Abstract
Purpose
Primary congenital glaucoma (PCG) is an autosomal recessive eye disorder, accounting for 0.01%–0.04% of blindness around the world. Unfortunately, the molecular characteristics concerning the pathogenic mechanisms of the disease remain poorly understood.
Methods
Here, for the first time, we employed gas chromatography coupled to time-of-flight mass spectrometry (GC/TOF MS) to reveal comprehensively the metabolic characteristics of PCG.
Results
First, 363 metabolites were detected in 50 aqueous humor (AH) samples from 30 patients with PCG, 10 patients with congenital cataracts (CCs), and 10 patients with aged-related cataracts (ARCs). Second, 290 metabolites in total were found in another 15 patients with PCG and 10 patients with primary open angle glaucoma (POAG). A further analysis suggested that patients with PCG had a significantly distinct metabolomics profile. Three amino acid-associated metabolites, including glycine, urea, and phenylalanine, were identified to be significantly different (p≤0.05) in relation to PCG. Meanwhile, three glaucoma-associated single nucleotide polymorphisms (SNPs), rs7114303, rs9364602, and rs2165241, were determined to be related to these three metabolites. The results here indicate that certain amino acid-associated metabolites and their metabolisms are key regulatory elements and metabolic pathways in the pathogenesis of PCG.
Conclusions
Collectively, this work not only extended our understanding of the molecular characteristics of PCG, but also presented glycine as a potential biomarker for earlier diagnosis and may provide new therapeutic strategies for the disease.
Introduction
Glaucoma is one of the most common causes of global irreversible blindness. The number of glaucoma patients was estimated at 64.3 million in 2013, and it is expected to increase to nearly 80 million globally by the year 2020 [1]. Generally, there are three subtypes of primary glaucoma according to the age of onset, etiology, and anatomy of the anterior chamber: primary open angle glaucoma (POAG), primary congenital glaucoma (PCG), and primary angle closure glaucoma (PACG) [2]. Among them, POAG is the most common form, accounting for 80% of glaucomatous diseases, while PCG is recognized as another severe form of glaucoma in newborns and infants, despite its low incidence [2,3].
The disease of glaucoma, including PCG, remains poorly understood, and more importantly, there are no biomarkers for diagnosis and prognosis, as well as a small range of therapeutic options [4]. Genomic, proteomic, and metabolomics techniques have recently offered the potential to identify molecular biomarkers in glaucoma due to increased sensitivity and accuracy [5]. For example, several genetic biomarkers have been determined, including CDKN2B-AS1 genomic region polymorphisms, which display a strong association with POAG [5]. Moreover, Funke et al. (2016) collected retina samples of glaucoma and non-glaucoma control donors to uncover proteomic changes using a state-of-the-art mass spectrometry (MS) workflow [6]. The results finally identified new molecular players, including adenine nucleotide translocase 3 (ANT3), methyl-CpG binding protein 2 (MeCp2), and dense fine speckles 70 (DFS70), as significantly associated with glaucoma. González-Iglesias et al. (2014) also conducted a comparative proteomic study of POAG, pseudoexfoliation glaucoma (PEXG), and healthy controls using two-dimensional fluorescence difference gel electrophoresis (2D-DIGE), and 17 proteins were determined to be most differentially altered, presenting new perspectives in biomarker discovery for glaucoma [7].
In addition, another advanced high-throughput approach, metabolomics, has been applied to extend our understanding of molecular changes in the pathogenesis and to provide potential biomarkers for various eye diseases, including age-related macular degeneration, high myopia, glaucoma, and diabetic retinopathy [4,8-11]. For instance, using gas chromatography-mass spectrometry (GC-MS), Rong et al. (2017) reported the targeted metabolite profiling of 86 serum samples from newly diagnosed PACG patients and controls [12]. Palmitoleic acid, γ-linolenic acid, and linoleic acid were determined to be potential biomarkers for the screening of PACG. Likewise, Ji et al. (2017) employed a non-targeted metabolomics technology, gas chromatography coupled to time-of-flight mass spectrometry (GC/TOF MS) to identify potential biomarkers in relation to high myopia [11]. In total, 242 metabolites were identified in 40 aqueous humor (AH) samples from patients with high myopia and the controls. Twenty-nine significantly changed metabolites and their regulatory aspects of the metabolic pathways were further determined to be key regulatory elements or pathways in the development of high myopia.
The AH plays a significant role in glaucoma pathogenesis, as efflux congestion of the AH mainly contributes to ocular hypertension in the disease [13]. However, limited studies on the molecular characteristics in relation to PCG have been previously published except in genetic linkage analysis. Here, taking advantage of the non-targeted metabolomics technology GC/TOF MS reported by Ji et al. (2017), for the first time, we comprehensively uncover metabolomics signatures in AH samples from patients with PCG and controls [11]. The results here may provide potential biomarkers and extend our understanding of the pathophysiology, allowing for a better understanding of the mechanisms of onset and for the determination of new therapeutic strategies for PCG.
Methods
Subjects
Four kinds of patients were recruited in the present study (Table 1): 45 patients with PCG, 10 patients with congenital cataracts (CCs), 10 patients with aged-related cataracts (ARCs), and 10 patients with POAG. All patients were diagnosed by the same ophthalmologist according to previous reports [14-17]. Especially for patients with PCG, the diagnostic criteria were: (1) intraocular pressure (IOP) >21 mmHg, (2) corneal diameter enlargement or corneal edema, and (3) increased cup-to-disc ratio. Meanwhile, the ARC and CC patients met the inclusion criteria with no ocular disease other than cataracts. In addition, we enrolled POAG patients with an age greater than 35 years, an open anterior chamber angle without morphological abnormalities, an IOP of more than 22 mmHg, and glaucomatous optic neuropathy upon funduscopy. All participants provided written informed consent, and the Ethics Committee of the Eye & ENT Hospital of Fudan University (Shanghai, China) reviewed and approved the study protocol. Meanwhile, all investigations were conducted in accordance with the tenets of the Declaration of Helsinki.
