Abstract
The Australian pterosaur record is poor by world standards, comprising fewer than 20 fragmentary specimens. Herein, we describe the new genus and species Ferrodraco lentoni gen. et sp. nov., based on the most complete pterosaur specimen ever found in Australia, and the first reported from the Winton Formation (Cenomanian–lower Turonian). The presence of premaxillary and mandibular crests, and spike-shaped teeth with subcircular bases, enable Ferrodraco to be referred to Anhangueria. Ferrodraco can be distinguished from all other anhanguerian pterosaurs based on two dental characters: the first premaxillary and mandibular tooth pairs are small; and the fourth–seventh tooth pairs are smaller than the third and eighth ones. Ferrodraco was included in a phylogenetic analysis of Pterosauria and resolved as the sister taxon to Mythunga camara (upper Albian Toolebuc Formation, Australia), with that clade occupying the most derived position within Ornithocheiridae. Ornithocheirus simus (Albian Cambridge Greensand, England), Coloborhynchus clavirostris (Valanginian Hastings Sands, England), and Tropeognathus mesembrinus (upper Aptian–lower Albian Romualdo Formation, Brazil) were resolved as successive sister taxa, which suggests that ornithocheirids were cosmopolitan during the Albian–Cenomanian. Furthermore, the stratigraphic age of Ferrodraco lentoni (Cenomanian–lower Turonian) implies that anhanguerians might have survived later in Australia than elsewhere.
Subject terms: Taxonomy, Phylogenetics, Palaeontology, Palaeontology, Taxonomy
Introduction
Pterosaurs are known from Mesozoic strata on every continent, with their fossil record spanning the Late Triassic–end Cretaceous1. Despite this, pterosaur remains are typically incomplete and fragmentary, primarily because their bones have thin cortices and are typically hollow2. Pterosaurs are rare in Australia, with their fossil record restricted to the Cretaceous and comprising isolated and fragmentary bones from Queensland3–9, New South Wales10, Western Australia11,12 and Victoria13,14. The Victorian pterosaur fossils remain undescribed14, whereas those from New South Wales can be identified no more precisely than Anhangueria indet10. By contrast, the few pterosaur fossils from Western Australia evidently represent an anhanguerian12 and an azhdarchid11,15.
All of the pterosaur remains known from Queensland derive from the Eromanga Basin, all but one are from the upper Albian Toolebuc Formation (the exception being a ctenochasmatoid from the upper Albian Mackunda Formation6), and most have been tentatively referred to the Ornithocheiridae sensu Unwin16,17. Although some authors (e.g. Unwin16,17) have considered the Anhangueridae as a junior synonym of the Ornithocheiridae, a recent phylogenetic analysis by Andres and Myers18 resolved Anhangueridae and Ornithocheiridae as sister taxa. Although it is possible that some of the Queensland ‘ornithocheirids’, particularly those identified as cf. Anhanguera6, might in fact be anhanguerids, this cannot be determined because of their incomplete preservation. The only named pterosaur species from Australia — Mythunga camara (Queensland Museum [QM, Brisbane, Queensland, Australia] F18896)5,9 and Aussiedraco molnari (QM F10613)3,8 — are from the upper Lower Cretaceous (upper Albian) Toolebuc Formation of Queensland. Mythunga is represented by an incomplete skull found near Hughenden, comprising incomplete premaxillae, maxillae, dentaries, and other cranial elements preserved in matrix within the nasoantorbital fenestra, and was originally interpreted as a short-snouted archaeopterodactyloid5. A recent redescription of this taxon demonstrated that it was not short-snouted9; moreover, the inclusion of Mythunga in a phylogenetic analysis (based on a modified version of the Andres et al.19 dataset) suggested that it was an anhanguerian, not an archaeopterodactyloid. However, the inclusion of Mythunga in this analysis reduced the resolution within Anhangueria to such a degree that its position within that clade could not be established9. By contrast, Aussiedraco is represented by a mandibular symphysis found near Boulia that was initially assigned to aff. Ornithocheirus3 but later classified more broadly as a member of Pteranodontoidea8.
Here we report on, and briefly describe, a new anhanguerian pterosaur species from the Cenomanian–lower Turonian (lower Upper Cretaceous) Winton Formation of Queensland, northeast Australia. This specimen — discovered in April 2017 by one of the authors (RAE) — is the first pterosaur reported from the Winton Formation, and is also the most complete pterosaur ever found in Australia.
Results
Systematic palaeontology
Pterosauria Kaup, 1834
Pterodactyloidea Plieninger, 1901
Ornithocheiroidea Seeley, 1891 sensu Kellner, 2003
Anhangueria Rodrigues and Kellner, 2013
Ornithocheirae Seeley, 1870
Ornithocheiridae Seeley, 1870
Ornithocheirinae Andres, Clark and Xu, 2014
Ferrodraco lentoni gen. et sp. nov.
Etymology
From the Latin ferrum (iron), in reference to the ironstone preservation of the holotype specimen, and the Latin draco (dragon). The species name honours former Winton Shire mayor Graham Thomas ‘Butch’ Lenton, in recognition of his years of service to the Winton community and support to the Australian Age of Dinosaurs Natural History Museum.
Holotype
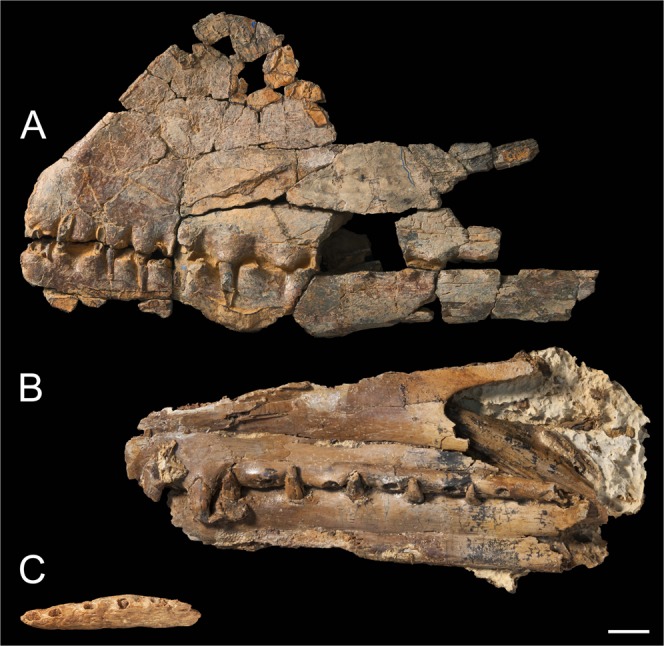
Australian Age of Dinosaurs Fossil (AODF, Winton, Queensland, Australia) 876 (‘Butch’): anterior portion of skull comprising partial premaxillae, maxillae and dentaries (including premaxillary and mandibular crests and the mandibular symphysis); partial left frontal; left mandibular articular region comprising the surangular, angular and articular; five partial cervical vertebrae; partial right scapulocoracoid; partial left ulna; partial left radius; left proximal and distal carpals; left metacarpal IV; proximal end of right metacarpal IV; fragmentary left non-wing manual phalanges; partial left first wing phalanx (IV-1); and associated fragments. Several elements, including the skull and mandible and many of the appendicular elements (based on key-fits between adherent matrix on anatomically adjacent elements) were clearly articulated post-fossilisation; however, erosion and soil rotation led to fragmentation of the specimen prior to its excavation.
