Abstract
Background
The number of periprosthetic joint infections (PJI) after total knee arthroplasty (TKA) is increasing annually. Animal models have been used to clarify their clinical characteristics and the infection mechanism of pathogenic bacteria, However, since the prosthesis design of animal models is not uniform, it is difficult to simulate the environment of clinical PJI.
Objectives
To retrospect the progress on the prosthesis design of animal models of PJI after TKA and to summarize the criteria for evaluating a clinically representative model of PJI.
Methods
This systematic review was reported on the basis of Systematic Reviews and Meta-Analyzes (PRISMA). Pubmed, EMbase, Cochrane Library, Web of Science, Wanfang Data and China National Knowledge Infrastructure were researched for animal models of PJI after TKA from database establishment to April 2019 according to Chinese and English retrieval words, including “periprosthetic joint infections and total knee arthroplasty,” “periprosthetic joint infections and model,” “periprosthetic joint infections and biofilm,” and “total knee arthroplasty and model.”
Results
A total of 12 quantitative studies were enrolled in our study finally: 8 representative studies described prosthesis designs used in PJI animal models, 4 studies described prosthesis designs in non-infected animal models which were suitable for infection models. The major problems need to be dealed with were prosthesis, installation location, material, the function of separating the articular and medullary cavity, fixation manner, and the procedure of preserving the posterior cruciate ligament.
Conclusion
A highly representative design of the animal prosthesis of PJI should meet the following criteria: the surface of the prosthesis is smooth with the formation of biofilm, composed of titanium-6Al-4V or cobalt-chromium-molybdenum alloy; prosthesis can bear weight and is highly stable; and it can connect the joint cavity and medullary cavity simultaneously. To reach a more reliable conclusion, further experiments and improvements are required.
Introduction
Periprosthetic joint infection (PJI) is one of the severe complications of total knee arthroplasty (TKA), accounting for 25%-38% of postoperative complications in TKA [1–4]. Although through a series of measures [5–8] (including the use of perioperative antibiotics, intraoperative antibiotic cement, intraoperative antibiotic calcium sulfate, improvement of surgical environment, and elimination of local bacterial colonization), the infection rate has been controlled at about 1%-3% [9, 10]. However, with the rapid growth of the population undergoing TKA in recent years, the number of people with PJI has also increased annually [11, 12]. Two-stage revision, involving an antibiotic-impregnated polymethylmethacrylate spacer, is thought to be the gold standard for treating PJI, but the current cure rate for two-stage revision is only 72%-95% according to several studies [7, 11, 13].
To better understand and recognize the diagnosis of PJI and develop novel treatment strategies, it is important to investigate its clinical characteristics and infection mechanism of pathogenic bacteria. Nowadays, many studies focus on in vivo and in vitro experiments. However, conclusions based on in vitro experiments do not guarantee the authenticity of the infection mechanism and the clinical effectiveness of various interventions. On the other hand, experiments in animals are also not able to completely simulate the clinical pathogenesis of PJI and transform our understanding of PJI due to synthetic factors, among which the prosthesis design of animal models acts as an important medium. Its clinical pathogenesis has been widely discussed in the past [14–22], which can help us better understand the colonization of bacteria, formation of biofilm, and adhesion process and resistance to the host immune response [23, 24]. The implant design was the first step of performing PJI models, if it is far different from clinical implant, then the next steps will be affected and the results may be lack of persuasion. Nevertheless, the current design of PJI models is still dissatisfying. They are not clinically representative and can barely reproduce the periprosthetic environment because of the lack of weight bearing, poor matching, only partial replacement, weak stability, inconsistent relevantly clinical material, rough surface, and non-anatomical appearance [14, 16, 25–30]. According to the International Consensus on Orthopedic Infections in 2019, the ideal prosthesis design of a PJI model has yet to be established [31].
In this review, we retrospectively collected information about previously established prostheses used in PJI animal models of knee replacement, and herein, we discuss their advantages and disadvantages in order to summarize the most clinically relevant and reproducible evaluation criteria of prostheses, and to promote the development of a novel animal prosthesis. Thus, our review may better a provide theoretical basis for clinical diagnosis, treatment, and prevention of PJI.
Methods
Data sources and search strategy
This systematic review was reported on the basis of Systematic Reviews and Meta-Analyzes (PRISMA) statements for prosthesis design of animal models of PJI following TKA [32].
One investigator (PD) design the search strategy and two investigators (WJF and JLC) completed the literature search independently. After cross-checking, the disagreements over the included articles were submitted to the third person (HRC) for arbitration. Studies associated with PJI models were identified using Pubmed, EMbase, Cochrane Library, Web of Science, Wanfang Data and China National Knowledge Infrastructure from database establishment to April 1st 2019 using the following keywords: (1) “periprosthetic joint infections and total knee arthroplasty,” (2) “periprosthetic joint Infections and model,” (3) “periprosthetic joint infections and biofilm,” and (4)“total knee arthroplasty and model.” In addition to electronic retrieval, all the relative references in the included studies were searched manually to avoid omitting undiscovered studies in the retrieval database.
Study selection and eligibility criteria
Two investigators (WJF and JLC) read the titles and abstracts of all downloaded literature, then preliminarily excluded the research that obviously did not meet the eligibility criteria, and finally carefully scanned the full text to screen out the studies that met the standards for data extraction. The eligibility criteria in this study were: (1) animal trials, (2) studies describing content of prosthesis design of PJI following TKA in detail, (3) the prosthesis that was clinically representative or used frequently by different researchers in the past or recently. The exclusion criteria were as follows: (1) Literature reviews, conference abstracts, letters to the editor, (2) studies describing prosthesis too briefly, (3) Repetitive prosthesis design, (4) Non-animal trial.
Data extraction and items
After scanning the database, we analyzed their advantages and disadvantages on the prosthesis design of animal models of PJI, including author, publication years, experimental subject, prosthesis and their characteristics(location, material, whether it was located in the weight-bearing area or not, separate the articular and medullary cavity or not), operative procedure(whether cement was used or not, PCL was preserved or not), inoculation bacteria and their details(species, inoculation location, dose and concentration). These are the international issues at present, which will be answered with‘yes’, ‘no’, ‘not mention’ or matching descriptions in Tables 1 and 2.
