Abstract
The objectives of this study were to assess the effects of Saccharomyces cerevisiae fermentation products (SCFP; NaturSafe, SCFPns; and Original XPC, XPC; Diamond V) on growth performance, carcass traits, immune response, and antimicrobial resistance in beef steers fed high-grain diets. Ninety Angus steers (initial body weight [BW], 533 ± 9.8 kg) were assigned to a randomized complete design with 6 treatments (n = 15/treatment): 1) control, 2) low (12 g SCFPns·steer−1·d−1), 3) medium (15 g SCFPns·steer−1·d−1), 4) high SCFP (18 g SCFPns·steer−1·d−1), 5) encapsulated XPC (eXPC; 7 g XPC·steer−1·d−1 encapsulated with 9 g capsule material), and 6) antibiotics (ANT; 330 mg monensin + 110 mg tylosin·steer−1·d−1). Steers were fed ad libitum a diet containing 10% barley silage and 90% barley grain concentrate mix (dry matter basis) for 105 d. Increasing SCFPns tended (P < 0.09) to linearly increase feed efficiency. Average daily gain (ADG) tended (P < 0.10) to be greater in steers supplemented with eXPC than control. The SCFPns also tended (P < 0.10) to linearly increase marbling score. Proportion of severely abscessed livers tended (P < 0.10) to be lower in steers supplemented with medium and high SCFPns, eXPC, or ANT. A treatment × days on feed interaction were noticed (P < 0.01) for blood glucose, blood urea nitrogen (BUN), and acute phase proteins. The concentration of blood glucose responded quadratically (P < 0.05) on days 28 and 56, whereas BUN linearly (P < 0.01) increased on day 105 with increasing SCFPns dose. The SCFPns linearly increased haptoglobin (P < 0.03) and serum amyloid A (SAA;P < 0.05) concentrations on day 105, and lipopolysaccharide binding protein (LBP;P < 0.01) on days 56 and 105. The percentage of erythromycin-resistant and erythromycin + tetracycline-resistant enterococci was greater (P < 0.05) with ANT than control, SCFPns, and eXPC, whereas no difference was observed among control, SCFPns, and eXPC. No treatment effect was detected on the percentage of tetracycline-resistant enterococci. These results indicate that feeding SCFPns and eXPC was beneficial in improving ADG, feed efficiency and decreasing liver abscesses in a manner comparable to ANT. Unlike antibiotics, SCFPns or eXPC did not increase antimicrobial resistance. Both SCFPns and eXPC are potential alternatives to in-feed antibiotics.
Keywords: antibiotics, beef steers, erythromycin resistance, growth performance, Saccharomyces cerevisiae fermentation products, tetracycline resistance
Introduction
Antibiotics like monensin and tylosin have been widely used in beef feedlot operations to improve feed efficiency and reduce the incidence of liver abscesses (Nagaraja and Chengappa, 1998; Meyer et al., 2009). However, increasing concern over the use of antibiotics in livestock leading to the emergence of antibiotic-resistant bacteria with adverse impacts on human health has resulted in their ban for growth promotion in many countries. Therefore, there is an increasing need for alternatives to antibiotics for use in livestock production. Saccharomyces cerevisiae fermentation products (SCFP) are produced through anaerobic fermentation and have been widely used in livestock feed. The SCFP contains B vitamins, amino acids, organic acids, and other nutritional elements that stimulate the growth of fiber-digesting and lactic acid-utilizing bacteria (Wiedmeier et al., 1987; Callaway and Martin, 1997). As with the antibiotic monensin (Tedeschi et al., 2003), SCFP has also been reported to improve feed efficiency in feedlot beef cattle (Wagner et al., 2016).
However, production responses to SCFP in beef cattle have been variable, possibly due to differences in dosage, and diet, particularly high-grain diets where the rumen pH is often well below the point that reduces fiber digestion (Swyers et al., 2014; Geng et al., 2016). Studies have reported a reduction (Swyers et al., 2014), and no difference (Geng et al., 2016) in the finishing performance of beef cattle fed diets supplemented with SCFP. Geng et al. (2016) explained that the failure of SCFP to improve the growth performance of finishing cattle was related to the inclusion of monensin in the diet. Wagner et al. (2016) concluded from a meta-analysis study that the marginal improvement in feed efficiency of finishing cattle fed SCFP reflected increases in nutrient digestibility and the ruminal production of propionate. Our previous study showed that feeding SCFP to finishing beef heifers improved ruminal and total tract digestibility of OM and NDF (Shen et al., 2018), with responses in rumen fermentation to SCFP often being dose-dependent (Lascano et al., 2012; Shen et al., 2018).
In addition, until recently, most research studies focused on evaluating the effects of SCFP on rumen fermentation and microbial activity. In studies with swine, SCFP have improved gut morphology and immune activity (Van der Peet-Schwering et al., 2007; Shen et al., 2009). The improvement in immune activity suggests that SCFP may confer health benefits to swine, but the possibility of this response has not been explored in beef cattle. Recently, Shen et al. (2018) evaluated the effect of SCFP on intestinal function by directly delivering it into the duodenum, a method of administration that tended to increase fecal IgA when compared with when it was orally fed. Elevated IgA is desired and suggests that SCFP may benefit intestinal mucosal immunity if it escapes ruminal degradation and remains biologically active in the lower digestive tract (Suzuki et al., 2004).
Feeding SCFP modulated ruminal microbial profiles (Mullins et al., 2013) and reduced detectable markers of virulence in Salmonella (Feye et al., 2016), which can alter the antibiotic-resistant profile of resident bacteria (Kirkup and Riley, 2004). We hypothesize that feeding SCFP will improve feed efficiency and reduce liver abscesses and antimicrobial-resistant enterococci in a dose-dependent manner in finishing beef steers. Thus, this study was conducted to 1) determine the effect of adding ruminally protected and unprotected SCFP at varying dosages on growth performance, feed efficiency, carcass traits, animal health, and immune response including fecal IgA and acute phase proteins in finishing beef steers; and 2) evaluate the effects of adding SCFP on antimicrobial resistance in enterococci.
Materials and Methods
Experiments with steers were reviewed and approved by the Institutional Animal Care and Use Committee at the Lethbridge Research and Development Center (#1731; Lethbridge, AB, Canada). Cattle were cared for and managed according to the guidelines of the Canadian Council on Animal Care (2009).
Encapsulation of SCFP
Two different SCFP products (NaturSafe, SCFPns; and Original XPC, XPC; Diamond V, Cedar Rapids, IW) were used. Both products were composed of dried S. cerevisiae fermentation products, but were different SCFP preparation. The XPC (eXPC) was encapsulated in a stearic acid and palm oil-based capsule material (King Techina Feed Co., Ltd, Hangzhou, China) as reported by Wang et al. (2011). The eXPC consisted of approximately 45% of XPC and 55% of capsule material. The stability of eXPC was assessed by incubating it in vitro with rumen inoculum for 6, 12, or 24 h and measuring DM disappearance (DMD). Similarly, the release of eXPC in the intestine was estimated by measuring DMD from bags incubated under simulated abomasal and intestinal conditions with the incubation solution containing lecithin, bile salts, bile acids, and trypsin (Shen et al., 2017). The DMD after 24 h of in vitro ruminal incubations was 82% for XPC and only 25% for eXPC. The sum of DMD in the simulated ruminal and intestinal models was 88% and 71% for XPC and eXPC, respectively. These results suggest that a large portion of eXPC would reach the intestinal tract. Although one cannot definitively determine whether the more soluble fermentation metabolite components within eXPC were made available in the rumen or in the intestine, a significant part of eXPC escapes rumen fermentation and would be expected to be active in the intestine.
Animals, Design, and Treatments
Ninety Angus steers (15 head/treatment) were purchased at local auction market from the same source and housed in individual feedlot pens (4.9 × 1.8 m) in a sheltered barn bedded with sawdust. Steers were gradually adapted to a high-grain finishing diet over 4 wk before starting the experiment, ear tagged, and vaccinated against infectious bovine rhinotracheitis (IBR), parainfluenza-3 (PI-3) virus, and Haemophilus somnus (Resvac 2/Somubac, Pfizer Animal Health, Parsippany-Troy Hills, NJ) and against Clostridium spp. (Tasvax 8, Schering-Plough Animal Health, Upper Hutt, NZ). Steers (initial BW, 533 ± 9.8 kg) were blocked by BW and assigned to a randomized complete design to 1 of 6 treatments. The treatments were 1) control (no antibiotics, no SCFP); 2) low (12 g SCFPns·steer−1·d−1); 3) medium (15 g SCFPns·steer−1·d−1); 4) high (18 g SCFPns·steer−1·d−1); 5) eXPC (16 g eXPC·steer−1·d−1); and 6) antibiotics (ANT; 330 mg monensin + 110 mg tylosin·steer−1·d−1). The dose of SCFPns used was based on the manufacture’s recommendation. All the treatments were mixed with 50 g ground barley and 8 g molasses, and top dressed onto feed once daily at feeding. Steers in the control group received 50 g ground barley and 8 g molasses.
