Abstract
Background
It remains unknown which is the optimal first-line treatment regimen for patients with advanced epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (NSCLC). We performed a network meta-analysis to address this important issue.
Methods
PubMed, Embase, Cochrane Library, Web of Science and major international scientific meetings were searched for relevant randomized controlled trials (RCTs). Progression-free survival (PFS) data was the primary outcome of interest, and overall survival (OS) and serious adverse events (SAEs) were the secondary outcomes of interests, reported as hazard ratio (HR) or odds ratio (OR) and 95% confidence intervals (CIs).
Results
25 RCTs with a total of 5005 patients randomized to receive seven treatments were included in the meta-analysis. Third-generation tyrosine kinase inhibitor (TKI) (osimertinib) and first-generation TKIs (F-TKIs) in combination with chemotherapy (F-TKIs+CT) were more effective than F-TKIs alone in terms of PFS (HR = 0.46, 95% CI: 0.22–0.93; P = 0.031 and HR = 0.62, 95% CI: 0.39–0.98; P = 0.041) and OS (HR = 0.63, 95% CI: 0.43–0.91; P = 0.014 and HR = 0.73, 95% CI: 0.57–0.92; P = 0.008). Second-generation TKIs (S-TKIs) showed significant OS advantage over F-TKIs (HR = 0.83, 95% CI: 0.70–0.99; P = 0.04). Based on treatment ranking in terms of PFS and OS, osimertinib had the highest probability of being the most effective treatment (89% and 86%) and with the best tolerability. F-TKIs+CT was ranked the second-most effective regimen, but with relatively high risk of SAEs.
Conclusions
Osimertinib seemed to be the most preferable first-line treatment in advanced EGFR-mutated NSCLC. However, limitations of the study including a single RCT investigating osimertinib and immature OS data need to be considered.
Introduction
Non-small cell lung cancer (NSCLC) remains the leading cause of cancer-associated mortality globally [1–2], and approximately 15% to 50% of NSCLC patients have an activating epidermal growth factor receptor (EGFR) mutation [3]. First-generation tyrosine kinase inhibitors (F-TKIs) (gefitinib, erlotinib, or icotinib) have consistently shown a progression-free survival (PFS) benefit compared to chemotherapy (CT) in first-line of treatment of advanced EGFR-mutated NSCLC [4–10]. Recently, the survival differences between F-TKIs, second-generation TKIs (S-TKIs) (afatinib or dacomitinib), and third-generation TKI (osimertinib) have been investigated in a number of trials [11–15]. Most of the trials have demonstrated the benefit of S-TKIs and osimertinib over F-TKIs in previously treated advanced EGFR-mutated NSCLC. Promising results also have been reported for F-TKIs in combination with CT (F-TKIs+CT) [16–19] or Bevacizumab (F-TKIs+Bev) [20–22]. However, direct comparison trials between S-TKIs, T-TKIs, and combination regimens involving TKIs are still lacking, and therefore, there are still unresolved questions around which is the optimal first-line treatment for patients with advanced EGFR-mutated NSCLC.
Two previous network meta-analyses [23–24] have evaluated first-line treatments in advanced EGFR-mutated NSCLC. However, first-line treatment of osimertinib has not been assessed in Lin et al’ study [23], while first-line treatment of combination regimens involving TKIs has not been evaluated in Batson et al’ study [24]. Moreover, since the two meta-analyses, several available RCTs [19, 22, 25] have been newly published. Thus, we performed a novel network meta-analysis, attempting to identify the most preferable first-line treatment regimen in patients with advanced EGFR-mutated NSCLC.
Methods
This study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria [26] (S1 Table).
Literature search strategy
Two investigators independently searched PubMed, Embase, Cochrane Library, Web of Science, and major international scientific meetings (American Society of Clinical Oncology, European Society for Medical Oncology, and World Conference on Lung Cancer) for the available trials published before March 1, 2019. The detailed strategies are shown in S2 Table.
Inclusion and exclusion criteria
Studies meeting the following criteria were included: (1) types of studies: randomized controlled trials (RCTs); (2) types of participants: advanced EGFR-mutated NSCLC patients; (3) types of interventions: at least one intervention was a TKI (F-TKIs, S-TKIs, or osimertinib), alone or in combination with other types of treatments; and (4) outcome: reported PFS or overall survival (OS) data. Studies which failed to meet the above criteria were excluded from the network meta-analysis.
Data extraction
Two investigators extracted the following data from each study independently: first author or RCT name, year of publication, duration of RCT, region, interventions, numbers of patients, hazard ratios (HRs) and their 95% confidence intervals (CIs) of PFS and OS, and odds ratios (ORs) and their 95% CIs of serious adverse events (SAEs). Crude HRs with 95% CIs for PFS and OS were either extracted directly from the original reports or calculated by the Kaplan–Meier curves based on the methods of Parmar et al. [27] and Tierney et al. [28]. Data on the overall numbers of patients with SAEs were directly extracted if they were reported in the published article. If only the numbers of individual SAEs were reported separately in articles, we pooled all numbers of them to represent the overall numbers of SAEs.
Quality assessment
Cochrane risk of bias tool [29] was used to evaluate the methodological quality of RCTs, which includes the following five domains: sequence generation, allocation concealment, blinding, incomplete data, and selective reporting. A RCT was rated as “low risk of bias” if all key domains indicated as low risk, was rated as “high risk of bias” if one or more key domains indicated as high risk, and was judged to be “unclear risk of bias” when more than three domains indicated as unclear risk.
Statistical analysis
The primary outcome was PFS, and the secondary outcomes were OS and SAEs. For direct comparisons, standard pairwise meta-analysis (PWMA) was performed. The heterogeneity between studies was assessed by chi-square (χ2) and I-square (I2) tests. A P value <0.10 or I2 > 50% was considered significant heterogeneity existing, and a random-effects analysis model was used; otherwise, a fixed-effects model was used. PWMA was performed using the software Review Manager 5.3 (Cochrane Collaboration, Oxford, UK).
