Abstract
Background
The high chances of getting latent tuberculosis infection (LTBI) among health care workers (HCWs) will an enormous problem in low and upper-middle-income countries.
Method
Search strategies were done through both national and international databases include SID, Barakat knowledge network system, Irandoc, Magiran, Iranian national library, web of science, Scopus, PubMed/MEDLINE, OVID, EMBASE, the Cochrane library, and Google Scholar search engine. The Persian and the English languages were used as the filter in national and international databases, respectively. Medical Subject Headings (MeSH) terms was used to controlling comprehensive vocabulary. The search terms were conducted without time limitation till January 01, 2019.
Results
The prevalence of LTBI in Iranian’s HCWs, based on the PPD test was 27.13% [CI95%: 18.64–37.7]. The highest prevalence of LTBI in Iranian’s HCWs were estimated 41.4% [CI95%: 25.4–59.5] in the north, and 33.8% [CI95%: 21.1–49.3] in the west. The lowest prevalence of LTBI was evaluated 18.2% [CI95%: 3.4–58.2] in the south of Iran. The prevalence of LTBI in Iranian’s HCWs who had work-experience more than 20 years old were estimated 20.49% [CI95%: 11–34.97]. In the PPD test, the prevalence of LTBI in Iranian’s HCWs who had received the Bacille Calmette–Guérin (BCG) was estimated 15% [CI95%: 3.6–47.73]. While, in the QFT, the prevalence of LTBI in Iranian’s HCWs in non-vaccinated was estimated 25.71% [CI95%: 13.96–42.49].
Conclusions
This meta-analysis shows the highest prevalence of LTBI in Iranian’s HCWs in the north and the west probably due to neighboring countries like Azerbaijan and Iraq, respectively. It seems that Iranian’s HCWs have not received the necessary training to prevent of TB. We also found that BCG was not able to protect Iranian’s HCWs from TB infections, completely.
1. Introduction
Latent tuberculosis infection (LTBI) is an immune response to Mycobacterium tuberculosis (Mtb) antigens without symptoms of active tuberculosis (TB) [1]. Mtb is able to colonize inside the alveolar macrophages and finally form granuloma. Mtb is ingested by phagocytosis by resident alveolar macrophages and tissue dendritic cells (DC) [2, 3]. The immune cells contribute and the pathological mark of TB, the granuloma, is formed. In the granuloma, macrophages differentiate into epithelial cells or foamy macrophages, or fuse to form giant cells, and become surrounded by lymphocytes, fibroblasts and extracellular matrix proteins. In such conditions, the Mtb will be surviving until the granuloma fails due to immunosuppression [4, 5]. Mtb use the granuloma as they are effective at initial infection level since they recruit new macrophages to allow the spread of infection between host cells [6]. At this stage, the LTBI is formed in the patient’s body [7].
There are several reports of TB outbreaks in Iran. According to the Iranian’s ministry of health, the incidence and the prevalence of TB are high in Sistan and Baluchestan, Khorasan, Mazandaran, Guilan, West and East Azerbaijan, Ardabil, Kurdistan, Khuzestan and southern coasts. Conversely, the incidence and the prevalence of TB are low in the central parts of Iran. The highest incidence and prevalence of TB belong to Golestan and Sistan-Baluchistan [8].
The risk of tuberculosis in health care workers (HCWs) is estimated to be twice as high in the general population, in high-income countries, and five times higher than the general population in countries with a low and middle income [9, 10]. In addition, one of the challenges in many countries is the transfer of tuberculosis from patients admitted to the hospital to HCWs [10]. Most importantly, the transfer of resistant Mycobacterium tuberculosis strains from admitted patients to HCWs has increased the importance of the subject [11].
According to the findings, direct exposure to HCWs in patients with tuberculosis, direct contact with phlegm specimens and blood products of suspected tuberculosis patients, and long hours of work in high-risk places increases the risk of tuberculosis infection [12, 13]. This means that direct contact is one of the most important and worrisome factor in the transmission of tuberculosis to HCWs [10–12, 14]. Work experience, age [15], occupational status [16], the use of personal protective equipment, ventilation [17], hospital infection control unit and infection control in isolation rooms can affect LTBI outbreaks in HCWs [9–12]. To diagnose LTBI, the mantoux tuberculin skin test (TST) and QuantiFERON-TB Gold (QFT) are used [18]. Studies have shown that QFT has a higher sensitivity and specificity in detecting LTBI [19, 20]. However, some researchers believe that QFT is not superior to TST in detecting LTBI [21–23].
The early detection of LTBI in controlling, treating and preventing Mtb is a key element in patients who preventive treatment can reduce the risk of active tuberculosis in patients by up to 90% [24]. So far, systematic review and meta-analysis has not been conducted to evaluate the prevalence and risk factors of LTBI among Iranian’s HCWs. In Iran, the Centers for Disease Control and Prevention (CDC) do not control the Mtb as a regular program, however, reports of LTBI outbreaks in HCWs attracts a high controversy [25]. Due to the highest level of evidence and an essential role in evidence-based decision-making of meta-analysis studies [26, 27]. This study estimated the prevalence and risk factors of LTBI among Iranian’s HCWs which can have vital information for policy-makers and planning at the country level.
2. Methods
2.1. Study protocol
This is the first study that was conducted based on the meta-analysis of observational studies according to epidemiology guidelines [27], and the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (S1 File) [28]. The study was achieved based on five steps; design and search strategy; collecting original articles; evaluating inclusion and exclusion criteria, and finally qualitative evaluation and statistical analysis of data. Two independent researchers (MH.YK & A.J) evaluated the data. The disagreements were solved by consensus between the team and a Bacteriologist (H.S.E). The review protocol was registered in International Prospective Register of Systematic Reviews (PROSPERO) (https://www.crd.york.ac.uk/PROSPERO/) Identifier: CRD42018117682 [29, 30] (S2 File).
2.2. Search strategy
In order to maximize its sensitivity, search strategy was lead through Persian (national) databases, include scientific information database (http://www.sid.ir), Barakat knowledge network system (http://health.barakatkns.com), Iranian research institute for information science and technology (https://irandoc.ac.ir), MagIran (http://www.magiran.com), Iranian national library (http://www.nlai.ir/). The international databases, including web of science, Scopus, PubMed/MEDLINE, OVID, EMBASE, the Cochrane Library (Cochrane Database of Systematic Reviews), and Google Scholar search engine. The Persian and the English languages were used as the filter in national and international databases, respectively. The search terms were adapted to international databases. To search a combination of words, Boolean operators (AND & OR) were used. Searching was done through medical subject heading (MeSH) terms. The search terms were conducted without any time limitation till January 01, 2019. The authors independently analyzed the manuscript contained in the title and abstract. For instance, PubMed search formula was provided in the appendix.
2.3. Inclusion and exclusion criteria
2.3.1. Inclusion criteria based on PICO (related to evidence-based medicine) [31, 32]
Inclusion criteria were determined based on PICO model. In this study, population was the population of Iranian’s HCWs who were residents in the geographic regions of Northern, Southern, Eastern, Western of Iran. Comparison was conducted on a population of HCWs who did not have signs of active TB disease and did not feel illness. Outcome was the overall prevalence of LTBI infection among Iranian’s HCWs.
2.3.2. Exclusion criteria
In this study, review articles, letters, editorial, case reports, conference papers, and comments were excluded. The studies which did not have a focus on the prevalence of LTBI in Iranian’s HCWs, duplicated papers, non-English full papers, non-Persian full papers, and non-accessible full-text papers were excluded. Likewise, the populations other than Iranian’s HCWs were excluded.
