Abstract
Background
The Brief Intervention for Weight Loss Trial enrolled 1882 consecutively attending primary care patients who were obese and participants were randomised to physicians opportunistically endorsing, offering, and facilitating a referral to a weight loss programme (support) or recommending weight loss (advice). After one year, the support group lost 1.4kg more (95%CI 0.9 to 2.0): 2.4 kg versus 1.0 kg. We use a cohort simulation to predict effects on disease incidence, quality of life, and healthcare costs over 20 years.
Methods
Randomly sampling from the trial population, we created a virtual cohort of 20 million adults and assigned baseline morbidity. We applied the weight loss observed in the trial and assumed weight regain over four years. Using epidemiological data, we assigned the incidence of 12 weight-related diseases depending on baseline disease status, age, gender, body mass index. From a healthcare perspective, we calculated the quality adjusted life years (QALYs) accruing and calculated the incremental difference between trial arms in costs expended in delivering the intervention and healthcare costs accruing. We discounted future costs and benefits at 1.5% over 20 years.
Results
Compared with advice, the support intervention reduced the cumulative incidence of weight-related disease by 722/100,000 people, 0.33% of all weight-related disease. The incremental cost of support over advice was £2.01million/100,000. However, the support intervention reduced health service costs by £5.86 million/100,000 leading to a net saving of £3.85 million/100,000. The support intervention produced 992 QALYs/100,000 people relative to advice.
Conclusions
A brief intervention in which physicians opportunistically endorse, offer, and facilitate a referral to a behavioural weight management service to patients with a BMI of at least 30kg/m2 reduces healthcare costs and improves health more than advising weight loss.
Introduction
Screening and brief intervention for obesity in primary care is effective. In the first randomised trial of such an intervention, called BWeL (Brief intervention for Weight Loss) ISRCTN26563137,1, 2 we screened consecutive patients attending 137 primary care physicians in England for obesity, defined as a BMI of at least 25kg/m2 for people of Asian ethnicity or 30kg/m2 for people of other ethnicities together with a raised body fat percentage for age and gender. Eighty-three percent of people who had a BMI at or above these limits agreed to participate and 1882 participants were enrolled. In the support intervention, physicians advised patients that the best way to lose weight was to attend one of two well-known UK commercial weight management programmes which are available as a 12-week group programme paid for by the English NHS and offered them a referral to the group. These were Slimming World and Rosemary Conley. If the patient agreed to this, s/he was given a specific appointment before leaving the practice. Of the 940 participants in this arm, 77% of the patients agreed to the referral and 40% attended. For the 942 participants in the advice arm, physicians advised the patient their health would benefit from weight loss. Patients welcomed the intervention; one in 500 thought the intervention inappropriate and unhelpful, while more than four in five thought it appropriate and helpful. The mean weight loss among all people offered the intervention at 12 months was 2.4kg in the support condition and 1.0kg in the advice condition, an adjusted difference (95% confidence interval) 1.43 (0.89 to 1.97). Twenty-five percent of the support condition lost 5% of their body weight, compared with 14% of the control, an odds ratio 2.11 (1.67 to 2.68).
It is uncertain whether this is a cost-effective intervention. Firstly, although the intervention is of modest cost, it yielded modest weight loss. Furthermore, while losing weight reduces the risk of diabetes and other obesity-related diseases,3 most people who lose weight regain it.4 The incidence of obesity-related disease is the same after lost weight is regained as it would have been without weight loss, even though the cumulative incidence is lower in the long-term.5 It therefore remains uncertain whether providing a brief intervention of this kind is a cost-effective intervention, which we investigate here by computing the incremental cost-effectiveness ratio for the support intervention relative to the advice intervention from the perspective of the healthcare system.
Methods
Model set-up
We modelled the cost-effectiveness of a one-off delivery of both the support and advice intervention in the trial. To do so we created virtual cohorts of 20 million people by randomly sampling with replacement from the adult population enrolled in the BWeL trial. We applied a BMI growth equation to the BMI of cohort members, simulated using a Monte Carlo process. The data on BMI change were projected forward based on past trends in BMI for age and gender-specific groups in the English population taken from the Health Survey for England from 2004 to 2014. The individuals in the virtual cohort model were followed in annual cycles for 20 years or until death from 2015 to 2035. In each year, an individual could develop obesity-related disease, recover from it, or die from it. The probabilities of these outcomes depended on age, sex, and BMI with the data for these probabilities derived from systematic reviews of the literature. If an individual developed disease, we modelled the QALY decrement that ensued and the healthcare costs, with the data again derived from the literature. The modelling used the UK Health Forum (UKHF) Microsimulation Model. This model was developed for the Foresight Tackling Obesities Report and has been used subsequently to predict the prevalence of obesity in various countries or specific cohorts, and its consequences on health outcomes, and the economic costs that these entail in the population.6–10
In the first year, we applied the mean weight loss observed in each trial arm to eligible individuals in each virtual cohort. We then assumed that in the second and subsequent four years, any weight lost was regained linearly back to where their starting BMI percentile would have been if the person was following natural trends for their age and gender. These trends were derived from the Health Survey for England.4 Thus individuals where there is average weight gain over time (typically younger groups) returned to a weight higher than baseline and those where there is average weight loss (typically older groups) returned to a point lower than baseline. After the fifth year of the simulation, individuals in both scenarios progressed along their BMI percentile, following national trends in BMI for their age and gender. We therefore assumed that weight trajectories diverged for five years and that all lost weight was regained and that the intervention did not recur.
