ABSTRACT
This state-of-the-art review aims to highlight the challenges in quantifying vitamin activity in foods that contain several vitamers of a group, using as examples the fat-soluble vitamins A and D as well as the water-soluble folate. The absorption, metabolism, and physiology of these examples are described along with the current analytical methodology, with an emphasis on approaches to standardization. Moreover, the major food sources for the vitamins are numerated. The article focuses particularly on outlining the so-called SLAMENGHI factors influencing a vitamer's’ ability to act as a vitamin, that is, molecular species, linkage, amount, matrix, effectors of absorption, nutrition status, genetics, host-related factors, and the interaction of these. After summarizing the current approaches to estimating the total content of each vitamin group, the review concludes by outlining the research gaps and future perspectives in vitamin analysis. There are no standardized methods for the quantification of the vitamers of vitamin A, vitamin D, and folate in foods. For folate and β-carotene, a difference in vitamer activity between foods and supplements has been confirmed, whereas no difference has been observed for vitamin D. For differences in vitamer activity between provitamin A carotenoids and retinol, and between 25-hydroxyvitamin D and vitamin D, international consensus is lacking. The challenges facing each of the specific vitamin communities are the gaps in knowledge about bioaccessibility and bioavailability for each of the various vitamers. The differences between the vitamins make it difficult to formulate a common strategy for assessing the quantitative differences between the vitamers. In the future, optimized stationary digestive models and the more advanced dynamic digestive models combined with in vitro models for bioavailability could more closely resemble in vivo results. New knowledge will enable us to transfer nutrient recommendations into improved dietary advice to increase public health throughout the human life cycle.
Keywords: vitamin A, vitamin D, folate, vitamer, total vitamin activity, foods
Introduction
Worldwide, the term “vitamin” describes organic compounds that the human body by itself cannot produce in sufficient amounts to prevent the development of deficiency diseases. The Polish biochemist Casimir Funk coined the word “vitamine” when he discovered that thiamine could cure polyneurosis (1). In 1941, Burk and Winzler introduced the word “vitamer,” when their investigation of biotin found that several chemical compounds possess biotin activity. At the Vitamin Conference on Gibson Island in July 1942, Burk and Winzler, together with their colleague Hickman, developed the definition of vitamer that embraced compounds that act in a similar way to overcome a given vitamin deficiency (2). The terms vitamine and vitamer have been challenged, and the spelling vitamine was later changed to vitamin, because not all the described compounds were amines like thiamine.
In total 13 vitamin activities have been identified, including the 4 fat-soluble vitamins A, D, E, and K and the 9 water-soluble vitamins B-1 (thiamine), B-2 (riboflavin), B-3 (niacin), B-5 (pantothenic acid), B-6 (pyridoxine), B-7 (biotin), B-9 (folate), B-12 (cobalamin), and C (ascorbic acid). An overview of the vitamers of each vitamin is provided in Table 1. Inadequate dietary intake of vitamins can cause lethal vitamin-specific deficiency diseases, which can also be cured by a sufficient intake, though visible signs of the deficiency period can persist. Vitamin intake requirements to prevent deficiency diseases have been established. However, due to challenges in identifying and quantifying the vitamers in foods, and because of the difficulty in understanding the complex bioactivity of vitamers in relation to food sources interacting with the host, it is still difficult to estimate the optimal intake for health through all stages of the lifecycle (3, 4).
TABLE 1.
Summary of the vitamers for each of the 13 vitamins, including information on availability of standardized methods for foods, CRMs for foods, and quantification principle for the state-of-the-art analytical method
| Vitamin | Vitamer | Standardized | CRM | State-of-the-art |
|---|---|---|---|---|
| method1, matrix2 | organization3, matrix4 | method and quantification | ||
| (organization) | principle5 | |||
| Vitamin A | Total retinol | Foods (CEN) | – | LC-UV |
| All-trans retinol | Foods (CEN) | – | ||
| 13-cis-retinol | Foods (CEN) | – | ||
| Retinol esters | IF, AN (ISO) | NISTy | ||
| Retinaldehyde | IF, AN (ISO) | – | ||
| Total β-carotene | – | ERMd | LC-VIS | |
| All-trans β-carotene | Foods (CEN) | ERMd | ||
| Total α-carotene | – | ERMd | ||
| All-trans α-carotene | – | ERMd | ||
| β-Cryptoxanthin | – | – | ||
| Vitamin D | Vitamin D-3 | Foods (CEN)/IF, AN (ISO) | ERMb/NISTy | LC-MS/MS |
| Vitamin D-2 | Foods (CEN)/IF, AN (ISO) | NISTn | ||
| 25-Hydroxyvitamin D-3 | – | NISTq | ||
| 25-Hydroxyvitamin D-2 | – | – | ||
| Vitamin E | α-Tocopherol, total | Foods (CEN/ISO) | ERMb/NISTy,m | LC-UV/LC-fluorescence |
| α-Tocopherol | IF, AN (ISO) | – | ||
| α-Tocopheryl acetate | IF, AN (ISO) | NISTy | ||
| Vitamin K | Phylloquinone, total | Foods (CEN) | NISTy | LC-fluorescence/LC-MS/MS |
| Phylloquinone, trans | Foods (CEN) | – | ||
| Phylloquinone, cis | Foods (CEN) | – | ||
| Menaquinone-4 | – | – | ||
| Menaquinone-n (MK-n) | – | – | ||
| Thiamine | Thiamine, total | Foods (CEN) | ERMa,d,e/NISTx,m | LC-fluorescence/LC-MS/MS |
| Thiamine, free | – | NISTz | ||
| Thiamine | – | – | ||
| Thiamine monophosphate | – | – | ||
| Thiamine diphosphate | – | – | ||
| Thiamine triphosphate | – | – | ||
| Riboflavin | Riboflavin, total | Foods (CEN) | ERMa,e/NISTx,n,p | LC-fluorescence/LC-MS/MS |
| Riboflavin | – | – | ||
| Flavin mononucleotide | – | – | ||
| Flavin adenine dinucleotide | – | – | ||
| Niacin | Niacin, total | Foods (CEN) | ERMc/NISTx,m,q | LC-MS/MS |
| Niacin, free | – | NISTz | ||
| Nicotinic acid (niacin) | – | NISTq | ||
| Nicotinamide | – | NISTx,y,m,p,q | ||
| Bound forms of niacin | – | – | ||
| Vitamin B-6 | Pyridoxine, total | Foods (CEN) | ERMd,e/NISTx,y,p | LC-fluorescence/LC-MS/MS |
| Pyridoxine, free | – | NISTz | ||
| Pyridoxine | – | – | ||
| Pyridoxal | – | NISTp | ||
| Pyridoxamine | – | NISTp | ||
| Pyridoxine-5′-phosphate | – | – | ||
| Pyridoxal-5′-phosphate | – | – | ||
| Pyridoxine-5′-d-glucoside | – | – | ||
| Pantothenic acid | Pantothenic acid, total | Foods (CEN) | NISTx,y,n,q | LC-MS/MS |
| Pantothenic acid | – | – | ||
| Coenzyme A | – | – | ||
| Biotin | Biotin, total | Foods (CEN) | NISTx,y | LC-MS/MS |
| Biotin | – | – | ||
| Biocytin (o-biotinyl lysine) | – | – | ||
| Folate | Folate, total | FOODs (CEN) | ERMa,d,e | LC-MS/MS |
| Folic acid | – | NISTy,m | ||
| Dihydrofolate | – | – | ||
| Tetrahydrofolate (THF) | – | – | ||
| 5-Methyltetrahydrofolate | – | – | ||
| 5-Formyltetrahydrofolate | – | – | ||
| 10-Formyltetrahydrofolate | – | – | ||
| 5,10-Methenyltetrahydrofolate | – | – | ||
| 5,10-Methylenetetrahydrofolate | – | – | ||
| 10-Formyldihydrofolate | – | – | ||
| 10-Formylfolic acid | – | – | ||
| Vitamin B-12 | Vitamin B-12, total | Foods (CEN)/IF, AN (ISO) | ERMe | LC-MS/MS |
| Cyanocobalamin | – | – | ||
| Methylcobalamin | – | – | ||
| Adenosylcobalamin | – | – | ||
| Hydroxycobalamin | – | – | ||
| Vitamin C | Vitamin C, total | IF, AN (ISO) | ERMc/NISTx,y,p | LC-UV |
| Ascorbic acid | – | – | ||
| Dehydroascorbic acid | – | – |
Standardised method in foodstuff for each vitamer or the total for a vitamin provided by CEN, European Committee for Standardisation and ISO, International Organization for Standardization; information on where to obtain (buy) CEN-methods: https://standards.cen.eu/dyn/www/f?p=CENWEB:5 or ISO-methods: https://www.iso.org/home.html
IF, Infant formula; AN, Adult nutritional
NIST, National Institute of Standards and Technology, ERM, European Reference material.
CRMs: aBCR-121, wholemeal flour; bBCR-122, margarine; cBCR-431, brussels sprouts; dBCR-485, mixed vegetables; eBCR-487, pig liver; fERM-BD600, wholemilk powder (more information at: https://crm.jrc.ec.europa.eu/). NIST: nSRM3235, soy milk; mSRM3233, fortified breakfast cereal; pSRM1549a, whole milk powder; qSRM1546a, meat homogenate; xSRM1869, infant/adult nutritional formula (milk/whey/soy-based); ySRM1849a, infant/adult nutritional formula I (milk-based); zSRM3287, blueberry (more information at: https://www-s.nist.gov/srmors/). SRM with reference values is provided in Supplemental Table 2.
LC-UV, Liquid chromatography-ultraviolet light detection; LC-VIS, Liquid chromatography-visible light detection; LC-MS/MS, Liquid chromatography-tandem mass spectrometry.
Historically, the methods for quantification of each of the vitamins have relied on either a biological assay (i.e., an animal model) for the fat-soluble vitamins or a microbiological assay (i.e., specific bacteria requiring the individual B-vitamins for growth). A chemical method for the quantification of vitamin C was introduced in the 1930s (5), and subsequently chemical methods have gradually been introduced for the quantification of the other vitamins. Information on standardized specific chemical methods is provided in Table 1. The use of specific chemical methods introduced new challenges, including how to convert the specific information on the quantity of each vitamer into vitamin activity, and which compounds are actually vitamers. To estimate total vitamin activity, the respective vitamin activity of each of the vitamers must be understood and estimated. This includes assessments of bioaccessibility and bioavailability. Bioaccessibility describes the amount of an ingested nutrient that is potentially available for absorption, and it depends on digestion and on the release of the vitamer from the food matrix in the gastrointestinal tract (i.e., oral, gastric, and small intestinal). As soon as a food has been digested, bioaccessibility can be measured as either solubility or through a static or dynamic gastrointestinal model. The analytical methods used to estimate the bioaccessible amount of a vitamer can be similar to the methods used for the quantification of vitamers in foods.
By contrast, bioavailability is defined as the amount of an ingested nutrient that is absorbed and available for use in normal physiological functioning and storage (6). In addition to digestion and the release of the vitamer from the food matrix, bioavailability depends on absorption by intestinal cells, transport to body cells, and conversion into the functional form. It may be estimated in vivo through use of, for example, 13C-labeled foods, or in vitro through, for example, the use of Caco-2 cell-line models.
The determinants of bioavailability were originally summarized for carotenoids in the mnemonic acronym “SLAMENGHI.” The letters of the acronym represent the molecular species of the nutrient (i.e., vitamer); its linkage (i.e., whether it is bound to a protein or present as a glucoside); the amount of vitamer in the meal consumed; the matrix in which the vitamer is present; the effectors of absorption (i.e., the presence of fat for fat-soluble vitamins); the nutrient status (i.e., the difference in uptake when a person is deficient); genetic factors (i.e., whether genetic variants have an effect on the absorption); host-related factors (i.e., whether sex and age affect absorption), and finally any interaction between the aforementioned factors (7).