2.4. Latent TB detection criteria
2.4.1. The Mantoux tuberculin skin test (TST)
To the Mantoux tuberculin skin test (TST), purified protein derivative (0.1 Ml) is used [33–35], and the induration at TST site is measured 72 hours later. TST reaction of ≥ 5 mm of induration is classified as negative but is considered as positive in patients receiving corticosteroid or patients with Acquired Immunodeficiency Syndrome (AIDS), diabetes mellitus, lymphoma, and leukemia. The induration of ≥10 mm is classified positive in; recent immigrants (< 5 years) from high-prevalence countries; residents and employees of high-risk congregate settings; mycobacteriology laboratory personnel; persons with clinical conditions that place them at high risk. The induration of ≥15 mm is considered positive in any person, including persons with no known risk factors for TB. Two-step testing methods were used for health care workers and nursing home residents [33–35].
2.4.2. Interferon-gamma release assays
Interferon-gamma release assays (IGRAs) show how the immune system reacts to the Mycobacteria that cause TB [36]. The IGRA has been approved by the U.S. Food and Drug Administration (FDA). Positive IGRA means that the person has been infected with TB bacteria. Negative IGRA means that the person’s blood did not react to the test and that latent TB infection or TB disease is not likely. IGRA is the preferred method of TB infection testing for people who have received the Bacille Calmette–Guérin (BCG) [30, 36–39].
2.5. Selection of studies
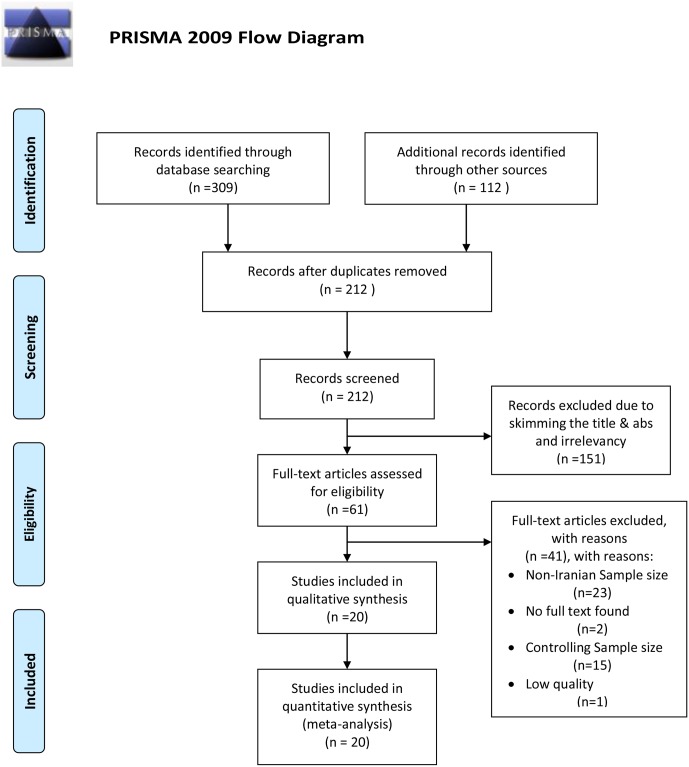
During the selection stage, duplicated studies were removed by the EndNote™ software Ver. X9 (Clarivate Analytics company). In the skimming and screening stage, co-authors, journals, and publishing years were evaluated by two experts based on inclusion and exclusion criteria (the eligibility stage), independently. The disagreements between the two were resolved through an expert bacteriologist (Fig 1).
Fig 1. A flow diagram following the PRISMA (depicted by MH-YK).
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Iterns for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097 For more information, visit www.prisma-statement.org.
2.6. Quality appraisal
In this stage, the irrelevant studies were excluded, and then the quality of each study was evaluated. To quality appraisal, the Newcastle-Ottawa Scale (NOS) checklist (S3 File) [40] was applied which is determined the quality of these studies based on three levels of scoring. The score of five or less defined a poor quality study; the score of five or six distinguished as the medium quality study, and the score of seven or eight determined as the high-quality study. Finally, the medium to high-quality studies were included in the data analysis (Fig 1).
2.7. Data extraction
The enter terms were author’s names, province, geographical regions, year of publishing, sample size, age, gender, history of BCG, history of exposure with tuberculosis, history of tuberculosis disease, laboratory diagnosis tests, job experience, duration of employment, workplaces, single-step or two-step TST, and history of hospitalization. The author’s name, institution, and the journal name were blinded, and then data was extracted through two researchers (MH.YK & A.J), independently. Only if necessary, the additional information/raw data was collected by phone call, mailing or fax.
2.8. Statistical analysis
The prevalence of LTBI in HCWs was considered as a binomial distribution probability, and the variance was calculated by a binomial distribution. To evaluate its heterogeneity, the Cochran test (Q) and I2 index were used [1, 41, 42]. The subgroup analysis was performed based on province, single-step or two-step TST, laboratory diagnosis tests, job, gender, history of TB disease, history of TB exposure, history of BCG, and geographical region. Sensitivity analysis was also achieved to evaluate the impact of each study, based on the results of the overall prevalence of LTBI in Iranian HCWs. The Begg's test and Egger's test were carried out using a funnel plot to examine publication bias. Data analysis was examined by the comprehensive meta-analysis (Ver. 2 Englewood, NJ 07631, USA), and the level of significance was considered as p<0.05.
3. Results
3.1. Study characteristics and methodological quality
In the primary search of study, 421 studies were found. After skimming and screening, 20 (4.75%) studies were eligible according to inclusions and exclusions criteria [43–62]. The total sample size was calculated 6453 Iranian’s HCWs (Fig 1) (S1 Table).
3.2. The overall prevalence LTBI in HCWs
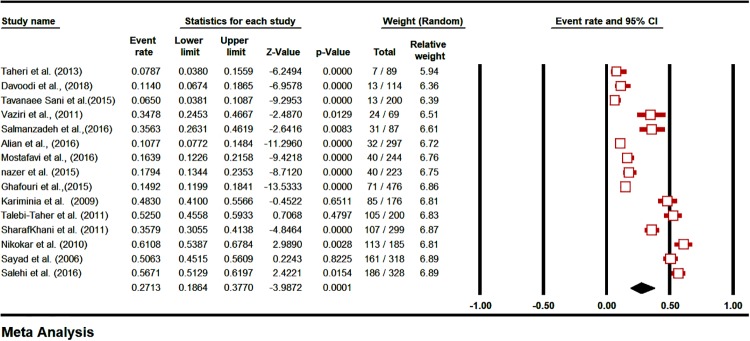
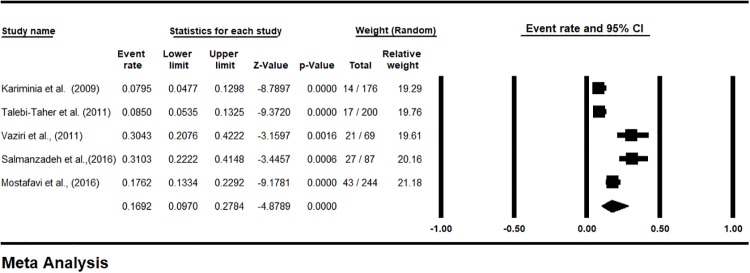
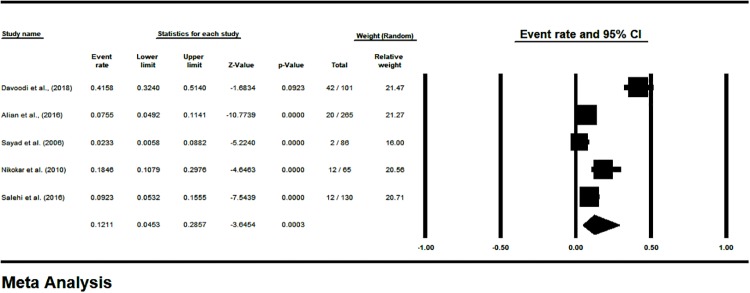
The prevalence of LTBI in HCWs, based on the PPD test (48 hours) was 27.13% [CI95%: 18.64–37.7] (Fig 2), and based on the QFT test was 16.92% [CI95%: 9.7–27.84] (Fig 3). The prevalence of LTBI was estimated 12.11% [CI95%: 4.53–28.57] in Iranian’s HCWs who had negative TST reaction (48 hours) in the first week (Fig 4). The prevalence of induration at TST site (48 h) was estimated <4 mm in 43.74% [CI 95%: 28.19–60.63], 5–9 mm in 17.52% [CI 95%: 9.73–29.5], 10–15 mm in 14.55% [CI 95%: 8.87–22.93] and >15 mm in 13.4% [CI 95%: 8.59–20.31] (S1 Fig).