Steers were fed a total mixed ration (TMR) ad libitum once daily at 0900 for 105 d. The TMR contained 10% barley silage, 87% dry-rolled barley, and 3% vitamin and mineral supplement (DM basis) to meet the nutrient requirements of finishing beef steers at a daily growth rate of 1.8 kg (NASEM, 2016; Table 1). The diets were prepared daily using a Data Ranger feed mixer (American Calan Inc., Northwood, NH). Fresh water was continuously available to all steers throughout the experiment.
Table 1.
Ingredient and chemical composition of the total mixed diet
| Item | |
|---|---|
| Ingredient, % DM | |
| Barley silage1 | 10.0 |
| Barley grain,2 dry-rolled | 87.0 |
| Supplement | |
| Barley, ground | 1.64 |
| Canola meal | 0.29 |
| Calcium carbonate | 0.73 |
| Molasses | 0.07 |
| Salt | 0.15 |
| Feedlot premix3 | 0.03 |
| Urea | 0.06 |
| Vitamin E (500,000 IU/kg) | 0.002 |
| Canola oil | 0.03 |
| Chemical composition, % DM | |
| DM | 79.8 |
| OM | 96.6 |
| NDF | 23.1 |
| ADF | 7.20 |
| Starch | 54.2 |
| CP | 13.2 |
1Composition (DM basis): 39.4% DM, 46.6% NDF, 24.7% ADF, 22.1% starch and 12.9% CP based on 4 samples composited by period.
2Composition (DM basis): 90.2% DM, 20.8% NDF, 5.3% ADF, 58.7% starch and 13.4% CP based on 4 samples composited by period.
3Supplied per kilogram of dietary DM: 15 mg Cu, 65 mg Zn, 28 mg Mn, 0.7 mg I, 0.2 mg Co, 0.3 mg Se, 6,000 IU vitamin A, 600 IU vitamin D, and 47 IU vitamin E.
The amount of feed offered was recorded daily for each steer and refusals were weighed weekly. Rolled barley, barley silage, TMR, and refusals were collected weekly and oven dried at 55 °C for 48 h to measure DM content. The daily DMI was calculated as the daily feed offered minus the weekly feed refused divided by 7 (DM basis). The steers were weighed on 2 consecutive days at the beginning and end of the experiment, and every 28 d in between. The ADG was calculated by subtracting initial BW from BW at the end of the experiment and dividing by the number of days on-feed. Feed efficiency (ratio of gain to feed, G:F) was determined as the ratio of ADG to daily DMI.
Blood Sampling and Analysis
Ten steers from each treatment were randomly selected for blood sampling. Blood samples were collected via jugular venipuncture on days 0, 28, 56, and 105. Two 10-mL vacuum tubes (Vacutainer, Becton Dickinson, Franklin Lakes, NJ) containing Na heparin or without additive were used to collect plasma and serum, respectively. Plasma samples were obtained by centrifuging at 3,000 × g for 20 min at 4 °C, and serum samples were obtained by centrifuging at 2,000 × g for 20 min at 4 °C. Both plasma and serum samples were stored at −20 °C. A subsample (1 mL) of the plasma was centrifuged at 16,000 × g for 2 min at 4 °C to remove fibrinogen, and the supernatants were analyzed for blood urea N (BUN), and blood glucose using a dry chemistry analyzer (VetTest analyzer, model 8008, IDEXX Lab, Westbrook, ME). The serum nonesterified fatty acid (NEFA) was determined using a commercial enzymatic colorimetric procedure (NEFA-HR 2, Wako Chemicals Inc., Richmond, VA). Concentrations of serum amyloid A (SAA; SEA885Bo), plasma haptoglobin (Hp; SEA817Bo), and lipopolysaccharide binding protein (LBP; SEB406Bo) were determined using bovine ELISA kits (Cloud-Clone Corp., Katy, TX) following the manufacturer’s instructions. The minimum detectable dose of SAA, Hp, and LBP is typically less than 0.067, 5.900, and 0.287 ng/mL, respectively.
Fecal Sampling and Analysis
Fecal samples (approximately 400 g wet) were collected concurrent with blood collection. After sampling, fecal pH was measured immediately using a pH meter (B20PI, SympHony Benchtop Meters; VWR, Edmonton, AB, Canada). Fecal samples were subdivided into 4 portions for the enumeration of generic Escherichia coli (E. coli), antimicrobial-resistant enterococci, estimation of fecal IgA concentration, and DM measurement. For enumeration of E. coli, 1 g of fresh feces was weighed into a 16 × 125 mm culture tube containing 9 mL of 0.1% peptone dilution water and vortexed vigorously. Serial dilutions of 10−4, 10−5, and 10−6 were prepared and 5 mL were then spread plated onto E. coli Petrifilm media (3M Canada, London, ON, Canada) and incubated at 35 °C for 24 h. Colonies that appeared blue and exhibited gas bubbles were counted as E. coli. Antimicrobial resistance in enterococci populations was determined as described by Beukers et al. (2015). Briefly, fecal samples were weighed (1.0 g) into sterile 4.0-mL tubes of 1 × phosphate buffered saline and vortexed for 30 s. Samples were serially diluted 10-fold and 100 µL of the 5−3, 5−4, and 5−5 were plated in duplicate onto Bile Esculin Azide (BEA) agar containing no antibiotics, and 5−1, 5−2, and 5−3 onto BEA amended with erythromycin (8 µg/mL; BEAE), BEA amended with tetracycline (16 µg/mL; BEAT), and BEA amended with a mixture of erythromycin and tetracycline (8 µg/mL; 16 µg/mL; BEAET). For greater feasibility, SCFPns at 15 g/d was not included in the AMR portion of this study, justified by including the upper (18 g/d) and lower (12 g/d) concentrations in feed. In addition, only the control, SCFPns at 18 g/d, and ANT treatment groups were enumerated on BEAT and BEAET. Antibiotic selective media was chosen based on importance in human medicine and observed resistance in enterococci in recent studies (Beukers et al., 2015). The concentration of antibiotics used in all plates was set at the breakpoint standards for defining resistance as described by the Clinical and Laboratory Standards Institute guidelines (2008). All plates were incubated for 48 h at 37 °C and isolates that grew on these plates were considered resistant to erythromycin (EryR), tetracycline (TetR), or both tetracycline and erythromycin (EryRTetR). The percentage of enterococci resistant to “X” on the selective plates was calculated according to Alexander et al. (2010), in which [(number of colonies on selective BEAX plates/total colonies on nonselective BEA plates) × 100%]. Fecal IgA was analyzed using ELISA kits (Bovine IgA ELISA Quantitation Set, Bethyl Laboratories, Montgomery, TX).
Carcass Traits
At the end of the experiment, steers were transported to a commercial abattoir for slaughter. Hot carcass weights (HCW, with kidneys removed), dressing percentage, 12th-rib back fat thickness (BFT), longissimus muscle (LM) area, marbling score, quality grade, saleable meat yield, and liver abscess score were recorded for each carcass. The percentage of HCW on final BW was calculated as dressing percentage. Marbling score was estimated according to pictorial standards from 1 (devoid) to 10 (abundant marbling; USDA, 1989). Canada AAA standards were used to estimate the quality grade with Canada grade A being equivalent to USDA Standard; AA to USDA Select; and AAA to USDA Choice. Saleable meat yield was calculated using an equation that considered the length, width, and fat cover over the rib eye muscle between the 11th and 12th ribs: Saleable meat (%) = (57.96 − 0.027 HCW + 0.202 LM area − 0.703 BFT)%. Liver abscess scores were generated according to the ranking scale used by the Canadian Beef Grading Agency as described by Brink et al. (1990). A liver was defined as abscessed if it had at least one abscess, and severe liver abscesses were defined as a liver with at least 4 small abscesses or at least 1 abscess with diameter larger than 2.5 cm.
Statistical Analysis
Data of DMI, BW, ADG, G:F, and carcass traits were analyzed using the Mixed procedure of SAS (Version 16.0.0, SAS Inst. Inc. Cary, NC) as a completely randomized design. The model included treatment as a fixed effect and steers within treatment as a random effect to account for blocking by initial BW. After a log transformation of E. coli counts and levels of antimicrobial resistance in enterococci, these indicators were analyzed together with blood metabolites, acute phase proteins, fecal pH, and IgA, using the Mixed procedure of SAS as a completely randomized design with treatment, days on-feed and their interaction as fixed effects, and steers within treatment as a random effect. The mixed model also included day 0 as covariate for analysis of blood metabolites and acute phase proteins. For repeated measures, various covariance structures were tested and AR(1) was selected based on the lowest value for Akaike’s information criteria. Orthogonal contrasts were conducted to determine linear and quadratic SCFPns dose responses. The GLIMMX procedure of SAS (version 16.0.0, SAS Inst. Inc., Cary, NC) was used to analyze meat quality and liver abscess data. Least square means were compared using the Tukey correction for multiple comparisons, and treatment effects were declared significant at P ≤ 0.05 and trends at 0.05 < P ≤ 0.10.
