Abstract
A study was conducted to determine the standardized total tract digestible phosphorus (STTD P) requirement for 24- to 130-kg finishing pigs housed under commercial conditions. A total of 1,130 barrows and gilts (PIC 359 × 1050, Hendersonville, TN; initially 24.2 kg) were used, with 26 to 27 pigs per pen with 7 replicates per treatment. Pens of pigs were allotted to treatments in a randomized complete block design with body weight (BW) as the blocking factor. The dietary treatments were fed in 4 phases and were formulated to contain 80%, 90%, 100%, 115%, 130%, and 150% of the National Research Council (NRC) requirement estimate for finishing pigs within each phase. Weight ranges for each phase were: 27 to 49, 49 to 76, 76 to 90, and 90 to 130 kg. Treatments were achieved by increasing the amount of monocalcium phosphate at the expense of corn in the diet with no added phytase. All diets were formulated to contain a similar 1.14:1 to 1.16:1 total Ca:P ratio across treatments in all phases. Increasing STTD P resulted in a quadratic response (P < 0.05) in average daily gain (ADG), gain-to-feed ratio (G:F), and final BW. The greatest improvement was observed with STTD P at 130% of NRC for ADG and final BW and at 115% STTD P for G:F. Average daily feed intake increased linearly (linear, P < 0.05) with the inclusion of STTD P. Increasing STTD P resulted in an increase (quadratic, P < 0.05) in hot carcass weight (HCW) and carcass ADG with the greatest response observed with STTD P at 130% of NRC. There was a marginally significant response (quadratic, P < 0.10) in carcass G:F, with the greatest improvement with STTD P at 115% of NRC. Carcass yield decreased (linear, P < 0.05) with increasing STTD P, while there was a marginally significant (linear, P < 0.10) decrease in backfat and increase in fat-free lean. At the end of the study, a metacarpal was collected and analyzed for bone ash. Increasing STTD P resulted in an increase (linear, P < 0.05) in bone ash weight and percentage ash. For ADG and G:F, the quadratic model demonstrated the best fit. The maximum response in ADG and G:F was estimated at 122% and 116% of NRC STTD P, respectively. The broken-line linear model best fit the data for percentage bone ash, with a plateau achieved at 131% of the NRC STTD P. In conclusion, the estimated STTD P requirement of 24 to 130 kg ranged from 116% to 131% of the NRC publication (2012) requirement estimate.
Keywords: bone mineralization, finishing pigs, growth, modeling, phosphorus
INTRODUCTION
Phosphorus (P) is an inorganic element that is essential for growth performance and development and maintenance of the skeletal system (Berndt and Kumar, 2009; NRC, 2012). Approximately two-thirds of the body concentration of P is found in the pig skeleton, while the remaining P is found in muscle tissues where it is involved in different biological functions (Crenshaw, 2001). Diets formulated with excess P can lead to an increase in P excretion, negatively effecting the environment. In addition, this mineral is the third most expensive component in swine diets after energy and protein (Fan et al., 2001). Thus, diets are typically formulated to avoid excess P, with low margins of safety.
In 2012, the National Research Council (NRC) started to report the requirements for P in a standardized total tract digestibility (STTD) basis, which are based on a factorial approach. The NRC (2012) emphasized a need for empirical data to validate the model-derived digestible P requirement. Moreover, the concentration of P is greater in muscle tissue compared to adipose tissue (Nielsen, 1973). This implies that improvements in the genetic potential for lean growth in pigs result in a greater P requirement. The highest final pig body weight (BW) from the empirical estimates reported in the NRC (2012) model was approximately 109 kg, it dates 8 yr before the NRC publication, and this is the only data point past 70 kg BW. Today, pigs are marketed at heavier BW, and the genetic selection for high protein deposition warrants a revaluation of the digestible P requirement.
Furthermore, current statistical capabilities for modeling dose-response studies has allowed for a more precise estimation of the concentration of P needed to optimize different response criteria. Therefore, the objective of this study was to determine the effects of STTD P on growth performance, carcass characteristics, and bone mineralization of 24- to 130-kg pigs housed under commercial conditions.
MATERIAL AND METHODS
The Kansas State University Institutional Animal Care and Use Committee (Manhattan, KS) approved all experimental procedures in this study.
Animals and Diets
The study was conducted at a commercial research-finishing site in southwestern Minnesota (New Horizon Farms, Pipestone, MN). The facility was naturally ventilated and double-curtain sided. One barn was used containing 42 pens (3.05 × 5.49 m2) with completely slatted concrete flooring and a deep pit for manure storage. Each pen was equipped with a 4-hole stainless steel, dry self-feeder (Thorp Equipment, Thorp, WI) and 1 cup waterer. The facility was equipped with a computerized feeding system (FeedPro; Feedlogic Corp., Willmar, MN) capable of measuring and recording daily feed additions to individual pens. Thirteen barrows and fourteen gilts (PIC 359 × 1050, Genus PIC, Hendersonville, TN) were housed in each pen and were allowed ad libitum access to feed and water throughout the experiment.
A total of 1,130 pigs (initial average BW of 24.2 kg) were used in a 111-d growth trial. After placement in the finishing facility, pigs were fed a common diet until the initiation of the trial. The common diet was formulated to be at the pigs’ STTD P requirement based on NRC (2012) estimates (0.33% STTD P). On day 0 of the trial, pens of pigs were sorted by average BW and randomly allotted to 1 of 6 dietary treatments in a randomized complete block design with BW as the blocking factor. There were 7 replicate pens per treatment with 26 or 27 pigs per pen.
All treatment diets were manufactured at the New Horizon Farms Feed Mill in Pipestone, MN, and fed in meal form. The experimental diets were corn-soybean meal based and were fed in 4 different phases (Table 1). The diets were formulated to contain 80%, 90%, 100%, 115%, 130%, and 150% of the NRC (2012) publication requirement for finishing pigs within each phase. The NRC (2012) requirement for phases 1 (25 to 50 kg), 2 (50 to 75 kg), 3 (75 to 100 kg), and 4 (100 to 135 kg), expressed as a percentage of the diet, are estimated as 0.31%, 0.27%, 0.24%, and 0.21% STTD P, respectively. Phase 1 diets were fed from day 0 to 29 (24.1 to 49.1 kg); phase 2 diets were fed from day 29 to 56 (49.1 to 75.5 kg); phase 3 diets were fed from day 56 to 70 (75.5 to 89.7 kg); and phase 4 diets were fed from day 70 to 111 (89.7 to 130.4 kg). The STTD P concentrations were achieved by increasing the amount of monocalcium phosphate at the expense of corn. There was no added phytase. Diets were formulated to an expected similar total Ca:P ratio of 1.14:1 to 1.16:1 across dietary treatments in all phases with the inclusion of limestone at the expense of corn.
Table 1.
