Abstract
Objectives
We hypothesized that short chain fatty acid (SCFA) production by oral pathogens is suppressed by exposure to cigarette smoke extract (CSE).
Background
Tobacco smoking is a major risk factor for plaque‐induced periodontal diseases. Despite increased disease susceptibility, overt oral inflammation is suppressed in smokers, presenting a diagnostic conundrum. Bacterial‐derived SCFAs can penetrate into oral tissues where they influence multiple components of immune and healing responses. Indeed, the SCFA burden has been correlated with the inflammatory condition of the gingiva. However, the influence of cigarette consumption on SCFA production is unknown.
Methods
GC/MS was employed to monitor the production of several SCFAs (propionic acid, isobutyric acid, butyric acid, and isovaleric acid) by representative anaerobic oral pathogens (Filifactor alocis 35896, Fusobacterium nucleatum 25586, Porphyromonas gingivalis 33277) that were exposed, or not, to a physiologically relevant dose of CSE (2000 ng/ml nicotine equivalents) generated from 3R4F reference cigarettes.
Results
The growth of all three bacterial species was unaffected by CSE. The capacity to produce SCFAs by these bacteria was highly varied. F alocis produced the highest concentration of a specific SCFA (butyrate); P gingivalis provided the most robust overall SCFA signal, while F alocis and F nucleatum did not release detectable levels of isobutyrate or isovalerate. As P gingivalis 33277 was the broadest SCFA producer, three low‐passage clinical isolates (10208C, 5607, and 10512) were also examined. Compared to unconditioned microbes, reduced SCFA release was apparent in CSE‐exposed low‐passage clinical isolates of P gingivalis which reached significance for one of the three isolates (propionic, isobutyric, butyric, and isovaleric acids, all P < 0.05).
Conclusions
There is high disparity in the SCFA profiles of variant chronic periodontitis‐associated bacteria, while CSE exposure reduces SCFA production by a specific clinical strain of P gingivalis. If the latter phenomenon occurs in vivo, a reduced SCFA burden may help explain the reduced vascular response to dental plaque in tobacco smokers.
Keywords: chronic periodontitis, Filifactor alocis, Fusobacterium nucleatum, Porphyromonas gingivalis, short chain fatty acids, tobacco smoking
1. INTRODUCTION
Cigarette use is a major risk factor for chronic periodontitis and other periodontal diseases.1 Smoking has been shown to increase susceptibility to destructive disease and to promote colonization by several pathogens, including Porphyromonas gingivalis and Fusobacterium nucleatum.1, 2 Smoking also reduces the efficacy of multiple treatment modalities like scaling and root planing 3; surgical interventions 4; adjunctive antimicrobial therapy 5, 6; and bone regeneration efforts,7 while smoking cessation has a positive impact on the periodontal tissues.8, 9, 10, 11 There is also increasing evidence that the ill effects of smoking on periodontal health are dose‐related 12 and that environmental tobacco smoke exposure may predispose to disease.13, 14, 15, 16, 17 Interestingly, gingival bleeding—an important clinical indicator for the presence of periodontal diseases—is suppressed in smokers. As we have recently reviewed, this discrepancy can be attributed to suppressed gingival angiogenesis in response to plaque.18 In a previous study, we were also able to show that P gingivalis becomes a less potent inducer of pro‐inflammatory responses from mononuclear cells upon exposure to cigarette smoke extract (CSE). This decrease in inflammatory potential could be due to differential regulation of several genes in CSE‐treated bacteria; for example, genes involved in the biosynthesis of the inflammatory capsule were downregulated.19 A reduced inflammatory response would be expected to facilitate the persistence of P gingivalis in its subgingival niche. However, the mechanisms of tobacco‐induced and/or tobacco‐exacerbated chronic periodontitis remain to be clarified.
