Abstract
The most common neurodegenerative diseases are Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease, frontotemporal lobar degeneration, and the motor neuron diseases, with AD affecting approximately 6% of people aged 65 years and older, and PD affecting approximately 1% of people aged over 60 years. Specific proteins are associated with these neurodegenerative diseases, as determined by both immunohistochemical studies on post-mortem tissue and genetic screening, where protein misfolding and aggregation are key hallmarks. Many of these proteins are shown to misfold and aggregate into soluble non-native oligomers and large insoluble protein deposits (fibrils and plaques), both of which may exert a toxic gain of function. Proteotoxicity has been examined intensively in cell culture and in in vivo models, and clinical trials of methods to attenuate proteotoxicity are relatively new. Therapies to enhance cellular protein quality control mechanisms such as upregulation of chaperones and clearance/degradation pathways, as well as immunotherapies against toxic protein conformations, are being actively pursued. In this article, we summarize the common pathophysiology of neurodegenerative disease, and review therapies in early-phase clinical trials that target the proteotoxic component of several neurodegenerative diseases.
Key Points
| Aberrant protein misfolding and aggregation is associated with neurodegenerative disease. |
| Biochemical pathways that suppress or remove aggregated proteins are only just now being targeted and examined in the clinic. |
Introduction
The Cost of Neurodegenerative Disease
Neurodegeneration is an umbrella term for an array of neurological diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD), as well as frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS). These conditions, which are estimated to affect over 40 million people worldwide [1], are characterized by the progressive structural and functional loss of neurons, which ultimately leads to the development of clinical features [2]. The symptoms are typically chronic in nature with profound impacts on the well-being of the affected individuals [3–5], as well as their families, friends and caregivers. Although neurodegenerative disease (ND) may affect people of all ages, its prevalence and incidence increases dramatically with age [6]. As life expectancy increases and the world’s population ages, the number of individuals suffering from these disorders will increase. In addition to human suffering, NDs pose an ever-increasing economic burden, estimated to cost over approximately US$300 billion to the US healthcare system alone per year [7], a value that is expected to increase due to the aging population. Therefore, the development of effective therapeutics is essential in decreasing the personal, social, and economic burdens of these devastating neurological disorders.
Protein Aggregation is a Common Pathological Hallmark of Neurodegenerative Disease
A common pathological hallmark of neurodegenerative disease (ND) is the deposition of specific proteins into insoluble proteinaceous deposits in and around affected tissues (e.g. Lewy bodies spread throughout the central, autonomic, and peripheral nervous systems in PD, and intracellular inclusions in upper and lower motor neurons in ALS) [8–10]. In most cases, the major constituent of the insoluble material is a disease-specific protein (Table 1), such as amyloid-β (Aβ) [11] and tau [12] in AD, α-synuclein in PD [9] and multiple system atrophy (MSA) [13–15], huntingtin in HD [16], and TDP-43 in ALS/FTD [10], although there is evidence of protein overlap emerging between syndromes [17]. The existence of these insoluble protein deposits suggests that protein misfolding and aggregation may play a key role in disease etiology and pathophysiology [18–21]. This is perhaps due to reduced protein homeostasis (proteostasis) concomitant with aging, genetic factors (mutations and polymorphisms) [22] and environmental factors (e.g. oxidative stress) [23], which can both lead to changes in protein folding quality control and result in, or catalyze, protein misfolding. Such dysregulation could then lead to the build-up of toxic oligomers, inclusion bodies, or aggregates that may be toxic in ND. Protein aggregation is defined as the accumulation of misfolded proteins into higher-order structures that can be either soluble or insoluble, and is considered an ‘off-folding’ pathway within the schema of protein folding theory [18], making protein processing an important focus of biomedical research.
Table 1.
Overview of aggregating proteins associated with neurodegenerative disease, and their biological and pathological roles
| Disease/protein | Physiological role | Deposit type | Location | Pathological characteristics |
|---|---|---|---|---|
| Alzheimer’s disease | ||||
| Amyloid-β |
Modulation of synaptic activity Innate immunity |
Plaque | Extracellular space | Amyloid |
| Tau | Stabilization of microtubules | Neurofibrillary tangles | Neurons |
Cytoplasmic Hyperphosphorylated Ubiquitinated |
| Parkinson’s disease and MSA | ||||
| α-Synuclein | Membrane remodelling |
Lewy bodies Lewy neurites Glial cytoplasmic inclusions (MSA) |
Neurons Glia |
Cytoplasmic Hyperphosphorylated Ubiquitinated |
| Huntington’s disease | ||||
| Huntingtin (Htt) | Unknown |
Inclusions IPOD |
Neurons (cortex striatum) |
Ubiquitinated Intranuclear Cytoplasmic |
| Frontotemporal lobar degeneration | ||||
| Tau | Stabilization of microtubules | Neurofibrillary tangles | Neurons |
Cytoplasmic Hyperphosphorylated Ubiquitinated |
| TDP-43 | RNA splicing and trafficking | Inclusions | Neurons |
Hyperphosphorylated Ubiquitinated Cytoplasmic C-terminal fragments (25 and 35 kDa) |
| FUS | RNA metabolism | Inclusions |
Motor and cortical neurons Glia |
Cytoplasmic Ubiquitinated |
| Amyotrophic lateral sclerosis | ||||
| SOD1 | Antioxidant enzyme |
Inclusions JUNQ |
Motor neurons (spinal) Glia (spinal) |
Ubiquitinated Cytoplasmic |
| TDP-43 | RNA splicing and trafficking | Inclusions |
Motor and cortical neurons Glia |
Hyperphosphorylated Ubiquitinated Cytoplasmic C-terminal fragments (25 and 35 kDa) |
| FUS | RNA metabolism | Inclusions |
Motor and cortical neurons Glia |
Cytoplasmic Ubiquitinated |
| Creutzfeld–Jakob | ||||
| Prion (PrPSC) |
Cell signalling Cell adhesion |
Plaque and synaptic | Synapses |
Amyloid Perivacuolar |
IPOD insoluble protein deposit, JUNQ juxtanuclear quality control compartment, MSA multiple system atrophy, SOD1 superoxide dismutase 1, FUS fused in sarcoma, TDP-43 TAR DNA binding protein of 43 kDa
Much research has been focused on understanding the abnormal processing of proteins implicated in neurodegeneration [24–26]. In the case of Aβ in AD, the transmembrane amyloid precursor protein (APP) is cleaved sequentially by β-secretase and then γ-secretase to generate highly aggregation-prone isoforms of Aβ polypeptide [27]. Multiple genetic factors can exacerbate this process [28]. The Aβ polypeptide is thought to self-associate into small soluble oligomers that proceed to grow in size until they form insoluble fibrillar structures, called amyloid fibrils, in the extracellular space [24]. Amyloid fibrils are defined by their highly stable cross-beta-sheet structure, which is thought to be a conformation accessible to all proteins [29–31]. Indeed, proteins in many diseases are capable of forming amyloid-like structures, such as amylin in diabetes [32], α-synuclein in PD [33], prion protein in transmissible spongiform encephalopathy [34], huntingtin in HD [35], transthyretin in familial amyloid polyneuropathy/transthyretin amyloidosis [36], β2-microglobulin in dialysis-related amyloidosis [37], and many others [30]. Amyloid structures are highly ordered and are therefore thought to result from general physicochemical properties of protein structure and topology [29]. In contrast to this, many proteins are also known to undergo aggregation into amorphous aggregates that do not have a defined a structure. Amorphous aggregates are associated with several degenerative diseases such as inclusion body myositis [38], cataract [39], and light-chain deposition disease [40]. Regardless of the final structure formed, the progression through smaller oligomeric complexes is thought to be critically important to cellular toxicity in diseases associated with protein aggregation [24].
Toxicity of Misfolded and Aggregated Proteins
Low molecular-weight soluble aggregates are associated with greater toxicity in ND models, perhaps due to their greater ability, over large aggregates, to freely diffuse within cells and cell to cell, as well as their high reactivity for aberrant interactions. It has even been suggested larger insoluble amyloid and amorphous aggregates are thought to act as protective ‘sinks’ that sequester the toxic soluble forms [41, 42]. However, there is also evidence that suggests that large aggregates sequester other molecules from their normal roles, and induce toxicity via loss or reduction of essential cellular functions [43, 44]. This discrepancy appears to be partially dependent on the type of protein that is aggregating and the type of aggregate that is forming. For example, Huntingtin protein (Htt) with expanded poly-Q repeats is invariably toxic, and a key feature of patient tissue and cellular expression is the formation of large perinuclear or intranuclear Htt inclusions [16].
Misfolded Htt is partitioned into a certain type of protein inclusion called an ‘Insoluble Protein Deposit’ (IPOD) [45]. Cells that form these IPOD inclusions appear to have greater survivability compared with cells that have large amounts of soluble Htt, suggesting that soluble monomers or oligomers are responsible for toxicity in this disease [46]. In contrast, expression of designed amyloidogenic proteins in HEK293T cells has been found to directly affect a population of metastable proteins and sequester them from healthy functioning [47]. More recently, the trafficking of protein and RNA from the nucleus to cytoplasm has been suggested to be dysregulated by misfolded and aggregated proteins in ND [48]. Regardless of the toxic species, key mechanisms of toxicity include interaction of misfolded species with various cellular components, sequestration of essential proteins from their normal function, and chronic impairment of stress-response mechanisms (Fig. 1).
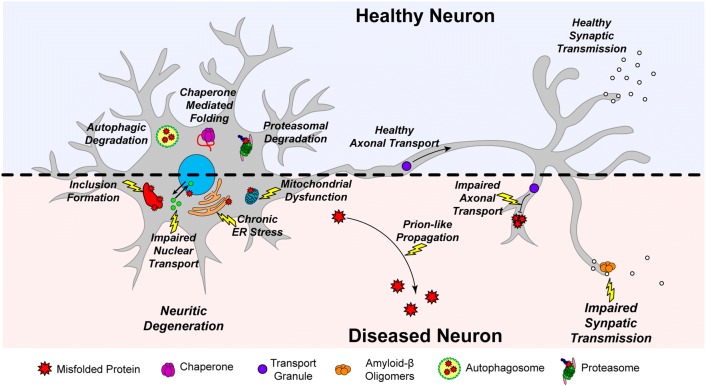
Fig. 1.
The effects of proteotoxicity and cellular mechanisms that prevent it. A healthy neuron is capable of maintaining the stability of its functional proteome through the maintenance of proper folding fidelity, and degradation of misfolded proteins via autophagy and the ubiquitin-proteasome system. Misfolded proteins can also be rescued and refolded through interactions with chaperone proteins. If these mechanisms fail or become less effective beyond critical thresholds, unstable and misfolded proteins can accumulate and form inclusions, and/or aberrantly interact with molecules essential to key cellular pathways such as nucleocytoplasmic transport and mitochondrial functioning. The endoplasmic reticulum can also become chronically stressed. Misfolded proteins can prevent proper transport of RNA and proteins along axons, leading to axonal dysfunction and eventually cell death. Aberrant interactions involving intracellular or extracellular misfolded proteins or aggregates can impair synaptic transmission, which is essential for proper neuronal functioning. Finally, misfolded and aggregated proteins have the capability to propagate to nearby cells, leading to progressive neuronal degeneration
The initial misfolding and subsequent formation of protein aggregates is thought to be a consequence of the inability of cellular protein quality control machinery to maintain protein homeostasis (proteostasis), either through a decline in effectiveness with age or by mutation in disease-associated proteins (reviewed by Yerbury et al. [49]). This is supported by evidence linking mutations in genes intimately involved in maintaining proteostasis being causative for ND (e.g. UBQLN2 in ALS [50] and PRKN (parkin) in PD [51]). Mechanisms by which cells ensure proteome stability include expression and regulation of molecular chaperone proteins, and degradation through the ubiquitin proteasome system (UPS) or autophagy. These mechanisms are currently the subject of studies attempting to develop effective therapies for NDs. Recent clinical trials of therapies targeting these cellular networks are discussed below.
Molecular Chaperone Proteins
Molecular chaperone proteins are abundantly expressed in cells and facilitate proper protein folding through association with nascent, intermediate-state, or damaged polypeptides [52]. Under healthy conditions, when a protein is misfolded, the chaperone machinery facilitates repair through protein refolding, or clearance through protein-degradation pathways [53, 54]. This protective effect is performed by an ATP-dependent active ‘folding’ mechanism regulated through HSP70, or a much more common ‘holding’ mechanism where an unfolded or misfolded intermediate is bound by a chaperone until it can be folded or degraded. The largest subclass of molecular chaperones, the heat shock proteins (HSPs), are named for their upregulated expression in response to heat stress [55], and play multiple roles within the maintenance of proteostasis [56] and other biochemical pathways throughout the cell [57].
HSPs act primarily to bind and stabilize misfolded protein conformers [54, 58], but can also stabilize large aggregates from breaking apart [59]. Another important aspect of protein folding is the processing of disulfide bonds, which occurs predominantly in the endoplasmic reticulum, by protein disulfide-isomerase and similar proteins. Owing to their role in protein folding and response to stress, chaperones are considered to be a ‘first line of defence’ in proteostasis, therefore playing an important role in proteinopathies, where they have been suggested to have great therapeutic potential. Indeed, several pharmacological approaches attempting to promote the upregulation of chaperone proteins or the heat shock response (HSR) have been examined in rodent models of ND [60–64] with varying degrees of success. This has prompted several approaches to upregulate chaperone proteins as a means to treat ND, discussed in detail below.
Arimoclomol to Activate the Heat Shock Response
Recently, the drug arimoclomol underwent a phase II/III clinical trial in patients suffering from superoxide dismutase 1-associated familial ALS (SOD1-fALS) [65], where the misfolding and deposition of mutant SOD1 into cytoplasmic inclusions in motor neurons and glia is a key hallmark of disease [66]. Arimoclomol is a potent activator of the HSR, which upregulates expression of HSPs when induced, and would therefore be expected to decrease the load of misfolded and aggregated SOD1 through increased chaperone-mediated folding. The mechanism of action of arimoclomol is thought to proceed through the ability of arimoclomol to bind strongly to heat shock transcription factor 1 (HSF1) for long periods, thus maintaining HSF1 in its activated state [67]. When activated, HSF1 induces the expression of multiple HSPs, and this activation can occur when cells are challenged by proteotoxic or other stressors [68]. The ability of arimoclomol to upregulate the HSR would be of particular use in the case of neurons, which appear to be deficient in upregulating the HSR in response to proteotoxic stress [69–76]. Preclinical studies in mice treated with arimoclomol have suggested that the HSR is upregulated as a result of treatment [77, 78].
