Abstract
Objective:
Acute kidney injury is a frequent complication following neonatal cardiac surgery and is associated with significant morbidity and mortality. The objectives of this study were to determine if plasma neutrophil gelatinase-associated lipocalin levels were associated with acute kidney injury and clinical outcomes in neonates with congenital heart disease undergoing cardiopulmonary bypass.
Design:
Retrospective single-center observational study
Setting:
A pediatric cardiac intensive care unit within a tertiary-care academic hospital.
Patients:
Patients age less than 30 days undergoing cardiac surgery requiring cardiopulmonary bypass.
Measurements and Main Results:
Plasma neutrophil gelatinase-associated lipocalin peaked at 12 hours post-cardiopulmonary bypass and more than doubled compared to preoperative levels. Higher preoperative and 24 hour postoperative neutrophil gelatinase-associated lipocalin levels were associated with acute kidney injury (r = 0.30, r = 0.49), longer duration of mechanical ventilation (r = 0.40, r = 0.51), intensive care unit (r = 0.32, r = 0.33) and hospital lengths of stay (r = 0.28, r = 0.32) and total hospital charges (r = 0.35, r = 0.30; all p values < 0.05).
Conclusions:
Both preoperative and 24 hour postoperative plasma neutrophil gelatinase-associated lipocalin levels are associated with acute kidney injury and worse clinical outcomes in neonates undergoing cardiac surgery. Plasma neutrophil gelatinase-associated lipocalin levels may have a role in risk stratification for predicting postoperative renal dysfunction as well as providing a potential clinical trajectory in the postoperative period.
Introduction:
Acute kidney injury (AKI) is a frequent complication after cardiac surgery and is associated with significant morbidity, including longer and more expensive hospital stays1, 2 and longer duration of mechanical ventilation.3, 4 Patients that develop AKI after cardiac surgery have a higher risk of mortality5 and even minimal increases in serum creatinine are associated with decreased postoperative survival.6
The diagnosis of AKI is dependent on an elevation in serum creatinine and decrease in urine output and is often based on the most recent Kidney Disease: Improving Global Outcomes (KDIGO) criteria.7 However, serum creatinine is a delayed marker of renal function and significant injury may have already occurred by the time a rise in serum creatinine is measured. Furthermore, it is affected by multiple patient characteristics including age, sex, weight, diet and hydration status.8 After the birth of a healthy newborn, serum creatinine should rapidly decrease in the first week of life, which reflects maturation of glomerular function.9
However, due to an elevated serum creatinine at birth, diagnosing AKI in the neonatal population can be particularly challenging. Various renal biomarkers have been studied in the adult and pediatric populations in an attempt to improve accuracy and timing of AKI diagnosis. Ideally, these biomarkers would increase rapidly in response to renal injury which would, ultimately, allow for interventions that could lessen or prevent the disease process.
One such renal biomarker is neutrophil gelatinase-associated lipocalin (NGAL). NGAL is upregulated in proximal tubule cells in the kidneys after ischemic or nephrotoxic injuries.10 Its levels rapidly increase after initial insult and can be sampled in both the urine and plasma.11 Multiple studies in both pediatric and adult populations have shown that it is a reliable predictive biomarker of AKI after cardiac surgery.8, 12–17 However, few studies have examined its reliability in neonatal populations.
The primary objective of this study was to determine the association between plasma NGAL and AKI in neonates undergoing cardiopulmonary bypass for cardiac surgery. In addition, the associations of plasma NGAL to clinical outcomes were examined.
