Abstract
The prenylation of peptides and proteins is an important post-translational modification observed in vivo. We report that the Pd-catalyzed Tsuji–Trost allylation with a Pd/BIPHEPHOS catalyst system allows the allylation of Cys-containing peptides and proteins with complete chemoselectivity and high n/i regioselectivity. In contrast to recently established methods, which use non-native connections, the Pd-catalyzed prenylation produces the natural n-prenylthioether bond. In addition, a variety of biophysical probes such as affinity handles and fluorescent tags can be introduced into Cys-containing peptides and proteins. Furthermore, peptides containing two cysteine residues can be stapled or cyclized using homobifunctional allylic carbonate reagents.
Introduction
Over the last three decades it has been recognized that post-translational modifications (PTMs) (glycosylation, phosphorylation, sulfation, acylation, lipidation, etc.) play an important role in controlling protein function and localization. Among the PTMs, prenylation is essential for associating certain proteins to specific membranes. A particularly intriguing example for this is the Ras superfamily of small GTPases involved in signal transduction processes that lead to cell growth and differentiation as well as in vesicular trafficking.1
For the biophysical and cell biological investigation of proteins in general and of PTMs in particular, chemoselective methods are needed that enable access to modified proteins via synthetic manipulations at the reactive side chains of proteinogenic amino acids using either chemical reagents or a transition-metal catalyst.2−5 The formation of new covalent bonds allows the attachment of affinity tags, fluorophores, click handles, or PET tracers. Cysteine represents an attractive handle for the introduction of such chemical modifications, as it is the second least frequent amino acid in proteins (1.7%)6 and shows a very strong inherent nucleophilicity, which makes it especially attractive for reactions with electrophilic reagents. Maleimides7 and iodoacetamides8 represent the earliest electrophiles used to alkylate Cys (Figure 1, A) and have been frequently applied to date. More recent developments include a variety of carbonylacrylic reagents as well as vinylpyridines.9 Since then numerous different bioconjugation strategies have been developed.2,10 One of them involves the transformation of Cys into dehydroalanine (Dha) upon treatment with 2,5-dibromohexanediamide (DBHDA)11 or O-mesitylenesulfonylhydroxylamine (MSH)12 (B), which is then reacted with a thiol nucleophile (C). A disadvantage of this method is that the formation of Dha is associated with racemization at the α-carbon because the diastereoselectivity of the thiol addition in simple Dha peptides is reported to be low.13 A more direct access to S-allylcysteine without epimerization can be accomplished by selenenylsulfide reductive rearrangement (D),14 followed by further derivatization either by olefin cross-metathesis15 or a Kirmse–Doyle reaction.16 Alternatively, allylic halides might be used to directly allylate Cys (E),14,17 although these highly reactive reagents are more difficult to handle and preclude more elaborate reagent structures. Very recently Buchwald et al. introduced arylpalladium reagents, which can be used for the arylation of Cys-containing peptides and proteins (F).18 This concept has been extended to Au(III) complexes.19 Pentelute et al. reported site-selective Cys conjugation with perfluoroarene reagents at the π-clamp motif FCPF (G)20 and ligation with cyclooctynes.21
Figure 1.
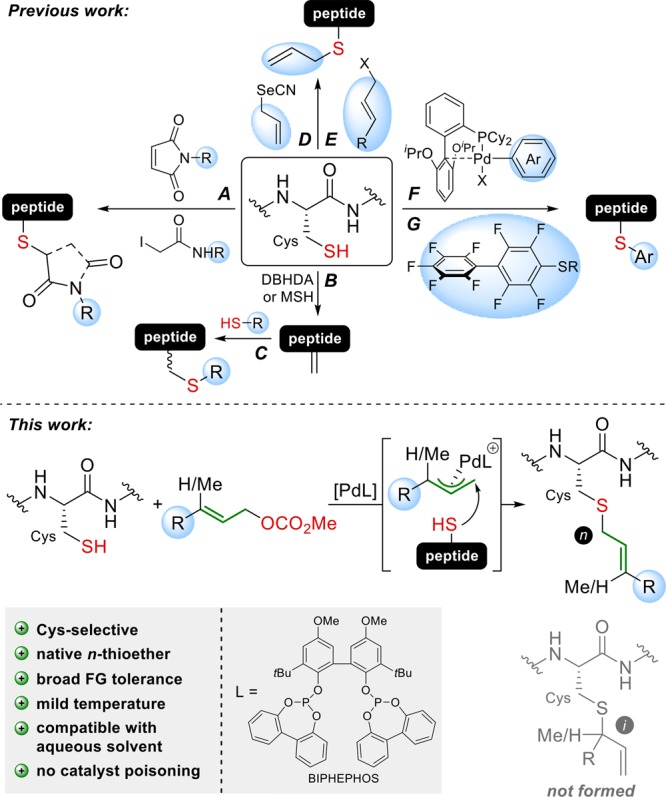
Selected examples of Cys modifications established to date (A–G) in comparison with the Pd-catalyzed Cys allylation described in this work.
