Abstract
This study was conducted to investigate the effects of Clostridium butyricum and Enterococcus faecalis on growth performance, immune function, inflammation-related pathways, and microflora community in weaned piglets challenged with lipopolysaccharide (LPS). One hundred and eighty 28-d-old weaned piglets were randomly divided into 3 treatments groups: piglets fed with a basal diet (Con), piglets fed with a basal diet containing 6 × 109 CFU C. butyricum·kg−1 (CB), and piglets fed with a basal diet containing 2 × 1010 CFU E. faecali·kg−1 (EF). At the end of trial, 1 pig was randomly selected from for each pen (6 pigs per treatment group) and these 18 piglets were orally challenged with LPS 25 μg·kg−1 body weight. The result showed that piglets fed C. butyricum and E. faecalis had greater final BW compared with the control piglets (P < 0.05). The C. butyricum and E. faecalis fed piglets had lower levels of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), IL-1β, tumor inflammatory factor-α (TNF-α), and had greater level of serum interferon-γ (IFN-γ) than control piglets at 1.5 and 3 h after injection with LPS (P < 0.05). Furthermore, piglets in the C. butyricum or E. faecalis treatment groups had a greater ratio of jejunal villus height to crypt depth (V/C) compared with control piglets after challenge with LPS for 3 h (P < 0.05). Compared with the control treatment, the CB and EF treatments significantly decreased the expression of inflammation-related pathway factors (TLR4, MyD88, and NF-κB) after challenge with LPS for 3 h (P < 0.05). High-throughput sequencing revealed that C. butyricum and E. faecalis modulated bacterial diversity in the colon. The species richness and alpha diversity (Shannon) of bacterial samples in CB or EF piglets challenged with LPS were higher than those in LPS-challenged control piglets. Furthermore, the relative abundance of Bacteroidales-Rikenellanceae in the CB group was higher than that in the control group (P < 0.05), whereas EF piglets had a higher relative abundance of Lactobacillus amylovorus and Lactobacillus gasseri (P < 0.05). In conclusion, dietary supplementation with C. butyricum or E. faecalis promoted growth performance, improved immunity, relieved intestinal villus damage and inflammation, and optimized the intestinal flora in LPS-challenged weaned piglets.
Keywords: Clostridium butyricum, Enterococcus faecalis, growth performance, jejunal morphology, microflora community, weaned piglets
© The Author(s) 2019. Published by Oxford University Press on behalf of the American Society of Animal Science. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Introduction
Since the early 1950s, studies have shown that antibiotics can promote the growth performance (Dibner et al., 2005; Huyghebaert et al., 2011) and reduce the rate of diarrhea in animal (Walsh et al., 2007; Andreas et al., 2016). However, the development of resistance to many antibiotics has necessitated the search for alternatives to antibiotics. Recently, the provision of probiotics has achieved notable results in improving growth performance, maintaining intestinal health, and enhancing immunity in animals (Truusalu et al., 2004; Asai et al., 2011; Dheilly et al., 2011). Clostridium butyricum, a gram-positive anaerobic bacterium, is found in healthy animals and the human gut (Finegold et al., 1983; Zhang et al., 2017), and has the characteristics of showing resistance to acidic pH, high temperature, and bile salts (Kong et al., 2011; Zhang et al., 2016). Previous studies have shown that C. butyricum has effects related to the improvement of broiler growth performance and immune function, and optimization of intestinal microflora structure (Cao et al., 2012; Yang et al., 2012; Liao et al., 2015), whereas the intestinal inflammatory response in mice is effectively alleviated after feeding with C. butyricum (Hayashi et al., 2013). Similarly, Sumon et al. (2018) have reported that dietary C. butyricum can improve the growth performance and immune response of Macrobrachium rosenbergii. Studies have also shown that C. butyricum can improve growth performance in weaned piglets (Takahashi et al., 2018) and optimizes the microbial community (Zhang et al., 2018). Studies on Enterococcus faecalis, a lactic acid bacterium that occurs in the intestines of healthy animals (Toit et al., 2000; Gaggìa et al., 2010), have shown that it can improve growth performance and reduce diarrhea in weaned piglets (Hu et al., 2015), whereas Tsukahara et al. (2011) have shown that E. faecalis improves villous atrophy in early-weaned mice and pigs. Although some previous studies have investigated the use of C. butyricum and E. faecalis as probiotics, there have been few studies on the effects of such probiotics on intestinal inflammation and microbial community structure in weaned piglets.
Lipopolysaccharide (LPS) is a class of endotoxin present in the outer membrane of gram-negative bacteria, which is essential for the physical integrity and function of the bacterial outer membrane (Moran et al., 1995). Many animal studies have used LPS to generate acute inflammation models (Lei et al., 2015; Wyns et al., 2015). However, few studies have investigated the effects of C. butyricum and E. faecalis on weaned piglets in response to exposure to LPS.
Therefore, the present study mainly investigated the effects of C. butyricum and E. faecalis on growth performance, immune function, intestinal structure, inflammation-related pathways, and intestinal flora structure in weaned piglets challenged with LPS.
Materials and Methods
Animals, Housing, and Experimental Design
The following procedures were approved by the Ethics Committee of Zhejiang Agricultural and Forestry University. A total of 180 28-d-old weaned piglets (Duroc × Landrace × Yorkshire) were randomly assigned to 3 treatments (6 replicate pens per treatment with 10 pigs in each pen). The treatments were as follows: piglets fed a basal diet (Con), piglets fed a basal diet supplemented with 6 × 109 CFU of C. butyricum·kg−1 (CB), and piglets fed a basal diet supplemented with 2 × 1010 CFU of E. faecalis·kg−1 (EF). At the end of trial, one pig was randomly selected from for each pen (6 pigs per treatment group) and these 18 piglets were challenged with LPS 25 μg·kg−1 BW. The basal diet was formulated to meet the nutrient requirements suggested by the National Research Counci (1998) and contained no antibiotics (Table 1). Water and feed were provided ad libitum. Piglets were housed in a temperature-controlled room maintained at 25.0 to 28.0 °C.
Table 1.
Composition and nutrient levels of the basal diet (air-dry basis) %
| Ingredients | Content | Nurtient level2 | |
|---|---|---|---|
| Corn | 52.00 | DE | 13.52 |
| Wheat midding | 8.90 | CP | 20.96 |
| Soybean meal | 10.00 | Lys | 0.98 |
| Extruded soybean | 8.00 | Met+Cys | 0.58 |
| Imported fish meal | 8.00 | Thr | 0.59 |
| Whey | 7.00 | Ile | 0.67 |
| Choline chloride | 0.10 | Ca | 0.82 |
| Phospholipid | 2.00 | TP | 0.60 |
| Premix1 | 4.00 | AP | 0.42 |
| Total | 100.00 |
1Supplied the following per kg of diet: vitamin A, 10,500 IU; vitamin D3, 450 IU;vitamin E, 10 mg; pantothenic acid, 20 mg; vitamin B6, 2 mg; biotin, 0.3 mg; folic acid, 5 mg; vitamin B12, 0.009 mg; ascorbic acid, 50 mg; Fe, 160 mg; Cu, 140 mg; Mn, 50 mg; Zn, 130 mg; I, 0.5 mg; Se, 0.3 mg.
2Values for net energy were calculated, the contents of ether extract, crude protein, acid-detergent fibre, neutral-detergent fibre, ash, Ca, and P were analyzed. DM, dry matter; T, Total; AA, amino acid.
Bacterial Preparations
The commercial preparations of C. butyricum and E. faecalis were provided by Zhejiang Vegamax Biological Technology Co., Ltd. China. The basal diet was formulated to contain either 6 × 109 CFU C. butyricum·kg−1 or 1 × 1010 CFU E. faecalis·kg−1.
