Abstract
This study was conducted to compare the effects of adding sodium butyrate (SB), medium-chain fatty acids (MCFAs), or n-3 polyunsaturated fatty acids (n-3 PUFAs) to the diet of sows during late gestation and lactation on the reproductive performance of sows and the growth performance and intestinal health of suckling piglets. Twenty-four sows (Landrace × Large-White hybrid; third parity; 200 ± 15 kg) were randomly assigned to receive 1 of 4 diets: basal diet (control group), basal diet + 1 g SB/kg (SB group), basal diet + 7.75 g MCFA/kg (MCFA group), or basal diet + 68.2 g n-3 PUFA/kg (n-3 PUFA group). The experiment began on day 85 of gestation and ended day 22 of lactation. Colostrum samples were collected from each sow. After the experiment, blood and tissue samples were collected from 1 randomly selected piglet. The results showed that the weaning-to-estrus interval of sows in the SB, MCFA, and n-3 PUFA groups was shorter than that of sows in the control group (P < 0.05). The incidence of diarrhea in suckling piglets in the SB, MCFA, and n-3 PUFA groups was lower than that of piglets in the control group (P < 0.05). The fat, protein, IgA, IgG, and IgM concentration in colostrum from sows increased following dietary supplementation with SB, MCFA, or n-3 PUFA (P < 0.05). Comparison with the control group, the mRNA expression of claudin-1, zona occludens 1, and interleukin-10 increased in the jejunum mucosa of suckling piglets in the SB, MCFA, and n-3 PUFA groups, while that of TLR4 decreased (P < 0.05). Compared with the control group, the Chao1 and ACE indexes of microbial flora in the colon contents of piglets in the SB, MCFA, and MCFA groups increased (P < 0.05), while the relative abundance of Firmicutes, Actinobacteria, and Synergistetes decreased at the phylum level (P < 0.05). In conclusion, during late pregnancy and lactation, dietary SB supplementation had a greater effect on intestinal health and caused a greater decrease in preweaning mortality of suckling piglets than did dietary MCFA or n-3 PUFA supplementation; dietary MCFA supplementation shortened the weaning-to-estrus interval of sows to a greater extent than did dietary SB or n-3 PUFA supplementation; and dietary n-3 PUFA supplementation increased the fat and protein content in the colostrum to the greatest extent.
Keywords: medium-chain fatty acid, n-3 polyunsaturated fatty acid, sodium butyrate, sow, suckling piglet
Introduction
The reproductive performance of sows and growth performance of newborn piglets are fundamental to the development of pig farming. Three-quarters of fetal weight is gained in the last quarter of pregnancy, during which sufficient energy is required to meet the nutritional needs of sows. Sows with inadequate nutrient intake during lactation will utilize body fat and protein to meet the nutritional needs of breastfeeding, resulting in weight loss, a prolonged weaning-to-estrus interval or loss of estrus, and shortened productive life.
Recently, studies have found that fatty acids act as a source of energy, and have several unique roles, such as metabolic regulation, antibacterial activity, and anti-inflammatory effects (Liu, 2016). Several studies have reported that the addition of fatty acids to the daily feed rations of sows during late pregnancy and/or lactation can reduce body weight loss during lactation, shorten the weaning-to-estrus interval, and increase the fat content of milk. Furthermore, the type and amount of fat in daily feed rations also affect the immunoglobulin and fatty acid composition in milk and can improve the survival rate and daily weight gain of newborn piglets when ingested through the breast milk (Lauridsen et al., 2004; Shen et al., 2015; Jin et al., 2017). Butyric acid is a short-chain fatty acid; as a new feed additive, its sodium salt, i.e., sodium butyrate (SB), can significantly enhance the growth performance of piglets by improving the morphology of the small intestine, promoting the proliferation of beneficial bacteria, and boosting the immune system (Huang et al., 2015). Medium-chain fatty acids (MCFAs) are saturated fatty acids with 6- to 12-carbon chains. Medium-chain fatty acids can provide the body with a rapid supply of energy because they have good stability and low energetic value, and can be rapidly digested, absorbed, and oxidatively metabolized in the body (Zentek et al., 2011). Azain 1993) reported that the addition of 10% medium-chain triglycerides (MCTs) to the daily feed rations of sows from late pregnancy to 7-d postpartum can improve the MCFA content in milk and the survival rate of piglets weighing < 900 g. n-3 polyunsaturated fatty acids (n-3 PUFAs), including α-linolenic acid, eicosapentaenoic acid, and docosahexaenoic acid, are long-chain fatty acids containing more than 2 double bonds and 16- to 22-carbon chains. They play important roles in growth and development, as well as enhancing the immune system in animals. A study carried out by McAfee et al. (2016) revealed that the addition of 1% fish oil to the daily feed rations of sows during late pregnancy and lactation can promote the growth of newborn piglets and reduce weaning stress.
Numerous studies have investigated the effects of SCFA, MCFA, and n-3 PUFA on the reproductive performance of sows or growth performance of piglets; however, studies comparing the functions of SB, MCFA, and n-3 PUFA on sow and piglet performance are limited. In the present study, we compared the effects of dietary SB, MCFA, and n-3 PUFA supplementation during late pregnancy and lactation on the reproductive performance of sows and growth performance of piglets, to provide a theoretical reference for the reasonable use of SB, MCFA, and n-3 PUFA in sow reproduction.
Materials and Methods
Animal Use and Care
All experimental procedures were approved by the Southwest University Animal Care and Use Committee.
Twenty-four tested sows (Landrace × Large-White hybrid; third parity; 200 ± 15 kg) were provided by the Sichuan Giastar Group Co., Ltd. Sows were individually housed in gestation crates (0.60 by 2.15 m) with partially slatted floors until day 110 of gestation when they were transferred to individual farrowing crates (1.20 by 2.15 m) with a cast iron sow floor and plastic creep floor.
Experimental Diets and Design
Twenty-four sows were randomly assigned to 1 of 4 diets: basal diet (control group), basal diet + 1 g coated SB/kg (SB group), basal diet + 7.75 g coated MCFA/kg (MCFA group), and basal diet + 68.2 g coated n-3 PUFA/kg (n-3 PUFA group). Each dietary treatment included 6 replicates with 1 sow per replicate. Of note, sows were selected based on their reproductive performance at the second parity in order to prevent a false lack of effect. The basic daily feed ration (Table 1) for sows during late pregnancy and lactation was formulated according to NRC (1998). Coated SBs, MCFAs, and long-chain n-3 PUFAs used in the present study were provided by Singao Agribusiness Development Co., Ltd. (Xiamen, Fujian, China). Sodium butyrate is the active ingredient of coated SB, accounting for more than 98% of its content; the active ingredient of coated MCFA is MCT, which accounts for 70.0% of its content; the main active ingredients of coated n-3 PUFA include α-linolenic acid, eicosapentaenoic acid, and docosahexaenoic acid, which account for 20% of its content. The experiment began on day 85 of gestation and ended on day 21 of lactation. During the test period from day 85 of gestation until farrowing, each sow was provided 2.70 kg/d diet. On the day of farrowing, each sow was provided 1.5 kg diet; the amount was then being successively increased (2 kg/d on days 1 and 2 of lactation; 4 kg/d on days 3 to 7 of lactation; 5.5 kg/d from days 8 to 14; 6.0 kg/d from days 15 to 21). The feed was divided into 2 equal portions, provided at 0800 and 1600 h. Water was provided from nipple drinker systems throughout the whole feeding period.
Table 1.
Ingredients and composition of basal diets (air-dry basis)
| Stages | ||
|---|---|---|
| Late gestation | Lactation | |
| Ingredients (%) | ||
| Corn | 50.04 | 47.65 |
| Barley | 17.40 | 18.00 |
| Soybean meal | 17.20 | 19.00 |
| Expanded soybean | 6.00 | 6.00 |
| Soybean oil | 2.70 | 2.70 |
| Fish meal | 2.00 | 2.00 |
| Limestone | 1.60 | 1.60 |
| CaHPO4 | 1.40 | 1.40 |
| NaCl | 0.40 | 0.40 |
| Lys | 0.26 | 0.25 |
| Premix1 | 1.00 | 1.00 |
| Total | 100.00 | 100.00 |
| Composition2 (%) | ||
| DE (Mcal/kg) | 3.39 | 3.42 |
| CP | 15.40 | 15.90 |
| EE | 5.00 | 5.10 |
| Ash | 5.80 | 5.90 |
| CF | 3.90 | 3.50 |
| Ca | 1.07 | 1.20 |
| Total P | 0.63 | 0.74 |
| Available P | 0.50 | 0.59 |
| Lys | 1.14 | 1.17 |
1The premix provided the following per kilogram of the diet for late gestation: Cu 5 mg, I 0.15 mg, Fe 83 mg, Mn 20 mg, Zn 128 mg, VA 13,400 IU, VD3 2,800 IU, choline chloride 1,000 mg, VE 22.4 mg, VK3 3 mg. The premix provided the following per kilogram of the diet for lactation: Cu 15 mg, I 0.13 mg, Fe 82 mg, Mn 20 mg, Zn 128 mg, VA 10,000 IU, VD3 2,000 IU, VE 30 mg, and VK3 1.5 mg.
2DE is calculated value and others are measured values.
Recording and Sample Collection
The daily food intake of sows was recorded throughout the lactation period. The total born litter size, number of piglets born alive, number of piglets stillborn, individual birth weight, and litter birth weight were recorded. To confirm that a piglet is stillborn, animals that appear to be stillborn should be dissected to determine whether their lungs float; this is because stillborn individuals (intrapartum) look the same as their living littermates, but do not breath (Mota-Rojas et al., 2006). The feed intake of each sow during lactation was recorded daily and average daily feed intake was calculated. Within 24 h of farrowing, the litter size of these sows was standardized to 11 piglets per litter within the same dietary treatment group to eliminate the effect of litter size on milk production. Piglets removed and added to sows were randomly selected. The piglets were weaned at 22 d of age, after which the sows were transferred to the breeding facility. Estrous was detected with a boar once daily, and the interval from weaning to first estrus was recorded.
