Abstract
Stool is not just a simple waste material. Some stool tests can be easily used in primary care in the differential diagnosis of disorders such as gastrointestinal infections, malabsorption syndromes, and inflammatory bowel diseases. Stool tests can prevent unnecessary laboratory investigations. Stool analyses include microscopic examination, chemical, immunologic, and microbiologic tests. Stool samples can be examined for leukocytes, occult blood, fat, sugars (reducing substances), pH, pancreatic enzymes, alpha-1 antitrypsin, calprotectin, and infectious causes (bacteria, viruses, and parasites). Stool should also be macroscopically checked in terms of color, consistency, quantity, shape, odor, and mucus.
Keywords: Children, gastrointestinal disorders, stool tests
Abstract
Dışkı yalnızca basit bir atık değildir. Bazı dışkı testleri sindirim sistemi enfeksiyonları, malabsorpsiyon sendromları ve inflamatuvar bağırsak hastalıkları gibi hastalıkların ayırıcı tanısında birinci basamak sağlık hizmetlerinde kolaylıkla kullanılabilir. Dışkı testleri sayesinde gereksiz laboratuvar incelemeleri önlenebilir. Dışkı incelemeleri arasında mikroskobik inceleme, kimyasal, immünolojik ve mikrobiyolojik testler vardır. Dışkı örneği; lökosit, gizli kan, yağ, indirgeyici maddeler olarak adlandırılan şekerler, pH, pankreas enzimleri, alfa-1 antitripsin, kalprotektin ve enfeksiyöz nedenler (bakteri, virüs ve parazitler) açısından incelenebilir. Dışkı renk, kıvam, miktar, şekil, koku ve mukus varlığı açısından da makroskobik olarak kontrol edilmelidir.
Introduction
Important information about diseases that affect the gastrointestinal system can be obtained with stool examinations. Stool can be examined macroscopically, microscopically, chemically, immunologically, and microbiologically. Stool samples to be examined should be collected in a clean container, fresh or kept under appropriate conditions.
The aim of this review was to present the most up-to-date information about stool tests that have an important place in the diagnosis and follow-up of childhood gastrointestinal diseases.
Macroscopic examination of the stool
Stool samples should be evaluated macroscopically in terms of color, consistency, quantity, form, odor, and presence of mucus. The presence of a small amount of mucus in stool is normal. However, the presence of copious mucus or bloody mucus is abnormal. The normal color is tawny due to the presence of bilirubin and bile. In infants, the stool may be green, its consistency may be watery or pasty. Stool color varies greatly depending on diet. Clay-colored or putty colored stool is observed in biliary obstructions. If more than 100 mL blood is lost from the upper gastrointestinal system, black, tarry stool is observed. Besides bleeding, black-colored stool may also be observed due to iron or bismuth treatment. Red-colored stool is observed in lower gastrointestinal tract bleeding.
It has been shown that evaluation of stool color using a ‘stool color card’ in newborn babies (Fig. 1) increases awareness of biliary atresia (1, 2). The Modified Bristol visual stool scale, which evaluates stool consistency (Fig. 2), has been found to be beneficial in monitoring treatment efficiency in functional constipation (3).
Figure 1.
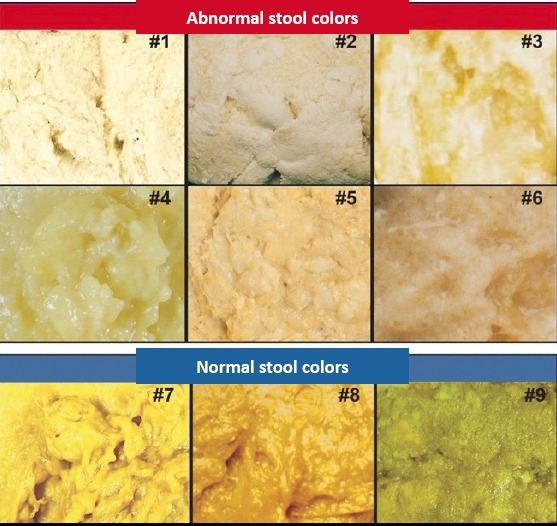
Stool color card used for screening biliary atresia in infants
Figure 2.

Modified Bristol stool scale
Microscopic examination of the stool
The most important step in the detection of stool abnormalities and intestinal problems is microscopic examination of the stool. Microscopic examination is a diagnostic tool for defining protozoa, helminths, and fecal leukocytes. Erythrocytes and leukocytes are not observed in normal stool. In order to see leukocytes, examinations should be performed in stool samples obtained from the area with mucus. Leukocytes are generally observed in bacterial infections. They are not observed in diarrheas caused by viruses and parasites. The presence of leukocytes in the stool is not a sensitive test in the diagnosis of inflammatory diarrhea because its ability to detect inflammatory diarrhea varies greatly (4).