Table 1. Summary of human aqueous humor samples.
| Group | Patient ID | Gender | Age (Years old) | Ethnicity | Disease |
|---|---|---|---|---|---|
| 1 |
XB-5_1 |
Male |
5 |
Han |
Congenital cataract |
| XB-10_1 |
Male |
4 |
Han |
Congenital cataract |
|
| XB-8_1 |
Male |
5 |
Han |
Congenital cataract |
|
| XB-9_1 |
Male |
7 |
Han |
Congenital cataract |
|
| XB-2_1 |
Female |
3 |
Han |
Congenital cataract |
|
| XB-6_1 |
Male |
3 |
Han |
Congenital cataract |
|
| XB-7_1 |
Male |
6 |
Han |
Congenital cataract |
|
| XB-3_1 |
Male |
7 |
Han |
Congenital cataract |
|
| XB-4_1 |
Female |
6 |
Han |
Congenital cataract |
|
| XB-1_1 |
Female |
5 |
Han |
Congenital cataract |
|
| LB-10_1 |
Male |
61 |
Han |
Aged-related cataract |
|
| LB-7_1 |
Female |
56 |
Han |
Aged-related cataract |
|
| LB-4_1 |
Female |
63 |
Han |
Aged-related cataract |
|
| LB-5_1 |
Female |
66 |
Han |
Aged-related cataract |
|
| LB-6_1 |
Male |
62 |
Han |
Aged-related cataract |
|
| LB-9_1 |
Male |
63 |
Han |
Aged-related cataract |
|
| LB-3_1 |
Female |
65 |
Han |
Aged-related cataract |
|
| LB-8_1 |
Male |
61 |
Han |
Aged-related cataract |
|
| LB-1_1 |
Female |
60 |
Han |
Aged-related cataract |
|
| LB-2_1 |
Male |
63 |
Han |
Aged-related cataract |
|
| C416–1_1 |
Male |
7 |
Han |
Primary congenital glaucoma |
|
| C467_1 |
Female |
9 |
Han |
Primary congenital glaucoma |
|
| C420–2_1 |
Male |
7 |
Han |
Primary congenital glaucoma |
|
| C412_1 |
Male |
5 |
Han |
Primary congenital glaucoma |
|
| C426_1 |
Male |
6 |
Han |
Primary congenital glaucoma |
|
| C473_1 |
Male |
5 |
Han |
Primary congenital glaucoma |
|
| C424–1_1 |
Female |
4 |
Han |
Primary congenital glaucoma |
|
| C461_1 |
Male |
7 |
Han |
Primary congenital glaucoma |
|
| C401_1 |
Male |
7 |
Han |
Primary congenital glaucoma |
|
| C472–2_1 |
Male |
5 |
Han |
Primary congenital glaucoma |
|
| C411_1 |
Female |
4 |
Han |
Primary congenital glaucoma |
|
| C413_1 |
Female |
4 |
Han |
Primary congenital glaucoma |
|
| C423_1 |
Male |
8 |
Han |
Primary congenital glaucoma |
|
| C362_1 |
Male |
5 |
Han |
Primary congenital glaucoma |
|
| C456–1_1 |
Male |
5 |
Han |
Primary congenital glaucoma |
|
| C393_1 |
Male |
5 |
Han |
Primary congenital glaucoma |
|
| C444_1 |
Male |
8 |
Han |
Primary congenital glaucoma |
|
| C469–1_1 |
Male |
5 |
Han |
Primary congenital glaucoma |
|
| C454_1 |
Male |
4 |
Han |
Primary congenital glaucoma |
|
| C469–2_1 |
Male |
4 |
Han |
Primary congenital glaucoma |
|
| C462_1 |
Male |
3 |
Hui |
Primary congenital glaucoma |
|
| C456–2_1 |
Female |
4 |
Han |
Primary congenital glaucoma |
|
| C468_1 |
Female |
4 |
Han |
Primary congenital glaucoma |
|
| C416–2_1 |
Male |
4 |
Han |
Primary congenital glaucoma |
|
| C474_1 |
Male |
7 |
Han |
Primary congenital glaucoma |
|
| C424–2_1 |
Male |
7 |
Han |
Primary congenital glaucoma |
|
| C415_1 |
Male |
3 |
Han |
Primary congenital glaucoma |
|
| C420–1_1 |
Male |
3 |
Han |
Primary congenital glaucoma |
|
| C472–1_1 |
Male |
3 |
Han |
Primary congenital glaucoma |
|
| C419_1 |
Male |
3 |
Han |
Primary congenital glaucoma |
|
| 2 |
C478_1 |
Male |
3 |
Han |
Primary congenital glaucoma |
| C510_1 |
Female |
4 |
Han |
Primary congenital glaucoma |
|
| C506_1 |
Male |
3 |
Han |
Primary congenital glaucoma |
|
| C493_1 |
Female |
5 |
Han |
Primary congenital glaucoma |
|
| C481_1 |
Female |
9 |
Han |
Primary congenital glaucoma |
|
| C477_1 |
Male |
14 |
Han |
Primary congenital glaucoma |
|
| C485_1 |
Male |
5 |
Han |
Primary congenital glaucoma |
|
| C505_1 |
Female |
3 |
Han |
Primary congenital glaucoma |
|
| C513_1 |
Male |
3 |
Han |
Primary congenital glaucoma |
|
| C515_1 |
Male |
4 |
Han |
Primary congenital glaucoma |
|
| C476_1 |
Male |
3 |
Han |
Primary congenital glaucoma |
|
| C474_1 |
Male |
3 |
Han |
Primary congenital glaucoma |
|
| C520_1 |
Male |
4 |
Han |
Primary congenital glaucoma |
|
| C487_1 |
Male |
3 |
Han |
Primary congenital glaucoma |
|
| C507_1 |
Male |
3 |
Han |
Primary congenital glaucoma |
|
| K899_1 |
Female |
55 |
Han |
Primary open angle glaucoma |
|
| K2077–1_1 |
Female |
61 |
Han |
Primary open angle glaucoma |
|
| K175_1 |
Male |
44 |
Han |
Primary open angle glaucoma |
|
| K301_1 |
Male |
77 |
Han |
Primary open angle glaucoma |
|
| K174_1 |
Male |
77 |
Han |
Primary open angle glaucoma |
|
| K2085_1 |
Male |
23 |
Han |
Primary open angle glaucoma |
|
| K2053_1 |
Male |
45 |
Han |
Primary open angle glaucoma |
|
| K1013–2_1 |
Male |
68 |
Han |
Primary open angle glaucoma |
|
| K2077–2_1 |
Male |
68 |
Han |
Primary open angle glaucoma |
|
| K1013–1_1 | Male | 35 | Han | Primary open angle glaucoma |
AH samples
AH samples from patients were obtained after topical anesthesia and disinfection before routine cataract surgery [18]. Paracentesis was employed to ensure minimal contact with other intraocular structures and, more importantly, to prevent the potential influences on metabolites in the AH samples. The extraction of AH samples was conducted as previously reported [11]. Afterwards, AH samples were transferred to dust-free Eppendorf tubes immediately and centrifuged twice at 4 °C at 16,000 ×g for 15 min. Finally, the supernatant was collected and rapidly stored at −80 °C until further metabolomics analysis.