Fig 2. The prevalence subgroup analysis based on TST/PPD induration diameter (48 hrs.) in Iranian’s HCWs with LTBI (forest plot-random effect model).
Fig 3. The prevalence subgroup analysis based on QFT in Iranian’s HCWs with LTBI (forest plot—Random effect model).
Fig 4. The prevalence subgroup analysis based on TST/PPD induration diameter after one week in Iranian’s HCWs with LTBI (forest plot—Random effect model).
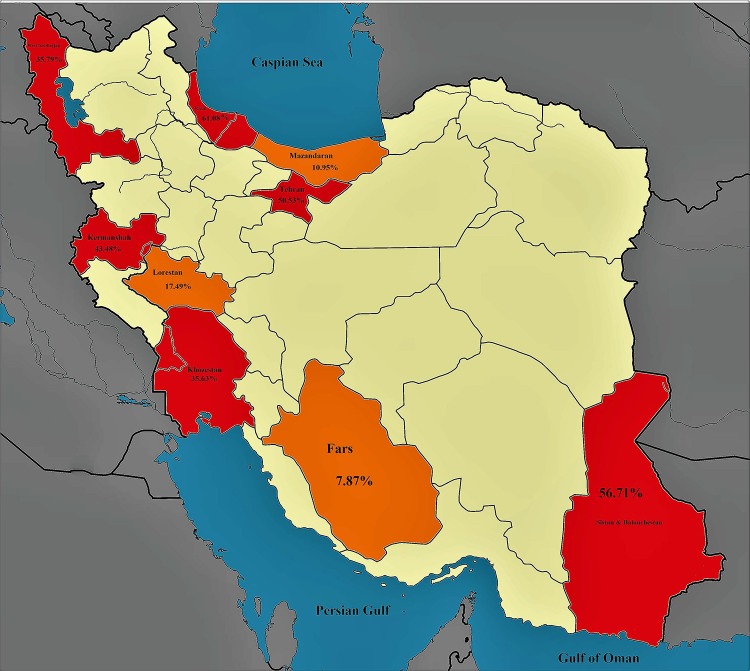
3.3. The prevalence of LTBI in Iranian HCWs based on geographical region of Iran
The highest prevalence of LTBI in Iranian’s HCWs was estimated 41.4% [CL95%: 25.4–59.5] in the north, and 33.8% [CI95%: 21.1–49.3] in the west of Iran. The lowest prevalence of LTBI was found 18.2% [CI95%: 3.4–58.2] in the south of Iran. These results showed a significant relationship between LTBI prevalence in Iranian’s HCWs and the geographic location in Iran (p <0.0001) (S2 Fig) (Fig 5).
Fig 5. Demonstrating LTBI in Iranian’s HCWs based on geographical classification (random effect model).
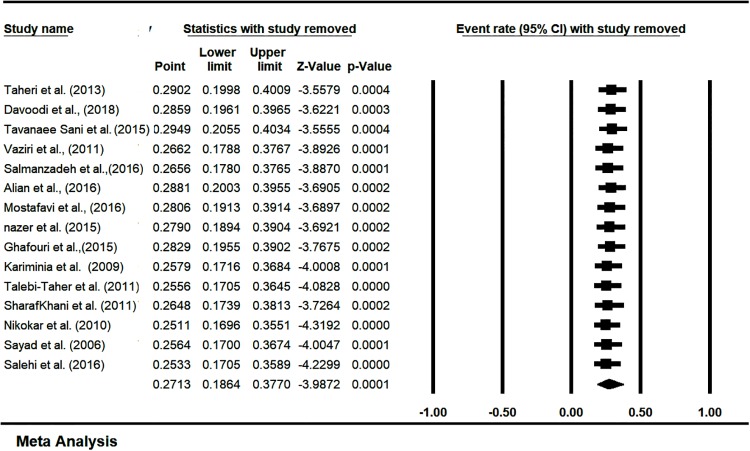
3.4. Sensitivity analysis and cumulative meta-analysis
Sensitivity analysis of prevalence of LTBI in Iranian’s HCWs was estimated with a 95% confidence interval. It showed that there is no significant effect on the overall prevalence of LTBI in Iranian’s HCWs (Fig 6). The overall prevalence of LTBI in Iranian’s HCWs based on the publication year was estimated by cumulative meta-analysis and represented in (S3 Fig). The sub group analysis of the quality of studies was showed in (S4 Fig).
Fig 6. Sensitivity analysis to prevalence of LTBI in Iranian’s HCWs (one study removed test).
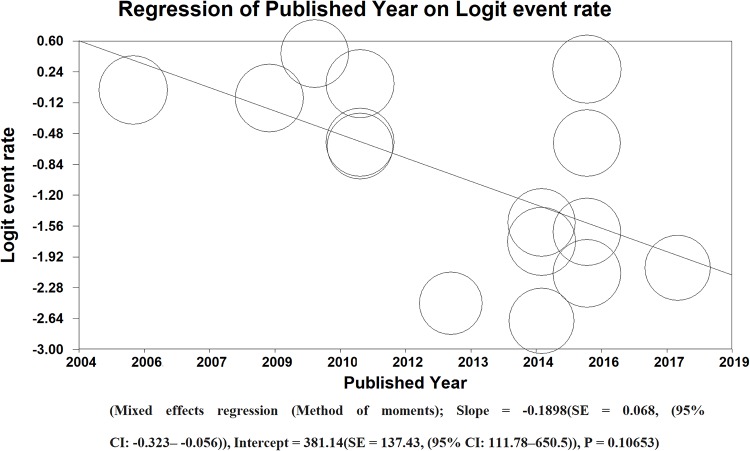
3.5. Meta-regression
Base on the PPD (48 hours) results, the prevalence of publishing manuscripts about identification of LTBI in Iranian’s HCWs has decreased in Iran. There was no significant relationship between publishing years. (Mixed effects regression (Method of moments); Slope = -0.1898(SE = 0.068, (95% CI: -0.323– -0.056)), Intercept = 381.14 (SE = 137.43, (95% CI: 111.78–650.5)), P = 0.10653) (Fig 7).