Diet composition for phases 1 to 4 diets (as-fed basis)1
| Item | Phase 1 | Phase 2 | Phase 3 | Phase 4 |
|---|---|---|---|---|
| Ingredient, % | ||||
| Corn | 66.71–65.25 | 77.45–76.17 | 84.24–83.09 | 84.91–83.93 |
| Soybean meal, 46.5% CP | 30.78–30.88 | 20.15–20.24 | 13.58–13.66 | 13.13–13.20 |
| Limestone | 0.95–1.18 | 0.90–1.10 | 0.85–1.03 | 0.83–0.98 |
| Monocalcium phosphate, 21% P | 0.45–1.59 | 0.43–1.42 | 0.38–1.27 | 0.26–1.03 |
| Sodium chloride | 0.35 | 0.35 | 0.35 | 0.35 |
| L-lysine HCl | 0.35 | 0.35 | 0.30 | 0.25 |
| DL-methionine | 0.12 | 0.07 | 0.02 | 0.01 |
| L-threonine | 0.11 | 0.12 | 0.10 | 0.08 |
| L-tryptophan | 0.01 | 0.02 | 0.02 | 0.01 |
| Vitamin premix2 | 0.08 | 0.08 | 0.08 | 0.08 |
| Trace mineral premix3 | 0.10 | 0.10 | 0.10 | 0.10 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Calculated analysis | ||||
| Standardized ileal digestible amino acids, % | ||||
| Lysine | 1.21 | 0.95 | 0.75 | 0.70 |
| Isoleucine:lysine | 61 | 59 | 60 | 64 |
| Leucine:lysine | 127 | 136 | 151 | 161 |
| Methionine:lysine | 33 | 32 | 30 | 31 |
| Methionine and cysteine:lysine | 56 | 57 | 58 | 60 |
| Threonine:lysine | 61 | 63 | 65 | 67 |
| Tryptophan:lysine | 18.7 | 18.2 | 18.2 | 18.5 |
| Valine:lysine | 66 | 67 | 70 | 74 |
| Total lysine, % | 1.35 | 1.07 | 0.85 | 0.80 |
| Net energy, kcal/kg | 2,445–2,407 | 2,509–2,476 | 2,549–2,520 | 2,555–2,529 |
| Crude protein, % | 20.7 | 16.5 | 13.8 | 13.6 |
| Calcium, % | 0.56–0.84 | 0.50–0.75 | 0.45–0.67 | 0.42–0.61 |
| Phosphorus, % | 0.49–0.73 | 0.44–0.65 | 0.40–0.59 | 0.37–0.53 |
| STTD P, %4 | 0.25–0.46 | 0.22–0.40 | 0.19–0.36 | 0.17–0.31 |
| Available phosphorus, % | 0.17–0.42 | 0.15–0.37 | 0.13–0.32 | 0.11–0.27 |
| Calcium:phosphorus | 1.15 | 1.15 | 1.14 | 1.15 |
1Treatment were formulated to contain 80%, 90%, 100%, 115%, 130%, and 150% of NRC (2012) STTD P estimates across dietary phases (0.31%, 0.31%, 0.24%, 0.21% for phases 1, 2, 3, and 4, respectively). Phase 1 diets were fed from day 0 to 29 (24.1 to 49.1 kg), phase 2 from day 29 to 56 (49.1 to 75.5 kg), phase 3 from day 56 to 70 (75.5 to 89.7 kg), and phase 4 from day 70 to 111 (89.7 to 130.4 kg).
2Provided per kg of premix: 8,818,490 IU vitamin A; 1,102,311 IU vitamin D; 35,273 IU vitamin E; 3,527.4 mg vitamin K; 30.9 mg vitamin B12; 39,683 mg niacin; 22,046 mg pantothenic acid; and 6,614 mg riboflavin.
3Provided per kg of premix: 165 g Zn from Zn sulfate; 165 g Fe from iron sulfate; 40 g Mn from manganese oxide; 17 g Cu from copper sulfate; 0.3 g I from calcium iodate; and 0.3 g Se from sodium selenite.
4Standardized total tract digestible phosphorus.
Pens of pigs were weighed and feed disappearance was recorded on days 0, 29, 56, 70, 99, and 111 to determine average daily gain (ADG), average daily feed intake (ADFI), gain-to-feed ratio (G:F), grams of STTD P intake per day, and grams of STTD P intake per kilogram of gain. The STTD P, based on formulated values, was multiplied by ADFI to calculate grams of STTD P intake per day. The total grams of STTD P intake, based on formulated values, were divided by total BW gain to calculate the grams of STTD P intake per kilogram of gain.
Carcass and Bone Data Collection
On day 99, the 2 heaviest pigs in each pen were selected, weighed, and sold according to standard farm procedures. These pigs were used in calculation of pen growth performance but not carcass characteristics. On day 111, final pen weights were taken and one barrow and one gilt with intermediate weights were selected and tattooed with a pen identification. These pigs were transported to a commercial abattoir in northwest Iowa (Natural Food Holdings Inc., Sioux Center, IA) for processing and collection of metacarpal bones. Following processing, the left front feet were separated at the junction of carpals and radius and ulna and individually placed in a zip-lock plastic bag with a permanent identification tag within the bag. These feet were transferred on dry ice to the Kansas State University Swine Laboratory and stored at −20 ℃ until analysis of bone mineral content.
The remaining pigs were individually tattooed with the specific pen identity on the shoulder to allow for carcass measurements to be recorded on a pen basis. These pigs were transported to a commercial packing plant in southwestern Minnesota (JBS Swift and Company, Worthington, MN) for processing and carcass data collection. Carcass measurements included hot carcass weight (HCW), loin depth, backfat depth, and percentage lean. Fat depth and loin depth were measured with an optical probe inserted between the third and fourth last rib (counting from the ham end of the carcass) at a distance approximately 7 cm from the dorsal midline. Percentage carcass yield was calculated by dividing the average pen HCW collected at the plant by the average final live weight at the farm before transport. Carcass ADG was calculated by multiplying the overall ADG by percentage carcass yield. Carcass G:F was calculated by dividing the overall ADFI by carcass ADG.
Bone Ash Analysis
After thawing overnight, the feet were autoclaved for 1 h at 121 ℃. The third and fourth metacarpals of each foot were removed. These bones were cleaned of extraneous soft tissue and refrozen. The third metacarpal was dried at ambient temperature for 24 h and cut in half and weighed. They were wrapped in cheesecloth to keep their tag ID and defatted by petroleum ether using a Soxhlet apparatus for 7 d. Defatted metacarpals were placed in a 105-℃ drying oven for 24 h to determine the dry fat-free weight. Bones were then ashed in a muffle furnace at 600 ℃ for another 24 h to determine percentage ash. Ash is expressed as a percentage of dried fat-free bone weight.
Chemical Analysis
Representative diet samples were obtained from 6 feeders of each treatment approximately 3 d after the beginning and 3 d before the end of the phase and delivered to the Kansas State University Swine Laboratory, Manhattan, KS, and stored at −20 °C until analysis. Samples of the diets were combined within dietary treatment, and a composite sample from each treatment was analyzed in duplicate (Ward Laboratories, Inc., Kearney, NE; Table 2). Samples were analyzed for Ca and P (method 985.01; AOAC International, 1990).
Table 2.
Chemical analysis of experimental diets (as-fed-basis)1
| STTD P, % of NRC (2012)2–5 | ||||||
|---|---|---|---|---|---|---|
| Item | 80 | 90 | 100 | 115 | 130 | 150 |
| Total calcium (Ca), % | ||||||
| Phase 1 | 0.74 | 0.74 | 0.76 | 0.79 | 0.73 | 0.91 |
| Phase 2 | 0.51 | 0.55 | 0.59 | 0.74 | 0.91 | 0.84 |
| Phase 3 | 0.58 | 0.54 | 0.57 | 0.59 | 1.11 | 0.84 |
| Phase 4 | 0.53 | 0.58 | 0.85 | 0.89 | 0.67 | 0.69 |
| Total phosphorus (P), % | ||||||
| Phase 1 | 0.40 | 0.43 | 0.47 | 0.49 | 0.56 | 0.62 |
| Phase 2 | 0.31 | 0.43 | 0.48 | 0.49 | 0.57 | 0.57 |
| Phase 3 | 0.33 | 0.36 | 0.36 | 0.44 | 0.46 | 0.57 |
| Phase 4 | 0.31 | 0.39 | 0.48 | 0.53 | 0.54 | 0.58 |
1Representative samples of treatment diets were taken from 6 feeders per dietary treatment 3 d after the beginning and 3 d before the end of the phase and stored at −20 °C. After blending, subsamples were submitted to Ward Laboratories, Inc. (Kearney, NE) for analyses.
