Preface
Mobile Health or mHealth - The application of mobile devices, their components and related technologies to healthcare is improving patients’ access to treatment and advice. Now, in combination with connected diagnostic devices it offers new possibilities to diagnose, track and control infectious diseases and improve health system efficiencies. In this context we look at these technologies and highlight their promise but also the challenges in realising their potential to increase patient access to testing, aid in their treatment and improve the capability of public health authorities to monitor outbreaks, implement responses, and assess the impact of interventions across the world.
1. Introduction
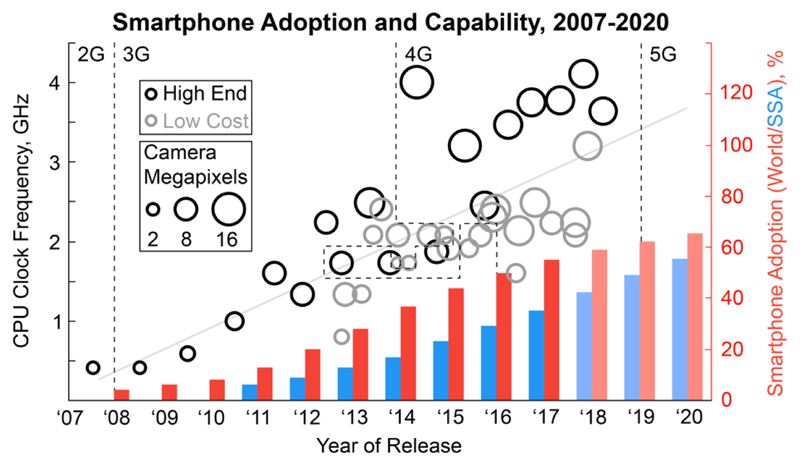
Rapid advances in portable communications technologies and digital computing have improved the speed of, and efficiency with which data can be processed and exchanged. In particular the arrival of the smartphone and the networks needed to support it, are rapidly reducing the costs of data acquisition and transfer worldwide. As of 2016, global smartphone adoption (the percentage of all mobile phone connections that come from a smartphone) has reached 51 %1 and by 2020 in sub-Saharan Africa it is forecast to reach 55 %2 (Fig. 1). This powerful pocket computer with built-in sensors and wireless connectivity provides researchers and health systems with new opportunities to capture and handle data3. The adoption and capabilities of smartphones and their related technologies in resource-rich settings and resource-limited settings are continually growing with low cost smartphones reducing the affordability barrier4 while offering similar sensing and processing capabilities to more costly “high-end” devices (Fig. 1).
Figure 1.
The growing power of low cost smartphones. Smartphone adoption (the percentage of global (red) or sub-Saharan Africa (blue) mobile phone connections that come from a smartphone), processor speed (CPU clock frequency) and camera resolution (primary camera megapixels) of the flagship models of popular smartphone manufacturers. The stated processor speed takes into account multiple processor cores at a parallelisation of 50 %. “High End” models retail for between approximately € 610-1000 whereas “Low Cost” models retail for between € 60-16090.
The way that we detect and respond to disease is also continually advancing. Step-changes in the development of sensitive and specific immunological and molecular based diagnostics as well as genetic sequencing have allowed the detection and staging of an increasing number of diseases. This has had a large influence on our ability to understand the burden and transmission dynamics of infectious agents as well as to guide clinical decision making and control - no better illustrated than in the field of infectious diseases5–7. Recent advances in nanotechnology, microfluidics and microarray-based systems have brought closer than ever the promise of simple, yet highly sensitive and specific devices, which can be used outside of the laboratory. These areas of use are often described as at or near the “point-of-care” which include places such as the patient’s own home, a primary care setting or by their bedside in a hospital. In pursuit of this, the new generation of nucleic acid tests have been developed that require less than five minutes of hands-on time8 and deep sequencing is now possible on a small hand-held device.9 These advances, combined with changing consumer attitudes towards self-testing, and increased appetite for wearable biosensors, are enticing healthcare providers to begin to shift towards the new paradigm of P4 Medicine which is predictive, pre-emptive, personalized and participatory10.
These developments have occurred alongside the emergence of the field of mobile health (or mHealth defined by the World Health Organisation as medical and public health practice supported by mobile devices11) and this has spurred the co-development of diagnostics and mobile devices to create connected diagnostics. mHealth interventions have been used to address a range of challenges from disease surveillance to health systems strengthening and health education12,13 and the combination of these with these connected diagnostics will impact all areas of healthcare, including those related to infectious diseases.
2. Potential for Infectious Disease Response
Diagnosis and monitoring of disease are key to clinical management. The control of infectious diseases represents a unique challenge because infections can be transmitted to others and thus require a focus on early detection and treatment, surveillance and outbreak control. Diagnostic and monitoring tools must therefore be integrated with effective surveillance and control measures to limit the spread of infection. mHealth approaches (Table 1) could improve the efficiency, speed and interconnectedness of an integrated clinical and public health response via two major mechanisms: (i) increased access to healthcare outside of care settings (e.g. by improved self-testing) and (ii) the real-time or close to real-time reporting of diagnostic results to patients and healthcare professionals to elicit rapid and appropriate clinical and public health responses to both endemic infections and outbreaks of epidemic potential. To date, most mHealth interventions, as exemplified in Box 1 have focused on using established mobile technologies (such as text messages and calls) to connect healthcare workers and patients to each other and to test results14. Combining this with portable diagnostic devices that connect and report results automatically has the potential to streamline this process.
Table 1. Examples of mHealth enhancement of infectious disease diagnosis, treatment and control.
| Area | Function | mHealth Opportunities |
|---|---|---|
| Outbreak Identification | Novel outbreak monitoring | Electronic collection of epidemiological and clinical data |
| Diagnosis | Community or self-testing | Early detection, automated result capture and analysis |
| Disease characterisation | Portable genetic sequencing of samples (e.g. to identify emerging drug resistance) | |
| Syndromic surveillance | Multi source data capture and passive reporting (e.g. activity levels and location history) | |
| Treatment and Patient Management | Linkage to clinical care | Mobile connection to clinical care (decision trees, electronic prescribing) |
| More efficient and effective use of stakeholder time | Automated report generation and supply chain management, reduce transcription errors in reporting | |
| Chronic infection monitoring and response to therapy | Long term biomarker reporting and analysis to guide community-based medication and care | |
| Disease Control and Elimination | Cluster ‘hot spot’ identification Outbreak response Epidemic control |
Rapid geospatial and phylogenetic mapping Real time reporting to public health agencies to implement control strategies via connected clinical and public health systems Social media queries capture and mapping during outbreaks Electronic implementation of control measures Data visualisation for epidemiological and clinical mapping, contact management and monitoring the effectiveness of interventions Targeted information dissemination |
Box 1. An mHealth approach to HIV self-testing.