Fig 7. Meta-regression of LTBI in Iranian’s HCWs according to publishing year of studies (method of moments).
3.6. The prevalence of LTBI in HCWs based on term of employment
Base on the PPD results, the prevalence of LTBI in Iranian’s HCWs with more than 10 years old work-experience was evaluated 51%. The prevalence of LTBI in Iranian’s HCWs with less than 10 years old work-experience was estimated at 29.30%.
Base on the QFT results, the prevalence of LTBI in Iranian’s HCWs with more than 20 years old work-experience was calculated 20.49% [CI95%: 11–34.97], which showed a significant relationship between the duration of employment (P <0.0001) (S5 Fig).
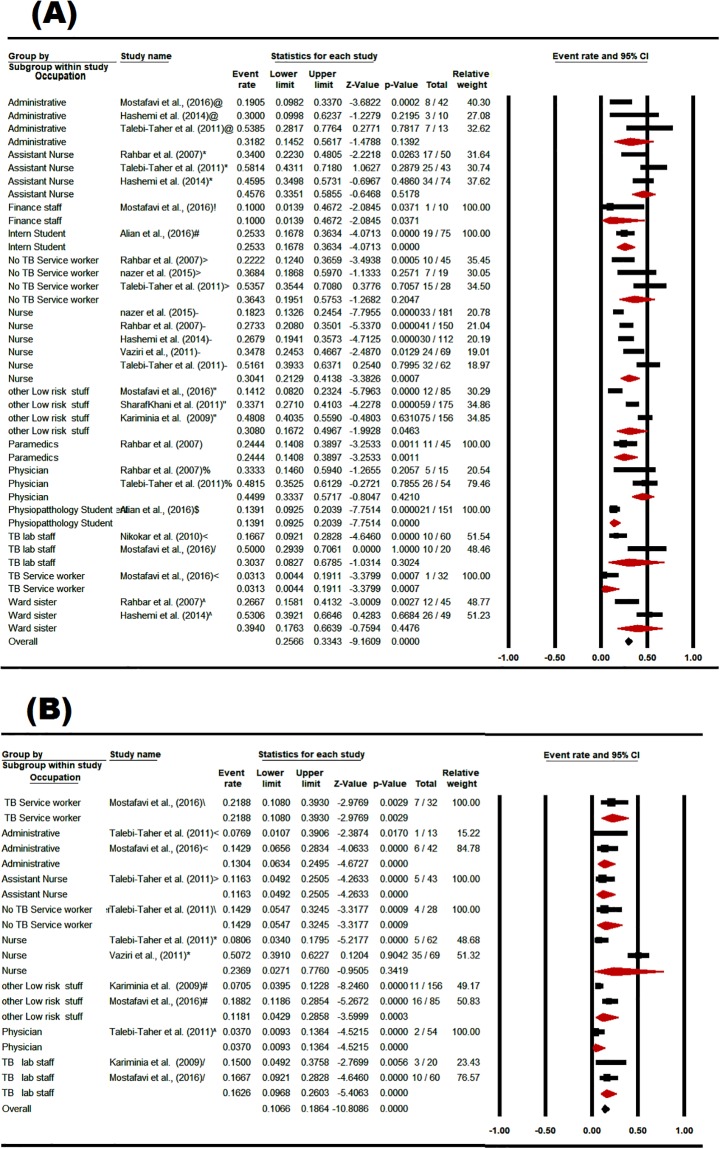
3.7. The prevalence of LTBI in Iranian HCWs based on occupation and wards
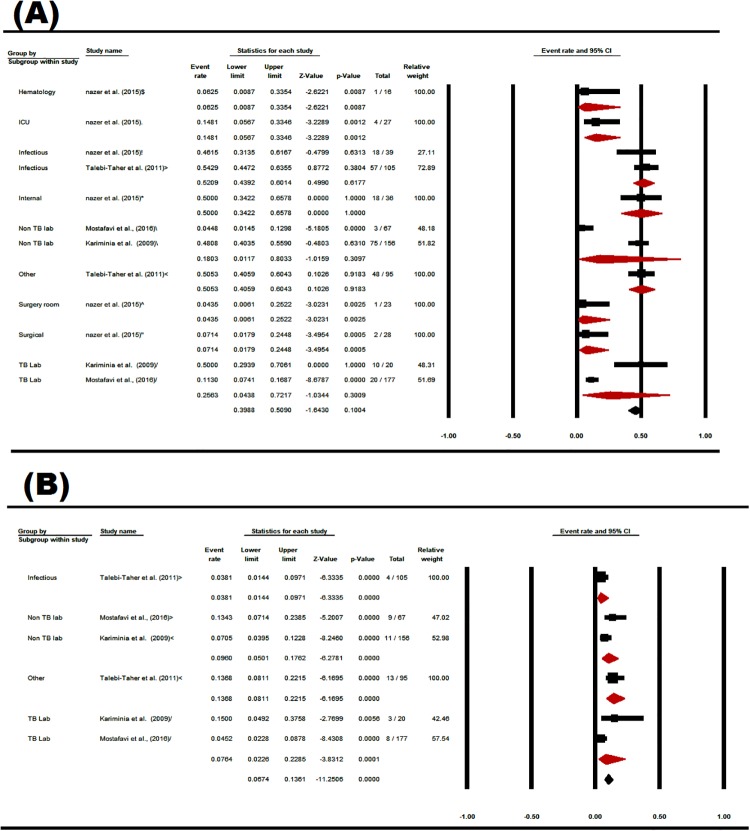
Base on the PPD results, the prevalence of LTBI in assistant nurses was estimated 45.76% [CI 95%: 33.51–58.55], in physicians was estimated 44.99% [CI95%: 33.37–57.17], in ward nurses was calculated 39.4% [CI95%: 17.63–66.39], and in service workers was estimated 36.43% [CI95%: 19.51–57.53]. Base on the QFT results, the prevalence of LTBI in both nurses and TB service workers was higher than other occupations (Fig 8).
Fig 8. The prevalence subgroup analysis of occupational based on PPD (A), and QFT (B) in Iranian’s HCWs with LTBI (forest plot—Random effect model).
The prevalence of LTBI in the infectious ward was estimated 52.09% [CI95%: 43.92–60.14], and in the internal ward was evaluated 50% [CI95%: 34.22–65.78]. The lowest prevalence of LTBI was estimated in the infectious wards based on QFT. There was a significant relationship between the prevalence of LTBI in Iranian’s HCWs, and hospital wards (p <0.0001) (Fig 9).
Fig 9. The prevalence subgroup analysis of ward based on PPD (A), and QFT (B) in Iranian’s HCWs with LTBI (forest plot—Random effect model).
3.8. The prevalence of LTBI in Iranian’s HCWs based on gender and age
The prevalence of LTBI was estimated at 42.16% [CI95%: 26.41–59.69] in male Iranian’s HCWs based on the PPD test. The prevalence of LTBI in Iranian’s HCWs and type of gender based on PPD test (P<0.051). In QFT, however, a significant relationship was showed between the prevalence of LTBI in Iranian’s HCWs, and the gender (P <0.0001) (S6 Fig).
The highest prevalence of LTBI in Iranian’s HCWs who were more than 40 years old was estimated 44% [CI 95%: 26.47–63.16] in the PPD test.
The highest prevalence of LTBI in Iranian’s HCWs aged 30 years old was estimated 22.52% [CI95%: 3.7–68.34] in the QFT. In both PPD test and QFT, it was evaluated that there was significant relationship between the prevalence of LTBI in Iranian’s HCWs, and age of HCWs (P<0.0001) (S7 Fig).