In a recent small-scale phase II/III clinical trial, 38 patients were treated with arimoclomol 200 mg three times daily for up to 12 months with no significant adverse effects reported, indicating the safety and tolerability of the drug. Importantly, patients were screened for SOD1 mutations and an A4V mutation subgroup was created owing to the rapidly progressing ALS this mutation causes [79]. However, patient outcomes, determined by the ALS Functional Rating Scale (ALSFRS) [80] and forced expiratory volume at 6 s (FEV6) [81] rates of decline, were not significantly improved for the arimoclomol group compared with placebo, even in the case of the A4V subgroup, but there was a trend towards arimoclomol-treated patients having slightly better outcomes (ALSFRS and FEV6) at all time points examined. This failure to significantly halt disease progression could be due to the fact that upon diagnosis of ALS, a patient has already undergone significant denervation [82] and has lost a significant proportion of their motor neurons, where higher efficacy could potentially be achieved with earlier treatment. A phase III trial of arimoclomol in ALS is currently underway (ClinicalTrials.gov identifier: NCT03491462), with this trial involving a greater number of patients who suffer from either familial or sporadic ALS.
Arimoclomol was also recently tested for safety in a small trial involving the muscle-wasting disease inclusion body myositis (iBM) [83]. iBM is a muscle inflammatory disease that results in extensive skeletal muscle weakness, potentially affecting respiratory muscles, which predominantly occurs in people above 50 years of age [84–86]. Hallmarks of the disease related to inflammatory features include high levels of major histocompatibility complex class I and invasion of local tissues by mononuclear cells [87]. Hallmarks that suggest proteostasis decline include the presence of inclusion bodies that are composed of multiple proteins such as p62 [88], hyperphosphorylated tau [89], APP [90], TAR DNA-binding protein 43 [91, 92], and heterogeneous nuclear ribonucleoproteins A1 and A2B1 (hnRNPA1 and hnRNPA2B1) [93].
After finding that arimoclomol reduced pathology and decreased muscle defects in a rat model of iBM [83], researchers performed a small-scale trial of the drug in patients with sporadic iBM. Similar to the previous ALS trial, the drug was found to be well tolerated by patients (n = 24), with only minor adverse effects that were mainly gastrointestinal in nature. Also, similar to the above ALS trial, arimoclomol was not found to have any significant effects on markers of disease progression, as measured by the Inclusion Body Myositosis-Functional Rating Scale (IBMFRS) [94]. The authors of this study do note that there was a trend towards better outcomes (IBMFRS) in the arimoclomol-treated group compared with placebo control, and that a longer trial (> 4 months) with more patients may result in significant differences. A phase II trial is now currently underway to examine arimoclomol with more statistical power in regard to halting or treating iBM (ClinicalTrials.gov identifier: NCT02753530).
Effective Strategies in Chaperone Upregulation
When considering an effective method of treatment in ND disease, one has to take into account the specific protein responsible for pathology. Evidence supporting this notion exists within preclinical studies which suggest that specific targets within the chaperone network are effective only in certain cases of ND. For example, transgenic mice expressing ALS-associated mutant SOD1 were crossed with mice overexpressing either Hsp70 [95], Hsp27 [96, 97], HSJ1 [98], or the HSR regulating protein SIRT1 [99], resulting in variable protection and rescue of viability in these models. Neither Hsp70 nor Hsp27 upregulation was found to affect disease onset or progression, whereas SIRT1 overexpression was found to extend lifespan in mice expressing low levels of mutant SOD1 [99]. In contrast, targeting the HSR with pharmacological compounds (withaferin A, celastrol, arimoclomol) showed a positive effect in mutant SOD1 mouse models [77, 100–102]. This highlights that targeting specific components of the chaperone regulatory network may not be a viable approach and that general upregulation of chaperones and other stress response proteins is potentially the most effective treatment option.
Viral-Based Genetic Therapy to Increase Chaperone Capacity
Beyond pharmacological approaches, viral-delivered gene therapies are of growing interest to researchers due to their effectiveness at introducing genetic material into post-mitotic neurons [103], which are generally the most susceptible cells to proteotoxicity [104]. Most evidence of the efficacy of gene delivery of chaperones has been conducted in preclinical models, showing a powerful ability to reduce the levels of misfolded and aggregated proteins in PD models and inhibit death in dopaminergic neurons in in vivo models [105, 106]. Although clinical trials related to the delivery of genes to rebalance or upregulate components of proteostasis are yet to be carried out, viral-based gene therapy has been examined in clinical trials of PD [107, 108].
In one such trial, a small number of patients were administered an adeno-associated virus (AAV) carrying the gene encoding the glial-derived neurotrophic factor neurturin, which is essential for neuron health and growth, as well as being shown to rescue neuron function in PD model rats [109]. The aim was to deliver the AAV to the substantia nigra in patients in the hope that neurturin would be expressed and protect neurons from degeneration [108]. Encouragingly, there were no unexpected adverse effects from the surgery and infusion of AAV, and follow-up of patients showed no adverse effects up to 2 years after treatment [110]. Additionally, decline in patient motor function was stalled or slowed. Several of the patients enrolled in this trial passed away from unrelated causes and were autopsied to examine the expression of neurturin, finding however that it was only present at levels moderately higher than control PD patients [111]. The ability to deliver a gene to affected areas in the brain for long-term expression and protection is desirable compared with continual injection with other gene delivery methods, although there are considerations with the safety of viral particles.
Targeting the Ubiquitin Proteasome System (UPS)
If proteins fail to fold properly they can become targets for selective degradation by the UPS. As well as being involved in the degradation of misfolded proteins within cells, ubiquitination is deeply involved in cellular signalling [112]. The system is composed of multiple ligation enzymes that act in a cascade to label a protein with single or poly-ubiquitin via isopeptide bonds at lysine residues. Precise control of this system is necessary as the structure of a ubiquitin chain modulates its signalling purpose (e.g. K27 linkages on ubiquitin are associated with the DNA damage response, whereas K48 linkages are associated with proteasomal degradation) [113]. Following attachment of a K48 ubiquitin chain, proteins are shuttled to the 26S proteasome, where, upon delivery, the ubiquitin chain is cleaved by deubiquitinating enzymes, and the cargo is unfolded and transferred into the proteasome for degradation [114]. The balance between ubiquitination and deubiquitination is tightly controlled to maintain a pool of free ubiquitin for use in signalling or degradation pathways, and it is suggested that tipping the balance of this system may be a cause for neurodegeneration [49]. Although the UPS has been the subject of intensive research in cancer therapy [115, 116], it has seen less interest as a therapeutic target for neurodegeneration. In cases where the UPS has been targeted, the focus is on upregulation of ubiquitin ligases, or inhibition of deubiquitinating enzymes [117].
Cilostazol to Activate the UPS in Alzheimer’s Disease (AD)
It has recently been suggested that the UPS is dysfunctional in AD [118, 119] and dementia in general [11, 120, 121]. In the case of AD, hyperphosphorylated and ubiquitinated tau is found in intraneuronal neurofibrillary tangles in patients [12, 122] and mouse models [123, 124], suggesting that the UPS may be the main degradation pathway for tau. A small molecule called cilostazol has historically been used to treat patients suffering from peripheral vascular disease [125], and is a phosphodiesterase 3 inhibitor that increases UPS function through a cAMP/protein kinase A-dependent mechanism. Recently, some small-scale studies of this UPS-activating small molecule were carried out to determine its effectiveness in patients with mild cognitive impairment (MCI) [126] and AD [127, 128].
In an initial study, 20 patients were enrolled to receive cilostazol 100 mg/day for 6 months, where it was determined that there were no significant differences in patient decline (Mini-Mental State Examination [MMSE], Alzheimer’s Disease Assessment Scale–Cognitive Subscale Japanese version [ADAS-Jcog], Trail Making Test-A [TMT-A] or Revised Wechsler Memory Scale [WMS-R] logical memory-I tests), although diagnosis of some patients in this study was not fully confirmed [127]. Furthermore, patients who received cilostazol were found to have increased regional cerebral blood flow in the right anterior cingulate lobe. Although no change in decline was observed here, increased blood flow to the brain is a current potential treatment for AD, considering that reduced cerebral blood flow is found in AD patients [129]. Another small-scale study (n = 30 patients) carried out in Taiwan [128] determined that cilostazol reduced the odds of clinical deterioration of cognitive decline (measured by MMSE and Clinical Dementia Rating Scale Sum of Boxes [CDR-SB] at 12 months after treatment start). Importantly, cilostazol was administered as an add-on therapy to patients already being administered acetylcholinesterase inhibitors.
No adverse events were recorded in the above studies, although, given the fact that cilostazol has been US FDA approved for use in the treatment of peripheral vascular disease [125], adverse events would not be expected. In the case of the ongoing cilostazol MCI trial (NCT02491268), a greater number of patients (n = 200) who were more rigorously screened (physical examination, laboratory tests, MMSE, CDR, Alzheimer's Disease Assessment Scale-cognitive subscale [ADAS-cog], WMS-R, TMT, Free and Cued Selective Reminding Test [FCSRT], Alzheimer’s disease co-operative study ADL scale for mild cognitive impairment [ADCS-MCI-ADL], magnetic resonance imaging [MRI]) have been enrolled to take placebo or cilostazol 50 mg twice daily for 96 weeks, with outcomes measured at 4, 24, 48, 72, and 96 weeks [126]. It will be interesting to see if cilostazol is found to be effective at the conclusion of this study. At the very least, its lack of adverse effects makes it a possible supplement for all AD patients.
Autophagy
Although the UPS plays an important role in protein degradation, autophagy-mediated degradation is the main pathway through which aggregated protein is degraded in non-dividing neuronal cells [130]. Suppression of this system leads to the accumulation of protein aggregates [131, 132]. Autophagy is the process by which large structures, which can include large protein aggregates and organelles, are delivered to lysosomes for degradation. A basic mechanistic overview of autophagy is that an initial double membrane structure, called a phagophore, forms, which elongates and engulfs cytoplasmic material for degradation. Once the phagophore has closed around cargo, it becomes an autophagosome, which can fuse with a lysosome to form an autolysosome, in which the cargo is digested and recycled back into the cytoplasm (reviewed in detail by Bento et al. [133]). Currently, there are understood to be three types of autophagy: chaperone-mediated autophagy, macroautophagy, and microautophagy, each characterized by different methods of cargo delivery to the autophagosome. Although there are several pathways by which autophagy can be initiated or cargo can be delivered, several key regulators of autophagy that can be targeted to induce autophagy have been identified as having therapeutic potential.
Transcription Factor EB Targeting
Transcription factor EB (TFEB) is a regulator of autophagy that promotes the expression of a gene network called the ‘coordinated lysosomal expression and regulation network’ (CLEAR) [134]. Upon starvation or stress, TFEB will translocate from the cytoplasm to the nucleus to upregulate expression of CLEAR, effectively stimulating autophagy to occur and clear the cell of any unnecessary materials. The drug 2-hydroxypropyl-β-cyclodextrin (HPbCD) was recently found to upregulate TFEB and assist in the clearance of aggregated α-synuclein in a cell-based PD model [135]. Although not specifically used in clinical trials of proteinopathies, HPbCD has seen recent use in phase I/II clinical trials in the treatment of Niemann–Pick disease type C, which affects the intracellular transport of cholesterol [136]. In this study, HPbCD was administered intrathecally at dosages of 50–1200 mg once monthly, or 400 mg every 2 weeks. Findings indicated that even doses of 1200 mg were well tolerated. No serious reactions to the drug were reported; however, other expected adverse events were recorded, including hearing loss, post-lumbar headache, and post-injection fatigue, the latter two of which were strictly related to the lumbar injections. Hearing loss was found to be dose-limiting in patients. Disease progression was slowed in treated patients and examination of biomarkers for efficacy related to cell health and cholesterol homeostasis suggested a neuroprotective effect most likely dependent on mobilization of cholesterol within the CNS. The success of this trial marks HPbCD as a possible treatment in proteinopathies as a method to promote degradation of toxic protein aggregates via autophagy.
Rapamycin to Inhibit the Mechanistic Target of Rapamycin
The compound rapamycin (RAPA) was previously shown to interact with and inhibit the mechanistic target of rapamycin (mTOR) [137, 138]. Inhibition of mTOR through caloric restriction leads to upregulation of autophagy in eukaryotes [139, 140], and this is also seen with rapamycin treatment [141]. Indeed, rapamycin treatment has previously been shown to be protective in animal models of HD [142], PD [143], and AD [144, 145], making it a prospective therapeutic for these disorders.
A phase I clinical trial is currently recruiting with the aim of determining the safety and efficacy of treatment of ALS with rapamycin (NCT03359538). Patients taking part in this clinical trial will be split into three different groups—a placebo group, a 1 mg/m2 daily-dose group, and a 2 mg/m2 daily-dose group, where plasma rapamycin levels will be examined so that dosages can be adjusted accordingly. Rapamycin will be administered orally in tablet form. The primary outcome to be measured will be patient stress response in the form of regulatory T-cell (Treg) number. Secondary outcomes will include the number of serious adverse events, and adverse events, the ability of rapamycin to cross the blood–brain barrier, changes in the ALSFRS-revised between placebo and control, and other outcomes. Although this trial is still recruiting, a completed trial of rapamycin on older humans has recently been completed [146], showing good safety outcomes with few adverse effects. The aim of this study was to determine the safety of rapamycin treatment in older humans, where it was found that rapamycin was well tolerated. Rapamycin could potentially be a supplement that all sufferers of neurodegeneration could take in conjunction with other medications, however, further trials would have to be conducted to ensure safety and efficacy. Other possible complications arising from rapamycin use could involve reduced wound healing [147] and immune suppression [148]. Furthermore, rapamycin is considered to have only mildly beneficial effects in some previous clinical trials including diseases such as cancer and diabetes [149].
Nilotinib to Upregulate Autophagy in Neurodegenerative Disease
A promising small compound approach to decreasing the levels of cellular α-synuclein has been found with the FDA-approved tyrosine kinase inhibitor nilotinib, which is currently approved for the treatment of chronic myeloid leukaemia [150]. Nilotinib is capable of inducing autophagy via inhibition of the Abelson tyrosine kinase [151], which has been shown to result in the increased degradation of α-synuclein [152] and amyloid [153] in preclinical models. A small proof-of-concept study involving 12 subjects diagnosed with PD was initially carried out to examine the safety and tolerability of nilotinib at dosages (150–300 mg daily for 24 weeks, oral dose) lower than that used in cancer treatment [154]. Although the study did not have a placebo control group, the reported adverse effects within the cohort was low and was not considered to be related to treatment. These events included urinary tract infections, myocardial infarction, pneumonia, headaches, back pain, coughing, nausea, and irritation. Considering the low level of adverse events and good pharmacokinetic data [154], nilotinib has been moved into a clinical trial with more statistical power (randomized, double-blind, placebo-controlled investigation with 75 patients [NCT02954978]), with dosages similar to those previously reported [154]. Studies investigating nilotinib in AD (NCT02947893) and HD (NCT03764215) are also currently underway.
Balancing Synthesis and Processing of Proteins
Even though the upregulation of protein degradation machinery is showing promise in some cases, the lack of specificity in these systems, and their tight biochemical regulation, makes them difficult to drug. An alternative to increasing degradation is to alter synthesis or processing of specific proteins involved in disease. For example, SOD1-fALS is particularly suitable for knockdown due to the absence of severe adverse effects when the SOD1 gene is knocked out in mouse models [155]. Knockdown of protein expression can be achieved with small molecules, however it is typically achieved using antisense therapy.