Growth Performance and Occurrence of Diarrhea
On the day of weaning and on day 28 postweaning, all piglets were weighed individually. Feed consumption per pen was measured throughout the experiment. Average daily feed intake (ADFI), ADG, and feed to gain (F: G) were calculated for the piglets in each pen. Instances of the diarrhea in each piglet were recorded daily in order to calculate the rate of diarrhea
Sample Collection
On day 28 postweaning, 1 pig was randomly selected from for each pen (6 pigs per treatment group) and these 18 piglets were injected with 25 μg·kg−1 LPS. Vascular blood (5 mL) was collected from each pig at 1.5 and 3 h after injection. Piglets were anesthetized and killed at 4 h after injection. Blood samples were allowed to clot 4 °C and then centrifuged at 5,000 × g for 10 min. The resulting serum was collected and stored at −20 °C for further analysis. Jejunum segments were preserved in 4% paraformaldehyde and stored at 4 °C, and samples of the empty field mucosa were collected in Eppendorf tubes and stored at −80 °C.
Serum Parameter Analysis
The concentrations of serum factors, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), IgA, IgM and IgG, IL-1β, tumor inflammatory factor-α (TNF-α), and interferon-γ (IFN-γ), were measured using a multifunction microplate reader and kits purchased from the Jiancheng Biological Engineering Research Institute, Nanjing, China.
Jejunal Morphological Analysis
Jejunal samples were washed with normal saline, fixed with 4% paraformaldehyde phosphate buffer for 48 h. The tissues were then cut into 4 μm sections, which were routinely dehydrated, dipped in wax, embedded in paraffin, and sectioned. The processed samples were subsequently dewaxed with xylene, stained with hematoxylin and eosin, dehydrated with ethanol, and covered with coverslips. The villus height and crypt depth were observed under an optical microscope (NIKON Eclipse ci, Japan) and photographed (NIKON digital sight DS-FI2, Japan). Ten fields of view were selected for the measurement of villus height and crypt depth.
Immunohistochemical Analysis
Immunohistochemistry for the detection of Toll-like receptors 4 (TLR4), Myeloid differentiation factor 88 (MyD88), and Nuclear factor-kappa B (NF-κB) was performed using 4-μm thick paraffin sections of jejunal tissues prepared as described previously. Sections were incubated with primary antibodies against TLR4, MyD88, and NF-κB, and then incubated with secondary antibody and diaminobenzidine. The sections were observed under an optical microscope and photographed, and the cumulative optical density (IOD) in the photograph was calculated and averaged. Cells colored brown-yellow when viewed under the microscope were identified as positive cells.
16S rRNA Sequencing of Colonic Microflora
Following the procedures described in our previous study (Yang et al., 2018), the colonic microbiota was analyzed by high-throughput sequencing using the colonic contents of the different treatment groups. The main methods included DNA extraction, PCR amplification of 16S rRNA, amplicon sequencing, and sequence data processing. Total genomic DNA was extracted using a QIAamp DNA stool Mini Kit, followed by the measurement of DNA concentration and purity. Genomic DNA integrity was determined by electrophoresis on a 1% agarose gel. 16S rRNA gene sequencing and related biological analyses was performed by Novogene (Beijing, China) using on the Illumina HiSeq platform. The composition and abundance of the microflora were determined by further alpha and beta diversity analyses.
Statistical Analysis
SPSS Statistics 21.0 (SPSS Inc., USA) and GraphPad Prism 7 (GraphPad Software Inc., USA) were used for statistical analyses. One-way ANOVA was performed and differences among the means of different treatment were compared using least significant difference tests. An alpha value of 0.05 was used to assess the significance among means.
RESULTS
Growth Performance
The results showed that inclusion of C. butyricum and E. faecalis in the diet increased the ADG (P < 0.05) and G:F ratio (P < 0.05) of weaned piglets. Furthermore, we found that the rate of diarrhea among piglets in the probiotic groups was lower than that in control group animals (P < 0.05) (Table 2).
Table 2.
Effects of C. butyricum and E. faecalis on growth performance of the weaned piglets1
| Item | Treatment | SEM2 | P Value | ||
|---|---|---|---|---|---|
| Control | CB | EF | |||
| Initial BW, kg | 8.86 | 9.10 | 9.04 | 0.10 | 0.47 |
| Final BW, kg | 17.61b | 19.60a | 18.44a | 0.24 | 0.02 |
| ADG, g | 312.76b | 375.00a | 347.96a | 6.85 | <0.01 |
| ADFI, g | 641.88b | 654.17a | 626.29c | 2.93 | <0.01 |
| F:G | 2.05a | 1.75c | 1.81b | 0.04 | <0.01 |
| Diarrhea rate, % | 6.67a | 0.50c | 1.30b | 0.00 | <0.01 |
1Control, CB and EF represents the piglets supplemented with basal diet, piglets supplemented with the C. butyricum and piglets supplemented with E. faecalis, respectively. Piglets were regarded as the experimental units.
2Pooled SEM; n = 6 per treatment.
a,bmeans within the same raw with different superscripts differ significantly (P < 0.05).
Liver Function
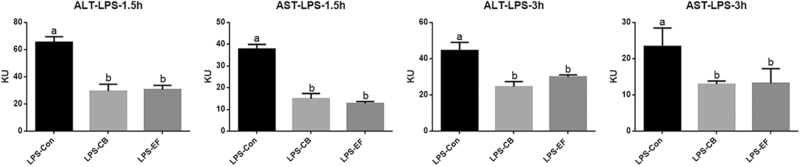
Compared with piglets fed the control diet, those fed diets supplemented with C. butyricum or E. faecalis showed reduced levels of serum AST and ALT at 1.5 and 3 h postchallenge with LPS (P < 0.05) (Fig. 1). These results indicated that C. butyricum and E. faecalis can alleviate the liver damage caused by stress.
Figure 1.
Effects of C. butyricum and E. faecalis on AST and ALT in weaned piglets challenged with LPS involved 1.5 h and 3 h. LPS-Con represents the control piglets challenged with LPS on day 30; LPS-CB represents the piglets supplemented with C. butyricum and challenged with lipopolysaccharide on day 30; LPS-EF represents the piglets supplemented with E. faecalis and challenged with LPS on day 30; Small superscript letter indicates differences (P < 0.05). Values means n = 6 for the analysis of liver function indexes.
Serum Inflammatory Factors
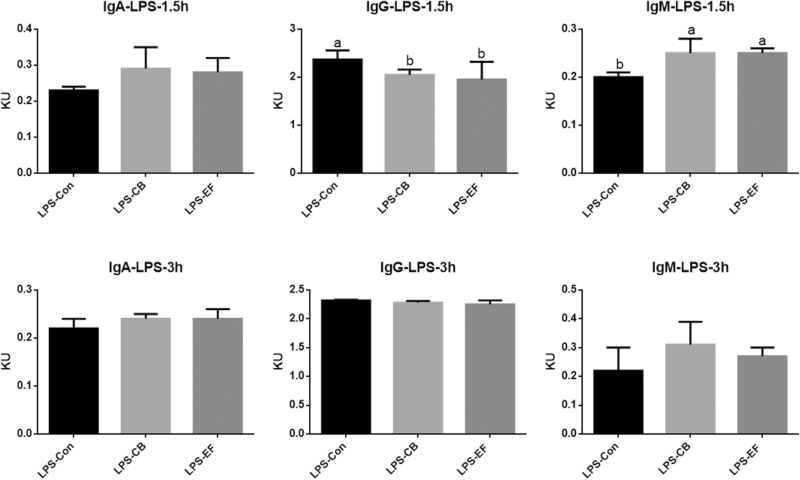
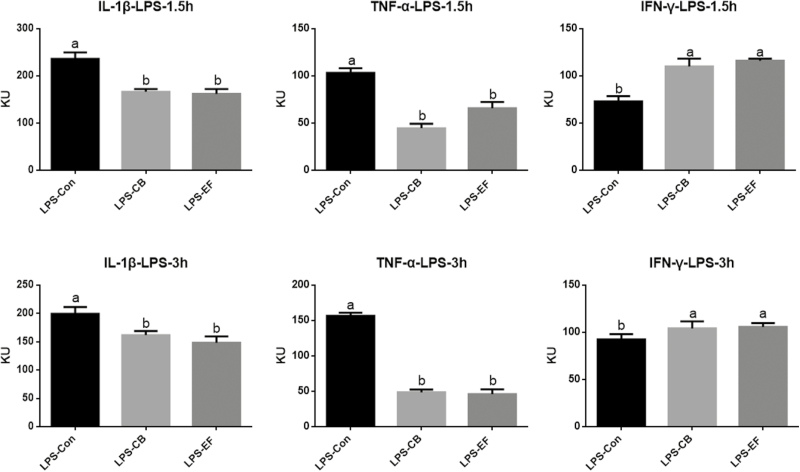
Piglets fed C. butyricum or E. faecalis had higher concentrations of serum IgM than those fed the basal diet at 1.5 h postchallenge with LPS (P < 0.05) (Fig. 2). Compared with the control group piglets, those in the probiotic groups had lower serum concentrations of IL-1β and TNF-α at 1.5 and 3 h postchallenge with LPS (P < 0.05), whereas the concentration of IFN-γ was increased (Fig. 3).
