Through 2017, roughly 40 years since HIV/AIDS became a global health crisis, the world has experienced devastating consequences: an estimated 35 million HIV-infected persons have died, 17 million children have been orphaned, and 37 million persons are living with the virus [1,2]. With sustained commitment to address the epidemic, control and virtual elimination of HIV is within reach in some countries. In 2014, the Joint United Nations Programme on HIV/AIDS released ambitious treatment targets to reach HIV epidemic control by 2030, calling for 90% of all persons living with HIV (PLHIV) to be diagnosed, 90% of diagnosed PLHIV to be on HIV treatment, and 90% of treated PLHIV to be virally suppressed by 2020 (90-90-90) [3]. By 2017, 75% of PLHIV knew their HIV status, 79% of these were on treatment, and 81% of these achieved viral suppression [1]. Still millions of PLHIV who require diagnosis and treatment are not yet reached.
As countries near universal coverage of diagnoses, treatment, and viral suppression among PLHIV a strong national surveillance system serves as a bedrock for monitoring attainment and sustainment of HIV epidemic control and is the primary system for management of public health response efforts. Through infectious disease outbreaks, we know that rapid identification of newly infected persons has a profound impact on breaking the transmission chain and ending epidemics. With new developments in laboratory technology, rapid detection of recent HIV infection (i.e. HIV-antibody seroconversion occurring on average in the past 6 months) is now possible using a validated rapid test for recent infection (RTRI) that diagnoses HIV and detects recent infection within minutes [4-6]. The RTRI allows person, place, and time to be described for all new HIV diagnoses at the point of HIV testing services (HTS), forming the basis of a real-time surveillance and public health response system that accelerates the surveillance to care continuum. As the system generates routine information on trends in new diagnoses, it can simultaneously provide signals at the most granular level to inform programs where to focus interventions to reach those that need them the most.
In such a system, RTRI data are used in conjunction with geographic data to identify clusters of recent infections, triggering a public health response. Cluster investigations in the concerning area will vary in scope but can include review of existing epidemiological and programmatic data to verify trends; implementation of standardized questionnaires to confirm epidemiological links; extending case finding strategies to locate and treat undiagnosed PLHIV; active identification and re-engagement of those out of care; and delivery of preexposure prophylaxis and other prevention services to HIV-negative contacts at high risk of infection in a transmission network. Once linked to treatment, achieving HIV disease control will require person-centered interventions that focus on rapid treatment; optimal drug regimens; access to routine viral load testing; viral load suppression; and supportive adherence and retention strategies for persons on treatment.
In Fiscal Year 2019, the United States President’s Emergency Plan for AIDS Relief funded 16 countries (El Salvador, Eswatini, Ethiopia, Guatemala, Kenya, Lesotho, Malawi, Namibia, Nicaragua, Panama, Rwanda, Tanzania, Uganda, Vietnam, Zambia, Zimbabwe) nearing the 90–90–90 targets to introduce the TRACE initiative (Tracking with Recency Assays to Control the Epidemic). Through TRACE, the RTRI is conducted as a supplementary test in HTS to detect recent infection among confirmed newly diagnosed PLHIV. Upon regulatory approval, the test may be considered part of routine national HIV testing algorithms to concurrently diagnose HIV and determine recency of infection. Unlike population-based HIV household surveys where laboratory-based recency testing is applied to HIV-positive blood from a crosssectional sample of individuals, the integration of RTRI in routine HTS offers access to recency testing to persons with new HIV diagnoses in all facility-and community-based testing sites in a country. As the surveillance system matures, its sensitivity will improve with adoption of RTRI in national HIV testing algorithms, increased access to viral load confirmatory testing, and improvements in patient health information systems (HIS) to detect prior diagnoses among test seekers. Additionally, the system’s ability to reach undiagnosed PLHIV who may not seek HTS is expected to expand with the immediate use of surveillance data to direct high throughput case finding strategies in identified clusters.
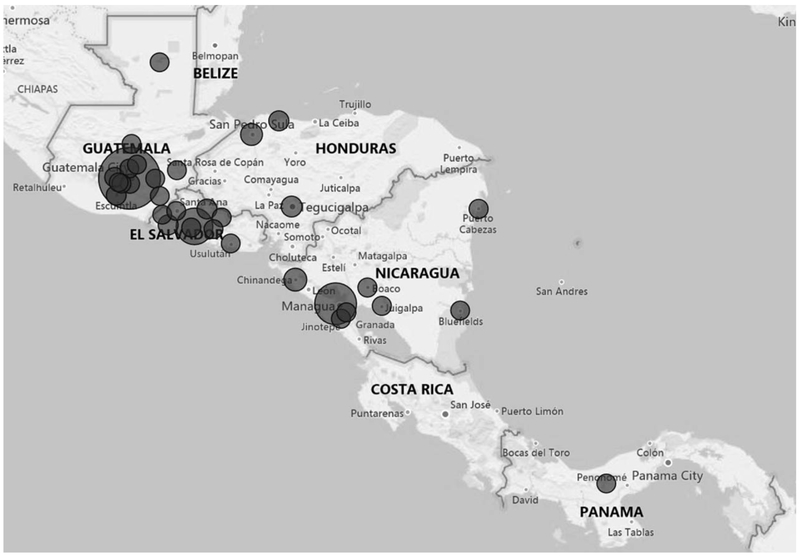
Electronic HIS with automated analysis and dashboard interfaces can facilitate real-time data monitoring and use at all levels from the site, sub-national units, and national level. Figure 1 shows an example of a visualization dashboard from Central America where the RTRI was deployed in several countries (more information available at: https://www.pepfarsolutions.org/emerging-technologies-innovations/2018/7/11/surveillance-of-recent-hiv-infections-using-point-of-care-recency-tests-to-rapidly-detect-and-respond-to-recent-infections). Routine data review allows Ministries of Health to leverage limited public health resources to investigate, identify and confirm transmission networks, and target interventions to interrupt transmission. Given that sensitive patient-level information is collected, national policies are needed to protect the confidentiality, security, and privacy of this information. These protections are particularly relevant for countries planning to integrate RTRI results into HIV case surveillance systems, which monitor individuals for their health outcomes throughout the course of their HIV disease to characterize the HIV epidemic and guide public health and clinical decision-making. Strong government commitment and community partnerships will be needed to acknowledge the legal and social environments around HIV and to establish risk mitigation strategies to protect individuals and populations against potential misuse of these surveillance data.
