Abstract
The objective of this study was to investigate the effects of diets supplemented with sodium stearoyl-2-lactylate (SSL), polyglycerol fatty acid ester (PGFE), and combined emulsifiers (0.02% SSL and 0.08% PGFE) on growth performance, nutrient digestibility, and plasma lipid profiles in weaned piglets and to further evaluate the possible effects of feeding exogenous emulsifiers on digestive enzyme activities and liver bile acid (BA) metabolism. Twenty-eight barrows (age at 35 d, Duroc × Landrace × Yorkshire) with an initial BW of 10.13 ± 0.16 kg were randomly assigned to 4 dietary treatment groups (7 pigs/treatment). Dietary treatment groups included the following: 1) basal diet (Control, CTR); 2) basal diet with 0.1% SSL (SSL); 3) basal diet with 0.1% PGFE (PGFE); and 4) basal diet with 0.08% PGFE+0.02% SSL (PG-SL). SSL diet increased ADG and ADFI of piglets during day 0 to 17 (P < 0.05) compared with the CTR treatment. Piglets fed emulsifier diets experienced a significant improvement in the digestibility of nutrients (DM, CP, ether extract, energy, calcium, and phosphorus) during the first 17 d (P < 0.05). The level of low-density lipoprotein cholesterol (LDL-C) was lower in the PGFE and PG-SL treatment groups than in the CTR treatment group (P < 0.05). Feeding emulsifier diets increased the lipase activity of the pancreas when compared with the CTR diet (P < 0.05). Moreover, the emulsifier diets significantly increased the mRNA expression of FXR (P < 0.05) and decreased the mRNA expression of CYP27A1 (P < 0.05) in the liver. In conclusion, the addition of emulsifiers improved nutrient digestibility and increased the mRNA expression of FXR BA receptors while inhibiting the mRNA expression of BA biosynthesis by CYP27A1 in weanling piglets.
Keywords: digestive enzyme, emulsifiers, growth performance, nutrient digestibility, piglets
Introduction
Lipids are recognized as the second energy source in piglet diets. Piglets have a low feed intake after early weaning, and the inclusion of soybean oil as a vegetable fat in diets to meet the energy requirements is of potential interest (Soares and Lopez, 2002). Previous research has indicated that the addition of fats to piglet diets has improved their ADG and feed efficiency in the later period primarily after weanling, as opposed to in earlier stages (Cera et al., 1988; Li et al., 1990; Howard et al., 1990). It may be that digestive limitations affect the secretion of bile acids (BA) in weaned piglets and result in poor lipid digestion (Lewis et al., 2000). The biosynthesis of BA occurs in the liver and is released from the gallbladder into the duodenum under the stimulation of cholecystokinin (Fang et al., 2018). Definitive evidence has shown that adding exogenous emulsifiers to piglet diets alleviates the negative effects of poor fat digestion caused by low hepatic bile acid synthesis (Jones et al., 1992; Zhao et al., 2015).
Emulsifiers are molecular surfactants with both hydrophilic and hydrophobic groups. In the presence of an emulsifier, oil droplets are distributed in oil-water emulsions, which lead to effective fat digestion and absorption. Sodium stearoyl-2-lactylate (SSL), an emulsifier, is a sodium salt that can be neutralized by stearic acid and lactic acid and has been extensively studied and utilized in the modern food industry (Gómez et al., 2004). Data from several sources have demonstrated that feeding diets with SSL to animals improved growth performance and nutrient digestibility (Wang et al., 2016; Wang et al., 2017; Yin et al., 2019). Polyglycerol fatty acid ester (PGFE) is another emulsifier that is esterified by polyglycerol and fatty acids (Wang et al., 2016). The stability and emulsification properties of suitable combined emulsifiers, as opposed to individual emulsifiers, can be increased (McClements and Jafari, 2018). Recently, Upadhaya et al. (2018) observed that the growth performance and fat digestibility in broilers were improved when the diet was supplemented with combined emulsifiers. However, there has been no research on PGFE in the feed industry. Therefore, we hypothesized that diets supplemented with combined emulsifiers, namely, SSL and PGFE, which have better stability and emulsification properties, would increase growth performance and fat digestibility in piglets.
The aim of this study was to evaluate the effect of diets supplemented with SSL, PGFE, and combined emulsifiers (0.02% SSL and 0.08% PGFE) on growth performance, nutrient digestibility, and plasma lipid profiles in weaned piglets. We further investigated the possible effects of these diets on the activities of digestive enzymes in the pancreas and intestines and observed the effect of feeding exogenous emulsifiers on the metabolism of liver bile acid.
Materials and Methods
Animal care and treatment complied with the standards described in the guidelines for the care and use of laboratory animals of the Northeast Agricultural University (NEAU-[2011]-9).
Sodium stearoyl-2-lactylate and polyglycerol fatty acid ester were purchased from Sheng Xing Biological Technology Co. Ltd. (Henan, China). SSL had a hydrophilic lipophilic balance value of 8.3, and PGFE had a hydrophilic lipophilic balance value of 5.5.
