Abstract
This experiment compared physiological and productive responses in finishing beef cattle managed under heat stress conditions, and supplemented (SUPP) or not (CON) with an immunomodulatory feed ingredient (Omnigen-AF; Phibro Animal Health, Teaneck, NJ). Crossbred yearling cattle (¾ Bos taurus × ¼ Bos indicus; 64 heifers and 64 steers) were ranked by initial body weight (BW) (440 ± 3 kg) and sex, and allocated to 1 of 16 unshaded drylot pens (8 heifers or steers/pen). Pens within sex were randomly assigned to receive SUPP or CON (n = 8/treatment). Cattle received a total-mixed ration (91% concentrate inclusion and 1.21 Mcal/kg of net energy for gain; dry matter [DM basis]) during the experiment (day 0 to 106). The immunomodulatory feed was offered as a top-dress to SUPP pens (56 g/d per animal; as-fed basis) beginning on day 7. Cattle BW were recorded on day 0, 14, 28, 42, 56, 70, 84, 98, and 106. Feed intake was evaluated from each pen by recording feed offer daily and refusals biweekly. Intravaginal temperature of heifers was recorded hourly from day 1 to 6, 29 to 41, and 85 to 97. Environmental temperature humidity index (THI) was also recorded hourly throughout the experiment, and averaged 79.8 ± 0.6. Concurrently with BW assessment, hair samples from the tail-switch were collected (3 animals/pen) for analysis of hair cortisol concentrations. Blood samples were collected on day 0, 28, 56, 84, and 106 from all animals for plasma extraction. Whole blood was collected on day 0, 56, and 106 (3 animals/pen) for analysis of heat shock protein (HSP) 70 and HSP72 mRNA expression. Cattle were slaughtered on day 107 at a commercial packing facility. Results obtained prior to day 7 served as independent covariate for each respective analysis. Heifers receiving SUPP had less (P ≤ 0.05) vaginal temperature from 1500 to 1900 h across sampling days (treatment × hour, P < 0.01; 39.05 vs. 39.19 °C, respectively; SEM = 0.04), when THI ranged from 85.3 to 90.1. Expression of HSP70 and HSP72 was less (P ≥ 0.03) for SUPP cattle on day 106 (22.6- vs. 51.5-fold effect for HSP70, SEM = 9.7, and 11.0- vs. 32.8-fold effect for HSP72; treatment × day, P ≤ 0.04). No treatment effects were detected (P ≥ 0.22) for performance, carcass traits, plasma concentrations of cortisol and haptoglobin, or hair cortisol concentrations. Results from this study suggest that SUPP ameliorated hyperthermia in finishing cattle exposed to heat stress conditions, but such benefit was not sufficient to improve productive responses.
Keywords: feedlot cattle, heat stress, immunomodulation, performance, temperature
INTRODUCTION
Heat stress is one of the main challenges to feedlot systems during the summer months, particularly those located in subtropical and tropical environments (Grandin, 2016). St-Pierre et al. (2003) estimated that heat stress costs the U.S. feedlot industry approximately $300 million annually, with decreased production efficiency being a major contributor to this outcome. Hyperthermia impacts BW gain by reducing feed intake, and altering metabolic processes associated with feed efficiency and welfare (Collier et al., 2008; Baumgard and Rhoads, 2013). Several research efforts have investigated management strategies to alleviate hyperthermia in feedlot systems, including shade utilization, cooling systems, and selection of heat-tolerant cattle (Summer et al., 2019). Nonetheless, precision methods to alleviate heat stress in finishing cattle are still warranted to optimize profitability of feedlot industries worldwide (Brown-Brandi, 2018).
Omnigen-AF (OMN) is a patented, proprietary-branded product recently shown to alleviate hyperthermia while improving feed intake and body condition score in heat-stressed lactating dairy cows (Leiva et al., 2017). Others have reported similar outcomes in lactating and nonlactating dairy cows exposed to elevated thermal and humidity load (Brandão et al. 2016; Fabris et al., 2016; Gandra et al., 2019). Collectively, these results suggest OMN as a novel, nutritional approach to enhance thermoregulation of cattle under heat stress conditions. Nevertheless, research is warranted to verify OMN potential thermoregulatory capabilities in finishing beef cattle, including assessment of biomarkers associated with hyperthermia and chronic stress (Leiva et al., 2017). Based on the presented, we hypothesized that supplementing OMN would alleviate hyperthermia and its physiological consequences, resulting in improved performance of finishing cattle exposed to heat stress. Therefore, this experiment compared body temperature, physiological, and productive responses in heat-stressed finishing cattle supplemented or not with OMN.
MATERIALS AND METHODS
This experiment was conducted from May to August 2018 at the Texas A&M - McGregor Research Center (McGregor, TX). All animals were cared for in accordance with acceptable practices and experimental protocols reviewed and approved by the Texas A&M AgriLife Research, Agriculture Animal Care and Use Committee (#2018-010A).
Animals and Treatments
One hundred and twenty-eight yearling cattle (¾ Angus × ¼ Nelore; 64 heifers and 64 steers), born and raised at the McGregor Research Center, were utilized in this experiment (day 0 to 106). On day 0, cattle were ranked by initial BW (440 ± 3 kg) and sex, and allocated to 1 of 16 drylot pens (8 heifers or steers/pen). Pens were 50 × 10 m with no shade available and contained 8 m of linear bunk space, and a circular watering trough with 2-m diameter was shared between adjacent pens. Pens within sex were randomly assigned to receive (SUPP; n = 8) or not (CON; n = 8) OMN supplementation (56 g/d per animal; as-fed basis) beginning on day 7. According to the manufacturer (Phibro Animal Health, Teaneck, NJ), OMN contains a mixture of active dried Saccharomyces cerevisiae, dried Trichoderma longibrachiatum fermentation product, niacin, vitamin B12, riboflavin-5-phosphate, d-calcium pantothenate, choline chloride, biotin, thiamine monohydrate, pyridoxine hydrochloride, menodione dimethylpyrimidinol bisulfate, folic acid, calcium aluminosilicate, sodium aluminosilicate, diatomaceous earth, calcium carbonate, rice hulls, and mineral oil (full formulation is proprietary). The OMN dosage was based on previous research with lactating dairy cows (Leiva et al., 2017), which had equivalent BW and elevated nutritional requirements as cattle evaluated in this experiment.