Intervention effectiveness
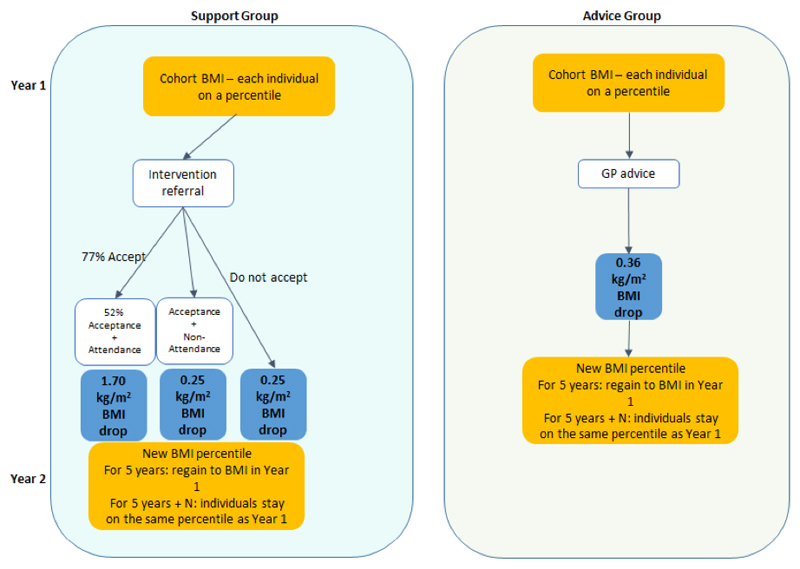
We applied the weight loss of each intervention to the cohort representing the participants in each arm of the trial in year 1 of the modelling (Appendix Table 1 and Figure 1). In the intervention group, the effect of the intervention depended on whether or not a patient attended or did not attend the weight loss programme when it was offered and this was allocated at random in the model. Those that did attend (40% of the trial arm) lost a mean of 4.7kg and those that did not attend lost a mean of 0.7kg.1 In the advice group, we applied a uniform effect to each member of the cohort, representative of the mean weight loss in the advice arm of the BWeL trial (1.0kg).
Figure 1. The two scenarios for modelling the effectiveness of interventions.
Data on obesity-related disease
In the second stage of modelling, we assessed the incidence of 12 obesity-related diseases in the cohort. These were breast cancer, cardiovascular disease, colorectal cancer, type 2 diabetes mellitus, endometrial cancer, hypertension, knee osteoarthritis, oesophageal cancer, ovarian cancer, pancreatic cancer, renal cancer, and stroke. At baseline, we applied baseline prevalence based on a person’s age, gender, and BMI using data from the literature (Appendix Table 2). In each year of the 20-year period, the model calculated an individual’s transition probability using a Monte Carlo process of developing disease based on their age, sex, current disease state, medical history and risk factor (BMI). The incidence was assumed to depend solely on current BMI, which, at least for diabetes, appears to be the case.5 For the stochastic transitions used in the microsimulation, this probability was derived from an application-generated random number to determine if the transition takes place for a particular individual. The data on the incidence of disease and the relation of that to BMI was derived from published sources which in turn were derived from periodically updated systematic searching and appraisal (Table 2 Appendix). In addition, in each subsequent year of the modelling, the disease could remit, continue, or lead to death and those data too were derived from epidemiological sources and applied stochastically to cohort members. In each year of the modelling, we calculated the incidence of each obesity-related disease in each arm of the model, calculated as the number of new cases of disease occurring in the cohort that were alive in that year. We totalled these incidences to give the cumulative incidence over the 20-year period and expressed these as per 100,000 persons as different numbers were alive in each year of the cohort. This represents the total number of new cases of weight-related disease occurring in this cohort during this time. As people are at risk of all diseases and because these typically co-occur e.g. many people develop both diabetes and hypertension, this is different from the number of people with a disease.