Materials and Methods:
Subject Recruitment
This study is a secondary analysis of a prospective randomized controlled trial examining preoperative steroid therapy in neonates undergoing cardiac surgery requiring cardiopulmonary bypass (ClinicalTrials.gov Identifier ). Patient selection, enrollment, and randomization have been previously described.18, 19 Briefly, all inpatient neonates (≤ 30 days of age) scheduled to undergo cardiac surgery involving CPB were eligible. Exclusion criteria included prematurity defined as less than 37 weeks post gestational age at the time of surgery, steroids within the 2 days prior to surgery, suspected infection or a hypersensitivity that would be a contraindication to methylprednisolone or use of mechanical circulatory support or active resuscitation at the time of proposed randomization. The protocol was approved by the institutional review board, and written informed consent was obtained from a parent/guardian before randomization.
Study Design and Measurements
Subjects were randomly assigned to either preoperative placebo (approximately 8 hours preoperatively) and intraoperative methylprednisolone at 30 mg/kg of body weight (single-dose group) or preoperative and intraoperative methylprednisolone (two-dose group) within strata, according to planned corrective or palliative operation. Whole blood samples were collected in ethylenediaminetetraacetic acid tubes at 5 perioperative time points. Plasma was isolated by centrifugation, decanted into aliquots, and stored at −80°C until processed for immunoassays.
Subjects enrolled in this secondary analysis were included if they had adequate volume of plasma for measurement of NGAL at 5 perioperative time points:
1) prior to skin incision, 2) immediately after the completion of modified ultrafiltration at the end of CPB, 3) at 4, 4) 12 and 5) 24 hours postoperatively. All samples were batched and run simultaneously to avoid potential laboratory assay variance. To measure NGAL levels, an enzyme-linked immunoassay approach was utilized. Levels were examined following the manufacturer’s protocol (Quantikine Human Lipocalin-2/NGAL Immunoassay; DLCN20; R&D Systems). In general terms, plasma (diluted 1:20) was added to a 96-well plate containing the targeted antisera, following rigorous washing steps and conjugation with a secondary detection antisera. The resultant colorimetric reaction was read by scanning spectrophotometry (VersaMax, Molecular Devices, Sunnyvale, CA). The specific concentration of the NGAL level in each sample was then determined by comparison to a standard curve generated from known concentrations of NGAL. The sensitivity for the NGAL assay (minimum detectable dose) was 0.012 ng/mL. NGAL quantities were calculated using SoftMax Pro Software 5.4 and expressed as absolute concentration in ng/mL.
The primary outcome measure was AKI defined as an increase in serum creatinine by ≥ 0.3 mg/dL from preoperative levels within 48 hours. This is equivalent to stage I AKI according to the most recent KDIGO criteria and has been used in a similar study.8, 20 Additional clinical outcome measures were urine output for 36 hours post-CPB, postoperative duration of mechanical ventilation, ICU, and hospital lengths of stay and total hospital charges.
Statistical Analysis
Standard descriptive statistics were used to summarize the general demographic and clinical data. Continuous data are listed as medians with associated interquartile ranges. Categorical data are expressed as number and percentage of subjects. Pearson correlation was used to correlate NGAL levels to clinical outcomes. Independent t-tests were used to compare NGAL levels at each of the five time points in AKI vs no AKI groups as well as the single-dose vs two-dose steroid groups. Linear mixed effects models were used to evaluate differences in plasma NGAL levels over time between AKI and no AKI groups. Due to the exploratory nature of this analysis we did not adjust for multiple comparisons and used a 0.05 level to judge significance. Statistical analyses were performed with SAS, version 9.2 (SAS Institute, Inc, Cary, NC).
Results:
Of the 76 subjects in the parent study, 63 had adequate volume of plasma samples for NGAL analysis at all 5 perioperative time points and comprise this study cohort. Demographic, surgical and clinical characteristics for the cohort overall and separated by those with and without AKI are described in Table 1. AKI occurred in 15/63 (24%) patients. Serum creatinine levels were 0.6 vs 0.5 mg/dL preoperatively, 0.6 vs 0.5 mg/dL upon arrival to the ICU, and 0.9 vs 0.5 mg/dL 36 hours postoperatively in the group with and without AKI, respectively.
Table 1.