2Phase 1 calculated STTD P were 0.25%, 0.28%, 0.31%, 0.36%, 0.40%, and 0.46%, and calculated total P were 0.49%, 0.52%, 0.56%, 0.61%, 0.66%, and 0.73% for 80%, 90%, 100%, 115%, 130%, and 150% of the NRC (2012) estimate, respectively.
3Phase 2 calculated STTD P were 0.22%, 0.24%, 0.27%, 0.31%, 0.35%, and 0.40%, and calculated total P were 0.44%, 0.46%, 0.49%, 0.54%, 0.59%, and 0.65% for 80%, 90%, 100%, 115%, 130%, and 150% of the NRC (2012) estimate, respectively.
4Phase 3 calculated STTD P were 0.19%, 0.22%, 0.24%, 0.28%, 0.31%, and 0.36%, and calculated total P were 0.40%, 0.42%, 0.45%, 0.49%, 0.53%, and 0.59 % for 80%, 90%, 100%, 115%, 130%, and 150% of the NRC (2012) estimate, respectively.
5Phase 4 calculated STTD P were 0.17%, 0.19%, 0.21%, 0.24%, 0.27%, and 0.31%, and calculated total P were 0.37%, 0.39%, 0.42%, 0.45%, 0.49%, and 0.53% for 80%, 90%, 100%, 115%, 130%, and 150% of the NRC (2012) estimate, respectively.
Statistical Analysis
Experimental growth data were analyzed as a randomized complete block design with block as a random effect and pen as the experimental unit. The study was structured as a split-plot design in a randomized complete block design for the bone data. The whole-plot treatments included the different STTD P concentrations. Within each of the dietary treatments, there was a one-way treatment structure with gender as the factor level. A random effect of block by treatment was used to identify the pair of pigs (one barrow and one gilts) within each pen as the experimental unit for gender. The two-way interaction between dietary treatments and gender was tested, and no significant interactions were observed. Response variables were analyzed using generalized linear and nonlinear mixed models. Polynomial contrasts were implemented to evaluate the functional form of the dose response to increasing dietary STTD P on ADG, ADFI, G:F, BW, grams of STTD P intake per day, grams of STTD P intake per kilogram of gain, HCW, carcass ADG, carcass G:F, carcass yield, carcass backfat, carcass fat-free lean, and carcass loin depth. Backfat depth, loin depth, and percentage lean were adjusted to a common HCW. The Kenward–Roger method was used to adjust the denominator degrees of freedom and correct the standard errors for bias (Littell et al., 2006). The coefficients for the unequally spaced linear and quadratic contrasts were derived using the interactive matrix language (IML) procedure in SAS (Version 9.3, SAS Institute Inc., Cary, NC). Statistical models were fit using GLIMMIX procedure of SAS. Results were considered significant at P ≤ 0.05 and marginally significant at 0.05 < P ≤ 0.10.
In addition, the effects of the STTD P levels on overall ADG, G:F, and percentage bone ash were fit using procedures described by Gonçalves et al. (2016). Models were expanded to account for heterogeneous residual variances when needed. Competing statistical models included a linear (LM), quadratic polynomial (QP), broken-line linear (BLL), and broken-line quadratic (BLQ). Dose-response models were compared based on the Bayesian information criterion (BIC), where the smaller the value, the better (Milliken and Johnson, 2009). A decrease in BIC greater than 2 was considered a significant improvement in model fit. The 95% CI of the estimated requirement to reach maximum performance or to reach plateau performance was computed. Results reported correspond to inferences yielded by the best fitting models.
RESULTS
Chemical Analysis
The average values of analyzed P across dietary phases were approximately 20%, 10%, 6%, 5%, 5%, and 5% lower than formulated values for treatments that represented 80%, 90%, 100%, 115%, 130%, and 150% of NRC estimates across phases, respectively (Table 2). These values are still within the acceptable analytical variation based on the Association of American Feed Control Officials (AAFCO)’s sample program (AAFCO, 2015) with the exception of the lowest P treatment. Although variation in analyzed P existed, analyzed P content still increased linearly with increasing STTD P treatments. The average values of analyzed Ca across dietary phases were approximately 22%, 18%, 27%, 27%, 32%, and 15% greater than formulated values for treatments that represented 80%, 90%, 100%, 115%, 130%, and 150% of NRC estimates across phases, respectively. Average values of analyzed Ca were approximately 23% higher than formulated values. Chemical analysis of dietary Ca is typically more variable, with a higher coefficient of variation than P (Wu et al., 2018). According to the AAFCO’s sample program, the acceptable variability in the laboratory analyses of Ca is approximately 30% given the formulated Ca levels used in this study (AAFCO, 2015). According to the analyzed values of Ca and P, the analyzed Ca:P ratio in the final diets were greater than formulated, with the average across phases varying from 1.40:1 to 1.74:1 among treatments.
Growth performance
From day 0 to 56 (phases 1 and 2), which corresponded to the grower period, increasing the STTD P increased ADG (quadratic, P < 0.05; Table 3) driven by an increase in ADFI (quadratic, P < 0.05). The greatest improvement occurred as the STTD P increased from 80% to 115% of the NRC requirement estimate, starting to decrease at the highest STTD P concentration of 150%. Feed efficiency was not affected by dietary treatment (P > 0.10). From day 56 to 111 (phases 3 and 4), which corresponded to the finisher period, increasing STTD P increased ADG (quadratic, P < 0.05) driven by an improvement in G:F (quadratic, P < 0.05). The greatest improvement in G:F occurred as the STTD P increased from 80% to 115% of the NRC requirement estimate and started to decrease at higher levels. For ADG, the greatest increase was observed from 80% to 130% of the NRC requirement estimate and then it decreased at the highest STTD P. During this period, feed intake was not affected (P > 0.10) by dietary treatment. The grams of STTD P intake per day increased in a quadratic fashion (P < 0.05) during the grower period and in a linear manner during the finisher period (P < 0.05). The grams of STTD P intake per kilogram of gain increased linearly (P < 0.05) for the grower and finisher periods, with a marginal quadratic response during the finisher period (P < 0.10).
Table 3.