Health economics data
We assigned a QALY value to each of the disease states (Table 2- Appendix) using data on EQ-5D, as recommended by the English healthcare decision making body, NICE. The majority of the EQ-5D measures were obtained from Sullivan et al 11 and otherwise from searching of the literature. Following English guidance (NICE), we discounted future benefits at 1.5%.12
Data on direct costs only were obtained from the NHS England programme budgeting cost database 2012-2013. Expenditure figures included only healthcare costs incurred by the NHS reflecting that the analysis was undertaken from the healthcare perspective.13 The intervention costs were derived from the trial (Appendix Table 3). The costs included the costs of a person weighing themselves before the consultation (the scale depreciation cost along with minimal staff supervision time), the time of the physician in delivering the brief intervention, which applied in both arms. In the support arm, the additional costs were the time of the physician’s receptionist booking an appointment at the weight management programme, depending on the participants response. The largest cost was that of the weight loss programme itself (£49.50), which was the NHS contracting cost applicable in 2015, the base year of the modelling, if the participant chose to take up this offer. The costs of healthcare for incident disease modelled comprised primary care costs (staff and prescriptions); secondary care (inpatient: elective, non-elective, outpatient and other secondary care); urgent care/emergency care costs (ambulance and accident and emergency); community care costs; and cost of care provided in other settings: In the NHS budget cost database, only the total healthcare expenditure of diabetes, as opposed to type 2 diabetes was available. Based on epidemiological data, we assumed that 90% of diabetes prevalence and associated costs were attributable to type 2 diabetes.14 There were no UK data on hypertension costs so data from the Netherlands were used. We followed a standard approach by discounting future costs and benefits at 1.5% per year, as recommended for economic outputs by NICE.12
Incremental cost-effectiveness of the support intervention relative to advice
The difference in costs and health outcomes between the two brief interventions was used to calculate the incremental cost-effectiveness ratio (ICER) of the support intervention compared with the advice arm: the difference in costs (net difference between healthcare and intervention costs incurred) divided by the difference in health outcomes (quality adjusted life years (QALY) gained). The ICER was compared to a threshold, which is the “price” society is willing to pay for an extra unit of health “output”, in this case a QALY, which helps determine whether the intervention tested is good value for money.15 We used NICE’s threshold of £20,000/QALY.
Results
Epidemiological results of the long-term cost effectiveness of the BWeL study
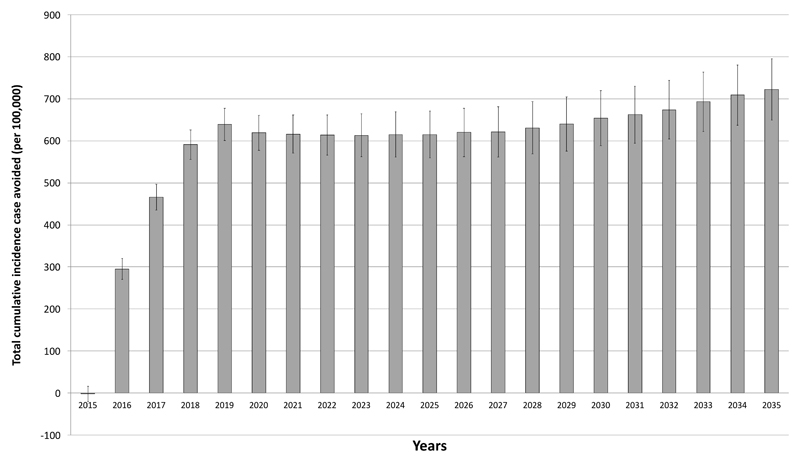
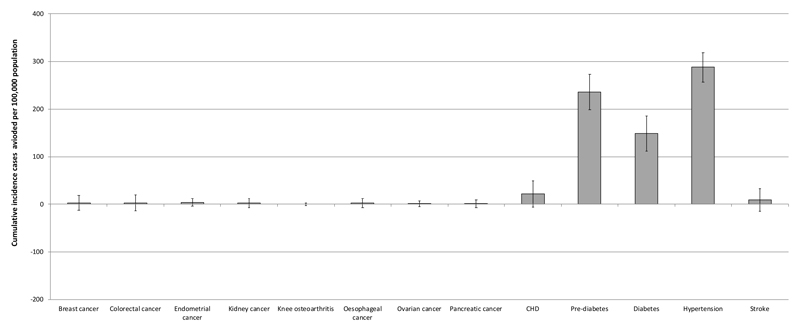
In the advice arm, by 2035, the cumulative incidence of obesity-related disease was predicted to be 219,249 cases per 100,000 i.e. on average people developed two obesity-related conditions over the 20 years. The largest contributors were pre-diabetes (64,470), diabetes (34,824), and hypertension (79,764), with 14,472 cases of heart disease and 10,115 cases of stroke. In the support arm, the cumulative total incidence was 218,707 cases per 100,000 people, a reduction in cumulative incidence of obesity-related disease of 0.33%. The predicted number of cases (% reduction) of prediabetes was 64,534 (0.36%), diabetes 34,675 (0.43%), hypertension 79,476 (0.36%), heart disease 14,450 (0.15%), and stroke 10,016 (0.09%) per 100,000 (Figures 2 and 3).