Antisense Therapy
In antisense therapy, small DNA or RNA strands called antisense oligonucleotides (ASOs) are administered to patients. The ASO is synthesised to be complementary to the messenger RNA (mRNA) sequence of the protein targeted for knockdown so that it binds with high affinity, either blocking translation or resulting in mRNA degradation (ASO pharmacology is reviewed in Bennett et al. [156]), effectively lowering expression [157]. This strategy was recently employed in the case of SOD1-fALS in a phase I trial for safety and efficacy [158], based on findings in the SOD1-fALS mouse model [159]. Patients with various SOD1-fALS mutations were enrolled for a single intrathecal injection of SOD1 ASO (ISIS 333611) at doses ranging from 0 to 3 mg. Recorded adverse events were mostly related to the invasiveness of lumbar puncture (back pain, nausea, headache, vomiting, falling), although these occurred infrequently. The ASO was deemed to be safe and capable of being used at higher doses in future.
Measurement of the levels of SOD1 in cerebrospinal fluid (CSF) showed small decreases in SOD1 expression for most mutants over the course of 16 months (but modest increases for the A4V and N139K mutants). The authors claim that concentration decreases could be enhanced with more infusions at greater concentrations. Currently, a different ASO against SOD1 (BIIB067; ClinicalTrials.gov identifier: NCT02623699) is undergoing trials to determine the safety, efficacy, and pharmacokinetics of the ASO, with secondary outcomes assessing its effectiveness at reducing SOD1 levels in patients suffering from SOD1-fALS. The study involves 84 SOD1-fALS patients who will receive ascending doses (single or multiple) of ASO or placebo control across a 169-day period. Primary outcomes will include measuring adverse events, and measurements of laboratory, clinical, physical and neurological outcomes.
ASO therapy is also being actively pursued in the case of HD, with a recently finished phase I/II clinical trial (ClinicalTrials.gov identifier: NCT02519036). The trial was carried out following the development and demonstrated success of ASOs in HD mouse models, which showed that ASOs targeting Htt mRNA were effective at delaying the progression of disease [160]. ASOs for HD therapy were further developed in mouse models by designing them to have greater specificity for mutant Htt mRNA [161]. Considering the success in preclinical experiments, an Htt targeting ASO, IONIS-HTTRx, was examined in a phase I/II clinical trial [162]. This study involved 46 patients diagnosed with early HD who were allotted to receive monthly intrathecal injections of the ASO (or placebo control) for 4 months. Following completion, the study reported that no adverse events that occurred were related to the drug, and that the reported adverse events were mild. They also reported that significant reductions in mutant Htt levels in the CSF were observed. There is yet to be a full publication made available for this particular study, however, what has been reported appears to show promise for sufferers of HD.
Finally, there is a current early-phase clinical trial examining the safety and tolerability of an ASO targeting the microtubule associated protein tau (MAPT) gene in patients diagnosed with mild AD (Clinical Trials.gov identifier: NCT03186989). This trial involves the enrolment of 44 participants who will be subject to monthly intrathecal injections of the ASO ‘IONIS MAPTRx’ for 4 months. This particular ASO targets MAPT mRNA to decrease the amount of tau protein irrespective of its isoform. The main outcomes will be the number of adverse events related to the drug, and also the efficacy of reducing the levels of tau protein in the CSF of participants. Considering the preclinical evidence that supports the effectiveness of MAPT targeting ASOs to reduce the levels of tau protein [163], it will be interesting to see the outcomes of this method to treat patients with mild AD. Indeed, if this ASO is shown to have a good safety and tolerability profile, it could be rapidly expanded for use in other tauopathies.
Small Molecules to Decrease Protein Expression
Another attempt to reduce SOD1 levels was performed using the antimalarial drug pyrimethamine, which has been shown to decrease SOD1 expression in cultured human cells [164], was tested in a pilot phase I trial in SOD1-associated fALS, and later in a phase I/II trial to determine safety. The initial pilot trial [165] sought to measure the effectiveness of SOD1 decrease in patients by examining the level of SOD1 expression in leukocytes. Patients were enrolled into an oral dosing regimen beginning at 25 mg/week and increasing over 4 weeks to reach 100 mg/week, which was the maximum dose held for the remaining 6 weeks of the study. The maximum dose was mostly not well tolerated in most patients (nausea and headaches were common), however, the 75 mg dose was well tolerated across patients. Serious adverse effects included one patient developing a severe rash that required corticosteroid therapy, and another patient suffering from a seizure shortly after administration of a pyrimethamine 100 mg dose. Less severe effects included headaches, general malaise, gastrointestinal defects, dizziness and tinnitus. Determination of SOD1 expression and enzymatic activity in patient leukocytes showed a decrease for both measurements after the first dose, with this decrease being maintained throughout the trial.
These results, combined with pyrimethamine being well tolerated at these doses, prompted further study on dose-ranging safety [166], which determined similar adverse effects as the previous study (nausea, headaches, malaise). Measurement of the SOD1 content of patient CSF showed a general decrease in SOD1 levels throughout the trial, however, patient outcomes were difficult to quantify due to the variable survival associated with SOD1-fALS patients carrying different mutations. These studies show that small compound-based knockdown of SOD1, and perhaps other aggregation-prone proteins, may be worth further exploration.
β-Secretase Inhibitors in AD
In regard to therapeutic targeting of protein processing, Aβ is a key target for this approach in AD due to its production being dependent on sequential enzymatic cleavage of the transmembrane APP [167]. There are two pathways of cleavage, one being termed non-amyloidogenic and the other amyloidogenic. The non-amyloidogenic pathway is a result of initial APP cleavage by α-secretase to generate a soluble extracellular fragment (sAPPα), and then cleavage of the membrane domain of the remaining APP by γ-secretase to form the P3 fragment (Aβ1–40) and APP intracellular domain. In the amyloidogenic pathway, the extracellular portion APP is cleaved first by β-secretase 1 (BACE1) to generate extracellular sAPPß, after which the membrane domain is cleaved by γ-secretase to generate the more amyloidogenic Aβ1–42 peptide associated with AD. Owing to the correlation of Aβ plaque load increasing with age [168], it would be reasonable to assume that blocking Aβ1–42 generation through inhibition of β-secretase-driven APP cleavage would be a viable therapeutic pathway.
Several recent trials have been conducted using BACE1 inhibitors, including verubecestat (NCT01739348, NCT01953601) [169], atabecestat (NCT02569398) [170], lanabecestat (NCT02972658, NCT02245737, NCT02783573) [171], and elenbecestat (NCT02956486, NCT03036280, NCT02322021) [172]. Collectively, BACE inhibitor trials have not performed well in the clinic, where many of the trials have been prematurely terminated due to futility or off-target effects (NCT01739348, NCT01953601, NCT02972658, NCT02783573). There are several major challenges associated with BACE inhibition, including the difficulty in developing highly specific compounds that have no off-target effects [173], along with a currently incomplete understanding of the contribution of BACE1 to neurological functioning [174]. Furthermore, recent evidence using mouse models of AD have shown that the time of drug administration is important [175].
Plaque in mice brains was directly imaged during a treatment regimen of the BACE1 inhibitor NB-360, where it was found that the growth of existing plaque was similar to that in no treatment, but the formation of new plaque was reduced with BACE1 inhibition [175]. This suggests that early detection and subsequent administration of BACE1 inhibitors may be a viable therapeutic option. Furthermore, an effective therapeutic approach to AD may involve a combinatorial approach of the careful arrest of production of Aβ monomers and the clearance of aggregated Aβ via immunotherapy or upregulation of clearance mechanisms. A caveat to this approach can be found in the evidence that cognitively normal people can have abundant Aβ-positive plaques [176], implying that modulation of plaque levels may not be important in AD.
Small-Molecule Tau Aggregation Inhibitors
Other than Aβ plaques, another neuropathological hallmark of AD is observed with the aberrant aggregation of the microtubule stabilizing protein tau [12]. In contrast to Aβ plaques, tau aggregates are intracellular and are termed as neurofibrillary tangles. Although the bulk of therapeutics that have been designed to treat AD have targeted Aβ, tau has also been a focus of therapeutic intervention through various approaches, including small molecule aggregation inhibitors. One such inhibitor is the compound methylthioninium (MT) [177, 178], which is used mostly to treat methemoglobinaemia [179] or as a dye to stain tissues [180]. It has been suggested that MT can directly interact with and inhibit tau–tau interactions that are responsible for oligomerization [178], and that MT can also induce autophagy as another mechanism of tau aggregate clearance [181]. Considering MT is safe and has excellent pharmacokinetics in humans [182], and is shown to be effective in both in vitro [177, 183, 184] and in vivo [185–187] preclinical studies, MT and some derivatives have recently been examined in early-phase clinical trials [188].
Administration of MT [188] at dosages of either 69, 138, or 228 mg/day for 102 weeks to a subset of mild and moderate AD patients (n = 170) was found to have very few adverse effects, in line with previously validated pharmacokinetics of the drug [182]. The clinical outcomes were measured at 24 weeks of treatment using the ADAS-cog [189], finding that the 138 mg/day treatment was statistically effective at preventing cognitive decline in mild sufferers, but was found to have no impact on moderate AD patients. Currently, a derivative of MT, TRx2037 (also known as LMTM or LMTX; see below for details), created by TauRx Therapeutics (Singapore) is being assessed in several phase III clinical trials of dementias, including mild AD (NCT01689233) and behavioural variant FTD, for safety and efficacy (NCT02245568, NCT01626378). The study design of these trials improves upon the previously mentioned MT trial, with a longer time period and more primary outcome measures (NCT01689233: 200 mg/day, 78-week time frame, ADAS-cog11, Alzheimer’s Disease Cooperative Study–Activities of Daily Living 23-item [ADCS-ADL23]; NCT02245568: 200 mg/day, 152-week time frame, comparison of serious and non-serious events, change from baseline tests [haematology, serum, weight, respiration, blood pressure, pulse, electrocardiograms]; NCT01626378: 200 mg/day, 52-week time frame, Addenbrooke’s Cognitive Examination–Revised [ACE-R], Functional Assessment Questionnaire [FAQ], brain MRI).
TRx2037, which is also known as leuco-methylthioninium bis[hydromethanesulfonate] (LMTM), was developed as a reduced form of MT to improve its stability, and was found to be effective in vitro and in vivo at reducing tau aggregation and pathology [183, 185]. LMTM was used in a recent phase III trial for safety and efficacy in sufferers of mild to moderate AD [190]. Patients (n = 891) were administered LMTM 75 or 125 mg twice daily (placebo control was LMTM 4 mg for discolouration of urine and faeces) for 15 months. The primary outcomes of the study were ADAS-Cog and ADCS-ADL [191], which were assessed at 65 weeks after the start of treatment. Adverse events were mostly gastrointestinal and urinary in nature, but also included anaemia, folate deficiency, and coughing, although these were considered not serious enough for discontinuation. Adverse events appeared to occur at equal rates in the placebo (84%), 75 mg (84%), and 125 mg (87%) groups. However, none of the treatment groups were found to show a significant improvement in either ADAS-Cog or ADCS-ADL, unlike the previous study of MT [188]. On the other hand, the authors note that in this study, LMTM was not used as a monotherapy, and, as a result of patients potentially taking other AD therapeutics, could confound the analysis owing to variable rates of decline.
Stabilization of Native Conformations of α-Synuclein
The misfolding and aberrant aggregation of α-synuclein is associated with both PD [9] and MSA [13–15]. Similar to Aβ, α-synuclein can be considered as an intrinsically disordered protein that is capable of adopting toxic oligomeric and fibrillar conformations [192]. Considering this, there have been attempts to stabilize the native conformations of the protein (either disordered monomer or native tetramer) to prevent it from adopting toxic conformations, and also to increase the intracellular clearance of aggregated α-synuclein. The small molecule NPT200-11 (developed as a collaboration between Neuropore Therapies and UCB), which is reported to stabilize α-synuclein and prevent aggregation, showed good efficacy in preclinical models [193]. Specifically, it was capable of being orally administered, crossing the blood–brain barrier, while significantly reducing pathology, motor phenotypes, and behavioural phenotypes. This success prompted a phase I trial, which was conducted only on healthy subjects, to determine the maximum safe dose (NCT02606682). This trial was completed in early 2016 and any results have yet to be published or reported.
Antibody-Based Therapies
Another promising method by which toxic misfolded and aggregated protein may be cleared is the use of monoclonal antibodies (mAbs), or manipulation of the immune system to produce antibodies that recognize the toxic species. This type of approach has several advantages owing to the high binding selectivity of antibodies to specific epitopes. Since misfolded and aggregated proteins typically have a substantially altered structure from their native conformation(s), antibodies can be generated that target the misfolded forms through rational design approaches [194–196], or by library screening methods [197]. Administration of an antigen to a patient to promote the generation of antibodies by the immune system is called active immunization, whereas administration of a recombinantly produced mAb to a patient is termed passive immunization. Passive immunization has the advantage of the produced antibody being generated as an mAb, meaning the selectivity of binding is significantly increased. As a result of the increased binding selectivity, typically less off-target effects are observed with this approach [197].
Immunotherapies for Amyloid-β in AD
Active immunization has shown some success in AD mouse models [198–200], however, an early active immunotherapy using Aβ1–42 as an immunogen resulted in a small proportion of patients suffering from meningoencephalitis [201, 202]. Regardless, patients from this trial who did not develop meningoencephalitis were found to have significantly improved cognitive capabilities compared with control patients in a long-term follow-up [203]. More recent immunopathological investigation of some of the immunized patients showed that plaque removal was persistent for 14 years after immunization [204]. A more recent clinical trial is underway involving a vaccine called ACI-24 that is composed of the last 15 residues of Aβ1–42 modified to insert into liposomes and present a beta-sheet conformation [205]. Preclinical trials in transgenic APPxPS-1 mice showed good immunogenicity, improved cognitive capabilities, reduced Aβ pathology, and showed no signs of increased inflammation [206]. Phase I/II trials of the vaccine were carried out involving 198 patients with mild-moderate AD, with a 340–460 mg dose of vaccine injected subcutaneously (EU clinical trials number 2008-006257-40). More recently, ACI-24 is being examined in a phase I clinical trial aimed at determining safety and tolerability in patients with Down syndrome (NCT02738450). Patients will be subcutaneously injected with ACI-24 seven times over 12 months at either high or low doses, where follow-up will occur over 12 months after the final injection. Primary outcomes will include monitoring of adverse events and measurement of antibody titres, while secondary outcomes will include measures of amyloid using positron emission tomography imaging and CSF extraction.