Experimental Design, Animals, and Diets
A total of 28 healthy crossbred barrows (age at 35 d, Duroc × Landrace × Yorkshire) with an initial BW of 10.13 ± 0.16 kg were randomly assigned to 4 dietary treatment groups, with each treatment group composed of 7 piglets. Dietary treatment groups included the following: 1) basal diet (CTR); 2) basal diet supplemented with 0.1% SSL (SSL); 3) basal diet supplemented with 0.1% PGFE (PGFE); and 4) basal diet supplemented with 0.1% combined emulsifier (0.08% PGFE + 0.02% SSL) (PG-SL). All diets were formulated according to the requirements for weaned piglets as recommended by the NRC (2012) and fed as mash. The ingredients are shown in Table 1. Piglets were individually housed in metabolic cages (1.6 × 0.6 × 1.0 m). A stainless steel self-feeder and nipple drinker were installed in each cage. Piglets had free access to diet and water throughout the 5-wk study period. BW was measured after fasting overnight on day 0, day 18, and day 36 to obtain ADG. Feed intake was recorded weekly to calculate the ADFI and Gain:feed (G:F).
Table 1.
Basic diet ingredients and nutrient levels
| Basic diet ingredients | Content, % |
|---|---|
| Corn | 62.00 |
| Soybean meal, 46.2% CP | 16.20 |
| Corn gluten meal, 62.70% CP | 2.00 |
| Full-fat soybean | 9.00 |
| Dried whey | 3.00 |
| Imported fish meal, 64% CP | 3.00 |
| Soybean oil | 1.50 |
| L-Lysine, 98% | 0.17 |
| Calcium hydro phosphate | 0.84 |
| Mountain flour | 0.89 |
| Salt | 0.40 |
| Premix1 | 1.00 |
| Total | 100.00 |
| Nutrient levels 2 | |
| Metabolic energy, Mcal/kg | 3.19 |
| Crude protein | 19.54 |
| Crude fat | 6.13 |
| Lysine | 1.15 |
| Methionine | 0.34 |
| Threonine | 0.75 |
| Tryptophan | 0.22 |
| Calcium | 0.79 |
| Total phosphorus | 0.69 |
| Available phosphorus | 0.36 |
| Sodium | 0.25 |
| Chlorine | 0.25 |
1Provided the following per kilogram of diet: Fe, 190 mg; Cu, 190 mg; Mn, 45 mg; Zn, 140 mg; Se, 0.4 mg; I, 0.5 mg; vitamin A, 45, 000, 000 IU; vitamin D3, 8, 500, 000 IU; vitamin E, 80, 000 mg; vitamin K3, 5, 000 mg; vitamin B1, 8, 000 mg; vitamin B2, 20, 000 mg; vitamin B6, 8, 000 mg; vitamin B12, 100 mg; niacin, 100, 000 mg; D-pantothenic acid, 45, 000 mg; D-biotin, 500 mg; and folate, 4000 mg.
2Crude protein, crude fat, calcium, and total phosphorus were analyzed values, the rest were calculated values according to the NRC (2012).
Feces Collection and Chemical Analysis
Four piglets were selected from each treatment group to assess the apparent total tract digestibility (ATTD). Fecal samples were collected during phase 1 (day 14, 15, and 16 of the experiment) and phase 2 (day 31, 32, and 33 of the experiment) according to published procedures (Ren et al., 2011). Twice a day at 08:00 and 20:00 h, individual feces were collected and weighed in plastic bags. To avoid ammonia loss, 10 mL of H2SO4 (10% v/w) was added and the feces were then stored at −20 °C. Before analysis, the fecal samples of each piglet were thawed and homogenized. A sample of 200 g was removed and left to dry in a hot air oven at 60 ± 5 °C for 48 to 72 h and then ground through a 1-mm screen. The feed offered to each piglet was weighed daily to calculate the ATTD. Dry matter, CP, ether extract (EE), energy, calcium (Ca), and phosphorus (P) in both the feces and diets were assessed according to the method described by Fan et al. (2017).
Sample Collection
On day 36, blood samples (10 mL) were collected using heparin tubes from 16 piglets (each treatment group contained 4 pigs with similar BW) by venous puncture after fasting overnight. The samples were then centrifuged at 3,000 g for 15 min at 4 °C. The plasma was separated and immediately stored at −80 °C until analysis. After blood sampling, the pigs were sacrificed using electricity (250 V, 0.5 A, for 5 to 6 s), with subsequent jugular exsanguination. The pancreas, duodenal mucosa, and liver were obtained immediately, rinsed with physiological saline, frozen in liquid nitrogen, and preserved at −80 °C for future analysis.
Plasma Lipids
Triglycerides (Livak and Schmittgen), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured using the Unicel DxC 800 Synchron (Clinical System, Beckman Coulter, Fullerton, CA).
Activities of Digestive Enzymes
To determine the activities of digestive enzyme, 0.5 g of pancreatic or duodenal mucosal tissue samples was placed in an ice-cold 0.9% sodium chloride solution (1:9, wt/vol) and then thawed and homogenized for 60 s, centrifuged at 13,800 g for 20 min at 4 °C, and the supernatant then transferred to several 1.5-mL Eppendorf tubes for assaying. Amylase (EC 3.2.1.1) activity was measured according to a procedure from a previous study (Somogyi, 1960). Lipase (EC 3.1.1.3) and trypsin (EC 3.4.21.4) activities were analyzed using a method described by Erlanson-Albertsson et al. (1987). The protein concentration of the pancreas was assessed using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), following the method of Bradford according to the manufacturer’s guidelines. Enzyme activities were expressed in units per milligram or gram of protein.
