Abstract
Negative symptoms are a core clinical feature of schizophrenia which are only marginally responsive to current treatments. Recent work suggests that deficits in reinforcement learning and anticipatory responses to reward may be two mechanisms that help explain impairments in motivation in those with schizophrenia. The present study utilized a reinforcement-learning paradigm, which allowed us to examine both reward anticipation and reinforcement learning. Twenty-eight people with schizophrenia and 30 healthy controls completed a reinforcement-learning task while undergoing functional magnetic resonance imaging. Participants with schizophrenia also completed a weeklong ecological momentary assessment protocol reporting anticipated motivation and pleasure in their daily activities. Unexpectedly, we found no significant group differences in performance or neural response in reinforcement-learning. However, we found that poorer reward learning was associated with greater clinician ratings of negative symptoms and EMA assessed anticipatory motivation and pleasure negative symptoms. In regards to anticipatory responses, we found that people with schizophrenia showed blunted activation in the anterior cingulate, insula, caudate and putamen while anticipating reward. Further, BOLD response in reward related regions during anticipation of reward was significantly related to both clinician rated motivation and pleasure deficits as well as daily reports of motivation and pleasure. Our results provide further evidence of deficits during reward anticipation in individuals with schizophrenia, particularly for those with severe negative symptoms and some evidence for worse reward learning among those with greater negative symptoms. Moreover, our findings suggest that these deficits show important relationships with emotional and motivational functioning in everyday life.
Keywords: reward anticipation, ecological momentary assessment, fMRI, reinforcement learning, schizophrenia
General Scientific Summary:
This study confirms previous research showing deficits in reward anticipation in schizophrenia across both self-report and neural response in striatal regions and the insula. The study also demonstrates an important link between deficits in laboratory measures of reward anticipation and anticipated motivation and pleasure in daily life as measured via ecological momentary assessment.
Introduction
Deficits in motivation and pleasure are two aspects of what is commonly referred to as negative symptoms in schizophrenia. These deficits are only marginally responsive to available treatments (Buchanan et al., 2007), thus delineating mechanisms that may serve to maintain these symptoms is vital. Recent work has highlighted different mechanisms that may underlie these deficits (Barch & Dowd, 2010; Gold, Waltz, Prentice, Morris, & Heerey, 2008; Kring & Barch, 2014). For example, Gold and colleagues (Gold et al., 2012) proposed a model of motivational deficits driven by individuals with schizophrenia showing impairments in the ability to represent positive expected values and use these mental representations to guide behavior and learn from reward. The current study sought to investigate two related mechanisms to clarify motivational impairments in schizophrenia: reward anticipation and reinforcement learning (RL), both of which require the ability to represent and use positive expected values. Both mechanisms are thought to be disrupted in schizophrenia and have important links to negative symptoms; however, no studies have examined both processes across patients utilizing multiple methods of measurement.
Anticipation in Schizophrenia
The anticipation of future rewards guides both behavior and learning (Montague & Berns, 2002; O’Doherty, 2004; Schultz, Dayan, & Montague, 1997). While the bulk of the literature assessing consummatory response to pleasure in schizophrenia suggests that responses are largely intact (Cohen & Minor, 2010; Kring & Moran, 2008), self-reported anticipation of reward when reward is not present has been shown to be reduced in those with chronic schizophrenia (Gard, Gard, Kring, & John, 2006; Kring, Siegel, & Barrett, 2014; Moran & Kring, 2018; Wynn, Horan, Kring, Simons, & Green, 2010), first-episode (Mote, Minzenberg, Carter, & Kring, 2014), and those at high-risk (Schlosser et al., 2014) relative to healthy controls (but see (Strauss, Wilbur, Warren, August, & Gold, 2011)). Neuroimaging studies have also highlighted reward anticipation impairments in schizophrenia. Studies in patient groups have shown hypoactivation in response to cues predicting reward in ventral and dorsal striatal regions in unmedicated schizophrenia patients (Esslinger et al., 2012; Juckel, Schlagenhauf, Koslowski, Wüstenberg, et al., 2006; Nielsen, Rostrup, Wulff, Bak, Lublin, et al., 2012; Schlagenhauf et al., 2009), first-episode schizophrenia patients (Hanssen et al., 2015), chronic medicated schizophrenia patients taking typical and atypical antipsychotics (Arrondo et al., 2015; Kirsch, Ronshausen, Mier, & Gallhofer, 2007; Li et al., 2018; Simon et al., 2010; Subramaniam et al., 2015) and in unaffected first-degree relatives (de Leeuw, Kahn, & Vink, 2014; Oliver Grimm et al., 2014; Li et al., 2018). However, others have found comparable levels of BOLD response during anticipatory reward between healthy controls and individuals with schizophrenia taking only atypical antipsychotics (Juckel, Schlagenhauf, Koslowski, Filonov, et al., 2006; Nielsen, Rostrup, Wulff, Bak, Broberg, et al., 2012; Schlagenhauf et al., 2008).
There is also evidence that clinician ratings of amotivation and anhedonia relate to BOLD response in the ventral and dorsal striatal regions during reward anticipation (Kirschner et al., 2016; Kluge et al., 2018; Radua et al., 2015; Stepien et al., 2018) This relationship appears to be specific to the experiential deficits (amotivation and anhedonia) of negative symptoms rather than expressive deficits which were not associated with BOLD activity during anticipation of reward (Kirschner et al., 2016; Kluge et al., 2018; Stepien et al., 2018). In addition, we have previously seen relationships between anhedonia and anticipatory responses in the ventral striatum, dorsolateral prefrontal cortex, VMPFC and inferior frontal cortex (Dowd & Barch, 2012). However, others have found no relationship between anticipatory responses and negative symptoms (Esslinger et al., 2012; O Grimm, Vollstädt-Klein, Krebs, Zink, & Smolka, 2012; Li et al., 2018). Work connecting daily life activity and BOLD response during anticipation is limited, however, Kluge and colleagues (2017) conducted a study examining motor activity assessed via actigraphy, as a measure of apathy (Kluge et al., 2018). They found that motor activity in daily life, was related to hypoactivation of the inferior frontal gyrus during reward anticipation, but not in the ventral striatum. Thus, BOLD response during reward anticipation appears to be linked specifically to experiential deficits assessed via clinician ratings and to activity in daily life. However, more work is needed to connect these responses to daily life measures of motivation and pleasure.
Reinforcement Learning in Schizophrenia
Reinforcement learning (RL) involves two components: 1) positive reinforcement to learn associations that lead to reward and 2) punishment to learn to avoid loss. In RL tasks, participants are typically presented with pairs of stimuli and asked to select the stimulus that allows them to win money or avoid losing money. Over time participants learn to associate stimuli with reward or loss avoidance. This ability to learn from reinforcement is thought to be mediated by ventral and dorsal regions of the striatum as well as cognitive control regions such as the orbital frontal cortex and the dorsolateral prefrontal cortex (Frank & Claus, 2006; Gold et al., 2012).
Studies assessing behavioral differences in RL between individuals with schizophrenia and controls suggest impairments in ability to learn from reward (Barch et al., 2017; Cicero, Martin, Becker, & Kerns, 2014; Dowd, Frank, Collins, Gold, & Barch, 2016; Fervaha et al., 2013; Gold et al., 2012; Hartmann-Riemer et al., 2017; Strauss, Frank, et al., 2011; Waltz, Frank, Robinson, & Gold, 2007). While some find that learning to avoid punishment may be intact in schizophrenia (Gold et al., 2012; Hartmann-Riemer et al., 2017; Reinen et al., 2016; Waltz et al., 2007), some find impairments avoiding loss (Barch et al., 2017; Fervaha et al., 2013; Moustafa et al., 2015). Imaging studies are mixed. Using an RL task during fMRI, Dowd et al., and colleagues (2016) found that patients with schizophrenia showed hypoactivation compared to controls during early learning in the dorsolateral prefrontal cortex and anterior insula, but similar activation in dorsal and ventral striatal regions. Similarly, Culbreth and colleagues (Culbreth, Westbrook, Xu, Barch, & Waltz, 2017) showed intact prediction error related ventral striatal activations in medicated patients. However, other studies have shown hypoactivation of ventral and dorsal striatal regions during the experience positive prediction error (receiving an unexpected reward) in individuals with schizophrenia relative to controls (Murray et al., 2008; Schlagenhauf et al., 2014).