Passive immunotherapies of mAbs have been undergoing late-stage clinical trials, where the administered mAbs have been raised against specific epitopes of Aβ (Table 2). Some trials are ongoing (gantenerumab—NCT02051608), however, those that have results reported for phase III and II clinical trials (aducanumab—NCT02484547; bapineuzumab—NCT00575055 and NCT00574132; crenezumab—NCT01343966; ponezumab—NCT00722046; solanezumab—NCT00905372 and NCT00904683) showed no significant effects on the cognitive decline in patients, as measured using ADAS-cog. These trials mostly showed minor amyloid-related imaging abnormalities (ARIA) in contrast to the previously mentioned active immunization (Table 2). The inability of these mAbs to improve outcomes for patients with mild–moderate AD has made some call into question the amyloid cascade hypothesis [207–210], suggesting that other targets may lead to more beneficial outcomes in clinical trials.
Table 2.
Clinical trials of passive immunotherapies targeted at Aβ
| mAb | Epitope | Binding selectivity | Phase | Patients | Dosage | Outcomes | ClinicalTrials.gov identifier | References |
|---|---|---|---|---|---|---|---|---|
| Aducanumab | aa3–7 |
Mon: × Olig: ✔ Fib: ✔ |
III |
1605 Mild–moderate AD |
Low* High* IV |
Terminated as of 19 March 2019 due to futility | NCT02484547 | [217] |
| BAN2401 | Protofib |
Mon: – Olig: – Fib: – |
II |
800 Mild–moderate AD |
2.5, 5, 10 mg/kg biweekly or monthly IV |
Effective in phase II testing, as announced at the Alzheimers International Conference 2018 | NCT02094729 | [218] |
| Bapineuzumab | aa1–5 |
Mon: ✔ Olig: ✔ Fib: ✔ |
III |
2452 (1121 ApoE4+) (1331 ApoE4-) Mild–moderate AD |
0.5, 1.0 mg/kg every 13 weeks IV |
ADAS-cog11: No difference DAD score: No difference ARIA-E: Observed in some patients |
[219] | |
| Crenezumab | aa13–24 |
Mon: ✔ Olig: ✔ Fib: ✔ |
II |
431 Mild–moderate AD |
300 mg SC injection every 2 weeks, or 15 mg/kg IV every month |
ADAS-cog12: No difference CDR-SB: No difference ADCS-ADL: No difference ARIA-E: One case |
NCT01343966 | [220] |
| Gantenerumab | aa3–12, 18–27 |
Mon: ✔ Olig: ✔ Fib: ✔ |
III |
389 Mild–moderate AD |
Monthly SC injection | (Ongoing) | NCT02051608 | [221, 222] |
| Ponezumab | aa30–40 |
Mon: ✔ Olig: × Fib: × |
II |
198 Mild–moderate AD |
0.1, 0.5, 1 mg/kg, or 3, 8.5 mg/kg IV injection every 50 days |
ADAS-cog: No difference DAD: No difference ARIA-E: 13.8% drug-treated |
NCT00722046 | [223] |
| Solanezumab | aa16–26 |
Mon: ✔ Olig: × Fib: × |
III |
2040 Mild–moderate AD |
400 mg IV injection Monthly |
ADAS-cog11: No difference ADAS-cog14: No difference ARIA-E: 4.9% for solanezumab-treated patients |
[224] |
Aβ amyloid-β, AD Alzheimer’s disease, ADAS-cog Alzheimer’s Disease Assessment Scale–cognitive subscale, ADCS-ADL Alzheimer’s Disease Cooperative Study-Activities of Daily Living, ApoE apolipoprotein E, ARIA amyloid-related imaging abnormalities, CDR-SB Clinical Dementia Rating Scale Sum of Boxes, DAD Disability Assessment for Dementia, Fib fibrils, IV intravenous, Mon monomers, Olig oligomers, SC subcutaneous, ✔ positive binding to this species, × negative binding to this species
*No specific dosages are listed for this clinical trial
Others have argued that a consideration for AD (and neurodegeneration in general) is the time at which therapeutic intervention should occur, as amyloid pathology and changes to brain function are measurable years prior to cognitive decline [211, 212], and there are suggestions that amyloid burden can predict decline [213]. A potential key drawback to the above mAbs is their non-selectivity for the toxic Aβ oligomers. The non-selective nature of binding is likely to result in target distraction, leading to binding with functional Aβ monomer that can reduce the effective concentration of the therapeutic, or binding with Aβ plaques, which can result in ARIA-related dose limitations. Regardless, new clinical trials are currently being carried out with solanezumab where the aim is to test if the mAb can be used to prevent or reduce cognitive decline in individuals who are positive for Aβ plaques, as measured by brain scans, but do not show clinical presentation of the disease (NCT02008357). Although these mAbs were ineffective at slowing decline, they were all well tolerated and exhibited low levels of ARIA (Table 2). A more rational approach, targeting not just the amino acid sequence but also structural conformation, could reduce the ARIA observed even further and make mAbs targeting toxic Aβ even more potent [194, 214]. More recently, there has been a focus on targeting the soluble oligomeric forms of Aβ [194] as it is thought to be the major toxic species within the amyloid cascade hypothesis [215, 216]. Notably, Aβ oligomer-specific antibodies have not yet been tested in patients.
Immunotherapies for Tau in AD
In AD, tau pathology has been observed to be more strongly correlated with clinical decline than Aβ pathology. Immunotherapies targeting tau have thus been intensively researched, focusing on pathologic tau which, as mentioned previously, is abnormally phosphorylated [12]. Similar to Aβ, tau immunotherapies have included both active and passive immunization. Active immunization clinical trials targeting tau include ACI-35 [205] and AADvac-1 [225]. ACI-35 is a vaccine, similar to ACI-24, consisting of several fragments of the tau peptide sequence encompassing phosphorylated serine residues 396 and 404 incorporated into a liposomal delivery system [205]. Administration of this vaccine to transgenic mice expressing mutant human tau P301L, found that treated mice had reduced levels of tau aggregates and improved survival [226]. A phase I trial of ACI-35 was carried out to assess tolerance and efficacy (ISRCTN13033912), where patients were injected with low, medium, or high doses two to five times over 6 months. The primary outcomes included monitoring of adverse events and measurement of antibody titre in sera, while secondary outcomes included measurements of biomarkers and cognitive decline. No publication has resulted from this trial, although it finished in 2017.
Another active immunotherapy for tau currently in clinical trials is AADvac-1, which is a KLH (keyhole limpet haemocyanin)-conjugated peptide composed of amino acids 294–395 [225, 227]. The aim was to target the region of tau responsible for aberrant tau–tau interactions rather than phosphorylation sites. Injection of the vaccine into a rat AD model showed that treated animals had reduced tau oligomers, neurofibrillary tangles, and phosphorylation, while also having improved clinical phenotypes [225]. This success led to AADvac-1 being examined in a phase I clinical trial to assess the immunogenicity and safety of the vaccine in humans [228] (NCT01850238). The trial involved 30 patients who were injected with AADvac-1 40 μg/mL once per month for 6 months, where the primary outcomes were measurements of any adverse events. The vaccine was well tolerated, where the most common adverse event was injection site reactions (observed in 53% participants), which were only minor. The vaccine elicited no aberrant immune response or microhaemorrhages akin to previously discussed Aβ vaccines.
A follow-up phase I open-label study was also performed on participants in this trial 72 weeks after conclusion (NCT02031198) [229]. In this study, 26 of the previous participants were enrolled and were injected similarly to the previous trial, except that injections occurred three times a month. Again, the most common adverse event observed was injection site reaction (50% of participants), and no aberrant immune responses were reported, except for microhaemorrhages observed in one patient. Cognitive decline, as measured by baseline ADAS-cog11 value, was shown to be significantly reduced in treated patients compared with placebo control. This safety and tolerance profile prompted AADvac-1 to move into a phase II clinical trial that is currently ongoing (NCT02579252). This trial has enrolled 208 participants with mild AD who will be monitored over a 24-month period where patients will receive a single dose of the vaccine per month for 6 months, after which five booster shots will be administered over a 15-month period.
There are currently eight ongoing clinical trials involving passive immunotherapies targeting tau, with several others in late-stage preclinical development [230]. These clinical trials have been reviewed in detail by Congdon and Sigurdsson [231]. BMS-986168 is an antibody targeting residues 9–18 near the N-terminus of tau; in animal models this antibody reduces levels of tau in the interstitial space and soluble Aβ1–40 in the brain [232]. Several clinical trials involving this antibody are either completed or are underway (NCT02460094, NCT02294851, NCT03068468, NCT02658916), including a phase II trial to study the antibody’s clinical efficacy in 400 patients with progressive supranuclear palsy (PSP; NCT03352557). C2N-8E12 recognizes amino acids 25–30 of the tau protein. In cell culture, this antibody prevented pathological tau seeding caused by exogenous tau aggregates [233].
Two phase II trials are expected to be completed in 2019 and 2020; one involves 330 patients with PSP and the other involves 400 patients with early-stage AD. The antibody RO7105705 likely targets pSer409 on tau, although the epitope has not been disclosed [234]. RO7105705 is currently being assessed for safety and tolerability. The antibody LY3303560 possibly targets a conformational epitope on tau, although this information has also not been officially disclosed. Phase I trials to study safety and pharmacokinetics have recently been completed for MCI, and are expected to finish in 2020 for AD. UCB0107 and JNJ-63733657 are antibodies designed to prevent the seeding and spreading of pathological tau. UCB0107 binds to amino acids 235–246 in the proline-rich region of tau, and JNJ-63733657 likely binds to the mid-region of tau [235]. Antibodies targeting pathological hyperphosphorylated tau have also recently entered clinical development for the treatment of AD [236]. Tau immunotherapy continues to develop rapidly, with several new trials likely to start in the near future.
Immunotherapies for α-Synuclein in PD and Multiple System Atrophy
Immunotherapies targeting the protein misfolding and aggregation component of PD and MSA are also currently being pursued, with a focus on the primary aggregating protein in these syndromes, i.e. α-synuclein. In comparison to the previously mentioned tau and Aβ therapies (Sects. 6.1 and 6.2, respectively), the α-synuclein targeting therapies are less developed. Two active immunotherapies (PD01A—NCT01568099, NCT01885494, NCT02216188, NCT02618941; and PD03A—NCT02270489, NCT02267434) are currently being investigated as part of the SYMPATH initiative. Immunization of multiple transgenic α-synuclein mouse models with either PD01A or PD03A epitopes resulted in decreases in pathology and motor deficits [237, 238]. In regard to clinical trials, the SYMPATH initiative has reported (no scientific publications currently available) that the PD01A and PD03A vaccinations were well tolerated. Interestingly, the epitopes were designed to mimic specific parts of α-synuclein in such a way as to not elicit humoral or T-cell immune responses. This technology was initially validated in AD patients [239], finding minimal adverse effects and, most importantly, no unwanted immune response. Currently, the results from the clinical trials of the PD01A and PD03A vaccinations are unpublished, however there are plans to make this information available to the wider scientific community.
Several passive immunotherapies are being produced for the synucleopathies, including BIIB054 (NCT02459886, NCT03716570, NCT03318523) [240], prasinezumab (NCT02157714, NCT02095171, NCT03100149) [241], BAN0805 (BioArctic, recently approved by the FDA for phase I trials as of 2019), and MEDI1341 (NCT03272165). The purpose of these immunotherapies is considered to be the targeting of extracellular α-synuclein to prevent the prion-like cell-to-cell transfer of misfolded or aggregated species [242–244]. Of these passive immunotherapies, BIIB054 and prasinezumab have both passed early-phase trials for safety tolerability. BIIB054 was intravenously administered at a single dose of 15 or 45 mg/kg to 18 patients suffering from PD. Encouragingly, most adverse events recorded were not associated with drug administration [245]. Likewise, prasinezumab was found to elicit no severe adverse events relating to drug administration [241]. In this study, prasinezumab was intravenously administered to six cohorts of patients in ascending doses (0.3, 1.0, 3.0, 10, 30, or 60 mg/kg, or placebo). More severe events were recorded for the patients receiving prasinezumab comparative to placebo control, where these events included mostly bowel-related problems (constipation, diarrhoea) and other effects related to diagnostic tests (post-lumbar puncture syndrome). Overall, only 13% of patients reported treatment-related adverse events, therefore prasinezumab was considered to be safe and well tolerated [241].
The current early-phase trials of passive immunotherapies against α-synuclein, including BAN0805 and MEDI1341, have very little publicly available data. BAN0805 is suggested to target protofibrillar and oligomeric α-synuclein species, and MEDI1341 is suggested to have a significantly lower effector function than other similar antibodies; however, the safety and tolerability of these two promising candidates remain to determined [246].
Translation of Preclinical Trials to the Clinic
A major difficulty for the development of effective therapeutics for proteopathic NDs is the disconnect between preclinical success and clinical success. Treatments first have to show efficacy and safety in animal models of disease prior to being utilized in a clinical trial, and the current success rate of clinical trials aiming to treat ND has been very low. The reasons for this difficulty stem from many factors, including a lack of understanding of disease aetiology, difficulty in diagnosing disease onset, the genetic and lifestyle diversity of the populations suffering from ND, and the heterogeneity of ND pathology.
Currently, our understanding of neurodegeneration disease aetiology is incomplete. Although this review discusses the toxic nature of protein misfolding and aggregation, it must be stated that it is still unknown whether aggregation is a symptom, whether it is causative, or even whether it is a protective measure in proteopathic ND. In addition to protein aggregation, inflammation is another hallmark pathology of ND that is potentially causative [247]. It is highly likely that ND is a result of many different molecular events and that each will need to be alleviated if disease is to be treated. A single-target approach with therapeutics may not be sufficient for successful treatment, and administration of multiple therapeutics could potentially offer better prospects, as has been the de facto standard in cancer therapy [248].
Biomarkers of ND are notoriously difficult to identify owing to the invasiveness of procedures to procure them, often requiring samples of CSF. The development of sensitive and easy-to-procure biomarkers is not only necessary for effectively tracking decline and changes to patients in clinical trials but also for earlier diagnoses. However, sensitivity of immunological (including blood analytes) and neuroimaging techniques has improved significantly over the last decade [249, 250]. The translation of improved imaging and immunological measurements of disease pathology, in combination with a better understanding of disease aetiology, will provide a useful means by which to detect and diagnose those people at risk of developing ND, which can lead to effective preventative measures being taken. Many of the previously discarded therapeutics could potentially have a positive effect if administered prior to the typically detected events that signal neurodegeneration, such as cognitive decline, which occur well after damage has occurred to the central nervous system.
Finally, the genetic and pathological heterogeneity of patients is a significant consideration for the success of clinical trials, as a therapeutic effective for one class of patient may not be effective in others. Therefore, each individual case should be monitored carefully for genetic background and important markers of pathology [251]. The majority of ND cases are sporadic, which can be significantly influenced by genetic risk factors, as well as environmental factors. It is therefore critical to understand each individual’s molecular and physiological signatures. In some cases, it could be possible to stratify or group patients on the basis of their molecular signature (RNA/DNA/protein expression profiles) so that cases in which therapeutics would be effective can be determined and not masked by large sample sizes of a diverse patient population. The rapid increase in technology, and medical interest in patient-derived stem cell models, would prove valuable as an initial step to examine not only drug response but also patient pathology diversity [252].