For moving organisms, fresh stool can be examined immediately. If it is not possible to examine the stool immediately, it may be kept in 10% formalin for helminths and protozoa. The smallest amount of stool required for examination is 2–5 grams. At least three consecutive stool samples are required for the examination of parasites. Contamination of stool with urine should be avoided. Giardia can be detected in a single sample in 50–70% of cases and in a third sample in 90%. In the diagnosis of intestinal amoebiasis, cysts and trophozoites belonging to Entamoeba histolytica (E. histolytica) are investigated through microscopy examinations. However, the person who performs this examination should be an expert in this area. In addition, microscopy examinations cannot differentiate E. histolytica strains from Entamoeba dispar (E. dispar) and Entamoeba moshkovskii strains. Three samples may be needed to be sent on separate days to detect infection because excretion of cysts and trophozoites is variable. The samples are intensified and stained with iodine in order to detect cysts. Staining with iron hematoxylin and/or Wheatley trichrome should be performed to search for trophozoites. In invasive intestinal amoebiasis, blood is generally present in stool samples. The presence of phagocyted erythrocytes is not diagnostic for E. histolytica infection. Phagocyted erythrocytes may also be seen with E. dispar. Leukocytes may not always be observed in the stool because they may be disintegrated by parasitic organisms. A rapid antigen test is more useful compared with microscopy examinations in the diagnosis of Giardia, Cryptosporidium, and Entamoeba infection (5).
Occult blood in the stool
In peroxidase-based tests, peroxidase-like activity of hematin and/or hemoglobin transforms the catalyzer to blue. A restrictive diet may not be needed during the test. A systematic review demonstrated that sticking to a restrictive diet did not decrease the rate of occult blood positivity. Iron preparations ingested orally do not cause a positive hemoccult test. Ingestion of large amounts of vitamin C causes false-negative results and intake of vitamin C should be limited to less than 250 mg daily for at least three days before sampling. Before examination, stool samples should not be diluted again. Dilution increases the test’s sensitivity, but causes an increase in false-positive results. Aspirin and non-steroidal anti-inflammatory drugs may lead to false positivity by causing minor bleeding in the gastrointestinal mucosa. In addition, the test should be repeated with two samples daily for three days to increase the test’s accuracy. A loss of about 2–5 mL blood daily is normal in the intestines. Hemorrhages above this limit can be detected in the hemoccult test (6). Immunohistochemical occult blood tests were developed in order to measure human hemoglobin directly in the stool using monoclonal or polyclonal antibodies against the globin part of the human hemoglobin. It has high sensitivity and specificity in detecting lower gastrointestinal hemorrhages. In upper gastrointestinal hemorrhages, disintegration of the globin chain by proteases decreases the sensitivity. No special diet is required before the tests (7).
Detection of fat in the stool
In healthy humans, daily excretion of fat in the stool is less than 6 g and this amount remains constant even if daily fat consumption is 100–125 g. Excretion of fat in the stool may moderately increase in the absence of fat malabsorption in patients with diarrhea. Values up to 14 g/day were reported in volunteers whose diarrheas were induced by laxatives and in patients whose stool weights were more than 1000 g/day. Therefore, a moderate increase in excretion of fat in the stool in a patient with diarrhea does not indicate that malabsorption is the primary cause and other investigations should be performed to determine the cause of the diarrhea (8).
Various tests may be used to detect fat malabsorption (steatorrhea). The gold standard in the diagnosis of steatorrhea is quantitative calculation of stool fat. For this objective, the stool is collected for 72 hours while the patient is on a diet containing 100 g fat daily. However, qualitative tests are also used as a screening tool for steatorrhea because it is considerably difficult to collect stool for 72 hours. The Sudan III stain and acid steatocrit tests are among these tests. These tests can be performed more easily and rapidly compared with the detection of fat in a 72-hour stool sample, but they could not be substituted for the 72-hour fecal fat test (9).
a) Seventy-two–hour fecal fat test
This method requires collection of stool for 72 hours following a 6-day high-fat diet. A 3-day stool collection is ideal because it will decrease potential errors and variability that could arise when a shorter collection time period is used. A prolonged stool collection time also enables estimation of daily fecal weight. This is generally higher than 200 g/day in patients with steatorrhea. Patients should consume 70–120 g fat daily for an accurate estimation. More than 6 g/day fat in the stool is pathological. In patients with steatorrhea, however, more than 20 g fat is generally found in the stool daily. The test does not give an accurate result if less than 60 g fat is consumed daily (8).