Real-Time Quantitative PCR (RT-PCR)
Analysis of the mRNA expression of genes related to the metabolism of liver BA included the BA receptor: FXR; BA biosynthesis: CYP7A1, CYP27A1, and CYP8B1; and BA transporters: BSEP, MRP2, SLCO1A2, and SLC10A1. Primers were designed using Primer Premier 5.0 and are shown in Table 2. The mRNA abundance in the liver was detected by real-time PCR, as described previously (Peng et al., 2016). The 2-ΔΔCt method was used to calculate relative expression levels, with β-action used as the housekeeping gene (Livak and Schmittgen, 2001).
Table 2.
Pairs of primers used for quantitative real-time PCR assay1
| Gene | Primer sequence (5′-3′) | Accession NO. |
|---|---|---|
| β-actin | F:ATGCTTCTAGGCGGACTGT | AY550069 |
| R:CCATCCAACCGACTGCT | ||
| FXR | F:AAGGACCGAGAGGCAGTAGAGAAG | NM_001287412.1 |
| R:TCTGCGTGGTGATGGTTGAATGTC | ||
| CYP7A1 | F:GAAAGAGAGACCACATCTCGG | NM_001005352.3 |
| R:GAATGGTGTTGGCTTGCGAT | ||
| CYP27A1 | F:ACTGAAGACCGCGATGAAAC | NM_001243304.1 |
| R:CAAAGGCGAATCAGGAAGGG | ||
| CYP8B1 | F:TTCCGCAAGTTCGACCGCATG | NM_214426.1 |
| R:GCTGCTTATGCCGTGCCTCTC | ||
| BSEP | F:GGAGCAAGAGCCAGTTCTGTTCTC | GQ169125.1 |
| R:CCTTCTTGGCAGCGTGGATGAC | ||
| MRP2 | F:TCCAACAGGTGGCTTGCAGTTC | AF403247.1 |
| R:GTCATCCTCACCAGCCAGTTCAG | ||
| SLC10A1 | F:TGACCACCTGCTCCACCTTCC | XM_001927695.5 |
| R:AGAGCACTGTGACAGCAATAGCG | ||
| SLCO1A2 | F:ATGTGGTGAGTCAGGGGCAT | NM_001256595.1 |
| R:CCTCTTAGTGCTGCTGGCAA |
1FXR = farnesoid X receptor; CYP7A1 = cholesterol 7α-hydroxylase; CYP27A1 = sterol 27-hydroxylase; CYP8B1 = sterol 12α-Hydroxylase; BSEP = bile acid response element; MRP2 = multidrug resistance associated protein 2; SLC10A1 = solute carrier family 10 member 1; SLCO1A2 = solute carrier organic anion transporter family member 1A2.
Statistical Analyses
Statistical analyses were performed using SPSS 20.0 (IBM-SPSS Inc., Chicago, IL). One-way ANOVA was performed, and multiple comparisons were made using the Duncan test. Mean values and SEM are presented. The statistical model accounted for the effects of the emulsifiers. Each pig was considered an experimental unit. Mean values were considered significant when P < 0.05, and trends were considered when 0.05 ≤ P ≤ 0.10.
Results
Growth Performance
The growth performance of piglets supplemented with different emulsifiers is summarized in Table 3. Emulsifier supplementation numerically increased BW on day 18 and 35. However, compared with the CTR group, the differences were not significant (P > 0.05). The SSL diet supplement increased ADG and ADFI when compared with CTR treatment during day 0 to 17 (P < 0.05), but there were no significant differences from day 18 to 35 and overall (P > 0.05). In addition, ADG, ADFI, and G:F for the PGFE and PG-SL groups were not improved in the current study (P > 0.05).
Table 3.
Effect of different emulsifier supplementation on growth performance in piglets1,2
| Items | Treatments3 | SEM | P-value | |||
|---|---|---|---|---|---|---|
| CTR | SSL | PGFE | PG-SL | |||
| BW, kg | ||||||
| Day 0 | 10.06 | 10.06 | 10.06 | 10.05 | 0.14 | 1.000 |
| Day 17 | 16.84 | 18.43 | 17.54 | 17.10 | 0.35 | 0.400 |
| Day 35 | 29.68 | 31.05 | 31.36 | 29.13 | 0.62 | 0.536 |
| ADG, kg/d | ||||||
| Day 0–17 | 0.40b | 0.49a | 0.44ab | 0.41ab | 0.02 | 0.144 |
| Day 17–35 | 0.71 | 0.70 | 0.77 | 0.67 | 0.02 | 0.300 |
| Day 0–35 | 0.56 | 0.60 | 0.61 | 0.55 | 0.02 | 0.398 |
| ADFI, kg/d | ||||||
| Day 0–17 | 0.79b | 0.94a | 0.86ab | 0.81ab | 0.02 | 0.137 |
| Day 17–35 | 1.46 | 1.49 | 1.58 | 1.40 | 0.04 | 0.473 |
| Day 0–35 | 1.14 | 1.22 | 1.23 | 1.12 | 0.03 | 0.477 |
| G:F | ||||||
| Day 0–17 | 0.50 | 0.52 | 0.52 | 0.51 | 0.01 | 0.894 |
| Day 17–35 | 0.49 | 0.47 | 0.49 | 0.48 | 0.01 | 0.745 |
| Day 0–35 | 0.49 | 0.49 | 0.50 | 0.49 | 0.01 | 0.947 |
1G:F = Gain: feed.
2 n = 7 pigs per treatment.
3CTR = basal diet; SSL = basal diet + 0.1% SSL; PGFE = basal diet + PGFE; PG-SL = basal diet + 0.08% PGFE + 0.02% SSL.
a,bMean values within a row without a common superscript differ significantly (P < 0.05).