Results
Growth Performance and Carcass Traits
Across treatments, DMI averaged 12.0 kg/d and did not differ among treatments (Table 2). Final BW and ADG were not affected by either SCFP supplementation or monensin and tylosin, whereas the ADG tended (P < 0.10; P value not shown in table) to be greater with eXPC than control. Feed efficiency tended (P < 0.09) to be greater with eXPC than control and linearly improved with increasing SCFPns in the diet.
Table 2.
Growth and carcass traits of finishing steers fed a diet supplemented with, Saccharomyces cerevisiae fermentation product (SCFPns), encapsulated Original XPC (eXPC) or antibiotics (ANT)1
| SCFPns, g/d | P-value2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item | 0 | 12 | 15 | 18 | eXPC | ANT | SEM | Treat | L | Q |
| No. of steers | 15 | 15 | 15 | 15 | 15 | 15 | ||||
| Growth | ||||||||||
| Initial BW, kg | 533 | 533 | 533 | 533 | 533 | 533 | 9.8 | 1.00 | 0.99 | 0.96 |
| Final BW, kg | 703 | 719 | 721 | 709 | 722 | 716 | 13.4 | 0.36 | 0.59 | 0.45 |
| DMI, kg/d | 11.8 | 12.2 | 11.9 | 11.6 | 12.5 | 11.9 | 0.36 | 0.84 | 0.95 | 0.23 |
| ADG, kg/d | 1.62 | 1.77 | 1.79 | 1.68 | 1.80 | 1.75 | 0.08 | 0.49 | 0.26 | 0.15 |
| G:F, g/kg | 137 | 145 | 150 | 146 | 144 | 147 | 4.9 | 0.54 | 0.09 | 0.42 |
| Carcass traits | ||||||||||
| HCW, kg | 414 | 423 | 420 | 417 | 421 | 421 | 8.4 | 0.97 | 0.64 | 0.49 |
| Dressing, % | 58.9 | 58.8 | 58.2 | 58.8 | 58.5 | 59.0 | 0.37 | 0.73 | 0.56 | 0.66 |
| Back fat, mm | 20.4 | 22.0 | 20.8 | 20.1 | 19.3 | 20.8 | 1.71 | 0.92 | 0.97 | 0.40 |
| LM area, cm3 | 80.3 | 80.3 | 81.1 | 78.5 | 80.2 | 77.1 | 2.64 | 0.75 | 0.74 | 0.53 |
| Marbling score3 | 5.51 | 6.33 | 5.96 | 6.00 | 5.96 | 5.75 | 0.22 | 0.29 | 0.10 | 0.15 |
| Saleable meat4, % | 48.7 | 47.3 | 48.3 | 48.4 | 49.2 | 47.5 | 1.46 | 0.93 | 0.84 | 0.53 |
| Quality grade5, % | 80 | 100 | 80 | 87 | 93 | 87 | – | 0.97 | ||
| Liver score, % | ||||||||||
| Abscessed6 | 66.7 | 60.0 | 53.3 | 60.0 | 53.3 | 60.0 | – | 0.97 | ||
| Severely7 | 53.3 | 33.3 | 20.0 | 20.0 | 20.0 | 6.7 | – | 0.10 | ||
1ANT = 330 mg monensin + 110 mg tylosin/d; eXPC = 16 g eXPC/d.
2Treat = treatment; L, Q = linear and quadratic effects of SCFPns (0, 12, 15, and 18 g/d).
3According to pictorial standards (from 1 = devoid to 10 = abundant marbling; USDA, 1989).
4Estimated lean yield = 57.96 − 0.027 HCW + 0.202 LM area − 0.703 Back fat.
5Canada grade AAA = equivalent to USDA choice.
6The percentage of liver with at least 1 abscess.
7The percentage of liver with at least 4 small abscesses or at least 1 abscess with a diameter greater than 2.5 cm.
Treatments had no effect on carcass traits, except that marbling score tended (P < 0.10) to linearly increase with increasing SCFPns (Table 2). Although the proportion of total abscessed livers did not differ, abscesses in steers supplemented with SCFPns at 15 and 18 g/d, eXPC or ANT tended (P < 0.10) to be less severe than in control steers.
Blood Metabolites and Acute Phase Proteins
Concentration of blood glucose did not differ among treatments on days 0 and 105, but responded quadratically on days 28 (P < 0.01) and 56 (P < 0.05) to increased SCFPns (Table 3). Blood glucose was also higher (P < 0.03) with the low dose of SCFPns than ANT. On day 28, steers receiving eXPC tended (P < 0.07) to have lower blood glucose concentration than control, without differing from ANT. Serum NEFA concentration did not differ among treatments throughout the feeding period, except for a quadratic (P < 0.08) change in NEFA concentration on day 28 with increasing SCFPns. No treatment effect on BUN concentration was observed on days 0, 28, or 56, whereas concentration of BUN linearly (P < 0.01) increased with increasing SCFPns on day 105. Steers supplemented with eXPC or ANT also had greater (P < 0.04) BUN concentrations than control steers on day 105. The concentration of BUN did not differ between eXPC and ANT steers over the entire feeding period.
Table 3.
Blood metabolites of finishing steers fed a diet supplemented with, Saccharomyces cerevisiae fermentation product (SCFPns), encapsulated Original XPC (eXPC) or antibiotics (ANT)1
| SCFPns, g/d | P-value 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item | 0 | 12 | 15 | 18 | eXPC | ANT | SEM | Treat | L | Q |
| No. of steers | 10 | 10 | 10 | 10 | 10 | 10 | ||||
| Glucose, mg/dL | ||||||||||
| Day 0 | 94.3 | 97.3 | 91.1 | 94.4 | 98.1 | 90.4 | 4.99 | 0.85 | 0.89 | 0.77 |
| Day 28 | 101.6ab | 109.9a | 98.2ab | 87.0b | 87.3b | 92.0b | 5.43 | 0.03 | 0.16 | 0.01 |
| Day 56 | 106.2 | 90.0 | 98.7 | 105.2 | 100.7 | 94.7 | 6.04 | 0.40 | 0.58 | 0.05 |
| Day 105 | 108.6 | 92.3 | 96.5 | 105.2 | 95.0 | 100.0 | 7.46 | 0.62 | 0.43 | 0.12 |
| NEFA, µM | ||||||||||
| Day 0 | 146 | 125 | 164 | 201 | 209 | 204 | 40.0 | 0.58 | 0.43 | 0.26 |
| Day 28 | 105 | 120 | 105 | 79 | 113 | 142 | 15.4 | 0.12 | 0.44 | 0.08 |
| Day 56 | 85 | 86 | 78 | 97 | 77 | 90 | 19.9 | 0.98 | 0.83 | 0.68 |
| Day 105 | 125 | 139 | 152 | 132 | 137 | 136 | 16.8 | 0.93 | 0.50 | 0.53 |
| BUN3, mg/dL | ||||||||||
| Day 0 | 15.0 | 14.3 | 14.2 | 13.5 | 15.6 | 13.2 | 1.51 | 0.87 | 0.52 | 0.86 |
| Day 28 | 20.6 | 20.1 | 16.7 | 23.6 | 22.5 | 19.0 | 1.83 | 0.13 | 0.82 | 0.11 |
| Day 56 | 19.6 | 19.5 | 19.3 | 21.0 | 24.4 | 21.9 | 1.57 | 0.18 | 0.71 | 0.51 |
| Day 105 | 22.5b | 27.4ab | 26.7ab | 30.8a | 30.2a | 30.8a | 1.56 | 0.01 | 0.01 | 0.53 |
1ANT = 330 mg monensin + 110 mg tylosin/d; eXPC = 16 g eXPC/d.
2Treat = treatment; L, Q = linear and quadratic effects of SCFPns (0, 12, 15, and 18 g/d); treatment × day was significant (P < 0.01) for glucose and BUN concentration.
3BUN = Blood urea N.
a,bMeans within a row with different superscripts differ (P < 0.05).
There was a treatment × days on feed interaction (P < 0.01) for plasma Hp, SAA, and LBP concentration (Table 4). Blood concentrations of Hp, LBP, and SAA differed among treatments at day 0; thus, these values were used as covariate to analyze the data for days 28, 56, and 105. There were no differences in plasma Hp concentration among treatments on days 28 and 56, but it quadratically (P < 0.03) increased with increasing SCFPns on day 105. Steers fed eXPC on day 105 had less (P < 0.01) Hp than steers receiving the medium or high dose of SCFPns, and tended to have lower (P < 0.10) Hp concentration than control and ANT. SCFPns linearly increased plasma LBP concentration on days 56 (P < 0.05) and 105 (P < 0.09). Plasma SAA concentration responded quadratically (P < 0.01) on days 28 and 56 or linearly (P < 0.01) on days 105 to increasing SCFPns in the diet. The SAA concentration tended (P < 0.10) to be greater with eXPC than control on days 28 and 105, and also greater (P < 0.01) than ANT on day 28.
