Abstract
Two performance studies were conducted to investigate the effects of 3 different sources of Cu on production parameters of piglets. A total of 256 piglets weaned at 24 ± 2 d were randomly allocated into 4 treatments with 10 or 8 replicates per treatment of 4 or 3 piglets per pen in Exp. 1 and 2, respectively. The experimental period was divided into 3 feeding phases: Phase 1 (24 to 35 d), Phase 2 (36 to 49 d), and Phase 3 (50 to 70 d). Treatments included a Control group (fed 10 mg/kg of Cu from CuSO4), a group fed 160 mg/kg of either CuSO4 (CuSO4-160) or tri-basic copper chloride (TBCC), and a group fed Cu methionine hydroxy analogue chelated (Cu-MHAC) at 150, 80, and 50 mg/kg in Phases 1, 2, and 3, respectively. The methionine value of Cu-MHAC was accounted during diet formulation to achieve the same levels of methionine across treatments. Phases 1 and 2 diets contained 2,200 and 1,500 ppm of ZnO, respectively; and antibiotics were used as growth promoters. Performance parameters were analyzed as completely randomized block design, in which each experiment was considered as a block. In trial 2, blood serum and mucosal samples, from the fundic region of the stomach, were collected from 1 piglet per replicate at day 70 and tested for serum growth hormone levels (GH) and ghrelin mRNA expression, respectively. The contrast between Cu-MHAC vs. CuSO4-160 + TBCC showed that piglets fed Cu-MHAC exhibited better feed conversion ratio (FCR) in all feeding phases compared with feeding inorganic Cu (P < 0.05). Overall, feeding Cu-MHAC improved body weight (BW), BW gain, feed intake (FI), and FCR vs. Control diet fed piglets; yet, it improved BW and FCR vs. TBCC fed piglets, and improved BW, BW gain, and FI vs. CuSO4-160 fed piglets (P < 0.05). Feeding TBCC promoted similar performance than feeding CuSO4-160, regardless of age (P > 0.05). Both ghrelin expression and growth hormone serum levels were significantly increased by feeding Cu-MHAC vs. Control diet fed animals (P < 0.01). Feeding CuSO4-160 upregulated ghrelin expression vs. Control (P < 0.01) while GH serum levels and ghrelin expression did no change by feeding TBCC compared with Control diet fed animals (P > 0.05). It was concluded that feeding Cu-MHAC at the levels tested herein can improve growth performance of piglets beyond feeding 160 ppm of either CuSO4 or TBCC, which may be partially explained by the increased expression of ghrelin and GH serum levels.
Keywords: copper, ghrelin, growth hormone, pigs
Introduction
Although the requirement of copper as a nutrient is low, with concentrations recommended by NRC (2012) estimated to be around 5 to 6 mg/kg for nursery pigs, high levels of CuSO4 (125 to 250 mg/kg) are typically used in the swine industry as a growth promoter. The mechanism of action by which Cu can promote growth performance in monogastric animals most likely include its antibacterial and systemic effect.
Studies have shown a favorable impact of Cu in terms of microbiota modulation (Shurson et al., 1990) as well as improvements in gut health and absorptive capacity (Zhao et al., 2007), besides its effect in reducing intestinal immune challenge (Arias and Koutsos, 2006) of animals supplemented with high levels of Cu even in diets with antibiotics (Cromwell, 2001). This antibacterial effect might be related to both Cu from feed that reached the intestine directly and Cu that was already absorbed and returned to the intestine through bile duct (enterohepato-biliar-enteric pathway).
Regarding the systemic effects, after ingestion, Cu can attain several tissues with influence on growth. For example, high Cu levels may induce a higher concentration of ghrelin at the fundic region of stomach stimulating growth hormone (GH) secretion (Yang et al., 2012). Zhou et al. (1994a) observed that intravenous injection of Cu histidinate, which bypass the lumen, increased the concentration of Cu in liver, serum, and brain, which was associated with better weight gain, feed conversion, and muscle development in piglets. The mechanism of the growth promoting effect of Cu may also be related to its ability of stimulating the release of hypothalamic appetite regulators (Zhu et al., 2011), enhancement of enzyme activity (Luo and Dove, 1996), and stimulation of serum mitogenic activity (Zhou et al., 1994b).
