Abstract
Free and glycosylated sterols are both structural components of the plasma membrane that regulate their biophysical properties and consequently different plasma membrane-associated processes such as plant adaptation to stress or signaling. Several reports relate changes in glycosylated sterols levels with the plant response to abiotic stress, but the information about the role of these compounds in the response to biotic stress is scarce. In this work, we have studied the response to the necrotrophic fungus Botrytis cinerea in an Arabidopsis mutant that is severely impaired in steryl glycosides biosynthesis due to the inactivation of the two sterol glucosyltransferases (UGT80A2 and UGT80B1) reported in this plant. This mutant exhibits enhanced resistance against B. cinerea when compared to wild-type plants, which correlates with increased levels of jasmonic acid (JA) and up-regulation of two marker genes (PDF1.2 and PR4) of the ERF branch of the JA signaling pathway. Upon B. cinerea infection, the ugt80A2;B1 double mutant also accumulates higher levels of camalexin, the major Arabidopsis phytoalexin, than wild-type plants. Camalexin accumulation correlates with enhanced transcript levels of several cytochrome P450 camalexin biosynthetic genes, as well as of their transcriptional regulators WRKY33, ANAC042, and MYB51, suggesting that the Botrytis-induced accumulation of camalexin is coordinately regulated at the transcriptional level. After fungus infection, the expression of genes involved in the indole glucosinolate biosynthesis is also up-regulated at a higher degree in the ugt80A2;B1 mutant than in wild-type plants. Altogether, the results of this study show that glycosylated sterols play an important role in the regulation of Arabidopsis response to B. cinerea infection and suggest that this occurs through signaling pathways involving the canonical stress-hormone JA and the tryptophan-derived secondary metabolites camalexin and possibly also indole glucosinolates.
Keywords: Arabidopsis, biotic stress, Botrytis cinerea, camalexin, indole glucosinolates, JA signaling pathway, steryl glycosides
Introduction
Steryl glycosides (SGs) are conjugated forms of sterols resulting from the attachment through a glycosidic bond of a sugar residue, most commonly a single glucose monomer, to the free hydroxyl group at C3 position of the sterol backbone (Ferrer et al., 2017). This reaction is catalyzed by UDP-glucose:sterol glycosyltransferase (SGT; E.C. 2.4.1.173), an enzyme that has been cloned and functionally characterized from different organisms (Grille et al., 2010) including several plant species (Warnecke et al., 1997; DeBolt et al., 2009; Chaturvedi et al., 2012; Li et al., 2014; Tiwari et al., 2014; Ramírez-Estrada et al., 2017). The hydroxyl group at C6 position of the sugar moiety can in turn be esterified with a long-chain fatty acid to form acyl steryl glycosides (ASG), although the enzyme responsible for this transformation has not been identified yet (Ferrer et al., 2017). The content of glycosylated sterols (SG + ASG) differs among plant species and tissues, but in general, these compounds are relatively minor components ranging from 10% to 30% of the total sterol fraction, although there are some exceptions in the Solanaceae family, as for instance tomato and potato, in which glycosylated sterols are the predominant form of sterols (Moreau et al., 2002; Furt et al., 2010; Nyström et al., 2012).
The role of free sterols (FSs) as key structural components of the plasma membrane has been known for a long time. Free sterols help to modulate the plasma membrane biophysical properties and hence its biological function and the activity of membrane-bound proteins (Carruthers and Melchior, 1986; Cooke and Burden, 1990; Grandmougin-Ferjani et al., 1997). Free sterols have also been recognized as important modulators of plant growth and development (Schrick et al., 2000; Schrick et al., 2002; He et al., 2003; Carland et al., 2010; Ovecka et al., 2010; Qian et al., 2013; Nakamoto et al., 2015), and glycosylated sterols are also emerging as important players in determining plasma membrane organization and functionality (Moreau et al., 2002; Grosjean et al., 2015; Cassim et al., 2019). Like FSs, glycosylated sterols are unevenly distributed in the plasma membrane, and it is currently accepted that SG and ASG are also highly enriched alongside with sterols, sphingolipids and selected proteins in liquid-ordered phase domains referred to as membrane rafts or DRM (sterol-enriched detergent-resistant membrane fraction). These dynamic assemblies of lipids and proteins appear to be involved in different plant cell processes including polarized cell growth, cell-to-cell communication, intracellular membrane trafficking, and signal transduction cascades enabling plants to respond to environmental changes (Mongrand et al., 2010; Zauber et al., 2014; Gronnier et al., 2018). However, the specific role of glycosylated sterols in regulating membrane properties and function still remains uncertain, although some experimental evidences support the view that a proper ratio of the glycosylated versus free forms of sterols in cell membranes is essential for normal plant cell function and overall plant performance. Thus, an Arabidopsis null mutant defective in the two SGTs present in this species, namely, UGT80A2 and UGT80B1 (DeBolt et al., 2009), displays highly reduced levels of glycosylated sterols in different plant organs that lead to multiple morphological and biochemical seed phenotypes (DeBolt et al., 2009), defects in the male gametophyte (Choi et al., 2014), and aberrant root epidermal cell patterning (Pook et al., 2017). Also, down-regulation of SGTs in agroinfiltrated Withania somnifera leaves leads to shortened plant height and leaf area compared to control plants (Singh et al., 2016).
Forward- and reverse-genetic approaches have also shown that changes in SGT expression levels are associated to altered responses of different plant species to abiotic stress conditions. An increased sensitivity to heat and cold stress has been reported in Arabidopsis and W. somnifera plants with reduced levels of SGT (Mishra et al., 2015; Singh et al., 2017), whereas enhanced tolerance to heat, cold, and salt stress has been associated to overexpression of SGT in Arabidopsis, tobacco, and W. somnifera, respectively (Mishra et al., 2013; Pandey et al., 2014; Saema et al., 2016). These observations are consistent with the induction of SGT genes in response to abiotic stress reported in tomato (Ramírez-Estrada et al., 2017), W. somnifera (Chaturvedi et al., 2012), and cotton (Li et al., 2014), and also with changes observed in the relative proportions of glycosylated sterols in the plasma membrane of oat, rye, and potato in association with cold acclimation and freezing tolerance (Palta et al., 1993; Takahashi et al., 2016), during tomato and apple fruit chilling and after tomato fruit rewarming (Whitaker, 1991; Whitaker, 1994; Rudell et al., 2011), in wheat leaves under high day and night temperature (Narayanan et al., 2016), and in Arabidopsis under drought stress conditions (Tarazona et al., 2015). On the contrary, the experimental evidence supporting a role for glycosylated sterols in mediating plant responses against biotic stress is far more limited. Arabidopsis and tobacco plants overexpressing W. somnifera SGT show increased resistance toward Alternaria brassicicola and Spodoptera litura, respectively (Pandey et al., 2014; Mishra et al., 2017), and basal immunity in W. somnifera plants is compromised after silencing of several members of the SGT gene family (Singh et al., 2016). However, it is still unclear whether these effects are due to the altered levels of glycosylated sterols or are actually triggered by the concomitant changes in the contents of other bioactive specialized plant defense compounds present in these species (Pandey et al., 2014; Singh et al., 2016; Mishra et al., 2017). The marked induction of specific members of the tomato and W. somnifera SGT gene families in response to methyl jasmonate (MeJA) further suggests a role for sterol glycosylation in plant response to biotic stress imposed by necrotrophic pathogens. However, the impact of this transcriptional response on the levels of steroidal glycoalkaloids in tomato and whitanolides in W. somnifera remains to be established. These defense compounds are not produced in the model plant Arabidopsis thaliana, which presents a rather scarce secondary metabolism. Consequently, the Arabidopsis double mutant ugt80A2;B1 impaired in the SGs biosynthesis (DeBolt et al., 2009) is a very suitable tool to study the role of this kind of conjugated sterols in the plant defense response to pathogen attack, which involves changes at the transcriptional, biochemical, and physiological levels (AbuQamar et al., 2017).
When a pathogen is detected by the plant, it activates different layers of defense depending of the pathogen invasion stage. A first layer is constituted by a repertoire of plasma membrane pattern recognition receptors that perceive signals produced by the pathogen, known as pathogen- or microbe-associated molecular patterns (MAMPs), or plant-derived damage-associated molecular patterns (DAMPs) produced by the host upon pathogen infection (Bohm et al., 2014; Zipfel, 2014). This induces a basal disease resistance response called pattern-triggered immunity that protects the plant against most nonadapted pathogens (Couto and Zipfel, 2016). Conversely, pathogens try to overcome plant defenses by releasing effectors that alternatively can also be recognized by cytoplasmic receptors (Cui et al., 2015; Couto and Zipfel, 2016). Following either PAMPs or effector recognition, plant immune responses involve a complex network of signaling pathways that can be modulated by phytohormones (Pieterse et al., 2012). Salicylic acid (SA) and jasmonic acid (JA) are recognized as the two major defense hormones, and their response pathways are usually considered effective against biotrophic and necrotrophic pathogens, respectively (Pieterse et al., 2012). Other phytohormones, mainly ethylene and abscisic acid (ABA), are also involved in the defense response interacting synergically or antagonistically (Shigenaga and Argueso, 2016; Berens et al., 2017). In the case of Arabidopsis, other key components of the innate immune system are tryptophan-derived secondary metabolites such as the phytoalexin camalexin and the indole glucosinolates (IGs) (Bednarek, 2012). The biosynthesis of these compounds is induced in response to different pathogens, including bacteria and fungi (Clay et al., 2009; Ahuja et al., 2012), and their role in the immune response has been confirmed by analysis of different biosynthetic mutants (Tsuji et al., 1992; Glazebrook and Ausubel, 1994; Thomma et al., 1999; Lipka et al., 2005; Bednarek et al., 2009; Clay et al., 2009). It is important to note that JA has been acknowledged as a regulator of the Trp derivatives biosynthesis (Guo et al., 2013). Simultaneous applications of glucose and JA have a dramatic impact on both aliphatic and indolic glucosinolates accumulation, although the latter ones seem to be more sensitive to the treatments.
As a first step to elucidate the role of glycosylated sterols in the plant response to biotic stress, we have assayed the response of the Arabidopsis double mutant ugt80A2;B1 against Botrytis cinerea infection, which is considered the second most important plant pathogen (Dean et al., 2012). This fungus produces several toxic compounds and cell wall degrading enzymes that can kill the host cells and decompose the plant tissue (Williamson et al., 2007). In Arabidopsis, global transcriptional analyses of B. cinerea–infected plants have identified thousands of transcripts whose expression is altered upon infection (AbuQamar et al., 2006; Birkenbihl and Somssich, 2011; Mulema and Denby, 2012; Windram et al., 2012). These data, together with genetic studies, have shown that several groups of transcription factor families, including ERFs (Huang et al., 2016; Zhang et al., 2016), WRKYs (Birkenbihl et al., 2012; Jiang and Yu, 2016), MYBs (Ramírez et al., 2011; Mengiste, 2012), and NACs (Wang et al., 2009; Nuruzzaman et al., 2013), have a major role in coordinating these changes, but only few target genes or upstream regulators have been identified (Windram et al., 2012). An exception is WRKY33, which targets multiple signaling pathways simultaneous upon B. cinerea infection, acting as a dual transcription factor in a promoter-dependent manner (Liu et al., 2015) because it binds directly to the promoter of genes involved in JA signaling (JAZ1 and JAZ5), ET-JA crosstalk (ORA59), and camalexin biosynthesis (PAD3 and CYP71A13) up-regulating their expression, but down-regulates the expression of other targets, as some ABA biosynthetic genes (NCED3 and NCED5) (Birkenbihl et al., 2012; Liu et al., 2015). In addition, Pangesti et al. (2016) already suggested that the JA-responsive transcription factor ORA59 is related to the camalexin accumulation during ISR.
Here we report that the ugt80A2;B1 mutant shows increased resistance against B. cinerea infection, which is paralleled by an increase in the levels of JA and camalexin, and a concomitant up-regulation of several genes involved in the defense JA signaling pathway and the biosynthesis of camalexin, as well as of some of the transcription factors mentioned above, suggesting that the resistance phenotype observed in the mutant is the result of these transcriptional and metabolic changes.