Table 1. Animal models of prosthetic joint infection.
| Author, Year | Animal | Prosthesis | Preserving PCL or not | Bacteria | |||||
|---|---|---|---|---|---|---|---|---|---|
| Implant, Location, Material | Located in the weight -bearing area or not |
Separating the articular and medullary cavity or not | Using cement or not |
Species | Inoculation location | Dose, concentration (cfu/ml) |
|||
| Bernthal 2010[14] |
Mouse | Femoral medullary cavity, stainless steel K-wire | No | No | No | Yes | S.aureus | Knee cavity |
2ul, 5 ×102 to 104 |
| Carli 2017[15] |
Mouse | Tibial component, tantalum | Yes | Yes | No | Yes | S.aureus | Knee cavity |
2ul, 3×105 |
| Craig 2005[16] |
Rabbit | Lateral femoral condyle, stainless steel hollow nail + UHMWPE insert | No | No | Yes | Yes | MRSA | Knee cavity |
0.1ml, 1×102 to 103 |
| Saleh-Mghir 2011[30] |
Rabbit | Tibial component, silicone | Yes | Yes | NM | NM | MRSA | Knee cavity |
0.5ml, 5×107 |
| Poultsides 2010[34] |
Rabbit | ①Tibial medullary cavity, Cylinder, Tantalum ②Proximal tibia, circular silicone | Yes | Yes | NM | NM | MRSA | Femoral artery | 1ml, 3–5×108 |
| Kalteis 2006[37] |
Rat | Femoral medullary cavity, hollow nail(NM materials) | No | No | No | NM | S.aureus | Femoral medullary cavity |
100μL, 1×108 |
| Petty 1985[38] |
Dog | Femoral medullary cavity, Cylinder,①stainless steel, ②cobalt-chromium alloy ③polymer polyethylene ④cement |
No | YES | ①②③NO, ④Yes | NM |
S.aureus, S.epidermidis E. coli |
Femoral medullary cavity |
1×102 to 108 |
| Schurman 1975[39] |
Rabbit | Suprapatellar bursa, stainless steel particles | No | No | - | - | S.aureus | Suprapatellar bursa | - |
NOTE: MRSA = methicillin-resistant staphylococcus aureus, S.aureus = Staphylococcus aureus, UHMWPE = Ultra-High Molecular Weight Polyethylene
S.Epidermidis = Staphylococcus epidermidis, E. Coli = Escherichia coli, K-wire = Kirschner wire, PCL = Posterior Cruciate Ligament, NM = not mention
Table 2. Non-infective animal models.
| Author, Year | Animal | Prosthesis | Preserving PCL or not | |||
|---|---|---|---|---|---|---|
| Implant, Location, Material | Located in the weight-bearing area or not | separating the intra-articular and medullary cavity or not | Using cement or not |
|||
| Turner 1989[19] |
Dog | Femoral component, cobalt-chromium alloy Tibial component, tantalum alloy+50% dense fibrous metal plate Insert, HMWPE |
Yes | Yes | Yes | Yes |
| Yan Zhi Qiang 2014[22] |
Rabbit | Femoral component, Co-Cr-Mo alloy ②Tibial component, UHMWPE |
Yes | Yes | Yes | Yes |
| Xu Yang 2015[35] |
Mouse | Tibial component, Tantalum alloy | Yes | Yes | No | Yes |
| Zampelis 2013[36] |
Rabbit | Tibial component, NM materials | Yes | Yes | No | NM |
NOTE: UHMWPE = Ultra-High Molecular Weight Polyethylene, HMWPE = High Molecular Weight Polyethylene, PCL = Posterior Cruciate Ligament, NM = not mention
Risk of bias within studies
The STAIR [33] (the initial Stroke Therapy Academic Industry Roundtable) risk of bias tool was used to independently assess the methodological and reporting quality of included studies by two investigators (WJF and JLC), and the divergences were submitted to the third person(HRC) for arbitration after checking each other. The included studies were assessed across the following factors: sample size calculation, inclusion and exclusion criteria, randomization, allocation concealment, reporting of animals excluded from analysis, blinded assessment of outcome, reporting potential conflicts of interest and study funding. Finally, the quality score was calculated according to above information. The total scores were 7 points, and a study of more than or equal to 3 points were defined as high quality research.
Results
Search results
A total of 4299 records were searched through English and Chinese database. After removing duplicates and screening topics and abstracts, 481 records remained. Then the full text and their references were carefully read and analyzed. According to the eligibility and exclusion criteria, 12 articles were finally selected, 8 of which representative studies described prosthesis designs used in PJI animal models and 4 described prosthesis designs in non-infected animal models. The PRISMA flow diagram was presented in detail in Fig 1.
Fig 1. Study screening flow.
Assessment of methodological and reporting quality
Based on the STAIR tool, all the details of quality of 12 studies were presented in Table 3. 6 articles were assessed as high quality [16, 22, 30, 34–36]. All 12 studies referred to potential conflicts of interest and study funding [14–16, 19, 22, 30, 34–39], while no studies reported the sample size calculation. Over a half of the included studies (58%, 7/12) reported animals excluded from analysis [16, 22, 30, 34–36, 39]. In 42% (5/12) of the included studies, randomization of the experiment was reported [16, 30, 35–37]. Only 8% (1/12) of the included studies described the inclusion and exclusion criteria [22], and 17% (2/12) described allocation concealment [16, 35], and 17% (2/12) described blinded assessment of outcome [34, 35].
Table 3. Risk of bias.
| Study | Sample size calculation | Inclusion and exclusion criteria | Randomization | Allocation concealment | Reporting of animals excluded from analysis | Blinded assessment of outcome | Reporting potential conflicts of interest and study funding | Quality score |
|---|---|---|---|---|---|---|---|---|
| Bernthal 2010[14] |
N/A | N/A | N/A | N/A | N/A | N/A | M | 1 |
| Carli 2017[15] |
N/A | N/A | N/A | N/A | N/A | N/A | M | 1 |
| Craig 2005[16] |
N/A | N/A | M | M | M | N/A | M | 4 |
| Turner 1989[19] |
N/A | N/A | N/A | N/A | N/A | N/A | M | 1 |
| Yan Zhi Qiang 2014[22] |
N/A | M | N/A | N/A | M | N/A | M | 3 |
| Saleh-Mghir 2011[30] |
N/A | N/A | M | N/A | M | N/A | M | 3 |
| Poultsides 2008[34] |
N/A | N/A | N/A | N/A | M | M | M | 3 |
| Xu Yang 2015[35] |
N/A | N/A | M | M | M | M | M | 5 |
| Zampelis 2013[36] |
N/A | N/A | M | N/A | M | N/A | M | 3 |
| Kalteis 2006[37] |
N/A | N/A | M | N/A | N/A | N/A | M | 2 |
| Petty 1985[38] |
N/A | N/A | N/A | N/A | N/A | N/A | M | 1 |
| Schurman 1975[39] |
N/A | N/A | N/A | N/A | M | N/A | M | 2 |
NOTE: N/A = not available; M = mentioned.