Despite the benefits, some undesirable effects are associated with supra nutritional levels of Cu depending on the sources. The use of some Cu sources may reduce the efficiency of phytase by forming resistant Cu-phytate insoluble complexes. Banks et al. (2004) reported a reduction of 8.11% and 14.49% in apparent P retention with 250 ppm of Cu supplemented as CuSO4 or Cu-citrate, respectively. Other negative effects include oxidation of labile organic compounds such as vitamin E (Luo et al., 2005), environmental excretion (Huang et al., 2010; Manangi et al., 2012), reduction of nutrients utilization due to antagonism (Pang and Applegate, 2007), and presence of contaminants in commercial products (i.e., dioxins).
Therefore, it is important to understand the characteristics of each mineral source and how they affect parameters of commercial interest. The objective of this study was to evaluate different Cu sources as growth promoter on performance, ghrelin gene expression, and serum GH levels of nursery pigs.
Material and Methods
General
Two trials were conducted at Instituto Federal do Espírito Santo and all the procedures were approved by the Ethics Committee on Animal Use of Centro de Tecnologia Animal. Cu Methionine Hydroxy Analogue Chelate (Cu-MHAC, Mintrex Cu, Novus International, Inc., St Charles, MO) is a chelate of 1 Cu atom connected with 2 molecules of 2-hydroxy-4-methylthio butanoic acid (HMTBa) using coordinate covalent bonds. Methionine activity from Cu-MHAC (78% Met activity) was accounted for and all diets were adjusted to be equal in methionine across treatments. Tribasic Cu chloride (TBCC, Cu2[OH]3Cl) is a more concentrated form of inorganic Cu (58%). Copper sulfate pentahydrate (CuSO4.5H2O) represents an inorganic source of Cu typically used in commercial swine production.
Animals and Experimental Design
Two performance trials of equal design were conducted sequentially in the same open-side barn piglet facility with slatted-floor pens, nipple drinkers, and semi-automatic feeders. The barn used was not cleaned before the beginning of the trials to create some environmental challenge. A total of 256 Agroceres PIC piglets weaned at 24 ± 2 d were used. Exp. 1 included 160 piglets (80 barrows and 80 gilts) with body weight (BW) of 5.43 ± 0.90 kg. In Exp. 2, 96 piglets (48 barrows and 48 gilts) with initial BW of 4.73 ± 0.95 kg were used. Pigs were allocated to 4 treatments in a completely randomized block design with 10 replicates per treatment and 4 pigs per replicate (Exp. 1) or 8 replicates per treatment and 3 pigs per replicate (Exp. 2). The effect of sex was blocked in both trials and it was not significant for any parameter tested (P > 0.1).
Treatments and Performance
Corn, soybean meal, and dairy byproducts were the main components of the experimental diets (Table 1). The rations were formulated to be iso-nutritional across treatments except for Cu levels, to meet or exceed the nutritional requirements of piglets in agreement with Rostagno et al. (2011). Corn and soybean meal samples were subjected to proximate analysis, and amino acids and ME analysis were performed by Near Infrared Spectroscopy before diet formulation. The experimental period was divided into 3 feeding phases: Phase 1 (24 to 35 d), Phase 2 (36 to 49 d), and Phase 3 (50 to 70 d). Four treatments were used: 1) a group fed 10 mg/kg Cu from CuSO4 (Control) in all 3 feeding phases; 2) animals fed 160 mg/kg Cu from CuSO4 (CuSO4-160) throughout the trial; 3) a group fed Cu from Cu-MHAC at 150, 80, and 50 mg/kg for Phases 1, 2, and 3, respectively; and 4) a group fed 160 mg/kg of Cu from TBCC in all feeding phases (Table 2). Cu-MHAC was included at lower levels due to its supposed greater bioavailability and according to providers’ recommendation.
Table 1.