This state-of-the-art review aims to describe current advances in the quantification of total vitamin activity from food, focusing on vitamin A, vitamin D, and folate. A short description will be given of each vitamin group's metabolism and function in the human body, followed by the state-of-the-art analytical methods used for their quantification in food (including efforts at standardization). The review then briefly enumerates the important food sources of the vitamers and current insights into their vitamin activity. Lastly we discuss current research gaps and offer perspectives on quantifying total vitamin activity in our food in the future.
Vitamin A
Retinol refers to a group of closely related fat-soluble compounds present in our diet, that is, all-trans retinol, 13-cis retinol, retinyl esters, and retinaldehyde. In addition, 3 carotenoids with provitamin A activity have been identified: α-carotene, β-carotene, and β-cryptoxanthin (Figure 1). Of these, β-carotene is the predominant carotenoid in the human diet and also believed to have the greatest vitamin A activity, with 12 μg of β-carotene from food being equivalent to 1 μg of retinol, or 1 μg retinol activity equivalent (RAE). For α-carotene and β-cryptoxanthin the amount is double, thus 24 μg of each vitamer is equivalent to 1 μg RAE (8).
FIGURE 1.
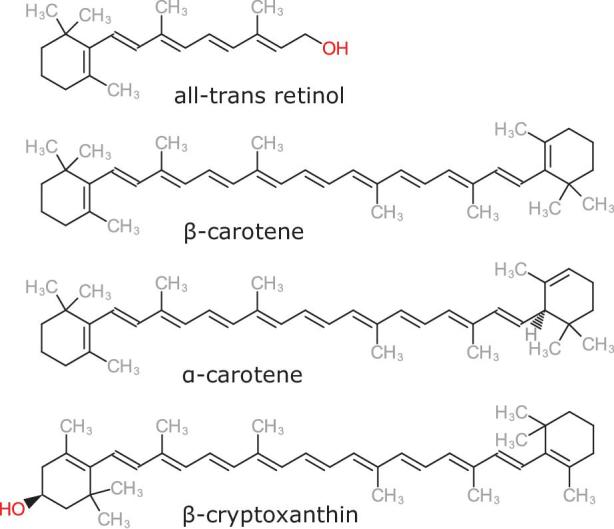
Chemical structures of vitamin A and its vitamers present in our diet.
Metabolism and function of vitamin A in the human body
Upon entering the small intestine, retinol and carotenoids follow slightly different routes of absorption. Retinol is absorbed together with lipids and other fat-soluble vitamins and packaged in chylomicrons as retinyl esters or retinyl palmitate before being released into the bloodstream or liver (9). Absorption of carotenoids is regulated by scavenger receptor class B type I, which can be repressed by the intestinal transcription factor intestine specific homeobox (ISX) (10, 11). Once absorbed, cytosolic β,β-carotene 15,15′-monooxygenase (BCO1) cleaves β-carotene to retinal, after which it is further converted to retinol and incorporated into chylomicrons and transported into the lymph duct (12, 13). Uncleaved β-carotene remaining in the enterocyte is also incorporated into the chylomicrons to be transported to the liver and peripheral tissues. Retinol is stored in the liver stellate cells as retinyl esters, for the body to call on when needed.
Retinol plays an important role in vision for most animals. In humans, retinal forms part of rhodopsin, which is a photochemical reacting to light exposure. One of the clinical consequences of vitamin A deficiency is therefore impaired vision in dim light, with severe deficiency leading to irreversible blindness (14). Retinol can be irreversibly converted to retinoic acid, which is a major ligand for retinoic acid receptors retinoic acid receptor/retinoid X receptor (RAR/RXR) that bind to promoter regions in DNA to initiate gene transcription (15). This process underlies many metabolic pathways and explains the multiple functionality of vitamin A in, amongst others, embryogenesis, cellular differentiation of mucus-secreting cells, synthesis of glycoproteins, and lipid metabolism.
Intake of retinol in large quantities can lead to hypervitaminosis A and can be toxic because the human body is not equipped with a feedback mechanism that regulates absorption of retinol (16). In contrast, β-carotene absorption is regulated by retinoic acid in a feedback mechanism under control of the homeobox ISX (17). Whereas low concentrations of retinoic acid stimulate absorption, high concentrations inhibit uptake of β-carotene. Therefore, no upper limit of intake has been defined for β-carotene, whereas there is such a limit for preformed retinol.
In recent years, it has become clear that the bioconversion of provitamin A carotenoids is not restricted to enterocytes, because BCO1 is also expressed in peripheral tissue including the liver, kidney, eyes, skin, uterus, and testes (17, 18). It is still to be elucidated how much the local production of retinol from provitamin A precursors contributes to overall vitamin A requirements.
Analytical methods used for quantification of vitamin A vitamers in food
Methods for quantitative assessment of carotenoids were comprehensively reviewed in 2014 by Amorim-Carrilho et al. (19). There are no standard methods for carotenoid extraction, but it is mostly done with a water-miscible solvent such as acetone, methanol, ethanol, or tetrahydrofuran, followed by partitioning to hexane, petroleum ether, diethyl ether, or dichloromethane. In case chlorophyll or lipids need to be removed from the sample, a saponification step is required. However, this can lead to loss of some of the more polar carotenoids. Reversed-phase HPLC is the preferred method for carotenoid separation coupled with either a diode array (DAD), a visible spectroscopic detector, or an MS detector. The most common mobile-phase solvents used for carotenoids are acetonitrile and methanol, with small amounts of other solvents being added for optimal carotenoid recovery. Because foods are usually composed of a variety of carotenoids, modification of the chromatographic system, e.g. the mobile phase, may be needed for different types of foods. Some standardization in methodology for the analysis of retinol and β-carotene in foods has been undertaken (e.g. Szpylka & De Vries, 2005; Blake, 2007). Some standardization in methodology for the analysis of retinol and β-carotene in foods has been undertaken (20, 21). Although hampered by the large range of food matrices, official methods have been published for quantifying retinol and β-carotene in foods, which include information on foods included in the validation (22–25). Major critical steps in the procedure are sample collection and sample preparation, due to the vulnerability of vitamers to heat, light, acid, and oxygen exposure. The complexity of assessing retinol and provitamin A carotenoids in food is evidenced by relatively large interlaboratory variability, which appears to be mostly driven by sample preparation and to a lesser extent by the chromatographic system (26, 27). A few of the problems concern the instability of carotenoid internal standards and the lack of a labeled international standard usable in quantification by liquid chromatography–tandem mass spectroscopy (LC-MS/MS). Table 1 provides information on available reference materials. These are limited to retinol and α- and β-carotene, which hampers objective interlaboratory quality control.
Content of vitamin A vitamers in foods
So-called “preformed retinol” is only derived from animal-based or fortified foods, whereas provitamin A carotenoids are mostly derived from brightly colored fruits and vegetables, with dairy products such as margarine and cheese as modest animal-based sources. Abundant β-carotene (values in μg/100 g) is present in sweet potatoes (9444), carrots (8332), and spinach (6288) among the vegetables, and in dried apricots (2163), cantaloupe (2020), and wild plums (1930) among the fruits. Butternut squash (3116) contains an appreciable amount of β-cryptoxanthin, whereas carrots (14,251) are a rich source of α-carotene (28). Over the last decade, biofortified staple crops with increased β-carotene content have been developed through breeding, such as orange-fleshed sweet potato, orange maize, and yellow cassava, which are primarily marketed in low-income countries (29). Although the latter 2 contain relatively low concentrations of β-carotene, the typically large intake in certain populations make them good sources of provitamin A. Transgenic crops with enhanced provitamin A carotenoids, such as rice, wheat, sorghum, soybean, potato, cassava, cauliflower, tomato, and banana, are still undergoing research (30). The final content of provitamin A vitamers in food at the point of consumption largely depends on retention during food processing, storage, and preparation, which is determined by the duration of storage and the intensity of processing and preparation (31, 32).
Methods to estimate activity of vitamin A vitamers
Bioavailability of retinol from food is generally considered to be high, although this has been based on studies using oil as a matrix. The bioefficacy of provitamin A carotenoids from food is determined by the multiplier of bioavailability and bioconversion to vitamin A, usually referred to as the conversion factor. Whereas bioavailability is highly influenced by the food matrix, bioconversion is more driven by factors related to the host. SLAMENGHI factors were first described for carotenoids by West and Castenmiller in 1998 (7). Here we give only a brief summary of some new insights.
Species—It has been suggested that β-cryptoxanthin might be better absorbed compared with β-carotene (33); however, this claim has not yet been substantiated by thorough studies. Recently, vitamin A activity has also been reported for some ketocarotenoids, namely, sapotexanthin and cryptocapsin (34).
Linkage—Carotenoid esters are frequently found in fruits and vegetables, but to date it is not known how these affect bioavailability.
Amount—The fraction of carotenoids absorbed decreases with increasing dose, as is seen for many nutrients (7); however, the total amount absorbed can still be higher from a larger dose.
Matrix—Liberation of carotenoids from the food matrix is 1 of the main limiting steps in their bioavailability. Green leafy vegetables, such as spinach and kale, are rich in carotenoids, but only around 5–10% of the total carotenoid content is bioavailable. In contrast, carotenoids from fruits show higher bioavailability despite their relatively lower carotenoid content (35–37).
Effectors of absorption—Fat content of the diet is the most important enhancer of carotenoid absorption (38–40), whereas fiber present in the diet can reduce absorption efficiency (41).
Nutrient status—Vitamin A status of the host affects absorption of β-carotene by a negative feedback loop via homeobox ISX (17).
Genetic factors—Common variants in the BCO1 gene have been shown to reduce the conversion of β-carotene by ≤69% (42), but several other genes might also influence absorption and conversion (43).
Host-related factors—Iron and zinc deficiency both can affect bioconversion of carotenoids to retinol, and vitamin A metabolism in general. In addition, any disease affecting fat absorption will also affect the absorption of carotenoids.
Interactions—No studies have looked into interactions between the factors stated above, but such interactions might exist.
Vast discrepancy exists between conversion factors reported for different foods and across settings (44, 45). A relatively small number of studies have attempted to assess the conversion factor of β-carotene from an oil-based matrix to retinol, which overall ranged from 2.1 to 3.8. Conversion factors are expressed as the amount (μg) of ingested provitamin A required to form 1 μg of retinol. Until 2001, the average conversion factor of β-carotene from food was considered to be 6, whereas it was 12 for other provitamin A carotenoids. This difference was based on chemical structure, which allows central cleavage of β-carotene to produce 2 retinol molecules, whereas α-carotene and β-cryptoxanthin will only yield 1 retinol molecule. This has resulted in reporting of provitamin A concentrations as retinol equivalents (REs) in food composition tables, taking these conversion factors into account. Based on a range of studies conducted at the end of the previous century, conversion factors for specific fruits and vegetables were shown to vary from 3 to 12 for tubers and fruits, and from 10 to 77 for cooked and raw vegetables (not including tubers) (44, 45). This prompted the Institute of Medicine in the United States to increase the conversion factors to 12 for β-carotene and 24 for other provitamin A carotenoids (RAEs). This was based on food patterns estimated to deliver ∼20% of provitamin A carotenoids from fruit and ∼80% from vegetables, thereby weighing conversion factors for vegetables more heavily than for fruit. Although many countries have followed suit by using the RAE rather than RE in their national food composition tables and nutrient intake recommendations, the WHO has not yet renewed its intake recommendations published in 2004 and therefore still uses the outdated RE (46). Even so, the revised vitamin A intake recommendations for Europe by the European Food Safety Authority in 2015 retained the old conversion factors (47). This is confusing for anyone trying to assess adequacy of dietary vitamin A intake, and especially relevant for populations with a high dependency on provitamin A intake to fulfill their vitamin A requirements (48).