Digestibility of Nutrients
As shown in Table 4, the ATTD of DM, CP, EE, and energy were increased when SSL, PGFE or the blend of both was added to the diets in phase 1 (day 14 to 16) (P < 0.05). In addition, the digestibility of Ca and P was greater than that in the CTR group (P < 0.05). However, there was no significant difference in ATTD of nutrient after supplementation with emulsifiers in phase 2 (day 31 to 33) (P > 0.05).
Table 4.
Effect of different emulsifier supplementation on nutrient digestibility in piglets1,2
| Items | Treatments3 | SEM | P-value | |||
|---|---|---|---|---|---|---|
| CTR | SSL | PGFE | PG-SL | |||
| Day 14–16 | ||||||
| DM, % | 85.71b | 89.34a | 90.75a | 89.99a | 0.71 | 0.021 |
| CP, % | 78.93b | 85.08a | 85.57a | 85.97a | 1.13 | 0.055 |
| EE, % | 55.56b | 67.61a | 71.70a | 69.68a | 2.49 | 0.014 |
| Energy, % | 85.42b | 90.32a | 90.71a | 89.79a | 0.74 | 0.021 |
| Ca, % | 52.84b | 71.10a | 72.57a | 69.27a | 2.92 | 0.010 |
| P % | 61.63b | 72.55a | 76.95a | 72.79a | 1.92 | 0.004 |
| Day 31–33 | ||||||
| DM, % | 89.00 | 90.60 | 90.61 | 89.51 | 0.37 | 0.343 |
| CP, % | 84.89 | 88.06 | 87.96 | 86.46 | 0.66 | 0.244 |
| EE, % | 62.43 | 64.44 | 66.34 | 67.26 | 1.57 | 0.745 |
| Energy, % | 89.21 | 90.70 | 90.63 | 89.22 | 0.40 | 0.391 |
| Ca, % | 67.60 | 71.84 | 72.15 | 72.90 | 1.28 | 0.449 |
| P, % | 62.66 | 66.97 | 68.26 | 66.21 | 1.23 | 0.422 |
1EE = ether extract, Ca = calcium, P = phosphorus.
2 n = 4 pigs per treatment.
3CTR = basal diet; SSL = basal diet + 0.1% SSL; PGFE = basal diet + PGFE; PG-SL = basal diet + 0.08% PGFE + 0.02% SSL.
a,bMean values within a row without a common superscript differ significantly (P < 0.05).
Plasma Lipid Profiles
The LDL-C concentration was decreased (P < 0.05) in the PGFE and PG-SL groups compared with piglets fed the RET diet (Table 5). However, no significant differences were observed between the dietary treatments for the levels of CHOL, TG, and HDL-C in plasma (P > 0.05).
Table 5.
Effect of different emulsifier supplementation on plasma lipid metabolism in piglets1,2
| Items | Treatments3 | SEM | P-value | |||
|---|---|---|---|---|---|---|
| CTR | SSL | PGFE | PG-SL | |||
| CHOL, mmol /L | 2.60 | 2.79 | 2.39 | 2.54 | 0.07 | 0.196 |
| TG, mmol /L | 0.51 | 0.39 | 0.49 | 0.40 | 0.03 | 0.344 |
| HDL-C, mmol /L | 2.45 | 2.22 | 2.04 | 2.67 | 0.16 | 0.562 |
| LDL-C, mmol /L | 1.37a | 1.15ab | 0.87b | 0.89b | 0.08 | 0.064 |
| HDL-C/LDL-C | 1.90 | 1.96 | 2.67 | 3.31 | 0.30 | 0.296 |
1CHOL = cholesterol, TG = triglyceride, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol.
2 n = 4 pigs per treatment.
3CTR = basal diet; SSL = basal diet + 0.1% SSL; PGFE = basal diet + PGFE; PG-SL = basal diet + 0.08% PGFE + 0.02% SSL.
a,bMean values within a row without a common superscript differ significantly (P < 0.05).
Activities of Digestive Enzymes
Digestive enzyme activities are reported in Table 6. During the experimental period, the lipase activities of the pancreas of piglets fed emulsifiers were greater than in the CTR treatment group (P < 0.05), but similar results were not found in duodenal mucosal tissue (P > 0.05). In addition, trypsin and amylase activity in pancreatic and duodenal mucosa were not significantly different between the dietary supplemental SSL, PGFE, or the blend of both (P > 0.05).
Table 6.
Effect of different emulsifier supplementation on digestive enzyme activities in piglets1
| Items | Treatments2 | SEM | P-value | |||
|---|---|---|---|---|---|---|
| CTR | SSL | PGFE | PG-SL | |||
| Pancreas | ||||||
| Lipase U/mg prot | 8.62b | 10.35a | 10.14a | 9.94a | 0.27 | 0.039 |
| Trypsin U/mg prot | 91.17 | 83.67 | 83.56 | 88.16 | 4.01 | 0.920 |
| Amylase U/mg prot | 450.19 | 402.56 | 442.34 | 386.45 | 15.54 | 0.456 |
| Duodenum | ||||||
| Lipase U/g prot | 82.98 | 100.76 | 101.25 | 103.84 | 6.09 | 0.686 |
| Trypsin U/mg prot | 55.39 | 60.53 | 51.88 | 60.20 | 2.54 | 0.619 |
| Amylase U/mg prot | 0.92 | 1.12 | 1.26 | 1.28 | 0.11 | 0.641 |
1 n = 4 pigs per treatment.