The percentage of absorbed fat (fractional fat absorption) can be calculated after determining the mean daily fat intake. Fractional fat absorption is calculated with the following formula:
(Fat intake - fat excreted)/fat intake x 100.
The percentage for normal fractional fat absorption is 94%. Quantitative estimation of the amount of fat in the stool does not enable differentiation of the causes of steatorrhea (8).
b) Detection of fecal fat using Sudan III staining
Qualitative tests including the Sudan III stain continue to be used in clinical practice because collection of stool for 72 hours is difficult. If applied appropriately, Sudan III staining can detect more than 90% of patients who have clinically significant steatorrhea. Variability in the test’s performance and interpretation limit general sensitivity and reliability. Microscopy examination is not sensitive enough especially in inexperienced hands. The Sudan III test has been reported to have a sensitivity of 77% and a specificity of 98% (9).
Following staining of stool samples with Sudan III, neutral fats and fatty acids can be specified. For the detection of neutral fats, a small piece of stool is put on a microscope slide and 2 drops of water and 2 drops of 95% ethyl alcohol are added on the slide. Three-to-four drops of Sudan III dye are added. The presence of free triglycerides and soaps is investigated. These are generally observed as bundles or plaques and rarely as globules or crystals. The stain dark orange. For the detection of fatty acids, 2–3 drops of 36% glacial acetic acid are spattered onto the preparation. Three-to-four drops of Sudan III dye are added. Flame heating is performed. A microscopy examination is performed. Orange-colored fat drop globules are counted and recorded as fatty acids. Normally, the number of neutral fat particles should be <50 and the number of fatty acids should be <100. The differentiation of neutral fat and fatty acids using the Sudan III method is not effective in the differentiation of digestive disorder from absorption disorder (9, 10).
It has been shown that a method applied with Sudan III dye using a special approach directed to counting fat globules and measuring their dimensions on fecal fat microscopic examination (fecal qualitative fat microscopic examination) has a close relationship with chemically measured fetal fat excretion, and a high diagnostic accuracy. Accordingly, observation of 10–20 globules with a diameter of 10 µm and above is considered (+), 20–100 globules with a diameter of 10–50 µm is considered (++), and more than 100 fat globules with large diameters is considered (+++) (11).
c) Acid steatocrit test
This examination is performed on a small stool sample and is based on measurement of weight. It is a simple, rapid, inexpensive, and reliable method. When compared with the 72-hour fat collection test, which is considered the gold standard, it has a sensitivity of 100%, a specificity of 95%, and a positive predictive value of 90% (12, 13).
Acid steatocrit %= fat layer/(fat layer+solid layer) x 100
The fat content in the stool can be measured in the following way:
Fecal fat= -0.43+[0.45 (acid steatocrit %)] g/24 hours
d) Near-infrared reflectance analysis: NIRA
This analysis enables simultaneous measurement of fat, nitrogen, and carbohydrates in a single fecal sample. It is equally accurate as the 72-hour fecal fat test. It is a simple, rapid, and reliable method in the measurement of steatorrhea. A small amount of fecal sample is enough. Fecal samples should be studied immediately after collection or kept for a few days at most (14–16).
Fecal pH, electrolytes, and reducing substances
After a fresh and watery fecal sample is homogenized and centrifuged, pH and electrolyte intensities are measured in the watery part of the feces. Fecal pH is measured in a fresh fecal sample using nitrazine paper. Normally, the fecal pH ranges between 7.0 and 7.5. A fecal pH below 5.5 indicates acidic feces. In babies fed with breastmilk, the fecal pH is mildly acidic. The fecal osmolarity is equal to serum osmolarity (290 mosmol/kg). The osmotic gap is obtained by multiplying the sum of Na and K values in the fecal water by two and subtracting this value from the fecal osmolarity [osmotic gap= 290 - (Na + K) x 2]. Specifying the osmotic gap in the stool is important in patients with osmotic diarrhea. The osmotic gap is high in osmotic diarrhea (>125 mosmol/kg), and is small in secretory diarrhea (>125 mosmol/kg). This formula is preferred to direct measurement of fecal osmolarity because bacterial fermentation or contamination of fecal samples with concentrated urine after collection of the stool may lead to a falsely high osmolarity (17).