Estimation of total content of vitamin A
Given the interlaboratory variation, it can be expected that values for food carotenoid concentrations are approximations rather than accurate figures in many cases. One of the pitfalls is the loss of vitamers or conversion of the trans to the cis configuration during sample preparation. Reliable measurement requires well-trained and experienced laboratory staff, in addition to proper laboratory facilities. Moreover, when the content of provitamin A carotenoids is expressed as vitamin A activity, it should be clear which conversion factors have been used. Lastly, although β-carotene concentration in foods is well characterized, less is known about the concentrations and vitamin activity of α-carotene and β-cryptoxanthin.
Gaps and future perspectives for estimation of vitamin A activity
The large interindividual variation in the bioconversion of β-carotene to vitamin A determines conversion factors—and thereby vitamin A activity—to a large extent. Better insight into the factors related to this variation is required. It might not be appropriate to use food-based conversion factors when bioconversion depends in large part on the host, and it would therefore be more logical to disentangle a bioavailability factor based on the food (food composition tables) and a conversion factor based on the host (nutrient requirements). This implies also that actual concentrations of vitamers should be reported and not be expressed as RE or RAE. Another aspect that needs attention is the lack of knowledge of the vitamin A activity of β-cryptoxanthin, which is suggested to be higher than assumed so far because of better bioavailability in comparison with β-carotene (33). Therefore, studies on conversion factors for β-cryptoxanthin, either synthetic or from food, are required.
Vitamin D
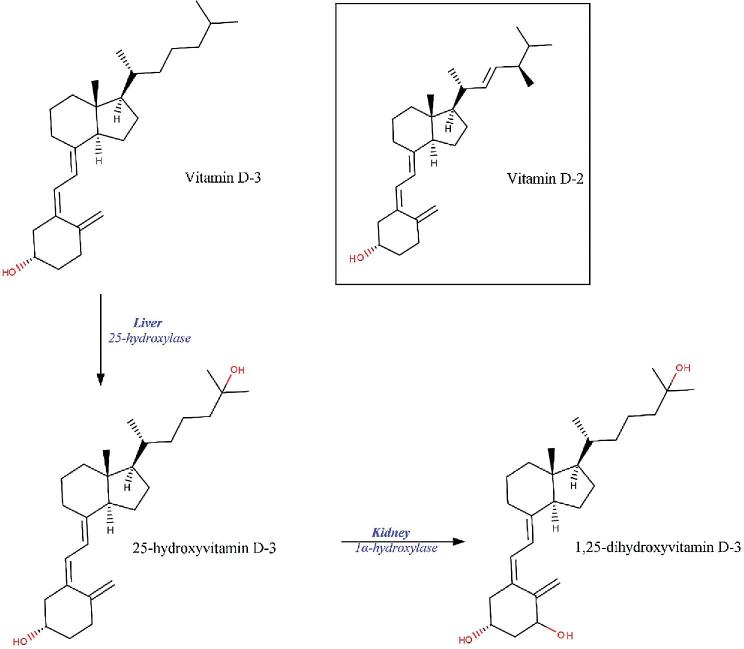
The vitamin D–active compounds are vitamin D-3, 25-hydroxyvitamin D-3 [25(OH)D3], vitamin D-2, 25-hydroxyvitamin D-2 [25(OH)D2], as well as the corresponding metabolites of 1,25-dihydroxyvitamin D [1,25(OH)2D]. Vitamin D-3 is synthesized in the skin of vertebrates from 7-dehydrocholesterol by UVB exposure, and vitamin D-2 is synthesized from ergosterol in fungi and in certain plants contaminated with fungi. Vitamin D compounds are called secosteroids, which means that they are steroids where 1 ring (the B ring between 9 and 10) is open; the hydroxylated vitamers 25(OH)D3 and 1,25(OH)2D are present due to hydroxylation primarily in the liver and in the kidney, respectively (Figure 2). Vitamin D-3 and vitamin D-2 differ only in the side chain, with vitamin D-2 having a double bond (between carbons 22 and 23) and an additional methyl group.
FIGURE 2.
Chemical structures of vitamin D-3 and vitamin D-2, and the hydroxylation of vitamin D-3 to 25-hydroxy vitamin D-3 and 1,25-dihydroxyvitamin D-3 in the liver and in the kidney, respectively.
Metabolism and function of vitamin D in the human body
Studies in rats have shown that the parent vitamer, vitamin D, is absorbed by passive diffusion and transported in chylomicrons to be hydroxylated in the liver, but investigation in human cell lines has shown that the absorption of vitamin D is far more complicated and uptake involves proteins in the intestinal cell membrane. Absorption in humans is between 55% to 99% (49). In contrast, 25(OH)D is absorbed via the portal route, but there is no information on amount absorbed (50).
Vitamin D plays an essential role for the homeostasis of calcium and phosphorus, and the diseases rickets in children and osteomalacia in the elderly are the outcomes of vitamin D deficiency. The accepted biomarker for vitamin D status is the concentration of 25(OH)D in serum, which represents the amount of vitamin D-3 generated by UVB exposure of the skin and vitamin D and 25(OH)D from the diet. The concentrations for insufficiency and deficiency are disputed, but 50 nmol/L and 30 nmol/L, respectively, of 25(OH)D are generally accepted (51, 52). The prevalence for deficiency depends on age and country, and is reported to be between 4% and 18% in Germany, the United Kingdom, the United States, and Canada (52), but ≤80% in Middle Eastern countries (53).
Besides bone health, vitamin D status has been associated with nonskeletal diseases, but the recent results from 3 intervention studies, which investigated the effect of supplementation of vitamin D-3 at levels of 2000 IU daily (54), 100,000 IU monthly (55), and 4000 IU daily (56), did not show decreased risk for development of cancer (54, 55), cardiovascular incident (54, 55), falls (55), fractures (55), or type 2 diabetes (56). However, it is argued that these studies lacked a criterion for vitamin D status, which at the start was between 64 and 77 nmol/L. Thus, a majority of the participants had a vitamin D status that is regarded as sufficient. A recent meta-analysis identified association between low vitamin D status and increased risk of all-cause mortality (57).
Analytical methods used for quantification of vitamin D vitamers in food
Content of vitamin D in food was originally assessed by biological assay until the introduction of chemical methods in the 1980s. The biological assay measures the ability to cure rickets in deficient rats, whereas chickens were used to differentiate between vitamin D-3 vitamers and vitamin D-2 vitamers (58, 59). The chemical methods introduced in the 1980 s focused on the parent forms vitamin D-3 and vitamin D-2, and comparing the 2 methods showed similar results for fortified food with vitamin D-3 (60). Since the mid-1990s the contents of 25(OH)D vitamers in meat, offal, eggs, and dairy products through the use of LC-UV/DAD methods have been published (61–63), and more recently LC-MS/MS methods for milk, pork, and liver have been reported (64–66). The principle of all the chemical methods is an alkaline saponification during which an antioxidant is added to protect vitamin D. The nonsaponifiable extracts include the vitamin D vitamers and is subsequently cleaned up using liquid-liquid extraction with an organic solvent, for example, a combination of n-heptane and ethylacetate or iso-octane. Further clean-up steps can include solid-phase extraction (SPE) and/or preparative LC before detection and quantification by use of reversed-phase LC-UV/DAD. If reversed-phase LC-MS/MS is used for quantification, the matrix and the concentration determine if a solid-phase extraction is necessary. Additionally, a derivatization step utilizing a Diels–Alder reaction with, for example, 4-phenyl-1,2,4-triazoline-3,5-dionediene, is generally applied to enhance ionization, thereby increasing specificity and lowering the limit of quantification. However, there are no reports comparing the biological and chemical methods. Standardized methods for quantification of vitamin D-3 and vitamin D-2 in foods are available (67, 68), and recently, an LC-MS/MS method for infant formula and adult nutritionals was published (69, 70). Thus, no standardized method is available for the content of 25(OH)D3 and 25(OH)D2, but an interlaboratory trial, where the participating laboratories (5 in total) used their own in-house, single-laboratory validated methods, reported reproducibility between 8% and 24% in a variety of food matrices (71).
A few certified reference materials like meat homogenate, whole milk powder, and eggs are available (72, 73); see Table 1 for more information.
Content of vitamin D vitamers in foods
Only a limited number of foods naturally contain vitamin D. Vitamin D-3 is primarily found in food of animal origin, with the highest content in fish, and decreasing content in eggs, offal, meat, and dairy products. Except for fish and eggs, the concentration of vitamin D-3 is generally <1 µg/100 g, and decreasing amounts are found in liver, chicken, beef, pork, and dairy products. It should be noted that in Atlantic salmon (Salmo salar), the vitamin D-3 concentration in wild salmon is reported as 6.7–26.6 µg/100 g whereas it is lower in farmed salmon (2.3–9.1 µg/100 g), although data are limited (74). In general vitamin D-3 is accompanied by the vitamer 25(OH)D3, though in salmon to a lesser degree than in eggs, pork, beef, and dairy products, being ∼5%, ∼25%, 20–150%, ∼250%, and 50–90% of the concentration of vitamin D-3, respectively (63, 74–77). In general, lean cuts of meat have a lower concentration of vitamin D and 25(OH)D than fatty parts (63, 66, 76). However, for wild fish, for example, mackerel and herring, in which fat content varies through the season, no association between fat and vitamin D-3 occurs (78). The vitamers, vitamin D-2 and 25(OH)D2, are also present in dairy products and beef (63, 77). In wild mushrooms and UVB-treated mushrooms vitamin D-2 is the major vitamer (79).
Increased concentrations of vitamin D-3 in feed for hens, pigs, and cattle yield products with higher concentrations of vitamin D-3, as in eggs, pork, and beef (62, 80, 81). Exposure to sun or artificial UVB will similarly increase the content of vitamin D-3 in dairy products and pork (66, 82, 83).
Feed regulations in the European Union have approved the use of 25(OH)D3 in feed for poultry, pigs, and cattle (84, 85). If 25(OH)D3 is used in the feeding of hens and pigs the content of 25(OH)D3 will increase while content of vitamin D-3 will decrease in eggs and pork, respectively (62, 80). If the animal is fed 25(OH)D3 from birth to slaughter, the content of vitamin D-3 in meat, for example, is negligible (86). Apart from vitamin D and 25(OH)D, the dihydroxyvitamin D vitamer 1,25(OH)2D has been reported in milk from humans and cows (65).
In many countries certain foods, especially milk and margarine, are either voluntarily or mandatorily fortified (51), either with vitamin D-3 or vitamin D-2.
Methods to estimate activity of vitamin D vitamers
The implementation of feeding systems that increase the content of 25(OH)D3 in food products or use of UVB exposure in mushrooms to generate products containing vitamin D-2 highlights the need to know the contribution to vitamin D activity from metabolites other than vitamin D-3. Based on recent reviews the SLAMENGHI factors affecting the absorption can be summarized (49, 50, 87, 88) as follows:
Species —The difference between the vitamers, 25(OH)D3 and vitamin D-2, compared with vitamin D-3 is thoroughly covered due to use of different estimates in food databases. The difference between the vitamers has been investigated in animal, human, and recently in vitro models.
25(OH)D3 v ersus vitamin D - 3
In a review of data estimating the biological activity of 25(OH)D3 compared with vitamin D-3 using animal models it was concluded, “Based on calcification score testing in rachitic rats the biological activity of 25OHD is about 1.5 times greater than that of vitamin D. Based on the ability to enhance calcium absorption studies have demonstrated 25OHD3 to be 5 times as active as vitamin D3. These kinds of studies measure the acute effect of vitamin and can differentiate more precisely between effects of native vitamin D and metabolites. However, intestinal absorption of calcium is not a clinical end point parameter” (89). Testing of 25(OH)D3 in the animal assay estimated 25(OH)D3 to have 1.7 times greater vitamin activity than vitamin D-3 (90).