Materials and Methods
Plant Material and Growth Conditions
All A. thaliana plants used in this study were of the Wassilewskija (Ws-0) ecotype. The generation of the ugt80A2;B1 double mutant by crossing two single mutants carrying homozygous T-DNA insertions in the UGT80A2 and UGT80B1 genes and the subsequent characterization of the single and double mutant lines have been previously reported by DeBolt et al. (2009). Seeds of the double mutant were kindly provided by Dr. DeBolt (University of Kentucky, USA). Mutants and wild-type (WT) seeds were stratified at 4°C for 3 days and sown in jiffy7 peat pellets (Clause-Tezier Ibérica, http://www.clausetezier.com/). Plants were grown in a chamber with a light intensity of 150 to 200 μEm−2 s−1 at 23°C under 10-h light/14-h dark cycles and 60% humidity.
Botrytis cinerea Infection
For B. cinerea infections, six fully expanded leaves of 5-week-old plants were inoculated as described by Coego et al. (2005) with 6 ml droplets of a fungal spore suspension containing 2.5 × 104 spores microliters in potato dextrose broth (PDA) (12 g L−1, Difco). Plants exposed to the same treatment but without fungal spores were used as control (mock). All the treated plants were covered with transparent plastic to maintain 100% relative humidity and returned to the growth chamber. Four biological replicates with 12 to 15 WT or mutant plants were performed for each treatment (infected or mock). Disease symptoms were evaluated by determining the lesion diameter of at least 50 lesions 3 days after inoculation. Three more biological replicates (15–20 plants per treatment) were performed to analyze changes in gene expression and metabolite levels (hormones and camalexin) induced by fungal infection. For this, infected or mock-treated rosette leaves from WT and mutant plants were harvested before (0 h) and after infection (24 and 48 h), pooled (five to six plants per time point and treatment), frozen in liquid nitrogen, lyophilized, and stored until used.
High-Throughput Reverse Transcription–Quantitative Polymerase Chain Reaction Analyses of Gene Expression
Lyophilized rosette leaf samples (10 mg) from Arabidopsis WT and mutant plants obtained as described above were used for total RNA extraction using a Maxwell 16 LEV Plant RNA kit (Promega) and a Maxwell® 16 Instrument (Promega) according to manufacturer’s instructions. The cDNA samples for reverse transcription–quantitative polymerase chain reaction (RT-qPCR) gene expression analyses were prepared from 1 microgram of total RNA using SuperScript III Reverse Transcriptase (Invitrogen) and oligo(dT) primers according to the manufacturer’s instructions. The expression of the different genes analyzed in this work was quantified by real-time PCR using the Biomark™ instrument (Fluidigm Corporation, San Francisco, USA) and 2 × SsoFast™ EvaGreen® Supermix with low Rox (Bio-Rad, www.bio-rad.com) as previously reported (Manzano et al., 2016), using PP2AA3 (At1g13320) (Hong et al., 2010) and UBC (At5g25760) (Czechowski et al., 2005) as housekeeping reference genes and specific primers for each analyzed gene ( Supplemental Table 1 ). Data for each WT and ugt80A2;B1 mutant samples, infected or treated with mock, are expressed as normalized quantity values versus the housekeeping genes. Expression was calculated using Data Analysis Gene Expression software (http://www.dagexpression.com/dage.zip) (Ballester et al., 2013). Quantification of transcript levels was done in three independent biological replicates, and for each biological replicate, two technical replicates were performed.
Determination of Hormones and Camalexin Levels
Hormones and camalexin were extracted from the same samples used for gene expression analysis as described by Sánchez-Bel et al. (2017). Briefly, 30 mg of dry material was extracted with 1 ml of H2O:MeOH (90:10) with 0.01% of HCOOH with a mix of internal standards. After centrifugation and filtration of the supernatant with 0.22-µm filter of regenerated cellulose, 20 µl was injected into a Waters Acquity UPLC coupled with a triple quadrupole tandem mass spectrometer (Waters), and the separation of compounds was performed with a Kinetex C18 analytical column (Phenomenex), 5 µm of particle size and 2.1 × 100 mm. Before the analysis, external calibration curves with pure chemical standards were obtained for each tested compound complemented with heavy isotopes of each hormone as internal standards. The MassLynx 4.1 software (Waters) was used to process the quantitative data from calibration standards and plant samples.
Results
Impairment of SGs Biosynthesis Leads to Enhanced Resistance of Arabidopsis to B. cinerea Infection
The current knowledge about the specific contribution of glycosylated sterols to plant biotic stress response is scarce. To gain some insight about the role of these compounds in the plant response to this kind of stress, we checked the effect of B. cinerea infection, a common necrotrophic fungal pathogen, in Arabidopsis WT plants (Ws-0) and the previously generated double mutant ugt80A2;B1, which has inactivated the two genes reported to encode SGT in Arabidopsis (UGT80A2 and UGT80B1) and presents reduced levels of glycosylated sterols in different plant organs, including the rosette leaves (DeBolt et al., 2009). To this end, leaves of WT and mutant plants were inoculated with a B. cinerea spore suspension, and the size of the resulting lesions was measured 3 days after inoculation. The results from four independent experiments showed that the average diameter of the lesions in the ugt80A2;B1 mutant plants was significantly smaller (about one half) than in the WT plants ( Figure 1 ). These results indicate that the simultaneous inactivation of Arabidopsis UGT80A2 and UGT80B1 genes results in increased resistance against B. cinerea infection. Interestingly, infection with this necrotrophic fungus did not affect the expression of UGT80A2 and UGT80B1 genes in the WT plants because their transcript levels at 24 and 48 h postinoculation (hpi) remained unchanged compared to the noninfected plants ( Figure 2 ).
Figure 1.
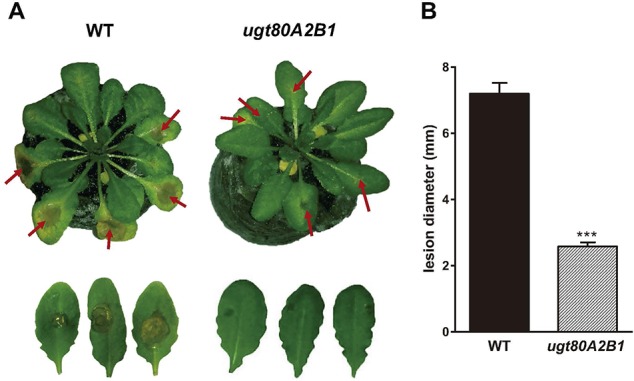
The ugt80A2;B1 mutant impaired in steryl glycosides biosynthesis shows enhanced resistance to B. cinerea infection. (A) Symptoms of infection in leaves of wild-type (WT) and ugt80A2;B1 mutant plants 3 days after inoculation with B. cinerea. Red arrows point to B. cinerea inoculation sites (B) diameter of the resulting lesions. Data represent the average ± SEM of at least 50 lesions in one experiment. The experiment was repeated three more times with similar results. Asterisks indicate significant differences between WT and mutant plants according to Student t test (***P < 0.001).
Figure 2.
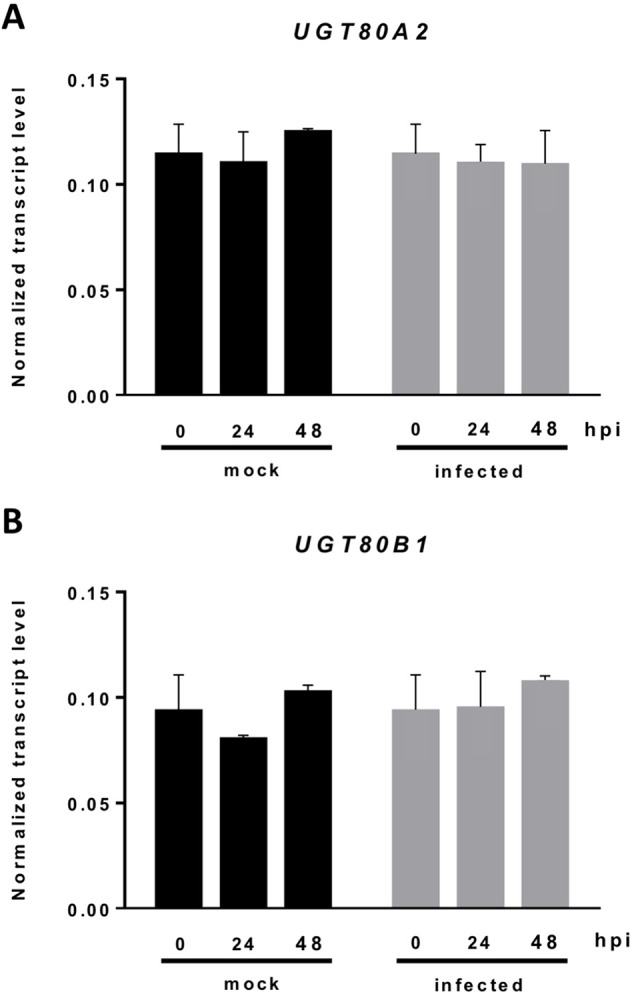
Expression of the UGT80A2 and UGT80B1 genes remains unchanged upon B. cinerea infection. The transcript levels of UGT80A2 (A) and UGT80B1 (B) were measured by reverse transcription–quantitative polymerase chain reaction using RNA extracted from rosette leaves of Arabidopsis WT plants inoculated (infected) or not (mock) with B. cinerea at time points 0, 24, and 48 h. Data for each WT sample, infected or treated with mock, are expressed as normalized quantity values using two independent housekeeping genes (UBC and PP2A). Values are means ± SEM of three independent biological experiments.
Resistance of the Arabidopsis ugt80A2;B1 Mutant to B. cinerea Involves JA Signaling
The JA-mediated defense pathway is assumed to have a central role in plant resistance against necrotrophic pathogens (Rowe et al., 2010). In order to determine if the resistance to the B. cinerea observed in the ugt80A2;B1 mutant was associated to this pathway, we analyzed the expression of some JA-responsive marker genes of the two major branches recognized in the Arabidopsis JA signaling pathway, the ERF and the MYC branches (Pieterse et al., 2012), in plants infected or not with the pathogenic fungus at different time points. The expression of PDF1.2, a JA-responsive gene representative of the ERF branch, was significantly induced after infection with B. cinerea both in the WT and the mutant plants, but at 48 hpi, the induction in the ugt80A2;B1 mutant was about twice that in the WT plants ( Figure 3A ). A similar expression pattern was observed for PR4, another JA-responsive gene of the ERF branch, but in this case, the transcript levels were more than twofold higher in the mutant than in the WT ( Figure 3B ). On the contrary, the expression of the MYC-branch representative gene VSP2 was not significantly affected by the infection neither in the WT plants nor in the mutant ( Figure 3C ). This was not unexpected because activation of the MYC branch has been related with defense against chewing insects, while defense against necrotrophic pathogens is mediated by the ERF one (Pieterse et al., 2012). In addition to genetic responses, plants usually experience important hormonal changes after pathogen attack. Thus, we measured the levels of JA in the same tissue samples used for the gene expression analysis. As shown in Figure 3D , JA levels increased after infection with the fungal pathogen in both WT and ugt80A2;B1 mutant plants. However, JA levels were markedly higher in the mutant than in the WT, with values that were approximately twofold and threefold higher at 24 and 48 hpi, respectively ( Figure 3D ). It is worth to mention that 48 h after B. cinerea infection the expression of ACS6, a gene involved in ethylene biosynthesis (Li et al., 2012), increased more than twofold in the WT plants and about fourfold in the ugt80A2;B1 mutant compared with the mock treatment at the same time point ( Figure S1 ). This hormone interacts synergistically with JA in the ERF branch (Pieterse et al., 2012). However, the expression of NCED3 and RAB18, two genes involved, respectively, in the biosynthesis and response to ABA, a hormone that interacts with JA in the MYC branch (Anderson et al., 2004), was not affected by B. cinerea treatment neither in the WT nor in the mutant plants ( Figure S2 ).These results indicate that the resistance of the ugt80A2;B1 mutant to B. cinerea is mediated by the ERF branch of the JA pathway, mainly as a result of an increased accumulation of this hormone in the infected mutant.