Colostrum samples (20 mL) were collected from the functional glands of each sow within 2 h of the first piglet’s birth before suckling after 20 IU of oxytocin injection (Qilu Limited Company, Shandong, China). Colostrum samples (10 mL) were centrifuged at 4 °C and 3,000 × g for 20 min, and the supernatant was harvested and stored at −80 °C for analysis of immunoglobulin A (IgA), IgG, and IgM. The other 10 mL sample was used to analyze colostrum composition.
The weights of suckling piglets were recorded in the morning at day 22 of lactation. During the lactation period, the fecal consistency score of piglets was determined daily according to the following criteria: 5, normal, feces firm and well formed; 3 to 4, soft consistency, feces soft and formed; 1 to 2, fluid feces, usually yellowish; and 0, feces watery and projectile. When the fecal consistency was scored at 0, 1, or 2, the piglets were considered to have diarrhea. The incidence of diarrhea in piglets (%) was determined as: number of cumulative piglets with diarrhea/(total number of suckling piglets × number of breastfeeding days) × 100%. The preweaning mortality of piglets was recorded, whereby the survival rate of piglets at weaning (%) = number of weaned piglets per litter/the adjusted litter size × 100%.
On day 22 after delivery, 1 piglet from each litter was randomly selected for slaughter. Before slaughter, a 5-mL blood sample was collected via jugular puncture into a 10-mL tube treated with sodium heparin, and centrifuged at 3,000 × g and 4 °C for 20 min. Plasma was harvested and stored at −20 °C. Then, piglets were anaesthetized with an intravenous injection of sodium pentobarbital (50 mg/kg BW) and exsanguinated by severing the carotid artery and jugular vein. The abdominal cavity was opened, and the viscera were removed. Samples of the jejunum mucosa (about 4 cm2 size) were taken from the midpoint of the jejunum and placed in a 4% formalin solution. Approximately 20 g of digesta (wet weight) was taken from the caecum. The mucosa of 20 cm jejunum and colon were collected and immediately frozen in liquid nitrogen and stored at −80 °C.
Chemical Analysis
The composition of colostrum, including fat, nonfat solids, protein, and lactose, was analyzed with an automatic milk analyzer (MilkoScan FT120; Foss Electric A/S, Hillerød, Denmark). The concentrations of IgA, IgG, and IgM in the supernatant and plasma were determined using porcine-specific commercial ELISA kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China). Intra- and inter-assay coefficients of variations (CVs) were 3.0 and 4.51% for IgA, 2.76 and 4.45% for IgG, and 3.2 and 4.80% for IgM, respectively. The levels of plasma total protein, albumin, globulin, glucose, urinary nitrogen, triglycerides, free fatty acids, cholesterol, high-, low-, and very low-density lipoprotein, and the activities of plasma glutamic oxaloacetic transaminase, glutamic pyruvic transaminase, and alkaline phosphatase were determined using porcine-specific commercial ELISA kits (Nanjing Jiancheng Bioengineering Institute). Intra- and inter-assay CVs were: total protein, 1.7 and 3.52%; albumin, 1.9 and 3.25%; globulin, 3.5 and 5.6%; glucose, 1.1 and 3.7%; urinary nitrogen, 2.0 and 1.7%; triglycerides, 1.82 and 1.9%; free fatty acids, 5.0 and 8.23%; cholesterol, 0.8 and 1.1%; high-density lipoprotein, 4.7 and 5.1%; low-density lipoprotein, 2.6 and 3.7%; very low-density lipoprotein, 8 and 4.6%; glutamic oxaloacetic transaminase, 1.8 and 4.0%; glutamic pyruvic transaminase, 2.0 and 3.91%; alkaline phosphatase, 2.4 and 3.8%, respectively.
The morphology of jejunum mucosa samples was analyzed as described by Sun et al. (2013). The jejunum villus height and crypt depth were measured under a microscope with 40× combined magnification. At least 10 well-oriented intact villi, and the associated crypt depth of each section, were measured in each piglet.
Tissue samples were homogenized using 10 mL TRIzol (Invitrogen, Carlsbad, CA) and a mechanical tissue disruptor. Then, nucleic acid was extracted with 2 mL of chloroform and RNA was precipitated with ethanol. Total RNA was further purified and concentrated (PureLink RNA Mini Kit; Invitrogen), and the concentration and quality were determined by measuring absorbance at 260 and 280 nm using a NanoDrop spectrophotometer, respectively (Thermo Fisher Scientific Inc., Waltham, MA). All RNA samples contained a 260:280 nm ratio >1.8. First-strand complementary DNA (cDNA) was synthesized using a RevertAide First-Strand cDNA Synthesis Kit (K1622; Fermentas Inc., Burlington, Ontario, Canada). To quantify the mRNA levels of glyceraldehyde 3-phosphate dehydrogenase (GAPDH; a housekeeping gene), tight junction proteins, and cytokines, real-time PCR was performed using cDNA. Primer sequences for all genes are listed in Table 2. Primer specificity was tested with a BLAST analysis against the genomic NCBI database. Real-time PCR was performed using the SYBR Green method on the ABI 7900 Sequence Detection System (Applied Biosystems, Grand Island, NY). Analyses were performed in triplicate, and mean values were calculated. Data were collected and analyzed using the “fit point” option of the LightCycler software (version 3.5; Idaho Technology Inc., Salt Lake, UT). A calibration curve was generated via amplification of serially diluted cDNA using the fit point option of the software for target genes and using GAPDH gene as an internal reference. The fluorescence was determined within the geometric region of the semi-log view of the amplification plot. The relative expression of the target gene was calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Table 2.
The sequences of primers
| Gene1 | Sequence number | Product length, bp | Primer sequences, 5′→3′ | T m value |
|---|---|---|---|---|
| ZO-1 | XM_003353439.1 | 169 | F: GAGGATGGTCACACCGTGGT R: GGAGGATGCTGTTGTCTCGG |
60 ℃ |
| Occludin | NM_001163647.2 | 105 | F: TGGGTTAAAAACGTGTCGGC R: CACTTTCCCGTTGGACGAGT |
60 ℃ |
| Claudin-1 | NM_001161635.1 | 155 | F: ACCCCAGTCAATGCCAGATA R: GGCGAAGGTTTTGGATAGG |
58 ℃ |
| TLR4 | AB232527 | 113 | F: CAGATAAGCGAGGCCGTCATT R: TTGCAGCCCACAAAAAGCA |
55 ℃ |
| MγD88 | AB292176.1 | 148 | F: GATGGTAGCGGTTGTCTCTGAT R: GATGCTGGGGAACTCTTTCTTC |
60 ℃ |
| NF-κB p65 | EU399817.1 | 133 | F: CAGCCCTATCCCTTTACG R: GCCACAGCCTGAGCAA |
60 ℃ |
| TNF-α | NM_214022.1 | 168 | F: CCACGCTCTTCTGCCTACTGC R: GCTGTCCCTCGGCTTTGAC |
61 ℃ |
| IL-6 | NM_9405217033 | 146 | F: TCAGTCCAGTCGCCTTCT R: CCTTTGGCATCTTCTTCC |
56 ℃ |
| IL-10 | NM_214041.1 | 136 | F: CACTGCTCTATTGCCTGATCTTCC R: AAACTCTTCACTGGGCCGAAG |
56 ℃ |
| IL-1β | NM_9405217038 | 165 | F: CAAGGAAGTGATGGCTAA R: ACCAAGGTCCAGGTTTT |
54 ℃ |
| GAPDH | AF017079.1 | 178 | F: ACATCAAGAAGGTGGTGAAG R: ATTGTCGTACCAGGAAATGAG |
60 ℃ |
1ZO-1, zonula occludens-1; TLR4, toll-like receptor 4; MγD88, myeloid differentiation factor 88; NF-κB p65, nuclear factor-kappa B p65; TNF-α, tumor necrosis factor; IL-6, interleukin-6; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
The mucosa was placed in chilled lysis buffer (1× Tris-buffered saline, 1.5% Triton X-100, 0.5% deoxycholic acid sodium salt, 0.1% SDS, protease inhibitor cocktail, and 1 mM PMSF). Homogenate was placed on ice for 25 min, and then centrifuged at 12,000 × g at 4 °C for 25 min. The supernatant was collected and protein expression was analyzed by western blotting. Primary antibodies used in the experiment included anti-β-actin (ab8226; Abcam, Shanghai, China), anti-toll-like receptor 4 (TLR4; ab22048; Abcam), and anti-occludin (ab31721; Abcam). Secondary anti-rabbit (#7074; Cell Signaling Technology) or anti-mouse (#7076; Cell Signaling Technology) IgG horseradish peroxidase-conjugated antibody was used for detection and diluted to 1:2000.