Table 4.
Plasma acute phase protein concentration in steers fed a diet supplemented with, Saccharomyces cerevisiae fermentation product (SCFPns), encapsulated Original XPC (eXPC) or antibiotics (ANT)1
| SCFPns, g/d | P-value3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item2 | 0 | 12 | 15 | 18 | eXPC | ANT | SEM | Treat | L | Q |
| No. of steers | 10 | 10 | 10 | 10 | 10 | 10 | ||||
| Hp, μg/mL | ||||||||||
| Day 0 | 1535b | 1094c | 1243c | 1173c | 1176c | 1898a | 123.5 | 0.01 | 0.03 | 0.28 |
| Day 28 | 140 | 141 | 128 | 132 | 136 | 128 | 6.3 | 0.50 | 0.27 | 0.52 |
| Day 56 | 146 | 133 | 130 | 146 | 124 | 124 | 9.3 | 0.31 | 0.59 | 0.19 |
| Day 105 | 124bc | 126bc | 128b | 146a | 113c | 126bc | 5.5 | 0.01 | 0.04 | 0.03 |
| LBP, μg/mL | ||||||||||
| Day 0 | 743a | 516b | 413bc | 336c | 335c | 753a | 67.6 | 0.01 | 0.01 | 0.01 |
| Day 28 | 135 | 145 | 161 | 109 | 104 | 97 | 35.3 | 0.72 | 0.89 | 0.41 |
| Day 56 | 57 | 101 | 106 | 84 | 80 | 95 | 13.7 | 0.14 | 0.05 | 0.09 |
| Day 105 | 79 | 83 | 103 | 112 | 87 | 103 | 12.6 | 0.35 | 0.09 | 0.30 |
| SAA, μg/mL | ||||||||||
| Day 0 | 84.7 | 74.0 | 46.1 | 63.5 | 62.0 | 76.6 | 11.81 | 0.28 | 0.07 | 0.89 |
| Day 28 | 7.2bc | 18.7a | 9.0bc | 10.2b | 11.8b | 3.0c | 2.27 | 0.01 | 0.26 | 0.01 |
| Day 56 | 9.5c | 23.9ab | 31.9a | 10.6c | 9.2c | 13.5bc | 4.71 | 0.01 | 0.13 | 0.01 |
| Day 105 | 13.2 | 19.4 | 28.6 | 29.5 | 22.2 | 22.4 | 4.46 | 0.14 | 0.01 | 0.50 |
1ANT = 330 mg monensin + 110 mg tylosin/d; eXPC = 16 g eXPC/d.
2Hp = haptoglobin; SAA = serum amyloid A; LBP = lipopolysaccharide binding protein.
3Treat = treatment; L, Q = linear and quadratic effects of SCFPns (0, 12, 15, and 18 g/d); treatment × day was significant (P < 0.01).
a-cMeans within a row with different superscripts differ (P < 0.05).
Fecal pH, IgA, and Total E. coli
The fecal pH, IgA concentration, and total E. coli counts did not differ among treatments. A treatment × days on feed interaction was significant (P < 0.01) for all fecal variables measured (Table 5). Fecal pH linearly (P < 0.04) increased with increasing SCFPns on day 28. The eXPC tended (P < 0.09) to increase fecal pH compared with control on days 28 and 105. No treatment effect was observed on fecal IgA on days 28 and 56, whereas steers receiving eXPC had lower fecal IgA than control (P < 0.08) and other treatments (P < 0.05). Fecal E. coli counts linearly decreased with increasing SCFPns on days 56 (P < 0.07) and 105 (P < 0.02). Steers supplemented with eXPC had lower (P < 0.05) E. coli compared with control and ANT on day 105.
Table 5.
Fecal pH, IgA, and E. coli counts in finishing steers fed a diet supplemented with Saccharomyces cerevisiae fermentation product (SCFPns), encapsulated Original XPC (eXPC) or antibiotics (ANT)1
| SCFPns, g/d | P-value2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item | 0 | 12 | 15 | 18 | eXPC | ANT | SEM | Treat | L | Q |
| No. of steers | 10 | 10 | 10 | 10 | 10 | 10 | ||||
| Fecal pH | ||||||||||
| Day 0 | 6.19 | 6.40 | 6.49 | 6.25 | 6.28 | 6.23 | 0.12 | 0.42 | 0.28 | 0.15 |
| Day 28 | 6.22b | 6.37b | 6.22b | 6.63a | 6.49ab | 6.74a | 0.10 | 0.01 | 0.04 | 0.13 |
| Day 56 | 6.23 | 6.17 | 6.38 | 6.30 | 6.54 | 6.63 | 0.14 | 0.18 | 0.61 | 0.71 |
| Day 105 | 6.37 | 6.38 | 6.60 | 6.52 | 6.60 | 6.66 | 0.10 | 0.17 | 0.16 | 0.65 |
| IgA, µg/g | ||||||||||
| Day 0 | 2.41 | 2.34 | 2.29 | 1.28 | 1.07 | 1.78 | 0.50 | 0.25 | 0.24 | 0.20 |
| Day 28 | 1.39 | 2.11 | 1.93 | 1.92 | 1.17 | 1.18 | 0.44 | 0.49 | 0.31 | 0.54 |
| Day 56 | 5.29 | 5.67 | 7.61 | 6.18 | 5.26 | 5.09 | 1.18 | 0.66 | 0.36 | 0.90 |
| Day 105 | 3.78ab | 4.42a | 3.28ab | 4.56a | 1.82b | 5.12a | 0.78 | 0.05 | 0.71 | 0.23 |
| E. coli counts3 | ||||||||||
| Day 0 | 7.15 | 7.21 | 7.45 | 7.32 | 7.39 | 7.43 | 0.14 | 0.58 | 0.24 | 0.97 |
| Day 28 | 7.28bc | 7.09c | 7.61a | 7.24bc | 7.44ab | 7.51ab | 0.13 | 0.05 | 0.62 | 0.72 |
| Day 56 | 7.42a | 6.93b | 7.30ab | 7.14b | 7.25b | 7.43a | 0.11 | 0.02 | 0.07 | 0.08 |
| Day 105 | 7.50 | 7.14 | 7.15 | 7.17 | 7.18 | 7.31 | 0.12 | 0.23 | 0.02 | 0.35 |
1ANT = 330 mg monensin + 110 mg tylosin/d; eXPC = 16 g eXPC/d.
2Treat = treatment; L, Q = linear and quadratic effects of SCFPns (0, 12, 15, and 18 g/d); treatment × day was significant (P < 0.01).
3 E. coli counts showed on log10 basis.
a-cMeans within a row with different superscripts differ (P < 0.05).
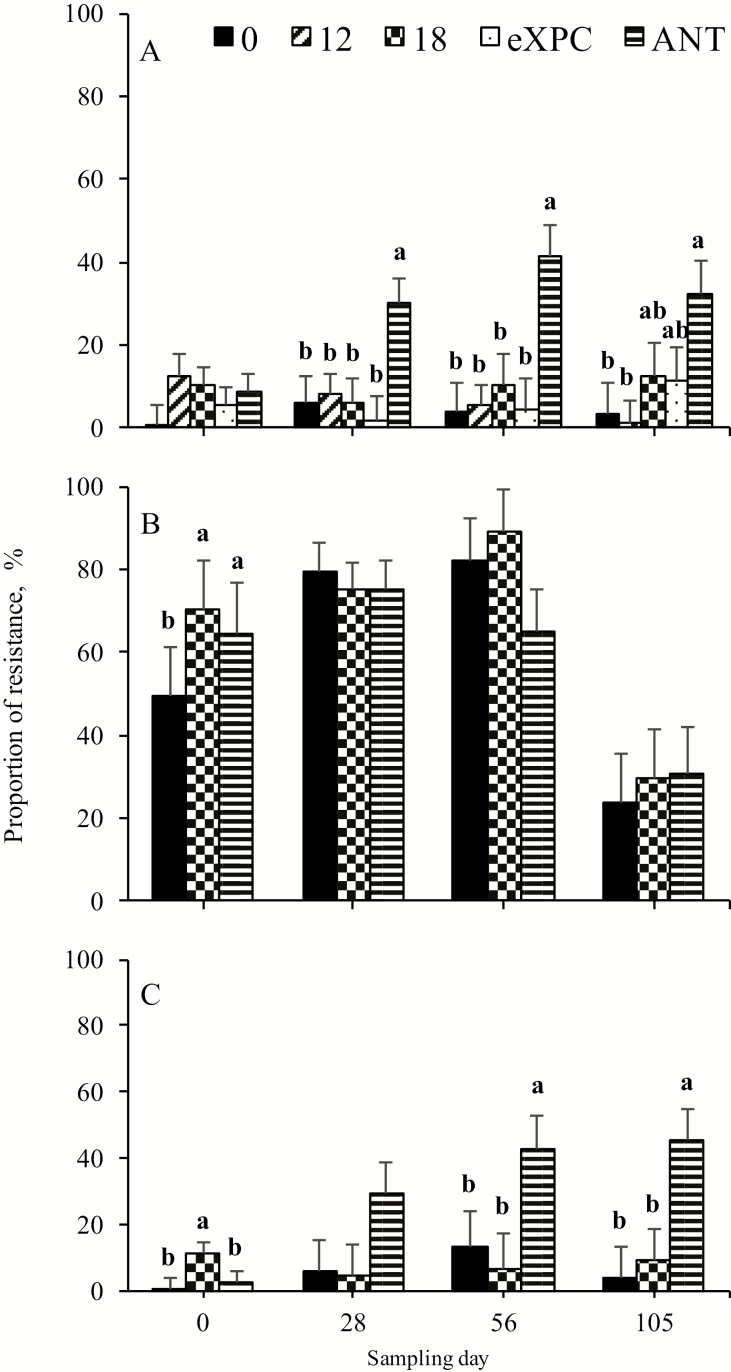
Antimicrobial Resistance of Enterococci
The percentage of EryR enterococci did not differ among treatments on day 0 (Table 6; Figure 1). However, on days 28 and 56, the percentage of EryR enterococci was greater (P < 0.02) in steers receiving antibiotics compared with other treatments. On day 105, although no significant treatment effects were observed, the percentage of EryR enterococci in steers receiving antibiotics tended (P < 0.06) to be greater than other treatments. The percentage of TetR enterococci was greater (P < 0.01) for steers supplemented with SCFPns and antibiotics than control on day 0, whereas no difference among treatments was observed from days 28 to 105. The percentage of EryRTetR enterococci was greater (P < 0.02) for steers receiving SCFPns compared with control and antibiotics on day 0, with no difference on day 28. From days 56 to 105, there was an increase of EryRTetR (P < 0.05) in steers administered antibiotics when compared with control and SCFPns steers.