Cattle had free-choice access to water and a total-mixed ration (TMR) during the experimental period (Table 1). The TMR was offered once a day (0800 h) based on the McGregor Research Center managerial guidelines, in a manner to yield 5% residual orts (as-fed basis; Erickson et al., 2003). The OMN was top-dressed promptly after TMR delivery to SUPP pens from day 7 to 106 to allow immediate consumption. On day 0, steers were implanted with Revalor-S (Merck Animal Health, Kenilworth, NJ) and heifers were implanted with Revalor-H (Merck Animal Health). All cattle were slaughtered on day 107 (Cargill Meat Solutions, Friona, TX).
Table 1.
Composition and nutritional profile of the total mixed ration offered for ad libitum consumption to cattle during the experiment
| Item | Component |
|---|---|
| Composition, as-fed basis | |
| Rolled corn, % | 60.5 |
| Dried distillers grain, % | 22.0 |
| Sorghum stalks, % | 9.0 |
| Liquid molasses, % | 6.0 |
| Mineral mix1, % | 2.5 |
| Nutritional profile,2 DM basis | |
| Net energy for maintenance, Mcal/kg | 1.94 |
| Net energy for gain,, Mcal/kg | 1.21 |
| Total digestible nutrients, % | 76.7 |
| Acid detergent fiber, % | 10.8 |
| Crude protein, % | 13.2 |
| Ether extract, % | 4.67 |
| Ca, % | 0.676 |
| P, % | 0.420 |
1Containing 21% Ca, 0.01% P, 21% NaCl, 0.20% K, 0.10% Mg, 0.045% Cu, 0.001% Se, 0.280% Zn, 220,000 IU/kg of vitamin A, 19,800 IU/kg of vitamin D3, and 3,500 IU/kg of vitamin E (Anipro Xtraperformance Feeds, College Station, TX). Also contained sodium monensin (Rumensin; Elanco Animal Health, Greenfield, IN) at 1320 g/ton.
2Based on wet chemistry procedures by a commercial laboratory (SDK Laboratories, Hutchinson, KS). Calculations for net energy for maintenance and gain used the equations proposed by the NRC (2000).
Sampling
Samples of offered TMR were collected monthly and analyzed for nutrient content by a commercial laboratory (SDK Laboratories, Hutchinson, KS) via wet chemistry procedures (Table 1). Calculations for net energy for maintenance and gain used the equations proposed by the NRC (2000). Nutritional profile of the TMR is described in Table 1.
Cattle were weighed on day 0, 14, 28, 42, 56, 70, 84, 98, and 106 before the TMR feeding (0700 h). Average daily gain (ADG) was calculated using BW from day 0 and 106. Moreover, growth rate of each animal was modeled by linear regression of BW against sampling days, and each regression coefficient was used as individual growth response. Intake of TMR (DM basis) from each pen was evaluated by recording daily TMR offer, and collecting the nonconsumed TMR on day 14, 28, 42, 56, 70, 84, 98, and 106. The nonconsumed TMR from each pen was discarded, and samples from each pen were dried for 96 h at 50 °C in forced-air ovens for DM calculation. Intake of TMR of each pen was divided by the number of sampling days and cattle within each pen, and expressed as kg per animal/d. Total BW gain (in grams, based on initial and final BW) and total TMR intake (in kg, DM basis) of each pen during the experimental period were used for feed efficiency (G:F) calculation, and reported as grams of BW gained per kg of DM consumed.
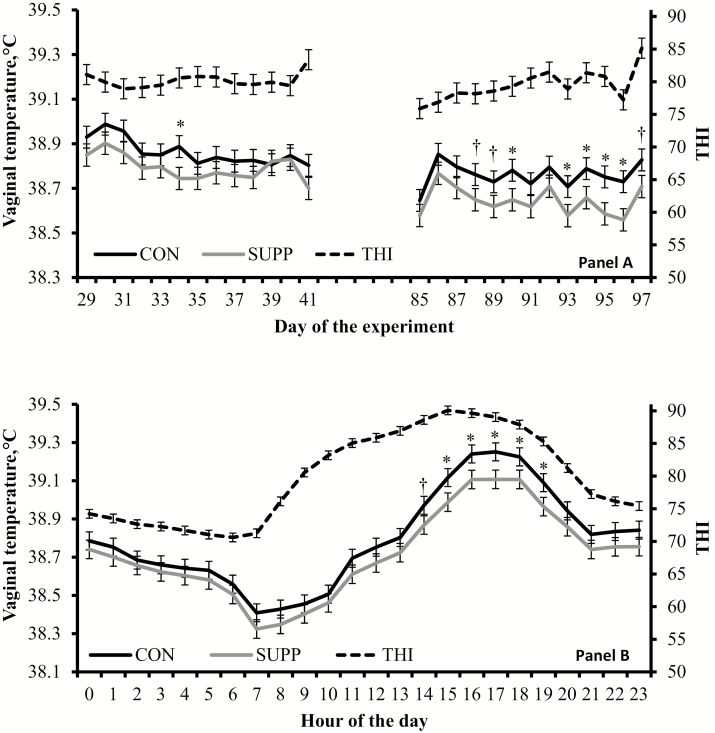
From day 0 to 14, 28 to 42, and 84 to 98, all heifers were fitted intravaginally with a thermometer (iButton temperature loggers DS1922L, Maxim Integrated, San Jose, CA) attached to a controlled internal drug-releasing device (CIDR, Zoetis, Florham Park, NJ) that did not contain hormones. Heifer vaginal temperature was recorded hourly from day 1 to 6 (PR1), day 29 to 41 (PR2), and day 85 to 97 (PR3). Length (14 d) and interval (≥ 14 d) among thermometer insertion were planned to prevent vaginal disorders, whereas data recorded during handling days were discarded (day 0, 14, 28, 42, 84, and 98). Prior to the first use, all thermometers were incubated for 48 h at 37 °C (Symphony Incubating Orbital Shaker Model 5000I; Troemner, LLC, Thorofare, NJ) and temperature was recorded hourly. Average temperature during the 48 h incubation period was documented for each individual thermometer. Average ± SEM temperature during incubation among all thermometers was 37.03 ± 0.09 °C. Environmental temperature, relative humidity, and temperature humidity index (THI; Willard et al., 2003) were also recorded hourly throughout the experiment using a hygrochron temperature and humidity logger (iButton logger DS1923-F5, Maxim Integrated), which was centrally located among pens with no protection from the sun or environmental conditions. Data were summarized (mean ± SE) as daily THI, and hourly THI across days (Fig. 1).