Figure 2.
Effects of C. butyricum and E. faecalis on IgA, IgG, IgM in weaned piglets challenged with LPS involved 1.5 h and 3 h. LPS-Con represents the control piglets challenged with LPS on day 30; LPS-CB represents the piglets supplemented with C. butyricum and challenged with LPS on day 30; LPS-EF represents the piglets supplemented with E. faecalis and challenged with LPS on day 30; Small superscript letter indicates differences (P < 0.05). Values means n = 6 for the analysis of serum inflammatory factor indexes.
Figure 3.
Effects of C. butyricum and E. faecalis on serum IL-1β, TNF-α, IFN-γ in weaned piglets challenged with LPS involved 1.5 h and 3 h. LPS-Con represents the control piglets challenged with LPS on day 30; LPS-CB represents the piglets supplemented with C. butyricum and challenged with LPS on day 30; LPS-EF represents the piglets supplemented with E. faecalis and challenged with LPS on day 30; Small superscript letter indicates differences (P < 0.05). Values means n = 6 for the analysis of serum inflammatory factor indexes.
Jejunal Morphology Analysis
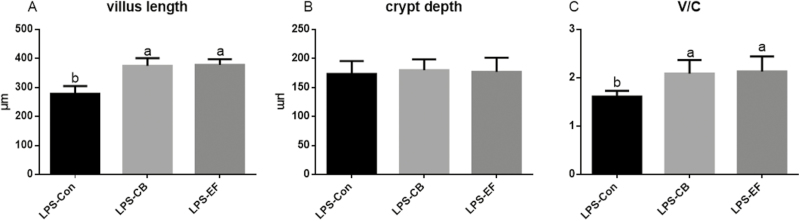
Although piglets fed C. butyricum or E. faecalis had higher jejunum villus lengths than the control piglets when challenged with LPS (P < 0.05), they showed no significant increase in crypt depth. Moreover, compared with control group piglets, those fed C. butyricum or E. faecalis had an increased the villus length to crypt depth ratio (V/C) following LPS challenge (P < 0.05) (Fig. 4).
Figure 4.
Effects of C. butyricum and E. faecalis on the jejunal villus height and the depth of jejunal crypt and the jejunal V/C in weaned piglets challenged with LPS. (A) The villus length in the jejunum of weaned piglets; (B) the crypt depth in the jejunum of weaned piglets; (C) the ratio of the villus length and crypt depth. LPS-Con represents the control piglets challenged with LPS on day 30; LPS-CB represents the piglets supplemented with C. butyricum and challenged with LPS on day 30; LPS-EF represents the piglets supplemented with E. faecalis and challenged with LPS on day 30; small superscript letter indicates differences (P < 0.05). Values means n = 10 for the analysis of the jejunum form.
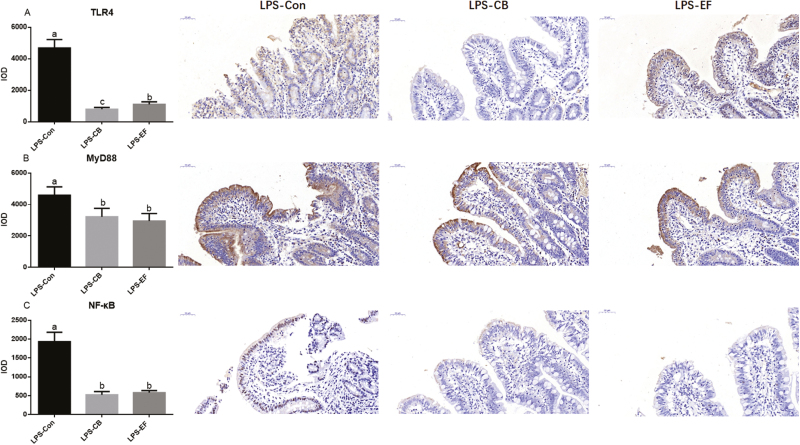
Expression of Jejunal TLR4, MyD88, and NF-κB
Compared with the control piglets, CB and EF supplementation decreased the protein expression of TLR4, MyD88, and NF-κB following challenge with LPS (P < 0.05) (Fig. 5). Clostridium butyricum or E. faecalis therefore appeared to have a significant effect on suppressing inflammation.
Figure 5.
Effects of C. butyricum and E. faecalis on jejunal about TLR4, MyD88, and NF-κB in weaned piglets challenged with LPS. This is the result of immunohistochemistry in jejunal mucosa. (A) Immunohistochemical results of TLR4; (B) immunohistochemical results of MyD88; (C) immunohistochemical results of NF-κB. LPS-Con represents the control piglets challenged with LPS on day 30; LPS-CB represents the piglets supplemented with C. butyricum and challenged with LPS on day 30; LPS-EF represents the piglets supplemented with E. faecalis and challenged with LPS on day 30; Small superscript letter indicates differences (P < 0.05). Values means n = 6 for the analysis of jejunal mucosa.