Least square means for growth performance and carcass characteristics of growing–finishing pigs fed increasing STTD P from 24 to 130 kg BW1
| STTD P, % of NRC (2012) requirement2 | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | 80 | 90 | 100 | 115 | 130 | 150 | SEM | Linear | Quadratic |
| Grower period (day 0 to 56) | |||||||||
| ADG, g | 886 | 910 | 904 | 933 | 930 | 917 | 11.7 | 0.001 | 0.003 |
| ADFI, g | 1,874 | 1,922 | 1,919 | 1,984 | 1,983 | 1,930 | 35.5 | 0.017 | 0.004 |
| G:F, g/kg | 473 | 474 | 471 | 470 | 469 | 476 | 4.9 | 0.958 | 0.271 |
| STTD P intake, g/d | 4.3 | 5.0 | 5.5 | 6.6 | 7.4 | 8.3 | 0.12 | <0.001 | 0.009 |
| STTD P intake, g/kg gain | 4.9 | 5.4 | 6.1 | 7.0 | 7.9 | 9.1 | 0.07 | <0.001 | 0.351 |
| Finisher period (day 56 to 111) | |||||||||
| ADG, g | 1,000 | 1,001 | 1,010 | 1,020 | 1,045 | 998 | 12.7 | 0.208 | 0.006 |
| ADFI, kg | 2,899 | 2,912 | 2,910 | 2,873 | 2,958 | 2,926 | 26.5 | 0.254 | 0.792 |
| G:F, g/kg | 345 | 344 | 347 | 355 | 353 | 341 | 3.5 | 0.818 | <0.001 |
| STTD P intake, g/d | 5.0 | 5.7 | 6.5 | 7.1 | 8.3 | 9.5 | 0.07 | <0.001 | 0.842 |
| STTD P intake, g/kg gain | 5.0 | 5.7 | 6.4 | 7.1 | 7.9 | 9.4 | 0.09 | <0.001 | 0.063 |
| Overall period (day 0 to 111) | |||||||||
| ADG, g | 941 | 955 | 956 | 975 | 986 | 956 | 6.3 | 0.001 | <0.001 |
| ADFI, g | 2,374 | 2,407 | 2,401 | 2,417 | 2,456 | 2,415 | 23.2 | 0.033 | 0.102 |
| G:F, g/kg | 397 | 397 | 398 | 404 | 401 | 396 | 3.2 | 0.574 | 0.002 |
| BW, kg | |||||||||
| Day 0 | 24.2 | 24.1 | 24.2 | 24.2 | 24.1 | 24.2 | 0.73 | 0.992 | 0.954 |
| Day 56 | 73.8 | 75.1 | 75.0 | 76.7 | 77.0 | 75.6 | 1.26 | <0.001 | <0.001 |
| Day 111 | 127.9 | 129.3 | 130.1 | 131.9 | 133.6 | 129.7 | 1.12 | <0.001 | <0.001 |
| Carcass characteristics3 | |||||||||
| HCW, kg | 93.0 | 94.5 | 94.5 | 95.4 | 96.5 | 93.9 | 0.72 | 0.012 | <0.001 |
| Carcass ADG, g4 | 685 | 698 | 694 | 706 | 712 | 692 | 4.6 | 0.029 | <0.001 |
| Carcass G:F, g/kg5 | 289 | 290 | 289 | 292 | 290 | 287 | 2.4 | 0.469 | 0.063 |
| Carcass yield, % | 72.8 | 73.1 | 72.6 | 72.3 | 72.2 | 72.4 | 0.24 | 0.027 | 0.368 |
| Backfat, mm6,7 | 19.0 | 18.4 | 18.3 | 18.6 | 18.0 | 18.1 | — | 0.073 | 0.580 |
| Fat-free lean, %6,7 | 55.0 | 55.4 | 55.4 | 55.3 | 55.6 | 55.5 | — | 0.097 | 0.519 |
| Loin depth, mm6,7 | 64.1 | 64.7 | 64.2 | 64.5 | 64.7 | 64.3 | — | 0.796 | 0.651 |
1A total of 1,130 pigs (337 × 1050, PIC, initially 24.1 kg BW) were used in a 111-d growth trial with 26 to 27 pigs per pen and 7 pens per treatment.
2All treatments contain variable concentrations of STTD P that represent 80%, 90%, 100%, 115%, 130%, and 150% of the NRC (2012) requirement for pigs within phases.
3Eight hundred seventy-seven pigs were transported to a commercial packing plant for processing and data collection (Swift and Company, Worthington, MN).
4Carcass average daily gain = overall average daily gain × carcass yield.
5Carcass G:F = carcass ADG/overall ADFI.
6SEM for backfat were 0.370, 0370, 0.376, 0.373, and 0.367; SEM for % lean were 0.247, 0.247, 0.251, 0.244, 0.249, and 0.245; and SEM for loin depth were 6.17, 6.17, 6.29, 6.08, 6.23 and 6.10 for 80%, 90%, 100%, 115%, 130%, and 150% of the NRC (2012) requirement, respectively.
7Adjusted for HCW.
For the overall study, increasing STTD P increased ADG and final BW (quadratic, P < 0.05). The greatest increase in ADG and final BW was observed as STTD P increased from 80% to 130% of NRC requirement estimates, with both ADG and final BW decreasing at the highest STTD P concentration of 150% of NRC estimates. Similarly, feed efficiency improved (quadratic, P < 0.05) as STTD P increased from 80% to 115% of the NRC requirement and started to worsen thereafter. ADFI increased (linear, P < 0.05) as STTD P increased, however, with the greatest feed intake observed at 130% STTD P of the NRC requirement estimate.
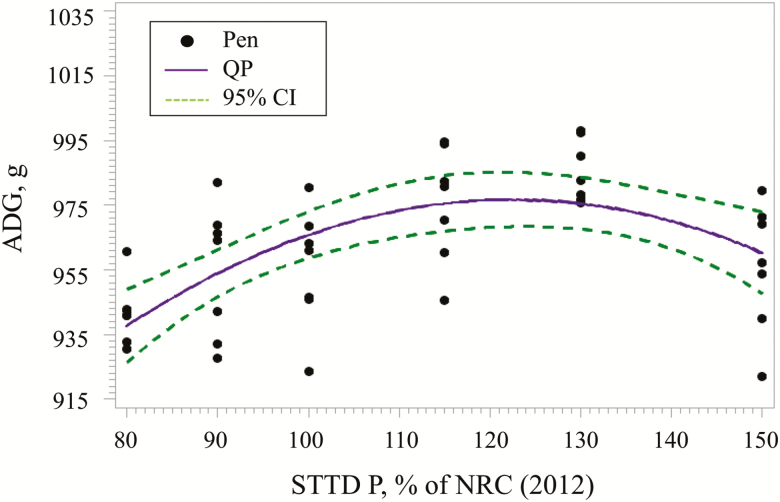
Homogeneous variance was used for ADG and heterogeneous variance was used for G:F models. For ADG (Figure 1), the best fitting model was the QP. Based on the best fitting model, the STTD P concentration for maximum ADG was estimated at 122% (95% CI: [104, 143%]) of the NRC (2012) requirement estimates within phases. The estimated QP regression equation was:
Figure 1.
Fitted QP regression model for ADG as a function of increasing STTD P in 24- to 130-kg pigs. The QP model estimated the maximum mean ADG at 122% (95% CI: [104, 143%]) of the NRC (2012) recommendations within phases. Based on the best fitting model, the estimated regression equation was ADG, g = 651.36 + 531.33 × (STTD P) – 216.90 × (STTD P)2.
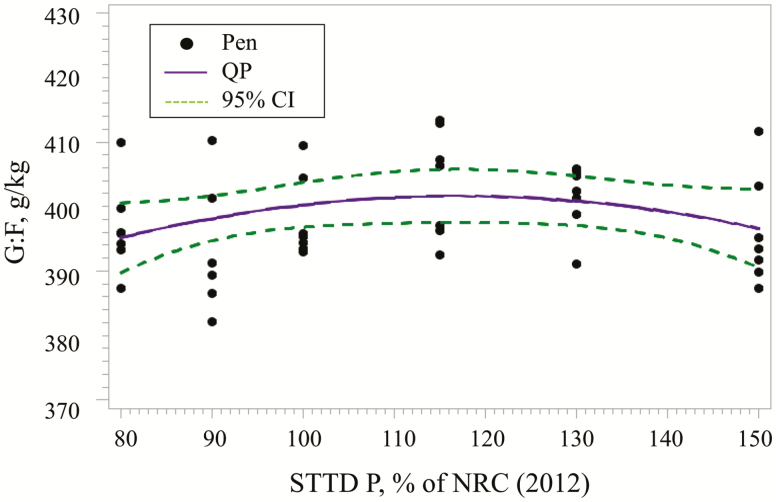
Similarly, the best fitting model for G:F (Figure 2) was the QP. The STTD P concentration for maximum G:F was estimated at 116% (95% CI: [90, >150%]) of the NRC (2012) requirement for each phase. Based on this model, the estimated regression equation was:
Figure 2.