Studies are increasingly demonstrating the feasibility of self-testing at large scale in resource-limited settings. Choko et al. demonstrated that HIV self-testing is safe, accurate and acceptable to over 76.5 % of the 16,600 residents contacted from 14 urban neighbourhoods in Malawi94. However as the authors and others95 mention, linkage to care remains an issue. mHealth can address this and Pai et al. have conducted a small scale study estimating the feasibility of HIV self-testing and remote follow up in South Africa96. Here participants performed a HIV rapid diagnostic test (RDT) themselves and were then linked to counselling and treatment. Although the test was not connected, mobile phone calls, internet and text messages enabled post-test linkage to care. Results for the 251 healthcare workers at the Groote Schuur hospital in Cape Town showed a high completion rate of 99.2 % with a 100 % acceptance rate of post-test counselling and referral by mobile phone for those identified as sero-positive (9 patients). This linkage to counselling and advice for patients testing positive was substantially higher than the 56.3 % reported in Malawi however, now larger scale and longer term studies that demonstrate cost-effective integration into a healthcare system are needed84.
The global risk of antimicrobial resistant infections is potentially catastrophic, demanding improved diagnostics to better guide antimicrobial therapy15. Connected diagnostics that can simultaneously detect a pathogen and identify antimicrobial sensitivity and resistance can enable selection of appropriate therapies whilst reporting the required data to surveillance centres. Likewise, for infections leading to chronicity, particularly those requiring long term therapy, mHealth provides major opportunities for home or community-based monitoring. In resource-limited settings, where health services may already be overwhelmed, these approaches are particularly useful. Taking diagnostics outside of formal health facilities and linking the output of testing into pathways of clinical and preventative care that may be delivered in the community could yield more cost effective and user-friendly healthcare. In principle these interventions will increase patient access to precision medicine and in resource-rich settings, connected healthcare systems such as Box 2 are beginning to be used to stratify individual patients into remote treatment and response monitoring programs.
Box 2. eSexual Health Clinic.
The eSexual Health Clinic97 demonstrates the viability and acceptability of online clinical and public health interventions for infectious disease. In this UK study, the first demonstrating a fully developed online diagnosis, care and prevention programme, patients with chlamydia were diagnosed and medically managed via an online clinical consultation. 2340 patients were texted a link to access their results online along with, if applicable, links that lead to antibiotic collection from a pharmacy. Discreet partner notification, health promotion and automated surveillance data capture were also integrated into the system to aid prevention for an infection which is asymptomatic, sexually transmitted and potentially stigmatised. While this study relied on a self-swab or urine sample which was posted to a central laboratory for testing or attendance at a genitourinary medicine clinic, there is a clear potential for this to be replaced by a connected diagnostic test which automatically links results. This would act to improve ease of use, time to results and further facilitate the remote testing and the acquisition of treatment, advice and follow up outside of conventional healthcare systems.
mHealth can also increase system efficiency, by reducing workload and errors associated with paper reporting and preventing stock-outs, through the increased automation of inventory and supply chain management systems16. Additionally, phone-based decision trees can assist less well trained users in decision making and can play an important role in diagnosis, monitoring or for data gathering more generally17. Furthermore, in disease surveillance, there is an urgent requirement to more rapidly detect and intervene for newly emerging epidemics (e.g. Ebola or Zika), as well as for increased sentinel surveillance for existing ones18. The use of connected diagnostics and symptom reporting apps, combined with standardised electronic collection of key epidemiological and clinical data have the potential to greatly enhance the efficiency and speed of management of both epidemic and endemic infections including the management of contacts where appropriate19. The real time reporting of diagnostic test results can enable this surveillance through the geospatial mapping of infections via geotagged test results20 or social network and internet search analysis, providing new tools for assimilation into outbreak control21,22.
As an example, the burden of HIV in sub-Saharan Africa remains unacceptably high. In areas such as KwaZulu-Natal, South Africa, HIV prevalence in the population reaches over 30 % overall, and higher in women23. This is despite a public health approach to HIV testing and anti-retroviral treatment for all. Reasons for a continuing high rate of new infections are many fold. The high level of associated stigma leads to poor rates of testing, as well as poor attendance at treatment clinics. High population mobility impedes chronic disease care, as well as being a risk for infection. In this context, there is a need for a precision public health approach to HIV care.24 mHealth provides the ideal framework in which to achieve this through, targeted behavioural change (through interactive apps), care roadmaps (whether community based or clinic) and connected diagnostic monitoring (HIV testing and viral load monitoring). Some examples of this are emerging. The HIVSmart! app25 has been developed from the internet based form used by Pia et al. in Box 1 to work with HIV RDTs to support patient linkage to and retention in care worldwide. Now further work is needed to fully develop these and other such interventions and overcome the challenges of integrating connected diagnostics into them.
3. Challenges of delivery
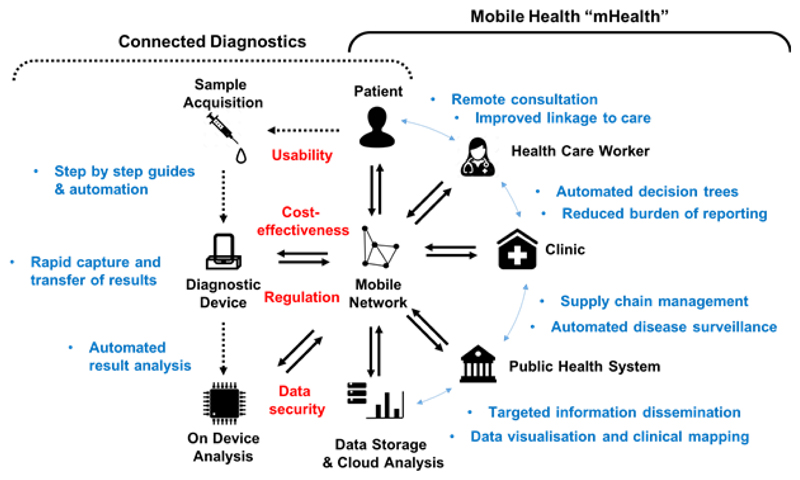
Despite the potential benefits and numerous connectable diagnostic devices being reported, as far as we are aware an mHealth intervention featuring a connected diagnostic linked to a clinical care pathway and/or surveillance system for an infectious disease has yet to be deployed. To fully implement this, systems must be in place for the secure transfer, analysis (either in situ or remotely) and storage of the data generated. Once analysed any relevant decisions or conclusions derived from it must be reported to and acted upon by either the patient, healthcare professional or relevant institution along with linkage to a suitable care pathway (Fig. 2). These stages and their challenges will be discussed further below but all have a bearing on how such a device and intervention is developed, regulated and deployed. Moreover, the potential for the misuse of confidential health and personal data requires that, to be acceptable and effective, mHealth approaches must be underpinned by the highest level of community/patient confidence and well-regulated clinical pathways.
Figure 2.
Deploying an mHealth connected diagnostic. The stages, stakeholders, example outcomes (blue) and challenges (red) to deploying an effective mHealth intervention that employs a connected diagnostic device.