The Bayesian network-meta analysis (NMA) for all outcomes were performed in a random-effect model [Generalized Linear Model (GLM)] using Markov chain Monte Carlo methods [30–31] in JAGS and the GeMTC package in R (https://drugis.org/software/r-packages/gemtc). OS and PFS were analyzed with GLM with a normal likelihood incorporating log hazard ratio statistics from individual trials to calculate HR between competing treatments. Count statistics of SAEs was analyzed with GLM with a binomial likelihood to calculate relative treatment effects expressed as OR between different treatments. For each outcome measure, four independent Markov chains were simultaneously run for 20,000 burn-ins and 100,000 inference iterations per chain to obtain the posterior distribution. The traces plot and Brooks-Gelman-Rubin method were used to assess the convergence of model [32]. Treatment effects were estimated by HR/OR and corresponding 95% CI. Network consistency was assessed with node-split models by statistically testing between direct and indirect estimates within treatment loop [33]. To rank probabilities of all available treatments, the surfaces under the cumulative ranking curve (SUCRAs) were calculated [34]. SUCRA equals one if the treatment is certain to be the best and zero if it’s certain to be the worst [34]. To jointly compare the efficacy and tolerability of each treatment and to assess their benefit-risk ratios, we ranked them based simultaneously on the SUCRA value of PFS and tolerability (1-SUCRASAEs) in the ranking plot. Lastly, comparison-adjusted funnel plot was used to detect the presence of small-study effects or publication bias.
Results
Literature search results and characteristics of included studies
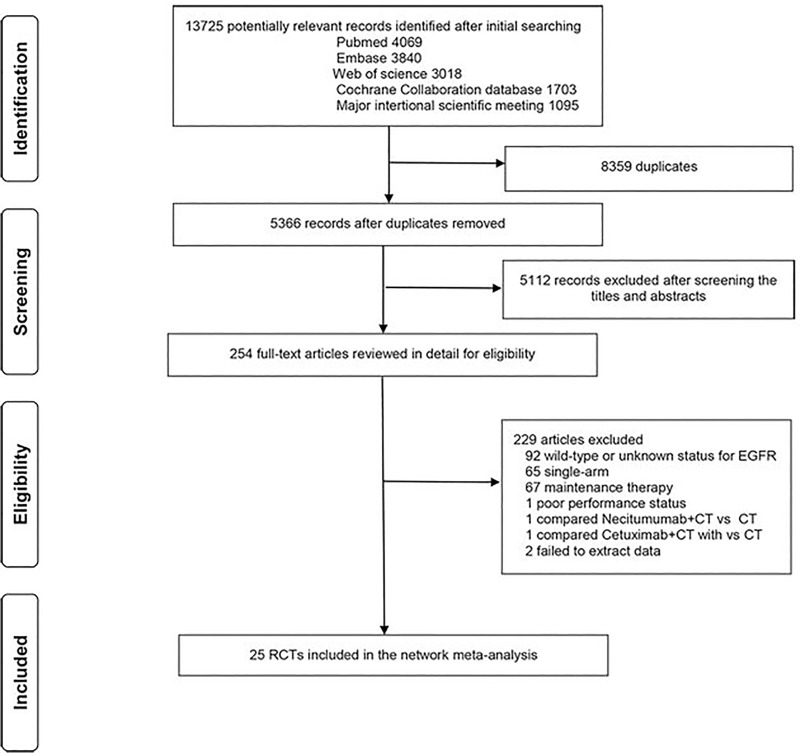
Fig 1 shows the flow diagram for study selection. The preliminary literature search identified 13725 studies. 13471 of them were excluded after removing the duplicates and an abstract review. The remaining 254 studies were screened through a full-text review for further eligibility. All relevant references were also reviewed. Finally, 25 RCTs [4–22, 25, 35–47] with 5005 patients were included in the network meta-analysis and compared seven treatments including F-TKIs, S-TKIs, osimertinib, CT, F-TKIs+CT, F-TKIs+Bev, and F-TKIs+Linsitinib (F-TKIs+Lin). The clinical characteristics are shown in Table 1.
Fig 1. Literature search and selection.
Table 1. Clinical characteristics of included trials.
| Trial | Design | Time | Region | Primary | Treatment | Sample | Median follow-up |
|---|---|---|---|---|---|---|---|
| Range | Endpoint | Size | (months) | ||||
| NEJ002/2010[4–5] | III | 2006–2009 | Multicenter | PFS | F-TKIs | 114 | >17 |
| CT | 114 | ||||||
| WJTOG3405/2010[6–7] | III | 2006–2009 | Japan | PFS | F-TKIs | 86 | 34 |
| CT | 86 | ||||||
| EURTAC/2012[8] | III | 2007–2011 | Multicenter | PFS | F-TKIs | 86 | 18.9 |
| CT | 87 | 14.4 | |||||
| OPTIMAL/2011[9–10] | III | 2011–2014 | China | PFS | F-TKIs | 82 | 25.9 |
| CT | 72 | ||||||
| LUX-Lung7/2016[11–12] | II | 2011–2013 | Multicenter | PFS | S-TKIs | 160 | 42.6 |
| F-TKIs | 159 | ||||||
| ARCHER1050/2017[13–14] | III | 2013–2015 | Asian | PFS | S-TKIs | 227 | 22.1 |
| F-TKIs | 225 | ||||||
| FLAURA/2017[15] | III | 2014–2016 | Multicenter | PFS | Osimertinib | 279 | 15 |
| F-TKIs | 277 | 9.7 | |||||
| FASTACT-2/2013[16] | II | 2009–2010 | Asian | PFS | F-TKIs+CT | 49 | 27.6 |
| CT | 48 | ||||||
| Yu/2014[17] | II | 2010–2012 | China | ORR | F-TKIs+CT | 14 | NR |
| CT | 18 | ||||||
| Cheng/2016[18] | II | 2012–2013 | Asian | PFS | F-TKIs+CT | 126 | NR |
| F-TKIs | 65 | ||||||
| NEJ009/2018[19] | III | 2011–2014 | Japan | PFS | F-TKIs+CT | 172 | NR |
| F-TKIs | 170 | ||||||
| JO25567/2014[20–21] | II | 2011–2012 | Japan | PFS | F-TKIs+Bev | 75 | 25.9 |
| F-TKIs | 77 | 27 | |||||
| NEJ026/2018[22] | III | NR | Japan | PFS | F-TKIs+Bev | 112 | 12.4 |
| F-TKIs | 112 | ||||||
| CONVINCE/2017[25] | III | 2013–2014 | China | PFS | F-TKIs | 148 | 18 |
| CT | 137 | 15.7 | |||||
| IPASS/2009[35–36] | III | 2005–2008 | Asian | PFS | F-TKIs | 132 | 17 |
| CT | 129 | ||||||
| TORCH/2012[37] | III | 2006–2009 | Italy, Canada | OS | F-TKIs | 19 | 24.3 |
| CT | 20 | ||||||
| Chen/2012[38] | II | 2007–2008 | China | PFS | F-TKIs | 9 | NR |
| CT | 15 | ||||||