Study characteristics
The progress of prosthesis designs used in PJI animal models
The animal model is a reasonable and efficient approach to transform all kinds of results in in vitro into the clinical setting. At present, there is great controversy regarding the design of prostheses, and various prostheses were used in PJI animal models [14–16, 30, 34, 37–39], which may cause confusion among researchers. These studies chose the most classic, representative, and widely used prostheses to analyze their characteristics and explore the developmental direction of new models in the future. Details are shown in Table 1.
The PJI animal model was first proposed and designed by Schurman in 1975 [39]. In order to evaluate the susceptibility of prostheses to PJI, stainless steel particles and Staphylococcus aureus were implanted into a rabbit’s suprapatellar bursa. The prosthesis was suspended in saline rather than implanted in the bone so it did not simulate the bone-cement-prosthesis interface during TKA.
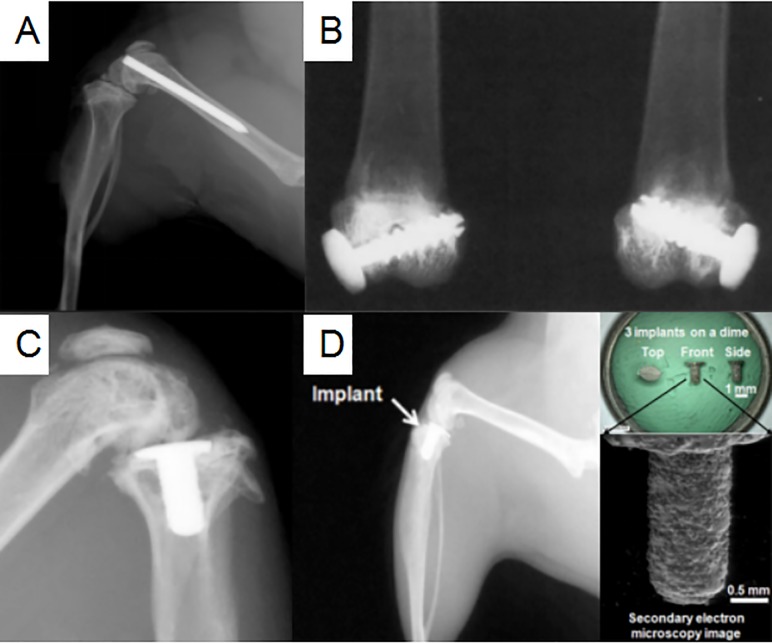
In 1985, Petty et al [38] recognized that there may be some correlations between the different implant materials and infection of pathogenic bacteria. After injecting different kinds of bacteria, including S. aureus, S. epidermidis, and Escherichia coli, into the medullary cavity at the distal end of the femur in dogs, different cylinders, composed of stainless steel alloy, cobalt-chromium alloy, high-density polyethylene, polymerized polymethylmethacrylate, or polymethylmethacrylate, respectively, were then introduced. Although larger animals like dogs have musculoskeletal and immunological systems similar to humans and can preferably mimic the environment of the human knee joint, their use will always be accompanied by more ethical challenges, financial costs, and lower throughput [25]. Recently, researchers had preferred to choose small animals, such as rabbits, rats, and mice, in PJI models. In 2010, Bernthal et al [14] used the same method in that a stainless steel Kirschner wire was retrogradely injected into the distal femur of mice for PJI modeling (Fig 2A). The implant in the medullary cavity was originally designed to imitate PJI after TKA, but in fact, it might confuse osteomyelitis models with PJI models [40, 41]. Because of its simplicity and reproducibility of manipulation, many researchers still used this method for PJI modeling [17, 18, 20, 21, 42]. However, the prosthesis had the following obvious defects. Only stainless steel materials were used, which were different from the current commonly titanium (Ti)-6Al-4V and cobalt-chromium-molybdenum alloy in TKA. In addition, it can not achieve the load-bearing state, and the authors did not use bone cement to fix the prosthesis. Although the minimal infecting dose was low, it cannot reproduce the periprosthetic environment. All these factors affected the formation of biofilm on the surface of the prosthesis [43]. Bernthal et al [14] also suggested that the cartilage in femur and tibia, which were not removed intraoperatively, may interact with the pathogenic bacteria.
Fig 2. The design of four prostheses.
(The figures were derived from references.). (A) A stainless steel Kirschner wire retrogradely inserted into the distal femur of a mouse model [14].(B) A full threaded stainless steel hollow nail and UHMWPE washer implanted in the lateral femoral condyle and anterior to the lateral collateral ligament of a rabbit model [16]. (C) A 3-dimensionally printed Ti-6Al-4V prosthesis implanted in the tibial plateau of a mouse model [15].(D) A 3-dimensional printed prosthesis implanted in the tibia of a murine model [35].
In another study reported by Craig et al in 2005 [16], after drilling a hole in the lateral femoral condyle anterior to the lateral collateral ligament, 0.1-ml bone cement, a fully threaded stainless steel hollow nail, and ultra-high molecular weight polyethylene (UHMWPE) washer were implanted successively (Fig 2B). The three materials used in this study were closer in similarity to TKA materials used in clinical practice, and they had increased stability; thus, they were also favored by many researchers [44–46]. In terms of anatomical location, the materials were located on the outside of the knee joint so that they can bear only compressive stress in the vertical direction, not rotational stress. Furthermore, the materials did not separate the medullary cavity and joint cavity, and no articular cartilage was removed intraoperatively. All these factors limited their further application in the future.
In 2017, Carli et al [15] first applied a three-dimensional (3D) printed tibial prosthesis with the Ti-6Al-4V in a PJI mice model (Fig 2C). This method was a great breakthrough in the development of the prosthesis design. Initially, this prosthesis originated from a non-infected model, and was first proposed and applied by Xu Yang et al in 2015 [35]. In order to assess the effect of recombinant human parathyroid hormone on cancellous bone integration, they developed an uncemented tibial prosthesis for a mouse model, whose surface was rough (Fig 2D). This prosthesis combined the advantages of several prostheses aforementioned [14, 16, 38, 39], including the ability to bear weight, separate the intra-articular and intramedullary cavities, use clinically relevant materials, and require simple manipulation. It was more similar to the tibial component replacement in clinical TKA and more representative than other prostheses of PJI. The former also met four criteria of clinically representative PJI models proposed by Carli et al in 2016 [25]. The criteria included the following conditions: (1) Biofilm can be formed on the surface of the prosthesis; (2) Prosthetic materials should be similar to clinical materials, bear weight, and create an intra-articular environment; (3) The animals chosen for models should have musculoskeletal and immunological system compared to human beings; (4) The bacteria, biofilm, and host immune response can be measured quantitatively. Among them, the first and second sections were aimed at the prosthesis design. In fact, in addition to the use of uncemented fixation and unknown stability, only tibial replacement had been performed without femoral replacement, which was also one of the problems that needs improved in the future.