4. Target Identification and Sample Acquisition
Identifying relevant diagnostic targets for infectious diseases and developing testing technologies aimed at distinguishing the pathogen or host response to the pathogen from commensal infections or non-specific immune responses is a well-established field26. Targets should be selected both on the predictive capacity for disease identification but also on suitability for use at the point-of-care. This requires careful consideration of what the true requirements and limitations are in each setting. The needs for self-testing at home compared to within a clinical setting are often quite different. The challenges of sample acquisition and processing that are met with ease in a primary care facility are considerably harder to meet in the field or at home. For example sampling using a venous draw requires more specialised equipment and expertise for effective and safe acquisition than a finger-prick blood sample, urine or a swab although these too can be challenging for untrained users27. Some assay systems based on plasma will require a blood centrifugation step prior to testing and swabs or urine samples require pre-processing. Ensuring simplicity of sample processing in the test is key. The development of paper-based centrifugation systems provide promise in this respect28 and controlling the tests via a mobile phone has the potential to help further by lowering the training needed to perform and interpret the test. This can be achieved by displaying step-by-step guides or via automating steps previously performed by the user.
5. Developing Connected Point of Care Infectious Disease Diagnostic Technology
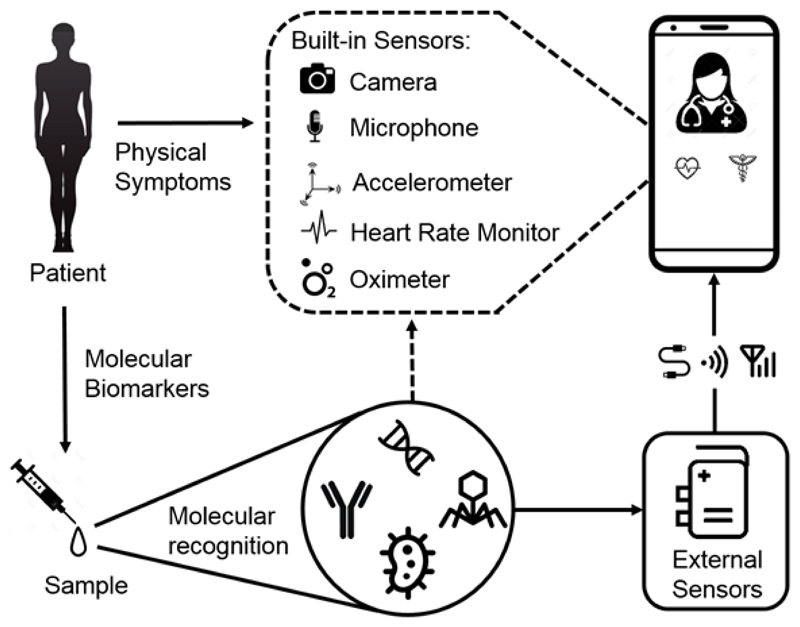
The World Health Organization’s ASSURED29 criteria defines the paradigm of an ideal point-of-care diagnostic and key design principles are extensively discussed elsewhere27,30. A connected device has an additional requirement whereby the signal generated must be transduced into digital information ready for transmission. Systems for this have been developed31 and these technologies are increasingly being used to create connected point-of-care diagnostics with a number of excellent recent reviews on the subject32–34. These systems, either capitalise upon the sensors already built into the phone or use sensors external to the phone and exploit its computational and connective power to create a connected diagnostic (Fig. 3).
Figure 3.
Diagnosis by device. How a connected diagnostic test can be used to detect disease markers - from the physical to the molecular – via sensors either built into or external to the phone.
In principle, a mobile phone camera can take the place of advanced laboratory-based spectrometers and match their quantitation and multiplexing capability35–37 via innovative engineering. These efforts are acting to democratise access to otherwise costly laboratory equipment and reduce the training needed to interpret test results e.g. via automated RDT measurements (Box 3). This approach capitalises on mobile phone imaging38 or video capture39 to enable the quantitative40 assessment of results with the potential for geotagging41 and rapid transmission of results to healthcare systems42. Similarly, mobile phone-based microscopy43 is now gaining traction in detecting microscopic parasitic infections44, and is rapidly approaching that of laboratory based microscopes with a significantly reduced upfront cost. Mobile phone-based microscopy is even yielding portable, handheld routes towards fluorescent imaging of viruses45 and DNA molecules46.
Box 3. Mobile phone-based rapid diagnostic test readers.
Dell et al. have developed GSID98 an open sourced mobile-phone based system for the capture, transmission and on device analysis of images of RDTs. Comprised of a reader, an app and a central, web-accessible database, it has been applied to the rapid diagnosis of malaria, in five hospitals and clinics in Zimbabwe99. User feedback was positive, with the healthcare workers even suggesting extending the range of data that could be inputted into the app to reduce the burden of reporting case notes. A revealing analysis of the frequency and delay in test data transmission, highlighted that on-device analysis and asynchronous data transfer is needed in areas with poor infrastructure.
Going beyond the capture and transmission of results the performance of RDT readers is such that they can be used to support and even improve the training of healthcare workers as shown recently by Laktabai et al. for the diagnosis of malaria in Kenya100. Furthermore, Brangel et al. have recently developed a smartphone app and RDTs to detect patients’ antibodies to different ebola subtypes in serum. The app analysed and geotagged captured images of the RDT’s test line(s) and could generate a map of test results indicating the survivors’ serological state. This prototype has been evaluated in laboratory settings only but has the potential to aid in: survivor identification in clinics, the epidemiological study of neglected tropical disease, and may enable the faster and cheaper evaluation of vaccine effectiveness under outbreak conditions especially in remote areas.
Other sensors found in mobile phones have also been explored in a broader context including the accelerometer for monitoring the body’s motion which correlates with certain diseases including Parkinson’s47 and even the microphone for monitoring lung function48. Ecology mapping of mosquitos is possible using the microphone49 highlighting the broader potential of smartphones to aid disease prevention. Now, new mobile phones and smart watches are actively embracing mHealth with purpose driven intrinsic sensors such as heart rate sensors being built into the back of the device50. In addition to this, manufacturers continue to add new sensors and imaging functionalities into the latest handsets. The potential diagnostic applications made possible by these remains to be seen however, 3D sensors such as the infrared depth sensor system found in the iPhone X may assist remote visual diagnosis.
External sensors can be engineered around any suitable biosensor or signal transduction system and connected to share data via mobile networks. Importantly, this can avoid the problem of interoperability between different phones and operating systems and reduces device or component heterogeneity which would otherwise hamper approval by regulators. For example, dedicated photodiodes or CMOS chips built into an external device along with a defined excitation source can yield controlled light environments for microscopy51,52 and sensing53.
Once these external sensors are built into a device, they must then transfer the data generated. Many manufacturers have begun to integrate internet connectivity directly into their laboratory based diagnostic equipment, giving users faster access to results and facile integration into laboratory information management systems. As these connected instruments decrease in size these devices are increasingly being deployed at or near to the point-of-care and recently have been deployed in response to the Ebola62and Zika63 epidemics.