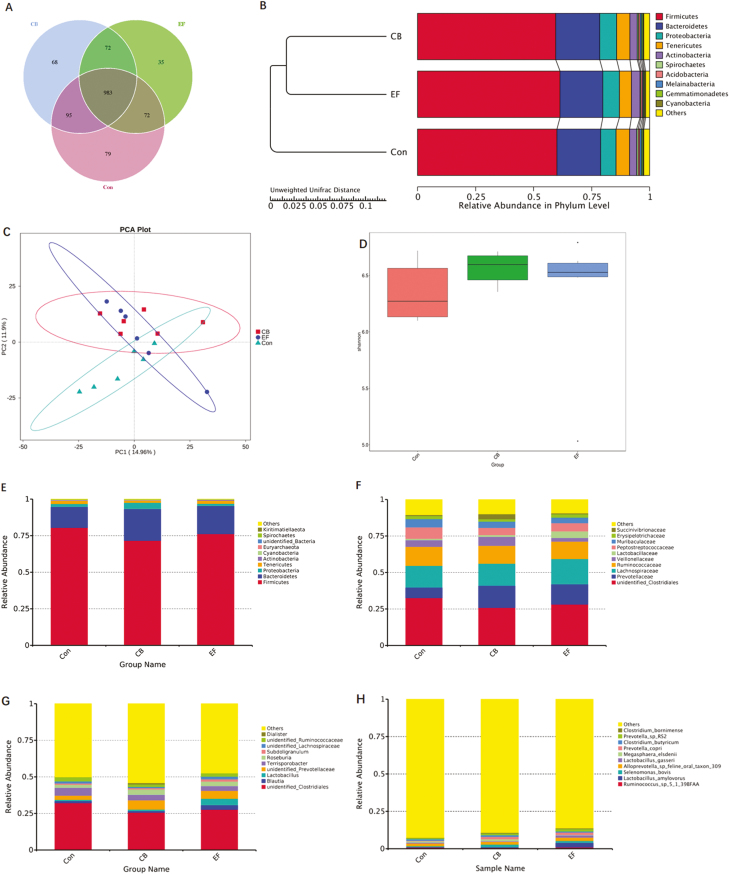
Analysis of Colon Microbial Community Structure
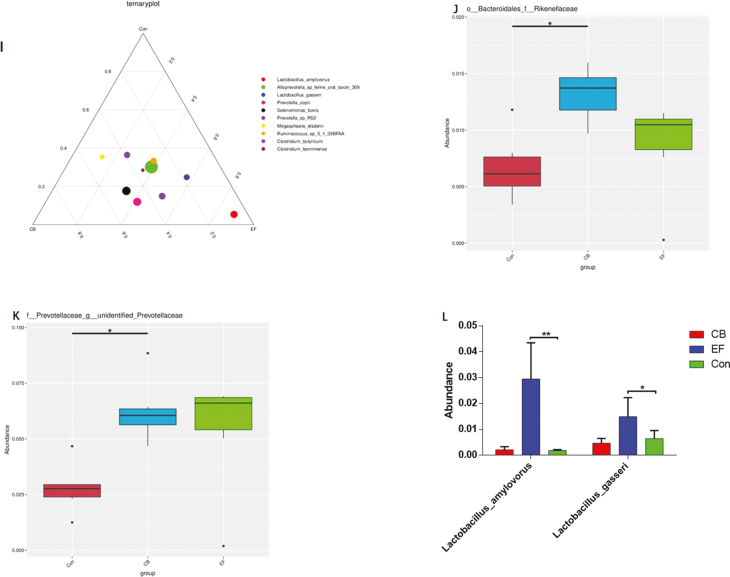
The abundance and diversity of microorganisms in colonic contents were determined based on high-throughput 16S-rRNA sequencing. A Venn diagram showed that a total of 1,404 operational taxonomic units (OTUs) were shared among the 3 treatment groups. The control group piglets had 79 unique OTUs, whereas CB and EF group piglets had 68 and 35 unique OTUs, respectively (Fig. 6A). Beta diversity (UPGMA cluster tree, Principal component analysis) analysis indicated that the microbial communities derived from CB or EF group animals showed varying degrees of difference from the control group piglets (Fig. 6B and C). The alpha diversity (Shannon) of bacteria in C. butyricum and E. faecalis piglets was found to be higher than that in control piglets (Fig. 6D). At the phylum level, the microbial composition of colonic contents in piglets was Firmicutes, Bacteroidetes, Proteobacteria, and Tenericutes (Fig. 6E), whereas at the family level, Clostridiaceae, Prevotellaceae, Lachnospiraceae, and Ruminococcaceae were predominant (Fig. 6F). We found that the relative abundance of Bacteroidales-Rikenellanceae in the CB group was significantly higher than that in the control group (P < 0.05) (Fig. 6J). At the genus level, Clostridium, Lactobacillus, Prevotellaceae, Terrisporobacter, and Roseburia were the dominant genera in all samples, with the abundance of Lactobacillus in EF group being higher than that in the control group (Fig. 6G). Furthermore, the relative abundance of unidentified Prevotellaceae in the CB group was higher than that in control group (P < 0.05) (Fig. 6L). At the species level, we found that Ruminococcus spp, Lactobacillus amylovorus, Selenomonas bovis, Alloprevotella spp, Lactobacillus gasseri, and Megasphaera elsdenii were the dominant species (Fig. 6H), and the abundance of L. amylovorus and L. gasseri in the EF group being were higher than that in the control group (Fig. 6L).
Figure 6.
Summary of microbial community in colon contents of weaned piglets. (A) The Venn diagram summarizing the numbers of common and unique OTUs in the microflora community in colonic contents of weaning piglets. (B) UPGMA Cluster Tree displaying the relative abundances of predominant bacteria at the species level in each group (unweighted UniFrac distance). (C) Principle component analysis plot about the colonic microflora. (D) Shannon index reflecting species diversity within and between groups. (E–H) The top 10 relative abundance of microflora community (level phylum, family, genus, species). (I) Differences of dominant species between groups (level species). (J–L) Species with significant differences between groups (level family, genus, species). Con represents the control piglets challenged with LPS; CB represents the piglets supplemented with the C. butyricum challenged with LPS; EF represents the piglets supplemented with E. faecalis challenged with LPS. OTU, operational taxonomic unit. Piglets were regarded as the experimental units, each treatment with n = 6. *Means different (P < 0.05), ** means significant difference (P < 0.01)
Discussion
In the past few decades, probiotics have been demonstrated to have positive effects in animals. However, previous studies have tended to report inconsistent observations regarding the effect of probiotics on growth performance. For example, whereas Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus have been shown to be beneficial in enhancing the growth performance in broilers (Mountzouris et al., 2007). Biernasiak et al. (2009) reported that probiotics have no significant effect on broiler growth performance. Similarly, although Yang et al. (2012) and Liao et al. (2015) have shown that C. butyricum promotes growth performance in broilers. Zhang et al. (2011) showed that supplementation with 1 × 109 CFU C. butyricum·kg−1 had no effect on the growth performance of these birds. In piglets, Lu et al. (2018) found that Enterococcus faecium, Bacillus subtilis, Saccharomyces cerevisiae, and Lactobacillus paracasei improved piglet growth performance and relieved diarrhea caused by weaning stress, whereas Liu et al. (2018) showed that Lactobacillus plantarum increased daily weight gain and feed conversion ratio. Zong et al. (2019) showed that feeding piglets with C. butyricum effectively improved growth performance and reduced the rate of diarrhea. Similarly, Chen et al. (2018a) showed that dietary supplementation with C. butyrium had positive effects on the growth of weaned piglets, although it had no significant effect on elevation of the G:F ratio. Furthermore, Hu et al. (2015) indicated that E. faecalis can promote the growth performance and reduce diarrhea in weaned piglets. In the present study, we found that the dietary supplementation with C. butyricum or E. faecalis led to a higher final BW and ADG. In addition, G:F ratio of animals in the groups supplemented with probiotics was reduced compared with that in control piglets. Moreover, the ratio of diarrhea piglets fed C. butyricum or E. faecalis was markedly reduced compared with of control group piglets.
Amino acid metabolic enzymes, such as AST and ALT, are highly active in the liver and are important indicators of liver function (Hijmans et al., 2014). Previous studies have indicated that probiotics have no significant effects on serum AST and ALT concentrations in broilers under either normal conditions or heat stress (Sohail et al., 2011). However, probiotics have been shown reduce serum AST and ALT concentrations in patients with alcoholic liver disease (Kirpich et al., 2008). Cao et al. (2019) showed that the Clostridium butyricum-based probiotics reduced the concentration of AST and ALT in serum, improved liver function. In the present study, we found that LPS-challenged piglets fed C. butyricum or E. faecalis had lower AST and ALT levels than LPS-challenged control piglets, which indicates that probiotic C. butyricum or E. faecalis can alleviate liver damage induced by LPS.