Figure 3.
Increased transcript levels of JA-responsive marker genes and JA levels in ugt80A2;B1 mutant plants compared to WT upon infection with B. cinerea. The transcript levels of PDF1.2 (A), PR4 (B), and VSP2 (C) were determined by reverse transcription–quantitative polymerase chain reaction using RNA extracted from rosette leaves of Arabidopsis WT and ugt80A2;B1 mutant plants infected or not (mock) with B. cinerea at time points 0, 24, and 48 h. Data for each WT and ugt80A2;B1 mutant samples, infected or treated with mock, are expressed as normalized quantity values calculated using two independent housekeeping genes (UBC and PP2A). JA was quantified in leaf extracts by ultraperformance liquid chromatography–mass spectrometer and expressed as µg/g of dry weight (D). Values are means ± SEM of three independent biological experiments. Asterisks represent significant differences determined by one-way analysis of variance (**P < 0.005, ***P < 0.001).
A crosstalk between hormone signaling pathways, particularly those mediated by SA and JA, has been found to contribute to plant resistance to different types of pathogens (Pieterse et al., 2012). Therefore, SA levels were determined in the same samples used for JA quantification. The levels of SA were similar in WT and ugt80A2;B1 mutant plants, and no significant changes were detected upon infection ( Figure S3A ). Furthermore, significant differences were neither observed between the WT and the mutant plants when the expression levels of NPR1, the gene encoding the main regulatory protein of the SA signaling pathway, were determined in plants infected or not with the pathogen ( Figure S3B ). The expression of PR1, a marker gene of the SA signaling pathway, increased about 10-fold upon fungus infection (48 hpi) either in the WT or in the ugt80A;2B1 mutant plants ( Figure S3C ). These results suggest that the SA-mediated defense pathway is not involved in the response of the ugt80A2;B1 mutant to B. cinerea infection.
The Synthesis of Camalexin and Indole Glucosinolates Is Induced in the ugt80A2;B1 Mutant Upon B. cinerea Infection
In response to pathogen attack, plants induce the biosynthesis of phytoalexins and other defense secondary metabolites, such as glucosinolates ( Figure 4 ). Because camalexin is the main phytoalexin accumulated in Arabidopsis after infection by fungi or bacteria, and its biosynthesis has been reported to be elicited by JA (De Geyter et al., 2012), we investigated if it could be involved in the resistance response observed in the Arabidopsis ugt80A2;B1 mutant infected with B. cinerea. To this end, the levels of camalexin were analyzed in the WT and mutant plant samples used for JA quantification. A marked accumulation of this compound was detected in WT and ugt80A2;B1 plants after 48 hpi with B. cinerea, but the levels in the mutant were significantly higher (about twofold) than in the WT ( Figure 5A ). The accumulation of camalexin in response to fungal infection was paralleled by an increase in the expression of several genes related to its biosynthesis ( Figures 5B–D ). The expression of the CYP79B2, CYP71A13, and CYP71B15 biosynthetic genes was strongly induced by fungal infection, particularly at 48 hpi, both in the WT and the ugt80A2;B1 mutant plants, but the transcript levels of these three genes were higher in the mutant than in the WT plants, specifically about threefold in the case of CYP79B2 ( Figure 5B ) and approximately 1.5-fold in CYP71A13 and CYP71B15 ( Figures 5C, D ).
Figure 4.
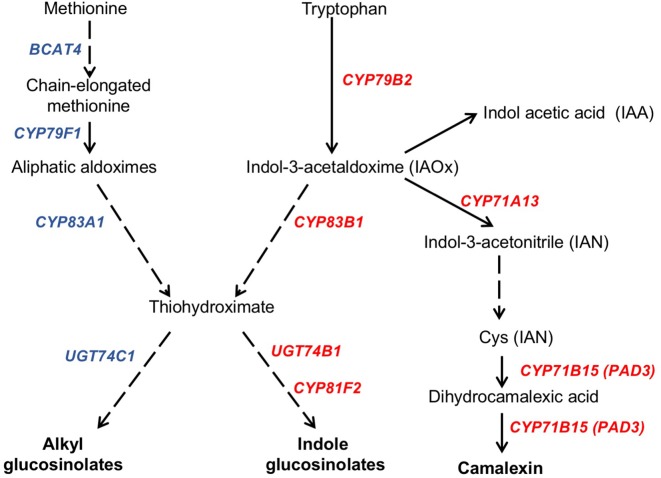
Schematic representation of camalexin and glucosinolate biosynthesis pathways. The biosynthesis of the tryptophan (camalexin, indole glucosinolates, and IAA) and methionine (alkylglucosinolates) derived compounds is indicated in a simplified form showing the biosynthetic steps mediated by genes whose expression levels have been quantified in this work. Genes whose expression increase in the ugt80A2;B1 mutant compared to WT upon B. cinerea infection are indicated in red, whereas those whose expression does not change are shown in blue. Solid arrows indicate single enzymatic steps, whereas dashed ones represent several enzymatic steps. Figure is based on previous representations of these pathways (Kliebenstein et al., 2005; Yatusevich et al., 2010; Frerigmann et al., 2016).
Figure 5.
The ugt80A2;B1 mutant displays camalexin accumulation and increased transcript levels of camalexin biosynthetic genes in comparison to WT upon infection with B. cinerea. Camalexin was quantified in leaf extracts of Arabidopsis WT and ugt80A2;B1 mutant plants infected or not (mock) with B. cinerea at time points 0, 24, and 48 h using ultraperformance liquid chromatography–mass spectrometer and is expressed as µg/g of dry weight (A). The transcript levels of CYP79B2 (A), CYP71A13 (B) and CYP71B15 (PAD3) (C) were determined by reverse transcription–quantitative polymerase chain reaction using RNA extracted from the same leaf samples used for camalexin quantification. Data for each WT and ugt80A2;B1 mutant samples, infected or treated with mock, are expressed as normalized quantity values calculated using two independent housekeeping genes (UBC and PP2A). Values are means ± SEM of three independent biological replicates. Asterisks represent significant differences determined by one-way analysis of variance (*P < 0.05, **P < 0.005, ***P < 0.001).
Camalexin biosynthesis involves the conversion of tryptophan to indole-3-acetaldoxime (IAOx), which is also the precursor of the phytohormone indole-3-acetic acid (IAA) and the plant defense secondary metabolites IGs ( Figure 4 ). Thus, we investigated if the inactivation of the two Arabidopsis SGTs also affected these biosynthetic pathways. While no relevant changes were observed in the IAA levels between WT and mutant plants ( Figure S4 ), the transcript levels of several genes encoding specific enzymes of the indole glucosinolate pathway, such as CYP83B1, UGT74B1, and CYP81F2 (Sønderby et al., 2010), were higher in the mutant than in the WT plants at 48 hpi ( Figure 6 ). The expression of CYP83B1 and UGT74B1 remained essentially unaltered in noninoculated leaves but was significantly up-regulated 48 h after Botrytis inoculation only in the ugt80A2;B1 mutant ( Figures 6A, B ). The transcript levels of CYP81F2, a gene specifically involved in the synthesis of 4-hydroxy-3-indolyl-methyl glucosinolates, increased significantly in the WT and mutant plants at 48 hpi, but this increase was significantly higher in the mutant than in control plants ( Figure 6C ). Altogether these data indicate that a transcriptional activation of the pathways involved in the synthesis of the Trp-derived defense compounds camalexin and indole glucosinolates is induced in the Arabidopsis ugt80A;2B1 mutant upon infection with B. cinerea. In agreement with the above results, the expression of some genes encoding transcriptional regulators of the camalexin and indole glucosinolates biosynthetic genes in the infected mutant was higher than in the infected WT plants. As shown in Figure 7A , the expression of the MYB51 transcription factor, a positive regulator of the biosynthetic steps required for the production of IAOx (Frerigmann et al., 2015), was significantly more expressed at 48 hpi in the ugt80A2;B1 mutant than in the WT plants, while the expression of ANAC042, a regulator of camalexin biosynthesis that acts downstream IAOx (Saga et al., 2012), increased in both WT and ugt80A2;B1 mutant plants upon infection, but at 48 hpi, this increase was significantly higher in the mutant ( Figure 7B ). A similar induction profile was observed for the transcript levels of WRKY33 ( Figure 7C ), a transcription factor activated by the mitogen-activated protein kinase cascade that has been well characterized as a camalexin biosynthesis inductor (Saga et al., 2012). All these results suggest that camalexin and, probably, also indole glucosinolates are actively involved in the enhanced resistance of the ugt80A2;B1 mutant to B. cinerea infection.
Figure 6.
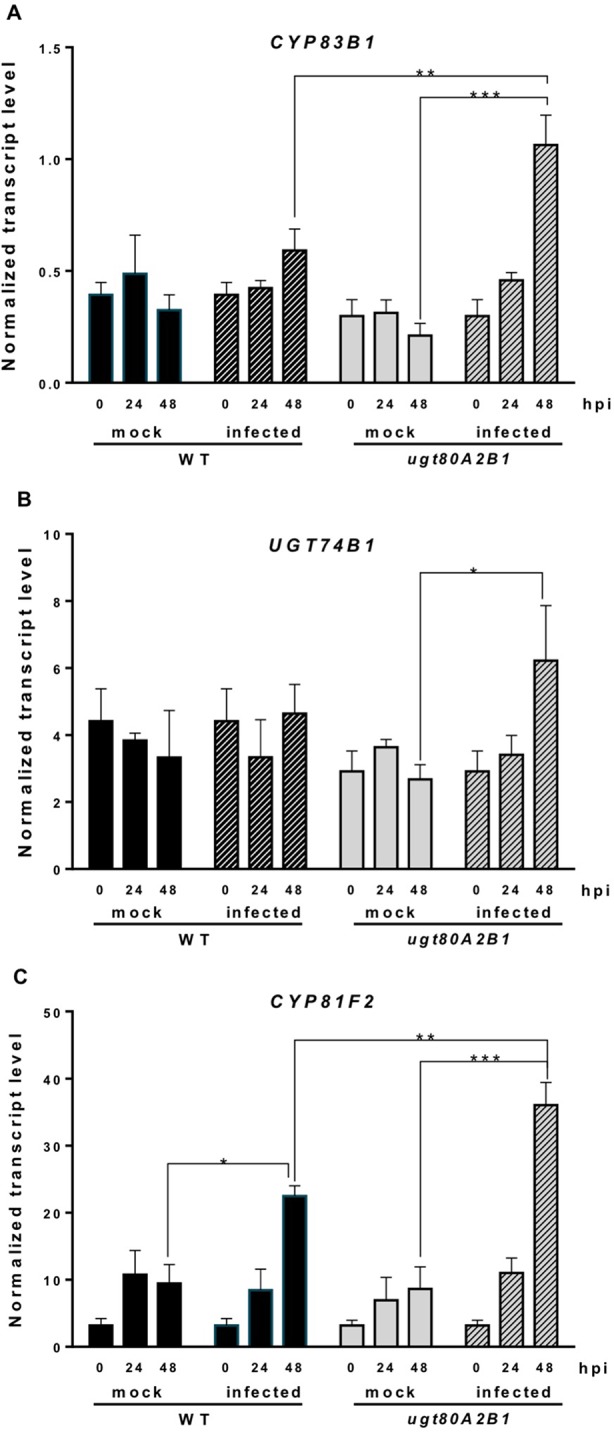
Increased transcript levels of indole glucosinolate biosynthetic genes in the ugt80A2;B1 mutant compared to WT upon infection with B. cinerea. The transcript levels of CYP83B1 (A), UGT74B1 (B), and CYP81F2 (C) were determined by reverse transcription–quantitative polymerase chain reaction using RNA extracted from rosette leaves of Arabidopsis WT and ugt80A2;B1 mutant infected or not (mock) with B. cinerea at different time points (0, 24, and 48 h). Data for each WT and ugt80A2;B1 mutant samples, infected or treated with mock, are expressed as normalized quantity values calculated using two independent housekeeping genes (UBC and PP2A). Values are means ± SEM of three independent biological replicates. Asterisks represent significant differences determined by one-way analysis of variance (*P < 0.05, **P < 0.005, ***P < 0.001).