Total bacterial DNA from approximately 0.50 g of caecum content was extracted using the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Bacterial community diversity and composition were determined in each caecum sample by high-throughput sequencing of microbial 16S ribosomal DNA genes. The extracted DNA was amplified using the 515F/860R universal prokaryote primer set (forward primer 515F, 5′-GTGCCAGCMGCCGCGGTAA-3′; reverse primer 806R, 5′-GGACTACHVGGGTWTCTAAT-3′) targeting the V4 hypervariable regions of bacterial 16S rRNA genes. A unique 5-8-base error-correcting barcode for each sample was added to the end of 515F, allowing sample multiplexing during sequencing. The PCR assay was performed in an ABI Gene Amp 9700 thermocycler under the following conditions: initial denaturation at 95 °C for 2 min; 30 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s. The PCR products were visualized on 1.5% (w/v) agarose gels to check for primer dimers and contaminant bands; amplicons with those issues were excluded. Paired-end sequencing was performed using the Illumina HiSeq2500 Platform (Novogene, Beijing, China). Raw 16S data sequences were obtained, and then screened and assembled using the QIIME and FLASH software packages. UPARSE was used to analyze these effective sequences and determine operational taxonomic units (OTUs). Reads were clustered to OTUs using uclust (Edgar, 2010) with a sequence identity threshold of 97%. Subsequently, high-quality sequences were compared against the Ribosomal Database Project classifier program to assign taxonomy (v.2.20) (Wang et al., 2007) at a 90% confidence threshold. Sequences were aligned and phylogenetic trees were obtained through uclust and FastTree, respectively. Chimeric sequences were removed with ChimeraSlayer (Haas et al., 2011). Singletons and OTUs below 0.005% were removed, as recommended by Bokulich et al. (2013). Alpha-diversity analysis including Shannon, ACE, Chao1, and Simpson was performed by Mothur software package (ver. 1.32.0) (Schloss et al., 2009).
Statistical Analysis
Data were analyzed using the GLM procedure of SAS (SAS Institute Inc., Cary, NC). Data were subjected to 1-way analysis of variance (ANOVA) in a randomized complete block design. Differences between treatment means were determined by Tukey’s multiple comparison tests. Results are reported as means ± standard error of the mean (SEM), and P < 0.05 was considered statistical significance. For data of reproductive performance, a power analysis was performed if the difference did not reach a significance of 0.05. When the power value > 0.75, a nonsignificant effect was considered to be the true absence of a treatment response.
Results
As shown in Table 3, the feed intake of sows in SB and MCFA groups was higher than that in the control group (P < 0.05); however, the feed intake of sows in n-3 PUFA group was lower (P < 0.05). The weaning-to-estrus intervals of sows in the SB, MCFA, and n-3 PUFA groups were lower than that of sows in the control group (P < 0.05). There were no differences in the numbers of piglets born, born alive, or stillborn per litter, the born alive rate, the weight of piglets at birth, or litter weight of piglets at birth among the 4 groups (P > 0.05). Of note, for the reproductive performance that did not reach as significance of 5%, all power values exceeded 0.75.
Table 3.
Effects of supplementing sow diet with SB, MCFA, or n-3 PUFA during late pregnancy and lactation on the reproductive performance of sows and growth performance of piglets until weaning at 22 d of age
| Treatments1 | ||||||
|---|---|---|---|---|---|---|
| CON | SB | MCFA | n-3 PUFA | SEM | P-value | |
| Daily feed intake per sow, kg/d | 6.46b | 7.58a | 7.33a | 5.75c | 0.22 | 0.010 |
| Daily digestable energy intake per sow, Mcal/d | 22.1b | 25.9a | 25.4a | 22.4b | 0.61 | <0.001 |
| Reproductive performance of sows | ||||||
| Number of piglets born per litter | 14.2 | 15.0 | 14.6 | 14.8 | 0.50 | 0.695 |
| Number of piglets born alive per litter | 13.4 | 14.2 | 13.6 | 13.8 | 0.51 | 0.685 |
| Number of piglets stillborn per litter | 0.83 | 0.83 | 1.00 | 1.00 | 0.25 | 0.932 |
| Born alive rate, % | 94.7 | 94.5 | 94.5 | 93.7 | 1.69 | 0.892 |
| Weight of piglets at birth, kg | 1.44 | 1.47 | 1.52 | 1.43 | 0.08 | 0.850 |
| Litter weight of piglets at birth, kg | 20.4 | 23.1 | 21.6 | 21.0 | 1.40 | 0.563 |
| Weaning-estrus interval, d | 6.60a | 5.40b | 4.00c | 5.40b | 0.54 | 0.030 |
| Growth performance of piglets | ||||||
| Survival rate of piglets at weaning, % | 82.0b | 91.3a | 87.5ab | 85.0ab | 2.17 | 0.045 |
| Weight of piglets at weaning, kg | 5.36 | 5.65 | 5.51 | 5.73 | 1.32 | 0.761 |
| Daily body weight gain of piglets, g/d | 186 | 198 | 190 | 204 | 8.27 | 0.302 |
| Incidence of diarrhea in piglets, % | 20.6a | 12.2d | 14.5c | 17.3b | 1.21 | <0.001 |
1CON, basal diet; SB, basal diet + 1 g/kg coated sodium butyrate; MCFA, basal diet + 7.75 g/kg coated medium-chain fatty acids; n-3PUFA, basal diet + coated 68.2 g/kg n-3 polyunsaturated fatty acids. Data are presented as mean ± SEM (n = 6).
a–cValues in the same row with different small letter superscripts mean significant difference (P < 0.05).
The survival rate of piglets at weaning in the SB group was higher than that of piglets in the control group (P < 0.05). There were no significant differences in the weight of piglets at weaning among the 4 groups (P > 0.05). Compared with the control group, the daily body weight gain of piglets in n-3 PUFA group tended to be higher (P = 0.095). The incidence of diarrhea in suckling piglets decreased following dietary supplementation with SB, MCFA, or n-3 PUFA (P < 0.05).
The composition of colostrum is presented in Table 4. Supplementing the sows’ diet with SB, MCFA, or n-3 PUFA increased the contents of fat, protein, IgA, IgG, and IgM in their colostrum (P < 0.05). The content of solids-not-fat in colostrum of sows receiving n-3 PUFA was higher than that of sows in the control and SB groups (P < 0.05). There was no difference in the lactose content of colostrum from sows among the 4 groups (P > 0.05).
Table 4.
Effects of supplementing sow diet with SB, MCFA, or n-3 PUFA on the colostrum composition of sows
| Treatments1 | ||||||
|---|---|---|---|---|---|---|
| CON | SB | MCFA | n-3 PUFA | SEM | P-value | |
| Fat, % | 3.67d | 4.09c | 4.47b | 5.07a | 0.20 | <0.001 |
| Protein, % | 12.3c | 16.9b | 15.3b | 19.2a | 0.63 | <0.001 |
| Lactose, % | 2.89 | 3.26 | 3.30 | 2.61 | 0.21 | 0.135 |
| Solids-not-fat, % | 23.1b | 22.6b | 24.5ab | 26.6a | 0.86 | 0.027 |
| IgA, μg/mL | 5.80c | 18.5a | 6.70b | 6.98b | 0.71 | <0.001 |
| IgG, μg/mL | 45.1c | 169a | 81.3b | 98.0b | 6.71 | <0.001 |
| IgM, μg/mL | 45.1c | 165a | 61.2b | 56.7b | 5.65 | <0.001 |
1CON, basal diet; SB, basal diet + 1 g/kg coated sodium butyrate; MCFA, basal diet + 7.75 g/kg coated medium-chain fatty acids; n-3PUFA, basal diet + coated 68.2 g/kg n-3 polyunsaturated fatty acids. Data are presented as mean ± SEM (n = 6).
a–cValues in the same row with different small letter superscripts mean significant difference (P < 0.05).
Plasma biochemical indexes for suckling piglets are presented in Table 5. Compared with the control group, the addition of SB to sow diets increased the content of plasma total protein, urinary nitrogen, triglycerides, free fatty acids, high-density lipoprotein, globulin, IgA, IgG, and IgM, and the activity of plasma alkaline phosphatase, and decreased the content of plasma cholesterol (P < 0.05). Compared with suckling piglets in the control group, the content of plasma glucose, urinary nitrogen, free fatty acids, high-density lipoprotein, IgG, and IgM, and the activities of plasma glutamic pyruvic transaminase and alkaline phosphatase were increased by MCFA supplementation. Conversely, the plasma albumin/globulin ratio, activity of plasma glutamic oxaloacetic transaminase, and content of plasma triglycerides and cholesterol decreased when compared with the control group (P < 0.05). Increased content of plasma urinary nitrogen, free fatty acids, high-density lipoprotein, globulin, and IgG, increased activities of plasma alkaline phosphatase, and decreased plasma albumin/globulin ratio and content of plasma albumin, triglycerides, and cholesterol were observed in piglets in the n-3 PUFA group when compared with the control group (P < 0.05).
Table 5.
Effects of supplementing sow diet with SB, MCFA, or n-3 PUFA during late pregnancy and lactation on the plasma biochemical indexes of suckling piglets at 22 d of age
| Treatments1 | ||||||
|---|---|---|---|---|---|---|
| CON | SB | MCFA | n-3 PUFA | SEM | P-value | |
| Total protein, g/L | 56.1b | 66.7a | 57.7b | 58.9b | 1.16 | <0.001 |
| Glucose, mmol/L | 2.24b | 2.56b | 3.49a | 2.31b | 0.12 | 0.020 |
| Plasma urinary nitrogen, mmol/L | 2.20b | 2.91a | 3.17a | 2.84a | 0.10 | <0.001 |
| Glutamic oxaloacetic transaminase, U/L | 69.0a | 68.4a | 46.8b | 64.3a | 3.03 | <0.001 |
| Glutamic pyruvic transaminase, U/L | 44.0b | 39.6b | 63.9a | 43.7b | 2.26 | <0.001 |
| Alkaline phosphatase, U/L | 411d | 594c | 734a | 689b | 13.1 | <0.001 |
| Triglycerides, mmol/L | 0.64b | 0.71a | 0.37c | 0.34c | 0.02 | <0.001 |
| Free fatty acids, μmol/L | 200d | 283c | 450b | 855a | 17.9 | <0.001 |
| Cholesterol, mmol/L | 4.22a | 3.15b | 2.37c | 1.79d | 0.09 | <0.001 |
| High-density lipoprotein, mmol/L | 1.14c | 1.80a | 2.11a | 1.36b | 0.06 | <0.001 |
| Low-density lipoprotein, mmol/L | 0.88 | 0.90 | 0.96 | 0.98 | 0.03 | 0.060 |
| Very low-density lipoprotein, mmol/L | 0.87 | 0.90 | 1.05 | 0.88 | 0.02 | 0.081 |
| Albumin, g/L | 22.8a | 21.0a | 22.3a | 17.3b | 0.65 | <0.001 |
| Globulin, g/L | 33.2b | 45.8a | 35.5b | 41.7a | 1.29 | <0.001 |
| Albumin/globulin | 0.70a | 0.46c | 0.63b | 0.42c | 0.03 | <0.001 |
| IgA, μg/mL | 8.85b | 12.5a | 9.04b | 10.3ab | 0.72 | <0.001 |
| IgG, μg/mL | 43.5c | 91.6a | 69.2b | 90.8a | 3.73 | <0.001 |
| IgM, μg/mL | 72.4c | 104a | 84.7b | 78.1bc | 4.60 | 0.002 |
1CON, basal diet; SB, basal diet + 1 g/kg coated sodium butyrate; MCFA, basal diet + 7.75 g/kg coated medium-chain fatty acids; n-3PUFA, basal diet + coated 68.2 g/kg n-3 polyunsaturated fatty acids. Data are presented as mean ± SEM (n = 6).
a–cValues in the same row with different letter superscripts mean significant difference (P < 0.05).