3.9. The prevalence of LTBI in Iranian’s HCWs based on the history of tuberculosis contact and the tuberculosis clinical symptoms
The results showed that 30.15% [CI 95%: 11–60.13] of Iranian’s HCWs directly contacted to patients with tuberculosis. The results also showed that 6.9% [CI 95%: 2.36–18.55] of Iranian’s HCWs had active tuberculosis symptoms (S8 and S9 Figs).
3.10. The prevalence of LTBI in Iranian’s HCWs based on the “BCG”
The prevalence of LTBI in Iranian’s HCWs who received the BCG was estimated 15% [CI 95%: 3.6–47.73] based on the PPD test. While the prevalence of LTBI in Iranian’s HCWs who did not receive the BCG was estimated at 25.71% [CI95%: 13.96–42.49] based on the QFT. In both PPD, and QFT, there was a significant relationship between those who did and those who did not receive the vaccine (P<0.0001) (S10 Fig).
3.11. Publication bias
The publication bias in this study was evaluated by Begg's and Egger's tests. The publication bias by Begg's test was calculated 0.06, and the Egger's test was calculated 0.028. The probability of the publication bias in this study was significant (S11 Fig).
4. Discussion
This study is the first systematic review and meta-analysis of LTBI outbreak been carried out in Iranian’s HCWs. According to results of the current meta-analysis, the prevalence of LTBI in Iranian’s HCWs is estimated at 27.1% [1]. Among the low and middle-income countries, the prevalence of LTBI in Kenya [63], Zimbabwe [64], Russia [65], Brazil [66], Vietnam [67], Rwanda [68], China [69] and South Africa [70] has been higher than in Iran [1]. The prevalence of LTBI in HCWs of Italy [71], Norway [72] and India [73] is reported to be equal to or less than Iran. Iran is a TB endemic country [25] and the treatment of LTBI is usually done by using a single medicine and only in high risk groups [74]. While in high-income countries, screening of pulmonary and lab staffs is recommended annually [75]. Also, it could be seen that training of Iranian’s HCWs is not sufficient to prevent tuberculosis [25].
According to the results of meta-analysis, the lowest prevalence of LTBI among Iranian’s HCWs was in southern Iran (18.2%). The highest prevalence of LTBI among Iranian’s HCWs was reported in northern and western Iran. The high prevalence of LTBI among Iranian’s HCWs may be due to neighboring Azerbaijan and Iraq [76]. Azerbaijan which is listed on the high burden countries has high prevalence of multidrug resistance MTB [76–78]. In fact, the northern neighbors of Iran, such as Kazakhstan, Azerbaijan, are among the high burden countries with a high prevalence of multi-drug resistant tuberculosis [80]. On the other hand, the name of the country's western neighbor of Iran–Iraq is not listed on the high burden countries [76–78] but according to reports from Ministry of Health—Iran Center for Medical Education and Treatment, Infectious Disease Control Center- the Iraqi state may have become a high-risk source for tuberculosis after undergoing its recent crisis [79, 80].
The current study showed that 15.5% of the Iranian’s HCWs had used before the BCG with at least a positive PPD test. According to studies, BCG does not protect adults from getting infected with tuberculosis, so the positive results of tuberculin testing in people vaccinated with BCG will be considered as a latent infection [81]. In other words, previous vaccination with BCG prevents tuberculin testing [82]. This may be due to a false positive reaction in PPD [25]. Iranian’s HCWs may respond to skin tests without being infected with mycobacterium [83]. The reason for these false-positive reactions may be due to contamination with non-tuberculosis mycobacterium, previous BCG, poor test performance or inappropriate interpretation of the test [83].
5. Limitations
Information about this meta-analysis was extracted from data published in Iranian databases as there was no access to the actual information of the control center of the Ministry of Health and Medical Education, so the exact prevalence of LTBI among Iranian’s HCWs could not be calculated. Selection bias is able to limit the generalization of these findings because the type of bacteria strains in a country could be different with the other countries and could be related to descent diversities.
On the other hands, patients may not respond to skin test tuberculosis, even if they are infected with Mycobacterium. It may be due to skin allergies, recent infections (recent contact for 8 to 10 weeks), chronic infection, recent vaccinations with live viruses, advanced tuberculosis, some viral diseases (measles and bile), misdiagnosis skin or incorrect interpretation of the reaction. Patients may also respond to skin tests, even without being infected with Mycobacterium. The reason for these reactions may be due to contamination with non-tuberculosis Mycobacterium, previous BCG, inappropriate test run or inappropriate interpretation of the test.
Despite the fact that the CDC updates the guidelines for the prevention and transmission of M. tuberculosis in health-care settings annually, the protocol for among Iranian’s HCWs has not yet been prepared. Also, workshops could be developed to train tuberculosis prevention and self-care among Iranian’s HCWs in the western regions.
National databases are not sensitive to operators “AND” and “OR” to search for the combinations. Also, some databases were not fully accessible because of using Guilan University of Medical Sciences’—Iran Ministry of Health & Medical Education- VPN.
6. Conclusion
This meta-analysis showed the prevalence of LTBI among Iranian’s HCWs and estimated at 27.1%. The prevalence of LTBI between HCWs of Italy, Norway and India is reported to be equal to or less than Iranian’s HCWs. On the other hand, the highest prevalence of LTBI among Iranian’s HCWs in the north and the west of Iran may due to neighboring with Azerbaijan and Iraq which has become a high-risk source for tuberculosis by overcoming its recent years of crisis. Meanwhile, it could be seen that training of Iranian’s HCWs is not sufficient to prevent tuberculosis. We also found that BCG was not able to protect Iranian’s HCWs from TB infectious, completely.
Supporting information
(DOC)
(PDF)
(PDF)
(XLSX)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Acknowledgments
We would like to thank the vice chancellor of research and technology, Guilan University of Medical Sciences (ID: IR.GUMS.REC.1397.513).
Appendix: PubMed search strategy
((Latent Tuberculosis) AND Iran AND Prevalence AND ((Health Personnel) OR (Healthcare Worker) OR (Health Care Provider)).