Table 6.
Antimicrobial resistance of enterococci in finishing steers fed a diet supplemented with, Saccharomyces cerevisiae fermentation product (SCFPns), encapsulated Original XPC (eXPC) or antibiotics (ANT)1
| SCFPns, g/d | P-value4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item2,3 | 0 | 12 | 18 | eXPC | ANT | SEM | Treat | L | Q | |
| BEA, cfu/g feces | ||||||||||
| Day 0 | 3.59 | 4.21 | 3.68 | 4.54 | 4.67 | 0.61 | 0.63 | 0.80 | 0.46 | |
| Day 28 | 4.59bc | 4.31c | 5.36b | 6.73a | 4.15c | 0.31 | 0.01 | 0.17 | 0.04 | |
| Day 56 | 3.97 | 4.15 | 4.36 | 4.17 | 3.09 | 0.33 | 0.08 | 0.43 | 0.85 | |
| Day 105 | 3.20 | 2.48 | 3.11 | 3.02 | 2.17 | 0.61 | 0.70 | 0.79 | 0.38 | |
| BEAE, cfu/g feces | ||||||||||
| Day 0 | 0.79 | 2.33 | 2.52 | 0.97 | 2.09 | 0.60 | 0.15 | 0.03 | 0.60 | |
| Day 28 | 2.99 | 2.11 | 3.79 | 3.86 | 2.56 | 0.56 | 0.14 | 0.53 | 0.05 | |
| Day 56 | 1.58 | 1.23 | 2.08 | 1.63 | 1.84 | 0.54 | 0.85 | 0.65 | 0.32 | |
| Day 105 | 0.88 | 0.64 | 2.06 | 0.92 | 1.36 | 0.45 | 0.21 | 0.13 | 0.08 | |
| BEAT, cfu/g feces | ||||||||||
| Day 0 | 3.17 | N/A4 | 4.49 | N/A | 4.40 | 0.73 | 0.15 | |||
| Day 28 | 4.49b | N/A | 5.24a | N/A | 4.01b | 0.32 | 0.04 | |||
| Day 56 | 3.99a | N/A | 4.32a | N/A | 2.64b | 0.40 | 0.02 | |||
| Day 105 | 2.17 | N/A | 2.35 | N/A | 1.63 | 0.57 | 0.66 | |||
| BEAET, cfu/g feces | ||||||||||
| Day 0 | 0.47 | N/A | 2.51 | N/A | 1.08 | 0.58 | 0.06 | |||
| Day 28 | 3.01 | N/A | 3.71 | N/A | 2.89 | 0.51 | 0.49 | |||
| Day 56 | 1.96 | N/A | 2.10 | N/A | 1.96 | 0.61 | 0.98 | |||
| Day 105 | 1.11 | N/A | 1.54 | N/A | 1.71 | 0.50 | 0.69 | |||
1ANT = 330 mg monensin + 110 mg tylosin/d; eXPC = 16 g eXPC/d.
2Media types: BEA = Bile Esculin Azide (BEA) agar (total enterococci); BEAE = BEA amended with erythromycin (8 µg/mL); BEAT = BEA amended with tetracycline (16 µg/mL); BEAET = BEA amended with a mixture of erythromycin and tetracycline (8 and 16 µg/mL).
3N/A: not applicable, treatment 12 g SCFPns and eXPC were not plated on tetracycline or erythromycin + tetracycline. For greater feasibility, SCFPns at 15 g/d was not included in the AMR portion of this study, justified by including the upper (18 g/d) and lower (12 g/d) concentrations in feed. In addition, only the control, SCFPns at 18 g/d, and ANT treatment groups were enumerated on BEAT and BEAET. No treatment × day effects occurred.
4Treat = treatment; L, Q = linear and quadratic effects of SCFPns (0, 12, and 18 g); treatment × day was significant (P < 0.01).
a-cMeans within a row with different superscripts differ (P < 0.05).
Figure 1.
Proportion of resistance to (A) erythromycin, (B) tetracycline, or (C) erythromycin and tetracycline in fecal enterococci isolates collected across sampling days. a,bMeans within a day with different superscripts differ (P < 0.05). ANT = 330 mg monensin + 110 mg tylosin/d; eXPC = 7 g XPC·steer−1·d−1 encapsulated with 9 g capsule material.
Discussion
Effects of SCFPns and eXPC on Growth Performance and Carcass Traits
The absence of an effect of SCFPns on DMI confirms our previous findings that DMI of beef heifers fed a high-barley grain diet with SCFPns did not differ from control heifers (Shen et al., 2018). Other studies also found no effect of SCFP on DMI in either beef cattle (Swyers et al., 2014) or dairy cows (Acharya et al., 2017). Wagner et al. (2016) conducted a meta-analysis by compiling 18 experiments evaluating the effects of SCFP (Diamond V, Cedar Rapids, IW) on feedlot performance and found that although cattle receiving SCFP had greater DMI, the difference in DMI between treatments was subtle (control vs. SCFP; 7.69 vs. 7.77 kg/d; P < 0.05). Allen (2000) proposed that ruminal propionate production played an important role in decreasing DMI. Ruminal propionate is absorbed across the rumen wall into liver where it is oxidized and yields sufficient ATP to evoke satiety by the vagus nerve, thus reducing DMI. Total VFA concentration and molar proportion of propionate were not affected by adding SCFPns to the high-barley grain diet in our previous study (Shen et al., 2018). This could partly explain the lack of SCFPns effect on DMI in the present study. However, compared with control cattle, there was a slight but consistently greater DMI with either duodenally delivered SCFPns (+0.8 kg DMI·d−1; Shen et al., 2018) and the rumen-protected eXPC in the present study (+0.7 kg DMI·d−1).
The trend of improved feed efficiency with SCFPns is consistent with the improvement in ruminal and total tract digestibility of OM, NDF, and CP observed in our previous study using beef heifers fed a high-grain diet and supplemented with the same SCFPns product (Shen et al., 2018). Swyers et al. (2014) indicated that the most consistent response to SCFP was a stimulation of rumen cellulolytic bacteria. Although the dietary fiber content of the high-grain diet in our study was relatively low (23.1% NDF), the improved feed efficiency is likely associated with the increased fiber and protein digestibility, as well as an increased in rumen pH (Shen et al., 2018). However, the trend of greater ADG with eXPC than control appeared to be primarily due to numerically greater DMI in steers fed eXPC (12.5 vs. 11.8 kg/d). Although the XPC was encapsulated to protect it from rumen degradation, approximately 25% of eXPC (including both capsule and XPC) was released in in vitro rumen fermentation as we described in the preparation of eXPC. Neither SCFPns nor eXPC had an effect on growth performance or G:F when compared with ANT, which is consistent with Scott et al. (2017). These authors reported that the growth performance did not differ between beef heifers fed a combination of monensin and tylosin and heifers fed 18 g/d of SCFPns. The lack of difference in growth performance of steers fed SCFPns and ANT compared with control in the current study appears to be inconsistent with previous findings in beef heifers provided SCFPns (Shen et al., 2018). In that study, ruminal and total tract digestibility of OM and NDF improved with supplementation of SCFPns (18 g/d) compared with ANT and control. Nevertheless, the energy provided by the present high-grain diet exceeded energy requirements (NASEM, 2016), and therefore any improvements in the total tract OM digestibility with SCFP vs. control (80.7 vs. 77.2%; Shen et al., 2018) may have limited the impact on the growth performance.