Figure 2. Total cumulative incidence of diseases (cases/100,000 (95%CI)) avoided by year.
Figure 3. Cumulative Incidence avoided (cases per 100,000 population) for active relative to support scenarios in the UK by 2035.
Health economics results of the long-term cost effectiveness of the BWeL study
The support intervention cost between £1.95 and £51.45 per person, depending on the patient’s response, while the advice intervention cost £1.95 (Appendix Table 3). The incremental cost of the support intervention over advice was £20.10/person or £2.01 million/100,000 persons. The main additional cost was the cost of the weight loss programme. However, it reduced health service costs by 5.86 million/100,000 leading to a net saving of £3.85 million/100,000.
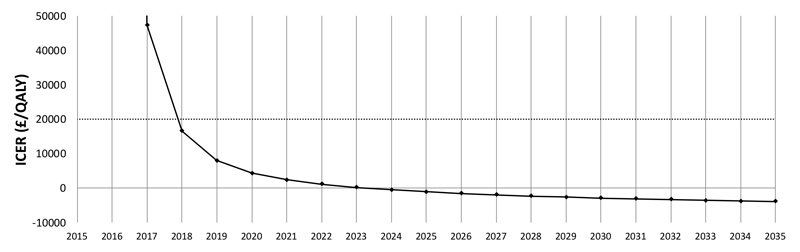
By 2035, the reduced incidence of disease from the support intervention produced an additional 992 QALYs/100,000 persons relative to advice. This resulted in a dominant result for the ICER: more effective and at lower cost. This result varied over the 20 years. In the third year, the intervention was cost-effective by NICE standards and cost-saving by the ninth year (Figure 4).
Figure 4. The ICER (£/QALY) for support versus advice over 20 years.
Discussion
Relative to advice to lose weight, a physician’s opportunistic offer of referral to group-based community weight loss support programme was more effective at reducing the incidence of obesity-related disease. Although it was costlier in the short-term, it was cost-effective from the third year after its implementation and cost-saving from year nine and over an entire 20-year period, while yielding an additional 992 QALYs/100,000 people.
The main strength of this study relates to the underpinning data from a trial and the model we used to model the long-term health impact. The randomised design of the trial, delivered at population-level rather than to a selected group of motivated participants makes it possible to estimate the population level impact of physician-led interventions. The model addresses the question relevant to policy: how the initial costs of offering treatment for obesity are balanced in relation to the disease prevented. The model was developed for the UK Foresight Report and has updated since then.16–18
We randomised people to a control intervention which was physician advice to lose weight to improve health, and this could be a limitation. In clinical practice, it is rare to make opportunistic interventions on weight,19, 20 and so advice to lose weight does not represent usual care. The cost of delivering the brief advice intervention would not ordinarily have been incurred and thus our analysis underestimates the cost difference between usual current care and the active intervention in the trial, thus overestimating the cost-effectiveness. However, the costs of the advice intervention were modest at £0.2 million/100,000 and even removing this cost entirely would not change the findings that the support intervention was cost-saving. Moreover, it is plausible that physician advice itself spurred action in some participants. On average, participants in this arm lost 1kg, about 700g more than epidemiological evidence might suggest.21 If this were so, this may have underestimated the ICER because it underestimated the weight gain difference and hence health benefits of weight loss. Neither issue seems likely to change the conclusion that the support intervention, involving endorsing, offering, and facilitating a referral to a modestly priced group-based weight management service is cost-saving and improves health to a greater extent than either simple advice or usual care, namely no intervention. A further limitation is that the results are based on a trial analysis and therefore apply directly only in the UK where physicians have access to referral to a programme that is modestly priced and effective. However, if physicians only have access to more costly weight loss programmes, then the cost saving would diminish, albeit the cost of the weight loss programme would have to be three times greater for the support arm to be cost neutral over 20 years. A final issue is that the microsimulation model is not currently able to conduct sensitivity analyses.
The analysis was conducted from the healthcare perspective, including only costs and benefits occurring in healthcare. A societal perspective would have included costs to the participants from attending the weight loss programme, for example, and the opportunity cost of the time spent doing so, which is considerable. We also excluded other costs that are arguably relevant including some weight-related diseases due to lack of data to model the impact and indirect and social care costs of the weight-related diseases in the model. Weight-related diseases are major risk factors for dependency in old age.22 However, we excluded costs that might arise from treatment and care for people with non-weight-related disease, which is likely to increase in incidence simply as a result of a longer life following weight loss23. which may reduce or eliminate the cost-savings. However, there is a debate about whether these additional costs should be included or not.24, 25 A lifetime societal perspective may have given a different estimate of the costs and benefits. However, our analysis was conducted in the same manner as other health economic analyses used to inform healthcare spending decisions, but the total net financial impact of this (or other healthcare) intervention on society remains unknown.