RL has been fairly reliably associated with motivation and pleasure. For example, Kasanova et al., and colleagues (2017) found relationships between dopamine release in the caudate, putamen and ventral striatum, during a reinforcement learning task and measures of daily engagement and enjoyment in healthy controls. In an EMA study in a community sample, reward prediction error signals in the putamen and nucleus accumbens was related to a greater discrepancy between anticipatory and consummatory pleasure during daily life (Bakker et al., 2018). Studies in individuals with schizophrenia have also linked RL learning, particularly reward learning, with negative symptoms (Barch et al., 2017; Farkas et al., 2008; Gold et al., 2012; Kasanova et al., 2017; Strauss, Frank, et al., 2011; Waltz et al., 2007) (however see (Dowd et al., 2016)). Further, we have shown (Moran, Culbreth, & Barch, 2017) that better RL performance relates to greater motivation and pleasure in daily life in individuals with schizophrenia. However, more work is needed to determine whether RL performance and neural activation for either learning to achieve reward or learning to avoid loss is related to motivation and pleasure in daily life in schizophrenia.
Current Study
Our first goal was to examine group differences in anticipatory responses to rewards in a behavioral task assessing self-reported pleasure and in an fMRI task assessing neural response to potential reward. We hypothesized that individuals with schizophrenia would self-report reduced anticipatory pleasure relative to healthy controls and show hypoactivation in regions such as the caudate, putamen, dorsolateral prefrontal cortex anterior cingulate, and insula during reward anticipation. Moreover, we hypothesized that anticipatory responses to reward (both self-report and BOLD activation) in those with schizophrenia would relate to daily anticipatory motivation and pleasure measured via EMA and clinician rated negative symptoms. Our second goal was to examine group differences in the behavioral and neural response to reinforcement learning and relate responses to individual differences in motivation and pleasure. We hypothesized that people with schizophrenia would show a deficit in their ability to learn from reward, however their ability to learn to avoid loss would be intact. Further, we hypothesized that reward learning in those with schizophrenia would be related to both clinician rated negative symptoms and daily reports of anticipatory motivation and pleasure negative symptoms.
Methods
Participants
Study participants included 31 stable outpatients with schizophrenia or schizoaffective disorder (SZ) as defined by the DSM-IV (American Psychiatric Association, 2000) and 32 healthy control participants (CON). Exclusion criteria included: (1) DSM-IV diagnosis of substance abuse or dependence in the past 6 months; (2) IQ less than 70 as measured by the Wechsler Test of Adult Reading (WTAR;(Wechsler, 2001)); (3) history of severe head trauma and/or loss of consciousness and (4) MRI contraindications. Participants completed and passed a urine drug screen before each research session. Additional criteria for the patient group included: (1) no medication changes in the two weeks prior to initial study participation or anticipated changes during study completion (2) stable outpatient or partial hospital status. Additional criteria for controls included: (1) no history of schizophrenia, schizoaffective disorder, or bipolar disorder (2) no current major depression (3) no immediate relative with a history of schizophrenia or schizoaffective disorder (4) no current psychotropic medication. Two individuals with SZ and one CON participant were excluded from analyses for not participating in all components of the study, and 1 SZ and 1 CON participant were excluded for not completing the imaging task. The final sample size included 28 SZ and 30 CON participants. All participants provided written informed consent to the protocol approved by the Washington University Institutional Review Board. Demographics are presented in Table 1. There were no group differences in age, gender, ethnicity, parental education or WTAR scores (ps > .56).
Table 1.
Participant Demographic and Clinical Measures
| CON (n = 30) |
SZ (n = 28) |
||||
|---|---|---|---|---|---|
| M | SD | M | SD | p-value | |
| Age | 35.48 | 10.37 | 37.18 | 12.25 | .57 |
| Sex (% Female) | 27% | 33% | .97 | ||
| Ethnicity, (n) | 59 | ||||
| African American | 19 | 15 | |||
| Asian | 2 | 1 | |||
| Caucasian | 9 | 11 | |||
| Education (years) | 15.26 | 2.18 | 12.75 | 2.95 | .001 |
| Parental Education (years) | 14.05 | 2.06 | 14.44 | 3.72 | .63 |
| WTAR | 95.58 | 18.06 | 93.25 | 20.48 | .64 |
| Relationship Status (%) | .18 | ||||
| Married / Partner | 31% | 18% | |||
| Divorced / Separated | 0% | 7% | |||
| Never Married | 69% | 78% | |||
| Housing Status (%) | .03 | ||||
| Alone | 34% | 21% | |||
| Parent/Family | 16% | 43% | |||
| Spouse/Partner | 31% | 14% | |||
| Friend/Roommate | 19% | 8% | |||
| Board and Care | 0% | 14% | |||
| Employment Status | <.001 | ||||
| Employed | 69% | 11% | |||
| Temporarily Unemployed | 22% | 14% | |||
| Disabled | 0% | 71% | |||
| Student | 9% | 4% | |||
| CAINS MAP | -- | 15.19 | 4.14 | -- | |
| CAINS EXP | -- | 5.39 | 4.04 | -- | |
| BPRS Positive Symptoms | -- | 7.65 | 4.14 | ||
| Medications (n) | -- | ||||
| Unmedicated | -- | 4 | |||
| Atypical antipsychotics | -- | 18 | |||
| Typical antipsychotics | -- | 5 | |||
| CPZ Equivalent | -- | 311.81 | 151.45 | -- | |
Note: WTAR = Wecshler Test of Adult Reading; CAINS MAP = CAINS Motivation and Pleasure Subscale; CAINS EXP = CAINS Expression Subscale; BPRS = Brief Psychiatric Rating Scale, Positive Symptom Scale; CPZ = Chlorpromazine Equivalent
Diagnostic and Clinical Assessment
Diagnostic status was confirmed using the Structured Clinical Interview for DSM-IV-TR conducted by masters level or Ph.D. level clinicians. Individuals with SZ were also assessed for general psychiatric symptoms using the Brief Psychiatric Rating Scale (BPRS; (Overall & Gorham, 1962)) which includes a positive symptom composite score. Negative symptoms were assessed using the Clinical Assessment Interview for Negative Symptoms (CAINS; (Kring, Gur, Blanchard, Horan, & Reise, 2013)) which includes a Motivation and Pleasure (MAP) and Expression (EXP) subscale, with higher scores indicating greater impairment.
Procedure
SZ participants completed 2 visits to the laboratory, 7 days of EMA assessment in between laboratory visits, and 1 fMRI-scanning visit. On the first visit, participants with SZ completed a diagnostic interview and were trained on using the smartphone. Following 1-week of EMA, participants returned to the laboratory and completed clinical symptom interviews to assess symptoms over the prior week and completed computerized laboratory tasks. They returned for an fMRI scan following completion of their 2nd laboratory visit. Control participants completed 1 visit to the laboratory completing a diagnostic interview and computerized laboratory tests. They returned for an fMRI scan following their 1st visit.
Ecological Momentary Assessment (EMA) Protocol and Questionnaire
Individuals with SZ were provided an Android-enabled smartphone to use during the EMA portion of the study. Participants were prompted to complete the EMA questionnaire 4 times per day for 7 days between the hours of 10:00 AM and 7:00 PM. The questionnaires occurred pseudorandomly approximately every 3 hours. Participants were allotted 15 minutes to begin the survey, after which their responses would not be counted. Participants were paid $1.75 for each EMA questionnaire they completed within 15 minutes of beep.