| ENSURE/2015[39] | III | 2011–2012 | Asian | PFS | F-TKIs | 110 | 28.9 |
| CT | 107 | 27.1 | |||||
| Han/2012[40] | III | 2005–2007 | Korea | OS | F-TKIs | 26 | 35 |
| CT | 16 | ||||||
| LUX-Lung3/2013[41–42] | III | 2009–2011 | Multicenter | PFS | S-TKIs | 230 | 41 |
| CT | 115 | ||||||
| LUX-Lung6/2014[42–43] | III | 2010–2011 | Asian | PFS | S-TKIs | 242 | 33 |
| CT | 122 | ||||||
| Hirsch/2011[44] | II | 2007–2008 | Multicenter | PFS | F-TKIs+CT | 6 | NR |
| F-TKIs | 9 | ||||||
| CALGB30406/2012[45] | II | 2005–2009 | United States | PFS | F-TKIs+CT | 33 | 38 |
| F-TKIs | 33 | ||||||
| TRIBUTE/2005[46] | III | 2001–2002 | United States | OS | F-TKIs+CT | 93 | NR |
| CT | 74 | NR | |||||
| Leighl/2017[47] | II | NR | Multicenter | PFS | F-TKIs+Lin | 44 | NR |
| F-TKIs | 44 |
Abbreviations: PFS, progression-free survival; OS, overall survival; ORR, overall response rate; NR, not reported; TKIs, tyrosine kinase inhibitor; F, first-generation; S, second-generation; Bev, bevacizumab; CT, chemotherapy; Lin, Linsitinib.
Assessment of included trial
The demographic characteristics of involved patients were generally well-balanced between different trials and different arms within each trial (see S3 Table). Median age ranged from 56 to 68 years. 22–44% of patients were male; and 0–14% were with squamous cell carcinoma. Most of patients were with Stage IV disease (73.2–100%), except patients included in WJTOG3405 trial (47.7%) [6–7]. EGFR mutations were mainly exon 19 deletions and 21 deletions mutations. In eleven studies, details of baseline demographic characteristics were not stated [16–17, 35–38, 40, 44–46]. The risk of bias in included RCTs was summarized in S1 Fig. Four RCTs [4–5, 17, 19, 22] were judged to be unclear risk of bias, as they had more than three domains indicating as unclear risk. The remaining RCTs were judged to be low risk of bias. No trial was rated with a high risk of bias. Funnel plot analysis in term of PFS did not indicate any evident risk of publication bias (S2 Fig).
Conventional pairwise meta-analysis
Results of individual trials are shown in S4 table. Results of PWMA are shown in Table 2. In terms of PFS, S-TKIs (HR = 0.65, 95% CI: 0.54–0.77, Pheterogeneity = 0.23), F-TKIs+CT (HR = 0.57, 95% CI: 0.47–0.70, Pheterogeneity = 0.22), and F-TKIs+Bev (HR = 0.56, 95% CI: 0.43–0.74, Pheterogeneity = 0.55) were more effective than F-TKIs. With regard to OS, S-TKIs showed significant advantage over F-TKIs (HR = 0.81, 95% CI: 0.67–0.97, Pheterogeneity = 0.52). As for overall SAEs, S-TKIs (OR = 2.29, 95% CI: 1.69–3.12, Pheterogeneity = 0.58), F-TKIs+CT (OR = 3.79, 95% CI: 2.58–5.56, Pheterogeneity = 0.58), and F-TKIs+Bev (OR = 4.05, 95% CI: 1.04–15.86, Pheterogeneity = 0.009) were more likely to cause SAEs than F-TKIs.
Table 2. Results of direct comparisons.
| Outcome | Treatment | No. of | No. of | HR/OR(95%CI) | Heterogeneity | |
|---|---|---|---|---|---|---|
| studies | patients | I2 | P | |||
| PFS | S-TKIs vs F-TKIs | 2 | 771 | HR 0.65(0.54–0.77) | 31% | 0.23 |
| F-TKIs+CT vs F-TKIs | 4 | 614 | HR 0.57(0.47–0.70) | 32% | 0.22 | |
| F-TKIs+Bev vs F-TKIs | 2 | 376 | HR 0.56(0.43–0.74) | 0 | 0.55 | |
| F-TKIs vs CT | 10 | 1595 | HR 0.46(0.29–0.72) | 93% | <0.001 | |
| F-TKIs+CT vs CT | 2 | 129 | HR 0.24(0.16–0.37) | 0 | 0.75 | |
| S-TKIs vs CT | 2 | 709 | HR 0.40(0.20–0.83) | 90% | 0.001 | |
| OS | S-TKIs vs F-TKIs | 2 | 771 | HR 0.81(0.67–0.97) | 0 | 0.52 |
| F-TKIs vs CT | 9 | 1423 | HR 1.01(0.88–1.15) | 0 | 0.87 | |
| S-TKIs vs CT | 2 | 709 | HR 0.91(0.74–1.10) | 0 | 0.78 | |
| F-TKIs+CT vs F-TKIs | 2 | 408 | HR 0.70(0.54–0.92) | 0 | 0.93 | |
| F-TKIs+CT vs CT | 2 | 264 | HR 0.71(0.35–1.46) | 78% | 0.03 | |
| SAEs | S-TKIs vs F-TKIs | 2 | 771 | OR 2.29(1.69–3.12) | 0 | 0.58 |
| F-TKIs+CT vs F-TKIs | 2 | 533 | OR 3.79(2.58–5.56) | 0 | 0.58 | |
| F-TKIs+Bev vs F-TKIs | 2 | 376 | OR 4.05(1.04–15.86) | 85% | 0.009 | |
| F-TKIs vs CT | 6 | 1229 | OR 0.30(0.21–0.43) | 48% | 0.09 | |
| S-TKIs vs CT | 2 | 709 | OR 0.68(0.21–2.22) | 92% | <0.001 | |
Abbreviations: No., number; HR, hazard ratio; CI, confidence interval; OR, odds ratio; PFS, progression-free survival; OS, overall survival; SAEs, serious adverse events; TKIs, tyrosine kinase inhibitor; F, first-generation; S, second-generation; Bev, bevacizumab; CT, chemotherapy.