If carbohydrate malabsorption is suspected, a reducing substance should be investigated in the stool using the Benedict or Fehling test. Glucose, lactose, and fructose are reducing sugars, but sucrose is not. Unabsorbed sucrose may be reduced to glucose and fructose by colonic bacteria and this causes a positive reducing substance test result. Benedict solution (Clinitest) is mixed with an equal amount of stool in a test tube and heated. If the supernatant fluid becomes green brown, a reducing substance is present (the test is positive). If the amount of reducing substance in the stool is <0.25 mg/dL, the test result is normal. If it is 0.25–0.5 mg/dL, the test result is suspicious. If it is >0.5 mg/dL, the test result is abnormal (18).
For the reducing substance test, the fecal sample should be fresh and reach the laboratory in 1/2 hours at the latest, because disintegration of lactose and other sugars that remain in the stool by way of enzymes continues for 2–14 hours. If the test is not performed early, sugars such as lactose are disintegrated and the result will be false. The stool should not come into contact with urine, water, toilet paper or diapers. The result will be false because most toilet papers contain sugar (e.g. cellulose) and diapers absorb water.
Fecal sugar chromatography
This test is used when a reducing substance is found in the stool. It enables evaluation of fecal sugars. It may be helpful in the diagnosis of classic galactosemia, sucrose malabsorption, lactose intolerance or fructosuria/hereditary fructose intolerance. In lactase deficiency, lactose, galactose, and glucose may be detected. In congenital glucose-galactose malabsorption, only glucose and galactose are detected (19).
Fecal alpha-1 antitrypsin test
Alpha-1 antitrypsin (alpha-1 AT) is a glycoprotein synthesized in the liver and the main component of alpha-1 globulins. Alpha-1 AT has a higher molecular weight compared with albumin and is excreted in the feces without breaking down because it is resistant to proteolysis and disintegration in the intestinal lumen. The normal excretion rate for fecal alpha-1 AT is lower than 2.6 mg/day and its intestinal clearance is lower than 13 mL/day. An increased alpha-1 AT clearance suggests that enteral protein loss is increased. The alpha-1 AT clearance test requires a 24-hour fecal sample and serum sample for simultaneous measurement of alpha-1 AT in the plasma.
Alpha-1 AT clearance = (fecal volume) x (fecal alpha-1 AT) / (serum alpha-1 AT)
Diarrhea may increase alpha-1 AT clearance in the absence of protein losing enteropathy.
The alpha-1 AT clearance value compatible with protein losing enteropathy is higher than 27 mL/day in patients without diarrhea and higher than 56 mL/day in patients with diarrhea. Alpha-1 AT clearance should be measured while acid suppression (omeprazole 40 mg/day) is administered in individuals who have suspicious hypertrophic secretory gastropathy or in individuals who have been found to have normal alpha-1 AT clearance despite known gastrointestinal protein loss because alpha-1 AT is disintegrated when the gastric acid pH reduces below 3.5 (20).
Indirect pancreatic function tests
Indirect tests measure the results of exocrine pancreatic insufficiency. Indirect tests are simpler, easier, and less expensive compared with direct pancreatic function tests. The basic function of these tests is to make the diagnosis of advanced exocrine pancreatic insufficiency. They are much less sensitive compared with the direct tests for the diagnosis of early phases of exocrine pancreatic insufficiency. Other disadvantages include false-positive results in non-pancreatic gastrointestinal disorders and the need for stool collection (21).
a) Fecal elastase-1 test
The most sensitive and specific indirect test for pancreatic function is fecal elastase. Elastase-1 is a pancreas-specific proteolytic enzyme that binds to bile salts and does not disintegrate while passing through the intestines, unlike the other pancreatic enzymes. It constitutes about 6% of all enzymes that are secreted by the pancreas. Measurement of fecal elastase-1 shows close correlation with the pancreatic output of pancreatic enzymes including elastase-1, amylase, lipase, and trypsin. A fecal elastase-1 level of <200 mcg/g is considered abnormal. Values between 200 and 250 mcg/g may be considered as a threshold and should be repeated. In patients with chronic pancreatitis, its sensitivity is 63%, 100%, and 100%, respectively, for mild, moderate, and severe exocrine pancreatic insufficiency. Its specificity is 93% in patients with exocrine pancreatic insufficiency. Watery diarrhea caused by non-pancreatic diseases or drugs may dilute fecal samples and lead to false-positive results. This problem can be overcome by lyophilization of fecal samples (21).
b) Fecal chymotrypsin test
Fecal chymotrypsin is an enzymatic product of pancreatic secretion that can be used to detect pancreatic insufficiency. However, chymotrypsin has a lower sensitivity and specificity for exocrine pancreatic insufficiency compared with fecal elastase-1. The sensitivity of fecal chymotrypsin for mild and, moderate-advanced pancreatic insufficiency is 49% and 85%, respectively. Chymotrypsin is variably affected during passage through the intestines and may be diluted in the presence of concurrent diarrhea. Exogenous enzyme intake should be discontinued in patients two days before the test because chymotrypsin is found also in enzyme preparations found on the market (21).