Metabolomics analysis
Gas chromatography (Agilent 7890A, Agilent) combined with a Pegasus 4D time-of-flight mass spectrometer (LECO ChromaTOF PEGASUS 4D, LECO) was employed for the metabolite profiling of AH samples, which is a popular technique for metabolite profiling due to its higher mass accuracy and mass resolution [11]. Briefly, 1 μl volume was injected into the Agilent DB-5MS capillary column (30 m × 250 μm × 0.25 μm, J&W Scientific, Folsom, CA), while the carrier gas, helium, was used at a constant flow rate (1 ml/min) through the column. Meanwhile, the initial temperature was 90 °C for 2 min, which then increased to 180 °C at a rate of 5 °C/min and finally to 285 °C at a rate of 15 °C/min. Temperatures for the injection, transfer line, and ion source were 280 °C, 270 °C, and 220 °C, respectively. The mass spectrometry data were obtained in full-scan mode at a rate of 100 spectra per second with an m/z range of 20–600 after a solvent delay of 492 s. Meanwhile, the resolution of the instrument was 1,000 (full width at half maximum [FWHM]) and the mass accuracy of the TOF attached to the GC was ±0.1 Da. In addition, the Chroma TOF4.3X software of the LECO Corporation was used here to acquire mass spectrometric data, including raw peak extraction, data baseline filtering and calibration, peak alignment, and deconvolution analysis. As with previous studies, the LECO/Fiehn Metabolomics Library was used to identify the compounds, which would give a similarity value for compound identification accuracy [11,19,20]. If the similarity value was more than 700, the metabolite identification was reliable. If the similarity value was between 200 and 700, the compound was considered a putative annotation. If the similarity value was less than 200, the compound was defined as an “analyte.”
Data analysis
Metabolic data were normalized and analyzed as previously reported [21]. Briefly, metabolites with no value in less than 50% of the samples were first removed, and then a data normalization step was performed by registering the median level of each compound to equal to one (1.00). Missing values (if any) were assumed below the limits of detection and were imputed with the observed minimum. A hierarchical cluster analysis was then performed using the MultiExperiment Viewer (Mev) 4.8 software. Meanwhile, the SIMCA-P software (v13.0, Umetrics, Malmö, Sweden) was employed here for both the principal component analysis (PCA) and partial least-squares discriminant analysis (PLS-DA). Together with the PLS-DA, the independent t test (statistical product and service solutions [SPSS] 17.0 software) was used to determine significantly different metabolites. Metabolites with both variable importance in the projection (VIP) values in the PLS-DA model greater than 1 and p values (in t tests) less than 0.05 were considered significant. Regression models were further used to adjust for age and gender in comparisons among the three groups (PCG versus CC versus ARC) and between the PCG and POAG groups. In addition, both the metabolic pathway and enrichment analysis of significantly different metabolites (adjusted p≤0.05) were conducted according to the MetaboAnalyst web server. Significant pathways were judged based on false discovery rate (FDR) threshold 0.05, whose raw p values are less than 0.005 [22].
Results
AH samples characteristics
In the present study, four kinds of patients were collected, all from the Han nationality (except one patient from the Hui nationality) in China: 45 patients with PCG, 10 patients with CCs, 10 patients with ARCs, and 10 patients with POAG. Notably, all 75 patients were then divided into two groups for two steps of metabolite profiling (Table 1): one group of 30 patients with PCG, 10 patients with CCs, and 10 patients with ARCs and a second group for the other 15 patients with PCG and 10 patients with POAG. Because it is hard to obtain normal samples for clinical use from the controls due to ethical issues, we first took two kinds of patients as the controls: patients with CCs and patients with ARCs. To investigate comprehensively the metabolic changes in patients especially with PCG, we further enrolled 15 patients with PCG and 10 patients with POAG for comparative metabolomics analyses at the second step. It should be pointed out that we analyzed the metabolic data during these two steps respectively, as the numbers of detected metabolites between them were different. We believe that the study is sufficient to reveal the metabolic characteristics of PCG.
Metabolic changes among patients with PCG, patients with CCs, and patients with ARCs
A non-targeted metabolomics technology, GC/TOF MS, was employed here to uncover comprehensively the human AH metabolome. In total, 363 metabolites (Appendix 1) were identified in the first group of 50 AH samples from 30 patients with PCG, 10 patients with CCs, and 10 patients with ARCs, which were classified according to the database from the Kyoto Encyclopedia of Genes and Genomes (KEGG) [16]. Among them, 173 metabolites were identified as mapping to the amino acid/carbohydrate/lipid/nucleotide super pathway and 132 metabolites were identified as analytes, while the remaining 58 metabolites were determined to be unknown.
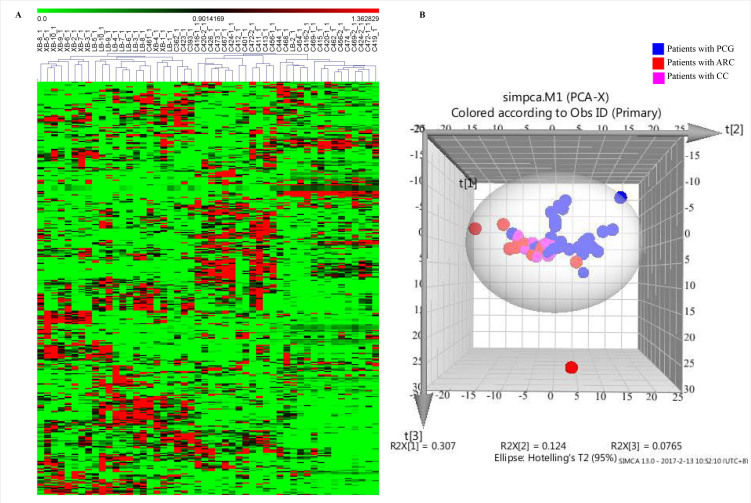
The hierarchical cluster analysis, an un-supervised approach, was then performed to investigate metabolic variations among the three kinds of patients (Figure 1A). Obviously, 29/30 samples from patients with PCG were clearly separated from the samples from patients with CCs and ARCs. We further employed another un-supervised approach, PCA, to determine the grouping of these 50 samples. As shown in Figure 1B, there was to some extent a separation between samples from patients with PCG and samples from the other two kinds of patients as some samples from three kinds of patients clustered together. The results here indicated that patients with PCG had a significantly distinct metabolomics profile.