Short chain fatty acids (SCFAs) are common metabolic products of bacteria. There are multiple potential mechanisms by which increased SCFA production could contribute to disease progression in a general population.20, 21, 22, 23 For example, SCFAs and other bacterial fermentation end products have been shown to drive epigenetic changes in eukaryotic cells 24 with negative effects on epithelial cell,25 fibroblast,26, 27 and T‐cell 28, 29 reproduction noted. Furthermore, SCFA has been shown to activate NF‐κB and stimulate pro‐inflammatory cytokine release 30, 31, 32; attract innate immune cells 33; promote vascular dilation 32; and endothelial proliferation.32, 34 SCFAs have been ascribed antimicrobial properties against some bacteria,35, 36 while they promote biofilm formation by others, including Actinomyces naeslundii.37 Thus, it is possible that alterations to the SCFA status quo, increases or decreases, could contribute to the microbial flux in subgingival plaque. SCFA concentrations in the gingival crevice correlate with the severity of inflammation, including periodontal bleeding and elevated subgingival temperature.34, 38
Because increased susceptibility to chronic periodontitis in tobacco users is accompanied by a decreased vascular response (ie, bleeding on probing, but also decreased production of gingival crevicular fluid) to dental plaque, we hypothesized that exposure to CSE may suppress SCFA production by P gingivalis and other relevant pathogens. Such reduced SCFA release would be consistent with the reduced vascular and inflammatory indices, including the suppressed bleeding response observed in human smokers with periodontal diseases.
To the best of our knowledge, the influence of smoking on SCFA production by oral pathogens has not been addressed. Thus, we aimed to evaluate levels of SCFAs (propionic acid CH3CH2COOH, isobutyric acid (CH3)2CHCOOH, butyric acid CH3(CH2)2COOH, and isovaleric acid (CH3)2CHCH2COOH) in culture supernatants of two established major SCFA‐producing gram‐negative pathogens, P gingivalis and F nucleatum,28 as well as the emerging gram‐positive pathogen, Filifactor alocis exposed to CSE.
2. MATERIAL AND METHODS
2.1. Materials
Gifu Anaerobe Medium was purchased from Nissui Pharmaceutical, Tokyo, Japan; brain heart infusion (BHI) came from Beckton Dickinson (Sparks, MD); 3R4F standard reference cigarettes from the Kentucky Tobacco Research and Development Center, Lexington, KY; dichloromethane came from Honeywell, Morristown, NJ; analytical standards for propionic, butyric, isobutyric, and isovaleric acid, sodium butyrate‐13C4, Na2SO4, menadione, hemin, arginine, and cysteine were purchased from Sigma‐Aldrich, St. Louis, MO; nicotine D4 came from Cerilliant, Round Rock, TX; NaOH, and HCl from Fisher Scientific, Pittsburgh, PA; fetal bovine serum (FBS) was bought from Atlanta Biologicals (Lawrenceville, GA, USA); helium was obtained from Welders Supply (Louisville, KY); while N‐Methyl‐N‐tert‐butyldimethylsilyltrifluoroacetamide (MTBSTFA) came from Regis Technologies, Morton Grove, IL.
2.2. Bacterial culture
Porphyromonas gingivalis 33277, F nucleatum 25586, and F alocis 35896, as well as the clinical P gingivalis isolates 10512, 5607, and 10208C from our own collection, were revived from frozen stocks. Briefly, the clinical isolates were obtained by sampling the subgingival microbiota of subjects with periodontitis with filter paper strips placed in deep periodontal pockets for 30 seconds. Microbiota was cultured anaerobically on blood agar containing gentamicin, and black‐pigmented colonies were screened with 16S rRNA probes specific for P gingivalis. Bacteria were grown in CSE‐conditioned and control GAM, BHI (supplemented with 5 μg/ml hemin and 1 μg/ml menadione), or BHI (supplemented with 0.1% cysteine, 20% arginine, 5% fetal bovine serum), respectively, under anaerobic conditions at 37˚C in a Coy Laboratories (Grass Lake Charter Township, MI) anaerobic chamber.
2.3. Preparation of CSE
CSE‐conditioned media were prepared using 3R4F reference cigarettes, as previously described 19 and employed at the physiologically relevant concentration of 2000 ng/ml nicotine equivalency,39, 40 as determined by gas chromatography–mass spectrometry (GC/MS). Briefly, 500 µl aliquots of analytical nicotine standards in control media (0‐20 000 ng/ml) were mixed with 20 µl nicotine D4 (final concentration 3846 ng/ml) and brought to pH of 13.0 by addition of 100 µl 2M NaOH. For the extraction of nicotine, 600 µl dichloromethane was added, samples were vortex‐mixed for 5 seconds, and layers allowed to separate. The organic phase was transferred into disposable glass cell culture vials containing the drying agent sodium sulfate. About 100 µl of the dehydrated solution was transferred into autosampler vials with low volume inserts. About 1 µl per sample was injected into the gas chromatograph for separation and nicotine concentration in CSE‐conditioned media determined by comparison to the standards.