The EMA questionnaire included questions assessing anticipated motivation and pleasure as they went about their daily activities. Participants were asked “in the next few hours which of the following activities do you think you will enjoy the most.” Participants were asked to select from among a number of potential behaviors including: eating/drinking; tv/radio/reading/computer; exercising; work/school; cleaning/cooking/chores; socializing; nothing in particular. Next they were asked to anticipate their pleasure: “How much do you think you will enjoy this activity.” Finally they were asked “how motivated do you think you will be in this activity.” Both pleasure and motivation levels were rated on a 5-point scale from “not at all” to “extremely.” Anticipated motivation (M=3.61, SD=.61) and anticipated pleasure (M=3.57, SD=.61) were highly correlated (r=.74, p<.001). Similar to composite motivation and pleasure ratings in the CAINS, we created a composite anticipated motivation and pleasure ratings (EMA-AMP) for each survey completed (mean EMA-AMP = 3.59, SD = .60), with higher scores representing greater anticipated motivation and pleasure. Consistent with previous EMA research (Myin-Germeys, van Os, Schwartz, Stone, & Delespaul, 2001), all participants completed at least 33% of surveys and thus were included in the present analyses. Mean response rate was 81% and a total of 710 responses were recorded across all participants.
Tasks
Gambling Task.
We adapted the Gambling Task (Delgado, Nystrom, Fissell, Noll, & Fiez, 2000; Forbes et al., 2009), a card-guessing paradigm, to assess self-reported anticipatory and consummatory emotion in response to reward (high and low) and loss (high and low) conditions. Participants completed this task outside the scanner during their initial behavioral session study visit. Ratings were completed on a 5-point scale going from 1 “unhappy” to 5 “happy” thus higher scores reflect higher anticipatory or consummatory pleasure. Each trial began with a guessing period wherein participants were asked to make a guess as to whether a card was higher or lower than 5. Following the guess, participants were presented with the trial type. Four trial types were included in the task: 1) low reward (50 cents) 2) high reward ($1 dollar) 3) low loss (25 cents) 4) high loss (50 cents). Next, participants predicted how much pleasure they would feel upon the outcome of the trial should they receive the outcome indicated by the cue. Participants were then given feedback on the outcome of their choice (i.e., win or not win on reward conditions; lose or not lose on loss conditions) and the amount of money they won/loss. Finally, participants rated how they felt upon receiving feedback regarding gains and losses. Ratings were completed on a 5-point scale going from 1 “unhappy” to 5 “happy.” Participants were unaware that outcomes were fixed and predetermined such that each participant received $5.25 upon completion of the task.
Probabilistic Incentive Learning Task.
We modified the Probabilistic Incentive Learning Task (PILT) (Gold et al., 2012) task which participants completed during imaging (see Figure 1). To modify the task to examine anticipation and RL, we added an anticipation phase to the beginning of each trial. Participants were first presented with a cue indicating upcoming trial type for 1000 ms indicating whether the upcoming trial type was a potential reward or loss condition. Next there was an anticipation phase which was between 2000–6000 ms. Next, stimuli were presented for 3000 ms. Stimuli consisted of 4 pairs of landscape pictures; 2 pairs associated with potential gain, and 2 pairs associated with potential loss. Participants were instructed to select the picture that was most likely to either (1) earn money (Reward trials) or (2) avoid losing money (Loss trials). Correct responses were reinforced on either 80% or 90% of trials. For the present study we have combined 80% and 90% trials reinforcement levels within each condition (reward, loss), however, the pattern of findings remain similar when examining reinforcement levels independently. The task consisted of a total of 4 runs of 20 trials each with 5 trials per condition in each run. Prior to the task participants completed a training session where participants completed at least one run (20 trials) of the task outside the scanner to ensure they were familiar with the task.
Figure 1.
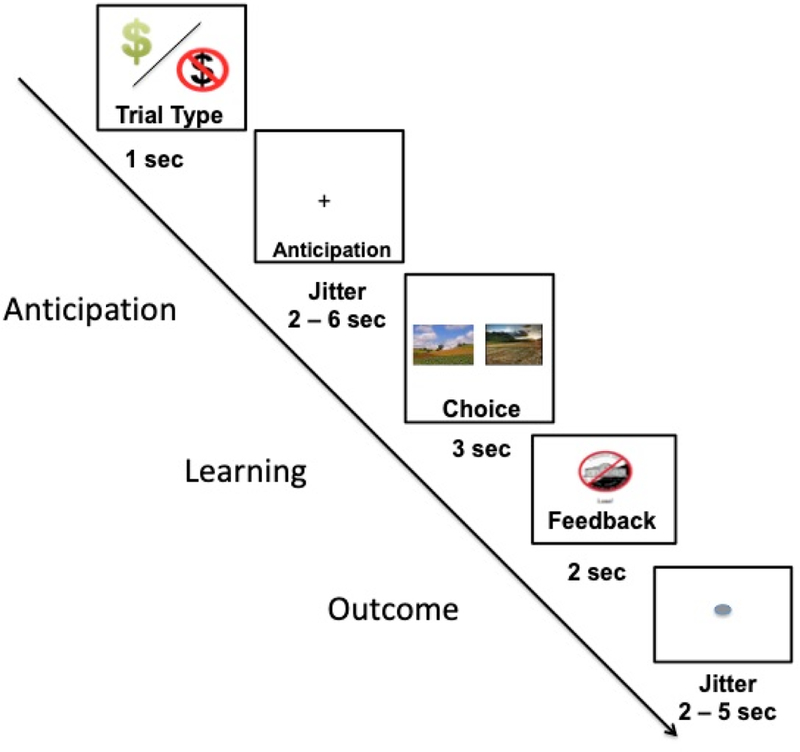
Schematic of a trial on the Probabilistic Incentive Learning Task completed during imaging.
Image Acquisition and Analysis
Images were acquired on a 3T Siemens Skyra system with a 32-channel head coil, which was customized and used for the Human Connectome Project (HCP). Structural scans (0.8 mm isotropic) as well as 4 functional runs of 407 frames using a multiband echo-planar sequence (TR=720ms, TE=33.1ms, flip angle =52°, 2.4 mm isotropic voxels, with a multi-band acceleration factor of 8). Each run was approximately 4 minutes and 30 seconds in length.
Imaging data was run through HCP minimal preprocessing pipelines (Glasser et al., 2013). Subsequently, data was analyzed using the Analysis of Functional Neuroimage software package (AFNI (Cox, 1996)). Binary masking was applied to each image to remove voxels outside the brain. The EPI datasets for each participant were smoothed using a 6-mm FWHM Gausian kernel to improve the signal to noise ratio. Six rigid body motion parameters were used as regressors to correct for motion. Movement estimates did not differ by group and were not associated with performance on RL task (rs<.13, ps>.15).
Each subject’s fMRI data was analyzed using a general linear model (GLM) in AFNI. GLM models included: two anticipatory cues (reward, loss),,4 choice outcomes (optimal choice during reward/loss; incorrect choice during reward/loss), and the 6 rigid body motion parameters with an assumed hemodynamic response (GAM function). We created contrasts comparing anticipation of reward - loss, and contrasts during choice comparing optimal choice - incorrect choice in both the reward and loss conditions. Given our a priori hypotheses, we conducted a Region of Interest (ROI) analysis in AFNI on the bilateral insula, anterior cingulate, caudate, and putamen. Regions of interest were defined using a single mask in standard MNI space and then applied to all individual EPI data. ROIs were defined using the AFNI Desai Atlas (Destrieux, Fischl, Dale, & Halgren, 2010). Mean percent signal change for each participant for each ROI and condition were extracted using the AFNI 3dmaskave program. Independent t-tests were computed to examine group differences in ROIs for each contrast of interest. Exploratory whole-brain analyses examining group differences in reward anticipation and relationships between whole-brain BOLD response and motivation and pleasure are presented in the supplement.
Behavioral and EMA Data Analysis
Behavioral Gambling task data was analyzed using a repeated measures ANOVA with Group (CON, SZ) as a between subject factor and within subject factors for Phase (anticipatory, consummatory) and Reward Level (high, low). Separate models were conducted for the reward and loss conditions. To analyze the behavioral data from the PILT scanner task we conducted a repeated measures ANOVA with Group as a between subject factor (CON, SZ) and within-subject factors for Condition (reward, loss) and Block (runs 1–4).
We used hierarchical linear modeling (HLM) in HLM 7.0 (Raudenbush, Bryk, Cheong, & Congdon, 2004) to investigate relationships of within-subject observations of EMA (Level 1) and between-subject observations (BOLD activity and task performance) (Level 2). We conducted separate models for BOLD activity within selected ROIs, PILT performance, and Anticipatory Pleasure Ratings (Level 2) to relate to daily anticipatory motivation and pleasure ratings as collected via EMA (Level 1). Finally, we conducted Spearman rank correlations between BOLD activity in selected ROIs and relationship to anticipatory ratings on the Gambling Task and clinical symptom ratings. False discovery rate corrections were used to correct for multiple comparisons (Benjamini & Hochberg, 1995).