Figure 1.
Metabolomics analysis of 50 AH samples, including 30 patients with PCG, 10 patients with CCs, and 10 patients with ARCs. A: Heat map of 363 metabolites detected in 50 AH samples by a hierarchical cluster analysis. Each line represents one metabolite. The darker the green color, the lower its content in the sample; likewise, the darker the red color, the higher its content in the sample. XB represents patients with CCs, and LB represents patients with ARCs, while C represents patients with PCG. B: 3D score plot for the first three components (t[1], t[2], and t[3]) in the PCA model of metabolomics data from 50 AH samples. PCG: primary congenital glaucoma; CCs: congenital cataracts; ARCs: aged-related cataracts.
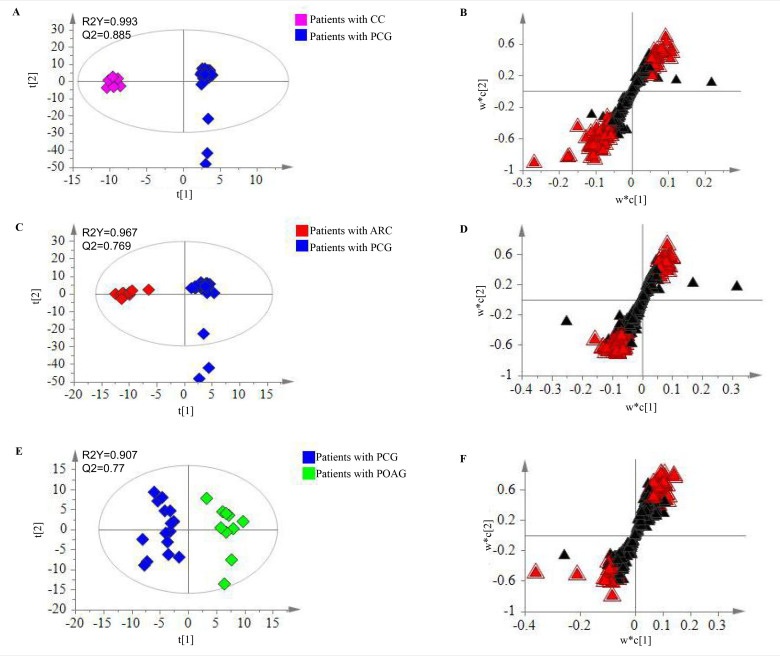
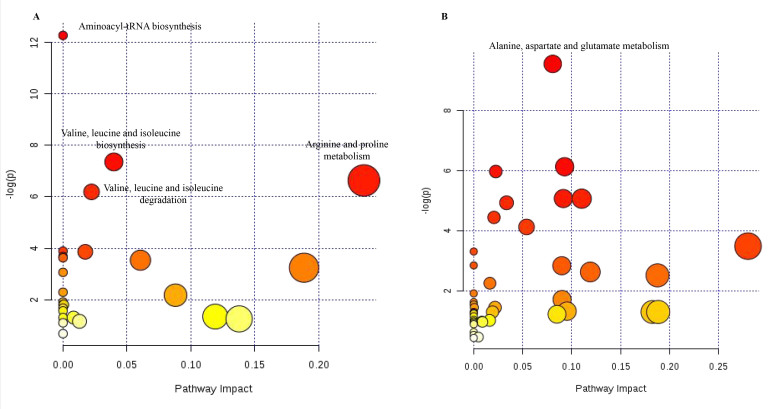
The supervised statistical method, PLS-DA, was used to determine significantly different metabolites. On the one hand, samples from patients with PCG were obviously separated from those with CCs in a score plot from the PLS-DA model with high R2Y and Q2 values (Figure 2A). Together with a dependent t test, 89 metabolites were determined to be significantly different (VIP ≥1 and p≤0.05), responsible for the separation (Figure 2B). On the other hand, samples from patients with PCG were also clearly separated from those with ARCs (Figure 2C). Similarly, 86 metabolites were found to be significantly changed (VIP ≥1 and p≤0.05), which played a role in the separation (Figure 2D). Altogether, 38 metabolites (Appendix 2) were finally identified to be significantly different (adjusted p≤0.05) among those three kinds of patients. A further metabolic pathway analysis of these 38 significantly different metabolites revealed that the 25 most relevant pathways (Appendix 3 and Figure 3A) were enriched, among which aminoacyl-tRNA biosynthesis; valine, leucine, and isoleucine biosynthesis; arginine and proline metabolism; and valine, leucine, and isoleucine degradation were the most significantly influenced pathways (FDR <0.05).
Figure 2.
Metabolomic analysis for patients with PCG. A: Score plot for the first two components (t[1] and t[2]) between patients with CCs and PCG in the PLS-DA model. B: S plot for the two first components (w*c [1] and w*c [2]) between patients with CCs and PCG in the PLS-DA model. C: Score plot for the first two components (t[1] and t[2]) between patients with ARCs and PCG in the PLS-DA model. D: S plot for the two first components (w*c [1] and w*c [2]) patients with ARCs and PCG in the PLS-DA model. E: Score plot for the first two components (t[1] and t[2]) between patients with PCG and POAG in the PLS-DA model. F: S plot for the two first components (w*c [1] and w*c [2]) patients with PCG and POAG in the PLS-DA model. Significantly changed metabolites were labeled with a red triangle. PCG: primary congenital glaucoma; CCs: congenital cataracts; ARCs: aged-related cataracts; POAG: primary open angle glaucoma.
Figure 3.
Metabolic pathway analysis for patients with PCG. A: Metabolic pathway analysis of 38 significantly different metabolites among patients with PCG, CCs, and ARCs using the MetaboAnalyst web server. B: Metabolic pathway analysis of 54 significantly different metabolites between patients with PCG and POAG by using the MetaboAnalyst web server. PCG: primary congenital glaucoma; CCs: congenital cataracts; ARCs: aged-related cataracts; POAG: primary open angle glaucoma. The color and size of each dot were associated with the -log (p) value and pathway impact value, respectively, where a small p value and high pathway impact value indicate the pathway is greatly influenced.