Network meta-analysis
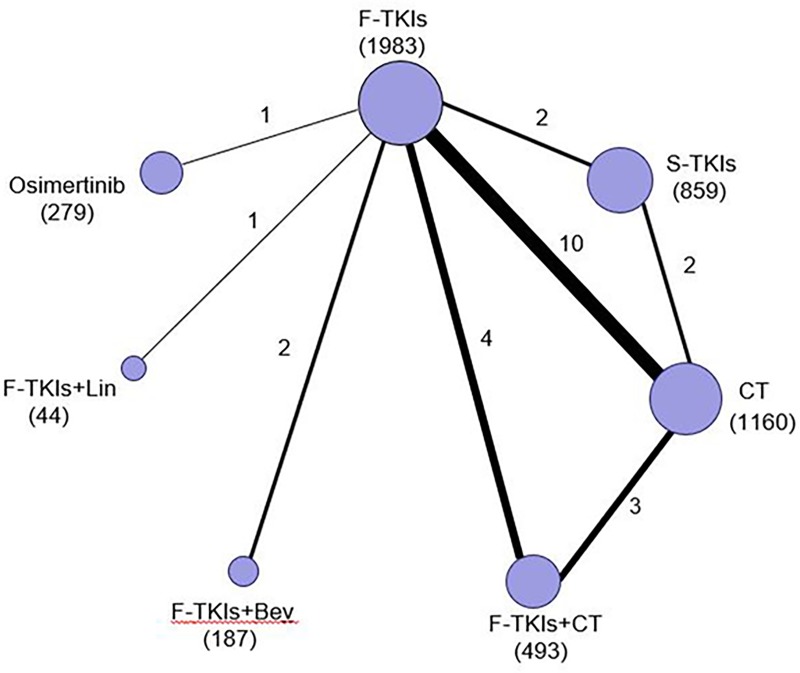
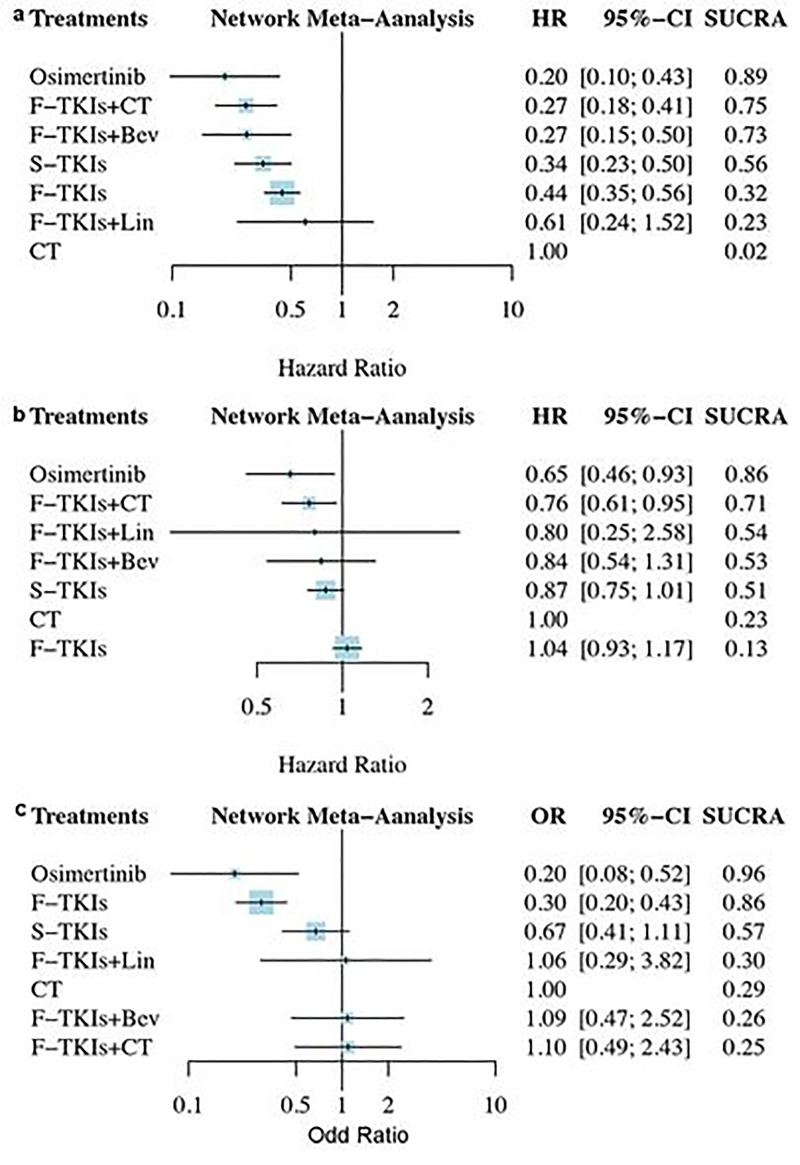
The network plot established for NMA is shown in Fig 2. Results of the NMA were presented in Table 3. Osimertinib and F-TKIs+CT were more effective than F-TKIs in terms of PFS (HR = 0.46, 95% CI: 0.23–0.93; P = 0.031 and HR = 0.62, 95% CI: 0.39–0.98; P = 0.041) and OS (HR = 0.63, 95% CI: 0.43–0.91; P = 0.014 and HR = 0.73, 95% CI: 0.57–0.92; P = 0.008). S-TKIs showed significant OS advantage over F-TKIs (HR = 0.83, 95% CI: 0.70–0.99; P = 0.04). Other comparisons among TKIs based regimens in terms of PFS or OS did not produce statistically significant differences. With regard to overall SAEs, osimertinib showed significantly lower risk of causing SAEs in comparison to each TKIs based regimens except F-TKIs; F-TKIs had significantly lower risk of causing SAEs than each TKIs based regimens except osimertinib. No significant differences were observed in other comparisons between TKIs based regimens in terms of SAEs.