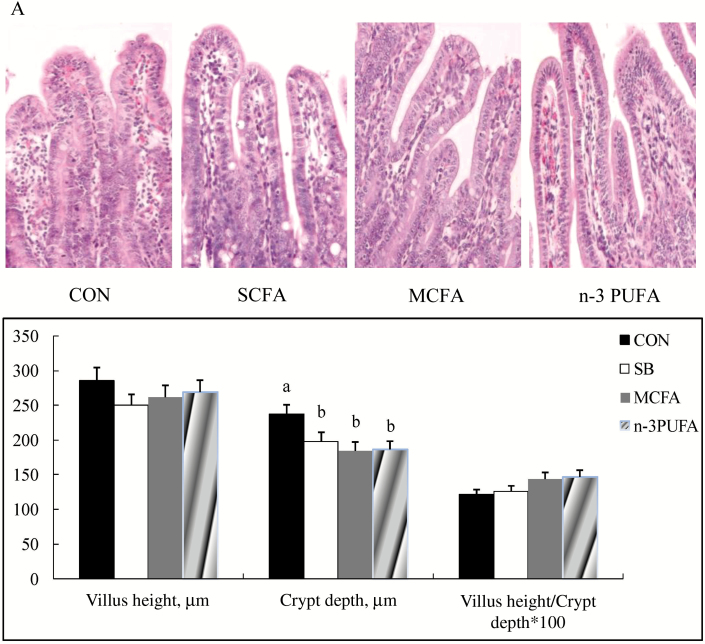
As shown in Fig. 1, there were no differences in jejunum villus height or villus height/crypt depth ratio among the 4 groups (P > 0.05); the jejunum crypt depth of suckling piglets in the SB, MCFA, and n-3 PUFA groups was lower than that of suckling piglets in the control group (P < 0.05).
Figure 1.
The effects of supplementing sow diet with SB, MCFA, or n-3 PUFA on the morphology of the jejunum mucosa of suckling piglets at 22 d of age. All data are mean ± SD (n = 6); a,bvalues with different letter superscripts within the same index mean significant difference (P < 0.05). CON, basal diet; SB, basal diet + 1 g/kg coated sodium butyrate; MCFA, basal diet + 7.75 g/kg coated medium-chain fatty acids; n-3PUFA, basal diet + coated 68.2 g/kg n-3 polyunsaturated fatty acids.
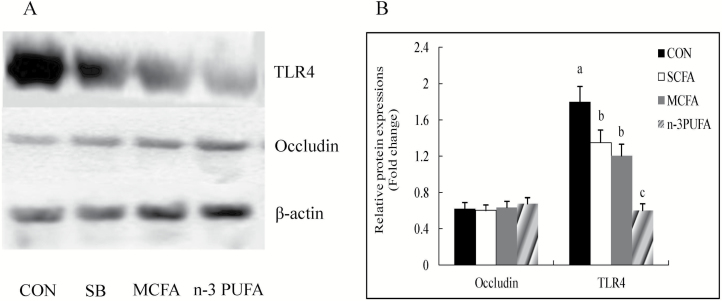
As shown in Table 6, compared with the control group, the expression of claudin-1, occludin, and zonula occludens-1 (ZO-1) mRNA was higher in the colon mucosa of suckling piglets fed diets supplemented with SB, MCFA, or n-3 PUFA (P < 0.05). Compared with control diet, supplementation with SB, MCFA, or n-3 PUFA decreased the mRNA expressions of TLR4, myeloid differentiation factor 88 (MγD88), interleukin-1β (IL-1β), and tumor necrosis factor (TNF-α) (P < 0.05), and increased the expression of IL-10 in the colon mucosa of suckling piglets (P < 0.05). The mRNA expression of NF-κB p65 in the colon mucosa of suckling piglets in the n-3 PUFA group was lower than that of piglets in the control, SB, and MCFA groups (P < 0.05). The relative protein expression of TLR4 in the jejunum mucosa of suckling piglets from the SB, MCFA, and n-3 PUFA groups was lower while that of occludin was higher compared the control group (P < 0.05) (Fig. 2).
Table 6.
Effects of supplementing sow diet with SB, MCFA, or n-3 PUFA on the mRNA expressions of tight junction proteins and inflammatory cytokines in the colon mucosa of suckling piglets at 22 d of age
| Treatments1 | ||||||
|---|---|---|---|---|---|---|
| Items2 | CON | SB | MCFA | n-3 PUFA | SEM | P-value |
| Tight junction proteins | ||||||
| Claudin-1 | 1.20b | 4.61a | 4.27a | 4.90a | 0.21 | <0.001 |
| Occludin | 1.06 | 1.31 | 1.22 | 1.20 | 0.12 | 0.340 |
| ZO-1 | 1.05d | 2.09a | 1.37c | 1.55b | 0.07 | <0.001 |
| Cytokines | ||||||
| TLR4 | 0.98a | 0.42b | 0.71c | 0.43b | 0.03 | <0.001 |
| MγD88 | 1.00a | 0.53b | 0.65b | 0.60b | 0.05 | 0.002 |
| NF-κB p65 | 1.13a | 0.94a | 1.22a | 0.41b | 0.05 | <0.001 |
| IL-6 | 0.94 | 1.25 | 1.11 | 1.03 | 0.06 | 0.150 |
| IL-10 | 1.04d | 5.57a | 1.42c | 2.11b | 0.17 | <0.001 |
| IL-1β | 1.02a | 0.57b | 0.69b | 0.61b | 0.02 | <0.001 |
| TNF-α | 0.90a | 0.25b | 0.23b | 0.27b | 0.04 | <0.001 |
1CON, basal diet; SB, basal diet + 1 g/kg coated sodium butyrate; MCFA, basal diet + 7.75 g/kg coated medium-chain fatty acids; n-3PUFA, basal diet + coated 68.2 g/kg n-3 polyunsaturated fatty acids. Data are presented as mean ± SEM (n = 6).
2ZO-1, zonula occludens-1; TLR4, toll-like receptor 4; MγD88, myeloid differentiation factor 88; NF-κB p65, nuclear factor-kappa B p65; IL-6, interleukin-6; TNF-α, tumor necrosis factor.
a–cValues in the same row with different letter superscripts mean significant difference (P < 0.05).
Figure 2.
The effects of supplementing sow diet with SB, MCFA, or n-3 PUFA on the relative protein expressions in the jejunum mucosa of suckling piglets at 22 d of age. (A and B) The western blotting analysis. n = 6 for A and B. All data are mean ± SD; a–cvalues with different letter superscripts within the same index mean significant difference (P < 0.05). CON, basal diet; SB, basal diet + 1 g/kg coated sodium butyrate; MCFA, basal diet + 7.75 g/kg coated medium-chain fatty acids; n-3 PUFA, basal diet + coated 68.2 g/kg n-3 polyunsaturated fatty acids; TLR4, toll-like receptor 4.
Overall, there were 1,157, 1,635, 1,769, and 1,822 OTUs in the caecum digesta of piglets in the control, SB, MCFA, and n-3 PUFA groups, respectively, while there were 326, 303, 366, and 270 unique OTUs, respectively. The Chao1 and ACE indexes in the caecum digesta of piglets in the SB, MCFA, and n-3 PUFA groups were higher than those of piglets in the control group (P < 0.05) (Table 7). There were no differences in the Simpson and Shannon indexes for the caecum digesta of piglets among the 4 groups (P < 0.05).
Table 7.