Fecal calprotectin
Calprotectin is a cytosolic protein that has immunomodulator, antimicrobial, and antiproliferative effects. The intensity of calprotectin increases in infections, inflammation, and malignancies. It is a zinc and calcium binding protein that is generally released by neutrophils and monocytes. It exerts its antimicrobial action with a zinc-binding effect by disintegrating zinc-dependent enzymes. It is found in tissue samples, body fluids, and in the stool. Therefore, it is a valuable marker showing neutrophil efficiency. In intestinal inflammation, the levels of fecal calprotectin increase. Therefore, it may be useful to differentiate inflammatory causes of chronic diarrhea from non-inflammatory causes. Fecal calprotectin increases in inflammatory bowel diseases (IBD). The amount of fecal calprotectin is correlated with infiltration of the intestinal mucosa by polymorphonuclear leukocytes (22).
Calprotectin is considerably correlated with clinical and histopathologic activity in IBDs. The sensitivity and specificity of fecal calprotectin in individuals with IBD have been found as 93% and 96% in adults, and 92% and 76% in children. It has been reported that calprotectin may be more useful for the exclusion of IBD diagnoses in patients who present with abdominal pain or diarrhea in primary care settings (conditions where the prevalence is low) and for making the diagnosis of IBD in patients in gastroenterology clinics (conditions where the prevalence is high). Accordingly, a negative fecal calprotectin result may be helpful for primary care physicians to exclude IBD. In more than 80% of individuals with a positive fecal calprotectin results in a primary care setting, a marked abnormality could not be shown in colonoscopy. Elevating the calprotectin threshold to 250 µg/d for colonoscopy indication decreases the sensitivity for the diagnosis of IBD. Fecal calprotectin may also be considered an assistive test in the differential diagnosis of chronic diarrheas. It may has potential areas of use including colorectal cancer screening and follow-up of clinical activity in IBD. These indications have not yet been included in routine clinical practice (23–25).
Fecal calprotectin levels vary by age. The threshold values in the first year of life (<350 µg/d) are higher compared with childhood (<275 µg/d) and adulthood (<50 µg/d). In studies conducted with children, different threshold values have been used for fecal calprotectin. The normal reference values for fecal calprotectin by age in children are shown in Table 1 (26).
Table 1.
Reference values for calprotectin in stool
| Age | Normal value (microgram/g) |
|---|---|
| 1–6 months | <538 |
| 7 months–3 years | <214 |
| 3–4 years | <75 |
| 4–49 years | <50 |
In recent years, it has become a stool test that is being frequently ordered in the diagnosis of cow’s milk allergy, especially in infants. However, fecal calprotectin examination has no place in the diagnosis of cow’s milk protein allergy. It may be useful in colitis associated with food allergy (27).
Fecal antigen tests
a) Helicobacter pylori (H. pylori) stool antigen test
Detection of H. pylori antigen in the stool indicates an ongoing infection. Therefore, a stool antigen test may be used for making the diagnosis of H. Pylori infection and for confirming eradication. The stool antigen test is the most cost-effective test among the diagnostic tests in areas where H. pylori prevalence is low-moderate. The sensitivity (94%) and specificity (97%) of monoclonal immunoassays (EIA) performed in laboratories are high, similar to the urea breath test. The stool antigen test is influenced by recent use of bismuth compounds, antibiotics, and proton pump inhibitors (PPI). Although some data have been reported suggesting that eradication can be predicted with the stool antigen test as early as 7 days following completion of treatment, patients should not use antibiotics for 4 weeks and PPIs for 1–2 weeks before the test to avoid false-negative results (28).
Active bleeding caused by peptic ulcers may decrease the specificity of the stool antigen test. However, the sensitivity of monoclonal EIA remains high in individuals who have had a recent peptic ulcer hemorrhage. The polyclonal EIA stool antigen test is not currently being used because its sensitivity is low. Office-type rapid monoclonal immunochromatographic stool antigen tests have high specificity (96%) and low sensitivity (50%). It should be kept in mind that the sensitivity and specificity of the H. pylori stool antigen test depends on the type of the commercial test used, the threshold chosen, and interpretation of weak-positive results (29, 30).