Fig 2. Network of eligible comparisons.
The size of the nodes is proportional to the number of patients (in parentheses) randomized to receive the treatment. The width of the lines is proportional to the number of trials (beside the line) comparing the connected treatments. TKIs, tyrosine kinase inhibitor; F, first-generation; S, second-generation; Bev, bevacizumab; CT, chemotherapy; Lin, Linsitinib.
Table 3. Results of network meta-analysis.
| a. Hazard ratios(HR) with 95% confidence interval (CI) for progression-free survival (PFS) | ||||||
| Osimertinib | ||||||
| 0.75(0.28–2.0) | F-TKIs+CT | |||||
| 0.74(0.25–2.3) | 0.99(0.45–2.2) | F-TKIs+Bev | ||||
| 0.60(0.22–1.6) | 0.80(0.43–1.5) | 0.80(0.36–1.8) | S-TKIs | |||
| 0.46(0.23–0.93) | 0.62(0.39–0.98) | 0.62(0.32–1.2) | 0.77(0.48–1.2) | F-TKIs | ||
| 0.34(0.09–1.3) | 0.45(0.15–1.4) | 0.46(0.13–1.5) | 0.57(0.18–1.7) | 0.73(0.26–2.0) | F-TKIs+Lin | |
| 0.20(0.10–0.43) | 0.27(0.18–0.41) | 0.27(0.15–0.50) | 0.34(0.23–0.50) | 0.44(0.35–0.56) | 0.61(0.24–1.52) | CT |
| b. Hazard ratios(HR) with 95% confidence interval (CI) for overall survival(OS) | ||||||
| Osimertinib | ||||||
| 0.87(0.56–1.3) | F-TKIs+CT | |||||
| 0.78(0.43–1.4) | 0.90(0.54–1.5) | F-TKIs+Bev | ||||
| 0.76(0.50–1.1) | 0.87(0.66–1.2) | 0.97(0.60–1.6) | S-TKIs | |||
| 0.63(0.43–0.91) | 0.73(0.57–0.92) | 0.81(0.51–1.3) | 0.83(0.70–0.99) | F-TKIs | ||
| 0.82(0.25–2.8) | 0.95(0.29–3.1) | 1.1(0.31–3.7) | 1.1(0.34–3.5) | 1.3(0.42–4.2) | F-TKIs+Lin | |
| 0.65(0.46–0.93) | 0.76(0.61–0.95) | 0.84(0.54–1.31) | 0.87(0.75–1.01) | 1.04(0.93–1.17) | 0.80(0.25–2.58) | CT |
| c. Odds ratios (OR) with 95% confidence interval (CI) for serious adverse events (SAEs) | ||||||
| Osimertinib | ||||||
| 0.18(0.06–0.56) | F-TKIs+CT | |||||
| 0.18(0.06–0.58) | 1.01(0.36–2.82) | F-TKIs+Bev | ||||
| 0.30(0.11–0.81) | 1.62(0.69–3.84) | 1.61(0.65–3.97) | S-TKIs | |||
| 0.67(0.28–1.61) | 3.68(1.83–7.41) | 3.65(1.72–7.74) | 2.27(1.38–3.73) | F-TKIs | ||
| 0.19(0.04–0.85) | 1.03(0.25–4.23) | 1.03(0.24–4.31) | 0.64(0.17–2.39) | 0.28(0.08–0.95) | F-TKIs+Lin | |
| 0.20(0.08–0.52) | 1.10(0.49–2.43) | 1.09(0.47–2.52) | 0.67(0.41–1.11) | 0.30(0.20–0.43) | 1.06(0.29–3.82) | CT |
Abbreviations: For survival outcomes (OS, PFS), an HR below 1 favors the column-defining treatment. For safety (SAEs), an OR below 1 favors the column-defining treatment. Comparisons with differences of statistical significance (p<0.05) are highlighted in bold format. TKIs, tyrosine kinase inhibitor; F, first-generation; S, second-generation; Bev, bevacizumab; CT, chemotherapy; Lin, Linsitinib.
Inconsistency assessment and treatment ranking
There were two independent closed loops in the network for PFS or OS: F-TKIs/S-TKIs/CT and F-TKIs/F-TKIs+CT/CT; one independent closed loop for SAEs: F-TKIs/S-TKIs/CT. Analysis of inconsistency showed that the NMA results were similar to the PWMA results for the three outcomes, which suggested the consistency between the direct and indirect evidence (S3 Fig).
The treatment rankings based on SUCRA are shown in Fig 3. In term of PFS (Fig 3A), osimertinib was the most effective treatment (0.89), followed by F-TKIs+CT (0.75), F-TKIs+Bev (0.73), and S-TKIs (0.56). With regard to OS (Fig 3B), osimertinib was still the most effective treatment (0.86), followed by F-TKIs+CT (0.71), F-TKIs+Lin (0.54), and F-TKIs+Bev (0.53). As for SAEs (Fig 3C), osimertinib was ranked as the least toxic regimen (0.96), followed by F-TKIs (0.86) and S-TKIs (0.57); F-TKIs+CT (0.25) was ranked as the highest toxic regimen.
Fig 3. The treatment rankings based on SUCRA.
(a) progression-free survival; (b) overall survival; (c) serious adverse events. SUCRA, surface under the cumulative ranking curves; TKIs, tyrosine kinase inhibitor; F, first-generation; S, second-generation; Bev, bevacizumab; CT, chemotherapy; Lin, Linsitinib.