Effects of supplementing sow diet with SB, MCFA, or n-3 PUFA on the microbial alpha diversity and microbial composition at the levels of phylum and genus in the caecum of suckling piglets at 22 d of age
| Treatments1 | ||||||
|---|---|---|---|---|---|---|
| CON | SB | MCFA | n-3PUFA | SEM | P-value | |
| Alpha diversity | ||||||
| Simpson index | 0.89 | 0.94 | 0.94 | 0.88 | 0.04 | 0.470 |
| Chao1 index | 587b | 755a | 883a | 815a | 34.3 | <0.001 |
| ACE index | 603b | 784a | 902a | 815a | 32.2 | <0.001 |
| Shannon index | 5.06 | 6.01 | 6.35 | 5.82 | 0.32 | 0.080 |
| At phylum level | ||||||
| Fiemicutes | 65.0a | 42.2b | 44.3b | 45.4b | 1.57 | <0.001 |
| Bacteriodetes | 14.2c | 31.5a | 32.3a | 33.9b | 2.17 | <0.001 |
| Proteobacteria | 16.8b | 19.9ab | 18.6b | 21.8a | 2.94 | 0.098 |
| Actinobacteria | 7.55a | 0.63b | 1.03b | 0.35b | 0.32 | <0.001 |
| Spirochaetes | 0.03c | 2.15a | 2.98a | 0.68b | 0.41 | <0.001 |
| Tenericutes | 0c | 0.03c | 1.33a | 0.48b | 0.10 | <0.001 |
| Verrucomicrobia | 0b | 0.05ab | 0.13ab | 0.23a | 0.05 | 0.030 |
| Deferribacteres | 0b | 0.18a | 0.05b | 0.03b | 0.03 | <0.001 |
| Synergistetes | 0.13a | 0.05b | 0.05b | 0.03b | 0.03 | 0.042 |
1CON, basal diet; SB, basal diet + 1 g/kg coated sodium butyrate; MCFA, basal diet + 7.75 g/kg coated medium-chain fatty acids; n-3PUFA, basal diet + coated 68.2 g/kg n-3 polyunsaturated fatty acids. Data are presented as mean ± SEM (n = 6).
a–cValues in the same row with different letter superscripts mean significant difference (P < 0.05).
As shown in Table 7, 9 microbial communities were identified in the caecum content of piglets with a richness of more than 0.1% at the phylum level, including Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Spirochaetes, Tenericutes, Verrucomicrobia, Deferribacteres, and Synergistetes, with Firmicutes being the most abundant. Supplementing the sow diet with SB, MCFA, or n-3 PUFA increased the relative richness of Firmicutes, Actinobacteria, and Synergistetes in the caecum content of suckling piglets (P < 0.05). The relative richness of Deferribacteres in the caecum content of suckling piglets in the SB group, Verrucomicrobia in the n-3 PUFA group, and Tenericutes in the MCFA group were also higher than those of piglets in the control group (P < 0.05). The addition of SB, MCFA, and n-3 PUFA reduced the relative richness of Bacteroidetes and Actinobacteria in the caecum content of suckling piglets (P < 0.05).
Discussion
Intake and Reproductive Performance of Sows
Previous studies have shown that the feed intake of sows fed a diet supplemented with fatty acids was related to several factors. First is the dose of fatty acids in the sow diet. Jang et al. (2017) reported that supplementing sows’ diet with 1% SB increased feed intake during late pregnancy and lactation, whereas supplementing the diet with 8% fish or coconut oil decreased feed intake by lactating sows but increased the total energy intake (Lauridsen and Danielsen, 2004). Gatlin et al. (2002) also observed that supplementing the diet with 10% MCTs decreased the feed intake of lactating sows. In this study, the feed intake of sows fed diets supplemented with SB and MCFA increased, whereas that of sows fed a diet supplemented with n-3 PUFA decreased compared with that of the control group. In addition, the average daily digestible energy intake of sows in the SB and MCFA groups (25.9 and 25.4 Mcal/d, respectively) was higher than that of sows in the control and n-3 PUFA groups (22.1 and 22.4 Mcal/d, respectively). The findings of these studies indicate that the feed intake of sows will increase if fatty acids are added to the diet at an appropriate ratio; otherwise, feed intake will decrease, although energy intake will not. Second is the fatty acid source. Dietary fat sources (palm oil, fish oil, and soybean oil) did not affect the feed intake of lactating sows (Jin et al., 2017). Eastwood et al. (2014) reported that plant-based n-6:n-3 PUFA ratios of 9:1 to 1:1 did not change the feed intake of lactating sows, whereas changes were observed with the use of plant- and fish-based n-3 PUFAs. Third is the product form of fatty acids. Sodium butyrate did not improve the feed intake of lactating sows (Biagi et al., 2007; Wang et al., 2014). The smell of SB may explain why its growth-promoting effects were negated; therefore, there may be a difference in the palatability and intake of sow diets containing coated or noncoated fatty acids (Wang et al., 2014). These explain the changes in feed intake by sows fed diets with SB, MCFA, or n-3 PUFA. In present study, the weights and back fat thickness of sows at farrowing and weaning were not measured. To our knowledge, supplementing diets with different fat or fatty acid sources does not affect the weights and back fat thickness of sows at farrowing and weaning (Jang et al., 2017; Yin et al., 2017; Lan and Kim, 2018).
Decreasing the weaning-to-estrus interval of sows is one approach used to enhance their reproductive performance. A relationship has been shown to exist between nutritional status and weaning-to-estrus interval. Sows that lose excessive amounts of body weight during lactation will have extended weaning-to-estrus intervals and experience increased duration of an estrus (Einarsson and Rojkittikhun, 1993). In this study, supplementing diets with SB, MCFA, and n-3 PUFA during late pregnancy and lactation shortened the weaning-to-estrus interval of sows. Rosero et al. (2016) also reported that the addition of α-linolenic acid to sow diets caused a rapid return to estrus. The shortened estrus interval in sows fed diets with SB, MCFA, or n-3 PUFA may be due to the fatty acids providing sufficient energy to sows, preventing deterioration of physical condition, which is beneficial for their postpartum recovery (Rosero et al., 2016). In addition, fatty acids have antibacterial and anti-inflammatory effects, can enhance immunity, and can prevent the occurrence of sow reproductive diseases (Gessner et al., 2015). Importantly, MCFA was the most effective dietary treatment to induce a rapid return to estrus.
Colostrum Composition
In the present study, the addition of SB, MCFA, or n-3 PUFA to sow diets increased fat content in colostrum. Previous studies have shown that the composition of colostrum and milk from sows was significantly influenced by the source of dietary fat or fatty acids. Azain (1993) found that supplementing diet with 10% MCT increased the contents of fat and MCT in the milk of sows. The addition of fish oil to sow diet during late pregnancy and lactation increased the contents of fat and n-3 PUFA in colostrum and milk, and decreased the content of saturated fatty acids in colostrum and milk, and monounsaturated fatty acids in colostrum (Shen et al., 2015). Jin et al. (2017) reported that the addition of fish oil to sow diets elevated milk fat content, and that the consumption of fish oil possibly benefits piglets via increasing n-3 PUFA availability. Yao et al. (2013) reported that the content of C18:n-3 and total n-3 PUFA in colostrum and milk were decreased by an increase in the n6:n3 PUFA ratio in lactating sow diets. Increasing the n-3 PUFA content in sow diets resulted in a higher content of fatty acids in milk and piglet blood (Eastwood et al., 2014). Peng et al. (2010) reported that supplementation in sow diets with conjugated linoleic acid during the late gestation and lactation stages increased the concentrations of conjugated linoleic acid isomers in the umbilical cord plasma, colostrum, milk, and plasma of neonatal and weanling piglets. Inconsistent reports exist in the literature. Jang et al. (2017) reported that dietary SB supplementation did not affect the milk composition, including the content of fat, protein, lactose, total solids, and solids (nonfat). In the present study, the SB, MCFA, and n-3 PUFA content in colostrum, milk, and piglet blood were not measured. Previous studies suggest that supplementing specific fatty acids will increase the transfer of these fatty acids to suckling piglets via colostrum and milk (Azain 1993; Yao et al., 2013; Eastwood et al., 2014; Shen et al., 2015; Jin et al., 2017).
In the present study, dietary SB, MCFA, or n-3 PUFA supplementation increased the protein content in colostrum. Fat sources (palm oil, fish oil, or soybean oil) did not change the protein content in colostrum and milk of sows (Jin et al., 2017). The protein content in sow milk was not changed by SB supplementation (Jang et al., 2017). To our knowledge, data on the mechanisms mediating the effects of fat or fatty acid sources on the protein content in sow colostrum are limited. We speculated that the increased protein content in sow colostrum might have resulted from the special functions of SB, MCFA, or n-3 PUFA in regulating metabolism, gut permeability and microbiota balance, and immunity (Hontecillas et al., 2002; Calder, 2008; Liu, 2016; Chiu et al., 2017).
In addition, feeding sows with SB, MCFA, or n-3 PUFA increased the content of IgG, IgM, and IgA in colostrum. Fatty acids modulate immunoglobulins in sow milk. Supplementing diets with 0.1% coated SBs during late pregnancy and lactation increased the content of IgG and IgA in colostrum (Jang et al., 2017). The content of IgG and IgA in sow milk was increased by dietary supplementation with fish oil (Jin et al., 2017). The findings of previous studies are inconsistent; for example, Eastwood et al. (2014) reported that increasing the ratio of n-3 PUFA in sow diets had no impact on the colostrum and piglet serum IgA and IgG concentrations. The mechanisms underlying the increased levels mammary immunoglobulins in sows with dietary fatty acid supplementation require further study.
The increased immunoglobulin, protein, and fat content in the colostrum further explains the improved survival and reduced incidence of diarrhea in suckling piglets from sows fed diets containing SB, MCFA, and n-3 PUFA. Notably, dietary n-3 PUFA supplementation was the most effective dietary treatment for increasing the content of fat and protein in colostrum; supplementing diets with SB was the most effective dietary treatment for increasing the content of IgG, IgM, and IgA in the colostrum.
Growth Performance of Suckling Piglets
In this study, supplementing sow diet with SB increased the survival rate of piglets at weaning. Previous studies have also reported the positive effects of fatty acids on the growth performance of suckling piglets. Supplementing sow diets with fatty acids during late pregnancy and lactation increased the average daily body weight gain of piglets at weaning (Lu et al., 2012; Tanghe et al., 2014; Balasubramanian et al., 2016). Gatlin et al. (2002) reported that supplementing sow diets with 10% MCTs, 8% fish or coconut oil increased the average daily body weight gain of suckling piglets (Lauridsen and Danielsen, 2004). Feed intake and milk composition in sows were the main factors limiting the growth performance of their nursing offspring (Jin et al., 2017). Therefore, the increased survival rate of piglets might be attributed to the changes in feed intake and milk composition in response to dietary SB supplementation.