Results and Discussion
Reaction Optimization
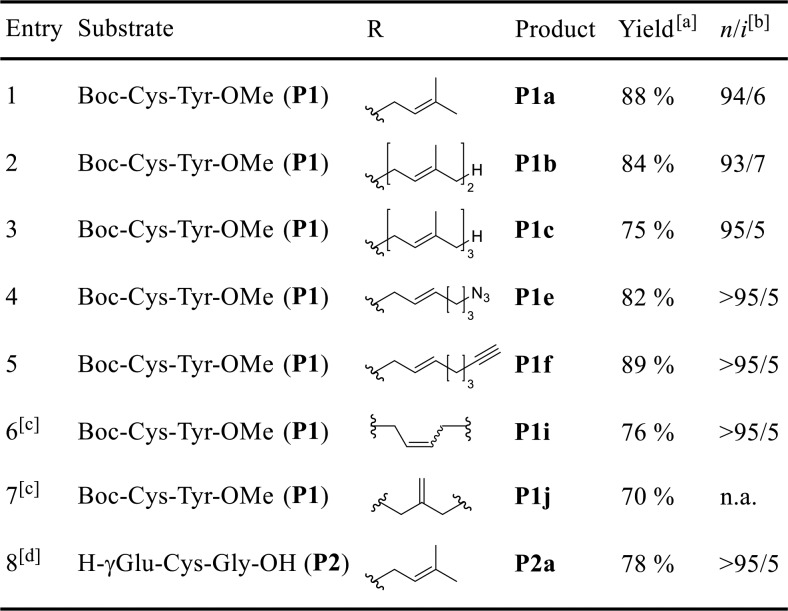
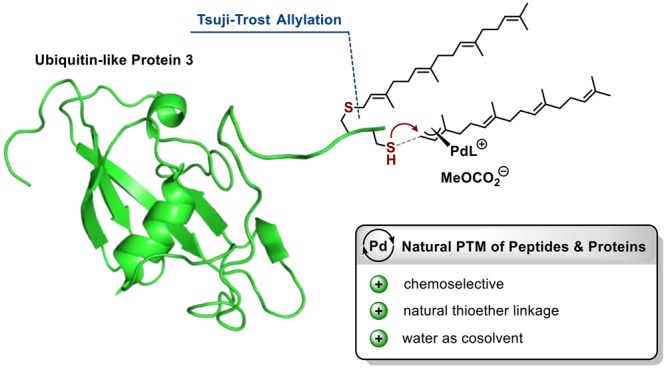
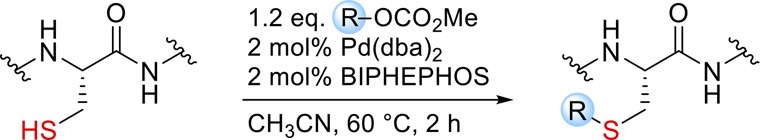
We envisioned that Cys could be selectively modified using the Pd-catalyzed Tsuji–Trost reaction, which would give rise to an allylthioether linkage, as present in naturally prenylated proteins, in a single step. The Tsuji–Trost allylation with C-, N-, or O-nucleophiles is well established in organic synthesis,22 and Francis et al.23 have impressively demonstrated the site-selective modification of proteins via Pd-catalyzed O-allylation of Tyr residues using the water-soluble phosphine ligand TPPTS. In contrast, the reaction with S-nucleophiles has been rarely studied,24,25 as it faces intrinsic difficulties: (a) S-nucleophiles can also function as efficient ligands for Pd and poison the catalyst and (b) thiols are easily oxidized and the reactions have to be carried out under exclusion of air. We hoped that with a prudent choice of ligand we could design a Pd catalyst system suitable for the allylation of Cys-containing peptides and proteins. In contrast to classic allylation procedures14,17 using an excess of highly reactive allylic halides, a Pd-mediated reaction would allow the use of easily accessible allylic carbonates as electrophiles. These reagents are much more versatile, as they are bench stable and can contain highly functionalized structural motifs. Furthermore, in situ activated electrophiles could be sterically controlled by the Pd complex so that the nucleophilic attack is directed to the terminal end of the η3-Pd-allyl complex intermediate to produce the n-allylation product in high selectivity, as the corresponding i product (resulting from internal attack) would be impossible to separate on the peptide or protein level. From a screening of a diverse set of mono- and bidentate phosphorus ligands we identified the bisphosphite ligand BIPHEPHOS as by far the most suitable ligand producing the desired n products in high selectivity. Furthermore, the n/i ratio was found to increase over time even when complete conversion was already reached, indicating the reversibility of this reaction (Table S1).
S-Allylation of Model Substrates
With these optimized conditions in hand, we wanted to apply the Pd-catalyzed S-allylation to a dipeptide substrate (P1) featuring Tyr as the second amino acid, which could give rise to O-allylation as described by Francis et al.23 before. Importantly, with our Pd/BIPHEPHOS catalyst system we observed exclusively S-allylation of Cys, as confirmed via NMR by HMBC experiments (Figure S1). With a series of allylation reagents (Figure 2) we could demonstrate that a diverse set of labeled peptides could be easily prepared by this method (Table 1, entries 1–7). Moreover, we successfully subjected unprotected glutathione (P2) to Pd-catalyzed S-prenylation in an aqueous solvent mixture (Table 1, entry 8), indicating a broader applicability of this method for the modification of longer peptides and proteins. This is corroborated by the fact that the reaction proceeds with fast kinetics. Full conversion of 10 mM Ac-Cys-OMe was observed within 10 min upon treatment with 2 equiv of Ra in the presence of 2.0 mol % of Pd/BIPHEPHOS at 35 °C. Even 0.5 mol % of the catalyst was found to be sufficient to obtain quantitative conversion after 30 min, demonstrating the high efficiency of the reaction (Figure S2). However, strictly oxygen free conditions were crucial for the activity of the catalyst.
Figure 2.
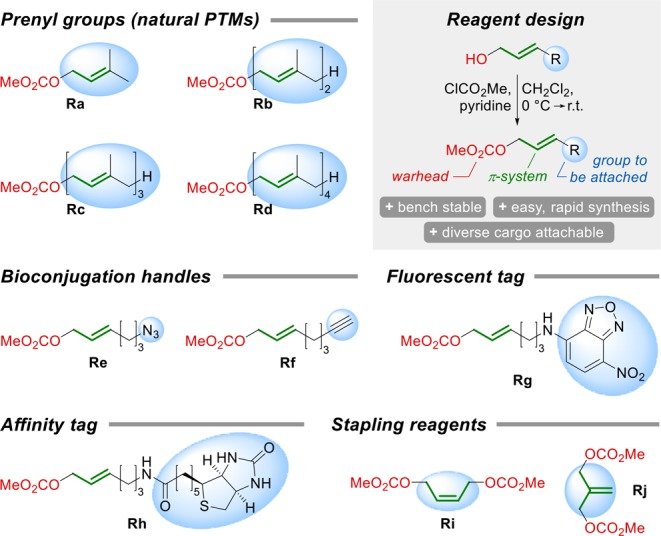
Allylic carbonate reagents prepared for the Pd-catalyzed Cys modification.
Table 1. Scope of the Pd-Catalyzed Allylation of Small Peptides.
Isolated yields after column chromatography.
Determined by 1H NMR spectroscopy; n.a. = not applicable.
0.5 equiv of bifunctional allylation reagent was used.
Reaction was performed in 2/1 CH3CN/H2O as the solvent for 18 h.