Type horizon and locality
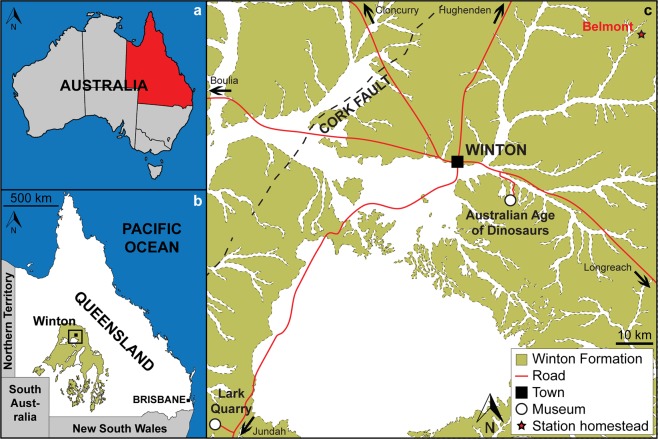
Winton Formation (Cenomanian–lower Turonian20,21); Australian Age of Dinosaurs Locality (AODL, Winton, Queensland, Australia) 245 (the ‘Pterosaur Site’), Belmont Station, Winton, Queensland, Australia (Fig. 1).
Figure 1.
Location of the Ferrodraco lentoni gen. et sp. nov. type locality (AODL 245). (a) Map of Australia showing the location of Queensland (modified from Poropat et al.60). (b) Map of Queensland showing the distribution of Winton Formation outcrop (modified from Poropat et al.60). (c) Map of the Winton area showing Winton Formation outcrop, the location of Belmont Station, and museums in the region. This map was drafted by S.F.P. in Adobe Illustrator CC 2017, and incorporates geological information from Vine61 and Vine & Casey62 [© Commonwealth of Australia (Geoscience Australia) 2019. This product is released under the Creative Commons Attribution 4.0 International Licence. http://creativecommons.org/licenses/by/4.0/legalcode].
Diagnosis
Anhanguerian pterodactyloid diagnosed by the following autapomorphies: (1) first tooth pair of the premaxilla and mandible smaller than other anterior teeth; (2) fourth up to seventh teeth smaller than third and eighth.
Description
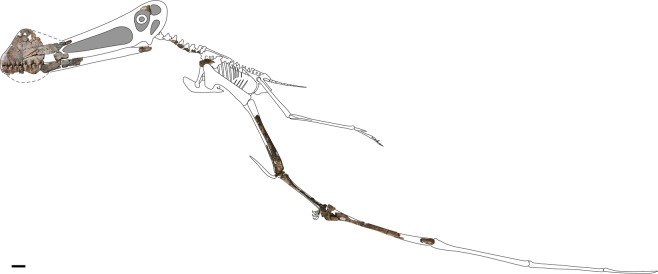
The holotype specimen of Ferrodraco lentoni (Fig. 2) is three-dimensionally preserved, with some elements crushed and distorted (e.g. ulna, radius, left metacarpal IV, manual phalanx IV-1). The fused scapulocoracoid and ossified extensor tendon process of wing phalanx IV-1 indicate that the type individual was ontogenetically mature; however, CT scans of the carpus demonstrate that the proximal and distal carpals were not sutured. Based on measurements of the type specimen (Table 1), and comparisons with other anhanguerian pterosaurs, the wingspan of Ferrodraco was approximately 4 m (see summary in Pentland and Poropat9).
Figure 2.
Ferrodraco lentoni gen. et sp. nov. holotype specimen AODF 876. All preserved elements were photographed and scaled to the same size, then articulated where possible. These were then used as the basis for the scaling of the skeletal reconstruction, the missing parts of which were based on the skeletal reconstruction of Tropeognathus mesembrinus by Witton63. Scale bar = 50 mm.
Table 1.
Measurements of Ferrodraco lentoni (AODF 876) in millimetres. Measurements based on incompletely preserved elements are indicated with an asterisk (*).
| Element | Anteroposterior length | Transverse width | Dorsoventral height | |||
|---|---|---|---|---|---|---|
| Upper jaw | 270* | 20* | 140* | |||
| Frontal | 40* | 19* | 38* | |||
| Lower jaw | 300* | 33* | 32* | |||
| Mandibular symphysis | 180* | 22* | 35* | |||
| Surangular, angular and articular | 50* | 23* | 20* | |||
| Cervical vertebra A | 27* | 18* | 18* | |||
| Cervical vertebra B | 28* | 19* | 9* | |||
| Cervical vertebra C | 21* | 11* | 21* | |||
| Cervical vertebra D | 16* | 15* | 7* | |||
| Cervical vertebra E | 13* | 14* | 5* | |||
| Measurement | Proximodistal length | Maximum width | Dorsoventral height | Proximodistal length | Maximum width | Dorsoventral height |
| Element | Left | Right | ||||
| Scapulocoracoid | — | — | — | 52* | 37* | — |
| Ulna | 235* | 57 | — | — | — | — |
| Radius | 110* | 24 | — | — | — | — |
| Carpus | 23 | 55 | — | — | — | — |
| Metacarpal IV | 205 | 37* | 19* | 100* | 45 | 27 |
| Manual phalanx IV—1 (proximal) | 310* | 54* | — | — | — | — |
| Manual phalanx IV-1 (distal) | 52* | 22* | — | — | — | — |
Assignment of Ferrodraco to the Anhangueria is supported by several cranial synapomorphies, including the presence of a mandibular groove, smooth and blade-like premaxillary and mandibular crests, and spike-shaped teeth22,23. Ferrodraco lentoni can be distinguished from other anhanguerians by the following combination of characters: anterior margin of the premaxilla flattened and triangular, first tooth pair of the premaxilla projecting vertically and slightly elevated relative to jawline; anterior portions of jaws not laterally expanded; vertically oriented teeth decreasing in size posteriorly; alveolar borders inflated relative to jawline; tall premaxillary crest level with anterior margin of skull, rises steeply at an angle of 60° with rounded dorsal margin.
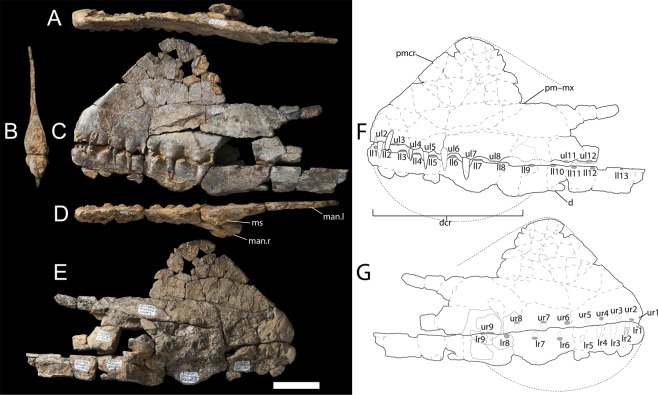
The premaxilla–maxilla, as preserved, is anteroposteriorly longer than it is dorsoventrally tall (Fig. 3; Table 1), with a transversely thin premaxillary crest (4 mm; Fig. 3). This comprises two thin lateral plates separated by trabeculae, as indicated by CT scans (Fig. 4A). The skull is not laterally expanded, as in other anhanguerian pterosaurs23. The premaxillary crest is incomplete posteriorly; however, the anterior margin is complete and confluent with the anterior margin of the skull, despite being inclined posterodorsally (Fig. 3C). The premaxillary crest is comparatively large, with a high and blunt anterior margin, as in Coloborhynchus clavirostris24 and Siroccopteryx moroccensis25. However, Ferrodraco differs from Coloborhynchus clavirostris because its first premaxillary teeth are smaller, vertically oriented, and more ventrally positioned. The premaxillary crest of Ferrodraco is dorsoventrally taller than that of Siroccopteryx25. The transverse width of the premaxillary crest in Ferrodraco is 2.5 mm, similar to that of Tropeognathus mesembrinus26 but divergent from the condition in Ornithocheirus simus27 wherein the crest is transversely wider. The crest of Ferrodraco, although broadly similar to that of Tropeognathus mesembrinus, differs in that it is less rounded overall, and in that its anterior margin slopes posterodorsally at an angle of 60° rather than ~70° (estimated based on published schematic in Wellnhofer26). As preserved, the premaxillary crest of Ferrodraco has an anteroposterior length of 131 mm and reaches its maximum dorsoventral height (128 mm, as measured from the ventral margin of the seventh alveolar rim) 99 mm posterior to the anterior margin of the skull, directly above the seventh upper alveolus.