Demographic, surgical and clinical characteristics of all patients and according to presence or absence of acute kidney injury.
| Clinical Characteristics | All (n=63) | AKI (n = 15) | No AKI (n = 48) |
|---|---|---|---|
| Male sex, n (%) | 32 (51%) | 6 (40%) | 26 (54%) |
| Weight, kg | 3.2 (2.9, 3.5) | 3.2 (2.6, 3.4) | 3.3 (2.9, 3.5) |
| Gestational age at surgery, weeks | 39.9 (39.1, 40.9) | 39.6 (38.9, 40.0) | 40.0 (39.4,41.0) |
| Age at surgery, days | 7.0 (6.0, 8.5) | 7.0 (5.0, 10.5) | 7.0 (6.0, 8.0) |
| Highest pre-op creatinine (mg/dL) | 0.8 (0.7, 1.0) | 0.9 (0.8, 1.0) | 0.8 (0.7, 0.9) |
| RACHS-1 score | |||
| 1 | 0 (0%) | 0 (0%) | 0 (0%) |
| 2 | 6 (10%) | 1 (7%) | 5 (10%) |
| 3 | 19 (30%) | 2 (13%) | 17 (35%) |
| 4 | 21 (33%) | 6 (40%) | 15 (31%) |
| 5 | 1 (2%) | 1 (7%) | 0 (0%) |
| 6 | 16 (25%) | 5 (33%) | 11 (23%) |
| CPB time, minutes | 155 (124, 178) | 163 (136, 186) | 155 (123, 178) |
| DHCA, n | 27 (43%) | 9 (60%) | 18 (38%) |
| Aortic cross clamp, minutes | 70 (43, 85) | 61 (42, 94) | 71 (43, 82) |
| Total fluid intake (36 hours), mLs | 588 (492, 672) | 619 (513, 669) | 578 (479, 670) |
| Total fluid output (36 hours), mLs | 573 (442, 688) | 425 (292, 573) | 620 (473, 776) |
| Total urine output (36 hours), mLs | 470 (310, 609) | 297 (159, 376) | 526 (401, 685) |
| Continuous furosemide drip, n (%) | 38 (60%) | 11 (73%) | 27 (56%) |
| Intermittent chlorothiazide, n (%) | 39 (62%) | 9 (60%) | 30 (63%) |
| Mechanical ventilation, days | 3.8 (2.0, 5.0) | 5.8 (4.1, 20.2) | 3.0 (1.9, 4.5) |
| ICU length of stay, days | 6.2 (4.8, 9.7) | 8.5 (6.4, 22.5) | 5.8 (4.2, 7.8) |
| Hospital length of stay, days | 17.0 (9.5, 24.0) | 24.0 (18.5, 41.0) | 15.5 (9.0, 22.0) |
| Hospital charges, thousands of dollars | 194 (148, 267) | 266 (214, 401) | 180 (140, 213) |
Data are reported as number (%) or median (interquartile range), as appropriate. ICU, intensive care unit; CPB, cardiopulmonary bypass; RACHS, risk adjustment for congenital heart surgery; DHCA, direct hypothermic circulatory arrest.
Prior to surgery, NGAL levels were low (57.9 ng/mL) and increased immediately following CPB (77.0 ng/mL). NGAL levels peaked at a two-fold increase at 12 hours postoperatively (130.7 ng/mL) followed by a slight decline by 24 hours postoperatively (125.5 ng/mL).