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Apriani L, McAllister S, Sharples K, Alisjahbana B, Ruslami R, Hill PC, et al. Latent tuberculosis infection in healthcare workers in low- and middle-income countries: an updated systematic review. The European respiratory journal. 2019;53(4). Epub 2019/02/23. 10.1183/13993003.01789-2018 . [DOI] [PubMed] [Google Scholar]
- 2.Korbel DS, Schneider BE, Schaible UE. Innate immunity in tuberculosis: myths and truth. Microbes Infect. 2008;10(9):995–1004. Epub 2008/09/03. 10.1016/j.micinf.2008.07.039 . [DOI] [PubMed] [Google Scholar]
- 3.North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. Epub 2004/03/23. 10.1146/annurev.immunol.22.012703.104635 . [DOI] [PubMed] [Google Scholar]
- 4.Russell DG. Who puts the tubercle in tuberculosis? Nat Rev Microbiol. 2007;5(1):39–47. Epub 2006/12/13. 10.1038/nrmicro1538 . [DOI] [PubMed] [Google Scholar]
- 5.De Chastellier C. The many niches and strategies used by pathogenic mycobacteria for survival within host macrophages. Immunobiology. 2009;214(7):526–42. 10.1016/j.imbio.2008.12.005 [DOI] [PubMed] [Google Scholar]
- 6.Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136(1):37–49. Epub 2009/01/13. 10.1016/j.cell.2008.11.014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houben RM, Dodd PJ. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med. 2016;13(10):e1002152 Epub 2016/10/26. 10.1371/journal.pmed.1002152 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azizi F, Hatami H, Janghorbani MJTEP. Epidemiology and control of common diseases in Iran. Tehran: Eshtiagh Publications; 2000:602–16. [Google Scholar]
- 9.Joshi R, Reingold AL, Menzies D, Pai MJPm. Tuberculosis among health-care workers in low-and middle-income countries: a systematic review. PLoS Med. 2006;3(12):e494 10.1371/journal.pmed.0030494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kassim S, Zuber P, Wiktor S, Diomande F, Coulibaly I, Coulibaly D, et al. Tuberculin skin testing to assess the occupational risk of Mycobacterium tuberculosis infection among health care workers in Abidjan, Cote d'Ivoire. Int J Tuberc Lung Dis. 2000;4(4):321–6. [PubMed] [Google Scholar]
- 11.Nosocomial TJIJT. Nosocomial tuberculosis in the era of drug resistant tuberculosis. Emerg Infect Dis. 2009; 56:59–61. [PubMed] [Google Scholar]
- 12.Yanai H, Limpakarnjanarat K, Uthaivoravit W, Mastro T, Mori T, Tappero J JTIJoT, et al. Risk of Mycobacterium tuberculosis infection and disease among health care workers, Chiang Rai, Thailand. Int J Tuberc Lung Dis. 2003;7(1):36–45. [PubMed] [Google Scholar]
- 13.Field MJ, editor. Tuberculosis in the Workplace Washington (DC): National Academies Press (US); 2001. C, The Occupational Tuberculosis Risk of Health Care Workers. https://www.ncbi.nlm.nih.gov/books/NBK222462/ [Google Scholar]
- 14.Azami M, Sayehmiri K, YektaKooshali MH, HafeziAhmadi MR. The prevalence of tuberculosis among Iranian elderly patients admitted to the infectious ward of hospital: A systematic review and meta-analysis. Int J Mycobacteriol. 2016;5:S199–S200. 10.1016/j.ijmyco.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 15.Christopher DJ, Daley P, Armstrong L, James P, Gupta R, Premkumar B, et al. Tuberculosis infection among young nursing trainees in South India. PLoS ONE. 2010;5(4):e10408 10.1371/journal.pone.0010408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garber E, San Gabriel P L L, Saiman L. A survey of latent tuberculosis infection among laboratory healthcare workers in New York City. Infection Control & Hospital Epidemiology. 2003;24(11):801–6. [DOI] [PubMed] [Google Scholar]
- 17.Jo K-W, Hong Y, Park JS, Bae I-G, Eom JS, Lee S-R, et al. Prevalence of latent tuberculosis infection among health care workers in South Korea: a multicenter study. Tuberc Respir Dis (Seoul) 2013;75(1):18–24. 10.4046/trd.2013.75.1.18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brock I, Weldingh K, Lillebaek T, Follmann F, Andersen P. Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am J Respir Crit Care Med. 2004;170(1):65–9. Epub 2004/04/17. 10.1164/rccm.200402-232OC . [DOI] [PubMed] [Google Scholar]
- 19.Kang YA, Lee HW, Yoon HI, Cho B, Han SK, Shim Y-S, et al. Discrepancy between the tuberculin skin test and the whole-blood interferon γ assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA. 2005;293(22):2756–61. 10.1001/jama.293.22.2756 [DOI] [PubMed] [Google Scholar]
- 20.Mori T, Sakatani M, Yamagishi F, Takashima T, Kawabe Y, Nagao K, et al. Specific detection of tuberculosis infection: an interferon-γ–based assay using new antigens. Am J Respir Crit Care Med. 2004;170(1):59–64. 10.1164/rccm.200402-179OC [DOI] [PubMed] [Google Scholar]
- 21.Bennett JE, Dolin R, Blaser MJ. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases: 2-Volume Set: Elsevier Health Sciences; 2014. [Google Scholar]
- 22.Menzies DJCID. What does tuberculin reactivity after bacille Calmette-Guérin vaccination tell us? Clin Infect Dis. 2000;31(Supplement_3):S71–S4. [DOI] [PubMed] [Google Scholar]
- 23.Menzies R, Vissandjee B. Effect of bacille Calmette-Guerin vaccination on tuberculin reactivity. Am Rev Respir Dis. 1992;145(3):621–5. Epub 1992/03/01. 10.1164/ajrccm/145.3.621 . [DOI] [PubMed] [Google Scholar]
- 24.Gazi MA, Islam MR, Kibria MG, Mahmud Z JEJoCM, Diseases I. General and advanced diagnostic tools to detect Mycobacterium tuberculosis and their drug susceptibility: a review. Eur J Clin Microbiol Infect Dis. 2015;34(5):851–61. 10.1007/s10096-014-2306-5 . [DOI] [PubMed] [Google Scholar]
- 25.Nasehi M, Hashemi-Shahraki A, Doosti-Irani A, Sharafi S, Mostafavi E JE, health. Prevalence of latent tuberculosis infection among tuberculosis laboratory workers in Iran. Epidemiol Health. 2017;30(39): e2017002 10.4178/epih.e2017002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liberati A, Taricco MJRiP, Books RMPMF. How to do and report systematic reviews and meta-analysis. 2010:137–64.