Figure 3.
Ferrodraco lentoni gen. et sp. nov. holotype skull and mandible AODF 876. (A) dorsal view; (B) anterior view; (C) left lateral view; (D) ventral view; (E) right lateral view; (F) schematic of left lateral view; and (G) schematic of right lateral view. Abbreviations: d, dentary; dcr, (preserved base of) dentary crest; ll#, lower left (alveolus number); lr#, lower right (alveolus number); man, mandibular ramus; ms, mandibular symphysis; pmcr, premaxillary crest; pmx-mx, premaxilla–maxilla; ul#, upper left (alveolus number); ur#, upper right (alveolus number). Scale bar = 50 mm.
Figure 4.
Ferrodraco lentoni gen. et sp. nov. holotype rostral sections AODF 876. Cross-section (A) internal structure of premaxillary crest; (B) weak mandibular groove (grey arrow) and internal structure indicates the mandible is incomplete ventrally; (C–I) demonstrate variation in the depth of the palatal ridge and corresponding mandibular groove, with ironstone matrix represented by shaded region; (H) demonstrates replacement tooth is located lingual to the functional tooth; (J) section through (H) demonstrating the replacement tooth is distal to the functional tooth. The location of the functional and replacement teeth was based on their pulp cavities (black). Co-ossified dentaries in (K) anterior and (L) dorsal (occlusal) views. (M) Co-ossified premaxillae and maxillae in ventral (occlusal) view. (N) Schematic of premaxillae and maxillae in ventral (occlusal) view showing the subtle longitudinal palatal ridge. Abbreviations: ft, functional tooth; ll#, lower left; prid, palatal ridge; rt, replacement tooth; ul#, upper left; ur#, upper right. Scale bars: 20 mm for all figures.
An anteroposteriorly elongate, transversely narrow palatal ridge, which occludes with the mandibular groove (observed in CT data), initiates posterior to the second pair of alveoli (Fig. 4B). The apparent longitudinal groove visible on the mandible in dorsal view (Fig. 4L) is an impression of the palatal ridge in adherent ironstone matrix. Concomitant with the palatal ridge, this groove (again, based on CT data) became deeper and more pronounced posteriorly (Fig. 4B–I), only terminating at the posterior margin of the mandibular symphysis (Fig. 4I), as in Tropeognathus mesembrinus26. The mandibular symphysis, formed by the complete coalescence of the dentaries anteriorly, is 180 mm long anteroposteriorly. This is significantly shorter than that of Anhanguera piscator (259 mm)28 and of the similarly sized specimen Staatliche Naturwissenschaftliche Sammlungen Bayerns–Bayerische Staatssammlung für Paläontologie und Geologie (SNSB–BSPG, Munich, Germany) 1987 I 47 (“Anhanguera robustus”)28. Although no precise length measurement for the mandibular symphysis of Tropeognathus mesembrinus has been published, Wellnhofer26 stated that it almost equal to one-third the total dentary length in that taxon (520 mm; so ~173 mm) — absolutely shorter than in Ferrodraco. The preserved portions of the diverging mandibular rami in Ferrodraco were relatively straight, although their morphology further posteriorly cannot be determined. The symphysis is not laterally expanded.
The anterior margin of the mandible is rounded in lateral view. The ventral margin of the anteriormost portion of the mandibular symphysis is convex, but is also mostly missing on the right side. Nevertheless, the preserved portion of the left dentary forms a laterally compressed plate of bone that is inclined ventromedially (Fig. 4B), and is interpreted here as evidence for a mandibular crest. CT scan data demonstrate that the internal texture of the dentary at its incomplete ventral margin is similar to that of the premaxillae. Furthermore, the anteroventral dip of the ventral margin of the dentaries, anterior to the mandibular symphysis, supports the existence of a mandibular crest. Given that it clearly did not extend posterior to the mandibular symphysis, the maximum length of this crest was 126 mm, whereas it was clearly taller dorsoventrally than the tallest preserved portion (10 mm). The preserved portion of the left dentary hosts thirteen alveoli, whereas nine alveoli are present on the preserved portion of the right one (Fig. 3F,G).
The left premaxilla–maxilla preserves the anteriormost eight consecutive alveoli and two additional more mesial alveoli interpreted as 11 and 12 based on interalveolar spacing and the number of teeth in the left dentary, which preserves thirteen alveoli in total (Fig. 3F). By contrast, the right premaxilla–maxilla preserves the nine anteriormost consecutive alveoli (Fig. 3G). The first pair of premaxillary teeth is positioned slightly dorsal to the rest of the tooth row, as in Tropeognathus mesembrinus26. Isolated jaw fragments preserving vacant alveoli were also recovered from the site, but their absolute positions within the jaws cannot be determined.
The lateral surfaces of the premaxillae–maxillae and dentaries show undulation, as a result of the inflation of the alveolar borders relative to the jawline. The degree of alveolar border inflation decreases posteriorly from the eighth alveolus on the left dentary. Based on their alveolar diameter, the maxillary teeth are subequal in size to, if not slightly larger than, the dentary teeth. In both the premaxillae and dentaries, the first tooth pair is situated on the anteriormost margin.
More than 40 isolated teeth and tooth fragments are present in the Ferrodraco lentoni holotype (see Supplementary Information) and, in some cases, their corresponding alveoli have been identified. The teeth are spike-shaped, vertically oriented and slightly lingually recurved, with the displacement of tooth curvature less than the tooth diameter. The transverse cross-sectional shape of the base of each tooth is oval, with each being longer mesiodistally than wide labiolingually, and the crown height of each tooth being less than four times its basal width. The spacing between successive teeth is greater than the diameter of either of those teeth, and the mesiodistal length of the interalveolar spaces increases posteriorly. CT scan data demonstrate that Ferrodraco had a distolingual pattern of tooth replacement (Fig. 4H–J), lending further support to the hypothesis that this mode of replacement was typical for dentulous pterosaurs29.