Figure 1.
Environmental THI (Willard et al., 2003) and vaginal temperature of feedlot cattle supplemented with an immunomodulatory feed ingredient (SUPP; n = 8) or not (CON; n = 8) during a 106-d finishing period. Values were recorded hourly (day 1 to 6, day 29 to 41, and day 85 to 97 of the experiment) using intravaginal thermometers (iButton temperature loggers DS1922L, Maxim Integrated, San Jose, CA) or an hygrochron temperature/humidity logger (iButton temperature loggers DS1923-F5, Maxim Integrated). Treatments were provided from day 7 to 106; hence, results from day 1 to 6 were averaged and included as independent covariate in each analysis. Treatment × day (panel A) and treatment × hour (panel B) interactions were detected (P ≤ 0.01). Values reported are covariately adjusted means. Within day or hour; * = P ≤ 0.05, † = P ≤ 0.10.
Three animals were randomly selected within each pen on d 0, and assigned to collection of tail-switch hair during the experimental period. Hair samples were clipped (Burnett et al., 2014; Cooke et al., 2017; Schubach et al., 2017) when cattle were restrained for BW assessment on day 0, 14, 28, 42, 56, 70, 84, 98, and 106. Hair was collected using scissors as close to the skin as possible, and the hair material closest to the skin (2.5 cm of length, 300 mg of weight) was stored at −20 °C. Blood samples were collected from all cattle immediately after BW assessment on day 0, 28, 56, 84, and 106 via jugular venipuncture into blood collection tubes (Vacutainer, 10 mL; Becton Dickinson, Franklin Lakes, NJ) containing freeze-dried sodium heparin. Blood samples were also collected from 3 animals/pen, which were randomly selected on day 0, into PAXgene tubes (BD Diagnostics, Sparks, MD) on day 0, 56, and 106 for whole blood RNA extraction. Hot carcass weight (HCW) was collected upon slaughter on day 107. After a 24-h chill, trained personnel assessed carcass characteristics including backfat thickness at the 12th-rib, marbling, and Longissimus muscle area.
Laboratorial Analysis
Plasma samples.
After collection, blood samples were placed immediately on ice, centrifuged (2,500 × g for 30 min; 4 °C) for plasma harvest, and stored at −80 °C on the same day of collection. Samples were analyzed for plasma cortisol (radioimmunoassay kit #07221106, MP Biomedicals, Santa Ana, CA; Burdick et al., 2009) and haptoglobin concentrations (Cooke and Arthington, 2013). The intra- and interassay CV were, respectively, 6.9 and 9.3% for cortisol, and 4.7 and 8.2% for haptoglobin.
Hair samples.
Cortisol was extracted from hair samples based on the procedures described by Moya et al. (2013). Briefly, hair samples were cleaned with warm water (37 °C) for 30 min, and dried at room temperature for 24 h. Hair samples were then washed twice with isopropanol, dried at room temperature for 120 h, and ground in a 10-mL stainless steel milling cup with a 12-mm stainless steel ball (Retsch Mixer Mill MM400 ball mill; Retsch, Hannover, Germany) for 5 min at a frequency of 30 repetitions/s. Twenty milligrams of ground hair and 1 mL of methanol were combined into a 7-mL glass scintillation vial, sonicated for 30 min, and incubated for 18 h at 50 °C and 100 rpm for steroid extraction. Upon incubation, 0.8 mL of methanol was transferred to a 2-mL microcentrifuge tube and evaporated at 45 °C. Samples were reconstituted in 100 μL of the PBS supplied with an ELISA cortisol kit (Salimetrics Expanded Range, High Sensitivity 1-E3002, State College, PA), and stored at −80 °C. Samples were analyzed for cortisol concentrations using the aforementioned ELISA kit, whereas intra- and interassay CV were, respectively, 4.8 and 7.1%.
PAXgene samples.
Total RNA was extracted using the PAXgene Blood RNA Kit (Qiagen, Valencia, CA). Quantity and quality of isolated RNA were assessed via UV absorbance (NanoDrop Lite; Thermo Fisher Scientific, Wilmington, DE) at 260 nm and 260/280 nm ratio, respectively (Fleige and Pfaffl, 2006). Reverse transcription of extracted RNA (400 ng) and real-time reverse transcription-PCR using gene specific primers (20 pM each; Table 2) and the QuantStudio 3 Real-time PCR system (Applied Biosystems; Foster City, CA) were performed as described by Rodrigues et al. (2015). Responses from the genes of interest were quantified based on the threshold cycle (CT), the number of PCR cycles required for target amplification to reach a predetermined threshold. The CT responses from genes of interest were normalized to the geometrical mean of CT values of β-actin and ribosomal protein L19 (Vandesompele et al., 2002). The CV for the geometrical mean of reference genes across all milk fat globule samples was 2.7%. Results are expressed as relative fold change (2−ΔΔCT; Ocón-Grove et al., 2008).
Table 2.
Primer sequences for all gene transcripts analyzed by quantitative reverse-transcriptase PCR
| Target gene | Primer sequence 5′ to 3′ | Accession no. | Reference |
|---|---|---|---|
| Heat shock protein 70 | |||
| Forward | CGGCTTAGTCCGTGAGAACA | BTU09861 | Liu et al. (2014) |
| Reverse | CCGCTCGGTATCGGTGAA | ||
| Heat shock protein 72 | |||
| Forward | AACATGAAGAGCGCCGTGGAGG | U02892 | Lacetera et al. (2006) |
| Reverse | GTTACACACCTGCTCCAGCTCC | ||
| β-actin | |||
| Forward | CTGGACTTCGAGCAGGAGAT | AY141970 | Gifford et al. (2007) |
| Reverse | GGATGTCGACGTCACACTTC | ||
| Ribosomal protein L19 | |||
| Forward | ATCGATCGCCACATGTATCA | NM_001040516 | Fricke et al. (2016) |
| Reverse | GCGTGCTTCCTTGGTCTTAG |
Statistical Analysis
Pen was considered the experimental unit for all analyses. Quantitative data were analyzed using the MIXED procedure of SAS (SAS Inst. Inc., Cary, NC), whereas binary data were analyzed using the GLIMMIX procedure of SAS (SAS Inst. Inc.) with a binomial distribution and logit link function. All data were analyzed using Satterthwaite approximation to determine the denominator df for tests of fixed effects, with pen(treatment × sex) and animal(pen) as random variables, whereas TMR intake and G:F used pen(treatment × sex) as the random variable. Model statements for initial and final BW, ADG, G:F, and carcass responses contained the effects of treatment, sex, and the resultant interaction. Model statements for TMR intake, BW, blood and hair variables contained the effects of treatment, time, sex, and all resultant interactions. Blood and hair variables were analyzed using results from day 0 as an independent covariate. The specified term for these repeated statements was time, with pen(treatment) as subject for TMR intake and animal(pen) as subject for all other analyses. The model statement used for analysis of vaginal temperature contained the effects of treatment, period (PR2 or PR3), day, hour, and all resultant interactions, whereas the specified term for the repeated statements was hour(day × period) with animal(period × day) as subject. Vaginal temperature analyses contained average thermometer incubation temperature, in addition to averaged results from PR1 as independent covariates. All results are reported as least square means, and separated using least square differences. Significance was set at P ≤ 0.05 and tendencies were determined if P > 0.05 and ≤ 0.10. Results are reported according to the main treatment effect if no interactions were significant, or according to the highest-order interaction detected that contained the effects of treatment.