2.4. Analysis of SCFA concentrations
Supernatants (14 000 g, 4˚C, 3 minutes) from late log or early stationary phase bacterial cultures were filtered through 0.2 µm filters and diluted 1/500 in deionized water prior to SCFA analysis. SCFAs were extracted from 1 ml culture supernatant aliquots with 10 µl sodium butyrate‐13C4 used as an internal standard. Samples were mixed with 5 µl 0.5 mol/L HCl and 1 ml dichloromethane. Layers were allowed to separate, and the organic phase was transferred into disposable glass cell culture vials containing sodium sulfate. About 100 µl of the dehydrated solution was transferred into autosampler vials with low volume inserts. Tert‐butyl dimethylsilyl (TBDMS) derivatives were allowed to form after the addition of 5 µl MTBSTFA for 20 minutes at 80˚C. About 1 µl of the mixture was injected into the gas chromatograph for separation. A model 5973 Hewlett‐Packard gas chromatography/mass spectrometry system with a 15 m × 0.25 mm DB‐5 UI column (Agilent Technologies, Santa Clara, CA) was used. The injection mode was splitless, oven temperatures were programmed from 60˚C to 120˚C at 10˚C/min after a 1 minute hold at the initial temperature, and, after a 1 minute hold at 120˚C, to 280˚C at 25˚C/min, with a 2 minutes hold at the final temperature. Helium was used as the carrier gas with a constant flow rate of 1.0 ml/min. All experiments were carried out in triplicate, unless otherwise stated.
2.5. Statistics
All statistical analyses were carried out using instat v3.06 (GraphPad, La Jolla, CA). Experiments were performed in triplicates. Differences in bacterial growth as well as interstrain variation in SCFA production were determined by ANOVA, while intrastrain variation in SCFA levels was determined by t test.
3. RESULTS
There were no significant differences in the growth characteristics of any bacterium in CSE‐conditioned versus control media (all P > 0.05), as measured spectrophotometrically at OD 600nm and as presented in Figure 1. While we have previously shown that P gingivalis can withstand high doses of CSE,19, 41, 42 this is the first report that F alocis and F nucleatum are tolerant to the complex mixture of toxins present in CSE, at least as assessed by replicative capacity.
Figure 1.
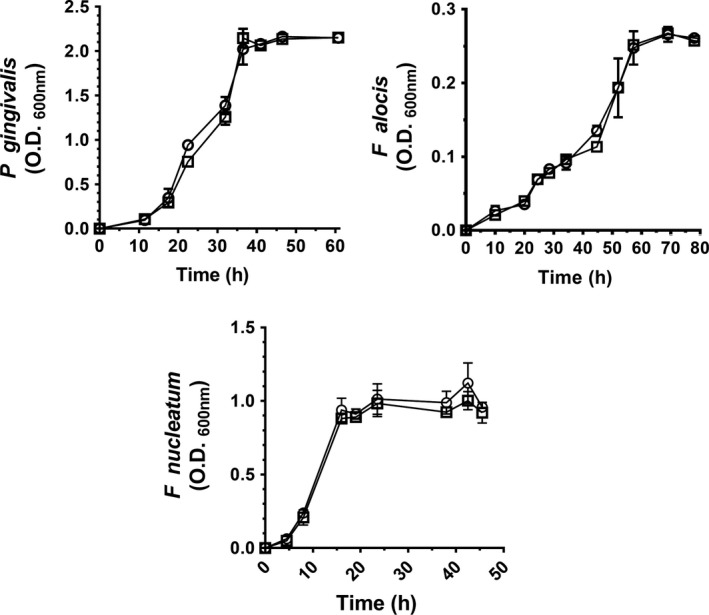
Cigarette smoke extract does not adversely influence the growth of oral pathogens. P gingivalis 33277 (top left), F nucleatum (top right), and F alocis (bottom) growth in control (○) and CSE‐conditioned (2000 ng/ml nicotine equivalents) media (□) was monitored spectrophotometrically at OD 600nm. Data are presented as mean values ± standard deviations. CSE exposure did not have a statistically significant influence on bacterial growth characteristics, as determined by ANOVA
As shown in Table 1, the SCFA signal from differing oral anaerobes was highly varied. Butyric acid was the predominant SCFA released by P gingivalis, F alocis, and F nucleatum, alike. F alocis produced the highest concentration of a specific SCFA (butyrate), while F alocis and F nucleatum did not release detectable levels of isobutyrate or isovalerate. The most robust, inclusive SCFA profile was produced by P gingivalis 33277. Therefore, we also examined SCFA production by several low‐passage clinical isolates of P gingivalis (10512, 5607 and 10208C). As presented in Figure 2, SCFA production by these strains was influenced by exposure to CSE. For the isolate 10502, a significant decrease in the levels of all four SCFAs was observed upon CSE exposure.