Designing PJI models based on non-infected animal models
As mentioned above, the design of the PJI animal model has made great progress, but there are still some differences from PJI after TKA in the clinical setting. How to improve the prosthesis is still the focus. With the advent of the age of multidisciplinary communication, some prostheses in non-infection models may be more suitable for infection models, which can accommodate for the shortcoming of PJI models and make the prostheses closer to perfect [15, 19, 35, 36]. Details are shown in Table 2.
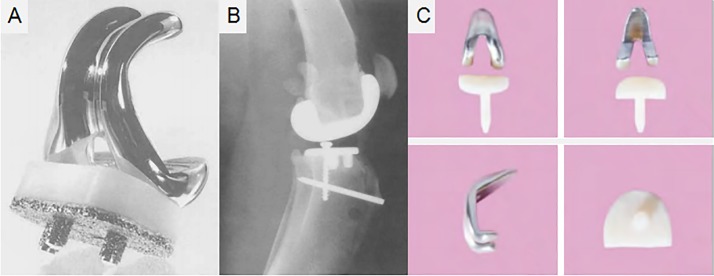
As early as 1989, Turner et al [19] designed an unrestricted posterior cruciate-retaining (CR) prosthesis for dogs to observe bone ingrowth of the tibial component in TKA. The posterior cruciate ligament was preserved as much as possible, and patella replacement was not performed during the operation (Fig 3A and 3B). This prosthesis consisted of three parts: the femoral prosthesis, insert, and tibial prosthesis similar to that used in clinical TKA. In terms of appearance and material science, the femoral prosthesis was made of a cobalt-chromium alloy, imitating the contour of the distal femur of dogs, and the insert was comprised of UHMWPE. The tibial component was made of titanium alloy, below which was a 50% dense fibrous metal plate and three cylindrical pegs coated with fiber metal, above which was the UHMWPE. In terms of stability, the femoral part was fixed with bone cement, tibial part was fixed by bone ingrowth, and a screw was inserted into the cancellous bone behind the tibial prosthesis to increase the stability. Regarding the degree of matching, the femoral anterior condyle, posterior condyle, and distal condyle underwent osteotomy with alignment and cutting saws, and the tibial posterior slope angle (TPSA) was maintained at about 25°. Moreover, a femoral trocar groove was designed in the femoral prosthesis to ensure matching with the patellofemoral joint after joint replacement.
Fig 3. The appearance of two prostheses.
(The figures were derived from references.) (A-B) An unrestricted posterior CR prosthesis implanted in a dog model [19].(C) An anatomical joint prosthesis implanted in a rabbit model [22].
Six months postoperatively in Turner et al’s study [19], the x-ray showed no changes in the position of the tibial or femoral prosthesis, and only a small amount of osteophytes was seen in the anterior, medial, and lateral sides of the tibial prosthesis. Unexpectedly, although cartilage wear and even subchondral invasion can be seen in the patellofemoral joint, it was stable. The prosthesis can be used for reference in the design of the PJI animal model, because it showed great advantages in implant stability, load-bearing, clinically relevant materials, which met the requirements for PJI models. However, it did not separate the intra-articular and intramedullary spaces. Furthermore, compared to small animals, dogs, sheep, and other large animals have simpler joint exposure, larger operating space, and relatively easier prosthesis installation, and the manipulation has less of an effect on postoperative function. However, it may be extremely difficult to apply the prosthesis to the knee joint of small animals. Because of the cross-domain application, the details need to be further adjusted in the future.
Not only in the field of infection, but also in other fields, the TKA prostheses of animal models are not uniform [19, 22, 36]. In order to provide standardized multi-model prostheses, in 2014, Yan et al [22] designed an anatomical joint prosthesis for rabbits (Fig 3C). First, computed tomography was used to scan the knee joints of rabbits with different weights and knee prostheses of humans. Then 3D reconstruction was performed to reduce the equal proportion of the prostheses of humans and adjust it according to the actual anatomic markers of the rabbit knee joint, including the internal-external and anteroposterior diameters of the femoral condyles and tibial plateau, diameters of the femoral and tibial medullary cavities, and so on. The tibial component was made of UHMWPE, and the femoral component was made of Co-Cr-Mo alloy. Cement was used for fixation. The results showed that the prosthesis was found to be in a good position, and no obvious loosening was found at 1 month postoperatively. The rate of excellent function postoperatively was 87% (13/15) within 7 days, flexion and extension of the knee joint on the operated side was more tense than that on the normal side, and 13 rabbits returned to normal crawling 7 days postoperatively. The design of the prosthesis in small animals is more challenging than that in large animals. It is not only difficult to expose the bone during operation, but more precise osteotomy and a higher degree of prosthesis matching are required. Although the authors used a fully anatomical prosthesis and did not install an insert, the knee joint on the operated side was still tight so as to affect their gait. The tibial component may be too thick to allow the knee joint activity. Eventually, 2 rabbits developed postoperative dysfunction. Other problems also existed, for example, the femoral component did not separate the intra-articular and intramedullary spaces, and it may demand higher surgical technique.
Discussion
Question 1: Should bone cement be used for fixation?
The achievement of implant stability plays an important part in the formation of bacterial biofilm and periprosthetic immune response. The instability of the prosthesis will not only affect the bone growth, resulting in loosening of the prosthesis [47], but it will also affect the adhesion of bacteria due to the unstable shear force [26]. In clinical practice, the application of cementless TKA is limited because it may be associated with higher loosening and revision rates than cemented TKA for lower initial stability [48]. Although cementless fixation is reliable according to many studies [49, 50], it may not be determinable by radiography whether the prosthesis has fretting within a short follow-up period. Furthermore, it has been reported that polymethylmethacrylate is also a good material for biofilm attachment [51, 52]. Other studies have shown that biofilm can even form on an antibiotic-loaded bone cement [53, 54], which is closely linked with the progression of PJI.
Question 2: Should one resect or preserve the posterior cruciate ligament?