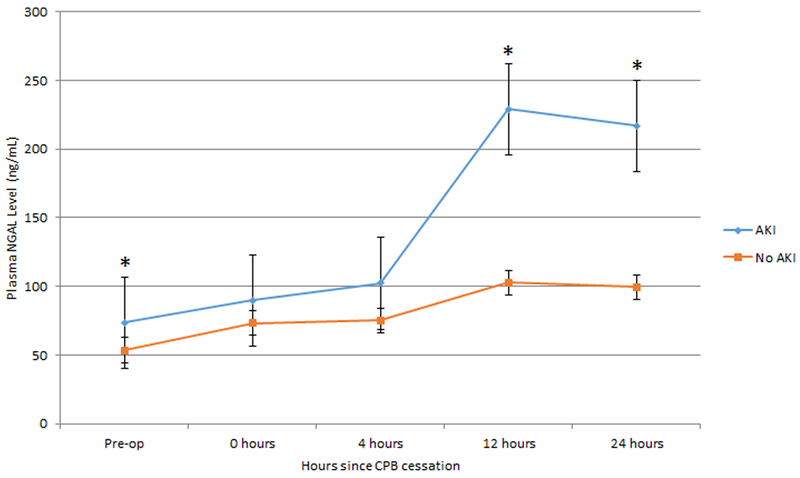
Plasma NGAL levels were higher in those with AKI compared to those without AKI preoperatively (p = 0.02), at 12 hours post-CPB (p < 0.001) and 24 hours post-CPB (p < 0.01) (Figure 1). Linear mixed effects models with generalized estimating equations that modeled NGAL values while controlling for time, AKI and the interaction between AKI and time demonstrated that the later time points have a significant difference in predicted NGAL between AKI groups. More specifically, there were significant interactions between time at 12 hours and AKI grouping (p=0.0003) and between time at 24 hours and AKI grouping (p=0.0025). The reference groups are pre-op and no AKI.
Figure 1.
Mean perioperative plasma NGAL levels with standard error bars in AKI vs no AKI groups. Asterisks denote statistically significant differences (p < 0.05).
Perioperative plasma NGAL levels and their association with outcomes are shown in Table 2. Higher preoperative and 24 hour postoperative NGAL levels were associated with worse urine output, longer postoperative duration of mechanical ventilation, ICU and hospital lengths of stay, and greater total hospital charges.
Table 2.
Correlation of Plasma NGAL levels with Clinical Outcomes
| Clinical Outcome | Pre-op | Post-CPB | 4 Hours | 12 Hours | 24 Hours |
|---|---|---|---|---|---|
| Urine output at 36 hrs | r = −0.26* | r = −0.10 | r = −0.21 | r = −0.45** | r = −0.48** |
| Mechanical ventilation | r = 0.40** | r = 0.28* | r = 0.16 | r = 0.29* | r = 0.51** |
| ICU length of stay | r = 0.32* | r = 0.21 | r = 0.08 | r = 0.18 | r = 0.33* |
| Hospital length of stay | r = 0.28* | r = 0.20 | r = 0.06 | r = 0.18 | r = 0.32* |
| Hospital charges | r = 0.35* | r = 0.25 | r = 0.11 | r = 0.22 | r = 0.30* |
Data reported as Pearson correlation coefficients, as appropriate. ICU, intensive care unit; NGAL, neutrophil gelatinase-associated lipocalin; CPB, cardiopulmonary bypass.
< 0.05
≤ 0.001
Given the potential confounding effects of the primary study’s steroid intervention on plasma NGAL levels, the differences in NGAL levels between the one and two-dose methylprednisolone groups were examined. Mean plasma NGAL levels preoperatively were 53.1 vs 62.6 ng/mL (p=0.18), immediately following CPB 66.3 vs 87.3 ng/mL (p=0.05), 4 hours post-CPB 84.3 vs 78.1 ng/mL (p=0.71), 12 hours post-CPB 135.7 vs 125.8 ng/mL (p=0.72), and 24 hours post-CPB 130.5 vs 120.7 ng/mL (p=0.70) in the one and two-dose methylprednisolone groups, respectively.
Discussion:
In this study of 63 neonates who underwent cardiac surgery requiring cardiopulmonary bypass, both preoperative and 24 hour postoperative plasma NGAL levels were associated with AKI, longer durations of mechanical ventilation and ICU and hospital lengths of stay. To our knowledge, this is the first study to suggest that preoperative NGAL levels are associated with important postoperative outcomes following neonatal cardiac surgery. This suggests that plasma NGAL may be a helpful tool preoperatively to identify those neonates at highest risk of developing AKI or experiencing a complicated postoperative course.