Table 1.
SCFA production by P gingivalis, F alocis, and F nucleatum species is highly variable
| SCFA (mmol/L ) | P gingivalis 33277 | F alocis 35896 | F nucleatum 25586 | |||
|---|---|---|---|---|---|---|
| Control | CSE | Control | CSE | Control | CSE | |
| Propionate | 0.85 (0.20) | 1.22 (0.12)* | 6.95 (9.26) | 20.13 (10.62)# | 2.06 (1.46) | 1.24 (0.39) |
| Isobutyrate | 0.58 (0.20)**/## | 0.46 (0.08)***/### | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
| Butyrate | 4.67 (1.03)**/# | 6.02 (0.90)**/# | 15.83 (0.70) | 15.83 (0.42) | 13.19 (4.41) | 12.76 (3.23) |
| Isovalerate | 1.34 (0.20)***/### | 1.48 (0.14)***/### | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
Oral bacteria were grown with or without cigarette smoke extract (CSE; 2000 ng/ml nicotine equivalents). SCFA release by cultures in late log or early stationary phase was monitored using GC/MS (normalized to cell number). Interspecies differences in SCFA release between control or CSE‐treated cultures were determined by ANOVA. Intraspecies differences in SCFA release from control vs CSE‐exposed cultures were determined by t test. Values are presented as mean ± standard deviation.
P < 0.05 (#/*); 0.01 (##/**) or 0.001 (###/***), respectively, compared to *F alocis or # F nucleatum.
Figure 2.
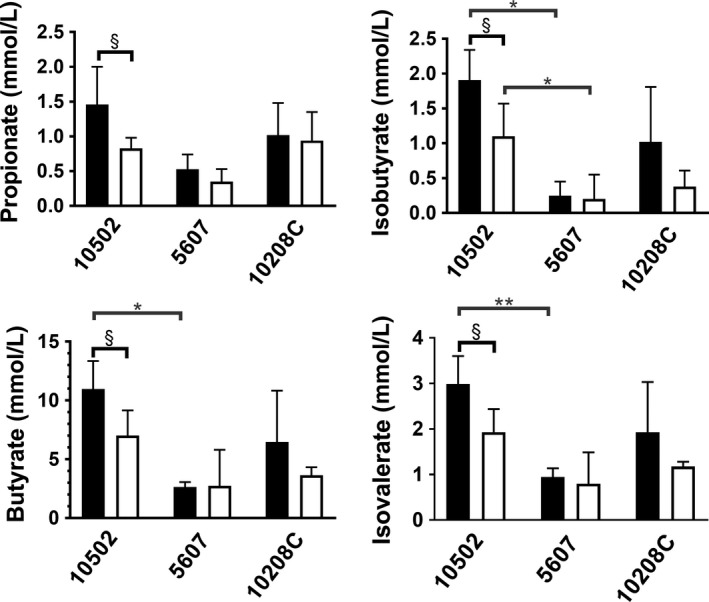
Cigarette smoke suppresses the production of SCFAs in P gingivalis 10502. Propionic acid (top left), isobutyric acid (top right), butyric acid (bottom left), and isovaleric acid (bottom right) production by P gingivalis 10502, 5607, and 10208C grown in control (black bars) or CSE‐conditioned (2000 ng/ml nicotine equivalents) medium (white bars) was determined by GCMS. SCFA release by CSE‐exposed strains compared to non‐exposed control bacteria (intrastrain variation) was determined by t test. Interstrain variation in the SCFA release was determined by ANOVA. Values are presented as mean ± standard deviation. P < 0.05 (*/§) or 0.01 (**), respectively
4. DISCUSSION
As noted earlier, SCFAs drive vasodilation, endothelial activation, and release of pro‐inflammatory cytokines. SCFAs, including propionic, butyric, and isovaleric acids, have been negatively correlated with periodontal inflammation. However, reports on bacterial SCFA production and plaque‐induced periodontal diseases are, in some ways, controversial. For example, the literature is in disagreement over whether or not increased SCFA concentrations at diseased sites reflect increased numbers of periodontal pathogens.21, 34 Since previous reports in this area have not addressed the smoking status of the subjects, variation in tobacco use may help explain such discrepancies.