The lack of an overall treatment effect on growth performance likely accounts for the similar carcass traits among treatments (Swyers et al., 2014; Geng et al., 2016). The greater marbling score with addition of SCFPns is in agreement with other studies that reported beneficial effects of feeding SCFP in beef steers. In a recent meta-analysis, supplementation of SCFP including different SCFP-based products developed by Diamond V such as YC, XP, and XPC, compared with beef cattle fed conventional diets, resulted in an improvement in quality grade (more USDA Choice and less USDA Select carcasses; Wagner et al., 2016). The improved marbling quality with feeding SCFP could be a result of increased ruminal propionate production, which is converted to glucose in the liver. Shen et al. (2018) showed that there tended to be a greater amount (P = 0.10) of OM (0.7 kg/d) fermented in the rumen with SCFP than control without a difference in the molar proportion of propionate, indicating that more propionate was produced with SCFP. Glucose is the preferred carbon source for fatty acid synthesis by intramuscular adipocytes (Smith and Crouse, 1984). The trend of reduced severely abscessed livers with SCFPns may be explained due to alleviation of rumen acidosis by feeding SCFPns as observed previously (Shen et al., 2018). Rapid starch digestion in the rumen of steers fed high-grain diets can lower ruminal pH and cause digestive disturbances such as acidosis and rumenitis, increasing the incidence of liver abscesses (McAllister et al., 1990; Nagaraja and Lechtenberg, 2007a, 2007b). Similarly, rapid starch digestion can also occur in the hindgut of steers fed high-grain diets, increasing the release of endotoxin and the translocation of pathogens (Li et al., 2012). The decrease in severely abscessed livers with eXPC addition suggests that feeding eXPC might have some potential benefits in decreasing the release of endotoxin and protect the cattle from translocating pathogens in the hindgut. In addition, although there was a trend (P < 0.09) for a linear effect of increasing SCFPns dosage on G:F and marbling score, differences in final BW, ADG, and carcass traits among low, medium, and high doses were minimal.
The lack of effect of ANT on carcass characteristics observed is consistent with previous studies that feeding monensin had no effect on carcass characteristics (Swyers et al., 2014; Ran et al., 2018). However, the reduction in severely abscessed livers in steers fed ANT was expected as a result of the inclusion of tylosin in the diet. Antibiotics, particularly tylosin, are commonly used to control liver abscesses in feedlot cattle by reducing acidosis and subsequent rumenitis (Nagaraja and Lechtenberg, 2007b; Amachawadi and Nagaraja, 2016). The fact that tylosin reduced the severity, but not the total number of abscessed livers, is consistent with our recent report of finishing cattle fed barley-based diet (Ran et al., 2018).
Effects of SCFPns and eXPC on Blood Metabolites and Immune Response
Blood glucose and NEFA concentrations are two important indicators for evaluating energy balance in cattle. Glucose can be synthesized from propionate in the liver or directly absorbed from the small intestine, whereas NEFA can be absorbed from feed, or mobilized from adipose tissue. Since lipid levels were low in the diet and no difference was observed in DMI, the exogenous NEFA should be similar between treatments. Blood glucose can be used to assess energy intake, whereas blood NEFA reflect the mobilization of body fat (Herdt, 2000). Usually a higher concentration of blood glucose is indicative of greater energy intake, reduced fat mobilization, and as a result, lower blood NEFA. In the present study, the energy provided by the finishing diet exceeded energy requirements for finishing beef cattle (NASEM, 2016), thus fat mobilization would be minimal. Hence, the absence of differences in blood NEFA concentration among treatments is not surprising. However, the biological impetus for quadratic changes of blood glucose concentration with increasing SCFPns on days 28 and 56 is difficult to explain. The trend of greater blood NEFA concentration with ANT on day 28 was consistent with previous reports that the NEFA concentration increased from 0.13 to 0.20 mM in beef steers supplemented with 330 mg monensin and 110 mg tylosin·steer−1·d−1 (Ran et al., 2018). We suggest that the greater serum NEFA concentration in steers supplemented with ANT was caused by lower DMI due to monensin.
The BUN is a useful indicator to evaluate protein balance in cattle, with the value being driven by protein digestibility and requirement (Kohn et al., 2005). The protein requirement depends on age and growth rate as more protein is required when cattle grow faster. In the present study, the obvious greater BUN on day 105 compared with days 28 and 56 is likely a reflection of steers depositing more fat and less muscle with increasing days of finishing. The linear increase in BUN with increasing SCFPns on day 105 suggests increased intestinal absorption of AA. In fact, feeding SCFPns to heifers fed a high-grain diet increased protein digestibility in the total digestive tract without changing ruminal NH3-N concentration or ruminal protein degradability (Shen et al., 2018). In addition, the greater BUN in cattle fed ANT than cattle fed the control diet may be indicative of reduced degradation of ruminal protein as a result of monensin, and an increase in feed protein digestion in the small intestine (Tedeschi et al., 2003). However, greater BUN with eXPC than control could result from either decreased or increased rumen protein degradability as we did not measure rumen NH3–N concentration with eXPC.
In ruminants, both external and internal stimuli including inflammatory response, tissue injury, and infection can trigger systemic reaction and release acute phase proteins like SAA, Hp, and LBP (Ceciliani et al., 2012; Tothova et al., 2014). Lipopolysaccharide (LPS) release increases from Gram-negative bacteria in cattle fed highly fermentable grain, and LPS is a strong pro-inflammatory agent that stimulates the acute phase response (Plaizier et al., 2012). Both LBP and SAA can bind LPS and play an important role in LPS clearance (Ceciliani et al., 2012). The linear or quadratic response in the concentration of LBP and SAA with increasing SCFPns suggests an improved immune response. Cattle fed high-grain diets are also at greater risk of hindgut acidosis, where LPS is easier to translocate into blood circulation than from rumen wall (Plaizier et al., 2012). The Hp is the principal scavenger of free hemoglobin and prevents oxidative damage (Ceciliani et al., 2012). However, greater blood Hp of steers administered the highest dose of SCFPns was not clear as blood hemoglobin concentration was unaffected (data not shown). Feeding eXPC did not result in differences in acute phase proteins compared with control or ANT, suggesting that eXPC did not elicit the same improvement in immunity as SCFPns. Blood acute phase proteins are also associated with elevated stress (Tothova et al., 2014). The greater concentration of SAA, Hp, and LBP on day 0 in the present study demonstrated that the steers were likely under additional immunological stress at the start of the study.
Effects of SCFPns and eXPC on Fecal Microflora and Immune Status
Fecal pH could be affected by the amount of feed digested in the hindgut. The linear increase in fecal pH with increasing SCFPns on day 28 with no difference in fecal pH on days 56 and 105 is consistent with our previous findings with short period feeding (28 d; Shen et al., 2018). We found that feeding SCFPns to beef heifers resulted in greater ruminal and lower intestinal digestion of OM (Shen et al., 2018). The absence of an effect of SCFPns on fecal IgA is consistent with our previous study (Shen et al., 2018) where a trend for greater fecal IgA concentration was found when SCFPns was added directly to the duodenum but not when it was included in the feed. In contrast, lower fecal IgA concentration was observed when eXPC was fed. The IgA secreted by the gut plays a crucial role in mucosal defense, and fecal IgA concentrations can be used as an indicator of mucosal immunity (Suzuki et al., 2004). The decreased fecal IgA concentration with eXPC suggests a reduction in the immune response or immune suppression. It appeared that adding either SCFPns or eXPC potentially decreased fecal total E. coli counts on days 56 and 105, suggesting possible beneficial effects of SCFPns and eXPC on the intestinal ecosystem. Although the reduction of E. coil counts was less than one log, it may still be biologically relevant.