Chemoselective Peptide Modification
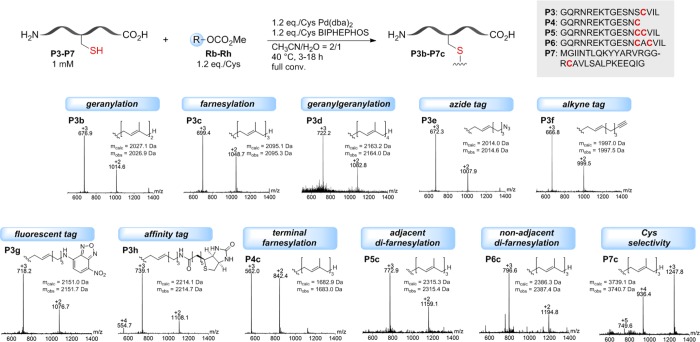
As a next step we tested the Pd-catalyzed Cys allylation on a series of more complex oligopeptides. For this purpose, the substrate concentration was reduced to 1 mM to account for the lower solubility of the peptides and the reaction temperature was adjusted to 40 °C to ensure peptide integrity. To compensate for slower kinetics under these conditions, the amounts of Pd and ligand were increased, which fully restored the reactivity of the system. As a relevant target protein for prenylation, we selected ubiquitin-like protein 3 (UBL3) and started out with modifying its C-terminal domain (peptide P3) with polyprenyl groups, bioconjugation handles, fluorescent and affinity tags, highlighting the versatility of this method (Figure 3). Furthermore, we could show that farnesylation is feasible at internal as well as terminal Cys and that this bioconjugation strategy offers access to adjacent and nonadjacent difarnesylated products, which are of special importance in naturally occurring proteins.26 The high chemoselectivity of this reaction was showcased on a 32aa polypeptide (P7) featuring nearly all functional group containing amino acids, which was found to undergo farnesylation exclusively on Cys, as proven by tryptic digest and mass spectrometric analysis (Figure S3).
Figure 3.
Peptide and reagent scope of the Pd-mediated allylation of oligopeptides. Peptide sequences containing internal, terminal, and multiple Cys residues were subjected to site-selective allylation, enabling the introduction of native prenyl groups and bioconjugation handles (azide/alkyne groups) as well as a fluorescent NBD tag and a biotin affinity tag. All modified peptides were purified, isolated, and characterized by LC-MS analysis.
Peptide Stapling/Cyclization
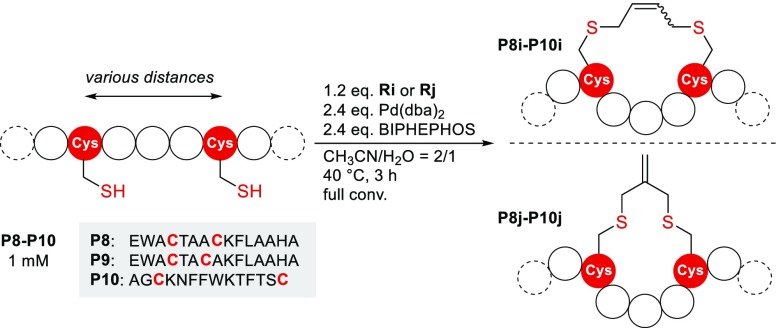
Having established a series of highly selective monofunctional allylic carbonate reagents that were successfully applied on a broad set of peptide substrates, we were eager to see if our methodology could also be extended to bifunctional allylation reagents. This would enable us to implement an additional type of a peptide stapling protocol,27 which is based on Pd-mediated S-allylation. To this end, we prepared two bifunctional allylic carbonates (Ri and Rj) with different geometries, which were subjected to Pd-mediated allylation using two α-helical peptides (P8 and P9)28 with cysteine residues spaced by i+3 and i+4 as well as peptide P10 with more distant residues (i+11)29 (Figure 4). Reactions leading to P8j, P9j, and P10i gave only one peak corresponding to the desired product, whereas for P8i and P9i two separate peaks with the expected mass occurred presumably due to the formation of E/Z isomers. Although the intramolecular reaction was favored for P10j, we observed also the dimer of P10j (approximately 10%), consisting of two peptides and two staples, as a side product. It is worth mentioning that the stapled products provide motifs for further functionalization by taking advantage of the double bond and that allylated peptides with identical staple motifs have recently been shown to function as substrates in decaging strategies using transition-metal catalysis as well.5
Figure 4.
Peptide stapling/cyclization using Pd-mediated allylation. Three model peptides with various distances (i+3, i+4, i+11) between the Cys residues were subjected to stapling/cyclization using two bifunctional reagents with different geometries.
Modifications of UBL3 Protein
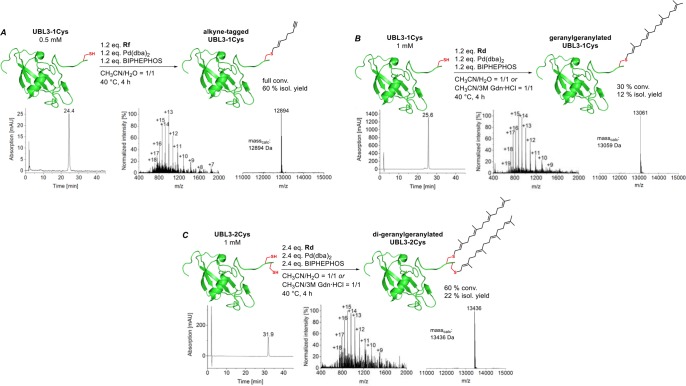
To further evaluate the potential of our method, we chose the full-length protein UBL3 as a substrate, which extends the application beyond classic reactions based on alkyl halides with peptide substrates.17 UBL3 undergoes post-translational geranylgeranylation in vivo, and direct access to such membrane-bound UBL3 variants will help to elucidate their so far unknown physiological role(s).30 Two variants, with one and two C-terminal Cys groups, were used here since mono- and dilipidation occur in nature. In order to find the appropriate reaction conditions for the Pd-mediated protein allylation, we first applied our alkyne-carrying reagent Rf to UBL3-1Cys using a 1/1 CH3CN/H2O mixture as the solvent to reconcile protein, reagent, and catalyst solubility. To our delight we observed full conversion in 4 h to the corresponding alkyne-tagged protein that could be isolated in 60% yield with >95% purity after HPLC purification (Figure 5A).
Figure 5.
Application of the Pd-mediated allylation for the modification of ubiquitin-like protein 3 (UBL3). Both UBL3 variants with one (A, B) and two (C) C-terminal Cys groups were successfully modified when they were treated with 1.2 equiv of allylation reagent per Cys residue. The HPLC traces (214 nm) and mass spectra of the purified products are depicted for UBL3-1Cys-alkyne (A), UBL3-1Cys-Gerger (B), and UBL3-2Cys-(Gerger)2 (C).
Having demonstrated that our methodology is suitable for the modification of proteins, we introduced the natively occurring geranylgeranyl group with reagent Rd into both UBL3 variants using similar conditions. These enabled geranylgeranylation of both UBL3 variants with a conversion of 30% in 4 h. A 1/1 mixture of 3 M aqueous Gdn·HCl with CH3CN was also tested and increased the conversion of UBL3-2Cys to 60%. After HPLC purification both variants were obtained in high purity (>95%) and with isolated yields of 12% for UBL3-1Cys and 22% for UBL3-2Cys, respectively (Figure 5B,C). Dialysis against a buffer containing 50 mM potassium phosphate at pH 7 gave folded, prenylated UBL3 variants, as confirmed by CD spectroscopy (Figure S4A).