Tobacco, as we have reported previously, induces multiple transcriptomic and structural alterations in P gingivalis that reduce the overall inflammatory potential of this bacterium.19, 41, 42 We proposed that components present in CSE may also suppress SCFA production by P gingivalis and other oral bacteria. This hypothesis appears to be true for a specific clinical P gingivalis isolate, but not for the type strain, or F nucleatum or F alocis.
A major limitation of our study is that only three clinical isolates of P gingivalis were available for analysis; even though a trend to reduced SCFA production was noted in all three clinical isolates, significance was only reached for strain 10502. However, should this phenomenon occur in a number of clinical strains in vivo, a further mechanism by which the pro‐angiogenic, pro‐inflammatory potential of this key bacterium may be dampened in smokers is established.
Increasing evidence suggests that an imbalance in SCFA production by the gut microbiome may play a role in the development of obesity, diabetes, inflammatory bowel disease, and even colorectal cancer.43, 44, 45 If tobacco use has the potential to affect bacterial SCFA production, this mechanism could have profound influence beyond the oral cavity.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
ACKNOWLEDGEMENTS
This work was kindly supported by NIDCR (R01DE019826 to DAS; R01DE017680 to DAS and the National Institute for General Medical Sciences (P20GM125504 to RJL).
Zeller I, Malovichko MV, Hurst HE, Renaud DE, Scott DA. Cigarette smoke reduces short chain fatty acid production by a Porphyromonas gingivalis clinical isolate. J Periodont Res. 2019;54:566–571. 10.1111/jre.12660
Iris Zeller and Marina V. Malovichko contributed equally to this manuscript.
The copyright line for this article was changed on 7 June 2019 after original online publication.
REFERENCES
- 1. Palmer RM, Wilson RF, Hasan AS, Scott DA. Mechanisms of action of environmental factors–tobacco smoking. J Clin Periodontol. 2005;32(suppl 6):180‐195. [DOI] [PubMed] [Google Scholar]
- 2. Bagaitkar J, Demuth DR, Scott DA. Tobacco use increases susceptibility to bacterial infection. Tob Induc Dis. 2008;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feres M, Bernal M, Matarazzo F, Faveri M, Duarte PM, Figueiredo LC. Subgingival bacterial recolonization after scaling and root planing in smokers with chronic periodontitis. Aust Dent J. 2015;60(2):225‐232. [DOI] [PubMed] [Google Scholar]
- 4. Kotsakis GA, Javed F, Hinrichs JE, Karoussis IK, Romanos GE. Impact of cigarette smoking on clinical outcomes of periodontal flap surgical procedures: a systematic review and meta‐analysis. J Periodontol. 2015;86(2):254‐263. [DOI] [PubMed] [Google Scholar]
- 5. Faveri M, Rebello A, de Oliveira Dias R, et al. Clinical and microbiologic effects of adjunctive metronidazole plus amoxicillin in the treatment of generalized chronic periodontitis: smokers versus non‐smokers. J Periodontol. 2014;85(4):581‐591. [DOI] [PubMed] [Google Scholar]
- 6. Javed F, Al‐Rasheed A, Almas K, Romanos GE, Al‐Hezaimi K. Effect of cigarette smoking on the clinical outcomes of periodontal surgical procedures. Am J Med Sci. 2012;343(1):78‐84. [DOI] [PubMed] [Google Scholar]
- 7. Patel RA, Wilson RF, Palmer RM. The effect of smoking on periodontal bone regeneration: a systematic review and meta‐analysis. J Periodontol. 2012;83(2):143‐155. [DOI] [PubMed] [Google Scholar]
- 8. Alexandridi F, Tsantila S, Pepelassi E. Smoking cessation and response to periodontal treatment. Aust Dent J. 2018;63(2):140‐149. [DOI] [PubMed] [Google Scholar]
- 9. Chambrone L, Preshaw PM, Rosa EF, et al. Effects of smoking cessation on the outcomes of non‐surgical periodontal therapy: a systematic review and individual patient data meta‐analysis. J Clin Periodontol. 2013;40(6):607‐615. [DOI] [PubMed] [Google Scholar]