Fitted QP regression model for G:F as a function of increasing STTD P in 24- to 130-kg pigs. The QP model estimated the maximum mean G:F at 116% (95% CI: [90, >150%]) of the NRC (2012) recommendations within phases. Based on the QP model, the estimated regression equation was G:F, g/kg = 338.34 + 108.98 × (STTD P) – 46.7864 × (STTD P)2.
Carcass characteristics
For carcass characteristics, HCW increased (quadratic, P < 0.05) as STTD P increased up to 130% of the NRC (2012) requirement estimate and, then, started to decrease at the higher STTD P concentration. Similarly, carcass ADG increased (quadratic, P < 0.05) with the greatest response observed with STTD P at 130% of the NRC (2012) requirement estimate. There was also a marginally significant response (quadratic, P < 0.10) in carcass G:F, with the greatest improvement observed as STTD P increased from 80% to 115% of the NRC requirement estimate, starting to worsen at higher STTD P levels. Carcass yield decreased (linear, P < 0.05) with increasing STTD P, while there was a marginally significant linear decrease (P < 0.10) in backfat and increase in fat-free lean. No statistically significant difference (P > 0.10) was observed for loin depth.
Bone Mineralization
For bone characteristics, increasing STTD P resulted in an improvement (linear, P < 0.05) in fat-free bone ash weight (Table 4). However, there was no evidence for difference (P > 0.10) in fat-free bone ash weight due to gender when the model was adjusted to account for differences in HCW between barrows and gilts. Similarly, ash as a percentage of fat-free dried bone increased (linear, P < 0.05) as STTD P increased, with diminishing returns in percentage bone ash at STTD P concentration greater than 130% of NRC estimates. In addition, barrows had significantly greater (P < 0.05) percentage bone ash than gilts.
Table 4.
Least square means for bone mineralization of growing–finishing pigs fed increasing STTD P from 24 to 130 kg BW1
| P-value2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| STTD P, % of NRC (2012)3 | Gender | Treatment | |||||||||
| Item4 | 80 | 90 | 100 | 115 | 130 | 150 | Barrow | Gilt | Linear | Quadratic | Gender |
| Ash bone weight, g | 8.47 | 8.75 | 9.05 | 9.25 | 9.56 | 10.03 | 9.24 | 9.13 | 0.001 | 0.840 | 0.501 |
| SEM | 0.177 | 0.178 | 0.180 | 0.188 | 0.179 | 0.178 | 0.108 | 0.115 | |||
| Ash, % | 60.8 | 60.7 | 61.1 | 61.5 | 61.9 | 61.9 | 61.5 | 61.2 | 0.001 | 0.373 | 0.036 |
| SEM | 0.18 | 0.19 | 0.19 | 0.20 | 0.19 | 0.19 | 0.107 | 0.116 | |||
1A total of 1,130 pigs (337 × 1050, PIC, initially 24.1 kg BW) were used in a 111-d growth trial with 26 to 27 pigs per pen and 7 pens per treatment. At the end of the study, 84 pigs (2 pigs/pen, 1 barrow/1gilt) nearest to the mean live weight of the pen were subsampled and shipped to a processing facility for collection of metacarpals for bone mineralization analysis (Natural Foods Holdings, Inc., Sioux Center, IA). The third metacarpals were autoclaved for 1 h. After cleaning, bones were placed in Soxhlets containing pretoleum ether for 7 d as a means of removing water and fat. They were then dried at 105 °C for 24 h and, then, ashed at 600 °C for 24 h.
2The two-way interaction was tested and no evidence for significant interactions were observed for ash bone weight and bone percentage ash.
3All treatments contain variable concentrations of STTD P that represent 80%, 90%, 100%, 115%, 130%, and 150% of the NRC (2012) requirement for pigs within phases.
4Adjusted for HCW.
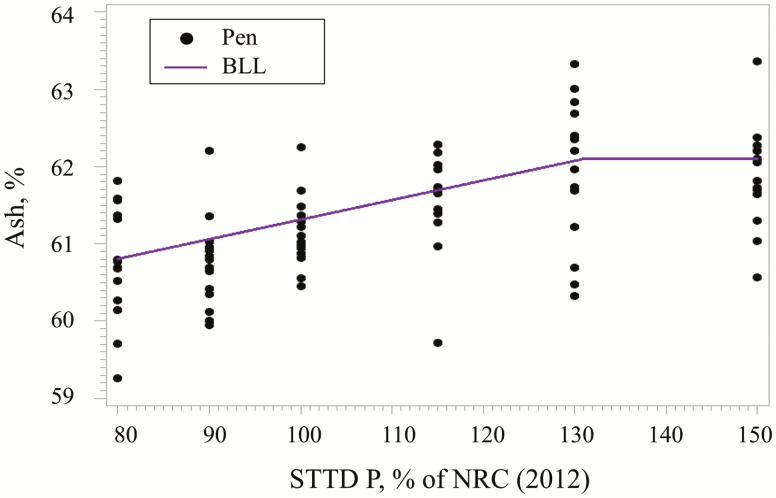
Homogeneous variance was used to model the bone mineralization data. The model that best fit the percentage bone ash (Figure 3) was the BLL. The STTD P concentration for maximum percentage bone ash was estimated at 131% (95% CI: [113, 148%]) of the NRC (2012) requirement estimate for each phase. The estimated regression equation was:
Figure 3.
Fitted BLL regression model for bone percentage ash as a function of increasing STTD P in 24- to 130-kg pigs. The BLL model estimated the maximum mean percentage ash at 131% (95% CI: [113, 148%]) of the NRC (2012) recommendations within phases. Based on the best fitting model, the estimated regression equation was bone ash, % = 62.1000 – 2.5374 × (1.31 – STTD P as % of NRC) if STTD P as % of NRC <1.31%, and bone ash, % = 62.100 if STTD P as % of NRC ≥1.31%.
DISCUSSION
Phosphorus requirement estimates for pigs reported in the latest NRC (2012) publication are expressed on a standardized total tract digestible basis. According to Almeida and Stein (2010), values for STTD P of feed ingredients are additive in mixed diets and can be used to formulate diets for pigs without compromising performance. However, the P requirements reported by the NRC are based on factorial estimates. Few empirical studies were considered appropriate to be included in the NRC model, and only three of them had an average pig BW greater than 60 kg, which dated from 8 to 31 yr before the NRC publication (Thomas and Kornegay, 1981; Hastad et al., 2004). It is well known that muscle tissue constitutes the second most abundant P reserve in the body after skeletal tissue, with minimum P found in the adipose tissue (Nielsen, 1973). Pig genotype can influence the extent of bone and muscle tissue deposition, which can lead to different dietary P requirements (Hittmeier et al., 2006; Alexander et al., 2008). In today’s pig production, pigs are not only marketed at heavier weights but are also highly selected for high lean tissue deposition. The current study was designed to provide more information on the STTD P requirement of modern genotype growing–finishing pigs and to validate NRC model requirement estimates.
Ekpe et al. (2002) conducted a study to estimate the STTD P requirement for 25- to 50-kg pigs. The authors have observed that the requirement to optimize growth rate and feed efficiency is approximately 113% to 127% of the NRC (2012) requirement estimates. Adeola et al. (2015) estimated the phosphorus requirement for 19- to 40-kg pigs through broken-line regression models. The breakpoints estimated in this study occurred at approximately 126% to 132% of the NRC (2012) estimates to maximize ADG and G:F. Quadratic improvements in ADG due to increasing available P concentrations were also reported in studies conducted by Stahly et al. (2001) and Arouca et al. (2012) with 9- to 119-kg and 95- to 120-kg pigs. The authors also reported quadratic improvements in feed efficiency of late finishing pigs.