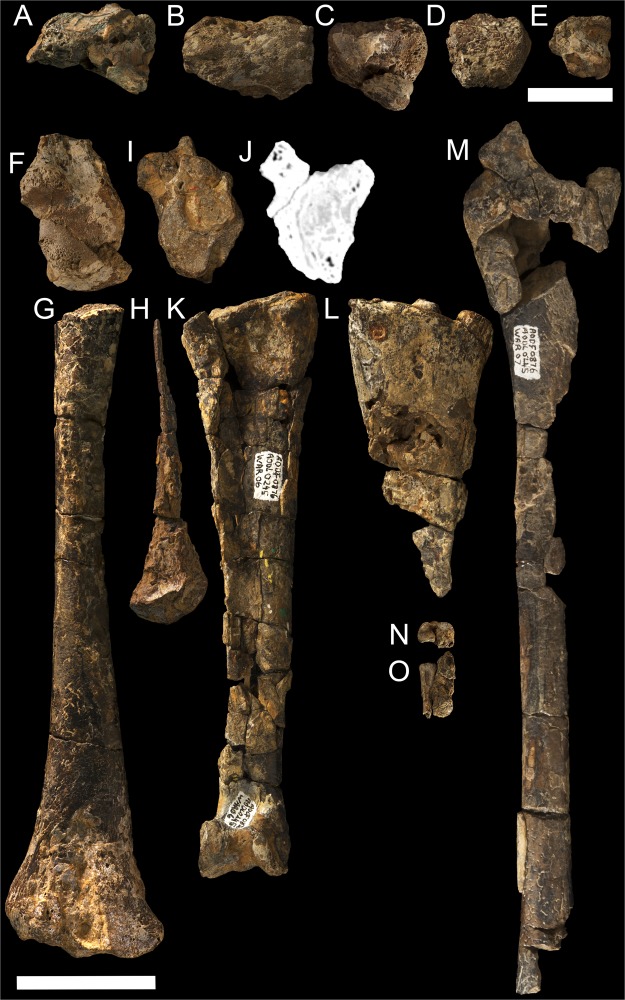
Five partial cervical vertebrae of Ferrodraco are preserved (Fig. 5A–E); however, their respective positions within the cervical series cannot be determined because of their fragmentary state. The right scapulocoracoid of Ferrodraco is represented only by part of the glenoid fossa, including the supraglenoidal buttress, the articular surface and the lower tubercle (Fig. 5F). The left ulna is incompletely preserved, being represented by most of the shaft and the distal end (Fig. 5G). The dorsal half of the distal articular surface of the ulna is saddle-shaped (concave dorsoventrally, convex anteroposteriorly), and the dorsal margin is a sharply-defined ridge. Centrally, the ulna bears a weakly developed tuberculum, and ventral to this the fovea carpalis is present. Only the distal portion of the left radius of Ferrodraco is preserved (Fig. 5H). The distal articular surface of the radius is incomplete but was clearly suboval in outline, as in other anhanguerians28,30, and dorsoventrally expanded. Although the preserved portion of the radius shaft is crushed and distorted, its diameter was clearly less than half that of the ulna.
Figure 5.
Ferrodraco lentoni gen. et sp. nov. holotype postcranial material AODF 876. (A–E) Cervical vertebrae A–E (all in ventral view); (F) Right scapulocoracoid (lateral view); (G) Left ulna preserving distal end (anterior view); (H) Left radius preserving distal end (posterior view); (I) Left syncarpus (distal view); (J) Internal structure of left syncarpus (ventral view), showing that the proximal (left) and distal (right) carpals were preserved together but were not fused (contact line indicated by red arrow); (K) Left metacarpal IV (posterior view); (L) Proximal end of right metacarpal IV (posterior view); (M) Proximal end of left wing phalanx I (dorsal view); (N,O) Proximal end of non-wing manual phalanx (with adherent matrix) in proximal (N) and (O) ventral views). Scale bars: A–E = 20 mm, F–O = 50 mm.
The left syncarpus is incomplete, and represented by the proximal and distal carpals preserved together but not fused; the lateral carpal is not preserved (Fig. 5I,J). The distal and proximal articular surfaces of the carpus are subequal in size, with the distal carpal triangular in outline. Both left and right fourth metacarpals are present in the holotype of Ferrodraco (Fig. 5J,K). Although only the proximal portion of right metacarpal IV has been recovered, it is better preserved than left metacarpal IV, which is complete but anteroposteriorly flattened. The proximal end of left metacarpal IV is less robust than the right, although this is clearly a result of distortion. Given that the right element is regarded as being more representative of the original morphology of metacarpal IV, it is clear that the proximal articular surface of this element was subrectangular in outline. The left metacarpal IV of Ferrodraco is proximodistally shorter than those of Anhanguera piscator (256 mm)28, Arthurdactylus conandoylei (227 mm)31, Staatliches Museum für Naturkunde Karlsruhe (SMNK, Karlsruhe, Germany) 1136 PAL (254 mm)31, and Museums Victoria (NMV, Melbourne, Australia) P197962 (212 mm)7. By contrast, it is proximodistally longer than those of Boreopterus cuiae (94 mm)32, SMNK 1134 PAL (165 mm)31 and SMNK 1135 PAL (175 mm)31. Despite the poor preservation of left metacarpal IV, it is clear that the dorsal and ventral distal condyles were separated by a median ridge. Only fragments of digits I–III and the proximal end of an indeterminate manual phalanx found in matrix has been recovered (Fig. 5M,N). Preliminary observation of the latter suggests that another, less well-preserved manual phalanx is also preserved. Although part of right metacarpal IV was recovered, the preserved portions of digits I–III are most likely from the left side, given that they were found in close proximity to left metacarpal IV. The manual phalanges are round to subtriangular in transverse cross-section. The left first wing phalanx is represented by the proximal portion and shaft (Fig. 5L), with the proximal articular surface (Table 1) dorsoventrally shorter than that of Anhanguera piscator (65 mm)28. The extensor tendon process also bears an oval pneumatic foramen, which is visible in ventral view.
Comparisons with australian pterosaurs
Comparisons between Ferrodraco and Mythunga
Given that only the anterior third of the skull and mandible (Fig. 6A), and the posteriormost portion of the left mandible, of Ferrodraco is preserved, there is little anatomical overlap between it and the holotype specimen of Mythunga camara5,9, which preserves only the mid-section (Fig. 6B). The CT scan data did not unequivocally demonstrate that any sections of the nasoantorbital fenestra margin were preserved in Ferrodraco; thus, the only anatomical landmark preserved in both it and Mythunga was the mandibular symphysis. Unfortunately, the position of the mandibular symphysis relative to the anterior margin of the nasoantorbital fenestra shows significant variation among anhanguerians9. Consequently, we restricted our comparisons between Ferrodraco and Mythunga to the teeth and alveoli.
Figure 6.
Australian pterosaur holotype cranial material. (A) Ferrodraco lentoni gen. et sp. nov. holotype skull and mandible (AODF 876); (B) Mythunga camara Molnar and Thulborn5 holotype skull and mandible (QM F18896); and (C) Aussiedraco molnari Kellner, Rodrigues and Costa8 holotype mandible (QM F10613). Scale bar = 20 mm.
The teeth of Mythunga and Ferrodraco are similar in that they are spike-shaped and labiolingually recurved; however, the teeth of Mythunga are much more robust, with the height to mesiodistal width ratio of complete teeth varying between 1.3 and 2.0 (see Table 2 in Pentland and Poropat9). By contrast, in Ferrodraco this ratio varies between 2.3 and 7.5 (see Supplementary Information). The fact that the teeth preserved are from the mid-section has probably impacted the degree of variation observed in this ratio, given that anterior teeth are larger than the posterior teeth in members of this clade. It is therefore likely that the anterior teeth of Mythunga would have been even larger and more robust than in Ferrodraco.
Ferrodraco and Mythunga are similar in that both have raised alveolar borders; however, these appear to be more prominent in the anteriormost portion of the Mythunga holotype. Unlike Mythunga, Ferrodraco does not appear to possess nutrient foramina in association with its alveolar borders, although this might be an artefact of preservation. CT scan data did not unequivocally demonstrate nutrient foramina were preserved in association with alveolar borders in Ferrodraco. Although the total tooth count cannot be determined for either Ferrodraco or Mythunga, provisional comparisons can be made based on the interalveolar spacing observed posterior to the mandibular symphysis. The interalveolar spacing increases posterior to the mandibular symphysis in Ferrodraco, whereas in Mythunga it does not decrease until four tooth positions posterior to the mandibular symphysis (see Table 3 in Pentland and Poropat9). These differences in interalveolar spacing and dentition imply that both tooth count and arrangement were different in these taxa.
Comparisons between Ferrodraco and Aussiedraco
The most striking difference between Aussiedraco (Fig. 6C) and Ferrodraco is that the latter possesses a mandibular crest; consequently, the mandible of Ferrodraco is relatively and absolutely taller dorsoventrally than that of Aussiedraco, despite the incomplete preservation of its ventral margin. However, given that some pterosaurs were evidently sexually dimorphic, with the most obvious manifestation of this being the development (or not) of the cranial crest(s)33, this character alone is insufficient to distinguish Ferrodraco from Aussiedraco.