Conclusions
It is abundantly clear that something must be done to alleviate the growing burden of neurological disorders worldwide. As it stands, the success rate of clinical trials in dementia is bewilderingly low, which cannot be allowed to persist. While attempts to specifically target toxic protein species are not new, methods to target and upregulate global protein homeostasis in cells are only just now moving into the clinic. The clinical trials discussed within this review hold promise to help treat and/or cure several NDs, although it remains to be seen how effective they will be on a wide scale. Future clinical trials into proteopathic NDs should include greater measures to group patients on the basis of their molecular pathology signatures. As the worldwide population ages, the need for effective means of combating age-related proteopathies is becoming increasingly urgent.
Author Contributions
LM wrote the initial manuscript. SSP and NRC revised the manuscript to its final form.
Compliance with Ethical Standards
Funding
The authors funded the open access fee. SSP acknowledges the Canadian Institutes of Health Research Transitional Operating Grant 2682, and the Alberta Prion Research Institute, Research Team Program Grant PTM13007. NRC acknowledges the Canadian Consortium for Neurodegeneration in Aging (CCNA) and Brain Canada.
Conflict of interest
Luke McAlary declares no conflicts of interest. Steven S. Plotkin is the Chief Physics Officer, and Neil R. Cashman is the founder and Chief Scientific Officer, of ProMIS Neurosciences.
Contributor Information
Luke McAlary, Email: lmcalary@phas.ubc.ca.
Steven S. Plotkin, Email: steve@phas.ubc.ca
Neil R. Cashman, Email: neil.cashman@vch.ca
References
- 1.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75.e2. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Bredesen DE, Rao RV, Mehlen P. Cell death in the nervous system. Nature. 2006;443(7113):796–802. doi: 10.1038/nature05293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kübler A, Winter S, Ludolph AC, Hautzinger M, Birbaumer N. Severity of depressive symptoms and quality of life in patients with amyotrophic lateral sclerosis. Neurorehabil Neural Repair. 2005;19(3):182–193. doi: 10.1177/1545968305276583. [DOI] [PubMed] [Google Scholar]
- 4.Ready RE, Mathews M, Leserman A, Paulsen JS. Patient and caregiver quality of life in Huntington’s disease. Mov Disord. 2008;23(5):721–726. doi: 10.1002/mds.21920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brod M, Stewart AL, Sands L, Walton P. Conceptualization and measurement of quality of life in dementia: the dementia quality of life instrument (DQoL) Gerontologist. 1999;39(1):25–36. doi: 10.1093/geront/39.1.25. [DOI] [PubMed] [Google Scholar]
- 6.Mayeux R. Epidemiology of neurodegeneration. Annu Rev Neurosci. 2003;26(1):81–104. doi: 10.1146/annurev.neuro.26.043002.094919. [DOI] [PubMed] [Google Scholar]
- 7.Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81(4):479–484. doi: 10.1002/ana.24897. [DOI] [PubMed] [Google Scholar]
- 8.Goedert M, Clavaguera F, Tolnay M. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci. 2010;33(7):317–325. doi: 10.1016/j.tins.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. α-Synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 10.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 11.Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120(3):885–890. doi: 10.1016/S0006-291X(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 12.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tu P-H, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, et al. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble α-synuclein. Ann Neurol. 1998;44(3):415–422. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- 14.Wakabayashi K, Yoshimoto M, Tsuji S, Takahashi H. α-Synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci Lett. 1998;249(2–3):180–182. doi: 10.1016/S0304-3940(98)00407-8. [DOI] [PubMed] [Google Scholar]
- 15.Spillantini MG, Anthony Crowther R, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous α-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett. 1998;251(3):205–208. doi: 10.1016/S0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- 16.DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277(5334):1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- 17.Jellinger KA. Interaction between pathogenic proteins in neurodegenerative disorders. J Cell Mol Med. 2012;16(6):1166–1183. doi: 10.1111/j.1582-4934.2011.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobson CM. Protein folding and misfolding. Nature. 2003;426(6968):884–890. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- 19.Hartl FU, Hayer-Hartl M. Converging concepts of protein folding in vitro and in vivo. Nat Struct Mol Biol. 2009;16(6):574–581. doi: 10.1038/nsmb.1591. [DOI] [PubMed] [Google Scholar]
- 20.Selkoe DJ. Folding proteins in fatal ways. Nature. 2003;426(6968):900–904. doi: 10.1038/nature02264. [DOI] [PubMed] [Google Scholar]
- 21.Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10(7):S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 22.Bertram L. The genetic epidemiology of neurodegenerative disease. J Clin Invest. 2005;115(6):1449–1457. doi: 10.1172/JCI24761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown RC, Lockwood AH, Sonawane BR. Neurodegenerative diseases: an overview of environmental risk factors. Environ Health Perspect. 2005;113(9):1250–1256. doi: 10.1289/ehp.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat Rev Mol Cell Biol. 2007;8(2):101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 25.Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443(7113):780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- 26.Koo EH, Lansbury PT, Kelly JW. Amyloid diseases: abnormal protein aggregation in neurodegeneration. Proc Natl Acad Sci USA. 1999;96(18):9989–9990. doi: 10.1073/pnas.96.18.9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 28.Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120(4):545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Dobson CM. Protein misfolding, evolution and disease. Trends Biochem Sci. 1999;24(9):329–332. doi: 10.1016/S0968-0004(99)01445-0. [DOI] [PubMed] [Google Scholar]
- 30.Goldschmidt L, Teng PK, Riek R, Eisenberg D. Identifying the amylome, proteins capable of forming amyloid-like fibrils. Proc Natl Acad Sci USA. 2010;107(8):3487–3492. doi: 10.1073/pnas.0915166107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75(1):333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 32.Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KB. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci USA. 1987;84(23):8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci USA. 1998;95(11):6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prusiner SB, McKinley MP, Bowman KA, Bolton DC, Bendheim PE, Groth DF, et al. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983;35(2 Pt 1):349–358. doi: 10.1016/0092-8674(83)90168-X. [DOI] [PubMed] [Google Scholar]
- 35.Scherzinger E, Sittler A, Schweiger K, Heiser V, Lurz R, Hasenbank R, et al. Self-assembly of polyglutamine-containing huntingtin fragments into amyloid-like fibrils: implications for Huntington’s disease pathology. Proc Natl Acad Sci USA. 1999;96(8):4604–4609. doi: 10.1073/pnas.96.8.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costa PP, Figueira AS, Bravo FR. Amyloid fibril protein related to prealbumin in familial amyloidotic polyneuropathy. Proc Natl Acad Sci USA. 1978;75(9):4499–4503. doi: 10.1073/pnas.75.9.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McParland VJ, Kad NM, Kalverda AP, Brown A, Kirwin-Jones P, Hunter MG, et al. Partially unfolded states of β2-microglobulin and amyloid formation in vitro. Biochemistry. 2000;39(30):8735–8746. doi: 10.1021/bi000276j. [DOI] [PubMed] [Google Scholar]
- 38.Greenberg SA. Theories of the pathogenesis of inclusion body myositis. Curr Rheumatol Rep. 2010;12(3):221–228. doi: 10.1007/s11926-010-0102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moreau KL, King JA. Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends Mol Med. 2012;18(5):273–282. doi: 10.1016/j.molmed.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gallo G, Goñi F, Boctor F, Vidal R, Kumar A, Stevens FJ, et al. Light chain cardiomyopathy. Structural analysis of the light chain tissue deposits. Am J Pathol. 1996;148(5):1397–1406. [PMC free article] [PubMed] [Google Scholar]
- 41.Yang T, Li S, Xu H, Walsh DM, Selkoe DJ. Large soluble oligomers of amyloid β-protein from alzheimer brain are far less neuroactive than the smaller oligomers to which they dissociate. J Neurosci. 2017;37(1):152–163. doi: 10.1523/JNEUROSCI.1698-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caughey B, Baron GS, Chesebro B, Jeffrey M. Getting a grip on prions: oligomers, amyloids, and pathological membrane interactions. Annu Rev Biochem. 2009;78:177–204. doi: 10.1146/annurev.biochem.78.082907.145410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kundra R, Ciryam P, Morimoto RI, Dobson CM, Vendruscolo M. Protein homeostasis of a metastable subproteome associated with Alzheimer’s disease. Proc Natl Acad Sci USA. 2017;114(28):E5703–E5711. doi: 10.1073/pnas.1618417114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ciryam P, Lambert-Smith IA, Bean DM, Freer R, Cid F, Tartaglia GG, et al. Spinal motor neuron protein supersaturation patterns are associated with inclusion body formation in ALS. Proc Natl Acad Sci USA. 2017;114(20):E3935–E3943. doi: 10.1073/pnas.1613854114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaganovich D, Kopito R, Frydman J. Misfolded proteins partition between two distinct quality control compartments. Nature. 2008;454(7208):1088–1095. doi: 10.1038/nature07195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431(7010):805–810. doi: 10.1038/nature02998. [DOI] [PubMed] [Google Scholar]
- 47.Olzscha H, Schermann SM, Woerner AC, Pinkert S, Hecht MH, Tartaglia GG, et al. Amyloid-like aggregates sequester numerous metastable proteins with essential cellular functions. Cell. 2011;144(1):67–78. doi: 10.1016/j.cell.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 48.Woerner AC, Frottin F, Hornburg D, Feng LR, Meissner F, Patra M, et al. Cytoplasmic protein aggregates interfere with nucleocytoplasmic transport of protein and RNA. Science. 2016;351(6269):173–176. doi: 10.1126/science.aad2033. [DOI] [PubMed] [Google Scholar]
- 49.Yerbury JJ, Ooi L, Dillin A, Saunders DN, Hatters DM, Beart PM, et al. Walking the tightrope: proteostasis and neurodegenerative disease. J Neurochem. 2016;137(4):489–505. doi: 10.1111/jnc.13575. [DOI] [PubMed] [Google Scholar]
- 50.Deng H-X, Chen W, Hong S-T, Boycott KM, Gorrie GH, Siddique N, et al. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature. 2011;477(7363):211–215. doi: 10.1038/nature10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392(6676):605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 52.Balchin D, Hayer-Hartl M, Hartl FU. In vivo aspects of protein folding and quality control. Science. 2016;353(6294):aac4354. doi: 10.1126/science.aac4354. [DOI] [PubMed] [Google Scholar]
- 53.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426(6968):895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 54.Hartl FU, Ulrich Hartl F, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475(7356):324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 55.Tissières A, Mitchell HK, Tracy UM. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974;84(3):389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- 56.Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268(3):1517–1520. [PubMed] [Google Scholar]
- 57.Haslbeck M, Vierling E. A first line of stress defense: small heat shock proteins and their function in protein homeostasis. J Mol Biol. 2015;427(7):1537–1548. doi: 10.1016/j.jmb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ungelenk S, Moayed F, Ho C-T, Grousl T, Scharf A, Mashaghi A, et al. Small heat shock proteins sequester misfolding proteins in near-native conformation for cellular protection and efficient refolding. Nat Commun. 2016;7:13673. doi: 10.1038/ncomms13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Binger KJ, Ecroyd H, Yang S, Carver JA, Howlett GJ, Griffin MDW. Avoiding the oligomeric state: αB-crystallin inhibits fragmentation and induces dissociation of apolipoprotein C-II amyloid fibrils. FASEB J. 2013;27(3):1214–1222. doi: 10.1096/fj.12-220657. [DOI] [PubMed] [Google Scholar]
- 60.Leak RK. Heat shock proteins in neurodegenerative disorders and aging. J Cell Commun Signal. 2014;8(4):293–310. doi: 10.1007/s12079-014-0243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neef DW, Jaeger AM, Thiele DJ. Heat shock transcription factor 1 as a therapeutic target in neurodegenerative diseases. Nat Rev Drug Discov. 2011;10(12):930–944. doi: 10.1038/nrd3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6(1):11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- 63.Pratt WB, Gestwicki JE, Osawa Y, Lieberman AP. Targeting Hsp90/Hsp70-based protein quality control for treatment of adult onset neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 2015;55:353–371. doi: 10.1146/annurev-pharmtox-010814-124332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bose S, Cho J. Targeting chaperones, heat shock factor-1, and unfolded protein response: Promising therapeutic approaches for neurodegenerative disorders. Ageing Res Rev. 2017;35:155–175. doi: 10.1016/j.arr.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 65.Benatar M, Wuu J, Andersen PM, Atassi N, David W, Cudkowicz M, et al. Randomized, double-blind, placebo-controlled trial of arimoclomol in rapidly progressive ALS. Neurology. 2018;90(7):e565–e574. doi: 10.1212/WNL.0000000000004960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 67.Hargitai J, Lewis H, Boros I, Rácz TM, Fiser A, Kurucz I, et al. Bimoclomol, a heat shock protein co-inducer, acts by the prolonged activation of heat shock factor-1. Biochem Biophys Res Commun. 2003;307(3):689–695. doi: 10.1016/S0006-291X(03)01254-3. [DOI] [PubMed] [Google Scholar]