RESULTS
Temperature Parameters
During the experimental period (day 0 to 106), environmental THI (± SEM) was 79.8 ± 0.6 and animals were exposed to THI > 72 during 82% of the time (2,096 h within a total of 2,544 h). Mean THI were similar (P = 0.31) between both periods of vaginal temperature evaluation (80.2 vs. 79.4 for PR2 and PR3, respectively; SEM = 0.5).
A treatment × day interaction was detected (P ≤ 0.01) for vaginal temperature, given that CON heifers had greater (P ≤ 0.05) temperatures on day 34, 90, 93, 94, 95, and 96 compared with SUPP cohorts, whereas tendencies for this outcome were noted (P ≤ 0.10) on day 88, 89, and 97 (Fig. 1a). A treatment × hour interaction was also detected (P < 0.01), as vaginal temperatures were greater in CON vs. SUPP heifers from 1500 to 1900 h (P ≤ 0.05), and a tendency for this outcome was noted (P = 0.09) at 1400 h (Fig. 1b).
Physiologic Parameters
Treatment × day interactions were noted (P ≤ 0.04) for mRNA expression HSP70 and HSP72. Expression of both genes in whole blood were similar (P ≥ 0.78) between treatments on day 56, but greater (P ≤ 0.03) in CON vs. SUPP cattle on day 106 (Table 3). No treatment differences were detected (P ≥ 0.67) for plasma concentrations of cortisol and haptoglobin, nor for cortisol concentration in tail-switch hair during the experimental period (Table 3). No interaction of sex with treatment or treatment × day were detected (P ≥ 0.37) for these physiological responses.
Table 3.
Physiological responses of feedlot cattle supplemented with an immunomodulatory feed ingredient (SUPP; n = 8) or not (CON; n = 8) during a 106-d finishing period1,2,3
| Item | CON | SUPP | SEM | P-value |
|---|---|---|---|---|
| Plasma cortisol, ng/mL | 62.5 | 63.4 | 1.9 | 0.76 |
| Plasma haptoglobin, mg/dL | 0.144 | 0.135 | 0.015 | 0.68 |
| Hair cortisol, pg/mg of hair | 3.26 | 3.14 | 0.19 | 0.67 |
| Heat shock protein 70, mRNA expression | ||||
| Day 56 | 21.1 | 17.3 | 9.7 | 0.78 |
| Day 106 | 51.5 | 22.6 | 9.7 | 0.03 |
| Heat shock protein 72, mRNA expression | ||||
| Day 56 | 13.6 | 10.5 | 6.5 | 0.73 |
| Day 106 | 32.8 | 11.0 | 6.5 | 0.02 |
1From day 7 to 106 of the experiment, 56 g/animal daily (as-fed basis) of Omnigen-AF (Phibro Animal Health, Teaneck, NJ) was mixed with the total mixed ration of SUPP cow at 0700 h. Animals were slaughtered on day 107 at a commercial packing facility (Cargill Meat Solutions; Friona, TX).
2Blood samples and hair samples from the tail switch were collected on day 0, 28, 56, 84, and 106. Values from day 0 were included as independent covariate in each respective analysis, hence, results reported are covariately adjusted least square means. Treatment × day interactions were noted (P ≤ 0.04) for mRNA expression of whole blood genes.
3Hair samples and whole blood for mRNA extraction were collected from 3 animals randomly selected from each pen throughout the experimental period. Moreover, mRNA expression of whole blood genes were analyzed in samples from day 0, 56, and 106, and reported as fold effect (Ocón-Grove et al., 2008).
Performance Parameters
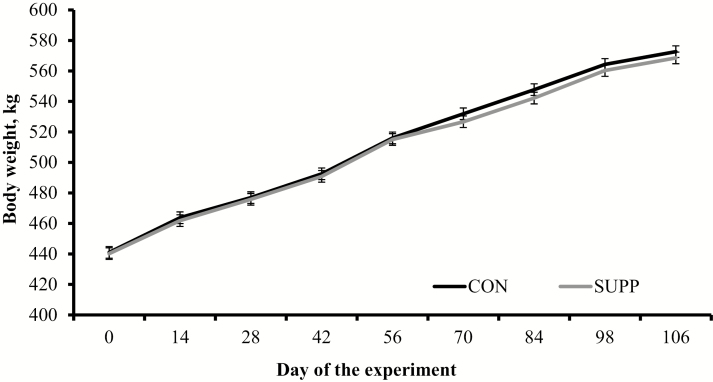
No treatment differences were detected (P ≥ 0.31) for feed intake, feed efficiency, ADG, and BW during the experimental period (Table 4). No treatment effects were also detected (P ≥ 0.27) when cattle BW and growth rate were analyzed based on 28-d samplings (Fig. 2). Carcass traits upon slaughter did not differ (P ≥ 0.22) between treatments (Table 5). No interaction of sex with treatment or treatment × day were detected (P ≥ 0.51) for performance responses.
Table 4.