b) Rotavirus stool antigen test
The methods that are used to detect rotavirus in the stool include immune-based tests [enzyme-linked immunosorbent assay (ELISA) and latex agglutination tests] and nucleic acid tests such as polymerase chain reaction (PCR). ELISA and latex agglutination are the most commonly used tests. Polymerase chain reaction is the most sensitive test. The virus can be detected 1–2 days before the onset of clinical disease by way of the ELISA test. Rotavirus can be detected with a rate of 94% 1–4 days after disease onset and with a rate of 76% 4–8 days after disease onset. It may sometimes be detected even 2 weeks after recovery of the disease (31).
c) Adenovirus stool antigen test
Enteric adenoviruses (type 40 or 41) may cause diarrhea for a longer time compared with rotavirus. Stool sample is used for adenovirus-specific ELISA analysis. It is a diagnostic test that can be used in primary care settings. ELISA has a sensitivity of 78% and a specificity of 100% (32, 33).
d) Giardia stool antigen test
A series of immunoserologic methods that use antibodies against cyst or trophozoite antigens have been developed for stool examination. Generally, these methods have higher sensitivity compared with non-traditional microscopy tests. The specificity and cost are relatively comparable. The direct immunofluorescence antigen test has the highest sensitivity. Immunoserologic methods have a limited area of use after treatment of infection. The disappearance of stool antigens after treatment suggests that the treatment was effective, but detection of antigen in the stool may be caused by excretion of dead parasites (34).
e) Entamoeba stool antigen test
Detection of entamoeba antigens in the stool is a sensitive, specific, rapid, and feasible method, and can differentiate E. histolytica and E. dispar. In the diagnosis of E. histolytica infection, commercial stool and serum antigen tests are available that use monoclonal antibodies to bind to the epitopes found on pathogenic E. histolytica strains. These epitopes are not found on non-pathogenic E. dispar strains. Kits that use ELISA, radioimmunoassay or immunofluorescence methods have been developed for antigen tests (34).
Measurement of Clostridium difficile (C. difficile) toxin in the stool
Stool samples of only patients with diarrhea should be studied and no checking should not be performed after treatment. Most C. difficile strains produce both A and B toxins, but some strains produce only A toxin or B toxin. Toxin B is clinically important. C. difficile-related disease caused by A toxin alone has not been observed. However, an EIA test performed for both toxins is more sensitive compared with the test performed for toxin B alone. The sensitivity of EIA for A and B toxins is about 75% and its specificity is high (99%). The test has a relatively high false-negative rate because 100–1000 pg toxins should be present for the test to be positive (35).
In children, it has a positive predictive value of 64%. The frequency of false negativity for EIA is higher in young children. Especially in young children, the detection of C. difficile in the stool does not necessarily indicate that diarrhea is caused by C. difficile (36).
Multiplex molecular panels
This method has been developed in order to detect gastrointestinal pathogens in stool samples by way of PCR. It enables the detection of numerous pathogens (more than 20 bacteria, viruses, and parasites) in a short period (one hour). It is rapid and sensitive for the diagnosis of infectious diarrheas (37, 38). The method is useful in the differentiation of acute graft versus host reaction from infectious etiologies, especially in immunocompromised patients presenting with diarrhea who have undergone organ transplantation. This method enables clinical diagnoses to be made in a timely fashion (39).
Conclusion
It should be kept in mind that stool tests give very useful information for physicians in the diagnosis and follow-up of gastrointestinal diseases, provided that they are interpreted appropriately.
Footnotes
Peer-review: Externally peer-reviewed.
Conflict of Interest: The author did not report any conflict of interest.
Financial Disclosure: The author stated that they did not receive any financial support for this study.
Hakem Değerlendirmesi: Dış bağımsız.
Çıkar Çatışması: Yazar çıkar çatışması bildirmemiştir.
Mali Destek: Yazar bu çalışma için mali destek almadığını beyan etmiştir.