The majority of the aforementioned studies were considered short-period studies compared to the current experiment, which evaluated the P requirement over the entire growing and finishing periods. Observations from our study have determined the requirements to optimize growth rate and feed efficiency at 116% to 122% of the NRC estimates, corroborating results from the earlier studies. Moreover, low dietary P concentrations can negatively affect ADG and G:F (Reinhart and Mahan, 1986), which is in agreement with the quadratic improvements in growth performance observed in the study herein. Conversely, Nieto et al. (2016) evaluated the effects of varying STTD P levels from 0.186% to 0.336% on performance of 48- to 80-kg pigs. The authors reported that ADG, final BW, and G:F were not affected by increasing digestible P. Similarly, O’Quinn et al. (1997) and Hastad et al. (2004), when evaluating the effects of increasing available P levels on performance of 25 to 118 kg and 88 to 109 kg, respectively, observed no effects of dietary P levels on ADG. Moreover, a quadratic effect of available P on feed efficiency was observed by O’Quinn et al. (1997), while no evidence of dietary P treatment effect on feed efficiency was observed in the study conducted by Hastad et al. (2004).
In the current study, the diets were formulated to maintain a similar Ca:P ratio across treatments. However, analyzed values of total Ca were higher and total P were lower than formulated values. This resulted in an average Ca:P ratio of 1.74:1 in the lowest STTD P treatment and an average of 1.40:1 in the highest STTD P treatment across phases. Previous studies have demonstrated that a wide Ca:P ratio can be detrimental to pig performance, which is more evident when diets are marginal or low in P (Reinhart and Mahan, 1986; Wu et al., 2018). According to González-Vega et al. (2016), growth performance of 25- to 50-kg pigs was reduced with increasing concentrations of STTD Ca, especially when diets contained low concentrations of P. Similarly, Merriman et al. (2017) have shown that diets containing a total Ca:P ratio greater than 1.18:1 resulted in reduced growth performance of 100- to 130-kg pigs fed diets at or below the P estimated requirement by NRC (2012). In the current study, the reduced growth performance observed in the low P treatments could be a result of a wide Ca:P ratio instead of purely due to P inadequacy. Therefore, this could lead to an overestimation of the P requirement.
During the grower period, the requirement of STTD P in grams of digestible P intake to support optimum growth rate was between 6.6 and 7.4 g/d. During the finisher period, the requirement of STTD P in grams of digestible P intake to support optimum growth rate was between 7.1 and 8.3 g/d. When published data are recalculated, with the exception of the study by Nieto et al. (2016), the highest digestible P intake in grams per day observed for 25- to 50-kg and 34- to 56-kg pigs in the studies by O’Quinn et al. (1997) and Hastad et al. (2004) were lower (6.7 and 5.9 g/d, respectively) than the values observed in our study. Similarly, the highest intake of digestible P in grams per day for 80- to 118-kg and 88- to 109-kg pigs in the studies by O’Quinn et al. (1997) and Hastad et al. (2004) were lower (6.9 and 6.1 g/d, respectively) than the values observed in our study. This may help explain the discrepancies in responses for ADG between the current study and those stated above. Furthermore, using ADG as response criteria, the digestible P intakes in grams per day observed herein are greater than NRC (2012) estimates (4.59 to 5.78 and 6.11 to 5.95 g/d during the grower and finisher periods, respectively). As the ratio of lean tissue growth increases, more P is required by the pig to support this growth (Jongbloed, 1987). Therefore, results from our study may reflect the changes over time in swine genetics and the improvements made in performance and lean tissue growth of pigs (Partanen et al., 2010).
In our study, pigs fed diets with increasing levels of STTD P showed an increase in feed intake, whereas Hastad et al. (2004), Arouca et al. (2012), and Nieto et al. (2016) observed no effects of dietary P concentration on ADFI. Phosphorus may be involved in appetite control (Ruan et al., 2007), and a P-deficiency could result in a reduction in feed intake by growing–finishing pigs (Aubel et al., 1936; Sørensen et al., 2018). However, it is worth noting that the feed intake responses in the current study could also have been due to an energy response as the diets were not balanced by energy across treatments. However, the dietary energy differences would not be expected to impact feed intake to the extent reported. Even though there was an energy dilution with increasing the STTD P concentrations, the caloric intake increased with increasing the P levels. This suggests that the energy concentration does not entirely explain the feed intake observed in this study.
Considerable research regarding the effects of increasing dietary P has observed no evidence of P concentration impacting carcass backfat thickness, muscle depth, and lean meat quantity and percentage (O’Quinn et al., 1997; Arouca et al., 2012; Nieto et al., 2016). These results are in agreement with the current study, in which no evidence of STTD P effect on loin depth was observed. However, our study demonstrated a marginal quadratic decrease in backfat and increase in fat-free lean as the digestible P concentration increased up to 130% of NRC estimates. It is hypothesized that when the P concentration is below the pig requirement, which was approximately 122% of NRC for growth rate in this study, the rate of muscle accretion is reduced (Bertram, 1995). According to Bertram (1995), when P is below the requirement, pigs also have a higher proportion of fat deposition in the carcass, which could explain the results observed in our study. Cromwell et al. (1970) also reported that pigs from 18- to 93-kg fed diets with the lowest P concentration had the greatest backfat thickness. A short study from 95 to 120 kg reported no effect of P concentration in the diet on carcass yield (Arouca et al., 2012). Similarly, results from a study conducted by O’Quinn et al. (1997) with 25- to 118-kg pigs have shown that carcass yield was not influenced by dietary P levels. Our study, however, demonstrated a linear decrease in percentage carcass yield with increasing dietary STTD P levels. The reason for the reduction in yield observed in our study is not well understood, but this reduction is consistent with observations from other studies with 26- to 127-kg pigs that evaluated different Ca to P ratios in diets adequate in STTD P (Vier et al., 2018a, 2018b).
Ca and P have specific requirements for bone mineralization (Létourneau-Montminy et al., 2012). Specifically, approximately 96% to 99% of Ca and 70% to 80% of P are found in the pig skeleton in the form of hydroxyapatite (Crenshaw, 2001). The remainder of the whole-body P is found in soft tissues, where both minerals have little or no common function. Thus, it is necessary to consider the ratio between Ca and P in diet formulation. Our study was structured to maintain a constant Ca:P ratio of 1.14:1 to 1.16:1 across dietary treatments. It has previously been reported that a wide Ca:P ratio is detrimental in diets with low P (González-Vega et al., 2016). The authors reported that approximately 1.05:1 to 1.61 Ca:P ratio is required to obtain optimum amount of bone ash in 25- to 50-kg pigs in diets with low to excess P (González-Vega et al., 2016). In our study, the analyzed Ca:P ratios in the final diets were greater than calculated and varied from 1.40:1 to 1.74:1 among treatments. Thus, a wide Ca:P ratio would have the potential to impair bone mineralization in pigs fed diets with low P concentration. However, bone ash of heavier pigs from 100- to 130-kg pigs linearly increased as the dietary concentration of Ca increased regardless of the concentration of P (Merriman et al., 2017). Therefore, the analyzed Ca:P ratio observed in final diets would not be expected to negatively impact bone mineralization in the current study.