Quesada-Gomez and Bouillon (2018) (87) reviewed the results from 4 human intervention studies (91–94), which included administration of 5–50 µg/d of 25(OH)D3 and vitamin D-3 in a parallel design. Administration of 1 µg of vitamin D-3 or 25(OH)D3 daily increased vitamin D status by either 1.5 nmol/L or 4.8 nmol/L, respectively. However, based on human intervention studies that administered 5–50 µg of vitamin D-3 daily, it is estimated that 1 µg/d of vitamin D-3 will increase vitamin D status by 2 nmol/L (95). Similarly, studies were identified that administered 25(OH)D3 (91–93, 96, 97). Combining the results from these studies revealed that administration of 1 µg/d of 25(OH)D3 increased vitamin D status by 3.9 nmol/L (range 1.8–5.0 nmol/L). Detailed information about the human intervention studies and the calculations are provided in Supplemental Table 1 and Supplemental Figure 1.
From a human intervention study using a crossover design, it was estimated that administration of 25(OH)D3 increased vitamin D status 1.5 times more than compared to the increase obtained by administration of vitamin D-3 (98). Furthermore, another study estimated the differences in the AUC after a bolus of the 2 metabolites; it showed that 25(OH)D3 increased vitamin D status 2–3 times more than vitamin D-3 (96). Recently, in a human cell line (Caco-2), 25(OH)D3 was taken up more efficiently than vitamin D-3, and more solubilized into mixed micelles by a factor of ∼1.8 (99).
Vitamin D - 2 v ersus vitamin D - 3
As mentioned in the Introduction, vitamin D-3 and vitamin D-2 are regarded as equal, even though this consensus has been challenged by findings that administering vitamin D-2 has less effect on vitamin D status than administration of vitamin D-3 (98, 100).
Linkage —Vitamin D and 25(OH)D in food are regarded as nonbound, which is the form absorbed, but esterified vitamin D can be present. This has not been properly investigated (49).
Amount —Although not investigated for vitamin D and 25OHD in foods, a study in human cell line model (Caco-2) indicates that absorption is depending on concentration of vitamin D, due to the involvement of proteins in the absorption process (50).
Matrix —Food matrix seems not to have an effect, except for bread baked with vitamin D-2–enriched yeast, which showed no bioavailability in humans (50, 101). However, this has not been investigated for 25(OH)D.
Effectors of absorption —These could include fat, dietary fiber, and other vitamin D vitamers. The length of fatty acids might influence the absorption, because MUFAs enhanced the increase in vitamin D status, whereas PUFA decreased it (49). However, no information on 25(OH)D is available, and more research is needed to investigate the how fat and other compounds possibly affect the absorption of D vitamers.
Nutrient status —The expectation would be a lower absorption with higher vitamin D status; however, only 1 specific study in rats has shown this effect (50). During the last 20 y vitamin D-3 has been included in a large number of human intervention studies, although these have not directly focused on absorption. Based on studies administering <30 µg/d vitamin D-3, a curvilinear association between daily intake and vitamin D status was estimated (102). Administration of 25(OH)D3 revealed no effect on vitamin D status (87). However, a steady state for vitamin D status occurs independent of the vitamer administered.
Genetic factors —These have been shown to have a significant impact on vitamin D status e.g. the polymorphisms of the vitamin D binding protein GC, which is essential for vitamin D transport and of the 25-hydroxylase CYP2R1, which is essential for the hydroxylation of vitamin D to 25(OH)D are associated with increased vitamin D status obtained by supplementation and UVB exposure (103). However, a genome-wide association study of 80,000 persons provided evidence against large interactions between common single-nucleotide polymorphisms and dietary vitamin D intake in the population studied (104).
Host-related factors —Few studies have investigated the effect of age, but apparently no differences were identified (50). No information exists for differences between sex, but in studies where women and men have been included no differences were observed (92). Vitamin D status is inversely related to BMI, but this has not been related to differences in absorption (50).
Interaction of the above factors —Not investigated.
Estimation of total content of vitamin D
Estimations of the content of vitamin D in food by the biological methods that quantify total vitamin D activity in deficient rats are limited (59). An IU of vitamin D is defined as 0.025 µg vitamin D-3, and vitamin D-2 is regarded as equivalent to vitamin D-3. For 25(OH)D, it could be valuable to transform the content to IU, but this is currently not possible because no international consensus is available; however, for this purpose it could be an advantage to establish a new unit, the vitamin D equivalent (VDE), where 1 VDE equals 1 µg vitamin D-3. As reviewed above, no method to estimate the difference between the 2 metabolites has been established, and in humans, 1 µg 25(OH)D3 has been estimated to be in the range 1–5 VDE. A factor of 5 (∼5 VDE) is currently used in some food databases (e.g., in the United Kingdom and Denmark), whereas a factor of 1 (∼1 VDE) for 25(OH)D3 is included in other food databases (e.g., in the Netherlands and Canada). No database differentiates between vitamin D-2 and vitamin D-3. In the Codex Alimentarius International Food Standards (CODEX), the issue regarding a difference between vitamin D-2 and vitamin D-3 has been on the agenda, but not the challenges for estimating the vitamin D activity of 25(OH)D3 (105).
Gaps and future perspectives for estimation of vitamin D activity
Data for contents of 25(OH)D metabolites in foods are steadily growing and being incorporated into food databases. In contrast, limited data are available for contents of 1,25-dihydroxy metabolites; the metabolites should be included in future analytical methods in order to investigate if the content of the metabolite contributes significantly to vitamin D activity.
However, the main gap is how to estimate the total vitamin D activity based on the content of mainly vitamin D-3, vitamin D-2, 25(OH)D3, and 25(OH)D2. The food matrix seems so far not to be important, but limited data are available, therefore different food matrices should be investigated. If the method of choice is a human intervention study, it should take into account that an increase in vitamin D status is host dependent, as well as dependent on the amount administered, therefore a crossover design should be prioritized. An alternative would be to investigate the use of human cell lines in an effort to estimate the difference between the parent vitamer and the 25-hydroxylated vitamers. Another technique would be to use 13C-labeled vitamin D vitamers, which have successfully been used for investigation of the metabolism and storage of vitamin D-3 in an animal model (106).
Until such results have been published, the vitamin D vitamers should be regarded as equal, that is, 1 µg 25(OH)D = 1 VDE, and the content of vitamin D is the sum of each of the vitamin D vitamers.
Folate
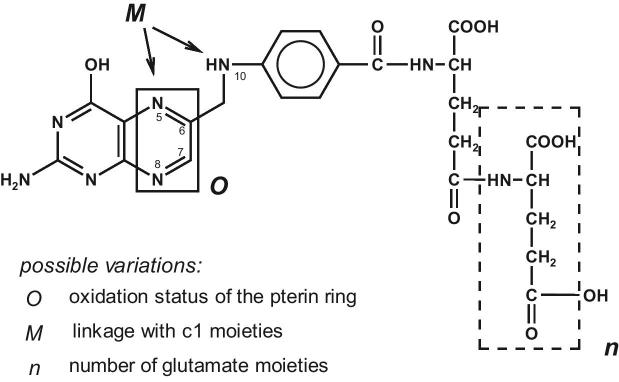
The folate group consists of a variety of compounds. Along with various pteroyl monoglutamic forms, folates in food primarily occur as pteroyl polyglutamates, which can contain 2 to 8 glutamyl residues in the molecule (Figure 3).
FIGURE 3.
Structural variety of folates with natural bioactive forms occurring as reduced tetrahydro forms at O and glutamate distribution ranging from n = 0 to 7.
Metabolism and function of folate in the human body
Folates are essential in metabolism primarily for the transfer of methyl and formyl groups, and they perform key functions in the metabolism of amino acids and nucleic acids. Prior to absorption, polyglutamate forms have to be converted into monoglutamates. This cleavage is performed by gamma-glutamyl hydrolase (GGH), which is either secreted from the pancreas or present in the intestinal brush border. After deconjugation, the monoglutamates are absorbed via transmembrane folate carrier proteins into intestinal epithelium cells, where they are reduced to tetrahydrofolate and transformed to 5-methyltetrahydrofolate (107). The latter is then transported in the blood circulation after passing through the liver via the portal vein. Surveys of folate status in several countries have been based on plasma or erythrocyte folate concentrations and revealed that often a significant part of the population had inadequate folate status. In Europe, for example, this applied to ∼10% of the French population, to 4% of pregnant women in Switzerland, and to >40% of a Swedish cohort of pregnant women (108). This form of dietary deficiency is particularly significant for pregnant women or women of childbearing age because it is strongly associated with an increased frequency of neural tube defects in the developing fetus. There is also increasing evidence that inadequate folate intake increases the susceptibility to cardiovascular disease, colorectal cancer, and Alzheimer disease (109).
Analytical methods used for quantification of folate vitamers in food
Various methods are now used to quantify total folate, of which microbiological and chromatography-based determinations constitute the most important ones. Microbiological assays (MAs) quantify folates by the growth of microorganisms, such as Lactobacillus casei, on food extracts containing folates. Due to its sensitivity and the need for only folic acid as calibrator, the MA is the gold standard in food folate analysis (110). Based on MAs, several reference materials such as mixed vegetables (73) or single food items ranging from meat to wheat flour (72) are commercially available and can assist in evaluating the assay's performance. More information is provided in Table 1. Despite the sensitivity of MAs for determining the overall folate content, they are not suited to the differential detection of individual vitamers. In addition, specific growth factors or inhibitors can provoke stimulation or inhibition of the microorganisms. Therefore, along with the variable response of the microorganisms to the single vitamers, the quantitative result can be compromised; the significant variations revealed by comparative studies can be attributed to these issues (111, 112).
Compared with the MA described above, the chemical analytical methods have the advantage of being able to distinguish between the individual folate vitamers. The most selective detection of folates in the field of LC methods is offered by MS. To compensate for losses of unstable folates during extraction and due to ionization interferences during MS, stable isotope dilution assays (SIDAs) have proved their strength in determining folate concentrations in food (113–116).
With SIDAs, stable isotopically labeled vitamers are used as internal standards (117) and LC-MS/MS allows accurate quantification of the single vitamers.
Several comparisons of these methods have been reported in the literature, often revealing higher total folate content from the MA than the chromatographic methods (118). A recent comparison between LC-MS/MS and MA supported these findings (119). For the latter comparative study, deconjugation of polyglutamates into monoglutamates was performed using pteroyl polyglutamate hydrolase (PPH) from chicken pancreas in combination with PPH from rat serum, at a pH of 5.0. After the experimental confirmation of enzyme activity with the test substrate pteroyl triglutamate, complete polyglutamate deconjugation in the sample matrix was to be expected. No significant differences between the methods were observed for broccoli, pea soup, or a reference material consisting of a mixture of vegetables. Substantial differences were seen with a wheat germ sample: the values from quantification by LC-MS/MS were ∼35% lower than those from the MA. Further analysis showed that deconjugation was not complete, and that diglutamates as well as residues of higher polyglutamates were detectable in their respective LC-MS/MS traces. These results were used to develop the first reported method for quantifying the diglutamates (119). Although this method returned higher values for quantification using LC-MS/MS, an optimized enzyme treatment made it possible to determine a concentration of 392 µg/100 g for the wheat germ, which offered a good approximation of the result from the MA. In addition, the dependency of the MA calibration on the choice of folate must be taken into account, because this can result in differences of ≤20% in the total folate content (119). Screening for diglutamates was therefore included into the method in order to verify the completeness of deconjugation. Novel developments target improving deconjugation efficiency by using genetically engineered plant GGH (115, 120).
Content of folate vitamers in foods
Staple foods such as wheat or corn products, potato, or rice contain only minuscule amounts of folates (121) unless they are fortified with synthetic folic acid, as mandated in >50 countries, mainly on the South and North American continents. A folate-rich diet requires careful selection of the food; in general, green vegetables, particularly legumes (122), and some fruits contain high concentrations of folates. Among the foods with highest folate content, cow peas are a very good source with >400 µg/100 g dry mass (123). This content is exceeded by the “king of fruit,” the durian, which has the highest concentration of any fruit, with >400 µg/100 g fresh mass (124); this is consumed almost exclusively in Southeast Asia and China. Tropical fruits (125) have high potential as folate sources, for example, the giant granadilla with 270 µg/100 g fresh mass (N Weber, Technical University of Munich, personal communication, 2019), along with fruits from regions of high biodiversity, for example, the Australian green plum (126). Green vegetables generally are good folate sources, with the prominent ones being spinach with >227 µg/100 g (113) or broccoli with 183 µg/100 g (119). Among fruits, strawberries as highly appreciated by consumers worldwide and can contain >150 µg/100 g (127). Further promising sources include microalgae or yeasts (128).