- 68.San Gil R, Ooi L, Yerbury JJ, Ecroyd H. The heat shock response in neurons and astroglia and its role in neurodegenerative diseases. Mol Neurodegener. 2017;12(1):65. doi: 10.1186/s13024-017-0208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang J, Oza J, Bridges K, Chen KY, Liu AYC. Neural differentiation and the attenuated heat shock response. Brain Res. 2008;1203:39–50. doi: 10.1016/j.brainres.2008.01.082. [DOI] [PubMed] [Google Scholar]
- 70.Nishimura RN, Dwyer BE, Clegg K, Cole R, de Vellis J. Comparison of the heat shock response in cultured cortical neurons and astrocytes. Mol Brain Res. 1991;9(1–2):39–45. doi: 10.1016/0169-328X(91)90128-K. [DOI] [PubMed] [Google Scholar]
- 71.Oza J, Yang J, Chen KY, Liu AYC. Changes in the regulation of heat shock gene expression in neuronal cell differentiation. Cell Stress Chaperones. 2008;13(1):73–84. doi: 10.1007/s12192-008-0013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pavlik A, Aneja IS. Cerebral neurons and glial cell types inducing heat shock protein Hsp70 following heat stress in the rat. Prog Brain Res. 2007;162:417–431. doi: 10.1016/S0079-6123(06)62020-7. [DOI] [PubMed] [Google Scholar]
- 73.Nishimura RN, Dwyer BE. Evidence for different mechanisms of induction of HSP70i: a comparison of cultured rat cortical neurons with astrocytes. Brain Res Mol Brain Res. 1996;36(2):227–239. doi: 10.1016/0169-328X(95)00261-P. [DOI] [PubMed] [Google Scholar]
- 74.Manzerra P, Rush SJ, Brown IR. Tissue-specific differences in heat shock protein hsc70 and hsp70 in the control and hyperthermic rabbit. J Cell Physiol. 1997;170(2):130–137. doi: 10.1002/(SICI)1097-4652(199702)170:2<130::AID-JCP4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 75.Manzerra P, Brown IR. Expression of heat shock genes (hsp70) in the rabbit spinal cord: localization of constitutive and hyperthermia-inducible mRNA species. J Neurosci Res. 1992;31(4):606–615. doi: 10.1002/jnr.490310404. [DOI] [PubMed] [Google Scholar]
- 76.Krueger AM, Armstrong JN, Plumier J, Robertson HA, Currie RW. Cell specific expression of Hsp70 in neurons and glia of the rat hippocampus after hyperthermia and kainic acid-induced seizure activity. Brain Res Mol Brain Res. 1999;71(2):265–278. doi: 10.1016/S0169-328X(99)00198-9. [DOI] [PubMed] [Google Scholar]
- 77.Kieran D, Kalmar B, Dick JRT, Riddoch-Contreras J, Burnstock G, Greensmith L. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat Med. 2004;10(4):402–405. doi: 10.1038/nm1021. [DOI] [PubMed] [Google Scholar]
- 78.Kalmar B, Novoselov S, Gray A, Cheetham ME, Margulis B, Greensmith L. Late stage treatment with arimoclomol delays disease progression and prevents protein aggregation in the SOD1 mouse model of ALS. J Neurochem. 2008;107(2):339–350. doi: 10.1111/j.1471-4159.2008.05595.x. [DOI] [PubMed] [Google Scholar]
- 79.Saeed M, Yang Y, Deng HX, Hung WY, Siddique N, Dellefave L, et al. Age and founder effect of SOD1 A4V mutation causing ALS. Neurology. 2009;72(19):1634–1639. doi: 10.1212/01.wnl.0000343509.76828.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169(1–2):13–21. doi: 10.1016/S0022-510X(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 81.Vandevoorde J, Verbanck S, Schuermans D, Kartounian J, Vincken W. FEV1/FEV6 and FEV6 as an alternative for FEV1/FVC and FVC in the spirometric detection of airway obstruction and restriction. Chest. 2005;127(5):1560–1564. doi: 10.1378/chest.127.5.1560. [DOI] [PubMed] [Google Scholar]
- 82.Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185(2):232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 83.Ahmed M, Machado PM, Miller A, Spicer C, Herbelin L, He J, et al. Targeting protein homeostasis in sporadic inclusion body myositis. Sci Transl Med. 2016;8(331):331ra41. doi: 10.1126/scitranslmed.aad4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dimachkie MM, Barohn RJ. Inclusion body myositis. Curr Neurol Neurosci Rep. 2013;13(1):321. doi: 10.1007/s11910-012-0321-4. [DOI] [PubMed] [Google Scholar]
- 85.Amato AA, Gronseth GS, Jackson CE, Wolfe GI, Katz JS, Bryan WW, et al. Inclusion body myositis: clinical and pathological boundaries. Ann Neurol. 1996;40(4):581–586. doi: 10.1002/ana.410400407. [DOI] [PubMed] [Google Scholar]
- 86.Benveniste O, Guiguet M, Freebody J, Dubourg O, Squier W, Maisonobe T, et al. Long-term observational study of sporadic inclusion body myositis. Brain. 2011;134(Pt 11):3176–3184. doi: 10.1093/brain/awr213. [DOI] [PubMed] [Google Scholar]
- 87.Needham M, Mastaglia FL. Inclusion body myositis: current pathogenetic concepts and diagnostic and therapeutic approaches. Lancet Neurol. 2007;6(7):620–631. doi: 10.1016/S1474-4422(07)70171-0. [DOI] [PubMed] [Google Scholar]
- 88.Nogalska A, Terracciano C, D’Agostino C, King Engel W, Askanas V. p62/SQSTM1 is overexpressed and prominently accumulated in inclusions of sporadic inclusion-body myositis muscle fibers, and can help differentiating it from polymyositis and dermatomyositis. Acta Neuropathol. 2009;118(3):407–413. doi: 10.1007/s00401-009-0564-6. [DOI] [PubMed] [Google Scholar]
- 89.Askanas V, Engel WK, Bilak M, Alvarez RB, Selkoe DJ. Twisted tubulofilaments of inclusion body myositis muscle resemble paired helical filaments of Alzheimer brain and contain hyperphosphorylated tau. Am J Pathol. 1994;144(1):177–187. [PMC free article] [PubMed] [Google Scholar]
- 90.Askanas V, Engel WK. Inclusion-body myositis: a myodegenerative conformational disorder associated with Abeta, protein misfolding, and proteasome inhibition. Neurology. 2006;66(2 Suppl 1):S39–S48. doi: 10.1212/01.wnl.0000192128.13875.1e. [DOI] [PubMed] [Google Scholar]
- 91.Salajegheh M, Pinkus JL, Taylor JP, Amato AA, Nazareno R, Baloh RH, et al. Sarcoplasmic redistribution of nuclear TDP-43 in inclusion body myositis. Muscle Nerve. 2009;40(1):19–31. doi: 10.1002/mus.21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weihl CC, Temiz P, Miller SE, Watts G, Smith C, Forman M, et al. TDP-43 accumulation in inclusion body myopathy muscle suggests a common pathogenic mechanism with frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2008;79(10):1186–1189. doi: 10.1136/jnnp.2007.131334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Izumi R, Warita H, Niihori T, Takahashi T, Tateyama M, Suzuki N, et al. Isolated inclusion body myopathy caused by a multisystem proteinopathy-linked hnRNPA1 mutation. Neurol Genet. 2015;1(3):e23. doi: 10.1212/NXG.0000000000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jackson CE, Barohn RJ, Gronseth G, Pandya S, Herbelin L, Muscle Study G Inclusion body myositis functional rating scale: a reliable and valid measure of disease severity. Muscle Nerve. 2008;37(4):473–476. doi: 10.1002/mus.20958. [DOI] [PubMed] [Google Scholar]
- 95.Liu J, Shinobu LA, Ward CM, Young D, Cleveland DW. Elevation of the Hsp70 chaperone does not effect toxicity in mouse models of familial amyotrophic lateral sclerosis. J Neurochem. 2005;93(4):875–882. doi: 10.1111/j.1471-4159.2005.03054.x. [DOI] [PubMed] [Google Scholar]
- 96.Krishnan J, Vannuvel K, Andries M, Waelkens E, Robberecht W, Van Den Bosch L. Overexpression of Hsp27 does not influence disease in the mutant SOD1G93Amouse model of amyotrophic lateral sclerosis. J Neurochem. 2008;106:2170–2183. doi: 10.1111/j.1471-4159.2008.05545.x. [DOI] [PubMed] [Google Scholar]
- 97.Sharp PS, Akbar MT, Bouri S, Senda A, Joshi K, Chen H-J, et al. Protective effects of heat shock protein 27 in a model of ALS occur in the early stages of disease progression. Neurobiol Dis. 2008;30(1):42–55. doi: 10.1016/j.nbd.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 98.Novoselov SS, Mustill WJ, Gray AL, Dick JR, Kanuga N, Kalmar B, et al. Molecular chaperone mediated late-stage neuroprotection in the SOD1G93A mouse model of amyotrophic lateral sclerosis. PLoS One. 2013;8(8):e73944. doi: 10.1371/journal.pone.0073944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Watanabe S, Ageta-Ishihara N, Nagatsu S, Takao K, Komine O, Endo F, et al. SIRT1 overexpression ameliorates a mouse model of SOD1-linked amyotrophic lateral sclerosis via HSF1/HSP70i chaperone system. Mol Brain. 2014;7:62. doi: 10.1186/s13041-014-0062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin P-Y, Simon SM, Koh W, Folorunso O, Samuel Umbaugh C, Pierce A. Heat shock factor 1 over-expression protects against exposure of hydrophobic residues on mutant SOD1 and early mortality in a mouse model of amyotrophic lateral sclerosis. Mol Neurodegener. 2013;8(1):43. doi: 10.1186/1750-1326-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Patel P, Julien J-P, Kriz J. Early-stage treatment with withaferin a reduces levels of misfolded superoxide dismutase 1 and extends lifespan in a mouse model of amyotrophic lateral sclerosis. Neurotherapeutics. 2014;12(1):217–233. doi: 10.1007/s13311-014-0311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kiaei M, Kipiani K, Petri S, Chen J, Calingasan NY, Beal MF. Celastrol blocks neuronal cell death and extends life in transgenic mouse model of amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2(5):246–254. doi: 10.1159/000090364. [DOI] [PubMed] [Google Scholar]
- 103.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4(5):346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 104.Saxena S, Caroni P. Selective neuronal vulnerability in neurodegenerative diseases: from stressor thresholds to degeneration. Neuron. 2011;71(1):35–48. doi: 10.1016/j.neuron.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 105.Moloney TC, Hyland R, O’Toole D, Paucard A, Kirik D, O’Doherty A, et al. Heat shock protein 70 reduces α-synuclein-induced predegenerative neuronal dystrophy in the α-synuclein viral gene transfer rat model of Parkinson’s disease. CNS Neurosci Ther. 2014;20(1):50–58. doi: 10.1111/cns.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dong Z, Wolfer DP, Lipp H-P, Büeler H. Hsp70 gene transfer by adeno-associated virus inhibits MPTP-induced nigrostriatal degeneration in the mouse model of Parkinson disease. Mol Ther. 2005;11(1):80–88. doi: 10.1016/j.ymthe.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 107.Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369(9579):2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 108.Bartus RT, Baumann TL, Siffert J, Herzog CD, Alterman R, Boulis N, et al. Safety/feasibility of targeting the substantia nigra with AAV2-neurturin in Parkinson patients. Neurology. 2013;80(18):1698–1701. doi: 10.1212/WNL.0b013e3182904faa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Horger BA, Nishimura MC, Armanini MP, Wang LC, Poulsen KT, Rosenblad C, et al. Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons. J Neurosci. 1998;18(13):4929–4937. doi: 10.1523/JNEUROSCI.18-13-04929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Marks WJ, Jr, Baumann TL, Bartus RT. Long-term safety of patients with Parkinson’s disease receiving rAAV2-Neurturin (CERE-120) gene transfer. Hum Gene Ther. 2016;27(7):522–527. doi: 10.1089/hum.2015.134. [DOI] [PubMed] [Google Scholar]
- 111.Bartus RT, Kordower JH, Johnson EM, Brown L, Kruegel BR, Chu Y, et al. Post-mortem assessment of the short and long-term effects of the trophic factor neurturin in patients with α-synucleinopathies. Neurobiol Dis. 2015;78:162–171. doi: 10.1016/j.nbd.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 112.Oh E, Akopian D, Rape M. Principles of ubiquitin-dependent signaling. Annu Rev Cell Dev Biol. 2018;34:137–162. doi: 10.1146/annurev-cellbio-100617-062802. [DOI] [PubMed] [Google Scholar]
- 113.Yau R, Rape M. The increasing complexity of the ubiquitin code. Nat Cell Biol. 2016;18(6):579–586. doi: 10.1038/ncb3358. [DOI] [PubMed] [Google Scholar]
- 114.Kleiger G, Mayor T. Perilous journey: a tour of the ubiquitin–proteasome system. Trends Cell Biol. 2014;24(6):352–359. doi: 10.1016/j.tcb.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Soave CL, Guerin T, Liu J, Dou QP. Targeting the ubiquitin-proteasome system for cancer treatment: discovering novel inhibitors from nature and drug repurposing. Cancer Metastasis Rev. 2017;36(4):717–736. doi: 10.1007/s10555-017-9705-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat Med. 2014;20(11):1242–1253. doi: 10.1038/nm.3739. [DOI] [PubMed] [Google Scholar]
- 117.Opattova A, Cente M, Novak M, Filipcik P. The ubiquitin proteasome system as a potential therapeutic target for treatment of neurodegenerative diseases. Gen Physiol Biophys. 2015;34(4):337–352. doi: 10.4149/gpb_2015024. [DOI] [PubMed] [Google Scholar]
- 118.Manavalan A, Mishra M, Feng L, Sze SK, Akatsu H, Heese K. Brain site-specific proteome changes in aging-related dementia. Exp Mol Med. 2013;45:e39. doi: 10.1038/emm.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang P, Joberty G, Buist A, Vanoosthuyse A, Stancu I-C, Vasconcelos B, et al. Tau interactome mapping based identification of Otub1 as Tau deubiquitinase involved in accumulation of pathological Tau forms in vitro and in vivo. Acta Neuropathol. 2017;133(5):731–749. doi: 10.1007/s00401-016-1663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zouambia M, Fischer DF, Hobo B, De Vos RAI, Hol EM, Varndell IM, et al. Proteasome subunit proteins and neuropathology in tauopathies and synucleinopathies: Consequences for proteomic analyses. Proteomics. 2008;8(6):1221–1236. doi: 10.1002/pmic.200700679. [DOI] [PubMed] [Google Scholar]