References
- 1.Lien TH, Chang MH, Wu JF, et al. Effects of the infant stool color card screening program on 5-year outcome of biliary atresia in Taiwan. Hepatology. 2011;53:202–8. doi: 10.1002/hep.24023. [DOI] [PubMed] [Google Scholar]
- 2.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–4. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 3.Chumpitazi BP, Lane MM, Czyzewski DI, et al. Creation and initial evaluation of a Stool Form Scale for children. J Pediatr. 2010;157:594–7. doi: 10.1016/j.jpeds.2010.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gill CJ, Lau J, Gorbach SL, et al. Diagnostic accuracy of stool assays for inflammatory bacterial gastroenteritis in developed and resource-poor countries. Clin Infect Dis. 2003;37:365–75. doi: 10.1086/375896. [DOI] [PubMed] [Google Scholar]
- 5.Vandenberg O, Van Laethem Y, Souayah H, et al. Improvement of routine diagnosis of intestinal parasites with multiple sampling and SAF-fixative in the triple-faeces-test. Acta Gastroenterol Belg. 2006;69:361–6. [PubMed] [Google Scholar]
- 6.Rosenthal P, Jennings MT. Comparison of fecal occult blood tests for detection of gastrointestinal bleeding in pediatric patients. Am J Gastroenterol. 1992;87:1575–9. [PubMed] [Google Scholar]
- 7.Robertson DJ, Lee JK, Boland CR, et al. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia:A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;152:1217–37. doi: 10.1053/j.gastro.2016.08.053. [DOI] [PubMed] [Google Scholar]
- 8.Fine KD, Fordtran JS. The effect of diarrhea on fecal fat excretion. Gastroenterology. 1992;102:1936–9. doi: 10.1016/0016-5085(92)90316-q. [DOI] [PubMed] [Google Scholar]
- 9.Simko V. Fecal fat microscopy. Acceptable predictive value in screening for steatorrhea. Am J Gastroenterol. 1981;75:204–8. [PubMed] [Google Scholar]
- 10.Khouri MR, Huang G, Shiau YF. Sudan stain of fecal fat:new insight into an old test. Gastroenterology. 1989;96:421–7. doi: 10.1016/0016-5085(89)91566-7. [DOI] [PubMed] [Google Scholar]
- 11.Fine KD, Ogunji F. A new method of quantitative fecal fat microscopy and its correlation with chemically measured fecal fat output. Am J Clin Pathol. 2000;113:528–34. doi: 10.1309/0T2W-NN7F-7T8Q-5N8C. [DOI] [PubMed] [Google Scholar]
- 12.Amann ST, Josephson SA, Toskes PP. Acid steatocrit:a simple, rapid gravimetric method to determine steatorrhea. Am J Gastroenterol. 1997;92:2280–4. [PubMed] [Google Scholar]
- 13.Bijoor AR, Geetha S, Venkatesh T. Faecal fat content in healthy adults by the 'acid steatocrit method'. Indian J Clin Biochem. 2004;19:20–2. doi: 10.1007/BF02894252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neumeister V, Henker J, Kaltenborn G, et al. Simultaneous determination of fecal fat, nitrogen, and water by near-infrared reflectance spectroscopy. J Pediatr Gastroenterol Nutr. 1997;25:388–93. doi: 10.1097/00005176-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Benini L, Caliari S, Guidi GC, et al. Near infrared spectrometry for faecal fat measurement:comparison with conventional gravimetric and titrimetric methods. Gut. 1989;30:1344–7. doi: 10.1136/gut.30.10.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein J, Purschian B, Zeuzem S, et al. Quantification of fecal carbohydrates by near-infrared reflectance analysis. Clin Chem. 1996;42:309–12. [PubMed] [Google Scholar]
- 17.Eherer AJ, Fordtran JS. Fecal osmotic gap and pH in experimental diarrhea of various causes. Gastroenterology. 1992;103:545–51. doi: 10.1016/0016-5085(92)90845-p. [DOI] [PubMed] [Google Scholar]
- 18.Caballero B, Solomons NW, Torún B. Fecal reducing substances and breath hydrogen excretion as indicators of carbohydrate malabsorption. J Pediatr Gastroenterol Nutr. 1983;2:487–90. doi: 10.1097/00005176-198302030-00016. [DOI] [PubMed] [Google Scholar]
- 19.Assiri A, Saeed A, Alnimri A, et al. Five Arab children with glucose-galactose malabsorption. Paediatr Int Child Health. 2013;33:108–10. doi: 10.1179/2046905513Y.0000000055. [DOI] [PubMed] [Google Scholar]
- 20.Strygler B, Nicar MJ, Santangelo WC, et al. Alpha 1-antitrypsin excretion in stool in normal subjects and in patients with gastrointestinal disorders. Gastroenterology. 1990;99:1380–7. doi: 10.1016/0016-5085(90)91165-3. [DOI] [PubMed] [Google Scholar]
- 21.Leeds JS, Oppong K, Sanders DS. The role of fecal elastase-1 in detecting exocrine pancreatic disease. Nat Rev Gastroenterol Hepatol. 2011;8:405–15. doi: 10.1038/nrgastro.2011.91. [DOI] [PubMed] [Google Scholar]