Figure 7.
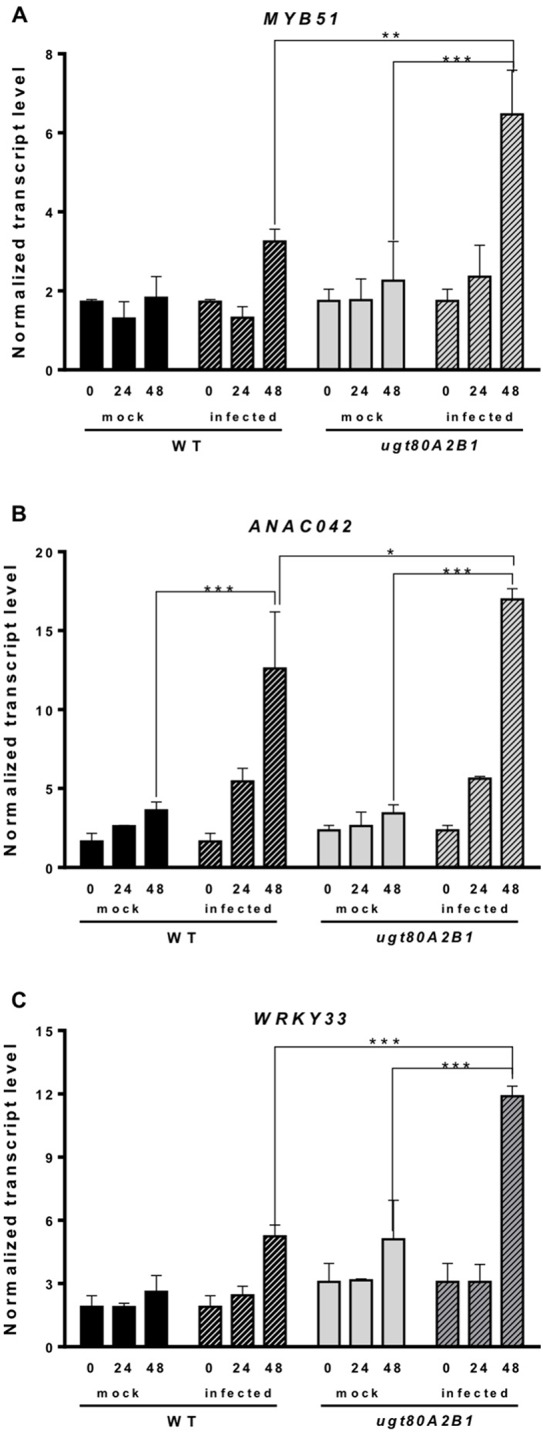
The ugt80A2;B1 mutant shows increased transcript levels of genes coding for transcription factors regulating camalexin and indole glucosinolate biosynthesis compared to WT upon infection with B. cinerea. The transcript levels of MYB51 (A), ANAC042 (B) and WRKY33 (C) were determined by reverse transcription–quantitative polymerase chain reaction using RNA extracted from rosette leaves of Arabidopsis WT and ugt80A2;B1 mutant infected or not (mock) with B. cinerea at different time points (0, 24, and 48 h). Data for each WT and ugt80A2;B1 mutant samples, infected or treated with mock, are expressed as normalized quantity values calculated using two independent housekeeping genes (UBC and PP2A). Values are means ± SEM of three independent biological replicates. Asterisks represent significant differences determined by one-way analysis of variance (*P < 0.05, **P < 0.005, ***P < 0.001).
The Synthesis of Alkylglucosinolates in the Arabidopsis ugt80A2;B1 Mutant Is Not Affected by B. cinerea Infection
Alkylglucosinolates (AGs) are also a class of plant defense secondary metabolites whose biosynthetic pathway is related to that of indole glucosinolates ( Figure 4 ), and it has been reported that both biosynthetic pathways may affect each other (Liu et al., 2016). This prompted us to investigate if the biosynthesis of this kind of glucosinolates could also be altered in the ugt80A2;B1 Arabidopsis mutant. To check this possibility, we analyzed the expression of several genes encoding enzymes involved in their biosynthetic pathway. The transcript levels of BCAT4 and CYP79F1 were similar in the WT and ugt80A2;B1 mutant plants infected or not with B. cinerea ( Figures S5A, B ). These genes are involved in the first stages of AGs biosynthesis, which involves the side-chain elongation of the precursor amino acid methionine and its subsequent oxidation to aldoxime (Sønderby et al., 2010) ( Figure 4 ). No significant changes were observed between WT and mutant plants in the transcript levels of CYP83A1 ( Figure S5C ) and UGT74C1 ( Figure S5D ), two genes involved, respectively, in the metabolism of the aldoxime to the corresponding alkylthiohydroximate and the subsequent conversion of this intermediate to AGs ( Figure 4 ). These results indicate that, unlike indole glucosinolates, the synthesis of AGs is not transcriptionally activated either in the ugt80A2;B1 mutant or in the WT upon infection with B. cinerea. This observation is further supported by the lack of induction of MYB28 and MYB29, two genes coding for transcription factors that positively regulate the expression of many alkyl glucosinolate biosynthetic genes (Yatusevich et al., 2010) whose transcript levels are similar in the WT and the mutant plants infected or not with the pathogen ( Figures S5E, F ). These results suggest that AGs are not involved in the defense response of Arabidopsis against infection by B. cinerea whether plants have normal or depleted levels of glycosylated sterols.
Discussion
Changes in the profile of glycosylated sterols have been widely related with the plant response to different abiotic stresses (Palta et al., 1993; Mishra et al., 2013; Pandey et al., 2014; Mishra et al., 2015; Saema et al., 2016; Takahashi et al., 2016; Singh et al., 2017). However, there are less experimental evidence supporting their involvement in biotic stress responses (Pandey et al., 2014; Singh et al., 2016; Mishra et al., 2017). Furthermore, most of the published data related with this issue were obtained using Solanaceae species with simultaneously altered levels of glycosylated sterols and glycosylated defense compounds, such as whithanolides in W. somnifera (Singh et al., 2016) and rutin in tobacco (Pandey et al., 2014), which could be ultimately responsible for the observed responses. Because A. thaliana lacks this kind of specialized secondary metabolites, it represents a suitable model to assess the role of conjugated sterols in plant defense against biotic agents. In this regard, Kopischke et al. (2013) reported a role for conjugated sterols in the plant response to Phytophthora infestans using the Arabidopsis psat1 mutant impaired in steryl ester (SE) biosynthesis. However, the observed response could not be correlated with changes in a specific sterol fraction because the levels of SE and ASG are reduced in the leaves of this mutant, whereas those of SGs are increased, and FSs remain unaltered. Because Phytophthora is a sterol-auxotroph pathogen, the altered profile of sterols in the mutant, together with the described capacity of pathogenesis-related 1 (PR-1) to inhibit pathogen growth by sequestering its sterols (Gamir et al., 2017), might explain the resistance phenotype of the psat1 mutant. Our results suggest that reduced levels of glycosylated sterols in the Arabidopsis ugt80A2;B1 mutant (DeBolt et al., 2009) confer resistance to the necrotrophic fungus B. cinerea ( Figure 1 ). The leaves of this mutant contain normal levels of nonglycosylated sterols (FSs + SE), whereas those of glycosylated sterols (SG + ASG) are markedly reduced, albeit not completely abolished (DeBolt et al., 2009). Thus, our results establish for the first time a direct link between reduced levels of glycosylated sterols and resistance against pathogen attack. We also show that expression of the two genes encoding the SGTs that synthesize the bulk of SGs in Arabidopsis remains unaltered upon infection with B. cinerea ( Figure 2 ). This observation supports the notion that SG biosynthesis is not induced in response to B. cinerea infection, although the possibility that a gene coding for an as yet unreported SGT potentially involved in the residual production of SGs and/or the synthesis of a specialized SG could be up-regulated cannot be entirely excluded. It is reported that although both enzymes display sterol glucosyltransferase activity, substrate specificity is apparent in that UGT80A2 is responsible for the accumulation of major SGs, while UGT80B1 is involved in accumulation of minor SGs and ASGs (Stucky et al., 2015).
In order to understand the molecular mechanism acting behind the resistance phenotype observed in the ugt80A2;B1 mutant plants, we first measured the levels of JA and SA in mutant and WT plants infected or not with B. cinerea because it is well known that these phytohormones act as primary signals in the regulation of plant responses to biotic stress (Santino et al., 2013). After infection with B. cinerea, JA content increased in both WT and mutant plants ( Figure 3D ), which is not surprising because an increase in the levels of this hormone has long been described in response to necrotrophic pathogen infection (Penninckx et al., 1996) and herbivore damage (Reymond et al., 2000). However, after 24 and 48 hpi, the JA levels were significantly higher in the mutant than in the WT ( Figure 3D ), and this differential increase correlated with a stronger up-regulation of some defense genes such as plant defensin 1.2 (PDF1.2) and the pathogenesis related protein 4 (PR4) in the infected ugt80A;2B mutant compared to WT ( Figures 3A, B ). These two genes are markers of the ERF branch of the JA signaling pathway that is activated upon necrotrophic pathogen attack (Santino et al., 2013), suggesting that this branch of the downstream JA signaling is activated in the ugt80A;2B mutant after Botrytis infection. Interestingly, the expression of ACS6 increased about twofold and fourfold, respectively, in WT and ugt80A2;B1 mutant plants 48 h after fungus infection ( Figure S1 ), which agrees with its previously reported induction by B. cinerea infection (Han et al., 2010; Li et al., 2012). ACS6 is one of the nine members of the Arabidopsis gene family encoding 1-amino-cyclopropane-1-carboxylic acid synthase, the rate-limiting enzyme in ethylene biosynthesis (Wang et al., 2002), a hormone that acts synergistically with JA on the expression of the ERF branch signaling pathway upon infection by necrotrophic pathogens (Pieterse et al., 2012). On the contrary, the JA signaling branch regulated by the MYC2 transcription factor, which has been reported to have a specific role in response to insect attack (Santino et al., 2013), was not activated after Botrytis infection neither in the WT nor in the mutant plants because no significant changes were observed in the expression of the MYC-branch marker gene vegetative storage protein 2 (VSP2) ( Figure 3C ). This is in accordance with the absence of changes observed after fungus infection in the expression of NCED3, one of the major genes encoding 9-cis-epoxycarotenoid dioxygenase, a key enzyme in the biosynthesis of ABA (Leng et al., 2014), and RAB18, an ABA-responsive gene, either in the WT or the ugt80A2;B1 mutant plants ( Figure S2 ), because ABA has been reported to act synergistically with JA on the expression of the MYC branch upon wounding or herbivory attack (Anderson et al., 2004). Similarly, the levels of SA, a hormone that usually interacts antagonistically with JA (Pieterse et al., 2012), and the expression of the NPR1 gene coding for a key transcriptional activator of the SA-dependent immune response (Pieterse et al., 2012) remained unaltered in infected and noninfected WT and mutant plants ( Figures S3A, B ). The expression of PR1, a marker gene of SA response (Pieterse et al., 2012), increased about 10-fold either in WT or mutant plants upon Botrytis infection ( Figure S3C ). This agrees with the results of Govrin and Levine (2002) indicating that PR1 induction by B. cinerea can be independent of SA. These results indicate that the SA signaling pathway is not involved in the response to B. cinerea either in the WT or in the mutant plants. All these observations indicate that depletion of glycosylated sterols content in the Arabidopsis ugt80A2;B1 mutant leads to an enhancement of Botrytis-induced JA levels that specifically activate the JA signaling pathway regulated by the ERF family of transcription factors. This finding further reinforces the hypothesis that changes in the relative proportions of sterols are perceived by plants as a stress signal that activates different hormone-related defensive responses in a sterol profile-dependent manner (Wang et al., 2012; Singh et al., 2015; Manzano et al., 2016).