2CTR = basal diet; SSL = basal diet + 0.1% SSL; PGFE = basal diet + PGFE; PG-SL = basal diet + 0.08% PGFE + 0.02% SSL.
a,bMean values within a row without a common superscript differ significantly (P < 0.05).
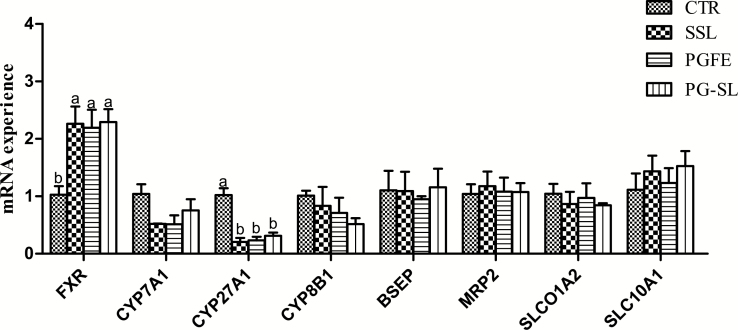
Gene Expression of Bile Acid Metabolism-Related Genes in the Liver
As shown in Fig. 1, the emulsifier diets significantly increased the mRNA expression of FXR (P < 0.05) and decreased the mRNA expression of CYP27A1 (P < 0.05). However, the mRNA abundance of the BA biosynthesis genes CYP7A1 and CYP8B1 exhibited no significant differences in piglets fed emulsifiers (P > 0.05). Additionally, there were no effects on BA transport-related genes of the liver (P > 0.05).
Figure 1.
Effect of different emulsifier supplementation on the relative mRNA expression levels of metabolism of liver BA1,2. a,bMean values within a row without a common superscript differ significantly (P < 0.05). 1FXR = farnesoid X receptor; CYP7A1 = cholesterol 7α-hydroxylase; CYP27A1 = sterol 27-hydroxylase; CYP8B1 = sterol 12α-Hydroxylase; BSEP = bile acid response element; MRP2 = multidrug resistance associated protein 2; SLC10A1 = solute carrier family 10 member 1; SLCO1A2 = solute carrier organic anion transporter family member 1A2. 2n = 4 pigs per treatment. 3CTR = basal diet; SSL = basal diet + 0.1% SSL; PGFE = basal diet + PGFE; PG-SL = basal diet + 0.08% PGFE + 0.02% SSL.
Discussion
Growth Performance
Current research has demonstrated an effective role for SSL-supplemented diets in increasing growth performance in broilers (Gheisar et al., 2015). In addition, Yin et al. (2019) reported that the ADG and ADFI were not different in growing pigs fed low-energy (ME = 3, 220 kcal/kg) diets supplemented with 0.05% SSL. In this study, we found that feeding a 0.1% SSL-supplemented diet to piglets improved the ADG and ADFI during day 0 to 17 but had no effect from day 18 to day 35, or day 0 to day 35. Danek et al. (2005) demonstrated that the ADG of experimental piglets fed 0.1% lecithin was improved by 26.3% compared with a control group in the first week but was reduced to 3.1% in the fourth week. Nevertheless, the growth performance of broilers can be enhanced during the starter phase (day 0 to day 21) for diets containing emulsifiers (Wang et al., 2016). However, Zhao et al. (2015) observed that the ADG of reduced energy treatments containing 0.1% lysophospholipids (LPL) was similar to the basal diet treatment during day 0 to 14, day 15 to 35, and day 0 to 35 in pigs. In this study, the growth performance of piglets was not improved when fed 0.1% PGFE or 0.02% SSL and 0.08% PGFE, which is consistent with previous reports that found that the addition of emulsifiers in piglet diets did not result in increases in growth performance (Jones et al., 1992; Øverland et al., 1994; Bontempo et al., 2016). From these inconsistent results, we speculate that dietary fat sources, emulsifier types, and feed palatability affect the growth performance of piglets.
Digestibility of Nutrients
Several in vitro studies on digestion have demonstrated that dietary emulsifiers can modulate direct contact between lipid substrates and lipases and promote lipid digestion (Mun et al., 2007; Liang et al., 2018). Previous studies have reported that the effect of SSL was limited to nutrient digestibility for broilers (Gheisar et al., 2015). However, Wang et al. (2017) observed that the digestibility of EE, CP, Ca, and P was improved when 0.1% SSL was fed to lactating sows. In this study, we found that emulsifiers fed to weaned piglets improved the digestibility of DM, EE, CP, Ca, P, and energy in phase 1 (day 14 to 16). One possible mechanism is that the digestive system of pigs is different with poultry. In agreement with our results, the effect of lecithin supplementation was greater in the early period after weanling than the later stages (Danek et al., 2005). One possible mechanism is that the development of digestive system tends to perfection with increasing temporal distance from weaning, which eventually increases nutrient digestibility (Soares and Lopez, 2002). Similar results were observed by Zhao et al. (2015) who demonstrated that LPL supplements in beef tallow diets enhanced the digestibility of DM, GE, N, and crude fat on day 14 in piglets, while nutrient digestibility also increased on day 35. Conversely, research from Xing et al. (2004) found that nutrient digestibility decreased when piglet diets were supplemented with LPL, who thought the level of nutrient digestibility may not be related to growth performance. In addition, several studies have also failed to observe that emulsifier diets are able to affect nutrient digestibility in animals (Øverland et al., 1994; Soares and Lopez, 2002). Increases in nutrient digestibility for pigs fed diets containing emulsifiers suggest that emulsifiers compensate for the lack of BA, which is synthesized in liver, can emulsify the lipid droplets and, ultimately, increase the use of dietary fat. Further experiments are needed to research the effects of emulsifiers on nutrient digestibility.