The mandible of Ferrodraco is more distally expanded and more robust than that of Aussiedraco. Furthermore, the left lateral surface of the mandible of Ferrodraco is slightly convex, whereas that of Aussiedraco is relatively straight. The occlusal surface of the mandibular symphysis in Ferrodraco is straight in lateral view, whereas in Aussiedraco it is markedly convex. In Aussiedraco, the anterior portion of the mandibular groove is more prominent than that of Ferrodraco, and appears most pronounced from the posterior margin of the third alveolus until the posterior margin of the fourth alveolus in the former taxon. However, in Ferrodraco the mandibular groove extends from the posterior margin of the 2nd tooth locus to the mandibular symphysis, which might not have been the case in Aussiedraco.
Aussiedraco differs from Ferrodraco (and Mythunga) in that its alveolar borders are not raised. As described by Kellner et al.8, the first four alveoli of Aussiedraco are oriented slightly more dorsolaterally than the fifth alveolus, which faces more dorsally. By contrast, all dentary alveoli anterior to the mandibular symphysis in Ferrodraco face dorsally. The mesiodistal lengths of the first two pairs of alveoli in Aussiedraco are greater than those of Ferrodraco. Although this might indicate that the anteriormost teeth of Aussiedraco were larger than those of Ferrodraco, it is possible that the Aussiedraco type specimen was slightly eroded prior to fossilisation, which would have exaggerated these features. Furthermore, if the suggestion that the first tooth pair in Aussiedraco was occupied by near-procumbent teeth is correct, then it differs from the condition in Ferrodraco, wherein the first tooth pair of the mandible are vertically oriented.
Perhaps most tellingly, two sulci were observed on the rostral tip of the mandible of Aussiedraco that were not present in Ferrodraco. Small sulci in this position on the mandible have not been reported in any other pterosaurs. However, it is possible that the Aussiedraco type specimen was slightly over-prepared (with acetic acid), in which case it is entirely possible that the two sulci might represent the bifurcating end of a mandibular groove. If this is correct, then this constitutes the second report of a bifurcating mandibular groove in anhanguerians. The first such report was made by Vila Nova et al.34 in their assessment of the Cearadactylus atrox holotype, which also lacks a mandibular crest35. This combination of characters, which has not been reported in any other pterosaurs, potentially represents a synapomorphy of Aussiedraco and Cearadactylus. However, Cearadactylus atrox differs from Aussiedraco in that it possesses a laterally expanded dentary.
Phylogenetic results
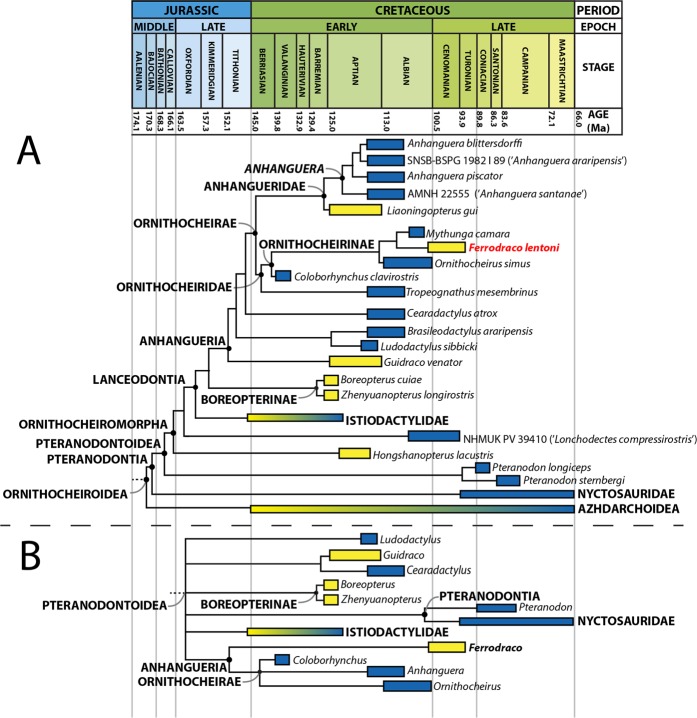
We included Ferrodraco lentoni in two phylogenetic analyses. In the first (modified from Pentland and Poropat9, which was in turn modified from Andres et al.19) Ferrodraco was resolved as the sister taxon to Mythunga camara (Fig. 7A). Ornithocheirus simus and Coloborhynchus clavirostris were resolved as the successive sister taxa to this clade within Ornithocheirinae, and Tropeognathus mesembrinus was resolved as sister taxon to Ornithocheirinae within Ornithocheiridae. In the second analysis (modified from Lü et al.36), Ferrodraco was resolved as the sister taxon to a polytomy (equivalent to Ornithocheirae sensu Andres et al.19) comprising Anhanguera, Coloborhynchus and Ornithocheirus (Fig. 7B); this clade fits the definition of Anhangueria sensu Andres et al.19.
Figure 7.
Time-calibrated phylogenetic trees of Ornithocheiroidea (Pterosauria: Pterodactyloidea), with some non-anhanguerian nodes collapsed for simplicity. The box next to each taxon demarcates its temporal range (including stratigraphic uncertainty), whereas the colour of each box reflects the palaeoenvironmental setting from which the taxon derives (yellow = terrestrial; blue = marine). (A) Tree based on the matrix of Andres et al.19, with Ferrodraco lentoni gen. et sp. nov. and Mythunga camara included; (B) Tree based on the matrix of Lü et al.36, with Ferrodraco lentoni gen. et sp. nov. included.
Discussion
Phylogenetic analysis indicates that Ferrodraco (Fig. 8) and Mythunga belong to the Ornithocheirinae sensu Andres et al.19, with two pterosaurs from England — Ornithocheirus simus from the Albian Cambridge Greensand and Coloborhynchus clavirostris from the Valanginian Hastings Beds — as successive sister taxa within that clade. The only non-ornithocheirine ornithocheirid in our analysis was Tropeognathus mesembrinus from the Albian Romualdo Formation of Brazil. Given that the Australian pterosaurs are more closely related to taxa from England than to forms from Brazil, it would seem that ornithocheirids were cosmopolitan during the Early–mid-Cretaceous, which contrasts with the Gondwanan provincialism shown in several terrestrial vertebrate clades during that time37–40. Although this result might be viewed as somewhat surprising, given the incompleteness of the English material, this result is consistent with previous interpretations that ornithocheirids could easily disperse across oceanic barriers41–43.
Figure 8.
Life restoration of Ferrodraco lentoni gen. et sp. nov. as an ornithocheirid pterosaur. Illustration by T.R.T.
Although our results do not support a specific biogeographic signal, many late Early Cretaceous pterosaur faunas that include anhanguerians also feature azhdarchoids. This includes the Barremian–Aptian Jehol Biota (summarised by Zhou44), the Aptian–Albian limestones of the Araripe Basin (summarised by Unwin and Martill42 and Leal et al.45), and the Albian–lower Cenomanian Kem Kem Beds (summarised by Jacobs et al.46). At present, Ornithostoma sedgwicki47 is the only edentulous pterosaur reported from the Cambridge Greensand; nevertheless, this consistent biogeographical pattern suggests that future excavations in the Winton Formation might also result in the discovery of azhdarchoids.