Performance parameters of feedlot cattle supplemented with an immunomodulatory feed ingredient (SUPP; n = 8) or not (CON; n = 8) during a 106-d finishing period1
| Item | CON | SUPP | SEM | P-value |
|---|---|---|---|---|
| Body weight parameters2 | ||||
| Initial (day 0), kg | 441 | 440 | 3 | 0.85 |
| Final (day 106), kg | 572 | 569 | 4 | 0.51 |
| Average daily gain, kg | 1.24 | 1.21 | 0.02 | 0.31 |
| Feed intake (DM),3 kg/d | 9.54 | 9.15 | 0.40 | 0.50 |
| Feed efficiency,4 g/kg | 132 | 133 | 4 | 0.93 |
1From day 7 to 106 of the experiment, 56 g/animal daily (as-fed basis) of Omnigen-AF (Phibro Animal Health, Teaneck, NJ) was mixed with the total mixed ration (TMR) of SUPP cow at 0700 h.
2Body weight was recorded prior to the first TMR feeding of the day, and ADG was calculated using initial and final weights.
3Feed intake was assessed by recording daily TMR offer, and collecting the nonconsumed TMR on day 14, 28, 42, 56, 70, 84, 98, and 106. Daily TMR intake of each pen was divided by the number of sampling days and cattle within each pen.
4Feed efficiency was calculated using total body weight gain (in grams) and total intake of the total mixed ration (in kg of DM) of each pen during the experimental period.
Figure 2.
Body weight of feedlot cattle supplemented with an immunomodulatory feed ingredient (SUPP; n = 8) or not (CON; n = 8) during a 106-d finishing period. Values were recorded prior to the first feeding of the day. Growth rate of each animal was modeled by linear regression of body weight against sampling days, and each regression coefficient was used as individual response. No treatment differences (P ≥ 0.27) in growth rate (1.23 vs. 1.20 kg/day for CON and SUPP, respectively; SEM = 0.02) or body weight were noted.
Table 5.
Carcass parameters of feedlot cattle supplemented with an immunomodulatory feed ingredient (SUPP; n = 8) or not (CON; n = 8) during a 106-d finishing period1,2
| Item | CON | SUPP | SEM | P-value |
|---|---|---|---|---|
| Hot carcass weight, kg | 357 | 352 | 3 | 0.22 |
| Backfat, cm | 1.58 | 1.65 | 0.07 | 0.47 |
| Area, cm2 | 90.5 | 90.8 | 1.1 | 0.86 |
| Marbling | 404 | 408 | 10 | 0.79 |
| Yield grade | 3.07 | 3.09 | 0.08 | 0.86 |
| Choice, % | 43.7 | 46.9 | 6.2 | 0.72 |
1From day 7 to 106 of the experiment, 56 g/animal daily (as-fed basis) of Omnigen-AF (Phibro Animal Health, Teaneck, NJ) was mixed with the total mixed ration of SUPP cow at 0700 h. Animals were slaughtered on day 107 at a commercial packing facility (Cargill Meat Solutions; Friona, TX).
2Backfat thickness measured at the 12th rib; marbling score: 400 = Small00, 500 = Modest00; yield grade calculated as reported by Lawrence et al. (2010).
DISCUSSION
Based on the THI values observed herein, cattle were exposed to heat stress conditions during the vast majority of the experimental period (Sullivan and Mader, 2018), which was expected based on the location and time of the year (St-Pierre et al., 2003). Corroborating our hypothesis and previous research in lactating dairy cows (Brandão et al. 2016; Leiva et al., 2017; Gandra et al., 2019), SUPP reduced vaginal temperatures in finishing beef heifers exposed to heat stress conditions. These outcomes were noted from 1400 to 1900 h across sampling days, despite THI being elevated throughout the day. Perhaps the THI threshold in which SUPP ameliorates hyperthermia in finishing heifers may be greater than the THI threshold commonly used to designate heat stress in feedlot systems (≥ 72; Sullivan and Mader, 2018). Leiva et al. (2017) also reported that SUPP lessened hyperthermia when daily THI was greater than 75, whereas THI ≥ 68 is the traditional heat stress threshold for lactating dairy cows (Zimbleman et al., 2009). Others have demonstrated that SUPP reduced hyperthermia in dairy cows during heat stress episodes, but not when cows are exposed to thermoneutral environments (Hall et al., 2014; Fabris et al., 2016). Nonetheless, SUPP reduced daily mean vaginal temperature mostly during PR3 (day 85 to 97), although mean THI was similar between PR2 and PR3. As discussed by Leiva et al. (2017), there may be a delay between SUPP feeding and thermoregulatory effects, and cattle need to be adapted to SUPP for several weeks before subsequent immunomodulatory properties are observed (Ryman et al., 2013; Nace et al., 2014). Yet, treatment effects on vaginal temperatures observed in this experiment corroborate SUPP as a nutritional alternative to alleviate hyperthermia in heat-stressed cattle. The exact mechanisms by which SUPP modulated thermoregulation still require investigation.
Cellular responses to hyperthermia result in synthesis of HSPs for protection against stress damage, which keep cellular proteins in a folding competent state to prevent irreversible aggregation (Gabriel et al., 2002). In cattle, HSP70 and HSP72 are often used as biomarkers of stress elicited by hyperthermia, and their mRNA expression is downregulated under thermoneutral conditions (Kristensen et al., 2004). Therefore, treatment differences noted for mRNA expression of HSP70 and HSP72 on day 106 corroborate that hyperthermia was alleviated in SUPP steers and heifers (Lacetera et al., 2006; Liu et al., 2014) toward the end of the experimental period. Moreover, SUPP may also have alleviated cell-mediated stress reactions given the ingredient’s immunomodulatory properties (Ryman et al., 2013; Nace et al., 2014; Brandão et al., 2016). Heat-stress conditions are also known to stimulate adrenocortical function in cattle, culminating in increased circulating concentrations of cortisol (Beede and Collier, 1986; Wise et al., 1988; De Rensis and Scaramuzzi, 2003). However, plasma cortisol concentrations were not impacted by treatments herein, nor in Leiva et al. (2017). In both studies, blood samples were collected in the morning, when vaginal temperatures were similar among SUPP and CON cattle and at their lowest value during the day along with THI. Handling cattle for blood sampling also elicits an acute stress response that rapidly increase circulating cortisol concentrations (Cook et al., 2000), which may prevent proper assessment of adrenocortical function. Hence, one could attribute lack of treatment effects on circulating cortisol to sampling schedule and cattle handling for blood collection (Cooke et al., 2017; Leiva et al., 2017).