The anatomical structure of dogs, rabbits, rats, and mice are similar to humans, and their knee joints are commonly selected as objects in experimental studies [14–20], because they have both anterior and posterior cruciate ligaments, medial and lateral collateral ligaments, and medial and lateral menisci. On the other hand, because of the obvious difference in walking gait, the anatomical characteristics of the lower extremity and limb alignment are different from those of humans. In theory, their hip-knee-ankle angle and TPSA in the sagittal position are much larger than those in humans. Relevant studies showed that the TPSA of dogs is about 23.6°-27.4°[55, 56], knee can reach about 138.5° in the standing position [57], and range of motion (ROM) is about 120° [58]. Whenever dogs walk or stand, the knee is usually at the position of flexion, and the posterior cruciate ligament is very important for maintaining stability of their knees [59].
In clinical practice, according to whether the posterior cruciate ligament is preserved, there are two kinds of prostheses, including posterior CR prostheses and posterior cruciate-stabilized prosthesis (PS) [60, 61]. The CR prosthesis can better simulate the rolling mechanism and biomechanical characteristics of the normal knee, and achieve higher proprioceptive sensation [28]. The PS prosthesis takes the place of the function of the posterior cruciate ligament, by creating an upright between the center of the tibial component and a cam between the posterior condyle of the femoral component, which may not fully restore the function of the intact posterior collateral ligament, especially in deep-flexion activities. Additionally, unlike the PS prosthesis for humans, the design of the PS prosthesis for animal models requires higher accuracy; thus, the CR prosthesis is more suitable than the PS prosthesis for animal models of deep knee flexion. Therefore, when designing the appearance of an animal prosthesis, not only the anatomical features of the bone structure but also the functional characteristics should be considered carefully.
Question 3: Should only tibial replacement be performed or should tibial and femoral replacement be performed simultaneously?
In a study by Carli et al [15], a stable biofilm could be formed after tibial plateau replacement following the injection of bacteria. Several studies have also shown that it is clinically representative [31, 53]. However, it does not satisfy the concept of the total anatomical prosthesis, which may result in a different periprosthetic environment while undergoing tibial replacement only or total condylar replacement, so it is difficult to meet the needs of basic clinical research. Although evidence is lacking, there may also be interactions between the preserved femoral articular cartilage and bacteria[14]. In contrast, performing femoral replacement simultaneously in animals will greatly increase the difficulty of surgery, which may lead to an increase of intraoperative bleeding, postoperative dysfunction, and so on [22]. Further experimental proof is needed to determine whether there is any difference between the two kinds of methods established in the PJI model.
Question 4: Should a UHMWPE insert be used or not?
Karbysheva et al [62] suggested that the microorganisms' ability to adhere and form a biofilm on different biomaterials of explanted joint prosthesis components might differs among biomaterials. The implant components in 40 patients diagnosed with PJI were retrieved to perform sonication cultures, which were analyzed qualitatively and quantitatively. The results demonstrated that the bacteria counts were larger in the polyethylene group than in the titanium alloy and the cobalt-chromium alloy groups, which indicated that the polyethylene implant had higher microbial adhesion affinity in vivo. Another factor that needs to be considered carefully is whether a UHMWPE insert will affect joint ROM. For large animals, it has been reported that the UHMWPE insert can be used in the process of TKA [19]. This technique is evolved, and there is no obvious limitation in flexion and extension of the knee joint after implanting the UHMWPE insert. However, for small animals, the tibiofemoral joint space may be too narrow to accommodate for such an insert. Even if the insert can be successfully implanted, there is still a high risk of low ROM. Presently, there are no reports on the implantation of a polyethylene insert during TKA in a small animal model.
Strengths and limitations
This is the first study concerning to the principles of implant design of the PJI models specially. We have not only systematically discussed implant stability, load-bearing, clinically relevant materials, separation of intra-articular and intramedullary spaces, but also the problems of fixation, posterior cruciate ligament, femoral replacement and UHMWPE insert.
Accompanied by evident strengths, several limitations also exist in this study. This study only focused on prosthesis design without other factors, however, a PJI model may be influenced by various complex factors, including the prosthesis design, selection of animal species, pathogenic bacteria, amount of bacteria implanted, immune environment around the prosthesis, method of implanting bacteria, and so on (Table 1), which will synthetically affect the density and quality of the biomembrane formed on the surface of the prosthesis. The reason why we chose this topic was that most influencing factors were based on the prosthesis design. Only by imitating the periprosthetic environment in the human body to the greatest extent can we create the most clinically representative model of PJI. Furthermore, the evaluation criterias recommended by us were established according to previous articles, so further experimental verification is required.
Overview
PJI following TKA will cause patients to suffer from enormous physical injury and financial burden, and it will present new severe challenges for the medical staff. The establishment of a PJI animal model has become one of the important ways to overcome this difficult problem. There is still no consensus on the criteria for these factors because of the individual modeling methods and concept resulting in bias in the studies’ results.Some achievements have been made in the design, but there are still many deficiencies; for example, the achievements can not be applied to clarify the clinical pathogenesis or to test the efficacy of drugs effectively. To reach a more reliable conclusion, the implant design needs to be improved and perfected.
Conclusion
In conclusion, when evaluating the high clinical representativeness of a prosthesis design of animal models of PJI following TKA, we recommend the following evaluation criterias, of which the first six are relatively more important:
The surface of the prosthesis should be smooth so as not to limit knee joint ROM.
The surface of the prosthesis should be found the formation of biofilm.
The implants are composed of Ti-6Al-4V or Co-Cr-Mo alloy, with or without UHMWPE, which are similar to clinical materials.
The implants can bear weight and separate the intra-articular and medullary cavities for reproducing the periprosthetic environment.
Since high stability is needed, such as the use of bone cement, the bone-cement-prosthesis interface can reduce the impact of wear particles on bone resorption.
Tibial replacement and femoral prosthesis replacement can be performed simultaneously as possible.
The posterior cruciate ligament can be preserved as much as possible to increase the stability of the knee. In other words, the femoral and tibial prostheses can accommodate the end point of the posterior cruciate ligament.
If allowed, good patella track and patellofemoral joint matching can be achieved.
Manipulation is simple and reproducible. Therefore, patella surface replacement should not be performed, as there is no evidence to support it.
Supporting information
(DOC)
The search strategies in each database and the inclusion and exclusion of articles.
(DOC)
Data Availability
The data can be found in Pubmed, EMbase, Cochrane Library, Web of Science, Wanfang Data and China National Knowledge Infrastructure.
Funding Statement
The current study received High-Level Hospital Construction Project of The First Affiliated Hospital of Guangzhou University of Chinese Medicine (Grant number 211010010121).