Results
Gambling Task – Behavioral Testing Session
As shown in Figure 2, when examining self-reported pleasure during reward trials we observed significant main effects of Phase (greater consummatory pleasure; F(1,56)=43.44, p<.001, ηp2 = 45), Reward Level (greater pleasure to high reward conditions; F(1,56)=25.47, p<.001ηp2 =.32) and Group (greater overall pleasure by controls; F(1,56)=33.36, p<.001, ηp2 =38). These main effects were qualified by a Group x Phase interaction (F(1, 56)=11.10, p<.01, ηp2 =.17). Follow-up t-tests revealed significant between group differences in anticipated pleasure during reward such that SZ participants predicted less pleasure than healthy controls in both the low and high reward conditions (ts>3.60, ps<.001). There were no group differences in consummatory pleasure (ps>.11). When examining loss conditions we found a main effect of Phase (less consummatory pleasure to loss; (F(1,56)=18.39, p<.001, ηp2=24) and Reward Level (less pleasure during high loss; F(1,56)=4.29, p<.05, ηp2 = 07). There was no main effect of Group (F(1,56)=.82, p=.67) or interactions.
Figure 2.
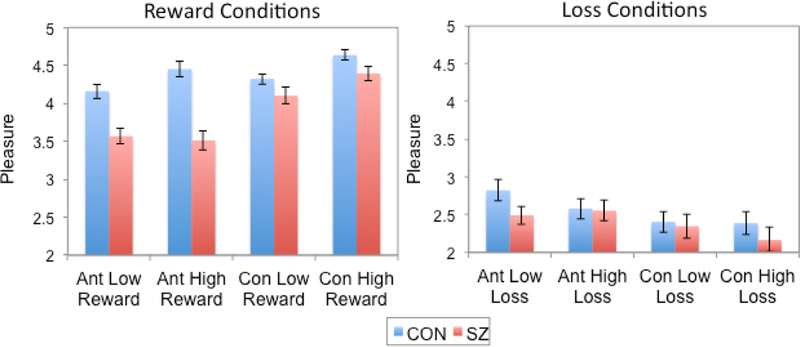
Self-reported pleasure ratings during reward and loss conditions of the Behavioral Gambling Task.
CON = control; SZ = schizophrenia; Ant Low = anticipated pleasure ratings on low conditions; Ant High = anticipated pleasure on high conditions; Con Low = consummatory pleasure ratings on low conditions; Con High = consummatory pleasure ratings on high conditions
When examining the relationship between self-reported anticipated pleasure on the Gambling Task with CAINS ratings, we found no significant relationship, suggesting that anticipated pleasure to either reward or loss was not related to clinician rated negative symptoms in SZ. However, as shown in Figure 3, we found that greater anticipated pleasure on reward trials of the Gambling task was related to greater daily anticipated motivation and pleasure as measured by EMA (b=.45, SE=.07, t=3.25, p<.05).
Figure 3.
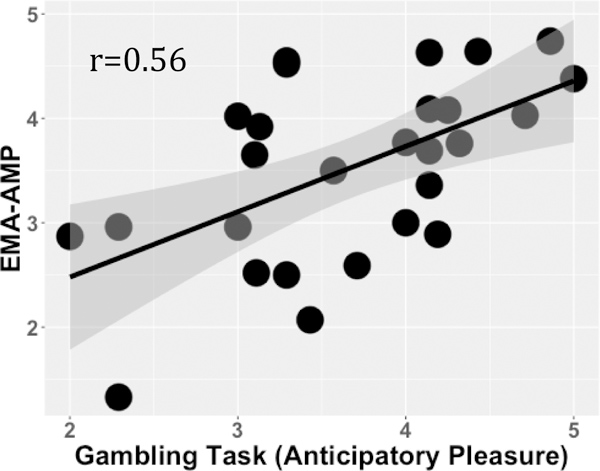
Graph illustrating the relationship between anticipated pleasure on reward trials of gambling task and mean daily anticipated motivation and pleasure ratings (EMA-AMP) in individuals with schizophrenia.
Probabilistic Incentive Learning Task – Imaging Session
Brain Response to Anticipation:
Independent samples t-tests comparing CON and SZ revealed significantly greater BOLD signal change for the anticipation of reward - punishment in CON relative to SZ participants (see Figure 4). Consistent with our hypotheses, we saw significant group differences in the anterior cingulate, caudate, insula, and putamen such that CON participants showed greater BOLD signal change during the anticipation of reward relative to SZ participants.
Figure 4.
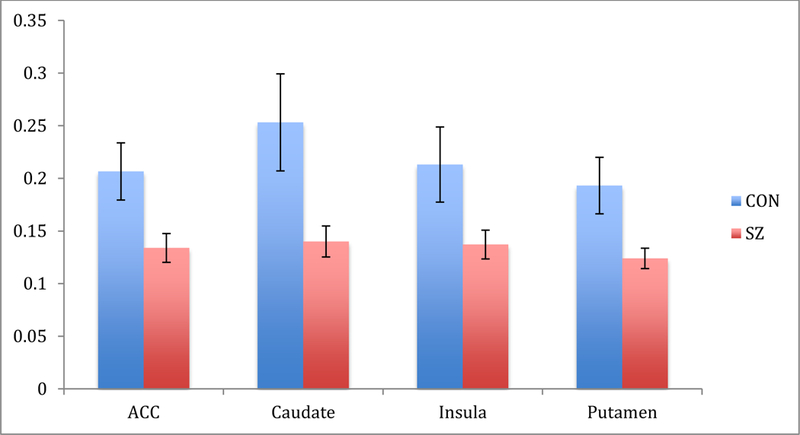
Percent signal change following cue predicting reward - loss during probabilistic incentive learning task (PILT) task in healthy control (CON) and schizophrenia participants (SZ) in apriori regions of interest.
Next, we examined the relationship between BOLD response during reward anticipation and CAINS-MAP ratings in individuals with schizophrenia. As shown in Figure 5, we found significant relationships between BOLD signal change during reward anticipation in the anterior cingulate (rs=−.43, p<.05) and insula (rs=−.46, p<.05) with CAINS-MAP ratings, suggesting that greater BOLD response during anticipation of reward was related to fewer clinician rated negative symptoms. We then examined the relationship between BOLD response during reward anticipation with daily ratings of anticipatory pleasure and motivation. BOLD response in the insula related to EMA-AMP scores, such that in individuals with SZ (b=.09, SE=.01, t=3.16, p<.01), greater signal change during reward anticipation related to greater anticipated motivation and pleasure in daily activities. Similarly, BOLD signal change in the anterior cingulate (b=.07, SE=.02, t=3.07, p<.05) and caudate (b=.06, t=2.93, p<.05) also related to EMA-AMP scores. Finally, in exploratory analyses, we examined the relationship between predicted pleasure as assessed on the Gambling task and BOLD response during reward anticipation on the RL task in the anterior cingulate, insula and caudate. We found that predicted pleasure on the gambling task was significantly related to BOLD response during reward anticipation in the anterior cingulate (rs=.51, p<.005), caudate (rs=.46, p<.05), and the insula (r=.43, p<.05).
Figure 5.
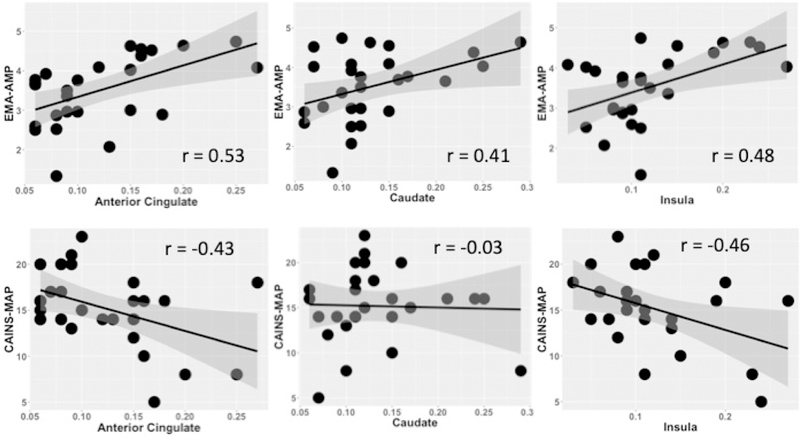
Graph illustrating relationship between BOLD signal during reward anticipation with mean daily anticipated motivation and pleasure (EMA-AMP) and clinician rated negative symptoms (CAINS-MAP) in individuals with schizophrenia.