Methods to estimate activity of folate vitamers
A first estimation of vitamin activity can be performed by MA, and it has already been shown that the MA reveals different responses to the vitamers (119). Interestingly, commonly used MAs are able to detect polyglutamates containing up to 3 glutamate residues with almost equivalent responses, which is a further asset of the MA (129). As conflicting results between the MA and chromatographic methods have also been attributed to additional compounds showing folate activity, the MA has also been used to assess folate derivatives modified during storage or food processing. In this regard, the oxidation product of 5-methyltetrahydrofolate, the pyrazino-s-triazine derivative of 4α-hydroxy-5-methyltetrahydrofolate (abbreviated as MeFox), revealed no response in an MA (119), and during testing the bioactivity of folate glycation products, L.casei revealed neither a positive response nor an inhibition of growth when exposed to N2-[1-(carboxy)ethyl]folic acid or N2-[1-(carboxy)methyl]folic acid (130).
As indicated by this short overview on the MA response to several vitamers, the SLAMENGHI factors mentioned before are also applicable to folate:
Species and Linkage —The vitamers differ in their biokinetics behavior; for example, 5-methyltetrahydrofolate applied in short-term human studies has an earlier tmax, i.e. its peak in plasma folate appears earlier than that of folic acid (131). The differences in overall bioavailability, in particular for the polyglutamylated forms, are still under discussion, with several studies giving inconclusive results (132).
Amount —Previous human studies indicate a linear relation between dose and plasma response after saturation of folate stores and below doses that will saturate the folate carriers. However, due to the uncertainties in response quantitation, this is an assumption.
Matrix —This factor has been assessed in more detail using bioaccessibility models investigating the influence of the food matrix on deconjugation or stability of folates during digestion. As a result of such in vitro studies a measure of bioaccessibility is obtained, which in the case of folates can be defined as the amount of monoglutamates obtained after simulation relative to the total amount of folates including polyglutamates initially present in the food. Several in vitro simulations of digestion have been applied, with probably the most prominent being the TNO (the Netherlands Organisation for Applied Scientific Research) - intestinal model, a dynamic computer-controlled digestion model. It simulates the transit time, peristaltic mixing, and diffusion-based absorption of in vivo studies (133). Other approaches have used a simulated digestion model with respect to folate stability and efficiency of deconjugation by applying porcine brush border membrane to realistically simulate digestion in the small intestine (134). When comparing the latter bioaccessibility results with those from a human bioavailability study, the impact of both stability and deconjugation on bioaccessibility was confirmed. The most labile vitamer, tetrahydrofolate, can be degraded and significant loss affects all vitamers unless stabilized by antioxidants like ascorbic acid.
When studying bioavailability in vivo, short-term studies often apply an AUC approach. Using AUC a defined amount of folates is administered followed by frequent blood sampling within a defined period of time. The bioavailability is then calculated relative to the AUC resulting after the ingestion of a reference substance. Generally, folic acid has been used as the reference, but recent studies favor 5-methyltetrahydrofolate as the reference due to its more similar kinetics to that of native food folates.
Effectors of absorption —Deconjugase inhibitors have to be named, and organic acids such as citric acid have been identified to impair deconjugation and absorption.
Nutrient status —There are no reliable data on this factor, which is a severe gap in bioavailability studies, where folate saturation of the subject is the usual protocol. However, it is not known how folate transport and absorption are affected.
Genetic factors —Several polymorphisms of genes involved in folate transport and metabolism can affect folate status (135, 136). These gene variants likely explain the large interindividual variations in bioavailability (sometimes with a CV >100%) reported in human studies (137). This finding highlights further the need to aim for individual dietary recommendations in the future.
Host-related factors —No data are available.
Interaction of the above factors —Not investigated.
The current dietary recommendations are based on the studies of Sauberlich et al. (138), who determined in a long-term study a 50% bioavailability of food folates relative to pteroylmonoglutamic acid. However, this general figure has to be questioned because recent human studies have revealed new bioavailability data for several foodstuffs, such as 73% for spinach and 33% for wheat germ. Moreover, even for the same type of food, highly divergent bioavailabilities have been reported, for example, 9% versus >64% for 2 different types of Camembert cheeses (137, 139).
The results clearly underline the importance of the food matrix, even within the same type of food product, in terms of folate bioavailability. Moreover, the human studies revealed a huge interindividual variability of folate bioavailability, which indicates the need for more individual than general recommendations. Nevertheless, the generally assumed 50% bioavailability of food folates led to the definition of dietary folate equivalents, which are equivalent to the amount of natural folates in a given food or, in case of food fortified with folic acid, multiplied by a factor of 1.7 (140).
Estimation of total content of folate
The result of the MA is considered to give a value for the total content of folate because the MA responds to all monoglutamates and partly to the polyglutamates. As discussed above, the somewhat different response of the microorganisms to the different vitamers has to be considered, and the addition of further PPH activity if polyglutamates consisting of more than 3 glutamate residues are expected. If the vitamer distribution is known approximately, the result of the MA can be more accurate if the major vitamer is used as calibrant (119). However, in laboratory comparisons, the MA revealed CVs >24%, which points to significant uncertainty (112).
For chromatographic methods, which usually measure the content of the vitamers after deconjugation to monoglutamates, total folate content is calculated as the sum of all resulting monoglutamates, and given as folic acid. A comprehensive chromatographic method to detect and accurately quantify all vitamers as they appear natively in foods is currently not available.
Gaps and future perspectives for estimation of folate activity
Notwithstanding the recent improvements in folate analysis, the sensitive and accurate quantitation of folates in foods is still challenging.
Although the MA is still the reference method for foods, the LC-MS/MS–based methods are increasingly being used for blood analysis and generally accepted (141). In infant foods and adult nutritionals a standardized LC-MS/MS method is available (142), and standardization of the recently published LC-MS/MS method in food (115) is currently in progress (J Jakobsen, Technical University of Denmark, personal communication, 2019).
A bottleneck for implementation of an LC-MS/MS method has been the availability and cost of the isotopically labeled internal standards, but this is becoming less relevant as several suppliers now provide almost all relevant vitamer isotopologues at reasonable prices. A series of labeled standards for starting with the LC-MS/MS method would currently cost around 2000€ (143) and would be sufficient for 2000 samples.
Nevertheless, LC-MS/MS requires thorough validation including accurate addition of isotopologic standards as well as ensuring complete deconjugation and consideration of endogenous vitamer contents in the application of enzymes during sample preparation.
To avoid the deconjugation issue, the development of methods to quantify native polyglutamates is even more challenging for the following reasons: 1) the polyglutamates are present in relatively low concentrations, which requires even more sensitive methods; 2) the lack of polyglutamic reference compounds; and 3) the lack of isotopically labeled polyglutamic vitamers—the polyglutamates show different binding behaviors than the monoglutamates on commonly used SPE materials for extract clean-up.
To unravel further the relations between native folate vitamers, their precursors, or metabolites, nontargeted metabolomics might offer some inroads, but lack of sensitivity of the applied high-resolution methodologies is still the roadblock to folate metabolomics. This is obvious from current physiological studies using metabolomics, where folates generally are not detected although they must be present (144).
Discussion and Conclusion
Of the 13 vitamins, 3 have been highlighted. These represent diverse aspects of the challenges in quantifying total vitamin activity. There are no standardized methods for quantification of the vitamers of vitamin A, vitamin D, and folate in foods, and it is troublesome and costly to perform a multilaboratory test, which is required for standardization. Through the AOAC Stakeholder Panel on Infant Formula and Adult Nutritional an effort has been underway since 2010 to establish standardized methods for the vitamins in the matrices of infant formula and adult nutritionals. These methods will provide a good starting point for the future standardization of methods for measuring the natural content of vitamins in foods. Because internationally standardized methods are lacking, the current data in food databases are established using single-laboratory validated methods, proficiency testing systems, certified reference materials, and/or the accreditation of a laboratory according to ISO17025.
For folate and β-carotene, a difference in vitamer activity between foods and supplements has been confirmed, whereas no difference has been observed for vitamin D. For differences in vitamer activity between provitamin A carotenoids and retinol, and between 25(OH)D and vitamin D, international consensus is lacking. To obtain this in the future, essential information on vitamers’ bioaccessibility and bioavailability from diverse foods and supplements is necessary. In addition, a new vitamin D unit is justified where the proposed unit is the VDE, which would be consistent in principle with the units for vitamin A and folate. The challenges facing each of the specific vitamin communities are the gaps in knowledge about bioaccessibility and bioavailability for each of the various vitamers. The differences between the vitamins make it difficult to formulate a common strategy for assessing the quantitative differences between the vitamers. In vivo studies in humans using foods labeled with stable isotopes are the best available option, but in the future, optimized stationary digestive models and the more advanced dynamic digestive models combined with in vitro models for bioavailability might more closely resemble in vivo results.
Lastly, knowledge of differences in absorption and bioconversion between individuals will contribute to the future estimation of nutrient recommendations as part of risk-benefit analyses. It is difficult to estimate the total vitamin activity in a food. However, we must take on this task and establish new models and methods that can reliably assess the vitamin activity of today's foods and of new foods that will be brought to consumers in the future. This knowledge will enable us to transfer nutrient recommendations into improved dietary advice to increase public health throughout the human life cycle.
Supplementary Material
Acknowledgements
The authors’ responsibilities were as follows—AM-B, JJ, MR: wrote the sections for vitamin A, vitamin D, and folate, respectively; and all authors: contributed to, read, and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: JJ, AM-B, MR, no conflicts of interest.
Supplemental Tables 1 and 2 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: BCO1, β,β-carotene 15,15′-monooxygenase; DAD, diode array detector; GGH, gamma-glutamyl hydrolase; ISX, intestine specific homeobox; LC-MS/MS, liquid chromatography–tandem mass spectrometry; MA, microbiological assay; PPH, pteroyl polyglutamate hydrolase; RAE, retinol activity equivalent; RAR, retinoic acid receptor; RE, retinol equivalent; RXR, retinoid X receptor; SIDA, stable isotope dilution assay; SPE, solid-phase extraction; VDE, vitamin D equivalent; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D; 25(OH)D2, 25-hydroxyvitamin D-2; 25(OH)D3, 25-hydroxyvitamin D-3.