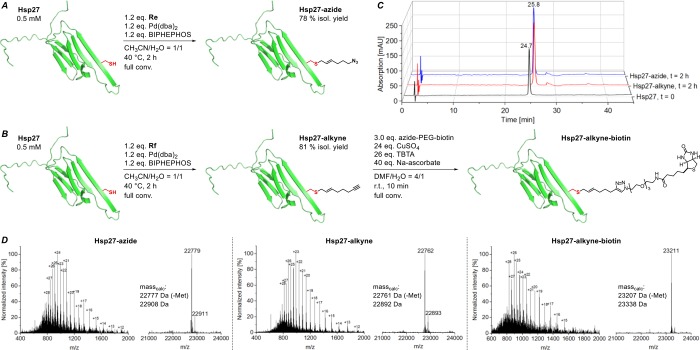
Modifications of Hsp27 Protein
In order to assess more general applications of our Pd-catalyzed protein allylation, we chose heat shock protein 27 (Hsp27) as our next target. It represents a more challenging protein target due to its higher molecular weight and its buried cysteine residue but led to similar prenylation results (Figure S5). Applying reagents Re and Rf, respectively, under conditions established above for UBL3, gave full conversion into the azide- as well as the alkyne-tagged protein conjugates in just 2 h (Figure 6A,B). The peak-to-peak conversion of Hsp27 is nicely illustrated by HPLC chromatograms at t = 0 and after 2 h (Figure 6C). Both modified Hsp27 variants were isolated in excellent yields (78% and 81%) and high purity (>95%). Direct dissolution of the obtained purified Hsp27 products in 50 mM phosphate buffer at pH 7 led to correctly folded proteins as demonstrated by CD measurements (Figure S4B). To demonstrate the utility of Hsp27-alkyne, we carried out a CuAAC reaction with a commercially available azido-biotin reagent, which led to full conversion into the desired product after only 10 min (Figure 6B).
Figure 6.
Attachment of azide as well as alkyne handles onto heat shock protein 27 (A, B), which can be employed for click derivatization to introduce labels (biotin). HPLC traces (214 nm) of substrate and crude reaction mixtures (after 2 h) of the Hsp27 modifications (C) and mass spectra of the purified Hsp27 with bioconjugation handles and crude CuAAC product are depicted (D).
Conclusion
In conclusion, we have developed a chemoselective method for the prenylation, functionalization, and stapling of Cys-containing peptides using Pd/BIPHEPHOS as a catalyst and readily accessible allylcarbonates as reagents. This method was applied to the modification of peptides and proteins for the installation of native prenyl groups as well as artificial bioconjugation handles. In contrast to many established peptide and protein modification reactions, our new Pd-catalyzed Cys-prenylation has the advantage that it forms natural allylthioether linkages as found in prenylated biomolecules and thus can be regarded as a chemical in vitro post-translational modification reaction, which is compatible with all proteinogenic amino acids. In addition, it is general regarding the allylic electrophiles that are applied in minimal excess (1.2 equiv) and therefore provides an efficient tool to introduce labels and tags as well as stabilizing staples into peptides and proteins, affording correctly folded products of high purity.
Acknowledgments
We gratefully acknowledge financial support by the Austrian Science Fund (FWF) (Project P29458), University of Vienna and NAWI Graz.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b08279.
Experimental procedures, characterization data, NMR spectra, chromatography traces, mass spectra, and circular dichroism spectra (PDF)
Author Contributions
§ T.S., J.K., and H.S. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- a Brunsveld L.; Kuhlmann J.; Alexandrov K.; Wittinghofer A.; Goody R. S.; Waldmann H. Lipidated Ras and Rab Peptides and Proteins - Synthesis, Structure, and Function. Angew. Chem., Int. Ed. 2006, 45, 6622–6646. 10.1002/anie.200600855. [DOI] [PubMed] [Google Scholar]; b Wennerberg K.; Rossman K. L.; Der C. J. The Ras superfamily at a glance. J. Cell Sci. 2005, 118, 843–846. 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]; c Stenmark H.; Olkkonen V. M. The Rab GTPase family. Genome Biology 2001, 2, reviews3007.1–reviews3007.7. 10.1186/gb-2001-2-5-reviews3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reviews on peptide/protein modifications:; a deGruyter J. N.; Malins L. R.; Baran P. S. Residue-Specific Peptide Modification: A Chemist’s Guide. Biochemistry 2017, 56, 3863–3873. 10.1021/acs.biochem.7b00536. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Boutureira O.; Bernardes G. J. L. Advances in Chemical Protein Modification. Chem. Rev. 2015, 115, 2174–2195. 10.1021/cr500399p. [DOI] [PubMed] [Google Scholar]; c Koniev O.; Wagner A. Developments and recent advancements in the field of endogenous amino acid selective bond forming reactions for bioconjugation. Chem. Soc. Rev. 2015, 44, 5495–5551. 10.1039/C5CS00048C. [DOI] [PubMed] [Google Scholar]; d Stenzel M. H. ACS Macro Lett. 2013, 2, 14–18. 10.1021/mz3005814. [DOI] [PubMed] [Google Scholar]; e Chalker J. M.; Gunnoo S. B.; Boutureira O.; Gerstberger S. C.; Fernandez-Gonzalez M.; Bernardes G. J. L.; Griffin L.; Hailu H.; Schofield C. J.; Davis B. G. Methods for converting cysteine to dehydroalanine on peptides and proteins. Chem. Sci. 2011, 2, 1666–1676. 10.1039/c1sc00185j. [DOI] [Google Scholar]; f Stephanopoulos N.; Francis M. B. Choosing an effective protein bioconjugation strategy. Nat. Chem. Biol. 2011, 7, 876–884. 10.1038/nchembio.720. [DOI] [PubMed] [Google Scholar]; g Basle E.; Joubert N.; Pucheault M. Protein Chemical Modification on Endogenous Amino Acids. Chem. Biol. 2010, 17, 213–227. 10.1016/j.chembiol.2010.02.008. [DOI] [PubMed] [Google Scholar]; h Chalker J. M.; Bernardes G. J. L.; Lin Y. A.; Davis B. G. Chemical Modification of Proteins at Cysteine: Opportunities in Chemistry and Biology. Chem. - Asian J. 2009, 4, 630–640. 10.1002/asia.200800427. [DOI] [PubMed] [Google Scholar]; i Sletten E. M.; Bertozzi C. R. Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew. Chem., Int. Ed. 2009, 48, 6974–6998. 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Hackenberger C. P. R.; Schwarzer D. Chemoselective Ligation and Modification Strategies for Peptides and Proteins. Angew. Chem., Int. Ed. 2008, 47, 10030–10074. 10.1002/anie.200801313. [DOI] [PubMed] [Google Scholar]