In the present study, the addition of SB, MCFA, or n-3 PUFA to sow diets decreased the incidence of diarrhea in suckling piglets. Previous studies have shown that fatty acids provide energy and also have important antibacterial and anti-inflammatory functions (Gatlin et al., 2002; Farmer et al., 2010; McAfee et al., 2016). Previous studies have also shown that supplementing sow diets with fatty acids during late pregnancy and lactation decreases the incidence of diarrhea in suckling piglets (Lu et al., 2012; Tanghe et al., 2014; Balasubramanian et al., 2016). The decreased incidence of diarrhea in suckling piglets is likely due to changes in the composition of breast milk, and is consistent with the survival results reported for piglets at weaning. Of note, dietary SB supplementation was the most effective dietary treatment for decreasing the diarrhea rate of suckling piglets.
Nutrient Metabolism of Suckling Piglets
In this study, the addition of SB to sow diets increased the plasma total protein content in suckling piglets, which indicates that protein synthesis in the liver is adequate (Wang et al., 2009). Meanwhile, the addition of MCFA increased the plasma glucose content in suckling piglets. This supports the findings of Newcomb et al. (1991) who reported that addition of MCT to sow diets during late pregnancy increased the plasma glucose content in neonatal piglets, indicating that the energy supply to tissues and organs is adequate. In addition, dietary SB, MCFA, or n-3 PUFA treatment increased the plasma urinary nitrogen content in suckling piglets, which indicates increased amino acid metabolism. Increased plasma urinary nitrogen has traditionally indicated an increase in nitrogen metabolism.
In the present study, the addition of SB to sow diets increased the plasma triglycerides content in suckling piglets, and dietary supplementation of SB, MCFA, or n-3 PUFA decreased the plasma cholesterol content in suckling piglets while increasing the content of plasma free fatty acids, high-density lipoprotein, and the activity of plasma alkaline phosphatase in suckling piglets. Previous studies have reported similar results. Yu et al. (2017) reported that addition of SB to sow diets inhibited lipid synthesis and promoted lipolysis in neonatal piglets, while the addition of MCT to weaned piglet diets decreased plasma cholesterol and triglyceride content (Li et al., 2015). Decreased plasma cholesterol and triglyceride levels indicate fat metabolism, while blood free fatty acids reflect the strength of lipolysis metabolism in the animal body. High-density lipoproteins mediate reverse cholesterol transport from peripheral cells to the liver for re-metabolizing. Combined with the results observed for milk fat, it can be inferred that the addition of SB, MCFA, or n-3 PUFA to sow diets increased the milk fat intake of piglets, resulting in increased fat metabolism and an increased concentration of plasma free fatty acids.
Intestinal Mucosal Morphology of Suckling Piglets
In this study, the addition of SB, MCFA, or n-3 PUFA to sow diets had no impact on the villus height, but decreased the jejunum crypt depth of suckling piglets. Chwen et al. (2013) reported that addition of MCT to sow diet increased the daily body weight gain and jejunum villus height of suckling piglets. Liu et al. (2012) reported that supplementing sow diets with fish oil increased the jejunum villus height of weaned piglets and decreased the jejunum villus height/crypt depth ratio. In general, increased intestinal villus height and decreased crypt depth are beneficial for nutrient absorption (Nabuurs et al., 1993). The mechanisms by which fatty acids improve intestinal morphology have been partly elucidated. Fatty acids can promote the development of the intestinal tract by supplying energy to small intestinal cells; in addition, fatty acids may also reduce the damage induced by harmful intestinal bacteria on the intestinal mucosa through sterilization or bacteriostasis, thereby improving the morphological structure and function of the intestines (Liu, 2016).
Immune Status of Suckling Piglets
In this study, dietary supplementation with n-3 PUFA decreased plasma albumin content, dietary supplementation with SB or n-3 PUFA increased the plasma globulin content, and dietary supplementation with SB, MCFA, or n-3 PUFA decreased the albumin/globulin ratio in the blood of suckling piglets. Plasma globulin, which is secreted by B cells following transformation into plasma cells, increases with increasing in antibody levels. The decreased albumin/globulin ratio in the blood, which reflects immune status, indicates that globulin synthesis increased and immunity improved, and the increased total protein content in the blood indicates that there is adequate protein synthesis in the liver (Wang et al., 2009).
In the present study, the plasma IgG content of piglets in the SB, MCFA, and n-3 PUFA groups, the plasma IgA content of piglets in the SB group, and the plasma IgM content of piglets in the SB and MCFA groups were higher than in piglets in the control group. Many studies have shown that dietary supplementation with fatty acids enhances blood immunoglobulin levels. Fang et al. (2014) reported that the addition of SB to weaned piglet diets enhanced the plasma IgG content, while dietary supplementation with SCFA and MCFA increased the plasma IgG content of weaned piglets (Kuang et al., 2015). Rooke and Bland (2002) reported that the plasma IgG content of piglets was positively correlated with the IgG content in sow milk. The results of the present study indicated that dietary SB, MCFA, or n-3 PUFA supplementation could, to some extent, regulate and enhance the immunity of suckling piglets. The increased plasma immunoglobulins in suckling piglets might be attributed to changes in the immunoglobulin content of sow colostrum in response to dietary SB, MCFA, or n-3 PUFA supplementation. Of note, similar to the results observed for immunoglobulins in the colostrum, SB supplementation was the most effective dietary treatment to enhance the immunoglobulin level in blood.
In this study, supplementing sow diets with SB, MCFA, or n-3 PUFA increased the mRNA expression of claudin and ZO-1 in the jejunum mucosa of suckling pigs. Tight junction, which are an important component of the intestinal mucosal barrier, are composed of transmembrane proteins (occludin and claudin) and a cytoplasmic protein (ZO), and play an important role in regulating the permeability of the intestinal mucosal barrier and preventing bacteria or endotoxins from entering the body. Sodium butyrate increased the mRNA expressions of occludin and ZO-1 in the porcine intestinal epithelial cells (IPEC-J2), and enhanced the protein expression of occludin in the jejunum mucosa of piglets (Huang et al., 2015). Dietary SB supplementation maintains the integrity of the intestinal barrier by inducing the expression of defensins in IPEC-J2 (Zeng et al., 2013). Yan et al. (2017) also reported that SB improved intestinal barrier function in IPEC-J2 cells through the selective upregulation of tight junction proteins and activation of the Akt signaling pathway.
The addition of SB, MCFA, and n-3 PUFA decreased the mRNA expression of TLR4 and its downstream effector MγD88 in the jejunum mucosa. TLR4 signals through MγD88-dependent and MγD88-independent pathways (Takeda et al., 2003). The MγD88-dependent pathway involves a series of signal transduction steps, which activate the nuclear transcription factor, NF-κB. Activated NF-κB rapidly transmits signals to the nucleus, enhancing the transcriptional expression of TNF-α and IL-1β, and simultaneously increases the secretion of pro-inflammatory cytokines such as IL-6 and IL-8, which further enhance the initial inflammatory signal and induces an inflammatory reaction (Pandey and Agrawal, 2006). In addition, compared with the control group, the mRNA expression of NF-κB p65 in the jejunum mucosa of piglets decreased in the n-3PUFA groups, the mRNA expression of anti-inflammatory IL-10 in the jejunum mucosa of piglets fed diets with SB, MCFA, and n-3 PUFA increased, and the mRNA expression of IL-1β and TNF-α in the jejunum mucosa of piglets in the MCFA and n-3PUFA groups decreased. Kuang et al. (2015) reported that the addition of SB and MCFA to the diets of weaned piglets decreased the mRNA expression IL-1β and TNF-α in the jejunum mucosa. The results of the present study indicate that dietary supplementation with fatty acids reduced inflammation by inhibiting TLR4 signaling, consequently improved intestinal health.
In general, supplementing sow diets with SB was better at regulating the immune function of suckling piglets than MCFA or n-3 PUFA.
Intestinal Microbiota of Suckling Piglets
Intestinal microorganisms participate in nutrient metabolism, and play an important role in intestinal morphology, immunity, digestion, and regulation of host gene expression (Bauer et al., 2006). An understanding of how fatty acid treatments affect intestinal bacterial communities may help to elucidate the association between their changes and improvements in growth performance and the intestinal health of suckling piglets. In this study, dietary supplementation with SB, MCFA, or n-3 PUFA increased the Chao1 and ACE indexes in the caecum content of suckling piglets. Alpha-diversity analysis includes Shannon, Simpson, Chao1, and ACE indexes, and other indicators. ACE and Chao1 indexes are used to evaluate the abundance of the microbial community, while the Shannon and Simpson indexes are commonly used to measure the diversity of the microbial community. Xu et al. (2016) reported that oral administration of SB increased the richness estimators (ACE and Chao) of the stomach, decreased the richness estimator (ACE) of the ileum, and increased the richness estimator (Chao) and the diversity of microbiota in the colon of neonatal piglets. Dietary SB decreased the ileal microbial diversity but increased that in the colon of weaned piglets (Huang et al., 2015). This discrepancy in response to fatty acid supplementation may be associated with the type and dosage of fatty acids or the different segments of gastrointestinal tract examined (Xu et al., 2016; Han et al., 2018).