For more portable systems, other sensors can also be employed. Biosensors that incorporate electrochemical transduction54 are eminently suited to digital interpretation and connection and many connected electrochemical biosensors have been developed55. Some of these sensors offer less invasive sample acquisition methods with some incorporated into wearable sensors held near the skin56,57 or used to analyse volatile organic compounds in a patient’s breath58. Other signal transduction methods including micro-cantilevers and surface acoustic wave detection59 offer the potential of ultra-rapid testing within 10 seconds60.
The advent of nanopore based sequencing61 has allowed devices that can sequence DNA to be miniaturised to the size of a USB stick offering the possibility of full genomic disease profiling in a handheld device9. However, this technology is still limited for use as a point-of-care diagnostic, in part by the need to perform multistep sample preparations unsuited to the untrained user, and because methods for securely handling, analysing, and interpreting the data generated are still in their infancy62. This problem is common to all connected diagnostics, but it is especially acute for the large amounts of genomic data generated by sequencing devices63.
6. Data Analysis: On-Phone and on Cloud
Automated result analysis has potential to reduce both trained and untrained user error when interpreting, recording and transmitting results of diagnostic tests. There are a number of methods to automate the visual interpretation of images and their suitability depends on the type of data captured, as well as the resources available in a given setting.
Cloud-based methods are most appropriate for more computationally expensive analysis, such as high-resolution image or video data, in settings where there is sufficient connectivity. However, on-phone feature extraction that reduces the size of the images before their transmission and cloud-based interpretation can mitigate this64. Cloud-based systems ensure connectivity to databases and allow algorithms to be updated centrally. It also removes the processing burden from mobile devices, increasing the range of compatible devices. Systems have been developed enabling hands-free automated analysis of HIV RDTs using Google Glass, which sent image data to a remote server65. Here a machine learning algorithm qualitatively classified the RDT before the result was sent back and displayed to the user.
On-phone analysis is more appropriate where less complex analysis is required or in remote settings with limited mobile network connectivity and bandwidth. On-phone image and video analysis has been used to detect the fluorescent products of microfluidic nucleic acid amplification66 and in cell67 and parasite68 counting. This allows the asynchronous transmission of data where the results are stored on-phone and uploaded once in range of mobile networks. Although this can reduce the transmission of patient data, on-phone storage poses its own security risks such as the loss or malfunction of the device. On-phone analysis is further enabled by the growing capability of mobile processing hardware (Fig. 1). The introduction of dedicated neural processing units and software frameworks for on-phone machine learning allow increasingly efficient and nuanced image classification69 and may improve inference when using defective equipment, or in poor lighting conditions70.
7. Data Connectivity to Health Systems
Optimally used mHealth applications have the potential to become the largest source of health data, for use in research and health improvement interventions71,72. However, unlike clinic-based services, where health information from diagnostic testing is currently acquired and stored in laboratory systems that are secure, the data collected from individual mHealth devices may not be securely stored or easily shared across multiple mHealth applications and connected to electronic health records73. Data sharing has been associated with contextual74 and ethical75 challenges including a lack of standardised data security to assure privacy. What is needed are methods to improve health data sharing and connectivity and develop a consensus on data governance76. Broad regulations such as the EU’s General Data Protection Regulation (GDPR) are beginning to address this, but more tailored approaches like the voluntary code of conduct on privacy for mHealth apps77 are needed. Moreover, recent ethics studies78 have highlighted the need for users to understand and consent to all aspects of how their data will be used.
Modular platforms to share information, standardize and coordinate data collection, and improve mHealth device connectivity are currently under development73. However, these platforms such as the Fast Healthcare Interoperability Resources (FHIR)79, will still require stakeholder collaboration and substantial standardisation of the interfaces between the hardware and software components in an mHealth system if they are to be put in place.
8. Challenges in mHealth diagnostic adoption
Regulation
In the past decade, regulatory development has not kept pace with technological innovation. Regulatory authorities, such as the US Food and Drug Administration and the UK Medicines and Healthcare Products Regulatory Agency, have taken a cautious risk-based approach to the regulation of mobile medical applications. Both these institutions are watching closely while exercising their “enforcement discretion” towards medical devices that pose a minimal risk to patients and consumers. This rapidly evolving field however, requires both the development of regulatory frameworks that consider the wide array of medical apps that are emerging and regulatory harmonization amongst different regulatory authorities to avoid the development of regulations that become barriers or disincentives to innovation.
Other challenges arise from the hardware itself. If the test is designed to be used with a range of mobile phones, then their variability in both hardware and software presents challenges for assessing risk in the regulatory review process. This uncertainty is pushing companies to either develop standalone devices with defined components or ship a standardised mobile phone with the diagnostic test and carefully control the software environment all of which influences cost.
Finally, the clinical governance of mHeath based care pathways must be considered. Here a patient may not see a clinician face-to-face and clinical records may be collected remotely via standardised questionnaire. Electronic clinical pathways can include a range of linked practitioners including doctors, nurses, pharmacists and other healthcare workers, and the pathway must allow escalation for face-to-face care and referral when needed. All this requires appropriate quality assurance of the clinical decision trees, care pathways and remote prescribing decisions as well as the development of a secure and user friendly interface for remote use80.
Cost and Clinical Effectiveness
The decision to employ an mHealth connected diagnostic and how best to integrate into an existing health system requires evidence of its clinical effectiveness and resulting cost effectiveness with particular scrutiny on the context in which it is to be used81 and how its implementation would function at scale. The cost and clinical effectiveness of point-of-care diagnostics82 and some mHealth strategies83,84 in infectious disease control have been assessed. However, a connected diagnostic and its associated mHealth intervention have yet to be analysed.
The challenge in this for mHealth connected diagnostics is that they are not a single intervention, but a systems approach involving a complex set of connected and mutually dependent interventions. For a full assessment of such a connected ecosystem all aspects must be considered. Health economic analyses are warranted85 to demonstrate value from the increased connectivity made possible through the connected diagnostics (which may not be necessarily cheaper) and how these can be employed as part of broader mHealth interventions. These evaluations need to account for the benefit and potential risk to patients, healthcare professionals and systems in terms of clinical and social outcomes (e.g. time saved or speed of access). Only with this demonstrated, can larger scale studies be employed to assess the impact of the associated increased testing and data generated on national healthcare systems.