The intricate immune response network evolved by plants to protect themselves against pathogens includes also the biosynthesis of different types of secondary metabolites that serve as defense compounds, such as phytoalexins and glucosinolates. Camalexin, a tryptophan-derived compound ( Figure 4 ), is the major phytoalexin of A. thaliana (Glawischnig, 2007). Interestingly, camalexin content was enhanced by B. cinerea infection in both WT and ugt80A2;B1 mutant plants ( Figure 5A ), but their levels were significantly higher in the mutant than in the WT plants ( Figure 5A ), suggesting a role of this phytoalexin in the resistance to the fungus observed in the mutant. A positive role of camalexin in plant resistance against pathogens, including several necrotrophic fungi, has previously been demonstrated by genetic approaches (Lemarié et al., 2015). Mutants with reduced camalexin levels show increased susceptibility to B. cinerea, while its accumulation has been correlated with resistance to the fungus (Ferrari et al., 2003; Kliebenstein et al., 2005; Ferrari et al., 2007; Van Baarlen et al., 2007). Because the secondary metabolites are derived from primary metabolic pathways, their biosynthesis should be temporally and spatially coordinated to maintain normal growth and plant development. Thus, the accumulation of camalexin in the proximity of the lesions induced by Botrytis is associated to a strong induction of tryptophan and camalexin biosynthetic genes in the same tissues (Schuhegger et al., 2006; Schuhegger et al., 2007). According to these observations, the increase in camalexin levels observed at 48 hpi ( Figure 5A ) correlates with significantly higher transcript levels of a set of cytochrome P450 genes encoding key enzymes of the camalexin biosynthetic pathway, such as CYP79B2, CYP71A13, and CYP71B15 (PAD3) ( Figures 5B–D ), and the WRKY33 and ANAC042 genes coding for transcriptional activators of camalexin biosynthesis ( Figures 7B, C ), being all these responses significantly more intense in the ugt80A;2B1 mutant than in WT plants ( Figures 5 and 7 ). It has been reported that WRKY33 binds to the promoters of CYP71B15 and CYP71A13 to induce camalexin biosynthesis (Petersen et al., 2008) during the early stages of pathogen infection (Birkenbihl et al., 2012), while upon induction of camalexin biosynthesis by treatment with AgNO3, the time course of ANAC042 expression parallels that of the biosynthetic genes CYP79B2, CYP71A12, and CYP71B15 (Saga et al., 2012). These observations indicate that camalexin biosynthesis induction in Arabidopsis leaves infected with B. cinerea is coordinately controlled at the transcriptional level similarly to what has been described in Arabidopsis roots treated with Flg22, where induction of camalexin biosynthesis was associated with the transcriptional induction of the PAD3, CYP71A12, and CYP71A13 biosynthetic genes (Millet et al., 2010). Moreover, these results support the hypothesis that the enhanced camalexin accumulation in the ugt80A2;B1 mutant infected with B. cinerea is due to a higher transcriptional up-regulation of its biosynthetic pathway compared to WT plants. Interestingly, ANAC042, CYP79B2, CYP71A12, and CYP71B15 genes have been previously included in a coexpression module closely related with another module comprising, among others, MYB51 (Saga et al., 2012), a gene reported to encode a positive regulator of both camalexin and IG biosynthesis whose expression is induced by B. cinerea infection (Frerigmann et al., 2015). Our results support these observations because the expression profile of MYB51 ( Figure 7A ) was similar to that of ANAC042 ( Figure 7B ), which, as mentioned above, correlated with that of some camalexin biosynthetic genes ( Figure 5 ).
Indole glucosinolates are small secondary metabolites involved in plant immunity (Bednarek et al., 2009; Clay et al., 2009) that share with camalexin the initial step of their biosynthetic pathways, the conversion of tryptophan to IAOx catalyzed by CYP79B2 ( Figure 4 ). MYB51, together with MYB122 and MYB34, regulates the IG biosynthesis in A. thaliana (Celenza et al., 2005; Gigolashvili et al., 2007), although the contribution of each MYB factor to IG production is different in shoots and roots, being MYB51 the main regulator in shoots (Frerigmann and Gigolashvili, 2014). In Arabidopsis WT and ugt80A2;B1 mutant plants, the expression of MYB51 increased after Botrytis infection, and its transcript levels were significantly higher in the mutant ( Figure 7A ). A similar expression pattern was observed for the genes involved in IG (CYP83B1, UGT74B1, and CYP81F2) ( Figure 6 ) and camalexin biosynthesis (CYP79B2, CYP71A13, and CYP71B15) ( Figures 5B–D ), which supports a role of MYB51 as a transcriptional regulator of the pathways leading to the synthesis of these kinds of defense compounds. In the case of camalexin biosynthesis, MYB51 would act in concert with WRKY33 and ANAC042 to activate the entire pathway because it is known that MYB51 induces the expression of CYP79B2 but not that of the downstream biosynthetic genes (Frerigmann et al., 2015), which as stated above would be activated by WRKY33 and ANAC042. Our results indicate that B. cinerea infection activates the expression of different Arabidopsis transcription factors (MYB51, WRKY33, and ANAC042) to enable camalexin biosynthesis. The higher expression of the genes coding for these transcriptional activators and the resulting higher accumulation of camalexin in the ugt80A2;B1 mutant compared to WT plants could be the reason of its resistance phenotype. Because the expression of the genes involved in the IG biosynthesis is also up-regulated in the infected mutant, it is reasonable to speculate that these compounds play also a role in this defense response. It is worth to mention that MYB51 is inducible by the ERF1 branch of the JA signaling pathway (Millet et al., 2010) whereas glucosinolate levels are reduced when the JA signaling is blocked (Mikkelsen et al., 2003; Mewis et al., 2005; Li et al., 2006). This, together with the fact that WRKY33 and ANAC042, can be regulated by JA (De Geyter et al., 2012) suggests that the different signaling pathways leading to the resistance phenotype against B. cinerea observed in the ugt80A2;B1 mutant might be activated by the increased JA levels detected in the mutant after infection with the fungus, compared to the WT ( Figure 3D ).
Alkylglucosinolates are a class of glucosinolates synthesized from methionine that are biosynthetically related with IGs because they share the common metabolic intermediate thiohydroxymate ( Figure 4 ). In fact, a crosstalk between both pathways has been reported. For instance, a cyp83a1 mutant produces lower levels of AG, but accumulates higher levels of IG than the corresponding WT (Hemm et al., 2003; Naur et al., 2003; Sønderby et al., 2010). However, in our experimental conditions, this kind of interaction does not seem to occur since, in contrast to the changes observed in the expression of the genes coding for the IG biosynthetic enzymes and the corresponding transcriptional activators ( Figures 6 and 7 ), no changes were detected between ugt80A2;B1 mutant and WT plants infected or not with B. cinerea either in the expression of the BCAT4, CYP79F1, CYP83A1, and UGT74C1 genes involved in AG biosynthesis ( Figures S5A–D ) or in the transcript levels of the genes coding for the transcription factors MYB28 and MYB29 reportedly involved in controlling the AG biosynthetic pathway in response to biotic and abiotic stress (Hirai et al., 2007; Sønderby et al., 2010) ( Figures S5E, F ). These results are in agreement with those obtained by Ferrari et al. (2007) in a full-genome expression analysis of Arabidopsis plants treated with B. cinerea, where the genes encoding enzymes involved in the biosynthesis of Trp and indole compounds were up-regulated, whereas most genes encoding enzymes involved in the biosynthesis of AG, like CYP79F1, REF2, and UGT74C1 (Hansen et al., 2001; Hemm et al., 2003; Gachon et al., 2005), were repressed or not significantly affected.
In conclusion, the results of this work show that an Arabidopsis ugt80A2;B1 mutant is more resistant to the infection by the necrotrophic fungus B. cinerea than the corresponding WT plants. This effective response against B. cinerea seems to be mediated by the enhanced levels of some defense secondary metabolites, such as camalexin and probably also IG, in the ugt80A2;B1 mutant compared to the WT. The biosynthesis of these compounds is regulated by a set of transcription factors that can be activated by the high levels of JA present in the mutant, which in turn would induce the expression of some defense genes, like PDF1.2 and PR4. However, the upstream mechanisms that trigger this response, including the membrane localized signal transduction steps, remain elusive. Steryl glycosides are enriched in the plasma membrane lipid rafts or DRM, which control dynamic protein interactions in a specific sterol-lipid environment (Zauber et al., 2014). The biological function of these microdomains has been linked to signaling and transport, since proteomic analysis have identified several proteins involved in these processes in the DRM (Shahollari and Berghöfer, 2004; Kierszniowska et al., 2009). Thus, it might be hypothesized that an altered composition of glycosylated sterols in the membrane rafts might affect their structure and function, resulting in an indirect differential modulation of some signaling pathways, such as those described in this work. The identification of some immunity-related proteins whose levels are increased in the DRM of the ugt80A2;B1 mutant (Zauber et al., 2014) would support this hypothesis. These proteins include PERK1, a membrane receptor-like kinase involved in the general perception and response to wounding and/or pathogen stimulus (Silva and Goring, 2002); PLC2 (phospholipase C2), a protein that plays a role in MAMP-triggered immunity by modulating ROS production (D’Ambrosio et al., 2017); and AtRBOHD, a protein required for ROS production induced by DAMPs and pathogen attack (Liu and He, 2016). An alternative possibility is that the resistance phenotype observed in the ugt80A2;B1 mutant could be due to a defect in a signaling role mediated directly by SGs, as described for the pleiotropic developmental phenotypes observed in different sterol biosynthesis mutants (Schrick et al., 2000; Schrick et al., 2002; He et al., 2003). The dissection of the activated transduction pathways and the identification of their different components will provide further insights about the mechanism of action by which glycosylated sterols may modulate the plant defense response against pathogen attack.
Data Availability Statement
All datasets generated for this study are included in the manuscript and the Supplementary Files .
Author Contributions
TA, AF, VF, and AB conceived and designed the research; NC and VP performed the infection studies and metabolites analysis; NC and ÁC conducted the gene expression analyses. NC, VP, MA, VF, AF, and TA collected and analyzed data. TA and AF wrote the manuscript.