- 121.Ciechanover A, Kwon YT. Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies. Exp Mol Med. 2015;47:e147. doi: 10.1038/emm.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mori H, Kondo J, Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer’s disease. Science. 1987;235(4796):1641–1644. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- 123.Tai H-C, Serrano-Pozo A, Hashimoto T, Frosch MP, Spires-Jones TL, Hyman BT. The synaptic accumulation of hyperphosphorylated tau oligomers in Alzheimer disease is associated with dysfunction of the ubiquitin-proteasome system. Am J Pathol. 2012;181(4):1426–1435. doi: 10.1016/j.ajpath.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Myeku N, Clelland CL, Emrani S, Kukushkin NV, Yu WH, Goldberg AL, et al. Tau-driven 26S proteasome impairment and cognitive dysfunction can be prevented early in disease by activating cAMP-PKA signaling. Nat Med. 2016;22(1):46–53. doi: 10.1038/nm.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Robless P, Mikhailidis DP, Stansby GP. Cilostazol for peripheral arterial disease. Cochrane Database Syst Rev. 2007. [DOI] [PubMed]
- 126.Saito S, Kojima S, Oishi N, Kakuta R, Maki T, Yasuno F, et al. A multicenter, randomized, placebo-controlled trial for cilostazol in patients with mild cognitive impairment: The COMCID study protocol. Alzheimers Dement. 2016;2(4):250–257. doi: 10.1016/j.trci.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sakurai H, Hanyu H, Sato T, Kume K, Hirao K, Kanetaka H, et al. Effects of cilostazol on cognition and regional cerebral blood flow in patients with Alzheimer’s disease and cerebrovascular disease: a pilot study. Geriatr Gerontol Int. 2012;13(1):90–97. doi: 10.1111/j.1447-0594.2012.00866.x. [DOI] [PubMed] [Google Scholar]
- 128.Tai S-Y, Chen C-H, Chien C-Y, Yang Y-H. Cilostazol as an add-on therapy for patients with Alzheimer’s disease in Taiwan: a case control study. BMC Neurol. 2017;17(1):40. doi: 10.1186/s12883-017-0800-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Torosyan N, Sethanandha C, Grill JD, Dilley ML, Lee J, Cummings JL, et al. Changes in regional cerebral blood flow associated with a 45 day course of the ketogenic agent, caprylidene, in patients with mild to moderate Alzheimer’s disease: results of a randomized, double-blinded, pilot study. Exp Gerontol. 2018;111:118–121. doi: 10.1016/j.exger.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 130.Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19(6):349–364. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 131.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J-I, Tanida I, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441(7095):880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 132.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441(7095):885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 133.Bento CF, Renna M, Ghislat G, Puri C, Ashkenazi A, Vicinanza M, et al. Mammalian autophagy: how does it work? Annu Rev Biochem. 2016;85(1):685–713. doi: 10.1146/annurev-biochem-060815-014556. [DOI] [PubMed] [Google Scholar]
- 134.Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, et al. A gene network regulating lysosomal biogenesis and function. Science. 2009;325(5939):473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 135.Kilpatrick K, Zeng Y, Hancock T, Segatori L. Genetic and chemical activation of TFEB mediates clearance of aggregated α-synuclein. PLoS One. 2015;10(3):e0120819. doi: 10.1371/journal.pone.0120819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ory DS, Ottinger EA, Farhat NY, King KA, Jiang X, Weissfeld L, et al. Intrathecal 2-hydroxypropyl-β-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: a non-randomised, open-label, phase 1–2 trial. Lancet. 2017;390(10104):1758–1768. doi: 10.1016/S0140-6736(17)31465-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369(6483):756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 138.Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78(1):35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 139.Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem. 1998;273(7):3963–3966. doi: 10.1074/jbc.273.7.3963. [DOI] [PubMed] [Google Scholar]
- 140.Scott RC, Schuldiner O, Neufeld TP. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev Cell. 2004;7(2):167–178. doi: 10.1016/j.devcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 141.Kamada Y, Funakoshi T, Shintani T, Nagano K, Ohsumi M, Ohsumi Y. Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J Cell Biol. 2000;150(6):1507–1513. doi: 10.1083/jcb.150.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Floto RA, Sarkar S, Perlstein EO, Kampmann B, Schreiber SL, Rubinsztein DC. Small molecule enhancers of rapamycin-induced TOR inhibition promote autophagy, reduce toxicity in huntington’s disease models and enhance killing of mycobacteria by macrophages. Autophagy. 2007;3(6):620–622. doi: 10.4161/auto.4898. [DOI] [PubMed] [Google Scholar]
- 143.Malagelada C, Jin ZH, Jackson-Lewis V, Przedborski S, Greene LA. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson’s disease. J Neurosci. 2010;30(3):1166–1175. doi: 10.1523/JNEUROSCI.3944-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tramutola A, Lanzillotta C, Barone E, Arena A, Zuliani I, Mosca L, et al. Intranasal rapamycin ameliorates Alzheimer-like cognitive decline in a mouse model of Down syndrome. Transl Neurodegener. 2018;7(1):28. doi: 10.1186/s40035-018-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-β levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;5(4):e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kraig E, Linehan LA, Liang H, Romo TQ, Liu Q, Wu Y, et al. A randomized control trial to establish the feasibility and safety of rapamycin treatment in an older human cohort: Immunological, physical performance, and cognitive effects. Exp Gerontol. 2018;105:53–69. doi: 10.1016/j.exger.2017.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nashan B, Citterio F. Wound healing complications and the use of mammalian target of rapamycin inhibitors in kidney transplantation: a critical review of the literature. Transplantation. 2012;94(6):547–561. doi: 10.1097/TP.0b013e3182551021. [DOI] [PubMed] [Google Scholar]
- 148.Saunders RN, Metcalfe MS, Nicholson ML. Rapamycin in transplantation: a review of the evidence. Kidney Int. 2001;59(1):3–16. doi: 10.1046/j.1523-1755.2001.00460.x. [DOI] [PubMed] [Google Scholar]
- 149.Li J, Kim SG, Blenis J. Rapamycin: one drug, many effects. Cell Metab. 2014;19(3):373–379. doi: 10.1016/j.cmet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.DeRemer DL, Ustun C, Natarajan K. Nilotinib: a second-generation tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia. Clin Ther. 2008;30(11):1956–1975. doi: 10.1016/j.clinthera.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 151.Manley PW, Cowan-Jacob SW, Mestan J. Advances in the structural biology, design and clinical development of Bcr-Abl kinase inhibitors for the treatment of chronic myeloid leukaemia. Biochimica et Biophysica Acta (BBA) Proteins Proteom. 2005;1754(1):3–13. doi: 10.1016/j.bbapap.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 152.Hebron ML, Lonskaya I, Moussa CEH. Nilotinib reverses loss of dopamine neurons and improves motor behavior via autophagic degradation of α-synuclein in Parkinson’s disease models. Hum Mol Genet. 2013;22(16):3315–3328. doi: 10.1093/hmg/ddt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lonskaya I, Hebron ML, Desforges NM, Franjie A, Moussa CEH. Tyrosine kinase inhibition increases functional parkin-Beclin-1 interaction and enhances amyloid clearance and cognitive performance. EMBO Mol Med. 2013;5(8):1247–1262. doi: 10.1002/emmm.201302771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pagan F, Hebron M, Valadez EH, Torres-Yaghi Y, Huang X, Mills RR, et al. Nilotinib effects in Parkinson’s disease and dementia with Lewy bodies. J Parkinsons Dis. 2016;6(3):503–517. doi: 10.3233/JPD-160867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gurney ME. Transgenic-mouse model of amyotrophic lateral sclerosis. N Engl J Med. 1994;331(25):1721–1722. doi: 10.1056/NEJM199412223312516. [DOI] [PubMed] [Google Scholar]
- 156.Bennett CF, Baker BF, Pham N, Swayze E, Geary RS. Pharmacology of Antisense Drugs. Annu Rev Pharmacol Toxicol. 2017;57(1):81–105. doi: 10.1146/annurev-pharmtox-010716-104846. [DOI] [PubMed] [Google Scholar]
- 157.Wurster CD, Ludolph AC. Antisense oligonucleotides in neurological disorders. Ther Adv Neurol Disord. 2018;11:175628641877693. doi: 10.1177/1756286418776932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Miller TM, Pestronk A, David W, Rothstein J, Simpson E, Appel SH, et al. An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: a phase 1, randomised, first-in-man study. Lancet Neurol. 2013;12(5):435–442. doi: 10.1016/S1474-4422(13)70061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Scott Ralph G, Radcliffe PA, Day DM, Carthy JM, Leroux MA, Lee DCP, et al. Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat Med. 2005;11(4):429. doi: 10.1038/nm1205. [DOI] [PubMed] [Google Scholar]
- 160.Kordasiewicz HB, Stanek LM, Wancewicz EV, Mazur C, McAlonis MM, Pytel KA, et al. Sustained therapeutic reversal of huntington’s disease by transient repression of Huntingtin synthesis. Neuron. 2012;74(6):1031–1044. doi: 10.1016/j.neuron.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Østergaard ME, Southwell AL, Kordasiewicz H, Watt AT, Skotte NH, Doty CN, et al. Rational design of antisense oligonucleotides targeting single nucleotide polymorphisms for potent and allele selective suppression of mutant Huntingtin in the CNS. Nucleic Acids Res. 2013;41(21):9634–9650. doi: 10.1093/nar/gkt725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tabrizi S, Leavitt B, Kordasiewicz H, Czech C, Swayze E, Norris DA, et al. Effects of IONIS-HTTRx in patients with early Huntington’s disease, results of the first HTT-lowering drug trial (CT.002) Neurology. 2018;90(15 Suppl):CT.002. [Google Scholar]
- 163.Schoch KM, DeVos SL, Miller RL, Chun SJ, Norrbom M, Wozniak DF, et al. Increased 4R-tau induces pathological changes in a human-tau mouse model. Neuron. 2016;90(5):941–947. doi: 10.1016/j.neuron.2016.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Benjamin D, Rex B, Kelly N, Scott S. Inhibition of SOD1 protein expression in the cell by pyrimethamine, an orally available small molecule. In: International symposium on ALS/MND2007. 2007.
- 165.Lange DJ, Andersen PM, Remanan R, Marklund S, Benjamin D. Pyrimethamine decreases levels of SOD1 in leukocytes and cerebrospinal fluid of ALS patients: a phase I pilot study. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(3):199–204. doi: 10.3109/17482968.2012.724074. [DOI] [PubMed] [Google Scholar]
- 166.Lange DJ, Shahbazi M, Silani V, Ludolph AC, Weishaupt JH, Ajroud-Driss S, et al. Pyrimethamine significantly lowers cerebrospinal fluid Cu/Zn superoxide dismutase in amyotrophic lateral sclerosis patients with SOD1 mutations. Ann Neurol. 2017;81(6):837–848. doi: 10.1002/ana.24950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.O’Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71(5):362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kennedy ME, Stamford AW, Chen X, Cox K, Cumming JN, Dockendorf MF, et al. The BACE1 inhibitor verubecestat (MK-8931) reduces CNS β-amyloid in animal models and in Alzheimer’s disease patients. Sci Transl Med. 2016;8(363):363ra150. doi: 10.1126/scitranslmed.aad9704. [DOI] [PubMed] [Google Scholar]
- 170.Timmers M, Streffer JR, Russu A, Tominaga Y, Shimizu H, Shiraishi A, et al. Pharmacodynamics of atabecestat (JNJ-54861911), an oral BACE1 inhibitor in patients with early Alzheimer’s disease: randomized, double-blind, placebo-controlled study. Alzheimers Res Ther. 2018;10(1):85. doi: 10.1186/s13195-018-0415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sakamoto K, Matsuki S, Matsuguma K, Yoshihara T, Uchida N, Azuma F, et al. BACE1 inhibitor lanabecestat (AZD3293) in a phase 1 study of healthy Japanese subjects: pharmacokinetics and effects on plasma and cerebrospinal fluid Aβ peptides. J Clin Pharmacol. 2017;57(11):1460–1471. doi: 10.1002/jcph.950. [DOI] [PubMed] [Google Scholar]
- 172.Panza F, Lozupone M, Solfrizzi V, Sardone R, Piccininni C, Dibello V, et al. BACE inhibitors in clinical development for the treatment of Alzheimer’s disease. Expert Rev Neurother. 2018;18(11):847–857. doi: 10.1080/14737175.2018.1531706. [DOI] [PubMed] [Google Scholar]
- 173.Coimbra JRM, Marques DFF, Baptista SJ, Pereira CMF, Moreira PI, Dinis TCP, et al. Highlights in BACE1 inhibitors for Alzheimer’s disease treatment. Front Chem. 2018;6:178. doi: 10.3389/fchem.2018.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vassar R. BACE1 inhibitor drugs in clinical trials for Alzheimer’s disease. Alzheimer’s Res Ther. 2014;6(9):89. doi: 10.1186/s13195-014-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Peters F, Salihoglu H, Rodrigues E, Herzog E, Blume T, Filser S, et al. BACE1 inhibition more effectively suppresses initiation than progression of β-amyloid pathology. Acta Neuropathol. 2018;135(5):695–710. doi: 10.1007/s00401-017-1804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci USA. 2009;106(16):6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Taniguchi S, Suzuki N, Masuda M, Hisanaga S-I, Iwatsubo T, Goedert M, et al. Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins. J Biol Chem. 2005;280(9):7614–7623. doi: 10.1074/jbc.M408714200. [DOI] [PubMed] [Google Scholar]
- 178.Wischik CM, Edwards PC, Lai RY, Roth M, Harrington CR. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc Natl Acad Sci USA. 1996;93(20):11213–11218. doi: 10.1073/pnas.93.20.11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mansouri A, Lurie AA. Concise review: methemoglobinemia. Am J Hematol. 1993;42(1):7–12. doi: 10.1002/ajh.2830420104. [DOI] [PubMed] [Google Scholar]
- 180.Prince CP. Chapter-10 methylene blue staining. Practical manual of medical microbiology. 2009. p. 45–6.