Digital Divide
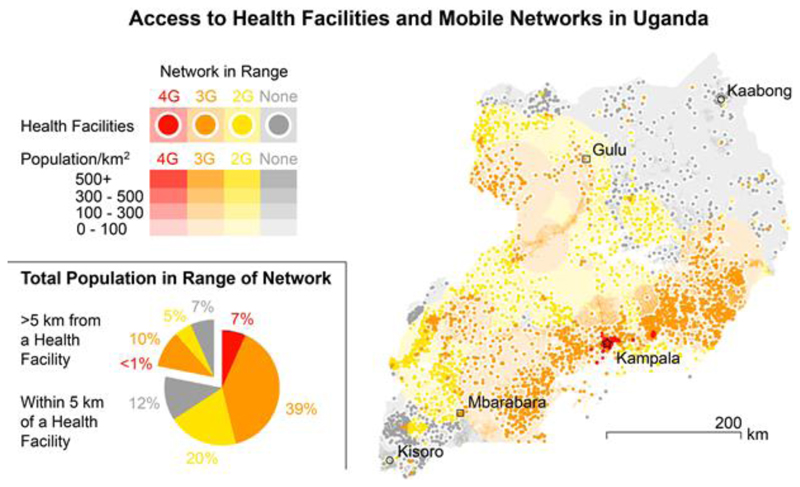
While mHealth technologies have the potential to widen access to testing for many health conditions, it is important to ensure that no one is left behind. Although trends are narrowing, today 35 % of the world’s population do not have access to mobile communications. This is predominantly because of lack of access in low and middle income countries1 although those of lower socio-economic status in resource-rich settings are also affected. Moreover, whilst the developed world awaits the roll out of high-speed 5G networks, many of the least developed countries in the world rely on 2G, and many of the remote and poor regions have no mobile phone coverage at all. To illustrate this, we have mapped network coverage and distance to healthcare facilities in Uganda, which is one of the three sub-Saharan African nations with publicly available datasets of these factors. We have collated data on healthcare facility location and cell tower location, range and generation, and correlated these to population density (Fig. 4). Here 22 % of the population do not live within 5 km of a healthcare facility (a target set by the Ugandan government86). Of this population 45 % live in range of 3G cell towers. An additional 23 % live in range of 2G cell towers, but 32 % are outside the range of cellular networks. Moreover, being in range of a cell tower does not necessarily indicate a good signal and other unforeseen factors, such as power supply interruption, may impact connectivity. Asynchronous data transfer may overcome this problem when reporting results, but further mHealth interventions, such as linkage to care, could be severely restricted. Compounding this are the considerable variations in digital literacy related to socio-economic position and education such that disadvantaged groups who might have the greatest need of a service are the most likely to be excluded.
Figure 4.
The digital and healthcare divide in Uganda. Map of healthcare facilities (2012)91 and the population density (2015)92 >5 km from healthcare facilities. Areas and healthcare facilities are coloured according to the generation of cellular network in range, where the range is calculated from cell tower location and the distance of the furthest measured signal93. The white circles around the health facilities indicate the 5 km radius threshold90.
In this digital era, age and gender gaps are also of concern - it has been estimated that 200 million fewer women than men own a mobile phone in low- and middle-income countries and that even when they do, they are less likely to use it for services, such as mobile internet, that could improve their health87. The Pew Research Centre has shown huge age disparities in smartphone ownership: for example 94 % of Chinese people aged between 18-35 own a smartphone compared to 30 % of people aged 50 and over88. Trend analysis suggests that these gaps are narrowing, but more needs to be done to ensure that mHealth devices, are made available to all, considering the unmet health need in women and the elderly.
9. Conclusion and Perspective
The convergence of infectious disease diagnostics with mobile phone-based connectivity provides opportunities to deliver potentially disruptive technologies to drive development of novel health systems. These should increase access to testing, diagnosis and treatment of infectious diseases, whilst improving outbreak detection, disease surveillance and guiding a precision public health response. With these technologies the potential for public participation is considerable, either through engagement in outbreak detection through crowd sourced or “citizen science” initiatives or via targeted prevention messaging so that individuals and communities can access digital care pathways for an integrated clinical and public health system of detection, care and disease control.
To realise these benefits, the development of connected diagnostics must be undertaken with an understanding of the context in which they are to be used such that they feed seamlessly into their associated mHealth systems. Those introduced in a resource-rich setting may be different and need a different surrounding technical ecosystem compared to those that are to be used in a resource-limited setting. Consumer demand in resource-rich settings may drive the commercial development of these devices in the near term, however they have the potential to transform care in all economic settings if appropriately tailored to local needs. To do this, investment needs to be directed towards the development of appropriate devices and systems in all socio-economic settings and towards addressing the ongoing digital divide.
In addition to this, if we are to harness the promise of mHealth interventions that use a connected diagnostic the intervention must be designed with a patient-centred focus which improves access and early intervention whilst reducing the burden on patient time and resources. This must be achieved whilst ensuring that overstretched health systems benefit by driving more effective and efficient care and linked public health responses.
The promise of automated or mobile-assisted self-testing with connected, efficient linkage to care is an exciting end-goal and the studies highlighted in boxes 1 to 3 demonstrate the elements of such a system. However, they are yet to be fully integrated and deployed or their effectiveness assessed at scale. For this to occur, we argue for a new approach to the regulation and governance of mHealth devices, interventions and applications, one that addresses the challenges of testing, digital access, data security and clinical governance – for without this user and institutional adoption and public confidence will be limited due to a lack of trust.
The future of diagnostics is likely to be increasingly digital and connected, accelerating changes in the way healthcare is provided. This provides researchers and policy makers with the exciting, if formidable, opportunity to transform healthcare systems in new ways to improve human health and wellbeing in future years to come89.
Supplementary Material
Data processing methods and supporting information are available online at DOI: 10.1038/s41586-019-0956-2
Acknowledgements
C.S.W. acknowledges funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement n° 747414). C.S.W., M.R.T., J.B, R.A.M. and M.M.S. acknowledge support from the i-sense Engineering and Physical Sciences Research Council (EPSRC) IRC in Early Warning Sensing Systems for Infectious Diseases (EP/K031953/1; www.i-sense.org.uk), M.R.T. and M.M.S. acknowledge support from the Medical Research Council (MRC) grant “m-Africa” (MR/P024378/1).
Footnotes
Author Contributions
All authors contributed to the writing of the manuscript
Author Information
The authors declare no competing financial interests.
References
- 1.GSMA. The Mobile Economy. 2017 https://www.gsma.com/mobileeconomy.
- 2.GSMA. Mobile Economy Sub-Saharan Africa. 2014 https://www.gsma.com/mobileeconomy/archive/GSMA_ME_SubSaharanAfrica_2014.
- 3.Perkel JM. Pocket laboratories. Nature. 2017;545:119–121. doi: 10.1038/545119a. [DOI] [PubMed] [Google Scholar]
- 4.GSMA. Global Mobile Trends. 2017 https://www.gsma.com/globalmobiletrends/index.html.