Funding
This work was funded by grants AGL2017-88842-R from FEDER/Ministerio de Ciencia, Innovación y Universidades-Agencia Estatal de Investigación (Spain), 2017SGR710 from the Generalitat de Catalunya, and by the CERCA Programme of the Generalitat de Catalunya. We also acknowledge financial support from the Spanish Ministerio de Economía y Competitividad through the “Severo Ochoa Programme for Centres of Excellence in R&D” 2016-2019 (SEV-2015- 0533). Research in VF laboratory has been funded by grant UJI-B2016-43 from the University Jaume I programme.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
NC and ÁC received PhD fellowships from the CONACYT (México). VP has been granted with the Juan de la Cierva project IJCI-2015-24527. We also thank the Genomics and NGS scientific service at the Centre for Research in Agricultural Genomics (CRAG) and the Scientific Instrumentation Service (SCIC) at the UJI.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2019.01162/full#supplementary-material
References
- AbuQamar S., Chen X., Dhawan R., Bluhm B., Salmeron J., Lam S., et al. (2006). Expression profiling and mutant analysis reveals complex regulatory networks involved in Arabidopsis response to Botrytis infection. Plant J. 48, 28–44. 10.1111/j.1365-313X.2006.02849.x [DOI] [PubMed] [Google Scholar]
- AbuQamar S., Moustafa K., Tran L. S. (2017). Mechanisms and strategies of plant defense against Botrytis cinerea. Crit. Rev. Biotech. 37, 262–274. 10.1080/07388551.2016.1271767 [DOI] [PubMed] [Google Scholar]
- Anderson J. P., Badruzsaufari E., Schenk P. M., Manners J. M., Desmond O. J., Ehlert C., et al. (2004). Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 16, 3460–3479. 10.1105/tpc.104.025833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja I., Kissen R., Bones A. M. (2012). Phytoalexins in defense against pathogens. Trends Plant Sci. 17, 73–90. 10.1016/j.tplants.2011.11.002 [DOI] [PubMed] [Google Scholar]
- Ballester M., Cordón R., Folch J. M. (2013). DAG expression: high-throughput gene expression analysis of real-time PCR data using standard curves for relative quantification. PLoS One 8, e80385. 10.1371/journal.pone.0080385.s002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek P., Pi’slewska-Bednarek M., Svatoš A., Schneider B., Doubský J., Mansurova M., et al. (2009). A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 232, 101–106. 10.1126/science.1163732 [DOI] [PubMed] [Google Scholar]
- Bednarek P. (2012). Chemical warfare or modulators of defence responses—the function of secondary metabolites in plant immunity. Curr. Opin. Plant Biol. 15, 407–414. 10.1016/j.pbi.2012.03.002 [DOI] [PubMed] [Google Scholar]
- Berens M. L., Berry H. M., Mine A., Arguesco C. T., Tsuda K. (2017). Evolution of hormone signaling networks in plant defense. Annu. Rev. Phytopathol. 55, 401–425. 10.1146/annurev-phyto-080516-035544 [DOI] [PubMed] [Google Scholar]
- Birkenbihl R. P., Diezel C., Somssich I. E. (2012). Arabidopsis WRKY33 is a key transcriptional regulator of hormonal and metabolic responses towards Botrytis cinerea infection. Plant Physiol. 159, 266–285. 10.1104/pp.111.192641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenbihl R. P., Somssich I. E. (2011). Transcriptional plant responses critical for resistance towards necrotrophic pathogens. Front. Plant Sci. 2, 76. 10.3389/fpls.2011.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm H., Albert I., Fan L., Reinhard A., Nurnberger T. (2014). Immune receptor complexes at the plant cell surface. Curr. Opin. Plant Biol. 20, 47–54. 10.1016/j.pbi.2014.04.007 [DOI] [PubMed] [Google Scholar]
- Carland F., Fujioka S., Nelson T. (2010). The sterol methyltransferases SMT1, SMT2, and SMT3 influence Arabidopsis development through nonbrassinosteroid products. Plant Physiol. 153, 741–756. 10.1104/pp.109.152587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers A., Melchior D. L. (1986). How bilayer lipids affect membrane-protein activity. Trends Biochem. Sci. 11, 331–335. 10.1016/0968-0004(86)90292-6 [DOI] [Google Scholar]
- Cassim A. M., Gouguet P., Gronnier J., Laurent N., Germain V., Grison M., et al. (2019). Plant lipids: key players of plasma membrane organization and function. Prog. Lipid Res. 73, 1–27. 10.1016/j.plipres.2018.11.002 [DOI] [PubMed] [Google Scholar]
- Celenza J. L., Quiel J. A., Smolen G. A., Merrikh H., Silvestro A. R., Normanly J., et al. (2005). The Arabidopsis ATR1 Myb transcription factor controls indolic glucosinolate homeostasis. Plant Physiol. 137, 253–262. 10.1104/pp.104.054395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi P., Mishra M., Akhtar N., Gupta P., Mishra P., Tuli R. (2012). Sterol glycosyltransferases—identification of members of gene family and their role in stress in Withania somnifera. Mol. Biol. Rep. 39, 9755–9764. 10.1007/s11033-012-1841-3 [DOI] [PubMed] [Google Scholar]
- Choi H., Ohyama K., Kim Y. Y., Jin J. Y., Lee S. B., Yamaoka Y., et al. (2014). The role of Arabidopsis ABCG9 and ABCG31 ATP binding cassette transporters in pollen fitness and the deposition of steryl glycosides on the pollen coat. Plant Cell 26, 310–324. 10.1105/tpc.113.118935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay N. K., Adio A. M., Denoux C., Jander G., Ausubel F. M. (2009). Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323, 95–101. 10.1126/science.1164627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coego A., Ramirez V., Gil M. J., Flors V., Mauch-Mani B., Vera P. (2005). An Arabidopsis homeodomain transcription factor, OVEREXPRESSOR OF CATIONIC PEROXIDASE 3, mediates resistance to infection by necrotrophic pathogens. Plant Cell 17, 2123–2137. 10.1105/tpc.105.032375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke D. T., Burden R. S. (1990). Lipid modulation of plasma membrane-bound ATPases. Physiol. Plant 78, 153–159. 10.1111/j.1399-3054.1990.tb08730.x [DOI] [Google Scholar]
- Couto D., Zipfel C. (2016). Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 16, 537–552. 10.1038/nri.2016.77 [DOI] [PubMed] [Google Scholar]
- Cui H., Tsuda K., Parker J. E. (2015). Effector-triggered immunity: from pathogen perception to robust defense. Annu. Rev. Plant Biol. 66, 487–511. 10.1146/annurev-arplant-050213-040012 [DOI] [PubMed] [Google Scholar]
- Czechowski T., Stitt M., Altmann T., Udvardi M. K., Scheible W. R. (2005). Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 139, 5–17. 10.1104/pp.105.063743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio J. M., Couto D., Fabro G., Scuffi D., Lamattina L., Munnik T., et al. (2017). Phospholipase C2 Affects MAMP-triggered immunity by modulating ROS production. Plant Physiol. 175, 970–981. 10.1104/pp.17.00173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R., Van Kan J. A. L., Pretorius Z. A., Hammond-Kosack K. E., Di Pietro A., Spanu P. D., et al. (2012). The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 13, 414–430. 10.1111/j.1364-3703.2011.00783.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBolt S., Scheible W., Schrick K., Auer M., Beisson F., Bischoff V., et al. (2009). Mutations in UDP-glucose:sterol glucosyltransferase in Arabidopsis cause transparent testa phenotype and suberization defects in seeds. Plant Physiol. 151, 78–87. 10.1104/pp.109.140582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Geyter N., Gholami A., Goormachtig S., Goossens A. (2012). Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends Plant Sci. 17, 349–359. 10.1016/j.tplants.2012.03.001 [DOI] [PubMed] [Google Scholar]
- Ferrari S., Plotnikova J. M., De Lorenzo G., Ausubel F. M. (2003). Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J. 35, 193–205. 10.1046/j.1365-313X.2003.01794.x [DOI] [PubMed] [Google Scholar]
- Ferrari S., Galletti R., Denoux C., De Lorenzo G., Ausubel F. M., Dewdney J. (2007). Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires phytoalexin deficient3. Plant Physiol. 144, 367–379. 10.1104/pp.107.095596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer A., Altabella T., Arró M., Boronat A. (2017). Emerging roles for conjugated sterols in plants. Prog. Lipid Res. 67, 27–37. 10.1016/j.plipres.2017.06.002 [DOI] [PubMed] [Google Scholar]
- Frerigmann H., Gigolashvili T. (2014). MYB34, MYB51 and MYB122 distinctly regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana. Mol. Plant 7, 814–828. 10.1093/mp/ssu004 [DOI] [PubMed] [Google Scholar]
- Frerigmann H., Glawischnig E., Gigolashvili T. (2015). The role of MYB34, MYB51 and MYB122 in the regulation of camalexin biosynthesis in Arabidopsis thaliana. Front. Plant Sci. 6, 654. 10.3389/fpls.2015.00654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerigmann H., Piślewska-Bednarek M., Sánchez-Vallet A., Molina A., Glawischnig E., Gigolashvili T., et al. (2016). Regulation of pathogen triggered tryptophan metabolism in Arabidopsis thaliana by MYB transcription factors and indole glucosinolate conversion products. Mol. Plant 9, 682–695. 10.1016/j.molp.2016.01.006 [DOI] [PubMed] [Google Scholar]
- Furt F., König S., Bessoule J.-J., Sargueil F., Zallot R., Stanislas T., et al. (2010). Polyphosphoinositides are enriched in plant membrane rafts and form microdomains in the plasma membrane. Plant Physiol. 152, 2173–2187. 10.1104/pp.109.149823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachon C. M., Langlois-Meurinne M., Henry Y., Saindrenan P. (2005). Transcriptional co-regulation of secondary metabolism enzymes in Arabidopsis: functional and evolutionary implications. Plant Mol. Biol. 58, 229–245. 10.1007/s11103-005-5346-5 [DOI] [PubMed] [Google Scholar]
- Gamir J., Darwiche R., Van’t Hof P., Choudhary V., Stumpe M., Schneiter R., et al. (2017). The sterol-binding activity of pathogenesis-related protein 1 reveals the mode of action of an antimicrobial protein. Plant J. 89, 502–509. 10.1111/tpj.13398 [DOI] [PubMed] [Google Scholar]
- Gigolashvili T., Berger B., Mock H. P., Müller C., Weisshaar B., Flugge U. I. (2007). The transcription factor HIG1/MYB51 regulates indolic glucosinolate biosynthesis in Arabidopsis thaliana. Plant J. 50, 886–901. 10.1111/j.1365-313X.2007.03099.x [DOI] [PubMed] [Google Scholar]
- Glawischnig E. (2007). Camalexin. Phytochemistry 68, 401–406. 10.1016/j.phytochem.2006.12.005 [DOI] [PubMed] [Google Scholar]
- Glazebrook J., Ausubel F. M. (1994). Isolation of phytoalexin deficient mutants of Arabidopsis thaliana and characterization of their interactions with bacterial pathogens. Proc. Natl. Acad. Sci. U.S.A. 91, 8955–8959. 10.1073/pnas.91.19.8955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govrin E. M., Levine A. (2002). Infection of Arabidopsis with a necrotrophic pathogen, Botrytis cinerea, elicits various defense responses but does not induce systemic acquired resistance (SAR). Plant Mol. Biol. 48, 267–276. 10.1023/A:1013323222095 [DOI] [PubMed] [Google Scholar]
- Grandmougin-Ferjani A., Schuler-Muller I., Hartmann M. A. (1997). Sterol modulation of the plasma membrane H+-ATPase activity from corn roots reconstituted into soybean lipids. Plant Physiol. 113, 163–174. 10.1104/pp.113.1.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grille S., Zaslawski A., Thiele S., Plat J., Warnecke D. (2010). The functions of steryl glycosides come to those who wait: recent advances in plants, fungi, bacteria and animals. Prog. Lipid Res. 49, 262–288. 10.1016/j.plipres.2010.02.001 [DOI] [PubMed] [Google Scholar]
- Gronnier J., Gerbeau-Pissot P., Germain V., Mongrand S., Simon-Plas F. (2018). Divide and rule: plant plasma membrane organization. Trends Plant Sci. 23, 899–917. 10.1016/j.tplants.2018.07.007 [DOI] [PubMed] [Google Scholar]