Bone mineralization, results from our trial determined the requirement for maximum bone ash as a percentage of dry, fat-free metacarpal at 131% of NRC estimates as opposed to 116% to 122% requirements for growth performance. These results are in agreement with the findings reported by Mahan et al. (1982), Hastad et al. (2004), Partanen et al. (2010), and Saraiva et al. (2012), in which the P requirement to maximize bone mineralization is greater than the level required to optimize growth performance. According to Crenshaw (2001), the deposition of P in bones continues after the deposition of P required for maximum muscle growth. We observed a greater percentage bone ash for barrows compared to gilts, but the magnitude of this difference is relatively small. Results from other research have reported no evidence for differences in bone ash content between sexes (Crenshaw et al., 1981; Teixeira et al., 2016), with only a tendency for boars to have lower percentage bone ash compared to gilts or barrows (Crenshaw et al., 1981).
Our study evaluated the bone mineralization as a percentage of dry, fat-free weight of metacarpals. It is known that the amount of water and fat in the bone can vary according to the age of the animal, type of bone, and nutritional status (Crenshaw et al., 2001). Thus, bone mineral content should be expressed as a percentage of the dry, fat-free weight (Crenshaw, 2001). The selection of bone type for the assessment of whole-body mineral content is more critical in young pigs than in older pigs (Crenshaw et al., 2009). The femur provided a better fit to dietary P inputs in 25- to 30-kg pigs compared to the fibula. However, in 40- to 120-kg pigs, no differences in femur, front feet, and hind feet were observed when evaluating such bones as predictors of whole-body mineral content (Crenshaw et al., 2009).
Standardized total tract digestible P requirement estimates of growing and finishing pigs in the current study were determined using different response criteria, including growth performance, carcass characteristics, and bone mineralization. Together, results from our study indicate that, as a percentage of the diet, NRC (2012) underestimates the STTD P requirement for growing–finishing pigs. Our results suggest that, depending on the response criteria, the STTD P level to optimize growth performance, carcass characteristics, and bone mineralization of 24- to 130-kg pigs ranged from 116% to 131% of NRC (2012) dietary percentage estimates across all phases.
Footnotes
Contribution number 19-266-J from the Kansas Agric. Exp. Stn., Manhattan, KS 66506–0210.
Appreciation is expressed to Genus PIC, North America (Hendersonville, TN) for partial funding. Special appreciation is also expressed to New Horizon Farms (Pipestone, MN) for use of the feed mill and animal facilities, and to Marty Heintz, Heath Houselog, and Whitney Adler for technical assistance.
LITERATURE CITED
- AAFCO 2015. AAFCO official publication. Champaign (IL): Association of American Feed Control Officials. [Google Scholar]
- Adeola O., Azain M. J., Carter S. D., Crenshaw T. D., Estienne M. J., Kerr B. J., Lindemann M. D., Maxwell C. V., Miller P. S., Shannon M. C., . et al. 2015. A cooperative study on the standardized total-tract digestible phosphorus requirement of twenty-kilogram pigs. J. Anim. Sci. 93:5743–5753. doi: 10.2527/jas.2015-9509. [DOI] [PubMed] [Google Scholar]
- Alexander L. S., Qu A., Cutler S. A., Mahajan A., Lonergan S. M., Rothschild M. F., Weber T. E., Kerr B. J., and Stahl C. H.. . 2008. Response to dietary phosphorus deficiency is affected by genetic background in growing pigs. J. Anim. Sci. 86:2585–2595. doi: 10.2527/jas.2007-0692. [DOI] [PubMed] [Google Scholar]
- Almeida F. N., and Stein H. H.. . 2010. Performance and phosphorus balance of pigs fed diets formulated on the basis of values for standardized total tract digestibility of phosphorus. J. Anim. Sci. 88:2968–2977. doi: 10.2527/jas.2009-2285. [DOI] [PubMed] [Google Scholar]
- AOAC International 1990. Official methods of analysis of AOAC International. 15th ed. Gaithersburg (MD): AOAC International. [Google Scholar]
- Arouca C. L. C., de F. C. Silva O., de D. Fontes O., Donzele J. L., de Oliveira R. F. M., Haese D., Kill J. L., and Paula E. de. 2012. Available phosphorus levels for 95 to 120 kg barrows genetically selected for lean gain. Rev. Bras. Zoo. 41:1433–1441. doi: 10.1590/S1516-35982012000600017. [DOI] [Google Scholar]
- Aubel C. E., Hughes J. S., and Lienhardt H. F.. . 1936. Phosphorus requirements in the ration of growing pigs. Kan. Agric. Exp. Sta. Tech. Bul. 41:5–82. [Google Scholar]
- Berndt T., and Kumar R.. . 2009. Novel mechanisms in the regulation of phosphorus homeostasis. Physiology (Bethesda). 24:17–25. doi: 10.1152/physiol.00034.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram M. J. 1995. The impact of dietary phosphorus regimen on muscle growth and quality in pig differing in lean growth capacity. Retrospective Theses and Dissertations. 10999. https://lib.dr.iastate.edu/rtd/10999 (Accessed 17 April 2019).
- Crenshaw T. D. 2001. Calcium, phosphorus, vitamin D, and vitamin K in swine nutrition. In: Lewis A. and Southern L. L., editors, Swine nutrition. 2nd ed. Boca Raton (FL): CRC Press; p. 196–221. [Google Scholar]
- Crenshaw T. D., Danielson J. R. Hoffman L. E., and Schneider D. K.. . 2009. Femurs are more accurate than fibulas as predictors of whole body bone mineral content in growing pigs. J. Anim. Sci, 87(E-Suppl 2):510. [Google Scholar]
- Crenshaw T. D., Peo E. R. Jr, Lewis A. J., Moser B. D., and Olson D.. . 1981. Influence of age, sex and calcium and phosphorus levels on the mechanical properties of various bones in swine. J. Anim. Sci. 52:1319–1329. doi: 10.2527/jas1981.5261319x. [DOI] [PubMed] [Google Scholar]
- Cromwell G. L., Hays V. W., Chaney C. H., and Overfield J. R.. . 1970. Effects of dietary phosphorus and calcium level on performance, bone mineralization and carcass characteristics of swine. J. Anim. Sci. 30(4):519–525. doi: https://doi.org/10.2527/jas1970.304519x. [Google Scholar]
- Ekpe E. D., Zijlstra R. T., and Patience J. F.. . 2002. Digestible phosphorus requirement of grower pigs. Can. J. Anim. Sci. 82:541–549. doi: 10.4141/A02-006. [DOI] [Google Scholar]
- Fan M. Z., Archbold T., Sauer W. C., Lackeyram D., Rideout T., Gao Y., de Lange C. F., and Hacker R. R.. . 2001. Novel methodology allows simultaneous measurement of true phosphorus digestibility and the gastrointestinal endogenous phosphorus outputs in studies with pigs. J. Nutr. 131:2388–2396. doi: 10.1093/jn/131.9.2388. [DOI] [PubMed] [Google Scholar]