Another limitation is that the trial followed up participants at three months and one year, during which time participants regained weight. Their experience was typical of trials in general and we assumed that they would regain all lost weight over the next four years.4 If weight regain were either more rapid or slower than this or if all weight lost was not regained, as occurred in the Diabetes Prevention Program,5 the cost-effectiveness would change. Our assumptions were based on a systematic review that estimated the rate of weight regain and found no evidence that the means by which weight loss occurred affected the rate of regain.26 Those few trials that have followed people for up to five years or longer have not observed that all weight lost has been regained.5, 27 Thus, as with most modelling, the assumptions are critical to the outcomes obtained. Validating this model would be difficult. In this study, all participants were recruited opportunistically and very briefly to conceal the nature of the intervention. By follow-up, many had forgotten being enrolled in a study and follow-up over 20 years, as would be required to directly estimate the costs of healthcare and assess quality of life would be difficult and, even in a trial of nearly 1800 people, would be subject to occasional high cost episodes of healthcare that can affect health-economic assessments, as we have observed previously.10 A final issue is that this economic analysis could not include a probabilistic sensitivity analysis. There are a large number of parameters in the model and a single run of the model takes several days. Running a sensitivity analysis would require a super computer.
The BWeL trial was the first trial to examine the effectiveness of physician-delivered opportunistic brief interventions. This is therefore the first cost-effectiveness evaluation of this kind of population-level intervention for weight-loss. However, although the central feature of BWeL was the physician-delivered brief intervention offered to a population not seeking support, 1 the biggest cost element and the element that delivered the weight loss was the 12-week commercial weight management programme to which primary care physicians referred the participants. This kind of service is available for physicians to refer to in many parts of England, and there is evidence that this is an effective programme.28, 29 The most recent such evaluation suggested that, compared with a self-help intervention (for which there is evidence of effectiveness),30 the 12-week programme was cost-saving.31 Our findings extend this evaluation because they included a delivery mechanism that has potentially a very high reach, certainly much higher than waiting for patients to ask for support, which is what happens in usual care. The large majority of people visit a primary care physician annually, making several visits a year.32, 33 Physicians could feasibly give a 30-second intervention sufficiently often to greatly expand the reach of effective weight loss programmes to have a real population impact and it would be valuable to assess this. That said, the same model was used in both cost-effectiveness evaluations. Although we have tried to be explicit about some assumptions in the model, it is possible that different models would produce different outputs and replicating these results in a different model would be helpful. A final issue is that this intervention, a brief opportunistic intervention by a physician, if implemented by policy, would be implemented recurrently. For example, English general practitioners have a requirement to update smoking status and provide support in how to quit to their patients that smoke every two years. Here we modelled the cost-effectiveness of the intervention as delivered only once, replicating a within-trial cost-effectiveness evaluation, but the impact of both recurrent costs and the impact on recurrent benefits remain unknown. Further modelling could usefully explore a range of scenarios to understand the possible impact of rollout of this programme.
Conclusions
A brief intervention where primary care physicians opportunistically endorse, offer, and facilitate a referral to weight management to consecutively attending patients who have a BMI of at least 30kg/m2 was cost-saving over a 20-year time horizon while improving health.
Supplementary Material
Footnotes
Competing interests
The trial was funded by the National Prevention Research Initiative of the UK, administered by the MRC. The funding partners are Alzheimer’s Research UK, Alzheimer’s Society, Biotechnology and Biological Sciences Research Council, British Heart Foundation, Cancer Research UK, Chief Scientist Office, Scottish Government Health Directorate, Department of Health, Diabetes UK, Economic and Social Research Council, Engineering and Physical Sciences Research Council, Health and Social Care Research Division, Public Health Agency, Northern Ireland, Medical Research Council, Stroke Association, Wellcome Trust, Welsh Government, and World Cancer Research Fund.
The weight loss programmes we used were provided by Slimming World and Rosemary Conley Health and Fitness Clubs. These are widely available through the English NHS at no cost to the patient and for which these organisations receive a fee. In this trial these 12-week programs were donated to the NHS by both these organisations and we are very grateful to them for this. Neither organisation had input into the protocol, the data analysis, or were involved in the decision to publish the findings. The investigators have no financial relationships with these companies.