Basal diet compositions and nutritional profile (Exp. 1 and 2)
| Ingredients, % | Phase 1 | Phase 1 | Phase 3 |
|---|---|---|---|
| Corn | 46.78 | 55.64 | 68.61 |
| Soybean meal | 25.00 | 25.99 | 27.31 |
| Dried milk | 20.00 | 12.50 | 0.000 |
| Sugar | 3.00 | 1.50 | 0.00 |
| Spray dried porcine plasma | 2.00 | 1.00 | 0.00 |
| Dicalcium phosphate | 1.56 | 1.51 | 1.51 |
| Soybean oil | 0.00 | 0.00 | 0.73 |
| Limestone | 0.47 | 0.63 | 0.79 |
| Salt | 0.35 | 0.48 | 0.46 |
| Zinc oxide | 0.22 | 0.15 | 0.00 |
| L-Lysine HCl (78%) | 0.25 | 0.26 | 0.35 |
| L-Threonine (98%) | 0.08 | 0.07 | 0.09 |
| L-Triptophan (98%) | 0.00 | 0.00 | 0.01 |
| MHA1 | 0.17 | 0.13 | 0.12 |
| Halquinol | 0.02 | 0.02 | 0.02 |
| Amoxilin | 0.00 | 0.03 | 0.00 |
| Vitamin Premix2 | 0.15 | 0.15 | 0.15 |
| Mineral Premix3 | 0.10 | 0.10 | 0.10 |
| Filler4 | 0.10 | 0.10 | 0.10 |
| Calculated nutrientes5 | |||
| ME, kcal/kg | 3544 | 3405 | 3230 |
| CP, % | 21.50 | 20.20 | 18.20 |
| SID Met+Cys, % | 0.78 | 0.70 | 0.61 |
| SID Lysine, % | 1.40 | 1.25 | 1.09 |
| SID Threonine, % | 0.88 | 0.79 | 0.69 |
| SID Triptophan, % | 0.25 | 0.22 | 0.20 |
| SID Isoleucine, % | 0.86 | 0.79 | 0.68 |
| SID Valine, % | 1.02 | 0.91 | 0.75 |
| Ca, % | 0.85 | 0.82 | 0.77 |
| Available P, % | 0.50 | 0.45 | 0.38 |
1MHA is a calcium salt of 2-hydroxy-4-methylthio butanoic acid. MHA amount was adjusted and replaced by filler according to the treatments to keep diets iso-methionine.
2Composition kg−1: Niacin 20.000 mg; vitamin A 6.000.000 UI; vitamin B2 4.000 mg; biotin 80.00 mg; Folic acid 800 mg; tiamin 1.350 mg; vitamin B12 20.000 µg; vitamin K 1.500 mg; pantothenic acid 9.350 mg; vitamin E 15.000 mg; vitamin D3 1.500.000 UI; vitamin B6 2000 mg.
3 Composition kg−1: Selenium 300 mg; manganese 40.000 mg; copper 10.000 mg; iron 100.000 mg; cobalt 1000 mg; iodine 1.500 mg; zinc 100.000 mg.
4Filler was replaced by Cu Methionine Hydroxy Analogue Chelate (15%), Tribasic Cu Chloride (58%) or Cu sulfate (25%) according to treatments.
5Based on Rostagno (2011) values for feed ingredients and on NIRS analyses for corn and soybean meal.
Table 2.
Supplemental Cu concentrations in dietary treatments
| Trt | Description | Phase 1 | Phase 2 | Phase 3 |
|---|---|---|---|---|
| 1 | Control | – | – | – |
| 2 | CuSO41 | 160 | 160 | 160 |
| 3 | Cu-MHAC2 | 150 | 80 | 50 |
| 4 | TBCC3 | 160 | 160 | 160 |
1Copper sulfate, 25% Cu.
2Cu Methionine Hydroxy Analogue Chelate, 15% Cu.
3Tribasic Cu chloride, 58% Cu.
Zinc oxide was included during the first 2 phases postweaning at 2,200 and 1,500 mg/kg as commonly used in piglets in Brazil, respectively. Feed and water were provided ad libitum throughout the entire experimental period and the diets contained antibiotics (halquinol at 200 g/ton in all phases and amoxilin at 255 g/ton in Phase 2 only). At the end of each feeding phase, BW, BW gain, feed intake, and feed conversion ratio were determined.
Ghrelin Gene Expression and Serum GH
In Exp. 2, a sample from the stomach mucosa at the fundic region was taken from one piglet per replicate on day 70, immediately snap frozen in liquid nitrogen, and then preserved at −80 °C for further analysis. Total RNA was extracted using Trizol (ThermoFisher Scientific) and quantified spectroscopically (Nano Vue—GE Healthcare). The RNA integrity was evaluated by electrophoresis in denaturant agarose gel. RNA samples were diluted at 500 ng/µL and equal volumes were combined to form 4 pools of RNA per treatment. Pooled RNA was treated with DNaseI (RNase-Free DNase Set—Qiagen) and then used to synthesize the first strand cDNA using the SuperScript III First-Strand Synthesis SuperMix kit (ThermoFisher Scientific) and an equal proportion mixture of random primers and oligo (dT). All procedures were performed according to the manufacturer recommendations.
Relative gene expression of ghrelin was carried out in a StepOnePlus thermocycler (ThermoFisher) using β-actin gene as the endogenous control, Universal Taqman Master mix (2X) and specific primers and probes (FAM-MGB), acquired from ThermoFisher Scientific with respective catalogue numbers 4351372 and 4331182. The relative gene expression was evaluated by ΔΔCT method (Livak and Schmittgen, 2001).