- 27.Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med. 1997;126(5):376–80. Epub 1997/03/01. 10.7326/0003-4819-126-5-199703010-00006 . [DOI] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG JAoim. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 29.Editors PLM. Best practice in systematic reviews: the importance of protocols and registration. PLoS Med. 2011;8(2):e1001009 Epub 2011/03/03. 10.1371/journal.pmed.1001009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasiri MJ, Pormohammad A, Goudarzi H, Mardani M, Zamani S, Migliori GB, et al. Latent tuberculosis infection in transplant candidates: a systematic review and meta-analysis on TST and IGRA. Infection. 2019;47(3):353–61. Epub 2019/02/26. 10.1007/s15010-019-01285-7 . [DOI] [PubMed] [Google Scholar]
- 31.JafariNezhad A, YektaKooshali MH. Lung cancer in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. PLoS One. 2018;13(8):e0202360 Epub 2018/08/17. 10.1371/journal.pone.0202360 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Costa Santos CM, de Mattos Pimenta CA, Nobre MR. The PICO strategy for the research question construction and evidence search. Rev Lat Am Enfermagem. 2007;15(3):508–11. Epub 2007/07/27. 10.1590/s0104-11692007000300023 . [DOI] [PubMed] [Google Scholar]
- 33.Krajewski W, Zdrojowy R, Grzegolka J, Krajewski P, Wrobel M, Luczak M, et al. Does Mantoux Test Result Predicts BCG Immunotherapy Efficiency and Severe ToXxicity in Non-Muscle Invasive Bladder Cancer. Urology journal. 2018. Epub 2018/11/25. 10.22037/uj.v0i0.4542 . [DOI] [PubMed] [Google Scholar]
- 34.Sargin G, Senturk T, Ceylan E, Telli M, Cildag S, Dogan H. TST, QuantiFERON-TB Gold test and T-SPOT.TB test for detecting latent tuberculosis infection in patients with rheumatic disease prior to anti-TNF therapy. Tuberkuloz ve toraks. 2018;66(2):136–43. Epub 2018/09/25. 10.5578/tt.66444 . [DOI] [PubMed] [Google Scholar]
- 35.Wang W, Liu HM, Zhou J, Wang YG, Feng X, Tang H, et al. Skin test of tuberculin purified protein derivatives with a dissolving microneedle-array patch. Drug delivery and translational research. 2019. Epub 2019/03/21. 10.1007/s13346-019-00629-y . [DOI] [PubMed] [Google Scholar]
- 36.Wigg AJ, Narayana SK, Anwar S, Ramachandran J, Muller K, Chen JW, et al. High rates of indeterminate interferon-gamma release assays for the diagnosis of latent tuberculosis infection in liver transplantation candidates. Transpl Infect Dis: an official journal of the Transplantation Society. 2019:e13087 Epub 2019/03/31. 10.1111/tid.13087 . [DOI] [PubMed] [Google Scholar]
- 37.Barton E, Gao Y, Ball D, Fidler K, Klein N, Curtis N, et al. Calcineurin Inhibitors and Variation in the Performance of Interferon-gamma Release Assays Used to Detect Tuberculosis Infection. Annals of the American Thoracic Society. 2019;16(6):771–5. Epub 2019/02/28. 10.1513/AnnalsATS.201811-784RL . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennet R, Nejat S, Eriksson M. Effective Tuberculosis Contact Investigation Using Interferon-Gamma Release Assays. The Pediatric infectious disease journal. 2019;38(4):e76–e8. Epub 2019/03/19. 10.1097/INF.0000000000002272 . [DOI] [PubMed] [Google Scholar]
- 39.Igari H, Akutsu N, Ishikawa S, Aoyama H, Otsuki K, Hasegawa M, et al. Positivity rate of interferon-gamma release assays for estimating the prevalence of latent tuberculosis infection in renal transplant recipients in Japan. J Infect Chemother: official journal of the Japan Society of Chemotherapy. 2019. Epub 2019/03/25. 10.1016/j.jiac.2019.02.018 . [DOI] [PubMed] [Google Scholar]
- 40.Poorolajal J, Cheraghi Z, Irani AD, Rezaeian S. Quality of Cohort Studies Reporting Post the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement. Epidemiol Health. 2011;33:e2011005 Epub 2011/07/01. 10.4178/epih/e2011005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. 2005;25(6):646–54. Epub 2005/11/12. 10.1177/0272989X05282643 . [DOI] [PubMed] [Google Scholar]
- 42.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. Epub 2010/04/01. 10.1002/jrsm.12 . [DOI] [PubMed] [Google Scholar]
- 43.Alian S, Dadashi A, Najafi N, Alikhani A, Davoudi A, Moosazadeh M, et al. Evaluation of Tuberculin Skin Test (TST) in Medical Students in Mazandaran University of Medical Sciences, Sari, Iran. Global Journal of Health Science. 2016;9(5):274. [Google Scholar]
- 44.Besharat M, Abbasi F. Tuberculin Skin Test among 1,424 Healthy Employees in Chaharmahal Province, Iran. Tanaffos. 2011;1(10):37–9. [PMC free article] [PubMed] [Google Scholar]
- 45.Davoodi L, Babamahmoodi F, Mirabi A, Mohammad Hosseini E. Evaluation of Tuberculin Skin Test Seroconversion Among the Staff in Qaemshahr Razi Hospital, 2015–2017. Journal of Mazandaran University of Medical Sciences. 2018;28(164):158–63. [Google Scholar]
- 46.Ghafouri M, Seyed Sharifi S. Prevalence of latent tuberculosis infections in Health care workers (HCW) in Mashhad Hospital. J North Khorasan Univ Med Sci. 2015;6(4):829–40. [Google Scholar]
- 47.Golchin M, Rostami M. Tuberculin test in nursing and human-sciences students. Journal of Research in Medical Sciences. 2005;10(3):172–6. [Google Scholar]
- 48.Hashemi SH, Mamani M, Alizadeh N, Nazari M, Sedighi I. Prevalence of tuberculosis infection among health-care workers in Hamadan, west of Iran. Avicenna J Clin Microbiol Infect. 2014;1(1):e19214. [Google Scholar]
- 49.Kariminia A, Sharifnia Z, Aghakhani A, Banifazl M, Eslamifar A, Hazrati M, et al. Comparison of QuantiFERON TB-G-test to TST for detecting latent tuberculosis infection in a high-incidence area containing BCG-vaccinated population. Journal of evaluation in clinical practice. 2009;15(1):148–51. Epub 2009/02/26. 10.1111/j.1365-2753.2008.00970.x . [DOI] [PubMed] [Google Scholar]
- 50.Mostafavi E, Nasehi M, Hashemi Shahraki A, Esmaeili S, Ghaderi E, Sharafi S, et al. Comparison of the tuberculin skin test and the QuantiFERON-TB Gold test in detecting latent tuberculosis in health care workers in Iran. Epidemiol Health. 2016;38:e2016032 Epub 2016/07/28. 10.4178/epih.e2016032 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nasehi M, Hashemi-Shahraki A, Doosti-Irani A, Sharafi S, Mostafavi E. Prevalence of latent tuberculosis infection among tuberculosis laboratory workers in Iran. Epidemiol Health. 2017;39:e2017002 Epub 2017/01/18. 10.4178/epih.e2017002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nazer M, Shahivand M, Zare S. The prevalence of latent tuberculosis (TB) infection in healthcare staff of Khorramabad Ashayer hospital in 2015. Yafte. 2015;17(2):23–31. [Google Scholar]
- 53.Nikokar I, Dadgran A, Mafozei L. A comparison of two-step tuberculin skin test between health-care workers and nonhospital employees. Iranian Journal of Medical Sciences. 2015;35(3):201–4. [Google Scholar]
- 54.Rahbar M, Karamyar M, Hajia M. Prevalence and determinant of tuberculin skin test among health care workers of Imam Khomeini Hospital of Uremia, Iran. Shiraz E-Medical Journal. 2007;8(4):162–7. [Google Scholar]
- 55.Salehi M, Mood BS, Metanat M. Positive Tuberculin Skin Test Among Health Care Workers: Prevalence and Risk Factors in Teaching Hospitals of a Highly Endemic Region for Tuberculosis, Zahedan, Iran. Int J Infect. 2016;3(3). [Google Scholar]