Kate Jolly is part-funded by the National Institute for Health Research (NIHR) and Collaboration for Leadership in Applied Health Research and Care (CLAHRC). Paul Aveyard and Susan Jebb are NIHR senior investigators and funded by the Oxford NIHR Biomedical Research Centre and CLAHRC. The views expressed are those of the authors and not necessarily those of the NIHR, the NHS or the Department of Health.
Susan Jebb and Paul Aveyard are investigators on an investigator-initiated randomised trial of Cambridge Weight Plan and funded by a research grant to the University of Oxford. None of the investigators have received personal financial payments from any of these research relationships.
Contributor Information
Lise Retat, Email: lise.retat@ukhealthforum.org.uk.
Laura Pimpin, Email: laura.pimpin@ukhealthforum.org.uk.
Laura Webber, Email: laura.webber@ukhealthforum.org.uk.
Abbygail Jaccard, Email: abbygail.jaccard@ukhealthforum.org.uk.
Amanda Lewis, Email: amanda.lewis@bristol.ac.uk.
Sarah Tearne, Email: sarah.tearne@phc.ox.ac.uk.
Kathryn Hood, Email: kathryn.hood@bristol.ac.uk.
Anna Christian-Brown, Email: annamaycbrown@gmail.com.
Peymane Adab, Email: p.adab@bham.ac.uk.
Rachna Begh, Email: rachna.begh@phc.ox.ac.uk.
Kate Jolly, Email: c.b.jolly@bham.ac.uk.
Amanda Daley, Email: a.daley@lboro.ac.uk.
Amanda Farley, Email: a.c.farley@bham.ac.uk.
Deborah Lycett, Email: deborah.lycett@coventry.ac.uk.
Alecia Nickless, Email: alecia.nickless@phc.ox.ac.uk.
Ly-Mee Yu, Email: ly-mee.yu@phc.ox.ac.uk.
Susan Jebb, Email: susan.jebb@phc.ox.ac.uk.
References
- 1.Aveyard P, Lewis A, Tearne S, Hood K, Christian-Brown A, Adab P, et al. Screening and brief intervention for obesity in primary care: a parallel, two-arm, randomised trial. The Lancet. 2016;388(10059):2492–2500. doi: 10.1016/S0140-6736(16)31893-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis A, Jolly K, Adab P, Daley A, Farley A, Jebb S, et al. A brief intervention for weight management in primary care: study protocol for a randomized controlled trial. Trials. 2013;14(1):393. doi: 10.1186/1745-6215-14-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zomer E, Gurusamy K, Leach R, Trimmer C, Lobstein T, Morris S, et al. Interventions that cause weight loss and the impact on cardiovascular risk factors: a systematic review and meta-analysis. Obes Rev. 2016;17(10):1001–11. doi: 10.1111/obr.12433. [DOI] [PubMed] [Google Scholar]
- 4.Dansinger ML, Tatsioni A, Wong JB, Chung M, Balk EM. Meta-analysis: the effect of dietary counseling for weight loss. Ann Intern Med. 2007;147(1):41–50. doi: 10.7326/0003-4819-147-1-200707030-00007. [DOI] [PubMed] [Google Scholar]
- 5.Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. The Lancet Diabetes & Endocrinology. 2015;3(11):866–875. doi: 10.1016/S2213-8587(15)00291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McPherson K, Marsh T, Brown M. Foresight tackling obesities: Future choices – modelling future trends in obesity and the impact on health. Foresight Tackling Obesities Future Choices. 2007.
- 7.Webber L, Divajeva D, Marsh T, McPherson K, Brown M, Galea G, et al. The future burden of obesity-related diseases in the 53 WHO European-Region countries and the impact of effective interventions: a modelling study. BMJ Open. 2014;4(7):e004787. doi: 10.1136/bmjopen-2014-004787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollingworth W, Hawkins J, Lawlor DA, Brown M, Marsh T, Kipping RR. Economic evaluation of lifestyle interventions to treat overweight or obesity in children. Int J Obes (Lond) 2012;36(4):559–66. doi: 10.1038/ijo.2011.272. [DOI] [PubMed] [Google Scholar]
- 9.National Institute for Clinical Excellence. Weight management: lifestyle services for overweight or obese adults. 2014.
- 10.Ahern AL, Wheeler GM, Aveyard P, Boyland EJ, Halford JC, Mander AP, et al. Extended and standard duration weight-loss programme referrals for adults in primary care (WRAP): a randomised controlled trial. The Lancet. 2017;389(10085):2214–2225. doi: 10.1016/S0140-6736(17)30647-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D Scores for the United Kingdom. Med Decis Making. 2011;31(6):800–804. doi: 10.1177/0272989X11401031. [DOI] [PubMed] [Google Scholar]
- 12.National Institute of Health and Clinical Excellence. Methods for the development of NICE public health guidance. third edition. London: NICE; 2012. [PubMed] [Google Scholar]
- 13.National Health Service. 2012-13 programme budgeting PCT benchmarking tool. 2013.