Plasma Lipid Profiles
The LDL-C concentration is an indicator of both decomposition and the transport of lipids in animals and humans. Furthermore, the HDL:LDL ratio is used to measure coronary heart disease and cardiovascular disease. In terms of plasma lipid parameters, a previous study by Jones et al. (1992) found that pigs fed lecithin or lysolecithin diets experienced a decreased concentration of LDL-C compared to treatments without emulsifiers, and the ratio of HDL:LDL was greater in the lecithin group than the lysolecithin group, especially when tallow lard or tallow was used as the fat source. In addition, recent research has shown that LDL-C levels decreased linearly when different levels of emulsifiers were added to broiler diets on day 20 (Roy et al., 2010). Similar results have also been observed by Upadhaya et al. (2018). In agreement with these results, we found that LDL-C concentrations decreased in the PGFE and PG-SL groups compared with piglets fed the CTR diet. One possible explanation is that the emulsifiers have a positive effect on promoting animal health (Upadhaya et al., 2018). However, Zhao et al. (2015) reported that the addition of emulsifiers in piglet diets decreased the level of TG compared with reduced-energy treatments but did not affect LDL-C, HDL-C, and CHOL concentrations. In contrast, Wang et al. (2016) did not observe different levels of TG, CHOL, LDL-C, or HDL-C in the serum of broilers fed SSL as part of a low-energy diet. We suggest that these inconsistent results may be caused by different fat sources or levels, emulsifier types, and animals.
Activities of Digestive Enzymes
Digestive enzyme activities aid in the evaluation of dietary nutrient utilization in the body. Fat is broken down into lipid droplets by the emulsifying action of BA, which increases the active surface of fats, thereby increasing the interface area with lipase, which breaks down the triacylglycerols into free fatty acids and mono- and di-acylglycerols (Gu and Li, 2003). Lai et al. (2017) reported that the addition of BA to the diet of broiler chickens significantly increased the activity of lipase in the duodenum on day 21 and day 42. Similarly, Guerreiro Neto et al. (2011) found that poultry fat or 50% soybean oil and 50% poultry fat diets supplemented with emulsifier (milk-derived casein) fed to broilers increased the lipase activity of the pancreas compared with that in the control group. In this study, we found that feed emulsifiers significantly increased the lipase activity of the pancreas in piglets and numerically increased lipase activity in duodenal mucosa tissue. The current study indicates that the positive influence of lipase activity was observed in SSL- and PGFE-supplemented diets. However, trypsin and amylase activities were not affected by emulsifiers. It seems unlikely that the activities of trypsin and amylase can be changed when diets are supplemented with emulsifiers. Further research is needed to clarify the mechanism of emulsifiers in modulating digestive enzyme activities in the body.
Genes Expression of Bile Acid Metabolism-Related Genes in the Liver
FXR senses changes in BA and can transcriptionally regulate the genes of BA biosynthesis (CYP7A1, CYP27A1, and CYP8B1) and the genes of BA transport (BSEP, MRP2, SLCO1A2, and SLC10A1) (Chiang, 2004; Modica et al., 2010). CYP7A1 is a critical regulatory gene in the classic pathway of BA synthesis that can regulate the genes of CYP8B1 to synthesize cholic acid. CYP27A1 is another key gene responsible for the synthesis of BA in the alternative pathway (Chiang, 2009; Liu et al., 2014). Information regarding the effects of feeding exogenous emulsifiers on the metabolism of liver BA in animals has not been reported. In this study, piglets fed emulsifier diets markedly increased the mRNA abundance of FXR and decreased the mRNA abundance of CYP27A1. We believe that the activation of FXR receptors depends on the supplementation of exogenous emulsifiers, which further to decrease the abundance of CYP27A1. Further experiments are needed to clarify the effect of emulsifiers on the metabolism of liver BA.
Conclusion
This study has demonstrated that supplementation with SSL, PGFE, and combined emulsifiers (0.02% SSL and 0.08% PGFE) in piglet diets has the potential to improve the growth performance and significantly increase nutrient digestibility during the early stages. Furthermore, the level of LDL-C was decreased in the PGFE and PG-SL treatment groups. Compared to diets with no emulsifier supplementation, the lipase activity of the pancreas and the mRNA abundance of BA metabolism genes in the liver were affected when emulsifiers were fed to piglets. Therefore, this study provides new insights that may facilitate the development of emulsifiers for the feed industry. However, better growth performance and fat digestibility were not observed in the PG-SL treatment; thus, further research on combined emulsifiers is still needed in the feed industry.