For these latter reasons, cortisol concentrations in hair from the tail switch were evaluated herein. This variable was recently identified as a biomarker of chronic stress in cattle, and not affected by the transient stress of handling (Burnett et al., 2014; Marti et al., 2015; Moya et al., 2015). Heightened adrenocortical function has also been positively associated with circulating haptoglobin concentrations in cattle (Cooke and Bohnert, 2011; Cooke et al., 2012), which is a key component of the bovine acute-phase response (Carroll and Forsberg, 2007). Relative to heat-stress, dairy goats exposed to hyperthermia had greater plasma haptoglobin concentrations compared with cohorts exposed to thermoneutral conditions (Hamzaoiu et al., 2013). Leiva et al. (2017) also reported that SUPP reduced serum haptoglobin concentrations in heat-stressed dairy cows. However, SUPP also failed to reduce concentrations of plasma haptoglobin and cortisol in tail-switch hair herein. Therefore, SUPP effects noted for body temperature and HSP were not sufficient to impact cattle adrenocortical and acute-phase reactions.
Hyperthermia is known to reduce voluntary feed intake (West, 2003; Rhoads et al., 2009) while increasing nutritional requirements, impairing feed efficiency and BW gain in cattle (Collier et al., 2008; Baumgard and Rhoads, 2013). Leiva et al. (2017) reported increased feed intake and body condition score in heat-stressed lactating dairy cows receiving SUPP. In this experiment, however, SUPP did not improve cattle TMR intake, G:F, and ADG during the experimental period, resulting in similar carcass quality upon slaughter. Values of THI observed in this experiment were greater than values reported by Leiva et al. (2017) and other research investigating SUPP to dairy cattle (Brandão et al. 2016; Fabris et al., 2016; Gandra et al., 2019), whereas the elevated metabolism of finishing cattle likely increases their sensitivity to heat stress (Sullivan and Mader, 2018). Nevertheless, mean vaginal temperature in CON heifers during each day and throughout the experimental period were, respectively, 0.09 °C and 0.08 °C greater than SUPP heifers, with a maximal difference of 0.17 °C. Perhaps these differences were not sufficient to result in enhanced production responses. The benefits of SUPP on hyperthermia were also noted toward the end of the experimental period, limiting the time for these effects to be translated into improved cattle performance. Alternatively, cattle utilized herein were born and raised at the experimental facility, from a herd selected for heat tolerance for several generations (Paschal et al., 1995). Hence, cattle were innately resilient and adapted to heat-stress conditions, which may have limited the thermoregulatory and productive benefits of SUPP.
Collectively, results from this experiment indicate that SUPP ameliorated hyperthermia in finishing cattle exposed to heat stress conditions, but such benefit was not sufficient to improve productive and carcass parameters. Based on heifer vaginal temperature and whole blood mRNA expression of HSP70 and HSP72 across sexes, SUPP benefits on thermoregulation were mostly noted towards the end of the 106-d experimental period. This outcome may be associated with a delay between SUPP feeding and its thermoregulatory effects, preventing substantial impacts of SUPP on cattle productive responses. Research is still warranted to determine the biological mechanisms by which SUPP modulates thermoregulation in heat-stressed animals. These should include evaluation of SUPP to finishing cattle not previously adapted to heat stress conditions, as well as different dosages of SUPP provision. Nonetheless, this experiment suggests SUPP as a nutritional approach to ameliorate hyperthermia and enhance welfare in feedlot systems with incidence of heat stress conditions
A.P.B. is supported by CAPES – Brazil (#88881. 128327/2016-01).
LITERATURE CITED
- Baumgard L. H., and Rhoads R. P. Jr. 2013. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 1:311–337. doi: 10.1146/annurev-animal-031412-103644. [DOI] [PubMed] [Google Scholar]
- Beede D. K., and Collier R. J.. . 1986. Potential nutritional strategies for intensively managed cattle during thermal stress. J. Anim. Sci. 62:543–554. doi: 10.2527/jas1986.622543x [DOI] [Google Scholar]
- Brandão A. P., Cooke R. F., Corrá F. N., Piccolo M. B., Gennari R., Leiva T., and Vasconcelos J. L. M.. . 2016. Physiologic, health, and production responses of dairy cows supplemented with an immunomodulatory feed ingredient during the transition period. J. Dairy Sci. 99:5562–5572. doi: 10.3168/jds.2015-10621. [DOI] [PubMed] [Google Scholar]
- Brown-Brandl T. 2018. Understanding heat stress in beef cattle. Rev. Bras. Zootec. 47: e20160414. doi: 10.1590/rbz4720160414 [DOI] [Google Scholar]
- Burdick N. C., Banta J. P., Neuendorff D. A., White J. C., Vann R. C., Laurenz J. C., Welsh T. H. Jr, and Randel R. D.. . 2009. Interrelationships among growth, endocrine, immune, and temperament variables in neonatal Brahman calves. J. Anim. Sci. 87:3202–3210. doi: 10.2527/jas.2009-1931. [DOI] [PubMed] [Google Scholar]
- Burnett T. A., Madureira A. M., Silper B. F., Nadalin A., Tahmasbi A., Veira D. M., and Cerri R. L.. . 2014. Short communication: Factors affecting hair cortisol concentrations in lactating dairy cows. J. Dairy Sci. 97:7685–7690. doi: 10.3168/jds.2014-8444. [DOI] [PubMed] [Google Scholar]
- Carroll J. A., and Forsberg N. E.. . 2007. Influence of stress and nutrition on cattle immunity. Vet. Clin. North Am. Food Anim. Pract. 23:105–149. doi: 10.1016/j.cvfa.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Collier R. J., Collier J. L., Rhoads R. P., and Baumgard L. H.. . 2008. Invited review: Genes involved in the bovine heat stress response. J. Dairy Sci. 91:445–454. doi: 10.3168/jds.2007-0540. [DOI] [PubMed] [Google Scholar]