- 14.Kanavos P, van der Aardweg S, Schurer W. Diabetes expenditure, burden of disease and management in 5 EU countries. LSE; London: 2012. [Google Scholar]
- 15.Vemer P, Rutten-van Mölken MPMH. Largely ignored: the impact of the threshold value for a QALY on the importance of a transferability factor. The European journal of health economics : HEPAC : health economics in prevention and care. 2011;12(5):397. doi: 10.1007/s10198-010-0253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown M, Marsh T, Retat L, Fordham R, Suhrcke M, Turner D, et al. Managing Overweight and Obesity among Adults. Report on Economic Modelling and Cost Consequence Analysis. National Institute for Clinical Excellence; London: 2013. [Google Scholar]
- 17.Hinde S, Bojke L, Richardson G, Retat L, Webber L. The cost-effectiveness of population Health Checks: have the NHS Health Checks been unfairly maligned? Journal of Public Health. 2017;25(4):425–431. doi: 10.1007/s10389-017-0801-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. The Lancet. 2011;378(9793):815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 19.Booth HP, Prevost AT, Gulliford MC. Access to weight reduction interventions for overweight and obese patients in UK primary care: population-based cohort study. BMJ Open. 2015;5(1):e006642. doi: 10.1136/bmjopen-2014-006642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noordman J, Verhaak P, van Dulmen S. Discussing patient's lifestyle choices in the consulting room: analysis of GP-patient consultations between 1975 and 2008. BMC Fam Pract. 2010;11:87. doi: 10.1186/1471-2296-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prospective Studies C. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramsay SE, Arianayagam DS, Whincup PH, Lennon LT, Cryer J, Papacosta AO, et al. Cardiovascular risk profile and frailty in a population-based study of older British men. Heart. 2015;101(8):616–622. doi: 10.1136/heartjnl-2014-306472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma C, Avenell A, Bolland M, Hudson J, Stewart F, Robertson C, et al. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ. 2017;359 doi: 10.1136/bmj.j4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee RH. Future costs in cost effectiveness analysis. J Health Econ. 2008;27(4):809–18. doi: 10.1016/j.jhealeco.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Garber AM, Phelps CE. Economic foundations of cost-effectiveness analysis. J Health Econ. 1997;16(1):1–31. doi: 10.1016/s0167-6296(96)00506-1. [DOI] [PubMed] [Google Scholar]
- 26.Dansinger ML, Tatsioni A, Wong JB, Chung M, Balk EM. Meta-analysis: The effect of dietary counseling for weight loss. Annals of Internal Medicine. 2007;147(1):41–50. doi: 10.7326/0003-4819-147-1-200707030-00007. [DOI] [PubMed] [Google Scholar]
- 27.Lindström J, Peltonen M, Eriksson JG, Ilanne-Parikka P, Aunola S, Keinänen-Kiukaanniemi S, et al. Improved lifestyle and decreased diabetes risk over 13 years: long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS) Diabetologia. 2013;56(2):284–293. doi: 10.1007/s00125-012-2752-5. [DOI] [PubMed] [Google Scholar]
- 28.Jolly K, Lewis A, Beach J, Denley J, Adab P, Deeks JJ, et al. Comparison of range of commercial or primary care led weight reduction programmes with minimal intervention control for weight loss in obesity: Lighten Up randomised controlled trial. BMJ. 2011;343 doi: 10.1136/bmj.d6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartmann-Boyce J, Johns DJ, Jebb SA, Summerbell C, Aveyard P, Behavioural Weight Management Review G Behavioural weight management programmes for adults assessed by trials conducted in everyday contexts: systematic review and meta-analysis. Obesity Reviews. 2014;15(11):920–932. doi: 10.1111/obr.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartmann-Boyce J, Jebb SA, Fletcher BR, Aveyard P. Self-Help for Weight Loss in Overweight and Obese Adults: Systematic Review and Meta-Analysis. American Journal of Public Health. 2015;105(3):e43–e57. doi: 10.2105/AJPH.2014.302389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahern AL, Wheeler GM, Aveyard P, Boyland EJ, Halford JCG, Mander AP, et al. Extended and standard duration weight-loss programme referrals for adults in primary care (WRAP): a randomised controlled trial. The Lancet. 2017;389(10085):2214–2225. doi: 10.1016/S0140-6736(17)30647-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleming DM. Morbidity registration and the fourth general practice morbidity survey in England and Wales. Scand J Prim Health Care Suppl. 1993;2:37–41. doi: 10.3109/02813439309045500. [DOI] [PubMed] [Google Scholar]
- 33.Hobbs FD, Bankhead C, Mukhtar T, Stevens S, Perera-Salazar R, Holt T, et al. Clinical workload in UK primary care: a retrospective analysis of 100 million consultations in England, 2007-14. Lancet. 2016;387(10035):2323–30. doi: 10.1016/S0140-6736(16)00620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smolina K, Wright FL, Rayner M, Goldacre MJ. Determinants of the decline in mortality from acute myocardial infarction in England between 2002 and 2010: linked national database study. BMJ (Clinical research ed.) 2012;344:d8059. doi: 10.1136/bmj.d8059. Corrected data on incidence and mortality in 2013 at http://www.bmj.com/content/347/bmj.f7379.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.British Heart Foundation. Cardiovascular Disease Statistics 2014. 2015.