Literature Cited
- Bontempo V., Comi M., and Jiang X. R.. . 2016. The effects of a novel synthetic emulsifier product on growth performance of chickens for fattening and weaned piglets. Animal. 10:592–597. doi: 10.1017/S1751731115002189 [DOI] [PubMed] [Google Scholar]
- Cera K. R., Mahan D. C., and Reinhart G. A.. . 1988. Weekly digestibilities of diets supplemented with corn oil, lard or tallow by weanling swine. J. Anim. Sci. 66:1430–1437. doi: 10.2527/jas1988.6661430x [DOI] [PubMed] [Google Scholar]
- Chiang J. Y. L. 2004. Regulation of bile acid synthesis: Pathways, nuclear receptors, and mechanisms. J. Hepatol. 40:0–551. doi: 10.1016/j.jhep.2003.11.006 [DOI] [PubMed] [Google Scholar]
- Chiang J. Y. 2009. Bile acids: Regulation of synthesis. J. Lipid Res. 50:1955–1966. doi: 10.1194/jlr.R900010-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danek P., Paseka A., Smola J., Ondráček J., Bečková R., and Rozkot M.. . 2005. Influence of lecithin emulsifier on the utilisation of nutrients and growth of piglets after weaning. Czech J. Anim. Sci. 50:459–465. doi: 10.17221/4245-CJAS [DOI] [Google Scholar]
- Erlanson-Albertsson C., Larsson A., and Duan R.. . 1987. Secretion of pancreatic lipase and colipase from rat pancreas. Pancreas. 2:531–535. https://insights.ovid.com/pubmed?pmid=3671348 [DOI] [PubMed] [Google Scholar]
- Fan Y. F., Yang Y. Y., Yang P., Xia T., and Ma Y. X.. . 2017. Available energy content, nutrients digestibility of chili meal and effects on performance of growing pigs. Anim. Feed Sci. Technol. 229:97–105. doi: 10.1016/j.anifeedsci.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W., Zhang L., Meng Q., Wu W., Lee Y. K., Xie J., and Zhang H.. . 2018. Effects of dietary pectin on the profile and transport of intestinal bile acids in young pigs. J. Anim. Sci. 96:4743–4754. doi: 10.1093/jas/sky327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheisar M. M., Hosseindoust A., Kim H. B., and Kim I. H.. . 2015. Effects of lysolecithin and sodium stearoyl-2-lactylate on growth performance and nutrient digestibility in broilers. Korean J.Poult. Sci. 42:133–137. doi: 10.5536/KJPS.2015.42.2.133 [DOI] [Google Scholar]
- Gómez M., Delreal S., Rosell C. M., Ronda F., Blanco F., Blanco C. A., and Caballero P. A.. . 2004. Functionality of different emulsifiers on the performance of breadmaking and wheat bread. Eu. F. Res. Tech. 219: 145–150. doi: 10.1007/s00217-004-0937-y [DOI] [Google Scholar]
- Gu X., and Li D.. . 2003. Fat nutrition and metabolism in piglets: A review. Anim. Feed Sci. Technol. 109: 151–170. doi: 10.1016/S0377-8401(03)00171-8 [DOI] [Google Scholar]
- Guerreiro Neto A. C., Pezzato A. C., Sartori J. R., Mori C., Cruz V. C., Fascina V. B., Pinheiro D. F., Madeira L. A., and Gonçalvez J. C.. . 2011. Emulsifier in broiler diets containing different fat sources. Braz. J. Poultry. Sci. 13: 119–125. doi: 10.1590/S1516-635X2011000200006 [DOI] [Google Scholar]
- Howard K. A., Forsyth D. M., and Cline T. R.. . 1990. The effect of an adaptation period to soybean oil additions in the diets of young pigs. J. Anim. Sci. 68:678–683. doi: 10.2527/1990.683678x [DOI] [PubMed] [Google Scholar]
- Jones D. B., Hancock J. D., Harmon D. L., and Walker C. E.. . 1992. Effects of exogenous emulsifiers and fat sources on nutrient digestibility, serum lipids, and growth performance in weanling pigs. J. Anim. Sci. 70:3473–3482. doi: 10.2527/1992.70113473x [DOI] [PubMed] [Google Scholar]
- Lai W., Huang W., Dong B., Cao A., Zhang W., Li J., Wu H., Zhang L.. . 2017. Effects of dietary supplemental bile acids on performance, carcass characteristics, serum lipid metabolites and intestinal enzyme activities of broiler chickens. Poult. Sci. 97:196–202. doi: 10.3382/ps/pex288 [DOI] [PubMed] [Google Scholar]
- Lewis D. S., Oren S., Wang X., Moyer M. L., Beitz D. C., Knight T. J., and Mott G. E.. . 2000. Developmental changes in cholesterol 7alpha- and 27-hydroxylases in the piglet. J. Anim. Sci. 78:943–951. doi: 10.2527/2000.784943x [DOI] [PubMed] [Google Scholar]
- Li D. F., Thaler R. C., Nelssen J. L., Harmon D. L., Allee G. L., and Weeden T. L.. . 1990. Effect of fat sources and combinations on starter pig performance, nutrient digestibility and intestinal morphology. J. Anim. Sci. 68:3694–3704. doi: 10.2527/1990.68113694x [DOI] [PubMed] [Google Scholar]
- Liang L., Zhang X., Wang X., Jin Q., and McClements D. J.. . 2018. Influence of dairy emulsifier type and lipid droplet size on gastrointestinal fate of model emulsions: in vitro digestion study. J. Agric. Food Chem. 66:9761–9769. doi: 10.1021/acs.jafc.8b02959 [DOI] [PubMed] [Google Scholar]