Learning:
We observed significant main effects of Condition (better performance on reward conditions; F(1,56)=9.09, p<.005, ηp2 = 14) and Block (better performance over time (F(3,160)=7.78, p<.001, ηp2 = .13), but no Condition x Block interaction (F(3,54)=1.17, p=.32). Inconsistent with our hypothesis (Figure 6), there was no main effect of Group (F(1,54)=.03, p=.86), Group x Condition, or Group x Time interaction (ps>.10).
Figure 6.
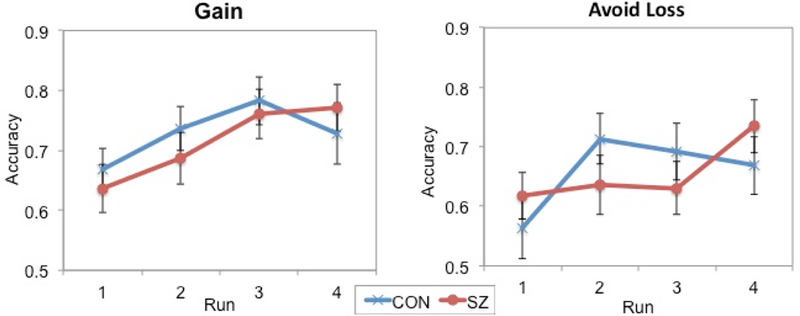
Performance on the reward learning (Gain) and loss avoidance (Avoid Loss) conditions across blocks of the Probabilistic Incentive Learning Task (PILT) demonstrating learning in control (CON) and individuals with schizophrenia (SZ).
However, as shown in Figure 7, we saw a significant correlation between ability to learn from reward and CAINS-MAP scores (rs=−.42, p<.05) suggesting that ability to learn from reward on the PILT was related to lower clinician rated negative symptoms. Moreover, accuracy on the reward condition of the PILT related to daily EMA-AMP ratings, suggesting that ability to learn from reward related to greater anticipated motivation and pleasure going about daily activities (b=.34, SE=.09, t=3.19, p<.01). As hypothesized, we saw no significant relationship between learning to avoid loss and clinician rated negative symptoms or EMA-AMP ratings (ps>.15).
Figure 7.
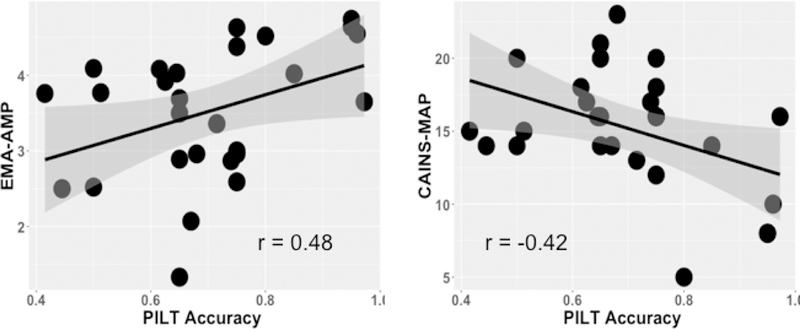
Graph illustrates relationship between accuracy on reward learning of the probabilistic incentive learning task (PILT) with mean daily anticipated motivation and pleasure ratings (EMA-AMP) ratings, and clinician rated negative symptoms (CAINS-MAP).
Choice Related Brain Activity:
Independent samples t-tests comparing CON and SZ on optimal choice vs. incorrect choice revealed no group differences for either the reward (ts<1.09, ps>.14) or loss (ts<1.19, ps>.18) condition. There were no significant relationships between BOLD signal change during choice and clinician rated negative symptoms. Moreover, we did not find a relationship between neural response during choice on the RL task and motivation and pleasure in daily life.
Relationship Between Anticipatory Responses and Learning
We conducted correlations to examine whether there was a relationship of either self-reported anticipation of reward or BOLD response during anticipation with average accuracy across all reward trials. There were no significant relationships (rs < .30; ps > .23) suggesting that anticipation of future reward and ability to learn from reward may be dissociable processes.
Relationship to Medication
Finally, we conducted correlations between chlorpromazine equivalents (CPZ) and measures of interest in the current study. First, we found no significant relationships between CPZ and behavior on the RL or Gambling tasks (rs<.17). Next, we found no relationship between BOLD signal change in ROIs and CPZ (rs<.19). Finally, CPZ did not relate to daily ratings of motivation and pleasure (b=−.08, SE=.05, t=−.98, p>.39) or clinician rated negative symptoms (CAINS-MAP, r=−.12, p=.64; CAINS-EXP, r = −.30, p=15).
Discussion
The goal of the current study was to examine two mechanisms related to motivational and pleasure deficits in schizophrenia: reward anticipation and reinforcement learning. Consistent with our hypotheses, whether examining self-reported anticipatory response or BOLD response during reward anticipation, we found that those with schizophrenia showed reduced response relative to controls. Further, specific deficits in anticipatory reward were linked to daily reports of anticipated motivation and pleasure. In regard to reinforcement learning, the present study confirms previous findings suggesting intact ability to learn to avoid loss in those with schizophrenia. We did not replicate previous research suggesting a deficit in either the behavior or neural response to reward learning in schizophrenia patients, though we did see an association between impaired reward learning and higher clinician related negative symptoms. The present findings suggest that higher negative symptom patients may show impairments in anticipating rewards and in reward learning relative to those with lower negative symptoms. Each of these findings is discussed in detail below.
Consistent with our hypotheses, we found evidence for impairments in reward anticipation in individuals with schizophrenia across multiple methods of measurement. For example, self-reported anticipatory pleasure to rewards on a laboratory task was reduced relative to controls. Further, when examining BOLD response, we found reduced activation in striatal regions and insula during the anticipation of reward among individuals with schizophrenia relative to controls. Our findings extend this literature by demonstrating this hypoactivation during reward anticipation in a task other than the commonly used MID task. Further, we found this altered response to reward anticipation in chronic patients with schizophrenia taking a mixture of typical and atypical antipsychotics, providing evidence consistent with a number of other studies in the literature (Li et al., 2018; Mucci et al., 2015; Simon et al., 2010) though not in others (Juckel, Schlagenhauf, Koslowski, Filonov, et al., 2006; Nielsen, Rostrup, Wulff, Bak, Lublin, et al., 2012). Taken together, these results add to the evidence for deficits in both the experience of anticipatory pleasure and neural activation associated with reward anticipation in schizophrenia.
We assessed motivation in schizophrenia via clinical interview and via EMA assessments of motivation and pleasure in daily life. We found that anticipatory responses (self-report and BOLD response in caudate, insula and anterior cingulate) related to daily anticipated motivation and pleasure in individuals with schizophrenia. These findings are consistent with prior work that has linked anticipatory pleasure with clinician ratings of negative symptoms and functioning (Juckel, Schlagenhauf, Koslowski, Wustenberg, et al., 2006; Li et al., 2018; Moran & Kring, 2018; Mucci et al., 2015; Simon et al., 2010). However, some other studies did not find this relationship (Esslinger et al., 2012; Nielsen, Rostrup, Wulff, Bak, Lublin, et al., 2012). It may be that mixed findings in the literature are due in part to the type of assessment of negative symptoms. For example, some studies relate anticipatory responses to dissociable measures of motivation and anhedonia (Arrondo et al., 2015; Kirschner et al., 2016; Mucci et al., 2015; Simon et al., 2010; Stepien et al., 2018; Subramaniam et al., 2015), while others examine the relationship with total negative symptoms (i.e., deficits in motivation, pleasure, and expression/alogia) (O Grimm et al., 2012; Juckel, Schlagenhauf, Koslowski, Filonov, et al., 2006; Juckel, Schlagenhauf, Koslowski, Wustenberg, et al., 2006; Nielsen, Rostrup, Wulff, Bak, Lublin, et al., 2012; Schlagenhauf et al., 2008). Our findings suggest that motivation and pleasure, and perhaps anticipatory motivation and pleasure in particular, are distinctly related to reward anticipation responses.