Neonates are at an increased risk for AKI due to immature renal development. For those neonates that undergo cardiac surgery, the risk is exponentially greater. Nearly a third of this cohort developed AKI, consistent with other reports.3, 8, 21 While the etiology is multifactorial, factors contributing to AKI include exposure to the cardiopulmonary bypass (CPB) circuit, which triggers a systemic inflammatory response, often prolonged and complex congenital heart surgery, small patient size in relation to bypass circuit, postoperative low cardiac output states that lead to poor renal perfusion as well as postoperative exposure to nephrotoxic medications.22–24
Despite the frequency and significance of AKI, the diagnosis remains challenging. Currently, serum creatinine is primarily used to diagnose AKI in the neonatal population. However, serum creatinine is a delayed marker of renal function and significant injury may have already occurred by the time a rise in serum creatinine is measured. Furthermore, it is affected by multiple patient characteristics and, due to the initial elevation of serum creatinine, which is reflective of maternal renal status, diagnosing AKI in the neonatal population is even more difficult. As was the case for many neonates in this study, cardiac surgery is often performed before serum creatinine levels completely reflect neonatal, not maternal, renal function. A biomarker that has excellent sensitivity and specificity and is quickly upregulated after a renal insult would be a more reliable diagnostic tool. The rate at which plasma NGAL rises allows for earlier diagnosis of AKI as compared to more traditional diagnostic techniques, which utilize rising serum creatinine and decreasing urine output. In previous studies, plasma NGAL levels reached peak concentrations 2–6 hours after CPB.8, 13, 15 In the current study, plasma NGAL levels peaked at 12 hours and remained elevated through 24 hours post-CPB. Obtaining plasma NGAL levels prior to surgery and in the early postoperative period may provide a means to stratify neonates and identify those at highest risk of significant postoperative AKI-related morbidity and mortality. Early identification of neonates at higher risk for AKI may inform perioperative decision-making and management regarding peritoneal dialysis catheter placement at the time of surgical intervention, postoperative dialysis utilization, avoidance of nephrotoxic medications and mean arterial pressure targets to support renal perfusion.
Previous studies that examined plasma NGAL levels and its correlation to clinical outcomes in pediatric and neonatal populations undergoing cardiopulmonary bypass did not report patient exposure to steroids. Little is known about the effect of steroids on plasma NGAL levels, but Pesonen et al. suggest that steroids decrease the release of NGAL by inhibiting degranulation of neutrophil-specific granules, thereby decreasing NGAL levels in those exposed to steroids.25 Their conclusion was that preoperative methylprednisolone administration is a confounding factor when interpreting plasma NGAL levels as they related to kidney injury. Although we cannot definitively comment on the impact of steroids on NGAL as all patients received at least a single dose of steroids, our data does not support a significant difference in NGAL between one and two doses of methylprednisolone.
The results of this study must be viewed in light of its limitations. By design this was a single center retrospective observational study. The diagnosis of AKI was based on an increase in serum creatinine and the inherit limitations of this described in detail above. Variations in postoperative care may also contribute to the differences observed in the AKI vs no AKI groups including exposure to nephrotoxic medications as well as diuretic dosing and frequency. Finally, only the immediate preoperative serum creatinine level and the highest preoperative level were collected prior to surgery, therefore we were unable to make a diagnosis of AKI preoperatively.
Future Directions
In order to build on the findings of this study, we plan to examine whether these findings can be replicated in a larger neonatal cohort. In addition, we will investigate whether urine NGAL is significantly correlated to AKI and clinical outcomes in this same patient population. Furthermore, we will be able to examine the effect of steroids on urine and plasma NGAL levels as half of the cohort in our future study will not be exposed to steroids.