- 10. Fiorini T, Musskopf ML, Oppermann RV, Susin C. Is there a positive effect of smoking cessation on periodontal health? A systematic review. J Periodontol. 2014;85(1):83‐91. [DOI] [PubMed] [Google Scholar]
- 11. Rosa EF, Corraini P, Inoue G, et al. Effect of smoking cessation on non‐surgical periodontal therapy: results after 24 months. J Clin Periodontol. 2014;41(12):1145‐1153. [DOI] [PubMed] [Google Scholar]
- 12. Tanaka K, Matsuse R, Miyake Y, Hanioka T, Arakawa M. Salivary cotinine concentrations and prevalence of periodontal disease in young Japanese women: the Kyushu Okinawa maternal and child health study. J Periodontol. 2013;84(12):1724‐1729. [DOI] [PubMed] [Google Scholar]
- 13. Erdemir EO, Sonmez IS, Oba AA, Bergstrom J, Caglayan O. Periodontal health in children exposed to passive smoking. J Clin Periodontol. 2010;37(2):160‐164. [DOI] [PubMed] [Google Scholar]
- 14. Javed F, Bashir Ahmed H, Romanos GE. Association between environmental tobacco smoke and periodontal disease: a systematic review. Environ Res. 2014;133:117‐122. [DOI] [PubMed] [Google Scholar]
- 15. Sanders A, Slade G. State cigarette excise tax, secondhand smoke exposure, and periodontitis in US nonsmokers. Am J Public Health. 2013;103(4):740‐746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sanders AE, Slade GD, Beck JD, Agustsdottir H. Secondhand smoke and periodontal disease: atherosclerosis risk in communities study. Am J Public Health. 2011;101(suppl 1):S339‐S346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sutton JD, Ranney LM, Wilder RS, Sanders AE. Environmental tobacco smoke and periodontitis in U.S. non‐smokers. J Dent Hyg. 2012;86(3):185‐194. [PubMed] [Google Scholar]
- 18. Buduneli N, Scott DA. Tobacco‐induced suppression of the vascular response to dental plaque. Mol Oral Microbiol. 2018;33(4):271‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bagaitkar J, Williams LR, Renaud DE, et al. Tobacco‐induced alterations to Porphyromonas gingivalis‐host interactions. Environ Microbiol. 2009;11(5):1242‐1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li QQ, Meng HX, Gao XJ, Wang ZH. Analysis of volatile fatty acids in gingival crevicular fluid of patients with chronic periodontitis. Zhonghua Kou Qiang Yi Xue Za Zhi. 2005;40(3):208‐210. [PubMed] [Google Scholar]
- 21. Lu RF, Feng L, Gao XJ, Meng HX, Feng XH. Relationship between volatile fatty acids and Porphyromonas gingivalis and Treponema denticola in gingival crevicular fluids of patients with aggressive periodontitis. Beijing Da Xue Xue Bao Yi Xue Ban. 2013;45(1):12‐16. [PubMed] [Google Scholar]
- 22. Lu RF, Meng HX, Gao XJ, Feng L, Xu L. Analysis of short chain fatty acids in gingival crevicular fluid of patients with aggressive periodontitis. Zhonghua Kou Qiang Yi Xue Za Zhi. 2008;43(11):664‐667. [PubMed] [Google Scholar]
- 23. Qiqiang L, Huanxin M, Xuejun G. Longitudinal study of volatile fatty acids in the gingival crevicular fluid of patients with periodontitis before and after nonsurgical therapy. J Periodontal Res. 2012;47(6):740‐749. [DOI] [PubMed] [Google Scholar]
- 24. Remely M, Aumueller E, Merold C, et al. Effects of short chain fatty acid producing bacteria on epigenetic regulation of FFAR3 in type 2 diabetes and obesity. Gene. 2014;537(1):85‐92. [DOI] [PubMed] [Google Scholar]
- 25. Sorkin BC, Niederman R. Short chain carboxylic acids decrease human gingival keratinocyte proliferation and increase apoptosis and necrosis. J Clin Periodontol. 1998;25(4):311‐315. [DOI] [PubMed] [Google Scholar]
- 26. Kurita‐Ochiai T, Seto S, Suzuki N, et al. Butyric acid induces apoptosis in inflamed fibroblasts. J Dent Res. 2008;87(1):51‐55. [DOI] [PubMed] [Google Scholar]