- Cook C. J., Mellor D. J., Harris P. J., Ingram J. R., and Matthews L. R.. . 2000. Hands-on and hands-off measurement of stress. In: Moberg G. P. and Mench J. A., editors, The biology of animal stress. CABI Publishing, Wallingford, UK: p. 123–146. [Google Scholar]
- Cooke R. F., and Arthington J. D.. . 2013. Concentrations of haptoglobin in bovine plasma determined by ELISA or a colorimetric method based on peroxidase activity. J. Anim. Physiol. Anim. Nutr. (Berl). 97:531–536. doi: 10.1111/j.1439-0396.2012.01298.x. [DOI] [PubMed] [Google Scholar]
- Cooke R. F., and Bohnert D. W.. . 2011. Bovine acute-phase response following corticotrophin-release hormone challenge. J. Anim. Sci. 89:252–257. doi: 10.2527/jas.2010-3131 [DOI] [PubMed] [Google Scholar]
- Cooke R. F., Carroll J. A., Dailey J., Cappellozza B. I., and Bohnert D. W.. . 2012. Bovine acute-phase response following different doses of corticotrophin-release hormone challenge. J. Anim. Sci. 90:2337–2344. doi: 10.2527/jas.2011-4608 [DOI] [PubMed] [Google Scholar]
- Cooke R. F., Schubach K. M., Marques R. S., Peres R. F. G., Silva L. G. T., Carvalho R. S., Cipriano R. S., Bohnert D. W., Pires A. V., and Vasconcelos J. L. M.. . 2017. Effects of temperament on physiological, productive, and reproductive responses in Bos indicus beef cows. J. Anim. Sci. 95:1–8. doi: 10.2527/jas.2016.1098 [DOI] [PubMed] [Google Scholar]
- De Rensis F., and Scaramuzzi R. J.. . 2003. Heat stress and seasonal effects on reproduction in the dairy cow–a review. Theriogenology. 60:1139–1151. [DOI] [PubMed] [Google Scholar]
- Erickson G. E., Milton C. T., Fanning K. C., Cooper R. J., Swingle R. S., Parrott J. C., Vogel G., and Klopfenstein T. J.. . 2003. Interaction between bunk management and monensin concentration on finishing performance, feeding behavior, and ruminal metabolism during an acidosis challenge with feedlot cattle. J. Anim. Sci. 81:2869–2879. doi: 10.2527/2003.81112869x [DOI] [PubMed] [Google Scholar]
- Fabris T., Laporta J., Correa F. N., Torres Y. M., Kirk D. J., McLean D. J., Chapman J. D., and Dahl G. E.. . 2016. Effect of OmniGen-AF® and heat stress during the dry period on subsequent performance of cows. J. Dairy Sci. 99(E-Suppl. 1):339. doi: 10.2527/jam2016-0723 [DOI] [PubMed] [Google Scholar]
- Fleige S., and Pfaffl M. W.. . 2006. RNA integrity and the effect on the real-time qRT-PCR performance. Mol. Aspects Med. 27:126–139. doi: 10.1016/j.mam.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Fricke P. M., Carvalho P. D., Lucy M. C., Curran F., Herlihy M. M., Waters S. M., Larkin J. A., Crowe M. A., and Butler S. T.. . 2016. Effect of manipulating progesterone before timed artificial insemination on reproductive and endocrine parameters in seasonal-calving, pasture-based Holstein-Friesian cows. J. Dairy Sci. 99:6780–6792. doi: 10.3168/jds.2016-11229 [DOI] [PubMed] [Google Scholar]
- Gabriel J. E., da Mota A. F., Boleli I. C., Macari M., and Coutinho L. L.. . 2002. Effect of moderate and severe heat stress on avian embryonic hsp70 gene expression. Growth. Dev. Aging. 66:27–33. [PubMed] [Google Scholar]
- Gandra J. R., Takiya C. S., Del Valle T. A., Orbach N. D., Ferraz I. R., Oliveira E. R., Goes R. H., Gandra E. R., Pereira T. L., Batista J. D., . et al. 2019. Influence of a feed additive containing vitamin B12 and yeast extract on milk production and body temperature of grazing dairy cows under high temperature-humidity index environment. Livest. Sci. 221:28–32. doi: 10.1016/j.livsci.2019.01.012 [DOI] [Google Scholar]
- Gifford C. A., Racicot K., Clark D. S., Austin K. J., Hansen T. R., Lucy M. C., Davies C. J., and Ott T. L.. . 2007. Regulation of interferon-stimulated genes in peripheral blood leukocytes in pregnant and bred, nonpregnant dairy cows. J. Dairy Sci. 90:274–280. doi: 10.3168/jds.S0022-0302(07)72628-0 [DOI] [PubMed] [Google Scholar]
- Grandin T. 2016. Evaluation of the welfare of cattle housed in outdoor feedlot pens. Vet. Anim. Sci. 1:23–28. doi: 10.1016/j.vas.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall L. W., Rivera F. A., Villar F., Chapman J. D., Long N. M., and Collier R. J.. . 2014. Evaluation of OmniGen-AF® in lactating heat-stressed Holstein cows. In 25th Annual Florida Ruminant Nutrition Symposium, University of Florida, Gainesville, FL: p. 16–26. [Google Scholar]
- Hamzaoui S., Salama A. A., Albanell E., Such X., and Caja G.. . 2013. Physiological responses and lactational performances of late-lactation dairy goats under heat stress conditions. J. Dairy Sci. 96:6355–6365. doi: 10.3168/jds.2013-6665 [DOI] [PubMed] [Google Scholar]
- Kristensen T. N., Løvendahl P., Berg P., and Loeschcke V.. . 2004. Hsp72 is present in plasma from Holstein-Friesian dairy cattle, and the concentration level is repeatable across days and age classes. Cell Stress Chaperones. 9:143–149. doi: 10.1379/csc-17.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacetera N., Bernabucci U., Scalia D., Basiricò L., Morera P., and Nardone A.. . 2006. Heat stress elicits different responses in peripheral blood mononuclear cells from Brown Swiss and Holstein cows. J. Dairy Sci. 89:4606–4612. doi: 10.3168/jds.S0022-0302(06)72510-3 [DOI] [PubMed] [Google Scholar]
- Lawrence T. E., Elam N. A., Miller M. F., Brooks J. C., Hilton G. G., VanOverbeke D. L., McKeith F. K., Killefer J., Montgomery T. H., Allen D. M., . et al. 2010. Predicting red meat yields in carcasses from beef-type and calf-fed Holstein steers using the United States Department of Agriculture calculated yield grade. J. Anim. Sci. 88:2139–2143. doi: 10.2527/jas.2009-2739 [DOI] [PubMed] [Google Scholar]