Blood samples were collected from 1 pig/pen (3 aliquots per animal) at day 70 and pooled to form 4 replicates per treatment. Serum GH concentrations were determined by the ELISA kit Asys LS-F5337 (LifeSpan Bioscience Inc., Seattle, WA) in agreement with the manufacturer.
Statistical Analysis
The performance data of both trials were analyzed together in a completely randomized block design using PROC GLM of SAS 9.3 (SAS Inc., NC, 2011) wherein each experiment was used as a block. Although initial BW was not different among treatments at the onset of the trial, it was shown to be significant when used as a covariate for many parameters and therefore used accordingly in the statistical analysis. The ghrelin RNA relative expression, and GH in serum data obtained from trial 2 were analyzed using a one-way ANOVA test. Differences among means were compared by Tukey’s least significant difference. Orthogonal contrasts were used to test relevant comparisons which included the effect of Cu-MHAC vs. feeding inorganic Cu (CuSO4-160 and TBCC-160) and CuSO4-160 vs TBCC-160. Statistical significance was considered with P ≤ 0.05, and P values between 0.05 and 0.10 were considered as a tendency.
Results
Performance
An effect of experiment (block) was observed for most of the performance parameters at the different feeding phases (P < 0.05), but no interaction between Experiment and Treatment was observed at any given point (P > 0.10). Therefore, performance from both Experiments was presented together.
No differences on performance parameters were observed among treatments during Phase I (P > 0.05, Table 3). However, orthogonal contrasts revealed an improvement in FCR when Cu-MHAC was supplemented compared with feeding 160 mg/kg of CuSO4 and TBCC (1.156 vs. 1.205 g/g, respectively).
Table 3.
Effect of copper supplementation on growth performance of nursery pigs1
| Treatment | 24–35 d of age | BW 35 d, kg |
24–49 d of age | BW 49 d, kg |
24–70 d of age | BW 70 d, kg |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BWG, kg |
FI, kg |
FCR, g/g |
BWG, kg |
FI, kg |
FCR, g/g |
BWG, kg |
FI, kg |
FCR, g/g |
||||
| Control | 3.97 | 4.69 | 1.185 | 9.37 | 11.53 | 15.84 | 1.384 | 16.94 | 24.52b | 40.17b | 1.663a | 29.94b |
| CuSO4 | 4.13 | 4.93 | 1.199 | 9.53 | 11.75 | 16.01 | 1.367 | 17.16 | 25.19b | 40.61b | 1.634ab | 30.61b |
| Cu-MHAC | 4.10 | 4.80 | 1.156 | 9.46 | 11.71 | 15.73 | 1.351 | 17.18 | 26.54a | 42.10a | 1.576b | 32.00a |
| TBCC | 3.91 | 4.80 | 1.210 | 9.40 | 11.46 | 15.96 | 1.405 | 16.88 | 25.41ab | 41.31ab | 1.651a | 30.82b |
| SEM | 0.05 | 0.06 | 0.009 | 0.05 | 0.12 | 0.13 | 0.009 | 0.12 | 0.37 | 0.42 | 0.009 | 0.37 |
| P | ||||||||||||
| Treatment | 0.32 | 0.42 | 0.15 | 0.68 | 0.55 | 0.78 | 0.06 | 0.47 | 0.01 | <0.05 | <0.05 | <0.01 |
| Initial weight | <0.01 | <0.01 | 0.84 | <0.01 | <0.01 | <0.01 | 0.84 | <0.01 | 0.10 | <0.01 | 0.54 | <0.01 |
| Experiment | 0.01 | 0.02 | 0.16 | 0.15 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Sex | 0.65 | 0.92 | 0.16 | 0.67 | 0.79 | 0.96 | 0.35 | 0.81 | 0.13 | 0.01 | 0.26 | 0.13 |
| Exp*Treat | 0.88 | 0.38 | 0.78 | 0.59 | 0.49 | 0.47 | 0.28 | 0.46 | 0.56 | 0.30 | 0.34 | 0.62 |
| P-value for orthogonal contrasts | ||||||||||||
| Control vs. Others | 0.47 | 0.20 | 0.88 | 0.43 | 0.56 | 0.82 | 0.57 | 0.51 | 0.02 | <0.05 | 0.12 | 0.01 |
| Cu-MHAC vs. (CuSO4 + TBCC) | 0.49 | 0.57 | 0.02 | 0.94 | 0.60 | 0.32 | 0.06 | 0.45 | 0.02 | 0.07 | 0.02 | 0.01 |
| CuSO4 vs. TBCC | 0.12 | 0.35 | 0.64 | 0.35 | 0.22 | 0.87 | 0.06 | 0.22 | 0.71 | 0.32 | 0.60 | 0.71 |
1BWG = body weight gain; FI = feed intake; FCR = feed conversion ratio.
a,b Means in a column followed by no common letter differ significantly (P < 0.05).