Metabolic changes between patients with PCG and POAG
Likewise, we used the same methodology for the metabolite profiling of the other 15 patients with PCG and 10 patients with POAG. The results here revealed that 290 metabolites (Appendix 4) in total were detected in all the 25 samples, which referred to the amino acid (19 metabolites)/carbohydrate (55 metabolites)/lipid (16 metabolites)/nucleotide (4 metabolites) super pathway, as well as another 196 metabolites. Likewise, a hierarchical cluster analysis of metabolic data showed that most samples (13/15) from patients with PCG were clearly separated from those with POAG (Appendix 5). A further statistical analysis showed that 54 metabolites (Appendix 6, Figure 2E,F) were significantly (adjusted p≤0.05) altered between the two groups. Among them, the levels of 17 biochemicals were increased, while the concentrations of 37 biochemicals were decreased. A metabolic pathway analysis of these 54 metabolites suggested that the 42 most relevant pathways (Appendix 7 and Figure 3B) were enriched. Especially, the pathway of alanine, aspartate, and glutamate metabolism was the most significantly influenced one (FDR <0.05).
Enrichment analysis of significantly changed metabolites in relation to PCG
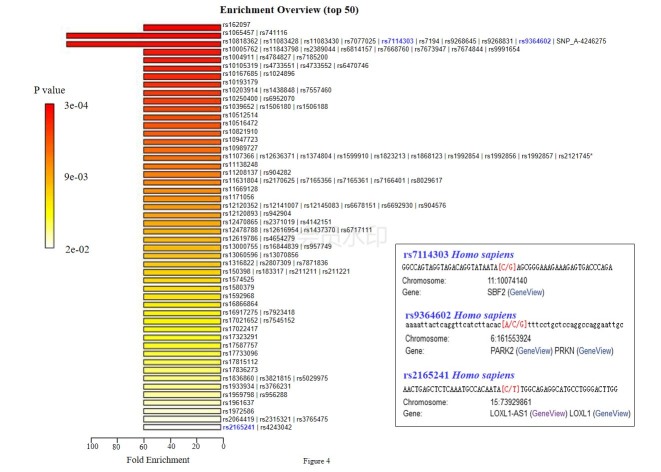
To determine the metabolic changes of AH related to PCG development, we then identified significantly changed metabolites among the abovementioned four kinds of patients. Interestingly, only three metabolites (Table 2) were significantly changed in common between patients with PCG and the three kinds of controls (patients with CCs/ARCs/POAG). Notably, glycine 2 was synchronously upregulated and urea was synchronously downregulated in patients with PCG when compared to the other three kinds of patients. We further submitted these three metabolites to the MetaboAnalyst web server for an enrichment analysis with the single nucleotide polymorphism (SNP)-associated metabolite set library (4,501 SNPs). The results displayed the top 50 groups of SNPs that were highly associated with these three metabolites (Appendix 8 and Figure 4). Among the top 50 groups of SNPs, three SNPs, including rs7114303, rs9364602, and rs2165241, were all reported to be involved in the development of glaucoma [23-25].
Table 2. List of metabolites significantly changed in common between patients with PCG and any other kind of patients (with CC/ARC/POAG).
| Biochemicals | Super Pathway | Ratio (PCG/POAG) | Ratio (PCG/CC) | *Adjust p value(PCG versus CC versus ARC) | Ratio (PCG/ARC) | *Adjust p value(PCG/POAG) |
|---|---|---|---|---|---|---|
| glycine 2 | Amino Acid | 3.86 | 8.39 | 0.0019 | 8.99 | 0.0018 |
| phenylalanine 1 | Amino Acid | 0.91 | 1.84 | 0.0007 | 1.53 | 0.0016 |
| urea | Amino Acid | 0.94 | 0.65 | 0.0000 | 0.82 | 0.0263 |
*Regression models were used to adjust for age and gender in comparisons among three groups (PCG versus CC versus ARC), and between groups of PCG and POAG. PCG: primary congenital glaucoma; CC: congenital cataract; ARC: aged-related cataract; POAG: primary open angle glaucoma.
Figure 4.
Top 50 groups of SNPs were highly associated with three metabolites significantly changed in common between patients with PCG and any other kind of patients (with CCs/ARCs/POAG) according to an enrichment analysis using the MetaboAnalyst web server with the SNP-associated metabolite set library (4501 SNPs). The details of three SNPs, including rs7114303, rs9364602, and rs2165241, are shown. PCG: primary congenital glaucoma; CCs: congenital cataracts; ARCs: aged-related cataracts; POAG: primary open angle glaucoma. *All SNPs in this group are shown in Appendix 8.
Discussion
Glaucoma is the second leading cause of irreversible blindness, affecting a significant and growing proportion of the world population [26]. However, it has a small range of therapeutic options due to the pathogenic mechanisms of the disease. PCG causes great harm to visual development in infancy, though it is a rare disease [27]. As is well known, PCG manifests in the first three years of life as an autosomal recessive trait, having resulted from abnormal development of the AH drainage structures [28]. Thus, the identification of the molecular characteristics in AH of PCG may improve clinical diagnosis and extend the understanding of the pathophysiology behind the disease [4]. In the present study, for the first time, we employed metabolomics technology to study the metabolic characterization of AH samples in relation to PCG, which may not only present effective biomarkers as diagnostic and progression detection tools for PCG, but also promote new knowledge of the molecular mechanism of PCG development and further provide new therapeutic options.
Ideally, the GC-MS method has sufficient specificity and sensitivity to profile for a metabolomics analysis [11]. However, to the best of our knowledge, there have been no published reports on metabolomics for PCG. Here, we employed the non-targeted technology GC-MS to uncover comprehensively the AH metabolome of patients with PCG and controls. In total, 363 and 290 metabolites were respectively identified at two steps of metabolite profiling for all 75 patients, covering most of the central metabolism pathways, including the amino acid/carbohydrate/lipid/nucleotide super pathway. There is no doubt that our study presented thus far the broadest AH metabolome for PCG, corroborating its power in uncovering the metabolic characteristics. Though GC-TOF/MS used here is a popular technique for metabolite profiling, it has several limitations. On the one hand, it is a good choice to employ tandem mass spectrometry or MS/MS for high-confidence detection. On the other, it is indeed better to perform a metabolomics analysis in combination with LC-MS/MS, as GC-MS cannot analyze non-volatile metabolites.