Effects of SCFPns and eXPC on Antimicrobial Resistance
Enterococci are ubiquitous in nature and occupy a wide range of environments including soil, water, and plants, as well as acting as commensals in animals and humans (Giraffa, 2002). Enterococci are widely used as “indicator” bacteria to determine antimicrobial resistance (AMR) in food-producing animals. The fact that they are easy to culture, can be readily isolated from healthy animals, and exhibit resistance to most clinical antibiotics makes them useful as an indicator of antimicrobial resistance. Tetracycline is the most widely used antibiotic in both humans and animals. Although tylosin is only administered in food producing animals, it is a member of the macrolides, the same family as erythromycin which is widely used for clinical treatment of humans (Zaheer et al., 2013). Antibiotic resistance to both erythromycin and tetracycline has been reported to be increasing worldwide (Inglis et al., 2006). In the present study, although the percentage of EryR and EryRTetR enterococci was considerably lower (<12%) on day 0, the TetR enterococci was quite high (50%–70%), likely reflecting the widespread bacterial resistance to tetracycline in a number of environments. In the present study, the percentage of TetR enterococci appeared to unexpectedly decrease during the experimental period, across all treatments. The percentage of EryR and EryRTetR enterococci was not affected by SCFPns or eXPC, whereas it was increased by adding ANT. In a previous study in Alberta (Beukers et al., 2015), the proportion of EryR enterococci (<10%) did not differ between the control or tylosin fed cattle upon arrival. However, with increasing days on feed, the proportion of EryR enterococci significantly increased in steers administered tylosin in feed. Tylosin promotes erythromycin resistance as both antibiotics are macrolides and members of the MLSB (macrolide–lincosamides–streptogramin B) superfamily, and as a result resistance to one antibiotic in this group can confer resistance to all members (Portillo et al., 2000). The proportion of EryR, TetR, and EryRTetR enterococci in beef cattle feces has not been previously documented, although the identification of resistant gene determinants in related cattle studies has been extensively researched (Chen et al., 2008; Amachawadi et al., 2015; Beukers et al., 2015). Enterococci are known for their genetic “promiscuity” due to their ability to acquire and transfer resistance genes (Werner et al., 2013). The macrolide and tetracycline resistance genes erm(B) and tet(M) are often linked, and evidence of these genes with individual fecal enterococci isolates has been described (Beukers et al., 2015). Although the cattle in our study were not administered tetracycline, co-selection for tetracycline resistance may have occurred as a linkage between tet and erm genes in the feces of tylosin-fed cattle has been identified (Chen et al., 2008). Therefore, administration of tylosin in-feed is not only problematic for selection for macrolide resistance, but also possibly for the co-selection of tetracycline resistance.
In conclusion, inclusion of SCFPns in a high-grain diet tended to linearly improve feed efficiency. As with the inclusion of tylosin in the diet, it also tended to reduce the number of severely abscessed livers, possibly as a result of a reduction in ruminal acidosis. The impact of SCFPns or eXPC supplementation on blood glucose and NEFA appeared minor and inconclusive, whereas the increase in BUN with SCFPns or eXPC suggests increased intestinal absorption of amino acids. The increased blood acute phase protein concentrations in steers supplemented with SCFPns or eXPC suggests that adding SCFPns or eXPC in high-grain diets may have improved immune responses. The effect of SCFPns addition on fecal IgA was not apparent, whereas the decreased fecal IgA concentration with eXPC suggests a reduction in the need for an immune response. Supplementing monensin and tylosin potentially increased the proportion of enterococci resistance to both erythromycin and tetracycline, whereas feeding SCFPns (12 or 18 g/d) or eXPC did not alter the level of antimicrobial resistant enterococci in the feces of finishing cattle. The present results suggest that feeding SCFPns or eXPC to feedlot steers may exert benefits in feed efficiency and health comparable to antibiotics, without increasing the level of antimicrobial resistance within fecal microbial populations. Thus, SCFPns may be used as an alternative to in-feed antibiotics in beef production systems.
Literature Cited
- Acharya S., Pretz J. P., Yoon I., Scott M. F., and Casper D. P.. . 2017. Effects of Saccharomyces cerevisiae fermentation products on the lactational performance of mid-lactation dairy cows. Transl. Anim. Sci. 1:221–228. doi: 10.2527/tas2017.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander T. W., Inglis G. D., Yanke L. J., Topp E., Read R. R., Reuter T., and McAllister T. A.. . 2010. Farm-to-fork characterization of Escherichia coli associated with feedlot cattle with a known history of antimicrobial use. Int. J. Food Microbiol. 137:40–48. doi: 10.1016/j.ijfoodmicro.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Allen M. S. 2000. Effects of diet on short-term regulation of feed intake by lactating dairy cattle. J. Dairy Sci. 83:1598–1624. doi: 10.3168/jds.S0022-0302(00)75030-2. [DOI] [PubMed] [Google Scholar]
- Amachawadi R. G., and Nagaraja T. G.. . 2016. Liver abscesses in cattle: a review of incidence in Holsteins and of bacteriology and vaccine approaches to control in feedlot cattle. J. Anim. Sci. 94:1620–1632. doi: 10.2527/jas.2015-0261. [DOI] [PubMed] [Google Scholar]
- Amachawadi R. G., Scott H. M., Aperce C., Vinasco J., Drouillard J. S., and Nagaraja T. G.. . 2015. Effects of in-feed copper and tylosin supplementations on copper and antimicrobial resistance in faecal enterococci of feedlot cattle. J. Appl. Microbiol. 118:1287–1297. doi: 10.1111/jam.12790. [DOI] [PubMed] [Google Scholar]
- Beukers A. G., Zaheer R., Cook S. R., Stanford K., Chaves A. V., Ward M. P., and McAllister T. A.. . 2015. Effect of in-feed administration and withdrawal of tylosin phosphate on antibiotic resistance in enterococci isolated from feedlot steers. Front. Microbiol. 6:483. doi: 10.3389/fmicb.2015.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink D. R., Lowry S. R., Stock R. A., and Parrott J. C.. . 1990. Severity of liver abscesses and efficiency of feed utilization of feedlot cattle. J. Anim. Sci. 68:1201–1207. doi: 10.2527/1990.6851201x. [DOI] [PubMed] [Google Scholar]
- Callaway E. S., and Martin S. A.. . 1997. Effects of a Saccharomyces cerevisiae culture on ruminal bacteria that utilize lactate and digest cellulose. J. Dairy Sci. 80:2035–2044. doi: 10.3168/jds.S0022-0302(97)76148-4. [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Care 2009. Guide to the care and use of farm animals in research teaching and testing. In: Olfert E. D., Cross B. M., and McWilliam A. A., editors, CCAC guidelines on: the care and use of farm animals in research, teaching and testing. Can. Counc. Anim. Care, Ottawa, Ontario, Canada. [Google Scholar]
- Ceciliani F., Ceron J. J., Eckersall P. D., and Sauerwein H.. . 2012. Acute phase proteins in ruminants. J. Proteomics 75:4207–4231. doi: 10.1016/j.jprot.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Chen J., Fluharty F. L., St-Pierre N., Morrison M., and Yu Z.. . 2008. Technical note: occurrence in fecal microbiota of genes conferring resistance to both macrolide-lincosamide-streptogramin B and tetracyclines concomitant with feeding of beef cattle with tylosin. J. Anim. Sci. 86:2385–2391. doi: 10.2527/jas.2007-0705. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute 2008. Performance standards for antimicrobial susceptibility testing, 18th informational supplement M100-S18. Wayne, PA. [Google Scholar]
- Feye K. M., Anderson K. L., Scott M. F., Henry D. L., Dorton K. L., Depenbusch B. E., and Carson S. A.. . 2016. Abrogation of Salmonella and E. coli O157:H7 in feedlot cattle fed a proprietary Saccharomyces cerevisiae fermentation prototype. J. Vet. Sci. Technol. 7:350–355. doi: 10.4172/2157-7579.1000350 [DOI] [Google Scholar]
- Geng C. Y., Ren L. P., Zhou Z. M., Chang Y., and Meng Q. X.. . 2016. Comparison of active dry yeast (Saccharomyces cerevisiae) and yeast culture for growth performance, carcass traits, meat quality and blood indexes in finishing bulls. Anim. Sci. J. 87:982–988. doi: 10.1111/asj.12522. [DOI] [PubMed] [Google Scholar]