- 5.Ginsburg GS, Willard HF. Genomic and Personalized Medicine. Vol. 1 Elsevier/Academic Press; Cambridge: 2013. [Google Scholar]
- 6.Peacock SJ, Weinstock GM. Microbial sequencing to improve individual and population health. Genome Med. 2014;6:103. doi: 10.1186/s13073-014-0103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bains RK. Human infectious diseases in the genomics era: where do we go from here? Genome Biol. 2014;15:529. doi: 10.1186/s13059-014-0529-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.UNITAID Secretariat. HIV/AIDS Diagnostics Technology Landscape. 5th Edition. 2015. https://aidsfree.usaid.gov/resources/hivaids-diagnostics-technology-landscape-5th-edition. [Google Scholar]
- 9.Jain M, et al. Nanopore sequencing and assembly of a human genome with ultra-long reads. Nat Biotechnol. 2018;36:338. doi: 10.1038/nbt.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hood L, Price ND. Demystifying Disease, Democratizing Health Care. Sci Transl Med. 2014;6:225ed225. doi: 10.1126/scitranslmed.3008665. [DOI] [PubMed] [Google Scholar]
- 11.Ali EE, Chew L, Yap KY-L. Evolution and current status of mhealth research: a systematic review. BMJ Innov. 2016;2:33. [A systematic overview of how mhealth research has changed over the last 10 years since the term was coined.] [Google Scholar]
- 12.Mackillop L, et al. Development of a real-time smartphone solution for the management of women with or at high risk of gestational diabetes. J Diabetes Sci Technol. 2014;8:1105–1114. doi: 10.1177/1932296814542271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. mHealth: New horizons for health through mobile technologies. 2011 https://apps.who.int/iris/handle/10665/44607.
- 14.Agarwal S, et al. Mobile Technology in Support of Frontline Health Workers: A comprehensive overview of the landscape, knowledge gaps and future directions. 2016 https://www.mhealthknowledge.org/resources/mobile-technology-support-frontline-health-workers-comprehensive-overview-landscape.
- 15.WHO. Antimicrobial Resistance Global Report on Surveillance. 2014 https://www.who.int/drugresistance/documents/surveillancereport/en/
- 16.Namisango E, Ntege C, Luyirika EBK, Kiyange F, Allsop MJ. Strengthening pharmaceutical systems for palliative care services in resource limited settings: piloting a mHealth application across a rural and urban setting in Uganda. BMC Palliative Care. 2016;15:20. doi: 10.1186/s12904-016-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haque F, et al. Evaluation of a Smartphone Decision-Support Tool for Diarrheal Disease Management in a Resource-Limited Setting. PLoS Negl Trop Dis. 2017;11:e0005290. doi: 10.1371/journal.pntd.0005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayward AC, et al. Comparative community burden and severity of seasonal and pandemic influenza: results of the Flu Watch cohort study. Lancet Respir Med. 2014;2:445–454. doi: 10.1016/S2213-2600(14)70034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fallah MP, et al. Bolstering Community Cooperation in Ebola Resurgence Protocols: Combining Field Blood Draw and Point-of-Care Diagnosis. PLoS Med. 2017;14:e1002227. doi: 10.1371/journal.pmed.1002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chunara R, Freifeld CC, Brownstein JS. New technologies for reporting real-time emergent infections. Parasitology. 2012;139:1843–1851. doi: 10.1017/S0031182012000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yom-Tov E, Johansson-Cox I, Lampos V, Hayward AC. Estimating the secondary attack rate and serial interval of influenza-like illnesses using social media. Influenza Other Respir Viruses. 2015;9:191–199. doi: 10.1111/irv.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lampos V, Miller AC, Crossan S, Stefansen C. Advances in nowcasting influenza-like illness rates using search query logs. Sci Rep. 2015;5:12760. doi: 10.1038/srep12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vandormael A, et al. Longitudinal Trends in the Prevalence of Detectable HIV Viremia: Population-Based Evidence From Rural KwaZulu-Natal, South Africa. Clin Infect Dis. 2018;66:1254–1260. doi: 10.1093/cid/cix976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dowell SF, Blazes D, Desmond-Hellmann S. Four steps to precision public health. Nature. 2016;540:189–191. [Google Scholar]
- 25.e-News, M. IAPAC, RI-MUHC, SYMPACT-X Announce Partnership to Implement HIVSmart!™ Self-Testing App in High HIV Burden Fast-Track Cities. 2017 https://publications.mcgill.ca/medenews/2017/09/18/apac-ri-muhc-sympact-x-announce-partnership-to-implement-hivsmart-self-testing-app-in-high-hiv-burden-fast-track-cities/
- 26.Bissonnette L, Bergeron MG. Portable devices and mobile instruments for infectious diseases point-of-care testing. Expert Rev Mol Diagn. 2017;17:471–494. doi: 10.1080/14737159.2017.1310619. [DOI] [PubMed] [Google Scholar]
- 27.Kozel TR, Burnham-Marusich AR. Point-of-Care Testing for Infectious Diseases: Past, Present, and Future. J Clin Microbiol. 2017;55:2313–2320. doi: 10.1128/JCM.00476-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhamla MS, et al. Hand-powered ultralow-cost paper centrifuge. Nat Biomed Eng. 2017;1:0009. [Google Scholar]
- 29.Mabey D, Peeling RW, Ustianowski A, Perkins MD. Tropical infectious diseases: Diagnostics for the developing world. Nat Rev Microbiol. 2004;2:231–240. doi: 10.1038/nrmicro841. [DOI] [PubMed] [Google Scholar]
- 30.Drain PK, et al. Evaluating Diagnostic Point-of-Care Tests in Resource-Limited Settings. Lancet Infect Dis. 2014;14:239–249. doi: 10.1016/S1473-3099(13)70250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo X, Davis JJ. Electrical biosensors and the label free detection of protein disease biomarkers. Chem Soc Rev. 2013;42:5944–5962. doi: 10.1039/c3cs60077g. [DOI] [PubMed] [Google Scholar]
- 32.Nayak S, Blumenfeld NR, Laksanasopin T, Sia SK. Point-of-Care Diagnostics: Recent Developments in a Connected Age. Anal Chem. 2017;89:102–123. doi: 10.1021/acs.analchem.6b04630. [A review of advances in POC diagnostics since 2014 with a view to enabling the development of connected diagnostics.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez-Barbosa N, Gamarra JD, Osma JF. The future point-of-care detection of disease and its data capture and handling. Anal Bioanal Chem. 2016;408:2827–2837. doi: 10.1007/s00216-015-9249-2. [DOI] [PubMed] [Google Scholar]
- 34.Kwon L, Long KD, Wan Y, Yu H, Cunningham BT. Medical diagnostics with mobile devices: Comparison of intrinsic and extrinsic sensing. Biotechnol Adv. 2016;34:291–304. doi: 10.1016/j.biotechadv.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Romeo A, Leung TS, Sanchez S. Smart biosensors for multiplexed and fully integrated point-of-care diagnostics. Lab Chip. 2016;16:1957–1961. doi: 10.1039/c6lc90046a. [DOI] [PubMed] [Google Scholar]
- 36.Kong JE, et al. Highly Stable and Sensitive Nucleic Acid Amplification and Cell-Phone-Based Readout. ACS Nano. 2017;11:2934–2943. doi: 10.1021/acsnano.6b08274. [DOI] [PubMed] [Google Scholar]