References
- 1. Piro A, Tagarelli G, Lagonia P, Tagarelli A, Quattrone A. Casimir Funk: his discovery of the vitamins and their deficiency disorders. Ann Nutr Metab 2010;57:85–8. [DOI] [PubMed] [Google Scholar]
- 2. Burk D, Winzler RJ. Heat-labile, avidin-uncombinable, species-specific and other vitamers of biotin. Science 1943;97:57–60. [DOI] [PubMed] [Google Scholar]
- 3. McCann JC, Ames B. Vitamin K, an example of triage theory: is micronutrient inadequacy linked to diseases of aging? Am J Clin Nutr 2009;90(4):889–907. [DOI] [PubMed] [Google Scholar]
- 4. Moyer MW. Vitamins on trial. Nature 2014;510:5–7. [DOI] [PubMed] [Google Scholar]
- 5. Tillmans J. Über das Wesen des C-Vitamins. Mitteilung aus dem Universitäts-Institut fü r Nahrungsmittelchemie in Frankfurt a. Main 1932;64:11–21. [Google Scholar]
- 6. Jackson MJ. The assessment of bioavailability of micronutrients: introduction. Eur J Clin Nutr 1997;51:S1–S2. [PubMed] [Google Scholar]
- 7. West CE, Castenmiller JJM. Quantification of the “SLAMENGHI” factors for carotenoid bioavailability and bioconversion. Int J Vitam Nutr Res 1998;68:371–77. [PubMed] [Google Scholar]
- 8. Institute of Medicine Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- 9. Gamble MV, Blaner WS. Factors affecting blood levels of vitamin A. In: Livrea MA, editor Vitamin A and retinoids: an update of biological aspects and clinical applications. Basel, Boston, Berlin: Birkhäuser Verlag-Springer; 2000. [Google Scholar]
- 10. Van Bennekum A, Werder M, Thuahnai ST, Han CH, Duong P, Williams DL, Wettstein P, Schulthess G, Phillips MC, Hauser H. Class B scavenger receptor-mediated intestinal absorption of dietary β-carotene and cholesterol. Biochemistry 2005;44:4517–25. [DOI] [PubMed] [Google Scholar]
- 11. Lobo GP, Hessel S, Eichinger A, Noy N, Moise AR, Wyss A, Palczewski K, von Lintig J. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal, β,β-carotene absorption and vitamin A production. FASEB J 2010;24:1656–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nagao A. Oxidative conversion of carotenoids to retinoids and other products. J Nutr 2004;134:237S–40S. [DOI] [PubMed] [Google Scholar]
- 13. Biesalski HK, Chichili GR, Frank J, von Lintig J, Nohr D. Conversion of beta-carotene to retinal pigment. Vitam Horm 2007;75:117–30. [DOI] [PubMed] [Google Scholar]
- 14. Tielsch JM, Sommer A. The epidemiology of vitamin A deficiency xerophthalmia. Ann Rev Nutr 1984;4:183–205. [DOI] [PubMed] [Google Scholar]
- 15. Bastien J, Rochette-Egly C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene 2004;328:1–16. [DOI] [PubMed] [Google Scholar]
- 16. Li Y, Wongsiriroj N, Blaner WS. The multifaceted nature of retinoid transport and metabolism. Hepatobiliary Surg Nutr 2014;3:126–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lobo GP, Amengual J, Palczewski G, Babino D, von Lintig J. Mammalian carotenoid-oxygenases: key players for carotenoid function and homeostasis. Biochim Biophys Acta 2012;1821:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Von Lintig J, Hessel S, Isken A, Kiefer C, Lampert JM, Voolstra O, Vogt K. Towards a better understanding of carotenoid metabolism in animals. Biochim Biophys Acta 2005;1740:122–31. [DOI] [PubMed] [Google Scholar]
- 19. Amorim-Carrilho KT, Cepeda A, Fente C, Regal P. Review of methods for analysis of carotenoids. Trends Anal Chem 2014;56:49–73. [Google Scholar]
- 20. Szpylka J, Devries JW. Determination of β-carotene in supplements and raw materials by reversed-phase high pressure liquid chromatography: collaborative study. J AOAC Int 2005;88:1279–91. [PMC free article] [PubMed] [Google Scholar]
- 21. Blake CJ. Status of methodology for the determination of fat-soluble vitamins in foods, dietary supplements, and vitamin premixes. J AOAC Int 2007;90:897–910. [PubMed] [Google Scholar]
- 22. CEN Foodstuffs - Determination Determination of vitamin A by high performance liquid chromatography - Part 1: Measurement of all-E-retinol and 13-Z-retinol. EN 12823-1:2014. Brussels: European Committee for Standardization (CEN), [Internet]. 2014. [cited August 15, 2019]. https://standards.cen.eu/dyn/www/f?p=204:110:0::::FSP_PROJECT,FSP_ORG_ID:27774,6256&cs=12328F54677CDAF7D1AF95500BD7CB38F. [Google Scholar]
- 23. CEN Foodstuffs - Determination Determination of vitamin A by high performance liquid chromatography - Part 2: Measurements of Beta-carotene. EN 12823-2:2000. Brussels: European Committee for Standardization (CEN), [Internet]. 2000. [cited August 15, 2019]. https://standards.cen.eu/dyn/www/f?p=204:110:0::::FSP_PROJECT,FSP_ORG_ID:13842,6256&cs=185FB2F96095FDE4F32138F14B38BE2E4. [Google Scholar]
- 24. ISO 12080-2:2009 [IDF 142-2:2009] Dried skimmed milk - Determination of vitamin A content. Part 2: Method using high-performance liquid chromatography. International Organization for Standardization, Geneva, Switzerland, [Internet]. 2009. [cited August 15, 2019]. https://www.iso.org/standard/53831.html [Google Scholar]
- 25. AOAC Authors. Official method 941.15 Total carotenoids in fresh plant materials and silages. Association of Analytical Communities, Gaithersburg, MD, 17th edition, 2006. [Google Scholar]
- 26. Scott KJ, Finglas PM, Scale OR, Hart DJ, de Froidmont-Giirtz I. Interlaboratory studies of HPLC procedures for the analysis of carotenoids in foods. Food Chem 1996;57:85–90. [Google Scholar]
- 27. Devries JW, Silvera KR. Determination of vitamins A (retinol) and E (alpha-tocopherol) in foods by liquid chromatography: collaborative study. J AOAC Int 2002;85:424–34. [PubMed] [Google Scholar]
- 28. US Department of Agriculture , Agricultural Research Service, Nutrient Data Laboratory. USDA National Nutrient Database for Standard Reference, [Internet]. Release 28, April 2018. https://ndb.nal.usda.gov/ndb/. [Google Scholar]
- 29. Bouis HE, Hotz C, McClafferty B, Meenakshi JV, Pfeiffer WH. Biofortification: a new tool to reduce micronutrient malnutrition. Food Nutr Bull 2011;32:S31–40. [DOI] [PubMed] [Google Scholar]
- 30. Garg M, Sharma N, Sharma S, Kapoor P, Kumar A, Chunduri V, Arora P. Biofortified crops generated by breeding, agronomy, and transgenic approaches are improving lives of millions of people around the world. Frontiers Nutr 2018;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maiani G, Periago Caston MJ, Catasta G, Toti E, Cambrodón IG, Bysted A, Granado-Lorencio F, Olmedilla-Alonso B, Knuthsen P, Valoti M et al.. Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol Nutr Food Res 2009;53:S194–218. [DOI] [PubMed] [Google Scholar]
- 32. Rodriguez-Amaya DB. Food carotenoids: analysis, composition and alterations during storage and processing of foods. Forum Nutr 2003;56:35–7. [PubMed] [Google Scholar]
- 33. Burri BJ, Chang JST, Neidlinger TR. β-Cryptoxanthin- and α-carotene-rich foods have greater apparent bioavailability than β-carotene-rich foods in Western diets. Brit J Nutr 2011;105:212–19. [DOI] [PubMed] [Google Scholar]
- 34. Chacón-Ordóñez T, Esquivel P, Quesada S, Jiménez RR, Cordero A, Carle R, Schweiggert R. Mamey sapote fruit and carotenoid formulations derived thereof are dietary sources of vitamin A: a comparative randomized cross-over study. Food Res Int 2019;122:340–47. [DOI] [PubMed] [Google Scholar]
- 35. van het Hof KH, Tijburg LB, Pietrzik K, Weststrate JA. Influence of feeding different vegetables on plasma levels of carotenoids, folate and vitamin C. Effect of disruption of the vegetable matrix. Br J Nutr 1999;82:203–12. [PubMed] [Google Scholar]
- 36. Castenmiller JJ, West CE, Linssen JP, van het Hof KH, Voragen AG. The food matrix of spinach is a limiting factor in determining the bioavailability of beta-carotene and to a lesser extent of lutein in humans. J Nutr 1999;129:349–55. [DOI] [PubMed] [Google Scholar]
- 37. Schweiggert RM, Mezger D, Schimpf F, Steingass CB, Carle R. Influence of chromoplast morphology on carotenoid bioaccessibility of carrot, mango, papaya, and tomato. Food Chem 2012;135:2736–42. [DOI] [PubMed] [Google Scholar]
- 38. Brown MJ, Ferruzzi MG, Nguyen ML, Cooper DA, Eldridge AL, Schwartz SJ, White WS. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. Am J Clin Nutr 2004;80:396–403. [DOI] [PubMed] [Google Scholar]
- 39. Goltz SR, Campbell WW, Chitchumroonchokchai C, Failla ML, Ferruzzi MG. Meal triacylglycerol profile modulates postprandial absorption of carotenoids in humans. Mol Nutr Food Res 2012;56:866–77. [DOI] [PubMed] [Google Scholar]
- 40. White WS, Zhou Y, Crane A, Dixon P, Quadt F, Flendrig LM. Modeling the dose effects of soybean oil in salad dressing on carotenoid and fat-soluble vitamin bioavailability in salad vegetables. Am J Clin Nutr 2017;106:1041–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Riedl J, Linseisen J, Hoffmann J, Wolfram G. Some dietary fibers reduce the absorption of carotenoids in women. J Nutr 1999;129:2170–6. [DOI] [PubMed] [Google Scholar]
- 42. Leung WC, Hessel S, Méplan C, Flint J, Oberhauser V, Tourniaire F, Hesketh JE, von Lintig J, Lietz G. Two common single nucleotide polymorphisms in the gene encoding beta-carotene 15,15′-monoxygenase alter beta-carotene metabolism in female volunteers. FASEB J 2009;23:1041–53. [DOI] [PubMed] [Google Scholar]
- 43. Borel P, Desmarchelier C. Genetic variations associated with vitamin A status and vitamin A bioavailability. Nutrients 2017;9:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tang G. Bioconversion of dietary provitamin A carotenoids to vitamin A in humans. Am J Clin Nutr 2010;91:1468S–73S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Van Loo-Bouwman CA, Naber TH, Schaafsma G. A review of vitamin A equivalency of β-carotene in various food matrices for human consumption. Br J Nutr 2014;111:2153–66. [DOI] [PubMed] [Google Scholar]
- 46. WHO/FAO Vitamin and mineral requirements in human nutrition. Report of a joint FAO/WHO expert consultation on human vitamin and mineral requirements 2nd ed Geneva: WHO;2004. [Google Scholar]
- 47. EFSA (European Food Safety Authority) Panel on Dietetic Products, Nutrition and Allergies Scientific opinion on dietary reference values for vitamin A. EFSA J 2015;13:4028–121. [Google Scholar]
- 48. Melse-Boonstra A, Vossenaar M, van Loo-Bouwman CA, Kraemer K, de Pee S, West KP, Russell RM, Solomons NW. Dietary vitamin A intake recommendations revisited: global confusion requires alignment of the units of conversion and expression. Public Health Nutr 2017;20:1903–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reboul E. Intestinal absorption of vitamin D: from the meal to the enterocyte. Food Funct 2015;6:356–62. [DOI] [PubMed] [Google Scholar]
- 50. Borel P, Caillaud D, Cano NJ. Vitamin D bioavailability: state of the art. Crit Rev Food Sci Nutr 2015;55:1193–205. [DOI] [PubMed] [Google Scholar]
- 51. Holick MF. Vitamin D and health: evolution, biologic functions, and recommended dietary intakes for vitamin D. Clin Rev Bone Miner Metab 2009;7:2–19. [Google Scholar]