Currently, the situation with the early diagnosis of early glaucoma, including PCG, has prompted researchers to determine potential biomarkers to diagnose and monitor the progress of the disease [29]. For instance, Fraenkl et al. (2011) revealed that plasma citrate levels were significantly reduced in Caucasian patients with glaucoma, as well as indicated plasma citrate as a promising biomarker for the diagnosis of glaucoma [30]. Moreover, Castany et al. (2011) investigated the presence of dinucleoside polyphosphates in AH samples from patients with or without glaucoma and found that levels of diadenosine tetraphosphate (Ap4A) were significantly higher (almost 15 times) in glaucoma patients than in the controls, suggesting its potential application as a marker of glaucoma [29]. In this study, three metabolites, including glycine, urea, and phenylalanine, were finally determined to be significantly changed in common between patients with PCG and any other kind of patient (with CCs/ARCs/POAG). The changes in the phenylalanine concentration in patients with glaucoma were also reported at an early stage in the study by Hannappel et al. [31]. Moreover, urea may play a critical role in the pathogenesis of PCG, as urea could be used in glaucoma as an osmotic ocular hypotensive agent with a long history [32]. Notably, another metabolite, glycine, displayed high levels in patients with PCG, and it has been reported as a potential biomarker for brain tumors [33]. Likewise, previous reports have already identified the role of glycine as a neural transmitter in the retina and meanwhile revealed that vitreous levels of glycine were associated with glaucoma [34,35]. Thus, glycine likely plays a significant role in the development of glaucoma (including PCG), which is characterized by the interruption in the communication between the brain and the retinal photoreceptors. A further enrichment analysis showed that rs7114303, rs9364602, and rs2165241 were associated with those three metabolites. PCG is a genetically heterogeneous disorder, while two candidate genes, cytochrome P450 (CYP1B1) and latent transforming growth factor-beta-binding protein 2 (LTBP2) have been reported to be responsible for congenital glaucoma [36]. Interestingly, the three identified SNPs here were all reported to be associated with glaucoma. For example, rs7114303 locates in the SET binding factor 2 (SBF2) gene, whose mutation was reported to cause Charcot-Marie-Tooth disease type 4B (CMT4B) with juvenile onset glaucoma [23]. Furthermore, rs9364602 (parkin RBR E3 ubiquitin protein ligase, PARK2), to some extent, is associated with both glaucoma and amyotrophic lateral sclerosis (ALS) [25]. In addition, rs2165241 (located in the lysyl oxidase-like protein 1 gene), was observed to confer a risk of glaucoma [24]. It is believed that glycine may be a promising biomarker, while these three amino acid-associated metabolites and three SNPs may play crucial roles in PCG development.
The role of amino acids has been explored in glaucomatous patients [37]. For example, Dreyer et al. (1996) showed that the level of glutamate was increased in the vitreous body of patients with glaucoma when compared with controls [38]. Meanwhile, in our study, 11 amino acid-associated metabolites, including cystine, glycine, and trans-4-hydroxy-L-proline, were significantly and greatly different in patients with PCG when compared with patients with CCs and ARCs. Especially, the above-mentioned three metabolites (glycine, urea, and phenylalanine) were also belonging to the amino acid super pathway. Moreover, a further metabolic pathway analysis revealed several significantly influenced pathways, all of which are involved in amino acid metabolism. The results here suggest amino acid metabolism may play an important role in the development of PCG, as well as in the progression of other various eye diseases, including high myopia and cataracts [11,39]. For example, aminoacyl-tRNA synthetases has been suggested to play a regulatory role in mitochondrial mutations that have reported in congenital glaucoma with low frequencies (10%–22.85%) [40,41]. This is highly consistent with our results that aminoacyl-tRNA biosynthesis was determined to be the most significantly influenced pathway in patients with PCG when compared to patients with CCs and ARCs.
Altogether, the present study fully revealed metabolic characteristics in relation to PCG. A significantly distinct metabolite profile was observed behind the disease with certain especially active amino acid-associated metabolites and their metabolisms. More importantly, glycine was supposed to be a potential biomarker for PCG screening. In the future, validation work should be done with a large number of clinical samples. In addition, three glaucoma-associated SNPs (rs7114303, rs9364602, and rs2165241) need further verification by genomic approaches in the next steps.
Acknowledgments
The Chinese samples used for the analyses described in this manuscript were obtained from the Biobank of Eye & Ear Nose Throat Hospital, Fudan University. We would like to thank all the participants for their valuable contribution to this research. This work was supported in part by the Funds for International Cooperation and Exchange of the National Natural Science Foundation of China (NSFC81790641) and National Natural Science Foundation of China (NSFC81770968).
Appendix 1.
List of metabolites detected in the 50 AH samples from 30 patients with PCG, 10 patients with CC and 10 patients with ARC. PCG: primary congenital glaucoma; CC: congenital cataract; ARC: aged-related cataract. To access the data, click or select the words “Appendix 1.”
Appendix 2.
List of 38 significantly different metabolites between patients with PCG and CC/ARC. PCG: primary congenital glaucoma; CC: congenital cataract; ARC: aged-related cataract. To access the data, click or select the words “Appendix 2.”
Appendix 3.
Metabolic pathway analysis of 38 significantly different metabolites between patients with PCG and CC/ARC by using MetaboAnalyst web server. PCG: primary congenital glaucoma; CC: congenital cataract; ARC: aged-related cataract. To access the data, click or select the words “Appendix 3.”
Appendix 4.
List of 290 metabolites detected in the 25 AH samples from 15 patients with PCG and 10 patients with POAG. PCG: primary congenital glaucoma; POAG: primary open angle glaucoma. To access the data, click or select the words “Appendix 4.”
Appendix 5.
Heat map of 290 metabolites detected in 25 AH samples by hierarchical cluster analysis. Each line represented one metabolite. The darker the green color, the lower its content in the sample; likewise, the darker the red color, the higher its content in the sample. K represented patients with POAG, while C represented patients with PCG. To access the data, click or select the words “Appendix 5.”
Appendix 6.
List of 54 significantly different metabolites between patients with PCG and POAG. PCG: primary congenital glaucoma; POAG: primary open angle glaucoma. To access the data, click or select the words “Appendix 6.”
Appendix 7.
Metabolic pathway analysis of 54 significantly different metabolites between patients with PCG and POAG by using MetaboAnalyst web server. PCG: primary congenital glaucoma; POAG: primary open angle glaucoma. To access the data, click or select the words “Appendix 7.”
Appendix 8.
Enrichment analysis of three metabolites significantly changed in common between patients with PCG and any other kind of patients (with CC/ARC/POAG) by using MetaboAnalyst web server with single nucleotide polymorphisms (SNPs)-associated metabolites set library. PCG: primary congenital glaucoma; CC: congenital cataract; ARC: aged-related cataract; POAG: primary open angle glaucoma. To access the data, click or select the words “Appendix 8.”