- 37.Feng S, Tseng D, Di Carlo D, Garner OB, Ozcan A. High-throughput and automated diagnosis of antimicrobial resistance using a cost-effective cellphone-based micro-plate reader. Sci Rep. 2016;6:39203. doi: 10.1038/srep39203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herbst de Cortina S, et al. Laboratory Evaluation of a Smartphone-Based Electronic Reader of Rapid Dual Point-of-Care Tests for Antibodies to Human Immunodeficiency Virus and Treponema pallidum Infections. Sex Transm Dis. 2017;44:412–416. doi: 10.1097/OLQ.0000000000000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller Benjamin S, et al. Quantifying Biomolecular Binding Constants Using Video Paper Analytical Devices. Chem Eur J. 2018;24:9783–9787. doi: 10.1002/chem.201802394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loynachan CN. Platinum Nanocatalyst Amplification: Redefining the Gold Standard for Lateral Flow Immunoassays with Ultra-Broad Dynamic Range. ACS Nano. 2017;12:279–288. doi: 10.1021/acsnano.7b06229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brangel P, et al. A Serological Point-of-Care Test for the Detection of IgG Antibodies against Ebola Virus in Human Survivors. ACS Nano. 2018;12:63–73. doi: 10.1021/acsnano.7b07021. [DOI] [PubMed] [Google Scholar]
- 42.Gous N, et al. The impact of digital technologies on point-of-care diagnostics in resource-limited settings. Expert Rev Mol Diagn. 2018;18:385–397. doi: 10.1080/14737159.2018.1460205. [An in depth look at how digital technology is impacting principally commercial POC diagnostics in resource-limited settings.] [DOI] [PubMed] [Google Scholar]
- 43.Contreras-Naranjo JC, Wei Q, Ozcan A. Mobile Phone-Based Microscopy, Sensing, and Diagnostics. IEEE J Sel Topics Quantum Electron. 2016;22:1–14. [Google Scholar]
- 44.Rajchgot J, et al. Mobile-phone and handheld microscopy for neglected tropical diseases. PLoS Negl Trop Dis. 2017;11:e0005550. doi: 10.1371/journal.pntd.0005550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mudanyali O, et al. Wide-field optical detection of nanoparticles using on-chip microscopy and self-assembled nanolenses. Nat Photonics. 2013;7:240–247. doi: 10.1038/nphoton.2012.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei Q, et al. Imaging and Sizing of Single DNA Molecules on a Mobile Phone. ACS Nano. 2014;8:12725–12733. doi: 10.1021/nn505821y. [DOI] [PubMed] [Google Scholar]
- 47.Albert MV, Toledo S, Shapiro M, Kording K. Using Mobile Phones for Activity Recognition in Parkinson’s Patients. Front Neurol. 2012;3:158. doi: 10.3389/fneur.2012.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larson EC, et al. Proceedings of the 2012 ACM Conference on Ubiquitous Computing. ACM; Pittsburgh, Pennsylvania: 2012. pp. 280–289. [Google Scholar]
- 49.Mukundarajan H, Hol FJH, Castillo EA, Newby C, Prakash M. Using mobile phones as acoustic sensors for high-throughput mosquito surveillance. eLife. 2017;6:e27854. doi: 10.7554/eLife.27854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan PH, et al. Diagnostic Performance of a Smartphone-Based Photoplethysmographic Application for Atrial Fibrillation Screening in a Primary Care Setting. J Am Heart Assoc. 2016;5:e004000. doi: 10.1161/JAHA.116.003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanz M, Picazo-Bueno JÁ, Granero L, García J, Micó V. Compact, cost-effective and field-portable microscope prototype based on MISHELF microscopy. Sci Rep. 2017;7:43291. doi: 10.1038/srep43291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greenbaum A, Akbari N, Feizi A, Luo W, Ozcan A. Field-Portable Pixel Super-Resolution Colour Microscope. PLoS ONE. 2013;8:e76475. doi: 10.1371/journal.pone.0076475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laksanasopin T, et al. A smartphone dongle for diagnosis of infectious diseases at the point of care. Sci Transl Med. 2015;7:273re271. doi: 10.1126/scitranslmed.aaa0056. [DOI] [PubMed] [Google Scholar]
- 54.Hammond J, Formisano N, Estrela P, Carrara S, Tkac J. Electrochemical biosensors and nanobiosensors. Essays Biochem. 2016;60:69–80. doi: 10.1042/EBC20150008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wen W, Yan X, Zhu C, Du D, Lin Y. Recent Advances in Electrochemical Immunosensors. Anal Chem. 2017;89:138–156. doi: 10.1021/acs.analchem.6b04281. [DOI] [PubMed] [Google Scholar]
- 56.Gao W, et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature. 2016;529:509–514. doi: 10.1038/nature16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee H, et al. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci Adv. 2017;3:e1601314. doi: 10.1126/sciadv.1601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu L, et al. Smartphone-based sensing system using ZnO and graphene modified electrodes for VOCs detection. Biosens Bioelectron. 2017;93:94–101. doi: 10.1016/j.bios.2016.09.084. [DOI] [PubMed] [Google Scholar]
- 59.Go DB, Atashbar MZ, Ramshani Z, Chang H-CY. Surface acoustic wave devices for chemical sensing and microfluidics: a review and perspective. Anal Methods. 2017;9:4112–4134. doi: 10.1039/C7AY00690J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turbé V, et al. Towards an ultra-rapid smartphone-connected test for infectious diseases. Sci Rep. 2017;7:11971. doi: 10.1038/s41598-017-11887-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clarke J, et al. Continuous base identification for single-molecule nanopore DNA sequencing. Nat Nanotechnol. 2009;4:265–270. doi: 10.1038/nnano.2009.12. [DOI] [PubMed] [Google Scholar]
- 62.Ginsburg G. Medical genomics: Gather and use genetic data in health care. Nature. 2014;508:451–453. doi: 10.1038/508451a. [DOI] [PubMed] [Google Scholar]
- 63.Quick J, et al. Real-time, portable genome sequencing for Ebola surveillance. Nature. 2016;530:228–232. doi: 10.1038/nature16996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alcantara MF, et al. Improving tuberculosis diagnostics using deep learning and mobile health technologies among resource-poor communities in Perú. Smart Health. 2017;1-2:66–76. [Google Scholar]
- 65.Feng S, et al. Immunochromatographic Diagnostic Test Analysis Using Google Glass. ACS Nano. 2014;8:3069–3079. doi: 10.1021/nn500614k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen W, et al. Mobile Platform for Multiplexed Detection and Differentiation of Disease-Specific Nucleic Acid Sequences, Using Microfluidic Loop-Mediated Isothermal Amplification and Smartphone Detection. Anal Chem. 2017;89:11219–11226. doi: 10.1021/acs.analchem.7b02478. [DOI] [PubMed] [Google Scholar]
- 67.Zhu H, et al. Cost-effective and rapid blood analysis on a cell-phone. Lab Chip. 2013;13:1282–1288. doi: 10.1039/c3lc41408f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.D’Ambrosio MV, et al. Point-of-care quantification of blood-borne filarial parasites with a mobile phone microscope. Sci Transl Med. 2015;7:286re284. doi: 10.1126/scitranslmed.aaa3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ravi S. ProjectionNet: Learning Efficient On-Device Deep Networks Using Neural Projections. 2017 Preprint at https://arxiv.org/abs/1708.00630.