- 52. Cashman KD. Vitamin D deficiency: defining, prevalence, causes, and strategies of addressing. Calcif Tissue Int [Internet] 2019. doi: 10.1007/s00223-019-00559-4. [DOI] [PubMed] [Google Scholar]
- 53. Lips P, Cashman KD, Lamberg-Allardt C, Bischoff-Ferrari HA, Obermayer-Pietsch B, Bianchi ML, Stepan J, Fuleihan GE-H, Bouillon R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol 2019;180:P23–54. [DOI] [PubMed] [Google Scholar]
- 54. Manson JE, Cook NR, Lee E-M, Christen W, Bassuk SS, Mora S, Gibson H, Gordon D, Copeland T, D'Agostino D et al.. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med 2019;380:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Scragg RKR. Overview of results from the vitamin D assessment (ViDA) study. J Endo Invest [Internet] 2019. Available from: 10.1007/s40618-019-01056-z. [DOI] [PubMed] [Google Scholar]
- 56. Pittas AG, Dawson-Hughes B, Sheehan P, Ware JW, Knowler WC, Aroda VR, Brodsky I, Ceglia L, Chadha C, Chatterjee R et al.. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med [Internet] 2019. doi: 10.1056/NEJMoa1900906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gaksch M, Jorde R, Grimnes G, Joakimsen R, Schirmer H, Wilsgaard T, Mathiesen EB, Njølstad I, Løchen M-L, März W et al.. Vitamin D and mortality: individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS One 2017;12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bills CE. Vitamin D assay—line test and chemical methods. Biol Symp 1947;12:409–34. [Google Scholar]
- 59. Søndergaard H, Leerbech E. The content of vitamin D in Danish foods. Publication 69 Statens Levnedsmiddelinstitut; Søborg, Denmark;1982. [Google Scholar]
- 60. Johnsson H, Hessel H. High performance liquid chromatographic determination of cholecalciferol vitamin D3 in food: a comparison with a bioassay method. Int J Vitam Nutr Res 1987;57:357–66. [PubMed] [Google Scholar]
- 61. Mattila PH, Piironen VI, Koivistoinen PE, Uusi-Rauva EJ. Contents of cholecalciferol, ergocalciferol, and their 25-hydroxylated metabolites in milk products and raw meat and liver as determined by HPLC. J Agric Food Chem 1995;43:2394–99. [Google Scholar]
- 62. Mattila P, Lehikoinen K, Kiiskinen T, Piironen V. Cholecalciferol and 25-hydroxycholecalciferol content of chicken egg yolk as affected by the cholecalciferol content of feed. J Agric Food Chem 1999;47:4089–92. [DOI] [PubMed] [Google Scholar]
- 63. Jakobsen J, Saxholt E. Vitamin D metabolites in bovine milk and butter. J Food Compos Anal 2009;22:472–8. [Google Scholar]
- 64. Strobel N, Buddhadasa S, Adorno P, Stockham K, Greenfield H. Vitamin D and 25-hydroxyvitamin D determination in meats by LC-IT-MS. Food Chem 2013;138:1042–47. [DOI] [PubMed] [Google Scholar]
- 65. Gomes FP, Shaw PN, Whitfield K, Hewavitharana AK. Simultaneous quantitative analysis of eight vitamin D analogues in milk using liquid chromatography-tandem mass spectrometry. Anal Chim Acta 2015;891:211–20. [DOI] [PubMed] [Google Scholar]
- 66. Barnkob LL, Petersen PM, Nielsen JP, Jakobsen J. Vitamin D enhanced pork from pigs exposed to artificial UVB light in indoor facilities. Eur Food Res Technol 2019;245:411–18. [Google Scholar]
- 67. NMKL 167 Kolekalciferol (vitamin D3) ock ergokalciferol (vitamin D2). Vatskekromatografisk bestämning i livsmedel. Nordisk Metodikkomite for Naeringsmidler, DTU Food, Kemitorvet, 2800 Kgs. Lyngby, Denmark, [Internet]. 2000. [cited May 12, 2019]. Available from: https://www.nmkl.org/index.php/en/publications/item/kolekalciferol-vitamin-d3-och-ergokalciferol-vitamin-d2-vatskekromatografisk-bestamning-i-livsmedel-nmkl-167-2000. [Google Scholar]
- 68. CEN Foodstuffs. Determination of vitamin D by high performance liquid chromatography. Measurement of cholecalciferol (D3) or ergocalciferol (D2). DS/EN 12821:2009 [Internet]. Brussels: European Committee for Standardization (CEN); 2009. [cited May 12, 2019]. Available from: https://standards.cen.eu/dyn/www/f?p=204:110:0::::FSP_PROJECT,FSP_ORG_ID:27772,6256&cs=1D597F7B81605B657692E8499FFEB199E. [Google Scholar]
- 69. Gill BD, Abernethy GA, Green RJ, Indyk HE. Analysis of vitamin D2 and vitamin D3 in fortified milk powders and infant and nutritional formulas by liquid chromatography–tandem mass spectrometry: single-laboratory validation, first action 2016.05. J AOAC Int 2016;99:1321–33. [DOI] [PubMed] [Google Scholar]
- 70. Gill BD, Indyk HE. Analysis of vitamin D2 and vitamin D3 in infant and adult nutritional formulas by liquid chromatography-tandem mass spectrometry: a multilaboratory testing study. J AOAC Int 2018;101:256–63. [DOI] [PubMed] [Google Scholar]
- 71. Roseland JM, Patterson KY, Andrews KW, Phillips KM, Phillips MM, Pehrsson PR, Dufresne GL, Jakobsen J, Gusev PA, Savarala S et al.. Interlaboratory trial for measurement of vitamin D and 25-hydroxyvitamin D [25(OH)D] in foods and a dietary supplement using liquid chromatography-mass spectrometry. J Agric Food Chem 2016;64:3167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. NIST (National Institute of Standards and Technology) Standard reference materials [Internet]. [cited May 10, 2019]. Available from: https://www.nist.gov/srm. [Google Scholar]
- 73. European Reference Materials (ERM) [Internet]. [cited May 10, 2019]. Available from: www.lgcstandards.com. [Google Scholar]
- 74. Jakobsen J, Smith C, Bysted A, Cashman KD. Vitamin D in wild and farmed Atlantic salmon (Salmosalar)—what do we know? Nutrients 2019;25:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. DTU Foods public food database [Internet]. [cited April 25, 2019]. Available from: https://frida.fooddata.dk/. [Google Scholar]
- 76. Clausen I, Jakobsen J, Leth T, Ovesen L. Vitamin D3 and 25-hydroxyvitamin D3 in raw and cooked pork cuts. J Food Compos Anal 2003;16:575–85. [Google Scholar]
- 77. Duffy SK, O'Doherty JV, Rajauria G, Clarke LC, Hayes A, Dowling KG, O'Grady MN, Kerry JP, Jakobsen J, Cashman KD et al.. Vitamin D-biofortified beef: a comparison of cholecalciferol with synthetic versus UVB-mushroom-derived ergosterol as feed source. Food Chem 2018;256:18–24. [DOI] [PubMed] [Google Scholar]
- 78. Jakobsen J, Jäpelt RB. Vitamin D. In: Leo ML, Nollet FT, editors. Analysis of active compounds in functional foods. 1st ed.Boca Raton: Taylor & Francis; 2013:219–51. [Google Scholar]
- 79. Teichmann A, Dutta PC, Staffas A, Jägerstad M. Sterol and vitamin D2 concentrations in cultivated and wild grown mushrooms: effects of UV irradiation. LWT—Food Sci Technol 2007;40:815–22. [Google Scholar]
- 80. Burild A, Lauridsen C, Faqir N, Sommer HM, Jakobsen J. Vitamin D3 and 25-hydroxyvitamin D3 in pork and their relationship to vitamin D status in pigs. J Nutr Sc [Internet] 2016. doi: 10.1017/jns.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Duffy SK, O'Doherty JV, Rajauria G, Clarke LC, Cashman KD, Hayes A, O'Grady MN, Kerry JP, Kelly A. Cholecalciferol supplementation of heifer diets increases beef vitamin D concentration and improves beef tenderness. Meat Sci 2017;134:103–10. [DOI] [PubMed] [Google Scholar]
- 82. Jakobsen J, Jensen SK, Hymøller L, Andersen EW, Kaas P, Burild A, Jäpelt RP. Short communication: artificial ultraviolet B light exposure increases vitamin D levels in cow plasma and milk. J Dairy Sci 2015;98:6492–8. [DOI] [PubMed] [Google Scholar]
- 83. Larson-Meyer DE, Ingold BC, Fensterseifer SR, Austin KJ, Wechsler PJ, Hollis BW, Makowski AJ, Alexander BM. Sun exposure in pigs increases the vitamin D nutritional quality of pork. PLoS One 2017;12:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. EFSA Commission regulation (EC) No 887/2009. European Food Safety Authority; 2009. [Google Scholar]
- 85. EFSA Commission regulation (EU) 2017/1492. European Food Safety Authority; 2017. [Google Scholar]
- 86. Jakobsen J, Maribo H, Bysted A, Sommer HM, Hels O. 25-Hydroxyvitamin D3 affects vitamin D status similar to vitamin D3 in pigs – but the meat produced has a lower content of vitamin D. Br J Nutr 2007;98:908–13. [DOI] [PubMed] [Google Scholar]
- 87. Quesada-Gomez JM, Bouillon R. Is calcifediol better than cholecalciferol for vitamin D supplementation? Osteoporos Int 2018;29:1697–711. [DOI] [PubMed] [Google Scholar]
- 88. Carlberg C. Nutrigenomics of vitamin D. Nutrients [Internet] 2019;11(3). doi: 10.3390/nu11030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ovesen L, Brot C, Jakobsen J. Food contents and biological activity of 25-hydroxyvitamin D: a vitamin D metabolite to be reckoned with? Ann Nutr Metab 2003;47:107–13. [DOI] [PubMed] [Google Scholar]
- 90. Jakobsen J. Bioavailability and bioactivity of vitamin D3 active compounds. Which potency should be used for 25-hydroxyvitamin D3? Int Congr Ser 2007;1297:133–42. [Google Scholar]
- 91. Barger-Lux MJ, Heaney RP, Dowell S, Chen TC, Holick MF. Vitamin D and its major metabolites: serum levels after graded oral dosing in healthy men. Osteoporos Int 1998;8:222–30. [DOI] [PubMed] [Google Scholar]
- 92. Cashman KD, Seamans KM, Lucey AJ, Stöcklin E, Weber P, Kiely M, Hill TR. Relative effectiveness of oral 25-hydroxyvitamin D3 and vitamin D3 in raising wintertime serum 25-hydroxyvitamin D in older adults. Am J Clin Nutr 2012;95:1350–6. [DOI] [PubMed] [Google Scholar]
- 93. Vaes AMM, Tieland M, de Regt MF, Wittwer J, van Loon LJC, de Groot LCPGM. Dose–response effects of supplementation with calcifediol on serum 25-hydroxyvitamin D status and its metabolites: a randomized controlled trial in older adults. Clin Nutr 2018;37:808–14. [DOI] [PubMed] [Google Scholar]
- 94. Navarro-Valverde C, Sosa-Henríquez M, Alhambra-Expósito MR, Quesada-Gómez JM. Vitamin D3and calcidiol are not equipotent. J Steroid Biochem Mol Biol 2016;164:205–8. [DOI] [PubMed] [Google Scholar]
- 95. McKenna MJ, Murray BF. Vitamin D dose response is underestimated by Endocrine Society's clinical practice guideline. Endocr Connect 2013;2:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bischoff-Ferrari HA, Dawson-Hughes B, Stöcklin E, Sidelnikov E, Willett WC, Edel JO, Stähelin HB, Wolfram S, Jetter A, Schwager J et al.. Oral supplementation with 25(OH)D3 versus vitamin D3: effects on 25(OH)D levels, lower extremity function, blood pressure, and markers of innate immunity. J Bone Miner Res 2012;27(1):160–9. [DOI] [PubMed] [Google Scholar]
- 97. Minisola S, Cianferotti L, Biondi P, Cipriani C, Fossi C, Franceschelli F, Giusti F, Leoncini G, Pepe J, Bischoff-Ferrari HA et al.. Correction of vitamin D status by calcidiol: pharmacokinetic profile, safety, and biochemical effects on bone and mineral metabolism of daily and weekly dosage regimens. Osteoporos Int 2017;28:3239–49. [DOI] [PubMed] [Google Scholar]
