7519(71)90010-5 [DOI] [PubMed] [Google Scholar]
- Lie O. 1977. Genetic variations in the antibody response in young bulls. Acta Vet. Scand. 18:572–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie O. 1979. Genetic analysis of some immunological traits in young bulls. Acta Vet. Scand. 20:372–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson U., Bossé J., Mallard B. A., Rosendal S., and Wilkie B. N.. . 1997. Antibody response to Actinobacillus pleuropneumoniae antigens after vaccination of pigs bred for high and low immune response. Vaccine 15:997–1000. doi: 10.1016/s0264-410x(96)00294-0 [DOI] [PubMed] [Google Scholar]
- Mallard B. A., Wilkie B. N., and Kennedy B. W.. . 1989. Genetic and other effects on antibody and cell mediated immune response in swine leucocyte antigen (SLA)-defined miniature pigs. Anim. Genet. 20:167–178. [DOI] [PubMed] [Google Scholar]
- Mallard B. A., Wilkie B. N., Kennedy B. W., and Quinton M.. . 1992. Use of estimated breeding values in a selection index to breed Yorkshire pigs for high and low immune and innate resistance factors. Anim. Biotechnol. 3:257–280. doi: 10.1080/10495399209525776 [DOI] [Google Scholar]
- Mallard B. A., Wagter L. C., Ireland M. J., and Dekkers J. C.. . 1997. Effects of growth hormone, insulin-like growth factor-I, and cortisol on periparturient antibody response profiles of dairy cattle. Vet. Immunol. Immunopathol. 60:61–76. doi: 10.1016/S0165-2427(97)00118-9 [DOI] [PubMed] [Google Scholar]
- Martin L. B., and Coon C. A.. . 2010. Immunology. Infection protection and natural selection. Science 330:602–603. doi: 10.1126/science.1198303 [DOI] [PubMed] [Google Scholar]
- Netea M. G., and van der Meer J. W.. . 2017. Trained immunity: An ancient way of remembering. Cell Host Microbe 21:297–300. doi: 10.1016/j.chom.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Newman M. J., Truax R. E., French D. D., Dietrich M. A., Franke D., and Stear M. J.. . 1996. Evidence for genetic control of vaccine-induced antibody responses in cattle. Vet. Immunol. Immunopathol. 50:43–54. doi: 10.1016/0165-2427(95)05483-9 [DOI] [PubMed] [Google Scholar]
- Oliphint R. A. 2006. Evaluation of the inter-relationships of temperament, stress responsiveness and immune function in beef calves. M.Sc. Thesis, Texas A&M Univ., College Station, TX. [Google Scholar]
- Petherick J. C., Holroyd R. G., Doogan V. J., and Venus B. K.. . 2002. Productivity, carcass and meat quality of lot-fed Bos indicus cross steers grouped according to temperament. Aust. J. Exp. Agric. 42:389–398. doi: 10.1071/EA01084 [DOI] [Google Scholar]
- Porto-Neto L. R., Reverter A., Prayaga K. C., Chan E. K., Johnston D. J., Hawken R. J., Fordyce G., Garcia J. F., Sonstegard T. S., Bolormaa S., . et al. 2014. The genetic architecture of climatic adaptation of tropical cattle. PLoS One 9:e113284. doi: 10.1371/journal.pone.0113284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R. E., Templeton J. W., Smith R. 3rd, and Adams L. G.. . 1990. Ability of mononuclear phagocytes from cattle naturally resistant or susceptible to brucellosis to control in vitro intracellular survival of Brucella abortus. Infect. Immun. 58:879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauw W. M., Kanis E., Noordhuizen-Stassen E. N., and Grommers F. J.. . 1998. Undesirable side effects of selection for high production efficiency in farm animals: A review. Liv. Prod. Sci. 56:15–33. doi: 10.1016/S0301-6226(98)00147-X [DOI] [Google Scholar]
- Rossi C. R., Kiesel G. K., Kramer T. T., and Hudson R. S.. . 1978. Cell-mediated and humoral immune responses of cattle to Brucella abortus, Mycobacterium bovis, and tetanus toxoid: Immunization of the fetus. Am. J. Vet. Res. 39:1742–1747. [PubMed] [Google Scholar]
- R Core Team 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Sackett D., Holmes P., Abbott K., Jephcott S., and Barber M.. . 2006. Assessing the economic cost of endemic disease on the profitability of Australian beef cattle and sheep producers. MLA Project AHW.087. Meat & Livestock Australia; https://www.mla.com.au/research-and-development/search-rd-reports/final-report-details/Animal-Health-and-Biosecurity/Assessing-the-economic-cost-of-endemic-disease-on-the-profitability-of-Australian-beef-cattle-and-sheep-producers/120. [Google Scholar]
- Skendros P., and Boura P.. . 2013. Immunity to brucellosis. Rev. Sci. Tech. 32:137–147. [DOI] [PubMed] [Google Scholar]
- Taylor J. D., Fulton R. W., Lehenbauer T. W., Step D. L., and Confer A. W.. . 2010. The epidemiology of bovine respiratory disease: What is the evidence for predisposing factors? Can. Vet. J. 51:1095–1102. [PMC free article] [PubMed] [Google Scholar]
- Tulloh N. M. 1961. Behaviour of cattle in yards. II. A study of temperament. Anim. Behav. 9:25–30. doi: 10.1016/0003-3472(61)90046-X [DOI] [Google Scholar]
- Thompson-Crispi K. A., Hine B., Quinton M., Miglior F., and Mallard B. A.. . 2012a. Short communication: Association of disease incidence and adaptive immune response in Holstein dairy cows. J. Dairy Sci. 95:3888–3893. doi: 10.3168/jds.2011-5201 [DOI] [PubMed] [Google Scholar]
- Thompson-Crispi K. A., Sewalem A., Miglior F., and Mallard B. A.. . 2012b. Genetic parameters of adaptive immune response traits in Canadian Holsteins. J. Dairy Sci. 95:401–409. doi: 10.3168/jds.2011-4452 [DOI] [PubMed] [Google Scholar]
- Van Wyk H. 2015. Antibiotic resistance. S. Afr. Pharm. J. 82:20–23. [Google Scholar]
- Voisinet B. D., Grandin T., Tatum J. D., O’Connor S. F., and Struthers J. J.. . 1997. Feedlot cattle with calm temperaments have higher average daily gains than cattle with excitable temperaments. J. Anim. Sci. 75:892–896. doi: 10.2527/1997.754892x [DOI] [PubMed] [Google Scholar]
- Watson D. L., and Gill H. S.. . 1991. Effect of weaning on antibody responses and nematode parasitism in Merino lambs. Res. Vet. Sci. 51:128–132. doi: 10.1016/0034-5288(91)90002-6 [DOI] [PubMed] [Google Scholar]
- Wilkie B., and Mallard B.. . 1999. Selection for high immune response: An alternative approach to animal health maintenance? Vet. Immunol. Immunopathol. 72:231–235. doi: 10.1016/S0165-2427(99)00136-1 [DOI] [PubMed] [Google Scholar]
- Yazdanbakhsh M., Kremsner P. G., van Ree R.. . 2002. Allergy, parasites, and the hygiene hypothesis. Science 296:490–494. doi: 10.1126/science.296.5567.490 [DOI] [PubMed] [Google Scholar]