- Reviews on metal-catalyzed modifications:; a Ohata J.; Martin S. C.; Ball Z. T. Metal-Mediated Functionalization of Natural Peptides and Proteins: Panning for Bioconjugation Gold. Angew. Chem., Int. Ed. 2019, 58, 6176–6199. 10.1002/anie.201807536. [DOI] [PubMed] [Google Scholar]; b Jbara M.; Maity S. K.; Brik A. Palladium in the Chemical Synthesis and Modification of Proteins. Angew. Chem., Int. Ed. 2017, 56, 10644–10655. 10.1002/anie.201702370. [DOI] [PubMed] [Google Scholar]; c Yang M.; Li J.; Chen P. R. Transition metal-mediated bioorthogonal protein chemistry in living cells. Chem. Soc. Rev. 2014, 43, 6511–6526. 10.1039/C4CS00117F. [DOI] [PubMed] [Google Scholar]; d Chankeshwara S. V.; Indrigo E.; Bradley M. Palladium-mediated chemistry in living cells. Curr. Opin. Chem. Biol. 2014, 21, 128–135. 10.1016/j.cbpa.2014.07.007. [DOI] [PubMed] [Google Scholar]; e Li J.; Chen P. R. Moving Pd-Mediated Protein Cross Coupling to Living Systems. ChemBioChem 2012, 13, 1728–1731. 10.1002/cbic.201200353. [DOI] [PubMed] [Google Scholar]; f Böhrsch V.; Hackenberger C. R. P. Suzuki–Miyaura Couplings on Proteins: A Simple and Ready-to-use Catalytic System in Water. ChemCatChem 2010, 2, 243–245. 10.1002/cctc.200900300. [DOI] [Google Scholar]; g Antos J. M.; Francis M. B. Transition metal catalyzed methods for site-selective protein modification. Curr. Opin. Chem. Biol. 2006, 10, 253–262. 10.1016/j.cbpa.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Selected recent examples:; a Liu J.; Cheng R.; Wu H.; Li S.; Wang P. G.; DeGrado W. F.; Rozovsky S.; Wang L. Building and Breaking Bonds via a Compact S-Propargyl-Cysteine to Chemically Control Enzymes and Modify Proteins. Angew. Chem., Int. Ed. 2018, 57, 12702–12706. 10.1002/anie.201806197. [DOI] [PMC free article] [PubMed] [Google Scholar]; b de Bruijn A. D.; Roelfes G. Catalytic Modification of Dehydroalanine in Peptides and Proteins by Palladium-Mediated Cross-Coupling. Chem. - Eur. J. 2018, 24, 12728–12733. 10.1002/chem.201802846. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Bloom S.; Liu C.; Kölmel D. K.; Qiao J. X.; Zhang Y.; Poss M. A.; Ewing W. R.; MacMillan D. W. C. Decarboxylative alkylation for site-selective bioconjugation of native proteins via oxidation potentials. Nat. Chem. 2018, 10, 205–211. 10.1038/nchem.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Bauer M.; Wang W.; Lorion M. M.; Dong C.; Ackermann L. Internal Peptide Late-Stage Diversification: Peptide-Isosteric Triazoles for Primary and Secondary C(sp3)–H Activation. Angew. Chem., Int. Ed. 2018, 57, 203–207. 10.1002/anie.201710136. [DOI] [PubMed] [Google Scholar]; e Lee H. G.; Lautrette G.; Pentelute B. L.; Buchwald S. L. Palladium-Mediated Arylation of Lysine in Unprotected Peptides. Angew. Chem., Int. Ed. 2017, 56, 3177–3181. 10.1002/anie.201611202. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Addy P. S.; Erickson S. B.; Italia J. S.; Chatterjee A. A Chemoselective Rapid Azo-Coupling Reaction (CRACR) for Unclickable Bioconjugation. J. Am. Chem. Soc. 2017, 139, 11670–11673. 10.1021/jacs.7b05125. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Lin S.; Yang X.; Jia S.; Weeks A. M.; Hornsby M.; Lee P. S.; Nichiporuk R. V.; Iavarone A. T.; Wells J. A.; Toste F. D.; Chang C. J. Redox-based reagents for chemoselective methionine bioconjugation. Science 2017, 355, 597–602. 10.1126/science.aal3316. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Willwacher J.; Raj R.; Mohammed S.; Davis B. G. Selective Metal-Site-Guided Arylation of Proteins. J. Am. Chem. Soc. 2016, 138, 8678–8681. 10.1021/jacs.6b04043. [DOI] [PubMed] [Google Scholar]; i Seki Y.; Ishiyama T.; Sasaki D.; Abe J.; Sohma Y.; Oisaki K.; Kanai M. Transition Metal-Free Tryptophan-Selective Bioconjugation of Proteins. J. Am. Chem. Soc. 2016, 138, 10798–10801. 10.1021/jacs.6b06692. [DOI] [PubMed] [Google Scholar]
- Metal-catalyzed uncaging-Review:; a Völker T.; Meggers E. Transition-metal-mediated uncaging in living human cells - an emerging alternative to photolabile protecting groups. Curr. Opin. Chem. Biol. 2015, 25, 48–54. 10.1016/j.cbpa.2014.12.021. [DOI] [PubMed] [Google Scholar]; Selected examples:; b Sun S.; Oliveira B. L.; Jiménez-Osés G.; Bernardes G. J. L. Radical-Mediated Thiol-Ene Strategy: Photoactivation of Thiol-Containing Drugs in Cancer Cells. Angew. Chem., Int. Ed. 2018, 57, 15832–15835. 10.1002/anie.201811338. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Jbara M.; Laps S.; Morgan M.; Kamnesky G.; Mann G.; Wolberger C.; Brik A. Palladium prompted on-demand cysteine chemistry for the synthesis of challenging and uniquely modified proteins. Nat. Commun. 2018, 9, 3154. 10.1038/s41467-018-05628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Liu J.; Zheng F.; Cheng R.; Li S.; Rozovsky S.; Wang Q.; Wang L. Site-Specific Incorporation of Selenocysteine Using an Expanded Genetic Code and Palladium-Mediated Chemical Deprotection. J. Am. Chem. Soc. 2018, 140, 8807–8816. 10.1021/jacs.8b04603. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Stenton B. J.; Oliveira B. L.; Matos M. J.; Sinatra L.; Bernardes G. J. L. A thioether-directed palladium-cleavable linker for targeted bioorthogonal drug decaging. Chem. Sci. 2018, 9, 4185–4189. 10.1039/C8SC00256H. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Jbara M.; Maity S. K.; Seenaiah M.; Brik A. Palladium Mediated Rapid Deprotection of N-Terminal Cysteine under Native Chemical Ligation Conditions for the Efficient Preparation of Synthetically Challenging Proteins. J. Am. Chem. Soc. 2016, 138, 5069–5075. 10.1021/jacs.5b13580. [DOI] [PubMed] [Google Scholar]; g Völker T.; Dempwolff F.; Graumann P. L.; Meggers E. Progress towards Bioorthogonal Catalysis with Organometallic Compounds. Angew. Chem., Int. Ed. 2014, 53, 10536–10540. 10.1002/anie.201404547. [DOI] [PubMed] [Google Scholar]; h Sasmal P. K.; Carregal-Romero S.; Parak W. J.; Meggers E. Light-Triggered Ruthenium-Catalyzed Allylcarbamate Cleavage in Biological Environments. Organometallics 2012, 31, 5968–5970. 10.1021/om3001668. [DOI] [Google Scholar]; i Streu C.; Meggers E. Ruthenium-Induced Allylcarbamate Cleavage in Living Cells. Angew. Chem., Int. Ed. 2006, 45, 5645–5648. 10.1002/anie.200601752. [DOI] [PubMed] [Google Scholar]
- Hermanson G. T.Bioconjugation Techniques, 3rd ed.; Academic Press: 2013. [Google Scholar]
- Moore J. E.; Ward W. H. Cross-linking of Bovine Plasma Albumin and Wool Keratin. J. Am. Chem. Soc. 1956, 78, 2414–2418. 10.1021/ja01592a020. [DOI] [Google Scholar]
- Goddard D. R.; Michaelis L. Derivatives of Keratin. J. Biol. Chem. 1935, 112, 361–371. [Google Scholar]
- a Matos M. J.; Navo C. D.; Hakala T.; Ferhati X.; Guerreiro A.; Hartmann D.; Bernardim C.; Saar K. L.; Compañón I.; Corzana F.; Knowles T. P. J.; Jiménez-Osés G.; Bernardes G. J. L. Quaternization of Vinyl/Alkynyl Pyridine Enables Ultrafast Cysteine-Selective Protein Modification and Charge Modulation. Angew. Chem., Int. Ed. 2019, 58, 6640–6644. 10.1002/anie.201901405. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Bernardim B.; Cal P. M. S. D.; Matos M. J.; Oliveira B. L.; Martínez-Sáez N.; Albuquerque I. S.; Perkins E.; Corzana F.; Burtoloso A. C. B.; Jiménez-Osés G.; Bernardes G. J. L. Stoichiometric and irreversible cysteine-selective protein modification using carbonylacrylic reagents. Nat. Commun. 2016, 7, 13128. 10.1038/ncomms13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamblin D. P.; van Kasteren S.; Bernardes G. J. L.; Chalker J. M.; Oldham N. J.; Fairbanks A. J.; Davis B. G. Chemical site-selective prenylation of proteins. Mol. BioSyst. 2008, 4, 558–561. 10.1039/b802199f. [DOI] [PubMed] [Google Scholar]
- a Morrison P. M.; Foley P. J.; Warriner S. L.; Webb M. E. Chemical generation and modification of peptides containing multiple dehydroalanines. Chem. Commun. 2015, 51, 13470–13473. 10.1039/C5CC05469A. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Nathani R.; Moody P.; Smith M. E. B.; Fitzmaurice R. J.; Caddick S. Bioconjugation of Green Fluorescent Protein via an Unexpectedly Stable Cyclic Sulfonium Intermediate. ChemBioChem 2012, 13, 1283–1285. 10.1002/cbic.201200231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardes G. J. L.; Chalker J. M.; Errey J. C.; Davis B. G. Facile Conversion of Cysteine and Alkyl Cysteines to Dehydroalanine on Protein Surfaces: Versatile and Switchable Access to Functionalized Proteins. J. Am. Chem. Soc. 2008, 130, 5052–5053. 10.1021/ja800800p. [DOI] [PubMed] [Google Scholar]
- a Galonic D. P.; van der Donk W. A.; Gin D. Y. Oligosaccharide–Peptide Ligation of Glycosyl Thiolates with Dehydropeptides: Synthesis of S-Linked Mucin-Related Glycopeptide Conjugates. Chem. - Eur. J. 2003, 9, 5997–6006. 10.1002/chem.200305290. [DOI] [PubMed] [Google Scholar]; b Zhu Y.; van der Donk W. A. Convergent Synthesis of Peptide Conjugates Using Dehydroalanines for Chemoselective Ligations. Org. Lett. 2001, 3, 1189–1192. 10.1021/ol015648a. [DOI] [PubMed] [Google Scholar]
- Chalker J. M.; Lin Y. A.; Boutureira O.; Davis B. G. Enabling olefin metathesis on proteins: chemical methods for installation of S-allyl cysteine. Chem. Commun. 2009, 3714–3716. 10.1039/b908004j. [DOI] [PubMed] [Google Scholar]
- Selected examples:; a Lin Y. A.; Boutureira O.; Lercher L.; Bhushan B.; Paton R. S.; Davis B. G. Rapid Cross-Metathesis for Reversible Protein Modifications via Chemical Access to Se-Allyl-selenocysteine in Proteins. J. Am. Chem. Soc. 2013, 135, 12156–12159. 10.1021/ja403191g. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lin Y. A.; Chalker J. M.; Davis B. G. Olefin Cross-Metathesis on Proteins: Investigation of Allylic Chalcogen Effects and Guiding Principles in Metathesis Partner Selection. J. Am. Chem. Soc. 2010, 132, 16805–16811. 10.1021/ja104994d. [DOI] [PubMed] [Google Scholar]; c Lin Y. A.; Chalker J. M.; Floyd N.; Bernardes G. J. L.; Davis B. G. Allyl Sulfides Are Privileged Substrates in Aqueous Cross-Metathesis: Application to Site-Selective Protein Modification. J. Am. Chem. Soc. 2008, 130, 9642–9643. 10.1021/ja8026168. [DOI] [PubMed] [Google Scholar]; Review:; d Lin Y. A.; Chalker J. M.; Davis B. G. Olefin Metathesis for Site-Selective Protein Modification. ChemBioChem 2009, 10, 959–969. 10.1002/cbic.200900002. [DOI] [PubMed] [Google Scholar]