It has been suggested that anhanguerians went extinct at the end of the Cenomanian, based on their apparent absence from post-Cenomanian strata43. Their apparent absence follows a period of major environmental disturbance characterised by an increase in atmospheric and oceanic surface temperatures48–50, an increase in atmospheric carbon dioxide51,52, a global oceanic anoxic event52–54, and marine transgression49,55,56. At present, the majority of anhanguerians are known from restricted lagoon environments, with stable isotopic analysis of isolated teeth derived from the Araripe Basin indicating a piscivorous diet comprising both freshwater and marine fish57. Given that marine vertebrates and invertebrates were most severely impacted during the Cenomanian–Turonian event58, we suggest that anhanguerians were also impacted by a disruption in trophic interactions. However, if the teeth sampled from the Araripe Basin are not diagenetically altered and are indicative of the feeding behaviours of this clade more broadly, it is possible that anhanguerians in terrestrial settings might have persisted beyond the Cenomanian.
The presence of an anhanguerian in the Winton Formation is not surprising, given that several pterosaurs referred to the Ornithocheiridae have been reported from the underlying upper Albian Toolebuc and Mackunda formations. However, recent analyses of detrital zircons obtained from localities in the Winton area by Tucker et al.20,21 suggest that deposition of the northern part of the Winton Formation — from which Ferrodraco lentoni derives — might have taken place as late as the early Turonian. Given that Ferrodraco derives from a locality northeast of Winton, it potentially represents a late-surviving member of the Ornithocheiridae specifically, and of Anhangueria more broadly. Another possible anhanguerian from the post-Cenomanian of Australia is represented by a jaw fragment bearing two raised alveoli (WAM 68.5.11), derived from the Molecap Greensand and described by Kear et al.12. The jaw fragment was tentatively assigned by those authors to the Ornithocheiridae (sensu Unwin17) or Anhangueridae (sensu Kellner23). As noted by Kear et al.12, the Molecap Greensand preserves pollen and dinocysts that vary in age from the Cenomanian through to Coniacian. Given that both the Molecap Greensand and the Ferrodraco type locality lack refined age constraints, considerable further work is required before it can be determined whether Australia was a refugium for anhanguerian pterosaurs during the Late Cretaceous. At the very least, however, a strong case can be made for their persistence until the end-Cenomanian.
Methods
CT scan analysis
The Ferrodraco lentoni holotype specimen, AODF 876, was CT scanned at St Vincent’s Hospital Melbourne (Victoria, Australia) using a Revolution CT scanner (GE Medical Systems). The specimen was scanned at 140 kV and 401.5 mA, obtaining 0.625 cm voxel size. The CT data was imported into Mimics 21 (Materialise, Belgium) to enable visualisation of internal features.
Nomenclatural acts
This published work and the nomenclatural acts it contains have been registered in ZooBank, the proposed online registration system for the International Code of Zoological Nomenclature. The ZooBank Life Science Identifiers (LSIDs) can be resolved and the associated information viewed by appending the LSIDs to the prefix http://zoobank.org/. The LSID for this publication is urn:lsid:zoobank.org:pub:5FC8D723-2340-4331-BD3A-87A3F5A95C80, and the LSIDs for the new erected groups and taxa are: urn:lsid:zoobank.org:act:435795CA-3D70-4895-98AD-AA277351C0E2 (Ferrodraco), and urn:lsid:zoobank.org:act:9A96B460-92A9-4259-AF2D-9A7C9FB6E75A (Ferrodraco lentoni).
Phylogenetic analysis
In order to constrain the phylogenetic position of Ferrodraco lentoni, we scored it for two phylogenetic datasets. The first was the modified version of the Andres et al.19 dataset employed by Pentland and Poropat9 to constrain the phylogenetic position of Mythunga camara; the second was that of Lü et al.36, for which we scored both Ferrodraco lentoni and Mythunga camara. In the latter dataset, we also changed the scores of characters 51–65 from ‘0’ to ‘−’ for all edentulous taxa (i.e. Nyctosaurus, Muzquizopteryx, Pteranodon, Tapejara, Tupandactylus, Sinopterus, Huaxiapterus, Shenzhoupterus, Chaoyangopterus, Tupuxuara, Thalassodromeus, Quetzalcoatlus, Zhejiangopterus and Azhdarcho); however, this had no discernible effect on the outcome.
Analyses of both datasets were conducted in TNT v. 1.5 (Tree analysis using New Technology)59. We analysed the modified Andres et al.19 matrix following the methodology used by the original authors. This resulted in a single most parsimonious tree (MPT) of 866.272 steps, which was almost completely resolved (except for the three taxa within Nyctosauridae, which formed a polytomy as in the original analysis). The inclusion of Ferrodraco lentoni stabilised the position of Mythunga camara within Anhangueria, such that this clade was fully resolved in this analysis (unlike in Pentland and Poropat9, wherein much of Anhangueria lacked resolution). We also analysed the modified Lü et al.36 following the methodology used by the original authors, although the inclusion of Mythunga camara proved sufficiently problematic that it was pruned. Analysis of the pruned matrix resulted in 31,116 MPTs of 517 steps. The topology of the strict consensus tree obtained from these 31,116 MPTs was almost identical to that of the original tree presented by Lü et al.36. However, in our tree Pteranodontia was slightly better resolved, with Elanodactylus, Beipiaopterus, Feilongus and Moganopterus basal to other pteranodontians.
Supplementary information
Acknowledgements
The authors would like to thank: all of the Australian Age of Dinosaurs Natural History Museum (AAOD) staff and volunteers who participated in the excavations at the ‘Pterosaur Site’; AAOD volunteer Ali Calvey for preparing AODF 876 (a.k.a. ‘Butch’); Suzanne McNestrie and her colleagues at St Vincent’s Hospital Melbourne for CT scanning AODF 876; Tim Ziegler (Museums Victoria) for helping to organise the CT scanning of AODF 876 and for allowing A.H.P and S.F.P. access to photographic equipment at Museums Victoria; and Olga Panagiotopoulou and Hyab Mehari Abraha (Monash University) for enabling S.F.P. to analyse the CT scan data of AODF 876 using Mimics. The authors would also like to thank: the Paleontological Society for an Arthur James Boucot Research Grant (awarded to S.F.P. in 2017), which enabled A.H.P. and S.F.P. to make firsthand observations of the Mythunga and Aussiedraco type specimens at Queensland Museum; Andrew Rozefelds, Scott Hocknull and Kristen Spring (all Queensland Museum) for allowing A.H.P. and S.F.P. access to the Mythunga and Aussiedraco holotype specimens; the Willi Hennig Society; and Patricia Vickers-Rich, Robin Beck and two anonymous reviewers for their helpful reviews that greatly improved the manuscript.
Author Contributions
A.H.P. and S.F.P. designed the project. A.H.P., T.S., R.A.E., H.A.E., J.A.E. and D.A.E. oversaw the collection, preparation and curation of the fossils. A.H.P. and S.F.P. described the specimens and scored them for the phylogenetic analyses, and S.F.P. ran the analyses. A.H.P., S.F.P. and T.R.T. assembled the figures. All authors contributed to the writing of the paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-49789-4.