While the current findings point to deficits in anticipation of future monetary reward and relate these deficits to daily life anticipation, we did not examine whether group differences in anticipatory pleasure extend to events in daily life. It may be that anticipation to other domains may be intact. In fact, EMA studies are mixed as to whether these deficits are seen in daily activities. For example, Gard and colleagues (2007) found reductions in anticipated pleasure relative to controls (Gard, Kring, Gard, Horan, & Green, 2007). However, in a follow-up study, Gard and colleagues (2014) showed that when asked to identify short-term goals and predict how much pleasure they will experience, people with schizophrenia showed elevated anticipatory pleasure relative to healthy controls (Gard et al., 2014). It may be that the process of generating these self-relevant goals allows individuals with schizophrenia to have a clear view of the goal in mind, which in turn may help in anticipating their future pleasure. It will be important for future research to examine group differences in anticipatory responses to every day life and to relate those back to laboratory measures of anticipatory reward including self-report and neural responses to get a clearer picture of how far these anticipatory deficits may extend.
The second mechanism investigated in the current study was reinforcement learning. The current findings support previous research suggesting that people with schizophrenia are able to learn to avoid loss (Gold et al., 2012; Juckel, Schlagenhauf, Koslowski, Filonov, et al., 2006; Strauss, Frank, et al., 2011; Waltz et al., 2007) thus arguing against an overall learning deficit in schizophrenia. Instead, people with schizophrenia appear to be sensitive to loss and have the ability to use this loss to guide their behavior to avoid continued loss. Indeed, findings from the present study suggest that both anticipatory responses to loss and ability to learn from loss are intact in schizophrenia and unrelated to motivation and pleasure deficits measured via clinician ratings or EMA ratings.
In contrast with our hypothesis, we did not see a deficit in the ability to learn from reward in the schizophrenia group as a whole. Instead, across both brain and behavior, we saw a similar pattern of findings in patients and controls. These findings are inconsistent with previous research, even research using a similar paradigm (Barch et al., 2017; Gold et al., 2012; Hartmann-Riemer et al., 2017). However, a number of prior studies analyzed data looking independently at schizophrenia groups based on high and low negative symptoms. Given the smaller sample size in the present study we were unable to examine whether those with negative symptoms showed a deficit in learning from reward. However, we found that individual differences in the ability to learn from reward significantly related to both clinician related motivation and pleasure symptoms and daily anticipatory motivation and pleasure deficits as measured by EMA among patients. Thus, the current findings, along with previous research, may suggest that reward learning is not impaired across all individuals with schizophrenia, rather it is an impairment seen in those who report greater impairments in motivation and pleasure. It may also be the case that the addition of cues in the current study, alerting individuals to potential reward or loss conditions, helped participants with schizophrenia to focus and learn the pictures more rapidly than in previous studies, potentially more so for those patients with fewer motivation and pleasure negative symptoms. Thus, despite having deficits in their anticipatory responses, the predictive cues may have given individuals with schizophrenia contextual support that aided in learning. This hypotheses is consistent with the fact that we did not see associations between behavioral or bold responses to anticipation and reward learning in this sample.
The present study had several limitations. First, the majority of our schizophrenia participants were taking antipsychotic (typical and atypical) medications, which influence dopamine, known to be important for both anticipatory responses and RL. While we did not find relationships between CPZ equivalents and task, brain response, or clinician symptom ratings, this does not rule out the possible impact medications may have had on the current findings. Second, while we had the power to detect significant relationships, our sample size was modest and would benefit from additional participants. This is especially notable given that we saw a relationship between negative symptoms and learning performance, but failed to find a group difference in learning. Indeed, in both the current study and work by Gold (2012), deficits in learning may be closely linked with negative symptoms and require larger samples to examine this. The current study had a reasonable range of negative symptom severity similar to that found in many previous studies (Barch et al., 2017; Catalano, Heerey, & Gold, 2018; Llerena, Wynn, Hajcak, Green, & Horan, 2016; McCarthy, Treadway, Bennett, & Blanchard, 2016; Moran et al., 2017; Moran & Kring, 2018; Reddy et al., 2015). Nonetheless, targeted recruitment of high and low negative symptom groups would allow us to better examine whether RL is a common deficit in schizophrenia or if it’s more tightly linked to those with significant motivational and pleasure deficits. Third, we did not model the learning data using reinforcement learning models, though the pattern of data shown in Figure 7 does not suggest that such modeling would reveal behavioral deficits and our prior work in two large samples did not find that using reinforcement learning models revealed changes in brain activation that were not present in more standard analyses (Culbreth et al., 2017). Finally, controls in the current study did not complete EMA ratings thus we were unable to examine whether relationships between neural response, behavioral task data, and daily ratings are seen in controls. Moreover, we are unable to examine potential group differences in anticipation during daily life.
Taken together, the current findings provide further evidence for impairments in anticipatory response to reward in individuals with schizophrenia across both self-report and neural response in striatal regions and the insula. Moreover, these anticipatory responses were linked to the motivation and pleasure individuals with schizophrenia anticipate when going about their daily activities. While we did not find group differences in the brain or behavior during reward learning, we did see that motivation and pleasure in daily life related to ability to learn from reward on our RL task. Thus, suggesting that reward learning may be preserved in those patients with low negative symptoms. These findings represent an important step towards identifying mechanisms related to motivational and emotional functioning in the daily lives of individuals with schizophrenia.
Supplementary Material
Acknowledgments
We thank the participants in this study who gave generously of their time. We also thank those that helped with all aspects of data collection including Julia M. Sheffeld, Maria Gehred, Lori Ingram, Anita Mahadevan, Lisa Gorham, and Callan Coghlan. Parts of this article have been reported in a presentation at the Society for Research in Psychopathology conference.
Funding: NIMH R37-MH066031
Footnotes
Financial Disclosures: Dr. Moran, Mr. Culbreth, Mr. Kandala and Dr. Barch report no conflicts of interest.