Lipopolysaccharide treatment causes an inflammatory reaction in hosts, via changes in various inflammatory factors in blood. Dietary supplementation with probiotics has been shown to enhance serum IgA, IgM, and IL-6 concentrations in broilers (Zhang et al., 2015), and compared with control broilers, birds fed C. butyricum have been found to have greater serum IgM at day 21(Han et al., 2018). In the present study, we showed that dietary supplementation with C. butyricum or E. faecalis increased the serum concentrations IgA and IgM and improved immune function. Previously, it has been shown that dietary supplementation with C. butyricum can prevent experimental acute colitis in mice through induction of IL-10 (Kanai et al., 2015), and can significantly reduce concentrations of TNF-α in broiler (Chen et al., 2018b). Furthermore, E. faecalis has been shown to induce the secretion of IL-6 and IFN-γ in the K mouse intestinal epithelial cell line (Hoffmann et al., 2011). Studies have also found that piglets infected with only enterotoxigenic Escherichia coli K88 (ETEC K88) have elevated mRNA levels of the proinflammatory cytokine IL-1β compared with untreated pigs at 6 and 24 h post-ETEC K88 infection (Li et al., 2018). In the present study, we found that concentrations of the proinflammatory factors IL-1β and TNF-α in the serum of CB or EF group piglets challenged with LPS were lower than those in control group piglets, whereas serum concentrations of the serum anti-inflammatory factor IFN-γ were significantly increased compared with the control group following LPS challenge. Collectively, these findings indicate that dietary supplementation with C. butyricum or E. faecalis effectively alleviates the LPS-induced inflammatory response.
The length of villi and depth of crypts in the intestine are considered primary indicators for detecting any perturbation of intestinal function. Previous studies in broilers have shown that probiotic supplementation can increased villus height and the ratio of villus height to crypt depth, whereas the crypt depth was reduced (Awad et al., 2009; Kim et al., 2012). Research has also shown that daily oral administration of E. faecalis can alleviate the intestinal damage in piglets or young rats due to weaning, and significantly increase the height of the jejunal villi (Tsukahara et al., 2011). Furthermore, broilers fed C. butyricum have been found to have a significantly increased ratio of villus height to crypt depth (Cao et al., 2012), and Zhang et al. (2016) have shown that the supplementation of basal diet with C. butyricum increased jejunal villus height and decreased crypt depth in broiler chickens challenged with E. coli K88. In the present study, we demonstrated that inclusion of C. butyricum or E. faecalis in the diet increased the ratio of jejunum villus height to crypt depth, thereby indicating that C. butyricum or E. faecalis can protect the intestinal development and facilitate the maintenance of normal intestinal function following LPS stimulation.
Toll-like receptors 4 (TLR4), one of the Toll-like receptors (TLRs), regulates the inflammatory response by recognizing LPS and mediating its signal transduction, thereby playing an important role in the immune system in response to against the invasion of gram-negative bacteria (Gordon, 2002; Akira et al., 2004). TLR4, the first member of TLR family to be discovered, belongs to the type I transmembrane receptors of the TLR interleukin super-family, and functions in proinflammatory and immune responses (Lin et al., 2017). Nonspecific recognition, combined with pathogen-associated molecular patterns, activates NF-κB-related protein pathways, and promotes inflammatory reactions (Rashidian et al., 2016). TLR-mediated myocardial inflammation causes severe damages to the myocardium, and the TLR4/MyD88/NF-κB signaling pathway plays an important role in coronary microembolism (CME)-induced myocardial injury (Heusch et al., 2016). It has been demonstrated that Lactobacillus can inhibit the NF-κB activation and TLR4 expression induced by 2,4,6-trinitrobenzenesulfonic acid (TNBS) in mice (Lee et al., 2009), and Lim et al. (2017) have shown that Bifidobacterium can ameliorate high-fat diet induced colitis in mice by inhibiting the activation of NF-κB and production of LPS. Furthermore, it has been reported that C. butyricum inhibits the phosphorylation of NF-κB, nuclear factor kappa-B RelA (NF-κB p65), and extracellular regulated protein kinases in the gastric tissues (Liu et al., 2016). Zhao et al. (2017) revealed that the inflammation of chickens were alleviated by C. butyricum via down-regulating TLR4, MyD88, and NF-κB-dependent pathways. Ushida et al. (2010) showed that E. faecalis promotes the production of inflammatory factor IL-12 by mediating MyD88 in mice. To date, however, there have been relatively few studies on the effects of C. butyricum and E. faecalis onTLR4 inflammation-related pathways in weaned piglets. In the present study, we found that among the piglets challenged with LPS, the protein expression levels of TLR4, MyD88, NF-κB in the jejunum of piglets fed C. butyricum or E. faecalis were significantly decreased compared with those in control piglets fed the basal diet. Both C. butyricum and E. faecalis were found to alleviate the inflammatory responses induced by LPS through modulating the TLR4 inflammatory signaling pathway.
The structure of the intestinal microbial community is extremely complex and plays a key role in feed utilization, digestive tract integrity, and animal health. In piglets, the colon is the main site of microbial colonization (Mazmanian et al., 2008; Kong et al., 2011; Luo et al., 2013). A study by Duan et al. (2018) confirmed that C. butyricum optimized the intestinal microbial structure of Penaeus vannamei, as determined by alpha diversity analysis (Shannon, abundance-based coverage estimator), which is consistent with the results of the present study. Also consistent with the findings of the present study, Hagihara et al. (2018) found that C. butyricum increased the abundance of the bacterial genera Bifidobacterium, Coprococcus, and Bacteroides. We detected a higher abundance of Bacteroidetes and Proteobacteria in the CB group piglets compared with those in the control group, as indicated by a species relative abundance map and UPGMA cluster tree. Miao et al. (2018) found that C. butyricum administered to breast-feeding maternal mice can regulate the balance of intestinal flora in their offspring. In our previous study, we demonstrated that C. butyricum reduces the number of E. coli in the intestines of broiler chickens and increases the number of Bifidobacterium, thereby regulating the intestinal flora (Cao et al., 2012). Zhang et al. (2014) showed that birds fed C. butyricum had decreased and increased populations of E. coli and Lactobacillus, respectively, compared with control birds challenged with E. coli K88, whereas Chen et al. (2018a) found that C. butyricum increased the levels of Bacillus and Ruminococcaceae in the intestines of LPS-challenged piglets, although there were no differences between CB group and control piglets with regards to the levels of Bacillus and Ruminococcaceae. In the present study, we found that among the piglets challenged with LPS, those receiving diets supplemented with C. butyricum showed enhanced growth performance compared with control group.
In addition, Samli et al. (2007) found that broilers fed with probiotic E. faecium were characterized by an increase in lactic acid bacteria colonization in the ileal content of chicks. Bacillus is one of the members of microbial that can be directly fed to animals (Gerritsen et al., 2011), and in this study, an increase in L. amylovorus and L. gasseri in LPS-challenged piglets fed an E. faecalis-supplemented diet indicates the beneficial effect of this probiotic bacterium. Oh et al. (2018) reported that L. gasseri has a strong ability to colonize the intestines, which has beneficial antioxidative, anti-inflammatory effects and also inhibits α-glucosidase activity and nitric oxide production in livestock. Moreover, L. gasseri has also been shown to inhibit the antiviral activity of respiratory syncytial virus, to upregulate the genes stimulated by interferon, and to increase the level of interferon (Eguchi et al., 2019), and effectively inhibits Staphylococcus aureus (Cifuentes et al., 2019). Moreover, Slavica et al. (2015) have reported that L. amylovorus converts glucose into lactate and acetate for absorption and utilization by host animals. Lactobacillus amylovorus has also been shown to protect the intestinal cells from ETEC K88 infection through inhibiting the ETEC-induced increase in the levels of TLR4 and MyD88, the phosphorylation of inhibitor of nuclear factor kappa-B kinase (IKK α), inhibitor of nuclear factor kappa-B kinase (IKK β), inhibitor of NF-κB (IκB α), and NF-κB p65, and over-production of the inflammatory cytokines IL-8 and IL-1β (Finamore et al., 2014). In the present study, we found that dietary supplementation with C. butyricum or E. faecalis increased the diversity of the intestinal microflora in LPS-challenged piglets, optimized intestinal flora structure, and effectively improved piglet health.