- Liu J., Lu H., Lu Y. F., Lei X., Cui J. Y., Ellis E., Strom S. C., and Klaassen C. D.. . 2014. Potency of individual bile acids to regulate bile acid synthesis and transport genes in primary human hepatocyte cultures. Toxicol. Sci. 141:538–546. doi: 10.1093/toxsci/kfu151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., and Schmittgen T. D.. . 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- McClements D. J., and Jafari S. M.. . 2018. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid Interface Sci. 251:55–79. doi: 10.1016/j.cis.2017.12.001 [DOI] [PubMed] [Google Scholar]
- Modica S., Gadaleta R. M., and Moschetta A.. . 2010. Deciphering the nuclear bile acid receptor FXR paradigm. Nucl. Recept. Signal. 8:e005. doi: 10.1621/nrs.08005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun S., Decker E. A., and McClements D. J.. . 2007. Influence of emulsifier type on in vitro digestibility of lipid droplets by pancreatic lipase. Food Res. Int. 40: 770–781. doi: 10.1016/j.foodres.2007.01.007 [DOI] [Google Scholar]
- NRC 2012. Nutrient requirements of swine, 11th rev. ed Nati. Acad. Press, Washington, DC. [Google Scholar]
- Overland M., Mroz Z., and Sundstøl F.. . 1994. Effect of lecithin on the apparent ileal and overall digestibility of crude fat and fatty acids in pigs. J. Anim. Sci. 72:2022–2028. doi: 10.2527/1994.7282022x [DOI] [PubMed] [Google Scholar]
- Peng X., Hu L., Liu Y., Yan C., Fang Z. F., Lin Y., Xu S. Y., Li J., Wu C. M., Chen D. W., . et al. 2016. Effects of low-protein diets supplemented with indispensable amino acids on growth performance, intestinal morphology and immunological parameters in 13 to 35 kg pigs. Animal. 10:1812–1820. doi: 10.1017/S1751731116000999 [DOI] [PubMed] [Google Scholar]
- Ren P., Zhu Z., Dong B., Zang J., and Gong L.. . 2011. Determination of energy and amino acid digestibility in growing pigs fed corn distillers’ dried grains with solubles containing different lipid levels. Arch. Anim. Nutr. 65:303–319. doi: 10.1080/1745039X.2011.588849 [DOI] [PubMed] [Google Scholar]
- Roy A., Haldar S., Mondal S., and Ghosh T. K.. . 2010. Effects of supplemental exogenous emulsifier on performance, nutrient metabolism, and serum lipid profile in broiler chickens. Vet. Med. Int. 2010:262604. doi: 10.4061/2010/262604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares M., and Lopez C.. . 2002. Effects of dietary lecithin and fat unsaturation on nutrient utilisation in weaned piglets. Anim. Feed Sci. Technol. 95:0–177. doi: 10.1016/s0377-8401(01)00324-8 [DOI] [Google Scholar]
- Somogyi M. 1960. Modifications of two methods for the assay of amylase. Clin. Chem. 6:23–35. http://clinchem.aaccjnls.org/content/6/1/23.long [PubMed] [Google Scholar]
- Upadhaya S. D., Lee J. S., Jung K. J., and Kim I. H.. . 2018. Influence of emulsifier blends having different hydrophilic-lipophilic balance value on growth performance, nutrient digestibility, serum lipid profiles, and meat quality of broilers. Poult. Sci. 97:255–261. doi: 10.3382/ps/pex303 [DOI] [PubMed] [Google Scholar]
- Wang C. Q., Bai Y. S., Zhao X., Shi B. M., Meng X. Y., and Shan A. S.. . 2017. Effects of feeding sodium stearoyl-2-lactylate diets to lactating sows on performance, digestibility of nutrients, composition, and fat globule size in milk. J. Anim. Sci. 95:5091–5099. doi: 10.2527/jas2017.1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F. C., and Marangoni A. G.. . 2016. Advances in the application of food emulsifier α-gel phases: Saturated monoglycerides, polyglycerol fatty acid esters, and their derivatives. J. Colloid Interface Sci. 483:394–403. doi: 10.1016/j.jcis.2016.08.012 [DOI] [PubMed] [Google Scholar]
- Wang J. P., Zhang Z. F., Yan L., and Kim I. H.. . 2016. Effects of dietary supplementation of emulsifier and carbohydrase on the growth performance, serum cholesterol and breast meat fatty acids profile of broiler chickens. Anim. Sci. J. 87:250–256. doi: 10.1111/asj.12412 [DOI] [PubMed] [Google Scholar]
- Xing J. J., van Heugten E., Lit D. F., Touchette K. J., Coalson J. A., Odgaard R. L., and Odle J.. . 2004. Effects of emulsification, fat encapsulation, and pelleting on weanling pig performance and nutrient digestibility. J. Anim. Sci. 82:2601–2609. doi: 10.2527/2004.8292601x [DOI] [PubMed] [Google Scholar]
- Yin J., Jiao Y., Kim Y. M., and Kim I. H.. . 2019. Effects of reducing dietary energy (tallow) in diets containing emulsifier blend on growth performance, nutrient digestibility, and blood profile in growing pigs. Can. J. Anim. Sci. 99: 206–209. doi: 10.1139/cjas-2017-0155 [DOI] [Google Scholar]
- Zhao P. Y., Li H. L., Hossain M. M., and Kim I. H.. . 2015. Effect of emulsifier (lysophospholipids) on growth performance, nutrient digestibility and blood profile in weanling pigs. Anim. Feed Sci. Technol. 207: 190–195. doi: 10.1016/j.anifeedsci.2015.06.007 [DOI] [Google Scholar]