From weaning to 49 d, BW gain and FI were not affected by treatment (P > 0.0.5). However, piglets fed Cu-MHAC tended to improve FCR when compared with CuSO4-160 and TBCC-160 (P = 0.06), and CuSO4-160 pigs tended to have better FCR than TBCC-160 (P = 0.06), when using orthogonal contrasts.
At the end of the experiment, piglets fed diets with Cu-MHAC had greater BW than all other treatments (P < 0.01). Adding Cu from Cu-MHAC increased BW gain and FI vs. Control or CuSO4-160 and the FCR was significantly better in animals fed Cu-MHAC vs. TBCC-160 and Control (P < 0.05).
By orthogonal contrasts, increasing Cu levels in the diets, regardless of Cu sources, significantly increased FI (40.17 vs. 41.34; P<0.05) and BW gain (24.52 vs. 25.71; P = 0.02) during days 24 to 70. The contrast between feeding Cu-MHAC vs. inorganic Cu (CuSO4-160 and TBCC-160) evidenced a significant improvement (P = 0.02) in BW (32.00 vs. 30.72) and in FCR (1.576 vs. 1.643) when feeding organic Cu, and a tendency to improve FI (P = 0.07). The TBCC-160 group was not different from CuSO4-160 or Control pigs for any production parameter tested at this time or at the end of any feeding phase (P > 0.10).
Ghrelin RNA Expression and Serum GH Concentration (Exp. 2)
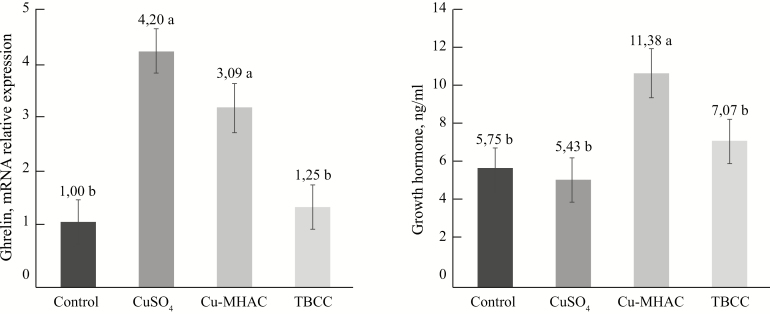
Ghrelin RNA expression in the gastric mucosa was upregulated in piglets fed CuSO4-160 or Cu-MHAC compared with piglets fed Control or TBCC-160 diets (P < 0.001, Figure 1). Serum GH levels were greater in piglets fed Cu-MHAC compared with those fed Control, CuSO4, and TBCC (P < 0.01, Figure 1). The supplementation with TBCC at 160 mg/kg did not change the ghrelin RNA expression nor serum GH compared with the Control diet fed piglets (P > 0.05).
Figure 1.
Ghrelin mRNA expression relative to actin (P < 0.001) and serum growth hormone level (P < 0.01) in Experiment 2. Significant orthogonal contrasts for ghrelin: Control vs. Others, P < 0.001; CuSOs4 vs. TBCC, P < 0.0001. Significant orthogonal contrasts for growth hormone: Cu-MHAC vs (CuSO4 + TBCC), P < 0.01.
Discussion
Copper is essential for a wide variety of health and performance-related functions in all animal species. In swine diets, it has been supplemented using inorganic salts such as Cu oxide (CuO) or, more commonly, CuSO4. However, the use of inorganic salts can result in poor bioavailability of the mineral, primarily because of the numerous nutrients and ingredient antagonisms that impair absorption (Underwood and Suttle, 2001). The advantage of organic trace minerals is that the binding of the organic ligand(s) to the mineral should provide stability to the complex in the upper gastrointestinal tract. It avoids mineral losses to antagonists and allows the intact molecule to be delivered to the absorptive epithelium of the small intestine (Leeson and Summers, 2001). Nevertheless, not all organic trace minerals are equally able to deliver more bioavailable mineral to the animals (Richards et al., 2008), since there are different ligands, number, type, and strength of bonds depending on sources.