Conclusion
In this study, we demonstrate that dietary supplementation with either C. butyricum or E. faecalis enhances the growth performance of weaned piglets. We also observed a tendency of C. butyricum and E. faecalis to enhance immune function by alleviating liver damage. Moreover, C. butyricum and E. faecalis improved intestinal morphology of LPS-challenged piglets, decreased the expression of inflammation-related pathway proteins, and increased the abundance and diversity of the colonic microbial population in LPS-challenged piglets.
Acknowledgments
The present research was supported by the Key Research Project of Zhejiang Province (No. 2017C02005) and the National Natural Science Foundation of China (No. 31501985). We also acknowledge Vegamax Biotechnology Co. Ltd. (Anji, Zhejiang, China) for providing the product of Clostridium butyricum and Enterococcus faecalis.
Conflict of interest statement.
None declared.
Literature Cited
- Akira S., and Takeda K.. . 2004. Toll-like receptor signaling. Nat. Rev. Immunol. 4:499–511. doi: 10.1074/jbc.R300028200 [DOI] [PubMed] [Google Scholar]
- Andreas P., Farfán-López C., Rondón Y., Mora F., Rossini M., and Araque H.. . 2016. Effect of using mannoproteins and antibiotics as growth promoters in diets for weaned piglets on performance. Rev. Cient-Fac. Cien. V. 26:26–32. http://www.redalyc.org/articulo.oa?id=95944832006 [Google Scholar]
- Asai T., Masani K., Sato C., Hiki M., Usui M., Baba K., Ozawa M., Harada K., Aoki H., and Sawada T.. 2011. Phylogenetic groups and cephalosporin resistance genes of Escherichia coli from diseased food-producing animals in japan. Acta Vet. Scand. 53:52. doi: 10.1186/1751-0147-53-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad W. A., Ghareeb K., Abdel-Raheem S., and Böhm J.. . 2009. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 88:49–56. doi: 10.3382/ps.2008-00244. [DOI] [PubMed] [Google Scholar]
- Biernasiak J., and Slizewska K.. . 2009. The effect of a new probiotic preparation on the performance and faecal microflora of broiler chickens. Veter. Med-Czech. 54:525–531. [Google Scholar]
- Cao G. T., Tao F., Hu Y. H., Li Z. M., Zhang Y., Deng B., and Zhan X. A.. . 2019. Positive effects of a Clostridium butyricum-based compound probiotic on growth performance, immune responses, intestinal morphology, hypothalamic neurotransmitters, and colonic microbiota in weaned piglets. Food. Funct. 10: 2926–2934. doi: 10.1039/c8fo02370k. [DOI] [PubMed] [Google Scholar]
- Cao G. T., Xiao Y. P., Yang C. M., Chen A. G., Liu T. T., Zhou L., Zhang L., and Ferket P. R.. . 2012. Effects of Clostridium butyricum on growth performance, nitrogen metabolism intestinal morphology and cecal microflora in broiler chickens. J. Anim. Vet. Adv. 11: 2665–2671. doi: 10.3923/javaa.2012.2665.2671 [DOI] [Google Scholar]
- Chen L., Li S., Zheng J., Li W., Jiang X. M., Zhao X. L., Li J., Che L. Q., Lin Y., Xu S. Y., . et al. 2018a. Effects of dietary Clostridium butyricum supplementation on growth performance, intestinal development, and immune response of weaned piglets challenged with lipopolysaccharide. J. Anim. Sci. Biotechno. 9:62. doi: 10.1186/s40104-018-0275-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. P., Zhang H., Cheng Y. F., Li Y., Wen C., and Zhou Y. M.. . 2018b. Dietary L-threonine supplementation attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier damage of broiler chickens at an early age. Brit. J. Nutr. 119: 1254–1262. doi: 10.1017/s0007114518000740 [DOI] [PubMed] [Google Scholar]
- Cifuentes G. C., Rodriguez-Palmero M., Jimenez J., and Moreno J. A.. . 2019. Antimicrobial activity against human pathogenic strain of Staphylococcus aureus CECT4013 by Lactobacillus gasseri strains. Ann. Nutr. Metab. 74 (Supplement.1):27. doi: 10.1159/000496759 [DOI] [Google Scholar]
- Dheilly A., Bouder A., Le Devendec L., Hellard G., and Kempf I.. . 2011. Clinical and microbial efficacy of antimicrobial treatments of exprimental avian colibacillosis. Vet. Microbiol. 149:422–429. doi: 10.1016/j.vetmic.2010.11.033 [DOI] [PubMed] [Google Scholar]
- Dibner J. J., and Richards J. D.. . 2005. Antibiotic growth promoters in agriculture: History and mode of action. Poult. Sci. 84:634–643. doi: 10.1093/ps/84.4.634 [DOI] [PubMed] [Google Scholar]
- Duan Y. F., Wang Y., Dong H. B., Ding X., Liu Q. S., Li H., Zhang J. S., and Xiong D. L.. . 2018. Changes in the intestine microbial, digestive, and immune-related genes of Litpoenaeus vannamei in response to dietary probiotic Clostridium butyricum supplementation. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi K., Fujitani N., Nakagawa H., and Miyazaki T.. . 2019. Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055. Sci. Rep. 9:4812. doi: 10.1038/s41598-019-39602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finamore A., Roselli M., Imbinto A., Seeboth J., Oswald I. P., and Mengheri E.. . 2014. Lactobacillus amylovorus inhibits the TLR4 inflammatory signaling triggered by enterotoxigenic Escherichia coli via modulation of the negative regulators and involvement of TLR2 in intestinal caco-2 cells and pig explants. Plos One 9:e94891. doi: 10.1371/journal.pone.0094891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold S. M., Sutter V. L., and Mathisen G. E.. . 1983. Normal indigenous intestinal flora. Human Intestinal Microflora in Health and Disease. New York: Academic Press; 3–31. doi: 10.1016/b978-0-12-341280-5.50007-0. [DOI] [Google Scholar]
- Gaggìa F., Mattarelli B., and Biavati B.. 2010. Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. 141:S15–S28. doi: 10.1016/j.ijfoodmicro.2010.02.031 [DOI] [PubMed] [Google Scholar]
- Gerritsen J., Smidt H., Rijkers G. T., and de Vos W. M.. 2011. Intestinal microbiota in human health and disease: The impact of probiotics. Genes Nutr. 6:209–240. doi: 10.1007/s12263-011-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. 2002. Pattern recognition receptors: Doubling up for the innate immune response. Cell 111:927–930. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- Hagihara M., Yamashita R., Matsumoto A., Mori T., Kuroki Y., Kudo H., Oka K., Takahashi M., Nonogaki T., Yamagishi Y., et al. 2018. The impact of clostridium butyricum MIYAIRI 588 on the murine gut microbiome and colonic tissue. Anaerobe 54:8–18. doi: 10.1016/j.anaerobe.2018.07.012. [DOI] [PubMed] [Google Scholar]
- Han J. F., Wang Y. W., Song D., Lu Z. X., Dong Z. L., Miao H. J., Wang W. W., He J. H., and Li A. K.. . 2018. Effects of Clostridium butyricum and Lactobacillus plantarum on growth performance, immune function and volatile fatty acid level of caecal digesta in broilers. Food. Agr. Immunol 29:797–807. doi: 10.1080/09540105.2018.1457013 [DOI] [Google Scholar]