In the present study, the dominant microflora in the piglet colon at the phylum level were Firmicutes, Bacteroidetes, and Proteobacteria. Firmicutes app. play an important role in carbohydrate metabolism (Walker et al., 2011), with Ruminococcus, Dorea, Sarcina, Megasphaera, Anaerovibrio, Coprococcus, and Blautia spp. belonging to Firmicutes. Ruminococcus has a multifunctional fibrous body with a complex structure, which degrades insoluble cellulose, such as xyloglucan and xylan, and as well as cellulose, and releases soluble oligosaccharides (Ravachol et al., 2016). Flavobacterium and Prevotella belong to Bacteroidetes and are mainly involved in the degradation of various soluble polysaccharides such as oligosaccharides, starch, and pectin (Larsbrink et al., 2014). Proteobacteria are mostly involved in protein fermentation (Cowieson and Bedford, 2009) and include Succinivibrio and Desulfovibrio. In this study, focusing on the phylum level, the addition of SB, MCFA, or n-3 PUFA to sow diets reduced the relative abundance of Firmicutes, Actinobacteria, and Synergistetes in the caecum content, whereas it increased the relative abundance of Bacteroidetes and Spirochaetes. The decreased relative abundance of Firmicutes in the caecum content of suckling piglets indicated reduced levels of carbohydrates and proteins entering the caecum. The increased relative abundance of Bacteroidetes in the caecum content of suckling piglets may help the host to obtain more energy from complex polysaccharides, which are resistant to the action of digestive enzymes. Synergistetes can induce diarrhea and vomiting in animals (Magalhaes et al., 2007). Therefore, the decreased relative abundance of Synergistetes in the caecum content of suckling piglets favors improvement of the intestinal microbiota and reduces the incidence of diarrhea in suckling piglets. This is consistent with the difference in the incidence of diarrhea in suckling piglets.
In conclusion, during late pregnancy and lactation, providing a sow diet containing MCFA was the most effective dietary treatment to shorten the weaning-to-estrus interval; dietary n-3 PUFA supplementation was the most effective treatment to increase the fat and protein content in the colostrum; and providing a sow diet with SB was most effective at promoting intestinal health and decreasing the preweaning mortality of suckling piglets. Dietary SB, MCFA, or n-3 PUFA supplementation favored improvements in the intestinal microbiota.
Acknowledgments
This study was funded by grants from the National Science and Technology Major Project of China (2018YFD0501000; 2017YFD0500504), the Fundamental Research Funds for the Central Universities (XDJK2019B014), the National Natural Science Foundation of China (31872370; 31772610), and the Natural Science Foundation Project of CQ CSTC (cstc2018jcyjAX0025). All authors read and approved the final manuscript.
Literature Cited
- Azain M. J. 1993. Effects of adding medium-chain triglycerides to sow diets during late gestation and early lactation on litter performance. J. Anim. Sci. 71:3011–3019. doi: 10.2527/1993.71113011x [DOI] [PubMed] [Google Scholar]
- Balasubramanian B., Park J. W., and Kim I. H.. 2016. Evaluation of the effectiveness of supplementing micro-encapsulated organic acids and essential oils in diets for sows and suckling piglets. Ital. J. Anim. Sci. 15:626–633. doi:10.1080/1828051X.2016.1222243 [Google Scholar]
- Bauer E., Williams B. A., Smidt H., Verstegen M. W., and Mosenthin R.. 2006. Influence of the gastrointestinal microbiota on development of the immune system in young animals. Curr. Issues Intest. Microbiol. 7:35–51. doi:http://dx.doi.org/ [PubMed] [Google Scholar]
- Biagi G., Piva A., Moschini M., Vezzali E., and Roth F. X.. 2007. Performance, intestinal microflora, and wall morphology of weanling pigs fed sodium butyrate. J. Anim. Sci. 85:1184–1191. doi: 10.2527/jas.2006-378 [DOI] [PubMed] [Google Scholar]
- Bokulich N. A., Subramanian S., Faith J. J., Gevers D., Gordon J. I., Knight R., Mills D. A., and Caporaso J. G.. 2013. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 10:57–59. doi: 10.1038/nmeth.2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder P. C. 2008. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol. Nutr. Food Res. 52:885–897. doi: 10.1002/mnfr.200700289 [DOI] [PubMed] [Google Scholar]
- Chiu S. C., Chao C. Y., Chiang E. I., Syu J. N., Rodriguez R. L., and Tang F. Y.. 2017. N-3 polyunsaturated fatty acids alleviate high glucose-mediated dysfunction of endothelial progenitor cells and prevent ischemic injuries both in vitro and in vivo. J. Nutr. Biochem. 42:172–181. doi: 10.1016/j.jnutbio.2017.01.009 [DOI] [PubMed] [Google Scholar]
- Chwen L. T., Foo H. L., Thanh N. T., and Choe D. W.. 2013. Growth performance, plasma fatty acids, villous height and crypt depth of preweaning piglets fed with medium chain triacylglycerol. Asian-Australas. J. Anim. Sci. 26:700–704. doi: 10.5713/ajas.2012.12561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowieson A. J., and Bedford M. R.. 2009. The effect of phytase and carbohydrase on ileal amino acid digestibility in monogastric diets: complimentary mode of action? World Poultry Sci. J. 2009:609–624. doi:10.1017/S0043933909000427 [Google Scholar]
- Eastwood L., Leterme P., and Beaulieu A. D.. 2014. Changing the omega-6 to omega-3 fatty acid ratio in sow diets alters serum, colostrum, and milk fatty acid profiles, but has minimal impact on reproductive performance. J. Anim. Sci. 92:5567–5582. doi: 10.2527/jas.2014-7836 [DOI] [PubMed] [Google Scholar]
- Edgar R. C. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. doi: 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- Einarsson S., and Rojkittikhun T.. 1993. Effects of nutrition on pregnant and lactating sows. J. Reprod. Fertil. Suppl. 48:229–239. doi:10.1007/BF01204450 [PubMed] [Google Scholar]
- Fang C. L., Sun H., Wu J., Niu H. H., and Feng J.. 2014. Effects of sodium butyrate on growth performance, haematological and immunological characteristics of weanling piglets. J. Anim. Physiol. Anim. Nutr. (Berl) 98:680–685. doi: 10.1111/jpn.12122 [DOI] [PubMed] [Google Scholar]
- Farmer C., Giguère A., and Lessard M.. 2010. Dietary supplementation with different forms of flax in late gestation and lactation: effects on sow and litter performances, endocrinology, and immune response. J. Anim. Sci. 88:225–237. doi: 10.2527/jas.2009-2023 [DOI] [PubMed] [Google Scholar]
- Gatlin L. A., Odle J., Soede J., and Hansent J. A.. 2002. Dietary medium- or long-chain triglycerides improve body condition of lean-genotype sows and increase suckling pig growth. J. Anim. Sci. 80:38–44. doi: 10.2527/2002.80138x [DOI] [PubMed] [Google Scholar]
- Gessner D. K., Gröne B., Couturier A., Rosenbaum S., Hillen S., Becker S., Erhardt G., Reiner G., Ringseis R., and Eder K.. 2015. Dietary fish oil inhibits pro-inflammatory and ER stress signalling pathways in the liver of sows during lactation. PLoS One 10:e0137684. doi: 10.1371/journal.pone.0137684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas B. J., Gevers D., Earl A. M., Feldgarden M., Ward D. V., Giannoukos G., Ciulla D., Tabbaa D., Highlander S. K., Sodergren E., . et al. ; Human Microbiome Consortium. 2011. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 21:494–504. doi: 10.1101/gr.112730.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y. S., Tang C. H., Zhao Q. Y., Zhan T. F., Zhang K., Han Y. M., and Zhang J. M.. 2018. Effects of dietary supplementation with combinations of organic and medium chain fatty acids as replacements for chlortetracycline on growth performance, serum immunity, and fecal microbiota of weaned piglets. Livest. Sci. 216:210–218. doi:10.1016/j.livsci.2018.08.013 [Google Scholar]
- Hontecillas R., Wannemeulher M. J., Zimmerman D. R., Hutto D. L., Wilson J. H., Ahn D. U., and Bassaganya-Riera J.. 2002. Nutritional regulation of porcine bacterial-induced colitis by conjugated linoleic acid. J. Nutr. 132:2019–2027. doi: 10.1093/jn/132.7.2019 [DOI] [PubMed] [Google Scholar]
- Huang C., Song P., Fan P., Hou C., Thacker P., and Ma X.. 2015. Dietary sodium butyrate decreases postweaning diarrhea by modulating intestinal permeability and changing the bacterial communities in weaned piglets. J. Nutr. 145:2774–2780. doi: 10.3945/jn.115.217406 [DOI] [PubMed] [Google Scholar]
- Jang Y. D., Lindemann M. D., Monegue H. J., and Monegue J. S.. 2017. The effect of coated sodium butyrate supplementation in sow and nursery diets on lactation performance and nursery pig growth performance. Livest. Sci. 195:13–20. doi:10.1016/j.livsci.2016.11.005 [Google Scholar]
- Jin C., Fang Z., Lin Y., Che L., Wu C., Xu S., Feng B., Li J., and Wu D.. 2017. Influence of dietary fat source on sow and litter performance, colostrum and milk fatty acid profile in late gestation and lactation. Anim. Sci. J. 88:1768–1778. doi: 10.1111/asj.12836 [DOI] [PubMed] [Google Scholar]
- Kuang Y., Wang Y., Zhang Y., Song Y., Zhang X., Lin Y., Che L., Xu S., Wu D., Xue B., . et al. 2015. Effects of dietary combinations of organic acids and medium chain fatty acids as a replacement of zinc oxide on growth, digestibility and immunity of weaned pigs. Anim. Feed Sci. Tech. 208:145–157. doi:10.1016/j.anifeedsci.2015.07.010 [Google Scholar]