- 22.Degraeuwe PL, Beld MP, Ashorn M, et al. Faecal calprotectin in suspected paediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2015;60:339–46. doi: 10.1097/MPG.0000000000000615. [DOI] [PubMed] [Google Scholar]
- 23.Holtman GA, Lisman-van Leeuwen Y, Kollen BJ, et al. Diagnostic Accuracy of Fecal Calprotectin for Pediatric Inflammatory Bowel Disease in Primary Care:A Prospective Cohort Study. Ann Fam Med. 2016;14:437–45. doi: 10.1370/afm.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conroy S, Hale MF, Cross SS, et al. Unrestricted faecal calprotectin testing performs poorly in the diagnosis of inflammatory bowel disease in patients in primary care. J Clin Pathol. 2018;71:316–22. doi: 10.1136/jclinpath-2017-204506. [DOI] [PubMed] [Google Scholar]
- 25.Manceau H, Chicha-Cattoir V, Puy H, et al. Fecal calprotectin in inflammatory bowel diseases:update and perspectives. Clin Chem Lab Med. 2017;55:474–83. doi: 10.1515/cclm-2016-0522. [DOI] [PubMed] [Google Scholar]
- 26.Li F, Ma J, Geng S, et al. Fecal calprotectin concentrations in healthy children aged 1-18 months. PLoS One. 2015;10:e0119574. doi: 10.1371/journal.pone.0119574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beşer OF, Sancak S, Erkan T, et al. Can Fecal Calprotectin Level Be Used as a Markers of Inflammation in the Diagnosis and Follow-Up of Cow's Milk Protein Allergy? Allergy Asthma Immunol Res. 2014;6:33–8. doi: 10.4168/aair.2014.6.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones NL, Koletzko S, Goodman K, et al. Joint ESPGHAN/NASPGHAN Guidelines for the Management of Helicobacter pylori in Children and Adolescents (Update 2016) J Pediatr Gastroenterol Nutr. 2017;64:991–1003. doi: 10.1097/MPG.0000000000001594. [DOI] [PubMed] [Google Scholar]
- 29.Shimoyama T. Stool antigen tests for the management of Helicobacter pylori infection. World J Gastroenterol. 2013;19:8188–91. doi: 10.3748/wjg.v19.i45.8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veijola L, Myllyluoma E, Korpela R, et al. Stool antigen tests in the diagnosis of Helicobacter pylori infection before and after eradication therapy. World J Gastroenterol. 2005;11:7340–4. doi: 10.3748/wjg.v11.i46.7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas EE, Puterman ML, Kawano E, et al. Evaluation of seven immunoassays for detection of rotavirus in pediatric stool samples. J Clin Microbiol. 1988;26:1189–93. doi: 10.1128/jcm.26.6.1189-1193.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meurman O, Ruuskanen O, Sarkkinen H. Immunoassay diagnosis of adenovirus infections in children. J Clin Microbiol. 1983;18:1190–5. doi: 10.1128/jcm.18.5.1190-1195.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin AL, Kudesia G. Enzyme linked immunosorbent assay for detecting adenoviruses in stool specimens:comparison with electron microscopy and isolation. J Clin Pathol. 1990;43:514–5. doi: 10.1136/jcp.43.6.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uyar Y, Taylan Ozkan A. [Antigen detection methods in diagnosis of amebiasis, giardiasis and cryptosporidiosis] [Article in Turkish] Turkiye Parazitol Derg. 2009;33:140–50. [PubMed] [Google Scholar]
- 35.Shim JO. Clostridium difficile in Children:To Treat or Not to Treat? Pediatr Gastroenterol Hepatol Nutr. 2014;17:80–4. doi: 10.5223/pghn.2014.17.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deshpande A, Pasupuleti V, Patel P, et al. Repeat stool testing to diagnose Clostridium difficile infection using enzyme immunoassay does not increase diagnostic yield. Clin Gastroenterol Hepatol. 2011;9:665–9. doi: 10.1016/j.cgh.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 37.Liesman RM, Binnicker MJ. The role of multiplex molecular panels for the diagnosis of gastrointestinal infections in immunocompromised patients. Curr Opin Infect Dis. 2016;29:359–65. doi: 10.1097/QCO.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 38.Binnicker MJ. Multiplex Molecular Panels for Diagnosis of Gastrointestinal Infection:Performance, Result Interpretation, and Cost-Effectiveness. J Clin Microbiol. 2015;53:3723–8. doi: 10.1128/JCM.02103-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alejo-Cancho I, Fernández Avilés F, Capón A, et al. Evaluation of a multiplex panel for the diagnosis of acute infectious diarrhea in immunocompromised hematologic patients. PLoS One. 2017;12:e0187458. doi: 10.1371/journal.pone.0187458. [DOI] [PMC free article] [PubMed] [Google Scholar]