- Hayashi A., Sato T., Kamada N., Mikami Y., Matsuoka K., Hisamatsu T., Hibi T., Roers A., Yagita H., Ohteki T., . et al. 2013. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe 13:711–722. doi: 10.1016/j.chom.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Heusch G., and Gersh B. J.. . 2016. The pathophysiology of acute myocardial infarction and of strategies of protection beyond reperfusion: A continual challenge. Eur. Heart J. 38:774–784. doi: 10.1093/eurheartj/ehw224 [DOI] [PubMed] [Google Scholar]
- Hijmans B. S., Grefhorst A., Oosterveer M. H., and Groen A. K.. 2014. Zonation of glucose and fatty acid metabolism in the liver: Mechanism and metabolic consequences. Biochimie 96:121–129. doi: 10.1016/j.biochi.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Messlik A., Kim S. C., Sartor R. B., and Haller D.. 2011. Impact of a probiotic Enterococcus faecalis in a gnotobiotic mouse model of experimental colitis. Mol. Nutr. Food Res. 55:703–713. doi: 10.1002/mnfr.201000361. [DOI] [PubMed] [Google Scholar]
- Hu Y., Dun Y., Li S., Zhang D., Peng N., Zhao S., and Liang Y.. 2015. Dietary Enterococcus faecalis LAB31 improves growth performance, reduces diarrhea, and increases fecal Lactobacillus number of weaned piglets. Plos One 10:e0116635. doi: 10.1371/journal.pone.0116635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyghebaert G., Ducatelle R., and Van Immerseel F.. . 2011. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 187:182–188. doi: 10.1016/j.tvjl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Kanai T., Mikami Y., and Hayashi A.. . 2015. A breakthrough in probiotics: Clostridium butyricum regulates gut homeostasis and anti-inflammatory response in inflammatory bowel disease. J. Gastroenterol. 50:928–939. doi: 10.1007/s00535-015-1084-x. [DOI] [PubMed] [Google Scholar]
- Kim J. S., Ingale S. L., Kim Y. W., Kim K. H., Sen S., Ryu M. H., Lohakare J. D., Kwon I. K., and Chae B. J.. 2012. Effect of supplementation of multi-microbe probiotic product on growth performance, apparent digestibility, cecal microbiota and small intestinal morphology of broilers. J. Anim. Physiol. Anim. Nutr. (Berl). 96:618–626. doi: 10.1111/j.1439-0396.2011.01187.x. [DOI] [PubMed] [Google Scholar]
- Kirpich I. A., Solovieva N. V., Leikhter S. N., Shidakova N. A., Lebedeva O. V., Sidorov P. I., Bazhukova T. A., Soloviev A. G., Barve S. S., McClain C. J., . et al. 2008. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: A pilot study. Alcohol 42:675–682. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q., He G. Q., Jia J. L., Zhu Q. L., and Ruan H.. 2011. Oral administration of Clostridium butyricum for modulating gastrointestinal microflora in mice. Curr. Microbiol. 62:512–517. doi: 10.1007/s00284-010-9737-8. [DOI] [PubMed] [Google Scholar]
- Lee J. H., Lee B., Lee H. S., Bae E. A., Lee H. Y., Ahn Y. T., Lim K. S., Huh C. S., and Kim D. H.. 2009. Lactobacillus suntoryeus inhibits pro-inflammatory cytokine expression and TLR-4-linked NF- κB activation in experimental colitis. Int. J. Colorectal Dis. 24:231–237. doi: 10.1007/s00384-008-0618-6 [DOI] [PubMed] [Google Scholar]
- Lei Y. C., Yang L. L., Li W., and Luo P.. 2015. Sphingosine kinase 1 dependent protein kinase C-δ activation plays an important role in acute liver failure in mice. World J. Gastroenterol. 21:13438–13446. doi: 10.3748/wjg.v21.i48.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. H., Li Y. P., Zhou Q., Qiao J. Y., and Wang W. J.. 2018. Dietary supplementation with Clostridium butyricum helps to improve the intestinal barrier function of weaned piglets challenged with enterotoxigenic Escherichia coli K88. J. Appl. Microbiol. 125:964–975. doi: 10.1111/jam.13936 [DOI] [PubMed] [Google Scholar]
- Liao X. D., Ma G., Cai J., Fu Y., Yan X. Y., Wei X. B., and Zhang R. J.. 2015. Effects of Clostridium butyricum on growth performance, antioxidation, and immune function of broilers. Poult. Sci. 94:662–667. doi: 10.3382/ps/pev038. [DOI] [PubMed] [Google Scholar]
- Lim S. M., and Kim D. H.. . 2017. Bifidobacterium adolescentis IM38 ameliorates high-fat diet-induced colitis in mice by inhibiting NF-κb activation and lipopolysaccharide production by gut microbiota. Nutr. Res. 41:86–96. doi: 10.1016/j.nutres.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Lin S., Li Y., Shen L., Zhang R., Yang L., Li M., Li K., and Fichna J.. 2017. The anti-inflammatory effect and intestinal barrier protection of HU210 differentially depend on TLR4 signaling in dextran sulfate sodium-induced murine colitis. Dig. Dis. Sci. 62:372–386. doi: 10.1007/s10620-016-4404-y. [DOI] [PubMed] [Google Scholar]
- Liu W. C., Kang J. S., and Kim I. H.. 2018. Dietary Lactobacillus plantarum GB805 supplementation improves growth performance and nutrient digestibility in weaning pigs. Indian. J. Anim. Res. 52:1313–1316. doi: 10.18805/ijar.B-852 [DOI] [Google Scholar]
- Liu J. M., Wang F. Y., Luo H. H., Liu A. H., Li K. X., Li C., and Jiang Y.. 2016. Protective effect of butyrate against ethanol-induced gastric ulcers in mice by promoting the anti-inflammatory, anti-oxidant and mucosal defense mechanisms. Int. Immunopharmacol. 30:179–187. doi: 10.1016/j.intimp.2015.11.018 [DOI] [PubMed] [Google Scholar]
- Lu X., Zhang M., Zhao L., Ge K., Wang Z., Jun L., and Ren F.. . 2018. Growth performance and post-weaning diarrhea in piglets fed a diet supplemented with probiotic complexes. J. Microbiol. Biotechnol. 28:1791–1799. doi: 10.4014/jmb.1807.07026. [DOI] [PubMed] [Google Scholar]
- Luo J., Zheng A., Meng K., Chang W., Bai Y., Li K., Cai H., Liu G., and Yao B.. . 2013. Proteome changes in the intestinal mucosa of broiler (Gallus gallus) activated by probiotic Enterococcus faecium. J. Proteomics 91:226–241. doi: 10.1016/j.jprot.2013.07.017. [DOI] [PubMed] [Google Scholar]
- Mazmanian S. K., Round J. L., and Kasper D. L.. 2008. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- Miao R. X., Zhu X. X., Wan C. M., Wang Z. L., Wen Y., and Li Y. Y.. 2018. Effect of Clostridium butyricum supplementation on the development of intestinal flora and the immune system of neonatal mice. Exp. Ther. Med. 15:1081–1086. doi: 10.3892/etm.2017.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A. P. 1995. Structure-bioactivity relationships of bacterial endotoxins. J. Toxicol-Toxin Rev. 14: 47–83. doi: 10.3109/15569549509089968 [DOI] [Google Scholar]
- Mountzouris K. C., Tsirtsikos P., Kalamara E., Nitsch S., Schatzmayr G., and Fegeros K.. . 2007. Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult. Sci. 86:309–317. doi: 10.1093/ps/86.2.309. [DOI] [PubMed] [Google Scholar]
- Oh N. S., Joung J. Y., Lee J. Y., and Kim Y.. 2018. Probiotic and anti-inflammatory potential of Lactobacillus rhamnosus 4B15 and Lactobacillus gasseri 4M13 isolated from infant feces. Plos One 13:e0192021. doi: 10.1371/journal.pone.0192021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashidian A., Muhammadnejad A., Dehpour A. R., Mehr S. E., Akhavan M. M., Shirkoohi R., Chamanara M., Mousavi S. E., and Rezayat S. M.. 2016. Atorvastatin attenuates TNBS-induced rat colitis: The involvement of the TLR4/NF-kb signaling pathway. Inflammopharmacology 24:109–118. doi: 10.1007/s10787-016-0263-6. [DOI] [PubMed] [Google Scholar]