References
- 1.Gomes HA, Moreira BS, Sampaio RF, Furtado SRC, Cronemberger S, Gomes RA, Kirkwood RN. Gait parameters, functional mobility and fall risk in individuals with early to moderate primary open angle glaucoma: a cross-sectional study. Braz J Phys Ther. 2018;22:376–82. doi: 10.1016/j.bjpt.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y, Zhang L, Li S, Zhu X, Sundaresan P. Candidate Gene Analysis Identifies Mutations in CYP1B1 and LTBP2 in Indian Families with Primary Congenital Glaucoma. Genet Test Mol Biomarkers. 2017;21:252–8. doi: 10.1089/gtmb.2016.0203. [DOI] [PubMed] [Google Scholar]
- 3.Jünemann A, Hohberger B, Rech J, Sheriff A, Fu Q, Schlötzer-Schrehardt U, Voll RE, Bartel S, Kalbacher H, Hoebeke J, Rejdak R, Horn F, Wallukat G, Kunze R, Herrmann M. Agonistic autoantibodies to the β2-adrenergic Rerceptor involved in the pathogenesis of open angle glaucoma. Front Immunol. 2018;9:145. doi: 10.3389/fimmu.2018.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbosa-Breda J, Himmelreich U, Ghesquière B, Rocha-Sousa A, Stalmans I. Clinical Metabolomics and Glaucoma. Ophthalmic Res. 2018;59:1–6. doi: 10.1159/000479158. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharya SK, Lee RK, Grus FH. Molecular biomarkers in glaucoma. Invest Ophthalmol Vis Sci. 2013;54:121–31. doi: 10.1167/iovs.12-11067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funke S, Perumal N, Beck S, Gabel-Scheurich S, Schmelter C, Teister J, Gerbig C, Gramlich OW, Pfeiffer N, Grus FH. Glaucoma related proteomic alterations in human retina samples. Sci Rep. 2016;6:29759. doi: 10.1038/srep29759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.González-Iglesias H, Álvarez L, García M, Escribano J, Rodríguez-Calvo PP, Fernández-Vega L, Coca-Prados M. Comparative proteomic study in serum of patients with primary open-angle glaucoma and pseudoexfoliation glaucoma. J Proteomics. 2014;98:65–78. doi: 10.1016/j.jprot.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Anders F, Teister J, Funke S, Pfeiffer N, Grus F, Solon T, Prokosch V. Proteomic profiling reveals crucial retinal protein alterations in the early phase of an experimental glaucoma model. Graefes Arch Clin Exp Ophthalmol. 2017;255:1395–407. doi: 10.1007/s00417-017-3678-x. [DOI] [PubMed] [Google Scholar]
- 9.Barbas-Bernardos C, Armitage EG, García A, Mérida S, Navea A, Bosch-Morell F, Barbas C. Looking into aqueous humor through metabolomics spectacles-exploring its metabolic characteristics in relation to myopia. J Pharm Biomed Anal. 2016;127:18–25. doi: 10.1016/j.jpba.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Cheng CY, Choi H, Ikram MK, Sabanayagam C, Tan GS, Tian D, Zhang L, Venkatesan G, Tai ES, Wang JJ, Mitchell P, Cheung CM, Beuerman RW, Zhou L, Chan EC, Wong TY. Plasma metabonomic profiling of diabetic retinopathy. Diabetes. 2016;65:1099–108. doi: 10.2337/db15-0661. [DOI] [PubMed] [Google Scholar]
- 11.Ji Y, Rao J, Rong X, Lou S, Zheng Z, Lu Y. Metabolic characterization of human aqueous humor in relation to high myopia. Exp Eye Res. 2017;159:147–55. doi: 10.1016/j.exer.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Rong S, Li Y, Guan Y, Zhu L, Zhou Q, Gao M, Pan H, Zou L, Chang D. Long-chain unsaturated fatty acids as possible important metabolites for primary angle-closure glaucoma based on targeted metabolomic analysis. Biomed Chromatogr. 2017;31:3963. doi: 10.1002/bmc.3963. [DOI] [PubMed] [Google Scholar]
- 13.Izzotti A, Longobardi M, Cartiglia C, Sacca SC. Proteome alterations in primary open angle glaucoma aqueous humor. J Proteome Res. 2010;9:4831–8. doi: 10.1021/pr1005372. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Chen Y, Wang L, Jiang D, Wang W, Xia M, Yu L, Sun X. CYP1B1 genotype influences the phenotype in primary congenital glaucoma and surgical treatment. Br J Ophthalmol. 2014;98:246–51. doi: 10.1136/bjophthalmol-2013-303821. [DOI] [PubMed] [Google Scholar]
- 15.Bennett TM, Maraini G, Jin C, Sun W, Hejtmancik JF, Shiels A. Noncoding variation of the gene for ferritin light chain in hereditary and age-related cataract. Mol Vis. 2013;19:835–44. [PMC free article] [PubMed] [Google Scholar]
- 16.Kondo Y, Saitsu H, Miyamoto T, Lee BJ, Nishiyama K, Nakashima M, Tsurusaki Y, Doi H, Miyake N, Kim JH, Yu YS, Matsumoto N. Pathogenic mutations in two families with congenital cataract identified with whole-exome sequencing. Mol Vis. 2013;19:384–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Hogewind BF, Gaplovska-Kysela K, Theelen T, Cremers FP, Yam GH, Hoyng CB, Mukhopadhyay A. Identification and functional characterization of a novel MYOC mutation in two primary open angle glaucoma families from The Netherlands. Mol Vis. 2007;13:1793–801. [PubMed] [Google Scholar]
- 18.Bennett KL, Funk M, Tschernutter M, Breitwieser FP, Planyavsky M, Ubaida Mohien C, Müller A, Trajanoski Z, Colinge J, Superti-Furga G, Schmidt-Erfurth U. Proteomic analysis of human cataract aqueous humour: Comparison of one-dimensional gel LCMS with two-dimensional LCMS of unlabelled and iTRAQ®-labelled specimens. J Proteomics. 2011;74:151–66. doi: 10.1016/j.jprot.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Kind T, Wohlgemuth G, Lee DY, Lu Y, Palazoglu M, Shahbaz S, Fiehn O. FiehnLib: mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal Chem. 2009;81:10038–48. doi: 10.1021/ac9019522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang C, Hao R, Du X, Yuewen D, Sun R, Wang Q. Metabolomics responses of pearl oysters (Pinctada fucata martensii) fed a formulated diet indoors and cultured with natural diet outdoors. Front Physiol. 2018;9:944. doi: 10.3389/fphys.2018.00944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rao J, Cheng F, Hu C, Quan S, Lin H, Wang J, Chen GH, Zhao XX, Alexander D, Guo LN, Wang GY, Lai JS, Zhang DB. Metabolic map of mature maize kernels. Metabolomics. 2014;10:775–87. [Google Scholar]