- 70.Chen C, Chen Q, Xu J, Koltun V. Learning to See in the Dark. 2018 Preprint at https://arxiv.org/abs/1805.01934.
- 71.Pisani E, et al. Beyond open data: realising the health benefits of sharing data. BMJ. 2016;355:i5295. doi: 10.1136/bmj.i5295. [An analysis of the benefits of clinical data sharing and a brief overview of the current attempts to do so.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tresp V, et al. Going Digital: A Survey on Digitalization and Large-Scale Data Analytics in Healthcare. Proc IEEE. 2016;104:2180–2206. [Google Scholar]
- 73.Chen C, et al. Making sense of mobile health data: an open architecture to improve individual- and population-level health. J Med Internet Res. 2012;14:e112. doi: 10.2196/jmir.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anane‐Sarpong E, et al. “You Cannot Collect Data Using Your Own Resources And Put It On Open Access”: Perspectives From Africa About Public Health Data‐Sharing. Dev World Bioeth. 2017;00:1–12. doi: 10.1111/dewb.12159. [DOI] [PubMed] [Google Scholar]
- 75.Luxton DD, Kayl RA, Mishkind MC. mHealth data security: The need for HIPAA-compliant standardization. Telemed J E Health. 2012;18:284–288. doi: 10.1089/tmj.2011.0180. [DOI] [PubMed] [Google Scholar]
- 76.Pisani E, AbouZahr C. Sharing health data: good intentions are not enough. Bull World Health Organ. 2010;88:462–466. doi: 10.2471/BLT.09.074393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Privacy Code of Conduct on mobile health apps. 2016 https://ec.europa.eu/digital-single-market/en/privacy-code-conduct-mobile-health-apps.
- 78.Rumbold B, Wenham C, Wilson J. Self-tests for influenza: an empirical ethics investigation. BMC Med Ethics. 2017;18:33. doi: 10.1186/s12910-017-0192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.HL7 FHIR Foundation. FHIR. 2018 http://hl7.org/fhir.
- 80.Gibbs J, et al. The eClinical Care Pathway Framework: a novel structure for creation of online complex clinical care pathways and its application in the management of sexually transmitted infections. BMC Med Inform Decis Mak. 2016;16:98. doi: 10.1186/s12911-016-0338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.WHO. mHealth: use of mobile wireless technologies for public health. 2016 http://apps.who.int/gb/ebwha/pdf_files/EB139/B139_8-en.pdf.
- 82.El-Osta A, et al. Does use of point-of-care testing improve cost-effectiveness of the NHS Health Check programme in the primary care setting? A cost-minimisation analysis. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-015494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iribarren SJ, Cato K, Falzon L, Stone PW. What is the economic evidence for mHealth? A systematic review of economic evaluations of mHealth solutions. PLoS ONE. 2017;12:e0170581. doi: 10.1371/journal.pone.0170581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marcolino SM, et al. The Impact of mHealth Interventions: Systematic Review of Systematic Reviews. JMIR Mhealth Uhealth. 2018;6:e23. doi: 10.2196/mhealth.8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.LeFevre AE, Shillcutt SD, Broomhead S, Labrique AB, Jones T. Defining a staged-based process for economic and financial evaluations of mHealth programs. Cost Effectiveness and Resource Allocation. 2017;15:5. doi: 10.1186/s12962-017-0067-6. [A recent attempt to map what is needed to effectively assess an mHealth intervention with a focus on the economic and financial considerations at different scales of evaluation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Uganda Bureau of Statistics. Uganda National Household Survey Report Chapter 5.0: Health. 2010 http://www.ubos.org/UNHS0910/chapter5_%20distance%20to%20health%20facility.html.
- 87.GSMA. Bridging the gender gap: Mobile access and usage in low- and middle-income countries. 2015 https://www.gsma.com/mobilefordevelopment/resources/bridging-gender-gap-mobile-access-usage-low-middle-income-countries/
- 88.Pew Research Centre. Spring 2016 Global Attitudes Survey, Q81. 2016 http://www.pewglobal.org/dataset/spring-2016-survey-data.
- 89.The Academy of Medical Sciences. Health of the Public in 2040. 2016 https://acmedsci.ac.uk/policy/policy-projects/health-of-the-public-in-2040.
- 90.Details of how this graph was generated are available online at DOI: 10.5281/zenodo.1320937.
- 91.Uganda Bureau of Statistics. Health Centres Uganda. [Accessed 08/17];2012 http://maps.data.ug/layers/geonode%3Ahealth_centres_ubos_and_others_merged.
- 92.Africa population count data: Linard C, Gilbert M, Snow RW, Noor AM, Tatem AJ. PLoS ONE. 2012;7(2):e31743. doi: 10.1371/journal.pone.0031743.
- 93.Unwired Labs. OpenCelliD Project licensed under a Creative Commons Attribution-ShareAlike 4.0 International License. 2017 http://opencellid.org.
- 94.Choko AT, et al. Uptake, Accuracy, Safety, and Linkage into Care over Two Years of Promoting Annual Self-Testing for HIV in Blantyre, Malawi: A Community-Based Prospective Study. PLoS Med. 2015;12:e1001873. doi: 10.1371/journal.pmed.1001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martínez Pérez G, et al. ‘I Know that I Do Have HIV but Nobody Saw Me’: Oral HIV Self-Testing in an Informal Settlement in South Africa. PLoS ONE. 2016;11:e0152653. doi: 10.1371/journal.pone.0152653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pant Pai N, et al. Will an Unsupervised Self-Testing Strategy for HIV Work in Health Care Workers of South Africa? A Cross Sectional Pilot Feasibility Study. PLoS ONE. 2013;8:e79772. doi: 10.1371/journal.pone.0079772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Estcourt CS, et al. The eSexual Health Clinic system for management, prevention, and control of sexually transmitted infections: exploratory studies in people testing for Chlamydia trachomatis. Lancet Public Health. 2:e182–e190. doi: 10.1016/S2468-2667(17)30034-8. [DOI] [PubMed] [Google Scholar]
- 98.The GSID system. 2017 http://www.gsid.org/our_programs_surveillance_gsid_system_overview.html.
- 99.Dell N, Francis I, Sheppard H, Simbi R, Borriello G. Field evaluation of a camera-based mobile health system in low-resource settings. Proceedings of the 16th international conference on Human-computer interaction with mobile devices and services; 2014. pp. 33–42. [Google Scholar]
- 100.Laktabai J, et al. A mobile health technology platform for quality assurance and quality improvement of malaria diagnosis by community health workers. PLoS ONE. 2018;13:e0191968. doi: 10.1371/journal.pone.0191968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.