- Gonçalves M. A., Bello N. M., Dritz S. S., Tokach M. D., DeRouchey J. M., Woodworth J. C., and Goodband R. D.. . 2016. An update on modeling dose-response relationships: Accounting for correlated data structure and heterogeneous error variance in linear and nonlinear mixed models. J. Anim. Sci. 94:1940–1950. doi: 10.2527/jas.2015-0106. [DOI] [PubMed] [Google Scholar]
- González-Vega J. C., Walk C. L., Murphy M. R., and Stein H. H.. . 2016. Requirement for digestible calcium by 25 to 50 kg pigs at different dietary concentrations of phosphorus as indicated by growth performance, bone ash concentration, and calcium and phosphorus balances. J. Anim. Sci. 94:5272–5285. doi: 10.2527/jas.2016-0751. [DOI] [PubMed] [Google Scholar]
- Hastad C. W., Dritz S. S., Tokach M. D., Goodband R. D., Nelssen J. L., DeRouchey J. M., Boyd R. D., and Johnston M. E.. . 2004. Phosphorus requirements of growing-finishing pigs reared in a commercial environment. J. Anim. Sci. 82:2945–2952. doi: 10.2527/2004.82102945x. [DOI] [PubMed] [Google Scholar]
- Hittmeier L. J., Grapes L., Lensing R. L., Rothschild M. F., and Stahl C. H.. . 2006. Genetic background influences metabolic response to dietary phosphorus restriction. J. Nutr. Biochem. 17:385–395. doi: 10.1016/j.jnutbio.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Jongbloed A. W. 1987. Phosphorus in the feeding of swine: effect of diet on the absorption and retention of phosphorus by growing pigs. Lelystad, The Netherlands: Drukkeri Deboer. [Google Scholar]
- Létourneau-Montminy M. P., Jondreville C., Sauvant D., and Narcy A.. . 2012. Meta-analysis of phosphorus utilization by growing pigs: effect of dietary phosphorus, calcium and exogenous phytase. Animal 6:1590–1600. doi: 10.1017/S1751731112000560. [DOI] [PubMed] [Google Scholar]
- Littell R. C., Milliken G. A., Stroup W. W., Wolfinger R. D., and Schabenberger O.. . 2006. SAS® for mixed models, 2nd ed. Cary (NC): SAS Institute Inc. [Google Scholar]
- Mahan D. C. 1982. Dietary calcium and phosphorus levels for weanling swine. J. Anim. Sci. 54:559–564. doi: 10.2527/jas1982.543559x. [DOI] [PubMed] [Google Scholar]
- Merriman L. A., Walk C. L., Murphy M. R., Parsons C. M., and Stein H. H.. . 2017. Inclusion of excess dietary calcium in diets for 100- to 130-kg growing pigs reduces feed intake and daily gain if dietary phosphorus is at or below the requirement. J. Anim. Sci. 95:5439–5446. doi: 10.2527/jas2017.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milliken G. A., and Johnson. D. E.. 2009. Analysis of messy data: designed experiments. Vol. 1, 2nd ed. Boca Raton (FL): CRC Press; p. 21–557. [Google Scholar]
- Nielsen A. J. 1973. Anatomical and chemical composition of danish landrace pigs slaughtered at 90 kilograms live weight in relation to litter, sex and feed composition. J. Anim. Sci. 36:476–483. doi: 10.2527/jas1973.363476x. [DOI] [Google Scholar]
- Nieto V. M. O. S., Kiefer C., de Souza K. M. R., Gonçalves L. M. P., Bonin M. de N., dos Santos T. M. B., Carvalho K. C. N., and dos Santos A. P.. . 2016. Digestible phosphorus levels for barrows from 50 to 80 kg. Rev. Bras. Zoo. 45:242–249. doi: 10.1590/S1806-92902016000500006. [DOI] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed. Washington (DC): National Academies Press. [Google Scholar]
- O’Quinn P. R., Knabe D. A., and Gregg E. J.. . 1997. Digestible phosphorus needs of terminal-cross growing-finishing pigs. J. Anim. Sci. 75:1308–1318. doi: 10.2527/1997.7551308x. [DOI] [PubMed] [Google Scholar]
- Partanen K., Siljander-Rasi H., Karhapää M., Ylivainio K., and Tupasela. T.. 2010. Responses of growing pigs to different levels of dietary phosphorus—performance, bone characteristics, and solubility of faecal phosphorus. Liv. Sci. 134:109–112. doi: 10.1016/j.livsci.2010.06.113. [DOI] [Google Scholar]
- Reinhart G. A., and Mahan D. C.. . 1986. Effect of various calcium:phosphorus ratios at low and high dietary phosphorus for starter, grower and finishing swine. J. Anim. Sci. 63:457–466. doi: 10.2527/jas1986.632457x. [DOI] [Google Scholar]
- Ruan Z., Zhang Y.-G., Yin Y.-L., Li T.-J., Huang R.-L., Kim S. W., Wu G. Y., and Deng Z. Y.. 2007. Dietary requirement of true digestible phosphorus and total calcium for growing pigs. Asian-Austr. J. Anim. Sci. 20:1236–1242. doi: 10.5713/ajas.2007.1236. [DOI] [Google Scholar]
- Saraiva A., Donzele J. L., Oliveira R. F., Abreu M. L., Silva F. C., Guimarães S. E., and Kim S. W.. . 2012. Phosphorus requirements for 60- to 100-kg pigs selected for high lean deposition under different thermal environments. J. Anim. Sci. 90:1499–1505. doi: 10.2527/jas.2011-3623. [DOI] [PubMed] [Google Scholar]
- Sørensen K. U., Tauson A. H., and Poulsen H. D.. . 2018. Long term differentiated phosphorus supply from below to above requirement affects nutrient balance and retention, body weight gain and bone growth in growing-finishing pigs. Livest. Sci. 211:14–20. doi: 10.1016/j.livsci.2018.03.002. [DOI] [Google Scholar]
- Stahly T. S., Lutz T. R., and Clayton R. D. 2001. Dietary available phosphorus needs of high lean pigs fed from 9 to 119 kg body weight Swine Research Report, 2000. 4. https://lib.dr.iastate.edu/swinereports_2000/4 (Accessed 17 April 2019). [Google Scholar]
- Teixeira A. O., Corassa A., Moreira L. M., Nogueira E. T., Lopes J. B., Rocha Junior C. M., Ferreira V. P. A. 2016. Bone characteristics of pigs fed different sources of phosphorus. Rev. Col. Cienc. Pec. 29:245–254. doi: 10.17533/udea.rccp.v29n4a01. [DOI] [Google Scholar]
- Thomas H. R., and Kornegay E. T.. . 1981. Phosphorus in swine. I. Influence of dietary calcium and phosphorus levels and growth rate on feedlot performance of barrows, gilts and boars. J. Anim. Sci. 52:1041–1048. doi: 10.2527/jas1981.5251041x. [DOI] [PubMed] [Google Scholar]
- Vier C. M., Dritz S. S., Tokach M. D., Gonçalves M. A. D., Orlando M. A. U. A., Bergstrom J. R., Woodworth J. C., Goodband R. D., and DeRouchey. J. M.. 2018a. Effects of dietary total calcium to total phosphorus ratio on growth performance, carcass characteristics, bone mineralization, and economics in 58- to 281-lb pigs. Kan. Agric. Exp. Sta. Res. Rep. 4(9):18. doi: 10.4148/2378-5977.7666. [DOI] [Google Scholar]
- Vier C. M., Dritz S. S., Tokach M. D., Gonçalves M. A. D., Orlando U. A., Cast W., Bergstrom J. R., Woodworth J. C., Goodband R. D., and DeRouchey J. M.. . 2018b. Effects of analyzed calcium to phosphorus ratio on growth performance, carcass characteristics, bone mineralization, and economics of 56- to 279-lb pigs fed diets containing phytase. Kan. Agric. Exp. Sta. Res. Rep. 4(9):19. doi: 10.4148/2378-5977.7667. [DOI] [Google Scholar]
- Wu F., Woodworth J. C., Tokach M. D., DeRouchey J. M., Dritz S. S., and Goodband R. D.. 2018. Standardized total tract digestible phosphorus requirement of 13- to 28-lb pigs fed diets with or without phytase. Kan. Agric. Exp. Sta. Res. Rep. 4(9):17. doi: 10.4148/2378-5977.7665. [DOI] [Google Scholar]