- 36.Office for National Statistics. Deaths Registrations Summary Statistics, England and Wales. 2014.
- 37.World Obesity Federation. Relative risk Assessments IASO; Prepared for DYNAMO-HIA project. [Google Scholar]
- 38.Laires PA, Ejzykowicz F, Hsu TY, Ambegaonkar B, Davies G. Cost-effectiveness of adding ezetimibe to atorvastatin vs switching to rosuvastatin therapy in Portugal. J Med Econ. 2015;18(8):565–72. doi: 10.3111/13696998.2015.1031794. [DOI] [PubMed] [Google Scholar]
- 39.British Heart Foundation. Stroke Statistics 2009. 2009.
- 40.Rivero-Arias O, Ouellet M, Gray A, Wolstenholme J, Rothwell PM, Luengo-Fernandez R. Mapping the modified Rankin scale (mRS) measurement into the generic EuroQol (EQ-5D) health outcome. Med Decis Making. 2010;30(3):341–54. doi: 10.1177/0272989X09349961. [DOI] [PubMed] [Google Scholar]
- 41.Health and Social Care Information Centre. Health Survey for England 2012. 2012. [Google Scholar]
- 42.Polder JJ, Bonneux L, Meerding WJ, van der Maas PJ. Age-specific increases in health care costs. Eur J Public Health. 2002;12(1):57–62. doi: 10.1093/eurpub/12.1.57. [DOI] [PubMed] [Google Scholar]
- 43.International Diabetes Federation. Diabetes Atlas. 2014.
- 44.Arthritis Research UK. Musculoskeletal Calculator. 2016. [Google Scholar]
- 45.Zheng H, Chen C. Body mass index and risk of knee osteoarthritis: systematic review and meta-analysis of prospective studies. BMJ open. 2015;5(12):e007568. doi: 10.1136/bmjopen-2014-007568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conner-Spady BL, Marshall DA, Bohm E, Dunbar MJ, Loucks L, Al Khudairy A, et al. Reliability and validity of the EQ-5D-5L compared to the EQ-5D-3L in patients with osteoarthritis referred for hip and knee replacement. Qual Life Res. 2015;24(7):1775–84. doi: 10.1007/s11136-014-0910-6. [DOI] [PubMed] [Google Scholar]
- 47.Oxford Economics. The economic cost of arthritis for the UK economy - Final report. 2010.
- 48.Cancer Research UK. Statistics by cancer type - Average Number of New Cases Per Year and Age-Specific Incidence Rates per 100,000 Population, UK 2011-2013. 2016. [Google Scholar]
- 49.Cancer Research UK. Statistics by cancer type - Average Number of Deaths per Year and Age-Specific Mortality Rates, UK 2010-2012. 2016. [Google Scholar]
- 50.Office for National Statistics. Cancer Survival in England-Adults Diagnosed: 2009 to 2013, followed up to 2014. 2015.
- 51.Office for National Statistics. Cancer Survival in England: 10 year survival rates adults diagnosed between 2010-2011 and followed up to 2012. 2013.
- 52.Aune D, Navarro Rosenblatt DA, Chan DSM, Abar L, Vingeliene S, Vieira AR, et al. Anthropometric factors and ovarian cancer risk: A systematic review and nonlinear dose-response meta-analysis of prospective studies. International Journal of Cancer. 2015;136(8):1888–1898. doi: 10.1002/ijc.29207. [DOI] [PubMed] [Google Scholar]
- 53.World Cancer Research Fund. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. 2007.
- 54.Romanus D, Kindler HL, Archer L, Basch E, Niedzwiecki D, Weeks J, et al. Does health-related quality of life improve for advanced pancreatic cancer patients who respond to gemcitabine? Analysis of a randomized phase III trial of the cancer and leukemia group B (CALGB 80303) J Pain Symptom Manage. 2012;43(2):205–17. doi: 10.1016/j.jpainsymman.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Personal Social Services Research Unit, University of Kent; Canterbury: 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.