- Grosjean K., Mongrand S., Beney L., Simon-Plas F., Gerbeau-Pissot P. (2015). Differential effect of plant lipids on membrane organization: specificities of phytosphingolipids and phytosterols. J. Biol. Chem. 290, 5810–5825. 10.1074/jbc.M114.598805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Yang H., Zhang X., Yang S. (2013). Lipid transfer protein 3 as a target of MYB96 mediates freezing and drought stress in Arabidopsis. J. Exp. Bot. 64, 1755–1767. 10.1093/jxb/ert040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L., Li G. J., Yang K. Y., Mao G., Wang R., Liu Y., et al. (2010). Mitogen-activated protein kinase 3 and 6 regulate Botrytis cinerea–induced ethylene production in Arabidopsis. Plant J. 64, 114–127. 10.1111/j.1365-313X.2010.04318.x [DOI] [PubMed] [Google Scholar]
- Hansen C. H., Wittstock U., Olsen C. E., Hick A. J., Pickett J. A., Halkier B. A. (2001). Cytochrome p450 CYP79F1 from Arabidopsis catalyzes the conversion of dihomomethionine and trihomomethionine to the corresponding aldoximes in the biosynthesis of aliphatic glucosinolates. J. Biol. Chem. 276, 11078–11085. 10.1074/jbc.M010123200 [DOI] [PubMed] [Google Scholar]
- He J.-X., Fujioka S., Li T.-C., Kang S. G., Seto H., Takatsuto S., et al. (2003). Sterols regulate development and gene expression in Arabidopsis. Plant Physiol. 131, 1258–1269. 10.1104/pp.014605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemm M. R., Ruegger M. O., Chapple C. (2003). The Arabidopsis ref2 mutant is defective in the gene encoding CYP83A1 and shows both phenylpropanoid and glucosinolate phenotypes. Plant Cell 15, 179–194. 10.1105/tpc.006544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai M. Y., Sugiyama K., Sawada Y., Tohge T., Obayashi T., Suzuki A., et al. (2007). Omics-based identification of Arabidopsis Myb transcription factors regulating aliphatic glucosinolate biosynthesis. Proc. Natl. Acad. Sci. U. S. A. 104, 6478–6483. 10.1073/pnas.0611629104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. M., Bahn S. C., Lyu A., Jung H. S., Ahn J. H. (2010). Identification and testing of superior reference genes for a starting pool of transcript normalization in Arabidopsis. Plant Cell Physiol. 51, 1694–1706. 10.1093/pcp/pcq128 [DOI] [PubMed] [Google Scholar]
- Huang P.-Y., Catinot J., Zimmerli L. (2016). Ethylene response factors in Arabidopsis immunity. J. Exp. Bot. 67, 1231–1241. 10.1093/jxb/erv518 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Yu D. (2016). TheWRKY57 transcription factor affects the expression of jasmonate ZIM-domain genes transcriptionally to compromise Botrytis cinerea resistance. Plant Phys. 171, 2771–2782. 10.1104/pp.16.00747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszniowska S., Seiwert B., Schulze W. X. (2009). Definition of Arabidopsis sterol-rich membrane microdomains by differential treatment with methyl-beta-cyclodextrin and quantitative proteomics. Mol. Cell. Proteomics 8, 612–623. 10.1074/mcp.M800346-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein D. J., Rowe H. C., Denby K. J. (2005). Secondary metabolites influence Arabidopsis/Botrytis interactions: variation in host production and pathogen sensitivity. Plant J. 44, 25–36. 10.1111/j.1365-313X.2005.02508.x [DOI] [PubMed] [Google Scholar]
- Kopischke M., Westphal L., Schneeberger K., Clark R., Ossowski S., Wewer V., et al. (2013). Impaired sterol ester synthesis alters the response of Arabidopsis thaliana to Phytophthora infestans. Plant J. 73, 456–468. 10.1111/tpj.12046 [DOI] [PubMed] [Google Scholar]
- Lemarié S., Robert-Seilaniantz A., Lariagon C., Lemoine J., Marnet N., Levrel A., et al. (2015). Camalexin contributes to the partial resistance of Arabidopsis thaliana to the biotrophic soilborne protist Plasmodiophora brassicae. Front. Plant Sci. 6, 539. 10.3389/fpls.2015.00539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng P., Yuan B., Guo Y., Chen P. (2014). The role of abscisic acid in fruit ripening and responses to abiotic stress. J. Exp. Bot. 65, 4577–4588. 10.1093/jxb/eru204 [DOI] [PubMed] [Google Scholar]
- Li G., Meng X., Wang R., Mao G., Han L., Liu Y., et al. (2012). Dual-level regulation of ACC synthase activity by MPK3/MPK6 cascade and its downstream WRKY transcription factor during ethylene induction in Arabidopsis. PLoS Gen. 8, e1002767. 10.1371/journal.pgen.1002767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Brader G., Kariola T., Palva E. T. (2006). WRKY70 modulates the selection of signaling pathways in plant defense. Plant J. 46, 477–491. 10.1111/j.1365-313X.2006.02712.x [DOI] [PubMed] [Google Scholar]
- Li X., Xia T., Huang J., Guo K., Liu X., Chen T., et al. (2014). Distinct biochemical activities and heat shock responses of two UDP-glucose sterol glucosyltransferases in cotton. Plant Sci. 219-220, 1–8. 10.1016/j.plantsci.2013.12.013 [DOI] [PubMed] [Google Scholar]
- Lipka V., Dittgen J., Bednarek P., Bhat R., Wiermer M., Stein M., et al. (2005). Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310, 1180–1183. 10.1126/science.1119409 [DOI] [PubMed] [Google Scholar]
- Liu S., Bartnikas L. M., Volko S. M., Ausubel F. M., Tang D. (2016). Mutation of the glucosinolate biosynthesis enzyme cytochrome P450 83A1 monooxygenase increases camalexin accumulation and Powdery Mildew resistance. Front. Plant Sci. 7, 227. 10.3389/fpls.2016.00227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Kracher B., Ziegler J., Birkenbihl R. P., Somssich I. E. (2015). Negative regulation of ABA signaling by WRKY33 is critical for Arabidopsis immunity towards Botrytis cinerea 2100. Elife 4, e07295. 10.7554/eLife.07295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., He C. (2016). Regulation of plant reactive oxygen species (ROS) in stress responses: learning from AtRBOHD. Plant Cell Rep. 35, 995–1007. 10.1007/s00299-016-1950-x [DOI] [PubMed] [Google Scholar]
- Manzano D., Andrade P., Caudepón D., Altabella T., Arró M., Ferrer A. (2016). Suppressing farnesyl diphosphate synthase alters chloroplast development and triggers sterol-dependent induction of jasmonate- and Fe-related responses. Plant Physiol. 172, 93–117. 10.1104/pp.16.00431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengiste T. (2012). Plant immunity to necrotrophs. Ann. Rev. Phyto. 50, 267–294. 10.1146/annurev-phyto-081211-172955 [DOI] [PubMed] [Google Scholar]
- Mewis I., Appel H. M., Hom A., Raina R., Schultz J. C. (2005). Major signaling pathways modulate Arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiol. 138, 1149–1162. 10.1104/pp.104.053389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen M. D., Petersen B. L., Glawischnig E., Jensen A. B., Andreasson E., Halkier B. A. (2003). Modulation of CYP79 genes and glucosinolate profiles in Arabidopsis by defense signaling pathways. Plant Physiol. 131, 298–308. 10.1104/pp.011015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet Y. A., Danna C. H., Clay N. K., Songnuan W., Simon M. D., Werck-Reichhart D., et al. (2010). Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns. Plant Cell 22, 973–990. 10.1105/tpc.109.069658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M. K., Chaturvedi P., Singh R., Singh G., Sharma L. K., Pandey V., et al. (2013). Overexpression of WsSGTL1 gene of Withania somnifera enhances salt tolerance, heat tolerance and cold acclimation ability in transgenic Arabidopsis plants. PLoS One 8, e63064. 10.1371/journal.pone.0063064.s015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M. K., Singh G., Tiwari S., Singh R., Kumari N., Misra P. (2015). Characterization of Arabidopsis sterol glycosyltransferase TTG15/UGT80B1 role during freeze and heat stress. Plant Signal. Behav. 10, e1075682. 10.1080/15592324.2015.1075682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M. K., Srivastava M., Singh G., Tiwari S., Niranjan A., Kumari N., et al. (2017). Overexpression of Withania somnifera SGTL1 gene resists the interaction of fungus Alternaria brassicicola in Arabidopsis thaliana. Physiol. Mol. Plant Pathol. 97, 11–19. 10.1016/j.pmpp.2016.11.003 [DOI] [Google Scholar]
- Mongrand S., Stanislas T., Bayer E. M. F., Lherminier J., Simon-Plas F. (2010). Membrane rafts in plant cells. Trends Plant Sci. 15, 656–663. 10.1016/j.tplants.2010.09.003 [DOI] [PubMed] [Google Scholar]
- Moreau R. A., Whitaker B. D., Hicks K. B. (2002). Phytosterols, phytostanols, and their conjugates in foods: structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res. 41, 457–500. 10.1016/S0163-7827(02)00006-1 [DOI] [PubMed] [Google Scholar]
- Mulema J. M., Denby K. J. (2012). Spatial and temporal transcriptomic analysis of the Arabidopsis thaliana–Botrytis cinerea interaction. Mol. Biol. Rep. 39, 4039–4049. 10.1007/s11033-011-1185-4 [DOI] [PubMed] [Google Scholar]
- Nakamoto M., Schmit A.-C., Heintz D., Schaller H., Ohta D. (2015). Diversification of sterol methyltransferase enzymes in plants and a role for β-sitosterol in oriented cell plate formation and polarized growth. Plant J. 84, 860–874. 10.1111/tpj.13043 [DOI] [PubMed] [Google Scholar]
- Narayanan S., Tamura P. J., Roth M. R., Prasad P. V. V., Welti R. (2016). Wheat leaf lipids during heat stress: high day and night temperatures result in major lipid alterations. Plant Cell Environ. 39, 787–803. 10.1104/pp.112.202846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naur P., Petersen B. L., Mikkelsen M. D., Bak S., Rasmussen H., Olsen C. E., et al. (2003). CYP83A1 and CYP83B1, two nonredundant cytochrome P450 enzymes metabolizing oximes in the biosynthesis of glucosinolates in Arabidopsis. Plant Physiol. 133, 63–72. 10.1104/pp.102.019240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuruzzaman M., Sharoni A. M., Kikuchi S. (2013). Roles of NAC transcription factors in the regulation of biotic and abiotic stress responses in plants. Front. Microbiol. 4, 248. 10.3389/fmicb.2013.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström L., Schär A., Lampi A.-M. (2012). Steryl glycosides and acylated steryl glycosides in plant foods reflect unique sterol patterns. Eur. J. Lipid Sci. Technol. 114, 656–669. 10.1002/ejlt.201200033 [DOI] [Google Scholar]
- Ovecka M., Berson T., Beck M., Derksen J., Samaj J., Baluska F., et al. (2010). Structural sterols are involved in both the initiation and tip growth of root hairs in Arabidopsis thaliana. Plant Cell 22, 2999–3019. 10.1105/tpc.109.069880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palta J. P., Whitaker B. D., Weiss L. S. (1993). Plasma membrane lipids associated with genetic variability in freezing tolerance and cold acclimation of Solanum species. Plant Physiol. 103, 793–803. 10.1104/pp.103.3.793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey V., Niranjan A., Atri N., Chandrashekhar K., Mishra M. K., Trivedi P. K., et al. (2014). WsSGTL1 gene from Withania somnifera, modulates glycosylation profile, antioxidant system and confers biotic and salt stress tolerance in transgenic tobacco. Planta 239, 1217–1231. 10.1007/s00425-014-2046-x [DOI] [PubMed] [Google Scholar]
- Pangesti N., Reichelt M., de Mortel J. E., Kapsomenou E., Gershenzon J., van Loon J. J., et al. (2016). Jasmonic acid and ethylene signaling pathways regulate glucosinolate levels in plants during rhizobacteria-induced systemic resistance against a leaf-chewing herbivore. J. Chem. Ecol. 42, 1212–1225. 10.1007/s10886-016-0787-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx I. A., Eggermont K., Terras F. R., Thomma B. P., De Samblanx G. W., Buchala A., et al. (1996). Pathogen induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid–independent pathway. Plant Cell 8, 2309–2323. 10.1105/tpc.8.12.2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen K., Fiil B. K., Mundy J., Petersen M. (2008). Downstream targets of WRKY33. Plant Signal Behav. 3, 1033–1034. 10.4161/psb.6878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse C. M. J., Van der Does D., Zamioudis C., Leon-Reyes A., Van Wees S. C. M. (2012). Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 28, 489–521. 10.1146/annurev-cellbio-092910-154055 [DOI] [PubMed] [Google Scholar]