- a Crich D.; Brebion F.; Krishnamurthy V. Allylic Disulfide Rearrangement and Desulfurization: Mild, Electrophile-Free Thioether Formation from Thiols. Org. Lett. 2006, 8, 3593–3596. 10.1021/ol061381+. [DOI] [PubMed] [Google Scholar]; b Crich D.; Krishnamurthy V.; Hutton T. K. Allylic Selenosulfide Rearrangement: A Method for Chemical Ligation to Cysteine and Other Thiols. J. Am. Chem. Soc. 2006, 128, 2544–2545. 10.1021/ja057521c. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Crich D.; Zou Y.; Brebion F. Sigmatropic Rearrangements as Tools for Amino Acid and Peptide Modification: Application of the Allylic Sulfur Ylide Rearrangement to the Preparation of Neoglycoconjugates and Other Conjugates. J. Org. Chem. 2006, 71, 9172–9177. 10.1021/jo061439y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Calce E.; De Luca S. The Cysteine S-Alkylation Reaction as a Synthetic Method to Covalently Modify Peptide Sequences. Chem. - Eur. J. 2017, 23, 224–233. 10.1002/chem.201602694. [DOI] [PubMed] [Google Scholar]; b Wollack J. W.; Zeliadt N. A.; Mullen D. G.; Amundson G.; Geier S.; Falkum S.; Wattenberg E. V.; Barany G.; Distefano M. D. Multifunctional Prenylated Peptides for Live Cell Analysis. J. Am. Chem. Soc. 2009, 131, 7293–7303. 10.1021/ja805174z. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Xue C.-B.; Becker J. M.; Naider F. Efficient regioselective isoprenylation of peptides in acidic aqueous solution using zinc acetate as catalyst. Tetrahedron Lett. 1992, 33, 1435–1438. 10.1016/S0040-4039(00)91640-X. [DOI] [Google Scholar]
- a Kubota K.; Dai P.; Pentelute B. L.; Buchwald S. L. Palladium Oxidative Addition Complexes for Peptide and Protein Cross-linking. J. Am. Chem. Soc. 2018, 140, 3128–3133. 10.1021/jacs.8b00172. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Rojas A. J.; Pentelute B. L.; Buchwald S. L. Water-Soluble Palladium Reagents for Cysteine S-Arylation under Ambient Aqueous Conditions. Org. Lett. 2017, 19, 4263–4266. 10.1021/acs.orglett.7b01911. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Rojas A. J.; Zhang C.; Vinogradova E. V.; Buchwald N. H.; Reilly J.; Pentelute B. L.; Buchwald S. L. Divergent unprotected peptide macrocyclisation by palladium-mediated cysteine arylation. Chem. Sci. 2017, 8, 4257–4263. 10.1039/C6SC05454D. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Vinogradova E. V.; Zhang C.; Spokoyny A. M.; Pentelute B. L.; Buchwald S. L. Organometallic palladium reagents for cysteine bioconjugation. Nature 2015, 526, 687–691. 10.1038/nature15739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina M. S.; Stauber J. M.; Waddington M. A.; Rheingold A. L.; Maynard H. D.; Spokoyny A. M. Organometallic Gold(III) Reagents for Cysteine Arylation. J. Am. Chem. Soc. 2018, 140, 7065–7069. 10.1021/jacs.8b04115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Zhang C.; Welborn M.; Zhu T.; Yang N. J.; Santos M. S.; Van Voorhis T.; Pentelute B. L. π-Clamp-mediated cysteine conjugation. Nat. Chem. 2016, 8, 120–128. 10.1038/nchem.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Dai P.; Zhang C.; Welborn M.; Shepherd J. J.; Zhu T.; Van Voorhis T.; Pentelute B. L. Salt Effect Accelerates Site-Selective Cysteine Bioconjugation. ACS Cent. Sci. 2016, 2, 637–646. 10.1021/acscentsci.6b00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.; Dai P.; Vinogradov A. A.; Gates Z. P.; Pentelute B. L. Site-Selective Cysteine-Cyclooctyne Conjugation. Angew. Chem., Int. Ed. 2018, 57, 6459–6463. 10.1002/anie.201800860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reviews:; a Hong A. Y.; Stoltz B. M. The Construction of All-Carbon Quaternary Stereocenters by Use of Pd-Catalyzed Asymmetric Allylic Alkylation Reactions in Total Synthesis. Eur. J. Org. Chem. 2013, 2013, 2745–2759. 10.1002/ejoc.201201761. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Trost B. M. Pd- and Mo-Catalyzed Asymmetric Allylic Alkylation. Org. Process Res. Dev. 2012, 16, 185–194. 10.1021/op200294r. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Trost B. M.; Zhang T.; Sieber J. D. Catalytic asymmetric allylic alkylation employing heteroatom nucleophiles: a powerful method for C–X bond formation. Chem. Sci. 2010, 1, 427–440. 10.1039/c0sc00234h. [DOI] [Google Scholar]; d Dieguez M.; Pamies O. Biaryl Phosphites: New Efficient Adaptative Ligands for Pd-Catalyzed Asymmetric Allylic Substitution Reactions. Acc. Chem. Res. 2010, 43, 312–322. 10.1021/ar9002152. [DOI] [PubMed] [Google Scholar]; e Helmchen G.; Dahnz A.; Duebon P.; Schelwies M.; Weihofen R. Iridium-catalysed asymmetricallylic substitutions. Chem. Commun. 2007, 675–691. 10.1039/B614169B. [DOI] [PubMed] [Google Scholar]; f Trost B. M.; Van Vranken D. L. Asymmetric Transition Metal-Catalyzed Allylic Alkylations. Chem. Rev. 1996, 96, 395–422. 10.1021/cr9409804. [DOI] [PubMed] [Google Scholar]; Recent contributions from our laboratory:; g Kljajic M.; Schlatzer T.; Breinbauer R. Synthesis of 2-Pyrrolidinones by Palladium-Catalyzed [3 + 2] Cycloaddition of Isocyanates. Synlett 2019, 30, 581–585. 10.1055/s-0037-1610692. [DOI] [Google Scholar]; h Kljajic M.; Puschnig J. G.; Weber H.; Breinbauer R. Additive-Free Pd-Catalyzed α-Allylation of Imine-Containing Heterocycles. Org. Lett. 2017, 19, 126–129. 10.1021/acs.orglett.6b03407. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Brehm E.; Breinbauer R. Investigation of the origin and synthetic application of the pseudodilution effect for Pd-catalyzed macrocyclisations in concentrated solutions with immobilized catalysts. Org. Biomol. Chem. 2013, 11, 4750–4756. 10.1039/c3ob41020j. [DOI] [PubMed] [Google Scholar]