References:
- American Psychiatric Association. (2000). DSM-IV. Diagnostic and Statistical Manual of Mental Disorders 4th edition TR. [Google Scholar]
- Arrondo G, Segarra N, Metastasio A, Ziauddeen H, Spencer J, Reinders NR, … Murray GK. (2015). Reduction in ventral striatal activity when anticipating a reward in depression and schizophrenia: a replicated cross-diagnostic finding. Frontiers in Psychology, 6, 1280 10.3389/fpsyg.2015.01280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker JM, Goossens L, Kumar P, Lange IMJ, Michielse S, Schruers K, … Wichers M. (2018). From laboratory to life: associating brain reward processing with real-life motivated behaviour and symptoms of depression in non-help-seeking young adults. Psychological Medicine, 1–11. 10.1017/S0033291718003446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Gold JM, Johnson SL, Kring AM, MacDonald AW III, … Strauss ME. (2017). Explicit and implicit reinforcement learning across the psychosis spectrum. Journal of Abnormal Psychology, 126(5), 694–711. 10.1037/abn0000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, & Dowd EC (2010). Goal representations and motivational drive in schizophrenia: The role of prefrontal-striatal interactions. Schizophrenia Bulletin, 36(1), 919–934. 10.1093/schbul/sbq068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B (Methodological), 57(1), 289–300. https://doi.org/10.1111yj.2517-6161.1995.tb02031.x [Google Scholar]
- Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, … Carpenter WT. (2007). The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): The Efficacy of Glutamatergic Agents for Negative Symptoms and Cognitive Impairments. American Journal of Psychiatry, 164(10), 1593–1602. 10.1176/appi.ajp.2007.06081358 [DOI] [PubMed] [Google Scholar]
- Catalano LT, Heerey EA, & Gold JM (2018). The valuation of social rewards in schizophrenia. Journal of Abnormal Psychology, 127(6), 602–611. 10.1037/abn0000366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero DC, Martin EA, Becker TM, & Kerns JG (2014). Reinforcement learning deficits in people with schizophrenia persist after extended trials. Psychiatry Research, 220(3), 760–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, & Minor KS (2010). Emotional experience in patients with schizophrenia revisited: Meta-analysis of laboratory studies. Schizophrenia Bulletin, 36(1), 143–150. 10.1093/schbul/sbn061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research, 29(3), 162–173. [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, & Taylor PA (2017). FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connectivity, 7(3), 152–171. 10.1089/brain.2016.0475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbreth AJ, Westbrook A, Xu Z, Barch DM, & Waltz JA (2017). Intact Ventral Striatal Prediction Error Signaling in Medicated Schizophrenia Patients. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 1(5), 474–483. https://doi.org/10.1016Zj.bpsc.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw M, Kahn RS, & Vink M (2014). Fronto-striatal Dysfunction During Reward Processing in Unaffected Siblings of Schizophrenia Patients. Schizophrenia Bulletin, 41, 94–103. 10.1093/schbul/sbu153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, & Fiez JA (2000). Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology, 84(6), 3072–3077. Retrieved from http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=11110834&retmode=ref&cmd=prlinks [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, & Halgren E (2010). Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage, 53(1), 1–15. 10.1016/J.NEUROIMAGE.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd EC, & Barch DM (2012). Pavlovian reward prediction and receipt in schizophrenia: Relationship to anhedonia. PLoS ONE, 7(5), 1–12. 10.1371/journal.pone.0035622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd EC, Frank MJ, Collins A, Gold JM, & Barch DM (2016). Probabilistic Reinforcement Learning in Patients With Schizophrenia: Relationships to Anhedonia and Avolition. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 1(5), 460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esslinger C, Englisch S, Inta D, Rausch F, Schirmbeck F, Mier D, … Zink M. (2012). Ventral striatal activation during attribution of stimulus saliency and reward anticipation is correlated in unmedicated first episode schizophrenia patients. Schizophrenia Research, 140(1), 114–121. [DOI] [PubMed] [Google Scholar]
- Farkas M, Polgár P, Kelemen O, Réthelyi J, Bitter I, Myers CE, … Kéri S.(2008). Associative learning in deficit and nondeficit schizophrenia. NeuroReport, 19(1), 55–58. 10.1097/WNR.0b013e3282f2dff6 [DOI] [PubMed] [Google Scholar]
- Fervaha G, Graff-Guerrero A, Zakzanis KK, Foussias G, Agid O, & Remington G (2013). Incentive motivation deficits in schizophrenia reflect effort computation impairments during cost-benefit decision-making. Journal of Psychiatric Research, 47(11), 1590–1596. 10.1016/jjpsychires.2013.08.003 [DOI] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, … Dahl RE. (2009). Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry, 166(1), 64–73. 10.1176/appi.ajp.2008.07081336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, & Claus ED (2006). Anatomy of a decision: Striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychological Review, 113(2), 300–326. 10.1037/0033-295X.113.2.300 [DOI] [PubMed] [Google Scholar]
- Gard DE, Gard MG, Kring AM, & John OP (2006). Anticipatory and consummatory components of the experience of pleasure: A scale development study. Journal of Research in Personality, 40(6), 1086–1102. 10.1016/j.jrp.2005.11.001 [DOI] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, & Green MF (2007). Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schizophrenia Research, 93(1–3), 253–260. https://doi.org/10.1016Zj.schres.2007.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Sanchez AH, Cooper K, Fisher M, Garrett C, & Vinogradov S (2014). Do people with schizophrenia have difficulty anticipating pleasure, engaging in effortful behavior, or both? Journal of Abnormal Psychology, 123(4), 771–782. 10.1037/abn0000005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, … Jenkinson M. (2013). The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage, 80, 105–124. 10.1016/j.neuroimage.2013.04.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz J. a., Matveeva TM, Kasanova Z, Strauss GP, Herbener ES, … Frank MJ. (2012). Negative symptoms and the failure to represent the expected reward value of actions: behavioral and computational modeling evidence. Archives of General Psychiatry, 69(2), 129–138. 10.1001/archgenpsychiatry.2011.1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, & Heerey EA (2008). Reward Processing in Schizophrenia: A Deficit in the Representation of Value. Schizophrenia Bulletin, 34(5), 835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm O, Vollstädt-Klein S, Krebs L, Zink M, & Smolka MN (2012). Reduced striatal activation during reward anticipation due to appetite-provoking cues in chronic schizophrenia: A fMRI study. Schizophrenia Research, 134(2), 151–157. [DOI] [PubMed] [Google Scholar]
- Grimm Oliver, Heinz A., Walter H., Kirsch P., Erk S., Haddad L., … Meyer-Lindenberg A. (2014). Striatal Response to Reward Anticipation. JAMA Psychiatry, 71(5), 531 10.1001/jamapsychiatry.2014.9 [DOI] [PubMed] [Google Scholar]
- Hanssen E, van der Velde J, Gromann PM, Shergill SS, de Haan L, Bruggeman R, … van Atteveldt N. (2015). Neural correlates of reward processing in healthy siblings of patients with schizophrenia. Frontiers in Human Neuroscience, 9, 504 10.3389/fnhum.2015.00504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann-Riemer MN, Aschenbrenner S, Bossert M, Westermann C, Seifritz E, Tobler PN, … Kaiser S. (2017). Deficits in reinforcement learning but no link to apathy in patients with schizophrenia. Scientific Reports, 7, 40352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Filonov D, Wüstenberg T, Villringer A, . Heinz A. (2006). Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology, 187(2), 222–228. 10.1007/s00213-006-0405-4 [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Wüstenberg T, Villringer A, Knutson B, … Heinz A. (2006). Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage, 29(2), 409–416. https://doi.org/10.1016Zj.neuroimage.2005.07.051 [DOI] [PubMed] [Google Scholar]
- Kasanova Z, Ceccarini J, Frank MJ, Amelsvoort T. van Booij J, Heinzel A., … Myin-Germeys I (2017). Striatal dopaminergic modulation of reinforcement learning predicts reward—oriented behavior in daily life. Biological Psychology, 127, 1–9. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Ronshausen S, Mier D, & Gallhofer B (2007). The Influence of Antipsychotic Treatment on Brain Reward System Reactivity in Schizophrenia Patients. Pharmacopsychiatry, 40(5), 196–198. 10.1055/s-2007-984463 [DOI] [PubMed] [Google Scholar]