References
- 1.Srivastava K, Bozic KJ, Silverton C, Nelson AJ, Makhni EC, Davis JJJ. Reconsidering Strategies for Managing Chronic Periprosthetic Joint Infection in Total Knee Arthroplasty: Using Decision Analytics to Find the Optimal Strategy Between One-Stage and Two-Stage Total Knee Revision. JBJS. 2019;101(1):14–24. 10.2106/JBJS.17.00874 . [DOI] [PubMed] [Google Scholar]
- 2.Preobrazhensky PM, Bozhkova SA, Kazemirsky AV, Tikhilov RM, Kulaba TA, Kornilov NNJ. Functional outcome of two-stage reimplantation in patients with periprosthetic joint infection after primary total knee arthroplasty. International orthopaedics. 2019:1–7. 10.1007/s00264-018-4276-1 . [DOI] [PubMed] [Google Scholar]
- 3.Koh CK, Zeng I, Ravi S, Zhu M, Vince KG, Young SWJ. Periprosthetic joint infection is the main cause of failure for modern knee arthroplasty: an analysis of 11,134 knees. Clinical Orthopaedics Related Research. 2017;475(9):2194–201. 10.1007/s11999-017-5396-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi JJ. Prosthetic joint infection risk after TKA in the Medicare population. Clinical Orthopaedics Related Research. 2010;468(1):52–6. 10.1007/s11999-009-1013-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumbach SF, Prall WC, Scharpf AM, Hererich V, Schmidt M, Suedkamp NP, et al. Significant increase of pathogen detection rate by dry arthroscopic biopsies at suspected low-grade infection following total knee arthroplasty: a prospective observational study. Archives of orthopaedic trauma surgery. 2018;138(11):1583–90. 10.1007/s00402-018-3032-8 . [DOI] [PubMed] [Google Scholar]
- 6.Gwam CU, George NE, Etcheson JI, Tarazi JM, Han G-r, Griffith KM, et al. Clostridium difficile infection in the USA: incidence and associated factors in revision total knee arthroplasty patients. European Journal of Orthopaedic Surgery Traumatology. 2019;29(3):667–74. 10.1007/s00590-018-2319-3 . [DOI] [PubMed] [Google Scholar]
- 7.Lee YS, Chen AFJ. Two-Stage Reimplantation in Infected Total Knee Arthroplasty. Knee surgery related research. 2018;30(2):107 10.5792/ksrr.17.095 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang F-D, Wang Y-P, Chen C-F, Chen H-PJ. The incidence rate, trend and microbiological aetiology of prosthetic joint infection after total knee arthroplasty: A 13 years’ experience from a tertiary medical center in Taiwan. Journal of Microbiology, Immunology and Infection. 2018;51(6):717–22. 10.1016/j.jmii.2018.08.011 . [DOI] [PubMed] [Google Scholar]
- 9.Petis SM, Perry KI, Mabry TM, Hanssen AD, Berry DJ, Abdel MPJ. Two-Stage Exchange Protocol for Periprosthetic Joint Infection Following Total Knee Arthroplasty in 245 Knees without Prior Treatment for Infection. JBJS. 2019;101(3):239–49. 10.2106/JBJS.18.00356 . [DOI] [PubMed] [Google Scholar]
- 10.Siu K, Ng F, Chan P, Fu HC, Yan C, Chiu KJ. Bacteriology and risk factors associated with periprosthetic joint infection after primary total knee arthroplasty: retrospective study of 2543 cases. Hong Kong Med J. 2018;24(2):152–7. 10.12809/hkmj176885 . [DOI] [PubMed] [Google Scholar]
- 11.Yaghmour KM, Chisari E, Khan WSJ. Single-Stage Revision Surgery in Infected Total Knee Arthroplasty: A PRISMA Systematic Review. Journal of clinical medicine. 2019;8(2):174 10.3390/jcm8020174 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J-K, Lee D-Y, Kang D-W, Ro D-H, Lee MC, Han H-SJ. Efficacy of antifungal-impregnated cement spacer against chronic fungal periprosthetic joint infections after total knee arthroplasty. The Knee. 2018;25(4):631–7. 10.1016/j.knee.2018.04.004 . [DOI] [PubMed] [Google Scholar]
- 13.Kim K, Zhu M, Cavadino A, Munro JT, Young SWJ. Failed Debridement and Implant Retention Does Not Compromise the Success of Subsequent Staged Revision in Infected Total Knee Arthroplasty. The Journal of arthroplasty. 2019. 10.1016/j.arth.2019.01.066 . [DOI] [PubMed] [Google Scholar]
- 14.Bernthal NM, Stavrakis AI, Billi F, Cho JS, Kremen TJ, Simon SI, et al. A mouse model of post-arthroplasty Staphylococcus aureus joint infection to evaluate in vivo the efficacy of antimicrobial implant coatings. PloS one. 2010;5(9):e12580 10.1371/journal.pone.0012580 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carli AV, Bhimani S, Yang X, Shirley MB, de Mesy Bentley KL, Ross FP, et al. Quantification of peri-implant bacterial load and in vivo biofilm formation in an innovative, clinically representative mouse model of periprosthetic joint infection. 10.2106/JBJS.16.00815 2017;99(6):e25 . [DOI] [PubMed] [Google Scholar]
- 16.Craig MR, Poelstra KA, Sherrell JC, Kwon MS, Belzile EL, Brown TEJ. A novel total knee arthroplasty infection model in rabbits. Journal of orthopaedic research. 2005;23(5):1100–4. 10.1016/j.orthres.2005.03.007 . [DOI] [PubMed] [Google Scholar]
- 17.Hegde V, Dworsky EM, Stavrakis AI, Loftin AH, Zoller SD, Park HY, et al. Single-dose, preoperative vitamin-D supplementation decreases infection in a mouse model of periprosthetic joint infection. JBJS. 2017;99(20):1737–44. 10.2106/JBJS.16.01598 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heim CE, Vidlak D, Scherr TD, Hartman CW, Garvin KL, Kielian TJ. IL-12 promotes myeloid-derived suppressor cell recruitment and bacterial persistence during Staphylococcus aureus orthopedic implant infection. The Journal of Immunology. 2015;194(8):3861–72. 10.4049/jimmunol.1402689 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner TM, Urban RM, Sumner DR, Skipor AK, Galante JOJ. Bone ingrowth into the tibial component of a canine total condylar knee replacement prosthesis. Journal of Orthopaedic Research. 1989;7(6):893–901. 10.1002/jor.1100070616 . [DOI] [PubMed] [Google Scholar]