- Tilley S. D.; Francis M. B. Tyrosine-Selective Protein Alkylation Using π-Allylpalladium Complexes. J. Am. Chem. Soc. 2006, 128, 1080–1081. 10.1021/ja057106k. [DOI] [PubMed] [Google Scholar]
- Reviews:; a Liu W.; Zhao X. Carbon-Sulfur Bond Formation via Metal-Catalyzed Allylations of Sulfur Nucleophiles. Synthesis 2013, 45, 2051–2069. 10.1055/s-0033-1339176. [DOI] [Google Scholar]; b Gais D. In From Asymmetric Synthesis with Chemical and Biological Methods; Enders H. J., Jaeger K.-E., Eds.; Wiley-VCH: Weinheim, 2007; pp 215–250. [Google Scholar]
- Selected examples for S-allylation:; a Roggen M.; Carreira E. M. Enantioselective Allylic Thioetherification: The Effect of Phosphoric Acid Diester on Iridium-Catalyzed Enantioconvergent Transformations. Angew. Chem., Int. Ed. 2012, 51, 8652–8655. 10.1002/anie.201202092. [DOI] [PubMed] [Google Scholar]; b Wu X.-S.; Chen Y.; Li M.-B.; Zhou M.-G.; Tian S.-K. Direct Substitution of Primary Allylic Amines with Sulfinate Salts. J. Am. Chem. Soc. 2012, 134, 14694–14697. 10.1021/ja306407x. [DOI] [PubMed] [Google Scholar]; c Holzwarth M. S.; Frey W.; Plietker B. Binuclear Fe-complexes as catalysts for the ligand-free regioselective allylic sulfenylation. Chem. Commun. 2011, 47, 11113–11115. 10.1039/c1cc14599a. [DOI] [PubMed] [Google Scholar]; d Gao N.; Zheng S.-C.; Yang W.-K.; Zhao X.-M. Carbon-Sulfur Bond Formation via Iridium-Catalyzed Asymmetric Allylation of Aliphatic Thiols. Org. Lett. 2011, 13, 1514–1516. 10.1021/ol200197v. [DOI] [PubMed] [Google Scholar]; e Gais H.-J.; Jagusch T.; Spalthoff N.; Gerhards F.; Frank M.; Raabe G. Highly Selective Palladium Catalyzed Kinetic Resolution and Enantioselective Substitution of Racemic Allylic Carbonates with Sulfur Nucleophiles: Asymmetric Synthesis of Allylic Sulfides, Allylic Sulfones, and Allylic Alcohols. Chem. - Eur. J. 2003, 9, 4202–4221. 10.1002/chem.200204657. [DOI] [PubMed] [Google Scholar]; f Frank M.; Gais H.-J. Asymmetric synthesis of allylic sulfides via palladium-mediated allylation of thiols. Tetrahedron: Asymmetry 1998, 9, 3353–3357. 10.1016/S0957-4166(98)00345-0. [DOI] [Google Scholar]; g Goux C.; Lhoste P.; Sinou D. Palladium(0)-catalyzed alkylation of thiols. Tetrahedron 1994, 50, 10321–10330. 10.1016/S0040-4020(01)81764-6. [DOI] [Google Scholar]; h Genet J. P.; Blart E.; Savignac M.; Lemeune S.; Lemair-Audoire S.; Bernard J. M. A General and Simple Removal of the Allyloxycarbonyl Protecting Group by Palladium-Catalyzed Reactions Using Nitrogen and Sulfur Nucleophiles. Synlett 1993, 1993, 680–682. 10.1055/s-1993-22570. [DOI] [Google Scholar]; i Arredondo Y.; Moreno-Manas M.; Pleixats R.; Villarroya M. Palladium-catalyzed allylation of 5-membered heterocyclic ambident sulfur nucleophiles. Tetrahedron 1993, 49, 1465–1470. 10.1016/S0040-4020(01)90198-X. [DOI] [Google Scholar]; j Goux C.; Lhoste P.; Sinou D. Synthesis of allyl aryl sulphides by palladium(0)-mediated alkylation of thiols. Tetrahedron Lett. 1992, 33, 8099–8102. 10.1016/S0040-4039(00)74729-0. [DOI] [Google Scholar]; k Trost B. M.; Scanlan T. S. Synthesis of allyl sulfides via a palladium mediated allylation. Tetrahedron Lett. 1986, 27, 4141–4144. 10.1016/S0040-4039(00)84931-X. [DOI] [Google Scholar]; l Auburn P. R.; Whelan J.; Bosnich B. Homogeneous catalysis. Production of allyl alkyl sulphides by palladium mediated allylation. J. Chem. Soc., Chem. Commun. 1986, 146–147. 10.1039/c39860000146. [DOI] [Google Scholar]
- Wang M.; Casey P. J. Protein prenylation: unique fats make their mark on biology. Nat. Rev. Mol. Cell Biol. 2016, 17, 110–122. 10.1038/nrm.2015.11. [DOI] [PubMed] [Google Scholar]
- a Ohata J.; Ball Z. T. A Hexa-rhodium Metallopeptide Catalyst for Site-Specific Functionalization of Natural Antibodies. J. Am. Chem. Soc. 2017, 139, 12617–12622. 10.1021/jacs.7b06428. [DOI] [PubMed] [Google Scholar]; b Chu Q.; Moellering R. E.; Hilinski G. J.; Kim Y.-W.; Grossmann T. N.; Yehab J. T.-H.; Verdine G. L. Towards understanding cell penetration by stapled peptides. MedChemComm 2015, 6, 111–119. 10.1039/C4MD00131A. [DOI] [Google Scholar]; c Walensky L. D.; Bird G. H. Hydrocarbon-Stapled Peptides: Principles, Practice, and Progress. J. Med. Chem. 2014, 57, 6275–6288. 10.1021/jm4011675. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Chen Z.; Vohidov F.; Coughlin J. M.; Stagg L. J.; Arold S. T.; Ladbury J. E.; Ball Z. T. Catalytic Protein Modification with Dirhodium Metallopeptides: Specificity in Designed and Natural Systems. J. Am. Chem. Soc. 2012, 134, 10138–10145. 10.1021/ja302284p. [DOI] [PubMed] [Google Scholar]
- Shim S. Y.; Kim Y.-W.; Verdine G. L. A New i, i + 3 Peptide Stapling System for α-Helix Stabilization. Chem. Biol. Drug Des. 2013, 82, 635–642. 10.1111/cbdd.12231. [DOI] [PubMed] [Google Scholar]
- Brown S. P.; Smith A. B. Peptide/Protein Stapling and Unstapling: Introduction of s-Tetrazine, Photochemical Release, and Regeneration of the Peptide/Protein. J. Am. Chem. Soc. 2015, 137, 4034–4037. 10.1021/ja512880g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes B. P.; Saracco S. A.; Sook Lee S.; Crowell S. N.; Vierstra R. D. MUBs, a Family of Ubiquitin-fold Proteins That Are Plasma Membrane-anchored by Prenylation. J. Biol. Chem. 2006, 281, 27145–27157. 10.1074/jbc.M602283200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.