- Giraffa G. 2002. Enterococci from foods. FEMS Microbiol. Rev. 26:163–171. doi: 10.1111/j.1574-6976.2002.tb00608.x. [DOI] [PubMed] [Google Scholar]
- Herdt T. H. 2000. Ruminant adaptation to negative energy balance. Influences on the etiology of ketosis and fatty liver. Vet. Clin. North Am. Food Anim. Pract. 16:215–30, v. [DOI] [PubMed] [Google Scholar]
- Inglis G. D., Morck D. W., McAllister T. A., Entz T., Olson M. E., Yanke L. J., and Read R. R.. . 2006. Temporal prevalence of antimicrobial resistance in Campylobacter spp. from beef cattle in Alberta feedlots. Appl. Environ. Microbiol. 72:4088–4095. doi: 10.1128/AEM.02830-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkup B. C., and Riley M. A.. . 2004. Antibiotic-mediated antagonism leads to a bacterial game of rock-paper-scissors in vivo. Nature 428:412–414. doi: 10.1038/nature02429. [DOI] [PubMed] [Google Scholar]
- Kohn R. A., Dinneen M. M., and Russek-Cohen E.. . 2005. Using blood urea nitrogen to predict nitrogen excretion and efficiency of nitrogen utilization in cattle, sheep, goats, horses, pigs, and rats. J. Anim. Sci. 83:879–889. doi: 10.2527/2005.834879x. [DOI] [PubMed] [Google Scholar]
- Lascano G. J., Heinrichs A. J., and Tricarico J. M.. . 2012. Substitution of starch by soluble fiber and Saccharomyces cerevisiae dose response on nutrient digestion and blood metabolites for precision-fed dairy heifers. J. Dairy Sci. 95:3298–3309. doi: 10.3168/jds.2011-5047. [DOI] [PubMed] [Google Scholar]
- Li S., Khafipour E., Krause D. O., Kroeker A., Rodriguez-Lecompte J. C., Gozho G. N., and Plaizier J. C.. . 2012. Effects of subacute ruminal acidosis challenges on fermentation and endotoxins in the rumen and hindgut of dairy cows. J. Dairy Sci. 95:294–303. doi: 10.3168/jds.2011-4447. [DOI] [PubMed] [Google Scholar]
- McAllister T. A., Cheng K. J., Road L. M., and Buchanan-Smith J. G.. . 1990. Use of formaldehyde to regulate digestion of barley starch. Can. J. Anim. Sci. 70:581–589. doi: 10.4141/cjas90-070 [DOI] [Google Scholar]
- Meyer N. F., Erickson G. E., Klopfenstein T. J., Greenquist M. A., Luebbe M. K., Williams P., and Engstrom M. A.. . 2009. Effect of essential oils, tylosin, and monensin on finishing steer performance, carcass characteristics, liver abscesses, ruminal fermentation, and digestibility. J. Anim. Sci. 87:2346–2354. doi: 10.2527/jas.2008-1493. [DOI] [PubMed] [Google Scholar]
- Mullins C. R., Mamedova L. K., Carpenter A. J., Ying Y., Allen M. S., Yoon I., and Bradford B. J.. . 2013. Analysis of rumen microbial populations in lactating dairy cattle fed diets varying in carbohydrate profiles and Saccharomyces cerevisiae fermentation product. J. Dairy Sci. 96:5872–5881. doi: 10.3168/jds.2013-6775. [DOI] [PubMed] [Google Scholar]
- Nagaraja T. G., and Chengappa M. M.. . 1998. Liver abscesses in feedlot cattle: a review. J. Anim. Sci. 76:287–298. doi: 10.2527/1998.761287x. [DOI] [PubMed] [Google Scholar]
- Nagaraja T. G., and Lechtenberg K. F.. . 2007a. Acidosis in feedlot cattle. Vet. Clin. North Am. Food Anim. Pract. 23:333–350. doi: 10.1016/j.cvfa.2007.04.002 [DOI] [PubMed] [Google Scholar]
- Nagaraja T. G., and Lechtenberg K. F.. . 2007b. Liver abscesses in feedlot cattle. Vet. Clin. North Am. Food Anim. Pract. 23:351–69, ix. doi: 10.1016/j.cvfa.2007.05.002. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (NASEM). 2016. Nutrient requirements of beef cattle. 8th rev. ed Natl. Acad. Press, Washington, DC. [Google Scholar]
- Plaizier J. C., Khafipour E., Li S., Gozho G. N., and Krause D. O.. . 2012. Subacute ruminal acidosis (SARA), endotoxins and health consequences. Anim. Feed Sci. Technol. 172:9–21. doi: 10.1016/j.anifeedsci.2011.12.004 [DOI] [Google Scholar]
- Portillo A., Ruiz-Larrea F., Zarazaga M., Alonso A., Martinez J. L., and Torres C.. . 2000. Macrolide resistance genes in Enterococcus spp. Antimicrob. Agents Ch. 44:967–971. doi: 10.1128/AAC.44.4.967-971.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran T., Shen Y. Z., Saleem A. M., AlZahal O., Beauchemin K. A., and Yang W. Z.. . 2018. Using ruminally protected and nonprotected active dried yeast as alternatives to antibiotics in finishing beef steers: growth performance, carcass traits, blood metabolites, and fecal Escherichia coli. J. Anim. Sci. 96:4385–4397. doi: 10.1093/jas/sky272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. F., Dorton K. L., Henry D. L., Belknap C. R., Hanson D. L., and Depenbusch B. E.. . 2017. Effects of feeding a Saccharomyces cerevisiae fermentation prototype on performance, carcass characteristics, and liver abscess prevalence of beef heifers at a commercial feedlot. Prof. Anim. Scient. 33:320–326. doi.: 10.15232/pas.2016-01580 [DOI] [Google Scholar]
- Shen Y. Z., Jiao P. X., Wang H. R., Chen L. M., Walker N. D., and Yang W. Z.. . 2017. Validation of micro-encapsulation method to protect probiotics and feed enzyme from rumen degradation. J. Anim. Sci. 95 (Suppl. 4):P317 (Abstr.). [Google Scholar]
- Shen Y. B., Piao X. S., Kim S. W., Wang L., Liu P., Yoon I., and Zhen Y. G.. . 2009. Effects of yeast culture supplementation on growth performance, intestinal health, and immune response of nursery pigs. J. Anim. Sci. 87:2614–2624. doi: 10.2527/jas.2008-1512. [DOI] [PubMed] [Google Scholar]
- Shen Y. Z., Wang H. R., Ran T., Yoon I., Saleem A. M., and Yang W. Z.. . 2018. Influence of yeast culture and feed antibiotics on ruminal fermentation and site and extent of digestion in beef heifers fed high grain rations. J. Anim. Sci. 96:3916–3927. doi: 10.1093/jas/sky249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. B., and Crouse J. D.. . 1984. Relative contributions of acetate, lactate and glucose to lipogenesis in bovine intramuscular and subcutaneous adipose tissue. J. Nutr. 114:792–800. doi: 10.1093/jn/114.4.792. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Meek B., Doi Y., Muramatsu M., Chiba T., Honjo T., and Fagarasan S.. . 2004. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc. Natl. Acad. Sci. U. S. A. 101:1981–1986. doi: 10.1073/pnas.0307317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swyers K. L., Wagner J. J., Dorton K. L., and Archibeque S. L.. . 2014. Evaluation of Saccharomyces cerevisiae fermentation product as an alternative to monensin on growth performance, cost of gain, and carcass characteristics of heavy-weight yearling beef steers. J. Anim. Sci. 92:2538–2545. doi: 10.2527/jas.2013-7559. [DOI] [PubMed] [Google Scholar]
- Tedeschi L. O., Fox D. G., and Tylutki T. P.. . 2003. Potential environmental benefits of ionophores in ruminant diets. J. Environ. Qual. 32:1591–1602. [DOI] [PubMed] [Google Scholar]
- Tothova C., Nagy O., and Kovac G.. . 2014. Acute phase proteins and their use in the diagnosis of diseases in ruminants: a review. Vet. Med. (Praha) 59:163–180. doi: 10.17221/7478-VETMED [DOI] [Google Scholar]
- USDA. . 1989. Official united states standards for grades of carcass beef. Agric. Mark. Serv. USDA, Washington, DC. [Google Scholar]
- Van der Peet-Schwering C. M. C., Jansman A. J. M., Smidt H., and Yoon I.. . 2007. Effects of yeast culture on performance, gut integrity, and blood cell composition of weanling pigs. J. Anim. Sci. 85:3099–3109. doi: 10.2527/jas.2007-0110 [DOI] [PubMed] [Google Scholar]
- Wagner J. J., Engle T. E., Belknap C. R., and Dorton K. L.. . 2016. Meta-analysis examining the effects of Saccharomyces cerevisiae fermentation products on feedlot performance and carcass traits. Prof. Anim. Scient. 32:172–182. doi: 10.15232/pas.2015-01438 [DOI] [Google Scholar]
- Wang R., Tian Z., and Chen L.. . 2011. A novel process for microencapsulation of fish oil with barley protein. Food Res. Int. 44:2735–2741. doi: 10.1016/j.foodres.2011.06.013 [DOI] [Google Scholar]
- Werner G., Coque T. M., Franz C. M., Grohmann E., Hegstad K., Jensen L., van Schaik W., and Weaver K.. . 2013. Antibiotic resistant enterococci-tales of a drug resistance gene trafficker. Int. J. Med. Microbiol. 303:360–379. doi: 10.1016/j.ijmm.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Wiedmeier R. D., Arambel M. J., and Walters J. L.. . 1987. Effect of yeast culture and Aspergillus oryzae fermentation extract on ruminal characteristics and nutrient digestibility. J. Dairy Sci. 70:2063–2068. doi: 10.3168/jds.S0022-0302(87)80254-0. [DOI] [PubMed] [Google Scholar]
- Zaheer R., Cook S. R., Klima C. L., Stanford K., Alexander T., Topp E., Read R. R., and McAllister T. A.. . 2013. Effect of subtherapeutic vs. therapeutic administration of macrolides on antimicrobial resistance in Mannheimia haemolytica and enterococci isolated from beef cattle. Front. Microbiol. 4:133. doi: 10.3389/fmicb.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]