- 20.Vidlak D, Kielian TJ. Infectious dose dictates the host response during Staphylococcus aureus orthopedic-implant biofilm infection. Infection and immunity. 2016;84(7):1957–65. 10.1128/IAI.00117-16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Thompson JM, Ashbaugh AG, Khodakivskyi P, Budin G, Sinisi R, et al. Preclinical evaluation of photoacoustic imaging as a novel noninvasive approach to detect an orthopaedic implant infection. The Journal of the American Academy of Orthopaedic Surgeons. 2017;25(Suppl 1):S7 10.5435/JAAOS-D-16-00630 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhi-Qiang Y LZ, Xin-Liang W, et al. The design of anatomic knee prostheses for rabbits with computer aided design. Guangdong Medical Journal. 2014;35(13):1997–2001. [Google Scholar]
- 23.Malhotra R, Dhawan B, Garg B, Shankar V, Nag TCJ. A comparison of bacterial adhesion and biofilm formation on commonly used orthopaedic metal implant materials: An In vitro study. Indian journal of orthopaedics. 2019;53(1):148 10.4103/ortho.IJOrtho_66_18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricciardi BF, Muthukrishnan G, Masters E, Ninomiya M, Lee CC, Schwarz EMJ. Staphylococcus aureus evasion of host immunity in the setting of prosthetic joint infection: biofilm and beyond. Current Reviews in Musculoskeletal Medicine. 2018;11(3):389–400. 10.1007/s12178-018-9501-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carli AV, Ross FP, Bhimani SJ, Nodzo SR, Bostrom MPJ. Developing a clinically representative model of periprosthetic joint infection. JBJS. 2016;98(19):1666–76. 10.2106/JBJS.15.01432 . [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Tay JHJ. Metabolic response of biofilm to shear stress in fixed‐film culture. Journal of applied microbiology. 2001;90(3):337–42. 10.1046/j.1365-2672.2001.01244.x . [DOI] [PubMed] [Google Scholar]
- 27.Singh AV, Vyas V, Patil R, Sharma V, Scopelliti PE, Bongiorno G, et al. Quantitative characterization of the influence of the nanoscale morphology of nanostructured surfaces on bacterial adhesion and biofilm formation. PloS one. 2011;6(9):e25029 10.1371/journal.pone.0025029 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conditt MA, Noble PC, Bertolusso R, Woody J, Parsley BSJ. The PCL significantly affects the functional outcome of total knee arthroplasty. The Journal of arthroplasty. 2004;19(7):107–12. 10.1016/j.arth.2004.06.006 . [DOI] [PubMed] [Google Scholar]
- 29.Teughels W, Van Assche N, Sliepen I, Quirynen MJ. Effect of material characteristics and/or surface topography on biofilm development. Clinical oral implants research. 2006;17(S2):68–81. 10.1111/j.1600-0501.2006.01353.x . [DOI] [PubMed] [Google Scholar]
- 30.Saleh-Mghir A, Muller-Serieys C, Dinh A, Massias L, Crémieux A-CJ. Adjunctive rifampin is crucial to optimizing daptomycin efficacy against rabbit prosthetic joint infection due to methicillin-resistant Staphylococcus aureus. Antimicrobial agents and chemotherapy. 2011;55(10):4589–93. 10.1128/AAC.00675-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bargon R, J, Carli A, Fabritius M, Goel R, Goswami K, et al. General Assembly, Research Caveats: Proceedings of International Consensus on Orthopedic Infections. The Journal of arthroplasty. 2019;34(2S):S245 10.1016/j.arth.2018.09.076 . [DOI] [PubMed] [Google Scholar]
- 32.Moher D, Liberati A, Tetzlaff J, Altman DGJ. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine. 2009;151(4):264–9. 10.7326/0003-4819-151-4-200908180-00135 . [DOI] [PubMed] [Google Scholar]
- 33.Stroke TAIRSJ. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30(12):2752 10.1161/01.str.30.12.2752 . [DOI] [PubMed] [Google Scholar]
- 34.Poultsides LA, Papatheodorou LK, Karachalios TS, Khaldi L, Maniatis A, Petinaki E, et al. Novel model for studying hematogenous infection in an experimental setting of implant‐related infection by a community‐acquired methicillin‐resistant S. aureus Strain. Journal of Orthopaedic Research. 2008;26(10):1355–62. 10.1002/jor.20608 . [DOI] [PubMed] [Google Scholar]
- 35.Yang X, Ricciardi BF, Dvorzhinskiy A, Brial C, Lane Z, Bhimani S, et al. Intermittent parathyroid hormone enhances cancellous osseointegration of a novel murine tibial implant. The Journal of bone joint surgery American volume. 2015;97(13):1074 10.2106/JBJS.N.01052 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zampelis V, Tägil M, Lidgren L, Isaksson H, Atroshi I, Wang J-SJ. The effect of a biphasic injectable bone substitute on the interface strength in a rabbit knee prosthesis model. Journal of orthopaedic surgery research. 2013;8(1):25 10.1186/1749-799X-8-25 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalteis T, Beckmann J, Schröder H-J, Schaumburger J, Linde H-J, Lerch K, et al. Treatment of implant-associated infections with moxifloxacin: an animal study. International journal of antimicrobial agents. 2006;27(5):444–8. 10.1016/j.ijantimicag.2005.12.003 . [DOI] [PubMed] [Google Scholar]
- 38.Petty W, Spanier S, Shuster J, Silverthorne CJ. The influence of skeletal implants on incidence of infection. Experiments in a canine model. The Journal of bone & joint surgery American volume. 1985;67(8):1236–44. . [PubMed] [Google Scholar]
- 39.Schurman DJ, Johnson B Jr, Amstutz HJ. Knee joint infections with Staphylococcus aureus and Micrococcus species. 1975;57(1):40–9. [PubMed] [Google Scholar]
- 40.Kussmann M, Obermueller M, Berndl F, Reischer V, Veletzky L, Burgmann H, et al. Dalbavancin for treatment of implant-related methicillin-resistant Staphylococcus aureus osteomyelitis in an experimental rat model. Scientific reports. 2018;8(1):9661 10.1038/s41598-018-28006-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lankinen P, Lehtimäki K, Hakanen AJ, Roivainen A, Aro HTJ. A comparative 18 F-FDG PET/CT imaging of experimental Staphylococcus aureus osteomyelitis and Staphylococcus epidermidis foreign-body-associated infection in the rabbit tibia. EJNMMI research. 2012;2(1):41 10.1186/2191-219X-2-41 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edelstein AI, Weiner JA, Cook RW, Chun DS, Monroe E, Mitchell SM, et al. Intra-articular vancomycin powder eliminates methicillin-resistant S. aureus in a rat model of a contaminated intra-articular implant. JBJS. 2017;99(3):232–8. 10.2106/JBJS.16.00127 [DOI] [PubMed] [Google Scholar]