- Kirschner M, Hager OM, Bischof M, Hartmann MN, Kluge A, Seifritz E, … Kaiser S. (2016). Ventral striatal hypoactivation is associated with apathy but not diminished expression in patients with schizophrenia. Journal of Psychiatry & Neuroscience: JPN, 41(3), 152 10.1503/JPN.140383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluge A, Kirschner M, Hager OM, Bischof M, Habermeyer B, Seifritz E, … Kaiser S. (2018). Combining actigraphy, ecological momentary assessment and neuroimaging to study apathy in patients with schizophrenia. Schizophrenia Research, 195, 176–182. 10.1016/J.SCHRES.2017.09.034 [DOI] [PubMed] [Google Scholar]
- Kring AM, & Barch DM (2014). The motivation and pleasure dimension of negative symptoms: Neural substrates and behavioral outputs. European Neuropsychopharmacology, 24(5), 725–736. 10.1016/j.euroneuro.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Gur RE, Blanchard JJ, Horan WP, & Reise SP (2013). The Clinical Assessment Interview for Negative Symptoms (CAINS): Final development and validation. American Journal of Psychiatry, 170(2), 165–172. 10.1176/appi.ajp.2012.12010109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, & Moran EK (2008). Emotional response deficits in schizophrenia: Insights from affective science. Schizophrenia Bulletin, 34(5), 819–834. 10.1093/schbul/sbn071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Siegel EH, & Barrett LF (2014). Unseen Affective Faces Influence Person Perception Judgments in Schizophrenia. Clinical Psychological Science, 2(4), 443–454. 10.1177/2167702614536161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Yan C, Lv Q, Yi Z, Zhang J, Wang J, … Chan RCK. (2018). Striatal dysfunction in patients with schizophrenia and their unaffected first-degree relatives. Schizophrenia Research, 195, 215–221. [DOI] [PubMed] [Google Scholar]
- Llerena K, Wynn JK, Hajcak G, Green MF, & Horan WP (2016). Patterns and reliability of EEG during error monitoring for internal versus external feedback in schizophrenia. International Journal of Psychophysiology, 105, 39–46. 10.1016/J.IJPSYCHO.2016.04.012 [DOI] [PubMed] [Google Scholar]
- McCarthy JM, Treadway MT, Bennett ME, & Blanchard JJ (2016). Inefficient effort allocation and negative symptoms in individuals with schizophrenia. Schizophrenia Research. https://doi.org/10.1016Zj.schres.2015.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, & Berns GS (2002). Neural Economics and the Biological Substrates of Valuation. Neuron, 36(2), 265–284. [DOI] [PubMed] [Google Scholar]
- Moran EK, Culbreth AJ, & Barch DM (2017). Ecological momentary assessment of negative symptoms in schizophrenia: Relationships to effort-based decision making and reinforcement learning. Journal of Abnormal Psychology, 126(1), 96–105. 10.1037/abn0000240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran EK, & Kring AM (2018). Anticipatory Emotion in Schizophrenia. Clinical Psychological Science, 6(1), 63–75. 10.1177/2167702617730877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mote J, Minzenberg MJ, Carter CS, & Kring AM (2014). Deficits in anticipatory but not consummatory pleasure in people with recent-onset schizophrenia spectrum disorders. Schizophrenia Research, 159(1), 76–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa AA, Kéri S, Somlai Z, Balsdon T, Frydecka D, Misiak B, & White C(2015). Drift diffusion model of reward and punishment learning in schizophrenia: Modeling and experimental data. Behavioural Brain Research, 291, 147–154. [DOI] [PubMed] [Google Scholar]
- Mucci a., Dima D., Soricelli A., Volpe U., Bucci P., Frangou S., … Maj M. (2015). Is avolition in schizophrenia associated with a deficit of dorsal caudate activity? A functional magnetic resonance imaging study during reward anticipation and feedback. Psychological Medicine, 1–14. 10.1017/S0033291714002943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray GK, Cheng F, Clark L, Barnett JH, Blackwell AD, Fletcher PC, … Jones PB. (2008). Reinforcement and Reversal Learning in First-Episode Psychosis. Schizophrenia Bulletin, 34(5), 848–855. 10.1093/schbul/sbn078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myin-Germeys I, van Os J, Schwartz JE, Stone AA, & Delespaul PA (2001). Emotional reactivity to daily life stress in psychosis. Archives of General Psychiatry, 58(12), 1137–1144. 10.1001/archpsyc.58.12.1137 [DOI] [PubMed] [Google Scholar]
- Nielsen MO, Rostrup E, Wulff S, Bak N, Broberg BV, Lublin H, … Glenthoj B. (2012). Improvement of Brain Reward Abnormalities by Antipsychotic Monotherapy in Schizophrenia. Archives of General Psychiatry, 69(12), 1195 10.1001/archgenpsychiatry.2012.847 [DOI] [PubMed] [Google Scholar]
- Nielsen MO, Rostrup E, Wulff S, Bak N, Lublin H, Kapur S, & Glenthoj B (2012). Alterations of the Brain Reward System in Antipsychotic Naive Schizophrenia Patients. Biological Psychiatry, 71(10), 898–905. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP (2004). Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology, 14(6), 769–776. [DOI] [PubMed] [Google Scholar]
- Overall JE, & Gorham DR (1962). The brief psychiatric rating scale. Psychological Reports, 10(3), 799–812. 10.2466/pr0.1962.m3.799 [DOI] [Google Scholar]
- Radua J, Schmidt A, Borgwardt S, Heinz A, Schlagenhauf F, McGuire P, & Fusar-Poli P (2015). Ventral striatal activation during reward processing in psychosis a neurofunctional meta-analysis. JAMA Psychiatry, 72(12), 1243–1251. 10.1001/jamapsychiatry.2015.2196 [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS, Cheong YF, & Congdon RT (2004). HLM 6: Hierarchical linear and nonlinear modeling. 2008. [Google Scholar]
- Reddy LF, Horan WP, Barch DM, Buchanan RW, Dunayevich E, Gold JM, … Green MF. (2015). Effort-based decision-making paradigms for clinical trials in schizophrenia: Part 1 - Psychometric characteristics of 5 paradigms. Schizophrenia Bulletin, 41(5), 1045–1054. 10.1093/schbul/sbv089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinen JM, Van Snellenberg JX, Horga G, Abi-Dargham A, Daw ND, & Shohamy D (2016). Motivational Context Modulates Prediction Error Response in Schizophrenia. Schizophrenia Bulletin, 42(6), 1467–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlagenhauf F, Huys QJM, Deserno L, Rapp M. a., Beck A, Heinze H-JJ, … Heinz A. (2014). Striatal dysfunction during reversal learning in unmedicated schizophrenia patients. NeuroImage, 89, 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlagenhauf F, Juckel G, Koslowski M, Kahnt T, Knutson B, Dembler T, … Heinz A. (2008). Reward system activation in schizophrenic patients switched from typical neuroleptics to olanzapine. Psychopharmacology, 196(4), 673–684. 10.1007/s00213-007-1016-4 [DOI] [PubMed] [Google Scholar]
- Schlagenhauf F, Sterzer P, Schmack K, Ballmaier M, Rapp M, Wrase J, … Heinz A. (2009). Reward Feedback Alterations in Unmedicated Schizophrenia Patients: Relevance for Delusions. Biological Psychiatry, 65(12), 1032–1039. [DOI] [PubMed] [Google Scholar]
- Schlosser D.a, Fisher M., Gard D., Fulford D., Loewy RL., & Vinogradov S. (2014). Motivational deficits in individuals at-risk for psychosis and across the course of schizophrenia. Schizophrenia Research, 158(1–3), 52–57. https://doi.org/10.1016Zj.schres.2014.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, & Montague PR (1997). A Neural Substrate of Prediction and Reward. Science, 275(5306), 1593–1599. [DOI] [PubMed] [Google Scholar]
- Simon JJ, Biller A, Walther S, Roesch-Ely D, Stippich C, Weisbrod M, & Kaiser S (2010). Neural correlates of reward processing in schizophrenia — Relationship to apathy and depression. Schizophrenia Research, 118(1), 154–161. [DOI] [PubMed] [Google Scholar]
- Stepien M, Manoliu A, Kubli R, Schneider K, Tobler PN, Seifritz E, … Kirschner M. (2018). Investigating the association of ventral and dorsal striatal dysfunction during reward anticipation with negative symptoms in patients with schizophrenia and healthy individuals. PLOS ONE, 13(6), e0198215. 10.1371/journal.pone.0198215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Frank MJ, Waltz JA, Kasanova Z, Herbener ES, & Gold JM (2011). Deficits in positive reinforcement learning and uncertainty-driven exploration are associated with distinct aspects of negative symptoms in schizophrenia. Biological Psychiatry, 69(5), 424–431. https://doi.org/10.1016Zj.biopsych.2010.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Wilbur RC, Warren KR, August SM, & Gold JM (2011). Anticipatory vs. consummatory pleasure: What is the nature of hedonic deficits in schizophrenia? Psychiatry Research, 187, 36–41. 10.1016/j.psychres.2011.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam K, Hooker CI, Biagianti B, Fisher M, Nagarajan S, & Vinogradov S. (2015). Neural signal during immediate reward anticipation in schizophrenia: Relationship to real-world motivation and function. NeuroImage: Clinical, 9, 153–163. 10.1016/J.NICL.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Frank MJ, Robinson BM, & Gold JM (2007). Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biological Psychiatry, 62(7), 756–764. 10.1016/j.biopsych.2006.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2001). Wechsler: Wechsler Test of Adult Reading: WTAR. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wynn JK, Horan WP, Kring AM, Simons RF, & Green MF (2010). Impaired anticipatory event-related potentials in schizophrenia. International Journal of Psychophysiology, 77(2), 141–149. 10.1016/j.ijpsycho.2010.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