References
- 1.Barrett PM, Butler RJ, Edwards NP, Milner AR. Pterosaur distribution in time and space: an atlas. Zitteliana B. 2008;28:61–107. [Google Scholar]
- 2.Steel L. The palaeohistology of pterosaur bone: an overview. Zitteliana B. 2008;28:109–125. [Google Scholar]
- 3.Molnar RE, Thulborn RA. First pterosaur from Australia. Nature. 1980;288:361–363. doi: 10.1038/288361a0. [DOI] [Google Scholar]
- 4.Molnar RE. A pterosaur pelvis from western Queensland, Australia. Alcheringa. 1987;11:87–94. doi: 10.1080/03115518708618981. [DOI] [Google Scholar]
- 5.Molnar RE, Thulborn RA. An incomplete pterosaur skull from the Cretaceous of north-central Queensland, Australia. Arquivos do Museu Nacional, Rio de Janeiro. 2007;65:461–470. [Google Scholar]
- 6.Fletcher TL, Salisbury SW. New pterosaur fossils from the Early Cretaceous (Albian) of Queensland, Australia. Journal of Vertebrate Paleontology. 2010;30:1747–1759. doi: 10.1080/02724634.2010.521929. [DOI] [Google Scholar]
- 7.Kellner AWA, et al. New isolated pterodactyloid bones from the Albian Toolebuc Formation (western Queensland, Australia) with comments on the Australian pterosaur fauna. Alcheringa. 2010;34:219–230. doi: 10.1080/03115511003656552. [DOI] [Google Scholar]
- 8.Kellner AWA, Rodrigues T, Costa FR. Short note on a pteranodontoid pterosaur (Pterodactyloidea) from western Queensland, Australia. Anais da Academia Brasileira de Ciências. 2011;83:301–308. doi: 10.1590/S0001-37652011000100018. [DOI] [PubMed] [Google Scholar]
- 9.Pentland AH, Poropat SF. Reappraisal of Mythunga camara Molnar & Thulborn, 2007 (Pterosauria, Pterodactyloidea, Anhangueria) from the upper Albian Toolebuc Formation of Queensland, Australia. Cretaceous Research. 2019;93:151–169. doi: 10.1016/j.cretres.2018.09.011. [DOI] [Google Scholar]
- 10.Brougham T, Smith ET, Bell PR. Isolated teeth of Anhangueria (Pterosauria: Pterodactyloidea) from the Lower Cretaceous of Lightning Ridge, New South Wales, Australia. PeerJ. 2017;5:e3256. doi: 10.7717/peerj.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett SC, Long JA. A large pterodactyloid pterosaur from the Late Cretaceous (late Maastrichtian) of Western Australia. Records of the Western Australian Museum. 1991;15:435–443. [Google Scholar]
- 12.Kear BP, Deacon GL, Siverson M. Remains of a Late Cretaceous pterosaur from the Molecap Greensand of Western Australia. Alcheringa. 2010;34:273–279. doi: 10.1080/03115511003661651. [DOI] [Google Scholar]
- 13.Rich THV, Rich PV. Polar dinosaurs and biotas of the Early Cretaceous of southeastern Australia. National Geographic. Research. 1989;5:15–53. [Google Scholar]
- 14.Poropat SF, et al. Early Cretaceous polar biotas of Victoria, southeastern Australia—an overview of research to date. Alcheringa. 2018;42:157–229. doi: 10.1080/03115518.2018.1453085. [DOI] [Google Scholar]
- 15.Averianov A. Review of taxonomy, geographic distribution, and paleoenvironments of Azhdarchidae (Pterosauria) ZooKeys. 2014;432:1–107. doi: 10.3897/zookeys.432.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unwin DM. An overview of the pterosaur assemblage from the Cambridge Greensand (Cretaceous) of Eastern England. Mitteilungen aus dem Museum für Naturkunde in Berlin, Geowissenschaftliche Reihe. 2001;4:189–221. doi: 10.1002/mmng.20010040112. [DOI] [Google Scholar]
- 17.Unwin, D. M. In Evolution and Palaeobiology of Pterosaurs; Geological Society Special Publication 217 (eds Buffetaut, E. & Mazin, J.-M.), 139–190 (Geological Society, 2003).
- 18.Andres B, Myers TS. Lone star pterosaurs. Earth and Environmental Science Transactions of the Royal Society of Edinburgh. 2013;103:383–398. doi: 10.1017/S1755691013000303. [DOI] [Google Scholar]
- 19.Andres B, Clark J, Xu X. The earliest pterodactyloid and the origin of the group. Current Biology. 2014;24:1011–1016. doi: 10.1016/j.cub.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 20.Tucker RT, Roberts EM, Hu Y, Kemp AIS, Salisbury SW. Detrital zircon age constraints for the Winton Formation, Queensland: contextualizing Australia’s Late Cretaceous dinosaur faunas. Gondwana Research. 2013;24:767–779. doi: 10.1016/j.gr.2012.12.009. [DOI] [Google Scholar]
- 21.Tucker RT, Roberts EM, Darlington V, Salisbury SW. Investigating the stratigraphy and palaeoenvironments for a suite of newly discovered mid-Cretaceous vertebrate fossil-localities in the Winton Formation, Queensland, Australia. Sedimentary Geology. 2017;358:210–229. doi: 10.1016/j.sedgeo.2017.05.004. [DOI] [Google Scholar]
- 22.Campos DDA, Kellner A. Panorama of the flying reptiles study in Brazil and South America. Anais da Academia Brasileira de Ciências. 1985;57:453–466. [Google Scholar]
- 23.Kellner, A. W. A. In Evolution and Palaeobiology of Pterosaurs; Geological Society Special Publication 217 (eds Buffetaut, E. & Mazin, J.-M.) 105–137 (Geological Society, 2003).
- 24.Owen R. Monograph of the fossil Reptilia of the Mesozoic formations. Part I. Pterosauria (Pterodactylus). (Gault-Lias) Palaeontographical Society Monographs. 1874;27:1–14. [Google Scholar]
- 25.Mader BJ, Kellner AWA. A new anhanguerid pterosaur from the Cretaceous of Morocco. Boletim do Museu Nacional, Nova Serie, Geologia. 1999;45:1–11. [Google Scholar]
- 26.Wellnhofer P. New crested pterosaurs from the Lower Cretaceous of Brazil. Mitteilungen der Bayerischen Staatssammlung für Paläontologie und Historische. Geologie. 1987;27:175–186. [Google Scholar]
- 27.Rodrigues T, Kellner AWA. Taxonomic review of the Ornithocheirus complex (Pterosauria) from the Cretaceous of England. ZooKeys. 2013;308:1–112. doi: 10.3897/zookeys.308.5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kellner AWA, Tomida Y. Description of a new species of Anhangueridae (Pterodactyloidea) with comments on the pterosaur fauna from the Santana Formation (Aptian–Albian), Northeastern Brazil. National Science Museum Monographs. 2000;17:1–135. doi: 10.1159/000061634. [DOI] [Google Scholar]
- 29.Fastnacht M. Tooth replacement pattern of Coloborhynchus robustus (Pterosauria) from the Lower Cretaceous of Brazil. Journal of Morphology. 2008;269:332–348. doi: 10.1002/jmor.10591. [DOI] [PubMed] [Google Scholar]
- 30.Wellnhofer P. Neue Pterosaurier aus der Santana-Formation der Chapada do Araripe, Brasilien. Palaeontographica Abteilung A. 1985;187:105–182. [Google Scholar]
- 31.Frey E, Martill DM. A new pterosaur from the Crato Formation (Lower Cretaceous, Aptian) of Brazil. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen. 1994;194:379–412. [Google Scholar]
- 32.Lü J, Ji Q. A new ornithocheirid from the Early Cretaceous of Liaoning Province, China. Acta Geol. Sin. 2005;79:157–163. doi: 10.1111/j.1755-6724.2005.tb00877.x. [DOI] [Google Scholar]
- 33.Wang X, et al. Sexually dimorphic tridimensionally preserved pterosaurs and their eggs from China. Current Biology. 2014;24:1323–1330. doi: 10.1016/j.cub.2014.04.054. [DOI] [PubMed] [Google Scholar]