- 43.Wijeyekoon S, Mino T, Satoh H, Matsuo TJ. Effects of substrate loading rate on biofilm structure. Water Research. 2004;38(10):2479–88. 10.1016/j.watres.2004.03.005 . [DOI] [PubMed] [Google Scholar]
- 44.Zhai H, Pan J, Pang E, Bai BJ. Lavage with allicin in combination with vancomycin inhibits biofilm formation by Staphylococcus epidermidis in a rabbit model of prosthetic joint infection. PLoS One. 2014;9(7):e102760 10.1371/journal.pone.0102760 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gatin L, Saleh-Mghir A, Tasse J, Ghout I, Laurent F, Crémieux A-CJ. Ceftaroline-Fosamil efficacy against methicillin-resistant Staphylococcus aureus in a rabbit prosthetic joint infection model. Antimicrobial agents and chemotherapy. 2014;58(11):6496–500. 10.1128/AAC.03600-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarda L, Saleh-Mghir A, Peker C, Meulemans A, Crémieux A-C, Le Guludec DJ. Evaluation of 99mTc-ciprofloxacin scintigraphy in a rabbit model of Staphylococcus aureus prosthetic joint infection. Journal of Nuclear Medicine. 2002;43(2):239–45. [PubMed] [Google Scholar]
- 47.Abu-Amer Y, Darwech I, Clohisy JCJ. Aseptic loosening of total joint replacements: mechanisms underlying osteolysis and potential therapies. Arthritis research & therapy. 2007;9(1):S6 10.1186/ar2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crook PD, Owen JR, Hess SR, Al-Humadi SM, Wayne JS, Jiranek WAJTJoa. Initial stability of cemented vs cementless tibial components under cyclic load. 2017;32(8):2556–62. 10.1016/j.arth.2017.03.039 . [DOI] [PubMed] [Google Scholar]
- 49.Harwin SF, Kester MA, Malkani AL, Manley MTJ. Excellent fixation achieved with cementless posteriorly stabilized total knee arthroplasty. The Journal of arthroplasty. 2013;28(1):7–13. 10.1016/j.arth.2012.06.006 . [DOI] [PubMed] [Google Scholar]
- 50.Park J-W, Kim Y-HJ. Simultaneous cemented and cementless total knee replacement in the same patients: a prospective comparison of long-term outcomes using an identical design of NexGen prosthesis. The Journal of bone joint surgery British volume. 2011;93(11):1479–86. 10.1302/0301-620X.93B11.27507 . [DOI] [PubMed] [Google Scholar]
- 51.Kendall RW, Duncan CP, Smith JA, Ngui-Yen JHJ. Persistence of bacteria on antibiotic loaded acrylic depots: a reason for caution. Clinical Orthopaedics & Related Research. 1996;329:273–80. 10.1097/00003086-199608000-00034 . [DOI] [PubMed] [Google Scholar]
- 52.Aalirezaie A, Abolghasemian M, Busato T, Dennis D, Ghazavi M, Holst DC, et al. Hip and Knee Section, Treatment, Two-Stage Exchange: Proceedings of International Consensus on Orthopedic Infections. The Journal of arthroplasty. 2019;34(2S):S439 10.1016/j.arth.2018.09.028 . [DOI] [PubMed] [Google Scholar]
- 53.Carli AV, Bhimani S, Yang X, de Mesy Bentley KL, Ross FP, Bostrom MPJ. Vancomycin-Loaded Polymethylmethacrylate Spacers Fail to Eradicate Periprosthetic Joint Infection in a Clinically Representative Mouse Model. JBJS. 2018;100(11):e76 10.2106/JBJS.17.01100 . [DOI] [PubMed] [Google Scholar]
- 54.Sorlí L, Puig L, Torres-Claramunt R, González A, Alier A, Knobel H, et al. The relationship between microbiology results in the second of a two-stage exchange procedure using cement spacers and the outcome after revision total joint replacement for infection: the use of sonication to aid bacteriological analysis. The Journal of bone joint surgery British volume. 2012;94(2):249–53. 10.1302/0301-620X.94B2.27779 . [DOI] [PubMed] [Google Scholar]
- 55.Sabanci SS, Ocal MKJ. Comparison of goniometric measurements of the stifle joint in seven breeds of normal dogs. Vet Comp Orthop Traumatol. 2016;29(03):214–9. 10.3415/VCOT-15-05-0090 . [DOI] [PubMed] [Google Scholar]
- 56.Tomlinson J, Fox D, Cook JL, Keller GGJ. Measurement of femoral angles in four dog breeds. Veterinary Surgery. 2007;36(6):593–8. 10.1111/j.1532-950X.2007.00309.x . [DOI] [PubMed] [Google Scholar]
- 57.Watson C, Rochat M, Payton MJ. Effect of weight bearing on the joint angles of the fore-and hind limb of the dog. Veterinary and Comparative Orthopaedics and Traumatology. 2003;16(04):250–4. [Google Scholar]
- 58.Thomas TM, Marcellin-Little DJ, Roe SC, Lascelles BDX, Brosey BPJ. Comparison of measurements obtained by use of an electrogoniometer and a universal plastic goniometer for the assessment of joint motion in dogs. American journal of veterinary research. 2006;67(12):1974–9. 10.2460/ajvr.67.12.1974 . [DOI] [PubMed] [Google Scholar]
- 59.Maeda E, Noguchi H, Tohyama H, Yasuda K, Hayashi KJ. Biomechanical study of healing of patellar tendon after resection of the central one-third in an adult-mature rabbit model. Bio-medical materials and engineering. 2013;23(3):173–81. 10.3233/BME-130742 . [DOI] [PubMed] [Google Scholar]
- 60.Spekenbrink-Spooren A, Van Steenbergen LN, Denissen GA, Swierstra BA, Poolman RW, Nelissen RGJ. Higher mid-term revision rates of posterior stabilized compared with cruciate retaining total knee arthroplasties: 133,841 cemented arthroplasties for osteoarthritis in the Netherlands in 2007–2016. Acta orthopaedica. 2018;89(6):640–5. 10.1080/17453674.2018.1518570 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kolisek FR, McGrath MS, Marker DR, Jessup N, Seyler TM, Mont MA, et al. Posterior-stabilized versus posterior cruciate ligament-retaining total knee arthroplasty. The Iowa orthopaedic journal. 2009;29:23 [PMC free article] [PubMed] [Google Scholar]
- 62.Karbysheva S, Grigoricheva L, Golnik V, Popov S, Renz N, Trampuz AJ. Influence of retrieved hip-and knee-prosthesis biomaterials on microbial detection by sonication. European cells materials. 2019;37:16–22. 10.22203/eCM.v037a02 . [DOI] [PubMed] [Google Scholar]