- Pook V. G., Nair M., Ryu K., Arpin J. C., Schiefelbein J., Schrick K., et al. (2017). Positioning of the scrambled receptor requires UDP-Glc:sterol glucosyltransferase 80B1 in Arabidopsis roots. Sci. Rep. 7, 1–10. 10.1038/s41598-017-05925-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian P., Han B., Forestier E., Hu Z., Gao N., Lu W., et al. (2013). Sterols are required for cell-fate commitment and maintenance of the stomatal lineage in Arabidopsis. Plant J. 74, 1029–1044. 10.1111/tpj.12190 [DOI] [PubMed] [Google Scholar]
- Ramírez V., Agorio A., Coego A., García-Andrade J., Hernández M. J., Balaguer B., et al. (2011). MYB46 modulates disease susceptibility to Botrytis cinerea in Arabidopsis. Plant Physiol. 155, 1920–1935. 10.1104/pp.110.171843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Estrada K., Castillo N., Lara J. A., Arró M., Boronat A., Ferrer A., et al. (2017). Tomato UDP-glucose sterol glycosyltransferases: a family of developmental and stress regulated genes that encode cytosolic and membrane-associated forms of the enzyme. Front. Plant Sci. 8, 1–21. 10.3389/fpls.2017.00984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P., Weber H., Damond M., Farmer E. E. (2000). Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12, 707–719. 10.1105/tpc.12.5.707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe H. C., Walley J. W., Corwin J., Chan E. K., Dehesh K., Kliebenstein D. J. (2010). Deficiencies in jasmonate-mediated plant defense reveal quantitative variation in Botrytis cinerea pathogenesis. PLoS Pathog. 6, e1000861. 10.1371/journal.ppat.1000861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudell D. R., Buchanan D. A., Leisso R. S., Whitaker B. D., Mattheis J. P., Zhu Y., et al. (2011). Ripening, storage temperature, ethylene action, and oxidative stress alter apple peel phytosterol metabolism. Phytochemistry 72, 1328–1340. 10.1016/j.phytochem.2011.04.018 [DOI] [PubMed] [Google Scholar]
- Sánchez-Bel P., Sanmartín N., Pastor V., Mateu D., Cerezo M., Vidal-Albalat A., et al. (2017). Mycorrhizal tomato plants fine tunes the growth-defence balance upon N depleted root environments. Plant Cell Environ. 41, 406–420. 10.1111/pce.13105 [DOI] [PubMed] [Google Scholar]
- Saema S., Rahman L. U., Singh R., Niranjan A., Ahmad I. Z., Misra P. (2016). Ectopic overexpression of WsSGTL1, a sterol glucosyltransferase gene in Withania somnifera, promotes growth, enhances glycowithanolide and provides tolerance to abiotic and biotic stresses. Plant Cell Rep. 35, 195–211. 10.1007/s00299-015-1879-5 [DOI] [PubMed] [Google Scholar]
- Saga H., Ogawa T., Kai K., Suzuki H., Ogata Y., Sakurai N., et al. (2012). Identification and characterization of ANAC042, a transcription factor family gene involved in the regulation of camalexin biosynthesis in Arabidopsis. Mol. Plant Microbe Interact. 25, 684–696. 10.1094/MPMI-09-11-0244 [DOI] [PubMed] [Google Scholar]
- Santino A., Taurino M., De Domenico S., Bonsegna S., Poltronieri P., Pastor V., et al. (2013). Jasmonate signalling in plant defense response to multiple abiotic stresses. Plant Cell Rep. 32, 1085–1098. 10.1007/s00299-013-1441-2 [DOI] [PubMed] [Google Scholar]
- Schrick K., Mayer U., Horrichs A., Kuhnt C., Bellini C., Dangl J., et al. (2000). FACKEL is a sterol C-14 reductase required for organized cell division and expansion in Arabidopsis embryogenesis. Genes Dev. 14, 1471–1484. 10.1101/gad.14.2.1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrick K., Mayer U., Martin G., Bellini C., Kuhnt C., Schmidt J., et al. (2002). Interactions between sterol biosynthesis genes in embryonic development of Arabidopsis. Plant J. 31, 61–73. 10.1046/j.1365-313X.2002.01333.x [DOI] [PubMed] [Google Scholar]
- Schuhegger R., Nafisi M., Mansourova M., Petersen B. L., Olsen C. E., Svatos A., et al. (2006). CYP71B15 (PAD3) catalyzes the final step in camalexin biosynthesis. Plant Physiol. 141, 1248–1254. 10.1104/pp.106.082024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhegger R., Rauhut T., Glawischnig E. (2007). Regulatory variability of camalexin biosynthesis. J. Plant Physiol. 164, 636–644. 10.1016/j.jplph.2006.04.012 [DOI] [PubMed] [Google Scholar]
- Shahollari B., Berghöfer T. P. (2004). Receptor kinases with leucine-rich repeats are enriched in Triton X-100 insoluble plasma membrane microdomains from plants. Physiol. Plant 122, 397–403. 10.1111/j.1399-3054.2004.00414.x [DOI] [Google Scholar]
- Shigenaga A. M., Argueso C. T. (2016). No hormone to rule them all: interactions of plant hormones during the responses of plants to pathogens. Semin. Cell Dev. Biol. 56, 174–189. 10.1016/j.semcdb.2016.06.005 [DOI] [PubMed] [Google Scholar]
- Silva N. F., Goring D. R. (2002). The proline-rich, extensin-like receptor kinase-1 (PERK1) gene is rapidly induced by wounding. Plant Mol. Biol. 50, 667–685. 10.1023/A:1019951120788 [DOI] [PubMed] [Google Scholar]
- Singh A. K., Dwivedi V., Rai A., Pal S., Reddy S. G. E., Rao D. K. V., et al. (2015). Virus-induced gene silencing of Withania somnifera squalene synthase negatively regulates sterol and defence-related genes resulting in reduced withanolides and biotic stress tolerance. Plant Biotechnol. J. 13, 1287–1299. 10.1111/pbi.12347 [DOI] [PubMed] [Google Scholar]
- Singh G., Tiwari M., Singh S. P., Singh S., Trivedi P. K., Misra P. (2016). Silencing of sterol glycosyltransferases modulates the withanolide biosynthesis and leads to compromised basal immunity of Withania somnifera. Sci. Rep. 6, 25562. 10.1038/srep25562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G., Tiwari M., Singh S. P., Singh R., Singh S., Shirke P. A., et al. (2017). Sterol glycosyltransferases required for adaptation of Withania somnifera at high temperature. Physiol. Plant 160, 297–311. 10.1111/ppl.12563 [DOI] [PubMed] [Google Scholar]
- Sønderby I. E., Geu-Flores F., Halkier B. A. (2010). Biosynthesis of glucosinolates—gene discovery and beyond. Trends Plant Sci. 15, 283–290. 10.1016/j.tplants.2010.02.005 [DOI] [PubMed] [Google Scholar]
- Stucky D. F., Arpin J. C., Schrick K. (2015). Functional diversification of two UGT80 enzymes required for steryl glucoside synthesis in Arabidopsis. J. Exp. Bot. 66, 189–201. 10.1093/jxb/eru410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi D., Imai H., Kawamura Y., Uemura M. (2016). Lipid profiles of detergent resistant fractions of the plasma membrane in oat and rye in association with cold acclimation and freezing tolerance. Cryobiology 72, 123–134. 10.1016/j.cryobiol.2016.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazona P., Feussner K., Feussner I. (2015). An enhanced plant lipidomics method based on multiplexed liquid chromatography–mass spectrometry reveals additional insights into cold- and drought-induced membrane remodeling. Plant J. 84, 621–633. 10.1111/tpj.13013 [DOI] [PubMed] [Google Scholar]
- Thomma B. P., Nelissen I., Eggermont K., Broekaert W. F. (1999). Deficiency in phytoalexin production causes enhanced susceptibility of Arabidopsis thaliana to the fungus Alternaria brassicicola. Plant J. 19, 163–171. 10.1046/j.1365-313X.1999.00513.x [DOI] [PubMed] [Google Scholar]
- Tiwari P., Sangwan R. S., Asha, Mishra B. N., Sabir F., Sangwan N. S. (2014). Molecular cloning and biochemical characterization of a recombinant sterol 3-O-glucosyltransferase from Gymnema sylvestre R.Br. catalyzing biosynthesis of steryl glucosides. BioMed. Res. Int. 2014, 934351. 10.1155/2014/934351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji J., Jackson E. P., Gage D. A., Hammerschmidt R., Somerville S. C. (1992). Phytoalexin accumulation in Arabidopsis thaliana during the hypersensitive reaction to Pseudomonas syringae pv syringae. Plant Physiol. 98, 1304–1309. 10.1104/pp.98.4.1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Baarlen P., Woltering E. J., Staats M., van Kan J. A. (2007). Histochemical and genetic analysis of host and non-host interactions of Arabidopsis with three Botrytis species: an important role for cell death control. Mol. Plant Pathol. 8, 41–54. 10.1111/j.1364-3703.2006.00367.x [DOI] [PubMed] [Google Scholar]
- Wang K., Senthil-Kumar M., Ryu C. M., Kang L., Mysore K. S. (2012). Phytosterols play a key role in plant innate immunity against bacterial pathogens by regulating nutrient efflux into the apoplast. Plant Physiol. 158, 1789–1802. 10.1104/pp.111.189217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. L. C., Li H., Ecker J. R. (2002). Ethylene biosynthesis and signaling networks. Plant Cell 14, S131–S151. 10.1105/tpc.001768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Basnayake B. M. V. S., Zhang H., Li G., Li W., Virk N., et al. (2009). The Arabidopsis ATAF1, a NAC transcription factor, is a negative regulator of defense responses against necrotrophic fungal and bacterial pathogens. Mol. Plant Microbe Interact. 22, 1227–1238. 10.1094/MPMI-22-10-1227 [DOI] [PubMed] [Google Scholar]
- Warnecke D. C., Baltrusch M., Buck F., Wolter F. P., Heinz E. (1997). UDP-glucose:sterol glucosyltransferase: cloning and functional expression in Escherichia coli. Plant Mol. Biol. 35, 597–603. 10.1023/A:1005806119807 [DOI] [PubMed] [Google Scholar]
- Whitaker B. D. (1991). Changes in lipids of tomato fruit stored at chilling and non-chilling temperatures. Phytochemistry 30, 757–761. 10.1016/0031-9422(91)85247-W [DOI] [Google Scholar]
- Whitaker B. D. (1994). Lipid changes in mature-green tomatoes during ripening, during chilling, and after rewarming subsequent to chilling. J. Amer. Soc. Hort. Sci. 119, 994–999. 10.21273/JASHS.119.5.994 [DOI] [Google Scholar]
- Williamson B., Tudzynski B., Tudzynski P., van Kan J. A. L. (2007). Botrytis cinerea: the cause of grey mould disease. Mol. Plant Pathol. 8, 561–580. 10.1111/j.1364-3703.2007.00417.x [DOI] [PubMed] [Google Scholar]
- Windram O., Madhou P., Mchattie S., Hill C., Hickman R., Cooke E., et al. (2012). Arabidopsis defense against Botrytis cinerea: chronology and regulation deciphered by high-resolution temporal transcriptomic analysis. Plant Cell 24, 3530–3557. 10.1105/tpc.112.102046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatusevich R., Mugford S. G., Matthewman C., Gigolashvili T., Frerigmann H., Delaney S., et al. (2010). Genes of primary sulfate assimilation are part of the glucosinolate biosynthetic network in Arabidopsis thaliana. Plant J. 62, 1–11. 10.1111/j.1365-313X.2009.04118.x [DOI] [PubMed] [Google Scholar]
- Zauber H., Burgos A., Garapati P., Schulze W. X. (2014). Plasma membrane lipid–protein interactions affect signaling processes in sterol-biosynthesis mutants in Arabidopsis thaliana. Front. Plant Sci. 5, 78. 10.3389/fpls.2014.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Hong Y., Huang L., Li D., Song F. (2016). Arabidopsis AtERF014 acts as a dual regulator that differentially modulates immunity against Pseudomonas syringae pv. tomato and Botrytis cinerea. Sci. Rep. 6, 30251. 10.1038/srep30251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C. (2014). Plant pattern-recognition receptors. Trends Immunol. 35, 345–351. 10.1016/j.it.2014.05.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study are included in the manuscript and the Supplementary Files .