- 34.Vila Nova BC, Sayão JM, Neumann VHML, Kellner AWA. Redescription of Cearadactylus atrox (Pterosauria, Pterodactyloidea) from the Early Cretaceous Romualdo Formation (Santana Group) of the Araripe Basin, Brazil. Journal of Vertebrate Paleontology. 2014;34:126–134. doi: 10.1080/02724634.2013.793694. [DOI] [Google Scholar]
- 35.Leonardi G, Borgomanero G. Cearadactylus atrox nov. gen., nov. sp.: novo Pterosauria (Pterodactyloidea) da Chapada do Araripe, Ceara, Brasil. Trabalhos apresentados no VIII Congresso Brasileiro de Paleontologia. 1985;2:75–80. [Google Scholar]
- 36.Lü Junchang, Meng Qingjin, Wang Baopeng, Liu Di, Shen Caizhi, Zhang Yuguang. Short note on a new anurognathid pterosaur with evidence of perching behaviour from Jianchang of Liaoning Province, China. Geological Society, London, Special Publications. 2017;455(1):95–104. doi: 10.1144/SP455.16. [DOI] [Google Scholar]
- 37.Smith ND, et al. A Megaraptor-like theropod (Dinosauria: Tetanurae) in Australia: support for faunal exchange across eastern and western Gondwana in the Mid-Cretaceous. Proceedings of the Royal Society B. 2008;275:2085–2093. doi: 10.1098/rspb.2008.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agnolin FL, Ezcurra MD, Pais DF, Salisbury SW. A reappraisal of the Cretaceous non-avian dinosaur faunas from Australia and New Zealand: evidence for their Gondwanan affinities. Journal of Systematic Palaeontology. 2010;8:257–300. doi: 10.1080/14772011003594870. [DOI] [Google Scholar]
- 39.Herne MC, Nair JP, Salisbury SW. Comment on “A southern tyrant reptile”. Science. 2010;329:1013–c. doi: 10.1126/science.1190100. [DOI] [PubMed] [Google Scholar]
- 40.Novas FE, Agnolín FL, Ezcurra MD, Porfiri J, Canale JI. Evolution of the carnivorous dinosaurs during the Cretaceous: the evidence from Patagonia. Cretaceous Research. 2013;45:174–215. doi: 10.1016/j.cretres.2013.04.001. [DOI] [Google Scholar]
- 41.Wellnhofer P. Weitere Pterosaurierfunde aus der Santana-Formation (Apt) der Chapada do Araripe, Brasilien. Palaeontographica Abteilung A. 1991;215:43–101. [Google Scholar]
- 42.Unwin, D. M. & Martill, D. M. In The Crato Fossil Beds of Brazil: Window into an Ancient World (eds Martill, D. M., Bechly, G. & Loveridge, R. F.) 475–524 (Cambridge University Press, 2007).
- 43.Upchurch P, Andres B, Butler RJ, Barrett PM. An analysis of pterosaurian biogeography: implications for the evolutionary history and fossil record quality of the first flying vertebrates. Historical Biology. 2015;27:697–717. doi: 10.1080/08912963.2014.939077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Z. The Jehol Biota, an Early Cretaceous terrestrial Lagerstätte: new discoveries and implications. National Science Review. 2014;1:543–559. doi: 10.1093/nsr/nwu055. [DOI] [Google Scholar]
- 45.Leal Maria E. C., Pêgas Rodrigo V., Bonde Niels, Kellner Alexander W. A. Cervical vertebrae of an enigmatic pterosaur from the Crato Formation (Lower Cretaceous, Araripe Basin, NE Brazil) Geological Society, London, Special Publications. 2017;455(1):195–208. doi: 10.1144/SP455.15. [DOI] [Google Scholar]
- 46.Jacobs ML, Martill DM, Ibrahim N, Longrich N. A new species of Coloborhynchus (Pterosauria, Ornithocheiridae) from the mid-Cretaceous of North Africa. Cretaceous Research. 2019;95:77–88. doi: 10.1016/j.cretres.2018.10.018. [DOI] [Google Scholar]
- 47.Averianov AO. Ornithostoma sedgwicki — valid taxon of azhdarchoid pterosaurs. Proceedings of the Zoological Institute RAS. 2012;316:40–49. doi: 10.1134/S0031030112050036. [DOI] [Google Scholar]
- 48.Barron EJ. A warm, equable Cretaceous: the nature of the problem. Earth-Science Reviews. 1983;19:305–338. doi: 10.1016/0012-8252(83)90001-6. [DOI] [Google Scholar]
- 49.Kerr AC. Oceanic plateau formation: a cause of mass extinction and black shale deposition around the Cenomanian–Turonian boundary? Journal of the Geological Society. 1998;155:619–626. doi: 10.1144/gsjgs.155.4.0619. [DOI] [Google Scholar]
- 50.Huber, B. T., Norris, R. D. & MacLeod, K. G. Deep-sea paleotemperature record of extreme warmth during the Cretaceous. Geology30, 123–126, doi: 10.1130/0091-7613(2002)030<0123:DSPROE>2.0.CO;2 (2002).
- 51.Hallam A. A review of Mesozoic climates. Journal of the Geological Society. 1985;142:433–445. doi: 10.1144/gsjgs.142.3.0433. [DOI] [Google Scholar]
- 52.Arthur M. A., Schlanger S. O., Jenkyns H. C. The Cenomanian-Turonian Oceanic Anoxic Event, II. Palaeoceanographic controls on organic-matter production and preservation. Geological Society, London, Special Publications. 1987;26(1):401–420. doi: 10.1144/GSL.SP.1987.026.01.25. [DOI] [Google Scholar]
- 53.Schlanger SO, Jenkyns HC. Cretaceous oceanic anoxic events: causes and consequences. Geologie en Mijnbouw. 1976;55:179–184. [Google Scholar]
- 54.Leckie RM, Bralower TJ, Cashman R. Oceanic anoxic events and plankton evolution: Biotic response to tectonic forcing during the mid‐Cretaceous. Paleoceanography. 2002;17:13-1–13-29. doi: 10.1029/2001PA000623. [DOI] [Google Scholar]
- 55.Hancock JM, Kauffman EG. The great transgressions of the Late Cretaceous. Journal of the Geological Society. 1979;136:175–186. doi: 10.1144/gsjgs.136.2.0175. [DOI] [Google Scholar]
- 56.Hallam A. The case for sea-level change as a dominant causal factor in mass extinction of marine invertebrates. Philosophical Transactions of the Royal Society of London B. 1989;325:437–455. doi: 10.1098/rstb.1989.0098. [DOI] [Google Scholar]
- 57.Amiot R, et al. Oxygen and carbon isotope compositions of middle Cretaceous vertebrates from North Africa and Brazil: Ecological and environmental significance. Palaeogeography, Palaeoclimatology, Palaeoecology. 2010;297:439–451. doi: 10.1016/j.palaeo.2010.08.027. [DOI] [Google Scholar]
- 58.Sepkoski, J. In Patterns and Processes in the History of Life (eds Raup, D. M. & Jablonski, D.), 277–295 (Springer, 1986).
- 59.Goloboff PA, Farris JS, Nixon KC. TNT, a free program for phylogenetic analysis. Cladistics. 2008;24:774–786. doi: 10.1111/j.1096-0031.2008.00217.x. [DOI] [Google Scholar]
- 60.Poropat SF, et al. New Australian sauropods shed light on Cretaceous dinosaur palaeobiogeography. Scientific Reports. 2016;6:34467. doi: 10.1038/srep34467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vine, R. R. Mackunda, Q. 1:250 000 Geological Series Sheet SF54-11 (Bureau of Mineral Resources, Geology and Geophysics, Canberra, Australia, 1964).
- 62.Vine, R. R. & Casey, D. J. Richmond, Queensland. 1:250 000 Geological Series Sheet SF 54-4 (Bureau of Mineral Resources, Geology and Geophysics, Canberra, Australia, 1967).
- 63.Witton, M. P. Pterosaurs: Natural History, Evolution, Anatomy, 291 pp. (Princeton University Press, 2013).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.