Conclusions:
In conclusion, plasma NGAL levels peaked at 12 hours post-CPB and more than doubled compared to preoperative levels. Higher preoperative and 24 hour postoperative NGAL levels were associated with acute kidney injury, longer duration of mechanical ventilation, intensive care unit and hospital lengths of stay and total hospital charges. Plasma NGAL levels may have a role in risk stratification for predicting postoperative renal dysfunction as well as providing a potential clinical trajectory in the postoperative period.
Sources of Funding:
This work was supported in part by grants HL112968 and HL00726041 from the National Heart, Lung, and Blood Institute (NHLBI) as well as a Career Development Award from the American College of Cardiology Foundation/Pfizer Scholarship. This work is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or NIH.
Copyright Disclosure Statement:
Drs. Schroeder and Graham received support for article research from the National Institutes of Health (NIH). Dr. Buckley’s institution received funding from Grifols and National Heart, Lung, and Blood Institute (NHLBI) grant (awarded to Dr. Eric Graham to the institution). Dr. Stroud received funding from the NIH. Dr. Graham’s institution received funding from the NIH/NHLBI and the American College of Cardiology Foundation, and he received funding from Bayer Pharmaceuticals (research consultant). The remaining authors have disclosed that they do not have any potential conflicts of interest.
Abbreviations:
- NGAL
neutrophil gelatinase-associated lipocalin
- CPB
cardiopulmonary bypass
- ICU
intensive care unit
- AKI
acute kidney injury
- KDIGO
kidney disease: improving global outcomes
Footnotes
Clinical Trial Registry Number:
References:
- 1.Collister D, Pannu N, Ye F, James M, Hemmelgarn B, Chui B, Manns B and Klarenbach S. Health Care Costs Associated with AKI. Clin J Am Soc Nephrol. 2017;12:1733–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chertow GM, Burdick E, Honour M, Bonventre JV and Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–70. [DOI] [PubMed] [Google Scholar]
- 3.Li S, Krawczeski CD, Zappitelli M, Devarajan P, Thiessen-Philbrook H, Coca SG, Kim RW and Parikh CR. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med. 2011;39:1493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alkandari O, Eddington KA, Hyder A, Gauvin F, Ducruet T, Gottesman R, Phan V and Zappitelli M. Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Crit Care. 2011;15:R146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chertow GM, Levy EM, Hammermeister KE, Grover F and Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104:343–8. [DOI] [PubMed] [Google Scholar]
- 6.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P and Hiesmayr M. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15:1597–605. [DOI] [PubMed] [Google Scholar]
- 7.Selewski DT, Cornell TT, Heung M, Troost JP, Ehrmann BJ, Lombel RM, Blatt NB, Luckritz K, Hieber S, Gajarski R, Kershaw DB, Shanley TP and Gipson DS. Validation of the KDIGO acute kidney injury criteria in a pediatric critical care population. Intensive Care Med. 2014;40:1481–8. [DOI] [PubMed] [Google Scholar]
- 8.Krawczeski CD, Woo JG, Wang Y, Bennett MR, Ma Q and Devarajan P. Neutrophil gelatinase-associated lipocalin concentrations predict development of acute kidney injury in neonates and children after cardiopulmonary bypass. J Pediatr. 2011;158:1009–1015.e1. [DOI] [PubMed] [Google Scholar]
- 9.Gordjani N, Burghard R, Leititis JU and Brandis M. Serum creatinine and creatinine clearance in healthy neonates and prematures during the first 10 days of life. Eur J Pediatr. 1988;148:143–5. [DOI] [PubMed] [Google Scholar]
- 10.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J and Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–43. [DOI] [PubMed] [Google Scholar]