- 181.Congdon EE, Wu JW, Myeku N, Figueroa YH, Herman M, Marinec PS, et al. Methylthioninium chloride (methylene blue) induces autophagy and attenuates tauopathy in vitro and in vivo. Autophagy. 2012;8(4):609–622. doi: 10.4161/auto.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Peter C, Hongwan D, Küpfer A, Lauterburg BH. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur J Clin Pharmacol. 2000;56(3):247–250. doi: 10.1007/s002280000124. [DOI] [PubMed] [Google Scholar]
- 183.Harrington CR, Storey JMD, Clunas S, Harrington KA, Horsley D, Ishaq A, et al. Cellular models of aggregation-dependent template-directed proteolysis to characterize tau aggregation inhibitors for treatment of Alzheimer disease. J Biol Chem. 2015;290(17):10862–10875. doi: 10.1074/jbc.M114.616029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chang E, Congdon EE, Honson NS, Duff KE, Kuret J. Structure-activity relationship of cyanine tau aggregation inhibitors. J Med Chem. 2009;52(11):3539–3547. doi: 10.1021/jm900116d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Melis V, Magbagbeolu M, Rickard JE, Horsley D, Davidson K, Harrington KA, et al. Effects of oxidized and reduced forms of methylthioninium in two transgenic mouse tauopathy models. Behav Pharmacol. 2015;26(4):353–368. doi: 10.1097/FBP.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hochgräfe K, Sydow A, Matenia D, Cadinu D, Könen S, Petrova O, et al. Preventive methylene blue treatment preserves cognition in mice expressing full-length pro-aggregant human Tau. Acta Neuropathol Commun. 2015;3:25. doi: 10.1186/s40478-015-0204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hosokawa M, Arai T, Masuda-Suzukake M, Nonaka T, Yamashita M, Akiyama H, et al. Methylene blue reduced abnormal tau accumulation in P301L tau transgenic mice. PLoS One. 2012;7(12):e52389. doi: 10.1371/journal.pone.0052389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wischik CM, Roger TS, Wischik DJ, Bentham P, Murray AD, Storey JMD, et al. Tau aggregation inhibitor therapy: an exploratory phase 2 study in mild or moderate Alzheimer’s disease. J Alzheimers Dis. 2015;44(2):705–720. doi: 10.3233/JAD-142874. [DOI] [PubMed] [Google Scholar]
- 189.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141(11):1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 190.Gauthier S, Feldman HH, Schneider LS, Wilcock GK, Frisoni GB, Hardlund JH, et al. Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer’s disease: a randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet. 2016;388(10062):2873–2884. doi: 10.1016/S0140-6736(16)31275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S33–S39. doi: 10.1097/00002093-199700112-00005. [DOI] [PubMed] [Google Scholar]
- 192.Recasens A, Dehay B. Alpha-synuclein spreading in Parkinson’s disease. Front Neuroanat. 2014;8:159. doi: 10.3389/fnana.2014.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Price DL, Koike MA, Khan A, Wrasidlo W, Rockenstein E, Masliah E, et al. The small molecule alpha-synuclein misfolding inhibitor, NPT200-11, produces multiple benefits in an animal model of Parkinson’s disease. Sci Rep. 2018;8(1):16165. doi: 10.1038/s41598-018-34490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Silverman JM, Gibbs E, Peng X, Martens KM, Balducci C, Wang J, et al. A rational structured epitope defines a distinct subclass of toxic amyloid-beta oligomers. ACS Chem Neurosci. 2018;9(7):1591–1606. doi: 10.1021/acschemneuro.7b00469. [DOI] [PubMed] [Google Scholar]
- 195.Peng X, Cashman NR, Plotkin SS. Prediction of misfolding-specific epitopes in SOD1 using collective coordinates. J Phys Chem B. 2018;122(49):11662–11676. doi: 10.1021/acs.jpcb.8b07680. [DOI] [PubMed] [Google Scholar]
- 196.Gibbs E, Silverman JM, Zhao B, Peng X, Wang J, Wellington CL, et al. A rationally designed humanized antibody selective for amyloid beta oligomers in Alzheimer’s disease. Sci Rep. 2019;9(1):9870. doi: 10.1038/s41598-019-46306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hoogenboom HR. Selecting and screening recombinant antibody libraries. Nat Biotechnol. 2005;23(9):1105–1116. doi: 10.1038/nbt1126. [DOI] [PubMed] [Google Scholar]
- 198.Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, et al. Aβ peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408(6815):979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 199.Dodart J-C, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, et al. Immunization reverses memory deficits without reducing brain Aβ burden in Alzheimer’s disease model. Nat Neurosci. 2002;5(5):452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- 200.Asami-Odaka A, Obayashi-Adachi Y, Matsumoto Y, Takahashi H, Fukumoto H, Horiguchi T, et al. Passive immunization of the Aβ42(43) C-terminal-specific antibody BC05 in a mouse model of Alzheimer’s disease. Neurodegener Dis. 2005;2(1):36–43. doi: 10.1159/000086429. [DOI] [PubMed] [Google Scholar]
- 201.Ferrer I, Boada Rovira M, Sánchez Guerra ML, Rey MJ, Costa-Jussá F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathol. 2004;14(1):11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nicoll JAR, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: a case report. Nat Med. 2003;9(4):448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 203.Vellas B, Black R, Thal LJ, Fox NC, Daniels M, McLennan G, et al. Long-term follow-up of patients immunized with AN1792: reduced functional decline in antibody responders. Curr Alzheimer Res. 2009;6(2):144–151. doi: 10.2174/156720509787602852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nicoll JAR, Buckland GR, Harrison CH, Page A, Harris S, Love S, et al. Persistent neuropathological effects 14 years following amyloid-β immunization in Alzheimer’s disease. Brain. 2019;142(7):2113–2126. doi: 10.1093/brain/awz142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hickman DT, López-Deber MP, Ndao DM, Silva AB, Nand D, Pihlgren M, et al. Sequence-independent control of peptide conformation in liposomal vaccines for targeting protein misfolding diseases. J Biol Chem. 2011;286(16):13966–13976. doi: 10.1074/jbc.M110.186338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Muhs A, Hickman DT, Pihlgren M, Chuard N, Giriens V, Meerschman C, et al. Liposomal vaccines with conformation-specific amyloid peptide antigens define immune response and efficacy in APP transgenic mice. Proc Natl Acad Sci USA. 2007;104(23):9810–9815. doi: 10.1073/pnas.0703137104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Herrup K. The case for rejecting the amyloid cascade hypothesis. Nat Neurosci. 2015;18(6):794–799. doi: 10.1038/nn.4017. [DOI] [PubMed] [Google Scholar]
- 208.Karran E, De Strooper B. The amyloid cascade hypothesis: are we poised for success or failure? J Neurochem. 2016;139:237–252. doi: 10.1111/jnc.13632. [DOI] [PubMed] [Google Scholar]
- 209.Ricciarelli R, Fedele E. The amyloid cascade hypothesis in Alzheimer’s disease: it’s time to change our mind. Curr Neuropharmacol. 2017;15(6):926–935. doi: 10.2174/1570159X15666170116143743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65(11):1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Beason-Held LL, Goh JO, An Y, Kraut MA, O’Brien RJ, Ferrucci L, et al. Changes in brain function occur years before the onset of cognitive impairment. J Neurosci. 2013;33(46):18008–18014. doi: 10.1523/JNEUROSCI.1402-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Farrell ME, Kennedy KM, Rodrigue KM, Wig G, Bischof GN, Rieck JR, et al. Association of longitudinal cognitive decline with amyloid burden in middle-aged and older adults: evidence for a dose-response relationship. JAMA Neurol. 2017;74(7):830–838. doi: 10.1001/jamaneurol.2017.0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.van Dyck CH. Anti-amyloid-β monoclonal antibodies for Alzheimer’s disease: pitfalls and promise. Biol Psychiatry. 2018;83(4):311–319. doi: 10.1016/j.biopsych.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhang S, Fox DM, Urbanc B. Elucidating the role of hydroxylated phenylalanine in the formation and structure of cross-linked Aβ oligomers. J Phys Chem B. 2019;123(5):1068–1084. doi: 10.1021/acs.jpcb.8b12120. [DOI] [PubMed] [Google Scholar]
- 216.Mroczko B, Groblewska M, Litman-Zawadzka A, Kornhuber J, Lewczuk P. Amyloid β oligomers (AβOs) in Alzheimer’s disease. J Neural Transm. 2018;125(2):177–191. doi: 10.1007/s00702-017-1820-x. [DOI] [PubMed] [Google Scholar]
- 217.Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 218.Logovinsky V, Satlin A, Lai R, Swanson C, Kaplow J, Osswald G, et al. Safety and tolerability of BAN2401—a clinical study in Alzheimer’s disease with a protofibril selective Aβ antibody. Alzheimer’s Res Ther. 2016;8(1):14. doi: 10.1186/s13195-016-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370(4):322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cummings JL, Cohen S, van Dyck CH, Brody M, Curtis C, Cho W, et al. ABBY: a phase 2 randomized trial of crenezumab in mild to moderate Alzheimer disease. Neurology. 2018;90(21):e1889–e1897. doi: 10.1212/WNL.0000000000005550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ostrowitzki S, Deptula D, Thurfjell L, Barkhof F, Bohrmann B, Brooks DJ, et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol. 2012;69(2):198–207. doi: 10.1001/archneurol.2011.1538. [DOI] [PubMed] [Google Scholar]
- 222.Bohrmann B, Baumann K, Benz J, Gerber F, Huber W, Knoflach F, et al. Gantenerumab: a novel human anti-Aβ antibody demonstrates sustained cerebral amyloid-β binding and elicits cell-mediated removal of human amyloid-β. J Alzheimers Dis. 2012;28(1):49–69. doi: 10.3233/JAD-2011-110977. [DOI] [PubMed] [Google Scholar]
- 223.Landen JW, Cohen S, Billing CB, Jr, Cronenberger C, Styren S, Burstein AH, et al. Multiple-dose ponezumab for mild-to-moderate Alzheimer’s disease: safety and efficacy. Alzheimers Dement. 2017;3(3):339–347. doi: 10.1016/j.trci.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370(4):311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 225.Kontsekova E, Zilka N, Kovacech B, Novak P, Novak M. First-in-man tau vaccine targeting structural determinants essential for pathological tau–tau interaction reduces tau oligomerisation and neurofibrillary degeneration in an Alzheimer’s disease model. Alzheimers Res Ther. 2014;6(4):44. doi: 10.1186/alzrt278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Crespo-Biel N, Gafner V, Pihlgren M, López-Deber MP, Reis P, et al. Efficacy and safety of a liposome-based vaccine against protein Tau, assessed in tau.P301L mice that model tauopathy. PLoS One. 2013;8(8):e72301. doi: 10.1371/journal.pone.0072301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kontsekova E, Zilka N, Kovacech B, Skrabana R, Novak M. Identification of structural determinants on tau protein essential for its pathological function: novel therapeutic target for tau immunotherapy in Alzheimer’s disease. Alzheimers Res Ther. 2014;6(4):45. doi: 10.1186/alzrt277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Novak P, Schmidt R, Kontsekova E, Zilka N, Kovacech B, Skrabana R, et al. Safety and immunogenicity of the tau vaccine AADvac1 in patients with Alzheimer’s disease: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Neurol. 2017;16(2):123–134. doi: 10.1016/S1474-4422(16)30331-3. [DOI] [PubMed] [Google Scholar]
- 229.Novak P, Schmidt R, Kontsekova E, Kovacech B, Smolek T, Katina S, et al. FUNDAMANT: an interventional 72-week phase 1 follow-up study of AADvac1, an active immunotherapy against tau protein pathology in Alzheimer’s disease. Alzheimers Res Ther. 2018;10(1):108. doi: 10.1186/s13195-018-0436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sigurdsson EM. Tau immunotherapies for Alzheimer’s disease and related tauopathies: progress and potential pitfalls. J Alzheimer’s Dis. 2018;66(2):855–856. doi: 10.3233/JAD-189010. [DOI] [PubMed] [Google Scholar]
- 231.Congdon EE, Sigurdsson EM. Tau-targeting therapies for Alzheimer disease. Nat Rev Neurol. 2018;14(7):399–415. doi: 10.1038/s41582-018-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bright J, Hussain S, Dang V, Wright S, Cooper B, Byun T, et al. Human secreted tau increases amyloid-beta production. Neurobiol Aging. 2015;36(2):693–709. doi: 10.1016/j.neurobiolaging.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 233.Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI. Trans-cellular propagation of Tau aggregation by fibrillar species. J Biol Chem. 2012;287(23):19440–19451. doi: 10.1074/jbc.M112.346072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lee S-H, Le Pichon CE, Adolfsson O, Gafner V, Pihlgren M, Lin H, et al. Antibody-mediated targeting of tau in vivo does not require effector function and microglial engagement. Cell Rep. 2016;16(6):1690–1700. doi: 10.1016/j.celrep.2016.06.099. [DOI] [PubMed] [Google Scholar]
- 235.Rogers M. To block tau’s proteopathic spread, antibody must attack its mid-region. 2018. Available at: https://www.alzforum.org/news/conference-coverage/block-taus-proteopathic-spread-antibody-must-attack-its-mid-region. Accessed 5 Apr 2019.
- 236.Rosenqvist N, Asuni AA, Andersson CR, Christensen S, Daechsel JA, Egebjerg J, et al. Highly specific and selective anti-pS396-tau antibody C10.2 targets seeding-competent tau. Alzheimers Dement. 2018;4:521–534. doi: 10.1016/j.trci.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Mandler M, Valera E, Rockenstein E, Weninger H, Patrick C, Adame A, et al. Next-generation active immunization approach for synucleinopathies: implications for Parkinson’s disease clinical trials. Acta Neuropathol. 2014;127(6):861–879. doi: 10.1007/s00401-014-1256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Mandler M, Valera E, Rockenstein E, Mante M, Weninger H, Patrick C, et al. Active immunization against alpha-synuclein ameliorates the degenerative pathology and prevents demyelination in a model of multiple system atrophy. Mol Neurodegener. 2015;10(1):10. doi: 10.1186/s13024-015-0008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Schneeberger A, Mandler M, Otawa O, Zauner W, Mattner F, Schmidt W. Development of AFFITOPE vaccines for Alzheimer’s disease (AD)—from concept to clinical testing. J Nutr Health Aging. 2009;13(3):264–267. doi: 10.1007/s12603-009-0070-5. [DOI] [PubMed] [Google Scholar]
- 240.Weihofen A, Liu Y, Arndt JW, Huy C, Quan C, Smith BA, et al. Development of an aggregate-selective, human-derived α-synuclein antibody BIIB054 that ameliorates disease phenotypes in Parkinson’s disease models. Neurobiol Dis. 2019;124:276–288. doi: 10.1016/j.nbd.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 241.Jankovic J, Goodman I, Safirstein B, Marmon TK, Schenk DB, Koller M, et al. Safety and tolerability of multiple ascending doses of PRX002/RG7935, an anti-α-synuclein monoclonal antibody, in patients with parkinson disease: a randomized clinical trial. JAMA Neurol. 2018;75(10):1206–1214. doi: 10.1001/jamaneurol.2018.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Angot E, Steiner JA, Lema Tomé CM, Ekström P, Mattsson B, Björklund A, et al. Alpha-synuclein cell-to-cell transfer and seeding in grafted dopaminergic neurons in vivo. PLoS One. 2012;7(6):e39465. doi: 10.1371/journal.pone.0039465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hansen C, Angot E, Bergström A-L, Steiner JA, Pieri L, Paul G, et al. α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121(2):715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kordower JH, Dodiya HB, Kordower AM, Terpstra B, Paumier K, Madhavan L, et al. Transfer of host-derived alpha synuclein to grafted dopaminergic neurons in rat. Neurobiol Dis. 2011;43(3):552–557. doi: 10.1016/j.nbd.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Brys M, Ellenbogen A, Fanning L, Penner N, Yang M, Welch M, et al. Randomized, double-blind, placebo-controlled, single ascending dose study of anti-alpha-synuclein antibody BIIB054 in patients with Parkinson’s disease (S26.001) Neurology. 2018;90(15 Suppl):S26.001. [Google Scholar]
- 246.Sardi SP, Cedarbaum JM, Brundin P. Targeted therapies for Parkinson’s disease: from genetics to the clinic. Mov Disord. 2018;33(5):684–696. doi: 10.1002/mds.27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Amor S, Peferoen LAN, Vogel DYS, Breur M, van der Valk P, Baker D, et al. Inflammation in neurodegenerative diseases—an update. Immunology. 2014;142(2):151–166. doi: 10.1111/imm.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Al-Lazikani B, Banerji U, Workman P. Combinatorial drug therapy for cancer in the post-genomic era. Nat Biotechnol. 2012;30(7):679–692. doi: 10.1038/nbt.2284. [DOI] [PubMed] [Google Scholar]
- 249.Lewczuk P, Riederer P, O’Bryant SE, Verbeek MM, Dubois B, Visser PJ, et al. Cerebrospinal fluid and blood biomarkers for neurodegenerative dementias: an update of the Consensus of the Task Force on Biological Markers in Psychiatry of the World Federation of Societies of Biological Psychiatry. World J Biol Psychiatry. 2018;19(4):244–328. doi: 10.1080/15622975.2017.1375556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Beach TG. A review of biomarkers for neurodegenerative disease: will they swing us across the valley? Neurol Ther. 2017;6(Suppl 1):5–13. doi: 10.1007/s40120-017-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, Berg S, et al. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry. 2006;63(2):168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- 252.Fujimori K, Ishikawa M, Otomo A, Atsuta N, Nakamura R, Akiyama T, et al. Modeling sporadic ALS in iPSC-derived motor neurons identifies a potential therapeutic agent. Nat Med. 2018;24(10):1579–1589. doi: 10.1038/s41591-018-0140-5. [DOI] [PubMed] [Google Scholar]