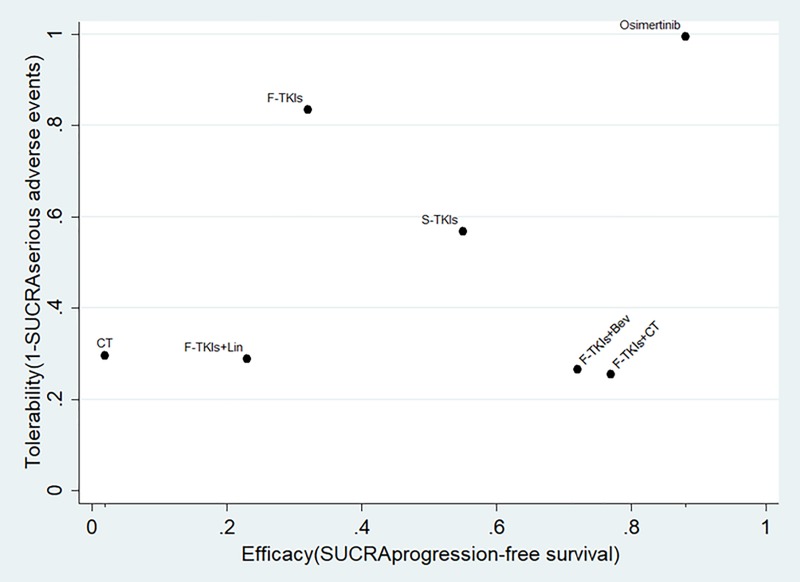
To further assess the benefit-risk ratios of the seven treatments simultaneously, we ranked them based on the SUCRA values of PFS and tolerability (31-SUCRA SAE) in the ranking plot (Fig 4). Osimertinib was likely to be the optimal treatment because it had the most efficacy and best tolerability. F-TKIs+CT and F-TKIs+Bev were also two more effective regimens, but with relatively high risk of causing SAEs. S-TKIs achieved relatively good efficacy with moderate tolerability.
Fig 4. Ranking plot based simultaneously on efficacy (x-axis: SUCRA value of overall survival) and tolerability (y-axis: 1-SUCRA value of serious adverse events).
SUCRA, surface under the cumulative ranking curves; TKIs, tyrosine kinase inhibitor; F, first-generation; S, second-generation; Bev, bevacizumab; CT, chemotherapy; Lin, Linsitinib.
Discussion
This novel network meta-analysis assessed the comparative efficacy and tolerability of all major TKIs based first-line treatments (including first-, second-, third-generation TKIs, and combination regimens involving TKIs) in advanced EGFR-mutated NSCLC. It showed that osimertinib provided significant PFS and OS advantage over F-TKIs, and had the highest probability of being the most effective treatment in improving PFS and OS, and with the best tolerability. In the FLAURA study [15] comparing first-line osimertinib with gefitinib or erlotinib in patients with advanced EGFR-mutated NSCLC, the median PFS (mPFS) was significantly longer with osimertinib than with standard F-TKIs (18.9 versus 10.0 months, P < 0.001). Overall SAEs were less frequent with osimertinib than with F-TKIs (34% vs. 45%).
F-TKIs+CT and F-TKIs+Bev were another two more effective regimens in our NMA. Based on treatment ranking, they were ranked second- and third- most effective regimen respectively, but with relatively high risk of causing SAEs. The NEJ009 study, a phase III trial evaluated the efficacy of a combination of gefitinib and CT in advanced NSCLC patients with EGFR mutations [18]. The combination arm demonstrated significantly improved mPFS (20.9 versus 11.2 months, P < 0.001) and median OS (mOS) (52.2 versus 38.8 months, P = 0.013) compared with gefitinib alone arm. A phase II study [20–21] comparing combination of erlotinib and bevacizumab with erlotinib alone in this patient population reported results with significant benefit on mPFS (16.4 versus 9.8 months, P = 0.0005). Similar improved mPFS was also observed in a phase III trial (NEJ026) [22] (16.9 months in erlotinib+bevacizumab arm versus 13.3 months in erlotinib alone arm, P < 0.001). Although both the two combination regimens were associated with higher incidence of grade 3 toxicities, few patients required dose reduction or withdrawal.
The survival difference between F-TKIs and S-TKIs has been investigated in two trials. LUX-LUNG 7 trial [11–12] showed no clinically meaningful survival benefit with afatinib versus with gefitinib in patients with advanced EGFR-mutated NSCLC. However, in another phase III trial [13–14] comparing dacomitinib with gefitinib in patients with EGFR-mutated NSCLC without brain metastases, dacomitinib was associated with significant improvement in mPFS (14.7 versus 9.2 months, P < 0.001) and mOS (34.1 months versus 26.8 months, P = 0.044) compared with gefitinib, but with increased grade 3 toxicities. In our NMA, S-TKIs was ranked fourth-most effective regimen with moderate risk of causing SAEs.
Based on the findings of this NMA, osimertinib seemed to be the preferable first-line treatment for patients with advanced EGFR-mutated NSCLC. However, the OS data were immature in the FLAURA study [15]. The survival rate at 18 months was not significantly longer with osimertinib than with F-TKIs (83% versus 71%) in the interim analysis [15]. More recently, postprogression outcomes of the FLAURA study have been reported [48]. Median second PFS was not reached [95% CI, 23.7-not calculable (NC)] in the osimertinib arm and 20.0 months (95% CI, 18.2-NC) in the standard-of-care (SoC) EGFR-TKI (gefitinib or erlotinib) arm [HR = 0.58, 95% CI: 0.44–0.78; P = 0.0004]. This suggested that osimertinib preserved clinical benefit after first progression. Moreover, median time to discontinuation of any EGFR-TKI or death was 23.0 months (95% CI, 19.5-NC) in the osimertinib arm and 16.0 months (95% CI, 14.8–18.6) in the SoC EGFR-TKI arm. These exploratory postprogression outcomes showed consistent improvements of osimertinib compared to SoC EGFR-TKI, and provide further confidence in the interim OS data.
There are several limitations in this network meta-analysis. First, in common with other meta-analyses, data were collected and analyzed basis of results reported from trials, and not on individual patient data. Therefore, effects of potential prognostic factors could not be accounted for. Second, most of included trials reported an immature OS data, and follow-up times across trials were different and generally short. Moreover, only one RCT investigated efficacy of osimertinib vs F-TKIs. These limitations do not allow us to reach a definitive conclusion about the superiority of one treatment over another. Third, this network meta-analysis included most of RCTs with Asians (15/25). We combined treatment effects for Asians and other groups assuming that there is no racial difference in the treatment effects. However, there was still no evidence supporting that the effect of TKIs among Asians is comparable to that among other racial groups, and we could not investigate such a racial difference in the treatment effect using this network meta-analysis data. Fourth, TKIs efficacy may be associated with patient characteristics (such as gender, race, and smoking status), tumor pathology, EGFR mutation types, and developing brain metastasis or not. However, we could not perform the subgroup-analyses because of insufficient data in the individual trials. Finally, some HRs of PFS or OS were calculated from the Kaplan–Meier curve due to that they were not directly reported in the articles. This may result in bias.