- 98. Jakobsen J, Wreford Andersen EA, Christensen T, Andersen R, Bügel S. Vitamin D vitamers affect vitamin D status differently in young healthy males. Nutrients [Internet] 2018;10. doi: 10.3390/nu10010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Desmarchelier C, Margier M, Prévéraud DP, Nowicki M, Rosilio V, Borel P, Reboul E. Comparison of the micellar incorporation and the intestinal cell uptake of cholecalciferol, 25-hydroxycholecalciferol and 1-α-hydroxycholecalciferol. Nutrients [Internet] 2017;9. doi: 10.3390/nu9101152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S, Chope G, Hyppon̈en E, Berry J, Vieth R et al.. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr 2012;95:1357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Itkonen ST, Skaffari E, Saaristo P, Saarnio EM, Erkkola M, Jakobsen J, Cashman KD, Lamberg-Allardt C. Effects of vitamin D2-fortified bread v. supplementation with vitamin D2 or D3 on serum 25-hydroxyvitamin D metabolites: an 8-week randomised-controlled trial in young adult Finnish women. Br J Nutr 2016;115:1332–39. [DOI] [PubMed] [Google Scholar]
- 102. The Nordic Council and Nordic Council of Ministers. Nordic nutrition recommendations 2012 [Internet]. [cited May 12, 2019]. Available from: https://www.norden.org/en/publication/nordic-nutrition-recommendations-2012. [Google Scholar]
- 103. Nissen J, Rasmussen LB, Ravn-Haren G, Wreford Andersen E, Hansen B, Andersen R, Mejborn H, Howarth Madsen K, Vogel U. Common variants in CYP2R1 and GC genes predict vitamin D concentrations in healthy Danish children. PLoS One 2014;9:e89907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Jiang X, O'Reilly PF, Aschard H, Hsu YH, Richards JB, Dupuis J, Ingelsson E, Karasik D, Pilz S, Berry D et al.. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat Commun 2018;9:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. FAO/WHO Report of the fortieth session of the CODEX Committee on Nutrition and Foods for Special Dietary Uses [Internet]. [cited April 25, 2019]. Available from: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-720-40%252FREPORT%252FREP19_NFSDUe.pdf. [Google Scholar]
- 106. Burild A, Frandsen HL, Poulsen M, Jakobsen J. Tissue content of vitamin D3 and 25-hydroxy vitamin D3 in minipigs after cutaneous synthesis, supplementation and deprivation of vitamin D3. Steroids 2015;98:72–79. [DOI] [PubMed] [Google Scholar]
- 107. McNulty H, Pentieva K. Folate bioavailability. In: Bailey LB, editor Folate in health and disease. Clinical nutrition in health and disease. 2nd ed Boca Raton, FL: Taylor & Francis; 2010:25–47. [Google Scholar]
- 108. McLean E, Benoist Bd, Allen LH. Review of the magnitude of folate and vitamin B12 deficiencies worldwide. Food Nutr Bull 2008;29:S38–51. [DOI] [PubMed] [Google Scholar]
- 109. Obeid R, Pietrzik K, Oakley GP Jr, Kancherla V, Holzgreve W, Wieser S. Preventable spina bifida and anencephaly in Europe. Birth Defects Res A Clin Mol Teratol 2015;103:763–71. [DOI] [PubMed] [Google Scholar]
- 110. CEN Foodstuffs. Determination of folate by microbiological assay. EN 14131:2003 [Internet]. Brussels: European Committee for Standardization (CEN);2003. [cited March 28, 2019]. Available from: https://standards.cen.eu/dyn/www/f?p=204:110:0::::FSP_PROJECT,FSP_ORG_ID:13861,6256&cs=147089C449E4C188F83301C820E611186. [Google Scholar]
- 111. Puwastien P, Pinprapai N, Judprasong K, Tamura T. International inter-laboratory analyses of food folate. J Food Comp Anal 2005;18:387–97. [Google Scholar]
- 112. Koontz JL, Phillips KM, Wunderlich KM, Exler J, Holden JM, Gebhardt SE et al.. Comparison of total folate concentrations in foods determined by microbiological assay at several experienced US commercial laboratories. J AOAC Int 2005;88:805–13. [PubMed] [Google Scholar]
- 113. Ringling C, Rychlik M. Analysis of seven folates in food by LC–MS/MS to improve accuracy of total folate data. Eur Food Res Technol 2013;236:17–28. [Google Scholar]
- 114. Rychlik M. Quantitation of folates by stable isotope dilution assays. In: Preedy VR, editor. B vitamins and folate: chemistry, analysis, function and effects. Cambridge, UK: Royal Society of Chemistry, 2012;396–418. [Google Scholar]
- 115. Ložnjak P, García-Salinas C, Díaz de la Garza RI, Bysted A, Jakobsen J. The use of a plant enzyme for rapid and sensitive analysis of naturally-occurring folates in food by liquid chromatography-tandem mass spectrometry. J Chrom A 2019;1594:34–44. [DOI] [PubMed] [Google Scholar]
- 116. Vishnumohan S, Arcot J, Pickford R. Naturally-occurring folates in foods: method development and analysis using liquid chromatography–tandem mass spectrometry (LC–MS/MS). Food Chem 2011;125:736–42. [Google Scholar]
- 117. Freisleben A, Schieberle P, Rychlik M. Syntheses of labeled vitamers of folic acid to be used as internal standards in stable isotope dilution assays. J Agric Food Chem 2002;50:4760–8. [DOI] [PubMed] [Google Scholar]
- 118. Konings EJM, Roomans HHS, Dorant E, Goldbohm RA, Saris WHM, van den Brandt PA. Folate intake of the Dutch population according to newly established liquid chromatography data for foods. Am J Clin Nutr 2001;73:765–76. [DOI] [PubMed] [Google Scholar]
- 119. Ringling C, Rychlik M. Origins of the difference between food folate analysis results obtained by LC-MS/MS and microbiological assays. Anal Bioanal Chem 2017;409:1815–25. [DOI] [PubMed] [Google Scholar]
- 120. Ramos-Parra PA, Urrea-López R, La Díaz de Garza RI. Folate analysis in complex food matrices: use of a recombinant Arabidopsis γ-glutamyl hydrolase for folate deglutamylation. Food Res Int 2013;54:177–85. [Google Scholar]
- 121. Rychlik M. Revised folate content of foods determined by stable isotope dilution assays. J Food Comp Anal 2004;17:475–83. [Google Scholar]
- 122. Rychlik M, Englert K, Kapfer S, Kirchhoff E. Folate contents of legumes determined by optimized enzyme treatment and stable isotope dilution assays. J Food Comp Anal 2007;20:411–9. [Google Scholar]
- 123. Coffigniez F, Rychlik M, Sanier C, Mestres C, Striegel L, Bohuon P, Briffaz A. Localisation and modeling of reaction and diffusion to explain folate behavior during soaking of cowpea. J Food Eng 2019;253:49–58. [Google Scholar]
- 124. Striegel L, Chebib S, Dumler C, Lu Y, Huang D, Rychlik M. Durian fruits discovered as superior folate sources, Front Nutr [Internet] 2018;5.Available from: https://www.frontiersin.org/article/10.3389/fnut.2018.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Akilanathan L, Vishnumohan S, Arcot J, Uthira L, Ramachandran S. Total folate: diversity within fruit varieties commonly consumed in India. Int J Food Sci Nutr 2010;61:463–72. [DOI] [PubMed] [Google Scholar]
- 126. Fyfe SE, Netzel ME, Tinggi U, Biehl EM, Sultanbawa Y. Buchanania obovata: an Australian indigenous food for diet diversification. Nutr Dietetics 2018;75:527–32. [DOI] [PubMed] [Google Scholar]
- 127. Striegel L, Chebib S, Netzel M E, Rychlik M. Improved stable isotope dilution assay for dietary folates using LC-MS/MS and its application to strawberries. Front Chem [Internet] 2018;6. doi: 10.3389/fchem.2018.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Jacob FF, Striegel L, Rychlik M, Hutzler M, Methner F-J. Yeast extract production using spent yeast from top-fermenting beer manufacture: influence of industrially applicable disruption methods on selected substance groups with physiological and process technology relevance. Eur Food Res Technol 2019;245:1169–82. [Google Scholar]
- 129. Tamura T, Shin YS, Williams MA, Stokstad ELR. Lactobacillus casei response to pteroylpolyglutamates. Anal Biochem 1972;49:517–21. [DOI] [PubMed] [Google Scholar]
- 130. Rychlik M, Roth-Meier D. Studies on new folates in fortified foods and assessment of their bioavailability and bioactivity. In: Rychlik M, editor. Fortified foods with vitamins. Weinheim: VCH-Wiley; 2011:143–54. [Google Scholar]
- 131. Büttner BE, Öhrvik VE, Witthöft CM, Rychlik M. Quantification of isotope-labelled and unlabelled folates in plasma, ileostomy and food samples. Anal Bioanal Chem 2011;399:429–39. [DOI] [PubMed] [Google Scholar]
- 132. McKillop DJ, McNulty H, Scott JM, McPartlin JM, Strain JJ, Bradbury I, Girvan J, Hoey L, McCreedy R, Alexander J et al.. The rate of intestinal absorption of natural food folates is not related to the extent of folate conjugation. Am J Clin Nutr 2006;84:167–73. [DOI] [PubMed] [Google Scholar]
- 133. Minekus M, Smeets-Peeters M, Bernalier A, Marol-Bonnin S, Havenaar R, Marteau P, Alric M, Fonty G, Veld JH. A computer-controlled system to simulate conditions of the large intestine with peristaltic mixing, water absorption and absorption of fermentation products. Appl Microbiol Biotechnol 1999;53:108–14. [DOI] [PubMed] [Google Scholar]
- 134. Ringling C, Rychlik M. Simulation of food folate digestion and bioavailability of an oxidation product of 5-methyltetrahydrofolate. Nutrients [Internet] 2017;9:969. doi: 10.3390/nu9090969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Zhao R, Matherly LH, Goldman ID. Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Exp Rev Mol Med 2009;11:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kang SS, Zhou J, Wong PW, Kowalisyn J, Strokosch G. Intermediate homocysteinemia: a thermolabile variant of methylenetetrahydrofolate reductase. Am J Hum Genet 1988;43: 414–21. [PMC free article] [PubMed] [Google Scholar]
- 137. Mönch S, Netzel M, Netzel G, Ott U, Frank T, Rychlik M. Folate bioavailability from foods rich in folates assessed in a short term human study using stable isotope dilution assays. Food Funct 2015;6:242–8. [DOI] [PubMed] [Google Scholar]
- 138. Sauberlich HE, Kretsch MJ, Skala JH, Johnson HL, Taylor PC. Folate requirement and metabolism in nonpregnant women. Am J Clin Nutr 1987;46:1016–28. [DOI] [PubMed] [Google Scholar]
- 139. Mönch S, Netzel M, Netzel G, Ott U, Frank T, Rychlik M. Pilot study on folate bioavailability from a Camembert cheese reveals contradictory findings to recent results from a human short-term study. Front Nutr 2016;3:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Mönch S, Netzel M, Netzel G, Rychlik M. Quantitation of folates and their catabolites in blood plasma, erythrocytes, and urine by stable isotope dilution assays. Anal Biochem 2010;398:150–60. [DOI] [PubMed] [Google Scholar]
- 141. Bhandari SD, Ming G, Szpylka J. Total folate in infant formula and adult nutritionals by trienzyme extraction and LC-MS/MS quantitation: a multilaboratory testing study, Final Action 2011.06. J AOAC Int 2018;101:1881–94. [DOI] [PubMed] [Google Scholar]
- 142. Merck. Research and development of folates [Internet] [cited May 10, 2019]. Available from: http://www.merckmillipore.com/DE/de/products/small-molecule-pharmaceuticals/bulk-api/folates/research-and-development-of-folates/yU2b.qB.I94AAAFNKh1ItHt_,nav. [Google Scholar]
- 143. Rychlik M, Kanawati B, Roullier-Gall C, Hemmler D, Liu Y, Alexandre H, Gougeon R D, Lena Gmelch L, Gotthardt M, Schmitt-Kopplin P. Foodomics assessed by Fourier transform mass spectrometry. In: Schmitt-Kopplin P, editor Fundamentals and applications of Fourier transform mass spectrometry. Elsevier; Forthcoming2019. [Google Scholar]
- 144. Bailey LB. Dietary reference intakes for folate: the debut of dietary folate equivalents. Nutr Rev 1998;56:294–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.