Different sources of Cu may be used to promote performance of postweaning piglets, but recommended levels and maximum performance reached can vary. Apgar et al. (1995) reported that growth performance was linearly improved as the dietary level of Cu increased from 0 to 200 mg/kg; however, no differences were observed between the 2 Cu sources tested in weanling piglets (CuSO4 and organic Cu lysine complex). On the other hand, studies suggested that chelated minerals are more bioavailable and better at avoiding antagonism in vivo based on digestibility, tissue mineral, gene expression, excretion, and functional assays compared with inorganic trace minerals (Richards et al., 2010; Zhao et al., 2010; Liu et al., 2014). Because of its higher availability, less Cu-MHAC can be used to replace high levels of inorganic sources and have similar or better growth promoter effects in pigs (Zhao et al., 2014). Gonzalez-Esquerra et al. (2017) reported that Cu-MHAC can optimize performance at every Cu level tested compared with CuSO4 in broilers. Ma et al. (2015) observed different response curves from Cu sources in nursery pigs, suggesting that different mechanisms of action are responsible for the differences in bioavailability between Cu-MHAC and CuSO4.
In the current study, decremental dosages (150, 80, and 50 mg/kg Cu in Phases 1, 2, and 3, respectively) of Cu-MHAC were more effective in improving performance than feeding 160 mg/kg from either CuSO4 or TBCC. Zhao et al. (2014) reported that the use of Cu-MHAC yielded better BW gain and FCR than feeding CuSO4 to nursery and grower/finisher pigs. In agreement with the current work, whereby pharmacological levels of Zn and antibiotics were added, Ma et al. (2015) reported in a meta-analysis of 6 experiments that Cu-MHAC improved growth performance in nursery pigs in diets with high levels of ZnO and the presence of antibacterial agents.
No performance improvements over the Control group were observed when feeding CuSO4-160. Although feeding high doses of CuSO4 is a common practice in piglets when local legislation allows it, variable results from feeding high levels of Cu from sulphate sources have frequently been reported. Additionally, as shown in a meta-analysis by Ma et al. (2015), feeding Cu as Cu-MHAC is more effective than CuSO4 in improving feed efficiency.
The use of TBCC as a more bioavailable and less reactive form of inorganic Cu when combined with vitamins has been proposed to improve performance (Luo et al., 2005). Cromwell et al. (1998) reported that TBCC was nearly as efficacious as CuSO4 when both were supplemented at 200 ppm. In the present study, TBCC-160 generated similar growth responses vs. either Control or CuSO4-160 piglets.
The antimicrobial properties of Cu have long been recognized. The generation of reactive oxygen species may partially explain its capacity to kill bacteria. Macrophage phagosome accumulates Cu during bacterial infection, which may constitute an important mechanism of killing microorganisms (Hodgkinson and Petris, 2012). Aside from its antimicrobial properties, Cu promotes relevant systemic effects in pigs (Zhou et al., 1994a, 1994b; Zhu et al., 2011).
Ghrelin is an GH-stimulating hormone produced and secreted primarily from the stomach (Kojima et al., 1999). Yang et al. (2012) reported that high Cu levels induced a higher concentration of ghrelin at the fundic region of the stomach stimulating GH secretion, without differences between the sources tested (CuSO4 and copper methionine). Salfen et al. (2004) provided support to the idea that ghrelin acts as a GH secretagogue in the hypothalamus of pigs by reporting a significant increase in GH following the initial administration of exogenous ghrelin. In the current study, pigs fed CuSO4-160 showed an increase in ghrelin RNA expression, but not on GH, whereas feeding Cu-MHAC increased both gastric ghrelin RNA expression and serum GH which may correlate with the performance improvements observed with the latter group of pigs.
In conclusion, feeding Cu-MHAC at the levels tested improved the overall performance of piglets while feeding either CuSO4 or TBCC at 160 mg/kg was shown to be ineffective under the conditions tested herein. Greater ghrelin mRNA expression in the gastric mucosa along with higher levels of serum GH was found in piglets fed lower Cu from Cu-MHAC, which may be associated with Cu-MHAC’s greater bioavailability and at least partially with the benefits observed in performance.