Conclusions
Osimertinib seemed to be the most preferable first-line treatment in advanced EGFR-mutated NSCLC. However, limitations of the study including a single RCT investigating osimertinib and immature OS data need to be considered.
Supporting information
A: Methodological quality graph: authors’ judgment about each methodological quality item presented as percentages across all included studies; B: Methodological quality summary: authors’ judgment about each methodological quality item for each included study, “+” low risk of bias; “?” unclear risk of bias; “-” high risk of bias.
(TIF)
TKIs, tyrosine kinase inhibitor; F, first-generation; S, second-generation; Bev, bevacizumab; CT, chemotherapy; Lin, Linsitinib.
(TIF)
(a) progression-free survival; (b) overall survival; (c) serious adverse events. TKIs, tyrosine kinase inhibitor; F, first-generation; S, second-generation; CT, chemotherapy.
(TIF)
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Non-small cell lung cancer, version 2.2019 (November 21, 2018). Available from: https://www.nccn.org/professionals/physician_gls [Google Scholar]
- 2.De Marinis F, Ciardiello F, Baas P, Crinò L, Giaccone G, Grossi F, et al. 30 Immunotherapy in advanced NSCLC-from the 'tsunami' of therapeutic knowledge to a clinical practice algorithm: results from an international expert panel meeting of the Italian Association of Thoracic Oncology (AIOT). ESMO Open. 2018;3: e000298 10.1136/esmoopen-2017-000298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reck M, Rabe KF. Precision diagnosis and treatment for advanced nonsmall- cell lung cancer. N Engl J Med. 2017;377: 849–861. 10.1056/NEJMra1703413 [DOI] [PubMed] [Google Scholar]
- 4.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. ; North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362: 2380–2388. 10.1056/NEJMoa0909530 [DOI] [PubMed] [Google Scholar]
- 5.Inoue A, Kobayashi K, Maemondo M, Sugawara S, Oizumi S, Isobe H, et al. ; North-East Japan Study Group. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol. 2013;24: 54–59. 10.1093/annonc/mds214 [DOI] [PubMed] [Google Scholar]
- 6.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. ; West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11: 121–128. 10.1016/S1470-2045(09)70364-X [DOI] [PubMed] [Google Scholar]
- 7.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Seto T, et al. Updated overall survival results of WJTOG 3405, a randomized phase III trial comparing gefitinib (G) with cisplatin plus docetaxel (CD) as the first-line treatment for patients with non-small cell lung cancer harboring mutations of the epidermal growth factor receptor (EGFR). Journal of Clinical Oncology. 2012;30(15 SUPPL.1). [Google Scholar]
- 8.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. ; Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13: 239–246. 10.1016/S1470-2045(11)70393-X [DOI] [PubMed] [Google Scholar]
- 9.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as firstline treatment for patients with advanced EGFR mutation-positive nonsmall-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, openlabel, randomised, phase 3 study. Lancet Oncol. 2011;12: 735–742. 10.1016/S1470-2045(11)70184-X [DOI] [PubMed] [Google Scholar]
- 10.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol. 2015;26: 1877–1883. 10.1093/annonc/mdv276 [DOI] [PubMed] [Google Scholar]
- 11.Park K, Tan EH, O'Byrne K, Zhang L, Boyer M, Mok T, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17: 577–589. 10.1016/S1470-2045(16)30033-X [DOI] [PubMed] [Google Scholar]
- 12.Paz-Ares L, Tan EH, O'Byrne K, Zhang L, Hirsh V, Boyer M, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol. 2017;28: 270–277. 10.1093/annonc/mdw611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18: 1454–1466. 10.1016/S1470-2045(17)30608-3 [DOI] [PubMed] [Google Scholar]
- 14.Mok TS, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Improvement in Overall Survival in a Randomized Study That Compared Dacomitinib With Gefitinib in Patients With Advanced Non-Small-Cell Lung Cancer and EGFR-Activating Mutations. J Clin Oncol. 2018;36: 2244–2250. 10.1200/JCO.2018.78.7994 [DOI] [PubMed] [Google Scholar]
- 15.Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. ; FLAURA Investigators. Osimertinib in untreated EGFRmutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378: 113–125. 10.1056/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- 16.Wu YL, Lee JS, Thongprasert S, Yu CJ, Zhang L, Ladrera G, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol. 2013;14: 777–786. 10.1016/S1470-2045(13)70254-7 [DOI] [PubMed] [Google Scholar]
- 17.Yu H, Zhang J, Wu X, Luo Z, Wang H, Sun S, et al. A phase II randomized trial evaluating gefitinib intercalated with pemetrexed/platinum chemotherapy or pemetrexed/platinum chemotherapy alone in unselected patients with advanced non-squamous non-small cell lung cancer. Cancer Biol Ther. 2014;15: 832–839. 10.4161/cbt.28874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng Y, Murakami H, Yang PC, He J, Nakagawa K, Kang JH, et al. Randomized Phase II Trial of Gefitinib With and Without Pemetrexed as First-Line Therapy in Patients With Advanced Nonsquamous Non-Small-Cell Lung Cancer With Activating Epidermal Growth Factor Receptor Mutations. J Clin Oncol. 2016;34: 3258–3266. 10.1200/JCO.2016.66.9218 [DOI] [PubMed] [Google Scholar]
- 19.Nakamura A, Inoue A, Morita S, Hosomi Y, Kato T, Fukuhara T, et al. Phase III study comparing gefitinib monotherapy (G) to combination therapy with gefitinib, carboplatin, and pemetrexed (GCP) for untreated patients (pts) with advanced non-small cell lung cancer (NSCLC) with EGFR mutations (NEJ009). J Clin Oncol: 2018. American Society of Clinical Oncology, ASCO. 2018;36(SUPPL): abstr 9005. [Google Scholar]
- 20.Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-smallcell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15: 1236–1244. 10.1016/S1470-2045(14)70381-X [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto N, Seto T, Nishio M, Goto K, Okamoto I, Yamanaka T, et al. Erlotinib plus bevacizumab (EB) versus erlotinib alone (E) as first-line treatment for advanced EGFR mutationpositive non-squamous non-small-cell lung cancer (NSCLC): survival follow-up results of JO25567. J Clin Oncol: 2018. American Society of Clinical Oncology, ASCO. 2018;36(SUPPL): abstr 9007. [Google Scholar]