- 11.Parikh CR, Devarajan P, Zappitelli M, Sint K, Thiessen-Philbrook H, Li S, Kim RW, Koyner JL, Coca SG, Edelstein CL, Shlipak MG, Garg AX and Krawczeski CD. Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol. 2011;22:1737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alcaraz AJ, Gil-Ruiz MA, Castillo A, Lopez J, Romero C, Fernandez SN and Carrillo A. Postoperative neutrophil gelatinase-associated lipocalin predicts acute kidney injury after pediatric cardiac surgery*. Pediatr Crit Care Med. 2014;15:121–30. [DOI] [PubMed] [Google Scholar]
- 13.Dent CL, Ma Q, Dastrala S, Bennett M, Mitsnefes MM, Barasch J and Devarajan P. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Crit Care. 2007;11:R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J and Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–8. [DOI] [PubMed] [Google Scholar]
- 15.Wagener G, Jan M, Kim M, Mori K, Barasch JM, Sladen RN and Lee HT. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology. 2006;105:485–91. [DOI] [PubMed] [Google Scholar]
- 16.Bojan M, Vicca S, Lopez-Lopez V, Mogenet A, Pouard P, Falissard B and Journois D. Predictive performance of urine neutrophil gelatinase-associated lipocalin for dialysis requirement and death following cardiac surgery in neonates and infants. Clin J Am Soc Nephrol. 2014;9:285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haase-Fielitz A, Bellomo R, Devarajan P, Bennett M, Story D, Matalanis G, Frei U, Dragun D and Haase M. The predictive performance of plasma neutrophil gelatinase-associated lipocalin (NGAL) increases with grade of acute kidney injury. Nephrol Dial Transplant. 2009;24:3349–54. [DOI] [PubMed] [Google Scholar]
- 18.Graham EM, Atz AM, Butts RJ, Baker NL, Zyblewski SC, Deardorff RL, DeSantis SM, Reeves ST, Bradley SM and Spinale FG. Standardized preoperative corticosteroid treatment in neonates undergoing cardiac surgery: results from a randomized trial. J Thorac Cardiovasc Surg. 2011;142:1523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham EM, Atz AM, McHugh KE, Butts RJ, Baker NL, Stroud RE, Reeves ST, Bradley SM, McGowan FX Jr. and Spinale FG Preoperative steroid treatment does not improve markers of inflammation after cardiac surgery in neonates: results from a randomized trial. J Thorac Cardiovasc Surg. 2014;147:902–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, Herzog CA, Joannidis M, Kribben A, Levey AS, MacLeod AM, Mehta RL, Murray PT, Naicker S, Opal SM, Schaefer F, Schetz M and Uchino S. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney International Supplements. 2012;2:1–138. [Google Scholar]
- 21.Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE, Chishti AS, Woroniecki R, Mammen C, Swanson JR, Sridhar S, Wong CS, Kupferman JC, Griffin RL and Askenazi DJ. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health. 2017;1:184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozik DJ and Tweddell JS. Characterizing the inflammatory response to cardiopulmonary bypass in children. Ann Thorac Surg. 2006;81:S2347–54. [DOI] [PubMed] [Google Scholar]
- 23.Brix-Christensen V The systemic inflammatory response after cardiac surgery with cardiopulmonary bypass in children. Acta Anaesthesiologica Scandinavica. 2001;45:671–679. [DOI] [PubMed] [Google Scholar]
- 24.Bellomo R, Auriemma S, Fabbri A, D’Onofrio A, Katz N, McCullough PA, Ricci Z, Shaw A and Ronco C. The pathophysiology of cardiac surgery-associated acute kidney injury (CSA-AKI). The International journal of artificial organs. 2008;31:166–78. [DOI] [PubMed] [Google Scholar]
- 25.Pesonen EJ, Suominen PK, Keski-Nisula J, Mattila IP, Rautiainen P and Jahnukainen T. The Effect of Methylprednisolone on Plasma Concentrations of Neutrophil Gelatinase-Associated Lipocalin in Pediatric Heart Surgery. Pediatr Crit Care Med. 2016;17:121–7. [DOI] [PubMed] [Google Scholar]