- Samli H. E., Senkoylu N., Koc F., Kanter M., and Agma A.. . 2007. Effects of Enterococcus faecium and dried whey on broiler performance, gut histomorphology and intestinal microbiota. Arch. Anim. Nutr. 61:42–49. doi: 10.1080/17450390601106655. [DOI] [PubMed] [Google Scholar]
- Slavica A., Trontel A., Jelovac N., Kosovec Ž., Šantek B., and Novak S.. 2015. Production of lactate and acetate by lactobacillus coryniformis subsp. Torquens DSM 20004(T) in comparison with Lactobacillus amylovorus DSM 20531(T). J. Biotechnol. 202:50–59. doi: 10.1016/j.jbiotec.2015.01.014. [DOI] [PubMed] [Google Scholar]
- Sohail M. U., Rahman Z. U., Ijaz A., Yousaf M. S., Ashraf K., Yaqub T., Zaneb H., Anwar H., and Rehman H.. 2011. Single or combined effects of mannan-oligosaccharides and probiotic supplements on the total oxidants, total antioxidants, enzymatic antioxidants, liver enzymes, and serum trace minerals in cyclic heat-stressed broilers. Poult. Sci. 90:2573–2577. doi: 10.3382/ps.2011-01502. [DOI] [PubMed] [Google Scholar]
- Sumon M. S., Ahmmed F., Khushi S. S., Ahmmed M. K., Rouf M. A., Chisty M. A. H., . et al. 2018. Growth performance, digestive enzyme activity and immune response of Macrobrachium rosenbergii fed with probiotic Clostridium butyricum incorporated diets. JKSUS. 30: 21–28. doi: 10.1016/j.jksus.2016.11.003 [DOI] [Google Scholar]
- Takahashi M., McCartney E., Knox A., Francesch M., Oka K., Wada K., Ideno M., Uno K., Kozłowski K., Jankowski J., et al. 2018. Effects of the butyric acid-producing strain Clostridium butyricum MIYAIRI 588 on broiler and piglet zootechnical performance and prevention of necrotic enteritis. Anim. Sci. J. 89:895–905. doi: 10.1111/asj.13006. [DOI] [PubMed] [Google Scholar]
- Toit M. D., Franz C. M. A. P., Dicks L. M. T., and Holzapfel W. H.. 2000. Preliminary characterization of bacteriocins produced by Enterococcus faecium and Enterococcus faecalis isolated from pig faeces. J. Appl. Microbiol. 88:482–494. doi: 10.1046/j.1365-2672.2000.00986.x [DOI] [PubMed] [Google Scholar]
- Truusalu K., Naaber P., Kullisaar T., Tamm H., and Mikelsaar M.. 2004. The influence of antibacterial and antioxidative probiotic Lactobacilli on gut mucosa in a mouse model of Salmonella infection. Microb. Ecol. Health Dis. 16:180–187. doi: 10.1080/08910600410021783 [DOI] [Google Scholar]
- Tsukahara T., Yoshida Y., Tsushima T., Watanabe T., Matsubara N., Inoue R., and Ushida K.. 2011. Evaluation of the heat-killed and dried cell preparation of Enterococcus faecalis against villous atrophy in early-weaned mice and pigs. Anim. Sci. J. 82:302–306. doi: 10.1111/j.1740-0929.2010.00829.x. [DOI] [PubMed] [Google Scholar]
- Ushida K., Yoshida Y., Tsukahara T., Watanabe T., and Inoue R.. 2010. Oral administration of Enterococcus faecalis EC-12 cell preparation improves villous atrophy after weaning through enhancement of growth factor expression in mice. Biomedical. Res-Tokyo. 31:191–198. doi: 10.2220/biomedres.31.191. [DOI] [PubMed] [Google Scholar]
- Walsh M. C., Sholly D. M., Hinson R. B., Saddoris K. L., Sutton A. L., Radcliffe J. S., Odgaard R., Murphy J., and Richert B. T.. 2007. Effects of water and diet acidification with and without antibiotics on weanling pig growth and microbial shedding. J. Anim. Sci. 85:1799–1808. doi: 10.2527/jas.2006-049. [DOI] [PubMed] [Google Scholar]
- Wyns H., Meyer E., Plessers E., Watteyn A., van Bergen T., Schauvliege S., De Baere S., Devreese M., De Backer P., and Croubels S.. 2015. Modulation by gamithromycin and ketoprofen of in vitro and in vivo porcine lipopolysaccharide-induced inflammation. Vet. Immunol. Immunopathol. 168:211–222. doi: 10.1016/j.vetimm.2015.09.014. [DOI] [PubMed] [Google Scholar]
- Yang C. M., Cao G. T., Ferket P. R., Liu T. T., Zhou L., Zhang L., Xiao Y. P., and Chen A. G.. 2012. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poult. Sci. 91:2121–2129. doi: 10.3382/ps.2011-02131. [DOI] [PubMed] [Google Scholar]
- Yang C. M., Zhang L. L., Cao G. T., Feng J., Yue M., Xu Y. L., Dai B., Han Q. J., and Guo X.Q.. 2018. Effects of dietary supplementation with essential oils and organic acids on the growth performance, immune system, faecal volatile fatty acids and microflora community in weaned piglets. J. Anim. Sci. 97:133–143. doi: 10.1093/jas/sky426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Cao G. T., Zeng X. F., Zhou L., Ferket P. R., Xiao Y. P., Chen A. G., and Yang C. M.. 2014. Effects of Clostridium butyricum on growth performance, immune function, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult. Sci. 93:46–53. doi: 10.3382/ps.2013-03412. [DOI] [PubMed] [Google Scholar]
- Zhang L., Li J., Yun T. T., Qi W. T., Liang X. X., Wang Y. W., and Li A. K.. 2015. Effects of pre-encapsulated and pro-encapsulated Enterococcus faecalis on growth performance, blood characteristics, and cecal microflora in broiler chickens. Poultry. Sci. 94:2821–2830. doi: 10.3382/ps/pev262 [DOI] [PubMed] [Google Scholar]
- Zhang J., Shi Z. L., Zeng M. H., Wei C., Wu H. X., Liu M. Y., Huang J. Q., Zheng Y. J., and Sun X.. 2017. Oral administration of Clostridium butyricum CGMCC0313-1 reduces ovalbumin-induced allergic airway inflammation in mice. Respirology. 22: 898–904. doi: 10.1111/resp.12985 [DOI] [PubMed] [Google Scholar]
- Zhang J., Sun J., Chen X. Y., Nie C. X., Zhao J. B., Guan W. Y., Lei L. H., He T., Chen Y. Q., Johnston L. J., et al. 2018. Combination of Clostridium butyricum and Corn Bran Optimized Intestinal Microbial Fermentation Using a Weaned Pig Model. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.03091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Yang X., Guo Y., and Long F.. 2011. Effects of dietary lipids and Clostridium butyricum on the performance and the digestive tract of broiler chickens. Arch. Anim. Nutr. 65:329–339. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang L. L., Zhan X. A., Zeng X. F., Zhou L., Cao G. T., Chen A. G., and Yang C. M.. 2016. Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J. Anim. Sci. Biotechno. 7. doi: 10.1186/s40104-016-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. N., Yang J., Wang L. L., Lin H., and Sun S. H.. 2017. Protection mechanism of Clostridium butyricum against Salmonella Enteritidis infection in broilers. Front. Microbio. 8. doi: 10.3389/fmicb.2017.01523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong X., Wang T. H., Lu Z. Q., Song D. G., Zhao J., and Wang Y. Z.. 2019. Effects of Clostridium butyricum or in combination with Bacillus licheniformis on the growth performance, blood indexes, and intestinal barrier function of weanling piglets. Livest. Sci. 220:137–142. dio: 10.1016/j.livsci.2018.12.024 [DOI] [Google Scholar]