- Leiva T., Cooke R. F., Brandão A. P., Schubach K. M., Batista L. F. D., Miranda M. F., Colombo E. A., Rodrigues R. O., Junior J. R. G., Cerri R. L. A., . et al. 2017. Supplementing an immunomodulatory feed ingredient to modulate thermoregulation, physiologic, and production responses in lactating dairy cows under heat stress conditions. J. Dairy Sci. 100:4829–4838. doi: 10.3168/jds.2016-12258 [DOI] [PubMed] [Google Scholar]
- Liu J., Ye G., Zhou Y., Liu Y., Zhao L., Liu Y., Chen X., Huang D., Liao S. F., and Huang K.. . 2014. Feeding glycerol-enriched yeast culture improves performance, energy status, and heat shock protein gene expression of lactating Holstein cows under heat stress. J. Anim. Sci. 92:2494–2502. doi: 10.2527/jas.2013-7152 [DOI] [PubMed] [Google Scholar]
- Marti S., Devant M., Amatayakul-Chantler S., Jackson J. A., Lopez E., Janzen E. D., and Schwartzkopf-Genswein K. S.. . 2015. Effect of anti-gonadotropin-releasing factor vaccine and band castration on indicators of welfare in beef cattle. J. Anim. Sci. 93:1581–1591. doi: 10.2527/jas.2014-8346 [DOI] [PubMed] [Google Scholar]
- Moya D., He M. L., Jin L., Wang Y., Penner G. B., Schwartzkopf-Genswein K. S. and McAllister T. A.. . 2015. Effect of grain type and processing index on growth performance, carcass quality, feeding behavior, and stress response of feedlot steers. J. Anim. Sci. 93:3091–3100. doi: 10.2527/jas.2014-8680 [DOI] [PubMed] [Google Scholar]
- Moya D., Schwatzkopf-Genswein K. S., and Veira D. M.. . 2013. Standarization of a non-invasive methodology to measure cortisol in hair of beef cattle. Livest. Sci. 158:138–144. doi: 10.1016/j.livsci.2013.10.007 [DOI] [Google Scholar]
- Nace E. L., Nickerson S. C., Kautz F. M., Breidling S., Wochele D., Ely L. O., and Hurley D. J.. . 2014. Modulation of innate immune function and phenotype in bred dairy heifers during the periparturient period induced by feeding an immunostimulant for 60 days prior to delivery. Vet. Immunol. Immunopathol. 161:240–250. doi: 10.1016/j.vetimm.2014.08.013 [DOI] [PubMed] [Google Scholar]
- NRC 2000. Nutrient Requirements of Beef Cattle. 7th ed Natl. Acad. Press, Washington, DC. [Google Scholar]
- Ocón-Grove O. M., Cooke F. N. T., Alvarez I. M., Johnson S. E., Ott T. L., and Ealy A. D.. . 2008. Ovine endometrial expression of fibroblast growth factor (FGF) 2 and conceptus expression of FGF receptors during early pregnancy. Domest. Anim. Endocrinol. 34:135–145. doi: 10.1016/j.domaniend.2006.12.002 [DOI] [PubMed] [Google Scholar]
- Paschal J. C., Sanders J. O., Kerr J. L., Lunt D. K., and Herring A. D.. . 1995. Postweaning and feedlot growth and carcass characteristics of Angus-, gray Brahman-, Gir-, Indu-Brazil-, Nellore-, and red Brahman-sired F1 calves. J. Anim. Sci. 73:373–380. doi: 10.2527/1995.732373x [DOI] [PubMed] [Google Scholar]
- Rhoads M. L., Rhoads R. P., VanBaale M. J., Collier R. J., Sanders S. R., Weber W. J., Crooker B. A., and Baumgard L. H.. . 2009. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 92:1986–1997. doi: 10.3168/jds.2008-1641 [DOI] [PubMed] [Google Scholar]
- Rodrigues M. C., Cooke R. F., Marques R. S, Cappellozza B. I., Arispe S. A., Keisler D. H., and Bohnert D. W.. . 2015. Effects of vaccination against respiratory pathogens on feed intake, metabolic, and inflammatory responses in beef heifers. J. Anim. Sci. 93:4443–4452. doi: 10.2527/jas.2015-9277 [DOI] [PubMed] [Google Scholar]
- Ryman V. E., Nickerson S. C., Kautz F. M., Hurley D. J., Ely L. O., Wang Y. Q., and Forsberg N. E.. . 2013. Effect of dietary supplementation on the antimicrobial activity of blood leukocytes isolated from Holstein heifers. Res. Vet. Sci. 95:969–974. doi: 10.1016/j.rvsc.2013.09.009 [DOI] [PubMed] [Google Scholar]
- Schubach K. M., Cooke R. F., Brandão A. P., Lippolis K. D., Silva L. G. T., Marques R. S., and Bohnert D. W.. . 2017. Impacts of stocking density on development and puberty attainment of replacement beef heifers. Animal. 11:2260–2267. doi: 10.1017/S1751731117001070 [DOI] [PubMed] [Google Scholar]
- St-Pierre N. R., Cobanov B., and Schnitkey G.. . 2003. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 86:E52–E77. doi:10.3168/jds.S0022-0302(03)74040–5 [Google Scholar]
- Sullivan K. F., and Mader T. L.. . 2018. Managing heat stress episodes in confined cattle. Vet. Clin. North Am. Food Anim. Pract. 34:325–339. doi: 10.1016/j.cvfa.2018.05.001 [DOI] [PubMed] [Google Scholar]
- Summer A., Lora I., Formaggioni P., and Gottardo F.. . 2019. Impact of heat stress on milk and meat production. Anim. Front. 9:39–46. doi: 10.1093/af/vfy026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., and Speleman F.. . 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J. W. 2003. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 86:2131–2144. doi: 10.3168/jds.S0022-0302(03)73803-X [DOI] [PubMed] [Google Scholar]
- Willard S., Gandy S., Bowers S., Graves K., Elias A., and Whisnant C.. . 2003. The effects of GnRH administration postinsemination on serum concentrations of progesterone and pregnancy rates in dairy cattle exposed to mild summer heat stress. Theriogenology. 59:1799–1810. [DOI] [PubMed] [Google Scholar]
- Wise M. E., Armstrong D. V., Huber J. T., Hunter R., and Wiersma F.. . 1988. Hormonal alterations in the lactating dairy cow in response to thermal stress. J. Dairy Sci. 71:2480–2485. doi: 10.3168/jds.S0022-0302(88)79834-3. [DOI] [PubMed] [Google Scholar]
- Zimbleman R. B., Rhoads R. P., Baumgard L. H., and Collier R. J.. . 2009. Revised temperature humidity index (THI) for high producing dairy cows. J. Dairy Sci. 92(E-Suppl. 1):347. [Google Scholar]