Literature Cited
- Apgar G. A., Kornegay E. T., Lindemann M. D., and Notter D. R.. . 1995. Evaluation of copper sulfate and a copper lysine complex as growth promoters for weanling swine. J. Anim. Sci. 73:2640–2646. doi: 10.2527/1995.7392640x. [DOI] [PubMed] [Google Scholar]
- Arias V. J., and Koutsos E. A.. . 2006. Effects of copper source and level on intestinal physiology and growth of broiler chickens. Poult. Sci. 85:999–1007. doi: 10.1093/ps/85.6.999 [DOI] [PubMed] [Google Scholar]
- Banks K. M., Thompson K. L., Rush J. K., and Applegate T. J.. . 2004. Effects of copper source on phosphorus retention in broiler chicks and laying hens. Poult. Sci. 83:990–996. doi: 10.1093/ps/83.6.990 [DOI] [PubMed] [Google Scholar]
- Cromwell G. L. 2001. Antimicrobial and promicrobial agents. In: Lewis A. J and Southern L. L., editors, Swine nutrition. 2nd ed CRC press, Boca Raton, FL: p. 401–446. [Google Scholar]
- Cromwell G. L., Lindemann M. D., Monegue H. J., Hall D. D., and Orr D. E. Jr. 1998. Tribasic copper chloride and copper sulfate as copper sources for weanling pigs. J. Anim. Sci. 76:118–123. doi: 10.2527/1998.761118x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Esquerra R., Araujo R. B., Lima C. G., Arce J. M., Lopez-Coello C., and Ávilla-González E.. . 2017. Differential performance response of broilers when fed Cu from Cu - methionine-hydroxy-analogue chelate vs sulfate sources. Proceedings of 2017 International Poultry Scientific Forum, Georgia World Congress Center; January 30–31; Atlanta, GeorgSSSia, p 55. [Google Scholar]
- Hodgkinson V., and Petris M. J.. . 2012. Interface copper homeostasis at the host-pathogen. J. Biol. Chem. 287:13549–13555. doi: 10.1074/jbc.R111.316406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Yoo J. S., Kim H. J., Wang Y., Chen Y. J., Cho J. H., and Kim I. H.. . 2010. The effects of different copper (inorganic and organic) and energy (tallow and glycerol) sources on growth performance, nutrient digestibility, and fecal excretion profiles in growing pigs. Asian-Australas. J. Anim. Sci. 23:573–579. doi: 10.5713/ajas.2010.80436 [DOI] [Google Scholar]
- Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., and Kangawa K.. . 1999. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–60. doi: 10.1038/45230 [DOI] [PubMed] [Google Scholar]
- Leeson S., and Summers J.. . 2001. Scott’s nutrition of the chicken. University Books, Guelph, Ontario, Canada. [Google Scholar]
- Liu Y., Ma Y. L., Zhao J. M., Vazquez-Añón M., and Stein H. H.. . 2014. Digestibility and retention of zinc, copper, manganese, iron, calcium, and phosphorus in pigs fed diets containing inorganic or organic minerals. J. Anim. Sci. 92:3407–3415. doi.org/ 10.2527/jas.2013-7080 [DOI] [PubMed] [Google Scholar]
- Livak K. J., and Schmittgen T. D.. . 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(ΔΔC(T)) method. Methods. 25(4):402–8. Epub 2002/02/16. [DOI] [PubMed] [Google Scholar]
- Luo X. G., and Dove C. R.. . 1996. Effect of dietary copper and fat on nutrient utilization, digestive enzyme activities, and tissue mineral levels in weanling pigs. J. Anim. Sci. 74:1888–1896. doi: 10.2527/1996.7481888x. [DOI] [PubMed] [Google Scholar]
- Luo X. G., Ji F., Lin Y. X., Steward F. A., Lu L., Liu B., and Yu S. X.. . 2005. Effects of dietary supplementation with copper sulfate or tribasic copper chloride on broiler performance, relative copper bioavailability, and oxidation stability of vitamin E in feed. Poult. Sci. 84:888–893. doi: 10.1093/ps/84.6.888 [DOI] [PubMed] [Google Scholar]
- Ma Y. L., Zanton G. I., Zhao J., Wedekind K., Escobar J., and Vazquez-Añón M.. . 2015. Multitrial analysis of the effects of copper level and source on performance in nursery pigs. J. Anim. Sci. 93:606–614. doi: 10.2527/jas.2014-7796. [DOI] [PubMed] [Google Scholar]
- Manangi M. K., Vazquez-Añon M., Richards J. D., Carter S., Buresh R. E., and Christensen K. D.. . 2012. Impact of feeding lower levels of chelated trace minerals versus industry levels of inorganic trace minerals on broiler performance, yield, footpad health, and litter mineral concentration. J. Appl. Poult. Res. 21:881–890. doi: 10.3382/japr.2012-00531 [DOI] [Google Scholar]