- 22.Ioannidis JP. The proposal to lower p value thresholds to. 005. JAMA. 2018;319:1429–30. doi: 10.1001/jama.2018.1536. [DOI] [PubMed] [Google Scholar]
- 23.Hirano R, Takashima H, Umehara F, Arimura H, Michizono K, Okamoto Y, Nakagawa M, Boerkoel CF, Lupski JR, Osame M, Arimura K. SET binding factor 2 (SBF2) mutation causes CMT4B with juvenile onset glaucoma. Neurology. 2004;63:577–80. doi: 10.1212/01.wnl.0000133211.40288.9a. [DOI] [PubMed] [Google Scholar]
- 24.Thorleifsson G, Magnusson KP, Sulem P, Walters GB, Gudbjartsson DF, Stefansson H, Jonsson T, Jonasdottir A, Jonasdottir A, Stefansdottir G, Masson G, Hardarson GA, Petursson H, Arnarsson A, Motallebipour M, Wallerman O, Wadelius C, Gulcher JR, Thorsteinsdottir U, Kong A, Jonasson F, Stefansson K. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science. 2007;317:1397–400. doi: 10.1126/science.1146554. [DOI] [PubMed] [Google Scholar]
- 25.Wong YC, Holzbaur EL. Temporal dynamics of PARK2/parkin and OPTN/optineurin recruitment during the mitophagy of damaged mitochondria. Autophagy. 2015;11:422–4. doi: 10.1080/15548627.2015.1009792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doganay S, Cankaya C, Alkan A. Evaluation of corpus geniculatum laterale and vitreous fluid by magnetic resonance spectroscopy in patients with glaucoma; a preliminary study. Eye (Lond) 2012;26:1044. doi: 10.1038/eye.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gothwal VK, Bharani S, Mandal AK. Impact of surgery on the quality of life of caregivers of children with congenital glaucoma. Ophthalmology. 2016;123:1161–2. doi: 10.1016/j.ophtha.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 28.Reddy V, Freedman S, Strickland S, Chen-Hsin Y, Kuchtey RW, Kuchtey J, Allingham RR, Hauser MA. In search of novel CYP1B1 mutations that cause primary congenital glaucoma. Invest Ophthalmol Vis Sci. 2015;56:2540–2540. [Google Scholar]
- 29.Castany M, Jordi I, Catala J, Gual A, Morales M, Gasull X, Pintor J. Glaucoma patients present increased levels of diadenosine tetraphosphate, Ap4A, in the aqueous humour. Exp Eye Res. 2011;92:221–6. doi: 10.1016/j.exer.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Fraenkl SA, Muser J, Groell R, Reinhard G, Orgul S, Flammer J, Goldblum D. Plasma citrate levels as a potential biomarker for glaucoma. J Ocul Pharmacol Ther. 2011;27:577–80. doi: 10.1089/jop.2011.0062. [DOI] [PubMed] [Google Scholar]
- 31.Hannappel E, Pankow G, Grassl F, Brand K, Naumann GOH. Amino acid pattern in human aqueous humor of patients with senile cataract and primary open-angle glaucoma. Ophthalmic Res. 1985;17:341–3. doi: 10.1159/000265398. [DOI] [PubMed] [Google Scholar]
- 32.Galin MA, Aizawa F, McLean JM. Urea as an osmotic ocular hypotensive agent in glaucoma. Arch Ophthalmol. 1959;62:347–52. doi: 10.1001/archopht.1959.04220030003001. [DOI] [PubMed] [Google Scholar]
- 33.Righi V, Andronesi OC, Mintzopoulos D, Black PM, Tzika AA. High-resolution magic angle spinning magnetic resonance spectroscopy detects glycine as a biomarker in brain tumors. Int J Oncol. 2010;36:301–6. doi: 10.3892/ijo_00000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brooks DE, Garcia GA, Dreyer EB, Zurakowski D, Franco-Bourland RE. Vitreous body glutamate concentration in dogs with glaucoma. Am J Vet Res. 1997;58:864–7. [PubMed] [Google Scholar]
- 35.Coull BM, Cutler RW. Light-evoked release of endogenous glycine into the perfused vitreous of the intact rat eye. Invest Ophthalmol Vis Sci. 1978;17:682–4. [PubMed] [Google Scholar]
- 36.Kaur K, Mandal AK, Chakrabarti S. Primary congenital glaucoma and the involvement of CYP1B1. Middle East Afr J Ophthalmol. 2011;18:7. doi: 10.4103/0974-9233.75878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wamsley S. B’Ann TG, Dahl DB, Case GL, Sherwood RW, May CA, Hernandez MR, Kaufman PL. Vitreous glutamate concentration and axon loss in monkeys with experimental glaucoma. Arch Ophthalmol. 2005;123:64–70. doi: 10.1001/archopht.123.1.64. [DOI] [PubMed] [Google Scholar]
- 38.Dreyer EB, Zurakowski D, Schumer RA, Podos SM, Lipton SA. Elevated glutamate levels in the vitreous body of humans and monkeys with glaucoma. Arch Ophthalmol. 1996;114:299–305. doi: 10.1001/archopht.1996.01100130295012. [DOI] [PubMed] [Google Scholar]
- 39.Ling CA, Weiter JJ, Buzney SM, Lashkari K. Competing theories of cataractogenesis after pars plana vitrectomy and the nutrient theory of cataractogenesis: a function of altered aqueous fluid dynamics. Int Ophthalmol Clin. 2005;45:173–98. doi: 10.1097/01.iio.0000176366.09135.63. [DOI] [PubMed] [Google Scholar]
- 40.Boczonadi V, Horvath R. Mitochondria: impaired mitochondrial translation in human disease. Int J Biochem Cell Biol. 2014;48:77–84. doi: 10.1016/j.biocel.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sundaresan P, Simpson DA, Sambare C, Duffy S, Lechner J, Dastane A, Dervan EW, Vallabh N, Chelerkar V, Deshpande M, O’Brien C, McKnight AJ, Willoughby CE. Whole-mitochondrial genome sequencing in primary open-angle glaucoma using massively parallel sequencing identifies novel and known pathogenic variants. Genet Med. 2015;17:279–84. doi: 10.1038/gim.2014.121. [DOI] [PubMed] [Google Scholar]