- 22.Furuya N, Fukuhara T, Saito H, Watanabe K, Sugawara S, Iwasawa S, et al. Phase III study comparing bevacizumab plus erlotinib to erlotinib in patients with untreated NSCLC harboring activating EGFR-mutations:NEJ026. J Clin Oncol: 2018. American Society of Clinical Oncology, ASCO. 2018;36(SUPPL): abstr 9006. [Google Scholar]
- 23.Lin JZ, Ma SK, Wu SX, Yu SH, Li XY. A network meta-analysis of nonsmall-cell lung cancer patients with an activating EGFR mutation: Should osimertinib be the first-line treatment? Medicine (Baltimore). 2018;97: e11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batson S, Mitchell SA, Windisch R, Damonte E, Munk VC, Reguart N. Tyrosine kinase inhibitor combination therapy in first-line treatment of non-small-cell lung cancer: systematic review and network meta-analysis. Onco Targets Ther. 2017;10: 2473–2482. 10.2147/OTT.S134382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi YK, Wang L, Han BH, Li W, Yu P, Liu YP, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28: 2443–2450. 10.1093/annonc/mdx359 [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8: 336–341. 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 27.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17: 2815–234. [DOI] [PubMed] [Google Scholar]
- 28.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007; 8: 16 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomized trials. BMJ. 2011;343: d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gelman A, Rubin D. Inference from iterative simulation using multiple sequences. Statist Sci. 1992;7: 457–511. [Google Scholar]
- 31.Neupane B, Richer D, Bonner AJ, Kibret T, Beyene J. Network meta-analysis using R: a review of currently available automated packages. PLoS One. 2014;9: e115065 10.1371/journal.pone.0115065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brooks S, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7: 434–455. [Google Scholar]
- 33.van Valkenhoef G, Dias S, Ades AE, Welton NJ. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods. 2016;7: 80–93. 10.1002/jrsm.1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8: e76654 10.1371/journal.pone.0076654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361: 947–957. 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 36.Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29: 2866–2874. 10.1200/JCO.2010.33.4235 [DOI] [PubMed] [Google Scholar]
- 37.Gridelli C, Ciardiello F, Gallo C, Feld R, Butts C, Gebbia V, et al. First-line erlotinib followed by second-line cisplatin-gemcitabine chemotherapy in advanced non-small-cell lung cancer: the TORCH randomized trial. J Clin Oncol. 2012;30: 3002–3011. 10.1200/JCO.2011.41.2056 [DOI] [PubMed] [Google Scholar]
- 38.Chen YM, Tsai CM, Fan WC, Shih JF, Liu SH, Wu CH, et al. Phase II randomized trial of erlotinib or vinorelbine in chemonaive, advanced, non-small cell lung cancer patients aged 70 years or older. J Thorac Oncol. 2012;7: 412–418. 10.1097/JTO.0b013e31823a39e8 [DOI] [PubMed] [Google Scholar]
- 39.Wu YL, Zhou C, Liam CK, Wu G, Liu X, Zhong Z, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26: 1883–1889. 10.1093/annonc/mdv270 [DOI] [PubMed] [Google Scholar]
- 40.Han JY, Park K, Kim SW, Lee DH, Kim HY, Kim HT, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30: 1122–1128. 10.1200/JCO.2011.36.8456 [DOI] [PubMed] [Google Scholar]
- 41.Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31: 3327–3334. 10.1200/JCO.2012.44.2806 [DOI] [PubMed] [Google Scholar]
- 42.Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16: 141–151. 10.1016/S1470-2045(14)71173-8 [DOI] [PubMed] [Google Scholar]
- 43.Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15: 213–222. 10.1016/S1470-2045(13)70604-1 [DOI] [PubMed] [Google Scholar]
- 44.Hirsch FR, Kabbinavar F, Eisen T, Martins R, Schnell FM, Dziadziuszko R, et al. A randomized, phase II, biomarker-selected study comparing erlotinib to erlotinib intercalated with chemotherapy in first-line therapy for advanced non-small-cell lung cancer. J Clin Oncol. 2011;29: 3567–3573. 10.1200/JCO.2010.34.4929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jänne PA, Wang X, Socinski MA, Crawford J, Stinchcombe TE, Gu L, et al. Randomized phase II trial of erlotinib alone or with carboplatin and paclitaxel in patients who were never or light former smokers with advanced lung adenocarcinoma: CALGB 30406 trial. J Clin Oncol. 2012;30: 2063–2069. 10.1200/JCO.2011.40.1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, et al. ; TRIBUTE Investigator Group. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23: 5892–5899. 10.1200/JCO.2005.02.840 [DOI] [PubMed] [Google Scholar]
- 47.Leighl NB, Rizvi NA, de Lima LG Jr, Arpornwirat W, Rudin CM, Chiappori AA, et al. Phase 2 Study of Erlotinib in Combination With Linsitinib (OSI-906) or Placebo in Chemotherapy-Naive Patients With Non-Small-Cell Lung Cancer and Activating Epidermal Growth Factor Receptor Mutations. Clin Lung Cancer. 2017;18: 34–42. 10.1016/j.cllc.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Planchard D, Boyer MJ, Lee JS, Dechaphunkul A, Cheema PK, Takahashi T, et al. Postprogression Outcomes for Osimertinib versus Standard-of-care EGFR-TKI in patients with Previously Untreated EGFR-mutated Advanced Non-Small Cell Lung Cancer. Clin Cancer Res. 2019;25: 2058–2063. 10.1158/1078-0432.CCR-18-3325 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A: Methodological quality graph: authors’ judgment about each methodological quality item presented as percentages across all included studies; B: Methodological quality summary: authors’ judgment about each methodological quality item for each included study, “+” low risk of bias; “?” unclear risk of bias; “-” high risk of bias.
(TIF)
TKIs, tyrosine kinase inhibitor; F, first-generation; S, second-generation; Bev, bevacizumab; CT, chemotherapy; Lin, Linsitinib.
(TIF)
(a) progression-free survival; (b) overall survival; (c) serious adverse events. TKIs, tyrosine kinase inhibitor; F, first-generation; S, second-generation; CT, chemotherapy.
(TIF)
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.