- NRC 2012. Nutrition requirements of swine. 11th ed Natl. Acad. Press, Washington, DC. [Google Scholar]
- Pang Y., and Applegate T. J.. . 2007. Effects of dietary copper supplementation and copper source on digesta pH, calcium, zinc, and copper complex size in the gastrointestinal tract of the broiler chicken. Poult. Sci. 86:531–537. doi: 10.1093/ps/86.3.531 [DOI] [PubMed] [Google Scholar]
- Richards J. D., Bowman G. R., and Vázquez-Añón M.. . 2008. Organic trace minerals: bioavailability and functional effects in animals. Proceedings 2008, Intermountain Nutrition Conference, Dairy Nutritional Strategies to Meet Economic and Environmental Challenges, 10th Annual Meeting; January 29–30, 2008; Salt Lake City, UT. [Google Scholar]
- Richards J. D., Zhao J., Harrell R. J., Atwell C. A., and Dibner J. J.. . 2010. Trace mineral nutrition in poultry and swine. Asian Australas. J. Anim. Sci. 23:1527–1534. doi.org/ 10.5713/ajas.2010.r.07 [DOI] [Google Scholar]
- Rostagno H. S., Albino L. F. T., Donzele J. L., Gomes P. C., Oliveira R. F., Lopes D. C., Ferreira A. S., Barreto S. L. T.. . 2011. Tabelas brasileiras para aves e suínos: composição de alimentos e exigências nutricionais. 3rd ed UFV, Departamento de Zootecnia, Viçosa: p. 252. [Google Scholar]
- Salfen B. E., Carroll J. A., Keisler D. H., and Strauch T. A.. . 2004. Effects of exogenous ghrelin on feed intake, weight gain, behavior, and endocrine responses in weanling pigs. J. Anim. Sci. 82:1957–1966. doi: 10.2527/2004.8271957x. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc 2011. SAS OnlineDoc® 9.3. SAS Institute Inc, Cary, NC.. [Google Scholar]
- Shurson G. C., Ku P. K., Waxler G. L., Yokoyama M. T., and Miller E. R.. . 1990. Physiological relationships between microbiological status and dietary copper levels in the pig. J. Anim. Sci. 68:1061–1071. doi: 10.2527/1990.6841061x. [DOI] [PubMed] [Google Scholar]
- Underwood E., and Suttle N.. . 2001. The mineral nutrition of livestock. CABI Publishing, London, United Kingdom. [Google Scholar]
- Yang W., Wang J., Zhu X., Gao Y., Liu Z., Zhang L., Chen H., Shi X., Yang L., and Liu G.. . 2012. High lever dietary copper promote ghrelin gene expression in the fundic gland of growing pigs. Biol. Trace Elem. Res. 150:154–157. doi: 10.1007/s12011-012-9477-7. [DOI] [PubMed] [Google Scholar]
- Zhao J., Allee G., Gerlemann G., Ma L., Gracia M. I., Parker D., Vazquez-Anon M., and Harrell R. J.. . 2014. Effects of a chelated copper as growth promoter on performance and carcass traits in pigs. Asian Australas. J. Anim. Sci. 27:965–973. doi: 10.5713/ajas.2013.13416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Harper A. F., Estienne M. J., Webb K. E. Jr, McElroy A. P., and Denbow D. M.. . 2007. Growth performance and intestinal morphology responses in early weaned pigs to supplementation of antibiotic-free diets with an organic copper complex and spray-dried plasma protein in sanitary and nonsanitary environments. J. Anim. Sci. 85:1302–1310. doi: 10.2527/jas.2006-434. [DOI] [PubMed] [Google Scholar]
- Zhao J., Shirley R. B., Vazquez-Anon M., Dibner J. J., Richards J. D., Fisher P., Hampton V., Christensen K. D., Allard J. P., and Giesen A. F.. . 2010. Effects of chelated trace minerals on growth performance, breast meat yield and foot pad health in commercial meat broilers. J. Appl. Poult. Res. 19:365–372. doi.org/ 10.3382/japr.2009-00020 [DOI] [Google Scholar]
- Zhou W., Kornegay E. T., Lindemann M. D., Swinkels J. W., Welten M. K., and Wong E. A.. . 1994a. Stimulation of growth by intravenous injection of copper in weanling pigs. J. Anim. Sci. 72:2395–2403. doi: 10.2527/1994.7292395x. [DOI] [PubMed] [Google Scholar]
- Zhou W., Kornegay E. T., and Storrie B.. . 1994b. A microplate-based bioassay system for measuring porcine serum mitogenic activity. J. Anim. Sci. 72:2378–2384. doi: 10.2527/1994.7292378x. [DOI] [PubMed] [Google Scholar]
- Zhu D., Yu B., Ju C., Mei S., and Chen D.. . 2011. Effect of high dietary copper on the expression of hypothalamic appetite regulators in weanling pigs. J. Anim. Feed Sci. 20:60–70. doi: 10.22358/jafs/66158/2011 [DOI] [Google Scholar]