- 27. Singer RE, Buckner BA. Butyrate and propionate: important components of toxic dental plaque extracts. Infect Immun. 1981;32(2):458‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kurita‐Ochiai T, Fukushima K, Ochiai K. Volatile fatty acids, metabolic by‐products of periodontopathic bacteria, inhibit lymphocyte proliferation and cytokine production. J Dent Res. 1995;74(7):1367‐1373. [DOI] [PubMed] [Google Scholar]
- 29. Kurita‐Ochiai T, Ochiai K. Butyric acid induces apoptosis via oxidative stress in Jurkat T‐cells. J Dent Res. 2010;89(7):689‐694. [DOI] [PubMed] [Google Scholar]
- 30. Cueno ME, Ochiai K. Re‐discovering periodontal butyric acid: New insights on an old metabolite. Microb Pathog. 2016;94:48‐53. [DOI] [PubMed] [Google Scholar]
- 31. Cueno ME, Saito Y, Ochiai K. Periodontal disease level‐butyric acid amounts locally administered in the rat gingival mucosa induce ER stress in the systemic blood. Microb Pathog. 2016;94:70‐75. [DOI] [PubMed] [Google Scholar]
- 32. Niederman R, Zhang J, Kashket S. Short‐chain carboxylic‐acid‐stimulated, PMN‐mediated gingival inflammation. Crit Rev Oral Biol Med. 1997;8(3):269‐290. [DOI] [PubMed] [Google Scholar]
- 33. Vinolo M, Rodrigues H, Hatanaka E, Hebeda C, Farsky S, Curi R. Short‐chain fatty acids stimulate the migration of neutrophils to inflammatory sites. Clin Sci (Lond). 2009;117(9):331‐338. [DOI] [PubMed] [Google Scholar]
- 34. Niederman R, Buyle‐Bodin Y, Lu BY, Robinson P, Naleway C. Short‐chain carboxylic acid concentration in human gingival crevicular fluid. J Dent Res. 1997;76(1):575‐579. [DOI] [PubMed] [Google Scholar]
- 35. Huang CB, Alimova Y, Myers TM, Ebersole JL. Short‐ and medium‐chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch Oral Biol. 2011;56(7):650‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yonezawa H, Osaki T, Hanawa T, et al. Destructive effects of butyrate on the cell envelope of Helicobacter pylori . J Med Microbiol. 2012;61(Pt 4):582‐589. [DOI] [PubMed] [Google Scholar]
- 37. Yoneda S, Kawarai T, Narisawa N, et al. Effects of short‐chain fatty acids on Actinomyces naeslundii biofilm formation. Mol Oral Microbiol. 2013;28(5):354‐365. [DOI] [PubMed] [Google Scholar]
- 38. Kashket S, Zhang J, Niederman R. Gingival inflammation induced by food and short‐chain carboxylic acids. J Dent Res. 1998;77(2):412‐417. [DOI] [PubMed] [Google Scholar]
- 39. Chen X, Wolff L, Aeppli D, et al. Cigarette smoking, salivary/gingival crevicular fluid cotinine and periodontal status. A 10‐year longitudinal study. J Clin Periodontol. 2001;28(4):331‐339. [DOI] [PubMed] [Google Scholar]
- 40. Fraser HS, Palmer RM, Wilson RF, Coward PY, Scott DA. Elevated systemic concentrations of soluble ICAM‐1 (sICAM) are not reflected in the gingival crevicular fluid of smokers with periodontitis. J Dent Res. 2001;80(7):1643‐1647. [DOI] [PubMed] [Google Scholar]
- 41. Bagaitkar J, Daep CA, Patel CK, et al. Tobacco smoke augments Porphyromonas gingivalis‐Streptococcus gordonii biofilm formation. PLoS ONE. 2011;6(11):e27386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bagaitkar J, Demuth DR, Daep CA, et al. Tobacco upregulates P. gingivalis fimbrial proteins which induce TLR2 hyposensitivity. PLoS ONE. 2010;5(5):e9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bultman SJ. The microbiome and its potential as a cancer preventive intervention. Semin Oncol. 2016;43(1):97‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Murugesan S, Nirmalkar K, Hoyo‐Vadillo C, García‐Espitia M, Ramírez‐Sánchez D, García‐Mena J. Gut microbiome production of short‐chain fatty acids and obesity in children. Eur J Clin Microbiol Infect Dis. 2018;37(4):621‐625. [DOI] [PubMed] [Google Scholar]
- 45. Sun M, Wu W, Liu Z, Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol. 2017;52(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]