Fig. 1. Distribution of confirmed recent HIV infections, Central America, October 2017 – April 2019.
Circle size reflects recent infection case volume by sub-national government unit (i.e. department). Small circles represent units with one to five recent cases. Medium circles represent units with 70 to 90 recent cases, and the large circle represents unit with 167 cases
We stand on the precipice of achieving HIV epidemic control but getting there and reaching virtual elimination of HIV will require creative thinking on how to monitor epidemic control, identify those not reached, and interrupt further transmission. Rapid identification and treatment of new infections are key to control of infectious diseases; with emerging technology to identify networks of recent transmission, this goal is now attainable for HIV. Understanding that much of HIV transmission goes unrecognized, HIV phylogenetic testing can serve as an additional tool to infer links in transmission networks to fine-tune the public health response [7]. By making HIV surveillance data widely available and actionable in real-time, our prevention and treatment impact will be maximized and virtual elimination may be a reality by 2030.
Acknowledgements
We would like to acknowledge the Ministries of Health in El Salvador, Guatemala, Honduras, Nicaragua, and Panama and Universidad de Valle de Guatemala for their leadership in the establishment of a surveillance and public health response system using RTRIs in Central America. We also thank PEPFAR’s Community of Practice for TRACE which include staff and technical teams from the United States (US) Centers for Disease Control and Prevention, the US Agency for International Development, the US Department of Defense; PEPFAR implementing partners; Country Ministries of Health; and TRACE global partners: the International Center for AIDS Care and Treatment Programs at Columbia University, and the University of California San Francisco.
Disclaimers: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies. As an inventor of a HIV rapid test for recent infection and as per policy of US government, B.S.P. receives royalties from the sale of this assay. There are no patents relating to this assay. This does not alter the authors’ adherence to all AIDS policies on sharing data and materials.
Footnotes
Attribution of support: This publication was made possible by support from the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) through the U.S. Centers for Disease Control and Prevention (CDC), Division of Global HIV and Tuberculosis (DGHT).
Conflicts of interest
There are no conflicts of interest.
References
- 1.The Joint United Nations Programme on HIV/AIDS (UNAIDS). Fact Sheet - World AIDS Day 2018: 2017 Global HIV Statistics. Geneva: UNAIDS, 2018. Available at: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. [Accessed 15 September 2018]. [Google Scholar]
- 2.United States Agency for International Development (USAID). Orphans and Vulnerable Children Affected by HIV and AIDS. Washington DC: USAID, 2016. Available at: https://www.usaid.-gov/what-we-do/global-health/hiv-and-aids/technical-areas/or-phans-and-vulnerable-children-affected-hiv. [Accessed 21 December 2018]. [Google Scholar]
- 3.UNAIDS. Ambitious Treatment Targets: writing the final chapter of the AIDS Epidemic. Geneva: UNAIDS, 2014. Available at: http://www.unaids.org/en/resources/documents/2017/90–90-90. [Accessed 15 September 2018]. [Google Scholar]
- 4.Granade TC, Nguyen S, Kuehl DS, Parekh BS. Development of a novel rapid HIV test for simultaneous detection of recent or long-term HIV type 1 infection using a single testing device. AIDS Res Hum Retroviruses 2013;29:61–67. [DOI] [PubMed] [Google Scholar]
- 5.Parekh BS, Detorio M, Shanmugam V, Yufenyuy E, Dobbs T, Kim AA, et al. Performance evaluation of Asante Rapid Recency Assay for HIV diagnosis and detection of recent infection: potential for surveillance and prevention. Abstract TUPECO849. 9th International AIDS Society Conference on HIV Science;23–26 July 2017. [Google Scholar]
- 6.Agyemang E, Dobbs T, Zungu I, Kim A, Adhikari A, Kim E, et al. Performance of a novel point-of-care HIV recency test among newly diagnosed pregnant adolescent girls and young women - Malawi, 2017. Abstract THPEC200. 22nd International AIDS Conference; 23–27 July 2017. [Google Scholar]
- 7.Oster AM, France AM, Panneer N, Ocfemia MCB, Campbell E, Dasgupta S, et al. Identifying clusters of recent and rapid HIV transmission through analysis of molecular surveillance data. J Acquir Immune Defic Syndr 2018;79:543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]