- Lan R., and Kim I.. 2018. Effects of organic acid and medium chain fatty acid blends on the performance of sows and their piglets. Anim. Sci. J. 89:1673–1679. doi: 10.1111/asj.13111 [DOI] [PubMed] [Google Scholar]
- Larsbrink J., Rogers T. E., Hemsworth G. R., McKee L. S., Tauzin A. S., Spadiut O., Klinter S., Pudlo N. A., Urs K., Koropatkin N. M., . et al. 2014. A discrete genetic locus confers xyloglucan metabolism in select human gut Bacteroidetes. Nature 506:498–502. doi: 10.1038/nature12907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauridsen C., and Danielsen V.. 2004. Lactational dietary fat levels and sources influence milk composition and performance of sows and their progeny. Livest. Sci. 91:95–105. doi:10.1016/j.livprodsci.2004.07.014 [Google Scholar]
- Li Y., Zhang H., Yang L., Zhang L., and Wang T.. 2015. Effect of medium-chain triglycerides on growth performance, nutrient digestibility, plasma metabolites and antioxidant capacity in weanling pigs. Anim. Nutr. 1:12–18. doi: 10.1016/j.aninu.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. 2016. Fatty acids, inflammation and intestinal health in pigs. J. Anim. Sci. Biotechnol. 6:321–329. doi:10.1186/s40104-015-0040-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Chen F., Odle J., Lin X., Jacobi S. K., Zhu H., Wu Z., and Hou Y.. 2012. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J. Nutr. 142:2017–2024. doi: 10.3945/jn.112.164947 [DOI] [PubMed] [Google Scholar]
- Livak K. J., and Schmittgen T. D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lu H., Su S., and Ajuwon K. M.. 2012. Butyrate supplementation to gestating sows and piglets induces muscle and adipose tissue oxidative genes and improves growth performance. J. Anim. Sci. 90 (Suppl. 4):430–432. doi: 10.2527/jas.53817 [DOI] [PubMed] [Google Scholar]
- Magalhaes J. G., Tattoli I., and Girardin S. E.. 2007. The intestinal epithelial barrier: how to distinguish between the microbial flora and pathogens. Semin. Immunol. 19:106–115. doi: 10.1016/j.smim.2006.12.006 [DOI] [PubMed] [Google Scholar]
- McAfee J. M., Kattesh H. G., Lindemann M. D., Voy B. H., Kojima C. J., Carroll J. A., Burdick Sanchez N. C., Gillespie B. E., and Saxton A. M.. 2016. Effect of omega-3 polyunsaturated fatty acid (n-3 PUFA) supplementation to lactating sows on growth and indicators of stress in the post-weaned pig. J. Anim. Sci. 95 (Suppl. 1):30. doi:10.2527/ssasas2017.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota-Rojas D., Trujillo M. E., Martínez J., Rosales A. M., Orozco H., Ramírez R., Sumano H., and Alonso-Spilsbury M.. 2006. Comparative routes of oxytocin administration in crated farrowing sows and its effects on fetal and postnatal asphyxia. Anim. Reprod. Sci. 92:123–143. doi: 10.1016/j.anireprosci.2005.04.012 [DOI] [PubMed] [Google Scholar]
- Nabuurs M. J., Hoogendoorn A., van der Molen E. J., and van Osta A. L.. 1993. Villus height and crypt depth in weaned and unweaned pigs, reared under various circumstances in The Netherlands. Res. Vet. Sci. 55:78–84. doi:10.1016/0034-5288(93)90038-H [DOI] [PubMed] [Google Scholar]
- Newcomb M. D., Harmon D. L., Nelssen J. L., Thulin A. J., and Allee G. L.. 1991. Effect of energy source fed to sows during late gestation on neonatal blood metabolite homeostasis, energy stores and composition. J. Anim. Sci. 69:230–236. doi: 10.2527/1991.691230x [DOI] [PubMed] [Google Scholar]
- NRC 1998. Nutrient requirements of swine. 10th ed. Natl. Acad. Press., Washington, DC. [Google Scholar]
- Pandey S., and Agrawal D. K.. 2006. Immunobiology of toll-like receptors: emerging trends. Immunol. Cell Biol. 84:333–341. doi: 10.1111/j.1440-1711.2006.01444.x [DOI] [PubMed] [Google Scholar]
- Peng Y., Ren F., Yin J. D., Fang Q., Li F. N., and Li D. F.. 2010. Transfer of conjugated linoleic acid from sows to their offspring and its impact on the fatty acid profiles of plasma, muscle, and subcutaneous fat in piglets. J. Anim. Sci. 88:1741–1751. doi: 10.2527/jas.2009-2354 [DOI] [PubMed] [Google Scholar]
- Ravachol J., de Philip P., Borne R., Mansuelle P., Maté M. J., Perret S., and Fierobe H. P.. 2016. Mechanisms involved in xyloglucan catabolism by the cellulosome-producing bacterium Ruminiclostridium cellulolyticum. Sci. Rep. 6:22770. doi: 10.1038/srep22770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooke J. A., and Bland I. M.. 2002. The acquisition of passive immunity in the new-born piglet. Livest. Prod. Sci. 78:13–23. doi:10.1016/s0301-6226(02)00182-3 [Google Scholar]
- Rosero D. S., Boyd R. D., McCulley M., Odle J., and van Heugten E.. 2016. Essential fatty acid supplementation during lactation is required to maximize the subsequent reproductive performance of the modern sow. Anim. Reprod. Sci. 168:151–163. doi: 10.1016/j.anireprosci.2016.03.010 [DOI] [PubMed] [Google Scholar]
- Schloss P. D., Westcott S. L., Ryabin T., Hall J. R., Hartmann M., Hollister E. B., Lesniewski R. A., Oakley B. B., Parks D. H., Robinson C. J., . et al. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75:7537–7541. doi: 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Wan H., Zhu J., Fang Z., Che L., Xu S., Lin Y., Li J., and Wu D.. 2015. Fish oil and olive oil supplementation in late pregnancy and lactation differentially affect oxidative stress and inflammation in sows and piglets. Lipids 50:647–658. doi: 10.1007/s11745-015-4024-x [DOI] [PubMed] [Google Scholar]
- Sun Z. H., He Z. X., Zhang Q. L., Tan Z. L., Han X. F., Tang S. X., Zhou C. S., Wang M., and Yan Q. X.. 2013. Effects of energy and protein restriction, followed by nutritional recovery on morphological development of the gastrointestinal tract of weaned kids. J. Anim. Sci. 91:4336–4344. doi: 10.2527/jas.2011-4500 [DOI] [PubMed] [Google Scholar]
- Takeda K., Kaisho T., and Akira S.. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126 [DOI] [PubMed] [Google Scholar]
- Tanghe S., Missotten J., Raes K., Vangeyte J., and De Smet S.. 2014. Diverse effects of linseed oil and fish oil in diets for sows on reproductive performance and pre-weaning growth of piglets. Livest. Sci. 164:109–118. doi:10.1016/j.livsci.2014.03.009 [Google Scholar]
- Walker A. W., Ince J., Duncan S. H., Webster L. M., Holtrop G., Ze X., Brown D., Stares M. D., Scott P., Bergerat A., . et al. 2011. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 5:220–230. doi: 10.1038/ismej.2010.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Garrity G. M., Tiedje J. M., and Cole J. R.. 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73:5261–5267. doi: 10.1128/AEM.00062-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Yang M., Xu S., Lin Y., Che L., Fang Z., and Wu D.. 2014. Comparative effects of sodium butyrate and flavors on feed intake of lactating sows and growth performance of piglets. Anim. Sci. J. 85:683–689. doi: 10.1111/asj.12193 [DOI] [PubMed] [Google Scholar]
- Wang J. P., Yoo J. S., Kim H. J., Lee J. H., and Kim I. H.. 2009. Nutrient digestibility, blood profiles and fecal microbiota are influenced by chitooligosaccharide supplementation of growing pigs. Livest. Sci. 125:298–303. doi:10.1016/j.livsci.2009.05.011 [Google Scholar]
- Xu J., Chen X., Yu S., Su Y., and Zhu W.. 2016. Effects of early intervention with sodium butyrate on gut microbiota and the expression of inflammatory cytokines in neonatal piglets. PLoS One 11:e0162461. doi: 10.1371/journal.pone.0162461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H., and Ajuwon K. M.. 2017. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS One 12:e0179586. doi: 10.1371/journal.pone.0179586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W., Li J., Wang J. J., Zhou W., Wang Q., Zhu R., Wang F., and Thacker P.. 2013. Effects of dietary ratio of n-6 to n-3 polyunsaturated fatty acids on immunoglobulins, cytokines, fatty acid composition, and performance of lactating sows and suckling piglets. J. Anim. Sci. Biotechnol. 3:1–8. doi:10.1186/2049-1891-3-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Lee K. Y., Kim J. K., and Kim I. H.. 2017. Effects of different n-6 to n-3 polyunsaturated fatty acids ratio on reproductive performance, fecal microbiota and nutrient digestibility of gestation-lactating sows and suckling piglets. Anim. Sci. J. 88:1744–1752. doi: 10.1111/asj.12819 [DOI] [PubMed] [Google Scholar]
- Yu S., Ren E., Xu J., and Zhu W.. 2017. Effects of early intervention with sodium butyrate on lipid metabolism-related gene expression and liver metabolite profiles in neonatal piglets. Livest. Sci. 195:80–86. doi:10.1016/j.livsci.2016.11.013 [Google Scholar]
- Zeng X., Sunkara L. T., Jiang W., Bible M., Carter S., Ma X., Qiao S., and Zhang G.. 2013. Induction of porcine host defense peptide gene expression by short-chain fatty acids and their analogs. PLoS One 8:e72922. doi: 10.1371/journal.pone.0072922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentek J., Buchheit-Renko S., Ferrara F., Vahjen W., Van Kessel A. G., and Pieper R.. 2011. Nutritional and physiological role of medium-chain triglycerides and medium-chain fatty acids in piglets. Anim. Health Res. Rev. 12:83–93. doi: 10.1017/S1466252311000089 [DOI] [PubMed] [Google Scholar]