- 56.Salmanzadeh S, Abbasissifar H, Alavi SM. Comparison study of QuantiFERON test with tuberculin skin testing to diagnose latent tuberculosis infection among nurses working in teaching hospitals of Ahvaz, Iran. Caspian journal of internal medicine. 2016;7(2):82–7. Epub 2016/07/08. . [PMC free article] [PubMed] [Google Scholar]
- 57.Sayad B, Zarpeyma A, Janbakhsh A. Tuberculin Skin Test Results in Health Care Workers of Imam Khomeini Hospital (Kermanshah 2004). J Kermamnshah Univ Med Sci (Behbood). 2006;10(3):258–67. [Google Scholar]
- 58.Sharafkhani R, Ahmadi N, Salarilak S, Rahimirad M, Khashabi J. prevalence of tuberculosis infection in health and office workers at Urmia University of Medical Sciences. J Urmia Univ Med Sci. 2011;22(2):119–22. [Google Scholar]
- 59.Taheri M, Bazrafkan H, Habibagahi M. Determining the Latent Tuberculosis Infection by IFN—gamma Elispot Assay in Healthcare Workers from University Hospitals of Shiraz, South West of Iran. Iran Red Crescent Med J. 2013;15(6):477–82. Epub 2013/12/19. 10.5812/ircmj.3635 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Talebi-Taher M, Javad-Moosavi SA, Entezari AH, Shekarabi M, Parhizkar B. Comparing the performance of QuantiFERON-TB Gold and Mantoux test in detecting latent tuberculosis infection among Iranian health care workers. Int J Occup Med Environ Health. 2011;24(4):359–66. Epub 2011/11/17. 10.2478/s13382-011-0046-7 . [DOI] [PubMed] [Google Scholar]
- 61.Tavanaee Sani A, Hajian S, Maryam S. Evaluation of PPD test in Medical Student of Mashhad University Medical Sciences in 2011–2013. Med J Mashhad Univ of Med Sci. 2015;58(8):441–5. [Google Scholar]
- 62.Vaziri S, Khazaei S, Neishaboori S, Kanani M, Madani S. The degree of agreement of quantiferon TB gold test and tuberculin skin test in nurses. J Gorgan Univ Med Sci. 2011;13(1):37–43. [Google Scholar]
- 63.Agaya J, Nnadi CD, Odhiambo J, Obonyo C, Obiero V, Lipke V, et al. Tuberculosis and latent tuberculosis infection among healthcare workers in Kisumu, Kenya. Trop Med Int Health. 2015;20(12):1797–804. 10.1111/tmi.12601 . [DOI] [PubMed] [Google Scholar]
- 64.Corbett EL, Marston B, Churchyard GJ, De Cock KM. Tuberculosis in sub-Saharan Africa: opportunities, challenges, and change in the era of antiretroviral treatment. Lancet. 2006;367(9514):926–37. Epub 2006/03/21. 10.1016/S0140-6736(06)68383-9 . [DOI] [PubMed] [Google Scholar]
- 65.Drobniewski F, Cooke M, Jordan J, Casali N, Mugwagwa T, Broda A, et al. Systematic review, meta-analysis and economic modelling of molecular diagnostic tests for antibiotic resistance in tuberculosis. Health Technol Assess. 2015;19(34):1–188, vii–viii. Epub 2015/05/09. 10.3310/hta19340 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Franco C, Zanetta D JTIJoT, Disease L. Assessing occupational exposure as risk for tuberculous infection at a teaching hospital in Sao Paulo, Brazil. Int J Tuberc Lung Dis. 2006;10(4):384–9. . [PubMed] [Google Scholar]
- 67.Powell K, Han D, Hung N, Vu T, Sy D, Trinh T, et al. Prevalence and risk factors for tuberculosis infection among personnel in two hospitals in Viet Nam. Int J Tuberc Lung Dis. 2011;15(12):1643–9. 10.5588/ijtld.11.0207 . [DOI] [PubMed] [Google Scholar]
- 68.Rutanga C, Lowrance DW, Oeltmann JE, Mutembayire G, Willis M, Uwizeye CB, et al. Latent tuberculosis infection and associated factors among Health Care Workers in Kigali, Rwanda. PLoS One.2015;10(4):e0124485 10.1371/journal.pone.0124485 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu S, Xia L, Yu S, Chen S, Zhang J. The burden and challenges of tuberculosis in China: findings from the Global Burden of Disease Study 2015. Sci Rep. 2017;7(1):14601 Epub 2017/11/04. 10.1038/s41598-017-15024-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Rie A, Beyers N, Gie RP, Kunneke M, Zietsman L, Donald PR. Childhood tuberculosis in an urban population in South Africa: burden and risk factor. Arch Dis Child. 1999;80(5):433–7. Epub 1999/04/20. 10.1136/adc.80.5.433 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Durando P, Sotgiu G, Spigno F, Piccinini M, Mazzarello G, Viscoli C, et al. Latent tuberculosis infection and associated risk factors among undergraduate healthcare students in Italy: a cross-sectional study. BMC Infectious Diseases. 2013;13(1):443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8(1):15 Epub 2008/01/16. 10.1186/1471-2458-8-15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prasad R, Singh A, Balasubramanian V, Gupta N. Extensively drug-resistant tuberculosis in India: Current evidence on diagnosis & management. Indian J Med Res. 2017;145(3):271–93. Epub 2017/07/28. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nasreen S, Shokoohi M, Malvankar-Mehta MS JPo. Prevalence of latent tuberculosis among health care workers in high burden countries: a systematic review and meta-analysis. PLoS One. 2016;11(10):e0164034 10.1371/journal.pone.0164034 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Apriani L, McAllister S, Sharples K, Alisjahbana B, Ruslami R, Hill PC, et al. Latent tuberculosis infection in health care workers in low and middle-income countries: an updated systematic review. Eur Respir J. 2019; 18;53(4):1801789 10.1183/13993003.01789-2018 [DOI] [PubMed] [Google Scholar]
- 76.Karadakhy K, Othman N, Ibrahimm F, Saeed AA, Amin AAH. Tuberculosis in Sulaimaniyah, Iraqi Kurdistan: A Detailed Analysis of Cases Registered in Treatment Centers. Tanaffos. 2016;15(4):197–204. Epub 2016/01/01. . [PMC free article] [PubMed] [Google Scholar]
- 77.Hassan DN, Hanna AJ. Tuberculosis and sudden death in Baghdad. Am J Forensic Med Pathol. 1984;5(2):169–74. Epub 1984/06/01. 10.1097/00000433-198406000-00013 . [DOI] [PubMed] [Google Scholar]
- 78.Sargazi A, Sepehri Z, Sagazi A, Jim PN, Kiani Z JAr, control i. Eastern Mediterranean region tuberculosis economic burden in 2014. Antimicrob Resist Infect Control. 2015;4(1):P102 10.1186/2047-2994-4-S1-P102 [DOI] [Google Scholar]
- 79.Sahebi L, Ansarin K, Maryam S, Monfaredan A, Sabbgh Jadid H. The factors associated with tuberculosis recurrence in the northwest and west of iran. Malays J Med Sci. 2014;21(6):27–35. Epub 2015/04/22. . [PMC free article] [PubMed] [Google Scholar]
- 80.Jimma W, Ghazisaeedi M, Shahmoradi L, Abdurahman AA, Kalhori SRN, Nasehi M, et al. Prevalence of and risk factors for multidrug-resistant tuberculosis in Iran and its neighboring countries: systematic review and meta-analysis. Rev Soc Bras Med Trop. 2017;50(3):287–95. Epub 2017/07/13. 10.1590/0037-8682-0002-2017 . [DOI] [PubMed] [Google Scholar]
- 81.Rezai MS, Abedi S, Afshari M, Moosazadeh M JOph, perspectives r. Estimating tuberculin skin test reactions among children and teenagers who received the bacillus Calmette-Guerin vaccination at birth: A meta-analysis. Osong Public Health Res Perspect. 2017;8(1):3–10. 10.24171/j.phrp.2017.8.1.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goscé L, Bitencourt J, Gupta R, Arruda S, Rodrigues L, Abubakar I JIJoID. BCG vaccination following latent TB treatment: Possible implications for different settings. Int J Infect Dis. 2019;80:S17–S9. 10.1016/j.ijid.2019.02.026 . [DOI] [PubMed] [Google Scholar]
- 83.Al-Orainey IO JAotm. Diagnosis of latent tuberculosis: Can we do better? Ann Thorac Med. 2009;4(1):5–10. 10.4103/1817-1737.44778 [DOI] [PMC free article] [PubMed] [Google Scholar]